- German Aerospace Center – DLR e.V., Institute of Engineering Thermodynamics, Cologne, Germany
Heat supply for residential buildings is still mainly based on fossil fuels and is thus a significant contributor to CO2 emissions. Renewable heating remains a technological challenge, often for the reason that during cold weather periods heat demand increases drastically whereas renewable production minimizes. Reactive solids produced by renewable energy can be an energy storage and carrier to flexibly leverage the often extreme seasonal discrepancy of residential heat demand. This work demonstrates a fully integrated thermochemical heating system based on calcium oxide and water operational for the first time in a real building environment. With the extraction of the thermochemically stored energy at a temperature level of 60°C we proved that the technology can be integrated into existing heating infrastructures of buildings, by replacing fossil fuel-based burners. This work advances the technology readiness level of thermochemical energy storage to validation in relevant environment.
1 Introduction
According to the International Energy Agency the global heat demand in 2022 accounted for almost half of the total final energy consumption and 38% of energy related CO2 emissions (IEA, 2024). The building sector contributes to almost half of this consumption with its demand for space and water heating (Li and Yao, 2021). The share of renewable heat supply for buildings did not significantly progress over the last years (IEA, 2024). One reason for this is, that the highest demand for space heating is during winter time at the same time when the renewable production is most likely minimal. The application of heat pumps helps to couple the renewable electricity sector with the heat supply for buildings. In combination with a thermal storage the systems provide some flexibility, but only in the range of hours or a couple of days at maximum. Therefore, this approach cannot completely resolve the problem of limited availability of renewables in the winter over longer periods, such as several weeks. Consequently, energy storage systems with a larger capacity able to store renewable energy for a longer period seem favourable (Dowling et al., 2020). However, in order to reach economic feasibility, the storage cost per kWh must be extremely low since long-term storage systems undergo only a very limited number of storage cycles per year (Guerra, 2021).
Thermochemical energy storage with gas-solid reactions is a promising approach for long-term thermal energy storage for two reasons: The chemically stored energy can be preserved free of thermal losses for a user-defined time period and some of the reactive materials are available at a very low cost. For example, zeolites, metal hydroxides or hydrates and their reaction with water vapour are considered. A recent review provides a good comparison between the different reaction systems and their performance for an application in buildings heat supply (Moulakhnif et al., 2024). A review of Li et al. provides the current state of technology for different salt hydrate-based gas-solid energy storage systems. The authors conclude that several optimisations on material, reactor and system level are still required before such systems can emerge from laboratory scale to engineering applications (Li et al., 2022). Salt hydrate-based systems offer potential volumetric storage densities from 94 to 308 kWh/m3 if water vapour is available (Hua et al., 2022). As most salt hydrates have charging temperatures around 100°C, charging with solar thermal energy or low-grade waste heat is possible. But, in order to reach above mentioned energy storage densities, water has to be evaporated first before the energy can be released. Even though, the energy for evaporation could be somehow supplied by the ambient (e.g., bore holes), the necessity to include such low (partial) pressures of water vapour in the system increase its complexity.
However, the expansion of renewable energy focuses mostly on the production of electricity from wind turbines and photovoltaics (IEA, 2024). This creates an opportunity of charging thermochemical storage systems with electricity. Such charging enables the use of different reactive materials, which require higher charging temperatures, for example, metal hydroxides. One interesting system in that regard is the reaction of calcium oxide(CaO) with water or water vapour to calcium hydroxide (Ca(OH)2):
Advantages are a good potential volumetric energy density of up to 215 kWh/m3 - using liquid water from the tap - and very low costs for the storage material itself (Schmidt and Linder, 2020).
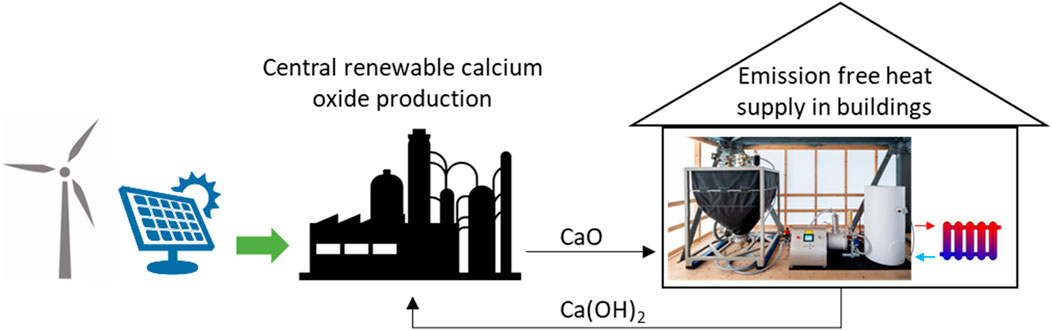
The graphical abstract shows an application concept for energy storage and heat supply for buildings which is the underlying idea of this work. Whenever excess renewable electricity is available, CaO is produced in a larger centralized production plant. A similar approach is currently developed for the electric calcination of lime stone at even higher temperatures (Fennell et al., 2021). The charged thermochemical material can be stored centrally and then distributed to smaller storage units of nearby buildings. In the building, thermal energy is released by performing the exothermal reaction between CaO and tap water on demand. The released heat is transferred to the building’s heating circuit, and replaces former burning of fossil fuels. The discharged material, Ca(OH)2, is transported back to the regeneration plant where it will be regenerated using renewable electricity. The reaction can be repeated for an unlimited number of cycles, enabling long-term, large capacity storage of renewable energy for heating purposes. Schmidt et al. analysed the concept for a long-term heat storage system in buildings in detail and presented energy balance equations and first sizing considerations (Schmidt and Linder, 2020).
Due to the promising potential, the reaction system has been investigated by various groups, on material, reactor and system integration level over the past decade. The reaction kinetics have been characterised (Criado et al., 2014; Gupta et al., 2021; Schaube et al., 2012) and the cycle stability has been proven (Dutta and Shirai, 1974). Current works on the material level address the specific enhancement of material or bulk properties, aiming e.g., for stabilization of pellets and granules (Valverde-Pizarro et al., 2020; Lucke et al., 2023; Jashari et al., 2023; Xia et al., 2020), enhancement of the thermal conductivity (Huang et al., 2019; Li et al., 2016; Funayama et al., 2023), avoidance of agglomeration (Xu et al., 2017; Roßkopf et al., 2014; Gollsch et al., 2020) and adjustment of the reaction temperature (Maruyama et al., 2020). Recent activities try to understand the impact of changes of the bulk properties during the thermochemical reaction (Xu et al., 2022). Simulation models have been developed based on available material data and partially validated with experimental data from laboratory scale reactors (Risthaus et al., 2020).
Despite a good understanding of the fundamentals, the development of a thermochemical reactor is still one of the major difficulties in bringing the technology into a first application. Agglomeration tendency, low thermal conductivity and high charging temperatures required pose challenges for the development of well-functioning reactors at reasonable costs. Indirectly operated fixed-bed reactors in which the storage material is firmly in contact with the heat exchanger surface have generally shown good functionality but have only limited scalability.
One intensively pursued approach is hence to decouple the storage capacity costs from the reactor costs by separating the material storage and reactor components. However, such a concept requires the storage material to be moved to and from the storage tank and through the reactor itself. The operation of gravimetrically moving bed reactors has proven difficult with the powder material (Schmidt et al., 2016). Fluidised bed reactors set specific requirements on bulk material properties and require large fluidisation volume flows, which in turn limits the achievable temperature levels during heat extraction and thus the possible applications (Marie et al., 2022). One reactor concept that proved to be fundamentally suitable for operation with the powder is the mechanically-induced fluidised bed. Risthaus et al. developed the reactor concept and were able to successfully demonstrate the charging and discharging of the thermochemical material (Risthaus et al., 2022). The concept prevents agglomeration of the powder and ensures unrestricted contact of the reaction gas with the particles.
Nevertheless, the reported experimental works on reactors are yet limited on characterising the reaction process with water vapour. What has not yet been addressed, is the operation and thermodynamic design of such a thermochemical reactor that enable the extraction of thermal energy at application-relevant temperature levels. In particular, neither the simpler operation with liquid water and coupled heat extraction has been investigated, nor a complete thermochemical system with separation of the reactor and storage units, including the material transport, has been successfully operated.
Therefore, this work presents the first fully operational thermochemical pilot system designed and thermodynamically optimised for the specific application of heat supply for buildings. The system includes all required components for the complete demonstration of the heat supply process: Storage tanks for the solid energy storage material, an automated material transport system, the thermochemical reactor as well as a domestic hot water storage tank. The coupling of the hot water storage tank and the reactor represents the final integration point into the heating circuits of existing buildings.
The reactor was thermodynamically designed to verify the extraction of useful heat at a temperature level of 50°C–60°C. Reaching this temperature level would enable the technology to be directly integrated in a majority of existing heating infrastructures in buildings, replacing fossil fuel burners. The entire system was recently integrated in an application environment in a research building and is therefore the first work that provides data of a CaO based thermochemical heating system operated under application conditions outside the laboratory.
2 Materials and system development
2.1 Thermochemical material
For the operation of the thermochemical heating system, commercially available calcium oxide is used. The company Lhoist Germany Rheinkalk GmbH supplied CaO with the product name “Weißfeinkalk CL 90 - Q Rapidquell”. According to performed TGA measurements, the material consists of 83.4% CaO. The material is grounded to a fine powder with an average particle size of 5 μm. The enthalpy of reaction with liquid water can be calculated by the standard enthalpies of formation presented in table books and accounts 65.1 kJ/mol, corresponding to a gravimetric energy density of 0.323 kWh/kgCaO based on CaO’s molar mass.
2.2 Pilot system development
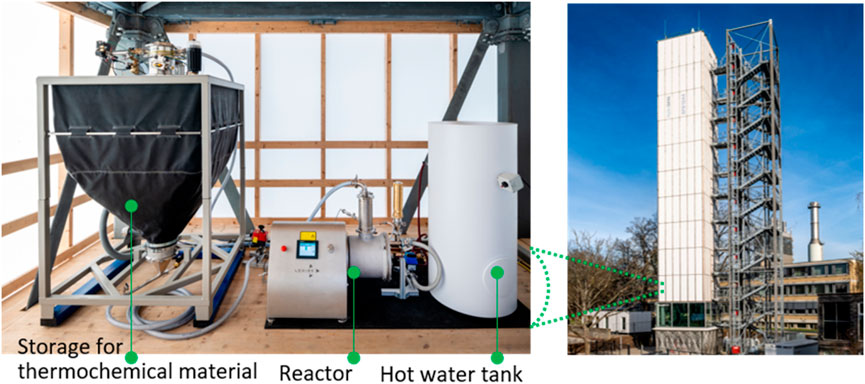
Figure 1 shows the conceptual process design of the developed pilot system for decentralized heat supply for buildings. The main idea behind the pilot system was to decouple the energy storage capacity from the power unit, allowing a compact reactor design and a system that can be decentralized and cost-efficiently up-scaled. As a result, the plant consists of three separated main components: the storage and conveying unit, the thermochemical reactor unit and the domestic hot water storage tank. An automated transport system can move the solid material between the storage tanks and the reactor. The reactor and hot water storage tank are thermally coupled by a water circuit with heat exchangers.

Figure 1. Process Flow Diagram: Conceptual design for a decentralized, long-term thermochemical storage system based on the reaction material calcium oxide.
For the storage unit, two containers have been designed, with a dust-tight textile suitable for the fine powder. The textile containers are easily transportable and scalable and the material’s flexibility helps prevent powder agglomeration within the containers. Each container has a volume of 1.1 m3, corresponding to app. 900 kg CaO powder and app. 275 kWh of chemically-stored thermal energy. The containers are connected to the reactor with an automatic conveying system that induces vacuum (s. VC1, Figure 1) to transport the material in batches in and out of the containers and the reactor.
Starting the operation of the system, CaO is transported inside the reactor by opening V1, V7 and V6 and closing V2, V3 and V4 (s. Figure 1). After the reactor is filled, V7 closes and the water required for the reaction is sprayed into the reaction chamber via a nozzle. This water mass flow rate directly influences the reaction temperature, and is controlled with pulsing a magnet valve (s. V5, Figure 1) coupled with a mass flow meter (s. MFM2, Figure 1). In order to transfer the generated reaction heat, a hot water storage tank is connected to the reactor by a water circuit. By controlling the mass flow (WP1 and MFM3, Figure 1) and measuring the inlet and outlet water temperature of the reactor, the transferred thermal power to the hot water storage tank can be determined. The hot water storage tank has a volume of 250 L and also allows for a targeted setting of the inlet water temperature of the reactor via the use of a conventional heating element in order to simulate different operation scenarios. By the end of the discharging cycle, the Ca(OH)2 batch is transferred from the reactor into the Ca(OH)2 container by opening V3, V4, V7 and closing V1, V2 and V6. Subsequently, a new CaO batch is transferred in the reactor or the experiment ends, depending on the targeted thermal power generation.
2.3 Reactor development
The reactor development was based on the concept of a mechanically-induced fluidised bed. Previous investigations with the concept proved that the rotating ploughshare mixer ensures very good mixing and fluidisation of the fine CaO particles. The fluidisation enables the largest possible contact surface between particles and a reaction partner ensuring an optimal reactivity of the solids in the reactor. The ploughshare mixer additionally helps that no agglomeration of the particles occurs during the processes.
With these advantages in mind, one aim was to design a reactor specifically optimised for the reaction of CaO with liquid water. So far, reactors have always been designed to carry out the charging or discharging reaction of metal hydroxides with water vapour. However, the process requirements with water vapour are fundamentally different and clearly more complex. By inducing liquid water as reaction partner, not only the necessity of a separate evaporation unit can be avoided but also the lower maximum operation temperature also enables the use of more cost-efficient construction materials and reduces the required complexity of the reactor design.
One target was to integrate the heat exchanger into the reactor and to design it thermodynamically that thermal energy at a temperature level of 50°C–60 °C can be extracted from the exothermal reaction. The target temperature level represents general circulation supply temperatures of buildings’ heating circuits. Figure 2 shows the developed reactor. The reaction chamber is (partially) filled with CaO powder and the rotation of the ploughshare mixer induces a fluidisation of the particles. As soon as water is added through a spray nozzle into the chamber the exothermal reaction in the reactor volume is initiated. The released thermal energy is controlled by the supplied mass of water and finally transferred to the reactor jacket. The reactor works in a semi-continuous batch operation. When a batch has completely reacted, the material is automatically conveyed out of the reactor and new material is filled in by the conveying system.
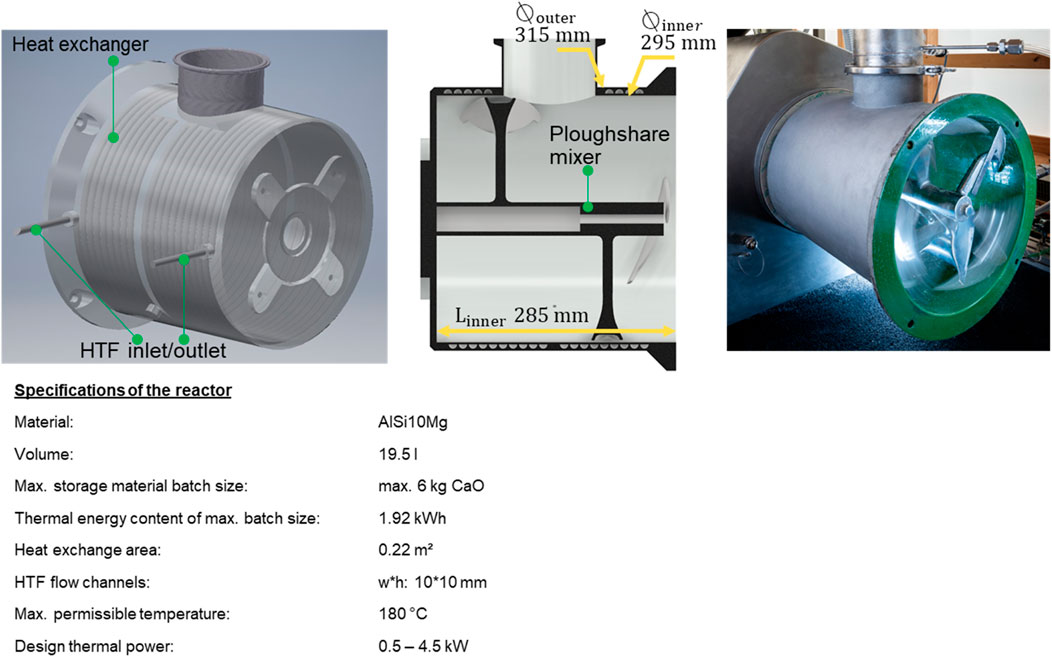
Figure 2. Thermochemical reactor for pilot scale heating system: CAD drawings, photo and technical data.
The thermodynamic design considerations are based on the calculation of the overall heat exchange,
The operation at the lower power level determines the minimal flow rate of the heat transfer fluid. Channels with 10 mm width were designed to ensure that even at the lowest mass flow rate the flow inside the channels is turbulent. By additive manufacturing the direct integration of the heat transfer fluid channel into the reactor jacket was possible. This channel runs from one side of the jacket over the total length to the other side, making use of the maximum available area.
AlSi10 Mg was chosen as construction material. The aluminium alloy offers three distinct advantages compared to stainless steel: it is lighter, less expensive and has higher thermal conductivity. However, aluminium can potentially cause side reactions when in contact with CaO and water, which could lead to degradation of the reactor jacket. To avoid this, a coating of the reactor with a 0.5 mm thick layer of Ethylene Tetrafluoroethylene (ETFE, green colour in Figure 2) was applied. The coating ensures complete chemical resistance of the reactor’s surfaces in contact with the reactive materials.
3 Results of integration and operation in real building environment
As part of the cooperation between the DLR’s Institute of Engineering Thermodynamics and the University of Stuttgart within the frame of the Collaborative Research Centre 1,244, we integrated the pilot system into the adaptive high-rise building, the D1244 on the University Campus. Figure 3 shows the installation of the pilot system in the building. From its operation in real boundary conditions such as outside temperatures, humidity - and even structural vibrations by actively controlled actuators integrated in some of the columns and cross bracings of the building (cf. CRC12441) - conclusions for necessary optimization, e.g., regarding its automated operation, can be drawn.
3.1 Thermochemical heat supply
For the heat supply operation, the following procedure was applied: 4.1 kg of material, corresponding to 3.41 kg CaO, was transported via the automatic conveying system from the container into the reactor. The amount of storage material occupies 60% of the reactors volume in order to leave enough space for the compensation of the volume expansion during the hydration from CaO to Ca(OH)2. The reactor was subsequently closed and a rotation of the ploughshare mixer at a set speed of 250 r/min was started. A mass flow of 25 L/h was adjusted in the water circuit between the reactor and the hot water storage tank. After reaching steady state conditions, water was added into the reaction chamber to initiate the reaction. The water supply rate was regulated to control the reaction temperature.
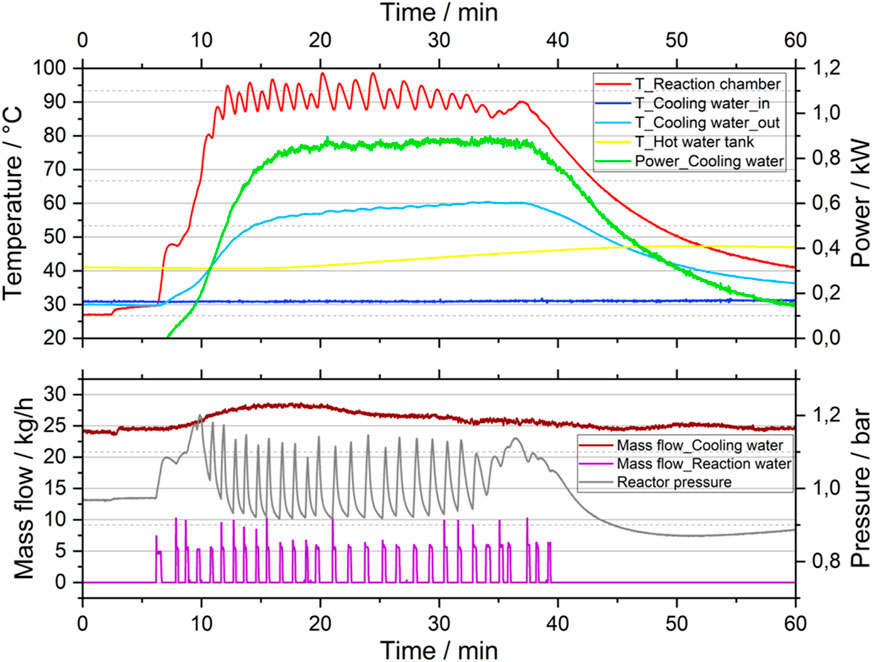
Figure 4 shows the experimental data of this representative discharging process. The upper part of the diagram shows the temperature trends of the material in the reactor and the cooling water inlet and outlet as well as the thermal power transferred to the cooling water during the process. The lower part of the graph shows the mass flow rate of the cooling water and the points in time when water was sprayed into the reaction chamber as well as the pressure in the reactor.
At the beginning of the experiment steady state conditions can be observed, with an inlet and outlet temperature of the cooling water of 31°C and 30°C respectively and a temperature of the solid material in the reactor of 27°C. At the seventh minute the water addition was initiated and as a result an immediate rise of the temperature in the reactor due to the material’s exothermal reaction occurred. In the following minutes water was sprayed from time to time. As a consequence, a steep temperature increase occurs in the reactor reaching 85°C–95°C within 5 min. Once we reached more than 90°C particle temperature we started controlling the water addition in a way to maintain temperatures below 100°C. By pulsing the water injection as soon as the temperature drops below 90°C a particle temperature fluctuation between 85°C and 95°C was maintained for the following 30 min.
The heat of reaction was transferred to the cooling water. During the flow through the reactor jacket the water temperature increased from 31°C at the inlet to 55°C–60°C at the outlet. The inlet temperature was maintained at 31°C in order to represent a recirculation temperature from a heating circuit. The outlet temperature was maintained within the range of 55°C–60°C for app. 25 min. That corresponds to a relatively constant achieved thermal power of 0.87 kW within that time (compare green line in Figure 4). A maximum power of 0.9 kW was reached at the 35th minute. At the 40th minute water was injected once more, yet not further temperature increase took place, what indicated that most of the CaO was converted and the discharging process was technically completed. In the residual time the reaction/material temperature declines as the sensible heat of the hot particles is transferred to the cooling water. The thermal power consequently drops until the experiment was stopped as soon as the material temperature reached 40°C.
As a last step, the reacted material, Ca(OH)2, was automatically transported out of the reactor into the container. The material was in dry, powder form, no agglomerates were visible. Samples of the Ca(OH)2 were taken from the reactor and TGA measurements prove a conversion between 80% and 87%. In principle a new batch of CaO would be introduced subsequently into the reactor to realize a semi-continuous operation. The short time in between the change of batches would not influence the thermal power of the heating system since it is buffered by the hot water storage tank.
The measured effective heat transfer coefficient accounted to app. 85 W/m2K which is in excellent agreement with the assumptions for the reactor development. By operating at a higher reaction temperature of e.g., 180°C this would translate in a specific power density of 11.2 kW/m2. The value underlines the potential of reaching compact reactor designs for the mechanically-induced fluidised bed reactor concept. For example, for a single-family building with 6 kW required thermal power, 0.5 m2 heat exchange surface would be sufficient.
The accumulated thermal energy transferred to the cooling water was measured to 0.525 kWh. 754 g of water was injected and reacted with the present CaO. The released chemical energy based on measured injected water mass accounted 0.756 kWh. Comparing this value to the aforementioned transferred energy, 69.4% of the released chemical energy has been successfully recovered. The remaining part accounts for thermal losses of the current system and will be addressed by ongoing optimization of the system.
4 Discussion
Reactors in kW scale have already been developed and operated for some thermochemical energy storage systems. However, up to now, reactors were mainly designed to investigate single aspects of the process, for example, the characterization of heat and mass transfer phenomena or the proof of the reaction in bulk scale. To our knowledge, none of the reported systems represent the operation of the thermochemical reaction, where the thermal energy released by the reaction is recovered at temperature levels required for the application.
In this work a pilot scale thermochemical heating system was developed and this first-of-its kind unit was installed and tested outside of the laboratory in a test building but without thermal requirements. By this approach the operation of the reaction of CaO with tap water and the extraction and transfer of the reaction heat at a temperature level of 60°C was demonstrated in a relevant building environment.
The achievement of 60°C supply temperature at a recirculation temperature of 31°C proves, that the technology is suitable for implementation in a majority of existing heating infrastructures. It could directly replace fossil fuel burners without the necessity of further adaptions of heating elements or the installation of floor heaters Although operation has not been performed at various ambient conditions, the required supply and recirculation temperatures would not change with different ambient temperatures. It is merely the required thermal power of the reactor that would need to be adjusted to the thermal load of the building. A further testing campaign to determine the adjustability of the pilot system to fluctuating thermal powers is planned.
One essential result of this work is the operation of the reaction of CaO with liquid water. Up to now, thermochemical systems based on hydroxides or hydrates have all been operated with water vapour. Such operation demands complex reactor designs, control of the gas flow, gas-solid separation and high energy demand as well as apparatus expenditure for the vapour supply. The reaction with liquid water enabled the development of a less complex, pressure-less, reactor and therefore made the application of aluminium alloy as lighter and cheaper construction material possible. It was shown that the reaction temperature can be controlled by the water supply rate and with an operation at 90°C reaction temperature a sufficient heating water supply temperature of 60°C was reached. An overall heat transfer coefficient of 85 kW/m2K was achieved. The concept of the mechanically fluidized bed proved to prevent agglomeration between particles and is capable to work with the material in powder form. A comprehensive analysis on the material stability over long term cycling and under different operation conditions is foreseen in the next steps.
Another main result of this work is the demonstration of the separation of the storage and the reactor unit. The storage of the solid in separated containers and the transport to and from the reactor worked well–also outside controlled laboratory conditions. The separation of the units and the material’s transport allows a high degree of conceptual design freedom for potential applications. One example could be a centralized larger charging plant and storage combined with distribution and decentralized discharging in the buildings of a surrounding residential area.
In summary, a fully integrated thermochemical heating system has been demonstrated in an application environment for the first time. Important process steps, like the transport of storage material, the heat supply from the thermochemical reaction and a facilitated reactor design and operation have been proven successfully. The technology can now be scaled into a variety of applications. Currently, improvements in regard to energy efficiency, specific power level, automated control and long-term operation are ongoing.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
MS: Project administration, Writing – original draft, Resources, Conceptualization, Methodology, Supervision, Writing – review and editing, Funding acquisition, Investigation. VS: Investigation, Data curation, Software, Visualization, Formal Analysis, Writing – original draft. VK: Writing – review and editing, Data curation, Investigation, Formal Analysis. ML: Funding acquisition, Resources, Methodology, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was partially funded by Helmoltzgemeinschaft (HGF) in the frame of the Helmholtz Validation Fund, Project- ID HVF-0092 and by Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) Project-ID 279064222 in the frame of the SFB 1244, A05.
Acknowledgments
The Authors thank Andreas Weigl and Christian Brack for their technical support in installation and operation of the pilot plant, as well as Rheinkalk GmbH (Lhoist group) for providing the bulk material.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
1Demonstrator | CRC1244 | University of Stuttgart (www.uni-stuttgart.de).
References
Criado, Y. A., Alonso, M., and Abanades, J. C. (2014). Kinetics of the CaO/Ca(OH)(2) hydration/dehydration reaction for thermochemical energy storage applications. Industrial and Eng. Chem. Res. 53 (32), 12594–12601. doi:10.1021/ie404246p
Dowling, J. A., Rinaldi, K. Z., Ruggles, T. H., Davis, S. J., Yuan, M., Tong, F., et al. (2020). Role of long-duration energy storage in variable renewable electricity systems. Joule 4 (9), 1907–1928. doi:10.1016/j.joule.2020.07.007
Dutta, S., and Shirai, T. (1974). Kinetics of drying and decomposition of calcium hydroxide. Chem. Eng. Sci. 29 (9), 2000–2003. doi:10.1016/0009-2509(74)85021-9
Fennell, P. S., Davis, S. J., and Mohammed, A. (2021). Decarbonizing cement production. Joule 5 (6), 1305–1311. doi:10.1016/j.joule.2021.04.011
Funayama, S., Schmidt, M., Mochizuki, K., Linder, M., Takasu, H., and Kato, Y. (2023). Calcium hydroxide and porous silicon-impregnated silicon carbide-based composites for thermochemical energy storage. Appl. Therm. Eng., 220. doi:10.1016/j.applthermaleng.2022.119675
Gollsch, M., Afflerbach, S., Angadi, B., and Linder, M. (2020). Investigation of calcium hydroxide powder for thermochemical storage modified with nanostructured flow agents. Sol. Energy 201, 810–818. doi:10.1016/j.solener.2020.03.033
Guerra, O. J. (2021). Beyond short-duration energy storage. Nat. Energy 6 (5), 460–461. doi:10.1038/s41560-021-00837-2
Gupta, A., Armatis, P. D., Sabharwall, P., Fronk, B. M., and Utgikar, V. (2021). Kinetics of Ca(OH)2 decomposition in pure Ca(OH)2 and Ca(OH)2-CaTiO3 composite pellets for application in thermochemical energy storage system. Chem. Eng. Sci. 246, 116986. doi:10.1016/j.ces.2021.116986
Hua, W., Yan, H., Zhang, X., Xu, X., Zhang, L., and Shi, Y. (2022). Review of salt hydrates-based thermochemical adsorption thermal storage technologies. J. Energy Storage 56, 106158. doi:10.1016/j.est.2022.106158
Huang, C., Xu, M., and Huai, X. (2019). Experimental investigation on thermodynamic and kinetic of calcium hydroxide dehydration with hexagonal boron nitride doping for thermochemical energy storage. Chem. Eng. Sci. 206, 518–526. doi:10.1016/j.ces.2019.06.002
IEA (2024). Renewables 2023. Available online at: https://www.iea.org/reports/renewables-2023. (Accessed June, 17 2025).
Jashari, A., Sandra, A., Klaus, A., and Wolfgang, K. (2023). Structural stabilization of granular Ca(OH)2 by coating with nanostructured additives for thermochemical cycling in a fixed reaction bed. Energy Convers. Manag. X, 18. doi:10.1016/j.ecmx.2023.100367
Li, S., Huang, H., Yang, X., Wang, C., Kobayashi, N., and Kubota, M. (2016). A facile method to construct graphene oxide–based magnesium hydroxide for chemical heat storage. Nanoscale Microscale Thermophys. Eng. 21 (1), 1–7. doi:10.1080/15567265.2016.1257673
Li, W., Klemeš, J. J., Wang, Q., and Zeng, M. (2022). Salt hydrate–based gas-solid thermochemical energy storage: current progress, challenges, and perspectives. Renew. Sustain. Energy Rev. 154, 111846. doi:10.1016/j.rser.2021.111846
Li, X., and Yao, R. (2021). Modelling heating and cooling energy demand for building stock using a hybrid approach. Energy Build., 235. doi:10.1016/j.enbuild.2021.110740
Lucke, B. W., Afflerbach, S., and Krumm, W. (2023). Optimization of the coating process of a Ca(OH)2-Based thermochemical energy storage material. Chem. Ing. Tech. 95 (12), 1951–1959. doi:10.1002/cite.202300081
Marie, L. F., Landini, S., Bae, D., Francia, V., and O'Donovan, T. (2022). Advances in thermochemical energy storage and fluidised beds for domestic heat. J. Energy Storage 53, 105242. doi:10.1016/j.est.2022.105242
Maruyama, A., Kurosawa, R., and Ryu, J. (2020). Effect of lithium compound addition on the dehydration and hydration of calcium hydroxide as a chemical heat storage material. ACS Omega 5 (17), 9820–9829. doi:10.1021/acsomega.9b04444
Moulakhnif, K., Ait Ousaleh, H., Sair, S., Bouhaj, Y., El Majd, A., Ghazoui, M., et al. (2024). Renewable approaches to building heat: exploring cutting-edge innovations in thermochemical energy storage for building heating. Energy Build. 318, 114421. doi:10.1016/j.enbuild.2024.114421
Risthaus, K., Bürger, I., Linder, M., and Schmidt, M. (2020). Numerical analysis of the hydration of calcium oxide in a fixed bed reactor based on lab-scale experiments. Appl. Energy 261, 114351. doi:10.1016/j.apenergy.2019.114351
Risthaus, K., Linder, M., and Schmidt, M. (2022). Experimental investigation of a novel mechanically fluidized bed reactor for thermochemical energy storage with calcium hydroxide/calcium oxide. Appl. Energy 315, 118976. doi:10.1016/j.apenergy.2022.118976
Roßkopf, C., Haas, M., Faik, A., Linder, M., and Wörner, A. (2014). Improving powder bed properties for thermochemical storage by adding nanoparticles. Energy Convers. Manag. 86, 93–98. doi:10.1016/j.enconman.2014.05.017
Schaube, F., Koch, L., Wörner, A., and Müller-Steinhagen, H. (2012). A thermodynamic and kinetic study of the de- and rehydration of Ca(OH)(2) at high H2O partial pressures for thermo-chemical heat storage. Thermochim. Acta 538, 9–20. doi:10.1016/j.tca.2012.03.003
Schmidt, M., Gollsch, M., Giger, F., Grün, M., and Linder, M. (2016). “Development of a moving bed pilot plant for thermochemical energy storage with CaO/Ca(OH)2,” in Solarpaces 2015: international conference on concentrating solar power and chemical energy systems, Cape Town, South Africa: AIP Conference Proceedings. doi:10.1063/1.4949139
Schmidt, M., and Linder, M. (2020). A novel thermochemical long term storage concept: balance of renewable electricity and heat demand in buildings. Front. Energy Res. 8. doi:10.3389/fenrg.2020.00137
Valverde-Pizarro, C. M., Briones, L., Sanz, E., Escola, J., Sanz, R., González-Aguilar, J., et al. (2020). Coating of Ca(OH)2/γ-Al2O3 pellets with mesoporous Al2O3 and its application in thermochemical heat storage for CSP plants. Renew. Energy 162, 587–595. doi:10.1016/j.renene.2020.08.095
Xia, B. Q., Zhao, C., Yan, J., and Khosa, A. (2020). Development of granular thermochemical heat storage composite based on calcium oxide. Renew. Energy 147, 969–978. doi:10.1016/j.renene.2019.09.065
Xu, M., Huai, X., and Cai, J. (2017). Agglomeration behavior of calcium hydroxide/calcium oxide as thermochemical heat storage material: a reactive molecular dynamics study. J. Phys. Chem. C 121 (5), 3025–3033. doi:10.1021/acs.jpcc.6b08615
Keywords: thermochemcial energy storage, seasonal energy storage, calcium hydroxide (Ca(OH)2), calcium oxide (CaO), thermal energy storage (TES) systems
Citation: Schmidt M, Sourmelis V, Kühl V and Linder M (2025) Zero emission heating with calcium oxide and water: development and demonstration of first pilot scale thermochemical heating system for buildings. Front. Energy Res. 13:1617554. doi: 10.3389/fenrg.2025.1617554
Received: 24 April 2025; Accepted: 16 June 2025;
Published: 26 June 2025.
Edited by:
Xinyu Huang, Xi’an Jiaotong University, ChinaReviewed by:
Abdalqader Ahmad, University of Birmingham, United KingdomAnnarita Spadoni, Italian National Agency for New Technologies, Energy and Sustainable Economic Development (ENEA), Italy
Amira Dellagi, Research and Technology Center of Energy, Tunisia
Copyright © 2025 Schmidt, Sourmelis, Kühl and Linder. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Matthias Schmidt, bWF0dGhpYXMuc2NobWlkdEBkbHIuZGU=
 Matthias Schmidt
Matthias Schmidt Venizelos Sourmelis
Venizelos Sourmelis Viktor Kühl
Viktor Kühl