- 1University of Life Sciences in Lublin, Lublin, Poland
- 2Department of Machinery Exploitation and Management of Production Processes, University of Life Sciences in Lublin, Lublin, Poland
This literature review examines the problems associated with ash deposition and deposit formation in low-power heating devices and identifies possible solutions. The combustion of herbaceous biomass, especially that from waste from the agri-food industry, causes a large increase in the amount of mineral deposits in heating devices at a low melting point of the compounds they are composed of (particularly due to the presence of potassium (K), sodium (Na), and sulfur (S) compounds). They affect not only the formation of deposits and sediments but also the emission of fly ash, its handling, and the possibilities for its use and disposal. Problems related to ash depend on the content of alkaline oxides, which vary in proportion across different types of biomass. A dominant percentage of silicon dioxide (SiO2) is characteristic of ash from grass, rice husks, miscanthus, and straw pellets. Grass ash also contains a significant amount of potassium oxide (K2O), while ash from poplar wood, willow, and soy pellets is notable for its high calcium oxide (CaO) content. The deposition of ash volatiles and aerosols on boiler surfaces reduces heat exchange efficiency. The solution to the ash problems during combustion is the use of mineral additives, with kaolin being the most promising because of its very good ability to capture problematic alkali-containing compounds during combustion and convert them into potassium aluminium silicates with a high melting point. In addition, kaolin exhibits certain catalytic properties and can act as a carrier for active catalytic components that can be used in the processes of reducing nitrogen oxides (NOx) and particulate matter (PM). Natural carrier materials, such as kaolin clays, are of considerable practical importance as acidic catalysts. Methods for measuring the surface acidity of solids - including Brønsted and Lewis acid centres - which play a key role in catalysis are therefore of significant interest. The introduction of such additives increases the heating efficiency of the plant and at the same time reduces carbon monoxide (CO) and NOx emissions (which is inconsistently confirmed in the literature).
1 Introduction
Biomass as an energy source contributes to reducing dependence on imported fossil fuels (coal, gas), while at the same time bringing benefits to countries where biomass fuels are an emerging source of renewable energy. Understanding the combustion behaviour of fuels is essential for the efficient and safe design and operation of equipment. Similarly, understanding the mineral content is crucial, as it significantly impacts the dynamics of the power generation system. The energetic use of biomass has many benefits, including social, economic and energy security. The considerable diversity of plant species useful in the energy sector is associated with a large diversity in terms of physical properties (hardness, specific gravity, moisture, porosity) and chemical composition of the raw materials obtained from them. This has a significant impact on the calorific value of the biomass but also on the combustion process and the condition of the heating equipment in which it is carried out. Although biomass is environmentally friendly, its use can create many problems that can lead to a sudden interruption of boiler operation, for example, as a result of the complex process of ash deposition on the convection surfaces of heat exchange. This process occurs in a wide range of flue gas temperatures, surfaces and depends on the properties of the ash as well as the design and operating conditions of the boiler. Problems associated with the presence of ash in biomass combustion systems (such as grate boilers and drop-in or retort burners) are linked to the formation of agglomerates from partially molten ash at high temperatures, as well as the deposition of ash in low-temperature zones of the boiler’s heat exchange surfaces, particularly in convective sections. These phenomena contribute to accelerated metal corrosion, increased emissions, and complications in the utilization and disposal of ash waste from such biomass-fired systems. Some negative effects of increased ash deposition as a result of biomass combustion on the efficiency and operation of the combustion system can be presented as follows:
1. Decrease in boiler efficiency. Increased ash deposition, along with changes in the properties of ash deposits containing low-melting compounds [K, Na, S, and calcium (Ca)], leads to the formation of a coating on the particles of bottom ash. This coating is partially molten, which at a later stage results in the sticking and adhesion of particles, ultimately leading to ash agglomeration in the combustion chamber. In addition, the deposition of melted or partially melted ash deposits on the heat exchange surfaces slows down the heat exchange in the boiler, which is manifested by a decrease in the thermal efficiency of the combustion chamber and its performance.
2. Boiler damage. The process of deposit formation may occur to such an extent that the flow of flue gases may be restricted. This may lead to mechanical damage to boiler components, which is also associated with the formation of corrosion. The accumulation and subsequent shedding of large ash deposits in the upper parts of the boiler and the surfaces of the steam pipes may restrict the flow of gas, causing damage to the combustion system components.
3. Maintenance problems. Very large deposits would require premature shutdown for maintenance. To remove ash deposits, shutdowns (often unplanned) are required to clean the accumulated deposits.
4. Furthermore, the accumulation of ash deposits on the heat exchange surfaces also leads to an increase in the temperature of the boiler exhaust gases, which results in a decrease in the efficiency of the combustion device and an increase in CO and NOx emissions.
Thanks to the knowledge of the chemical composition and physical properties of ash, it is possible to predict the tendency to form deposits on boiler elements and their potential for corrosion, erosion or abrasion. The behavior of ash in the system depends to a large extent on the fuel used. To achieve a stable energy generation system, it is essential to thoroughly understand the requirements of the fuel used in order to adapt the combustion technology — especially when it comes to biomass derived from industrial waste or energy crops, which due to their chemical composition exhibit different behavior during combustion than other types of biomass (Nunes et al., 2016; Wei et al., 2022).
The aim of this study is therefore to analyze and synthesize information related to the quantity and chemical composition of ash from various types of herbaceous biomass, as well as to examine the issues associated with deposit formation and ash deposition in heating systems that burn such biomass, based on the current state of knowledge in the relevant literature. These issues are related not only to the sintering and slagging of bottom ash, but also to the chemical composition of solid biofuels, which is of great importance in the case of using various types of biomass. This study may help to systematize knowledge regarding the impact of ash on the combustion process and emissions in heating systems, while also indicating solutions aimed at minimizing problems related to bed agglomeration and fouling of heat exchangers in heating equipment.
2 Characteristics of the chemical composition of ash
Ash is a solid residue resulting from the complete oxidation of fuel. It is formed from inorganic (mineral) components during combustion. Inorganic material contained in biomass can be divided into two fractions: by-products that are added to fuels during its processing (sand or clay from harvesting, storage) and inherent material that acts as nutrients fulfilling basic biological functions for plants and is more finely distributed in the fuel (atomically dispersed). The elements that make up ash include: aluminum (Al), Ca, iron (Fe), K, magnesium (Mg), Na, phosphorus (P), silicon (Si), copper (Cu). In the literature on the subject, the components of ash have been divided into macroelements: Ca, K, Si, Mg, P, Na, S and microelements: Al, Fe, manganese (Mn), lithium (Li), barium (Ba), boron (Br). These elements in ash occur in the form of oxide compounds: SiO2, aluminum oxide (Al2O3), iron oxide (Fe2O3), manganese oxide (Mn3O4), titanium oxide (TiO2), CaO, magnesium oxide (MgO), sulfur trioxide (SO3), phosphorus pentoxide (P2O5), sodium oxide (Na2O), K2O. Basic compounds of ash include: CaO, MgO, K2O, Na2O and Fe2O3. Examples of acidic components of ash are: SiO2, Al2O3, hydrochloric acid (HCl), S, sulfur suboxide (S2O), P2O5 and TiO2. When the acidic and basic components of ash react, a salt or mineral is formed. The chemical composition of ash is most commonly expressed as the percentage content of these oxides. The content of ash-forming elements in biomass fuels varies greatly depending on the fuel and its type. The presence and percentages of the above-mentioned oxides and elements in the ash are important in terms of their behavior in the combustion process of the biofuel in which they occur in the ash, which results in an impact on the operation of heating devices and pollutant emissions (Lokare, 2008; Odzijewicz et al., 2023; Tortosa-Masiá, 2010). In ash, we distinguish eutectic compounds, which are a mixture of two or more salts that together have a lower melting point than the compounds themselves. The molten alkali salt (KCl, potassium sulfate (K2SO4) is deposited on the heat exchanger surface and can transfer the metal oxide to the salt. They are characterized by the eutectic number, which quantitatively expresses how the alkali metals and alkaline earth metals present are related to each other (Stauber-Alfredsson, 2018). In the characterization of ash, it is also important that the alkali metals (responsible for the emission of fine particles) or alkaline earth metals (responsible for the increase in the melting temperature of the ash) (AAEMs-Alkali and Alkaline Earth Metals) form zeolites. This is a group of hydrated aluminosilicates of alkali or alkaline earth metals (mainly Na, K, Mg and Ca) with pores of uniform diameter, regular internal cavities and channels of discrete sizes and shapes. The Ca and Mg group is the strongest in bottom ash, constituting more than 50% in all fractions and fuels. Alkali metal and alkaline earth metal species (AAEMs) are the main components of biomass ash and can positively influence biomass combustion by acting as a catalyst. On the other hand, high concentrations of ash and alkali metals (K, Na, Ca, magnesium (Mg), etc.) in biomass cause ash-related problems through condensation and reactions, such as slagging (mainly related to alkali chlorides and sulfates), agglomeration (mainly dependent on the chemical and melting properties of the coating, which are sensitive to the relative amount of calcium and potassium in the fuel), corrosion (mainly related to flue gases containing chlorine (Cl2), HCl, NaCl (aerosol), KCl (aerosol) and other species that accelerate the oxidation of metal alloys) and contamination. Alkali metals such as K and Na together with Cl are the most important elements in biomass ash that affect undesirable processes. During fuel combustion, reactive compounds evaporate and alkali metals can form aerosols (potassium hydroxide (KOH), KCl, K2SO4, NaCl, sodium sulfate (Na2SO4) in the gas phase. Alkali metal aerosols can react with each other or other ash components (SiO2, Fe2O3) to form eutectic mixtures. Alkali metals can also occur in the solid phase in the form of silicates (potassium silicate (K2Si2O5), sodium metasilicate (Na2SiO3) and aluminosilicates (potassium aluminum silicate (KAISi3O8), nepheline (KAISiO4), albite (NaSi3AlO8) (Yu et al., 2021; Zhang et al., 2018; Royo R. et al., 2022; Jiao et al., 2023; Oladejo et al., 2020; Mlonka-Mędrala et al., 2020).
Ashes resulting from biomass combustion are classified into bottom ash and fly ash. Bottom ash captures evaporated potassium, which combines with silica present in the bed material, causing its agglomeration. Agglomeration occurs when ash particles form layers around the bed material particles (mainly quartz sand, which consists of silica, potassium and calcium). It occurs as a result of chemical reactions, the presence of a liquid phase and sintering in the solid state. Fly ash from biomass combustion, on the other hand, is very diverse in terms of chemical composition and the type of material from which it was obtained. In addition, the formation of SO3 also depends on its chemical composition. During combustion, inorganic compounds of biomass undergo chemical and physical changes. Volatile compounds (K and Na are volatile elements but Cl, carbon dioxide (CO2) and S are considered to be particularly volatile) are released into the gas phase (released ash is formed) while non-volatile (Al, Ca, Fe, Mg and Si) ash particles (these are usually large ash particles) remain on the furnace grate forming bottom ash or forming a coarse fraction of fly ash (Laxminarayan, 2018; Kowalczyk-Juśko, 2017). Elements contained in ash can also cause problems during combustion in the boiler. Despite the fact that the ash content in biomass is much lower than in coal, their different origins and differences in chemical composition affect the operation of heating devices causing increased slag and ash deposition in the furnace or increased wear of metal boiler elements due to corrosion. Therefore, it is a very important issue to assess the content of individual mineral components in the ashes resulting from the combustion of biomass (Zając et al., 2018). The characteristics of chemical compounds present in the ash are presented in Table 1.
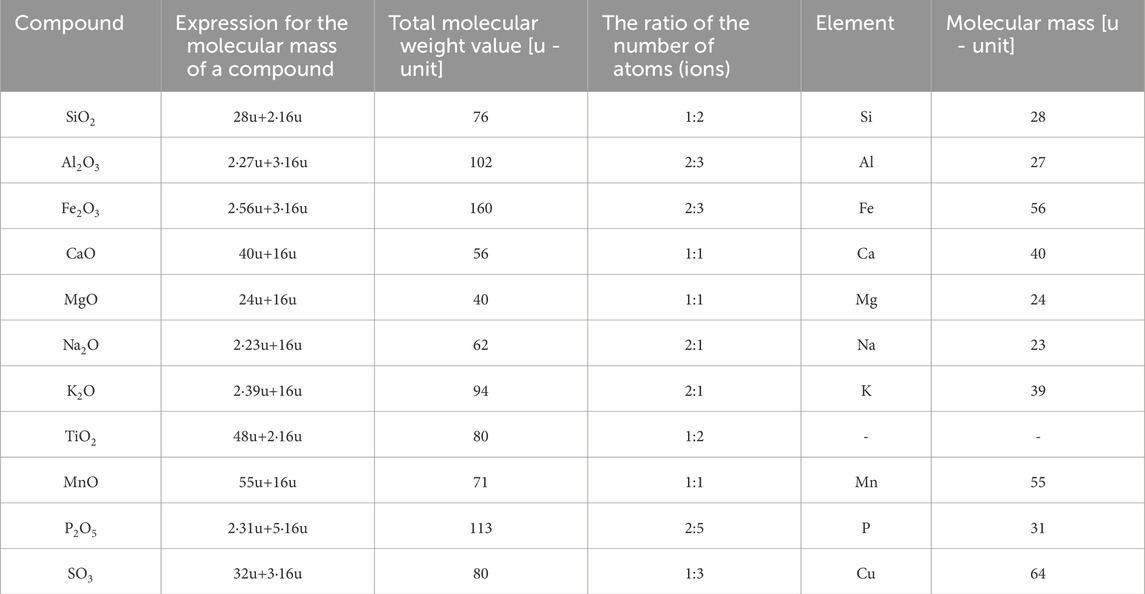
Table 1. Molecular weights of chemical compounds/elements present in biomass ash [Source: own study: Lide (2005)].
The sum of basic oxides is an important parameter for ash assessment. Based on the literature review, Tables 2, 3 present the % share of chemical compounds for different types of biomass.
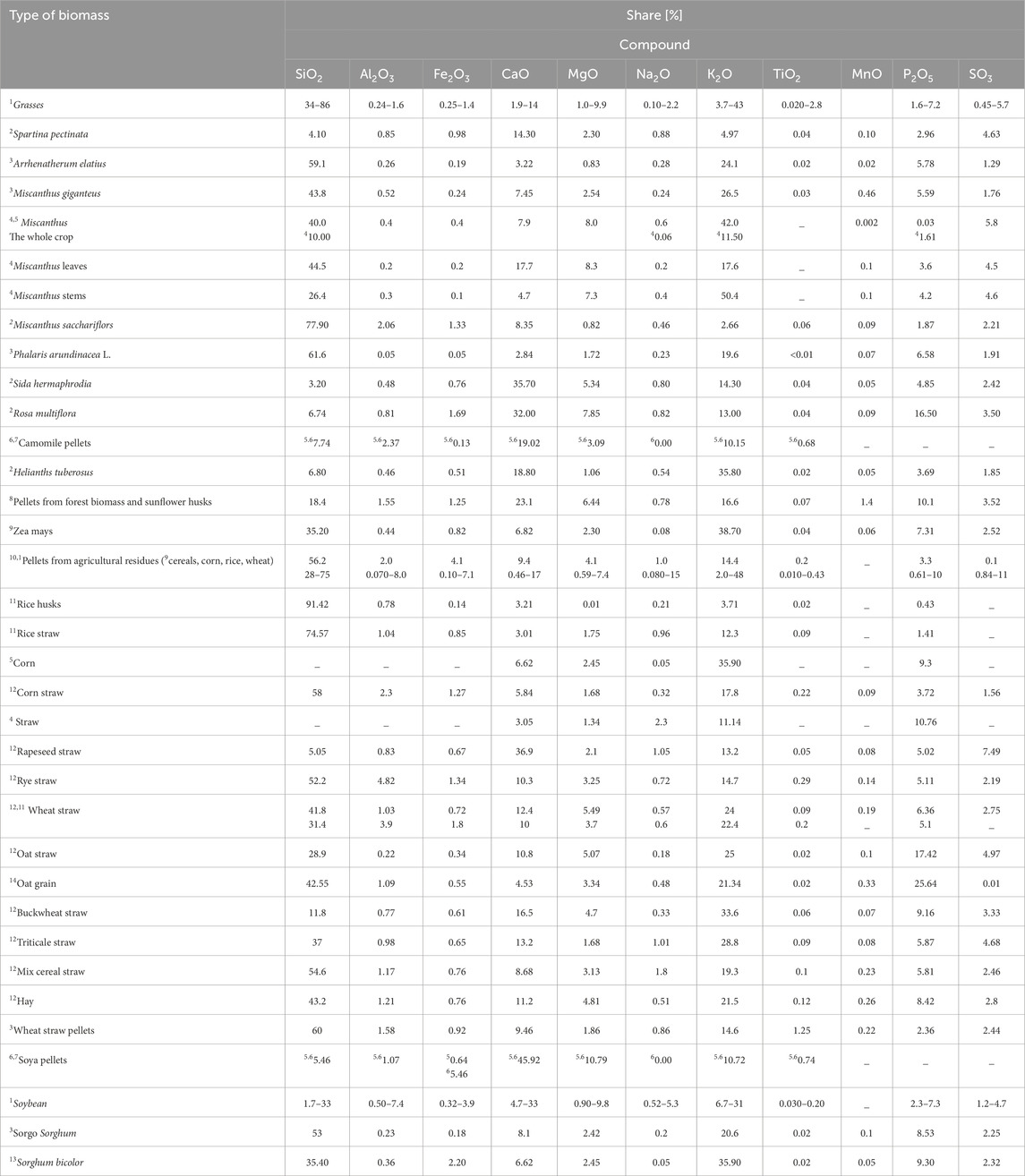
Table 2. % share of chemical compounds in herbaceous biomass [Source: own study: 1Zhai et al. (2021); 2Kowalczyk-Juśko (2009); 3Lalak et al. (2016); 4Baxter et al. (2014); 5Odzijewicz et al. (2023); 6Gvero et al. (2020); 7Botić et al. (2022); 8Kalisz et al. (2015); 9Kowalczyk-Juśko et al. (2015a); 10Sato et al. (2022); 11Dyjakon (2012); 12Kraszkiewicz et al. (2017); 13Kowalczyk-Juśko et al. (2015b); 14Kalisz (2023)].
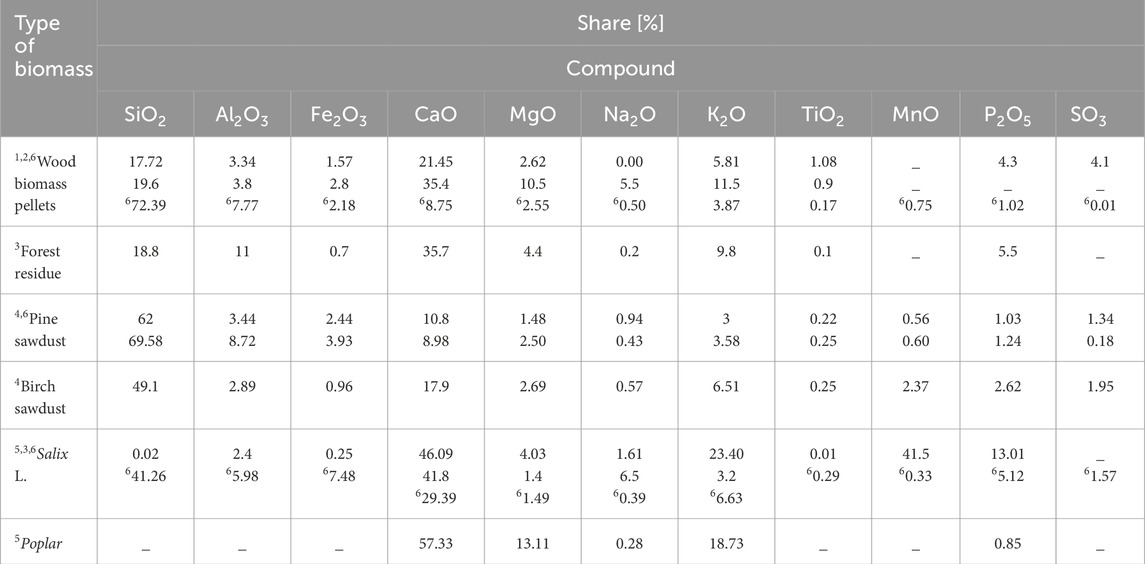
Table 3. % share of chemical compounds in wood biomass [Source: own study: 1Botić et al. (2022); 2Sato et al. (2022); 3Dyjakon (2012); 4Kraszkiewicz et al. (2017); 5Odzijewicz et al. (2023), 6Kalisz (2023)].
Based on the data presented in Tables 2, 3, it can be concluded that the chemical composition of biomass ash varies significantly. The composition of grass ash has the highest content of SiO2 (34%–86%) and K2O (50.4%) with moderate amounts of CaO (1.9%–17.7%) and P2O5 (0.03%–7.2%) and small amounts (<8%) of other elements. Rice husk ash has a similar content of SiO2 (91.42%) and rice straw (74.57%) to grass ash but a lower content of K2O (3.71%) and other chemical compounds. In addition, the lowest content of K2O is shown by miscanthus (2.66%), pine sawdust (3%) and woody biomass pellets (3.87%).
Willow, Virginia mallow, rapeseed straw, soybean pellets, multiflora rose, chamomile pellets contain much less SiO2 (respectively: 0.02%, 3.20%, 5.05%, 5.46%, 6.74%, 7.74%) than grasses, rice husks (91.42%), rice straw (74.57%), wheat straw pellets (60%) and agricultural residue pellets (28%–75%), but more CaO (45.92% - soybean pellets). However, the highest CaO content is found in poplar (57.33%) and willow (46.09%).
The intense internal reactions occurring in biomass ash at elevated temperatures generate a large amount of eutectic compounds, which significantly reduce the melting point of the ash resulting in an increased possibility of ash particles adhering from the gas flow. Additionally, ash formed from herbaceous biomass sinters at low temperatures (below 850 °C) and causes the formation of slags. Hence, wood-based products are the most commonly used biomass fuels, containing up to 40% less ash compared to other agricultural biomass raw materials, e.g., straw (Laxminarayan, 2018; Li et al., 2023; Generowicz et al., 2023; Rodriguez et al., 2020; Repić et al., 2019; Ion et al., 2023; Zapata et al., 2023; Dyjakon, 2012; Vaniyambadi, 2015; Yongtie et al., 2018; Zeng et al., 2018; Odzijewicz et al., 2023; Nzihou and Stanmore, 2013; Royo J. et al., 2022; Sarbassov et al., 2017; Schulze et al., 2020; Laxminarayan et al., 2017; Meka et al., 2022; Lalak et al., 2016; Xu et al., 2021; Thomas et al., 2018; Tesfaye et al., 2022; Niu et al., 2016; Zhai et al., 2021). The PN-EN ISO 21404:2020–08 standard (update of the CEN/TS 15370-1:2006 standard) is a document presenting a method for determining characteristic temperatures during the melting of complex inorganic components of solid biofuel ash at high temperatures. The characteristic temperatures defined in this standard are used to compare the tendency of ashes of different types and qualities of solid biofuels to form molten deposits or in assessing the susceptibility of the bed to agglomeration during heating (Stałe, 2025).
The composition of ash affects its softening and melting temperatures. The melting characteristics of the ash (Table 4) are an important factor in determining the propensity of fuels to form molten or partially melted slag deposits on the furnace wall surfaces of all combustion systems and can influence the nature of fouling deposits that occur on heat exchangers and other surfaces of biomass-fired boilers. The melting of most fuel ashes is a complex phenomenon that is best described by a melting curve, which shows the mass percentage of molten ash as a function of temperature. Two temperatures are considered in the ash melting curves and are used to describe the behavior of the ash:
- T15 temperature, at which 15% of the ash material melts. This is the temperature at which the surfaces of the particles or slag deposits begin to become sticky and susceptible to incoming particles,
- T70 temperature, at which 70% of the ash melts. This is the level from which the outer surface of the ash deposit on the vertical boiler tube will begin to flow.
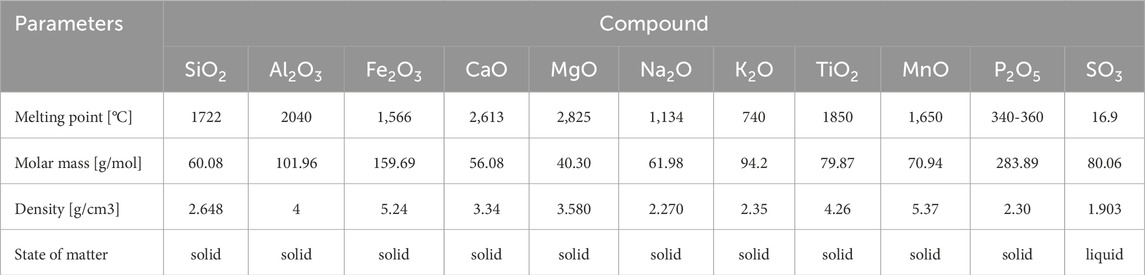
Table 4. Selected characteristics of ash components [Source: own study: Lide (2005)].
There are a number of complex thermodynamic models that can accurately predict the ash melting curve of a given fuel. For coal combustion, slagging and fouling indices have been developed to obtain and indicate the propensity of fuel ash to form deposits during combustion based on its chemical composition. When biomass is co-fired with coal, all the components of most biomass ash, mainly alkali metals and alkaline earth metals, are strong fluxing agents for aluminosilicate systems. The introduction of biomass can result in a lower melting point and therefore an increased slagging potential (Loo and Koppejan, 2008).
One of the very important parameters during each biomass combustion process is the temperature at which ash shrinkage (sintering) begins. This is the temperature at which ash shrinks, i.e., the temperature at which ash begins to show a tendency to slagging. The ash sintering temperature for different types of biomass varies as follows: wheat straw 840 °C, rapeseed straw 640 °C, sunflower husk pellets 810 °C, rye straw 730 °C, oat straw 770 °C, mustard straw 630 °C, corn straw 650 °C, hemp straw 640 °C, sorghum stalks 760 °C, tobacco stalks 570 °C (Zhu et al., 2014; Cichy, 2013; Chen et al., 2022).
The alkali content of biomass creates potential problems related to bed agglomeration as well as fouling and corrosion of heat exchangers. An example is herbaceous fuels, which contain silicon and potassium, the main components in fly ash formation. Compared to other biomass fuels, they are also rich in chlorine (Cl). These properties create problems with ash deposition and formation at high or moderate combustion temperatures. The main sources of these problems are:
1. Reaction of alkali with silica to form alkali silicates, which melt or soften at low temperatures (below 700 °C) depending on composition.
2. Reaction of alkali with sulfur to form alkali metal sulfates on the heat exchange surfaces of the combustion chamber.
The sintering and softening temperatures can differ significantly for the same type of herbaceous biomass, depending on the chemical composition of the ash, which is related to the plant species, cultivation method and soil type. Characteristic ash softening temperatures for some types of biomass are: rye straw 1,002 °C, wheat straw 998 °C, barley straw 980 °C. Alkaline material plays a major role in both processes. The mobility of the alkali material is defined as its ability to contact other materials and is measured using chemical extraction techniques. The presence of alkali metals and alkaline earth metals (AAEMs) in biomass has been found to have a significant effect on ash deposition, sintering, agglomeration, contamination, slagging and corrosion (Gogolev et al., 2021; Biedermann and Obernberger, 2005; Xu et al., 2020; Vassiliev et al., 2013a; Vassiliev et al., 2013b; Wang et al., 2020; Garba et al., 2012; Horvat and Dović, 2018; Hartmann et al., 2013; Baxter et al., 1998; Oladejo et al., 2020; Nzihou and Stanmore, 2013). Herbaceous biomass (straw, nut husks, fruit pits, grasses) causes more problems during combustion than wood. Compounds such as potassium, sodium, whether in the form of oxides, hydroxides or organometallic compounds, tend to lower the melting point of ash mixtures containing various minerals such as SiO2. Also, high chlorine content in the fuel increases the release of potassium in the gas phase at relatively low temperatures. This causes serious problems related to slagging, contamination and agglomeration of the bed in the boiler, which can lead to switching off the heating device (Dyjakon, 2012). In connection with the above, the chemical composition of the fuel affects the rate of ash deposition from biomass fuels. It was also found that the process of deposit formation from herbaceous grass or miscanthus is about six times faster than from wood. Woody biomass is rich in Ca, Si and K compounds. Determining the melting behavior and release to the gas phase of biomass fuel ash is of great importance, therefore it is important to study herbaceous biomass fuels in order to characterize the ash composition. From the point of view of biomass combustion, a high content of K and Ca is unfavorable, because they easily react with other elements (e.g., Si) to form alkalis with very low melting points (approx. 700 °C). In turn, an increased content of K can increase the slagging potential. In contrast, ashes with lower alkali and higher Ca content exhibit easier to manage slagging and contamination problems.
3 Ash in the operation of heating devices
During the operation of heating devices, the main problem is their contamination with ash during the process in which a deposit forms on the colder areas of the device as a result of the condensation of particles transferred from warmer to colder areas. Fouling means the process of deposit formation on convective heat exchange surfaces. The formation of large ash deposits is caused by a relatively large amount of compounds containing alkalis such as KCl and K2SO4 and the low melting point of many ash components. These deposits reduce the overall heat transfer coefficient, which leads to corrosion of metal surfaces. Additionally, the behavior of ash in the combustion chamber and furnace is problematic, as it forms slag and sinter due to the effects of high temperaturę. The mechanism of slag formation consists in the melting of ash, agglomeration of ash particles (also with fuel) and sintering. Slagging is a process during which deposits form on the heat exchange surfaces of the boiler and the refractory material as a result of adhesion of viscous particles. Slag deposits consist of an inner powder layer containing silicates and alkali compounds (Repić et al., 2019; Blondeau, 2013; Rodriguez et al., 2020; Ion et al., 2023; Wnorowska et al., 2020; Vega-Nieva et al., 2015).
Slagging is a very complex phenomenon, which is not only a physical and chemical reaction but also a serious problem in the use of biomass, which affects the operation of biomass combustion devices. Slagging and deposition reduce the efficiency of biomass combustion, which can lead to damage to the boiler, reducing its output, resulting in repair costs and, above all, difficulties in the daily use of biomass fuels (Yongtie et al., 2017; Rodríguez et al., 2021; Kowalczyk-Juśko, 2017). The parameter that determines the ability of ash to slagging is the content of iron oxides. The viscosity of particles is a key parameter in the case of ashes rich in silicates. High iron content causes a decrease in ash viscosity, which is manifested by a greater tendency to form deposits. Ashes with a Fe203 content of <6% are considered to be low-slagging (Kalisz et al., 2015; Kleinhans et al., 2018; Wu et al., 2018). Slag deposits on the boiler or furnace wall are formed as a result of the action of fine ash particles containing K and Cl. Chlorine promotes the release of potassium in the biomass, causing the formation of troublesome KCl, which condenses on the heating surfaces of the boiler. Problems with ash deposition depend on the presence of elements such as Na, K, Cl and Si and Al.
Potassium present in biomass as a result of oxidation processes during combustion leads to the formation of impermeable deposits on the surface of heating devices as well as agglomeration and corrosion (Deng et al., 2019; Miccio et al., 2019; Niu et al., 2016; Wang et al., 2018c). The more alkali metals and chlorides present in biomass, the lower the ash melting temperature and therefore the greater the tendency to form slag. The formation of slag in the furnace limits the access of air to the fuel, resulting in a decrease in temperature and an increase in the concentration of carbon monoxide in the exhaust gases. It is therefore necessary to ensure that the temperature in the furnace and combustion chamber in the case of grate furnaces exceeds 650 °C when burning agricultural biomass and, at the same time, is below the ash sintering temperature in the case of retort and horizontal-feed boilers or the ash softening temperature (in the case of heating appliances with a moving grate) (Pałaszyńska and Juszczak, 2018; Arvelakis et al., 2001; Livingston, 2016). Soluble potassium, which occurs in ionic form, is released during combustion as KOH, KCl and other forms, as a result of which it is easily available for further reaction with other flue gas compounds (Niu et al., 2016; Xu et al., 2021). According to Wang L. et al. (2012) and Kassman (2012), the main components of particulate matter present in the generated flue gases are potassium chloride (KCl) and potassium sulfate (K2SO4), whose melting points are 750 °C and 850 °C. Systems consisting of KCl and K2SO4 salts can melt at a low temperature of 550 °C or even lower (Lindberg et al., 2013; Funcia et al., 2020). Potassium and chlorine are the main components causing undesirable deposits and corrosion of superheater tubes in boilers fired with solid fuels (Ǻmand et al., 2006).
During co-combustion, leaching or the use of additives, an increase in the K + Cl and Si + Al ratio in the fuel can lead to an increase in the KCl concentration, which will manifest itself in greater slag formation. On the other hand, a decrease in the K + Cl and Si + Al ratio will lead to the capture of K by Si and Al, which will result in less slagging during combustion (Baxter et al., 1998; Kassman et al., 2013; Zhu et al., 2014). Ash deposition on the heating surface takes place as a result of four mechanisms: inertial collision, condensation of evaporated inorganic compounds, thermophoresis and chemical reactions (Veijonen et al., 2003; Hernik and Wnorowska, 2022; Jensen et al., 2019; Garba et al., 2012; Yongtie et al., 2018; Shao et al., 2012; Jensen et al., 2016; Stam, 2020; Yang et al., 2017; Livingston, 2016; Laxminarayan, 2018; Vaniyambadi, 2015).
In the initial stage of deposition, submicron ash particles are transferred to the cool surface under the influence of gas temperature, regardless of inertial impact. This process is known as thermophoresis. As a result of combustion, chemical reactions such as chlorination, oxidation and sulphation occur between solid and gaseous compounds and the deposition layer. An example is silica, which reacts with volatile alkali metal, i.e., potassium, to form low-melting compounds causing slagging and fouling at normal biomass boiler temperature (800 °C–900 °C). The produced alkali silicates or calcium chlorides tend to deposit on the boiler wall causing fouling or corrosion at low melting temperature 700 °C–750 °C (Veijonen et al., 2003; Baxter et al., 1998; Kleinhans et al., 2018; Shao et al., 2012; Funcia et al., 2020).
The main operational problem of biomass-fired boilers is the deposition of fly ash on the boiler surface. Alkalis, chlorine and silicates are the main fractions of ash deposits regardless of the type of fuel. Fouling and slagging are complex phenomena dependent on:
- Transformation of inorganic ash components in fuels,
- Chemical reactions between gas, liquid and solid phases in suspension and on surfaces,
- Reaction kinetics and species transport rates,
- Attachment of ash particles to surfaces and release of deposited liquids and solids.
The removal of deposits can occur by several mechanisms, namely,:
- Erosion,
- Detachment,
- Flow of molten slag,
- Thermal and mechanical stress in the deposits.
Erosion occurs when sharp-edged fly ash particles collide with unfused deposits, resulting in the removal of deposits by spalling or repeated deformation. Two main mechanisms are distinguished: brittle erosion and plastic erosion. The equivalence of both sub-mechanisms depends on the material properties of the particle and the wall material, the angle of impact, and the kinetic energy of the particle (Schulze et al., 2020; Stauber Alfredsson 2018; Livingston, 2016; Laxminarayan, 2018).
Debonding occurs when the stress generated (e.g., due to soot blowing or the weight of the deposit) exceeds the adhesion force at the interface of the depositing tube, causing the deposit to be removed from the tube surface. If the temperature of the outer layer of the bed is high enough to form low-viscosity slags, molten phases can flow out of the bed. Thermal shocks caused by temperature changes in the boiler can cause deposits to form due to differences in the thermal expansion coefficients of the tube, the corrosion layer, and the deposit (Laxminarayan, 2018).
It was also found that biomass with herbaceous raw materials causes increased agglomeration due to the high content of alkaline compounds. In turn, a small share of biomass in fuel ash (coal) has a significant effect on the microstructure and chemical composition of the sediments, increasing the rate at which the sediments consolidate and strengthen with increasing temperature. Alkali metals contained in biomass, i.e., K, which reacts with other ash-forming elements, while chlorine forms compounds containing alkalis, KCl causing problems related to fouling and slagging, corrosion and agglomeration on the superheater or boilers (Guo et al., 2020; Miccio et al., 2019; Shao et al., 2012; Loo and Koppejan, 2008; Kassman, 2012; Tortosa Masiá, 2010; Balint et al., 2024; Niu et al., 2016; Oladejo et al., 2020), which hinder heat transfer and thus reduce the thermal efficiency of the boiler (Guo et al., 2020; Mlonka-Mędrala et al., 2020). Excess alkali in biomass-based fuels can be released into KOH. The presence of KOH on heat exchange surfaces can be an important factor in the corrosion mechanisms of biomass combustion devices (Berlanga and Ruiz, 2013).
The influence of boiler design, operating conditions and fuel properties on ash deposit behaviour is seen by emphasizing or limiting the role of one or more of these mechanisms, thereby changing the composition, phase and properties of the deposit. Surfaces exposed to particle impact can accumulate both silica and alkali silicates at a very rapid rate. In contrast, surfaces exposed to flue gases and less to particle impact show signs of deposit accumulation, vapour condensation and sulphation of condensed vapours containing alkali. These processes lead to the formation of deposits with different properties, high reflectivity, low thickness and growth rate. Fuels containing little alkali or silica show less growth of deposits.
Studies of deposits on the heating surface of biomass boilers are most often carried out by analyzing mature deposits collected during boiler maintenance. The composition of the deposits varies depending on the distance from the boiler superheater surface. The innermost layer of deposits usually contains iron oxide, KCl and K2SO4. The intermediate layer contains molten KCl and other ash particles. In some deposits, a reaction between KCl and captured ash particles can occur, leading to the release of chlorine-containing gases. However, it is difficult to study the dynamic deposition process in detail by analyzing mature deposits (Zhang et al., 2020; Sandy Sharp, 2010).
The chemistry of inorganic transformations in boilers is quite complex and involves many physicochemical pathways between alkalis, alkaline earth and other inorganic and organic compounds in the fuel. Inorganic substances derived from biomass fuels are deposited in various forms on the boiler surface (Veijonen et al., 2003; Shao et al., 2012; Capablo, 2016; Repić et al., 2019; Rabaçal and Costa, 2015).
Studies indicate that the major release of alkaline material occurs as a result of combustion during the charring phase. The original form of the alkaline waste gases corresponds to thermodynamic estimates of product stability and vapor pressure. Deposits enriched in potassium, sulfur, and chlorine indicate that condensation and chemical reaction mechanisms play a significant role in deposit formation. The condensation mechanism enriches the deposit with condensable vapors such as chlorides. The chemical reaction mechanism converts alkali and alkaline earth metals to sulfates. Both mechanisms are dependent on the mass transfer rate. Differences between the boiler wall and convection channel deposits are evident and indicate different mechanisms responsible for ash deposition in different areas of the boiler.
4 Slagging and pollution indicators
In the literature on the subject, slagging and contamination indicators have been defined to determine the risk of deposits and agglomeration of biomass ash during combustion and co-combustion of biofuel in a boiler.
One of the most popular indices used as an indicator of the tendency for fuel ash contamination is the ratio of base to acid B/A to acid oxides in the ash:
If: B/A ratio <0.75 – contamination may occur; B/A < 0.4 or B/A > 0.7 – the tendency to sedimentation is low, but if 0.7 > B/A>0.4 – high (Bapat et al., 1997; Dyjakon, 2012).
In the case of biomass, the importance of potassium is also justified. Therefore, biomass of agricultural and herbaceous origin - straw, nutshells, fruit pits, weeds and grasses cause more problems than wood. The metals potassium and sodium, whether in the form of oxides, hydroxides or organometallic compounds, tend to lower the melting point of ash mixtures containing other various minerals, such as silica (SiO2). As a result, serious problems with slagging, fouling or agglomeration of the bed in the boiler arise, which can lead to its shutdown. Therefore, it is justified to introduce a new indicator based on the melting and sintering coefficient of SI oxides:
If: SI index >2 – the risk of slagging is low, SI < 2 – high (Bapat et al., 1997; Basu et al., 2000) and the Cm index, which expresses the ratio of basic to acidic oxides:
The SR slag viscosity index is also used to determine the tendency to deposition. Viscosity is one of the basic properties of liquid slags and is a key parameter for many industrial processes. It plays an important role in slag adhesion, ash fusion, viscous flow and other phenomena:
A high value of the index means a high content of slag viscosity. If: SR > 72 – the slag has a low tendency to slagging, 72 ≥ SR > 65 – medium, SR ≤ 65 – low, SR is 72–80 – the tendency to form impurities is low, SR is 65–73 – medium and 50-65 – very high (Bryers, 1996).
The dependence of melting temperatures on basic compounds (Fe2O3+CaO + MgO) shows a similar character for different fuels (coals, biomass and municipal waste).
Biomass is burned in various heating devices (pulverized fuel, grate, fluidized bed boilers) where particle agglomeration may occur. In order to estimate this risk, the bed agglomeration index BAI was developed, relating the ash composition to agglomeration (fluidized bed reactors):
If: BAI <0.15, then agglomerates are formed (Capablo et al., 2009).
Another IC index is based on the ratio of iron oxide and calcium oxide as a parameter to assess contamination:
If: IC < 0.3 or IC > 3.0 then the tendency to create pollutants is low, 0.3 < IC < 3.0 – high (Ściążko et al., 2006).
A similar tendency to form deposits is described by the quotient (SA) of silicon oxide and aluminum oxide (Bapat et al., 1997).
Another indicator is the sum of Fe and Ca oxides:
If the sum of Fe2O3+CaO does not exceed 10% then the fuel has a low tendency to form pollutants.
The final parameter is the Fu fouling index, which takes into account in particular the alkaline elements (sodium and potassium). This index is presented by the following equation:
The Fu index determines the tendency of the fuel to form and then sinter contaminants on heating surfaces and shows the tendency to form deposits that initiate slagging.
If: Fu ≤ 0.6 – the fuel is not susceptible to contamination, 0.6 < Fu ≤ 40 – it has a high tendency, Fu > 40 – very high tendency to the formation and sintering of contaminants (Bapat et al., 1997; Dyjakon, 2012; Kowalczyk-Juśko, 2017).
On the other hand, Miles et al. (1996) presented the alkali index in the following equation in order to monitor the amount of alkali in fuels and minimize the occurrence of slagging and contamination:
where: HHV (Higher Heating Value) is the higher calorific value (MJ/kg) at H = 0% and K2O and nitrous oxide (N2O) are expressed as a percentage of these components in the fuel composition in dry mass according to the index:
- Alkaline index >0.17 kg alkali/MJ – probable contamination.
- Alkaline index >0.34 kg alkali/MJ – contamination will definitely occur.
Whereas (Pronobis, 2005) presents the following values to reduce slagging and Cl based contamination in biomass fuels, where Cl is the chlorine content (%) in the dry mass of the sample:
- Cl < 0.2 – low tendency to slagging,
- 0.2 < Cl < 0.3 – moderate tendency to slagging,
- 0.3 < Cl < 0.5 – high tendency to slagging,
- Cl > 0.5 – very high tendency to slagging.
In the study conducted by Kowalczyk-Juśko (2017) for herbaceous biomass, the ratio of alkaline to acidic components, showing a tendency to ash deposition, was the lowest in the case of perennial grasses (Miscanthus sacchariflorus) and prairie knotweed (Spartina pectinata Boscex Link), and many times higher in the case of Virginia mallow. Ashes from the biomass of Chinese miscanthus (Miscanthus sacchariflorus) and Japanese knotweed (S. pectinata) were characterized by a low tendency to form impurities and sediments (Table 5). At the same time, in the case of ash from Virginia mallow (Sida hermaphrodita Rusby) and Jerusalem artichoke (Helianthus Tuberosus L.), a very high tendency to sintering, slagging and sedimentation was observed, which is evidenced by significantly higher Fu, SI, BAI values.
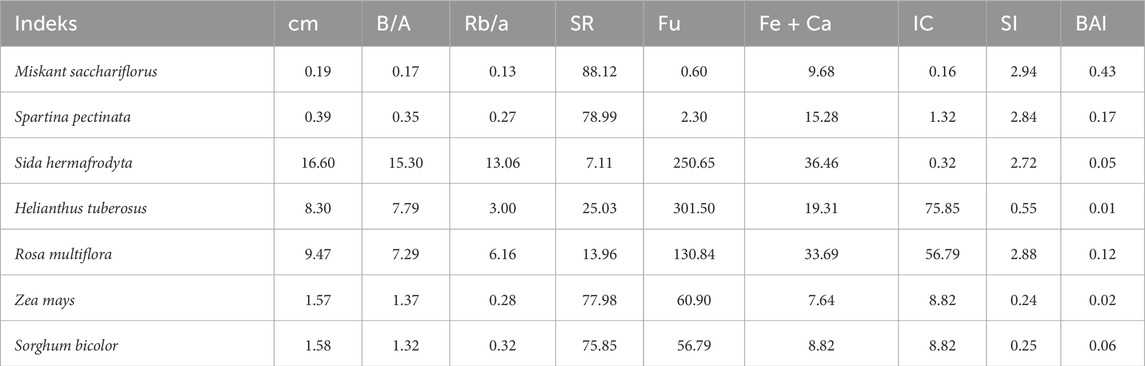
Table 5. Slagging and contamination indicators for ashes from the tested biomass [Source: Kowalczyk-Juśko (2017)].
In turn, the research conducted by Dyjakon (2012) for the slagging and contamination indicators of the boiler heating surfaces showed significant differences in the tested plant biomass, which is presented in Table 6.
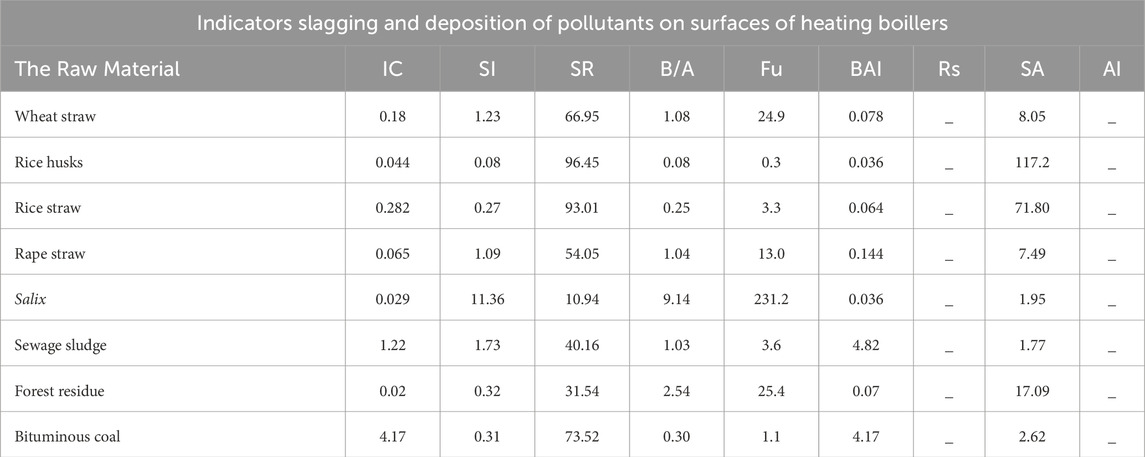
Table 6. Results of indicators slagging and deposition of pollutants on surfaces of heating boilers [Source: own study: Dyjakon (2012)].
In turn, in the study by Baxter et al. (2014) it was shown that the ratio of alkaline to acidic components in the leaves of Miscanthus giganteus ranged from 0.7 to 1.3, while in the stems these proportions were different. The B/A ratio was 1.3–3.2.
There are many empirical correlations that allow the assessment of ash behavior and its effect on deposition both on boiler walls (slagging) and on the edges of convection tubes (fouling). A number of slagging and fouling indices have been developed for coal fuels to obtain and indicate the tendency of fuel ash to form deposits in combustion chambers or boilers based on chemical composition, which exhibit a different behavior than biomass fuels. For example, the slagging indices for Serbian coal, which consists of 88% brown ash coals, their application to biomass fuels containing brown ash should confirm the tendency of this fuel to form deposits on boiler heating surfaces. Since most slagging indices are therefore based on the assessment of the melting properties of aluminosilicate ash from coal, their application to biomass ash systems, which are very different in chemical terms, may be problematic. In case of low-level biomass co-firing, the mixed ash still consists mainly of the aluminosilicate system, so that typical methods for assessing coal slagging can be applied. If biomass is co-fired with coal in larger quantities, all important components of most biomass ash, mainly alkali metals and alkaline earth metals, are strong fluxing agents for aluminosilicate systems. The introduction of biomass can result in a lower melting point and therefore an increase in the slagging potential. Pollution indicators of biomass ash are based on the total alkali content of the fuel. The main driving force for conventional pollutant formation in boilers is considered to be the deposition of Na and K compounds by oxidation/condensation mechanism (Loo and Koppejan, 2008; Repić et al., 2019).
5 Assessment of the possibilities of improving the thermal properties of ash in terms of their impact on exhaust gas emissions
Slagging, deposit formation and surface corrosion are ash-related problems that should be investigated when using fuel blends, as they can lead to reduced efficiency and availability of facilities, which results in increased energy costs. Proper selection of biomass fuels based on the chemical composition of the ash and mixing them in the right proportions can reduce the rate of ash deposition and corrosion. The mechanisms of release of inorganic compounds depend on the boiler operating conditions and staged combustion can cause local reducing conditions, which poses a risk of boiler corrosion. Corrosion is the process of dissolving metal into a more stable compound. This process occurs as a result of electrochemical reactions, oxidation and reduction reactions, involving ions and electrons, forming an oxide. The mechanism of the corrosion process caused by chlorine, HCl, Cl2, NaCl, KCl and alkali compounds (K, Na) is the process of steel oxidation accelerated by the presence of chlorine in the flue gases, called active oxidation. The corrosion rate is temperature dependent and depends on the volatilization of the metal chloride (Lokare, 2008; Stauber Alfredsson, 2018).
In the research conducted by Royo J. et al. (2022), it was shown that in order to compensate (at least partially) the negative impact of plant components in the mixtures, it is interesting to use a higher value of excess air (λ), because it allows to lower the combustion temperature (at λ > 1), which means reducing the degree of sintering and deposition, thus improving the ash behavior during pellet combustion.
In the study conducted by Naganuma et al. (2013) the mechanism of fouling formation was investigated and it was found that alkali sulfates play an important role in increasing the adhesion force. One possible solution to the fouling problem is to reduce the adhesion force. Therefore, thermal spraying of a nickel alloy onto the heating device pipes may help achieve this.
In turn, Pałaszyńska and Juszczak (2018) examined the effect of selected biomass types (coffee husk pellets or pellets combined with wheat straw pellets and cherry stones, sewage sludge pellets, rye and corn straw briquettes, and miscanthus briquettes) on the slag formation process. The studies showed that the boiler operated correctly during the combustion of the above-mentioned types of biomass. For all types of fuel, only brittle, porous slag was formed, which was easily fragmented by the reciprocating movement of the grate pushers and then removed from the furnace. This means that the temperature in the boiler was above the ash sintering temperature and below the ash softening temperature. In addition, a significant amount of brittle deposits was observed in the combustion chamber and on the heat exchange surfaces, which required cleaning the boiler after 40 h of operation. However, most of the deposits were created by burning coffee husk pellets.
On the other hand, herbaceous biomass fuel in the form of pellets from rice husks and corn stalks was commonly used in studies (He et al., 2022) on pollutant emissions and slag properties. The studies indicate opposite trends in the change of CO and NOx concentrations in exhaust gases. NOx emissions generated during the combustion of corn stalks as a result of multi-layer secondary air distribution are comparable to the combustion of rice husks. The obtained research results can contribute to efficient combustion and low pollutant emissions. Therefore, NOx emissions and ash slagging rates at the exhaust gas outlet are used as an assessment indicator to study the operating conditions for low NOx emissions and slagging rates during the combustion of corn stalks under air distribution conditions. On the other hand, slagging does not occur during the combustion of rice husks, therefore, NOx and CO emission concentrations serve as an assessment indicator when selecting optimal operating conditions.
5.1 Methods of modifying thermal properties of ash
One of the solutions to the problems related to the formation of ash during biomass combustion is the use of additives that reduce the negative impact on biomass ash and boiler operation. The additives are environmentally friendly and easy to use, which change the chemical composition of the ash, transforming it into a safe form and improve the ash melting temperature. To achieve better additive efficiency, it is required that the additive has a smaller particle size, which facilitates physical adsorption and chemical reactions. In biomass boilers, additives can be used that reduce the negative impact of biomass ash on boiler operation. The additive can act in two different ways by reacting with alkali metals such as KCl and KOH and binding the base into less aggressive compounds or by diluting it so that critical alkali forms occur in lower concentrations in fly ash (Vamvuka and Kakaras, 2011; Plaza et al., 2019; Jensen et al., 2016; Shao et al., 2012; Vamvuka et al., 2009; Kubica et al., 2016; Mroczek et al., 2011). Reagents are classified into: aluminosilicates (Al-Si), S, P and Ca, e.g., magnesite. Addition of sulfur-based additives (ammonium sulfate (NH)2SO4) to the combustion chamber reduces the rate of corrosion. Additives changing the physicochemical properties of ash, facilitate its removal by means of soot blowers, e.g., copper oxychloride (3CuO∙CuCl2∙4H2O), which through its action reduces slagging as a result of causing ash deposits to disintegrate and become brittle. However, repeated soot blowing leads to rapid erosion of the pipe surface. Additives based on aluminosilicates are used to transform evaporated inorganic substances into less harmful forms or to retain hazardous compounds in specific ash fractions, for example, by binding potassium. It has also been shown that aluminosilicate or SiO2 based additives react with gaseous alkali species (KCl, KOH, NaCl, sodium hydroxide (NaOH) and reduce the degree of fouling in the superheater area. Of all the types, Al-Si based additives are the best at capturing K present in biomass during combustion, coal fly ash, forming aluminosilicates with higher melting points. Coal fly ash with higher Si content and lower melting point captures KCl more effectively than fly ash from reference coal. This additive has been commercially used in full-scale biomass boilers for K capture (Jensen et al., 2019; Shao et al., 2012; Zhou et al., 2017; Wang et al., 2018a; Wang et al., 2018b; Wang et al., 2018c; Wang et al., 2019; Chen et al., 2022). Among the above-mentioned additives, Al-Si is the most effective Kaolin is preferred due to its availability and ability to capture K to form high-melting potassium aluminosilicates and thus better cope with the problems associated with biomass combustion and the relatively high content of alkali metals in the biomass ash, which leads to high contents of salts such as KCl, KOH and K2SO4 in the flue gases. Al-Si based additives are kaolin (Al2O3∙2SiO2), kaolinite (a component of kaolin), bentonite, bauxite, zeolite, halloysite, olivine, feldspar, mica Equation 11:
where Al2Si2O5(OH)4-halloysite, KAISiO4-kalsilite
More stable and high-melting forms can be created using monocalcium phosphate Equations 11, 12:
where CaK2P2O7-calcium dipotassium diphosphate.
On the other hand, alkali metal chlorides can be converted to the gas phase into alkali metal sulfates, which are characterized by a higher melting point and lower corrosive activity (Equations 11, 13) (Madhiyanon et al., 2020; Nørskov et al., 2010; Maj and Matus, 2023; Wang et al., 2018b; Wang et al., 2018c; Sandy Sharp, 2010; Wang et al., 2019; Li et al., 2023; Blomberg, 2007; Meng et al., 2017; Kassman, 2012; Plaza et al., 2019; Zhou et al., 2016).
As reported by Batir et al. (2019) and Wang et al. (2013), kaolin raises the melting temperature of biomass ash and is considered the most effective additive. Its main component is kaolinite, Al2Si2O5(OH)4, which, when heated to 450 °C, transforms into metakaolinite. Metakaolinite then reacts with potassium (K) to form crystalline products such as KAlSiO4 and leucite (KAlSi2O6), characterized by high melting points of approximately 1,600 °C and 1,500 °C, respectively. Kaolin can reduce the concentration of potassium chloride, which results in a reduction of the agglomeration process. The use of Al-Si-based additives has been considered the most appropriate additive to eliminate problems associated with biomass combustion (Wang X. et al., 2012; Davidsson et al., 2008; Jensen et al., 2019; Khalil et al., 2012; Zhu et al., 2014). The addition of kaolin plays a significant role in reducing the emission of fine particles. K and Na are believed to be released from the biomass particle in the form of volatile chloride vapors (KCl and NaCl) and hydroxide vapors (KOH and NaOH). The release of alkali in the form of chlorides is related to the rate of release of highly reactive HCl, which can act as a limiting or promoting factor. The addition of HCl to biomass combustion has been shown to increase the release of alkali metals.
On the other hand, under low HCl conditions, alkaline compounds tend to react with silicates (Rabaçal and Costa, 2015).
In contrast, the addition of mica showed the highest inhibition efficiency (90%) for both corn straw and poplar (Sandberg, 2011; Abioye et al., 2023). It has also been shown (Escobar and Műller, 2007) that clay minerals rich in SiO2, e.g., kaolinite irreversibly capture alkali. Clay minerals with different SiO2 and Al2O3 compositions were tested and it was found that the capture efficiency is influenced by the ratio of both components.
The addition of halloysite to biomass combustion causes:
- An increase in the characteristic temperatures of biomass ash, which has a positive effect on reducing the process of fly ash deposition on heat exchange surfaces,
- Reduction of contamination and slagging of the boiler heating surfaces,
- Reduction of bed agglomeration,
- Reduction of high-temperature corrosion,
- Extension of the boiler operation inter-repair periods,
- Change in the quality and composition of ash, expanding the possibilities of its economic use,
- Reduces the average height of sediment formation (39% decrease), sediment mass (37% decrease) and sedimentation rate (37% decrease) (Hernik and Wnorowska, 2022; Plaza et al., 2019).
In addition, halloysite in its raw form has additional layers of water molecules Al2Si2O5(OH)4∙2H2O, which distinguishes it from kaolin Al2Si2O5(OH)4. Differences also occur in the structure, as the halloysite structure reveals the presence of nanotubes, whereas kaolin forms a lamellar structure. While kaolin is an almost pure mineral (99.9% purity) in the form in which it is commercially available on the market, halloysite is a raw ore containing numerous impurities and trace amounts of other elements. Both aluminosilicates have an extensive specific surface area, thanks to which they are considered very good sorbents (Sobieraj and Kalisz, 2021).
Other sorbents, such as dolomite and clay, can also be used, which prevent the reaction between potassium and silica. There are many studies on the properties of sand and rock ilmenites, showing their ability to transport oxygen and interact with ash components derived from fuel. Other additives consisting mainly of iron ore are hematite (FeO) and titanium-iron ore - ilmenite (FeTiO3). Hematite has the ability to remove Hg from fly ash with a wide temperature range. In turn, ilmenite has the advantage of absorbing potassium molecules from ash, thus reducing agglomeration with the sand bed material used. Studies have shown that the addition of KCl, K2CO3 or K2SO4 increases the reactivity of ilmenite, while alkaline compounds such as potassium (III) phosphate (KPO3) have a negative effect on reactivity. Moreover, potassium salts: KCl, potassium carbonate (K2CO3) or K2SO4 as the main forms of potassium present in biomass ash, can catalytically affect biomass combustion (Corcoran et al., 2014; Corcoran et al., 2018; Sun et al., 2018; Zhou et al., 2017; Yang et al., 2017; Izquierdo et al., 2017; Gogolev et al., 2021; Andersson et al., 2024; Kaniowski et al., 2022). However, the use of bauxite (consists mainly of aluminium hydroxides – hydrargillite (Al2O3∙3H2O), boehmite (aluminium hydroxide Al(OH)3 or diaspore (aluminium hydroxide AlO(OH), contains clay minerals, silica, iron oxides and hydroxides) as an additive enriching bottom ash (ash from agricultural residues with an admixture of brown coal) with calcium compounds can capture K. Under combustion conditions no signs of ash deposition or bed agglomeration were observed (Vamvuka et al., 2009; Wolf et al., 2004).
In the case of materials used in a reactor, an ash layer may form on the bed particles due to the deposition of AAEM compounds released from the fuel (Marinkovic et al., 2015).
Table 7 summarizes the different cases of using aluminosilicate-based additives to reduce ash deposition by capturing K.
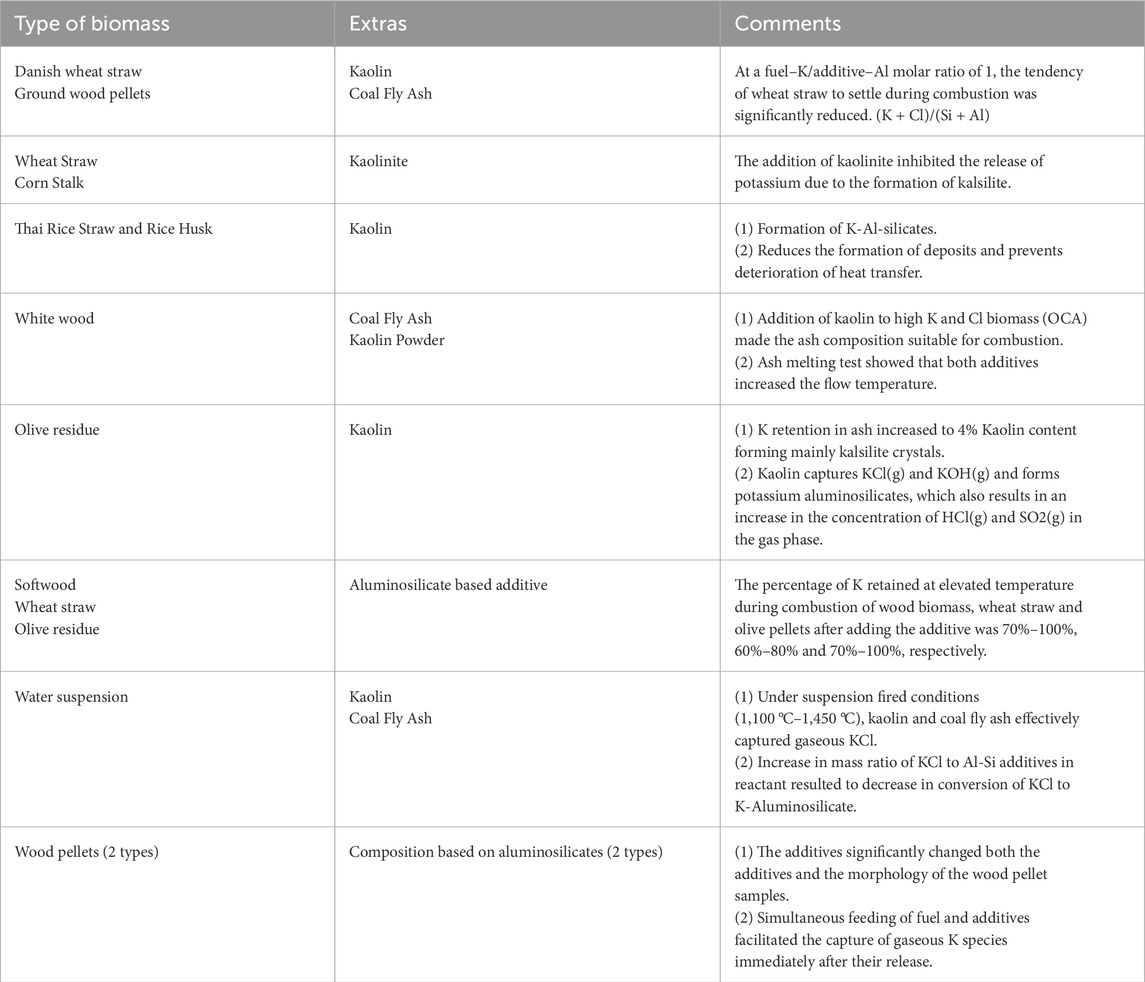
Table 7. List of additives based on aluminosilicates that capture potassium (K) during biomass combustion [Source: own study: Wang et al. (2022); Li et al. (2020); Madhiyanon et al. (2020); Roberts et al. (2019); Batir et al. (2019); Clery et al. (2018); Arendt et al. (2016); Paneru et al. (2016)].
5.2 The impact of modified biofuel with anti-caking additives on exhaust gas emissions – environmental and technological aspects
Sintering of biomass ash on heat exchangers reduces heat exchange efficiency and makes it difficult to clean the accumulated ash. In the case of heat exchangers, the ash deposits contain elements such as K, S and Cl (Dell’Antonia et al., 2013). Based on the research conducted (Botić et al., 2022) using herbaceous biomass (soy straw pellets and camomile processing waste) burned in a low-power heating device (<50 kW), the results show that the gas emission values (permissible values for: CO -750 mg/m3 and NOx - 200 mg/m3) during the combustion of biomass containing a higher percentage of nitrogen significantly exceed the recommended values. All types of additives (clay, kaolinite, bentonite) led to a reduction of CO emissions by about 50% during the combustion of wood pellets and pellets from camomile waste, while soy straw pellets increased their emissions. NOx emission values after the use of ash anti-caking additives did not change significantly in wood pellets (<200 mg/m3 compared to pellets from camomile waste). In turn, the study (Gehrig et al., 2019) focused on the use of energy willow (instead of forest biomass) and the additive - kaolin during combustion in a small boiler of 12 kW. The observed emission of particulate matter shows a clear decrease for fuel mixtures mixed with kaolin. The addition of kaolin reduces the K content in the tested particles regardless of the fuels used and confirms the K retention in the bottom ash. In addition, gaseous NOx emissions correlate with the N content of fuel blends and show an increase in NOx emissions with the increase in the share of energy willow, where kaolin has no major effect on NOx emissions. On the other hand, a study conducted (Gollmer et al., 2023) in a small-scale biomass combustion plant (33 kW) showed that the addition of 0.5% by weight of kaolin (especially the Kaolin III type with the smallest particle size) to wood chips significantly reduced CO emissions - from 96 mg/Nm3 (without addition) to 26 mg/Nm3. This reduction was greater than in the case of the use of an electrostatic precipitator (ESP), which reduced CO emissions to 96 mg/Nm3. The authors suggest that kaolin may act catalytically, although this mechanism has not yet been fully explained. In turn, in the experiment conducted (Nguyen et al., 2019) using a 0.1 MWth fluidized bed boiler (CFB) in an oxygen atmosphere, replacing quartz sand with kaolin as a bed material contributed to the stabilization of the combustion process and reduced CO and NOx emissions. Additionally, kaolin improved the fluidization properties and prevented particle agglomeration. Another study (Xu et al., 2018) conducted in a 1000 MW coal-fired power plant found that adding kaolin to the combustion chamber reduced the emission of fine PM2.5 particles by 36.72% after passing through an electrostatic precipitator. Although the main goal was to reduce particulate matter emissions, changes in ash composition were also observed, which may affect gaseous emissions, including CO and NOx. Similar studies were conducted (Norizam et al., 2024) investigating the effect of kaolin addition on the partitioning of chemical elements in particulate matter (PM) during the combustion of primary and waste wood biomass fuels in a 250 kW grate boiler. The results showed that the PM emission levels were significantly reduced by about 70%–76% and 60%–66% after the addition of kaolin for the primary and waste woody biomass, respectively, which inhibited the partitioning of alkaline species into fine and ultrafine PM. On the other hand, the concentration of non-volatile elements (SiO2 and Al2O3) significantly increased in the PM emissions after the addition of kaolin due to the adhesion and aggregation of particulate matter between airborne kaolin and fine and ultrafine PM. Moreover, the addition of 1.55 wt. % kaolin showed comparable effects with the addition of 2.5 wt. % on the chemical composition of PM emissions. KCl salts were also reduced after the addition of kaolin. The conducted computer simulations (Chen et al., 2021) showed that the addition of 3 wt. % kaolin during coal combustion reduced the emission of PM0.3 particles by 33.35%. Although the study focused on particulate matter, the authors suggest that kaolin may also affect gaseous emissions by adsorption of mineral precursors. In the study (Sommersacher et al., 2013) biomass fuels, i.e., spruce and straw and an additive (kaolin), were investigated. In the case of spruce, the addition of 0.2 wt. % kaolin was sufficient to reduce aerosol emissions by 87%. In addition, the melting point of the ash increased by 40 °C. The ash content of this mixture increased slightly and the increase in sulfur dioxide (SO2) and HCl emissions was also negligible. The addition of kaolin seems to be of particular interest for small (residential) pellet applications to significantly reduce particulate emissions, as these systems are not usually equipped with dust collectors. In the case of straw, the addition of kaolin in an amount of about 4 wt. % can be recommended to significantly reduce aerosol emissions and significantly improve the ash melting properties. The ash content of this fuel/kaolin mixture increases by about 69%. The addition of kaolin to straw is also applicable on a medium (>100 kW) and large scale, where an appropriate flue gas cleaning system is usually used. In the study (Huelsmann et al., 2019), an 8.6 kW pellet stove and a 12 kW pellet boiler were used to burn wood pellets with 0.5% and 1 wt. % kaolinite. The results show a similar particulate emission reduction effect for both combustion devices of 46% (pellet stove) and 48% (pellet boiler) using 0.5 wt. % kaolinite. However, the use of 1 wt. % kaolinite indicates different results. The total particulate emissions from the pellet boiler were reduced by approximately 69%, while emissions from the pellet boiler were only slightly reduced, by approximately 5%.
In turn, in the study (Wang et al., 2022), the results of combustion of wheat straw and wood with kaolin addition showed that at a dose of 3.13% of this additive, the tendency of wheat straw to sedimentation significantly decreased from 86% to 29%. It was also found that kaolin has a relatively higher Al content and the Al/Si molar ratio of 0.89 may indicate a higher K capture capacity of kaolin. Similar studies (Tissari et al., 2008) showed (combustion of oats and wood in a 20 kW pellet boiler) that K release during combustion was reduced in the case of oats, while it was increased in the case of wood. In addition, the role of kaolin in the formation of sediments during wood combustion was also investigated, where the addition of kaolin changed the fractionation of Cl so that it occurred mainly in the flue gases and not in the PM fraction. The corrosion potential was also found to be reduced by the addition of kaolin (Khalil et al., 2012). In contrast, in (Morris et al., 2022) pellets of miscanthus and wheat straw were used and tested in a 65 kW fluidized bed combustion chamber with different doses of dolomite and kaolin on a bed of quartz sand. The use of kaolin and dolomite at doses of 50%–150% by weight of ash prevented defluidization of the bed in the case of miscanthus. Dolomite released Ca content along with some Mg content into the ash, facilitating the formation of high melting Ca and Mg silicates. In contrast, kaolin absorbed potassium from the ash, facilitating the formation of high melting aluminosilicates. This absorption occurred to a depth of about 60 mm in the kaolin, although the typical depth was about 20 mm. In another study (Madhiyanon et al., 2013) it was shown that the addition of kaolin during combustion of empty fruit bunches allowed to maintain high temperature. Ӧhman et al. (2004) also showed how the addition of kaolin affects the tendency to slagging in case of combustion of pollutant-free and problematic (contaminated stem wood) wood fuel. It was observed that kaolin slightly reduced slagging in case of contaminated wood but significantly increased it in case of uncontaminated fuel. Increased slagging was associated with the formation of low-temperature Ca-Al-K silicates. In another study (Xiong et al., 2008) the effect of kaolin on the tendency to slagging during combustion of corn in a 50 kW boiler was assessed. It was observed that the addition of kaolin increased the concentration of KAISi2O6 in both slag and ash. In similar studies (Boström et al., 2009) oat grains mixed with kaolin were used for combustion. Several different ash fractions were characterized and it was found that in the unsintered bottom ash the emission of fine particulate matter was reduced and K was captured in the KAISiO4 and KAISi2O6 phases. Therefore, the main effects of ash transformation were attributed to the formation of two high melting point compounds KAISiO4 and KAISi2O6. It can therefore be concluded that kaolin reduces the amount of fine particles and substances forming deposits and thus prevents slag formation.
Another study Zhao et al. (2006) analyzed the effect of Na and Ca on NOx emission during coal combustion. The results showed that these minerals can significantly affect NOx emission, suggesting that the presence of various minerals, including kaolin, may have a significant effect on gaseous emissions. A similar study Xu et al. (2014) investigated the mechanism of alkali metal capture by kaolin by burning high-sodium lignite (a component of brown coal with relatively well-preserved wood structure) in a tube furnace, and the effects of kaolin content, reaction temperature, and kaolin and fuel particle sizes on the Na capture efficiency by kaolin. It was found that kaolin could chemically adsorb NaCl, the main sodium species in flue gas, to form high-melting sodium aluminosilicates such as nepheline (NaAlSiO4) and albite (NaAlSi3O8), and the nepheline formation reaction dominated the sorption mechanism. The larger addition of kaolin resulted in greater sodium binding in ash. The sodium capture efficiency decreased with the increase of temperature or the injection of larger kaolin particles. The sodium retention with 6 wt. % kaolin addition to fuel at 1,200 °C could reach 70% of the total sodium in the burning coal, which could greatly reduce the ash issues and facilitate the safe burning of high-alkali coal in boilers.
In addition, calcium additives containing CaO, calcium carbonate (CaCO3) and calcium hydroxide (Ca(OH)2) are more active during the combustion of biomass rich in K and P, helping to convert K vapours into high melting potassium silicates/phosphates. Accordingly, when biomass fuels contain high levels of K, Si and Ca, P-based additives can be used to reduce ash caking and bed agglomeration. A commonly used additive in Denmark is limestone (CaCO3) in an amount of 1%–2% to avoid caking and thus prevent deposits in biomass-fired heating devices. It has also been found that limestone addition can cause a shift from alkali chlorides to alkali sulphates in fly ash fines (Shao et al., 2012; Ronnback et al., 2008). Wolf et al. (2005) used Ca-based additives including limestone for the combustion of high-alkaline biomass, finding that the emission of gaseous SO2 could be reduced by up to 25%. It has been shown Bäfver et al. (2009) that the combustion of cereal grains (rye and wheat) with CaO in low-power heating devices reduces slagging by forming high-melting Ca-Mg-K phosphates, which may prevent the formation of fly ash particles due to the incorporation of potassium into calcium compounds that remain in the bottom ash. Furthermore, Bäfver et al. (2009) also found that the addition of limestone can cause a shift from alkali chlorides to alkali sulphates in the fine fly ash particles. He also observed that particle emissions could be reduced by adding kaolin to the combustion of oat grain in residential buildings (2% kaolin added to the fuel reduced particle emissions by 31% and 4% reduced emissions by 57%).
Composite additives containing 20%–50% of purge air, 25%–65% of kaolin, 20%–60% of MgO and 15%–30% of activated alumina may prove to be effective additives. These additives reduced the potassium content, which also reduced the sedimentation process. With the increase in combustion temperature, the additives showed better performance and the addition of CaO reduced the HCl emission (Liao et al., 2015).
In the studies Kalisz (2023) a mixture of halloysite and dry urea was used in a ratio of 1:3 using fuels (coal, biomass). The use of the urea-halloysite mixture ensured NOx reduction at an average level of 52%, which corresponds to emissions of 175 mg/m3. This result indicates the correctness of the stoichiometric excess coefficient of the additive SR = 2 used in the studies, along with maintaining the ammonia (NH3) level at a low level (<2 ppm).
In another work, Skodras et al. (2007) investigated the co-combustion of brown coal with olive stones, sawdust, almond shells, municipal sewage sludge and investigated the ability of urea to reduce the formation of toxic gases (polychlorinated dibenzo-p-dioxins (PCDD) and dibenzofurans (PCDF). In all cases, the addition of urea (CH4N2O) resulted in a reduction of emissions and it therefore can be used to prevent the formation of PCDD/F.
In order for the additive to be used in biomass burning devices, it should be inexpensive, non-damaging to the boiler or flue gas cleaning systems, and the use of product residues should be taken into account. Some additives that increase the sintering and ash softening temperature, e.g., halloysite, zeolite or kaolinite, can slightly reduce the amount of slag formed in the furnace and the amount of deposits in the boiler (Kubica et al., 2016; Mroczek et al., 2011).
NOx emission reduction systems are an integral part of the environmental protection system in the power unit. Among the operated installations, there are technologies that reduce NOx emissions at the combustion stage (primary methods) and their secondary reduction by the action of an additive such as urea on the exhaust gases in the appropriate temperature range. In secondary methods, two main reducing agents are used: aqueous solutions of ammonia or urea for which the reduction occurs according to the following reaction (Kuropka, 2001; KOBIZE, 2017):
where CO(NH2)2-urea, NO-nitrogen oxide, N2-nitrogen.
Natural silicates are an interesting raw material for the synthesis of catalysts and adsorbents. Catalysis is a phenomenon of changing the rate of chemical reactions as a result of the action of substances called catalysts on the reactants. A catalyst, on the other hand, is a substance that increases the rate at which a chemical reaction reaches equilibrium, while not being consumed in the process. Adsorption, on the other hand, consists in the separation and retention of gas components on the external and internal surface (in the pores) of a solid body called an adsorbent. The retention of molecules on the surface occurs as a result of the action of short-range physical and chemical forces. Catalysts are applied onto ceramic carriers, known as adsorbents. The carriers are characterized by a highly developed internal surface (channels) and often have a honeycomb structure and their task is to increase the surface of the active phase and increase the mechanical and thermal durability of the catalyst. It can also be a source of active centers of a different type than the active phase, or significantly modify its electronic structure. Examples of heterogeneous catalyst supports are: SiO2, Al2O3, chromium (III) oxide-Cr2O3, MgO, CaO. A heterogeneous catalyst consists of an active phase dispersed on a support and additionally consists of one or more promoters. Promoters are compounds that modify the physical and electronic structure of the active substance, are responsible for inhibiting its unfavorable phase transformations and facilitate regeneration. Promoters include: K, P, Mg, Ca, titanium (Ti), vanadium (V). Catalysis plays an important role in preventing and removing the effects of pollution resulting from the combustion of biomass, including herbaceous biomass. Catalytic reactions usually result in reduced energy demand and increased selectivity. Catalysis and catalytic processes are the basis for many industries. The use of natural material as a catalyst or substrate for the production of a catalyst not only reduces the costs associated with their production, but also makes the process used environmentally friendly and can make a significant contribution to sustainable development. Typically, the support materials must have strong adhesion to the catalytic material and provide an increase in surface area and adsorption capacity. Support materials for the ceramic industry, i.e., kaolin clays, have been recognized as useful materials for the preparation of TiO2 composites that enhance the removal of pollutants due to their adsorption properties and large surface area. TiO2 is a semiconducting oxide, known as one of the most commonly used photocatalysts due to its low cost, stability, high reactivity, and ability to react with a large number of pollutants (NOx) (Kuropka, 2000; Maia et al., 2025; Książek et al., 2017). Kaolin exhibits some catalytic properties and can act as a carrier for active catalytic components (metals, metal oxides) that can be used in NOx reduction processes. Like other aluminosilicates, kaolin can be modified to increase its adsorption capacity (NOx molecules are adsorbed on the kaolin surface, where adsorption is a key step that allows further chemical reactions) and catalytic capacity. The acidic nature of the catalyst surface includes issues related to both the nature (Lewis, Brønsted centers), concentration and strength of the acid centers. Acids are substances capable of donating a proton (Brønsted acids). According to the Lewis theory, an acid is a substance capable of accepting an electron pair. These definitions apply to liquids, but they also apply to solids, on the surface of which acid centers are present. The role of Lewis acid centers is played by coordinatively unsaturated cations. Anions containing hydrogen can dissociate to form protons - we then obtain Brønsted acid centers. Basic centers, which are surface anions, can also be present on the surface of the solid. Acid catalysts belong to one of the most important categories of heterogeneous catalysts, which consist of an active phase dispersed on a support. The support itself not only provides physical properties to facilitate high dispersion of active sites but also interacts with the active component, thus becoming an essential part of the catalytic material (Najbar, 2000).
Methods of reducing NOx emissions into the atmosphere using urea and aluminosilicates are divided into
1. Methods of reducing NOx emissions from combustion processes. This group of methods is referred to as primary or clean.
2. Methods of removing NOx from waste gases. This group includes secondary or cleaning methods, which in turn are divided into dry and wet.
Dry methods include: selective catalytic reduction, non-selective catalytic reduction, catalytic decomposition, adsorption. Adsorption involves binding gas phase molecules (adsorbate) to atoms on the surface of a solid (adsorbent) before the reaction that produces more desirable products. In catalysis, the reactants are adsorbates and the catalyst is the adsorbent. Wet methods, on the other hand, are absorption methods (absorption means uptake and refers to the process of receiving or taking in).
Among the dry methods, the selective catalytic reduction (SCR) method is particularly important. Its main principle is the reduction of particulate matter (PM) emissions and the decreased emission of nitrogen oxides (NOx) in the exhaust gases (Warych, 1994). SCR NOx catalysts are platinum group metals (Pt, rhodium (Rh), Pd) and transition metal oxides (V205, TiO2, MoO3), which have the advantage of being cheaper and less sensitive to poisoning than platinum group metals. Particularly interesting are catalysts containing V205 deposited on TiO2 and on a mixed oxide carrier TiO2-SiO2. The vanadium-titanium catalyst is characterized by high activity at low temperatures and high resistance to SO2 poisoning (Parvulesku et al., 1998).
The SCR mechanism involving Brønsted sites, using aluminosilicates (e.g., kaolin) as an example, is as follows:
1. Ammonia adsorption
The introduction of aluminum atoms into the SiO4 structure leads to the formation of acidic centers. Each trivalent aluminum atom introduced into the silicon-oxygen tetrahedra (SiO4) network is bound to four oxygen anions, the AlO4 tetrahedron has a charge of (−1). In order to maintain the electroneutrality of the aluminosilicate network, the presence of cations is necessary, compensating the negative charge. The simplest way to balance the charge is to introduce a proton, which leads to the formation of surface hydroxyl groups (OH) with the character of strong Brønsted acid centers. Their formation can be explained by assuming dissociative adsorption of a water molecule, occurring on the Al ion. The aluminum ion on the surface, surrounded by three tetravalent Si atoms, has an electrophilic character due to the shift of the charge from the Al3+ ion to the Si4+ ions. It can therefore accept a hydroxyl group, formed by donating a proton from a water molecule. This proton is bound to the neighboring oxide ion. The resulting Si-OH-Al group is strongly polarized and has the character of a strong acid. When aluminosilicates are heated above about 700 K, the surface dehydrates. Removing a water molecule from the Brønsted acid center leads to the exposure of an aluminum ion with the properties of an electron pair acceptor, i.e., to the formation of a Lewis acid center. On the surface of aluminosilicate catalysts at the temperature of catalytic reactions, we deal with both Brønsted and Lewis type centers. The ammonia molecule, reacting with Brønsted acid centers, forms ammonium ions (NH4+), and reacting with Lewis acid centers, forms complexes in which the ammonia electron pair forms a coordination bond with the Lewis electron acceptor center (NH3 L). At the Brønsted acid site (i.e., the–OH group in the kaolin structure), ammonia protonation occurs:
As a result, an adsorbed form of ammonia (ammonium ion) is created, ready to participate in subsequent reactions (Najbar, 2000).
2. Adsorption of NO and O2
The method of catalytic decomposition of NOx according to the reaction is:
Catalytic NO decomposition is a method of reducing NOx emissions because it does not require adding other reagents to the gas stream and potentially leads to the formation of only neutral products. Addition of reducing agents in reduction methods such as hydrocarbons, CO, H2 or ammonia can lead to secondary pollutants such as oxidized hydrocarbons, CO, CO2, N2O or ammonia. In the discussed method, except for N2O, there are no other pollutants. The best catalysts for NOx decomposition are zeolites doped with copper ions, especially Cu-ZSM-5, showing high activity. The catalyst shows maximum activity in the temperature range of 823–873 K. Higher temperatures deactivate the catalyst. The NOx decomposition reaction is carried out in the gas phase. The gas stream flows through the catalyst bed and nitrogen oxide diffuses from the gas to the catalyst surface. It is adsorbed on active centers, in this case metal atoms (e.g., Cu, Mn or Fe). As a result of interaction with the metal atom, a chemical reaction takes place. A two-stage mechanism of nitrogen oxide decomposition is postulated:
In the first stage, complexes are formed with the metal atoms of the catalyst. As a result of interactions, the bonds in the NO molecule break down and individual nitrogen and oxygen atoms become bound to the catalyst. On the catalyst surface, the atoms move and combine into new nitrogen and oxygen molecules. NO and molecular oxygen adsorb on adjacent sites of the catalyst (e.g., Fe3+ or Mn4+ centers, performing redox functions):
where NO2 - nitrogen dioxide.
In the presence of suitable catalysts, NOx can be reduced to less harmful substances, i.e., nitrogen (N2) and water (H2O) according to the reaction:
In this reaction, NO reacts with H2 in the presence of a catalyst to form N2 and H2O.
In addition, N2O emission is dependent on temperature and fuel chemical composition. In contrast to NOx, N2O concentration in exhaust gases decreases with increasing temperature. Higher O/N ratio of biomass has a positive effect on N2O emission. Large amounts of Ca, K and Na in biomass show catalytic effect on N2O reduction (Parvulesku et al., 1998; Veijonen et al., 2003).
3. Reaction between NH4+ and NOx
Among secondary methods, the most attention is currently paid to SCR, which consists of reducing NOx with ammonia at a temperature of 150 °C–450 °C in the presence of a catalyst. This process is referred to as selective, because ammonia has a greater chemical affinity for NOx than for O2. Among NOx contained in waste gases, 90%–95% is NO. In the presence of catalysts, the following reactions occur:
As a result of these reactions, nitrogen and water vapor are produced. Unfortunately, in these conditions, other reactions can occur leading to the production of undesirable products, e.g., N2O:
Another undesirable process is the direct oxidation of ammonia by oxygen:
This process is disadvantageous because harmful nitrogen oxides (NO, N2O, NO2) are formed and ammonia is consumed in reactions other than the DeNOx process. The catalyst used should be characterized by high activity in the DeNOx process and should demonstrate high selectivity to nitrogen. Small amounts of oxygen contained in the exhaust gases accelerate SCR NOx, but higher O2 contents have an unfavorable effect because they reduce the SCR rate. Secondary - dry methods of reducing NOx emissions also include adsorption on aluminosilicates (zeolites, activated carbon). The advantage of ammonia is the ease of diffusion even in very narrow pores of zeolitic catalysts. To check whether there are Brønsted or Lewis acid centers on the surface of the catalysts, ammonia should be adsorbed. The presence of NH4+ bands in the IR spectrum indicates the presence of Brønsted centers on the surface, while NH3L bands indicate the presence of Lewis centers. The adsorption method of purifying gases from NOx is characterized by high efficiency, but the cost of adsorbents is high and it requires regeneration of the column using steam or hot air (Najbar, 2000; Parvulesku et al., 1998).
A reaction takes place between the reduced form of ammonia and the oxidized nitrogen oxide on the catalyst surface:
Brønsted sites are important because:
1. They activate ammonia: they allow adsorption without oxidation (clean electron and proton transport).
2. They increase selectivity: the presence of too many Lewis sites can promote undesirable pathways (e.g., oxidation of NH3 to NO or N2O).
3. They compensate for the redox activity of metals: in many cases, the right combination of Brønsted sites and redox active centers determines the efficiency of the catalyst.
In the professional power industry, catalysts are used to improve the combustion process of coal fuel, and an example is the Reduxco catalyst from Dagas, which consists of organic compounds containing iron atoms. Desonox, a desulfurization and denitrification technology, should also be mentioned here. The role of the carrier for the metal catalyst is a synthetic zeolite. This process is based on the continuous elimination of SO3 from the system by binding it with combustion waste, e.g., ash and slag. This catalyst reduces the level of NOx in the reaction environment by reducing its amount in the exhaust gases as a result of catalyzing the high-temperature reaction of indirect CO oxidation using nitrogen oxides (Tic, 2014).
The advantage of using catalysts in the process of burning solid fuel is related to maintaining a constant high efficiency of the boiler and extending its operation and reducing costs related to repairs. Reducing the amount of hydrocarbons in the exhaust gases and unburned ash allows for more efficient fuel combustion and lack of deposition of unburned parts in the combustion chamber, which translates into greater boiler efficiency. And thanks to this, a reduction in harmful gas emissions into the atmosphere and reduction of corrosiveness of exhaust gases is also achieved. In addition, the introduction of a catalyst increases the heating efficiency of the installation and at the same time reduces CO and NOx emissions into the atmosphere.
6 Summary
Combustion of biomass, especially herbaceous biomass, can lead to the formation of deposits in heating devices burning biomass. Both straw and other herbaceous plant materials cause rapid contamination of heat exchange surfaces, also causing the formation of deposits on the surfaces of heating devices. However, this problem is not limited to straw raw materials. Other fuels that, due to their chemical composition, exhibit different combustion behavior than other types of biomass are biomass from industrial waste and energy plants, characterized by high ash content at a low melting point and high corrosion potential. The composition of the ash affects the softening and melting temperature of the ash. The value of the sintering and softening temperatures can differ significantly for the same type of herbaceous biomass, depending on the chemical composition of the ash, which is related to the plant species, cultivation method and soil type. The deposits are caused by fly ash, which is an undesirable effect of the combustion process. The chemical properties of the deposits correspond to KCl, the main compound forming the deposits. The formation of deposits also depends on the design and operation of the boiler. Also, high chlorine content in the fuel increases the release of potassium in the gas phase at relatively low temperatures. This causes serious problems related to slagging, fouling and agglomeration of the bed in the boiler, which can lead to the shutdown of the heating device. The parameter determining the ash’s slagging capacity is the iron oxide content. For coal fuels, a number of slagging and fouling indices have been developed to obtain and indicate the tendency of fuel ash to form deposits in combustion chambers or boilers based on the chemical composition, which shows a different behaviour than biomass fuels. In the case of co-firing of biomass at low levels, the mixed ash still consists mainly of aluminosilicate system, so that methods for assessing coal slagging can be used. Many existing biomass boilers are designed for exhaust gas temperatures of 900 °C or more. Lowering the combustion temperature to control deposits can lead to lower boiler operating parameters (heat exchange efficiency decreases), which has a negative impact on undesirable economic effects. Excessive fuel deposition is not only due to changes in the composition of the deposit depending on the type of raw material used, reflecting its structure, which is manifested in changing ash deposition mechanisms, but also from changes in the value of excess air during combustion. One way to reduce these undesirable phenomena is to use additives that can change the chemical composition of the ash, reduce the concentration of problematic compounds and increase the ash melting point. Natural silicates (e.g., kaolin clays) as a carrier are also an interesting raw material for the synthesis of catalysts and adsorbents. The use of natural material as a catalyst or substrate for the production of a catalyst not only reduces the costs associated with their production, but also makes the process used environmentally friendly and can make a significant contribution to sustainable development.
In recent years, a number of studies have been carried out on herbaceous plants aimed at better understanding the complex mechanism of ash transformation processes during the formation of slag in bottom ash, which also affects the release of solid particles during complete combustion. In addition, the ash melting behavior depends mainly on the relative concentrations of ash-forming elements, the total amount of critical compounds and their kinetic properties. In order to achieve sustainable and stable development, combustion technologies must be well understood and adapted to the requirements of a given fuel. Biomass, including herbaceous biomass, belongs to fuels characterized by a higher mineral content (K, P and Cl) and a high ash content at a low melting point and high corrosion potential.
Hence, as a result of the presented literature review, the following conclusions can be drawn:
1. Compounds such as K, Na, S, Ca in the form of oxides (K2O, Na2O, S2O, CaO) and hydroxides (KOH, NaOH, S(OH)2, Ca(OH)2) tend to lower the melting point of ashes containing minerals (SiO2), forming alkalis with very low melting points (approx. 700 °C).
2. Ash problems depend on the content of alkali oxides and their percentage share is different for different types of biomass. The percentage of SiO2 is dominant in grass ash, rice husks, sugar miscanthus and straw pellets, while K2O in grass ash and CaO (poplar, willow, soy pellets) and the sintering temperature affects the behavior of the ash.
3. Main causes of slag formation during biomass combustion include:
- Precipitation of crystals of alkali metal elements in biomass fuel,
- Ash melting, ash particle agglomeration (also with fuel) and sintering.
4. The chemical composition of the fuel and its origin influences the operation of heating devices, causing increased deposition of slag and ash in the furnace and thus increased wear of the metal components of the boiler.
5. The slagging indicators (SR), boiler heating surface contamination (B/A), oxide melting and sintering indicators (SI) are best suited for marking, which allows for the selection of the appropriate material for combustion and the composition of biomass raw material mixtures.
6. Among the additives, kaolin is the most effective, eliminating problems related to biomass combustion (it increases the melting point of biomass, its K capture capacity (higher Al content and Al/Si molar ratio of 0.89) reduces the concentration of potassium chlorides and the degree of contamination in the superheater area) and therefore reduces the amount of fine particles and deposit-forming substances, preventing slag formation. The main effects of ash transformation were attributed to the formation of two high-melting compounds KAISiO4 and KAISi2O6 (1,600 °C and 1,500 °C, respectively).
7. Kaolin has some catalytic properties and can act as a carrier for active catalytic components (metals, metal oxides) that can be used in NOx reduction processes. The versatility of kaolin applications, e.g., in the ceramic industry it is used for the production of ceramic-based products (ceramic plates, kiln charges, household articles) due to its favorable properties (temperature resistance, grain size and constant color).
8. The practical importance of natural support material (e.g., kaolin clays) as an acidic catalyst is a reason for great interest in methods for measuring the acidity of solid surfaces (Brønsted and Lewis acid sites), which are of fundamental importance for catalysis.
9. Particularly interesting are the catalysts containing V205 deposited on TiO2 and on a mixed oxide support TiO2-SiO2. In addition, the vanadium-titanium catalyst is characterized by high activity at low temperatures and high resistance to SO2 poisoning.
10. The introduction of a catalyst increases the heating efficiency of the installation and at the same time reduces CO and NOx emissions into the atmosphere.
In summary, available studies suggest that adding kaolin to solid fuels can reduce CO and NOx emissions, although the mechanisms of these processes are not yet fully understood. In the case of CO, a clear reduction in emissions is observed, which may be related to the catalytic effect of kaolin. The effect on NOx emissions is less clear and requires further research. Therefore, it would be advisable to conduct research on the effect of kaolin or urea in mixtures with selected herbal biomass on the combustion process and on exhaust gas emissions.
Author contributions
MD: Conceptualization, Formal Analysis, Methodology, Project administration, Validation, Visualization, Writing – original draft, Writing – review and editing. AK: Formal Analysis, Validation, Writing – review and editing, Project administration.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by the Polish Ministry of Science and Higher Education as part of statutory work number SUBB.WTR.19.042.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abioye, K. J., Noorfidza, Y. H., and Saeed, A. A. H. (2023). A brief review of solving biomass ash deposition with aluminum-silicate based additives and future perspective of kaolin. Chem. Eng. Trans. 98, 27–32. doi:10.3303/CET2398005
Ǻmand, L. E., Leckner, B., Eskilsson, D., and Tullin, C. (2006). Deposits on heat transfer tubes during co-combustion of biofuels and sewage sludge. Elsevier, Fuel 85, 1313–1322. doi:10.1016/j.fuel.2006.01.001
Andersson, V., Staničić, I., Kong, X., Leion, H., Mattisson, T., and Pettersson, J. B. C. (2024). Alkali desorption from ilmenite oxygen carrier particles used in biomass combustion. Elsevier, Fuel 359, 130400–130413. doi:10.1016/j.fuel.2023.130400
Arendt, P., Frandsen, J., Wang, G., Jensen, P. A., Wu, H., Frandsen, F. J., et al. (2016). Entrained flow reactor study of K-Capture by solid additives. Chem. Biochem. Eng., 762–766. doi:10.5071/24thEUBCE2016-2BV.1.50
Arvelakis, S., Vourliotis, P., Kakaras, E., and Koukios, E. G. (2001). Effect of leaching on the ash behavior of wheat straw and olive residue during fluidized bed combustion. Sci. Biomass Bioenergy 20 (6), 459–470. doi:10.1016/S0961-9534(01)00003-4
Bäfver, L. S., Rönnbäck, M., Leckner, B., Claesson, F., and Tullin, C. (2009). Particle emission from combustion of oat grain and its potential reduction by addition of limestone or kaolin. Fuel Process. Technol. 90, 353–359. doi:10.1016/j.fuproc.2008.10.006
Balint, R., Engblom, M., Niemi, J., Hupa, M., and Hupa, L. (2024). Superheater ash deposit ageing – impact of melt fraction on mophology and chemistry. Elsevier, Fuel 359, 1–20. doi:10.1016/j.fuel.2023.130386
Bapat, D. W., Kulkarni, S. V., and Bhandarkar, V. P. (1997). Proceedings of the 14th international conference on fluidized bed combustion. Vancouver, New York, 165–174. Available online at: https://www.osti.gov/biblio/355813 (Accessed April 12, 2024).
Basu, P., Kefa, C., and Jestin, L. (2000). Boilers and burners: design and theory. New York: Springer-Verlag.
Batir, O., Selçuk, N., and Kulah, G. (2019). Effect of kaolin addition on alkali capture capability during combustion of olive residue. Comb. Sci. Technol. 191 (1), 43–53. doi:10.1080/00102202.2018.1452376
Baxter, L. L., Miles, T. R., Miles, T. R., Jenkins, B. M., Milne, T., Dayton, D., et al. (1998). The behavior of inorganic material in biomass-fired power boilers: field and laboratory experiences. Fuel Process. Technol. 54, 47–78. doi:10.1016/S0378-3820(97)00060-X
Baxter, X. C., Darvell, L. I., Jones, J. M., Barraclough, T., Yates, N. Y., and Shield, I. (2014). Miscanthus combustion properties and variations with miscanthus agronomy. Fuel 117, 851–869. doi:10.1016/j.fuel.2013.09.003
Berlanga, C., and Ruiz, J. A. (2013). Study of corrosion in a biomass boiler. Hindawi. J. Chem. 1-8, 272090. doi:10.1155/2013/272090
Biedermann, F., and Obernberger, I. (2005). Ash-related during biomass combustion and possibilities for a sustainable ash utilisation. Inst. Process Part. Eng. 6690, 1–8.
Blomberg, T. E. (2007). Free alkali-Index for optimizing the fuel mixture in biomass Co-Firing. Heat Exch. Fouling Clean. VII, RP5 14, 97–102. Available online at: https://www.researchgate.net/publication/254591455 (Accessed March, 2024).
Blondeau, J. (2013). Ivestigation of pulverised biomass combustion: detailed modeling of particle pyrolysis and experimental analysis of ash deposition. Universit´e Cathol. Louvain, 1–296.
Boström, D., Grimm, A., Boman, Ch., Björnbom, E., and Öhman, M. (2009). Influence of kaolin and calcite additives on ash transformations in small-scale combustion of oat. Energy Fuels 23 (10), 5184–5190. doi:10.1021/ef900429f
Botić, T., Gvero, P., Drljača, D., Borković, A., Dragić, D., and Rakulj, S. (2022). Testing of small household biomass boilers from the aspect of waste gas emissions. STED J. 4 (2), 12–20. doi:10.7251/STED2202012B
Bryers, R. W. (1996). Fireside slagging, fouling, and high-temperature corrosion of heat-transfer surface due to impurities in steam-raising fuels. Prog. Energy Combust. Sci. 22, 29–120. doi:10.1016/0360-1285(95)00012-7
Capablo, J. (2016). Formation of alkali salt deposits in biomass combustion. Fuel Process. Technol. 153, 58–73. doi:10.1016/j.fuproc.2016.07.025
Capablo, J., Jensen, P. A., Pedersen, K. H., Hjuler, K., Nikolaisen, L., Backman, R., et al. (2009). Ash properties of alternative biomass. Energy Fuels 23, 1965–1976. doi:10.1021/ef8008426
Chen, S., Cheng, M., Xu, J., Liu, X., Yu, D., and Xu, M. (2021). Numerical analysis on reduction of ultrafine particulate matter by a kaolin additive during pulverized coal combustion. Energy Fuels 35, 9538–9549. doi:10.1021/acs.energyfuels.1c00773
Chen, Ch., Bi, Y., Feng, J., Huang, Y., Huang, J., and Huang, H. (2022). Study on the slagging tendency estimation of biomass fuel combustion with different additives and pretreatment processes. Elsevier, Energy 239, 122460. doi:10.1016/j.energy.2021.122460
Cichy, W. (2013). “Materiały lignocelulozowe jako alternatywne źródło biopaliw stałych,” in Wydawnictwo instytutu technologii drewna. Poznań.
Clery, D. S., Mason, P. E., Rayner, C. M., and Jones, J. M. (2018). The effects of an additive on the release of potassium in biomass combustion. Fuel 214, 647–655. doi:10.1016/j.fuel.2017.11.040
Corcoran, A., Marinkovic, J., Lind, F., Thunman, H., Knutsson, P., and Seemann, M. (2014). Ash properties of ilmenite used as bed material for combustion of biomass in a circulating fluidized bed boiler. Energy Fuel 28 (12), 7672–7679. doi:10.1021/ef501810u
Corcoran, A., Knutsson, P., Lind, F., and Thunman, H. (2018). Comparing the structural development of sand and rock ilmenite during long-term exposure in a biomass fired 12 MWth CFB-Boiler. Fuel Process. Technol. 171, 39–44. doi:10.1016/j.fuproc.2017.11.004
Davidsson, K. O., Åmand, L. E., Steenari, B. M., Elled, A. L., Eskilsson, D., and Leckner, B. (2008). Countermeasures against alkali-related problems during combustion in a circulating fluidized bed boiler. Elsevier, Chem. Eng. Sci. 21, 5314–5329. doi:10.1016/j.ces.2008.07.012
Dell’Antonia, D., Pergher, G., Cividino, S. R. S., Colantoni, A., Ceechini, M., Monarca, D., et al. (2013). Characterization of biomass emissions and potential reduction in small-scale pellet boiler. Univ. degli studi Udine, 1–17.
Deng, L., Jin, X., Long, J., and Che, D. (2019). Ash deposition behaviors during combustion of raw and water washed biomass fuels. J. Energy Inst. 92 (4), 959–970. doi:10.1016/j.joei.2018.07.009
Dyjakon, A. (2012). Analysis of slagging and fouling propensities of biofuels in terms of their combustion and Co-Combustion in the boilers. Agr. Eng. 4 (140), 5–18.
Escobar, I., and Műller, M. (2007). Alkali removal at about 1400 oC for the pressurized pulverized coal combustion combined cycle. Sorbents and sorption mechanisms. Am. Chem. Soc. Publ. Energy Fuels 21 (2), 735–743. doi:10.1021/ef060305k
Funcia, I., Bimbela, F., Gil, J., and Gandia, L. M. (2020). Application of a modeling tool to describe fly ash generation, composition, and melting behawior in a wheat straw fired commercial power plant. Processes 8 (1510), 1–20. doi:10.3390/pr8111510
Garba, M. U., Ingham, D. B., Ma, L., Porter, R. T. J., Pourkashnian, M., Tan, H. Z., et al. (2012). Prediction of potassium chloride sulfation and its effect on deposition in biomass-fired boilers. Energy Fuels 26 (11), 6501–6508. doi:10.1021/ef201681t
Gehrig, M., Wöhler, M., Pelz, S., Steinbrink, J., and Thorwarth, H. (2019). Kaolin as additive in wood pellet combustion with several mixtures of spruce and short-rotation-coppice willow and its influence on emissions and ashes. Fuel 235, 610–616. doi:10.1016/j.fuel.2018.08.028
Generowicz, N., Makara, A., Kowalski, Z., and Kulczycka, J. (2023). Removing scale deposits from heating systems. Pol. J. Environ. Stud. 32 (6), 5433–5445. doi:10.15244/pjoes/169563
Gogolev, I., Pikkarainen, T., Kauppinen, J., Linderholm, C., Steenari, B. M., and Lyngfelt, A. (2021). Investigation of biomass alkali release in a dual circulating fluidized bed chemical looping combustion system. Fuel 297, 120743–13. doi:10.1016/j.fuel.2021.120743
Gollmer, Ch., Weigel, V., and Kaltschmitt, M. (2023). Emission mitigation by aluminum-silicate-based fuel additivation of wood chips with kaolin and kaolinite. Energies 16, 3095–17. doi:10.3390/en16073095
Guo, Q., Cheng, Z., Chen, G., Yan, B., Hou, L., and Ronsse, F. (2020). Optimal strategy for clean and efficient biomass combustion based on ash deposition tendency and kinetic analysis. J. Clean. Prod. 271, 122529. doi:10.1016/j.jclepro.2020.122529
Gvero, P., Botić, T., Drljaca, D., Sinik, A., Dragic, D., and Rakulj, S. (2020). “Investigation of the possibilities for reduction of ash melting problems in biomass combustion,” in Conference: 4th SEE SDEWES conference. Sarajevo, 1–7.
Hartmann, H., Reisinger, K., Turowski, P., and Rossmann, P. (2013). Handbuch Bioenergie-Kleinanlagen, Bundesmin-isterium für Ernährung, Landwirtschaft und Verbraucherschutz. Gulzow-Pruzen Fachagentur Nachwachsende Rohrst. E.v. (FNR).
He, Z., Liu, S., Wang, S., Liu, W., Li, Y., and Feng, X. (2022). Reduced pollutant emissions and slagging rate of biomass pellet combustion by optimizing the multilayer distribution of secondary air. ACS Omega 7 (33), 28962–28973. doi:10.1021/acsomega.2c02587
Hernik, B., and Wnorowska, J. (2022). Numerical research on combustion processes and deposit formation on the deposition probe in the pulverized drop chamber. Renew. Energy 187, 1–13. doi:10.1016/j.renene.2022.01.057
Horvat, I., and Dović, D. (2018). Combustion of agricultural biomass – issues and solutions. Trans. Famena XLII, 75–86. doi:10.21278/TOF.42Si107
Huelsmann, T., Mack, R., Kaltschmitt, M., and Hartmann, H. (2019). Influence of kaolinite on the PM emissions from small-scale combustion. Biomass Convers. Biorefinery 9, 55–70. doi:10.1007/s13399-018-0316-8
Ion, I. V., Mahu, R., Popescu, F., Mocanu, G., and Chivu, R. (2023). Prediction of tar formation in a biomass heating boiler. Eng. Univ. Bull. 1 (57), 117–124.
Izquierdo, M. T., De las Obras-Loscertales, M., de Diego, L. F., García-Labiano, F., Mendiara, T., Abad, A., et al. (2017). Mercury emissions from coal combustion in fluidized beds under oxy-fuel and air conditions: influence of coal characteristics and O2 concentration. Elsevier, Fuel Process. Technol. 167, 695–701. doi:10.1016/j.fuproc.2017.08.021
Jensen, P. A., Frandsen, F. J., Wu, H., and Glaborg, P. (2016). A review: fly ash and deposit formation in PF fired biomass boilers. Denmark: Technical University of Denmark, 1–8.
Jensen, P. A., Laxminarayan, Y., Wang, G., Schwarzer, L., Sander, B., Holm, J. K., et al. (2019). Final report: flexible use of biomass on PF fired power plants. ForskEL project no. 12150/EUDP 64018-003.
Jiao, Y., Tian, L., Yu, S., Song, X., Wu, Z., Wei, J., et al. (2023). AAEM species migration/transformation during Co-Combustion of carbonaceous feedstocks and synergy behavior on Co-Combustion reactivity: a critical review. Energies 16, 7473–17. doi:10.3390/en16227473
Kalisz, S. (2023). Optymalizacja procesu spalania i waloryzacja ubocznych produktów spalania dla wypełnienia założeń gospodarki o obiegu zamkniętym. Praca zbiorowa. Monogr. Gliw. Wydaw. Politechniki Śląskiej. doi:10.34918/86707
Kalisz, S., Ciukaj, Sz., Tymoszuk, M., and Kubiczek, H. (2015). Fouling and its mitigation in PC boilers Co-Firing forestry and agricultural biomass. Heat. Trans. Eng. 36 (7-8), 763–770. doi:10.1080/01457632.2015.954962
Kaniowski, W., Taler, J., Wang, X., Kalemba-Rec, I., Gajek, M., Mędrala, M., et al. (2022). Investigation of biomass, RDF and coal ash-related problems: impact on metallic heat exchanger surfaces of boilers. Fuel 326, 125122–11. doi:10.1016/j.fuel.2022.125122
Kassman, H. (2012). Strategies to reduce gaseous KCl and chlorine in deposits during combustion of biomass in fluidised bed boilers. Sweden: Chalmers University of Technology.
Kassman, H., Pettersson, J., Steenari, B. M., and Åmand, L. E. (2013). Two strategies to reduce gaseous KCl and chlorine in deposits during biomass combustion – injection of ammonium sulphate and co-combustion with peat. Elsevier, Fuel Process. Technol. 105, 170–180. doi:10.1016/j.fuproc.2011.06.025
Khalil, R. A., Todorovic, D., Skreiberg, O., Becidan, M., Backman, R., Goile, F., et al. (2012). The effect of kaolin on the combustion of demolition wood under well – controlled conditions. International solid waste association. Waste Manag. 30, 643–644. doi:10.1177/0734242X11427942
Kleinhans, U., Wieland, Ch., Frandsen, F. J., and Spliethoff, H. (2018). Ash formation and deposition in coal and biomass fired combustion systems: progress and challenges in the field of ash particle sticking and rebound behavior. Elsevier, Prog. Energy Combust. Sci. 68, 65–168. doi:10.1016/j.pecs.2018.02.001
KOBiZE (2017). “Krajowy ośrodek bilansowania i zarządzania emisjami,” in Monitorowanie emisji powstałych z zastosowania mocznika w EU ETS (np. W procesach odazotowania spalin). Warszawa.
Kowalczyk-Juśko, A. (2009). Popiół z różnych roślin energetycznych. Proceedings of EC opole, 3:1, 159–164.
Kowalczyk-Juśko, A. (2017). The influence of the ash from the biomass on the power boiler pollution. J.Ecol. Eng. 18 (6), 200–204. doi:10.12911/22998993/76897
Kowalczyk-Juśko, A., Marczuk, A., Dach, J., Szmigielski, M., Zarajczyk, J., Jóźwiakowski, K., et al. (2015a). Thermochemical and bio-chemical maize biomass conversion for power en-gineering. Przem. Chem. 94 (2), 178–181. doi:10.15199/62.2015.2.9
Kowalczyk-Juśko, A., Kościk, B., Jóźwiakowski, K., Marczuk, A., Zarajczyk, J., Kowalczuk, J., et al. (2015b). Effects of biochemical and thermochemical conversion of sorghum biomass to usable energy. Przem. Chem. 94 (10), 1838–1840. doi:10.15199/62.2015.10.39
Kraszkiewicz, A., Niedziólka, I., and Kachel, M. (2017). The chemical composition of ash from the plant biomass in terms of indicators to assess slagging and pollution of surface heating equipment. Fresenius Environ. Bull. 26, 6383–6389.
Książek, S., Kida, M., and Poszelnik, P. (2017). Możliwości katalitycznego zastosowania materiałów odpadowych. J. Civ. Eng. Environ. Archit. XXXIV (64), 55–62. doi:10.7862/rb.2017.81
Kubica, K., Jewiarz, M., Kubica, R., and Szlęk, A. (2016). Straw combustion: pilot and laboratory studies on a straw-fired grate boiler. Energy Fuels 30, 4405–4410. doi:10.1021/acs.energyfuels.5b02693
Kuropka, J. (2000). Oczyszczanie gazów. Laboratorium. Wrocław: Oficyna wydawnicza politechniki wrocławskiej.
Kuropka, J. (2001). Redukcja niekatalityczna tlenków azotu w spalinach z kotłów rusztowych. Ochr. Środ. 2 (81), 25–27.
Lalak, J., Martyniak, D., Kasprzycka, A., Żurek, G., Moroń, W., Chmielewska, M., et al. (2016). Comparison of selected parameters of biomass and coal. Int. Agrophys. 30, 475–482. doi:10.1515/intag-2016-0021
Laxminarayan, Y. (2018). Formation, sintering and removal ash deposits. DTU library. Denmark: Technical University of Denmark, 1–197.
Laxminarayan, Y., Jensen, P. A., Wu, H., Frandsen, F. J., Sander, B., and Glarborg, P. (2017). Deposit shedding in biomass-fired boilers: shear adhesion strength measurements. Am. Chem. Soc. Publ. 31 (8), 8733–8741. doi:10.1021/acs.energyfuels.7b01312
Li, F., Yu, B., Li, J., Wang, Z., Guo, M., Fan, H., et al. (2020). Exploration of potassium migration behavior in straw ashes under reducing atmosphere and its modification by additives. Renew. Energy 145, 2286–2295. doi:10.1016/j.renene.2019.07.141
Li, X., Wang, X., Wang, H., and He, F. (2023). Low-Temperature-solid combustion technology of biomass for pollution reduction: potentials and necessary fundamentals. ACS Omega 8, 43433–43441. doi:10.1021/acsomega.3c07274
Liao, Y., Wu, S., Chen, T., Cao, Y., and Ma, X. (2015). The alkali metal characteristic during biomass combustion with additives. Energy Procedia 75, 124–129. doi:10.1016/j.egypro.2015.07.209
Lide, D. R. (2005). CRC handbook of chemistry and physics. 85th Edition. Printed in the United States of America: CRC Press.
Lindberg, D., Backman, R., Chartrand, P., and Hupa, M. (2013). Towards a comprehensive thermodynamic database for ash-forming elements in biomass and waste combustion - current situation and future developments. Fuel Process. Technol. 105, 129–141. doi:10.1016/j.fuproc.2011.08.008
Livingston, W. R. (2016). The status of large scale biomass firing. The milling and combustion of biomass materials in large pulverised coal boilers. IEA Energy Technol. Netw., 1–88.
Lokare, S. S. (2008). “Mechanistic investigation of ash deposition,” in Pulverized-coal and biomass combustion. Provo, Utah: Brigham Young University BYU Scholars Archive, 1–156.
Loo, van S., and Koppejan, J. (2008). “Biomass ash characteristics and behaviour in combustion systems,” in Book: the handbook of biomass combustion and Co-firing (London, Sterling, VA: Earthscan).
Madhiyanon, T., Sathitruangsak, P., Sungworagarn, S., Fukuda, S., and Tia, S. (2013). Ash and deposit characteristics from oil-palm empty-fruit-bunch (EFB) firing with kaolin additive in a pilot-scale grate-fired combustor. Elsevier, Fuel Process. Technol. 115, 182–191. doi:10.1016/j.fuproc.2013.05.018
Madhiyanon, T., Sathitruangsak, P., Sungworagarn, S., and Udomman, T. (2020). Investigation of rice-straw-ash fouling/slagging and countermeasures using supplementary additives and co-firing with Si–al-rich coal in a pilot-scale grate-fired combustor. J. Energy Inst. 93 (5), 1848–1867. doi:10.1016/j.joei.2020.04.001
Maia, M. B., Alves do Nascimento, J. L., Da Silva, A. S., and Garcia dos Santos, I. M. (2025). Kaolin waste applied as support for photocatalytic materials. Sustainability 17, 1–18. doi:10.3390/su17041605
Maj, I., and Matus, K. (2023). Aluminosilicate clay minerals: Kaolin, bentonite, and halloysite as fuel additives for thermal conversion of biomass and waste. Energies 16, 4359–17. doi:10.3390/en16114359
Marinkovic, J., Thunman, H., Knutsson, P., and Seemann, M. (2015). Characteristics of olivine as a bed material in an indirect biomass gasifier. Chem. Eng. J. 279, 555–566. doi:10.1016/j.cej.2015.05.061
Meka, W., Szuhanszki, J., Finney, K., Gudka, B., Jones, J., Pourkashanian, M., et al. (2022). Modeling and evaluation of ash-forming element fate and occurrence in woody biomass combustion in an entrained-flow burner. ACS Omega 7, 16306–16322. doi:10.1021/acsomega.1c06445
Meng, F., Gupta, S., Yu, J., Jiang, Y., Koshy, P., Sorrel, Ch., et al. (2017). Effects of kaolinite addition on the thermoplastic behaviour of coking coal during low temperature pyrolysis. Fuel Process. Technol. 167, 502–510. doi:10.1016/j.fuproc.2017.08.005
Miccio, F., Murri, A. N., Medri, V., and Landi, E. (2019). Utilization of fireclay for preventing fluidized-bed agglomeration during biomass thermochemical processing. Ind. Eng. Chem. Res. 58 (51), 23498–23507. doi:10.1021/acs.iecr.9b06253
Miles, T. R., Baxter, L. L., Bryers, R. W., Jenkins, B. M., and Oden, L. L. (1996). Boiler deposits from firing biomass fuels. Elsevier, Biomass Bioenergy 10 (2–3), 125–138. doi:10.1016/0961-9534(95)00067-4
Mlonka-Mędrala, A., Magdziarz, A., Gajek, M., Nowińska, K., and Nowak, W. (2020). Alkali metals association in biomass and their impact on ash melting behaviour. Elsevier, Fuel 261, 1–17. doi:10.1016/j.fuel.2019.116421
Morris, J. D., Daood, S. S., and Nimmo, W. (2022). The use of kaolin and dolomite bed additives as an agglomeration mitigation method for wheat straw and miscanthus biomass fuels in a pilot-scale fluidized bed combustor. Renew. Energy 196, 749–762. doi:10.1016/j.renene.2022.06.151
Mroczek, K., Kalisz, S., Pronobis, M., and Sołtys, J. (2011). The effect of halloysite additive on operation of boilers firing agricultural biomass. Fuel Process. Technol. 92, 845–855. doi:10.1016/j.fuproc.2010.11.020
Naganuma, H., Ikeda, N., Ito, T., Matsuura, M., Nunome, Y., Ueki, Y., et al. (2013). Reduction mechanisms of ash deposition in coal And/or biomass combustion boilers. Fuel 106, 303–309. doi:10.1016/j.fuel.2012.11.017
Najbar, M. (2000). Fizykochemiczne metody badań katalizatorów kontaktowych. Kraków: Wydawnictwo Uniwersytetu Jagiellońskiego.
Nguyen, H. K., Song, B., Moon, J., Lee, J. G., Mun, T.-Y., Bae, D., et al. (2019). The effect of kaolin as bed material in an Oxy-CFB combustor. Conf. Fluid. XVI.
Niu, Y., Tan, H., and Hui, S. (2016). Ash-related issues during biomass combustion: alkali-Induced slagging, silicate melt-induced slagging (ash fusion), agglomeration, corrosion, ash utilization, and related countermeasures. Elsevier. Prog. Energy Combust. Sci. 52, 1–61. doi:10.1016/j.pecs.2015.09.003
Norizam, N. N. A. N., Szuhánszki, J., Ahmed, I., Yang, X., Ingham, D., Milkowski, K., et al. (2024). Impact of the blending of kaolin on particulate matter (PM) emissions in a biomass field-scale 250 kW grate boiler. Elsevier, Fuel 374, 1–8. doi:10.1016/j.fuel.2024.132454
Nørskov, L., Telschow, S., and Wu, H. (2010). Effect of ammonium sulphate addition on the combustion of high chlorine and potassium biomass in a 12 MWth CFB – ash and species balance. Environ. Sci. Eng. Chem., 1–14.
Nunes, L. J. R., Matias, J. C. O., and Catalão, J. P. S. (2016). Biomass combustion systems: a review on the physical and chemical properties of tke ashes. Elsevier, Renew. Sustain. Energy Rev. 53, 235–242. doi:10.1016/j.rser.2015.08.053
Nzihou, A., and Stanmore, B. (2013). The fate of heavy metals during combustion and gasification of contaminated biomass-A brief review. J. Hazard. Mater. 256, 56–66. doi:10.1016/j.jhazmat.2013.02.050
Odzijewicz, J. I., Wołejko, E., Wydro, U., Wasil, M., and Jabłońska-Trypuć, A. (2023). Utilization of ashes from biomass combustion. Energies 15 (9653), 9653–16. doi:10.3390/en15249653
Ӧhman, M., Boström, D., and Nordin, A. (2004). Effect of kaolin and limestone addition on slag formation during combustion of wood fuels. Energy Fuels 18, 1370–1376. 1. doi:10.1021/ef040025
Oladejo, J. M., Adegbite, S., Pang, Ch., Liu, H., Lester, E., and Wu, T. (2020). In-situ monitoring of the transformation of ash upon heating and the prediction of ash fusion behaviour of coal/biomass blends. Elsevier, Energy 199, 117330. doi:10.1016/j.energy.2020.117330
Pałaszyńska, K., and Juszczak, M. (2018). Gaseous emissions during agricultural biomass combustion in a 50 kW moving step grate boiler. Chem. Process Eng. 3 (2), 197–208. doi:10.24425/119109
Paneru, M., Babat, S., Maier, J., and Scheffknecht, G. (2016). Role of potassium in deposit formation during wood pellets combustion. Fuel Process. Technol. 141, 266–275. doi:10.1016/j.fuproc.2015.10.008
Parvulesku, V. I., Grange, P., and Delmon, B. (1998). Catalytic removal of NO. Catal. Today 46, 233–316. doi:10.1016/s0920-5861(98)00399-x
Plaza, P., Maier, J., Maj, I., Gądek, W., and Kalisz, S. (2019). Potassium and chlorine distributions in high temperaturę halloysite formed deposits. ICBT Pol. 82 (01011), 1–9. doi:10.1051/e3sconf/20198201011
Pronobis, M. (2005). Evaluation of the influence of biomass co-combustion on boiler furnace slagging by means of fusibility correlations. Biomass Bioenergy 28, 375–383. doi:10.1016/j.biombioe.2004.11.003
Rabaçal, M., and Costa, M. (2015). Particulate emissions from the combustion of biomass pellets. Chapter 7. WIT Trans. State-of-the-art Sci. Eng. 85, 101–135. doi:10.2495/978-1-84566-062-8/007
Repić, B. S., Mladenović, M. R., and Marinković, A. D. (2019). Investigation of ash deposit formation on heat transfer surface of boilers using coals and biomass. Therm. Sci. 23 (Suppl. 5), 1575–1586. doi:10.2298/TSCI180413285R
Roberts, L. J., Mason, P. E., Jones, J. M., Gale, W. F., Williams, A., Hunt, A., et al. (2019). The impact of aluminosilicate-based additives upon the sintering and melting behaviour of biomass ash. Biomass Bioenergy 127, 105284. doi:10.1016/j.biombioe.2019.105284
Rodriguez, J. L., Álvarez, X., Valero, E., Ortiz, L., De la Torre-Rodriguez, N., and Acuña-Alonso, C. (2020). Design of solid biofuels blends to minimize the risk of sintering in biomass boilers. J. Energy Inst. 93, 2409–2414. doi:10.1016/j.joei.2020.07.0156
Rodríguez, J. L., Álvarez, X., Valero, E., Ortiz, L., De la Torre-Rodríguez, N., and Acuña-Alonso, C. (2021). Influence of ashes in the use of forest biomass as source of energy. Fuel 283, 119256. doi:10.1016/j.fuel.2020.119256
Ronnback, M., Johansson, L., Claesson, F., Johansson, M., and Tullin, C. (2008). “Emission from small-scale combustion of energy grain and use of additives to reduce particle emissions,” in Proceeding of 16th European biomass conference and exhibition. Valencia, 1429–1435.
Royo, J., Canalis, P., and Quintana, D. (2022). Chemical study of bottom ash sintering in combustion of pelletized residua agricultural biomass. Fuel 310, 1–14. doi:10.1016/j.fuel.2021.122145
Royo, R., Canalis, P., Zapata, S., Gómez, M., and Bartolomé, C. (2022). Article ash behaviour during combustion of agropellets produced by an agro industry - part 2: chemical characterization of sintering and deposition. Energies 15 (4), 1–20. doi:10.3390/en15041499
Sandberg, J. (2011). Fouling in biomass fired boilers. Mälardalen university press dissertations, No. 116. Sweden: Mälardalen University.
Sandy Sharp, W. B. A. (2010). Superheather corrosion in biomass boilers: today’s science and technology. Industrial Technologies Program, 1–105. doi:10.2172/1048711
Sarbassov, Y., Duan, L., Jeremias, M., Manovic, V., and Anthony, E. J. (2017). SO3 formation and the effect of fly ash in a bubbling fluidised bed under oxy-fuel combustion conditions. Elsevier, Fuel Process. Technol. 167, 314–321. doi:10.1016/j.fuproc.2017.07.015
Sato, N., Okuhara, H., Wada, C., Matsunaga, and Y., and Ohno, E. (2022). Effects and countermeasures on deposit ash of biomass single fired power plant. IHI. Eng. Rev. 55 (2622), 1–13.
Schulze, K., Scharler, R., Telian, M., and Obernberger, I. (2020). Advanced modeling of deposit formation in biomass furnaces – investigation of mechanisms and comparison with deposit measurements in a small-scale pellet boiler. Impacts Fuel Qual., 1–14.
Ściążko, M., Zuwała, J., and Pronobis, M. (2006). Advantages and disadvantages of co-combustion of biomass in power boilers on the background of operating experience of the first year of co-combustion of biomass on an industrial scale. Energetyka 3, 207–220.
Shao, Y., Wang, J., Preto, F., Zhu, J., and Xu, Ch. (2012). Ash deposition in biomass combustion or Co-Firing for power/heat generation. Energies 5, 5171–5189. doi:10.3390/en5125171
Skodras, G., Palladas, A., Kaldis, S. P., and Sakellaropoulos, G. P. (2007). Cleaner co-combustion of lignite–biomass–waste blends by utilising inhibiting compounds of toxic emissions. Elsevier, Chemosph. 67, 191–197. doi:10.1016/j.chemosphere.2006.05.099
Sobieraj, J., and Kalisz, S. (2021). Wpływ dodatków paliwowych Na emisję tlenków azotu przy spalaniu biomasy agrarnej. Źródła ciepła i energii elektrycznej. INSTAL 3 (21), 22–25. doi:10.36119/15.2021.3.2
Sommersacher, P., Brunner, T., Obernberger, I., Kienzl, N., and Kanzian, W. (2013). Application of novel and advanced fuel characterization tools for the combustion related characterization of different wood/kaolin and straw/kaolin mixtures. Energy Fuels 27, 5192–5206. doi:10.1021/ef400400n
Stam, A. (2020). Ash related problems with biomass combustion – application to co-firing in large scale boilers. Netherlands. doi:10.3990/1.9789036550277
Stauber Alfredsson, M. (2018). “Effects of different fuels on combustion boiler processes. The analysis of alternative fuel mixtures,” in Degree project in engineering chemistry. Second Cycle, 30. Credits Stockholm, Sweden, 1–82.
Sun, Z., Lu, D. Y., Hughes, R. W., and Filippou, D. (2018). O2 uncoupling behaviour of ilmenite and manganese-modified ilmenite as oxygen carriers. Fuel Process. Technol. 169, 15–23. doi:10.1016/j.fuproc.2017.08.025
Tesfaye, F., Lindberg, D., Moroz, M., Hupa, M., and Hupa, L. (2022). Copper in biomass fuels and its effect on combustion processes. Minerals, Metals Mater. Ser. 2, 13–20. doi:10.1007/978-3-030-92559-8_2
Thomas, J., Cottrill, S., and Summerfield, I. (2018). Measurement of the in-situ performance of solid biomass boilers. Full technical report. Kiwa. Department for business. Energy Ind. Strategy.
Tic, W. (2014). System poprawy efektywności energetycznej i ekologicznej spalania paliw stałych. Chemik 68, 850–855.
Tissari, J., Sippula, O., Kouki, J., Vuorio, K., and Jokiniemi, J. (2008). Fine particle and gas emissions from the combustion of agricultural fuels fired in a 20 kW burner. J. Energy Fuels 22 (3), 2033–2042. doi:10.1021/ef700766y
Tortosa Masiá, A. (2010). Characterisation and prediction of deposits in biomass co-combustion. España: Universidad de Sevilla.
Vamvuka, D., and Kakaras, E. (2011). Ash properties and environmental impact of various biomass and coal fuels and their blends. Fuel Process. Technol. 92, 570–581. doi:10.1016/j.fuproc.2010.11.013
Vamvuka, D., Pitharoulis, M., Alevizos, G., Repouskou, E., and Pentari, D. (2009). Ash effects during combustion of lignite/biomass blends in fluidized bed. Elsevier, Renew. Energy 34 (12), 2662–2671. doi:10.1016/j.renene.2009.05.005
Vaniyambadi, A. (2015). “A fundamental study of the effect of alkali salts on deposition,” in Biomass boilers (University of Toronto), 1–78.
Vassiliev, S. V., Baxter, D., Andersen, L. K., and Vassileva, C. G. (2013a). An overview of the composition and application of biomass ash. Part 1. Phase-mineral and chemical composition and classification. Fuel 105, 40–76. doi:10.1016/j.fuel.2012.09.041
Vassiliev, S. V., Baxter, D., Andersen, L. K., and Vassileva, C. G. (2013b). An overview of the composition and application of biomass ash. Fuel 105, 19–39. doi:10.1016/j.fuel.2012.10.001
Vega-Nieva, D., Garcia-Maraver, A., and Ortiz, L. (2015). Slagging and fouling risks derived from the combustion of solid biofuels. Chapter 85, 137–147. doi:10.2495/978-1-84566-062-8/008
Veijonen, K., Vainikka, P., Järvinen, T., and Alakangas, E. (2003). Biomass Co-Firing – an efficient way to reduce greenhouse gas emissions. Bioenergy, 1–26.
Wang, L., Becidan, M., and Skreiberg, Ø. (2013). Testing of zeolite and kaolin for preventing ash sintering and fouling during biomass combustion. Chem. Eng. Trans. 35, 1159–1164. doi:10.3303/CET1335193
Wang, G., Jensen, P. A., Wu, H., Frandsen, F. J., Sander, B., and Glarborg, P. (2018a). Potassium capture by kaolin, part 1: KOH. Energy Fuels 32, 1851–1862. doi:10.1021/acs.energyfuels.7b03645
Wang, G., Jensen, P. A., Wu, H., Frandsen, F. J., Sander, B., and Glarborg, P. (2018b). Potassium capture by kaolin, part 2: K2CO3, KCl and K2SO4. Energy Fuels 32, 3566–3578. doi:10.1021/acs.energyfuels.7b04055
Wang, G., Jensen, P. A., Wu, H., Frandsen, F. J., Laxminarayan, Y., Sander, B., et al. (2018c). Potassium capture by coal fly ash: K2CO3, KCl and K2SO4. Fuel Process. Technol. 194, 106115–106139. doi:10.1016/j.fuproc.2019.05.038
Wang, G., Jensen, P. A., Wu, H., Frandsen, F. J., Laxminarayan, Y., Sander, B., et al. (2019). KOH capture by coal fly ash. Fuel 242, 828–836. doi:10.1016/j.fuel.2018.12.088
Wang, J., Wei, B., Li, X., Yang, W., Zhang, Ch., Mian, I., et al. (2020). Study on reduction characteristics of Fe species in coal ash under SNCR condition. Elsevier, Fuel 277, 118231. doi:10.1016/j.fuel.2020.118231
Wang, G., Naimi Funch Poulsen, J. N., Naimi Funch Poulsen, S., Jensen, P. A., and Jappe, F. F. (2022). Influence of kaolin and coal fly ash addition on biomass ash deposition in an entrained flow reactor. Fuel 313, 123041–14. doi:10.1016/j.fuel.2021.123041
Wang, L., Hustad, J. E., Skreiberg, Ø., Skjevrak, G., and Grønli, M. (2012). A critical review on additives to reduce ash related operation problems in biomass combustion applications. Energy Procedia 20, 20–29. doi:10.1016/j.egypro.2012.03.004
Wang, X., Liu, Y., Tan, H., Ma, L., and Xu, T. (2012). Mechanism research on the development of ash deposits on the heating surface of biomass furnaces. Ind. Eng. Chem. Res. 51 (39), 12984–12992. doi:10.1021/ie302009m
Warych, J. (1994). Oczyszczanie przemysłowych gazów odlotowych. Środowisko-ochrona. Gazy odlotowe. Gazy odlotowe – oczyszczanie. Ochrona atmosfery, zanieczyszczenia gazowe, przemysłowe gazy odlotowe. Inżynieria chemiczna. Warszawa: Wydawnictwo Naukowo-Techniczne, 485.
Wei, L., Wang, S., Liu, G., Hao, W., Liang, K., Yang, X., et al. (2022). Corrosion behavior of high-cr-ni materials in biomass incineration atmospheres. ACS Omega 7 (25), 21546–21553. doi:10.1021/acsomega.2c01223
Wnorowska, J., Gądek, W., and Kalisz, S. (2020). Statistical model for prediction of ash fusion temperatures from additive doped biomass. Energies 13 (6543), 6543–21. doi:10.3390/en13246543
Wolf, K. J., Műller, M., Hilpert, K., and Singheiser, L. (2004). Alkali sorption in second – generation pressurized fluidized – bed combustion. American chemical society publications. Energy Fuels 18 (6), 1841–1850. doi:10.1021/ef040009c
Wolf, K. J., Smeda, A., Müller, M., and Hilpert, K. (2005). Investigations on the influence of additives for SO2 reduction during high alkaline biomass combustion. Energy Fuels 19, 820–824. doi:10.1021/ef040081a
Wu, G., Seebold, S., Yazhenskikh, E., Hack, K., and Műler, M. (2018). Viscosity model for oxide melts relevant to fuel slags. Part 3: the iron oxide containing low order systems in the system SiO2–Al2O3–CaO–MgO–Na2O–K2O–FeO–Fe2O3. Fuel Process. Technol. 171, 339–349. doi:10.1016/j.fuproc.2017.09.002
Xiong, S., Burvall, J., Örberg, H., Kalen, G., Thyrel, M., Öhman, M., et al. (2008). Slagging characteristics during combustion of corn stovers with and without kaolin and calcite. Energy Fuels 22 (5), 3465–3470. doi:10.1021/ef700718j
Xu, L., Liu, J., Kang, Y., Miao, Y., Ren, W., and Wang, T. (2014). Safely burning high alkali coal with kaolin additive in a pulverized fuel boiler. Energy Fuels 28, 5640–5648. doi:10.1021/ef501160f
Xu, Y., Liu, X., Wang, H., Zeng, X., Zhang, Y., Han, J., et al. (2018). Influences of In-Furnace kaolin addition on the formation and emission characteristics of PM2.5 in a 1000 MW coal-fired power station. Environ. Sci. Technol. 52, 8718–8724. doi:10.1021/acs.est.8b02251
Xu, J., Wang, J., Du, Ch., Li, S., and Liu, X. (2020). Understanding fusibility characteristics and flow properties of the biomass and biomass-coal ash samples. Elsevier, Renew. Energy 147, 1352–1357. doi:10.1016/j.renene.2019.09.066
Xu, Y., Liu, X., Qi, J., Zhang, T., Xu, M., Fei, F., et al. (2021). Compositional and structural study of ash deposits spatially distributed in superheaters of a large biomass-fired CFB boiler. Front. Energy 15 (2), 449–459. doi:10.1007/s11708-021-0734-3
Yang, J., Zhao, Y., Zhang, S., Liu, H., Chang, L., Ma, S., et al. (2017). Mercury removal from flue gas by magnetospheres present in fly ash: role of iron species and modification by HF. Elsevier, Fuel Process. Technol. 167, 263–270. doi:10.1016/j.fuproc.2017.07.016
Yongtie, C., Wenming, Y., Zhimin, Z., Mingchen, X., Boon, S. K., and Subbaiah, P. (2017). Modelling of ash deposition in biomass boilers: a review. Energy Procedia 143, 623–628. doi:10.1016/j.egypro.2017.12.737
Yongtie, C., Kunlin, T., Zhimin, Z., Wenming, Y., Hui, W., Guang, Z., et al. (2018). Modeling of ash formation and deposition processes in coal and biomass fired boilers: a comprehensive review. Elsevier, App. Energy 230, 1447–1544. doi:10.1016/j.apenergy.2018.08.084
Yu, J., Guo, Q., Gong, Y., Ding, L., Wang, J., and Yu, G. (2021). A review of the effects of alkali and alkaline earth metal species on biomass gasification. Fuel Process. Technol. 214, 106723. doi:10.1016/j.fuproc.2021.106723
Zając, G., Szyszlak-Bargłowicz, J., Gołębiowski, W., and Szczepanik, M. (2018). Chemical characteristics of biomass ashes. Energies 11, 1–15. doi:10.3390/en11112885
Zapata, S., Canalis, P., Royo, J., Gómez, M., and Bartolomé, C. (2023). Combustion performance of agropellets in an experimental fixed bed reactor versus a commercial grate boiler. Validation of ash behavior. ACS Omega 8, 29485–29499. doi:10.1021/acsomega.3c03201
Zeng, T., Pollex, A., Weller, N., Lenz, V., and Nelles, M. (2018). Blended biomass pellets as fuel for small scale combustion appliances: effect of blending on slag formation in the bottom ash and pre-evaluation options. Elsevier, Fuel 212, 108–116. doi:10.1016/j.fuel.2017.10.036
Zhai, J., Burke, I. T., and Stewart, D. I. (2021). Beneficial management of biomass combustion ashes. Renew. Sustain. Energy Rev. 151, 111555–18. doi:10.1016/j.rser.2021.111555
Zhang, Z., Yao, J. G., Boot-Handford, M. E., and Fennell, P. S. (2018). Pressurised chemical-looping combustion of an iron-based oxygen carrier: reduction kinetic measurements and modelling. Elsevier, Fuel Process. Technol. 171, 205–214. doi:10.1016/j.fuproc.2017.11.018
Zhang, H., Yu, Ch., Luo, Z., and Li, Y. (2020). Investigation of ash deposition dynamic process in an industrial biomass CFB boiler burning high-alkali and low-chlorine fuel. Energies 13 (1092), 1–11. doi:10.1039/D0RA04370B
Zhao, Z., Li, W., Qiu, J., Wang, X., and Li, B. (2006). Influence of Na and Ca on the emission of NOx during coal combustion. Elsevier, Fuel 85, 601–606. doi:10.1016/j.fuel.2005.09.001
Zhou, C., Rosén, C., and Engvall, K. (2016). Biomass oxygen/steam gasification in a pressurized bubbling fluidized bed: agglomeration behawior. Elsevier, App. Energy 172, 230–250. doi:10.1016/j.apenergy.2016.03.106
Zhou, Ch., Rosén, Ch., and Engvall, K. (2017). Selection of dolomite bed material for pressurized biomass gasification in BFB. Fuel Process. Technol. 167, 511–523. doi:10.1016/j.fuproc.2017.01.008
Keywords: biomass, heating devices, ash, deposits, slagging
Citation: Dula M and Kraszkiewicz A (2025) A review: problems related to ash deposition and deposit formation in low-power biomass-burning heating devices. Front. Energy Res. 13:1652415. doi: 10.3389/fenrg.2025.1652415
Received: 29 June 2025; Accepted: 25 September 2025;
Published: 17 October 2025.
Edited by:
Muhammad Aziz, The University of Tokyo, JapanCopyright © 2025 Dula and Kraszkiewicz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Małgorzata Dula, bWFsZ29yemF0YS5kdWxhQHVwLmx1Ymxpbi5wbA==
 Małgorzata Dula
Małgorzata Dula Artur Kraszkiewicz
Artur Kraszkiewicz