- National Renewable Energy Laboratory, Golden, CO, United States
Two-step thermochemical redox cycles are being developed as a potential pathway for the production of hydrogen and syngas. While there are many possible reactor and system configurations, moving oxide systems are considered promising in terms of the redox thermodynamics, due to the potential implementation of a countercurrent system that can achieve higher performance compared to other configurations. There is a lack of a robust thermodynamic modeling framework in the field, with multiple models incorporating incorrect thermodynamic assumptions that violate the second law of thermodynamics. We present in this work REDOTHERM, an open-source system model for moving oxides that incorporates the correct thermodynamic limits, as well as various options for the system auxiliary units including product separation, heat recovery, and oxygen removal. The model is agnostic to the energy source, and could be used for solar thermal or other configurations. We highlight the uses of this model, presenting some of the tradeoffs and challenges in redox-active material selection and how they affect the entire thermochemical hydrogen production process. This model could be easily adapted and used for material exploration, system/reactor design, and technoeconomic analysis.
1 Introduction
There is a significant growing interest in chemical looping processes where a metal oxide is used as an oxygen carrier in a redox cycle. These processes include chemical looping combustion, chemical looping reforming, chemical looping gasification, and thermochemical fuel production from water and
In a typical two-step non-stoichiometric metal oxide redox cycle, the endothermic reduction of the oxide is carried out as shown in Equation 1 where
This is followed by an exothermic oxidation step with either steam or
The endothermic reduction reaction is thermodynamically favored at high temperature and low oxygen partial pressure conditions, whereas the exothermic oxidation is thermodynamically favored by comparatively lower temperatures and high steam or
The techno-economic potential of chemical looping for
Coupling chemical looping redox cycles with concentrated solar thermal (CST) systems was first proposed as a potential pathway for the production of hydrogen during the 1970s (Nakamura, 1977). A myriad of widely-varying reactor concepts exist in the literature, including directly- and indirectly-irradiated designs as well as batch vs. flow-through designs (Steinfeld, 2005; Gokon et al., 2011; Romero and Steinfeld, 2012; Hathaway et al., 2016; Thanda et al., 2022; Budama et al., 2022). Many analyses pair a specific oxide material with a specific reactor design, and the resulting experimental or model-predicted performance combines the underlying material thermodynamics with heat and mass transfer limitations and characteristics that arise from the reactor design and/or selected operating conditions. These studies can provide valuable comprehensive assessments for the specific combination of oxide material, reactor, and operating conditions; however, there is little opportunity for extrapolation, direct comparison between oxide materials, or rapid iteration over wide ranges of temperature, material, and flow conditions for system-level performance optimization.
Most reactor designs can be conceptually categorized as fixed-bed batch reactors, fluidized-bed batch reactors, or flow-through designs employing parallel-flow (PF), mixed flow, or counter-current flow (CF) configurations. A general sketch of both PF and CF configurations is presented in Figure 1. Counter-current flow conditions are widely accepted as providing a theoretical upper limit on performance; however, as discussed by (Li et al., 2018b; Li et al., 2018a; Bulfin, 2019; de la Calle et al., 2022; and de la Calle et al., 2024), many past thermodynamic models for counter-current systems relied on inaccurate thermodynamic assumptions and thereby underestimate sweep gas and oxidizer flow requirements. This work aims to provide a simple thermodynamically-consistent model that can predict thermodynamic limits of
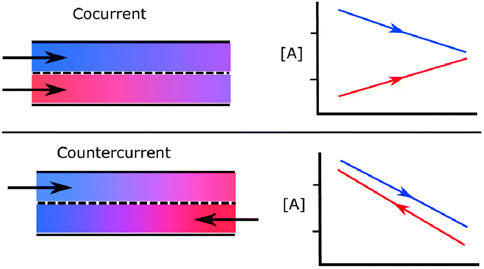
Figure 1. Comparison between parallel flow (cocurrent) and counterflow configurations with exchange of species A between the two streams (from Bulfin (2019) under CC-BY-NC license).
The paper follows to detail the modeling framework (Section 2), demonstrate the use of the modeling framework through an analysis of redox systems for thermochemical
2 Model
The thermodynamic model is based on the methodology developed and presented in (Bulfin, 2019), used in determining the thermodynamically-limited upper bounds of the process without considering transport limitations such as heat/mass transfer, or temperature and pressure gradients within the reactor system. We briefly present here the core methodology to allow the reader to follow our additional development of model extensions. For the full derivation the reader is referred to the original paper by (Bulfin, 2019).
2.1 Original thermodynamic model and its application to thermal reduction
The problem is formulated using an exchange coordinate
with A the species being exchanged,
with
while for counter-current flow the exchange coordinate of flow 2 is reversed, as shown in Equation 7 yielding
The thermodynamic upper limit for the exchange of A in a parallel flow (co-current) system is given by Equation 8:
with
and
In the case of a complete transfer of A from flow 1 to flow 2, neither Equation 9 nor Equation 10 are binding, but the fundamental thermodynamic condition of Equation 5 is met within the entirety of the reactor length. Two system parameters that are needed to calculate the
and the
with
with
2.2 Model extension
During the oxidation, a flow of
with the reactant and product mole fractions at the reactor effluent given by
The
considering that this time the oxide is absorbing
and the extent of reduction can be calculated as shown in Equation 18:
Assuming full selectivity, the conversion of
with
2.3 Redox thermodynamic formulation
To allow for a universal analysis of different redox materials, with different defect and vacancy formation mechanisms Zinkevich et al. (2006); Warren et al. (2022); Vieten et al. (2019); Wexler et al. (2023), we define the molar oxygen content fraction in the solid in Equation 20 as
with
with
2.4 System performance
The connection between the thermodynamics of a specific redox-active material and its performance in the redox cycle to an actual system is based on a previously developed model Lidor and Bulfin (2024) and is aligned with other works in the field Bulfin et al. (2021). However, one generalization presented here is the clear separation of heat and work requirements. While it is customary to convert the auxiliary work to heat equivalent in the field of solar thermochemistry Bulfin et al. (2021), there is increased interest in hybrid CST-PV systems that would be able to provide both heat and power. Moreover, the emerging field of high-temperature electric thermal energy storage (ETES) Ma et al. (2023) opens up the possibility of using renewable electricity to drive the thermochemical reaction. Hence, we have excluded the energy conversion penalties associated with the energy input source, whether via solar thermal or other sources. Instead, we have calculated the overall thermochemical conversion process efficiency based on the required heat and power per
with
with
The following energy terms are considered in this work:
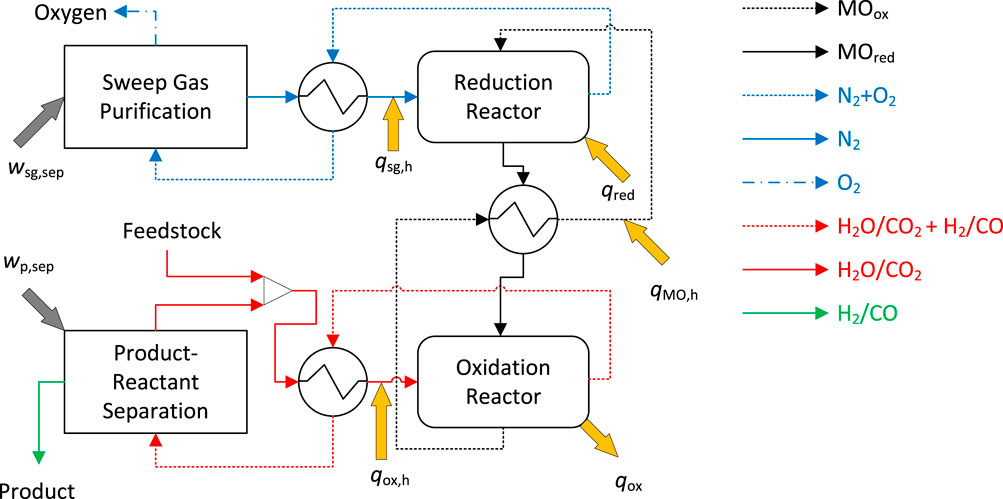
Figure 2. Process flow diagram for the generic moving oxide redox-based thermochemical system shown for
In addition, the effects of heat recovery, both in the gaseous streams (sweep gas and oxidizer gas) and in the solid stream, are evaluated by the implementation of heat recovery effectiveness values
The feedstock conversion extent is given by
with
which is in essence the moles of oxidizer that reacted per moles of redox-active material, over the moles of oxidizer fed into the reactor per moles of redox-active material.
2.5 Model structure
The model has been developed in MATLAB Version 2023b The MathWorks Inc. (2023) and is publicly available on a GitHub repository (https://github.com/NREL/REDOTHERM), including all the main scripts and required functions. The repository includes thermodynamic functions for several redox-active materials:
Thermodynamic properties of the fluids in the system are calculated using the CoolProp package (Bell et al., 2014) at the relevant process temperature and pressure, and the equilibrium composition of the oxidizer feed is calculated using the Gibbs energy minimization method implemented with Goodwin et al. (2023). The oxidizer gas can be selected as either
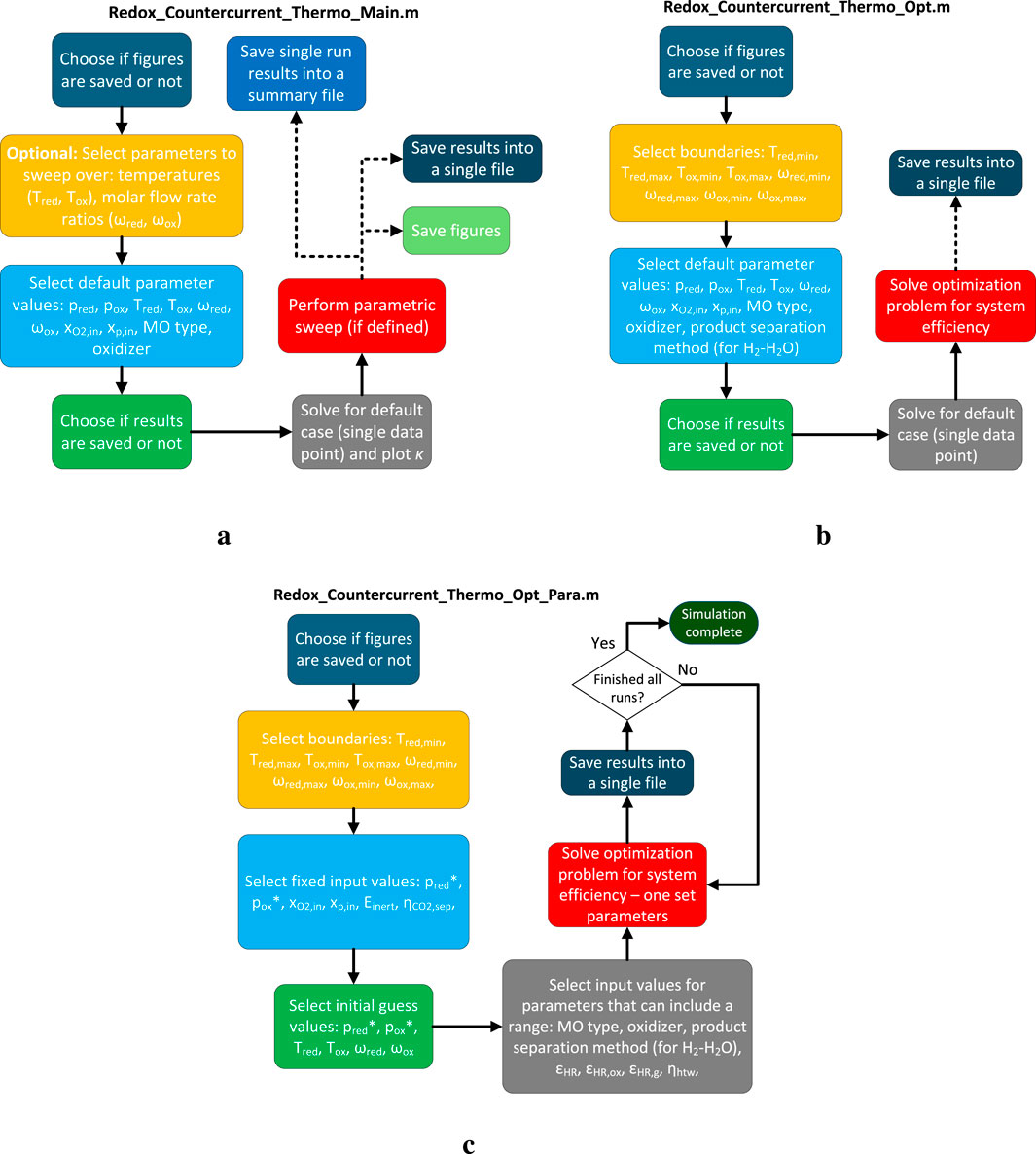
Figure 3. Code logic flowcharts for the three main scripts: (a) main code for single runs and parametric sweeps; (b) optimization code for a single run; and (c) optimization code for running multiple cases.
The reactor pressures during reduction
Cryogenic separation was selected for inert gas separation. Pressure swing adsorption (PSA) was implemented for product separation for
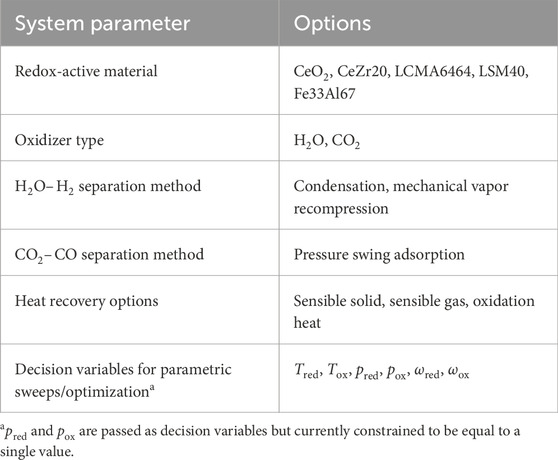
All the different options for the REDOTHERM code are provided in Table 1. The full details of the calculation methods for each term are given in the Supplementary Material.
3 Results
The basic capability of the model in predicting the redox performance of a water-splitting cycle is demonstrated for the case of
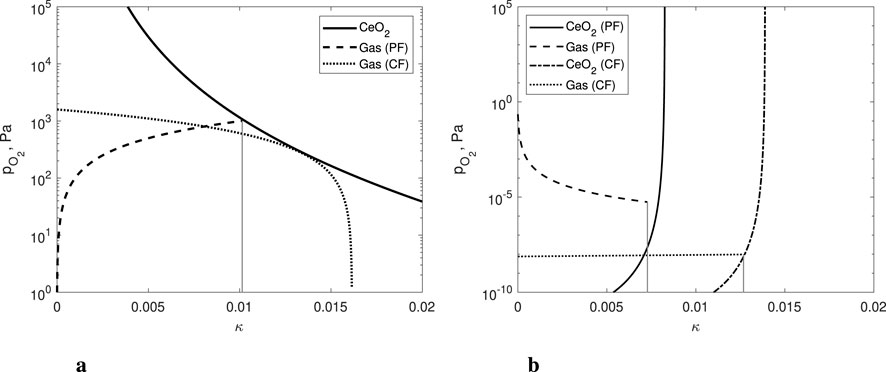
Figure 4. The
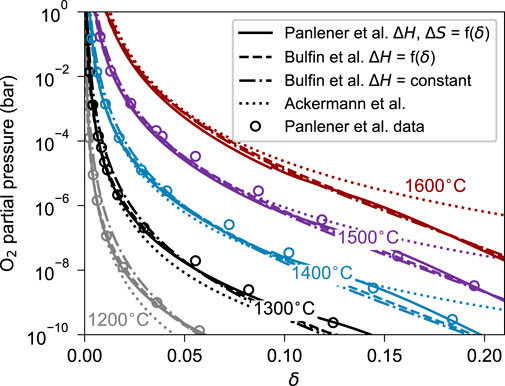
Figure 5. Comparison of the oxygen partial pressure
3.1 Parametric sweep capability
The parametric sweeps that are implemented in the main code (Redox_Countercurrent_Thermo_Main.m) are useful in investigating the effects of different operation and design parameters on all the different energy terms of the system as well as on its performance indicators. We present as an example the results of a parametric sweep over the molar flow rate ratios
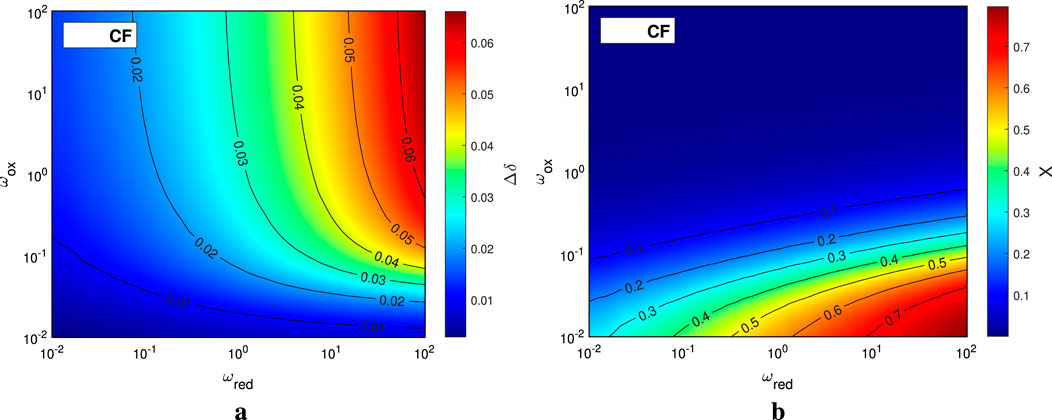
Figure 6. The reduction extent
We also calculate the system efficiency for these parametric sweep cases. The values used for the various heat recovery unit operations are
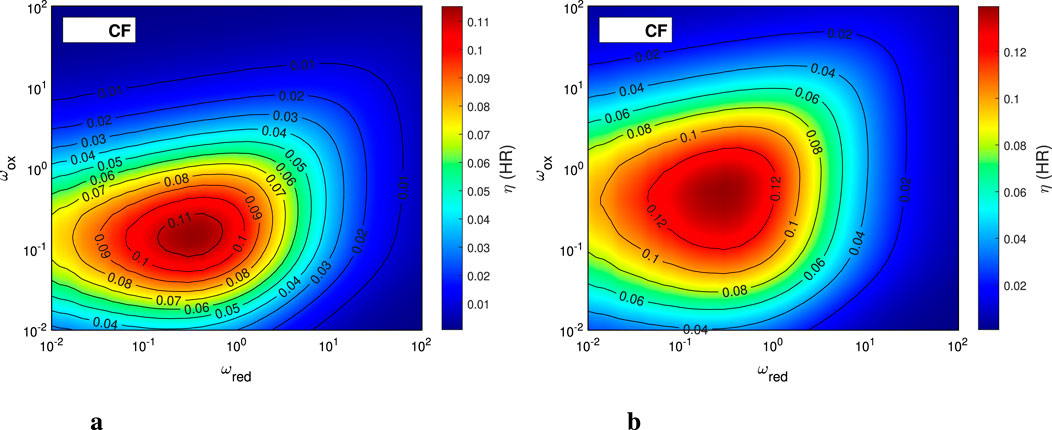
Figure 7. The cycle efficiency for CF case with
By examining the rest of the performance maps for the different energy fraction terms (sensible heating, etc.), one can obtain important insights into the specific thermochemical hydrogen (TCH) process that is evaluated, both from material and system perspective. The plots for this analysis are provided in the Supplementary Material.
3.2 Optimization capability
The optimization capability of the REDOTHERM code includes a single case optimization, as well as optimization for multiple cases, in which the model sweeps over a range of values for the different system parameters, such as redox-active material type, heat recovery effectiveness, and so forth. A sample usage is given here, demonstrating how it can be used to compare different redox-active materials, under the same operating conditions and boundaries. The input parameters, as well as the ranges of the decision variables, are given in Table 2. The optimization problem is solved using a direct search method (patternsearch in MATLAB) with the Nonuniform Pattern Search (NUPS) algorithm.
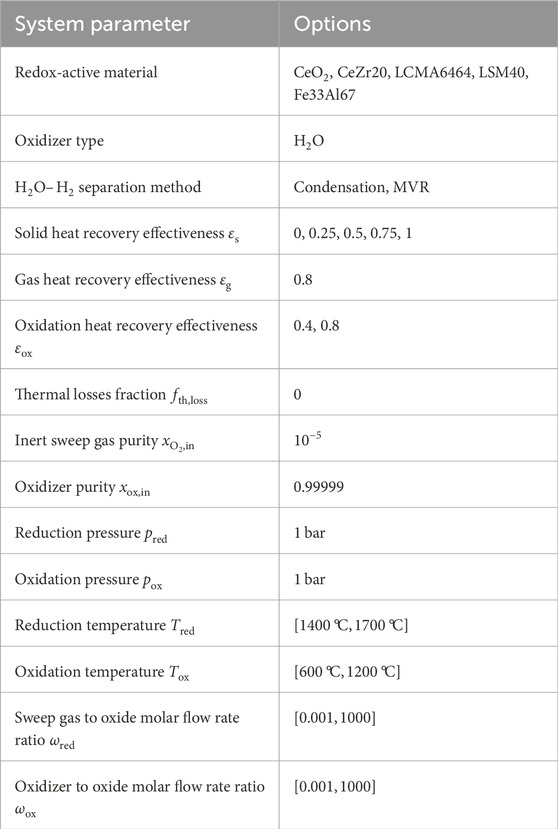
Table 2. Input parameters and ranges for decision variables for the REDOTHERM optimization analysis.
Five values for
The summary of the results from the optimization run is presented in Figure 8. We focus on a baseline case with
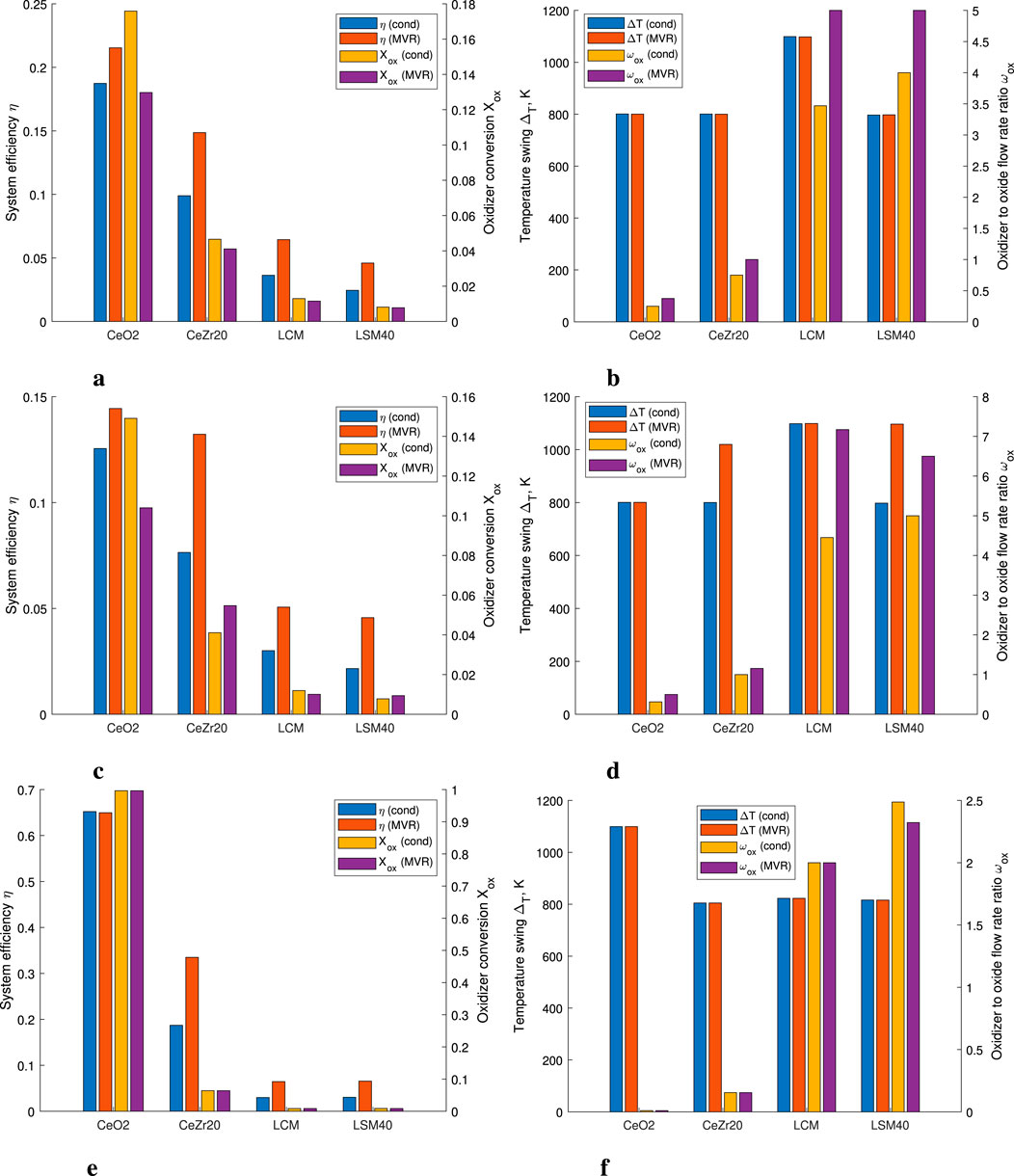
Figure 8. Comparison of the system efficiency, oxidizer conversion extent, temperature swing, and oxidizer to metal oxide flow rates ratio for different redox materials, using both condensation with reboiling and MVR as the
In Figure 8a we can see that the efficiency of
Examining a specific subset of results, we present in Figure 9 the specific energy terms for each of the evaluated redox-active materials for the base case optimized solution (i.e.,
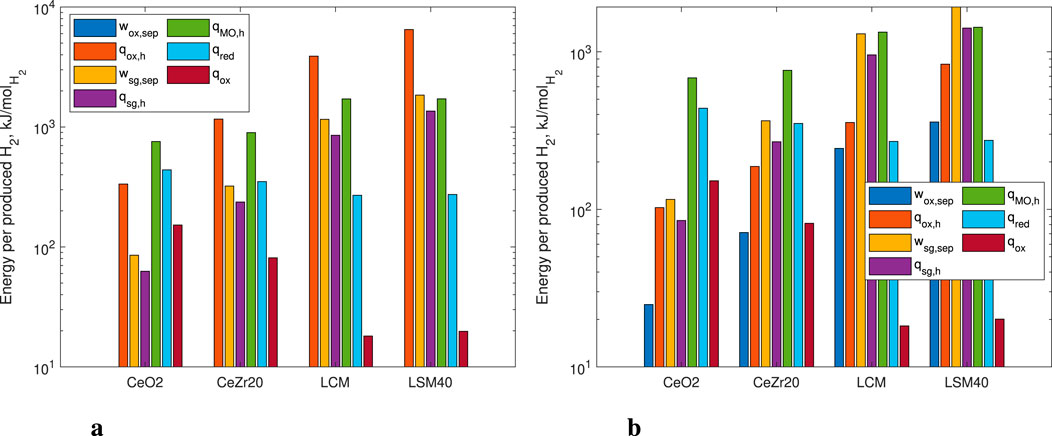
Figure 9. Energy terms for different redox-active materials normalized per mole of produced
The example cases shown in this section provide a brief overview of the capabilities of REDOTHERM. Extending upon them for new redox-active materials, both real and hypothetical, as well as adding more technology options for the auxiliary operations, is relatively straightforward and could support the field in the search for efficient and scalable designs.
3.3 Example of usage
In this subsection, we demonstrate the use of the REDOTHERM repository in a few types of analysis that can benefit different research activities within the TCH and chemical looping redox fields.
3.3.1 Productivity compared to efficiency and conversion
Many papers that deal with the discovery and characterization of TCH materials use the productivity (or yield) as the benchmarking performance indicator, defined as the amount of generated gaseous product (
In Figure 10 we present the productivity and conversion extent for
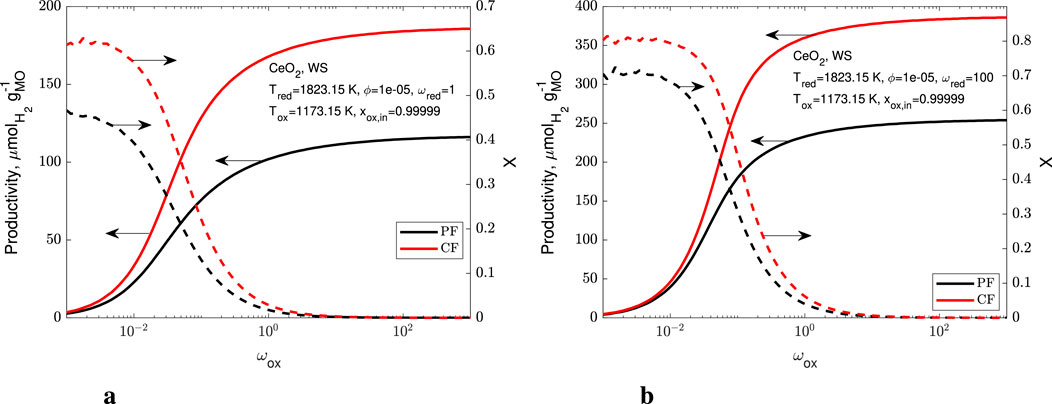
Figure 10. Productivity (solid lines) and conversion extent (dashed lines) as a function of the ratio of the oxidizer molar flow rate to the redox-active material molar flow rate for
In the same manner, REDOTHERM can be used to quickly evaluate the effects of the oxidizer purity on the redox performance. While in theory a feed of pure steam (or
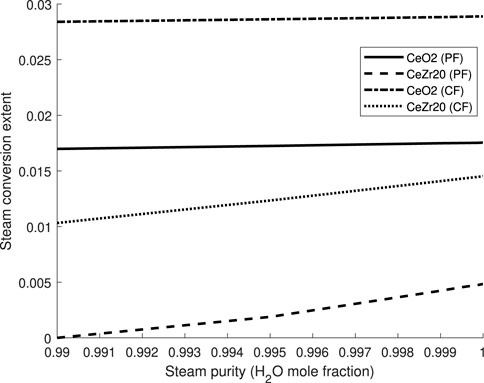
Figure 11. The effect of the oxidizer purity on the conversion extent for water splitting with
From these results, we can see that
3.3.2 Implementation for system and technoeconomic analysis of a specific case study
While all the performance indicators and results calculated by REDOTHERM are either dimensionless or specific values (per mole of redox-active material), the conversion to explicit values for a specific case study is straightforward. The use of REDOTHERM to size up a process for a given
with
All the specific energy terms
with i as the index specifying which term (i.e., inert gas separation, solid sensible heating, e.g.). From this, it is straightforward to perform sizing calculations for the auxiliary units, as well as sizing up the energy source, whether using CST or other. It can also be coupled to a hydrogen production financial model, such as ProFAST, to obtain a physics-based TEA framework (Kee and Penev, 2023).
4 Conclusion
The developed REDOTHERM model provides a comprehensive basis for analysis of different redox-based chemical looping systems for a variety of applications, with the initial focus on thermochemical hydrogen and syngas production. REDOTHERM is an open-source tool that can be used as a benchmarking tool for research and development work in this field. REDOTHERM can benefit the following activities:
1. Material discovery efforts: through quick prediction of the effects of design parameters on expected system performance, researchers could screen potential materials more efficiently, as well as couple system performance considerations to computational chemistry frameworks for designing materials with specific desired properties.
2. Reactor and system modeling: using REDOTHERM could allow reactor and system development efforts to quickly identify upper bound performance. While the model lacks detailed transport phenomena modeling, if the predicted performance for a system using REDOTHERM falls short of the target efficiency, an actual reactor/system would be unable to meet it.
3. Technoeconomic analysis (TEA): many TEA studies follow a simple ‘figure-of-merit’ approach, in which performance of different unit operations is assumed to meet a given value. While reasonable for established technologies with long operational data, when used for low technology readiness level (TRL) technologies such as redox-based chemical looping, there is not always a justification for selecting specific values. REDOTHERM could be coupled with a TEA framework to improve cost predictions and help in understanding the effects of different design and operating conditions on the cost of the product.
Natural future extensions and additions to REDOTHERM capabilities include adding models for the upstream energy generation, whether through solar thermal or other sources; including more auxiliary technology options; adding more reactor types, such as the mixed flow reactor; and adding sizing for the components, as a first step to incorporate TEA models. This open-source tool can help the research community in advancing this promising technology for various applications through a collaborative effort.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author contributions
AL: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Validation, Visualization, Writing – original draft, Writing – review and editing. JM: Investigation, Methodology, Validation, Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was authored by the National Renewable Energy Laboratory for the U.S. Department of Energy (DOE) under Contract No. DE-AC36-08GO28308. The authors gratefully acknowledge research support from the HydroGEN Advanced Water Splitting Materials Consortium, established as part of the Energy Materials Network under the U.S. Department of Energy, Office of Energy Efficiency and Renewable Energy, Fuel Cell Technologies Office, under Contract Numbers DE-EE0010732 and DE-EE0010729.
Acknowledgments
The authors would like to acknowledge the helpful comments and feedback on the model offered by Zahra Hosseinzadeh-Nik. AL would also like to thank Brendan Bulfin for the fruitful discussions on performance indicators and their relations to material properties.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fenrg.2025.1665986/full#supplementary-material
References
Abanades, S., and Flamant, G. (2006). Thermochemical hydrogen production from a two-step solar-driven water-splitting cycle based on cerium oxides. Sol. Energy 80, 1611–1623. doi:10.1016/j.solener.2005.12.005
Abanades, S., Legal, A., Cordier, A., Peraudeau, G., Flamant, G., and Julbe, A. (2010). Investigation of reactive cerium-based oxides for H2 production by thermochemical two-step water-splitting. J. Mater. Sci. 45, 4163–4173. doi:10.1007/s10853-010-4506-4
Ackermann, S., Takacs, M., Scheffe, J., and Steinfeld, A. (2017). Reticulated porous ceria undergoing thermochemical reduction with high-flux irradiation. Int. J. Heat Mass Transf. 107, 439–449. doi:10.1016/j.ijheatmasstransfer.2016.11.032
Adanez, J., Abad, A., Garcia-Labiano, F., Gayan, P., and De Diego, L. F. (2012). Progress in chemical-looping combustion and reforming technologies. Prog. energy Combust. Sci. 38, 215–282. doi:10.1016/j.pecs.2011.09.001
Bayon, A., de la Calle, A., Stechel, E. B., and Muhich, C. (2022). Operational limits of redox metal oxides performing thermochemical water splitting. Energy Technol. 10, 2100222–11. doi:10.1002/ente.202100222
Bell, I. H., Wronski, J., Quoilin, S., and Lemort, V. (2014). Pure and pseudo-pure fluid thermophysical property evaluation and the open-source thermophysical property library CoolProp. Industrial and Eng. Chem. Res. 53, 2498–2508. doi:10.1021/ie4033999
Budama, V. K., Brendelberger, S., Roeb, M., and Sattler, C. (2022). Performance analysis and optimization of solar thermochemical water-splitting cycle with single and multiple receivers. Energy Technol. 10, 2100220. doi:10.1002/ente.202100220
Bulfin, B. (2019). Thermodynamic limits of countercurrent reactor systems, with examples in membrane reactors and the ceria redox cycle. Phys. Chem. Chem. Phys. 21, 2186–2195. doi:10.1039/C8CP07077F
Bulfin, B., Call, F., Lange, M., Lübben, O., Sattler, C., Pitz-Paal, R., et al. (2015). Thermodynamics of CeO2 thermochemical fuel production. Energy and Fuels 29, 1001–1009. doi:10.1021/ef5019912
Bulfin, B., Hoffmann, L., de Oliveira, L., Knoblauch, N., Call, F., Roeb, M., et al. (2016). Statistical thermodynamics of non-stoichiometric ceria and ceria zirconia solid solutions. Phys. Chem. Chem. Phys. 18, 23147–23154. doi:10.1039/C6CP03158G
Bulfin, B., Miranda, M., and Steinfeld, A. (2021). Performance indicators for benchmarking solar thermochemical fuel processes and reactors. Front. Energy Res. 9, 677980–12. doi:10.3389/fenrg.2021.677980
Carrillo, R. J., and Scheffe, J. R. (2019). Beyond ceria: theoretical investigation of isothermal and near-isothermal redox cycling of perovskites for solar thermochemical fuel production. Energy and Fuels 33, 12871–12884. doi:10.1021/acs.energyfuels.9b02714
Chueh, W. C., Falter, C., Abbott, M., Scipio, D., Furler, P., Haile, S. M., et al. (2010). High-flux solar-driven thermochemical dissociation of CO2 and H2O using ceria redox reactions. Science 330, 1797–1801. doi:10.1126/science.1197834
de la Calle, A., Ermanoski, I., and Stechel, E. B. (2022). Towards chemical equilibrium in thermochemical water splitting. part 1: thermal reduction. Int. J. Hydrogen Energy 47, 10474–10482. doi:10.1016/j.ijhydene.2021.07.167
de la Calle, A., Ermanoski, I., Miller, J. E., and Stechel, E. B. (2024). Towards chemical equilibrium in thermochemical water splitting. Part 2: Re-oxidation. Int. J. Hydrogen Energy 72, 1159–1168. doi:10.1016/j.ijhydene.2024.05.298
Ehrhart, B. D., Muhich, C. L., Al-Shankiti, I., and Weimer, A. W. (2016). System efficiency for two-step metal oxide solar thermochemical hydrogen production – Part 2: impact of gas heat recuperation and separation temperatures. Int. J. Hydrogen Energy 41, 19894–19903. doi:10.1016/j.ijhydene.2016.07.110
Gokon, N., Mataga, T., Kondo, N., and Kodama, T. (2011). Thermochemical two-step water splitting by internally circulating fluidized bed of NiFe2O4 particles: successive reaction of thermal-reduction and water-decomposition steps. Int. J. Hydrogen Energy 36, 4757–4767. doi:10.1016/j.ijhydene.2011.01.076
Goodwin, D. G., Moffat, H. K., Schoegl, I., Speth, R. L., and Weber, B. W. (2023). Cantera: an object-oriented software toolkit for chemical kinetics, thermodynamics, and transport processes. doi:10.5281/zenodo.8137090
Hao, Y., Yang, C.-K., and Haile, S. M. (2014). Ceria–zirconia solid solutions (Ce1-xZrxO2-δ, x ≤ 0.2) for solar thermochemical water splitting: a thermodynamic study. Chem. Mater. 26, 6073–6082. doi:10.1021/cm503131p
Hathaway, B. J., Bala Chandran, R., Gladen, A. C., Chase, T. R., and Davidson, J. H. (2016). Demonstration of a solar reactor for carbon dioxide splitting via the isothermal ceria redox cycle and practical implications. Energy and Fuels 30, 6654–6661. doi:10.1021/acs.energyfuels.6b01265
Kathe, M., Empfield, A., Sandvik, P., Fryer, C., Zhang, Y., Blair, E., et al. (2017). Utilization of CO2 as a partial substitute for methane feedstock in chemical looping methane–steam redox processes for syngas production. Energy and Environ. Sci. 10, 1345–1349. doi:10.1039/c6ee03701a
Kee, J., and Penev, M. M. (2023). ProFAST production financial analysis scenario tool SWR-23-88. Golden, CO: Tech. rep., National Renewable Energy Laboratory.
Lany, S. (2024). Chemical potential analysis as an alternative to the van’t hoff method: hypothetical limits of solar thermochemical hydrogen. J. Am. Chem. Soc. 146, 14114–14127. doi:10.1021/jacs.4c02688
Li, S., Wheeler, V. M., Kreider, P. B., Bader, R., and Lipiński, W. (2018a). Thermodynamic analyses of fuel production via solar-driven non-stoichiometric metal oxide redox cycling. Part 2. Impact of solid–gas flow configurations and active material composition on system-level efficiency. Energy and Fuels 32, 10848–10863. doi:10.1021/acs.energyfuels.8b02082
Li, S., Wheeler, V. M., Kreider, P. B., and Lipiński, W. (2018b). Thermodynamic analyses of fuel production via solar-driven non-stoichiometric metal oxide redox cycling. Part 1. Revisiting flow and equilibrium assumptions. Energy and Fuels 32, 10838–10847. doi:10.1021/acs.energyfuels.8b02081
Lidor, A. (2024). Hydrogen–steam separation using mechanical vapor recompression cycle. Int. J. Hydrogen Energy 94, 664–668. doi:10.1016/j.ijhydene.2024.11.040
Lidor, A., and Bulfin, B. (2024). A critical perspective and analysis of two-step thermochemical fuel production cycles. Sol. Compass 11, 100077. doi:10.1016/j.solcom.2024.100077
Lidor, A., and Zimmermann, L. (2023). Experimental demonstration of high-temperature heat recovery in a solar reactor. Sol. Energy 262, 111915. doi:10.1016/j.solener.2023.111915
Lidor, A., Fend, T., Roeb, M., and Sattler, C. (2021). High performance solar receiver–reactor for hydrogen generation. Renew. Energy 179, 1217–1232. doi:10.1016/j.renene.2021.07.089
Lidor, A., Aschwanden, Y., Häseli, J., Reckinger, P., Haueter, P., and Steinfeld, A. (2023). High-temperature heat recovery from a solar reactor for the thermochemical redox splitting of H2O and CO2. Appl. Energy 329, 120211. doi:10.1016/j.apenergy.2022.120211
Ma, Z., Gifford, J., Wang, X., and Martinek, J. (2023). Electric-thermal energy storage using solid particles as storage media. Joule 7, 843–848. doi:10.1016/j.joule.2023.03.016
Mao, Y., Gao, Y., Dong, W., Wu, H., Song, Z., Zhao, X., et al. (2020). Hydrogen production via a two-step water splitting thermochemical cycle based on metal oxide–a review. Appl. energy 267, 114860. doi:10.1016/j.apenergy.2020.114860
McCord, D. C., Gager, E. J., Wang, X., Johnson, T. L., Beachy, J. S., King, K. A., et al. (2024). Solar thermochemical redox cycling using ga- and al-doped lsm perovskites for renewable hydrogen production. J. Phys. Chem. C 128, 15796–15806. doi:10.1021/acs.jpcc.4c02797
McDaniel, A. H., Miller, E. C., Arifin, D., Ambrosini, A., Coker, E. N., O’Hayre, R., et al. (2013). Sr- and Mn-doped LaAlO3-δ for solar thermochemical H 2 and CO production. Energy Environ. Sci. 6, 2424–2428. doi:10.1039/c3ee41372a
Nakamura, T. (1977). Hydrogen production from water utilizing solar heat at high temperatures. Sol. Energy 19, 467–475. doi:10.1016/0038-092X(77)90102-5
Panlener, R. J., Blumenthal, R. N., and Garnier, J. E. (1975). A thermodynamic study of nonstoichiometric cerium dioxide. J. Phys. Chem. Solids 36, 1213–1222. doi:10.1016/0022-3697(75)90192-4
Patankar, A. S., Wu, X.-Y., Choi, W., Tuller, H. L., and Ghoniem, A. F. (2022). A reactor train system for efficient solar thermochemical fuel production. J. Sol. Energy Eng. 144, 061014–12. doi:10.1115/1.4055298
Romero, M., and Steinfeld, A. (2012). Concentrating solar thermal power and thermochemical fuels. Energy Environ. Sci. 5, 9234–9245. doi:10.1039/c2ee21275g
Scheffe, J. R., and Steinfeld, A. (2014). Oxygen exchange materials for solar thermochemical splitting of H2O and CO2: a review. Mater. Today 17, 341–348. doi:10.1016/j.mattod.2014.04.025
Siegel, N. P., Miller, J. E., Ermanoski, I., Diver, R. B., and Stechel, E. B. (2013). Factors affecting the efficiency of solar driven metal oxide thermochemical cycles. Industrial and Eng. Chem. Res. 52, 3276–3286. doi:10.1021/ie400193q
Steinfeld, A. (2005). Solar thermochemical production of hydrogen - a review. Sol. Energy 78, 603–615. doi:10.1016/j.solener.2003.12.012
Thanda, V., Fend, T., Laaber, D., Lidor, A., von Storch, H., Säck, J., et al. (2022). Experimental investigation of the applicability of a 250 kW ceria receiver/reactor for solar thermochemical hydrogen generation. Renew. Energy 198, 389–398. doi:10.1016/j.renene.2022.08.010
Tran, J. T., Warren, K. J., Mejic, D., Anderson, R. L., Jones, L., Hauschulz, D. S., et al. (2023). Pressure-enhanced performance of metal oxides for thermochemical water and carbon dioxide splitting. Joule 7, 1759–1768. doi:10.1016/j.joule.2023.07.016
Vieten, J., Bulfin, B., Huck, P., Horton, M., Guban, D., Zhu, L., et al. (2019). Materials design of perovskite solid solutions for thermochemical applications. Energy and Environ. Sci. 12, 1369–1384. doi:10.1039/c9ee00085b
Warren, K. J., and Weimer, A. W. (2022). Solar thermochemical fuels: present status and future prospects. Sol. Compass 1, 100010. doi:10.1016/j.solcom.2022.100010
Warren, K. J., Tran, J. T., and Weimer, A. W. (2022). A thermochemical study of iron aluminate-based materials: a preferred class for isothermal water splitting. Energy Environ. Sci. 15, 806–821. doi:10.1039/d1ee02679h
Wexler, R. B., Gautam, G. S., Bell, R. T., Shulda, S., Strange, N. A., Trindell, J. A., et al. (2023). Multiple and nonlocal cation redox in ca–ce–ti–mn oxide perovskites for solar thermochemical applications. Energy and Environ. Sci. 16, 2550–2560. doi:10.1039/d3ee00234a
Wilson, S. A., Sarsam, P. W., Stechel, E. B., and Muhich, C. L. (2024). Extracting metal oxide redox thermodynamics from tga measurements requires moving beyond the linearized van ’t hoff approach. Front. Energy Res. 12, 1470010–1472024. doi:10.3389/fenrg.2024.1470010
Yang, C.-K., Yamazaki, Y., Aydin, A., and Haile, S. M. (2014). Thermodynamic and kinetic assessments of strontium-doped lanthanum manganite perovskites for two-step thermochemical water splitting. J. Mater. Chem. A 2, 13612–13623. doi:10.1039/C4TA02694B
Zang, G., Sun, P., Elgowainy, A. A., Bafana, A., and Wang, M. (2021). Performance and cost analysis of liquid fuel production from h2 and co2 based on the fischer-tropsch process. J. CO2 Util. 46, 101459. doi:10.1016/j.jcou.2021.101459
Zhao, X., Zhou, H., Sikarwar, V. S., Zhao, M., Park, A.-H. A., Fennell, P. S., et al. (2017). Biomass-based chemical looping technologies: the good, the bad and the future. Energy and Environ. Sci. 10, 1885–1910. doi:10.1039/c6ee03718f
Zinkevich, M., Djurovic, D., and Aldinger, F. (2006). Thermodynamic modelling of the cerium–oxygen system. Solid State Ionics 177, 989–1001. doi:10.1016/j.ssi.2006.02.044
Nomenclature
Roman symbols
HHV Higher heating value, J mol−1
Greek symbols
Subscripts
f final state
fuel Fuel
g gas phase
in Inlet
MO Metal oxide
out Outlet
ox Oxidation
prod Product
reac Reactant
red Reduction
req Required for the entire process
sg Sweep gas
s solid phase
Superscripts
ox Oxidation
red Reduction
Keywords: chemical looping, solar thermochemical hydrogen production, system modeling, advanced water splitting, thermodynamic modeling
Citation: Lidor A and Martinek J (2025) REDOTHERM: a thermodynamic modeling framework for redox-based thermochemical processes. Front. Energy Res. 13:1665986. doi: 10.3389/fenrg.2025.1665986
Received: 14 July 2025; Accepted: 15 September 2025;
Published: 07 October 2025.
Edited by:
Julie Mougin, CEA/LITEN, FranceReviewed by:
Abhishek Kumar Singh, University of Twente, NetherlandsAlberto De La Calle, Spanish National Research Council (CSIC), Spain
Copyright © 2025 Lidor and Martinek. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alon Lidor, YWxvbi5saWRvckBucmVsLmdvdg==
 Alon Lidor
Alon Lidor Janna Martinek
Janna Martinek