- 1Department of Biosciences, COMSATS University Islamabad, Islamabad, Pakistan
- 2Biofuel and Biodiversity Lab, Department of Plant Sciences, Quaid-i-Azam University, Islamabad, Pakistan
Introduction: In the current study, Lepidium apetalum oil (LAO) was identified as a novel source of feedstock for the production of methyl esters. The study aimed to find out the conversion rate of Lepidium apetalum seed oil into methyl esters using different variables, to get the most amounts possible.
Methods: In general, the novel sulfur doped nano-photocatalyst was synthesized and used in the most efficient way possible. Oil to methanol molar ratio, catalyst concentration and reaction temperature, were all used as variables.
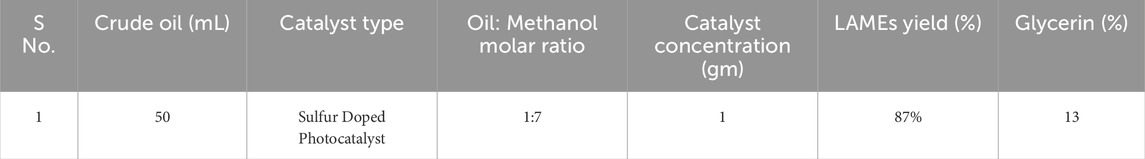
Results: The best yield Obtained was 92 percent Using 0.5 gm sulfur doped nano-photocatalyst, a temperature of 60°C, and a 1:6 molar ratio of oil to methanol.
Discussion: The methyl esters were characterized using GCMS, NMR, and Atomic Absorption Spectrum analytical techniques for structural and chemical analysis. The Color, density, flash point, cloud point, kinematic viscosity, sulfur content, pour point, and acid number of Lepidium apetalum methyl esters (LAMEs) were calculated and compared to ASTM standards.
1 Introduction
Because of rapid population and industrial growth in developing countries, global energy demand is projected to rise. Natural oil, coal, and natural gas are actually the most important energy options for meeting energy demand. The International Energy Agency’s statistical data also shows that traditional energy resources, such as fossil fuels, account for roughly 80% of total primary energy supply (Rizwanul Fattah et al., 2020; Passoth and Sandgren, 2019; Alavijeh et al., 2020).
Traditional energy sources are seen as important drivers of economic growth, but they are harmful to human health and the climate. A dramatic increase in greenhouse gas levels has resulted in the continued rise in fossil fuel consumption in the earth’s atmosphere over the last century. The depletion of natural energy supplies and the volatility of the oil market rekindled society’s interest in finding alternative fuels. In such conditions, our reliance on petro-carbon would not only result in a rise in environmental pollution, but also in a rapid increase in energy costs and foreign fuel price uncertainty.
Fossil, nuclear, and fissile fuels are the three main energy sources. Coal, Natural gas, oil shale, petroleum, and tar sands are some of the examples of fossil fuel sources. Wind, Biomass, solar, hydropower, and geothermal are all recorded sources of renewable energy. The fissile fuel sources are thorium and uranium which are well recognized. Since fossil fuels are rapidly depleting, we urgently need to find alternative energy sources to meet demand. Biodiesel is gaining interest as a possible substitute fuel (Moses et al., 2019).
Lepidium apetalum, also known as Pepper Cress or Lepidium ruderalectau, belongs to the Brassicaceae family (Raza et al., 2020). It comes from Europe originally, although it can be found all over the world in temperate climates. It grows up to 0.3 m (1 foot) tall and can be found at elevations of 400–4,800 m along hills, roadsides, ravines, waste sites, fields, and plains. Lepidium apetalum is a branched plant with minute hairs that can be glabrous or sparsely hairy. Uppermost leaves are linear or sub-entire, with short or narrow lobes on the basal and lower leaves. All leaves can be linear, short-lobed, or toothed in some cases. Almost every member of the family has a cruciform corolla, six stamens in a tetradynamous design and 20–30 flowering racemes of tiny white flowers. The flowers of Lepidium apetalum are hermaphrodite, meaning they have both male and female organs. They bloom from April to August. Reddish brown seeds of the Lepidium plant are used for oil extraction, which ripen between May and September. The seed fatty oil content is 40%, and it is renowned for being rich in unsaturated fatty acids (up to 70%) (Raza et al., 2020; Bhatia et al., 2020; Pattanaik et al., 2019; Al-Shehbaz et al., 2006; Jafri, 2015).
Currently, one solution is to find new biofuel feedstocks quickly, while another is to use new nano-catalyst to improve the performance of fatty acid methyl esters. Nano-catalysts have recently become more common as a result of their use in a variety of industries. Because of their large surface area and inability to use surface energy, base-catalysts are not suitable for catalysis. As a result of its influential catalytic operations, photo-catalyst has captivated the modern age. When two metals are alloyed, the catalytic activities of the photocatalyst are extremely strong. Because of the synergic influence of two metals, the combination of two metals took on a new characteristic. For the present study, sulfur-dope photo-catalyst was made in biofuel lab at Quaid-i-Azam University, Islamabad and National Centre for Physics, Islamabad to bring a change in conventional transesterification process by changing the nature of the catalyst. The stability and activities of a sulfur doped nano-photocatalyst in the synthesis of paper cress energy crop methyl esters were investigated in this study. In the energy crop paper cress methyl esters development, the novel nano-photocatalyst demonstrated optimum catalytic activities (Nehdi et al., 2012; Youzhi et al., 2016).
For the development of methyl esters, paper cress oil is a new source. Nehdi and co-workers, on the other hand, worked with Lepidium sativum oil and converted it to biodiesel at a rate of 96.8% (Nehdi et al., 2012). This finding has prompted the selection of Lepidium apetalum in order to determine its suitability for biodiesel processing. Locals are familiar with paper cress oil, but they are unaware of its biodiesel production potential. This energy crop thrives in non-productive soil and in saline, waterlogged soil. Furthermore, it is a non-edible energy crop with no effect on food security. In light of the above; the proposed energy crop appears to be a reasonable option with a lot of potential in the bioenergy industry (Pattanaik et al., 2019; Al-Shehbaz et al., 2006).
2 Materials and methods
2.1 Chemicals and reagents
In the present work, the chemicals and reagents used were chloroform (CHCL3), glycerin jelly, hydrochloric acid (HCL), isopropanol (C6H14), lactic acid (C3H6O3), nitric acid (HNO3), Molecular sieve (Na2O.Al2O3.2SiO2.9/2H2O gel), perchloric acid (HCLO4) and oxylic acid (C2H2O4). Analytical grade chemicals were used mainly in the experiments from Scharlau (Spain), Merk (Germany), and Sigma-Aldrich (US) (USA).
2.2 Oil extraction analysis
The primary step in making methyl esters is extracting oil from the feedstock source. Chemical and mechanical extraction methods were employed in the current methodology. Deionized and warmed water was used to wash the seed samples at 25°C to remove the impurities and dust particles It is then dried at the room temperature before oil extraction.
2.2.1 Mechanical oil extraction method
The dry seeds of the feedstock were washed thoroughly to eliminate impurities before being used in the mechanical oil extraction process. For oil extraction through mechanical means (KEK P0015, 10,127 Germany), the seeds were openly dried in the presence of sunlight and then processed. Physically, 5 L of crude oil were obtained from 13 kg of feedstock seeds after five rounds of continuous rotation.
2.2.2 Chemical oil extraction method
The oil expression in the feedstock is measured using the chemical oil extraction process. 3–5 gm paper cress seeds were dried in an oven at 60°C for 2–3 h. The dried seeds were crushed and turned into powder by using simple mortar and pestle. In a thimble of soxhlet, the powder was placed. A round bottom flask of soxhlet was used to add 250 mL petroleum ether as a solvent and was heated to 60°C. The solvent begins to boil, and the vapors come up into the soxhlet column. The solvent vapors were concentrated and lowered on the powder in the thimble by a condenser at the top of the soxhlet. Pores of the thimble allow the seepage of the dissolved oil contents to syphon tube and eventually into the round bottom flask. This procedure was carried out for a total of 9 hours. The material’s powder was then removed from the soxhlet and dried for 3 hours at 60°C to evaporate the solvent. Oil content was calculated Using Equation 1 (Al-Shehbaz et al., 2006; Jafri, 2015).
W1 = Weight of empty flask.
W2 = Sample powder + weight of empty flask.
W3 = Denotes the weight of sample.
W4 = Extraction oil weight.
2.3 Photocatalyst application status
In this method, with the presence of a new sulfur doped nano-photocatalyst, triglycerides are converted to fatty acid methyl esters.
2.3.1 Preparation of sulfur doped titania nano-photocatalyst
To prepare sulfur doped titania photocatalyst, 20 mL sulfuric acid (H2SO4) was mixed in 100 mL water. Then 22 mL Titanium Chloride (TiCl4) was dissolved in it by using a syringe. The solution was stirred for 30 min. Then added 2000 mL distilled water (DI) for dilution and kept for stirring to make solution 1. Then solution 2, i-e., 3 M sodium hydroxide (NaOH) solution (120 g NaOH/L of distilled water) was prepared to add this solution drop wise in solution one through burette to obtain pH 4. Then washed with distilled water for many times and dried it at 100°C for 4 hours. After sieving and grinding the dried sample, we got 300 g TiO2.
2.3.2 Transesterification mechanism using sulfur doped nano-photocatalyst
1 g of Nano-photocatalyst was mixed in 375 mL of methanol, 50 mL oil was applied to it, the solution was put in sunlight with the aid of four mirrors that concentrated the most sunlight on the solution, and the solution was fixed for stirring (Figure 1). The solution was allowed to settle over night after a 1-h stirring process.
2.4 Chemical and structural analysis of LAMEs
The physico-chemical properties of methyl esters were studied using existing protocols. The color, density, kinematic viscosity, sulfur content, total acid number, flash point, pour point, distillation recovery, cloud point, calorific value, cetane number, and phosphorus content of prepared methyl esters were all recorded and compared to high-speed diesel and ASTM standards.
2.4.1 Elemental analysis of LAMEs using atomic absorption spectrophotometer
For elemental analysis of the methyl esters the sample was prepared as required. 25 mL of it was transferred to a conical flask. Then added 10 mL HCL and 10 mL H2SO4 to it and boiled until the sample volume was reduced to 10 mL (Figure 2). Filtered the solution with Whatmann filter paper, and then applied distilled water to the sample to make it 25 mL in volume. The samples were transferred into plastic tubes (Figure 3) and the elemental analysis protocol was followed.
2.4.2 Lepidium apetalum methyl esters analysis using GC/MS machine
The chemical composition of prepared methyl esters was investigated using the GC-MS technique. A 1 mL sample of refined methyl esters was injected into a GC-MS model QP 2010 ultra (Shimadzu, Japan) using an external standard process with hexane as the solvent. Helium gas served as a vector. The temperature of the column was set at 50°C–300°C. Both the detector and injector were heated to 250°C.
2.4.3 NMR analysis
The LAMEs sample’s 1H and 13C NMR data were collected in deuterated chloroform with tetramethylsilane as a norm.
2.4.4 Physico-chemical properties of LAMEs
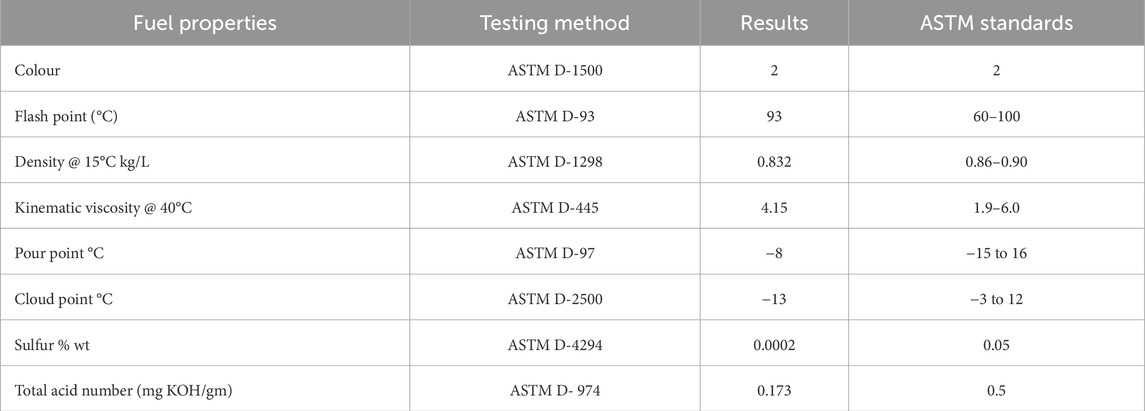
LAME fuel properties were quantitatively calculated and compared to ASTM requirements at Pakistan State Oil, Rawalpindi. Flash point (°C), Color, Density @ 15°C kg/L, Pour point °C, Cloud point °C, Sulfur percentage by weight, total acid number (mg KOH/gm) of methyl esters and Kinematic viscosity @ 40°C, were calculated using ASTM D-1500, D-93, D-1298, D-445, D-97, D-2500, D-4294.
3 Results and discussion
3.1 Lepidium seeds oil extraction
3.1.1 Mechanical oil extraction method
This technique is suitable for processing large volumes of oil. The feedstock seeds were thoroughly washed to eliminate impurities prior to mechanical oil extraction. The seeds were sun-dried before being put in an oil expeller (KEK P0015, 10,127, Germany). More than 5 L of crude oil were extracted from 30 kg of feedstock after five rounds of continuous rotation. The extracted oil was filtered and stored in a glass jar for further processing (Jafri, 2015).
3.1.2 Chemical oil extraction method
To assess the oil content in dried paper cress seeds, a chemical oil extraction method was used with a soxhlet apparatus. Oil content was discovered to be 31.32 percent. This was higher than the 26.77 percent observed by Nehdi et al. in the same Lepidium genus but separate species Lepidium sativum (Pattanaik et al., 2019; Al-Shehbaz et al., 2006).
3.2 Quantitative assessment of LAMEs yield
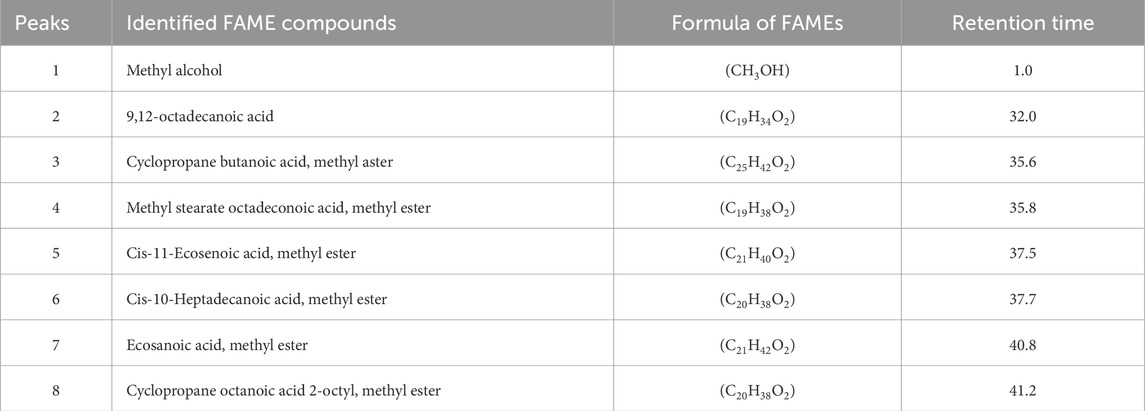
The present study is the first to use popular paper cress as a novel source for the development of methyl esters through the transesterification mechanism. GC/MS experiments confirmed that the methyl esters were synthesized, namely, Methyl alcohol, 9,12-octadecanoic acid, Cyclopropanebutanoic acid methyl aster, Methyl stearate octadeconoic acid methyl ester, Ecosanoic acid methyl ester, Cis-10-Heptadecanoic acid methyl ester, Cis-11-Ecosenoic acid methyl ester, and Cyclopropaneoctanoic acid 2-octyl metyl ester.
3.2.1 Paper cress free fatty acid contents
Free fatty acid (FFA) content in the crude oil must be determined before methyl esters can be synthesized. As a result, the FFA content of LAO was calculated and found to be 0.17 percent. LAO’s low FFA content allowed for direct transesterification without acid pretreatment to produce methyl esters (Jafri, 2015; Nehdi et al., 2012).
3.2.2 Transesterification mechanism via sulfur doped titania nano-photocatalyst
The technique of Hussain et al. was used to synthesize sulfur doped titania nano-photocatalyst in this study. A photocatalyst requires sunlight or UV rays to work properly and can significantly reduce reaction time. The transesterification reaction also does not need any temperature (Del Río et al., 2016; Cen et al., 2020; Mitsudome et al., 2008).
3.2.3 Preparation of sulfur doped titania nano-photocatalyst
20 mL sulfuric acid was mixed with 100 mL water and setteled to cool at room temperature. The dissolved 22 mL Titanium chloride was stirred in the solution for 30 min. For dilution, I added 2000 mL distilled water and held it for stirring. This was the first solution. Solution # 2, which was prepared as a 3 M sodium hydroxide solution (120 g NaOH/L of distilled water), was applied drop by drop to solution #1 to achieve a pH of 4. It was washed with distilled water several times and then dried at 100°C. Finally, after grinding and sieving the dried sample into powder form, 300 gm TiO2 was obtained. The sample was calcined by placing it in a 500oC furnace for more than 3 h. Ksibi et al. synthesized nitrogen and sulfur-doped TiO2 photocatalyst, as well as studying their behavior under near visible light and strong characterization. For the first time, Yu et al. show that sulfur doped TiO2 has bacterial effects on Micrococcus lylae in water under visible light (Ksibi et al., 2008; Yu et al., 2005).
3.2.4 X-ray diffraction (XRD) characterization
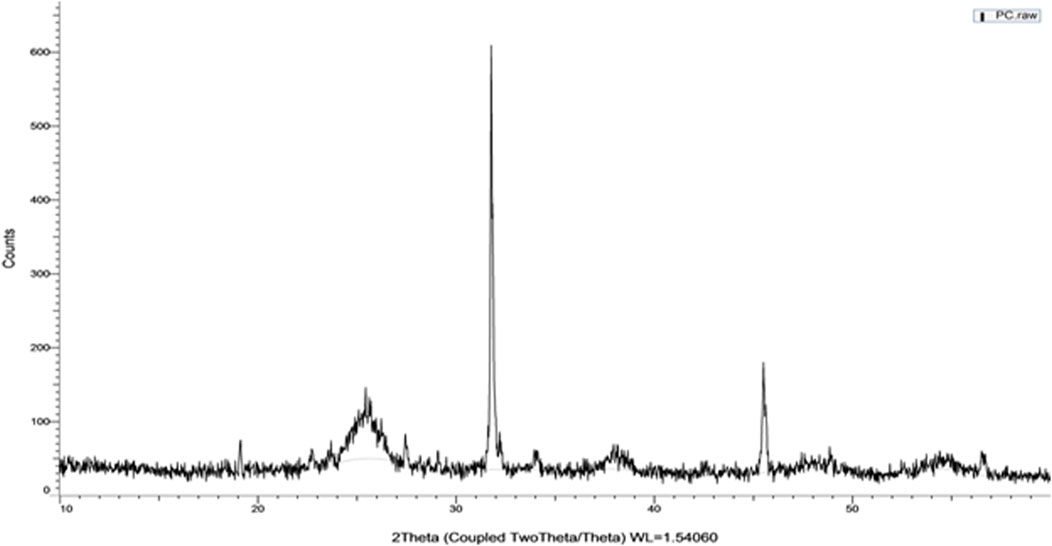
To analyze the crystalline phase of the powder that formed after the calcinations, Dust X-ray diffraction was used. Figure 4 shows high peaks at two theta (Ɵ) angles 18.9, 19, 25.6, 27.5, 32, 34, 38, 46, and 49. These peaks were matched to the Gao et al. XRD pattern (Figure 5) as a regular XRD pattern of sulfur doped TiO2 nano-photocatalyst and corrected the recognition of the engineered catalyst (Gao et al., 2011).
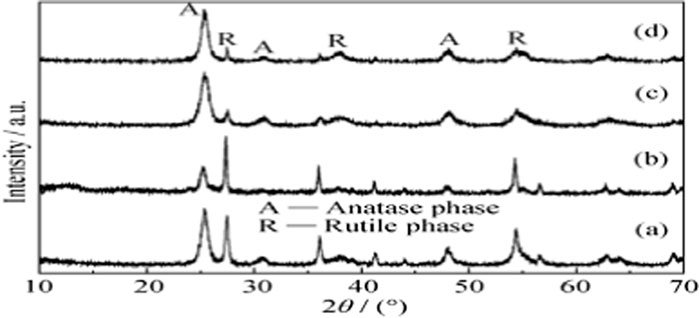
Figure 5. Sulfur doped TiO2 photocatalyst standard XRD pattern at (c) (Gao et al., 2011).
3.2.5 Transesterification mechanism
In 375 mL of methanol, 1 g of synthesized nano-photocatalyst was mixed and stirred for 30 min. In addition, 50 mL oil was added, and the solution was put in sunlight with the aid of four mirrors that focused the most sunlight on the solution, and the solution was held for stirring. A thermometer was placed next to the solution to record the temperature at the time, which was 35°C. The solution was allowed to settle overnight after 1 hour of stirring. The results were very promising, yielding 87 percent biodiesel in a very short period of time (Figure 6) without causing any temperature under normal atmospheric pressure (Table 1).
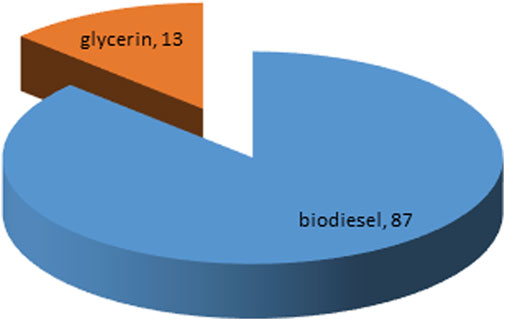
Figure 6. Biodiesel Production through Transesterification Process using Sulfur Doped Photocatalyst.
3.3 Chemical analysis
3.3.1 GC/MS analysis
Chemical composition of the LAMEs sample was investigated using the GC/MS technique. In this study, Different fatty acid methyl esters exhibited different peaks. Every single peak in the GC/MS chromatogram reflects a fatty acid methyl ester and was detected via library match software (NO. NIST11). Retention time data were used to classify various fatty acid methyl esters, which were analyzed and validated by mass spectrometric analysis (Shah et al., 2014).
In the methyl esters study, eight different peaks of different kinds of fatty acid methyl esters were observed (Figure 7). The experiment was conducted for 40 min at 300°C with helium as the carrier gas. Methyl alcohol (CH3OH) was found at peak number 1, followed by 9,12-octadecanoic acid (C19H34O2) at peak number 2, cyclopropanebutanoic acid, and methyl aster (C25H42O2) at peak number 3, Methyl stearate octadeconoic acid, methyl ester (C19H38O2) at peak number 4, Cis-11-Ecosenoic acid, methyl ester (C21H40O2) at peak number 5, Cis-10-Heptadecanoic acid, methyl ester (C20H38O2) at peak number 6, Ecosanoic acid, methyl ester (C21H42O2) at peak number 7, Cyclopropaneoctanoic acid 2-octyl, methyl ester (C20H38O2) at peak number 8as shown in Table 2.
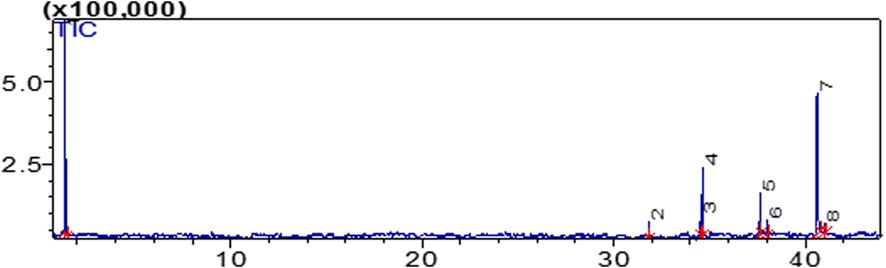
Figure 7. Eight Peaks of FAMEs Obtained through GC/MS Analysis using Photocatalyst as a Catalyst in Biodiesel Production.
Different researchers detected the fatty acid methyl esters described above in various types of biodiesel samples. By using efficient acid-catalyzed transesterification for biodiesel processing, Miao et al. discovered 9-0ctadecanoic acid methyl ester. By looking into high quality biodiesel production from Chlorella protothecoides, Hu et al. discovered eicosanoic and 9-0ctadecanoic acid methyl ester. Diya’uddeen et al. investigated the existence of Cis-11-Ecosanoic acid methyl esters while experimenting on Jatropha curcus. Similarly, Cis-11-Ecosanoic acid methyl esters were discovered in Jatropha curcus biodiesel by Becker and Moser. Pratas et al. studied the densities and viscosities of minority fatty acid methyl and ethyl esters found in biodiesel and discovered Docosanoic acid methyl esters in the study. Eicosanoic acid methyl ester was used in biodiesel made from Pongamia pinnata, according to Karmee and Chadha. The methyl ester of hexadecanoic acid was discovered in restaurant waste lipids by Canakci (Miao et al., 2009; Hu et al., 2011; Diya’uddeen et al., 2012; Moser et al., 2009; Cheng et al., 2009; Anwar et al., 2010; Pratas et al., 2011; Karmee and Chadha, 2005; Canakci, 2007).
3.3.2 13C NMR studies
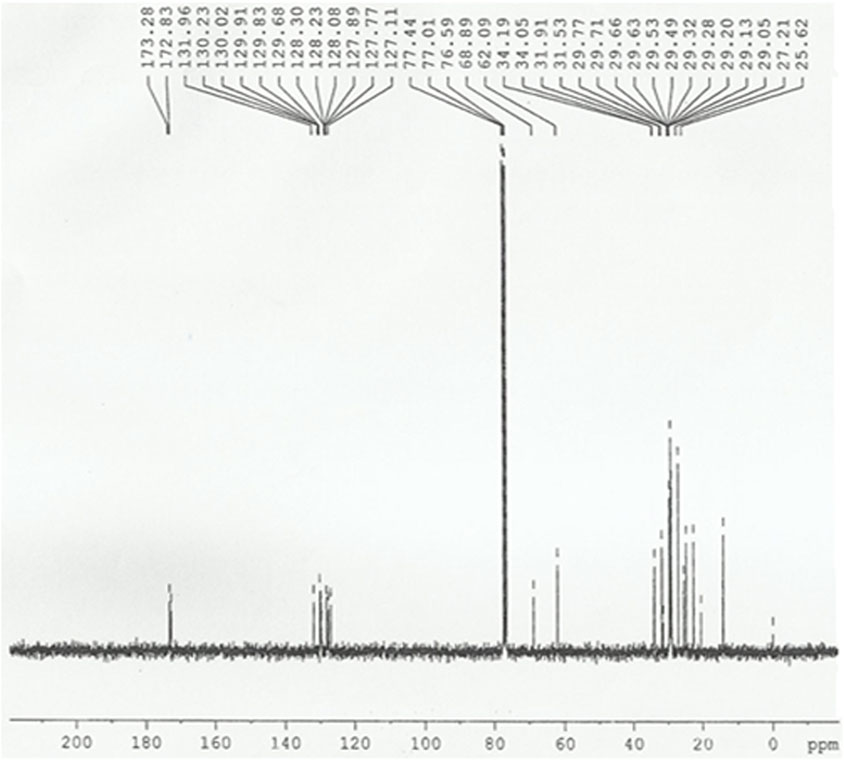
In deuterated chloroform, proton and 13C NMR data were collected with tetramethylsilane as a reference. The biodiesel was successfully synthesized, as demonstrated by the 13C NMR spectrum (Figure 8). The tasks for the following peaks are as follows. The carbonyl carbons in biodiesel peaked at 173.2 ppm and 172.8 ppm. The other peaks at 68.8–62.0 ppm may be due to the presence of methoxy and ethoxy carbons. Different aliphatic carbons in the sample trigger the peaks between 25.6 ppm and 34.1 ppm. The presence of an aromatic ring somewhere in the structure is also suggested by one or two peaks about 120 ppm. These results were very similar to those of the GC-MS study.
3.3.3 1H NMR studies
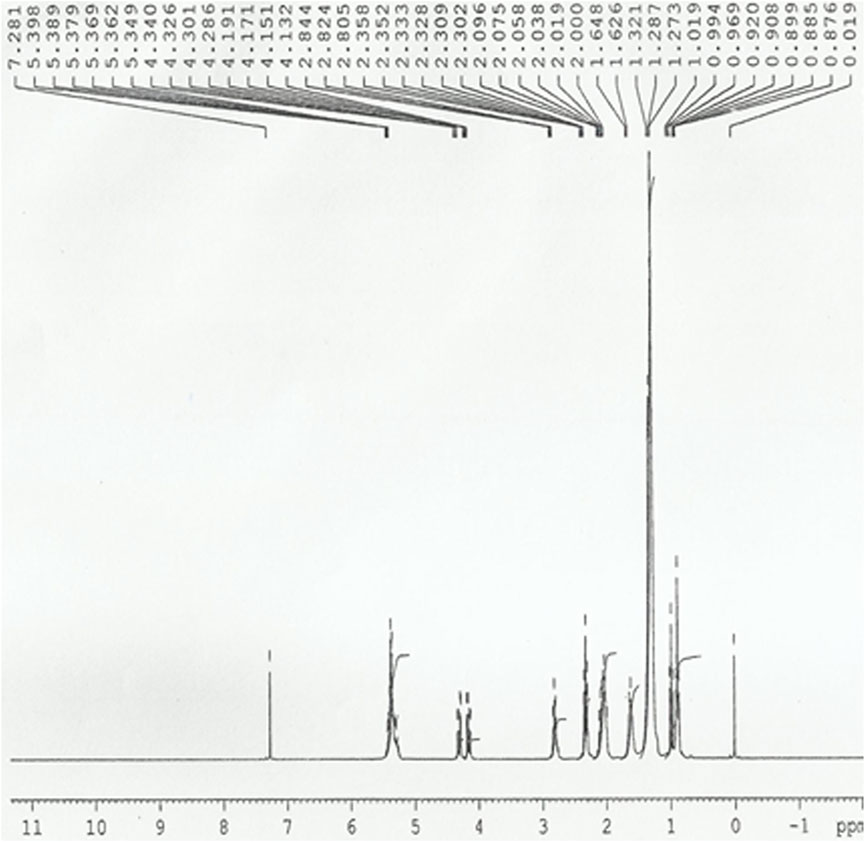
1H NMR may also be used to determine how much vegetable oil is converted to methyl esters during the transesterification process. In methyl esters, the relative signals chosen from integration were those of the methoxy group, aliphatic and aromatic protons. The existence of aliphatic protons is confirmed by peaks at 0.8–2.8 ppm. The presence of methoxy protons is estimated at 4.13–4.34 ppm. Peaks of 5–6 ppm indicate the existence of an NH group or an olefinic proton. Peaks 7–8 ppm (Figure 9) suggest the presence of aromatic protons, and these results are consistent with 13C NMR (Knothe, 2000; Knothe, 2005).
3.3.4 Comparative analysis of metals in LAMEs
Awareness of the trace elements present in biodiesel is critical because they can affect engine performance, because of environmental pollution, and cause engine corrosion. As a consequence, a biodiesel sample with a large concentration of these elements is often undesirable. Sodium (Na), potassium (K), and phosphorus (P) are the elements that must be regulated in biodiesel since they are primarily extracted from raw materials. Copper and iron, in particular, increase the formation of gum in biodiesel. Heavy metals released by vehicles, such as lead, are the primary cause of lead deposition in humans (Ruan et al., 2008; Ahn et al., 1995; Ma and Hanna, 1999).
The elemental analysis of biodiesel made by transesterification of LAO using nano-catalyst was tested in this report. The atomic absorption spectrum method was used to measure the concentration of elements K, Na, Mg, Ca, Fe, Ni, and Pb in the sample, which was then compared to high-speed diesel. The AAS results showed that the sample had lower concentrations of all of the above-mentioned elements than high-speed diesel, as shown in Table 3. This finding strongly indicates that LAMEs are a better alternative to mineral diesel, as well as being substantially less pollutant and toxic (Ullah and Bano, 2011).
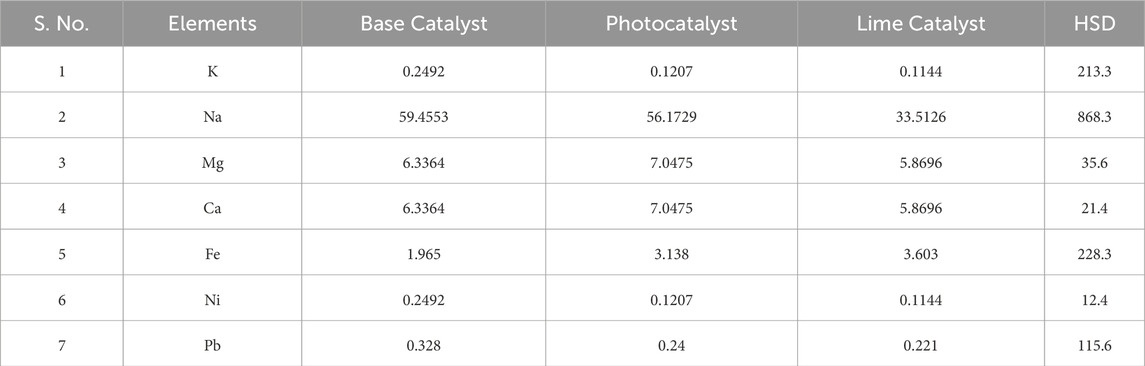
Table 3. Concentration of elements (µg/g) in three LAMEs samples in comparison with high speed diesel.
3.4 Physico-chemical properties of LAMEs
The quantitative properties of LAMEs were calculated and compared to ASTM standards. Flash point (°C), Color, Density @ 15°C kg/L, Pour point °C, Kinematic viscosity @ 40°C, Sulfur percentage by weight, Cloud point °C and Total Acid number (mg KOH/gm) of biodiesel were calculated using ASTM D-1500, 93, 1,298, 445, 97, 2500, 4,294, and 974, respectively.
3.4.1 Flash point
When a fuel is exposed to a flame, its flash point is the temperature at which it will ignite. It is a calculation of a substance’s proclivity to form flammable mixtures in the presence of air. The flash point is an important parameter in practice. When it comes to transportation, handling, and storage, a high flash point is considered secure. The flash point of rubber seed oil was measured at 120°C. Using the ASTM D-93 testing process, Karmee and Chadha found that Pongamia FAME has a flash point of 150°C. As shown in Table 4, LAMEs have a flash point of 93°C, which is within the range of ASTM D-93 specifications, making it a safer fuel to handle. The flash point of high-speed diesel was between 60 and 80°C (Karmee and Chadha, 2005; Demirbaş, 2003).
3.4.2 Density
When it comes to assessing the consistency of biodiesel, density is the key. Denser oils have more energy in them. Sesame oil biodiesel had a density of 0.871. A similar collection of findings was presented. They discovered that rice bran oil has a density of 0.877. The density of Sinapis alba oil biodiesel was discovered to be 0.872. LAMEs have a density of 0.832 at 15°C (Table 4), while high-speed diesel has a density of 0.834. This demonstrates that LAMEs have a lower density than high-speed diesel (Demirbaş, 2003; Ahmad et al., 2011).
3.4.3 Kinematic viscosity
Kinematic viscosity is a metric that measures how sticky a fuel is. Since low viscosity values in diesel can impair engine lubrication, biodiesel viscosity should be measured according to international testing standards. Morshad et al. discovered that the rubber oil biodiesel had a kinematic viscosity of 4.5 at 40°C, which was higher than the high-speed diesel value of 4.223. The sesame oil biodiesel had a kinematic viscosity of 5.77, according to Ahmad et al. Sinapis alba oil biodiesel had a kinematic viscosity of 5.45, which was higher than the ASTM requirements. The existence of high molecular weight compounds and large chemical structures may explain the increased viscosity. LAMEs have a kinematic viscosity of 4.15, which is exactly within ASTM requirements and lower than the high-speed diesel value of 4.22 (Saka and &Kusdiana, 2001).
3.4.4 Pour point and cloud point
The pour point and cloud point of a fuel are critical characteristics. When the fuel is cooled under specified conditions, the pour point is the lowest temperature at which it can flow, and the cloud point is the temperature at which paraffin starts to crystallise or detach from solution. Saka and Isayama developed a new catalyst-free process for producing biodiesel with a pour point of −16, which is similar to the pour point of high-speed diesel, i.e., −15 to 16. Rice bran oil biodiesel had pour point and cloud point values of −7 and 6, respectively. The cloud point and pour point values in corn oil biodiesel were found to be −2 and −16, respectively. Rocket seed oil has a pour point value of three and a cloud point value of −15, according to Rashid et al. LAMEs have a pour point of −8, which is below ASTM requirements, while the cloud point is −13, which is marginally higher than high-speed diesel (Saka and &Kusdiana, 2001; Rashid et al., 2009).
3.4.5 Sulfur contents
We may assess the presence of sulfated ash in biodiesel by igniting and burning it, then treating it with sulfuric acid. Fuel with low sulfur content is suitable for areas that are heavily contaminated. According to Karmee and Chadha, the sulfated ash content of Pongamia oil biodiesel was 0.005%, which was very low by ASTM standards (0.2 percent). Rocket seed oil contained 0.05 percent sulfur, according to Rashid et al. Sesame oil biodiesel contained 0.05 percent sulfur, according to the researchers. They discovered that biodiesel made from corn oil contains 0.01 percent sulfated ash. According to him, muskmelon seed oil biodiesel contains 0.021 percent sulfur. In terms of sulfur content, biodiesel outperforms high-speed diesel. Sulfur content in LAMEs was found to be 0.0002 percent by weight, which is extremely low and meets ASTM D-4294 requirements (Karmee and Chadha, 2005; Rashid et al., 2013).
3.4.6 Acid value
It refers to the amount of free fatty acids in a fresh fuel sample. For neutralizing 1 gm of fatty acid methyl esters, the acid value is expressed as mg KOH/gm. An engine’s acid content should be kept low. The maximum acid value for biodiesel, according to ASTDM-D 664, is 0.5 mg NaOH/gm. The total acid amount of S. alba oil biodiesel was 0.242 mg KOH/gm, according to researchers. Rashid et al. discovered that cotton seed oil has a total acid number of 0.16 mg KOH/gm. Rashid et al. investigated the acid value of 0.45 mg KOH/gm from muskmelon seed oil in another study. According to the ASTM D-974 testing process, the acid value of LAMEs was 0.17 mg KOH/gm, which is very low and makes LAMEs suitableto use (Rashid et al., 2008).
4 Conclusion
As the fossil fuels are depleting very fast, there is an urgent need to do research on alternative fuel to meet the world’s energy demands. One of the most environmentally friendly options for meeting this energy demand is Biodiesel. A novel photocatalyst was used to convert Lepedium apitalum oil into Lepedium apitalum methyl esters in this analysis. Using sulfur doped nano-photocatalyst, the best result was found to be 92.10 percent. In addition, different parameters affecting biodiesel yield were investigated. Using 1:6 oil to methanol ratio at 60°C and a 0.5 gm doped nano-photocatalyst, the best results were obtained. The LAMEs sample had fuel properties that were compatible with ASTM requirements. The chemistry of LAMEs was analyzed using various analytical techniques such as GC-MS, 13C NMR, 1H NMR, and Atomic Absorption spectrum (AAS), and the findings strongly indicate that Lapedium apitalum is a possible non-edible feedstock for biodiesel production in the future. Furthermore, this energy crop does not need a lot of water to grow and is very cost-effective. The physico-chemical nature of LAMEs indicates that it may be a bioenergy hotspot.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
SN: Conceptualization, Investigation, Methodology, Writing – original draft, Writing – review and editing. KU: Writing – review and editing, Data curation, Formal Analysis, Funding acquisition, Project administration, Supervision, Validation. MA: Project administration, Supervision, Validation, Writing – review and editing, Resources, Software. MZ: Resources, Software, Supervision, Validation, Writing – review and editing, Formal Analysis.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The authors of this study express their appreciation to COMSATS and Quaid i Azam University Islamabad for providing valuable opportunities and resources for conducting this research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ahmad, M., Ullah, K., Khan, M. A., Ali, S., Zafar, M., and Sultana, S. (2011). Quantitative and qualitative analysis of sesame oil biodiesel. Energy Sources, Part A Recovery, Util. Environ. Eff. 33 (13), 1239–1249. doi:10.1080/15567036.2010.531510
Ahn, E., Koncar, M., Mittelbach, M., and Marr, R. (1995). A low-waste process for the production of biodiesel. Sep. Sci. Technol. 30 (7-9), 2021–2033. doi:10.1080/01496399508010391
Alavijeh, R. S., Karimi, K., and van den Berg, C. (2020). An integrated and optimized process for cleaner production of ethanol and biodiesel from corn stover by Mucor indicus. J. Clean. Prod. 249, 119321. doi:10.1016/j.jclepro.2019.119321
Al-Shehbaz, I., Beilstein, M. A., and Kellogg, E. (2006). Systematics and phylogeny of the Brassicaceae (cruciferae): an overview. Plant Syst. Evol. 259 (2-4), 89–120. doi:10.1007/s00606-006-0415-z
Anwar, F., Rashid, U., Ashraf, M., and Nadeem, M. (2010). Okra (Hibiscus esculentus) seed oil for biodiesel production. Appl. Energy 87 (3), 779–785. doi:10.1016/j.apenergy.2009.09.020
Bhatia, S. K., Gurav, R., Choi, T.-R., Kim, H. J., Yang, S.-Y., Song, H.-S., et al. (2020). Conversion of waste cooking oil into biodiesel using heterogenous catalyst derived from cork biochar. Bioresour. Technol. 302, 122872. doi:10.1016/j.biortech.2020.122872
Canakci, M. (2007). The potential of restaurant waste lipids as biodiesel feedstocks. Bioresour. Technol. 98 (1), 183–190. doi:10.1016/j.biortech.2005.11.022
Cen, D., Ding, Y., Wei, R., Huang, X., Gao, G., Wu, G., et al. (2020). Synthesis of metal oxide/carbon nanofibers via biostructure confinement as high-capacity anode MaterialsACS. Appl. Mater Inter.
Cheng, Y., Zhou, W., Gao, C., Lan, K., Gao, Y., and Wu, Q. (2009). Biodiesel production from Jerusalem artichoke (Helianthus Tuberosus L.) tuber by heterotrophic microalgae Chlorella protothecoides. J. Chem. Technol. Biotechnol. 84 (5), 777–781. doi:10.1002/jctb.2111
Del Río, E., Hungría, A., Tinoco, M., Manzorro, R., Cauqui, M., Calvino, J., et al. (2016). CeO 2-modified Au/TiO 2 catalysts with outstanding stability under harsh CO oxidation conditions. Appl. Catal. B Environ. 197, 86–94. doi:10.1016/j.apcatb.2016.04.037
Demirbaş, A. (2003). Biodiesel fuels from vegetable oils via catalytic and non-catalytic supercritical alcohol transesterifications and other methods: a survey. Energy Convers. Manag. 44 (13), 2093–2109. doi:10.1016/s0196-8904(02)00234-0
Diya’uddeen, B. H., Abdul Aziz, A. R., Daud, W. M. A. W., and Chakrabarti, M. H. (2012). Performance evaluation of biodiesel from used domestic waste oils: a review. Process Saf. Environ. Prot. 90 (3), 164–179. doi:10.1016/j.psep.2012.02.005
Freire, J. G., Calderón-Cárdenas, A., Varela, H., and Gallas, J. A. (2020). Phase diagrams and dynamical evolution of the triple-pathway electro-oxidation of formic acid on platinum. Phys. Chem. 22 (3), 1078–1091.
Gao, H. T., Liu, Y. Y., Ding, C. H., Dai, D. M., and Liu, G. J. (2011). Synthesis, characterization, and theoretical study of N, S-codoped nano-TiO2 with photocatalytic activities. Int. J. Minerals, Metallurgy, Mater. 18 (5), 606–614. doi:10.1007/s12613-011-0485-y
Hossain, A., Boyce, A., Salleh, A., and Chandran, S. (2010). Impacts of alcohol type, ratio and stirring time on the biodiesel production from waste canola oil. Afr. J. Agric. Res. 5 (14), 1851–1859.
Hu, S., Guan, Y., Wang, Y., and Han, H. (2011). Nano-magnetic catalyst KF/CaO–Fe3 O4 for biodiesel production. Appl. Energy 88 (8), 2685–2690. doi:10.1016/j.apenergy.2011.02.012
Jafri, A. (2015). Developing and using a methodology for recovering and separating unsaponifables from cottonseed oil deodorizer distillate using a centrifugal molecular distillation process.
Karmakar, S., Ghosh, S., and Kumbhakar, P. (2020). Enhanced sunlight-driven photocatalytic and antibacterial activities of flower-like ZnO and MoS 2 nanocomposite. J. Nanopart Res. 22 (1), 1–20.
Karmee, S. K., and Chadha, A. (2005). Preparation of biodiesel from crude oil of Pongamia pinnata. Bioresour. Technol. 96 (13), 1425–1429. doi:10.1016/j.biortech.2004.12.011
Knothe, G. (2000). Monitoring a progressing transesterification reaction by fiber-optic near infrared spectroscopy with correlation to 1H nuclear magnetic resonance spectroscopy. J. Am. Oil Chemists' Soc. 77 (5), 489–493. doi:10.1007/s11746-000-0078-5
Knothe, G. (2005). Dependence of biodiesel fuel properties on the structure of fatty acid alkyl esters. Fuel Process. Technol. 86 (10), 1059–1070. doi:10.1016/j.fuproc.2004.11.002
Ksibi, M., Rossignol, S., Tatibouët, J. M., and Trapalis, C. (2008). Synthesis and solid characterization of nitrogen and sulfur-doped TiO2 photocatalysts active under near visible light. Mater. Lett. 62 (26), 4204–4206. doi:10.1016/j.matlet.2008.06.026
Miao, X., Li, R., and Yao, H. (2009). Effective acid-catalyzed transesterification for biodiesel production. Energy Convers. Manag. 50 (10), 2680–2684. doi:10.1016/j.enconman.2009.06.021
Mitsudome, T., Mikami, Y., Funai, H., Mizugaki, T., Jitsukawa, K., and Kaneda, K. (2008). Oxidant-free alcohol dehydrogenation using a reusable hydrotalcite-supported silver nanoparticle catalyst. Angew. Chem. 120 (1), 144–147. doi:10.1002/ange.200703161
Moser, B. R., Shah, S. N., Winkler-Moser, J. K., Vaughn, S. F., and Evangelista, R. L. (2009). Composition and physical properties of cress (Lepidium sativum L.) and field pennycress (Thlaspiarvense L.) oils. Industrial Crops Prod. 30 (2), 199–205. doi:10.1016/j.indcrop.2009.03.007
Moses, E., Thliza, B. A., and Deshi, J. J. (2019). Methanolysis reaction for production of biodiesel from palm-oil. Int. J. Res. Dev. 4 (7), 27–32.
Nehdi, I. A., Sbihi, H., Tan, C. P., and Al-Resayes, S. I. (2012). Garden cress (Lepidium sativum Linn.) seed oil as a potential feedstock for biodiesel production. Bioresour. Technol. 126, 193–197. doi:10.1016/j.biortech.2012.08.113
Passoth, V., and Sandgren, M. (2019). Biofuel production from straw hydrolysates: current achievements and perspectives. Appl. Microbiol. Biotechnol. 103 (13), 5105–5116. doi:10.1007/s00253-019-09863-3
Pattanaik, L., Pattnaik, F., Saxena, D. K., and Naik, S. N. (2019). Biofuels from agricultural wastes second and third generation of feedstocks. Elsevier, 103–142.
Pratas, M. J., Freitas, S., Oliveira, M. B., Monteiro, S. C., Lima, Á. S., and Coutinho, J. A. (2011). Densities and viscosities of minority fatty acid methyl and ethyl esters present in biodiesel. J. Chem. and Eng. Data 56 (5), 2175–2180. doi:10.1021/je1012235
Rashid, U., Anwar, F., Ansari, T. M., Arif, M., and Ahmad, M. (2009). Optimization of alkaline transesterification of rice bran oil for biodiesel production using response surface methodology. J. Chem. Technol. Biotechnol. 84 (9), 1364–1370. doi:10.1002/jctb.2191
Rashid, U., Anwar, F., Moser, B. R., and Knothe, G. (2008). Moringaoleifera oil: a possible source of biodiesel. Bioresour. Technol. 99 (17), 8175–8179. doi:10.1016/j.biortech.2008.03.066
Rashid, U., Ibrahim, M., Yasin, S., Yunus, R., Taufiq-Yap, Y. H., and Knothe, G. (2013). Biodiesel from Citrus reticulate (Mandarin orange) seed oil, a potential non-food feedstock. Industrial Crops Prod. 45, 355–359. doi:10.1016/j.indcrop.2012.12.039
Raza, A., Hafeez, M. B., Zahra, N., Shaukat, K., Umbreen, S., Tabassum, J., et al. (2020). The plant family brassicaceae: introduction, biology, and importance the plant family brassicaceae. Springer, 1–43.
Rizwanul Fattah, I., Ong, H., Mahlia, T., Mofijur, M., Silitonga, A., Rahman, S., et al. (2020). State of the art of catalysts for biodiesel production. Front. Energy Res. 8, 101. doi:10.3389/fenrg.2020.00101
Ruan, C. J., Li, H., Guo, Y. Q., Qin, P., Gallagher, J. L., Seliskar, D. M., et al. (2008). Kosteletzkyavirginica, an agroecoengineering halophytic species for alternative agricultural production in China's east coast: ecological adaptation and benefits, seed yield, oil content, fatty acid and biodiesel properties. Ecol. Eng. 32 (4), 320–328. doi:10.1016/j.ecoleng.2007.12.010
Saka, S., and Kusdiana, D. (2001). Biodiesel fuel from rapeseed oil as prepared in supercritical methanol. Fuel 80 (2), 225–231. doi:10.1016/s0016-2361(00)00083-1
Shah, M., Ali, S., Tariq, M., Khalid, N., Ahmad, F., and Khan, M. A. (2014). Catalytic conversion of jojoba oil into biodiesel by organotin catalysts, spectroscopic and chromatographic characterization. Fuel 118, 392–397. doi:10.1016/j.fuel.2013.11.010
Shahid, M., Farooqi, Z. H., Begum, R., Arif, M., Wu, W., and Irfan, A. (2019). Hybrid microgels for catalytic and photocatalytic removal of nitroarenes and organic dyes from aqueous medium: a review. Crit. Rev. Anal. Chem. 50, 513–537. doi:10.1080/10408347.2019.1663148
Shi, L., Xu, A., and Zhao, T. (2016). RuO2 monolayer: a promising bifunctional catalytic material for nonaqueous lithium–oxygen batteries. J. Phys. Chem. 120 (12), 6356–6362. doi:10.1021/acs.jpcc.6b00014
Tariq, M., Ali, S., Ahmad, F., Ahmad, M., Zafar, M., Khalid, N., et al. (2011). Identification, FT-IR, NMR (1H and 13C) and GC/MS studies of fatty acid methyl esters in biodiesel from rocket seed oil. Fuel Process. Technol. 92 (3), 336–341. doi:10.1016/j.fuproc.2010.09.025
Ullah, F., and Bano, A. (2011). Effect of plant growth regulators on oil yield and biodiesel production of safflower (Carthamus tinctorius L.). Braz. J. Plant Physiology 23 (1), 27–31. doi:10.1590/s1677-04202011000100005
Youzhi, G., Yang, H., Yiping, Z., Li, C., and Fanyong, Y. (2016). Fabrication of thermosensitive hydrogel-supported Ni nanoparticles with tunable catalytic activity for 4-nitrophenol. J. Mater. Sci. 51 (6), 3200–3210. doi:10.1007/s10853-015-9631-7
Keywords: energy crop Lepidium apetalum, non-edible seed oil, LAMEs, sulfur doped nano-photocatalyst, analytical studies
Citation: Naqvi SAH, Ullah K, Ahamd M and Zafar M (2025) Chemical conversion and characterization of paper cress seed oil to paper cress methyl esters via novel sulfur doped nano-photocatalyst. Front. Energy Res. 13:1412246. doi: 10.3389/fenrg.2025.1412246
Received: 04 April 2024; Accepted: 10 June 2025;
Published: 04 November 2025.
Edited by:
Paul Hellier, University College London, United KingdomReviewed by:
Reham Yahia El-Araby, National Research Centre, EgyptYiming Zhu, Shenyang Aerospace University, China
Copyright © 2025 Naqvi, Ullah, Ahamd and Zafar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kifayat Ullah, a2lmYXlhdC5kYXdhckBjb21zYXRzLmVkdS5waw==
 Syed Ali Hassan Naqvi
Syed Ali Hassan Naqvi Kifayat Ullah
Kifayat Ullah Mushtaq Ahamd
Mushtaq Ahamd Muhammad Zafar
Muhammad Zafar