- Department of Publication and Extension, Kampala International University, Kampala, Uganda
With the growing world demand for sustainable and carbon-neutral energy sources, microalgae have surfaced as a promising source of next-generation biofuels based on their high lipid content, fast growth rate, and their ability to grow on wastewater and carbon dioxide (CO2). Nonetheless, other constraints, including nutritional requirements, threats of contamination, and expensive production processes, make up-scaling challenging. Synthetic biology and microbial ecology have recently allowed engineers to develop, design, and grow synthetic microbiomes, custom microbe communities that can increase microalgal biomass yield, support nutrient reuse, and promote metabolic stability. This mini-review examines the synergistic concept of integrative hybrid biofactories, where microalgae are cultivated concomitantly with designed microbiomes in regulated photobioreactor cultures to realize better biofuel production and environmental sustainability. A particular focus is put on pathway modeling with the help of AI, co-metabolic interactions, and overall system optimization. Putting this discussion into the context of the greater circular carbon economy, the review shows new advances, techno-economic considerations, and prospects on how to scale hybrid systems up to industrial scale.
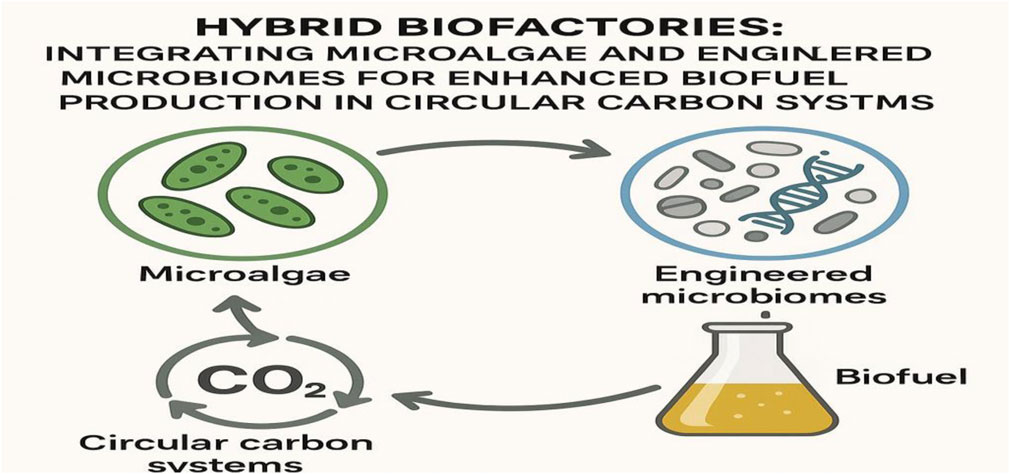
GRAPHICAL ABSTRACT | The graphical abstract illustrates the integration of microalgae and engineered microbiomes to form hybrid biofactories for enhanced biofuel production within a circular carbon system.
1 Introduction
The escalating global energy demand, coupled with the urgent need to reduce greenhouse gas emissions, has driven significant research into renewable and low-emission alternatives to conventional fossil fuels (Raimi and Newell, 2024). Among the bioenergy options, biodiesel stands out as a cleaner, renewable substitute widely applicable in compression ignition engines due to its favorable combustion properties and biodegradability (Balat, 2011). Next-generation biodiesel technologies are rapidly evolving to improve production efficiency, sustainability, and economic viability, addressing longstanding challenges associated with feedstock availability, energy input, and environmental impacts (Mehra et al., 2021). Recent techno-economic assessments reveal promising breakthroughs in integrating novel feedstocks, such as non-edible vegetable oils and waste-derived oils, alongside advanced conversion processes to enhance biodiesel yields while minimizing lifecycle carbon footprints (Singh et al., 2024). A variety of non-edible vegetable oils, including sesame oil, jatropha, and karanja, have been explored as alternative biodiesel feedstocks, demonstrating good engine compatibility and emissions profiles comparable to petroleum diesel (Patel et al., 2022; Sharma et al., 2023). For instance, biodiesel produced from sesame oil has undergone extensive characterization and performance evaluation, showing potential as a sustainable diesel substitute in compression ignition engines with reduced particulate emissions and improved lubricity (Mehra and Pant, 2021; Patel et al., 2022). Similarly, renewable biofuels such as n-octanol have been assessed for their energy, exergy, environmental, and sustainability metrics, highlighting their advantages in engine efficiency and lower pollutant emissions when used as blends or pure fuels (Mehra et al., 2023; Kumar et al., 2025).
Despite these advances, the sustainability of biodiesel production remains constrained by competition for arable land, food security concerns, and feedstock supply limitations inherent to first- and second-generation biofuels (Tilman et al., 2009; Naik et al., 2010). This has propelled the exploration of third-generation biofuels derived from microalgae, which offer superior photosynthetic efficiency, rapid biomass accumulation, and the ability to grow on non-arable land using saline or wastewater resources, thus avoiding direct competition with food crops (Chisti, 2007; Khan et al., 2018). Microalgae-based biodiesel production also aligns well with integrated waste management and carbon capture strategies, fostering circular bioeconomy approaches (Ugwu et al., 2025). Nonetheless, commercialization of microalgal biodiesel faces significant hurdles, including high capital and operational costs, substantial nutrient demands, and sensitivity to environmental fluctuations and contamination during scale-up (Shuba and Kifle, 2018). To overcome these limitations, hybrid biological systems that integrate microalgae with engineered microbial consortia are gaining attention (Mehra and Goel, 2025). These synthetic or natural microbiomes can enhance nutrient recycling, promote cooperative metabolic pathways, and suppress contamination, and increase lipid accumulation, thereby improving biofuel yields and system robustness (Mehra et al., 2025; Ramanan et al., 2016).
This review critically examines such hybrid biofactories within the framework of the Circular Carbon Economy (CCE), highlighting how advances in systems biology, synthetic biology, and artificial intelligence enable the design of optimized, resilient biofuel production platforms. By valorizing industrial and municipal waste streams, sequestering CO2 biologically, and creating synergistic microbial interactions, hybrid biofactories represent a promising paradigm for sustainable and scalable biofuel production aligned with global decarbonization goals (Zabala, 2021; Viswanathan et al., 2022; Arun et al., 2020).
2 Methodology
2.1 Review type
This mini-review follows a systematic-narrative literature review approach to ensure comprehensive, critical, and integrative coverage of the relevant literature addressing the integration of microalgae and engineered microbiomes for enhanced biofuel production. The method combines the systematic rigor of evidence-based literature identification with the narrative flexibility needed to synthesize interdisciplinary advancements in synthetic biology, systems engineering, and algal biotechnology.
2.2 Search strategy and keywords
To identify relevant studies, a structured search was conducted across major academic databases, including Scopus, Web of Science, PubMed, Science Direct, and Google Scholar. The literature search was limited to publications from 2010 to 2024 to ensure the inclusion of both foundational research and recent innovations in the field. Search queries were developed using a combination of controlled vocabulary (e.g., MeSH terms) and free-text keywords, applied with Boolean operators such as AND and OR to improve both precision and comprehensiveness. The search included terms such as “Microalgae” AND “biodiesel production,” “Genetic engineering” OR “CRISPR” AND “lipid accumulation,” “Transesterification” AND “microalgal oil,” and “Biofuel” AND “systems biology.” Additional terms included “Renewable energy” AND “microalgae optimization,” “Hybrid biofactories” AND “synthetic microbiomes,” “Algae–bacteria consortia” AND “carbon recycling,” and “AI modeling” AND “bioreactor optimization.” These search strings were adapted as necessary for each database to optimize the retrieval of relevant literature aligned with the scope of hybrid biofactory systems and circular carbon economy frameworks.
2.3 Inclusion and exclusion criteria
Studies were included if they met the following criteria: (i) peer-reviewed journal articles, reviews, or conference papers; (ii) focused on microalgae-based biofuel systems, particularly those involving co-cultivation with microbial consortia, synthetic microbiomes, or genetic enhancements aimed at increasing lipid yield; (iii) addressed aspects of bioreactor design, transesterification optimization, or metabolic modeling relevant to hybrid biofactories. Exclusion criteria were applied to eliminate sources that did not meet scientific or thematic relevance. These included non-English publications, unpublished theses, and non-peer-reviewed grey literature. Studies lacking experimental data, modeling results, or theoretical frameworks related to the integrative hybrid systems were also excluded.
3 Microalgae as a cornerstone of biofuel systems
3.1 Advantages over terrestrial crops
Microalgae have emerged as one of the most promising candidates for sustainable biofuel production, primarily due to their distinct advantages over traditional land-based crops. Unlike conventional biofuel feedstocks such as maize, soybean, or sugarcane, microalgae do not require arable land and can thrive in diverse environments, including freshwater, brackish water, seawater, and even wastewater (Singh and Gu, 2010). This flexibility makes them especially valuable in avoiding the long-standing food-versus-fuel dilemma that has limited the scalability of first-generation biofuels (Tilman et al., 2009). One of the most compelling attributes of microalgae is their high photosynthetic efficiency. They can convert solar energy into biomass much faster than terrestrial plants. Under ideal conditions, some microalgal species can double their biomass in just one to 3 days, whereas land-based crops often take several months to reach maturity (Chisti, 2007). Furthermore, microalgae are powerful tools for carbon capture. In integrated bioenergy systems, they are capable of absorbing and sequestering carbon dioxide at rates 10 to 50 times higher than terrestrial bioenergy crops (Wang et al., 2008). These unique features position microalgae as a cornerstone of next-generation biofuel technologies, particularly those aimed at climate resilience and circular carbon strategies. The key benefits of microalgae over conventional biofuel crops are summarized in Table 1.
3.2 Lipid productivity, growth kinetics, and wastewater valorization
Microalgae have emerged as a promising and sustainable source for biodiesel production, mainly due to their ability to accumulate high amounts of neutral lipids especially triacylglycerols (TAGs) which can be directly converted into biodiesel through transesterification. Under nutrient stress, certain strains like Nannochloropsis, Chlorella, and Botryococcus braunii can store over 50% of their dry cell weight as lipids, making them highly efficient lipid producers (Griffiths and Harrison, 2009; Hu et al., 2008). Compared to traditional oil crops, microalgae are incredibly productive. For example, Chlorella vulgaris and Nannochloropsis have been reported to yield between 20,000 and 80,000 L of oil per hectare annually, depending on cultivation conditions (Chisti, 2007; Mata et al., 2010). This dwarfs the yields from land-based crops: soybeans yield about 446 L/ha, rapeseed around 1,190 L/ha, and even oil palm, the most productive terrestrial crop, only yields roughly 5,950 L/ha (Brennan and Owende, 2010). These differences underscore the potential of microalgae as a scalable feedstock for biodiesel, particularly in regions where land and freshwater resources are limited.
In addition to their impressive productivity, microalgae offer flexibility in cultivation. Through systems like open raceway ponds and closed photobioreactors (PBRs), environmental conditions such as light, CO2 concentration, and nutrient availability can be carefully controlled to optimize biomass and lipid production (Mata et al., 2010). A recent study by Lopez-Rodriguez et al. (2023) demonstrated that Nannochloropsis gaditana grown in a vertical flat-panel PBR under high CO2 enrichment (5%) and optimized nitrogen dosing achieved a 52% increase in lipid content compared to non-optimized conditions. This shows how fine-tuning environmental factors, particularly using nutrient starvation strategies, can significantly enhance TAG accumulation, ideal for biodiesel applications. One of the most exciting developments in the field is the integration of microalgae with wastewater valorization. Microalgae can grow on various wastewater sources, municipal, industrial, and agricultural, by utilizing the nutrients present while simultaneously removing contaminants. This dual-purpose system supports both biofuel production and environmental remediation, aligning well with circular economy principles (Pittman et al., 2011; Wang et al., 2023; Zhang et al., 2024).
For instance, C. vulgaris and Scenedesmus obliquus cultivated in municipal wastewater have shown nitrogen removal efficiencies of up to 90%, along with significant reductions in phosphorus and pathogen levels (Li et al., 2022). Similarly, industrial wastewater, such as dairy and textile effluents, which are rich in organic matter has been successfully treated using Nannochloropsis species, while also producing lipids for biofuel (Patel et al., 2023). Even in agricultural runoff scenarios, microalgae help prevent eutrophication by absorbing excess fertilizers and degrading certain pesticides (Singh et al., 2024). Together, these capabilities make microalgae a central component of future sustainable biofuel platforms offering not just high yields and renewable energy, but also meaningful contributions to wastewater treatment and ecological restoration.
4 Engineered microbiomes in biofuel biotechnology
4.1 Synthetic consortia for nutrient recycling and lipid induction
In nature, microalgae rarely grow alone they often coexist with diverse communities of bacteria and fungi that can influence their growth, metabolism, and stress responses. This ecological insight has inspired the development of synthetically engineered microbial consortia deliberately assembled communities of algae and beneficial microbes designed to improve the performance of algal biofuel systems (Kazamia et al., 2012; Ramanan et al., 2016). These tailored consortia can perform crucial functions such as nitrogen fixation, phosphorus solubilization, and organic waste fermentation, significantly reducing the need for external nutrient inputs and improving the overall sustainability of biofuel production systems (Zhang et al., 2021).
Certain bacterial species including Azospirillum, Rhizobium, and Bacillus spp. have been shown to produce bioavailable nitrogen, growth hormones, and signaling molecules that enhance both biomass accumulation and lipid content in microalgae (Fuentes et al., 2016). These beneficial interactions lead to more robust cultures and higher yields of valuable bio-oil precursors. Interestingly, some microbial partners can also trigger stress responses such as nutrient starvation or oxidative stress that stimulate microalgae to produce more lipids, particularly triacylglycerols (TAGs), which are the key compounds for biodiesel production (Cho et al., 2015). By carefully engineering microbial consortia with specific metabolic traits, it’s possible to fine-tune algal cultivation systems for better nutrient efficiency, improved lipid yields, and increased resilience to environmental fluctuations. These synthetic microbiomes represent a powerful tool in optimizing next-generation biofuel platforms, bridging ecology, metabolic engineering, and sustainability.
4.2 Genetic and metabolic tools (CRISPR, quorum sensing, etc.)
Advances in genomic and metabolic technologies have greatly enhanced our ability to optimize microbial functions for biofuel production, especially at the consortium level. One of the most impactful tools is the CRISPR-Cas system, which has revolutionized precise genome editing in microalgae and bacteria. This technology allows researchers to introduce desirable traits such as improved carbon flow toward lipid synthesis, increased stress tolerance, and the secretion of beneficial metabolites (Ng et al., 2017; Nymark et al., 2016). For example, knocking out key starch biosynthesis genes in the model alga Chlamydomonas reinhardtii using CRISPR-Cas9 has successfully redirected energy storage from starch to lipid accumulation (Shin et al., 2016). In addition to genetic editing, synthetic microbial consortia can be controlled through quorum sensing (QS), a communication system that enables cells to coordinate gene expression based on population density or environmental signals. By engineering QS circuits, it is possible to synchronize behaviors such as nutrient exchange, optimizing light harvesting, or triggering lipid biosynthesis across the community (Simon et al., 2005).
Moreover, computational approaches like Flux Balance Analysis, a widely used systems biology tool, allow researchers to simulate and predict metabolic fluxes within complex multi-species networks under steady-state conditions. Genome-scale metabolic models help identify optimal pathways for metabolite production in these consortia (Zomorrodi and Segrè, 2016). Together, these technologies provide a powerful platform for designing robust, responsive, and high-performance synthetic microbiomes that complement microalgae in biofuel production. By leveraging the metabolic diversity of microbial communities along with precise genetic control, engineered consortia hold great promise for creating more efficient, sustainable, and scalable algal biofuel systems (Table 2).
5 Synergistic co-cultivation: microalgae–microbiome interactions
5.1 Mechanism behind the co-cultivation of algal consortia
Co-cultivation of algal consortia involves growing microalgae alongside other microorganisms such as bacteria, fungi, or even other algal species in the same environment. Instead of relying on a single species in isolation, this approach takes advantage of how different organisms naturally interact and support each other. These interactions can lead to better growth, more efficient nutrient use, increased tolerance to stress, and improved production of valuable compounds like lipids or pigments. One of the key mechanisms that makes co-cultivation effective is metabolic exchange. Microalgae, through photosynthesis, release oxygen and organic carbon compounds into the surrounding environment. In return, co-cultured bacteria often supply the algae with carbon dioxide through respiration, as well as essential nutrients such as nitrogen and phosphorus in forms that algae can easily absorb (Ramanan et al., 2016). Some bacteria even produce vitamins like vitamin B12 that many microalgae cannot synthesize on their own (Croft et al., 2005; Kazamia et al., 2012). This mutual support helps both organisms grow faster and survive better in changing or harsh conditions. Another important mechanism is nutrient recycling and enhancement. In co-cultures, bacteria can break down organic matter or complex nutrients in wastewater into simpler forms that algae can use for growth (Fuentes et al., 2016). Certain bacteria are also capable of fixing atmospheric nitrogen or solubilizing phosphate, which boosts nutrient availability without the need for chemical fertilizers (Xie et al., 2017). This is especially useful in large-scale algal production or wastewater-based cultivation systems.
Communication between microorganisms is also essential. Through a process called quorum sensing, microbes release signaling molecules to coordinate their behavior. These chemical signals can influence algal metabolism, including lipid biosynthesis, and may also trigger the formation of protective biofilms or other stress-resistance mechanisms (Subashchandrabose et al., 2011). For example, bacterial signals can increase algal resistance to oxidative stress or help stabilize the culture under nutrient-limited conditions. In addition to promoting growth and metabolic activity, co-cultivation systems offer greater ecological stability. In monocultures, algae are often vulnerable to contamination or invasion by harmful species, which can crash the entire culture. But in diverse consortia, the presence of multiple organisms competing for resources can suppress pathogens through a process known as competitive exclusion. Some bacteria also produce antimicrobial compounds that inhibit undesirable invaders (Brenner et al., 2008; Kim et al., 2020). More recently, researchers have started using synthetic biology tools to design engineered consortia with specific goals such as enhancing lipid production for biodiesel or boosting bioremediation capabilities. For example, engineered bacterial strains may be introduced to produce metabolic precursors that trigger higher lipid accumulation in algae or improve CO2 capture from industrial exhausts (Smith et al., 2010; Zhou et al., 2015). Therefore, the success of algal co-cultivation comes from how different organisms share resources, communicate chemically, and protect each other from environmental threats. These synergistic relationships not only improve biomass productivity but also make algal systems more sustainable and cost-effective for applications in biofuel production, wastewater treatment, and high-value bioproducts.
5.2 Effect of different types of wastewater on the cultivation of algal consortia
The type and composition of wastewater play a critical role in shaping the growth dynamics, biochemical profiles, and ecological interactions within algal consortia. Wastewaters vary widely in nutrient availability, organic and inorganic load, presence of toxicants, and microbial diversity. These factors can significantly influence how well algal consortia perform, particularly in terms of biomass productivity, nutrient removal efficiency, and value-added metabolite production. Municipal wastewater is one of the most commonly used types for algal cultivation. It typically contains high levels of nitrogen (mainly in the form of ammonium and nitrate), phosphorus, and organic carbon, making it a favorable medium for supporting both autotrophic algae and heterotrophic bacteria. The microbial diversity in municipal wastewater can further enhance synergistic interactions within the consortium, as many bacteria aid in nutrient solubilization or vitamin production essential for algal growth (Zhou et al., 2012). Studies have shown that co-cultivating microalgae with native bacteria in municipal effluents enhances nutrient uptake and biomass yield while reducing the chemical oxygen demand (COD) and biological oxygen demand (BOD) of the treated water (Ruiz-Marin et al., 2010; Ramanan et al., 2016).
In contrast, industrial wastewater such as that from textile, pharmaceutical, petrochemical, or food processing industries presents both opportunities and challenges. While certain types, such as dairy or brewery wastewater, are rich in biodegradable organic matter and nutrients, others may contain heavy metals, xenobiotics, or extreme pH values that can inhibit algal growth or disrupt microbial interactions (Liu et al., 2017). However, robust consortia with carefully selected or acclimated strains have demonstrated resilience under such stressors. For example, Chlorella and Scenedesmus species co-cultivated with metal-tolerant bacteria have shown promise in treating dye-laden textile effluents, simultaneously removing pollutants and accumulating lipids for biofuel production (Mahapatra et al., 2013). Agricultural wastewater, especially from livestock farms and aquaculture systems, contains high levels of ammonia, phosphate, and organic solids. These can stimulate rapid algal growth, but may also lead to inhibitory effects if the ammonia concentrations exceed tolerance levels. In co-cultivation systems, ammonia-oxidizing and denitrifying bacteria play a vital role in reducing nitrogen toxicity, thereby creating a more stable environment for algal photosynthesis (Santos et al., 2020). Moreover, algae-bacteria consortia in these systems can enhance the recovery of nutrients into biomass and reduce the eutrophication potential of effluents when discharged into natural water bodies (Wang et al., 2016).
Another emerging source is wastewater from anaerobic digestion (digestate), which is typically high in ammonium, volatile fatty acids, and residual organic carbon. Although digestate can be toxic to pure algal cultures, its complex composition is often better tolerated by microbial consortia. Certain bacterial species metabolize volatile fatty acids and generate CO2 that supports algal growth, while algae contribute oxygen to maintain aerobic niches (Coppens et al., 2016a; Coppens et al., 2016b). Co-cultivation in digestate can therefore be optimized by selecting strains with complementary metabolic profiles and tolerance thresholds. Lastly, synthetic or artificial wastewater is frequently used in experimental studies to simulate specific nutrient conditions or stress environments. These systems help researchers to systematically study algal-bacterial interactions without the variability of real wastewater. While not directly applicable to large-scale operations, findings from synthetic systems have informed the design of more resilient and efficient consortia for real-world applications (Chinnasamy et al., 2010). Therefore, the performance of algal consortia in wastewater-based cultivation systems is highly dependent on the type and quality of the wastewater used. Municipal and agricultural wastewaters are generally well-suited for consortia-based systems due to their balanced nutrient content, while industrial and digestate wastewaters require more careful selection of tolerant or engineered strains. Understanding these differences is essential for optimizing algal consortia for wastewater valorization, biomass production, and environmental sustainability.
5.3 Case studies of algae–bacteria/fungi consortia
Co-cultivating microalgae with bacteria or fungi in well-designed consortia is emerging as a practical and efficient strategy for improving biomass productivity, nutrient uptake, and lipid accumulation key parameters in biofuel production. These symbiotic systems mirror the ecological dynamics found in natural aquatic environments, where microalgae and microbes engage in reciprocal metabolic exchanges involving carbon compounds, nutrients, and signaling molecules (Ramanan et al., 2016). A notable example of this approach is the co-culture of Chlorella vulgaris with Azospirillum brasilense. In this system, the bacteria enhance nitrogen availability through diazotrophic activity (nitrogen fixation), which in turn supports increased algal growth and lipid storage two critical outcomes for biodiesel production (De-Bashan et al., 2004).
Similarly, Cho et al. (2015) demonstrated that co-culturing Scenedesmus obliquus with Bacillus species led to a significant increase in both biomass and chlorophyll content. This effect was largely attributed to bacterial production of indole-3-acetic acid (IAA), a natural plant hormone known to stimulate algal cell division and growth. Fungal-algal systems also offer unique advantages. For instance, Commault et al. (2013) reported that co-cultivating Chlorella pyrenoidosa with Aspergillus niger facilitated the spontaneous formation of pellets, which greatly simplified the harvesting process a typically resource-intensive step in microalgal cultivation. In addition to aiding separation, the fungus contributed to nutrient solubilization and reduced excess organic carbon in the system, thereby enhancing overall system stability. These examples show that engineered microbial consortia are more than just biological tools they act as bio-enhancers that help overcome major challenges in microalgal biofuel production. Whether by improving nutrient cycling, enhancing stress tolerance, or making harvesting more efficient, co-cultivation strategies bring us closer to scalable, cost-effective, and sustainable biofuel technologies.
5.4 Enhanced biomass, resilience, and process stability
The interactions between microalgae and microorganisms in co-cultivation systems can be highly synergistic, offering several advantages over traditional monoculture setups. When cultivated together, these organisms often enhance biomass production and improve system stability by recycling nutrients, producing antimicrobial compounds, and helping the system manage oxidative stress (Fuentes et al., 2016). One of the biggest drawbacks of monocultures whether algal or bacterial is their vulnerability to contamination, environmental stress, and nutrient imbalances. Co-cultures, on the other hand, create a more resilient system. They reduce the risk of process failure by distributing metabolic functions across different microbial partners, which helps buffer against fluctuations in pH, temperature, and light availability (Carney et al., 2014; Naseema Rasheed et al., 2023). A good example of this is the algae–bacteria consortia, where mutual metabolic support occurs. Algae, through photosynthesis, release oxygen and dissolved organic matter that bacteria can use. In return, bacteria provide carbon dioxide and, in some cases, fixed nitrogen to the algae creating a self-sustaining microenvironment with minimal external inputs (Kazamia et al., 2012). This mutualism lowers the need for additional fertilizers or gas inputs, reducing energy use and operational costs. Moreover, co-cultivation enhances the system’s adaptability to outdoor or large-scale environments, where conditions are often variable. The microbial community acts as a buffer, stabilizing the system during sudden shifts in environmental conditions, such as heat waves, nutrient drops, or pH changes. This robustness is crucial for reducing downtime and maintaining continuous production cycles (Naseema Rasheed et al., 2023). Therefore, designing and managing strategic algal–microbial partnerships is not just about increasing yields it's about building biologically efficient and environmentally consistent systems. These hybrid biofactories hold strong promise as the next-generation platform for sustainable biofuel production.
6 System integration and reactor design
6.1 Photobioreactor and bioreactor interfaces
The design and type of bioreactor used play a vital role in determining how productive and scalable an algal-microbial biofuel system can be. Among the different systems available, photobioreactors (PBRs) have become the industry standard for growing microalgae because they allow precise control over key factors like light distribution, CO2 delivery, temperature, and nutrient availability (Mata et al., 2010). PBRs come in open and closed formats. While open systems such as raceway ponds are cheaper to build and operate, they are more prone to contamination and environmental variability. In contrast, closed PBRs such as tubular, flat-panel, and column reactors offer better control over growing conditions, leading to higher biomass yields and reduced contamination risk, albeit at a higher cost (Ugwu et al., 2008). In hybrid biofactories that combine microalgae with bacteria or fungi, choosing the right bioreactor becomes even more critical. Microalgae need light to perform photosynthesis, while many bacteria or fungi grow best in dark, oxygen-rich environments. This contrast requires a more integrated reactor design, often involving a combination of systems. For instance, researchers have developed setups that use sequential photobioreactors followed by dark fermentation chambers, allowing different microbial communities to perform their metabolic functions in a coordinated and spatially separated way (Chen et al., 2022). This arrangement enhances synergy between phototrophic (light-loving) and heterotrophic (organic matter-consuming) organisms, improving overall system performance.
Recent innovations have taken things even further. Membrane photobioreactors (MPBRs) and hollow-fiber units have been developed to mimic the natural interactions between algae and bacteria more closely. These systems offer improved gas exchange, nutrient transfer, and biomass separation between compartments. They also allow for better control over variables like hydraulic retention time and nutrient gradients, which helps stabilize the system and boost its efficiency (Javed et al., 2024). In essence, selecting and customizing bioreactor designs for algal-microbial consortia isn’t just about maximizing algal growth it's about creating a balanced environment where different organisms can thrive together and contribute to sustainable, high-yield biofuel production.
6.2 Biofilm-based vs suspended systems
Choosing the right cultivation strategy, suspended versus biofilm systems, is a major design consideration in algal microbiome-based biofuel production. Suspended systems, like raceway ponds or photo bioreactors, allow algal and microbial cells to freely float in the culture medium. This configuration ensures uniform nutrient distribution and high mass transfer rates. However, it comes with a major drawback: harvesting. Because the biomass in these systems is highly diluted, separating it from the medium typically requires energy-intensive processes such as centrifugation or flocculation (Christenson and Sims, 2011). These steps significantly increase overall energy consumption and operational costs. On the other hand, biofilm-based systems offer some clear advantages. In these setups, microalgae and their microbial partners attach to surfaces, forming dense biofilms that are easier to harvest and require minimal dewatering (Sandar et al., 2019). This leads to greater biomass concentration and reduced energy input. Moreover, the close physical proximity of different microbial species within biofilms enhances metabolic exchange, promotes resilience against environmental stress, and reduces the risk of contamination (Wang et al., 2008). Innovations like Rotating Algal Biofilm Reactors (RABRs) and multilayer photogranular systems have demonstrated impressive performance, particularly in wastewater-based cultivation, where they’ve shown high lipid content and efficient nutrient removal (Zhou and Liu, 2014).
However, biofilm systems aren’t without their limitations. One key issue is that light penetration through thick biofilms can be light, reducing photosynthetic efficiency. They also tend to experience fouling and spatial heterogeneity, which can result in uneven growth and nutrient uptake. These problems may be addressed through the development of advanced materials and automation to maintain uniform biofilm growth and system efficiency. A broader challenge lies in the scalability of these systems. Suspended systems, while more productive per unit volume, scale horizontally and thus require more land and water. Biofilm systems, by contrast, scale vertically but are more complex to engineer. Both have trade-offs in terms of biomass quality and harvesting ease (Rajpoot et al., 2025). For example, Spirulina-derived biofuel blends with plastic oil have been found to reduce particulate matter and CO2 emissions, but they also increase nitrogen oxides (NOx) and lower brake thermal efficiency (BTE) (Rajpoot et al., 2024). In contrast, biodiesel from peppermint and tamarind sources has shown improvements in fuel economy and reductions in NOx, though performance varied depending on engine conditions (Rajpoot et al., 2024). These inconsistencies underscore the need for feedstock-specific assessments and robust life-cycle analyses. Current literature still lacks a comprehensive evaluation of the operational and economic implications of each cultivation mode. Ultimately, hybrid cultivation strategies combining the precise control of suspended systems with the harvesting benefits of biofilms may offer the best solution. When supported by techno-economic analysis and environmental impact assessments, such hybrid systems could strike the right balance between efficiency, scalability, and sustainability (Rajpoot et al., 2025; Rajpoot et al., 2024).
7 AI-driven optimization and metabolic modeling
7.1 Machine learning in pathway prediction and system control
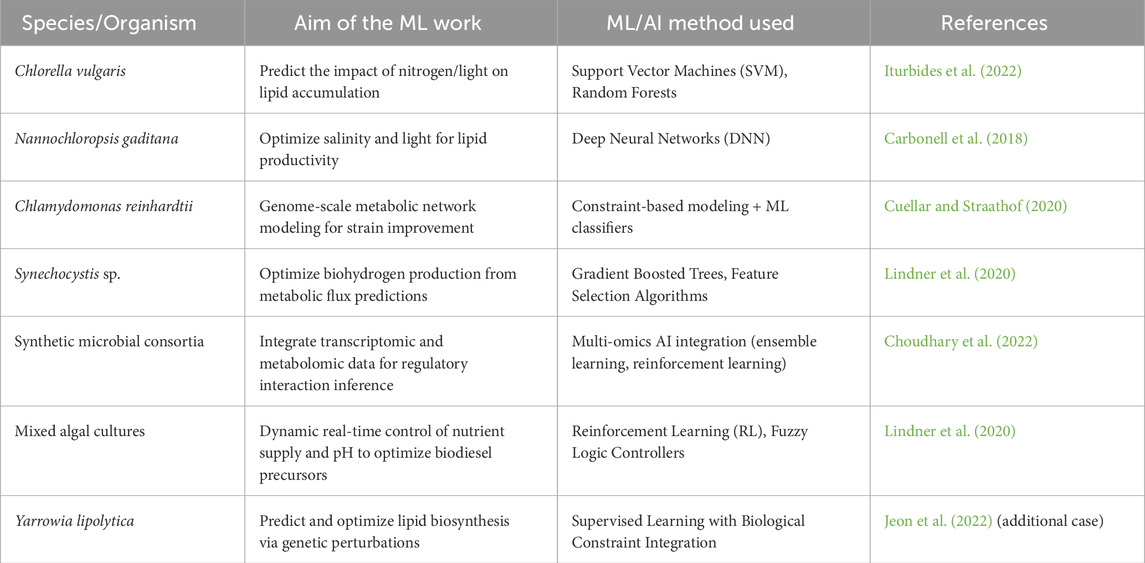
The integration of machine learning (ML) and artificial intelligence (AI) is significantly reshaping the way we design and optimize algal biofuel production systems. These technologies enable data-driven modeling, predictive analytics, and real-time adaptive control, making biological processes more efficient and responsive (Table 3). In the context of hybrid biofactories, particularly those that combine microalgae and engineered microbial consortia, AI plays a pivotal role in understanding and managing complex biological interactions. One of the most impactful applications of AI is in metabolic network prediction and optimization. By analyzing large datasets, machine learning algorithms can identify the best culture conditions and genetic modifications to maximize lipid production and nutrient uptake in microalgae. For instance, Carbonell et al. (2018) demonstrated how AI-assisted metabolic modeling could guide the selection of gene targets to boost lipid accumulation. Supervised learning models such as random forests, support vector machines (SVMs), and deep neural networks are increasingly used to predict how different environmental factors (e.g., light intensity or nitrogen availability), strain genotypes, and reactor configurations affect metabolic fluxes and lipid yields (Cuellar and Straathof, 2020). These models allow researchers to predict how changes in inputs will influence lipid output. A recent study by Iturbides et al. (2022) applied machine learning to predict the lipid response of C. vulgaris under nitrogen-depleted and varying light conditions, allowing for the identification of optimal cultivation parameters in real time. In addition to predictive modeling, reinforcement learning (RL) algorithms are being explored to automate bioreactor control. These algorithms learn to dynamically adjust parameters such as nutrient dosing, pH levels, and light cycles to maintain system balance and maximize biofuel productivity (Lindner et al., 2020). Moreover, AI enables the integration of multi-omics data, including transcriptomic, proteomic, metabolomic, and phenomic profiles, to uncover hidden regulatory mechanisms within microbial consortia. This systems-level insight helps inform the design of robust microbial communities and synthetic strains tailored to specific biofuel production goals (Choudhary et al., 2022). Together, these tools are making algal-microbial systems smarter, more adaptable, and scalable, offering a path forward in achieving efficient, data-informed, and climate-resilient biofuel production.
7.2 Digital twins for predictive system design
The emergence of digital twin technology marks a transformative shift in the way bioengineering systems are designed, monitored, and optimized. A digital twin is a virtual replica of a real-world system, such as a photobioreactor or algal cultivation unit, which uses real-time sensor data and predictive modeling to simulate, monitor, and optimize system performance. In the context of algal-microbial biofuel production, digital twins offer a powerful solution for enhancing process control, reducing trial-and-error experimentation, and improving the overall efficiency and sustainability of cultivation systems (Sanchez et al., 2021). By integrating real-time instrumentation with mechanistic and statistical models, digital twins can continuously monitor key parameters such as biomass accumulation, nutrient levels, gas exchange, and light distribution. These insights allow operators to make informed decisions or implement automated responses to fluctuations in environmental or biological conditions, ensuring stable and high-yield operation. For instance, digital twins can simulate how light intensity gradients or CO2 availability affect algal growth, enabling proactive adjustments that improve productivity and reduce energy waste (Schlüter et al., 2023). Perhaps more significantly, when combined with AI-driven feedback loops, digital twins enable dynamic process control. This means that the system can automatically react to internal and external changes, such as temperature shifts or microbial contamination, by adjusting cultivation parameters in real time. Such automation enhances resilience, reduces operational costs, and supports continuous optimization. Another frontier of this innovation is the integration of digital twins with genome-scale metabolic models (GEMs). These metabolic models help predict how algae or microbial consortia will respond to genetic modifications or environmental interventions. With such tools, researchers can perform in silico experiments testing thousands of scenarios virtually to identify optimal strain combinations and growth strategies before actual implementation, saving both time and resources (Zomorrodi and Segrè, 2016). Ultimately, the convergence of digital twins, AI, and systems biology is reshaping algal-microbial platforms into smart, adaptive biofactories. These technologies align seamlessly with the principles of the circular carbon economy, offering scalable solutions for sustainable energy production, carbon sequestration, and waste valorization.
8 Biofuel classification and environmental implications
Biofuels are broadly categorized into four generations based on the type of feedstock used and their technological maturity. First-generation biofuels, such as bioethanol and biodiesel derived from food crops (e.g., corn, sugarcane, soybean), are well-established but raise significant concerns related to food security, land use change, and water consumption (Naik et al., 2010; Tilman et al., 2009). Second-generation biofuels, produced from lignocellulosic biomass such as agricultural residues and forestry waste, attempt to mitigate these issues but require complex pretreatment processes and enzyme systems, limiting economic feasibility.
In contrast, third-generation biofuels, primarily derived from microalgae, offer a more sustainable and flexible platform due to their superior photosynthetic efficiency, faster growth rates, and ability to thrive on non-arable land using saline water or wastewater (Chisti, 2007; Khan et al., 2018). These characteristics eliminate direct competition with food production and reduce land-use pressures. Fourth-generation biofuels, still in developmental stages, incorporate synthetic biology and carbon capture technologies to enhance environmental performance and achieve carbon-negative bioenergy production (Arun et al., 2020). Among liquid biofuels, biodiesel is widely considered a practical substitute for conventional diesel, particularly for use in compression ignition (CI) engines. It can be produced via transesterification of triglyceride-rich feedstocks with alcohol in the presence of catalysts. Microalgae-derived biodiesel has shown promising energy content and combustion properties comparable to petroleum diesel, while also contributing to reduced greenhouse gas (GHG) emissions and pollutant outputs (Balat, 2011; Shuba and Kifle, 2018).
Recent studies have provided comprehensive assessments of biodiesel’s substitution potential. According to Atabani et al. (2012), biodiesel offers significant environmental advantages, including reductions in carbon monoxide (CO), unburned hydrocarbons (HC), and particulate matter (PM), although it may result in a marginal increase in nitrogen oxides (NOx). Further, Pandey et al. (2023) applied a multi-attribute decision-making framework to compare renewable diesel production pathways. Their findings emphasized that microalgae-based biodiesel systems, especially those integrated with wastewater reuse and CO2 capture, rank highly in terms of sustainability, when assessed across environmental, economic, and technical indicators. Moreover, experimental evaluations of biodiesel–diesel blends have demonstrated tangible reductions in harmful emissions without compromising engine performance. Patel et al. (2023) conducted combustion and emission tests using CI engines powered by various biodiesel blends, showing that optimized blends (e.g., B20–B40) can reduce CO and PM emissions significantly. Their study used the AHP-TOPSIS (Analytic Hierarchy Process and Technique for Order of Preference by Similarity to Ideal Solution) method to identify the most suitable biofuel blend under multiple criteria emphasizing the importance of balancing emission characteristics, cost-effectiveness, and fuel efficiency in biofuel selection. From an environmental standpoint, the use of microalgae-based biodiesel in hybrid biofactory systems further enhances sustainability. These systems enable closed-loop nutrient recycling, CO2 sequestration, and waste valorization, thereby aligning biofuel production with circular economy principles and reducing ecological footprints (Zabala, 2021; Viswanathan et al., 2022). Additionally, when designed using AI-driven process control and systems biology, these platforms offer reduced land and water requirements compared to traditional crop-based biofuels.
8.1 Techno-economic and environmental assessment
8.1.1 Life cycle analysis (LCA)
To understand the true environmental sustainability of algal-microbial biofuel systems, Life Cycle Assessment (LCA) is widely recognized as a critical evaluation tool. LCA analyzes the entire production chain from biomass cultivation and harvesting to lipid extraction, conversion into biofuels, and end-use combustion to estimate environmental impacts such as greenhouse gas (GHG) emissions, energy use, water consumption, and nutrient requirements (Lardon et al., 2009; Clarens et al., 2010). This cradle-to-grave perspective allows for meaningful comparisons between microalgal biofuels and both conventional fossil fuels and first- and second-generation biofuels. Numerous LCA studies have highlighted both the promise and limitations of algae-based biofuel systems. On one hand, microalgae offer unique advantages, including the ability to capture atmospheric CO2 and utilize wastewater for nutrient supply. On the other hand, energy-intensive steps such as biomass mixing, continuous illumination, and dewatering significantly affect the environmental footprint. For instance, Collet et al. (2011) reported that dewatering alone can account for up to 30% of total energy input, depending on the harvesting technology used. To improve LCA outcomes, researchers are increasingly turning to engineered microbial consortia. These microbiomes can fix nitrogen, degrade organic matter, and help recycle nutrients, thereby reducing the need for external chemical fertilizers and improving the system’s energy balance (Thakur et al., 2023). In one study, the integration of nitrogen-fixing bacteria reduced synthetic nitrogen input by up to 70%, significantly lowering the GHG emissions associated with algal cultivation. Another crucial aspect that influences LCA results is the definition of system boundaries and functional units. For example, systems that incorporate co-product valorization, such as using residual biomass to generate biogas or converting nutrient-rich effluent into biofertilizers, frequently demonstrate net environmental benefits. Subhadra and Edwards (2011) found that a hybrid biofuel system with co-product generation had a net GHG reduction of 78% compared to petroleum diesel. Thus, LCA not only reveals bottlenecks but also guides how to redesign algal-microbial biofactories for better environmental performance. When combined with techno-economic analysis (TEA), it offers a comprehensive foundation for planning sustainable, large-scale deployments.
8.1.2 Energy return on investment (EROI) and scalability
One of the most critical metrics for evaluating the sustainability of algal biofuel systems is the Energy Return on Investment (EROI), defined as the ratio of energy produced to the energy consumed during production. For traditional microalgal systems, this ratio has typically been below 1, with studies reporting values ranging from 0.13 to 0.75, largely due to the high energy costs associated with cultivation, harvesting, dewatering, and lipid extraction (Richardson et al., 2012; Clarens et al., 2010). This means that in many cases, more energy is consumed than is ultimately generated, challenging the viability of algae-based biofuels as a competitive renewable energy source. Recent technological progress, however, is beginning to shift this trend. For example, closed-loop photobioreactor (PBR) systems have improved light utilization efficiency by up to 50%–70% compared to open raceway ponds. Moreover, integrating engineered microbial consortia can significantly enhance nutrient cycling and lipid yields. In one study, microbial co-cultivation improved lipid productivity by up to 40%, while also reducing nitrogen input requirements by 20%–30% (Wang et al., 2008). Simultaneously, AI-enabled process optimization such as using machine learning to regulate light, CO2, and nutrient supply has shown promise in reducing operational energy costs by as much as 15%–20%, thus improving net energy gains.
Still, scalability remains a pressing concern. Systems that demonstrate promising lipid yields in laboratory conditions often experience a 30%–60% drop in productivity when transitioned to outdoor or industrial-scale settings. This is due to factors such as light attenuation, fluctuating temperature regimes, and increased risk of contamination, which are difficult to control outside laboratory environments (Quinn and Davis, 2015). One promising strategy to mitigate these challenges involves the waste-to-value approach. By incorporating engineered microbiomes, algal systems can utilize municipal or agricultural wastewater as a nutrient source, effectively reducing both input costs and environmental impact. For instance, substituting synthetic fertilizers with wastewater has been shown to cut nutrient costs by up to 90%, while simultaneously achieving comparable biomass yields (Lau et al., 1995; Rawat et al., 2011).
In addition to improving energy balances, economic viability is significantly enhanced by the co-production of value-added products. For example, a study by Davis et al. (2011) showed that coupling algal biodiesel production with the generation of bioelectricity and organic fertilizers can increase total revenue by 40%–60%, depending on market prices and regional waste streams. Such integrated biorefineries offer diversified income streams and reduce dependency on biodiesel as the sole product. To gain a more holistic understanding of algal-microbial systems, researchers advocate combining techno-economic analysis (TEA) with life cycle assessment (LCA). While TEA evaluates financial and technical viability, LCA accounts for environmental impacts such as greenhouse gas emissions, water use, and land footprint. For instance, a combined TEA-LCA approach applied to a wastewater-fed algal system showed a net GHG reduction of 68% compared to petroleum diesel and a projected cost of $5.50 per gallon of biodiesel approaching the commercial viability threshold (Collet et al., 2011). Ultimately, realizing the full potential of hybrid microalgae–microbiome systems will depend on interdisciplinary collaboration among synthetic biologists, engineers, economists, and environmental scientists. When integrated within the circular carbon economy, these platforms hold strong promise for achieving sustainable, scalable, and economically competitive biofuel production.
9 Challenges, knowledge gaps, and future directions
9.1 Challenges
9.1.1 Biological and engineering challenges
A primary biological constraint lies in the stability and resilience of synthetic or natural microbial consortia under fluctuating environmental and operational conditions. Microalgae–bacteria co-cultures are inherently dynamic and sensitive to changes in light intensity, temperature, nutrient availability, pH, and CO2 levels. Such fluctuations can disrupt synergistic interactions, shift microbial community composition, and reduce lipid productivity (Cheirsilp and Torpee, 2012; Ramanan et al., 2016). Furthermore, the ecological complexity of co-cultivation introduces competition, cross-feeding, and quorum sensing dynamics that are not yet fully understood or easily controlled. From an engineering perspective, the current design of photobioreactors and open pond systems is not fully optimized for managing multi-species cultures. Limitations include inadequate light distribution, gas exchange inefficiencies, and difficulty in scaling culture stability across spatial and temporal gradients. Additionally, the lack of integrated real-time monitoring and feedback systems impairs dynamic process control, particularly in large-scale operations where microbial responses can rapidly shift. There is a critical need for advanced bioprocess control strategies that incorporate artificial intelligence (AI), sensor networks, and automation to ensure consistent system performance (Carbonell et al., 2018).
9.1.2 Regulatory concerns
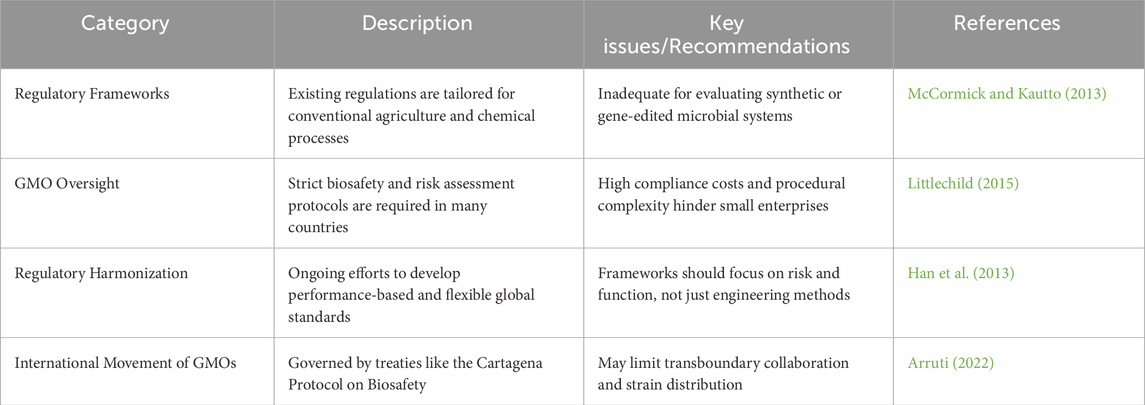
The advancement of hybrid biofactory systems combining microalgae with engineered microbial consortia offers significant promise for sustainable biofuel production. However, these innovations also bring about a range of regulatory challenges, particularly in terms of biosafety, environmental risk management, and product certification. Existing regulatory frameworks for biofuels have largely been developed with traditional agricultural systems and chemical refining in mind. As such, they are often ill-suited to assess the complexities introduced by genetically engineered microbial systems (McCormick and Kautto, 2013). One of the major concerns is regulatory uncertainty, which can significantly delay the commercialization of next-generation biofactories especially those involving synthetic organisms or gene-edited strains (see Tables 4, 5). In many countries, genetically modified organisms (GMOs) are subject to extensive risk assessments, which may include environmental containment protocols, ecological impact evaluations, and public consultations (Littlechild, 2015). These requirements are often prohibitively expensive for small research groups or early-stage start-ups, creating financial and procedural barriers that hinder innovation. At the international level, agreements such as the Cartagena Protocol on Biosafety further complicate the picture. This protocol aims to ensure the safe handling and transboundary movement of living modified organisms (LMOs), but it can also restrict the cross-border transfer of genetically engineered strains for research and commercial purposes (Arruti, 2022). There have been global efforts to modernize and harmonize regulatory systems by shifting towards performance-based frameworks which focus more on the actual risk and outcomes of a product rather than the specific engineering method used to create it (Han et al., 2013). Such frameworks emphasize traits like environmental persistence, horizontal gene transfer risk, or potential toxicity, rather than whether the organism was produced using CRISPR or other gene-editing tools. Nevertheless, the absence of a clear, flexible, and internationally consistent regulatory framework continues to pose a challenge. A well-defined yet adaptive system is crucial not only to protect public health and the environment but also to encourage responsible innovation in synthetic biology and bioenergy development.
9.1.3 Synthetic biology ethics and ecological considerations
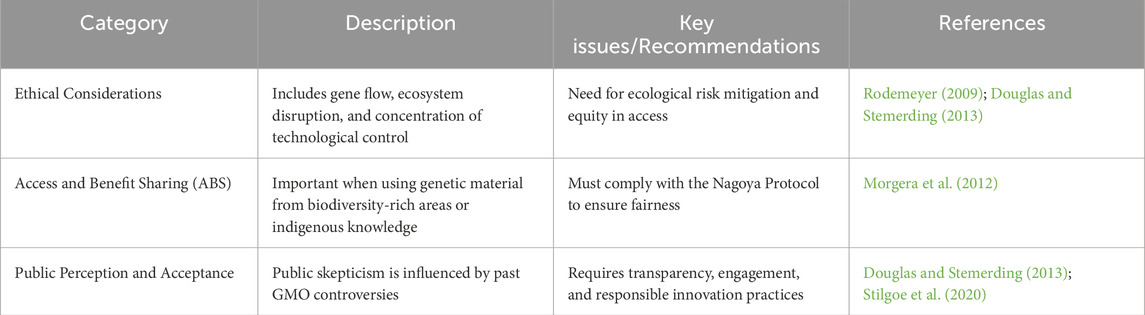
The incorporation of synthetic biology into biofuel production, particularly through hybrid biofactories involving engineered microalgae and microbial consortia, raises several ethical and environmental concerns that must be carefully considered. One major issue is the potential for gene flow from engineered strains into natural ecosystems. If containment strategies fail, these synthetic organisms could unintentionally disrupt local microbial communities, leading to ecological imbalances or even the displacement of native species (Rodemeyer, 2009). Public acceptance is another significant challenge. Societal attitudes toward synthetic organisms are still heavily influenced by the earlier controversies surrounding genetically modified organisms (GMOs). This lingering skepticism can affect public trust and hinder the broader adoption of hybrid biofactories (Douglas and Stemerding, 2013). In particular, communities may resist technologies perceived as risky, opaque, or developed without meaningful engagement. Beyond biosafety, the issues of ownership, equity, and access to biological resources raise further ethical questions. Concerns have been raised about the monopolization of bioresource technologies by a small number of powerful corporations. These concerns are amplified when engineered strains are developed from genetic material sourced from biodiversity-rich regions or based on traditional ecological knowledge, often without equitable sharing of benefits. The Nagoya Protocol on Access and Benefit-Sharing (ABS) plays a crucial role in promoting fairness and transparency in such cases by ensuring that communities and countries providing biological resources are properly acknowledged and compensated (Morgera et al., 2012).
To ensure that synthetic biology contributes positively to sustainable energy transitions, it must be aligned with broader principles of sustainability and justice. Technologies should avoid contributing to problems like land-use change, biodiversity loss, or social displacement issues that have plagued earlier generations of biofuels. Instead, hybrid biofactories should be guided by the framework of responsible innovation, which emphasizes transparency, public participation, anticipation of risks, and ethical design (Stilgoe et al., 2020). Ultimately, developing safe, equitable, and environmentally resilient biofuel systems will require interdisciplinary collaboration, including input from ecologists, ethicists, engineers, policymakers, and local communities. Long-term studies are especially needed to assess the ecological implications of releasing synthetic organisms and to develop robust mitigation strategies where needed.
9.2 Knowledge gaps
Despite increasing interest in hybrid consortia, significant gaps persist in understanding the mechanistic underpinnings of microalgae–microbiome interactions. Specifically, the metabolic pathways, gene regulation networks, and interspecies signaling mechanisms that drive mutualism or competition remain inadequately characterized. Multi-omics approaches such as metagenomics, transcriptomics, and metabolomics have not yet been fully utilized to elucidate these complex interactions at the systems level. Moreover, the techno-economic and life-cycle performance of hybrid biofactories is poorly documented. While laboratory and pilot-scale studies report promising improvements in lipid yields and nutrient recovery, few studies offer comprehensive assessments of energy return on investment (EROI), cost per liter of biodiesel, or net greenhouse gas (GHG) mitigation at industrial scale (Ugwu et al., 2025). In addition, the potential ecological risks associated with the deliberate release or unintended dispersal of genetically modified microbial strains in open systems present unresolved regulatory and biosafety challenges (McCormick and Kautto, 2013).
9.3 Future research directions
To advance the field and support the practical deployment of hybrid biofactories, several priority areas for future research can be identified.
a. Mechanistic Elucidation: Greater emphasis should be placed on decoding the molecular basis of algal-bacterial cooperation, including carbon fluxes, nitrogen cycling, vitamin exchange, and stress responses. This would require the integration of high-resolution omics tools and metabolic modeling frameworks.
b. AI-Assisted Process Control: The incorporation of AI-driven digital twins, machine learning algorithms, and predictive analytics can facilitate real-time optimization of culture conditions, early detection of contamination or instability, and intelligent strain selection for target metabolite synthesis (Nielsen and Keasling, 2016).
c. Strain Engineering: Advances in CRISPR-Cas genome editing and synthetic biology provide the tools to construct robust, metabolically engineered strains with enhanced lipid biosynthesis, stress tolerance, and compatibility with consortium partners.
d. Comprehensive Techno-Economic and Life-Cycle Assessments: Rigorous evaluation of capital and operational costs, energy consumption, environmental impacts, and market feasibility is necessary to inform scale-up decisions and attract industrial investment (Arun et al., 2020; Viswanathan et al., 2022).
e. Integration into Circular Carbon Systems: Future designs should emphasize integration with waste valorization platforms (e.g., municipal wastewater, flue gas CO2, agro-industrial effluents) to support resource circularity, carbon neutrality, and economic co-benefits.
f. Regulatory and Governance Frameworks: It is imperative to establish clear, evidence-based guidelines for the safe deployment of engineered consortia, including risk assessment protocols, environmental monitoring strategies, and public engagement mechanisms.
10 Conclusion
The integration of microalgae cultivation with engineered microbial consortia in hybrid biofactories represents a transformative advancement in the field of sustainable biofuel production. This synergistic model leverages metabolic complementarity between microalgae and microbiomes to enhance lipid accumulation, facilitate nutrient recycling, and optimize carbon utilization, major processes underpinning the circular carbon economy. Through innovations in reactor design, co-cultivation strategies, and AI-driven process optimization, hybrid systems offer promising solutions to the persistent challenges faced by conventional monoculture systems, such as limited scalability, high resource input, and operational instability. Despite these advancements, the transition from laboratory-scale proof-of-concept to commercial-scale hybrid biofactories remains contingent upon resolving several critical barriers. Technical challenges, such as ensuring stable consortia dynamics, improving biomass harvesting efficiency, and optimizing bioproduct selectivity, must be addressed. Equally pressing are regulatory and ethical concerns, particularly regarding the deployment of genetically engineered strains in open or semi-open systems. The development of clear regulatory frameworks grounded in responsible innovation principles will be essential to ensure environmental safety and public trust. Moreover, techno-economic assessments (TEAs) and life cycle analyses (LCAs) must be rigorously conducted to evaluate the feasibility and sustainability of these systems under various geographical and industrial contexts. Interdisciplinary collaboration spanning microbiology, systems biology, engineering, and computational sciences is vital to enhance understanding of microbe-microbe and microbe-algae interactions, as well as to refine predictive modeling tools that can guide reactor design and performance.
Ultimately, hybrid microalgae–microbiome systems exemplify a bioemulative paradigm, wherein principles of ecological cooperation are harnessed to build adaptive, circular, and robust biomanufacturing platforms. When embedded within global energy transition frameworks, these biofactories have the potential to contribute significantly to climate-resilient, decentralized, and resource-efficient bioeconomies. The long-term success of this paradigm will depend on targeted investment, policy support, and cross-sectoral partnerships that align scientific innovation with industrial scalability and societal needs. This review contributes to a comprehensive and interdisciplinary perspective on hybrid biofactories as next-generation biofuel platforms. By critically integrating recent advances in synthetic biology, microbial engineering, and circular carbon economy principles, the work provides a strategic roadmap for accelerating the development of sustainable, scalable, and AI-optimized algal biofuel systems. Its insights have implications for.
a. Research: Guiding future studies on microbiome dynamics, gene editing, and bioprocess modeling.
b. Industry: Informing bioreactor scale-up, co-product valorization, and investment strategies.
c. Policy: Supporting the development of regulation for synthetic consortia and green biotechnology in line with environmental sustainability goals.
Therefore, this work lays the foundation for redefining biofuel production as a circular, biologically intelligent, and ecologically integrated process, capable of contributing meaningfully to the global shift toward renewable energy.
Author contributions
UCN: Conceptualization, Formal Analysis, Investigation, Resources, Supervision, Writing – original draft, Writing – review and editing. OC: Conceptualization, Formal Analysis, Methodology, Resources, Supervision, Writing – original draft, Writing – review and editing. UJN: Conceptualization, Project administration, Supervision, Writing – original draft, Writing – review and editing. UP-C: Conceptualization, Project administration, Resources, Supervision, Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Arruti, C. I. G. (2022). The precautionary principle and the protection of biological diversity. Am. J. Appl. Sci. Res. 8 (1), 1–10. doi:10.11648/j.ajasr.20220801.11
Arun, J., Gopinath, K. P., SundarRajan, P., Malolan, R., and AjaySrinivaasan, P. (2020). Hydrothermal liquefaction and pyrolysis of Amphiroa fragilissima biomass: comparative study on oxygen content and storage stability parameters of bio-oil. Bioresour. Technol. Rep. 11, 100465. doi:10.1016/j.biteb.2020.100465
Atabani, A. E., Silitonga, A. S., Badruddin, I. A., Mahlia, T. M. I., Masjuki, H., Mekhilef, S., et al. (2012). A comprehensive review on biodiesel as an alternative energy resource and its characteristics. Renewable and sustainable energy reviews 16 (04), 2070–2093.
Brennan, L., and Owende, P. (2010). Biofuels from microalgae—a review of technologies for production, processing, and extractions of biofuels and co-products. Renew. Sustain. energy Rev. 14 (2), 557–577. doi:10.1016/j.rser.2009.10.009
Brenner, K., You, L., and Arnold, F. H. (2008). Engineering microbial consortia: a new frontier in synthetic biology. Trends Biotechnol. 26 (9), 483–489. doi:10.1016/j.tibtech.2008.05.004
Carbonell, P., Jervis, A. J., Robinson, C. J., Yan, C., Dunstan, M., Swainston, N., et al. (2018). An automated Design-Build-Test-Learn pipeline for enhanced microbial production of fine chemicals. Commun. Biol. 1 (1), 66. doi:10.1038/s42003-018-0076-9
Carney, L. T., Reinsch, S. S., Lane, P. D., Solberg, O. D., Jansen, L. S., Williams, K. P., et al. (2014). Microbiome analysis of a microalgal mass culture growing in municipal wastewater in a prototype OMEGA photobioreactor. Algal Res. 4, 52–61. doi:10.1016/j.algal.2013.11.006
Cheirsilp, B., and Torpee, S. (2012). Enhanced growth and lipid production of microalgae under mixotrophic culture condition: effect of light intensity, glucose concentration and fed-batch cultivation. Bioresour. Technol. 110, 510–516. doi:10.1016/j.biortech.2012.01.125
Chen, G., Wu, W., Xu, J., and Wang, Z. (2022). Corrigendum to “An anaerobic dynamic membrane bioreactor for enhancing sludge digestion: impact of solids retention time on digestion efficacy” [Bioresour. Technol. 329 (2021) 124864]. Bioresour. Technol. 344 (Pt A), 126216. doi:10.1016/j.biortech.2021.126216
Cheng, Y., Xie, W., Huang, R., Yan, X., and Wang, S. (2017). Extremely high N2O but unexpectedly low NO emissions from a highly organic and chemical fertilized peach orchard system in China. Agric. Ecosyst. and Environ. 246, 202–209. doi:10.1016/j.agee.2017.06.015
Chinnasamy, S., Bhatnagar, A., Hunt, R. W., and Das, K. C. (2010). Microalgae cultivation in a wastewater dominated by carpet mill effluents for biofuel applications. Bioresour. Technol. 101 (9), 3097–3105. doi:10.1016/j.biortech.2009.12.026
Chisti, Y. (2007). Biodiesel from microalgae. Biotechnol. Adv. 25 (3), 294–306. doi:10.1016/j.biotechadv.2007.02.001
Cho, D. H., Ramanan, R., Heo, J., Lee, J., Kim, B. H., Oh, H. M., et al. (2015). Enhancing microalgal biomass productivity by engineering a microalgal–bacterial community. Bioresour. Technol. 175, 578–585. doi:10.1016/j.biortech.2014.10.159
Choudhary, K., DeCost, B., Chen, C., Jain, A., Tavazza, F., Cohn, R., et al. (2022). Recent advances and applications of deep learning methods in materials science. npj Comput. Mater. 8 (1), 59. doi:10.1038/s41524-022-00734-6
Christenson, L., and Sims, R. (2011). Production and harvesting of microalgae for wastewater treatment, biofuels, and bioproducts. Biotechnol. Adv. 29 (6), 686–702. doi:10.1016/j.biotechadv.2011.05.015
Clarens, A. F., Resurreccion, E. P., White, M. A., and Colosi, L. M. (2010). Environmental life cycle comparison of algae to other bioenergy feedstocks. Environ. Sci. and Technol. 44 (5), 1813–1819. doi:10.1021/es902838n
Collet, P., Hélias, A., Lardon, L., Ras, M., Goy, R. A., and Steyer, J. P. (2011). Life-cycle assessment of microalgae culture coupled to biogas production. Bioresour. Technol. 102 (1), 207–214. doi:10.1016/j.biortech.2010.06.154
Commault, A. S., Lear, G., Packer, M. A., and Weld, R. J. (2013). Influence of anode potentials on selection of Geobacter strains in microbial electrolysis cells. Bioresour. Technol. 139, 226–234. doi:10.1016/j.biortech.2013.04.047
Coppens, J., Grunert, O., Van Den Hende, S., Vanhoutte, I., Boon, N., Haesaert, G., et al. (2016a). The use of microalgae as a high-value organic slow-release fertilizer results in tomatoes with increased carotenoid and sugar levels. J. Appl. Phycol. 28 (4), 2367–2377. doi:10.1007/s10811-015-0775-2
Coppens, J., Lindeboom, R., Muys, M., Coessens, W., Alloul, A., Meerbergen, K., et al. (2016b). Nitrification and microalgae cultivation for two-stage biological nutrient valorization from source separated urine. Bioresour. Technol. 211, 41–50. doi:10.1016/j.biortech.2016.03.001
Croft, M. T., Lawrence, A. D., Raux-Deery, E., Warren, M. J., and Smith, A. G. (2005). Algae acquire vitamin B12 through a symbiotic relationship with bacteria. Nature 438 (7064), 90–93. doi:10.1038/nature04056
Cuellar, M. C., and Straathof, A. J. (2020). Downstream of the bioreactor: advancements in recovering fuels and commodity chemicals. Curr. Opin. Biotechnol. 62, 189–195. doi:10.1016/j.copbio.2019.11.012
Davis, R., Aden, A., and Pienkos, P. T. (2011). Techno-economic analysis of autotrophic microalgae for fuel production. Appl. Energy 88 (10), 3524–3531. doi:10.1016/j.apenergy.2011.04.018
De-Bashan, L. E., Hernandez, J. P., Morey, T., and Bashan, Y. (2004). Microalgae growth-promoting bacteria as “helpers” for microalgae: a novel approach for removing ammonium and phosphorus from municipal wastewater. Water Res. 38 (2), 466–474. doi:10.1016/j.watres.2003.09.022
Douglas, C. M., and Stemerding, D. (2013). Governing synthetic biology for global health through responsible research and innovation. Syst. Synthetic Biol. 7, 139–150. doi:10.1007/s11693-013-9119-1
Fuentes, J. L., Garbayo, I., Cuaresma, M., Montero, Z., González-del-Valle, M., and Vílchez, C. (2016). Impact of microalgae-bacteria interactions on the production of algal biomass and associated compounds. Mar. drugs 14 (5), 100. doi:10.3390/md14050100
Griffiths, M. J., and Harrison, S. T. (2009). Lipid productivity as a key characteristic for choosing algal species for biodiesel production. J. Appl. Phycol. 21, 493–507. doi:10.1007/s10811-008-9392-7
Han, A., Hou, H., Li, L., Kim, H. S., and de Figueiredo, P. (2013). Microfabricated devices in microbial bioenergy sciences. Trends Biotechnol. 31 (4), 225–232. doi:10.1016/j.tibtech.2012.12.002
Hu, Q., Sommerfeld, M., Jarvis, E., Ghirardi, M., Posewitz, M., Seibert, M., et al. (2008). Microalgal triacylglycerols as feedstocks for biofuel production: perspectives and advances. plant J. 54 (4), 621–639. doi:10.1111/j.1365-313x.2008.03492.x
Iturbides, R. D. L. C., Haza, U. J., and Polaert, I. (2022). Recent technological innovations on continuous microwave assisted biomass pyrolysis and perspectives for industrial scale applications. Bioresour. Technol. Rep. 19, 101202. doi:10.1016/j.biteb.2022.101202
Javed, M. U., Mukhtar, H., Zieniuk, B., and Rashid, U. (2024). Algal-based hollow fiber membrane bioreactors for efficient wastewater treatment: a comprehensive review. Fermentation 10 (3), 131. doi:10.3390/fermentation10030131
Jeon, J., Cho, K., Kang, J., Park, S., Ada, O. U. E., Park, J., et al. (2022). Combined machine learning and biomolecular analysis for stability assessment of anaerobic ammonium oxidation under salt stress. Bioresour. Technol. 355, 127206. doi:10.1016/j.biortech.2022.127206
Kazamia, E., Czesnick, H., Nguyen, T. T. V., Croft, M. T., Sherwood, E., Sasso, S., et al. (2012). Mutualistic interactions between vitamin B12-dependent algae and heterotrophic bacteria exhibit regulation. Environ. Microbiol. 14 (6), 1466–1476. doi:10.1111/j.1462-2920.2012.02733.x
Khan, M. I., Shin, J. H., and Kim, J. D. (2018). The promising future of microalgae: current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb. Cell factories 17, 36–21. doi:10.1186/s12934-018-0879-x
Kim, E. K., Lee, K. A., Kyung, M., Jun, K. Y., Seo, S. H., Hwang, D., et al. (2020). Bacterial nucleoside catabolism controls quorum sensing and commensal-to-pathogen transition in the Drosophila gut. Cell Host and Microbe 27 (3), 345–357.e6. doi:10.1016/j.chom.2020.01.025
Kumar, P., Choudhary, A., Joshi, P. K., Kumar, R. P., and Bhatla, R. (2025). Machine learning models for estimating criteria pollutants and health risk-based air quality indices over eastern coast coal mine complex belts. Front. Environ. Sci. 13, 1589991. doi:10.3389/fenvs.2025.1589991
Lardon, L., Helias, A., Sialve, B., Steyer, J. P., and Bernard, O. (2009). Life-cycle assessment of biodiesel production from microalgae. Environ. Sci. and Technol. 43 (17), 6475–6481. doi:10.1021/es900705j
Lau, P. C., Kwong, T. L., and Yung, K. F. (2016). Effective heterogeneous transition metal glycerolates catalysts for one-step biodiesel production from low grade non-refined Jatropha oil and crude aqueous bioethanol. Scientific reports 6 (01), 23822.
Lee, H., Calvin, K., Dasgupta, D., Krinner, G., Mukherji, A., Thorne, P., et al. (2024). “Climate change 2023 synthesis report summary for policymakers,” in CLIMATE CHANGE 2023 synthesis report: summary for policymakers.
Li, P. D., Zhu, Z. R., Zhang, Y., Xu, J., Wang, H., Wang, Z., et al. (2022). The phyllosphere microbiome shifts toward combating melanose pathogen. Microbiome 10 (1), 56. doi:10.1186/s40168-022-01234-x
Lindner, J. P., Beck, T., Bos, U., and Albrecht, S. (2020). “Assessing land use and biodiversity impacts of industrial biotechnology,” in Sustainability and life cycle assessment in industrial biotechnology, 233–254.
Littlechild, J. A. (2015). Enzymes from extreme environments and their industrial applications. Front. Bioeng. Biotechnol. 3, 161. doi:10.3389/fbioe.2015.00161
Liu, L., Fan, H., Liu, Y., Liu, C., and Huang, X. (2017). Development of algae-bacteria granular consortia in photo-sequencing batch reactor. Bioresour. Technol. 232, 64–71. doi:10.1016/j.biortech.2017.02.025
Lopez-Rodriguez, N. A., Sanchez-Ortiz, L. K., Reynoso-Camacho, R., Riesgo-Escovar, J. R., and Loarca-Piña, G. (2023). Chronic consumption of moringa leaf powder (moringa oleifera) concentration-dependent effects in a Drosophila melanogaster type 2 diabetes model. J. Am. Nutr. Assoc. 42 (3), 285–294. doi:10.1080/07315724.2022.2034068
Mahapatra, D. M., Chanakya, H. N., and Ramachandra, T. V. (2013). Euglena sp. as a suitable source of lipids for potential use as biofuel and sustainable wastewater treatment. J. Appl. Phycol. 25 (3), 855–865. doi:10.1007/s10811-013-9979-5
Mata, T. M., Martins, A. A., and Caetano, N. S. (2010). Microalgae for biodiesel production and other applications: a review. Renew. Sustain. energy Rev. 14 (1), 217–232. doi:10.1016/j.rser.2009.07.020
McCormick, K., and Kautto, N. (2013). The bioeconomy in Europe: an overview. Sustainability 5 (6), 2589–2608. doi:10.3390/su5062589
Mehra, K. S., and Goel, V. (2025). Unveiling next-generation biodiesel Technologies: techno-Economic and energy breakthroughs for sustainable production. Biomass Bioenergy 199, 107910. doi:10.1016/j.biombioe.2025.107910
Mehra, K. S., and Pant, G. (2021). “Production of biofuel from sesame oil and its characterization as an alternative fuel for diesel Engine,” in IOP Conference Series: Materials Science and Engineering, (IOP Publishing), 1116, 1012076. doi:10.1088/1757-899x/1116/1/012076
Mehra, K. S., Singh, S., Singh, A. K., Kharkwal, H., and Avikal, S. (2021). Performance, energy, emission and cost analysis of Jatropha (Jatropha Curcas) oil as a biofuel for compression ignition engine. Mater. Today Proc. 43, 348–354. doi:10.1016/j.matpr.2020.11.675
Mehra, K. S., Pal, J., and Goel, V. (2023). A comprehensive review on the atomization and spray characteristics of renewable biofuels. Sustain. Energy Technol. Assessments 56, 103106. doi:10.1016/j.seta.2023.103106
Mehra, K. S., Abrar, I., Bhatia, R. K., and Goel, V. (2025). A comprehensive review of algae consortium for wastewater bioremediation and biodiesel production. Energy Convers. Manag. 325, 119428. doi:10.1016/j.enconman.2024.119428
Morgera, E., Buck, M., and Tsioumani, E. (2012). No need to reinvent the wheel for a human rights-based approach to tackling climate change: the contribution of international biodiversity law. In Climate Change and the Law. Dordrecht, Netherlands: Springer, 1.
Naik, S. N., Goud, V. V., Rout, P. K., and Dalai, A. K. (2010). Production of first and second generation biofuels: a comprehensive review. Renew. Sustain. energy Rev. 14 (2), 578–597. doi:10.1016/j.rser.2009.10.003
Naseema Rasheed, R., Pourbakhtiar, A., Mehdizadeh Allaf, M., Baharlooeian, M., Rafiei, N., Alishah Aratboni, H., et al. (2023). Microalgal co-cultivation-recent methods, trends in omic-studies, applications, and future challenges. Front. Bioeng. Biotechnol. 11, 1193424. doi:10.3389/fbioe.2023.1193424
Ng, I. S., Tan, S. I., Kao, P. H., Chang, Y. K., and Chang, J. S. (2017). Recent developments on genetic engineering of microalgae for biofuels and bio-based chemicals. Biotechnol. J. 12 (10), 1600644. doi:10.1002/biot.201600644
Nielsen, J., and Keasling, J. D. (2016). Engineering cellular metabolism. Cell 164 (6), 1185–1197. doi:10.1016/j.cell.2016.02.004
Nymark, M., Sharma, A. K., Sparstad, T., Bones, A. M., and Winge, P. (2016). A CRISPR/Cas9 system adapted for gene editing in marine algae. Sci. Rep. 6 (1), 24951. doi:10.1038/srep24951
Pandey, A., Kumar, D., and Patel, S. K. (2025). Major Engineering issues in conventional biofuel technologies. Optimizing biofuel production with artificial intelligence, 203–221.
Patel, K., and Singh, S. K. (2024). Sustainable biodiesel from used cooking oil: a comparative life cycle, energy, and uncertainty analysis. Environ. Dev. Sustain. 27, 14755–14780. doi:10.1007/s10668-024-05692-1
Patel, A. B., Vaghasiya, J. V., Chauhan, P., Sumesh, C. K., Patel, V., Soni, S. S., et al. (2022). Synergistic 2D MoSe 2@ WSe 2 nanohybrid heterostructure toward superior hydrogen evolution and flexible supercapacitor. Nanoscale 14 (17), 6636–6647. doi:10.1039/d2nr00632d
Patel, H. K., Patel, N. P., and Shah, M. P. (2023). “Microbial bioprospecting of biodiesel industry-derived crude glycerol waste conversion into value-added products,” in Green approach to alternative fuel for a sustainable future (Elsevier), 71–87.
Pittman, J. K., Dean, A. P., and Osundeko, O. (2011). The potential of sustainable algal biofuel production using wastewater resources. Bioresour. Technol. 102 (1), 17–25. doi:10.1016/j.biortech.2010.06.035
Quinn, J. C., and Davis, R. (2015). The potentials and challenges of algae based biofuels: a review of the techno-economic, life cycle, and resource assessment modeling. Bioresour. Technol. 184, 444–452. doi:10.1016/j.biortech.2014.10.075
Rajpoot, A. S., Choudhary, T., Chelladurai, H. M., Sinha, A. A., and Pachori, H. (2024). Comparative thermal assessment and emission analysis of various green biodiesel from novel feedstocks for CI engines: a sustainable approach towards emission reduction. Environ. Sci. Pollut. Res. 31 (27), 39650–39662. doi:10.1007/s11356-024-33817-6
Rajpoot, A. S., Shende, V., Chelladurai, H. M., Dwivedi, G., Verma, T. N., and Choudhary, T. (2025). Exploring the potential and progress of microalgae biomass production, harvesting, and pre-treatment for sustainable biofuel production: a comprehensive review. Environ. Dev. Sustain., 1–51. doi:10.1007/s10668-025-05984-0
Ramanan, R., Kim, B. H., Cho, D. H., Oh, H. M., and Kim, H. S. (2016). Algae–bacteria interactions: evolution, ecology and emerging applications. Biotechnol. Adv. 34 (1), 14–29. doi:10.1016/j.biotechadv.2015.12.003
Rawat, I., Kumar, R. R., Mutanda, T., and Bux, F. (2011). Dual role of microalgae: phycoremediation of domestic wastewater and biomass production for sustainable biofuels production. Applied energy 88 (10), 3411–3424.
Richardson, J. W., Johnson, M. D., and Outlaw, J. L. (2012). Economic comparison of open pond raceways to photo bio-reactors for profitable production of algae for transportation fuels in the Southwest. Algal Res. 1 (1), 93–100. doi:10.1016/j.algal.2012.04.001
Rodemeyer, J. D (2009). The regulation of environmental risks of GMOs in the United States. In Uncertain Risks Regulated. London: Routledge-Cavendish.
Ruiz-Marin, A., Mendoza-Espinosa, L. G., and Stephenson, T. (2010). Growth and nutrient removal in free and immobilized green algae in batch and semi-continuous cultures treating real wastewater. Bioresour. Technol. 101 (1), 58–64. doi:10.1016/j.biortech.2009.02.076
Sandar, S., Tibebu, D. T., Shromova, O. A., Kuittinen, S., Turunen, O., and Pappinen, A. (2019). Lake bottom biomass as a potential source for the biorefining industry. Bioresour. Technol. Rep. 7, 100282. doi:10.1016/j.biteb.2019.100282
Santana-Sánchez, A., Lynch, F., Sirin, S., and Allahverdiyeva, Y. (2021). Nordic cyanobacterial and algal lipids: triacylglycerol accumulation, chemotaxonomy and bioindustrial potential. Physiol. Plant. 173 (2), 591–602. doi:10.1111/ppl.13443
Sánchez-Cárdenas, M., Sánchez-Olmos, L. A., Kamaraj, S. K., Trejo-Zarraga, F., and Rodríguez-Valadez, F. J. (2021). Evaluation of the performance and atmospheric emissions in a diesel engine of the biofuel obtained by hydrodeoxygenation of oleic acid with Pt/γ-Al2O3 catalysts. Environmental Progress and Sustainable Energy 40 (4), e13582.
Santos, R. O., Varona, G., Avila, C. L., Lirman, D., and Collado-Vides, L. (2020). Implications of macroalgae blooms to the spatial structure of seagrass seascapes: the case of the Anadyomene spp.(Chlorophyta) bloom in Biscayne Bay, Florida. Mar. Pollut. Bull. 150, 110742. doi:10.1016/j.marpolbul.2019.110742
Schlüter, S., Lucas, M., Phalempin, M., Knecht, L., Langehenke, F., Deubel, A., et al. (2023). Organic farming systems affect carbon stocks but not soil structure and associated physical properties in a long-term farming trial on Chernozem. Geoderma 438, 116619. doi:10.1016/j.geoderma.2023.116619
Sharma, T., Singh, A., Kumar, N., Singh, D., and Chauhan, G. (2023). “Biofuel circular economy in environmental sustainability,” in Renewable energy in circular economy (Cham: Springer International Publishing), 199–218.
Shin, S. E., Lim, J. M., Koh, H. G., Kim, E. K., Kang, N. K., Jeon, S., et al. (2016). CRISPR/Cas9-induced knockout and knock-in mutations in Chlamydomonas reinhardtii. Sci. Rep. 6 (1), 27810. doi:10.1038/srep27810
Shuba, E. S., and Kifle, D. (2018). Microalgae to biofuels:‘Promising’alternative and renewable energy, review. Renew. Sustain. energy Rev. 81, 743–755. doi:10.1016/j.rser.2017.08.042
Simon, I., Siegfried, Z., Ernst, J., and Bar-Joseph, Z. (2005). Combined static and dynamic analysis for determining the quality of time-series expression profiles. Nat. Biotechnol. 23 (12), 1503–1508. doi:10.1038/nbt1164
Singh, J., and Gu, S. (2010). Commercialization potential of microalgae for biofuels production. Renew. Sustain. energy Rev. 14 (9), 2596–2610. doi:10.1016/j.rser.2010.06.014
Singh, V. P., Kumar, A., Srivastava, A., and Kumar, A. (2024). Advancing environmental sustainability: a comprehensive review on alleviating carbon footprint and its application in microalgal fermentation and bioremediation. Environ. Dev. Sustain., 1–46. doi:10.1007/s10668-024-05796-8
Smith, V. H., Sturm, B. S., Denoyelles, F. J., and Billings, S. A. (2010). The ecology of algal biodiesel production. Trends Ecol. and Evol. 25 (5), 301–309. doi:10.1016/j.tree.2009.11.007
Stilgoe, J., Owen, R., and Macnaghten, P. (2020). “Developing a framework for responsible innovation,” in The ethics of nanotechnology, geoengineering, and clean energy (Routledge), 347–359.
Subashchandrabose, S. R., Ramakrishnan, B., Megharaj, M., Venkateswarlu, K., and Naidu, R. (2011). Consortia of cyanobacteria/microalgae and bacteria: biotechnological potential. Biotechnol. Adv. 29 (6), 896–907. doi:10.1016/j.biotechadv.2011.07.009
Subhadra, B. G., and Edwards, M. (2011). Coproduct market analysis and water footprint of simulated commercial algal biorefineries. Appl. Energy 88 (10), 3515–3523. doi:10.1016/j.apenergy.2010.12.051
Tang, J., Abbas, A. K., Koka, N. A., Sadoon, N., Abbas, J. K., Abdalhuseen, R. A, et al. (2023). Optimization of thermal biofuel production from biomass using CaO-based catalyst through different algorithm-based machine learning approaches. Case Studies in Thermal Engineering 50, 103419.
Thakur, N., Nigam, M., Mann, N. A., Gupta, S., Hussain, C. M., Shukla, S. K., et al. (2023). Host-mediated gene engineering and microbiome-based technology optimization for sustainable agriculture and environment. Funct. and Integr. Genomics 23 (1), 57. doi:10.1007/s10142-023-00982-9
Tilman, D., Socolow, R., Foley, J. A., Hill, J., Larson, E., Lynd, L., et al. (2009). Beneficial biofuels—the food, energy, and environment trilemma. Science 325 (5938), 270–271. doi:10.1126/science.1177970
Ugwu, C. U., Aoyagi, H., and Uchiyama, H. (2008). Photobioreactors for mass cultivation of algae. Bioresour. Technol. 99 (10), 4021–4028. doi:10.1016/j.biortech.2007.01.046
Ugwu, T. N., Madubuike, H., Nwachukwu, A. A., Ukachukwu, C. O., Nwajagu, N., and Ogbulie, T. E. (2025). Harnessing bioenergy for sustainable and balanced energy generation in Nigeria. Acad. Green Energy 2 (2). doi:10.20935/acadenergy7593
Viswanaathan, S., Perumal, P. K., and Sundaram, S. (2022). Integrated approach for carbon sequestration and wastewater treatment using algal–bacterial consortia: opportunities and challenges. Sustainability 14 (3), 1075. doi:10.3390/su14031075
Viswanathan, V., Mongird, K., Franks, R., Li, X., Sprenkle, V., and Baxter, R. (2022). 2022 grid energy storage technology cost and performance assessment. Energy 2022, 1–151.
Wang, B., Li, Y., Wu, N., and Lan, C. Q. (2008). CO 2 bio-mitigation using microalgae. Appl. Microbiol. Biotechnol. 79, 707–718. doi:10.1007/s00253-008-1518-y
Wang, X., Yang, X., Chen, S., Li, Q., Wang, W., Hou, C., et al. (2016). Zinc oxide nanoparticles affect biomass accumulation and photosynthesis in Arabidopsis. Front. plant Sci. 6, 1243. doi:10.3389/fpls.2015.01243
Wang, L., Wang, H., Fan, J., and Han, Z. (2023). Synthesis, catalysts and enhancement technologies of biodiesel from oil feedstock–A review. Sci. total Environ. 904, 166982. doi:10.1016/j.scitotenv.2023.166982
Xie, X., Zhang, T., Wang, L., and Huang, Z. (2017). Regional water footprints of potential biofuel production in China. Biotechnology for biofuels10 (1), 95.
Zabala, A. (2021). The right to a sound environment. Nat. Sustain. 4 (9), 750–751. doi:10.1038/s41893-021-00733-3
Zhang, Q., Zhang, X., Mu, X., Wang, Z., Tian, R., Wang, X., et al. (2021). Recyclable waste image recognition based on deep learning. Resour. Conservation Recycl. 171, 105636. doi:10.1016/j.resconrec.2021.105636
Zhang, C., Fu, R., Kang, L., Ma, Y., Fan, D., and Fei, Q. (2024). An upcycling bioprocess for sustainable aviation fuel production from food waste-derived greenhouse gases: life cycle assessment and techno-economic analysis. Chem. Eng. J. 486, 150242. doi:10.1016/j.cej.2024.150242
Zhou, H., and Liu, R. (2014). ER stress and hepatic lipid metabolism. Front. Genet. 5, 112. doi:10.3389/fgene.2014.00112
Zhou, J., Xue, K., Xie, J., Deng, Y. E., Wu, L., Cheng, X., et al. (2012). Microbial mediation of carbon-cycle feedbacks to climate warming. Nat. Clim. Change 2 (2), 106–110. doi:10.1038/nclimate1331
Zhou, K., Liu, T., and Zhou, L. (2015). “Industry 4.0: towards future industrial opportunities and challenges,” in 2015 12th International conference on fuzzy systems and knowledge discovery (FSKD), Zhangjiajie, China, 15-17 August 2015 (IEEE), 2147–2152.
Keywords: microalgae biofuels, synthetic microbiomes, hybrid biofactories, circular carbon economy, AI-driven metabolic optimization
Citation: Nneoma UC, Chukwudi OF, Nnenna UJ and Paul-Chima UO (2025) Hybrid biofactories: integrating microalgae and engineered microbiomes for enhanced biofuel production in circular carbon systems. Front. Energy Res. 13:1654079. doi: 10.3389/fenrg.2025.1654079
Received: 25 June 2025; Accepted: 17 July 2025;
Published: 23 September 2025.
Edited by:
Kuppam Chandrasekhar, Kyungpook National University, Republic of KoreaReviewed by:
Aman Singh Rajpoot, PDPM Indian Institute of Information Technology, Design and Manufacturing, IndiaKuber Singh Mehra, Birla Institute of Applied Sciences, India
Copyright © 2025 Nneoma, Chukwudi, Nnenna and Paul-Chima. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ugwu Chinyere Nneoma, dWd3dW5Aa2l1LmFjLnVn
 Ugwu Chinyere Nneoma
Ugwu Chinyere Nneoma Ogenyi Fabian Chukwudi
Ogenyi Fabian Chukwudi Ugwu Jovita Nnenna
Ugwu Jovita Nnenna Ugwu Okechukwu Paul-Chima
Ugwu Okechukwu Paul-Chima