- 1Department of Mechanical Engineering, University of Alberta, Edmonton, AB, Canada
- 2Department of Mechanical Engineering, University of Benin, Benin City, Edo, Nigeria
Thermal energy storage (TES) technologies are emerging as key enablers of sustainable energy systems by providing flexibility and efficiency in managing thermal resources across diverse applications. This review comprehensively examines the latest advancements in TES mechanisms, materials, and structural designs, including sensible heat, latent heat, and thermochemical storage systems. Recent innovations in nano-enhanced phase change materials (PCMs), hybrid TES configurations, and intelligent system integration are highlighted. The role of advanced computational methods, such as digital twins and AI-based optimization, in enhancing TES performance is also explored. Applications in renewable energy systems, industrial processes, district heating networks, and green hydrogen production are discussed, along with associated challenges and future research directions. This review aims to synthesize current knowledge while identifying pathways for accelerating the development and practical deployment of next-generation TES technologies.
1 Introduction
Energy demand has continued to rise, with a notable 2.2% increase recorded in 2024, significantly above the 1.3% average annual growth observed over the past decade (IEA, 2025). This surge can be attributed to factors such as income growth, industrial expansion, structural shifts in the global economy, and the rapid proliferation of data centers and artificial intelligence technologies (Saouter and Gibon, 2024; Kwasi-Effah et al., 2022a). To meet rising global electricity demand, the energy sector has significantly increased investments in renewable and nuclear power. In 2023, renewable capacity additions reached a record ∼510 GW (mostly solar and wind), marking the 22nd consecutive year of growth (IRENA, 2025). Preliminary 2024 estimates suggest further growth, potentially nearing 600–700 GW (IEA, 2025). Nuclear power also expanded, with 5–10 GW of new capacity added in 2023, the highest in a decade as countries revisited nuclear for energy security (Fattouh et al., 2024). Together, renewables and nuclear accounted for about 80% of net electricity generation growth in 2023, pushing their combined share to about 38%–40% of global electricity (Energy Institute, 2024; IEA 2025).
Renewable energy sources such as solar and wind are inherently intermittent, as their availability depends on weather patterns and the time of day (Kwasi-Effah et al., 2022b; Qudrat-Ullah, 2024). To maintain a stable and reliable energy supply, effective energy storage solutions are essential. Although batteries, particularly lithium-ion are often at the forefront of these discussions, thermal energy storage (TES) is gaining traction as a vital technology, especially for industrial heating, concentrated solar power (CSP) systems, and grid stabilization (Pengonda et al., 2024; Verma et al., 2024). By heating or cooling a storage material, thermal energy storage (TES) technology stores thermal energy that can be used later for power generation, heating, or cooling. By storing surplus energy when it is available and releasing it when needed, TES systems assist the integration of renewable energy sources, enhance energy efficiency, and balance the supply and demand for energy (Mitali et al., 2022). Other forms of energy storage include mechanical storage, such as compressed air energy storage and pumped hydro storage; electrochemical storage, which involves lithium-ion and lead-acid batteries; electrical storage using supercapacitors; and chemical storage through hydrogen fuel systems (Kumi, 2023; Kwasi-Effah and Rabczuk, 2018).
Thermal energy storage (TES) differs from other energy storage methods primarily in its mechanism of storing energy as heat rather than electricity, mechanical energy, or chemical potential (Kwasi-Effah et al., 2024; Elkhatat and Al-Muhtaseb, 2023). TES devices store energy by heating or cooling a medium (sensible heat), taking advantage of phase shifts (latent heat), or initiating reversible chemical reactions (thermochemical storage), whereas electrochemical batteries (such as lithium-ion) store energy in chemical bonds and release it as electricity (Zhang J. et al., 2022). In contrast to pumped hydro or compressed air storage, which depend on gravitational potential or pressurized air, thermal energy storage demonstrates notable effectiveness in addressing heating and cooling requirements across buildings, industrial processes, and concentrated solar power plants (Alanazi, 2023).
A key advantage of TES over electrical storage is its ability to store large amounts of energy at relatively low cost, especially in high-temperature applications like industrial heat recovery and CSP (Agalit et al., 2020). Although, TES generally exhibits lower round trip efficiency than batteries, primarily due to energy losses during heat to electricity conversion, it offers several compelling advantages over lithium ion battery systems (Olympios et al., 2021). These include lower cost, greater scalability, longer operational lifespan, enhanced safety, and superior suitability for applications requiring thermal energy (Sarbu and Sebarchievici, 2018). Additionally, TES plays a vital role in enabling the integration of renewable energy, particularly in concentrated solar power (CSP) plants, by allowing for reliable power generation even during periods without sunlight (Alami et al., 2023; Palacios et al., 2020). Furthermore, TES systems often utilize abundant, non-toxic materials, making them a more environmentally sustainable alternative to certain electrochemical storage technologies that rely on scarce or hazardous components (Nadeem et al., 2019).
Thermal energy storage (TES) plays a vital role in the integration of renewable energy sources and in addressing supply-demand discrepancies, particularly within solar and industrial sectors (Zhao et al., 2023). Notable implementations include molten salt systems utilized in concentrated solar power (CSP) facilities, such as Spain’s Gemasolar plant, which facilitates continuous operation (Tamme et al., 2022; Zhao et al., 2023). Additionally, Phase change materials (PCMs) are gaining traction in building applications to enhance HVAC efficiency, while the recovery of industrial waste heat capitalizes on sensible heat storage (Sarbu and Sebarchievici, 2018). However, despite these advancements, challenges remain, including issues related to cost and material stability, especially in high-temperature scenarios (Zhao et al., 2023). The adoption of TES is on the rise, with market growth anticipated at approximately 10% CAGR, propelled by decarbonization initiatives (BTO, 2024).
Thermal Energy Storage (TES) has diverse and expanding applications across multiple sectors. In concentrated solar power (CSP) plants, molten salt TES enables continuous electricity generation beyond daylight hours, thereby enhancing grid reliability (Pelay et al., 2017). For industrial processes, high-temperature TES facilitates the recovery of waste heat from energy-intensive sectors such as steel and cement production, significantly reducing fossil fuel consumption (Sarbu and Sebarchievici, 2018). In buildings, phase change materials (PCMs) integrated into walls and HVAC systems help shift cooling loads and reduce peak electricity demand (Rai, 2021). District heating networks increasingly adopt large-scale water tanks to store surplus renewable heat seasonally, contributing to more stable and resilient energy systems (Sarbu et al., 2022). In the transportation sector, TES systems utilizing PCMs support refrigeration units by maintaining temperatures without continuous energy input (Fattouh et al., 2024).
Emerging applications further highlight the versatility of TES. Thermal batteries, solid-state systems using materials such as silicon or graphite are gaining traction for modular, high-energy-density storage in off-grid, residential, and defense contexts (Muthu et al., 2024). Additionally, electricity-to-heat-to-electricity (E2H2E) systems are being developed for long-duration storage, in which excess electricity is stored thermally and later converted back to electricity using advanced cycles such as thermophotovoltaic or Brayton systems (Olympios et al., 2022). TES is also being explored in green hydrogen production, where it helps stabilize thermal inputs for high-efficiency electrolysis (Pardo-Pina et al., 2024). Furthermore, TES supports efficient cooling in data centers and electronics, decarbonized district cooling using chilled water or ice storage, and renewable-powered desalination processes through solar thermal integration (Alkrush et al., 2024; Zheng et al., 2023). These advancements reflect TES’s critical role in enhancing energy flexibility, supporting decarbonization, and enabling sustainable energy transitions across sectors.
Several review papers have explored energy storage systems, including thermal energy storage (TES), across various applications beyond renewable energy integration. However, few offer a comprehensive analysis of TES technologies, mechanisms, and their diverse applications in sectors such as agriculture, buildings, smart grids, concentrated solar power, data centers, and hydrogen storage. For instance, Tan et al. (2021) presented a broad overview of energy storage systems including mechanical, electrical, electromagnetic, and thermal storage but offered only minimal coverage of TES, with no in-depth discussion of its mechanisms, material classifications, or sector-specific applications. Similarly, Chavan et al. (2022) explored TES in the context of waste heat recovery and its role in sustainable development, but the review was limited in scope, lacking coverage of recent technological advancements, emerging materials, and diverse application areas. Tawalbeh et al. (2023) conducted a material-focused review of TES, analyzing developments in sensible and latent heat storage materials, as well as encapsulation techniques. However, this review was narrowly centered on material science, omitting discussion on TES mechanisms, system integration, and innovative applications such as hybrid systems and hydrogen storage. Emrani and Berrada (2024) provided a techno-economic assessment of energy storage technologies with emphasis on renewable energy systems, but did not examine TES systems in detail, nor did it categorize TES types or discuss recent trends. Elalfy et al. (2024) reviewed energy storage methods across thermal, chemical, electrochemical, and hybrid systems, yet failed to address TES-specific mechanisms, materials innovation, or its expanding role in sustainable energy transitions.
In contrast, this review aims to fill these gaps by presenting a comprehensive synthesis of recent innovations in thermal energy storage. It focuses on the classification and operation of TES systems including sensible, latent, and thermochemical mechanisms while highlighting emerging materials such as PCM encapsulation, nano-enhanced PCMs, novel reactor designs, perovskite-based thermochemical compounds, and hybrid configurations. It further explores TES integration into advanced applications like concentrated solar power (CSP), green hydrogen production, industrial waste heat recovery, smart grids, and data center cooling. By combining technical insights with real-world case studies and techno-economic evaluations, this paper aims to accelerate the deployment of next-generation TES solutions for a resilient, low-carbon energy future.
2 Fundamentals of TES
Thermal Energy Storage (TES) systems operate on three primary mechanisms: sensible heat, latent heat, and thermochemical storage. Sensible heat storage is the process of increasing a material’s temperature without altering its phase. Energy is stored by common materials like water, oils, and molten salts because their intrinsic energy increases with temperature. The specific heat capacity, mass, and temperature change of the material all affect how much energy it can store (Sadeghi, 2022). The basis for latent heat storage is phase change materials (PCMs), such salt hydrates or paraffin wax, which absorb or release large amounts of heat during a phase transition (solid to liquid, for example) at a temperature that is almost constant (Aftab et al., 2021). Building applications and industrial heating benefit greatly from this process since it allows for a higher energy density within a more constrained temperature range (Zhang J. et al., 2022). Thermochemical storage uses reversible chemical reactions to store energy. While substantial research and development is still ongoing, these systems offer the maximum theoretical energy density and can allow long-duration storage with negligible losses over time (Bao and Ma, 2022). Figure 1 illustrate the various classification of thermal energy storage.
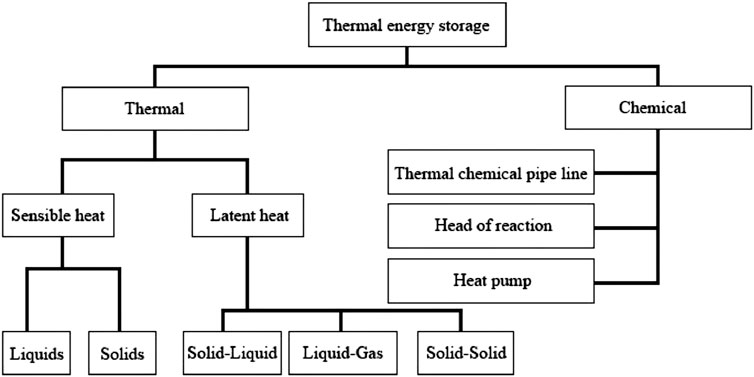
Figure 1. Classification of thermal energy storage (Sarbu and Sebarchievici, 2018).
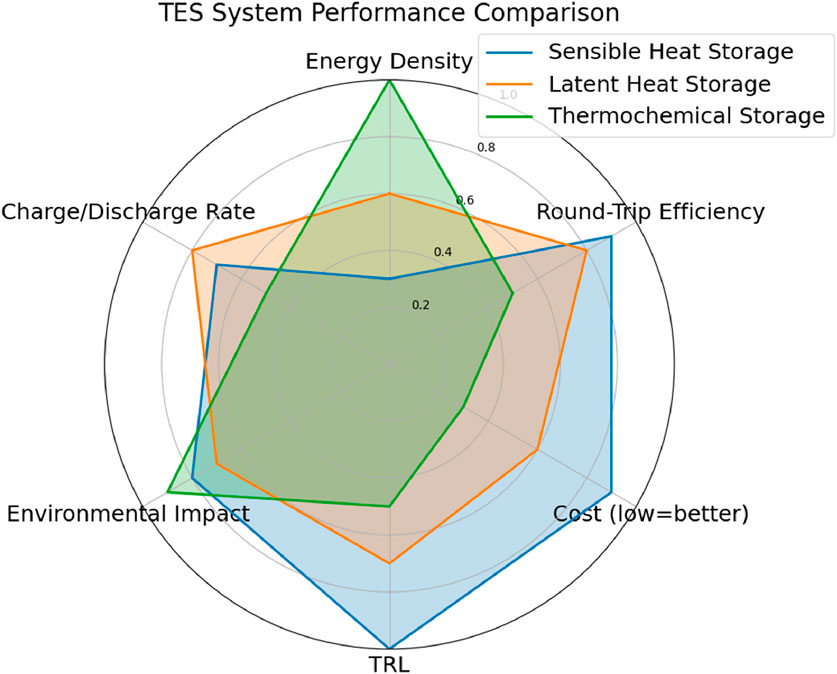
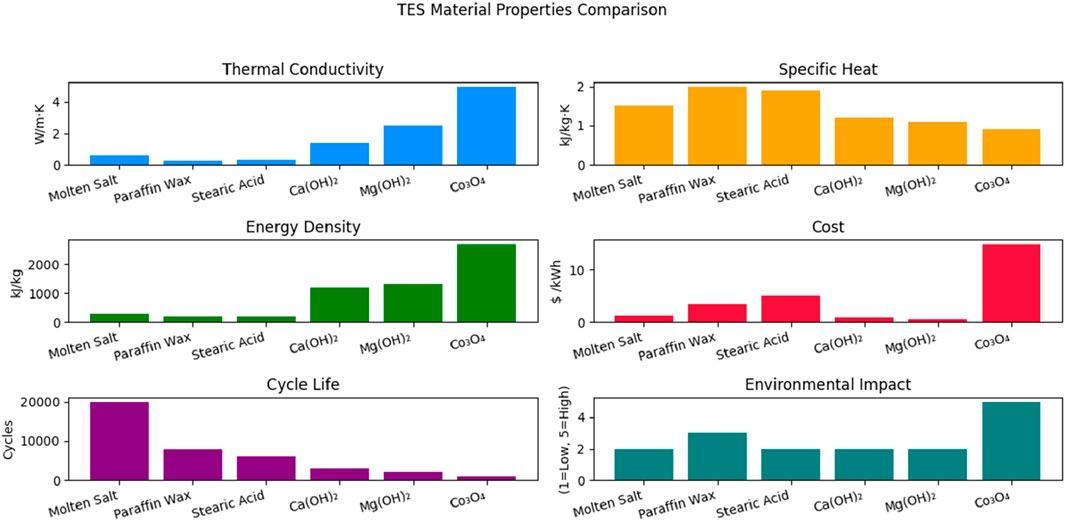
Thermal energy storage performance is characterized by several critical parameters that determine its efficiency, cost-effectiveness, and suitability for specific applications. Table 1 and Figure 2 illustrates the various thermal energy storage parameters and provides a comparison among sensible heat storage, latent heat storage, and thermochemical storage systems. Table 2 provides a concise comparison of sensible, latent, and thermochemical energy storage materials based on thermal properties, cost, durability, and environmental impact. It highlights the trade-offs between energy density and sustainability, offering insights for selecting materials tailored to specific TES applications. Below are the most important parameters, along with their significance and typical values for modern TES systems:
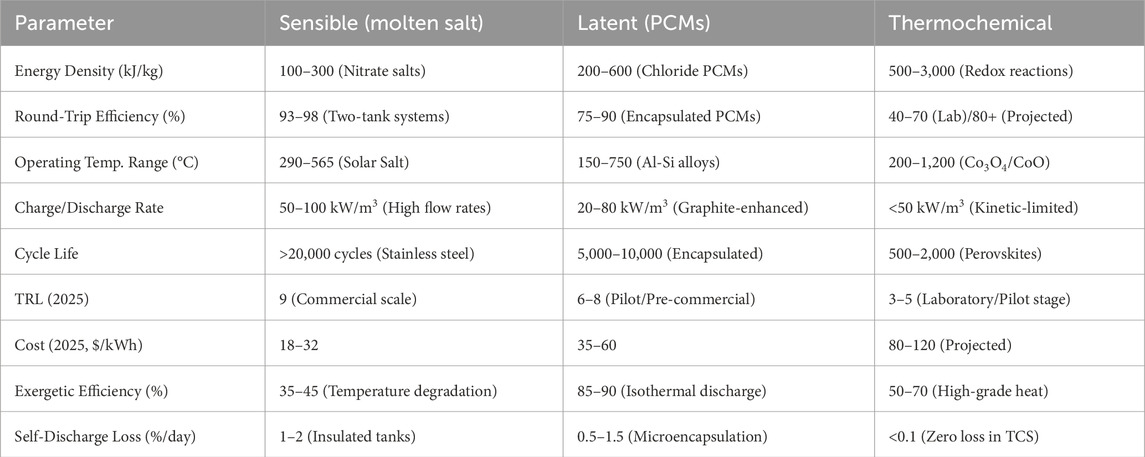
Table 1. Comparison of latent storage, sensible heat storage systems and thermochemical storage parameters (Zahid et al., 2022; Ochmann et al., 2023; Sarbu and Sebarchievici, 2018; Bauer, 2021).
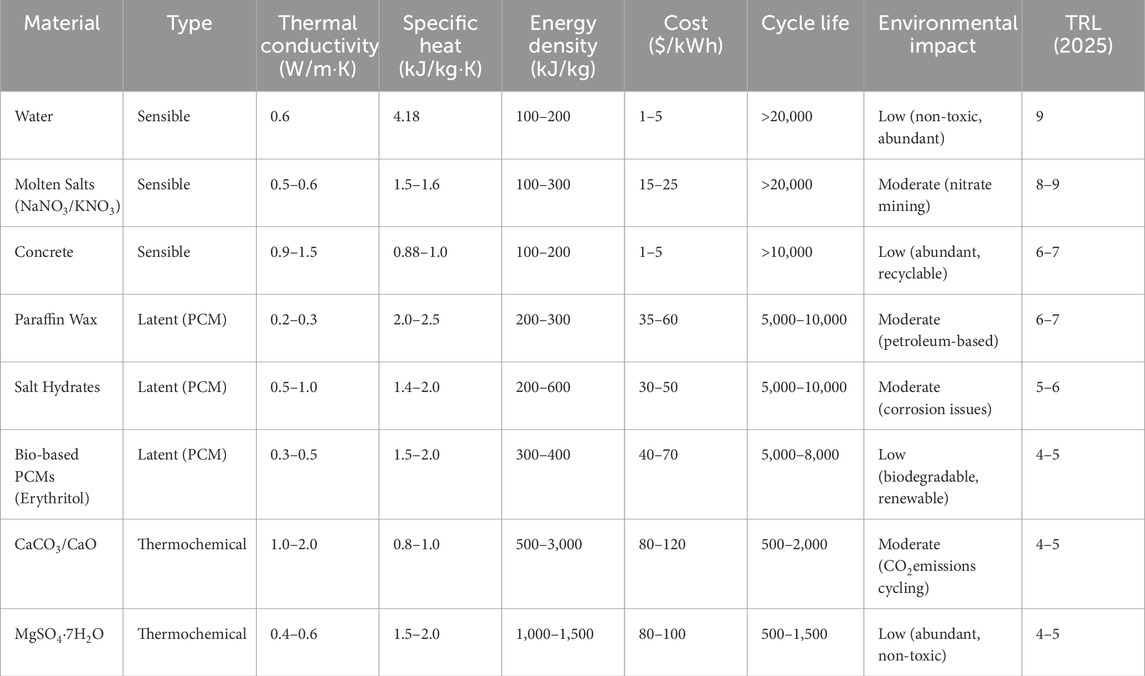
Table 2. Comparative overview of thermal energy storage materials by type and key performance metrics (Dixit et al., 2022; Liu Y. et al., 2024; Yuan et al., 2018).
Energy Density: Energy density indicates the amount of energy stored per unit volume or mass. It varies significantly among the three types. Sensible systems typically offer lower energy densities but have simpler and more mature designs. Latent systems provide higher energy density due to the enthalpy of phase change, while thermochemical systems can theoretically store more energy by leveraging endothermic and exothermic reactions (Aftab et al., 2021; Eze and Tamball, 2024).
Charge and Discharge cycle: The ability of a material to reversibly absorb and release heat over many cycles without significant degradation defines its charge and discharge performance (Wu et al., 2021). In sensible heat storage systems, the rates of charging and discharging are primarily influenced by the temperature gradient and the thermal conductivity of the storage medium. In latent heat systems, phase change processes can maintain a nearly constant temperature during charging and discharging, thereby enhancing overall system efficiency (Zahid et al., 2022).
Round Trip Efficiency: Round-trip efficiency measures how effectively the system can store and retrieve thermal energy. Sensible and latent systems often reach efficiencies of 70%–90%, depending on insulation and temperature losses, while thermochemical systems may be lower but offer better long-term storage potential (Vahidhosseini et al., 2024).
Operating Temperature Range: The TES media can efficiently store and release thermal energy within this temperature range without experiencing any degradation or loss of functionality (Ding et al., 2021). This specifies the usable heat for the intended use and needs to correspond with the thermal cycle of the system (for example, industrial waste heat recovery or CSP).
Cycle Life: This is the maximum number of full charge-discharge cycles that a TES system can have before seeing a noticeable decline in performance. It denotes robustness and long-term feasibility, particularly for everyday riding in industrial or renewable integration applications (Aghmadi and Mohammed, 2024).
Cost Metrics: The cost per unit of energy capacity ($/kWh) or power capacity ($/kW) is typically used to illustrate the capital and operating expenses related to putting in place a TES system (Elkhatat and Al-Muhtaseb, 2023). Economic viability requires a lower cost per kWh, particularly when compared to battery or pumped hydro systems (Walker and Duquette, 2022).
Exergetic Efficiency: This is the ratio of the input energy to the output useful energy (work potential). It takes into consideration thermal energy’s quality as well as its quantity (Akci Turgut and Dincer, 2023). In a thermodynamic sense, it aids in evaluating how well a TES system transforms stored heat back into work or useable energy.
Self-Discharge Loss: When the TES system is not actively charging or discharging, this is the gradual loss of thermal energy that has been stored. Caused by heat leaking through inadequate insulation; peak shaving and long-term storage require minimal self-discharge (Elalfy et al., 2024).
3 Sensible heat storage
Sensible heat storage (SHS), the most commercially mature thermal energy storage (TES) technology, stores thermal energy by changing the temperature of a solid or liquid medium without causing a phase change (Kwasi-Effah et al., 2023; Seyitini et al., 2023). It is extensively utilized in district heating systems, industrial waste heat recovery, and concentrated solar power (CSP) plants. The energy stored in SHS depends on the material’s mass, specific heat capacity, and temperature change (Aftab et al., 2021). With no phase change involved, the process is straightforward and predictable. Figure 3 demonstrates the process of sensible heat storage. The stored thermal energy is given in Equation 1.
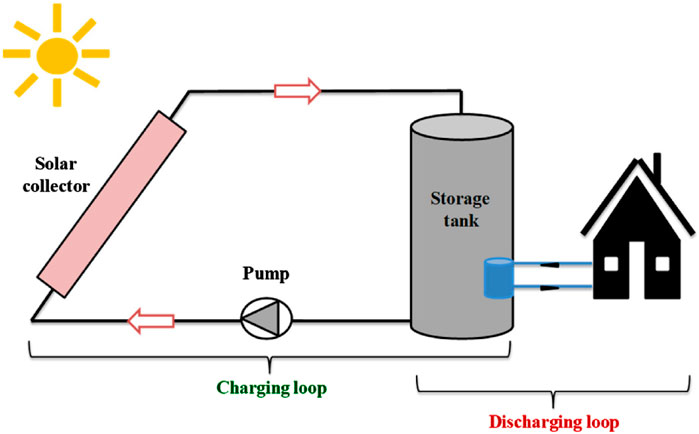
Figure 3. Sensible Heat storage system (Dincer and Ezan, 2018).
Where Q is the sensible heat stored (joules), m is mass of the substance (Kg),
3.1 Sensible heat materials
Table 3 presents commonly used materials for sensible heat storage (SHS), selected based on their specific heat capacity, operating temperature range, thermal conductivity, and density. These properties directly influence the energy storage capacity, charging/discharging rate, and system compactness. Water remains one of the most efficient SHS media due to its high specific heat capacity (4.18 kJ/kg·K), high density (∼1,000 kg/m3), and excellent thermal conductivity (0.6 W/m·K). However, its use is restricted to applications within 0°C–100°C due to phase change limitations. Concrete, widely used in building-integrated storage, has a specific heat between 0.88 and 1.0 kJ/kg·K and operates effectively up to 400°C. Its density (2,300–2,400 kg/m3) and moderate thermal conductivity (1.1–1.4 W/m·K) make it ideal for stationary systems. Cast iron offers a compact thermal storage solution with its high density (∼7,200 kg/m3), decent thermal conductivity (∼55 W/m·K), and operational stability up to 500°C, although its specific heat is relatively low at 0.46 kJ/kg·K. Molten salts such as sodium nitrate (NaNO3) and potassium nitrate (KNO3) are widely adopted in solar thermal plants due to their specific heat (1.5–1.6 kJ/kg·K), high operating range (200°C–600°C), and adequate thermal conductivity (0.5–0.6 W/m·K). Their densities typically range from 1,800 to 2,100 kg/m3. Rocks like basalt are valued for high-temperature storage, sustaining up to 1,000°C. With a specific heat of approximately 0.79 kJ/kg·K, density around 3,000 kg/m3, and thermal conductivity ranging from 1.5 to 3.0 W/m·K, they are ideal for packed-bed systems in industrial heat recovery and CSP plants.
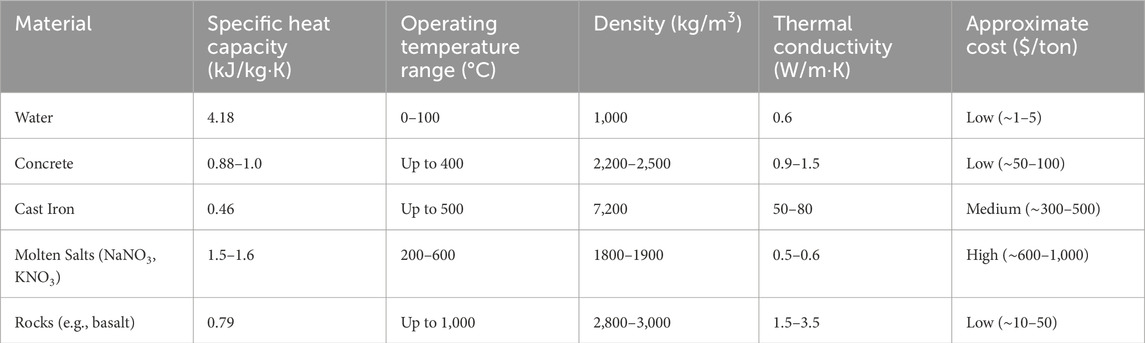
Table 3. Common sensible heat storage materials (Pompei et al., 2023; Yang et al., 2020).
3.2 Tank Based Storage Systems
Tank-based sensible heat storage (SHS) systems are well-established and widely used in residential and industrial applications. These systems typically employ fluids such as water, thermal oils, or molten salts contained within heavily insulated tanks to minimize heat losses (Khan et al., 2024). Without a phase change, the storing mechanism depends on raising or lowering the fluid’s temperature throughout cycles of charging and discharging. The development of efficient intake diffuser designs brought about by advances in computational fluid dynamics (CFD) has greatly improved thermal stratification and achieved stratification efficiencies surpassing 90%, which in turn has reduced mixing losses (Barrak et al., 2024; Chekifi and Boukraa, 2023). Water-based tank systems are commonly used in residential heating due to their low cost and their suitability for low-to-moderate temperature ranges (Kousksou et al., 2014). Synthetic thermal oils are frequently used as heat transfer fluids (HTFs) in industrial settings because of their capacity to function at higher temperatures, usually up to 400°C https://doi.org/10.3390/en14227486. Eutectic mixes of diphenyl and diphenyl oxide, marketed as Therminol VP-1 or Dowtherm A, are common synthetic thermal oils in the 12°C–400°C temperature range (Alva et al., 2018). They are stable and effective. In concentrated solar power (CSP) facilities and other industrial operations that need high-temperature heat transfer, these oils are very common. Innovations like polymer liners and ceramic internal coatings have been introduced to increase the longevity and durability of storage tanks operating at elevated temperatures. These materials improve resistance to corrosion and material degradation, guaranteeing the tanks’ structural integrity over extended periods of operation (Magliano et al., 2024). Overall, tank-based thermal energy storage systems offer the advantages of operational simplicity, high technology readiness levels, and cost-competitiveness. However, their energy density is generally lower compared to latent heat and thermochemical storage systems, which can limit their applicability in scenarios where space and weight are critical considerations (Mohammad, 2024). Figure 4 depicts a tank storage system integrated with a solar collector and boiler for supplying domestic hot water and space heating.
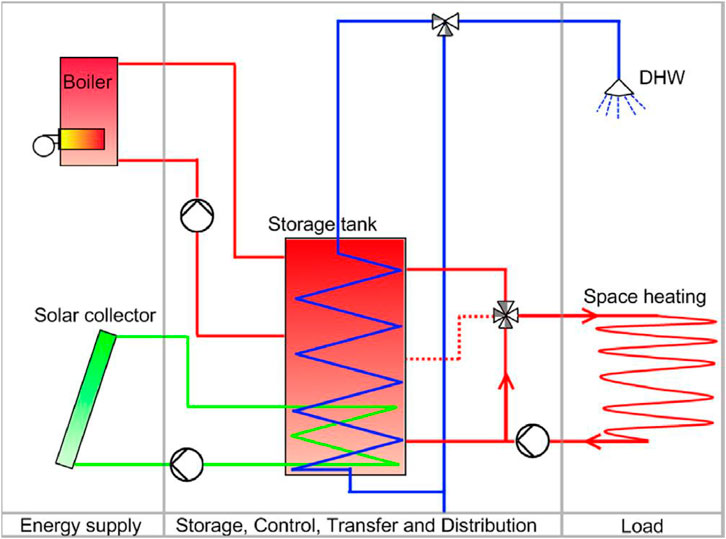
Figure 4. Tank based storage systems (Heier et al., 2015).
3.3 Packed-bed systems
The main heat storage medium of packed bed thermal energy storage (TES) systems is made of solid materials like alumina, ceramics, or natural rocks. As shown in Figure 5 these systems transmit thermal energy to or from the solid materials without causing a phase transition by passing a heat transfer fluid usually air or thermal oil through the packed bed. The inexpensive cost of materials and straightforward construction of packed bed systems make them especially appealing (Hrifech et al., 2020). According to Strasser and Selvam (2014), these systems typically operate in the 120°C–325°C temperature range, and they cost about $30 per kWh. The design configuration and operating factors, like flow rates and bed packing configurations, have a significant impact on efficiency.
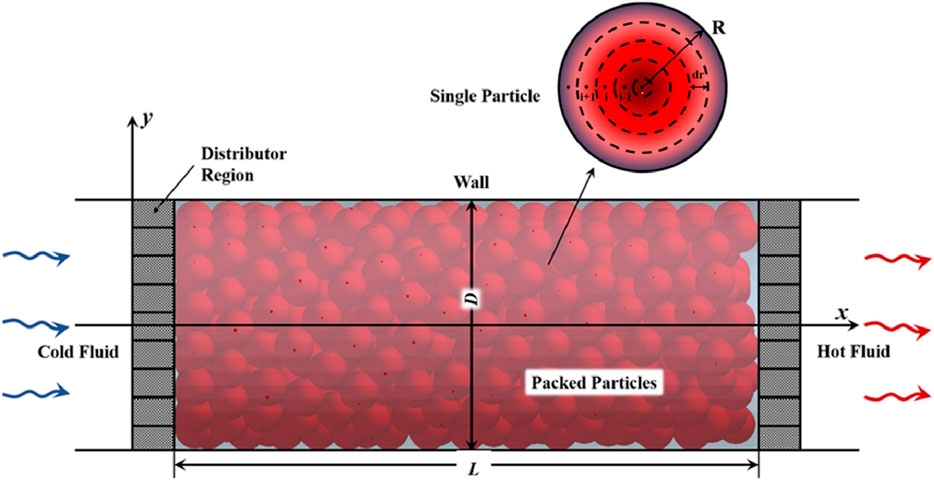
Figure 5. Packed bed storage (Ma et al., 2023a).
3.4 Concrete thermal energy storage
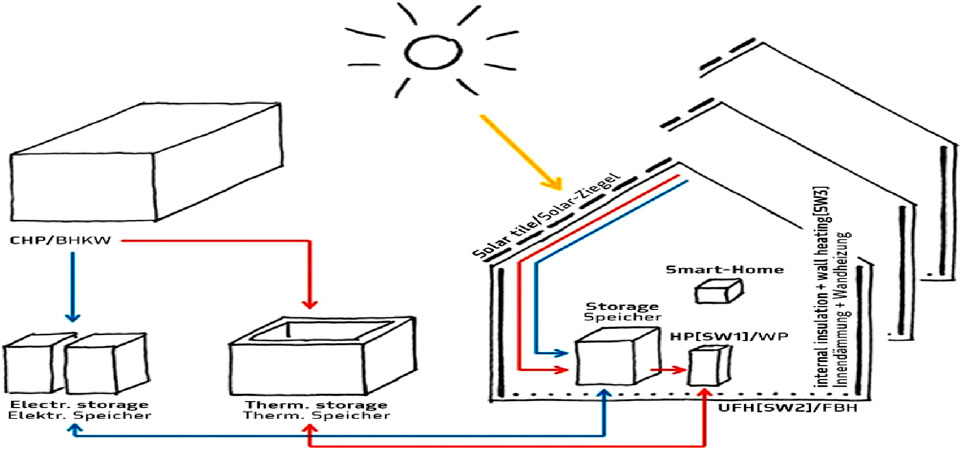
TES systems based on concrete heat vast amounts of specially prepared high-temperature concrete to store thermal energy. Concrete is suited for medium-to high-temperature applications because of its high specific heat capacity, which allows it to absorb and hold onto large amounts of thermal energy (Carrillo et al., 2019). At temperatures as high as 400°C, concrete storage systems have proven to operate dependably. Using concrete materials for storage has a relatively cheap cost of about $1 per kWh (Panneer Selvam et al., 2013). Depending on the insulation quality and system design, these systems can also have significant thermal efficiency. Concrete’s mechanical strength and thermal stability have been improved recently for long-term use (Hoivik et al., 2019). Figure 6 illustrates a smart home energy system incorporating concrete thermal energy storage for storing and distributing heat collected from solar tiles and a combined heat and power (CHP) unit.
3.5 Molten salts storage system
Molten salt systems represent a mature and highly efficient technology for large-scale thermal energy storage, particularly in concentrated solar power (CSP) applications. These systems typically employ mixtures of sodium nitrate (NaNO3), potassium nitrate (KNO3), and lithium nitrate (LiNO3) as the storage medium (Ayinla et al., 2024). The salts are heated to a molten state, allowing thermal storage at operational temperatures typically between 290°C and 565°C. At commercial stage, the cost of molten salt storage is estimated between $15–25 per kWh, offering a highly competitive solution relative to alternative storage technologies (Roper et al., 2022). Thermal efficiencies of molten salt systems are remarkably high, often ranging from 90% to 99%, depending on the heat exchange and insulation strategies employed (Roper et al., 2022). Figure 7 shows a molten salt thermal energy storage system integrated with a solar tower and Rankine cycle for efficient solar power generation.
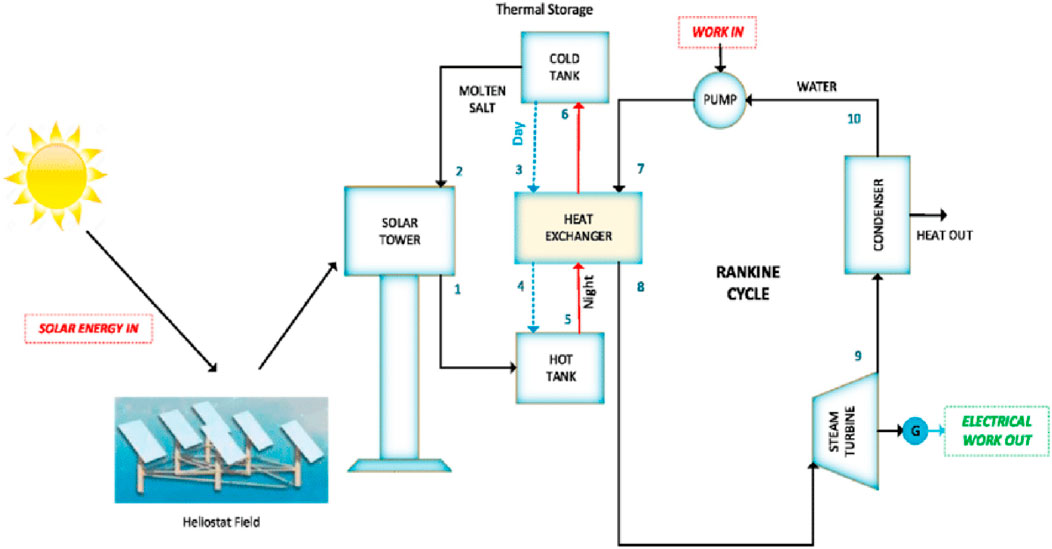
Figure 7. Molten salt storage systems (AlShafi and Bicer, 2021).
4 Latent heat
Latent heat storage (LHS) is an efficient method of thermal energy storage that utilizes phase change materials (PCMs) to store and release large amounts of energy during phase transitions (e.g., solid-liquid, liquid-gas). Unlike sensible heat storage, LHS occurs at a nearly constant temperature, making it ideal for applications requiring precise thermal regulation, such as solar energy systems, building thermal management, and electronic cooling. During the melting process, a material transitions from a solid to a liquid state by absorbing heat without a corresponding increase in temperature. This process is endothermic in nature, and the absorbed energy is stored as latent heat within the material’s molecular structure. Conversely, during freezing, the material undergoes a phase change from liquid back to solid, releasing the stored thermal energy into the surrounding environment. This exothermic process makes the latent heat available for energy recovery applications. Similarly, during vaporization, the phase transition from liquid to gas involves the absorption of a significant amount of thermal energy, known as the latent heat of vaporization. This mechanism requires a higher energy input compared to melting, due to the greater molecular separation involved in the liquid-to-gas transformation. The reverse process, condensation, entails the release of this stored energy as the material transitions from the gaseous to the liquid phase, also at a nearly constant temperature (Aljabair et al., 2023; Liu K. et al., 2024; Upadhyay and Singhal, 2023). Figure 8 depicts a solar collector system with a packed bed storage tank, illustrating the charging and discharging loops for latent heat storage, where hot air is loaded and cold air is returned to manage energy transfer efficiently.
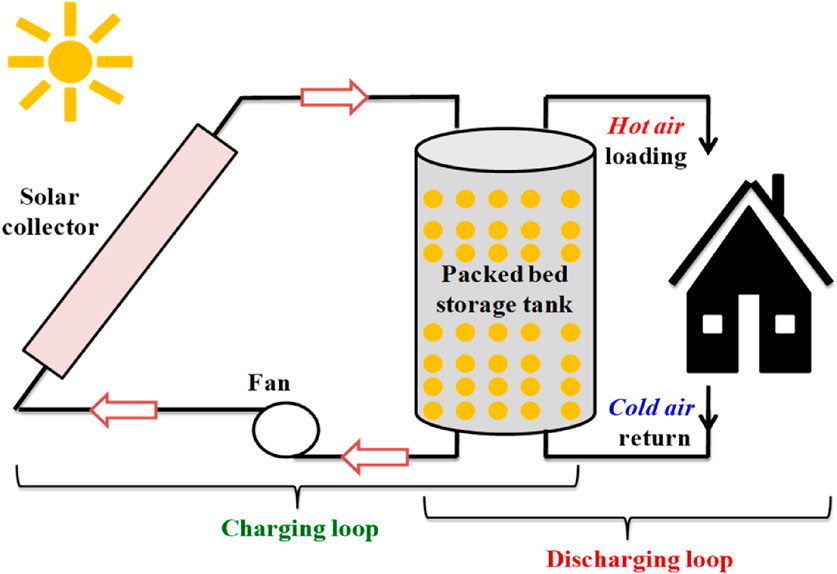
Figure 8. Latent heat storage systems (Dincer and Ezan, 2018).
These phase change processes enable latent heat storage systems to achieve high energy densities and maintain relatively stable operating temperatures, which are critical advantages for a range of thermal energy storage applications. The governing equations for these systems are derived from fundamental heat transfer principles, incorporating sensible heat, latent heat, and the interface motion during phase change. The energy conservation equation in a latent heat system, based on Fourier’s law of heat conduction as given by Equation 2 and the latent heat energy can be derived as shown from Equations 3–13 (Fortunato et al., 2012; Verma and Singal, 2008).
We will derive the total energy Q in a PCM System by integrating this equation.
Integrating both sides over the control volume V:
Simplifying terms, First Term (Sensible Heat Storage per unit volume):
Simplifying the second term (heat conduction):
Where S is the surface bonding of the volume V.
Simplifying the third term (Latent Heat absorption):
Combine and integrate over time:
Assuming no net heat loss/gain (adiabatic system), the conduction term vanishes, we get:
Sensible Heat Change:
Latent Heat Absorbed:
Total Energy = Sensible + Latent:
Where
Where
4.1 Phase change materials
Phase Change Materials (PCMs) are compounds that can undergo reversible phase changes, mainly between solid and liquid states, to store and release significant amounts of thermal energy. A PCM is very useful for thermal energy storage (TES) applications because it collects or releases a sizable amount of latent heat at a virtually constant temperature as it goes through a phase shift.
4.1.1 Key thermophysical properties of phase change materials
For latent heat thermal energy storage systems, the choice of appropriate Phase Change Materials (PCMs) depends on a number of crucial thermophysical characteristics that affect the systems’ dependability, performance, and safety (Kumar et al., 2025).
A PCM should possess a high latent heat of fusion to store a substantial amount of thermal energy per unit mass during the phase transition. This characteristic enhances the energy storage density of the system. For instance, polyethylene glycol (PEG) has been noted for its favorable latent heat properties, making it a candidate for thermal energy storage applications (Yang H. et al., 2024). A suitable phase change temperature is also necessary to guarantee compliance with the intended application’s intended operating temperature range. For charging and discharging cycles to be effective, the phase transition temperature and operating parameters must be closely matched. Another crucial characteristic is high thermal conductivity, which speeds up heat transmission throughout the melting and solidification processes and raises the thermal storage system’s total charging and discharging rates. However, many PCMs inherently exhibit low thermal conductivity. To address this, composite PCMs incorporating materials like Zn2Al-layered double hydroxide have been developed to enhance thermal conductivity (Yang H. et al., 2024). PCMs should exhibit minimal volumetric expansion or contraction during phase transitions to prevent mechanical stress and potential damage to the containment system. Materials with small volume changes during melting and solidification are preferred to maintain structural integrity (Thakare and Bhave, 2015). Furthermore, chemical stability is essential for preserving the PCM’s functionality across a large number of heat cycles. Long-term dependability and efficiency are ensured by stable PCMs’ resistance to subcooling, phase segregation, and breakdown. Lastly, PCMs’ non-toxicity and non-flammability are critical for maintaining operating safety, particularly in commercial, industrial, and residential settings. In general, materials that are safe for the environment and present few health hazards are favored.
Table 4 summarizes commonly used phase change materials (PCMs) for latent heat thermal energy storage. Paraffin wax and stearic acid are stable and easy to handle, but have relatively low thermal conductivity. Salt hydrates offer better conductivity and lower cost, though they can be corrosive and require careful containment. Erythritol, a bio-based PCM, provides high latent heat and better thermal performance, making it suitable for higher-temperature applications. These materials demonstrate a balance between cost, thermal performance, and environmental considerations, influencing their selection for specific use cases.
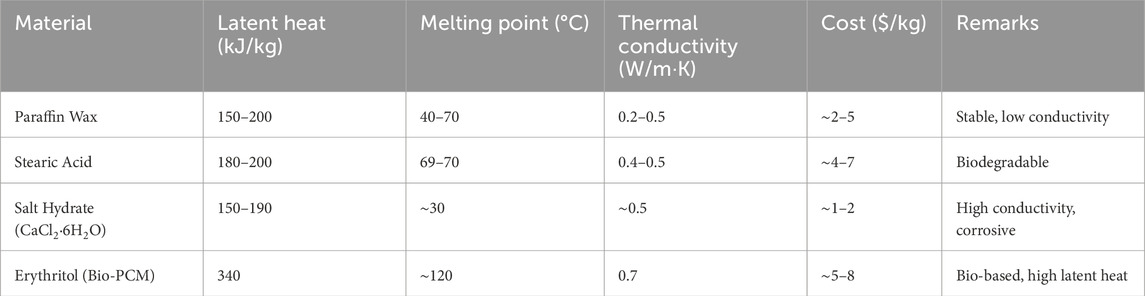
Table 4. Comparison of thermophysical properties of phase change materials (S. Kumar and Banerjee, 2024; Yang H. et al., 2024; Osibodu et al., 2024).
4.2 Classification of PCMs
Phase Change Materials (PCMs) are substances that store and release thermal energy during phase transitions, such as melting or freezing. They are classified into three main categories: Organic, Inorganic, and Eutectic. Organic PCMs include Paraffins and Non-Paraffins, with the latter further divided into Fatty Acids and Bio-Based PCMs like Plant Oils (e.g., coconut, soy), Animal Fats (e.g., beeswax), and Sugar Alcohols (e.g., erythritol). Inorganic PCMs consist of Salt Hydrates and Metallics, known for their high thermal conductivity. Eutectic PCMs are mixtures with a single melting point, categorized as Organic-Organic, Inorganic-Inorganic, Inorganic-Organic, and Bio-Based Eutectics. This classification highlights the diversity of PCMs for various applications, such as thermal energy storage and temperature regulation. Figure 9 effectively illustrates the various classifications of PCMs, providing a clear and structured overview of their categories and subcategories.
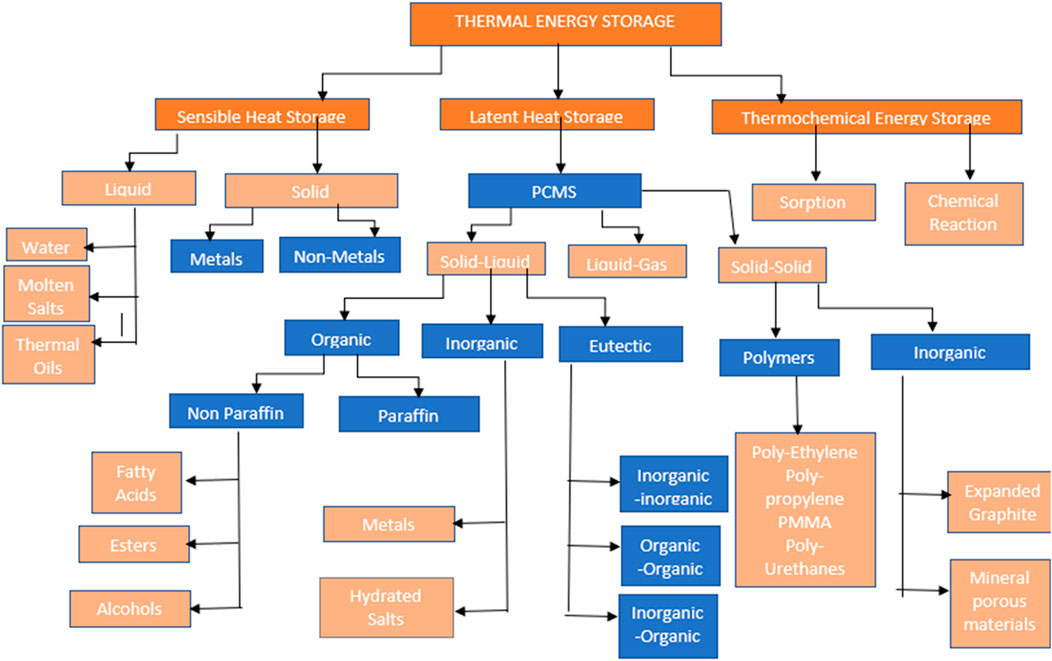
Figure 9. Classification of phase change materials (Podara et al., 2021).
4.2.1 Organic PCMs
Organic Phase Change Materials (PCMs) have drawn a lot of interest for use in thermal energy storage because of their advantageous thermophysical characteristics, which include congruent melting and solidification behavior, chemical stability, and non-corrosiveness (Kumar and Kumar, 2024). Organic PCMs are mainly carbon-based compounds and are broadly categorized into paraffin-based and non-paraffin-based materials.
4.2.1.1 Paraffin-based method
Paraffin PCMs consist of a mixture of straight-chain alkanes (CnH2n+2), exhibiting a relatively narrow melting range and excellent chemical and thermal stability over multiple thermal cycles. They have favorable characteristics like low vapor pressure in the melt, high latent heat of fusion, non-toxicity, and non-corrosiveness (Shu et al., 2017). However, their main drawbacks include low thermal conductivity and flammability, which limit their direct use in high-temperature or fire-sensitive applications.
4.2.1.2 Non parafin-based
Non-paraffin organic materials encompass compounds such as fatty acids, alcohols, esters, and glycols. Among these, fatty acids like stearic acid and palmitic acid exhibit distinct melting points, comparatively higher thermal conductivity than paraffins, and reduced tendencies for supercooling during phase transitions (Herrera et al., 2024). They also show significant latent heat levels and repeatable phase transition cycles. However, system designs must carefully handle problems including unpleasant odors, potential corrosiveness, and greater material prices (Sun et al., 2020).
Organic phase change materials (PCMs) present numerous advantages, including chemical stability and non-corrosiveness, which promote compatibility with a wide range of construction and storage materials. They possess high latent heat capacities, enabling the efficient storage and release of substantial thermal energy during phase transitions. Furthermore, organic PCMs demonstrate excellent cycling stability, maintaining performance over repeated phase change operations, thereby ensuring long-term reliability in thermal energy storage systems. Their non-toxic and environmentally friendly nature also makes them particularly well-suited for residential and commercial building applications. Despite their advantages, organic PCMs face challenges such as low thermal conductivity, which limits heat transfer efficiency. They are often flammable, raising safety concerns unless mitigated through encapsulation. Additionally, their higher cost compared to inorganic PCMs can hinder broader adoption. Issues like supercooling and volume changes during phase transitions further complicate system design and reliability.
4.2.2 Inorganic PCMs
Inorganic phase change materials (PCMs), primarily consisting of salt hydrates, metallics, and certain metal eutectics, are widely utilized in thermal energy storage applications due to their distinct thermophysical properties. These materials typically offer higher latent heat storage capacities and greater thermal conductivities compared to organic PCMs, facilitating faster charging and discharging cycles (Rathod and Banerjee, 2013; Sharma et al., 2009). One of the most extensively researched types of inorganic PCMs, salt hydrates, can be used in a wide range of settings, from high-temperature industrial processes to low-temperature building systems, because they can undergo phase transitions at different temperatures (Purohit and Sistla, 2021). Compared to their organic counterparts, inorganic PCMs offer greater operating safety because they are often non-flammable and show superior thermal stability at high temperatures (Sarbu and Sebarchievici, 2018). However, various obstacles limit the broader deployment of inorganic PCMs. The propensity for phase segregation and incongruent melting, in which the salt hydrates melt and separate into salt and water, is a serious problem that lowers thermal performance and stability over the long run (Zalba et al., 2003). Furthermore, a lot of inorganic PCMs undergo subcooling, which makes nucleating agents necessary to successfully start phase transitions (Beaupere et al., 2018). Another major technical challenge is the corrosion of containment materials by salt hydrates, which calls for the use of protective coatings or cautious material selection (Solé et al., 2015). Recent research has focused on overcoming these limitations through encapsulation techniques, additives to minimize supercooling, and the development of composite materials that combine the high energy density of inorganic PCMs with improved structural stability (Zhang H. et al., 2022).
4.2.3 Eutectic PCMs
Eutectic phase change materials (PCMs) are combinations of two or more substances that solidify at the same time at a single, sharp temperature (referred to as the eutectic point) at a certain composition (Sun et al., 2023). This behavior enables them to exhibit phase transitions at relatively constant temperatures, making them highly attractive for thermal energy storage applications requiring precise thermal management.
Eutectic PCMs are generally categorized into organic-organic, inorganic-inorganic, and organic-inorganic systems. Organic-organic eutectics, often composed of fatty acids or paraffins, offer low toxicity, strong chemical stability, and minimal supercooling. In contrast, inorganic-inorganic eutectics, typically based on salt mixtures, deliver higher thermal conductivity and greater volumetric energy density but are prone to corrosion and phase separation. Organic-inorganic eutectics aim to combine the strengths of both categories, though they can face compatibility challenges. The primary benefit of eutectic PCMs is their high melting and solidification points, which reduce temperature variations during energy storage and release and improve system efficiency (Atinafu et al., 2018). Additionally, by altering component ratios, their adjustable melting points can be customized for particular temperature ranges. However, unless stabilizers or encapsulation techniques are used, eutectic systems may be susceptible to phase separation during prolonged cycling, especially in inorganic-inorganic mixes (Huang et al., 2023). This could result in decreased storage performance. Eutectic PCMs are used in solar thermal systems, electronic cooling, and building thermal management, all of which depend on a restricted temperature range (Sun et al., 2023).
4.2.4 Bio-based PCMs
Bio-based phase change materials (PCMs) have emerged as a promising class of thermal storage media due to their sustainability, renewability, and environmental compatibility. They can be classified under non-paraffin organic phase change materials. These PCMs, which are made from natural sources including vegetable oils, animal fats, and bio-waxes, provide a sustainable substitute for conventional petroleum-based materials and are in line with the increasingly important concepts of the circular economy and low-carbon technology (Pielichowska et al., 2024).
The high latent heat of fusion of bio-based PCMs is one of their main benefits; it allows for significant energy storage per unit mass. Additionally, they have very small phase transition ranges, which makes them appropriate for temperature-sensitive uses including electronic thermal control, food preservation, and building heating and cooling. Additionally, compared to many synthetic organic PCMs, bio-based PCMs are typically less flammable, non-toxic, and biodegradable, which increases their attractiveness for both residential and commercial use (Benhorma et al., 2024; Simonsen et al., 2023). However, there are several difficulties with bio-based PCMs. Their generally low thermal conductivity can restrict the rate of heat transmission and call for the application of encapsulation techniques or conductivity boosters (Li D. et al., 2022). They may also operate differently if they have problems with phase separation, supercooling, and long-term chemical stability under frequent heat cycling. By combining bio-based PCMs with stabilizers, altering molecular architectures, or using sophisticated encapsulating techniques, these issues are frequently resolved and reliability and efficiency are increased.
4.3 PCM enhancement methods
The performance of Latent Heat Storage can be hindered by factors such as slow heat transfer rates, low thermal conductivity, phase segregation, and subcooling. Several strategies have been developed to address these challenges and enhance the efficiency and effectiveness of latent heat storage systems.
4.3.1 Encapsulation of phase change materials (PCMs)
One of the most widely used techniques to enhance latent heat storage systems’ performance is encapsulation. In this process, PCMs are encased in a protective shell composed of ceramics, metals, or polymers. Encapsulation of phase change materials (PCMs) enhances system reliability by preventing leakage during phase transitions, as PCMs often expand and contract. It also facilitates improved heat transfer by increasing the surface area of the PCM, thereby enhancing the heat exchange between the PCM and the surrounding medium. Additionally, encapsulation helps minimize phase separation, particularly in eutectic or organic PCMs, ensuring more consistent performance over multiple cycles (Huang et al., 2023; Zhang H. et al., 2022). To better understand the various encapsulation methods used in Phase Change Materials (PCMs), it is important to consider their impact on performance and durability. Table 5 provides a comparative analysis of three prominent encapsulation techniques; micro-encapsulation, macro-encapsulation, and shape-stabilization (SSPCMs). Each method is evaluated based on key factors such as heat transfer efficiency, durability, and cost, offering a clearer understanding of their respective advantages and limitations in the context of thermal energy storage systems.
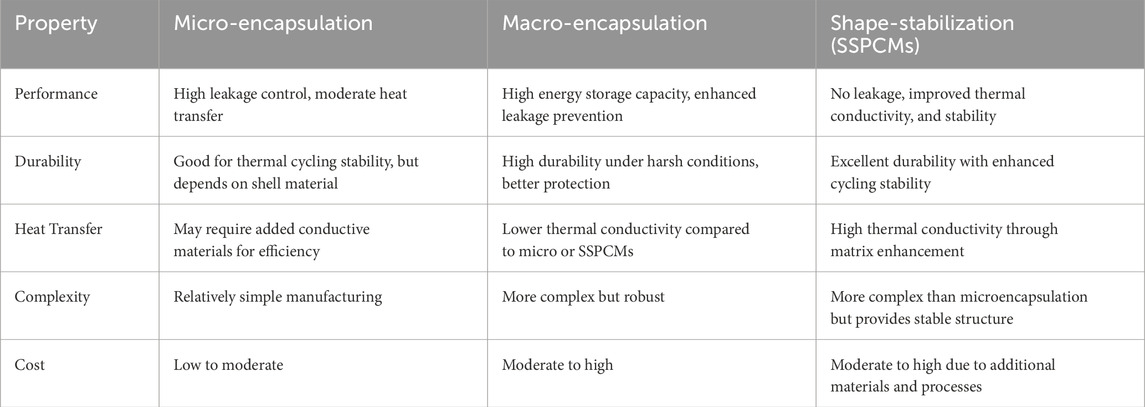
Table 5. Comparative analyses of PCM encapsulation (Ayyaril et al., 2023; Huang et al., 2023).
4.3.2 Addition of conductivity enhancers
Most PCMs have low thermal conductivity, which restricts the rate of heat transfer during charging and discharging and can drastically lower system efficiency. This is enhanced by adding materials with greater thermal conductivity to PCMs, such as metal foams, graphite, or carbon nanotubes (CNTs). These substances improve conductivity and speed up heat transfer (Wu et al., 2020).
4.3.3 Use of composite PCMs
Composite phase change materials (PCMs) combine multiple materials to enhance the performance of latent heat storage systems. By blending different PCMs, the properties of the material can be customized to meet specific application needs (Ghosh et al., 2022). This method is particularly effective for optimizing factors such as melting point, thermal conductivity, and heat storage capacity. Composite systems often combine organic PCMs, like paraffin wax, with inorganic materials, such as salt hydrates, to improve performance over a broader temperature range. Additionally, hybrid systems integrate latent heat storage with sensible heat storage, using materials like graphite or metal powders mixed with PCMs to boost both thermal storage capacity and heat transfer efficiency.
4.3.4 Thermal stratification enhancement
Improving thermal stratification in latent heat storage systems is crucial for maximizing the efficiency of energy storage and retrieval. During the charging process, thermal stratification ensures that higher-temperature regions are positioned at the top of the system, while cooler areas remain at the bottom. This arrangement reduces energy losses caused by mixing and ensures that energy is stored more effectively. Achieving optimal stratification can be accomplished by employing optimized inlet diffusers and buffer layers within the storage tank, which help maintain the desired temperature gradient and minimize mixing losses (Liang et al., 2022).
4.3.5 Improved encapsulation materials
Phase change materials (PCMs) are successfully protected by conventional encapsulation materials like metals and polymers, but research and development are always ongoing to create new materials with better qualities. Silica and carbon-based nanostructures are examples of contemporary encapsulation materials that improve the system’s overall strength and thermal conductivity (Lamy-Mendes et al., 2018). Furthermore, to further enhance the effectiveness and efficiency of latent heat storage systems, aerogels which are well-known for their superior insulating qualities are being investigated as encapsulation materials (Ma et al., 2023b).
4.3.6 Nano-enhanced PCMs
Nano-enhanced phase change materials (NePCMs) represent a significant advancement in thermal energy storage, where nanoparticles such as metals (Cu, Al, Ag), carbon-based materials (graphene, carbon nanotubes), or metal oxides (TiO2, Al2O3) are dispersed within conventional PCMs to enhance their thermophysical properties (Said et al., 2024; Tofani and Tiari, 2021). Nanoparticles are incorporated into phase change materials (PCMs) to significantly improve their thermal conductivity, enabling faster heat transfer and minimizing thermal lag during phase transition cycles (Tariq et al., 2020). Moreover, nanoparticles act as nucleation sites, which helps to reduce supercooling and ensures more consistent and reliable phase changes (Eanest Jebasingh and Valan Arasu, 2020). Research has also demonstrated that nanoparticle-enhanced PCMs (NePCMs) exhibit greater thermal stability and decreased phase separation, improving their long-term reliability and performance (Mebarek-Oudina and Chabani, 2023). For instance, Al2O3 (aluminum oxide) nanoparticles have been shown to enhance the thermal conductivity of paraffin wax-based PCMs by up to 20% (Li et al., 2024). Likewise, SiO2 (silicon dioxide) nanoparticles have been found to significantly reduce supercooling and facilitate stable nucleation during phase change processes (Singh et al., 2022). Additionally, studies have shown that CuO (copper oxide) nanoparticles enhance the thermal stability and maintain phase uniformity of PCMs across repeated heating and cooling cycles (Almeshaal and Altohamy, 2024).
In addition to experimental validations, computational studies have significantly advanced the understanding of nano-enhanced phase change materials (NePCMs) in thermal energy storage systems. For instance, molecular dynamics (MD) simulations have been used to study the dispersion of graphene oxide (GO) nanoparticles within paraffin wax. Zhang et al. (2020) conducted MD simulations and found that strong interactions between GO nanosheets and the paraffin matrix enhanced the overall thermal conductivity and created additional nucleation sites, accelerating the phase change process and reducing supercooling. Nandi and Biswas (2025) conducted a combined experimental and numerical investigation on a PCM-based energy storage system enhanced with CuO nanoparticles and modified fin structures under solar irradiation. Their study demonstrated that incorporating 0.01% weight fraction of CuO nanoparticles led to a 16.36% increase in energy storage capacity and accelerated the melting process across various fin configurations, thereby improving the system’s thermal efficiency. Similarly, Chavan et al. (2024) performed a computational analysis on hybrid nano-enhanced PCMs (HNePCMs) integrated into thermal energy storage systems. Their findings indicated that HNePCMs containing 10% mass fraction of highly conductive nanoparticles exhibited up to an 18.69% enhancement in energy storage capacity and a 20% reduction in melting time compared to base PCMs, highlighting the efficacy of nanoparticle integration in improving thermal performance. Furthermore, Teja et al. (2022) utilized numerical simulations to assess the impact of Al2O3 nanoparticles on the thermal characteristics of paraffin-based PCMs in a latent heat thermal energy storage system. Hussain and Hussain (2025) revealed that adding 2% and 5% volume fractions of Al2O3 nanoparticles significantly improved the melting and solidification processes, enhancing the overall thermal conductivity and energy storage efficiency of the system.
5 Thermochemical storage
Thermochemical Energy Storage (TCES) presents a viable approach for high-energy-density, long-term thermal energy storage. In contrast to sensible or latent heat storage methods, TCES utilizes reversible chemical reactions to efficiently store and release thermal energy while maintaining negligible energy losses over extended periods. Thermochemical Thermal Energy Storage (TCES) operates on the principle of reversible chemical reactions, where thermal energy is stored and released through endothermic and exothermic processes, respectively. The general reaction mechanism involves the decomposition of a compound into its constituents during the charging phase, which absorbs heat (endothermic), and the recombination of these constituents during discharging, which releases heat (exothermic). Figure 10 illustrates a thermochemical energy storage (TCES) system, where heat is stored and released through reversible chemical reactions typically involving salt hydrates. A typical TCES reaction is given by Equation 14 (Abanades, 2023).
where
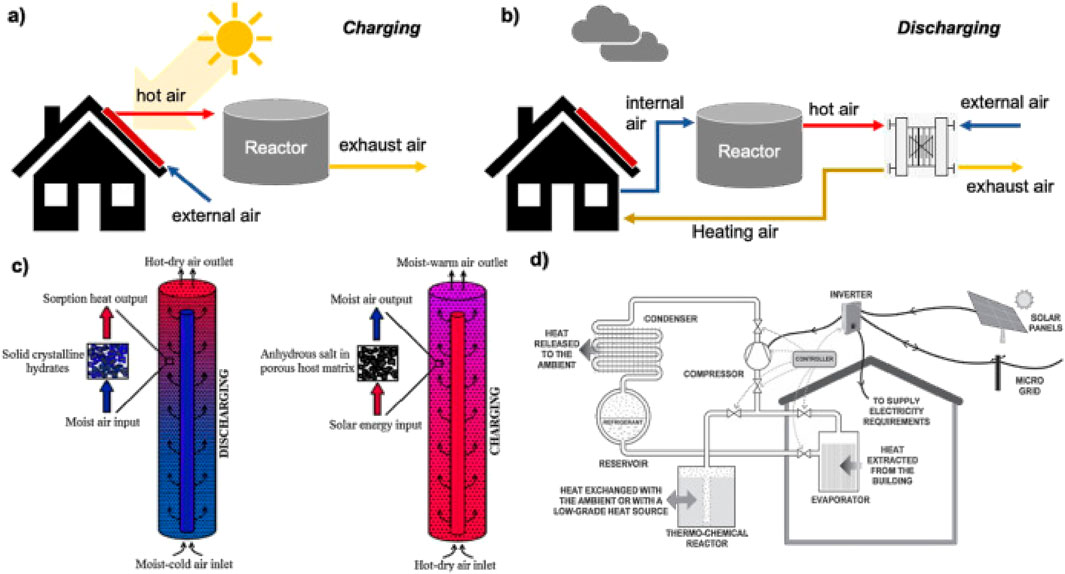
Figure 10. Thermochemical energy storage for building heating. (a) Charging: Solar-heated or externally heated dry air regenerates the reactor. Warm, dry air enters the reactor and moist exhaust air is vented. (b) Discharging: Indoor moist air passes through the reactor. Hydration releases heat and supplies hot air to the building via the heating coil, with exhaust air vented outside. (c) Packed-bed reactor operation: Left, discharging mode where moist air enters, forms solid crystalline hydrates, and exits as hot, dry air. Right, charging mode where hot, dry purge air regenerates the anhydrous salt in a porous host and exits as moist, warm air. (d) Integrated system schematic: Thermochemical reactor coupled to a vapor-compression loop and PV (Rathore and Shukla, 2021).
The energy stored (Q) as shown in Equation 15 in such systems is directly related to the reaction enthalpy.
Where n is the number of moles of the reacting substance. The reversibility of the reaction and favorable kinetics are critical for efficient cyclic operation.
5.1 Thermochemical materials for TES
Materials such as Materials such as salt hydrates (e.g., CaCl2·6H2O), metal oxides (e.g., MgO/Mg(OH)2), and carbonates (e.g., CaCO3/CaO) are commonly used due to their high reaction enthalpies and well-understood behavior. Table 6 presents a comparison of key thermochemical energy storage materials based on their reaction types and performance characteristics. While materials like Ca(OH)2 and Mg(OH)2 are relatively mature and low-cost, they suffer from issues such as sintering and agglomeration, which limit their cycle life. CaCO3 offers higher energy density but requires very high decomposition temperatures, making it less practical for some applications. Co3O4, though promising due to its high energy density, faces challenges related to cost, toxicity, and limited cycle life, indicating it is still largely at the research stage.
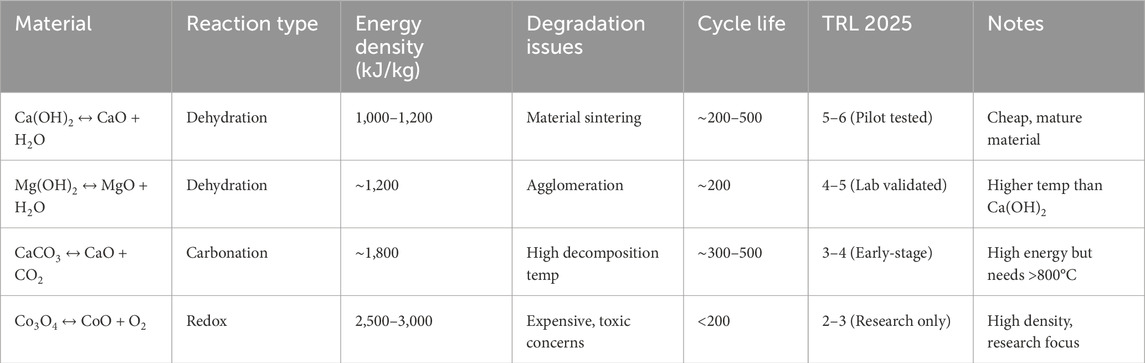
Table 6. Comparison of key thermochemical energy storage (TCES) materials and their performance characteristics (Feng et al., 2023; Raganati and Ammendola, 2023; Wang et al., 2022).
5.1.1 Salt hydrates
Salt hydrates are among the most widely researched thermochemical materials (TCMs) due to their moderate operating temperatures and high theoretical energy densities. Typical materials include magnesium sulfate (MgSO4·7H2O), calcium chloride (CaCl2·6H2O), and lithium chloride (LiCl·H2O), which store and release thermal energy via reversible hydration and dehydration reactions (Lin et al., 2021). Equations 16–18 gives some common reactions of salt hydrates. These reversible reactions enable heat absorption during dehydration (charging) and release during rehydration (discharging). However, challenges such as low cycling stability, hygroscopicity, and irreversible structural degradation impede their long-term application.
Recent advances have focused on improving the structural and thermal stability of salt hydrates by forming composites with porous host materials. For example, Liu (2023) demonstrated that embedding calcium chloride within a metal foam matrix enhanced the cyclic reversibility and thermal performance of the material. The porous framework improved water vapor transport and maintained dispersion, reducing salt agglomeration and promoting stable performance over multiple cycles. Similarly, Aarts et al. (2023) investigated the integration of salt hydrates into porous polymeric matrices to reduce deliquescence and improve structural integrity. Their study showed that the hybrid material maintained its energy storage capacity over extended thermal cycles. These findings indicate that structural stabilization through composite engineering is a promising strategy for enabling the practical deployment of salt hydrate-based thermochemical energy storage systems.
5.1.2 Metal oxide
Metal oxides store and release thermal energy through reversible redox reactions. The general reaction as shown in Equations 19, 20 involves the reduction of a metal oxide at high temperatures, releasing oxygen, and its re-oxidation at lower temperatures, absorbing oxygen:
These oxides exhibit excellent thermal stability and cycling durability, making them attractive for medium-to high-temperature TES applications. Recent research has focused on enhancing the redox properties of metal oxides for thermochemical energy storage applications. For instance, perovskite materials have shown promise due to their ability to undergo redox cycling over a wide temperature range. A study introduced sodium into the lattice structure of BaCoO3 to enhance oxygen vacancy formation and mobility, thereby improving its thermochemical energy storage performance (Ning et al., 2024). Additionally, layered transition metal oxides such as MoO3, WO3, Ga2O3, and V2O5 have been analyzed for their functions in energy conversion and storage systems (Bhojani et al., 2025). These materials exhibit favorable redox properties and structural stability, making them suitable candidates for thermochemical energy storage.
5.1.3 Carbonates
Carbonate-based TCMs, particularly calcium carbonate (CaCO3) and magnesium carbonate (MgCO3), leverage decomposition/carbonation cycles to store and release heat as given by Equations 21, 22.
During the charging phase, carbonates decompose into metal oxides and CO2 gas, absorbing significant amounts of energy. During discharge, recombination with CO2 releases stored heat. While they offer high energy storage capacities, practical challenges include slow carbonation rates and material sintering at elevated temperatures, which degrade performance over multiple cycles. To improve the practicality of carbonate-based thermochemical energy storage, researchers have explored methods to lower decomposition temperatures and enhance reaction reversibility. For example, a study introduced a reactive carbonate composite (RCC) where Fe2O3 was used to thermodynamically destabilize BaCO3, reducing its decomposition temperature from 1,400°C to 850°C. This modification makes the material more suitable for thermal energy storage applications (Williamson et al., 2023). Furthermore, combining barium carbonate with barium titanite has been investigated for ultra-high temperature thermochemical energy storage. This composite material exhibits improved thermodynamic characteristics and cycling stability, making it a promising candidate for high-temperature energy storage systems (Williamson et al., 2024).
TCES systems can be categorized based on how they handle reactants and products:
• Open Systems: These systems exchange reactants (e.g., water vapor or CO2) with the environment. They are simpler and more cost-effective but can be influenced by ambient conditions.
• Closed Systems: Reactants and products are contained within a sealed system, allowing for better control over reaction conditions and improved efficiency. However, they are generally more complex and expensive to implement.
5.2 Thermochemical energy storage reactor design
Thermochemical energy storage (TCES) reactors are critical components that facilitate reversible heat storage and release through chemical reactions. Effective reactor design must optimize heat and mass transfer, reaction kinetics, cyclability, and integration with energy systems. Table 7 compares different reactor types (fixed-bed, fluidized-bed, and moving-bed) based on their thermochemical materials, temperature ranges, energy density, and cycling stability for energy storage applications.
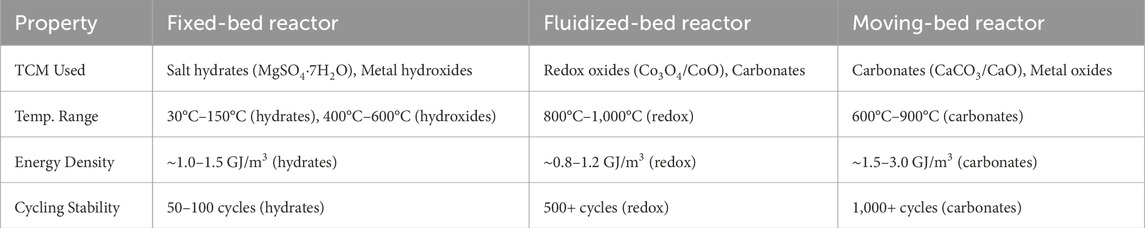
Table 7. Comparison of reactor types for thermochemical energy storage (Galazutdinova et al., 2024; Wuerth M. et al., 2019; Preisner and Linder, 2020).
5.2.1 Fixed bed reactors
Fixed bed reactors as shown in Figure 11 are characterized by a stationary bed of solid thermochemical materials through which the reactive gas flows. Their simplicity and ease of construction make them attractive for initial implementations. However, they often suffer from limitations in heat and mass transfer, leading to temperature gradients and reduced reaction efficiency. Recent studies have explored embedding heat exchangers within the fixed bed to enhance thermal conductivity and uniformity. For instance, integrating a honeycomb heat exchanger structure has shown improvements in heat distribution and storage capacity (Kant et al., 2021).
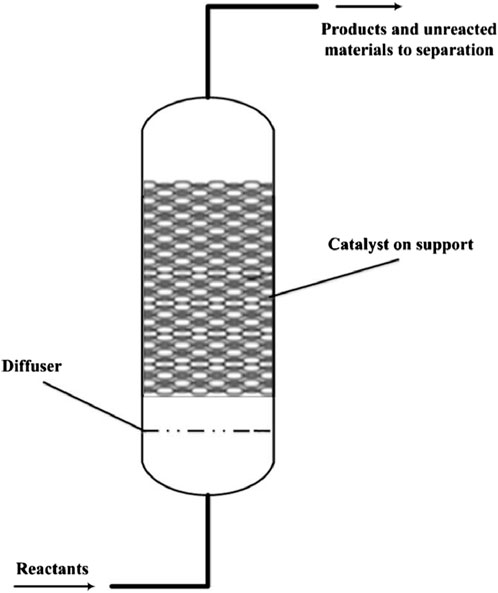
Figure 11. Diagram of a fixed based reactor (Santos, 2018).
5.2.2 Fluidized bed reactor
Fluidized bed reactors suspend solid particles in an upward flow of gas, creating a fluid-like state that enhances heat and mass transfer. Figure 12 demonstrates the design of a fluidized bed reactor. This configuration offers superior mixing properties, uniform temperature distribution, and scalability. Experimental investigations with CaO/Ca(OH)2 systems have demonstrated the feasibility of continuous operation and improved thermal performance in fluidized bed setups. However, challenges such as particle agglomeration and attrition need to be addressed for long-term stability (Wuerth M. et al., 2019).
5.2.3 Moving bed reactors
Moving bed reactors involve the continuous movement of solid particles through the reactor, allowing for steady-state operation and efficient heat exchange. This design as shown in Figure 13 is particularly beneficial for large-scale applications where continuous energy supply is required. Modeling studies have highlighted the advantages of counter-current flow arrangements in moving bed reactors, leading to enhanced thermal gradients and reaction rates. Nevertheless, mechanical complexities and potential for channeling remain as design considerations (Preisner and Linder, 2020).
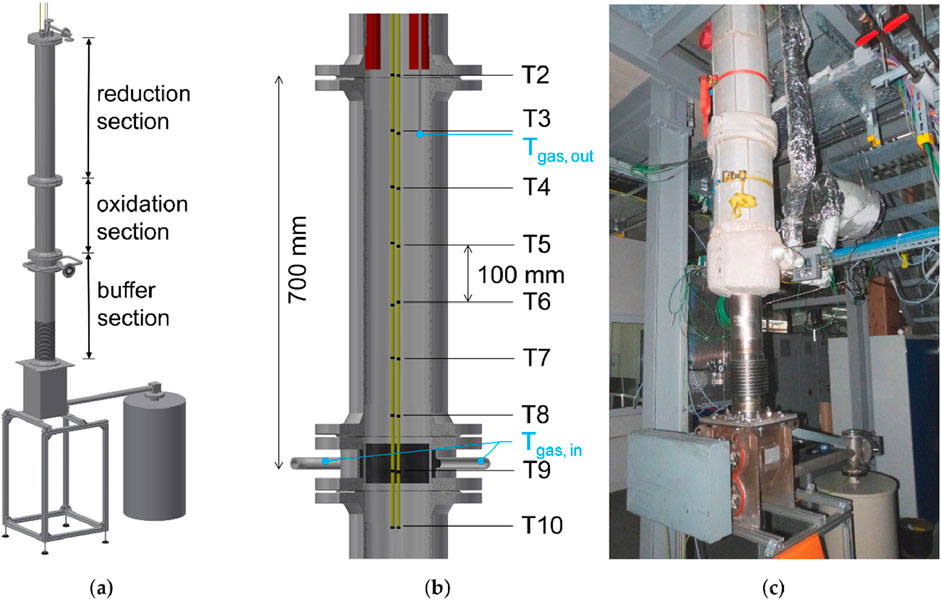
Figure 13. A moving bed reactor design for thermochemical storage systems. (a) Schematic diagram of the reactor showing the main sections: reduction section (top), oxidation section (middle), and buffer section (bottom). (b) Cross-sectional view indicating thermocouple (T2–T10) locations and gas inlet/outlet positions: T2–T10 represent temperature measurement points along the reactor height; vertical distance between T5 and T6 is 100 mm; total measured section height is 700 mm. (c) Photograph of the experimental setup showing the installed reactor and connected instrumentation (Korba et al., 2022).
5.2.4 Heat exchanger integration
Efficient heat exchanger integration is crucial for the performance of thermochemical energy storage (TCES) systems. Innovative designs, such as embedding heat exchangers within the reactive bed, have been explored to enhance heat transfer efficiency. For example, a recent study by Zhang Y. et al. (2024) investigated the performance of a TCES reactor embedded with a microchannel tube heat exchanger for water heating. The embedded design achieved a thermal efficiency of 82.35%, representing a 69-percentage point improvement over conventional systems due to enhanced heat transfer and reduced temperature gradients within the reactive bed. Similarly, Humbert and Sciacovelli (2024) applied topology optimization to develop advanced fin structures integrated within the reactive bed of a closed TCES reactor. Their optimized embedded structures yielded up to a 286% increase in heat transfer performance compared to traditional designs, demonstrating the potential of intelligent heat exchanger integration in maximizing energy efficiency.
5.2.5 Coupling with solar thermal systems
Integrating TCES systems with Concentrated Solar Power (CSP) plants offers a promising approach to address the intermittency of solar energy. By storing excess thermal energy during peak solar hours, TCES can provide a continuous energy supply during periods of low solar irradiance. Studies have proposed various integration strategies, including direct and indirect coupling of thermochemical reactors with CSP systems, to optimize energy conversion and storage efficiency (Pelay et al., 2019).
5.3 Reaction kinetics and transport phenomena
The efficiency of thermochemical energy storage (TCES) systems largely hinges on the reaction kinetics of their reversible chemical processes. This kinetics dictate the pace at which thermal energy is either stored or released during the system’s charge and discharge phases, thus directly influencing its dynamic response and operational efficiency. In gas-solid based TCES configurations, transport phenomena both mass and thermal are equally vital. Maintaining steady gas diffusion through the reactive bed is crucial to sustaining the reaction, especially for processes like hydration/dehydration and carbonation/decarbonation. In parallel, the thermal conductivity of the material and the heat transfer coefficient between system components determine the effectiveness of thermal energy delivery and extraction.
To better understand and optimize the interaction between reaction kinetics and transport mechanisms, researchers frequently employ simulation tools such as Computational Fluid Dynamics (CFD) and Finite Element Analysis (FEA). CFD helps visualize gas flow, temperature profiles, and species distributions within the system, enabling insight into reaction rates and thermal limitations. FEA, on the other hand, is instrumental in evaluating temperature-induced stress, material deformation, and structural reliability under repeated thermal cycles. These modeling approaches play a crucial role in refining TCES system designs and predicting their real-world performance under varied operating scenarios. A notable example is the work by Jiang et al. (2025) who formulated a numerical model to examine heat transfer and conversion dynamics in calcium carbonate (CaCO3) particles for TCES applications. Their study also assessed the potential of silicon carbide (SiC) additives in improving energy storage capacity and reaction behavior. Also, a study by Yaïci et al. (2013) developed a three-dimensional unsteady CFD model using COMSOL Multiphysics to simulate a seasonal solar thermochemical heat storage system for buildings.
5.4 Materials innovation/integration
Recent progress in thermochemical energy storage (TCES) has been largely driven by innovations in composite materials and nanotechnology, which effectively tackle critical issues such as material stability, thermal conductivity, and reaction kinetics.
5.4.1 Composite materials for enhanced stability
Incorporating hygroscopic salts into porous substrates like zeolites has proven effective in enhancing the stability and performance of TCES systems. For instance, a study by Chen et al. investigated LiCl/LiBr-zeolite composites and found that a 15 wt.% salt solution exhibited optimal adsorption rates and capacities. This composite achieved a heat storage density of up to 585.3 J/g, marking a 30.9% improvement over pure zeolite. The system also demonstrated a high energy discharge efficiency of 74.3% under specific conditions, highlighting its potential for long-term thermal energy storage applications (Chen et al., 2022).
5.4.2 Nano-enhanced salts for improved heat transfer and reaction kinetics
The addition of nanoparticles to molten salts has been shown to enhance their thermal properties significantly (Guo et al., 2021). Research indicates that incorporating nanoparticles such as SiO2, Al2O3, and TiO2 into NaNO3-KNO3 mixtures can increase specific heat capacities by 15%–57% in the solid phase and 1%–22% in the liquid phase (Chieruzzi et al., 2013; Guo et al., 2021; Guo et al., 2021). These enhancements are attributed to the formation of nanolayers on the surface of the nanoparticles, which increase the surface area and improve heat transfer rates. Furthermore, studies have demonstrated that the inclusion of MgO nanoparticles in salt hydrates can improve thermal conductivity and reduce solidification time, thereby enhancing the overall efficiency of TCES systems (Yang W. et al., 2024). For example, adding 0.25 wt.% MgO nanoparticles to NaNO3-KNO3 mixtures resulted in increased thermal diffusivity and reduced discharge time without significantly affecting latent heat values.
6 Techno-economic comparison of TES systems
The techno-economic performance of thermal energy storage (TES) systems is a critical factor in determining their practical applicability across sectors such as power generation, industrial heating, and building energy management. TES technologies namely sensible heat storage (SHS), latent heat storage (LHS), and thermochemical energy storage (TCES) differ significantly in terms of cost-effectiveness, energy density, technology readiness, and operational complexity.
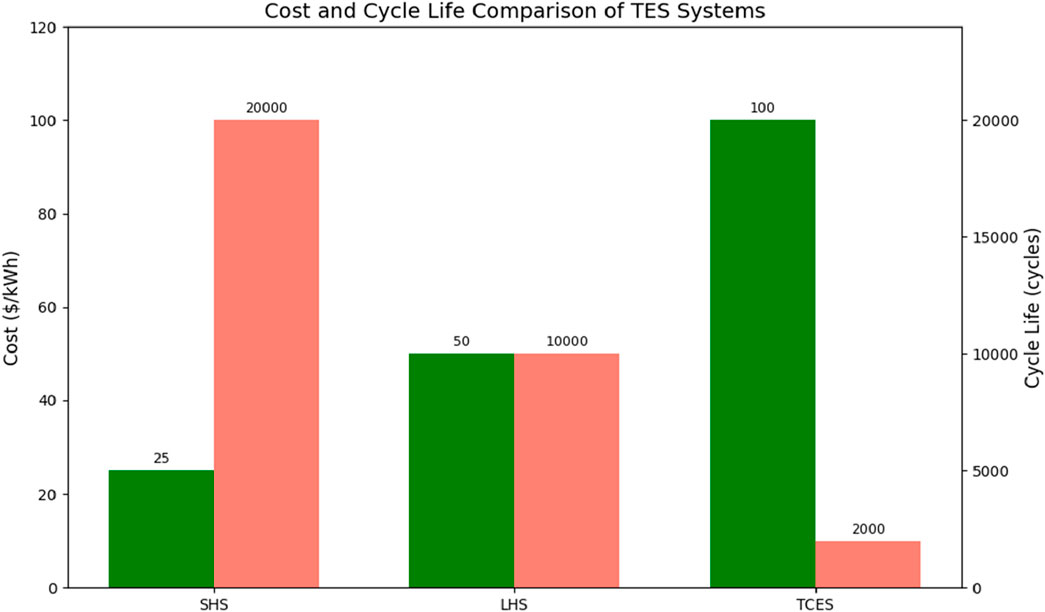
Sensible heat storage, particularly using water, molten salts, or concrete, remains the most commercially viable option due to its low cost per kWh (typically $1–25/kWh) and long cycle life (>10,000–20,000 cycles). These systems are mature (TRL 9) and have been deployed at scale, especially in CSP plants. However, they require large volumes due to low energy density and may have significant heat losses over time.
Latent heat storage systems (e.g., using paraffin wax, salt hydrates, or bio-based PCMs) offer higher energy densities and nearly isothermal behavior during phase change, making them compact and efficient for space-constrained applications. Despite moderate costs ($30–70/kWh) and reasonable cycling stability, LHS faces challenges such as low thermal conductivity and potential material degradation, which can impact long-term reliability.
Thermochemical storage systems, though still at low to mid TRL (3–6), promise the highest energy densities and negligible self-discharge, enabling long-duration storage and seasonal applications. However, their high initial costs ($80–150/kWh) are mostly based on laboratory scale projections, complex system design, and limited cycle life (typically <2,000 cycles) currently restrict their deployment to laboratory or pilot-scale projects.
From a techno-economic standpoint, SHS is preferred for large-scale, short-to-medium duration applications due to its maturity and low cost, while LHS is ideal for moderate-scale systems requiring compactness and higher energy density. TCES holds long-term potential for grid-scale and seasonal storage, particularly as material and system innovations reduce cost and improve cycling durability. Figures 14, 15 shows a comparison of the thermo-economic properties of thermal energy storage systems and the various thermal energy storage materials respectively. A hybrid approach that integrates these TES technologies based on use-case requirements may offer an optimal balance of cost, performance, and scalability.
7 Hybrid thermal energy storage
Hybrid Thermal Energy Storage (TES) systems have become increasingly important due to their ability to merge different energy storage technologies to improve efficiency and overall performance (Ding et al., 2021). These systems integrate thermochemical energy storage (TCES) with latent heat storage (such as phase change materials, PCMs) and sensible heat storage (for example, molten salts) in an effort to increase energy density, thermal stability, and efficiency. The main advantage of hybrid TES systems lies in their ability to combine multiple storage methods, each offering unique benefits. TCES systems, for instance, store energy through reversible chemical reactions like dehydration, rehydration, carbonation, and decarbonation, which enables them to achieve high energy densities. On the other hand, PCMs store energy by changing phases at specific temperatures, which helps maintain a stable temperature during the charging and discharging phases, thus improving the temperature regulation of the system. Moreover, when TCES is combined with sensible heat storage, such as molten salts, the system benefits from increased thermal power output and enhanced heat transfer efficiency during discharge cycles. This hybrid approach is especially advantageous for applications requiring high-temperature energy, like Concentrated Solar Power (CSP) plants, where it enables efficient energy capture and storage during peak sunlight periods (Elkelawy et al., 2024).
Hybrid TES systems are also known to improve system efficiency by decreasing response times. When coupled with electrochemical storage systems, such as batteries, these hybrid systems offer faster energy discharge, which helps overcome the slower kinetics typically seen with TCES (Ding et al., 2021). This feature makes hybrid TES systems ideal for renewable energy sources like wind and solar, which are characterized by intermittent energy production (Hanani et al., 2023). In terms of integration, hybrid TES systems provide a practical solution to reduce the cost of energy storage technologies by lowering the capital investment needed for large-scale thermochemical systems. By relying less on more expensive single-storage technologies, hybrid systems offer a more cost-effective approach to managing energy efficiently, especially in renewable energy contexts where energy supply and demand are constantly fluctuating.
Recent studies have demonstrated the effectiveness of hybrid TES systems in various applications. For instance, a study on a hybrid solar and waste heat thermal energy harvesting system showed that integrating a thermoelectric generator (TEG) with a photovoltaic (PV) system improved energy efficiency by utilizing both solar and waste heat (Hanani et al., 2023). Another research development in hybrid TES, such as the integration of phase change materials (PCMs) with solar thermal systems, have demonstrated enhanced energy storage performance and improved cost-effectiveness (Baghaei Oskouei et al., 2024). Overall, hybrid TES systems represent a major breakthrough in energy storage technology. By combining TCES, latent heat, and sensible heat storage, they offer a flexible and efficient solution to energy storage challenges, particularly for renewable energy systems. Continued advancements in this field are likely to further improve the performance, reliability, and cost-effectiveness of hybrid TES systems, making them a crucial component of sustainable energy solutions.
8 Application of thermal energy storage
Thermal energy storage (TES) plays a vital role across multiple sectors by improving energy efficiency and enabling renewable energy integration. In power generation, it supports CSP plants and waste heat recovery, while in industries it optimizes processes in steel, cement and chemical production. TES also enhances building HVAC systems, agricultural operations, smart grid stability, transport thermal management, data center cooling, and hydrogen storage solutions, demonstrating its versatility in energy optimization.
8.1 TES in concentrated solar power plants
Concentrated solar power (CSP) plants produce electricity by using mirrors or lenses to focus sunlight onto a central receiver, where a heat transfer fluid captures and carries the thermal energy. As shown in Figure 16, this heat is then used to produce steam that drives a turbine connected to a generator. Thermal energy storage (TES) is pivotal in enhancing the performance and reliability of concentrated solar power (CSP) systems by decoupling solar energy collection from electricity generation. Among the TES technologies employed in CSP, sensible heat storage remains the most widely adopted, particularly in commercial systems that use molten salt mixtures such as sodium nitrate and potassium nitrate. These salts exhibit high specific heat capacity and thermal stability, typically operating at temperatures up to 565°C. However, recent advancements have focused on replacing or enhancing molten salts with higher-temperature alternatives, including molten chlorides like magnesium chloride and potassium chloride, which can function above 700°C and offer improved cost-effectiveness and thermal efficiency. Caldwell et al. (2021) demonstrated that certain MgCl2-KCl-based molten salts are air-stable and less corrosive, enabling CSP systems to operate at temperatures exceeding 750°C without significant degradation, a notable improvement over traditional nitrate salt. Complementing these material developments, innovative diagnostic techniques have emerged to assess thermal properties in real time. Chung et al. (2023) introduced an in-situ method using modulated photothermal radiometry to measure the thermal conductivity of flowing molten chloride salts, providing key insights for optimizing heat transfer performance under operational conditions. Solid particle TES systems, using materials such as ceramic and sand particles, have also gained traction. These systems can operate at temperatures exceeding 1,000°C and serve as both heat transfer and storage media, making them especially suitable for next-generation CSP plants targeting higher thermodynamic efficiencies. Such systems offer high energy density and thermal stability, and their integration into CSP plants is increasingly being explored due to their scalability and cost-effectiveness (Barrasso et al., 2023).
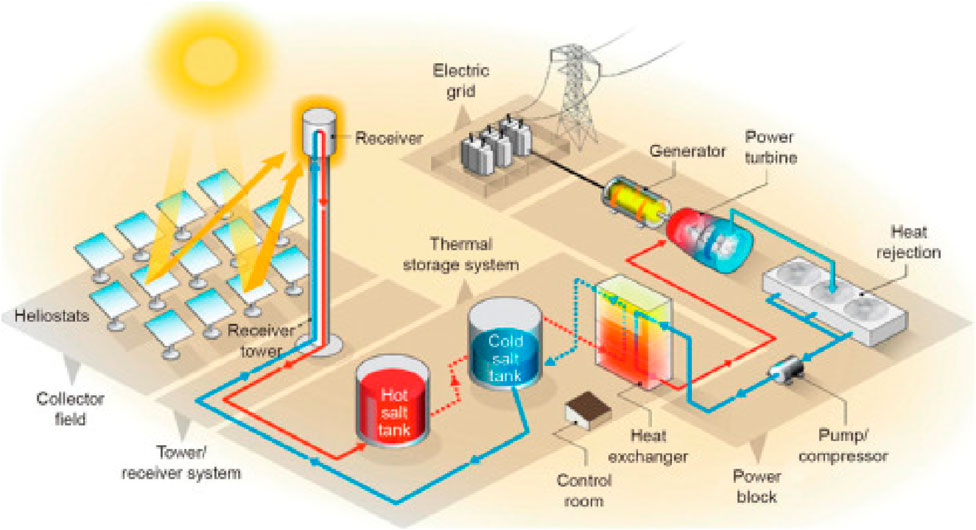
Figure 16. Thermal energy storage for concentrated solar power (Alnaimat and Rashid, 2023).
Thermochemical energy storage (TCES) stands out as a highly promising thermal energy storage (TES) approach for concentrated solar power (CSP) due to its superior energy density and capability for long-duration storage with minimal thermal losses. TES is critical for CSP plants, enabling consistent electricity generation by storing thermal energy during peak solar periods for use during low or no sunlight hours. For example, the Andasol CSP plant in Spain employs a two-tank molten salt system (60% NaNO3, 40% KNO3) to store 7.5 h of thermal energy at 565°C, providing 50 MW of dispatchable power with a round-trip efficiency of ∼93–98% and reducing the Levelized Cost of Electricity (LCOE) by ∼10–15% compared to CSP plants without TES. Emerging innovations, such as the solid particle TES system tested at Sandia National Laboratories’ Falling Particle Receiver, utilize ceramic particles to reach temperatures exceeding 1,000°C, boosting thermodynamic efficiency by ∼20% over conventional molten salts. However, issues like particle attrition and high capital costs (∼$20–30/kWh) remain. TCES systems, such as the CaO/CaCO3 looping in the GLASUNTES project, deliver high energy density (∼1.5–3.0 GJ/m3) but require advancements in catalysts to overcome slow reaction kinetics. Systems based on calcium looping (CaO/CaCO3) and magnesium-based redox cycles are under active development. For example, the GLASUNTES project recently demonstrated the use of molten glass in thermochemical systems at temperatures up to 1,300°C, significantly pushing the boundaries of operational temperature ranges in TES (GLASUNTES, 2016). Moreover, recent advancements include cascaded TCES configurations designed to optimize round-trip efficiency and exergy performance Lu et al. (2024) investigated such systems using coupled redox reactions, achieving better temperature control and reduced thermal degradation. System integration strategies have also seen significant refinement. Thermosyphon-based charging mechanisms in stratified single-medium storage tanks have shown enhanced efficiency in preserving thermal stratification without requiring mechanical pumps. Parida and Basu (2022) experimentally validated the efficacy of this approach in achieving rapid and uniform heat distribution, reducing exergy losses and simplifying system design.
From an economic perspective, integrating TES into CSP plants contributes significantly to lowering the Levelized Cost of Electricity (LCOE) by increasing plant capacity factors and reducing dependence on auxiliary fossil fuel systems Borge-Diez et al. (2022) conducted an economic assessment showing that TES-enhanced CSP plants are better equipped to respond to electricity price volatility, offering both technical and market-driven resilience. Additionally, life cycle assessments of CSP plants equipped with TES have indicated significant reductions in greenhouse gas emissions and water usage, reinforcing their role as a sustainable energy technology (Kuravi et al., 2013).
Looking ahead, hybrid CSP systems that integrate photovoltaic (PV) modules with TES are being investigated to enhance flexibility and dispatchability (Sumayli et al., 2023; Zapater and Vicente, 2023). These hybrid configurations allow for optimized use of solar resources across varying conditions, bolstering grid reliability. Continued research into novel high-temperature materials, cost-effective thermochemical cycles, and hybrid energy systems is expected to further solidify TES as a cornerstone of future CSP technologies and global renewable energy strategies.
8.2 TES in waste heat recovery in fossil/fuel plants
Thermal Energy Storage (TES) systems are pivotal in enhancing the efficiency of fossil fuel and nuclear power plants by capturing and reusing waste heat, thereby reducing fuel consumption and greenhouse gas emissions. These systems store surplus thermal energy from high-temperature exhaust gases or steam cycles, enabling better alignment of energy supply with demand, especially during peak loads or rapid fluctuations. For instance, a pilot TES system at the Drax Power Station in the UK utilizes molten salts to capture exhaust heat at ∼500°C, cutting coal use by ∼5% and CO2 emissions by ∼50,000 tons annually. At Iran’s Bushehr Nuclear Power Plant, integrating Organic Rankine Cycle (ORC) with TES boosts electrical output by 0.077% and reduces thermal discharge by 15%–20%. However, challenges such as corrosion in high-temperature systems and retrofit costs (∼$25–40/kWh) persist. Ongoing research focuses on optimizing heat exchanger designs and exploring cost-effective materials like basalt rocks to enhance TES scalability and adoption in power plants for a sustainable energy future.
Figure 17 depicts a thermal energy storage (TES) system integrated into fossil/fuel plants, where high-temperature exhaust gases (1,200°C) are captured, stored, and later discharged to preheat scrap, produce steam, or supply heat-demanding processes, improving energy efficiency and waste heat recovery.
Figure 17. Tes for waste recovery in fossil/fuel plant (Ortega et al., 2018).
Recent advancements have highlighted the integration of Thermal Energy Storage (TES) with Combined Heat and Power (CHP) systems and innovative thermodynamic cycles to optimize energy recovery in nuclear power plants. For instance, Salaudeen et al. (2025) explored the feasibility of using Organic Rankine Cycle (ORC) technology for waste heat recovery at Iran’s Bushehr Nuclear Power Plant. Their study demonstrated a modest 0.077% increase in electrical output and a significant 15%–20% reduction in thermal discharge into the Persian Gulf, showcasing both performance improvements and environmental benefits. In the nuclear sector, TES also contributes to peak shaving and enhances load-following capabilities. (Obara and Tanaka (2021) proposed a gas hydrate heat cycle for waste heat recovery, which showed higher conversion efficiency compared to conventional systems, indicating a promising approach for future energy recovery. Additionally Srivastava et al. (2024) conducted a thermo-economic feasibility study on ORC technology for waste heat recovery in Indian nuclear power plants. Their findings demonstrated improved efficiency and economic viability, further emphasizing the potential of advanced thermodynamic cycles in enhancing the sustainability of nuclear energy production. These studies demonstrate that Thermal Energy Storage (TES) technologies are increasingly being adopted in fossil fuel and nuclear power plants to capture waste heat, enhance efficiency, and reduce environmental impact. The integration of advanced thermodynamic cycles, such as Organic Rankine Cycle (ORC) and gas hydrate systems, has shown significant operational and ecological benefits, underscoring the potential of TES to improve both plant performance and sustainability.
8.3 TES in industrial processes
Thermal energy storage (TES) is increasingly vital for enhancing energy efficiency and lowering emissions in energy-intensive industrial processes such as steel production, cement manufacturing, and chemical processing. Innovative TES solutions are transforming these sectors. For example, at Sweden’s SSAB steel plant, a packed-bed TES system utilizing basalt rocks captures waste heat at 600°C to preheat scrap metal, reducing energy consumption by ∼10%. In cement production, Heidelberg Cement’s Lixhe plant employs calcium-based thermochemical energy storage (TCES) to store heat at 850°C, achieving ∼15% lower CO2 emissions. In chemical processing, metal hydride TES systems, such as those using MgH2, offer high energy density (∼2 GJ/m3) but are limited by slow hydrogen release kinetics. Ongoing research aims to improve reaction rates and reduce material costs to enhance the scalability and adoption of these emerging TES technologies, driving sustainable industrial operations toward a low-carbon future.
In the steel industry, molten salt TES systems are employed to capture and store waste heat from electric arc furnaces. This stored heat can be used to preheat raw materials or generate steam, which enhances the overall energy efficiency of steelmaking operations and reduces energy consumption (Klopčič et al., 2023). In the cement industry, calcium-based thermochemical energy storage (TCES) systems are gaining traction. These systems use calcium oxide (CaO) in reversible chemical reactions to store and release thermal energy, significantly improving energy efficiency during the high-temperature clinker production process. This approach helps reduce the overall energy consumption and CO2 emissions associated with cement manufacturing (Da et al., 2020; Hu et al., 2025). The chemical industry is also exploring the use of metal hydride-based TES systems. Metal hydrides absorb and release heat through reversible chemical reactions, making them suitable for chemical processes that require efficient heat management. These systems offer significant promise for improving heat storage and recovery, reducing energy demand and improving process sustainability (Chavan et al., 2022). These advancements demonstrate how TES technologies are improving the efficiency of industrial processes in multiple sectors, offering effective solutions for waste heat recovery and better energy utilization. Figure 18 depicts a thermal energy storage (TES) system for industrial processes, utilizing wind and solar energy, along with an optional heat source, to charge hot and cold storage tanks, which then discharge to power a turbine for electricity and optionally support district heating.
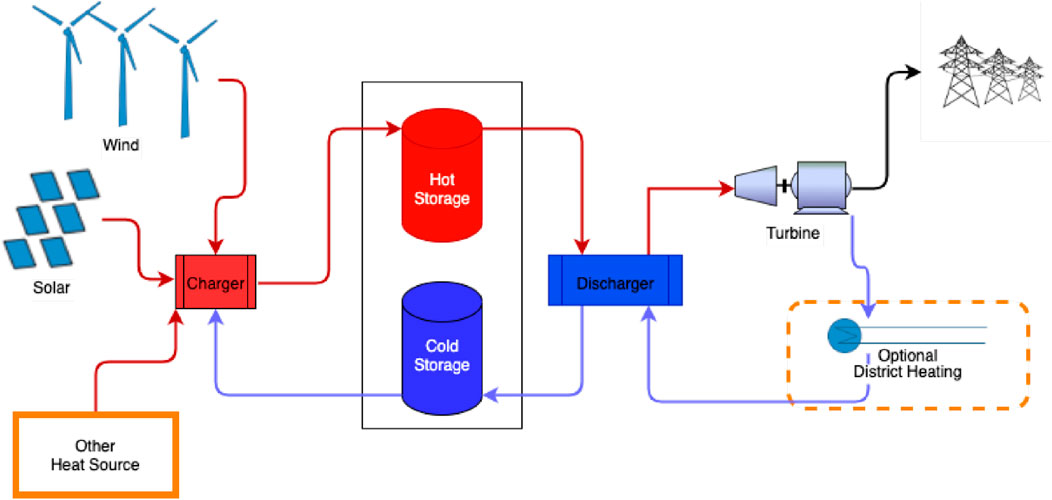
Figure 18. Thermal energy storage for industrial processes (Gautam et al., 2022).
8.4 TES in smart grids: Demand side management and renewable energy storage
Smart grids are advanced electricity networks that use digital technology to monitor and manage the generation, distribution, and consumption of electricity. The integration of Thermal Energy Storage (TES) into smart grids is emerging as a promising strategy to enhance demand-side management (DSM) and facilitate renewable energy storage. This can help stabilize energy supply, reduce peak demand, and improve the flexibility of the grid to accommodate intermittent renewable energy sources like solar and wind (Tan et al., 2021). For instance, Denmark’s Marstal District Heating System uses a 75,000 m3 water tank for seasonal TES, storing solar heat at 90°C and reducing fossil fuel use by ∼30%. PCM-based TES in residential smart grids, like those in Germany’s E. ON pilot projects, shifts cooling loads, reducing peak demand by ∼20%. Figure 19 illustrates a thermal energy storage (TES) system for a smart grid, where a solar generator powers a low voltage AC bus, supported by hydrogen storage, a fuel cell, and a battery, all connected through AC/DC converters to manage load and grid interactions via a transformer.
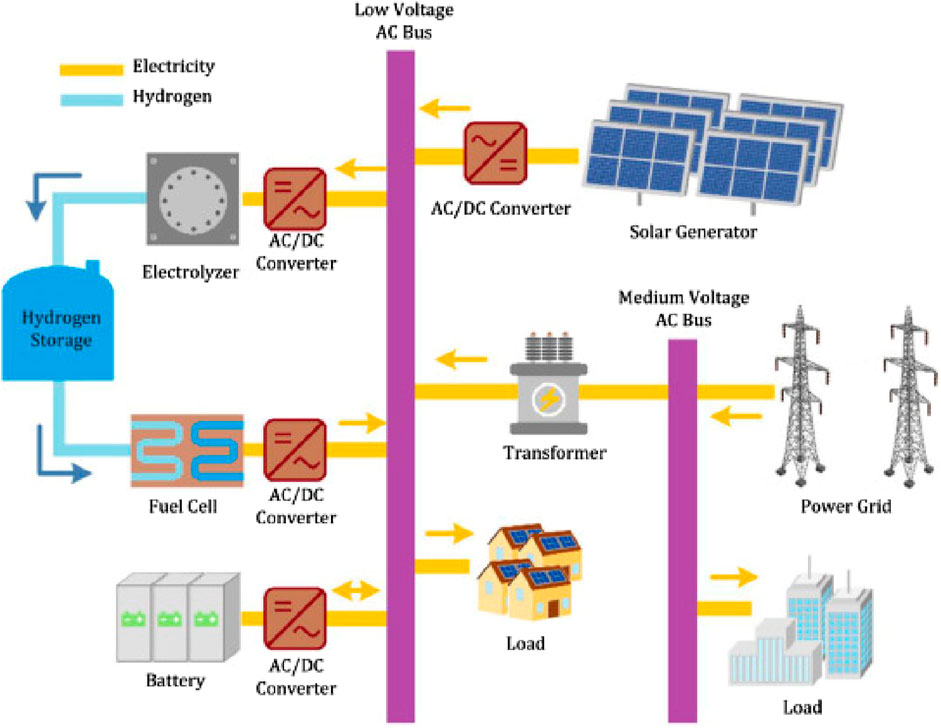
Figure 19. Thermal energy storage integration in a smart grid system (Tan et al., 2021).
Demand-side management (DSM) is a strategic approach to balancing electricity demand with supply, particularly during peak periods. Thermal Energy Storage (TES) systems play a crucial role in DSM by storing thermal energy during off-peak hours and releasing it when demand is high, thereby reducing the need for additional electricity generation and flattening demand curves for more efficient energy distribution. Phase Change Material (PCM)-based TES systems, for instance, can capture excess heat during low-demand periods such as at night or when solar energy production is high and release it during peak hours. This not only reduces the burden on the power grid but also optimizes energy use across sectors. In residential buildings, TES can be integrated through hot water tanks or thermally active building materials (e.g., walls and floors), which absorb and later release heat for space heating or cooling. This reduces dependence on grid electricity, contributes to energy conservation, and lowers utility costs. Moreover, TES significantly benefits consumers using heating, ventilation, and air conditioning (HVAC) systems by enabling load shifting. Systems like chilled water or ice storage can precool water at night when electricity is cheaper and demand is lower and use it for daytime cooling, thereby easing grid stress and enhancing cost efficiency (Groppi et al., 2021).
TES is also vital in supporting renewable energy penetration. When there is excess electricity generation (e.g., during peak solar hours), that energy can be converted into thermal form, stored as sensible or latent heat and used later when renewable generation is low. This strategy reduces curtailment and allows for better alignment between supply and demand (Kataray et al., 2023). Furthermore, in smart grid settings, TES systems are increasingly integrated with advanced energy management systems that use real-time grid data, dynamic pricing, and weather forecasts to optimize the timing and usage of stored thermal energy (Bakare et al., 2023).
8.5 TES in data centers
Data centers, consuming approximately 1%–1.5% of global electricity, rely heavily on energy-intensive cooling systems to maintain optimal server temperatures amid rising global demand for data storage and processing. Thermal energy storage (TES) offers innovative solutions to enhance energy efficiency in data center cooling. For instance, Google’s Hamina data center in Finland employs chilled water TES to store cooling capacity at night, reducing daytime energy consumption by ∼15%. Similarly, the Green Mountain Data Center in Norway utilizes underground TES (UTES), leveraging stable ground temperatures to cut cooling costs by ∼20%. Recent advancements in TES, such as nano-enhanced phase change materials (PCMs) and hybrid systems, show promise for further improving thermal performance and scalability. However, research gaps persist, including challenges in scaling UTES for smaller data centers and enhancing PCM encapsulation for high-cycle durability.
Data centers require continuous cooling, traditionally achieved through energy-intensive air-conditioning systems, with cooling accounting for a substantial share of total energy consumption. To address this, Thermal Energy Storage (TES) systems such as Phase Change Material (PCM)-based units or chilled water storage tanks are increasingly being integrated into data center operations. These systems can store excess cooling capacity during periods of low demand (e.g., nighttime) and release it during peak operational hours, when computational loads and cooling needs spike. This load shifting not only alleviates pressure on cooling equipment but also enables the use of lower-cost electricity during off-peak times, improving overall energy efficiency. Innovative approaches, such as Underground Thermal Energy Storage (UTES), are being explored to further enhance cooling efficiency. UTES systems leverage the earth’s stable underground temperatures to store and retrieve thermal energy, offering a sustainable and resilient cooling solution for data centers (Winick et al., 2025). The integration of Thermal Energy Storage in data center cooling strategies presents a promising avenue for enhancing energy efficiency, reducing operational costs, and ensuring reliable performance. As data centers continue to grow in size and energy demand, the adoption of TES technologies will be instrumental in achieving sustainable and resilient operations. Figure 20 shows a thermal energy storage (TES) system for data centers, utilizing a cold energy storage system with a chiller, pump, and plate heat exchanger to manage cooling for racks through indirect contact type cooling, supported by a cold room and optional hot room integration.
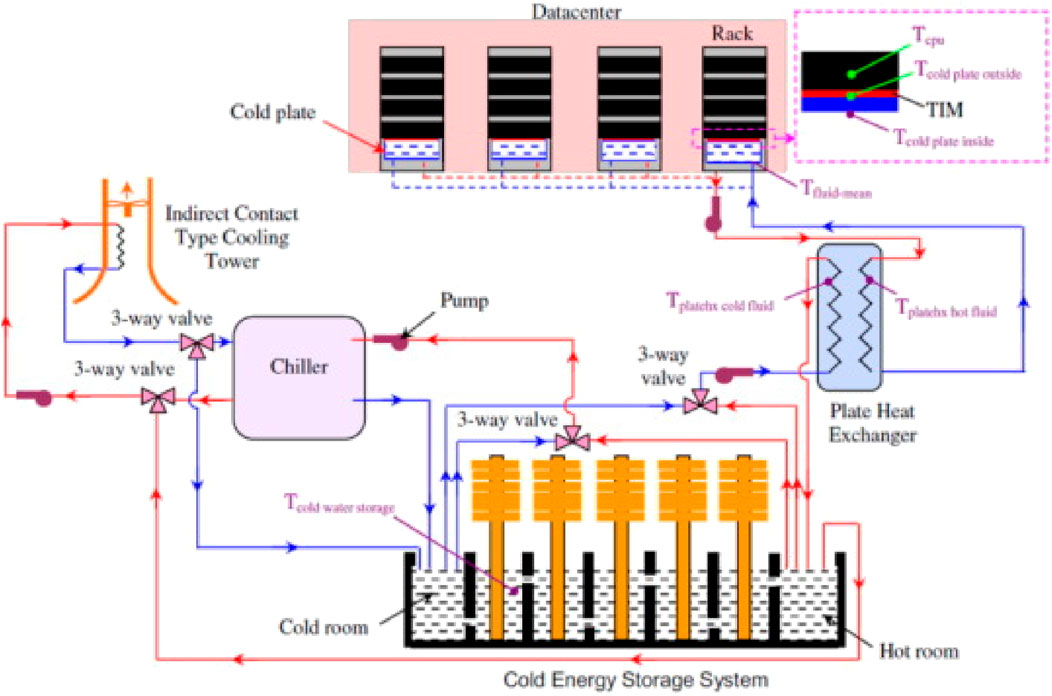
Figure 20. Thermal energy storage (TES) system for data centers (L. Liu et al., 2020).
8.6 TES in commercial and residential buildings
TES enhances building energy efficiency. In Denmark’s Drake Landing Solar Community, a borehole TES system stores solar heat seasonally, achieving 97% solar fraction for space heating. PCM-integrated walls in retrofit projects, like those in Spain’s Cubelles pilot, reduce cooling energy by ∼25%. Thermal Energy Storage (TES) plays a crucial role in boosting energy efficiency and promoting sustainability in both residential and commercial buildings. By capturing and storing thermal energy during off peak periods such as at night or when renewable energy generation is high TES systems can release this energy when demand increases thereby optimizing heating cooling and hot water supply. In building applications TES commonly uses mediums like water tanks Phase Change Materials (PCMs) or thermally massive structures such as concrete slabs to store and later distribute thermal energy. This not only helps shift heating and cooling loads but also lowers overall energy consumption and operating costs. For instance, integrating TES into building envelopes can regulate indoor temperatures more effectively by absorbing excess heat during the day and releasing it at night (Pavlov and Olesen, 2011). In domestic hot water systems, solar energy can be harnessed during the day to heat water which is then stored in insulated tanks for later use particularly at night or during cloudy weather reducing dependence on conventional energy sources (Seddegh et al., 2015). Likewise, underfloor heating systems equipped with TES circulate warm water through embedded pipes or heat retaining materials radiating heat evenly across the space. These systems are more energy efficient than conventional heating methods and benefit greatly from the thermal buffering that TES provides (Nair et al., 2022). Overall the integration of TES into buildings for space heating and cooling domestic hot water and underfloor heating significantly enhances energy efficiency lowers energy bills and contributes to a more sustainable built environment. Figure 21 demonstrates a thermal energy storage (TES) system for buildings, where solar panels power a hydrogen-based energy cycle (H12-NEC) with NEC dehydrogenation and hydrogenation, storing energy in an H12-NEC/NEC tank to provide heat and electricity, supplemented by an electronic fuel cell and grid connection.
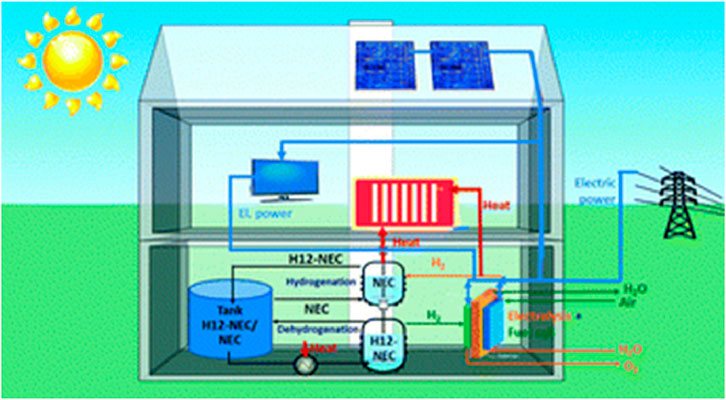
Figure 21. Thermal energy storage (TES) system for buildings with hydrogen integration (Teichmann et al., 2012).
8.7 TES in transportation
Thermal Energy Storage (TES) is becoming essential for improving the efficiency and sustainability of transportation, especially in electric vehicles (EVs) and refrigerated transport, with recent research highlighting its role in tackling energy management issues. TES improves thermal management in electric vehicles (EVs) and refrigerated transport. An example is Tesla’s Model S uses PCM-based battery thermal management systems (BTMS) to maintain battery temperatures, extending lifespan by ∼10%. In refrigerated trucks, PCM systems like those tested by Thermo King maintain 0°C–5°C, reducing energy use by ∼15%.
Effective thermal management systems (TMS), including air cooling, liquid cooling, and phase change material (PCM)-based cooling, are employed to maintain optimal battery temperatures, thereby enhancing efficiency and extending battery lifespan (Hwang et al., 2024). In electric vehicles, TES systems are being explored to optimize battery thermal management (Manisha et al., 2025). discusses the development of compact, lightweight, and high-performance Battery Thermal Management Systems (BTMS) achieved by constructing bi-functional heating-cooling plates, resulting in outstanding thermal control and energy storage density. Additionally, a novel synergetic cooling and charging strategy using a gallium-based liquid metal flexible charging connector has been developed to address the challenges of high-power direct current fast charging in EVs (Liu et al., 2025). Figure 22 describes a thermal energy storage (TES) system for electric vehicles, which includes a PCM heat exchanger combined with a dual-layer cabin heater. The system is regulated by an AC-powered PCM charger controller and is connected to the vehicle’s main system, featuring a PTC heater and a reservoir to maintain effective temperature management. In refrigerated transport systems, Thermal Energy Storage (TES) technologies, particularly those utilizing Phase Change Materials (PCMs), are instrumental in maintaining the required temperatures for perishable goods during transit. These systems absorb and release thermal energy to ensure temperature stability without continuous power supply, which is especially beneficial during power outages or transportation delays. TES systems enhance energy efficiency and thermal performance in cold chain logistics. For instance, a study by Calati et al. (2022) introduces a novel insulated wall concept for refrigerated trucks, integrating latent thermal energy storage with PCMs to improve temperature regulation. Furthermore, Selvnes et al. (2021) the application of cold thermal energy storage using PCMs in refrigeration systems, emphasizing their potential to reduce energy consumption and environmental impact. Recent research has focused on developing advanced TES materials, such as nano-encapsulated PCMs, which offer improved thermal conductivity and energy storage capacity. These materials enhance the performance of TES systems in transportation by providing more efficient thermal management and reducing the size and weight of storage systems. Such advancements are paving the way for more compact, lightweight, and efficient TES solutions, making them more viable for widespread adoption in various transportation applications.
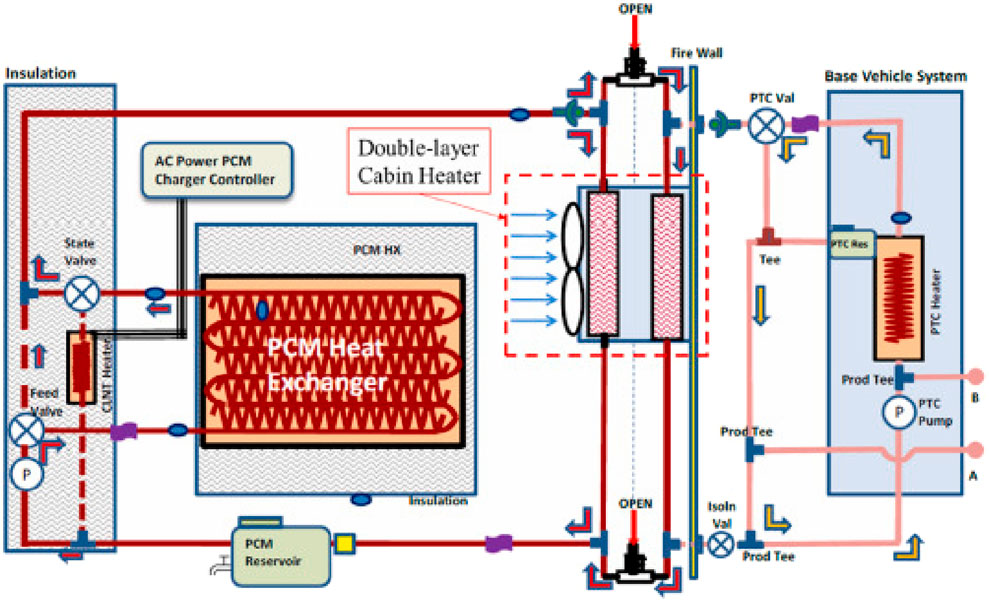
Figure 22. Thermal energy storage in electric vehicle (Xie et al., 2022).
8.8 TES in hydrogen storage
Hydrogen is a versatile, clean energy carrier with high energy content per unit mass (120 MJ/kg), but its low volumetric density at ambient conditions poses significant storage challenges. Efficient storage is crucial for applications in transportation, stationary power, and industrial sectors (Usman, 2022). TES enhances hydrogen storage efficiency in metal hydride systems. The H2M project in Germany integrates PCMs with MgH2, capturing heat at 300°C during hydrogen absorption and reusing it for desorption, improving efficiency by ∼25%. Challenges include slow kinetics and high material costs (∼$80–120/kWh) however research into complex hydrides like Na2Mg2NiH6 is addressing these issues.
Metal hydrides offer a promising solution by chemically absorbing hydrogen, enabling storage at lower pressures and ambient temperatures. However, the hydrogen absorption (hydriding) and desorption (dehydriding) processes in metal hydrides are inherently exothermic and endothermic, respectively, necessitating efficient thermal management to optimize performance (Davis Cortina et al., 2024). Integrating TES with metal hydride systems addresses this need by capturing the heat released during hydrogen absorption and reusing it during desorption, thereby enhancing the overall energy efficiency of the storage system.
Metal hydrides typically store hydrogen via reversible chemical reactions that either absorb or release heat during the processes of hydrogen absorption and desorption. Thermal Energy Storage (TES) systems help regulate this heat flow, thereby enhancing the overall energy efficiency and economic viability of the storage system. For example, the heat generated during the exothermic hydrogen absorption process can be captured by TES systems using sensible, latent, or thermochemical storage materials and subsequently reused to provide the heat needed for the endothermic hydrogen release phase. A comprehensive review by Davis Cortina et al. (2024) explores the integration of Thermal Energy Storage (TES) within metal hydride systems, emphasizing the potential of such combined systems to enhance thermal management and system efficiency. The study examines various TES materials, including sensible, latent, and thermochemical storage options, and assesses their compatibility with different metal hydrides. The authors highlight that coupling TES with metal hydride storage can lead to more compact, efficient, and cost-effective hydrogen storage solutions, particularly beneficial for stationary energy applications and industries with significant waste heat. The review also discusses the thermophysical, thermodynamic, and kinetic properties of metal hydride materials, providing insights into their suitability for integration with TES systems. Additionally, it presents a detailed analysis of notable metal hydride–TES coupled systems from the literature and assesses potential future developments in the field, offering guidance for researchers and engineers in advancing innovative and efficient hydrogen energy systems. Additionally, research into complex hydrides, such as Na2Mg2NiH6, has shown promise for dual applications in hydrogen and thermal energy storage (Humphries et al., 2018; Mohammadi et al., 2022) explores the thermal decomposition and hydrogen release characteristics of Na2Mg2NiH6, highlighting its suitability for reversible hydrogen storage and thermal energy applications. Figure 23 illustrates a hydrogen storage system for a steam power plant, utilizing high-temperature (HT) and low-temperature (LT) metal hydrides to store and release hydrogen, powered by a solar concentration system during sun availability, and driving a steam generator and turbine for continuous 24/7 operation. The material efficiently desorbs hydrogen at 278°C–350°C and maintains stable capacity across multiple hydrogenation cycles. In summary, the integration of TES with metal hydride hydrogen storage systems offers a synergistic approach to address the thermal management challenges inherent in hydrogen storage.
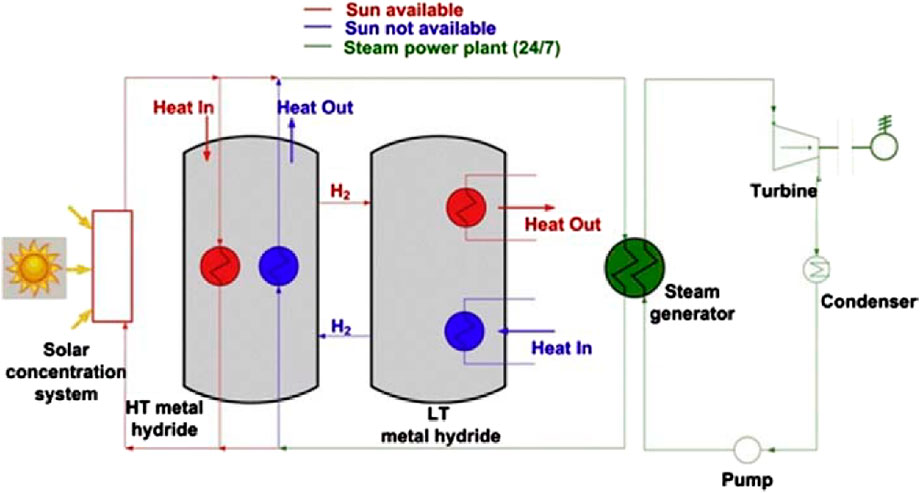
Figure 23. Hydrogen storage system for steam power plant (Li et al., 2018).
Integrating advanced computational methods with thermal energy storage (TES) systems has significantly enhanced design precision, operational efficiency, and material innovation. Below is a technically detailed analysis supported by case studies and verification methodologies.
8.9 Integration of computing with thermal energy storage systems
High-fidelity CFD and FEA enable precise prediction of thermal behavior in TES systems. For example, finite element models using COMSOL and MATLAB have been employed to simulate phase change material (PCM) melting dynamics, capturing interface movement and heat transfer rates in latent heat storage systems (Zeneli, et al., 2019). These models validate energy balance equations and ensure grid-independent solutions, critical for scaling simulations to real-world applications.
Machine learning algorithms optimize charge-discharge cycles by analyzing historical load data and weather patterns. For instance, AI controllers dynamically adjust HTF (heat transfer fluid) flow rates to balance supply-demand mismatches in hybrid TES systems, reducing energy waste by up to 20% (Guntreddi et al., 2024).
9 Future trends and challenges
The advancement of Thermal Energy Storage (TES) is being shaped by several transformative trends, including the hybridization of storage methods (combining sensible, latent, and thermochemical systems), the development of sustainable low-cost materials (e.g., bio-based PCMs), and the adoption of advanced manufacturing techniques such as additive manufacturing to improve heat transfer performance (Zhang et al., 2024c). Moreover, the integration of TES with AI-driven digital twins is enabling real-time monitoring, predictive control, and optimization of system performance (Ba et al., 2025; Mehraj et al., 2025). Despite these advances, challenges persist particularly the long-term stability of storage materials, economic barriers to large-scale deployment, and the lack of standardized testing protocols and lifecycle assessment frameworks. Overcoming these obstacles will require interdisciplinary collaboration between material scientists, thermal engineers, and industry stakeholders.
9.1 Sensible heat storage (SHS)
One of the major challenges in SHS systems is the low energy density of conventional materials such as water and concrete, which necessitate large storage volumes. To address this, researchers are focusing on the development of low-cost, high-specific-heat materials that can maintain thermal stability above 600°C. Additionally, improving thermal insulation and stratification in storage tanks is critical to minimizing heat loss. Promising alternatives such as recyclable basalt rock is being explored for industrial-scale energy storage applications (Li Y.-C. et al., 2022).
9.2 Latent heat storage (LHS)
LHS systems, which use phase change materials (PCMs) such as paraffin wax and salt hydrates, are limited by low thermal conductivity and issues like supercooling and phase segregation. To improve their performance, nano-enhanced PCMs with thermal conductivities greater than 1 W/m·K and cycling stability exceeding 10,000 cycles are being developed. Furthermore, bio-based PCMs like erythritol offer sustainable alternatives, but their costs must fall below $30/kWh to be commercially viable (Saqib and Andrzejczyk, 2023).
9.3 Thermochemical energy storage (TCES)
TCES systems are capable of storing energy at very high densities, but their deployment is hampered by material degradation due to sintering and agglomeration, especially in carbonate-based cycles like CaCO3/CaO and salt hydrates such as MgSO4·7H2O. Innovations in catalyst development and reactor design are necessary to improve reaction kinetics and cycling stability (targeting over 5,000 cycles), as well as to reduce decomposition temperatures (typically >800°C for CaCO3). These improvements are central to the viability of TCES in concentrated solar power (CSP) systems (Raganati and Ammendola, 2023).
10 Conclusion
This comprehensive review emphasizes the crucial role of Thermal Energy Storage (TES) technologies as a fundamental component of contemporary energy systems, meeting the growing need for improved energy efficiency, grid adaptability, and effective integration of renewable energy sources. By examining the fundamental principles and performance characteristics of sensible, latent, and thermochemical storage systems, the paper highlights the emerging trends and their diverse applications across sectors such as hydrogen storage, industrial processes, concentrated solar power (CSP), transportation, buildings, smart grids, and data centers. Sensible heat storage, exemplified by molten salts, remains the most mature and cost-effective approach, particularly in CSP plants, due to its high efficiency and scalability. Latent heat storage, leveraging phase change materials (PCMs), provides superior energy density and temperature stability, making it ideal for precise thermal regulation in building HVAC systems and transportation. Thermochemical energy storage (TCES), with its high energy density and long-term storage potential, shows significant promise for high-temperature industrial applications and hydrogen storage, despite challenges related to material stability and reaction kinetics.
The integration of TES with metal hydride systems for hydrogen storage, alongside its contributions to waste heat recovery, load shifting, and grid stabilization, demonstrates its versatility in addressing energy supply-demand mismatches. Hybrid TES systems, combining multiple storage mechanisms, further enhance efficiency, flexibility, and cost-effectiveness, offering innovative solutions for renewable energy integration. Economically, TES reduces the Levelized Cost of Electricity (LCOE) in CSP plants and improves renewable energy viability by enhancing capacity factors and grid reliability. Environmentally, it significantly lowers greenhouse gas emissions and resource consumption, aligning with global decarbonization objectives. However, challenges such as high material costs, thermal degradation, scalability, and integration with existing infrastructure persist. Ongoing advancements in composite materials, nano-enhanced PCMs, and advanced reactor designs are overcoming these barriers, paving the way for more robust, efficient, and durable TES solutions.
Looking ahead, several future trends are poised to further elevate TES’s impact. The development of bio-based and recyclable PCMs promises to enhance sustainability by reducing reliance on petroleum-derived materials, aligning with circular economy principles. Artificial intelligence (AI) and machine learning are increasingly being integrated into TES systems to optimize charging/discharging cycles, predict energy demand, and enhance system efficiency through real-time data analytics. Additionally, the emergence of modular and compact TES designs is expected to facilitate deployment in space-constrained environments, such as urban buildings and transportation. Advances in high-temperature thermochemical systems, particularly those leveraging abundant materials like calcium carbonate, are likely to expand TES applications in industrial decarbonization and long-duration energy storage. Furthermore, the integration of TES with emerging technologies, such as green hydrogen production and advanced nuclear reactors, holds potential to create synergies that enhance overall energy system resilience. As the global energy landscape shifts toward greater reliance on intermittent renewable sources, TES will remain an essential enabler of a resilient, sustainable, and low-carbon energy future, driven by these innovative trends and fostering the transition to renewable energy across energy-intensive sectors.
Author contributions
CK-E: Conceptualization, Supervision, Writing – review and editing. OO: Data curation, Investigation, Methodology, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. Generative AI tools were used to assist in language refinement, grammar correction, and improving the clarity and coherence of certain sections of the text. All technical content, analysis, interpretations, and conclusions were conceived and validated by the authors without reliance on AI. The final manuscript was thoroughly reviewed and approved by the authors to ensure accuracy and integrity.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aarts, J., van Ravensteijn, B., Fischer, H., Adan, O., and Huinink, H. (2023). Polymeric stabilization of salt hydrates for thermochemical energy storage. Appl. Energy 341, 121068. doi:10.1016/j.apenergy.2023.121068
Abanades, S. (2023). Thermochemical energy storage for high-temperature concentrating solar applications. Thermopedia. doi:10.1615/thermopedia.010258
Aftab, W., Usman, A., Shi, J., Yuan, K., Qin, M., and Zou, R. (2021). Phase change material-integrated latent heat storage systems for sustainable energy solutions. Energy Environ. Sci. 14 (8), 4268–4291. doi:10.1039/D1EE00527H
Agalit, H., Zari, N., and Maaroufi, M. (2020). Suitability of industrial wastes for application as high temperature thermal energy storage (TES) materials in solar tower power plants – a comprehensive review. Sol. Energy 208, 1151–1165. doi:10.1016/j.solener.2020.08.055
Aghmadi, A., and Mohammed, O. A. (2024). Energy storage systems: technologies and high-power applications. Batteries 10 (4), 141. doi:10.3390/batteries10040141
Akci Turgut, H. S., and Dincer, I. (2023). Assessment of a new binary geothermal based methanol synthesis plant with power, hydrogen and freshwater. Energy Convers. Manag. 294, 117576. doi:10.1016/j.enconman.2023.117576
Alami, A. H., Olabi, A. G., Mdallal, A., Rezk, A., Radwan, A., Rahman, S. M. A., et al. (2023). Concentrating solar power (CSP) technologies: status and analysis. Int. J. Thermofluids 18, 100340. doi:10.1016/j.ijft.2023.100340
Alanazi, A. (2023). Optimization of concentrated solar power systems with thermal storage for enhanced efficiency and cost-effectiveness in thermal power plants. Eng. Technol. Appl. Sci. Res. 13 (6), 12115–12129. doi:10.48084/etasr.6381
Aljabair, S., Alesbe, I., and Ibrahim, S. H. (2023). Review on latent thermal energy storage using phase change material. J. Therm. Eng. 9 (1), 247–256. doi:10.18186/thermal.1245298
Alkrush, A. A., Salem, M. S., Abdelrehim, O., and Hegazi, A. A. (2024). Data centers cooling: a critical review of techniques, challenges, and energy saving solutions. Int. J. Refrig. 160, 246–262. doi:10.1016/j.ijrefrig.2024.02.007
Almeshaal, M. A., and Altohamy, A. A. (2024). Experimental analysis of a photovoltaic thermal collector using phase change materials and copper oxide nanofluid. J. Energy Storage 93, 112265. doi:10.1016/j.est.2024.112265
Alnaimat, F., and Rashid, Y. (2023). “Thermal energy storage in concentrated solar power plants,” in Energy storage for multigeneration (Elsevier), 275–294. doi:10.1016/B978-0-12-821920-1.00001-7
AlShafi, M., and Bicer, Y. (2021). Thermodynamic performance comparison of various energy storage systems from source-to-electricity for renewable energy resources. Energy 219, 119626. doi:10.1016/j.energy.2020.119626
Alva, G., Lin, Y., and Fang, G. (2018). An overview of thermal energy storage systems. Energy 144, 341–378. doi:10.1016/j.energy.2017.12.037
Atinafu, D. G., Dong, W., Huang, X., Gao, H., and Wang, G. (2018). Introduction of organic-organic eutectic PCM in mesoporous N-doped carbons for enhanced thermal conductivity and energy storage capacity. Appl. Energy 211, 1203–1215. doi:10.1016/j.apenergy.2017.12.025
Ayinla, R. D., Kwasi-Effah, C. C., and Egware, H. O. (2024). Thermal conductivity enhancement of quaternary nitrate salt mixtures for thermal energy storage with Al2O3 nanoparticle doping. J. Sci. Technol. Res. 6(3), 253–278. doi:10.5281/zenodo.14020924
Ayyaril, S. S., Shanableh, A., Bhattacharjee, S., Rawas-Qalaji, M., Cagliani, R., Shabib, A. G., et al. (2023). Recent progress in micro and nano-encapsulation techniques for environmental applications: a review. Results Eng. 18, 101094. doi:10.1016/j.rineng.2023.101094
Ba, L., Tangour, F., El Abbassi, I., and Absi, R. (2025). Analysis of digital twin applications in energy efficiency: a systematic review. Sustainability 17 (8), 3560. doi:10.3390/su17083560
Baghaei Oskouei, S., Frate, G. F., Christodoulaki, R., Bayer, Ö., Akmandor, İ. S., Desideri, U., et al. (2024). Solar-powered hybrid energy storage system with phase change materials. Energy Convers. Manag. 302, 118117. doi:10.1016/j.enconman.2024.118117
Bakare, M. S., Abdulkarim, A., Zeeshan, M., and Shuaibu, A. N. (2023). A comprehensive overview on demand side energy management towards smart grids: challenges, solutions, and future direction. Energy Inf. 6 (1), 4. doi:10.1186/s42162-023-00262-7
Bao, H., and Ma, Z. (2022). “Thermochemical energy storage,” in Storing energy (Elsevier), 651–683. doi:10.1016/B978-0-12-824510-1.00028-3
Barrak, E. S., Hammodi, K. A., and Mouthanna, A. (2024). Thermal stratification in solar water storage tanks through inlet port diffuser optimization during charge and discharge processes. Eng. Headw. 8, 39–53. doi:10.4028/p-1r0MVz
Barrasso, M., Langella, G., Amoresano, A., and Iodice, P. (2023). Latest advances in thermal energy storage for solar plants. Processes 11 (6), 1832. doi:10.3390/pr11061832
Bauer, T. (2021). “Fundamentals of high-temperature thermal energy storage, transfer, and conversion,” in Ultra-high temperature thermal energy storage, transfer and conversion (Elsevier), 1–34. doi:10.1016/B978-0-12-819955-8.00001-6
Beaupere, N., Soupremanien, U., and Zalewski, L. (2018). Nucleation triggering methods in supercooled phase change materials (PCM), a review. Thermochim. Acta 670, 184–201. doi:10.1016/j.tca.2018.10.009
Benhorma, A., Bensenouci, A., Teggar, M., Ismail, K. A. R., Arıcı, M., Mezaache, E., et al. (2024). Prospects and challenges of bio-based phase change materials: an up to date review. J. Energy Storage 90, 111713. doi:10.1016/j.est.2024.111713
Bhojani, A. K., Jha, M., Joshi, A., Bhargava, K., Singh, G., Singh, D. K., et al. (2025). Layered transition metal oxides (MoO3, WO3, Ga2O3, V2O5) for energy conversion and storage: a comprehensive review. J. Energy Storage 107, 114979. doi:10.1016/j.est.2024.114979
Borge-Diez, D., Rosales-Asensio, E., Palmero-Marrero, A. I., and Acikkalp, E. (2022). Optimization of CSP plants with thermal energy storage for electricity price stability in spot markets. Energies 15 (5), 1672. doi:10.3390/en15051672
Calati, M., Zilio, C., Righetti, G., Longo, G. A., Hooman, K., and Mancin, S. (2022). Latent thermal energy storage for refrigerated trucks. Int. J. Refrig. 136, 124–133. doi:10.1016/j.ijrefrig.2022.01.018
Caldwell, A. S., Itskos, G., and Sandhage, K. H. (2021). Air-stable, earth-abundant molten chlorides and corrosion-resistant containment for chemically-robust, high-temperature thermal energy storage for concentrated solar power. Mater. Today 46, 9–17. doi:10.1016/j.mattod.2021.02.015
Carrillo, A. J., González-Aguilar, J., Romero, M., and Coronado, J. M. (2019). Solar energy on demand: a review on high temperature thermochemical heat storage systems and materials. Chem. Rev. 119 (7), 4777–4816. doi:10.1021/acs.chemrev.8b00315
Chavan, S., Rudrapati, R., and Manickam, S. (2022). A comprehensive review on current advances of thermal energy storage and its applications. Alexandria Eng. J. 61 (7), 5455–5463. doi:10.1016/j.aej.2021.11.003
Chavan, S., Venkateswarlu, B., Kim, S. C., and Joo, S. W. (2024). Designing next-generation thermal energy storage systems with nanoparticle-based hybrid phase change materials: a computational analysis. Energy Technol. 12 (4), 2301398. doi:10.1002/ente.202301398
Chekifi, T., and Boukraa, M. (2023). CFD applications for sensible heat storage: a comprehensive review of numerical studies. J. Energy Storage 68, 107893. doi:10.1016/j.est.2023.107893
Chen, D., Chen, X., Ma, Z., Wang, Y., Roskilly, A. P., and Zhou, J. (2022). Experimental study of LiCl/LiBr-zeolite composite adsorbent for thermochemical heat storage. Buildings 12 (11), 2001. doi:10.3390/buildings12112001
Chieruzzi, M., Cerritelli, G. F., Miliozzi, A., and Kenny, J. M. (2013). Effect of nanoparticles on heat capacity of nanofluids based on molten salts as PCM for thermal energy storage. Nanoscale Res. Lett. 8 (1), 448. doi:10.1186/1556-276X-8-448
Chung, K. M., Zhang, Y., Zeng, J., Haddad, F., Adapa, S., Feng, T., et al. (2023). In-situ thermophysical measurement of flowing molten chloride salt using modulated photothermal radiometry. Sol. Energy 265, 112124. doi:10.1016/j.solener.2023.112124
Da, Y., Xuan, Y., Teng, L., Zhang, K., Liu, X., and Ding, Y. (2020). Calcium-based composites for direct solar-thermal conversion and thermochemical energy storage. Chem. Eng. J. 382, 122815. doi:10.1016/j.cej.2019.122815
Davis Cortina, M., Romero de Terreros Aramburu, M., Neves, A. M., Hurtado, L., Jepsen, J., and Ulmer, U. (2024). The integration of thermal energy storage within metal hydride systems: a comprehensive review. Inorganics 12 (12), 313. doi:10.3390/inorganics12120313
Dincer, I., and Ezan, M. A. (2018). Heat storage: a unique solution for energy systems. Cham, Switzerland: Springer.
Ding, Z., Wu, W., and Leung, M. (2021). Advanced/hybrid thermal energy storage technology: material, cycle, system and perspective. Renew. Sustain. Energy Rev. 145, 111088. doi:10.1016/j.rser.2021.111088
Dixit, P., Reddy, V. J., Parvate, S., Balwani, A., Singh, J., Maiti, T. K., et al. (2022). Salt hydrate phase change materials: current state of art and the road ahead. J. Energy Storage 51, 104360. doi:10.1016/j.est.2022.104360
Eanest Jebasingh, B., and Valan Arasu, A. (2020). A detailed review on heat transfer rate, supercooling, thermal stability and reliability of nanoparticle dispersed organic phase change material for low-temperature applications. Mater. Today Energy 16, 100408. doi:10.1016/j.mtener.2020.100408
Elalfy, D. A., Gouda, E., Kotb, M. F., Bureš, V., and Sedhom, B. E. (2024). Comprehensive review of energy storage systems technologies, objectives, challenges, and future trends. Energy Strategy Rev. 54, 101482. doi:10.1016/j.esr.2024.101482
Elkelawy, M., El-Ashmawy, M., and Ahmed, S. M. (2024). State of the art in concentrated solar power: latest technological advancements and innovations in efficiency and energy storage. Pharos Eng. Sci. J. 1 (1), 17–28. doi:10.21608/pesj.2025.344983.1007
Elkhatat, A., and Al-Muhtaseb, S. A. (2023). Combined “renewable energy–thermal energy storage (RE–TES)” systems: a review. Energies 16 (11), 4471. doi:10.3390/en16114471
Emrani, A., and Berrada, A. (2024). A comprehensive review on techno-economic assessment of hybrid energy storage systems integrated with renewable energy. J. Energy Storage 84, 111010. doi:10.1016/j.est.2024.111010
Eze, V. H. U., and Tamball, J. S. (2024). Advanced modeling approaches for latent heat thermal energy storage systems. IAA J. Appl. Sci. 11 (1), 49–56. doi:10.59298/IAAJAS/2024/6.68.39.34
Fattouh, B., Vakhsouri, S., and Henserson, J. (2024). Nuclear energy in the global energy landscape: advancing sustainability and ensuring energy security?
Feng, Y., Li, X., Wu, H., Li, C., Zhang, M., and Yang, H. (2023). Critical review of Ca(OH)2/CaO thermochemical energy storage materials. Energies 16 (7), 3019. doi:10.3390/en16073019
Fortunato, B., Camporeale, S. M., Torresi, M., and Albano, M. (2012). Simple mathematical model of a thermal storage with PCM. AASRI Procedia 2, 241–248. doi:10.1016/j.aasri.2012.09.041
Galazutdinova, Y., Clark, R.-J., Al-Hallaj, S., Kaur, S., and Farid, M. (2024). New thermochemical salt hydrate system for energy storage in buildings. Energies 17 (20), 5228. doi:10.3390/en17205228
Gautam, K. R., Andresen, G. B., and Victoria, M. (2022). Review and techno-economic analysis of emerging thermo-mechanical energy storage technologies. Energies 15 (17), 6328. doi:10.3390/en15176328
Ghosh, D., Ghose, J., Datta, P., Kumari, P., and Paul, S. (2022). Strategies for phase change material application in latent heat thermal energy storage enhancement: status and prospect. J. Energy Storage 53, 105179. doi:10.1016/j.est.2022.105179
GLASUNTES (2016). Innovative high temperature thermal energy storage concept for CSP plants exceeding 50% efficiency. doi:10.3030/656753
Groppi, D., Pfeifer, A., Garcia, D. A., Krajačić, G., and Duić, N. (2021). A review on energy storage and demand side management solutions in smart energy islands. Renew. Sustain. Energy Rev. 135, 110183. doi:10.1016/j.rser.2020.110183
Guntreddi, V., Manoj, V., Reddy, M. R., Yegireddy, N. K., Swathi, A., and Raghutu, R. (2024). “Storage solutions for sustainable future: integrating batteries, supercapacitors, and thermal storage,” in E3S Web of Conferences. (EDP Sciences), doi:10.1051/e3sconf/202454703016
Guo, X., Hu, D., Yu, L., Xia, L., and Chen, G. Z. (2021). “Nanomaterials enhanced heat storage in molten salts,” in Energy-sustainable advanced materials (Springer International Publishing), 153–169. doi:10.1007/978-3-030-57492-5_6
Hanani, M. N., Sampe, J., Jaffar, J., and Mohd Yunus, N. H. (2023). Development of a hybrid solar and waste heat thermal energy harvesting system. Eng. Technol. Appl. Sci. Res. 13 (3), 10680–10684. doi:10.48084/etasr.5561
Heier, J., Bales, C., and Martin, V. (2015). Combining thermal energy storage with buildings—a review. Renew. Sustain. Energy Rev. 42, 1305–1325. doi:10.1016/j.rser.2014.11.031
Herrera, P., De la Hoz Siegler, H., and Clarke, M. (2024). Fatty acids as phase change materials for building applications: drawbacks and future developments. Energies 17 (19), 4880. doi:10.3390/en17194880
Hoivik, N., Greiner, C., Barragan, J., Iniesta, A. C., Skeie, G., Bergan, P., et al. (2019). Long-term performance results of concrete-based modular thermal energy storage system. J. Energy Storage 24, 100735. doi:10.1016/j.est.2019.04.009
Hrifech, S., Agalit, H., Jarni, A., Mouguina, E. M., Grosu, Y., Faik, A., et al. (2020). Characterization of natural rocks as filler materials for medium-temperature packed bed thermal energy storage system. J. Energy Storage 32, 101822. doi:10.1016/j.est.2020.101822
Hu, Q., Jiang, Y., and Wang, Z. (2025). Calcium-based composite materials for thermochemical heat storage: structure, performance and mechanisms. J. Alloys Compd. 1010, 177960. doi:10.1016/j.jallcom.2024.177960
Huang, Y., Stonehouse, A., and Abeykoon, C. (2023). Encapsulation methods for phase change materials – a critical review. Int. J. Heat Mass Transf. 200, 123458. doi:10.1016/j.ijheatmasstransfer.2022.123458
Humbert, G., and Sciacovelli, A. (2024). Design of effective heat transfer structures for performance maximization of a closed thermochemical energy storage reactor through topology optimization. Appl. Therm. Eng. 239, 122146. doi:10.1016/j.applthermaleng.2023.122146
Humphries, T. D., Sheppard, D. A., Li, G., Rowles, M. R., Paskevicius, M., Matsuo, M., et al. (2018). Complex hydrides as thermal energy storage materials: characterisation and thermal decomposition of Na 2 Mg 2 NiH 6. J. Mater. Chem. A 6 (19), 9099–9108. doi:10.1039/C8TA00822A
Hussain, E. S., and Hussain, I. Y. (2025). Numerical study of performance enhancement of phase change material (PCM) thermal energy storage (TES) system by using nanoparticles. Al-Nahrain J. Eng. Sci. 28 (1), 1–7. doi:10.29194/NJES.28010001
Hwang, F. S., Confrey, T., Reidy, C., Picovici, D., Callaghan, D., Culliton, D., et al. (2024). Review of battery thermal management systems in electric vehicles. Renew. Sustain. Energy Rev. 192, 114171. doi:10.1016/j.rser.2023.114171
IEA (2025). Global energy review 2025. Paris: IEA. Available online at: https://www.iea.org/reports/global-energy-review-2025.
Jiang, H., Li, X., and Liang, C. (2025). Heat-mass transfer, reaction and sintering characteristics in energy carrier for calcium-looping thermochemical energy storage. Energy 323, 135801. doi:10.1016/j.energy.2025.135801
Kant, K., Shukla, A., Smeulders, D. M. J., and Rindt, C. C. M. (2021). Performance analysis of a K2CO3-based thermochemical energy storage system using a honeycomb structured heat exchanger. J. Energy Storage 38, 102563. doi:10.1016/j.est.2021.102563
Kataray, T., Nitesh, B., Yarram, B., Sinha, S., Cuce, E., Shaik, S., et al. (2023). Integration of smart grid with renewable energy sources: opportunities and challenges – a comprehensive review. Sustain. Energy Technol. Assessments 58, 103363. doi:10.1016/j.seta.2023.103363
Khan, M. K., Raza, M., Shahbaz, M., Farooq, U., and Akram, M. U. (2024). Recent advancement in energy storage technologies and their applications. J. Energy Storage 92, 112112. doi:10.1016/j.est.2024.112112
Klopčič, N., Grimmer, I., Winkler, F., Sartory, M., and Trattner, A. (2023). A review on metal hydride materials for hydrogen storage. J. Energy Storage 72, 108456. doi:10.1016/j.est.2023.108456
Korba, D., Huang, W., Randhir, K., Petrasch, J., Klausner, J., AuYeung, N., et al. (2022). A continuum model for heat and mass transfer in moving-bed reactors for thermochemical energy storage. Appl. Energy 313, 118842. doi:10.1016/j.apenergy.2022.118842
Kousksou, T., Bruel, P., Jamil, A., El Rhafiki, T., and Zeraouli, Y. (2014). Energy storage: applications and challenges. Sol. Energy Mater. Sol. Cells 120, 59–80. doi:10.1016/j.solmat.2013.08.015
Kumar, S., and Banerjee, D. (2024). “A review on phase change materials for sustainability applications by leveraging machine learning,” in Energy consumption, conversion, storage, and efficiency (IntechOpen). doi:10.5772/intechopen.114380
Kumar, A., and Kumar, R. (2024). Enhancement and estimation of thermo-physical properties of organic-phase change materials (O-PCMs) and their applications in solar thermal technologies: a review. J. Energy Storage 101, 113741. doi:10.1016/j.est.2024.113741
Kumar, K. R., Raghutu, R. K., Venkata Padma, D., Sastry, S. V. A. R., and Hemalatha, R. (2025). Data-driven approaches to sustainable phase change material selection in latent heat storage systems. Int. J. Energy Water Resour. doi:10.1007/s42108-025-00347-x
Kumi, E. N. (2023). “Energy storage technologies,” in Pumped hydro energy storage for hybrid systems (Elsevier), 1–21. doi:10.1016/B978-0-12-818853-8.00002-9
Kuravi, S., Trahan, J., Goswami, D. Y., Rahman, M. M., and Stefanakos, E. K. (2013). Thermal energy storage technologies and systems for concentrating solar power plants. Prog. Energy Combust. Sci. 39 (4), 285–319. doi:10.1016/j.pecs.2013.02.001
Kwasi-Effah, C. C., and Rabczuk, T. (2018). Dimensional analysis and modelling of energy density of lithium-ion battery. J. Energy Storage 18, 308–315. doi:10.1016/j.est.2018.05.002
Kwasi-Effah, C. C., Ighodaro, O., Egware, H. O., and Obanor, A. I. (2022a). A novel empirical model for predicting the heat accumulation of a thermal energy storage medium for solar thermal applications. J. Energy Storage 56, 105969. doi:10.1016/j.est.2022.105969
Kwasi-Effah, C. C., Ighodaro, O., Egware, H. O., and Obanor, A. I. (2022b). Characterization an d comparison of the thermophysical property of ternary and quaternary salt mixture s for solar thermal power plant applications. Results Eng. 16, 100721. doi:10.1016/j.rineng.2022.100721
Kwasi-Effah, C. C., Egware, H. O., Obanor, A. I., and Ighodaro, O. O. (2023). Development and characterization of a quaternary nitrate based molten salt heat transfer fluid for concentrated solar power plant. Heliyon. 9 (5). doi:10.1016/j.heliyon.2023.e16096
Kwasi-Effah, C. C., Unuareokpa, O., Egware, H. O., Ighodaro, O., Obanor, A. I., Onoche, U., et al. (2024). Enhancing thermal conductivity of novel ternary nitrate salt mixtures for thermal energy storage (TES) fluid. Prog. Eng. Sci. 1 (4), 100020. doi:10.1016/j.pes.2024.100020
Lamy-Mendes, A., Silva, R. F., and Durães, L. (2018). Advances in carbon nanostructure–silica aerogel composites: a review. J. Mater. Chem. A 6 (4), 1340–1369. doi:10.1039/C7TA08959G
Li, B., Li, J., Shao, H., and He, L. (2018). Mg-based hydrogen absorbing materials for thermal energy storage—a review. Appl. Sci. 8 (8), 1375. doi:10.3390/app8081375
Li, D., Zhuang, B., Chen, Y., Li, B., Landry, V., Kaboorani, A., et al. (2022a). Incorporation technology of bio-based phase change materials for building envelope: a review. Energy Build. 260, 111920. doi:10.1016/j.enbuild.2022.111920
Li, Y.-C., Zhang, L., and Feng, B. (2022b). Optimization of thermal performance of high temperature sensible heat thermal energy storage system for direct steam generation: a simulation work. Appl. Therm. Eng. 217, 119225. doi:10.1016/j.applthermaleng.2022.119225
Li, D., Wang, Z., Wu, Y., Liu, C., and Arıcı, M. (2024). Experimental investigation on thermal properties of Al 2 O 3 nanoparticles dispersed paraffin for thermal energy storage applications. Energy Sources, Part A Recovery, Util. Environ. Eff. 46 (1), 8190–8200. doi:10.1080/15567036.2021.1916133
Liang, H., Liu, L., Zhong, Z., Gan, Y., Wu, J.-Y., and Niu, J. (2022). Towards idealized thermal stratification in a novel phase change emulsion storage tank. Appl. Energy 310, 118526. doi:10.1016/j.apenergy.2022.118526
Lin, J., Zhao, Q., Huang, H., Mao, H., Liu, Y., and Xiao, Y. (2021). Applications of low-temperature thermochemical energy storage systems for salt hydrates based on material classification: a review. Sol. Energy 214, 149–178. doi:10.1016/j.solener.2020.11.055
Liu, Y. (2023). Metal foam-salt hydrate composites for thermochemical energy storage. Solid mechanics. Institut Polytechnique de Paris.
Liu, L., Zhang, Q., Zhai, Z., Yue, C., and Ma, X. (2020). State-of-the-art on thermal energy storage technologies in data center. Energy Build. 226, 110345. doi:10.1016/j.enbuild.2020.110345
Liu, K., Wu, C., Gan, H., Liu, C., and Zhao, J. (2024a). Latent heat thermal energy storage: theory and practice in performance enhancement based on heat pipes. J. Energy Storage 97, 112844. doi:10.1016/j.est.2024.112844
Liu, Y., Li, X., Xu, Y., Xie, Y., Hu, T., and Tao, P. (2024b). Carbon-enhanced hydrated salt phase change materials for thermal management applications. Nanomaterials 14 (13), 1077. doi:10.3390/nano14131077
Liu, C., Li, M., Hu, D., Zheng, Y., Cao, L., and He, Z. (2025). Liquid metal-enabled synergetic cooling and charging of superhigh current. Engineering 47, 117–129. doi:10.1016/j.eng.2024.11.035
Lu, Y., Xuan, Y., Teng, L., Liu, J., and Wang, B. (2024). A cascaded thermochemical energy storage system enabling performance enhancement of concentrated solar power plants. Energy 288, 129749. doi:10.1016/j.energy.2023.129749
Ma, X., Fan, C., Shao, W., Cao, Q., and Cui, Z. (2023a). Numerical and experimental studies of packed bed thermal energy storage system based on a novel transient energy model. Energy Sci. Eng. 11 (2), 727–744. doi:10.1002/ese3.1358
Ma, X., Yang, C., Feng, X., Shang, H., Zhao, Y., and Zhang, B. (2023b). Halloysite-based aerogels for efficient encapsulation of phase change materials with excellent solar energy storage and retrieval performance. Appl. Energy 341, 121101. doi:10.1016/j.apenergy.2023.121101
Magliano, A., Perez Carrera, C., Pappalardo, C. M., Guida, D., and Berardi, V. P. (2024). A comprehensive literature review on hydrogen tanks: storage, safety, and structural integrity. Appl. Sci. 14 (20), 9348. doi:10.3390/app14209348
Manisha, M., Tiwari, S., Sahdev, R. K., Chhabra, D., Kumari, M., Ali, A., et al. (2025). Advancements and challenges in battery thermal management for electric vehicles. Renew. Sustain. Energy Rev. 209, 115089. doi:10.1016/j.rser.2024.115089
Mebarek-Oudina, F., and Chabani, I. (2023). Review on nano enhanced PCMs: insight on nePCM application in thermal management/storage systems. Energies 16 (3), 1066. doi:10.3390/en16031066
Mehraj, N., Mateu, C., and Cabeza, L. F. (2025). Artificial intelligence and thermal energy storage: a review of design techniques and applications. J. Energy Storage 124, 116870. doi:10.1016/j.est.2025.116870
Mitali, J., Dhinakaran, S., and Mohamad, A. A. (2022). Energy storage systems: a review. Energy Storage Sav. 1 (3), 166–216. doi:10.1016/j.enss.2022.07.002
Mohammad, A. (2024). “Techno-economic assessment of a flexible nuclear power plant coupled with a thermal energy storage unit,”. Doctoral dissertation (Khalifa University of Science).
Mohammadi, A., Ikeda, Y., Edalati, P., Mito, M., Grabowski, B., Li, H. W., et al. (2022). High-entropy hydrides for fast and reversible hydrogen storage at room temperature: binding-energy engineering via first-principles calculations and experiments. Acta Mater. 236, 118117. doi:10.1016/j.actamat.2022.118117
Muthu, P., Rajagopal, S., Saju, D., Kesavan, V., Dellus, A., Sadhasivam, L., et al. (2024). Review of transition metal chalcogenides and halides as electrode materials for thermal batteries and secondary energy storage systems. ACS Omega 9, 7357–7374. doi:10.1021/acsomega.3c08809
Nadeem, F., Hussain, S. M. S., Tiwari, P. K., Goswami, A. K., and Ustun, T. S. (2019). Comparative review of energy storage systems, their roles, and impacts on future power systems. IEEE Access 7, 4555–4585. doi:10.1109/ACCESS.2018.2888497
Nair, A. M., Wilson, C., Huang, M. J., Griffiths, P., and Hewitt, N. (2022). Phase change materials in building integrated space heating and domestic hot water applications: a review. J. Energy Storage 54, 105227. doi:10.1016/j.est.2022.105227
Nandi, A., and Biswas, N. (2025). Enhanced melting dynamics of phase change material (PCM) based energy storage system combining modified fin and nanoparticles under solar irradiation. Int. J. Numer. Methods Heat Fluid Flow 35, 2104–2143. doi:10.1108/HFF-08-2024-0643
Ning, Z., He, Y., Zhu, P., Chen, D., Yang, F., Zhou, J., et al. (2024). Enhancing thermochemical energy storage performance of perovskite with sodium ion incorporation. Inorganics 12 (10), 266. doi:10.3390/inorganics12100266
Obara, S., and Tanaka, R. (2021). Waste heat recovery system for nuclear power plants using the gas hydrate heat cycle. Appl. Energy 292, 116667. doi:10.1016/j.apenergy.2021.116667
Ochmann, J., Rusin, K., and Bartela, Ł. (2023). Comprehensive analytical model of energy and exergy performance of the thermal energy storage. Energy 283, 128783. doi:10.1016/j.energy.2023.128783
Olympios, A. V., McTigue, J. D., Farres-Antunez, P., Tafone, A., Romagnoli, A., Li, Y., et al. (2021). Progress and prospects of thermo-mechanical energy storage—a critical review. Prog. Energy 3 (2), 022001. doi:10.1088/2516-1083/abdbba
Olympios, A. V., McTigue, J. D., Sapin, P., and Markides, C. N. (2022). “Pumped-thermal electricity storage based on Brayton cycles,” in Encyclopedia of energy storage (Elsevier), 6–18. doi:10.1016/B978-0-12-819723-3.00086-X
Ortega, I., Uriz, I., Ortuondo, A., Hernandez, A.-B., Faik, A., Loroño, I., et al. (2018). Operation strategies guideline for packed bed thermal energy storage systems. Int. J. Energy Res. 43, 6211–6221. doi:10.1002/er.4291
Osibodu, S. J., Adeyinka, A. M., and Mbelu, O. V. (2024). Phase change material integration in concrete for thermal energy storage: techniques and applications in sustainable building. Sustain. Energy Res. 11 (1), 45. doi:10.1186/s40807-024-00138-8
Palacios, A., Barreneche, C., Navarro, M. E., and Ding, Y. (2020). Thermal energy storage technologies for concentrated solar power – a review from a materials perspective. Renew. Energy 156, 1244–1265. doi:10.1016/j.renene.2019.10.127
Pardo-Pina, S., Ferrández-Pastor, J., Rodríguez, F., and Cámara-Zapata, J. M. (2024). Analysis of an evaporative cooling pad connected to an air distribution system of perforated polyethylene tubes in a greenhouse. Agronomy 14 (6), 1187. doi:10.3390/agronomy14061187
Panneer Selvam, R., Hale, M., and Strasser, M. (2013). Development and performance evaluation of high temperature concrete for thermal energy storage for solar power generation. doi:10.2172/1072014
Parida, D. R., and Basu, S. (2022). Experimental investigation on the performance of thermosyphon charging of a single-medium stratified storage system for concentrated solar power applications. arXiv preprint arXiv:2211.16953.
Pavlov, G. K., and Olesen, B. W. (2011). “Building thermal energy storage - concepts and applications,” in In proceedings.
Pelay, U., Luo, L., Fan, Y., Stitou, D., and Rood, M. (2017). Thermal energy storage systems for concentrated solar power plants. Renew. Sustain. Energy Rev. 79, 82–100. doi:10.1016/j.rser.2017.03.139
Pelay, U., Luo, L., Fan, Y., Stitou, D., and Castelain, C. (2019). Integration of a thermochemical energy storage system in a Rankine cycle driven by concentrating solar power: energy and exergy analyses. Energy 167, 498–510. doi:10.1016/j.energy.2018.10.163
Pengonda, H. B., Rotte, N. K., Puttapati, S. K., and Yerramala, S. (2024). “Developing energy storage applications for next generation,” in New technologies for energy transition based on sustainable development goals (Singapore: Springer Nature), 297–317. doi:10.1007/978-981-97-2527-4_15
Pielichowska, K., Nowicka-Dunal, K., and Pielichowski, K. (2024). Bio-based polymers for environmentally friendly phase change materials. Polymers 16 (3), 328. doi:10.3390/polym16030328
Podara, C. V., Kartsonakis, I. A., and Charitidis, C. A. (2021). Towards phase change materials for thermal energy storage: classification, improvements and applications in the building sector. Appl. Sci. 11 (4), 1490. doi:10.3390/app11041490
Pompei, L., Nardecchia, F., and Miliozzi, A. (2023). Current, projected performance and costs of thermal energy storage. Processes 11 (3), 729. doi:10.3390/pr11030729
Preisner, N. C., and Linder, M. (2020). A moving bed reactor for thermochemical energy storage based on metal oxides. Energies 13 (5), 1232. doi:10.3390/en13051232
Purohit, B. K., and Sistla, V. S. (2021). Inorganic salt hydrate for thermal energy storage application: a review. Energy Storage 3 (2), e212. doi:10.1002/est2.212
Qudrat-Ullah, H. (2024). Myth: renewable energy is too intermittent to Be reliable? 17–47. doi:10.1007/978-3-031-59733-6_2
Raganati, F., and Ammendola, P. (2023). Review of carbonate-based systems for thermochemical energy storage for concentrating solar power applications: state-of-the-art and outlook. Energy Fuels 37 (3), 1777–1808. doi:10.1021/acs.energyfuels.2c03853
Rai, A. C. (2021). Energy performance of phase change materials integrated into brick masonry walls for cooling load management in residential buildings. Build. Environ. 199, 107930. doi:10.1016/j.buildenv.2021.107930
Rathod, M. K., and Banerjee, J. (2013). Thermal stability of phase change materials used in latent heat energy storage systems: a review. Renew. Sustain. Energy Rev. 18, 246–258. doi:10.1016/j.rser.2012.10.022
Rathore, P. K. S., and Shukla, S. K. (2021). Enhanced thermophysical properties of organic PCM through shape stabilization for thermal energy storage in buildings: a state of the art review. Energy Build. 236, 110799. doi:10.1016/j.enbuild.2021.110799
Roper, R., Harkema, M., Sabharwall, P., Riddle, C., Chisholm, B., Day, B., et al. (2022). Molten salt for advanced energy applications: a review. Ann. Nucl. Energy 169, 108924. doi:10.1016/j.anucene.2021.108924
Sadeghi, G. (2022). Energy storage on demand: thermal energy storage development, materials, design, and integration challenges. Energy Storage Mater. 46, 192–222. doi:10.1016/j.ensm.2022.01.017
Said, Z., Pandey, A. K., Tiwari, A. K., Kalidasan, B., Jamil, F., Thakur, A. K., et al. (2024). Nano-enhanced phase change materials: fundamentals and applications. Prog. Energy Combust. Sci. 104, 101162. doi:10.1016/j.pecs.2024.101162
Salaudeen, A., Bahiri, L., Das Rana, D., and Tomomewo, O. (2025). Waste heat recovery in nuclear power plants: a case study of Bushehr nu-clear power plant for improved efficiency. doi:10.2139/ssrn.5158805
Santos, E. (2018). Nanostructure materials as catalysts for the degradation of polyolefins. doi:10.13140/RG.2.2.12960.94726
Saouter, E., and Gibon, T. (2024). All you need to know about the next energy revolution. Switzerland: Springer Nature. doi:10.1007/978-3-031-51332-9
Saqib, M., and Andrzejczyk, R. (2023). A review of phase change materials and heat enhancement methodologies. WIREs Energy Environ. 12 (3), e467. doi:10.1002/wene.467
Sarbu, I., and Sebarchievici, C. (2018). A comprehensive review of thermal energy storage. Sustainability 10 (1), 191. doi:10.3390/su10010191
Sarbu, I., Mirza, M., and Muntean, D. (2022). Integration of renewable energy sources into low-temperature district heating systems: a review. Energies 15 (18), 6523. doi:10.3390/en15186523
Seddegh, S., Wang, X., Henderson, A. D., and Xing, Z. (2015). Solar domestic hot water systems using latent heat energy storage medium: a review. Renew. Sustain. Energy Rev. 49, 517–533. doi:10.1016/j.rser.2015.04.147
Selvnes, H., Allouche, Y., Manescu, R. I., and Hafner, A. (2021). Review on cold thermal energy storage applied to refrigeration systems using phase change materials. Therm. Sci. Eng. Prog. 22, 100807. doi:10.1016/j.tsep.2020.100807
Seyitini, L., Belgasim, B., and Enweremadu, C. C. (2023). Solid state sensible heat storage technology for industrial applications – a review. J. Energy Storage 62, 106919. doi:10.1016/j.est.2023.106919
Sharma, A., Tyagi, V. V., Chen, C. R., and Buddhi, D. (2009). Review on thermal energy storage with phase change materials and applications. Renew. Sustain. Energy Rev. 13 (2), 318–345. doi:10.1016/j.rser.2007.10.005
Shu, G., Liu, P., Tian, H., Wang, X., and Jing, D. (2017). Operational profile based thermal-economic analysis on an Organic Rankine cycle using for harvesting marine engine’s exhaust waste heat. Energy Convers. manage. 146, 107–123. doi:10.1016/j.enconman.2017.04.099
Simonsen, G., Ravotti, R., O’Neill, P., and Stamatiou, A. (2023). Biobased phase change materials in energy storage and thermal management technologies. Renew. Sustain. Energy Rev. 184, 113546. doi:10.1016/j.rser.2023.113546
Singh, S. K., Verma, S. K., and Kumar, R. (2022). Thermal performance and behavior analysis of SiO2, Al2O3 and MgO based nano-enhanced phase-changing materials, latent heat thermal energy storage system. J. Energy Storage 48, 103977. doi:10.1016/j.est.2022.103977
Solé, A., Miró, L., Barreneche, C., Martorell, I., and Cabeza, L. F. (2015). Corrosion of metals and salt hydrates used for thermochemical energy storage. Renew. Energy 75, 519–523. doi:10.1016/j.renene.2014.09.059
Srivastava, M., Sarkar, J., Sarkar, A., Maheshwari, N. K., and Antony, A. (2024). Thermo-economic feasibility study to utilize ORC technology for waste heat recovery from Indian nuclear power plants. Energy 298, 131338. doi:10.1016/j.energy.2024.131338
Strasser, M. N., and Selvam, R. P. (2014). A cost and performance comparison of packed bed and structured thermocline thermal energy storage systems. Sol. Energy 108, 390–402. doi:10.1016/j.solener.2014.07.023
Sumayli, H., El-Leathy, A., Danish, S. N., Al-Ansary, H., Almutairi, Z., Al-Suhaibani, Z., et al. (2023). Integrated CSP-PV hybrid solar power plant for two cities in Saudi Arabia. Case Stud. Therm. Eng. 44, 102835. doi:10.1016/j.csite.2023.102835
Sun, X., Liu, L., Mo, Y., Li, J., and Li, C. (2020). Enhanced thermal energy storage of a paraffin-based phase change material (PCM) using nano carbons. Appl. Therm. Eng. 181, 115992. doi:10.1016/j.applthermaleng.2020.115992
Sun, M., Liu, T., Sha, H., Li, M., Liu, T., Wang, X., et al. (2023). A review on thermal energy storage with eutectic phase change materials: fundamentals and applications. J. Energy Storage 68, 107713. doi:10.1016/j.est.2023.107713
Tamme, R., Laing, D., Steinmann, W.-D., and Bauer, T. (2022). Thermal energy storage, 285–313. doi:10.1007/978-1-0716-1422-8_684
Tan, K. M., Babu, T. S., Ramachandaramurthy, V. K., Kasinathan, P., Solanki, S. G., and Raveendran, S. K. (2021). Empowering smart grid: a comprehensive review of energy storage technology and application with renewable energy integration. J. Energy Storage 39, 102591. doi:10.1016/j.est.2021.102591
Tariq, S. L., Ali, H. M., Akram, M. A., Janjua, M. M., and Ahmadlouydarab, M. (2020). Nanoparticles enhanced phase change materials (NePCMs)-A recent review. Appl. Therm. Eng. 176, 115305. doi:10.1016/j.applthermaleng.2020.115305
Tawalbeh, M., Khan, H. A., Al-Othman, A., Almomani, F., and Ajith, S. (2023). A comprehensive review on the recent advances in materials for thermal energy storage applications. Int. J. Thermofluids 18, 100326. doi:10.1016/j.ijft.2023.100326
Teichmann, D., Stark, K., Müller, K., Zöttl, G., Wasserscheid, P., and Arlt, W. (2012). Energy storage in residential and commercial buildings via liquid organic hydrogen carriers (LOHC). Energy Environ. Sci. 5 (10), 9044. doi:10.1039/c2ee22070a
Teja, P. N. S., Gugulothu, S. K., Sastry, G. R., Burra, B., and Bhurat, S. S. (2022). Numerical analysis of nanomaterial-based sustainable latent heat thermal energy storage system by improving thermal characteristics of phase change material. Environ. Sci. Pollut. Res. 29 (34), 50937–50950. doi:10.1007/s11356-021-15485-y
Thakare, K. A., Vishwakarma, H. G., and Bhave, A. G. (2015). Experimental investigation of possible use of HDPE as thermal storage material in thermal storage type solar cookers. Int. J. Res. Eng. Technol. 4 (12), 92–99. Available online at: http://www.ijret.org.
Tofani, K., and Tiari, S. (2021). Nano-enhanced phase change materials in latent heat thermal energy storage systems: a review. Energies 14 (13), 3821. doi:10.3390/en14133821
Upadhyay, V. V., and Singhal, S. (2023). A review of current developments in the use of materials with latent heat phase changes for the storage of thermal energy. Mater. Today Proc. doi:10.1016/j.matpr.2023.02.187
Usman, M. R. (2022). Hydrogen storage methods: review and current status. Renew. Sustain. Energy Rev. 167, 112743. doi:10.1016/j.rser.2022.112743
Vahidhosseini, S. M., Rashidi, S., Hsu, S.-H., Yan, W.-M., and Rashidi, A. (2024). Integration of solar thermal collectors and heat pumps with thermal energy storage systems for building energy demand reduction: a comprehensive review. J. Energy Storage 95, 112568. doi:10.1016/j.est.2024.112568
Verma, P., Varun, V., and Singal, S. (2008). Review of mathematical modeling on latent heat thermal energy storage systems using phase-change material. Renew. Sustain. Energy Rev. 12 (4), 999–1031. doi:10.1016/j.rser.2006.11.002
Verma, V., Thangavel, S., Dutt, N., Kumar, A., and Weerasinghe, R. (2024). Highly efficient thermal renewable energy systems. CRC Press. doi:10.1201/9781003472629
Walker, T., and Duquette, J. (2022). Techno economic viability of hydroelectric energy storage systems for high-rise buildings. J. Energy Storage 53, 105044. doi:10.1016/j.est.2022.105044
Wang, K., Yan, T., Li, R. K., and Pan, W. G. (2022). A review for Ca(OH)2/CaO thermochemical energy storage systems. J. Energy Storage 50, 104612. doi:10.1016/j.est.2022.104612
Williamson, K., Møller, K. T., D’Angelo, A. M., Humphries, T. D., Paskevicius, M., and Buckley, C. E. (2023). Thermochemical energy storage in barium carbonate enhanced by iron(III) oxide. Phys. Chem. Chem. Phys. 25 (10), 7268–7277. doi:10.1039/D2CP05745J
Williamson, K., D’Angelo, A. M., Humphries, T. D., Paskevicius, M., and Buckley, C. E. (2024). Barium carbonate and barium titanate for ultra-high temperature thermochemical energy storage. J. Energy Storage 86, 111196. doi:10.1016/j.est.2024.111196
Winick, J. A., Zhu, G., Kitz, K., and Adams, A. (2025). Reducing data center peak cooling demand and energy costs with underground thermal energy storage (UTES) (No. NREL/CP-5700-93607). Golden, CO, United States: National Renewable Energy Laboratory.
Wu, S., Yan, T., Kuai, Z., and Pan, W. (2020). Thermal conductivity enhancement on phase change materials for thermal energy storage: a review. Energy Storage Mater. 25, 251–295. doi:10.1016/j.ensm.2019.10.010
Wu, Q., He, T., Zhang, Y., Zhang, J., Wang, Z., Liu, Y., et al. (2021). Cyclic stability of supercapacitors: materials, energy storage mechanism, test methods, and device. J. Mater. Chem. A 9 (43), 24094–24147. doi:10.1039/D1TA06815F
Wuerth, M., Spliethoff, H., Becker, M., Gleis, S., Vandersickel, A., and Talebi, E. (2019a). “Thermochemical energy storage employing fluidized bed technology: experimental,” in Fluidization XVI: Conference Type Fluidization.
Wuerth, M., Becker, M., Ostermeier, P., Gleis, S., and Spliethoff, H. (2019b). Development of a continuous fluidized bed reactor for thermochemical energy storage application. J. Energy Resour. Technol. 141 (7), 070710. doi:10.1115/1.4043629
Xie, P., Jin, L., Qiao, G., Lin, C., Barreneche, C., and Ding, Y. (2022). Thermal energy storage for electric vehicles at low temperatures: concepts, systems, devices and materials. Renew. Sustain. Energy Rev. 160, 112263. doi:10.1016/j.rser.2022.112263
Yaïci, W., Ghorab, M., Entchev, E., and Hayden, S. (2013). Three-dimensional unsteady CFD simulations of a thermal storage tank performance for optimum design. Appl. Therm. Eng. 60 (1–2), 152–163. doi:10.1016/j.applthermaleng.2013.07.001
Yang, T., Liu, W., Kramer, G., and Sun, Q. (2020). State of the art review of seasonal sensible heat storage. doi:10.46855/energy-proceedings-4255
Yang, H., Zou, Y., and Cui, H. (2024a). Advancements and challenges in enhancing salt hydrate phase change materials for building energy storage: optimization methodologies and mechanisms. Natl. Sci. Open 3 (3), 20230056. doi:10.1360/nso/20230056
Yang, W., Du, Y., Deng, Q., Li, S., Li, C., Tian, J., et al. (2024b). High thermal conductivity composite phase change material with Zn2+ metal organic gel and expanded graphite for battery thermal management. Appl. Therm. Eng. 249, 123358. doi:10.1016/j.applthermaleng.2024.123358
Yuan, Y., Li, Y., and Zhao, J. (2018). Development on thermochemical energy storage based on CaO-based materials: a review. Sustainability 10 (8), 2660. doi:10.3390/su10082660
Zahid, M. S., Ahmed, N., Qaisrani, M. A., Mahmood, M., Ali, M., Waqas, A., et al. (2022). Charging and discharging characterization of a novel combined sensible-latent heat thermal energy storage system by experimental investigations for medium temperature applications. J. Energy Storage 55, 105612. doi:10.1016/j.est.2022.105612
Zalba, B., Marin, J. M., Cabeza, L. F., and Mehling, H. (2003). Review on thermal energy storage with phase change: materials, heat transfer analysis and applications. Appl. Therm. Eng. 23 (3), 251–283. doi:10.1016/S1359-4311(02)00192-8
Zapater, M., and Vicente, J. (2023). Analysis of a hybrid PV-CSP plant integration in the electricity market.
Zeneli, M., Malgarinos, I., Nikolopoulos, A., Nikolopoulos, N., Grammelis, P., Karellas, S., et al. (2019). Numerical simulation of a silicon-based latent heat thermal energy storage system operating at ultra-high temperatures. Appl. Energy. 242, 837–853. doi:10.1016/j.apenergy.2019.03.147
Zhang, M., Wang, C., Luo, A., Liu, Z., and Zhang, X. (2020). Molecular dynamics simulation on thermophysics of paraffin/EVA/graphene nanocomposites as phase change materials. Appl. Therm. Eng. 166, 114639. doi:10.1016/j.applthermaleng.2019.114639
Zhang, H., Xu, C., and Fang, G. (2022a). Encapsulation of inorganic phase change thermal storage materials and its effect on thermophysical properties: a review. Sol. Energy Mater. Sol. Cells 241, 111747. doi:10.1016/j.solmat.2022.111747
Zhang, J., Shao, D., Jiang, L., Zhang, G., Wu, H., Day, R., et al. (2022b). Advanced thermal management system driven by phase change materials for power lithium-ion batteries: a review. Renew. Sustain. Energy Rev. 159, 112207. doi:10.1016/j.rser.2022.112207
Zhang, S., Ocłoń, P., Klemeš, J. J., Michorczyk, P., Pielichowska, K., and Pielichowski, K. (2022c). Renewable energy systems for building heating, cooling and electricity production with thermal energy storage. Renew. Sustain. Energy Rev. 165, 112560. doi:10.1016/j.rser.2022.112560
Zhang, S., Li, Y., and Yan, Y. (2024a). Hybrid sensible-latent heat thermal energy storage using natural stones to enhance heat transfer: energy, exergy, and economic analysis. Energy 286, 129530. doi:10.1016/j.energy.2023.129530
Zhang, Y., Hu, M., Chen, Z., Su, Y., and Riffat, S. (2024b). Performance study of a thermochemical energy storage reactor embedded with a microchannel tube heat exchanger for water heating. J. Energy Storage 78, 110043. doi:10.1016/j.est.2023.110043
Zhao, C., Yan, J., Tian, X., Xue, X., and Zhao, Y. (2023). Progress in thermal energy storage technologies for achieving carbon neutrality. Carbon Neutrality 2 (1), 10. doi:10.1007/s43979-023-00050-y
Keywords: thermal energy storage (TES), phase change materials (PCM), latent heat storage, sensible heat storage, thermochemical energy storage, nano-enhanced phase change materials, hybrid TES systems, renewable energy systems integration
Citation: Kwasi-Effaha CC and Okpako O (2025) Comprehensive review of emerging trends in thermal energy storage mechanisms, materials and applications. Front. Energy Res. 13:1651471. doi: 10.3389/fenrg.2025.1651471
Received: 21 June 2025; Accepted: 28 July 2025;
Published: 28 August 2025.
Edited by:
Yongping Huang, Southeast University, ChinaReviewed by:
Abdulhammed Hamzat, Wichita State University, United StatesMd. Abdullah, City University, Bangladesh
Copyright © 2025 Kwasi-Effaha and Okpako. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Collins Chike Kwasi-Effaha, Y29sbGluc2hpY2VudEBnbWFpbC5jb20=
 Collins Chike Kwasi-Effaha
Collins Chike Kwasi-Effaha Ogheneochuko Okpako
Ogheneochuko Okpako