- 1Department of Joint Surgery, Honghui Hospital, Xi'an Jiaotong University, Xi’an, Shanxi, China
- 2Department of Spine Surgery, First Affiliated Hospital of Shihezi University, Shihezi, Xinjiang, China
- 3Department of Urinary Surgery, Xi ‘an Jiaotong University Medical College Affiliated City Ninth Hospital, Xi’an, Shanxi, China
- 4Department of Spine Surgery, Honghui Hospital, Xi'an Jiaotong University, Xi’an, Shanxi, China
Intervertebral disc degeneration (IDD) is a prevalent and debilitating condition that affects millions worldwide, leading to chronic back pain and a reduced quality of life. This review shifts the focus to the pivotal role of the immune microenvironment in IDD, highlighting its dual functions—exacerbating degeneration through chronic inflammation while also offering protective mechanisms under certain conditions. Recent research highlights how immune cells such as macrophages, T cells, and B cells, along with cytokines like IL-1β, TNF-α, and IL-6, play dual roles in both exacerbating and potentially mitigating disc degeneration. Key signaling pathways, including NF-κB, MAPK, JAK-STAT, and the NLRP3 inflammasome, are discussed to illustrate their involvement in disc cell apoptosis, extracellular matrix degradation, and chronic inflammation. By synthesizing current research, this review underscores the potential of novel therapeutic strategies that target immune modulation. Anti-inflammatory drugs, biologics, stem cell therapy, and gene editing technologies are explored as promising avenues for treatment. Understanding the immune landscape of IDD not only enhances our knowledge of its pathogenesis but also opens new possibilities for effective, targeted therapies, aiming to improve patient outcomes and reduce the societal burden of this debilitating condition.
1 Introduction
Intervertebral disc degeneration (IDD) is a prevalent and far-reaching spinal disorder characterized by progressive degeneration of disc structure and function. The normal intervertebral disc consists of the annulus fibrosus, nucleus pulposus, and cartilaginous endplates, and its primary function is to absorb and distribute mechanical loads, thereby maintaining flexibility and stability of the spine. With age, the water content and elasticity of the intervertebral disc decreases, resulting in impairment of its load-carrying and distribution functions (1). This degenerative process involves multiple pathophysiologic mechanisms, including extracellular matrix (ECM) degradation, apoptosis, and inflammatory responses (2). Key enzymes, such as matrix metalloproteinases (MMPs) and deintegrin metalloproteinases bound to the structural domain of thrombopoietin (ADAMTS), play an important role in ECM degradation, destroying collagen and proteoglycans, thereby compromising the structural integrity of the disc. In addition, oxidative stress and nutritional deficiencies induce apoptosis and autophagy in intervertebral disc cells, further impairing their repair capacity (3, 4). Clinically, IDD mainly manifests as chronic lower back pain and sciatica, with symptoms that may be continuous or intermittent and are often accompanied by radiating pain and sensory abnormalities in the lower extremities. Severe herniated discs may also lead to spinal instability and limited mobility, significantly affecting the patient’s daily life and ability to work. (5).
The prevalence of IDD is high worldwide and is the leading cause of chronic lower back pain. Statistics show that approximately 80% of the population will experience lower back pain at least once in their lifetime, and the prevalence of IDD among people over the age of 40 reaches 60-80%. In the United States, IDD-related lower back pain is the leading cause of work absenteeism and decreased productivity, resulting in tens of billions of dollars in medical costs and indirect economic losses each year. (6). Therefore, elucidating the pathophysiological mechanisms of IDD and developing effective treatment strategies are crucial for improving patient quality of life and alleviating public health burdens. Investigating the immune microenvironment in IDD could unveil novel therapeutic targets, providing new insights and approaches for the prevention and treatment of this debilitating condition.
IDD has long been considered an irreversible degenerative disease primarily driven by age-related physiological changes (7, 8). Traditional treatments have focused on symptom relief and improving quality of life, including conservative and surgical treatments. Among conservative treatments, physical therapy, nonsteroidal anti-inflammatory drugs (NSAIDs), steroid injections, and lifestyle modifications can provide some pain relief, but cannot fundamentally reverse or stop disc degeneration (9). Surgical treatments (e.g., discectomy, spinal fusion, and artificial disc replacement), on the other hand, are aimed at severe degeneration cases, but these methods also have certain risks and limitations (10, 11).
The immune microenvironment plays a crucial role in maintaining tissue health and regulating disease processes, and includes a variety of immune cells (e.g., macrophages, T cells, and B cells) and secreted cytokines and chemokines. Under normal conditions, the immune microenvironment maintains tissue homeostasis by removing cellular debris, fighting pathogens, and promoting tissue repair. However, under pathological conditions, the immune microenvironment may undergo aberrant changes leading to chronic inflammation and tissue damage (12). In recent years, more and more studies have begun to focus on the role of the immune microenvironment in IDD, revealing the dual role of the immune response in the degenerative process. (13, 14) On the one hand, immune cells and inflammatory cytokines can play a protective role by removing degenerating cells and tissue debris, regulating ECM degradation, and promoting repair processes (15). On the other hand, excessive or sustained immune responses can lead to chronic inflammation, accelerated ECM degradation and apoptosis, and thus accelerated disc degeneration (16, 17). Specifically, the role of macrophages in IDD has garnered significant attention. Macrophages can polarize into functionally distinct M1 (pro-inflammatory) and M2 (anti-inflammatory) types (18). Studies have found that in the early stages of IDD, the number of M1 macrophages increases, secreting large amounts of pro-inflammatory cytokines (such as IL-1β and TNF-α), which not only directly damage the ECM but also activate other immune cells, further exacerbating the inflammatory response (19). In the repair stage, M2 macrophages promote ECM repair and regeneration by secreting anti-inflammatory cytokines and growth factors (such as IL-10 and TGF-β) (20). Additionally, the application of new technologies such as single-cell RNA sequencing has enabled researchers to more precisely analyze the cellular composition and gene expression profiles within disc tissues, uncovering the specific roles of different immune cell subsets in IDD (21). These research findings have deepened our understanding of the pathophysiological mechanisms of IDD and provided a theoretical foundation for developing novel immunomodulatory therapies. For instance, modulating macrophage polarization to inhibit excessive inflammation or promote tissue repair could be an effective strategy for future IDD treatments (22).
This review aims to systematically summarize the role of the immune system in the process of IDD and explore its therapeutic potential. In recent years, an increasing number of studies have shown that immune cells and inflammatory cytokines play critical roles in IDD, influencing both the progression of degeneration and tissue repair and regeneration. By reviewing the latest research advances, this review aims to reveal the dual role of the immune system in IDD and discuss the feasibility and prospects of innovative immunomodulatory therapeutic strategies.
2 Anatomy and immunological characteristics of the intervertebral disc
2.1 Structure and function of the intervertebral disc
The intervertebral disc, located between the vertebral bones, is composed of three main parts: the annulus fibrosus, the nucleus pulposus, and the cartilage endplates (23, 24). The annulus fibrosus is a tough outer ring composed of multiple layers of collagen fibers and chondrocytes, primarily functioning to provide structural support and restrict the movement of the nucleus pulposus (25). The nucleus pulposus, located at the center of the disc, contains a high-water content, proteoglycans, and collagen, serving mainly to cushion pressure and distribute loads. The cartilage endplates cover the upper and lower surfaces of the disc, composed of hyaline cartilage, protecting the vertebral bodies and transmitting loads (1). The primary function of the intervertebral disc is to absorb and distribute mechanical pressures generated by daily activities and movements (26). It achieves this through the gel-like properties of the nucleus pulposus and the tough structure of the annulus fibrosus, enabling the spine to flexibly move and maintain stability (27). Additionally, the intervertebral disc plays a crucial role in maintaining the height and shape of the spine, ensuring normal spinal curvature and rotation.
2.2 Composition of the immune microenvironment
The immune microenvironment of the intervertebral disc consists of various immune cells, cytokines, and other signaling molecules (28, 29). Under normal conditions, the disc contains very few immune cells due to its low blood supply and immune-privileged status (30). The main immune cells include macrophages, T cells, and B cells (15). Macrophages are key regulators of the disc’s immune response, playing vital roles in tissue repair and the clearance of cell debris (31). T cells and B cells, as components of the adaptive immune system, are crucial in chronic inflammation and immune memory (32). Additionally, neutrophils and natural killer (NK) cells also play important roles in acute and chronic inflammatory responses (33). Cytokines such as IL-1β, TNF-α, and IL-6 are critical in both healthy and pathological states of the disc. These cytokines influence disc function and health by regulating cell migration, inflammatory responses, and tissue repair (4, 34). Chemokines, on the other hand, guide immune cell migration to sites of damage or infection, thereby participating in the regulation of the immune microenvironment (30, 35).
2.3 Immunological characteristics of the intervertebral disc
Under normal conditions, the low oxygen environment and lack of vascular supply within the disc restrict the entry of immune cells (36). However, during disc degeneration, this immune privilege can be compromised, leading to immune cell infiltration and chronic inflammation. Common immune cells found in degenerated discs include macrophages, T cells, and neutrophils (37). Studies indicate that the increase in inflammatory cytokines and immune cells in degenerated discs is closely related to disc tissue degradation and pain (7, 38). For instance, the upregulation of cytokines such as IL-1β and TNF-α further enhances the activity of degradative enzymes like MMPs and ADAMTS, leading to ECM breakdown (39). These inflammatory cytokines not only directly damage the disc’s ECM components but also induce cell apoptosis and inhibit cell proliferation, thereby accelerating disc degeneration (40).
Understanding these immunological characteristics and their changes is crucial for developing new therapeutic strategies (41). Research indicates that modulating the immune response within the disc can reduce inflammation and tissue damage, thereby delaying or reversing disc degeneration (42). For example, using anti-inflammatory drugs and biologics can effectively inhibit the activity of key inflammatory cytokines, thereby alleviating pain and improving function (43, 44). Additionally, cell therapy and gene therapy have shown potential in modulating the disc’s immune microenvironment, promising new avenues for future IDD treatments (45). By further studying the immune microenvironment of the intervertebral disc and its changes during degeneration, we can better understand the mechanisms of IDD and explore the potential of immunomodulation in its treatment. This multidisciplinary research approach not only helps to elucidate the pathophysiological mechanisms of IDD but also provides new insights and methods for clinical treatment (43, 46).
3 Immune response in the process of intervertebral disc degeneration
3.1 Immune cells
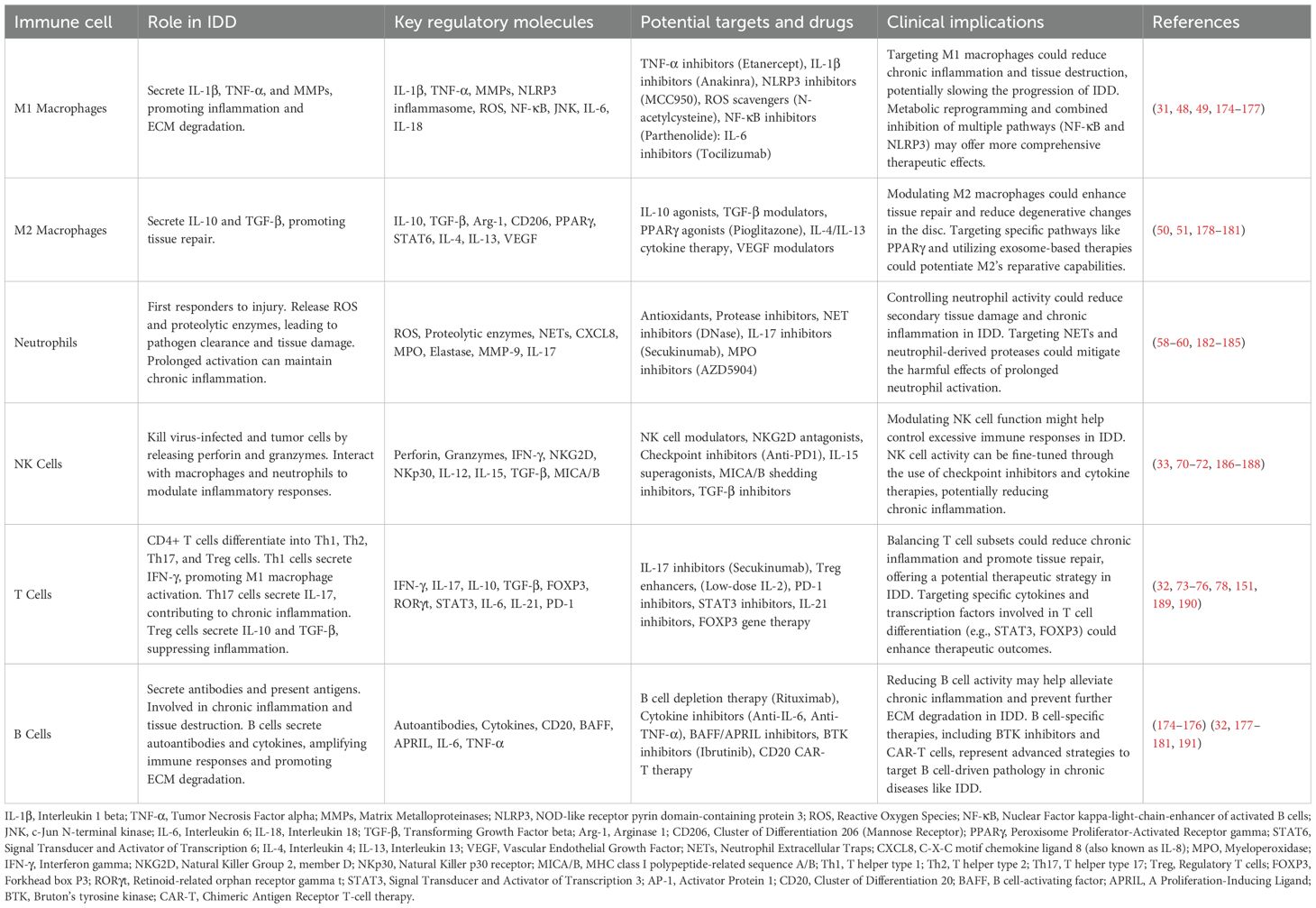
IDD is a complex pathological process involving multiple interacting factors, with immune responses playing a crucial role. Various immune cells, such as macrophages, neutrophils, natural killer (NK) cells, and lymphocytes (T cells and B cells), participate in this process, influencing the progression of disc degeneration through different mechanisms (Table 1).
3.1.1 Macrophages
Macrophages are among the earliest identified and studied immune cells in IDD. They are widely present in degenerated discs and regulate inflammatory responses and tissue remodeling by secreting various cytokines and enzymes (47). Macrophages play different roles in the early and late stages of disc degeneration. In the early stages, macrophages are primarily of the M1 type, secreting pro-inflammatory cytokines such as IL-1β and TNF-α, promoting inflammation and accelerating ECM degradation (31, 48). These pro-inflammatory macrophages also secrete MMPs, directly damaging the annulus fibrosus and nucleus pulposus structures (49). In the late stages of degeneration, the proportion of M2 macrophages increases. M2 macrophages secrete anti-inflammatory cytokines such as IL-10 and TGF-β, suppressing inflammation and promoting tissue repair (50). However, studies indicate that in chronic degeneration, the imbalance between M1 and M2 macrophages leads to persistent inflammation and tissue damage (51). Recent studies have further revealed the complex role of macrophages in IDD. Zhao et al. (34) found that M1 macrophages promote disc cell apoptosis and ECM degradation through the activation of the NLRP3 inflammasome (52). Additionally, Hou et al. discovered that the polarization state of macrophages can be regulated by specific microenvironmental factors such as hypoxia and high glucose, which are prevalent in degenerated discs (53). Another key finding is the interaction between macrophages and disc cells. In vitro co-culture experiments showed that disc cells could attract macrophages by secreting chemokines like CCL2 and regulate macrophage polarization by secreting cytokines such as IL-6 and TNF-α (54). This interaction plays an important role in maintaining the immune microenvironment of the disc. Recent findings have demonstrated that the metabolic features of the degenerated disc—particularly elevated lactate due to hypoxia and increased ROS—are potent modulators of macrophage phenotype (55). Lactate can stabilize HIF-1α and promote M2-like polarization, contributing to a reparative but potentially fibrotic environment (56). Conversely, high ROS levels activate the NF-κB pathway, skewing macrophages toward a pro-inflammatory M1 state (57). These metabolic cues may contribute to the imbalance between M1 and M2 macrophages observed in chronic IDD.
3.1.2 Neutrophils
Neutrophils, as key components of the innate immune system, also play critical roles at different stages of IDD. Neutrophils are among the first immune cells to respond rapidly to injury and infection (58, 59). In the acute stage of disc degeneration, neutrophils are quickly recruited to the lesion site, where they release reactive oxygen species (ROS) and proteolytic enzymes to directly kill pathogens and clear damaged cells (60). However, these molecules can also damage surrounding healthy tissues, further exacerbating disc degeneration (61). In the chronic stage, the persistent presence and activation of neutrophils lead to sustained low-grade inflammation, which is associated with chronic low back pain and further structural damage to the disc (62). Neutrophil migration is primarily regulated by chemokines such as CXCL8 (63). These chemokines are upregulated during disc degeneration, inducing neutrophil aggregation at the lesion site (64). Neutrophils further break down ECM components by releasing various effector molecules such as elastase and MMPs, damaging disc structure. (65). Recent studies indicate that neutrophils not only participate in the initial inflammatory response but also play important roles in chronic inflammation. For example, Dudli et al. (66) found that neutrophils form extracellular traps (NETs) in chronic disc degeneration, which capture and kill invading microbes but also cause self-tissue damage (67, 68). NETs can also interact with macrophages and other immune cells, further exacerbating the inflammatory response. In summary, neutrophils have multifaceted roles in IDD. Initially, they protect by responding rapidly to inflammatory signals, clearing pathogens, and damaged cells. However, the ROS and proteolytic enzymes released by neutrophils can also damage surrounding healthy tissues, especially during chronic inflammation (69). Therefore, careful regulation of neutrophil function is needed in treatment to balance pathogen clearance and tissue protection.
3.1.3 Natural killer cells
NK cells primarily kill virus-infected and tumor cells by releasing perforin and granzymes (70).In IDD, NK cells also participate in regulating local inflammatory responses (71). Although specific studies on NK cells in IDD are limited, there is evidence that NK cells can interact with macrophages and neutrophils to modulate the immune microenvironment in disc degeneration (72).
3.1.4 T cells
T cells play various roles in IDD, including promoting and regulating inflammatory responses (73). CD4+ helper T cells can differentiate into different subsets (such as Th1, Th2, Th17, and Treg cells), each playing distinct roles in inflammation and immune regulation (74). For instance, Th1 cells secrete IFN-γ, promoting M1 macrophage polarization and activation, thus enhancing inflammation. Th17 cells secrete pro-inflammatory cytokines such as IL-17, playing significant roles in autoimmune diseases and chronic inflammation (19). In contrast, Treg cells secrete anti-inflammatory cytokines such as IL-10 and TGF-β, suppressing inflammation and promoting tissue repair (75). The imbalance of T cells in IDD can lead to sustained inflammation and tissue damage (76). Recent studies show that T cells’ roles in IDD extend beyond local inflammatory responses. Weiler et al. (77) found significant T cell infiltration in the disc tissues of IDD patients, with these T cells secreting various cytokines and chemokines to regulate the local immune microenvironment (78). Additionally, T cells can amplify inflammatory responses by interacting with other immune cells such as macrophages and B cells, further exacerbating disc degeneration.
3.1.5 B cells
Despite limited studies on B cells in IDD, their potential roles should not be overlooked. B cells can regulate immune responses by secreting antibodies and presenting antigens (79). Studies show that in autoimmune diseases such as rheumatoid arthritis, B cells promote inflammation and tissue destruction by secreting autoantibodies. In IDD, B cells may participate in chronic inflammation through similar mechanisms. Recent research has further revealed specific roles of B cells in IDD. Significant B cell infiltration was found in degenerated discs, with these B cells promoting local inflammation by secreting autoantibodies and cytokines (80). Moreover, B cells can amplify immune responses by interacting with T cells, leading to further disc tissue damage (81).
3.1.6 Potential therapeutic targets
In chronic stages of IDD, the persistent activation and dysregulation of T cells and B cells lead to chronic inflammation and tissue destruction. Therefore, regulating the functions of T cells and B cells may be a potential strategy for treating IDD. For example, inhibitors targeting specific T cell subsets, such as IL-17A antibodies or Treg cell enhancers, may reduce chronic inflammation and promote tissue repair (82, 83). Clinical trials involving IL-17A inhibitors, like Secukinumab, have demonstrated their potential in treating inflammatory conditions such as ankylosing spondylitis, providing a rationale for their application in IDD (84).
Similarly, Treg cell enhancers, such as low-dose IL-2 therapy, have been explored in early-phase clinical trials for autoimmune diseases, showing promising results in enhancing Treg function and reducing pathological inflammation (85). These findings suggest that modulating Treg cells could be beneficial in IDD by restoring immune balance and promoting tissue repair. For B cells, depletion therapies like Rituximab, which targets CD20+ B cells, have been successful in treating rheumatoid arthritis by reducing B cell-mediated chronic inflammation (86). Although specific studies in IDD are limited, these results imply that B cell depletion could potentially control the chronic inflammation seen in advanced stages of IDD. Additionally, small molecule inhibitors targeting B cell activation pathways, such as Bruton’s tyrosine kinase (BTK) inhibitors, are currently under investigation in clinical trials for various autoimmune conditions, offering another avenue for potential IDD treatment (87).
In summary, T cells and B cells play critical roles in the progression of IDD by regulating the local immune microenvironment and amplifying inflammatory responses. Immunomodulatory therapies targeting these cells, supported by evidence from other inflammatory and autoimmune diseases, may represent novel strategies for treating IDD. Future research should focus on validating these approaches in the context of IDD through targeted clinical trials to improve patient outcomes (Figure 1).
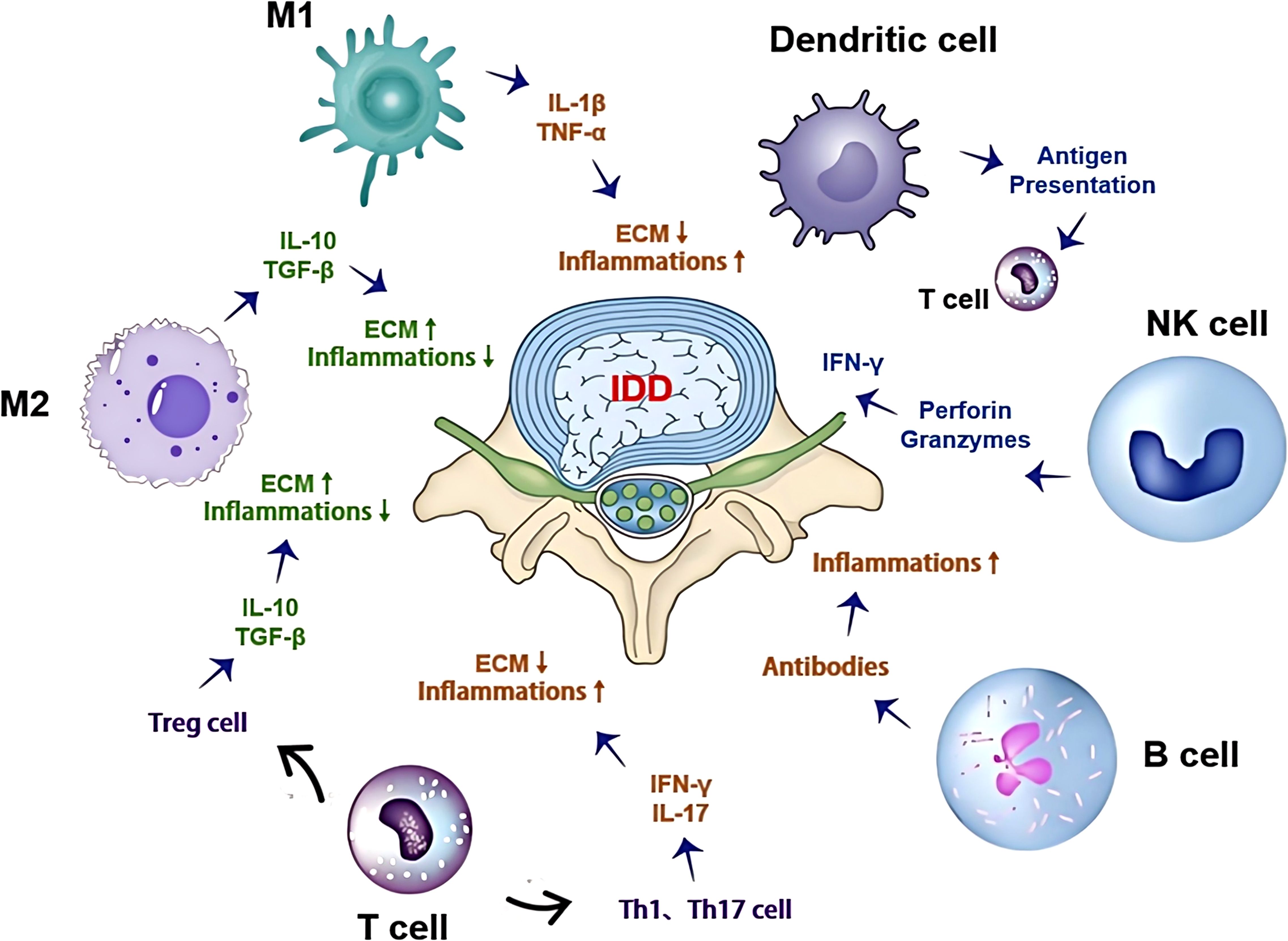
Figure 1. Complex interactions of the immune microenvironment with cell types, signaling pathways, and ECM degradation in intervertebral disc degeneration: This figure illustrates the complex interactions within the immune microenvironment during IDD. It includes various immune cells such as M1/M2 macrophages, T cells, B cells, NK cells, and dendritic cells, highlighting their roles in IDD. These cells secrete multiple cytokines and chemokines, which regulate the degradation and repair processes of the ECM. Arrows indicate the interactions and signaling pathways between these cells, illustrating the dual role of the immune microenvironment in either promoting or inhibiting disc degeneration.
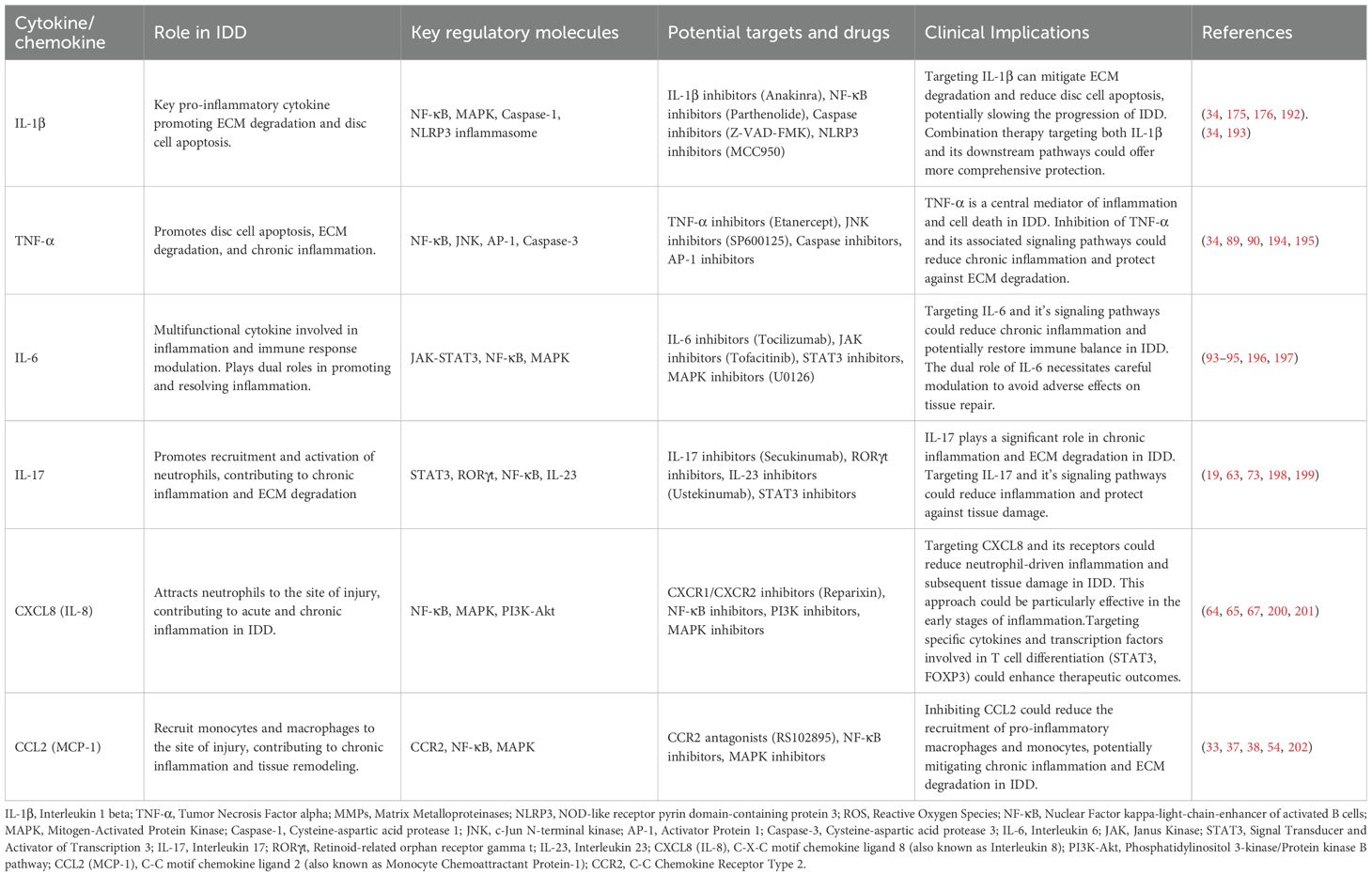
3.2 Cytokines and chemokines
During IDD, inflammatory factors play a crucial role. These factors mediate local inflammatory responses and affect disc cell survival, apoptosis, and matrix degradation (Table 2).
3.2.1 IL-1β
IL-1β is a key pro-inflammatory cytokine involved in IDD (4). It binds to its receptor IL-1R1 on the cell surface, activating downstream NF-κB and MAPK signaling pathways, thereby promoting the expression and activity of MMPs and accelerating the degradation of the disc matrix (83). Additionally, IL-1β induces oxidative stress and activates caspase signaling pathways, leading to the apoptosis of nucleus pulposus cells (NPCs) and annulus fibrosus cells (AFCs), further exacerbating disc degeneration (88).
3.2.2 TNF-α
TNF-α is another pro-inflammatory cytokine that plays a significant role in IDD (89). It exerts its effects through TNFR1 and TNFR2 receptors, activating NF-κB and JNK signaling pathways, and regulating the expression of various inflammatory mediators (90). Recent studies indicate that TNF-α increases disc cell death by inducing oxidative stress and is positively correlated with the severity of disc degeneration (34, 91). Moreover, TNF-α promotes the expression of MMPs and ADAMTSs, accelerating matrix degradation (92).
3.2.3 IL-6
IL-6 has a complex role in IDD. It binds to IL-6R and forms a complex with gp130, activating the JAK-STAT signaling pathway (93). As a pro-inflammatory cytokine, IL-6 promotes the expression of MMPs and ADAMTSs, accelerating matrix degradation (94). On the other hand, IL-6 is also considered to have anti-inflammatory effects, modulating immune cell responses through the STAT3 signaling pathway (95). This dual role makes IL-6’s function in disc degeneration complex and significant.
3.2.4 Chemokines
Chemokines primarily regulate the migration and localization of immune cells during IDD. For example, CCL5 (RANTES) mediates its effects through the CCR5 receptor, attracting macrophages and neutrophils to the lesion site, enhancing local inflammatory responses (96). CXCL8 (IL-8) mediates its effects through CXCR1 and CXCR2 receptors, attracting neutrophils to the site of inflammation and promoting acute inflammatory responses. This cell migration not only exacerbates local tissue damage but also contributes to the maintenance of chronic inflammation (97).
3.3 Regulation of disc cell survival and apoptosis by inflammatory mediators
Inflammatory mediators regulate disc cell survival and apoptosis through various pathways. Pro-inflammatory cytokines such as IL-1β and TNF-α can activate several apoptosis-related signaling pathways, such as the caspase family, inducing cell apoptosis (98). Additionally, oxidative stress and mitochondrial dysfunction are important mechanisms by which inflammatory mediators induce cell death.
3.4 Regulation of matrix degradation
Pro-inflammatory cytokines like IL-1β and TNF-α can activate the caspase signaling pathway, inducing cell apoptosis. Furthermore, these cytokines can increase apoptosis rates by inducing oxidative stress and mitochondrial dysfunction (99). MMPs and ADAMTSs are major enzymes responsible for disc matrix degradation, and their expression and activity are regulated by various inflammatory factors. IL-1β and TNF-α can significantly increase the expression of these degradative enzymes by activating NF-κB and MAPK signaling pathways, thus accelerating matrix degradation. For example, IL-1β binds to its receptor IL-1R1 on the cell surface, activating downstream NF-κB and MAPK signaling pathways, promoting the expression of MMP-3 and MMP-13 genes and proteins, leading to the breakdown of collagen and proteoglycans (100). TNF-α exerts its effects through TNFR1 and TNFR2 receptors, activating NF-κB and JNK signaling pathways, regulating the expression of ADAMTS-4 and ADAMTS-5 genes and proteins, further promoting matrix degradation (34, 101).
3.5 Prospects for the application of novel inflammatory modulators
With a deeper understanding of the mechanisms of IDD, significant progress has been made in developing novel inflammatory modulators. For instance, IL-1 receptor antagonists (Anakinra) and TNF-α inhibitors (such as Etanercept) have shown promising results in preclinical studies (102, 103). Additionally, novel small molecule inhibitors targeting NF-κB and MAPK signaling pathways also show potential therapeutic prospects. These new drugs precisely modulate inflammatory responses, not only alleviating symptoms but potentially delaying or reversing the progression of disc degeneration fundamentally.
In summary, the mechanisms by which inflammatory cytokines function in IDD are complex and diverse. In-depth research into these mechanisms and regulatory pathways not only aids in understanding the pathophysiology of IDD but also provides an important theoretical basis for developing novel therapeutic strategies.
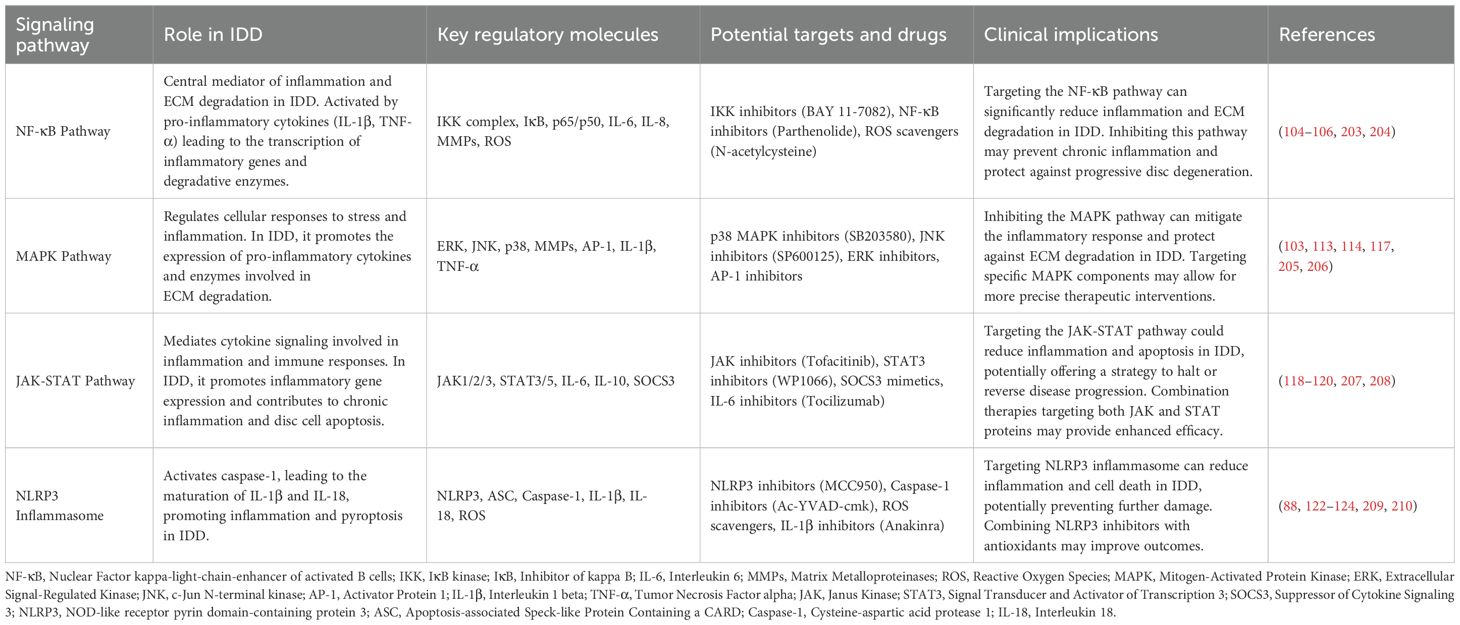
4 Signaling pathways of the immune system in intervertebral disc degeneration
4.1 NF-κB signaling pathway
The NF-κB signaling pathway plays a crucial role in disc degeneration and immune responses. NF-κB is a family of transcription factors that are key regulators of cellular stress and inflammatory responses (104). In IDD, NF-κB activation is primarily mediated by pro-inflammatory cytokines such as IL-1β and TNF-α (105). Upon binding to their receptors (IL-1R1 and TNFR1/TNFR2), these cytokines activate the IKK complex, leading to the phosphorylation and degradation of IκB proteins, thereby releasing NF-κB transcription factors. These factors then translocate to the nucleus and initiate the transcription of inflammatory genes (106). NF-κB activation leads to a cascade of downstream effects, including the upregulation of inflammatory cytokines (such as IL-6 and IL-8), chemokines (such as CCL5), and matrix metalloproteinases (MMP-3 and MMP-13) (107). These effects not only exacerbate the inflammatory response of disc cells but also accelerate matrix degradation (108). Moreover, NF-κB is linked to oxidative stress, mitochondrial dysfunction, and ferroptosis, which further promote cellular damage and matrix degradation (109). NF-κB also influences cell death pathways, including ferroptosis, cuproptosis, and pyroptosis, by regulating Bcl-2 family proteins (110). Given the pivotal role of NF-κB in disc degeneration, targeting this pathway presents significant therapeutic potential. Currently, some NF-κB inhibitors have shown promising results in preclinical studies. For instance, IKKβ inhibitors significantly slowed the progression of disc degeneration in animal models (111). Additionally, small-molecule drugs targeting the NF-κB pathway, such as BAY 11–7082 and Parthenolide, have demonstrated potential in inhibiting disc degeneration-related inflammatory responses (112).
4.2 MAPK signaling pathway
The activation of the MAPK signaling pathway in disc cells and its impact on immune responses have also garnered significant attention. The MAPK family includes three major pathways: ERK, JNK, and p38, which play critical roles in cell proliferation, differentiation, stress response, and death (103). In IDD, the MAPK signaling pathway is also activated by inflammatory cytokines such as IL-1β and TNF-α, influencing disc cell functions through various mechanisms (113). The ERK pathway is primarily involved in cell proliferation and differentiation, but its excessive activation in an inflammatory environment may lead to abnormal cell proliferation and matrix metabolic imbalance (114). The JNK and p38 pathways are mainly involved in stress responses, with their activation leading to increased expression of pro-inflammatory genes and promoting novel cell death pathways such as ferroptosis and cuproptosis by regulating caspase family proteins (115). Additionally, the MAPK signaling pathway is involved in epigenetic and post-transcriptional regulation, affecting gene expression and protein function by modulating histone modifications and RNA stability. Regulators targeting the MAPK pathway hold broad prospects in the treatment of disc degeneration (116). For example, the p38 MAPK inhibitor SB203580 has shown efficacy in suppressing inflammatory responses in disc cells in vitro, and the JNK inhibitor SP600125 has demonstrated potential in slowing disc degeneration in animal models (117).
4.3 JAK-STAT signaling pathway
The JAK-STAT pathway mediates signal transduction through cytokine receptors and plays a vital role in inflammation and immune responses. Cytokines such as IL-6 and IL-10 bind to their receptors, activating JAK kinases, which in turn activate STAT transcription factors. These STAT factors then translocate to the nucleus to regulate the expression of target genes (118). In IDD, the JAK-STAT signaling pathway contributes to disease progression by regulating the expression of inflammatory cytokines and the activation of immune cells. For example, IL-6 activates the JAK-STAT3 pathway, promoting the expression of pro-inflammatory genes, thereby exacerbating inflammation and matrix degradation in the disc (93). The JAK-STAT signaling pathway is also closely related to disc cell survival and death, influencing cell death pathways such as ferroptosis, cuproptosis, and pyroptosis by regulating the expression of Bcl-2 family proteins (119). Recent studies have also found that the JAK-STAT pathway plays an important role in epigenetic regulation, affecting gene expression through DNA methylation and histone modifications. JAK-STAT inhibitors show promising potential in the treatment of disc degeneration. For instance, Tofacitinib, a JAK inhibitor, has demonstrated efficacy in suppressing disc degeneration-related inflammatory responses in preclinical studies (120). Additionally, small-molecule inhibitors targeting STAT3 have shown potential in regulating inflammatory responses and slowing the degeneration process (121).
4.4 NLRP3 inflammasome
The NLRP3 inflammasome is a multiprotein complex that senses intracellular danger signals, activating caspase-1, which promotes the maturation and release of IL-1β and IL-18 (122). In IDD, danger signals such as oxidative stress, mitochondrial damage, and matrix degradation products can activate the NLRP3 inflammasome. Activation of the NLRP3 inflammasome leads to a strong local inflammatory response and cell death in the disc (34). The release of IL-1β not only promotes the expression of more inflammatory cytokines but also further activates the NF-κB and MAPK signaling pathways through a positive feedback mechanism, exacerbating disc degeneration. NLRP3 is also closely related to pyroptosis, an inflammatory form of cell death, which further intensifies the local inflammatory response (123). Activation of the NLRP3 inflammasome also involves ubiquitination processes that regulate protein degradation and function (124). The potential of NLRP3 inflammasome inhibitors in the treatment of disc degeneration is gradually becoming apparent. For example, MCC950, a specific NLRP3 inhibitor, significantly slowed the progression of disc degeneration in animal models (125). Additionally, other NLRP3 inhibitors such as Bavachin (BHB) and Berberine have shown efficacy in suppressing inflammatory responses and protecting the disc in preclinical studies (126).
In summary, signaling pathways such as NF-κB, MAPK, JAK-STAT, and NLRP3 inflammasome play critical roles in disc degeneration (Table 3). In-depth studies of the activation mechanisms and functions of these pathways not only aid in understanding the pathophysiological processes of disc degeneration but also provide an important theoretical basis for developing novel therapeutic strategies (Figure 2). Increasing evidence highlights the complex crosstalk among these signaling pathways. For instance, NF-κB activation primes the expression of NLRP3 inflammasome components, thus facilitating pyroptosis under stress (127). Meanwhile, IL-6-mediated activation of the JAK-STAT3 pathway can modulate NF-κB transcriptional output, amplifying inflammation (128). MAPK pathways (notably p38 and JNK) intersect with both NF-κB and NLRP3 activation cascades, creating a positive feedback loop (129). This synergy suggests that dual or multi-pathway inhibitors—such as those targeting both NF-κB and NLRP3—may offer enhanced therapeutic efficacy. Notably, intradiscal AAV-CRISPR/Cas9 knock-down of β-catenin not only preserves notochord-derived cells and annulus integrity but also attenuates NF-κB-driven NLRP3 priming, highlighting β-catenin as an upstream modulator of this inflammasome axis and positioning gene editing as a precise, multi-pathway regenerative strategy (130).
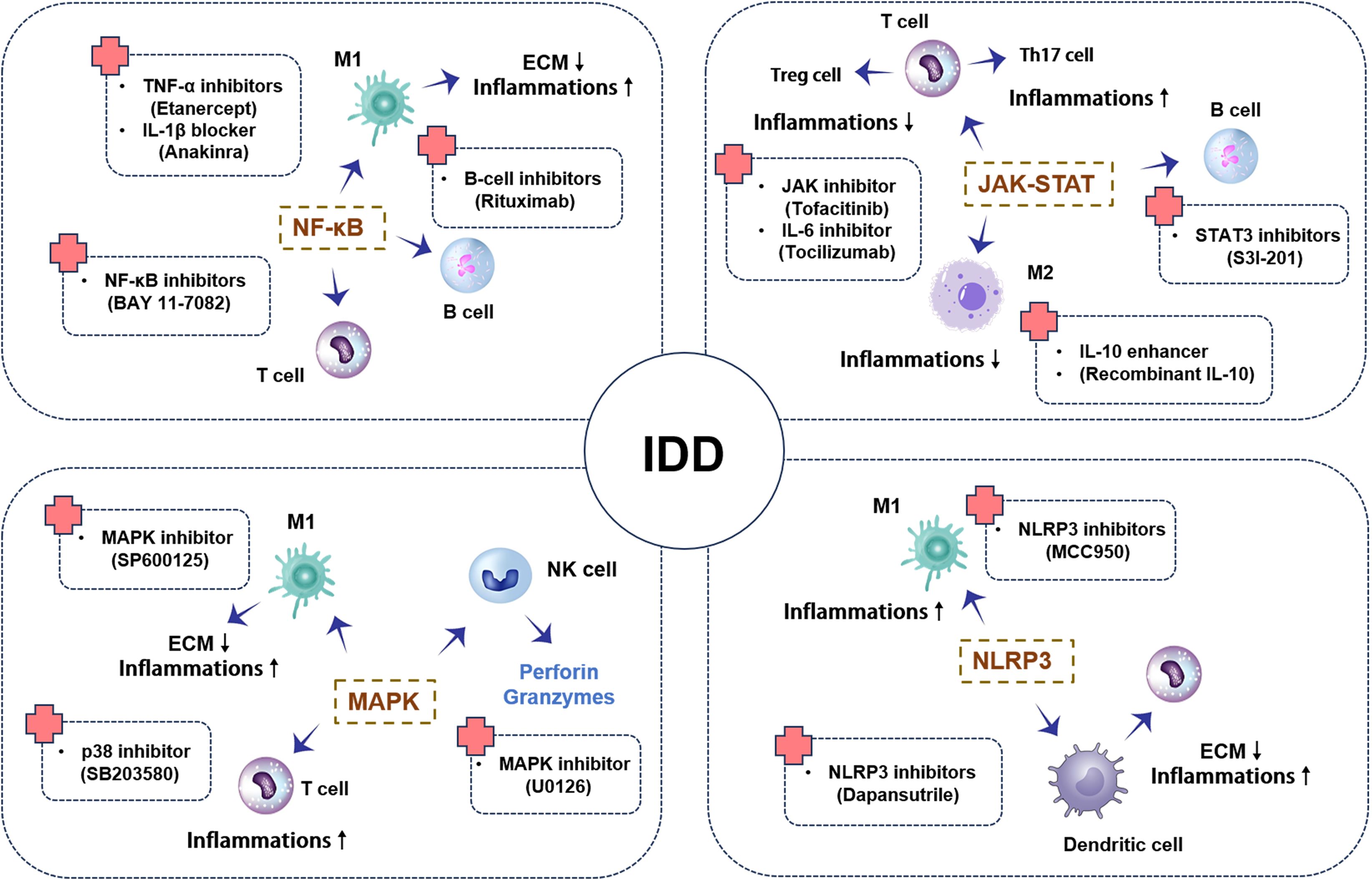
Figure 2. Schematic representation of key signaling pathways in intervertebral disc degeneration: This diagram depicts the activation and interactions of key signaling pathways, including NF-κB, MAPK, JAK-STAT, and NLRP3, during IDD. It includes pro-inflammatory actions, anti-inflammatory effects, and apoptosis. The schematic shows how these signaling pathways transmit signals between different immune cells, triggering inflammatory responses that further promote or inhibit the progression of disc degeneration.
5 The relationship between the immune microenvironment and ECM degradation
5.1 Role of inflammatory mediators in ECM degradation
The ECM, mainly composed of collagen, proteoglycans, and other matrix molecules, provides structural support and elasticity to the disc. Inflammatory mediators such as IL-1β and TNF-α significantly accelerate ECM degradation by upregulating the expression of MMPs and ADAMTS proteases (131). These inflammatory cytokines not only directly promote ECM degradation but also exacerbate the degenerative process by inducing mitochondrial autophagy in nucleus pulposus cells and altering the balance of ECM synthesis and degradation (132).
5.2 Role of miRNAs and lncRNAs in regulating inflammatory mediators and ECM degradation
During IDD, non-coding RNAs such as microRNAs (miRNAs) and long non-coding RNAs (lncRNAs) play increasingly recognized roles in regulating inflammatory mediators and ECM degradation. For example, miR-27b inhibits MMP-13 expression by directly targeting its 3’UTR region, thereby slowing ECM degradation (133). Studies indicate that miRNAs can regulate the expression of MMPs and ADAMTS through various pathways, influencing ECM degradation and the progression of disc degeneration. Furthermore, lncRNA HOTAIR, by binding to polycomb protein EZH2, promotes H3K27me3 modification, thereby inhibiting the expression of MMP-1 and MMP-3 and reducing matrix degradation (134). These molecular mechanisms illustrate the multi-layered regulatory roles of non-coding RNAs in ECM degradation. Additionally, miR-21 indirectly promotes the expression of MMP-3 and MMP-9 by downregulating tissue inhibitor of metalloproteinases 3 (TIMP3), leading to accelerated ECM degradation (135). LncRNA MEG3, through interaction with the tumor suppressor p53, inhibits the expression of MMP-2 and MMP-9, protecting the ECM from excessive degradation (136). Notably, non-coding RNAs not only play important roles in gene expression regulation but also influence signal transduction and intercellular communication, playing key roles in the pathogenesis of disc degeneration. For instance, lncRNA TUG1 regulates the Wnt/β-catenin signaling pathway, affecting inflammatory responses and ECM degradation (137). Future research should further elucidate the specific functions and mechanisms of non-coding RNAs in disc degeneration and explore their potential as therapeutic targets.
5.3 Immunoregulatory role of ECM degradation products
ECM degradation not only impacts the structure and function of the disc but also produces degradation products that have important immunoregulatory roles. ECM degradation products such as collagen fragments, proteoglycan fragments, and hyaluronic acid fragments can act as damage-associated molecular patterns (DAMPs), activating immune cells and triggering and sustaining inflammatory responses. For example, collagen fragments activate downstream NF-κB and MAPK signaling pathways through TLR2 and TLR4, inducing the expression of IL-1β, TNF-α, and IL-6, further exacerbating inflammation and ECM degradation (138). Hyaluronic acid fragments can similarly activate macrophages through the same pathways, enhancing their pro-inflammatory responses (55). Additionally, proteoglycan fragments such as aggrecan and versican fragments, by binding to the CD44 receptor, activate macrophages and dendritic cells, enhancing their pro-inflammatory and antigen-presenting functions (139).
Despite significant progress in the study of IDD and ECM degradation, many gaps and controversies remain. First, the molecular mechanisms of ECM degradation are not yet fully elucidated. Although many key proteases and signaling pathways have been identified, their specific roles and interactions at different pathological stages require further investigation. Additionally, the precise mechanisms by which inflammatory mediators and ECM degradation products regulate the immune microenvironment, especially in different types of immune cells (such as macrophages and T cells), remain unclear. Moreover, current animal models and in vitro systems have limitations in fully simulating the complex pathological processes of human IDD. Many findings have yet to be validated in clinical trials, and effective strategies for clinical treatment are still facing challenges. Therefore, future research needs to develop more precise and effective experimental models and adopt multidisciplinary approaches to further uncover the complex mechanisms of IDD. In conclusion, although progress has been made in understanding the relationship between IDD and ECM degradation, many scientific questions and technical challenges remain to be addressed to advance the field and provide more effective strategies for clinical treatment.
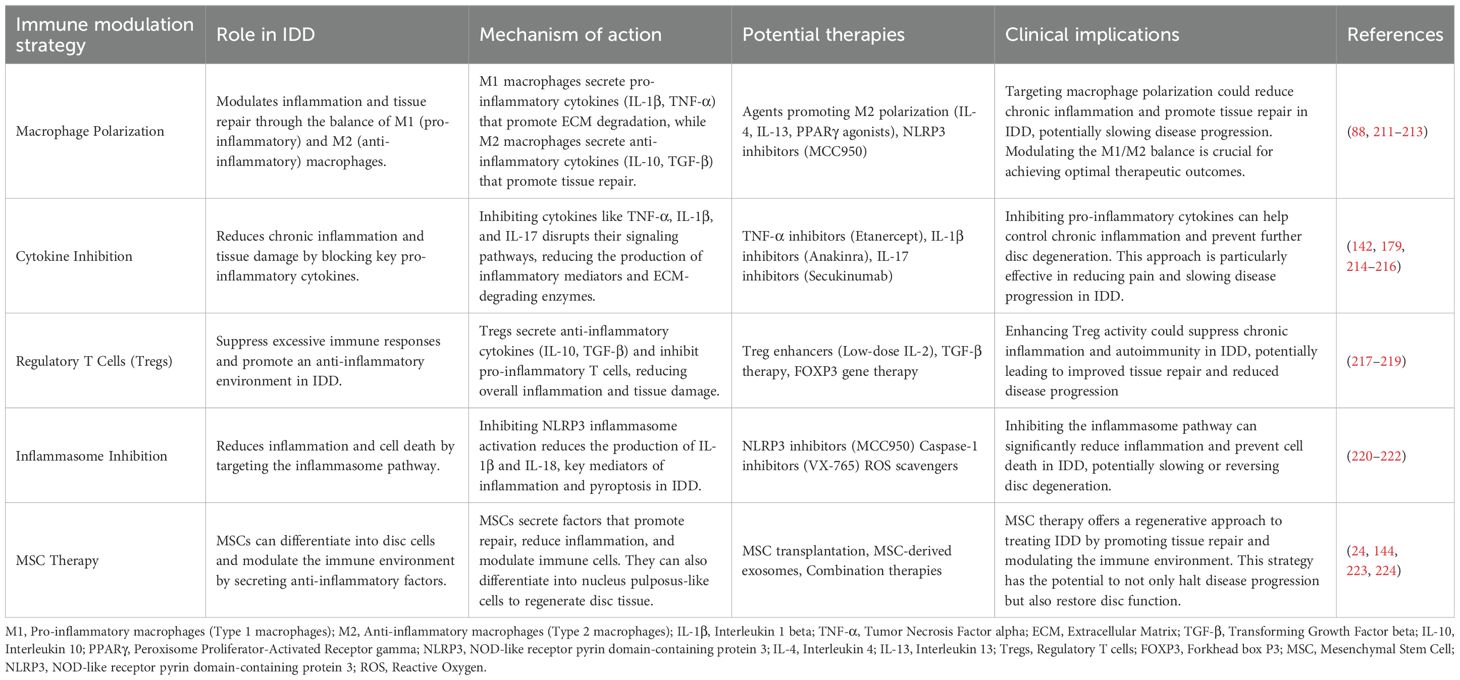
6 Potential of immune regulation in the treatment of intervertebral disc degeneration
6.1 Anti-inflammatory drugs
In recent years, the application of anti-inflammatory drugs in IDD has increased. Non-steroidal anti-inflammatory drugs (NSAIDs) are the most commonly used anti-inflammatory drugs, reducing prostaglandin synthesis by inhibiting cyclooxygenase (COX) to alleviate pain and inflammation. However, long-term use of NSAIDs can cause gastrointestinal and cardiovascular side effects (140). More targeted anti-inflammatory drugs, such as selective COX-2 inhibitors, TNF-α antagonists, and IL-1β inhibitors, have shown better therapeutic effects and fewer side effects. For example, studies have shown that the use of TNF-α antagonists (such as Etanercept) can effectively reduce pain and inflammation in patients with IDD (141). Additionally, IL-1β inhibitors (such as Anakinra) block the IL-1β signaling pathway, slowing the progression of IDD and improving patient symptoms (142). These novel anti-inflammatory drugs offer new directions for the treatment of IDD, but their long-term efficacy and safety need further study.
6.2 Biologics
Biologics also show great potential in the treatment of IDD. Biologics are protein-based drugs produced through genetic engineering techniques, capable of precisely targeting specific inflammatory factors or cells. Studies have shown that using monoclonal antibodies to block specific inflammatory factors can significantly slow the progression of IDD. For instance, anti-IL-6 receptor monoclonal antibody (such as Tocilizumab) has shown good anti-inflammatory effects in animal models, reducing disc tissue damage and inflammatory cell infiltration (143). Furthermore, stem cell therapy, as an emerging biological treatment, has shown potential in regenerating and repairing disc tissue (24, 101). Mesenchymal stem cells (MSCs) can regulate immune responses and promote disc cell proliferation and ECM synthesis by secreting various anti-inflammatory factors and growth factors (144). Clinical trials have shown that MSC injections can significantly improve symptoms in patients with IDD and promote disc tissue repair (145, 146). Despite their promise, MSC therapies face challenges in clinical application. The harsh, hypoxic, and nutrient-poor environment of the degenerated disc significantly limits MSC survival and function (147). Additionally, unmodified MSCs may have limited homing ability and paracrine activity. Recent advances, such as engineering MSCs with hypoxia-adaptive genes or embedding them in biomaterial scaffolds, have shown improved outcomes (148). Exosome-based delivery also emerges as a minimally immunogenic and stable alternative (145, 149).
6.3 Immunomodulatory therapy
Immunomodulatory therapy aims to modulate the immune system’s function, restore immune balance, and slow or reverse the progression of IDD. Recent studies on regulatory T cells (Tregs) and macrophages have shown significant therapeutic potential. Research indicates that Tregs can secrete anti-inflammatory cytokines (such as IL-10 and TGF-β), suppressing inflammatory responses and slowing IDD progression (150, 151). Additionally, the role of macrophages in IDD has gained considerable attention. M1 macrophages have pro-inflammatory effects, while M2 macrophages have anti-inflammatory and tissue repair functions. Promoting the polarization from M1 to M2 macrophages can significantly slow the progression of IDD (152, 153). An emerging immunomodulatory therapy involves using exosomes, which are nanoscale particles capable of delivering anti-inflammatory and reparative signals (Table 4). Studies have shown that exosomes derived from MSCs can modulate immune responses and slow disc degeneration (24).
7 Future research directions and challenges
7.1 Discovery of novel immune biomarkers
Identifying and validating new immune biomarkers is crucial for better understanding and monitoring the immune environment in IDD. Recent studies have emphasized the role of inflammatory cytokines like IL-6 and TNF-α, which are not only markers of inflammation but also active contributors to disc degeneration. For example, a 2021 study highlighted how elevated IL-6 and TNF-α levels in disc tissue correlate with increased MMP activity, leading to accelerated ECM degradation (154).
Beyond these traditional markers, emerging research has identified specific microRNAs (miRNAs) and long non-coding RNAs (lncRNAs) as promising biomarkers in IDD (144) (150, 155). Notably, miR-146a and miR-21 have been shown to regulate inflammatory responses and ECM homeostasis, with studies in 2022 and 2023 respectively demonstrating their potential as therapeutic targets in IDD (156). Additionally, lncRNAs like HOTAIR and MEG3 have been implicated in disc degeneration, with HOTAIR promoting ECM degradation and apoptosis, while MEG3 offers protective effects by inhibiting MMP expression (157). The discovery of these markers has been significantly advanced by high-throughput screening and systems biology approaches, such as single-cell RNA sequencing (scRNA-seq), which have enabled the identification of distinct immune cell subsets and molecular networks within degenerated disc tissues (21, 158). A 2022 study using scRNA-seq, for example, revealed distinct macrophage populations that play differential roles in inflammation and tissue repair (68). Furthermore, systems biology approaches integrating multi-omics data have provided comprehensive insights into the molecular landscape of IDD, as evidenced by a 2023 study that uncovered key regulatory networks involving miRNAs, lncRNAs, and cytokines (159). These advancements underscore the potential of novel biomarkers to improve early detection, monitor disease progression, and guide personalized treatment strategies in IDD.
7.2 Development of personalized immunotherapy
Developing personalized immunotherapy strategies tailored to patients’ unique immune profiles is a promising future direction in the treatment of IDD. For example, analyzing patients’ immune cell lineages and inflammatory cytokine levels can help design targeted treatment plans, such as using specific antibodies or small-molecule inhibitors to block key inflammatory pathways (160). Recent advancements have highlighted the potential of specific immune modulators and gene therapies to achieve precision medicine. By analyzing individual immune cell lineages and inflammatory cytokine levels, clinicians can design targeted treatment plans that are more effective and have fewer side effects. For example, a 2021 study demonstrated that patients with elevated Th17 cell levels responded favorably to IL-17 inhibitors, emphasizing the importance of immune profiling in therapy selection (161). Additionally, modulating macrophage polarization has shown potential, with research indicating that adjusting the M1/M2 balance through specific small-molecule inhibitors can reduce chronic inflammation and promote tissue repair in degenerative discs. These approaches underscore the value of personalized immunotherapy in addressing the specific immune dysfunctions associated with IDD (51).
Gene editing technologies, particularly CRISPR/Cas9, are at the forefront of personalized immunotherapy, offering precise modifications of genes involved in inflammatory responses. Recent studies have explored the use of CRISPR/Cas9 to edit genes within the NF-κB pathway, leading to reduced inflammation and slowed disease progression in IDD models (130). Moreover, correcting mutations responsible for excessive cytokine production has shown promise in reversing some of the pathological changes associated with the disease. The integration of multi-omics data, including genomics, transcriptomics, and proteomics, further enhances the development of personalized treatments by providing a comprehensive understanding of each patient’s unique disease biology. This data-driven approach enables the identification of key biomarkers and therapeutic targets, paving the way for more tailored and effective therapies. As personalized immunotherapy continues to evolve, future directions may include combination therapies that integrate multiple targeted approaches, potentially revolutionizing the management of IDD and improving patient outcomes.
7.3 Multidisciplinary collaboration
Promoting multidisciplinary collaboration is essential for advancing the understanding and treatment of IDD. The complexity of IDD, involving mechanical, biochemical, and immunological factors, necessitates an integrated approach drawing on expertise from fields such as immunology, molecular biology, bioengineering, and clinical medicine. By fostering collaboration across these disciplines, researchers can develop innovative technologies and therapeutic strategies that address the multifaceted nature of IDD. For example, bioengineering plays a crucial role in designing advanced biomaterials and drug delivery systems to enhance the therapeutic effects of immunomodulators. A 2021 study demonstrated the use of a novel hydrogel-based scaffold for the sustained release of anti-inflammatory drugs directly into the degenerated disc, significantly reducing local inflammation and promoting tissue regeneration (15, 162). These innovations highlight the importance of combining materials science, pharmacology, and clinical expertise to create biocompatible and effective treatments.
Moreover, integrating molecular biology with clinical research can accelerate the translation of basic science discoveries into therapeutic interventions. Identifying specific molecular targets, such as cytokines or signaling pathways involved in IDD, requires a deep understanding of both the underlying biology and the clinical manifestations of the disease. Collaborative efforts between molecular biologists and clinicians have led to the discovery of novel biomarkers for early-stage IDD, paving the way for personalized treatment plans. Additionally, collaboration between engineering and clinical medicine has the potential to revolutionize IDD treatment through the creation of advanced medical devices and surgical techniques. For instance, recent advancements in 3D bioprinting technology have enabled the development of anatomically accurate disc implants customized to fit individual patient anatomies, offering a promising alternative to traditional surgical interventions (163). Such breakthroughs are made possible through the joint efforts of engineers, biologists, and clinicians, who together bridge the gap between experimental technology and practical application in patient care.
7.4 Unresolved questions
The specific functions and regulatory mechanisms of immune cells in IDD remain incompletely understood, posing significant challenges to the development of targeted therapies. While existing studies have established that immune cells such as macrophages and T cells play pivotal roles in the progression of IDD, the precise mechanisms by which these cells contribute to the disease are still under investigation. For instance, macrophages, which can polarize into pro-inflammatory M1 and anti-inflammatory M2 phenotypes, exhibit dynamic and context-dependent behavior in IDD. A 2022 study by Zhang et al. indicated that M1 macrophages are predominant in the early stages of disc degeneration, contributing to tissue damage through the secretion of pro-inflammatory cytokines and matrix-degrading enzymes (50). However, the transition from M1 to M2 macrophages, which theoretically should promote tissue repair and resolution of inflammation, does not appear to occur effectively in many cases of chronic IDD (164). This suggests a potential dysregulation in macrophage polarization, which could be a critical factor in the persistence of inflammation and progression of the disease.
Moreover, the interaction networks between different immune cells, such as macrophages, T cells, and other immune components, are not yet fully mapped out. T cells, particularly the balance between pro-inflammatory Th1/Th17 cells and anti-inflammatory Treg cells, play a crucial role in modulating the immune environment within the degenerative disc (165). However, recent studies suggest that this balance is often disrupted in IDD, leading to a chronic inflammatory state that exacerbates tissue degradation. For example, a 2021 study highlighted that increased Th17 cell activity correlates with more severe disc degeneration, while Treg cell dysfunction may contribute to the failure of inflammation resolution (160). The specific molecular signals and pathways that govern these immune cell interactions in the disc microenvironment are still poorly understood, and unraveling these mechanisms could reveal new therapeutic targets. Additionally, the role of other immune cells, such as dendritic cells and B cells, in IDD remains underexplored. These cells could contribute to the chronicity of inflammation or interact with resident disc cells in ways that influence disease outcomes. In-depth research into these unresolved questions is essential for advancing our understanding of IDD pathophysiology and for the identification of novel, more effective therapeutic strategies.
7.5 Technical bottlenecks
Current research faces several technical challenges. For example, existing animal models cannot fully simulate the complex pathological processes of human IDD, limiting the extrapolation of research results (166). Gene editing approaches such as CRISPR/Cas9 offer precise modulation of inflammatory pathways. However, off-target mutations, immunogenicity of the delivery vector, and ethical concerns remain significant barriers to clinical adoption (167, 168). Strategies like high-fidelity Cas9 variants, exosome-based delivery systems, and transient editing protocols are being explored to mitigate these risks (169). Additionally, obtaining human samples poses certain difficulties, affecting the conduct of large-scale studies. To overcome these challenges, more advanced animal models and in vitro experimental systems need to be developed, utilizing high-throughput screening techniques to discover new immune regulatory factors.
7.6 Overcoming challenges
Recent research directions and technological advances provide new ideas and methods for advancing the study of interactions between IDD and the immune system. For example, single-cell RNA sequencing technology can accurately analyze different cell types and their interactions within disc tissue, helping understand immune cells’ functions at different pathological stages (170, 171). Additionally, the application of organoid technology is gradually emerging, allowing better simulation of IDD’s pathological processes in vitro, and screening potential therapeutic drugs (172). Furthermore, the application of artificial intelligence (AI) and machine learning (ML) technologies in biomedical research provides new tools for IDD research. These technologies can help analyze large datasets of genes, proteins, and metabolites, discovering new disease biomarkers and therapeutic targets (173). International cooperation and data-sharing platforms also help overcome sample acquisition limitations, promoting research progress through shared resources and technologies.
IDD is a prevalent condition marked by the progressive deterioration of disc structure and function. This review explores the pathophysiological mechanisms, emphasizing the role of ECM degradation, immune responses, and key signaling pathways like NF-κB, MAPK, and JAK-STAT. Advances in understanding immune microenvironments have highlighted potential therapeutic targets, including anti-inflammatory drugs, biologics, and immunomodulatory therapies. Future research should focus on identifying novel immune biomarkers, developing personalized treatments, and fostering multidisciplinary collaboration to enhance therapeutic strategies and improve patient outcomes.
Author contributions
QR: Writing – original draft, Writing – review & editing. LC: Writing – original draft, Writing – review & editing. YM: Investigation, Writing – original draft. YH: Investigation, Supervision, Writing – review & editing. SW: Conceptualization, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the Natural Science Foundation of China (grant no. 31760270).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. In this study, ChatGPT 4 was utilized to aid in conducting the literature review and optimizing text. However, AI played no role in the research design, data interpretation, or the formulation of academic perspectives. All produced content was meticulously reviewed and edited by the author.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
IDD, Intervertebral disc degeneration; ECM, extracellular matrix; MMPs, matrix metalloproteinases; NSAIDs, non-steroidal anti-inflammatory drugs; NK, natural killer; ADAMTS, A Disintegrin and Metalloproteinase with Thrombospondin motifs; ROS, reactive oxygen species; NETs, neutrophils form extracellular traps; AFCs, annulus fibrosus cells; NPCs, nucleus pulposus cells; Anakinra, IL-1 receptor antagonists; miRNAs, microRNAs; lncRNAs, long non-coding RNAs; DAMPs, damage-associated molecular patterns; COX, cyclooxygenase; MSCs, Mesenchymal stem cells; scRNA-seq, single-cell RNA sequencing.
References
1. Mohd Isa IL, Teoh SL, Mohd Nor NH, and Mokhtar SA. Discogenic low back pain: anatomy, pathophysiology and treatments of intervertebral disc degeneration. Int J Mol Sci. (2022) 24.
2. Mei Y, Wang L, Chen T, Song C, Cheng K, Cai W, et al. Ferroptosis: A new direction in the treatment of intervertebral disc degeneration. Cell Biochem Biophys. (2024). doi: 10.1007/s12013-024-01468-6
3. Benzakour T, Igoumenou V, Mavrogenis AF, and Benzakour A. Current concepts for lumbar disc herniation. Int Orthop. (2019) 43:841–51. doi: 10.1007/s00264-018-4247-6
4. Wang Y, Che M, Xin J, Zheng Z, Li J, and Zhang S. The role of IL-1β and TNF-α in intervertebral disc degeneration. BioMed Pharmacother. (2020) 131:110660. doi: 10.1016/j.biopha.2020.110660
5. Newell N, Little JP, Christou A, Adams MA, Adam CJ, and Masouros SD. Biomechanics of the human intervertebral disc: A review of testing techniques and results. J Mech Behav BioMed Mater. (2017) 69:420–34. doi: 10.1016/j.jmbbm.2017.01.037
6. Zhang AS, Xu A, Ansari K, Hardacker K, Anderson G, Alsoof D, et al. Lumbar disc herniation: diagnosis and management. Am J Med. (2023) 136:645–51. doi: 10.1016/j.amjmed.2023.03.024
7. Francisco V, Pino J, González-Gay M, Lago F, Karppinen J, Tervonen O, et al. A new immunometabolic perspective of intervertebral disc degeneration. Nat Rev Rheumatol. (2022) 18:47–60. doi: 10.1038/s41584-021-00713-z
8. Mohd Isa IL, Mokhtar SA, Abbah SA, Fauzi MB, Devitt A, and Pandit A. Intervertebral disc degeneration: biomaterials and tissue engineering strategies toward precision medicine. Adv Healthc Mater. (2022) 11:e2102530.
9. Xin J, Wang Y, Zheng Z, Wang S, Na S, and Zhang S. Treatment of intervertebral disc degeneration. Orthop Surg. (2022) 14:1271–80. doi: 10.1111/os.13254
10. Wu PH, Kim HS, and Jang IT. Intervertebral disc diseases PART 2: A review of the current diagnostic and treatment strategies for intervertebral disc disease. Int J Mol Sci. (2020) 21. doi: 10.3390/ijms21062135
11. Kim HS, Wu PH, and Jang IT. Lumbar degenerative disease part 1: anatomy and pathophysiology of intervertebral discogenic pain and radiofrequency ablation of basivertebral and sinuvertebral nerve treatment for chronic discogenic back pain: A prospective case series and review of literature. Int J Mol Sci. (2020) 21. doi: 10.3390/ijms21041483
12. Kao KC, Vilbois S, Tsai CH, and Ho PC. Metabolic communication in the tumour-immune microenvironment. Nat Cell Biol. (2022) 24:1574–83. doi: 10.1038/s41556-022-01002-x
13. Li M, Yin H, Yan Z, Li H, Wu J, Wang Y, et al. The immune microenvironment in cartilage injury and repair. Acta Biomater. (2022) 140:23–42. doi: 10.1016/j.actbio.2021.12.006
14. Haghikia A, Zimmermann F, Schumann P, Jasina A, Roessler J, Schmidt D, et al. Propionate attenuates atherosclerosis by immune-dependent regulation of intestinal cholesterol metabolism. Eur Heart J. (2022) 43:518–33. doi: 10.1093/eurheartj/ehab644
15. Xu H, Li J, Fei Q, and Jiang L. Contribution of immune cells to intervertebral disc degeneration and the potential of immunotherapy. Connect Tissue Res. (2023) 64:413–27. doi: 10.1080/03008207.2023.2212051
16. Karamanos NK, Theocharis AD, Piperigkou Z, Manou D, Passi A, Skandalis SS, et al. A guide to the composition and functions of the extracellular matrix. FEBS J. (2021) 288:6850–912. doi: 10.1111/febs.v288.24
17. Madhu V, Hernandez-Meadows M, Boneski PK, Qiu Y, Guntur AR, Kurland IJ, et al. The mitophagy receptor BNIP3 is critical for the regulation of metabolic homeostasis and mitochondrial function in the nucleus pulposus cells of the intervertebral disc. Autophagy. (2023) 19:1821–43. doi: 10.1080/15548627.2022.2162245
18. Cutolo M, Campitiello R, Gotelli E, and Soldano S. The role of M1/M2 macrophage polarization in rheumatoid arthritis synovitis. Front Immunol. (2022) 13:867260. doi: 10.3389/fimmu.2022.867260
19. Zhao X, Di Q, Liu H, Quan J, Ling J, Zhao Z, et al. MEF2C promotes M1 macrophage polarization and Th1 responses. Cell Mol Immunol. (2022) 19:540–53. doi: 10.1038/s41423-022-00841-w
20. Liu F, Qiu H, Xue M, Zhang S, Zhang X, Xu J, et al. MSC-secreted TGF-β regulates lipopolysaccharide-stimulated macrophage M2-like polarization via the Akt/FoxO1 pathway. Stem Cell Res Ther. (2019) 10:345. doi: 10.1186/s13287-019-1447-y
21. Hu X, Wang Z, Zhang H, Cui P, Li Y, Chen X, et al. Single-cell sequencing: New insights for intervertebral disc degeneration. BioMed Pharmacother. (2023) 165:115224. doi: 10.1016/j.biopha.2023.115224
22. Koroth J, Buko EO, Abbott R, Johnson CP, Ogle BM, Stone LS, et al. Macrophages and intervertebral disc degeneration. Int J Mol Sci. (2023) 24. doi: 10.3390/ijms24021367
23. Mirzaeipoueinak M, Mordechai HS, Bangar SS, Sharabi M, Tipper JL, and Tavakoli J. Structure-function characterization of the transition zone in the intervertebral disc. Acta Biomater. (2023) 160:164–75. doi: 10.1016/j.actbio.2023.02.019
24. Bhujel B, Shin HE, Choi DJ, and Han I. Mesenchymal stem cell-derived exosomes and intervertebral disc regeneration: review. Int J Mol Sci. (2022) 23. doi: 10.3390/ijms23137306
25. Sun K, Jiang J, Wang Y, Sun X, Zhu J, Xu X, et al. The role of nerve fibers and their neurotransmitters in regulating intervertebral disc degeneration. Ageing Res Rev. (2022) 81:101733. doi: 10.1016/j.arr.2022.101733
26. Desmoulin GT, Pradhan V, and Milner TE. Mechanical aspects of intervertebral disc injury and implications on biomechanics. Spine (Phila Pa 1976). (2020) 45:E457–e464. doi: 10.1097/BRS.0000000000003291
27. Gao B, Jiang B, Xing W, Xie Z, Luo Z, and Zou W. Discovery and application of postnatal nucleus pulposus progenitors essential for intervertebral disc homeostasis and degeneration. Adv Sci (Weinh). (2022) 9:e2104888. doi: 10.1002/advs.202104888
28. Wang L, He T, Liu J, Tai J, Wang B, Zhang L, et al. Revealing the immune infiltration landscape and identifying diagnostic biomarkers for lumbar disc herniation. Front Immunol. (2021) 12:666355. doi: 10.3389/fimmu.2021.666355
29. Hai B, Song Q, Du C, Mao T, Jia F, Liu Y, et al. Comprehensive bioinformatics analyses reveal immune genes responsible for altered immune microenvironment in intervertebral disc degeneration. Mol Genet Genomics. (2022) 297:1229–42. doi: 10.1007/s00438-022-01912-3
30. Bermudez-Lekerika P, Crump KB, Tseranidou S, NüESCH A, Kanelis E, Alminnawi A, et al. Immuno-modulatory effects of intervertebral disc cells. Front Cell Dev Biol. (2022) 10:924692. doi: 10.3389/fcell.2022.924692
31. Zhao X, Sun Z, Xu B, Duan W, Chang L, Lai K, et al. Degenerated nucleus pulposus cells derived exosome carrying miR-27a-3p aggravates intervertebral disc degeneration by inducing M1 polarization of macrophages. J Nanobiotechnology. (2023) 21:317. doi: 10.1186/s12951-023-02075-y
32. Zhang P, He J, Gan Y, Shang Q, Chen H, Zhao W, et al. Unravelling diagnostic clusters and immune landscapes of cuproptosis patterns in intervertebral disc degeneration through dry and wet experiments. Aging (Albany NY). (2023) 15:15599–623. doi: 10.18632/aging.205449
33. Liu S, Li K, He Y, Chen S, Yang W, Chen X, et al. PGC1α-inducing senomorphic nanotherapeutics functionalized with NKG2D-overexpressing cell membranes for intervertebral disc degeneration. Adv Sci (Weinh). (2024) 11:e2400749. doi: 10.1002/advs.202400749
34. Zhao Y, Qiu C, Wang W, Peng J, Cheng X, Shangguan Y, et al. Cortistatin protects against intervertebral disc degeneration through targeting mitochondrial ROS-dependent NLRP3 inflammasome activation. Theranostics. (2020) 10:7015–33. doi: 10.7150/thno.45359
35. Xue P, Wang Y, Lv L, Wang D, and Wang Y. Roles of chemokines in intervertebral disk degeneration. Curr Pain Headache Rep. (2024) 28:95–108. doi: 10.1007/s11916-023-01188-1
36. Song C, Zhou Y, Cheng K, Liu F, Cai W, Zhou D, et al. Cellular senescence - Molecular mechanisms of intervertebral disc degeneration from an immune perspective. BioMed Pharmacother. (2023) 162:114711. doi: 10.1016/j.biopha.2023.114711
37. Wang N, Mi Z, Chen S, Fang X, Xi Z, Xu W, et al. Analysis of global research hotspots and trends in immune cells in intervertebral disc degeneration: A bibliometric study. Hum Vaccin Immunother. (2023) 19:2274220. doi: 10.1080/21645515.2023.2274220
38. Zhang W, Li G, Luo R, Lei J, Song Y, Wang B, et al. Cytosolic escape of mitochondrial DNA triggers cGAS-STING-NLRP3 axis-dependent nucleus pulposus cell pyroptosis. Exp Mol Med. (2022) 54:129–42. doi: 10.1038/s12276-022-00729-9
39. Wang WJ, Yu XH, Wang C, Yang W, He WS, Zhang SJ, et al. MMPs and ADAMTSs in intervertebral disc degeneration. Clin Chim Acta. (2015) 448:238–46. doi: 10.1016/j.cca.2015.06.023
40. Binch ALA, Shapiro IM, and Risbud MV. Syndecan-4 in intervertebral disc and cartilage: Saint or synner? Matrix Biol. (2016) 52-54:355–62.
41. Hoogendoorn R, Doulabi BZ, Huang CL, Wuisman PI, Bank RA, and Helder MN. Molecular changes in the degenerated goat intervertebral disc. Spine (Phila Pa 1976). (2008) 33:1714–21. doi: 10.1097/BRS.0b013e31817d2468
42. Qin C, Chen M, Yu Q, Wang X, Hu T, Lei B, et al. Causal relationship between the blood immune cells and intervertebral disc degeneration: univariable, bidirectional and multivariable Mendelian randomization. Front Immunol. (2023) 14:1321295. doi: 10.3389/fimmu.2023.1321295
43. Kamali A, Ziadlou R, Lang G, Pfannkuche J, Cui S, Li Z, et al. Small molecule-based treatment approaches for intervertebral disc degeneration: Current options and future directions. Theranostics. (2021) 11:27–47. doi: 10.7150/thno.48987
44. Liu L, Wang W, Huang L, Xian Y, Ma W, Fan J, et al. Injectable pathological microenvironment-responsive anti-inflammatory hydrogels for ameliorating intervertebral disc degeneration. Biomaterials. (2024) 306:122509. doi: 10.1016/j.biomaterials.2024.122509
45. Dowdell J, Erwin M, Choma T, Vaccaro A, Iatridis J, and Cho SK. Intervertebral disk degeneration and repair. Neurosurgery. (2017) 80:S46–s54. doi: 10.1093/neuros/nyw078
46. Che H, Li J, Li Y, Ma C, Liu H, Qin J, et al. p16 deficiency attenuates intervertebral disc degeneration by adjusting oxidative stress and nucleus pulposus cell cycle. Elife. (2020) 9. doi: 10.7554/eLife.52570
47. Risbud MV and Shapiro IM. Role of cytokines in intervertebral disc degeneration: pain and disc content. Nat Rev Rheumatol. (2014) 10:44–56. doi: 10.1038/nrrheum.2013.160
48. Kawakubo A, Uchida K, Miyagi M, Nakawaki M, Satoh M, Sekiguchi H, et al. Investigation of resident and recruited macrophages following disc injury in mice. J Orthop Res. (2020) 38:1703–9. doi: 10.1002/jor.24590
49. Ribeiro-MaChado C, Santos SG, Amaral IA, Caldeira J, Pereira P, Barbosa MA, et al. Macrophage-based therapy for intervertebral disc herniation: preclinical proof-of-concept. NPJ Regener Med. (2023) 8:34. doi: 10.1038/s41536-023-00309-z
50. Li XC, Luo SJ, Fan W, Zhou TL, Tan DQ, Tan RX, et al. Macrophage polarization regulates intervertebral disc degeneration by modulating cell proliferation, inflammation mediator secretion, and extracellular matrix metabolism. Front Immunol. (2022) 13:922173. doi: 10.3389/fimmu.2022.922173
51. Zhao DW, Cheng Q, Geng H, Liu J, Zhang Y, Cui J, et al. Decoding macrophage subtypes to engineer modulating hydrogels for the alleviation of intervertebral disk degeneration. Adv Sci (Weinh). (2024) 11:e2304480. doi: 10.1002/advs.202304480
52. Zhao F, Guo Z, Hou F, Fan W, Wu B, and Qian Z. Magnoflorine alleviates “M1” Polarized macrophage-induced intervertebral disc degeneration through repressing the HMGB1/Myd88/NF-κB pathway and NLRP3 inflammasome. Front Pharmacol. (2021) 12:701087. doi: 10.3389/fphar.2021.701087
53. Hou Y, Shi G, Guo Y, and Shi J. Epigenetic modulation of macrophage polarization prevents lumbar disc degeneration. Aging (Albany NY). (2020) 12:6558–69. doi: 10.18632/aging.102909
54. Tian S, Chen X, Wu W, Lin H, Qing X, Liu S, et al. Nucleus pulposus cells regulate macrophages in degenerated intervertebral discs via the integrated stress response-mediated CCL2/7-CCR2 signaling pathway. Exp Mol Med. (2024) 56:408–21. doi: 10.1038/s12276-024-01168-4
55. Kozlov AM, Lone A, Betts DH, and Cumming RC. Lactate preconditioning promotes a HIF-1α-mediated metabolic shift from OXPHOS to glycolysis in normal human diploid fibroblasts. Sci Rep. (2020) 10:8388. doi: 10.1038/s41598-020-65193-9
56. Noe JT, Rendon BE, Geller AE, Conroy LR, Morrissey SM, Young LEA, et al. Lactate supports a metabolic-epigenetic link in macrophage polarization. Sci Adv. (2021) 7:eabi8602. doi: 10.1126/sciadv.abi8602
57. Tan H-Y, Wang N, Li S, Hong M, Wang X, and Feng Y. The reactive oxygen species in macrophage polarization: reflecting its dual role in progression and treatment of human diseases. Oxid Med Cell Longevity. (2016) 2016:2795090. doi: 10.1155/2016/2795090
58. de Oliveira S, Rosowski EE, and Huttenlocher A. Neutrophil migration in infection and wound repair: going forward in reverse. Nat Rev Immunol. (2016) 16:378–91. doi: 10.1038/nri.2016.49
59. Phillipson M and Kubes P. The healing power of neutrophils. Trends Immunol. (2019) 40:635–47. doi: 10.1016/j.it.2019.05.001
60. Zhang TL, Chen WK, Huang XP, Zheng BW, Wu PF, Zheng BY, et al. Single-cell RNA sequencing reveals the MIF/ACKR3 receptor-ligand interaction between neutrophils and nucleus pulposus cells in intervertebral disc degeneration. Transl Res. (2024) 272:1–18. doi: 10.1016/j.trsl.2024.05.011
61. Zhao W, Wei J, Ji X, Jia E, Li J, and Huo J. Machine learning algorithm predicts fibrosis-related blood diagnosis markers of intervertebral disc degeneration. BMC Med Genomics. (2023) 16:274. doi: 10.1186/s12920-023-01705-6
62. Firidin MN and Akyüz ME. Preoperative and postoperative diagnostic efficiency of multi-inflammatory index on pain scoring of degenerated intervertebral disc. Adv Clin Exp Med. (2022) 31:947–52. doi: 10.17219/acem/149336
63. Liew PX and Kubes P. The neutrophil’s role during health and disease. Physiol Rev. (2019) 99:1223–48. doi: 10.1152/physrev.00012.2018
64. Hwang MH, Kim KS, Yoo CM, Shin JH, Nam HG, Jeong JS, et al. Photobiomodulation on human annulus fibrosus cells during the intervertebral disk degeneration: extracellular matrix-modifying enzymes. Lasers Med Sci. (2016) 31:767–77. doi: 10.1007/s10103-016-1923-x
65. Kaplan MJ and Radic M. Neutrophil extracellular traps: double-edged swords of innate immunity. J Immunol. (2012) 189:2689–95. doi: 10.4049/jimmunol.1201719
66. Dudli S, Sing DC, Hu SS, Berven SH, Burch S, et al. Intervertebral disc/bone marrow cross-talk with Modic changes. European Spine Journal. (2017) 26(5):1362–1373. doi: 10.1007/s00586-017-4955-4
67. Mayadas TN, Cullere X, and Lowell CA. The multifaceted functions of neutrophils. Annu Rev Pathol. (2014) 9:181–218. doi: 10.1146/annurev-pathol-020712-164023
68. Ling Z, Liu Y, Wang Z, Zhang Z, Chen B, Yang J, et al. Single-cell RNA-seq analysis reveals macrophage involved in the progression of human intervertebral disc degeneration. Front Cell Dev Biol. (2021) 9:833420. doi: 10.3389/fcell.2021.833420
69. Euler M and Hoffmann MH. The double-edged role of neutrophil extracellular traps in inflammation. Biochem Soc Trans. (2019) 47:1921–30. doi: 10.1042/BST20190629
70. Chen Y, Lu D, Churov A, and Fu R. Research progress on NK cell receptors and their signaling pathways. Mediators Inflammation. (2020) 2020:6437057. doi: 10.1155/2020/6437057
71. Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, et al. Innate or adaptive immunity? The example of natural killer cells. Science. (2011) 331:44–9. doi: 10.1126/science.1198687
72. Song C, Zhou D, Cheng K, Liu F, Cai W, Mei Y, et al. Bioinformatics-based discovery of intervertebral disc degeneration biomarkers and immune-inflammatory infiltrates. JOR Spine. (2024) 7:e1311. doi: 10.1002/jsp2.1311
73. Yao Y, Xue H, Chen X, Cao Y, Yu J, Jiang X, et al. Polarization of helper T lymphocytes maybe involved in the pathogenesis of lumbar disc herniation. Iran J Allergy Asthma Immunol. (2017) 16:347–57.
74. Ruterbusch M, Pruner KB, Shehata L, and Pepper M. In vivo CD4(+) T cell differentiation and function: revisiting the Th1/Th2 paradigm. Annu Rev Immunol. (2020) 38:705–25. doi: 10.1146/annurev-immunol-103019-085803
75. Wang J, Zhao X, and Wan YY. Intricacies of TGF-β signaling in Treg and Th17 cell biology. Cell Mol Immunol. (2023) 20:1002–22. doi: 10.1038/s41423-023-01036-7
76. Zhu Z, He Z, Tang T, Wang F, Chen H, Li B, et al. Integrative bioinformatics analysis revealed mitochondrial dysfunction-related genes underlying intervertebral disc degeneration. Oxid Med Cell Longev. (2022) 2022:1372483. doi: 10.1155/2022/1372483
77. Weiler C, Schietzsch M, Kirchner T, Nerlich AG, Boos N, and Wuertz K. Age-related changes in human cervical, thoracic and lumbar intervertebral disc exhibit a strong intra-individual correlation. European Spine Journal. (2012) 21(Suppl 6):S810–S818. (e-published 12 Aug 2011). doi: 10.1007/s00586-011-1922-3
78. Weiler C, Nerlich AG, Zipperer J, Bachmeier BE, and Boos N. 2002 SSE Award Competition in Basic Science: expression of major matrix metalloproteinases is associated with intervertebral disc degradation and resorption. Eur Spine J. (2002) 11:308–20. doi: 10.1007/s00586-002-0472-0
79. Wang JC and Hammer JA. The role of actin and myosin in antigen extraction by B lymphocytes. Semin Cell Dev Biol. (2020) 102:90–104. doi: 10.1016/j.semcdb.2019.10.017
80. Stich S, Stolk M, Girod PP, Thomé C, Sittinger M, Ringe J, et al. Regenerative and immunogenic characteristics of cultured nucleus pulposus cells from human cervical intervertebral discs. PloS One. (2015) 10:e0126954. doi: 10.1371/journal.pone.0126954
81. Ramanathan S, Brilot F, Irani SR, and Dale RC. Origins and immunopathogenesis of autoimmune central nervous system disorders. Nat Rev Neurol. (2023) 19:172–90. doi: 10.1038/s41582-023-00776-4
82. Pavelka K, Kivitz A, Dokoupilova E, Blanco R, Maradiaga M, Tahir H, et al. Efficacy, safety, and tolerability of secukinumab in patients with active ankylosing spondylitis: a randomized, double-blind phase 3 study, MEASURE 3. Arthritis Res Ther. (2017) 19:285. doi: 10.1186/s13075-017-1490-y
83. Zhang Y, He F, Chen Z, Su Q, Yan M, Zhang Q, et al. Melatonin modulates IL-1β-induced extracellular matrix remodeling in human nucleus pulposus cells and attenuates rat intervertebral disc degeneration and inflammation. Aging (Albany NY). (2019) 11:10499–512. doi: 10.18632/aging.102472
84. Baeten D, Sieper J, Braun J, Baraliakos X, Dougados M, Emery P, et al. Secukinumab, an interleukin-17A inhibitor, in ankylosing spondylitis. N Engl J Med. (2015) 373:2534–48. doi: 10.1056/NEJMoa1505066
85. Rosenzwajg M, Churlaud G, Mallone R, Six A, DéRIAN N, Chaara W, et al. Low-dose interleukin-2 fosters a dose-dependent regulatory T cell tuned milieu in T1D patients. J Autoimmun. (2015) 58:48–58. doi: 10.1016/j.jaut.2015.01.001
86. Salles G, Barrett M, FOà R, Maurer J, O’brien S, Valente N, et al. Rituximab in B-cell hematologic Malignancies: A review of 20 years of clinical experience. Adv Ther. (2017) 34:2232–73. doi: 10.1007/s12325-017-0612-x
87. Sibaud V, Beylot-Barry M, Protin C, Vigarios E, Recher C, and Ysebaert L. Dermatological toxicities of Bruton’s tyrosine kinase inhibitors. Am J Clin Dermatol. (2020) 21:799–812. doi: 10.1007/s40257-020-00535-x
88. Hou Y, Shi J, Guo Y, and Shi G. DNMT1 regulates polarization of macrophage-induced intervertebral disc degeneration by modulating SIRT6 expression and promoting pyroptosis in vivo. Aging (Albany NY). (2023) 15:4288–303. doi: 10.18632/aging.204729
89. Meng Q, Liu K, Liu Z, Liu J, Tian Z, Qin S, et al. Digoxin protects against intervertebral disc degeneration via TNF/NF-κB and LRP4 signaling. Front Immunol. (2023) 14:1251517. doi: 10.3389/fimmu.2023.1251517
90. Hong J, Yan J, Chen J, Li S, Huang Y, Huang Z, et al. Identification of key potential targets for TNF-α/TNFR1-related intervertebral disc degeneration by bioinformatics analysis. Connect Tissue Res. (2021) 62:531–41. doi: 10.1080/03008207.2020.1797709
91. Zhao W, Li Y, Cheng X, Wei H, Li P, Fan L, et al. The antioxidant Glycitin protects against intervertebral disc degeneration through antagonizing inflammation and oxidative stress in nucleus pulposus cells. Aging (Albany NY). (2023) 15:13693–709. doi: 10.18632/aging.205251
92. Cheng Z, Xiang Q, Wang J, and Zhang Y. The potential role of melatonin in retarding intervertebral disc ageing and degeneration: A systematic review. Ageing Res Rev. (2021) 70:101394. doi: 10.1016/j.arr.2021.101394
93. Dong X, Hu F, Yi J, Zhang Y, Liu C, Geng P, et al. DPSCs protect architectural integrity and alleviate intervertebral disc degeneration by regulating nucleus pulposus immune status. Stem Cells Int. (2022) 2022:7590337. doi: 10.1155/2022/7590337
94. Chen J, Mei Z, Huang B, Zhang X, Liu J, Shan Z, et al. IL-6/YAP1/β-catenin signaling is involved in intervertebral disc degeneration. J Cell Physiol. (2019) 234:5964–71. doi: 10.1002/jcp.v234.5
95. Ji ML, Lu J, Shi PL, Zhang XJ, Wang SZ, Chang Q, et al. Dysregulated miR-98 contributes to extracellular matrix degradation by targeting IL-6/STAT3 signaling pathway in human intervertebral disc degeneration. J Bone Miner Res. (2016) 31:900–9. doi: 10.1002/jbmr.2753
96. Grad S, Bow C, Karppinen J, Luk KD, Cheung KM, Alini M, et al. Systemic blood plasma CCL5 and CXCL6: Potential biomarkers for human lumbar disc degeneration. Eur Cell Mater. (2016) 31:1–10. doi: 10.22203/eCM.v031a01
97. Willems N, Tellegen AR, Bergknut N, Creemers LB, Wolfswinkel J, Freudigmann C, et al. Inflammatory profiles in canine intervertebral disc degeneration. BMC Vet Res. (2016) 12:10. doi: 10.1186/s12917-016-0635-6
98. Tang G, Han X, Lin Z, Qian H, Chen B, Zhou C, et al. Propionibacterium acnes Accelerates Intervertebral Disc Degeneration by Inducing Pyroptosis of Nucleus Pulposus Cells via the ROS-NLRP3 Pathway. Oxid Med Cell Longev. (2021) 2021:4657014. doi: 10.1155/2021/4657014
99. Song C, Xu Y, Peng Q, Chen R, Zhou D, Cheng K, et al. Mitochondrial dysfunction: a new molecular mechanism of intervertebral disc degeneration. Inflammation Res. (2023) 72:2249–60. doi: 10.1007/s00011-023-01813-0
100. Liu ZM, Lu CC, Shen PC, Chou SH, Shih CL, Chen JC, et al. Suramin attenuates intervertebral disc degeneration by inhibiting NF-κB signalling pathway. Bone Joint Res. (2021) 10:498–513. doi: 10.1302/2046-3758.108.BJR-2020-0041.R3
101. Liao Z, Liu H, Ma L, Lei J, Tong B, Li G, et al. Engineering extracellular vesicles restore the impaired cellular uptake and attenuate intervertebral disc degeneration. ACS Nano. (2021) 15:14709–24. doi: 10.1021/acsnano.1c04514
102. Goupille P, Mulleman D, and Chevalier X. Is interleukin-1 a good target for therapeutic intervention in intervertebral disc degeneration: lessons from the osteoarthritic experience. Arthritis Res Ther. (2007) 9:110. doi: 10.1186/ar2324
103. Wang J, Zhang Y, Cao J, Wang Y, Anwar N, Zhang Z, et al. The role of autophagy in bone metabolism and clinical significance. Autophagy. (2023) 19:2409–27. doi: 10.1080/15548627.2023.2186112
104. Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol. (2009) 1:a001651.
105. Chen Z, Giotti B, Kaluzova M, Vallcorba MP, Rawat K, Price G, et al. A paracrine circuit of IL-1β/IL-1R1 between myeloid and tumor cells drives genotype-dependent glioblastoma progression. J Clin Invest. (2023) 133. doi: 10.1172/JCI163802
106. Yang M, Wang X, Wang L, Wang X, Li J, and Yang Z. IL-1α Up-regulates IL-6 expression in bovine granulosa cells via MAPKs and NF-κB signaling pathways. Cell Physiol Biochem. (2017) 41:265–73. doi: 10.1159/000456091
107. Glaeser JD, Salehi K, Kanim LEA, Napier Z, Kropf MA, Cuéllar JM, et al. NF-κB inhibitor, NEMO-binding domain peptide attenuates intervertebral disc degeneration. Spine J. (2020) 20:1480–91. doi: 10.1016/j.spinee.2020.04.025
108. Jiao Y, Yuan Y, Lin Y, Zhou Z, Zheng Y, Wu W, et al. Propionibacterium acnes induces discogenic low back pain via stimulating nucleus pulposus cells to secrete pro-algesic factor of IL-8/CINC-1 through TLR2-NF-κB p65 pathway. J Mol Med (Berl). (2019) 97:25–35. doi: 10.1007/s00109-018-1712-z
109. Zhang J, He L, Li Q, Gao J, Zhang E, and Feng H. EGR1 knockdown confers protection against ferroptosis and ameliorates intervertebral disc cartilage degeneration by inactivating the MAP3K14/NF-κB axis. Genomics. (2023) 115:110683. doi: 10.1016/j.ygeno.2023.110683
110. Tao X, Xue F, Xu J, and Wang W. Platelet-rich plasma-derived extracellular vesicles inhibit NF-κB/NLRP3 pathway-mediated pyroptosis in intervertebral disc degeneration via the MALAT1/microRNA-217/SIRT1 axis. Cell Signal. (2024) 117:111106. doi: 10.1016/j.cellsig.2024.111106
111. Wang H, Tian Y, Wang J, Phillips KLE, Binch ALA, Dunn S, et al. Inflammatory cytokines induce NOTCH signaling in nucleus pulposus cells: implications in intervertebral disc degeneration. J Biol Chem. (2013) 288:16761–74. doi: 10.1074/jbc.M112.446633
112. Guo Z, Gao WS, Wang YF, Gao F, Wang W, and Ding WY. MiR-502 suppresses TNF-α-induced nucleus pulposus cell apoptosis by targeting TARF2. BioMed Res Int. (2021) 2021:5558369. doi: 10.1155/2021/5558369
113. Fang W, Zhou X, Wang J, Xu L, Zhou L, Yu W, et al. Wogonin mitigates intervertebral disc degeneration through the Nrf2/ARE and MAPK signaling pathways. Int Immunopharmacol. (2018) 65:539–49. doi: 10.1016/j.intimp.2018.10.024
114. Liao Z, Luo R, Li G, Song Y, Zhan S, Zhao K, et al. Exosomes from mesenchymal stem cells modulate endoplasmic reticulum stress to protect against nucleus pulposus cell death and ameliorate intervertebral disc degeneration in vivo. Theranostics. (2019) 9:4084–100. doi: 10.7150/thno.33638
115. Lu S, Song Y, Luo R, Li S, Li G, Wang K, et al. Ferroportin-dependent iron homeostasis protects against oxidative stress-induced nucleus pulposus cell ferroptosis and ameliorates intervertebral disc degeneration in vivo. Oxid Med Cell Longev. (2021) 2021:6670497. doi: 10.1155/2021/6670497
116. Wang C, Chen Q, Chen S, Fan L, Gan Z, Zhao M, et al. Serine synthesis sustains macrophage IL-1β production via NAD(+)-dependent protein acetylation. Mol Cell. (2024) 84:744–759.e6. doi: 10.1016/j.molcel.2024.01.002
117. Zhang S, Liang W, Abulizi Y, Xu T, Cao R, Xun C, et al. Quercetin alleviates intervertebral disc degeneration by modulating p38 MAPK-mediated autophagy. BioMed Res Int. (2021) 2021:6631562. doi: 10.1155/2021/6631562
118. Chen B, Liu Y, Zhang Y, Li J, Cheng K, and Cheng L. IL-21 is positively associated with intervertebral disc degeneration by interaction with TNF-α Through the JAK-STAT signaling pathway. Inflammation. (2017) 40:612–22. doi: 10.1007/s10753-017-0508-6
119. Li J, Yu C, Ni S, and Duan Y. Identification of core genes and screening of potential targets in intervertebral disc degeneration using integrated bioinformatics analysis. Front Genet. (2022) 13:864100. doi: 10.3389/fgene.2022.864100
120. Suzuki S, Fujita N, Fujii T, Watanabe K, Yagi M, Tsuji T, et al. Potential involvement of the IL-6/JAK/STAT3 pathway in the pathogenesis of intervertebral disc degeneration. Spine (Phila Pa 1976). (2017) 42:E817–e824. doi: 10.1097/BRS.0000000000001982
121. Xu HW, Fang XY, Liu XW, Zhang SB, Yi YY, Chang SJ, et al. α-Ketoglutaric acid ameliorates intervertebral disk degeneration by blocking the IL-6/JAK2/STAT3 pathway. Am J Physiol Cell Physiol. (2023) 325:C1119–c1130. doi: 10.1152/ajpcell.00280.2023
122. Xu J and Núñez G. The NLRP3 inflammasome: activation and regulation. Trends Biochem Sci. (2023) 48:331–44. doi: 10.1016/j.tibs.2022.10.002
123. Xu H, Dai ZH, He GL, Cai HC, Chen XY, Chen YL, et al. Gamma-oryzanol alleviates intervertebral disc degeneration development by intercepting the IL-1β/NLRP3 inflammasome positive cycle. Phytomedicine. (2022) 102:154176. doi: 10.1016/j.phymed.2022.154176
124. Hai B, Mao T, Du C, Jia F, Liu Y, Song Q, et al. USP14 promotes pyroptosis of human annulus fibrosus cells derived from patients with intervertebral disc degeneration through deubiquitination of NLRP3. Acta Biochim Biophys Sin (Shanghai). (2022) 54:1720–30. doi: 10.3724/abbs.2022171
125. He D, Zhou M, Bai Z, Wen Y, Shen J, and Hu Z. Propionibacterium acnes induces intervertebral disc degeneration by promoting nucleus pulposus cell pyroptosis via NLRP3-dependent pathway. Biochem Biophys Res Commun. (2020) 526:772–9. doi: 10.1016/j.bbrc.2020.03.161
126. Chen Y, Zheng Z, Wang J, Tang C, Khor S, Chen J, et al. Berberine suppresses apoptosis and extracellular matrix (ECM) degradation in nucleus pulposus cells and ameliorates disc degeneration in a rodent model. Int J Biol Sci. (2018) 14:682–92. doi: 10.7150/ijbs.24081
127. McKee CM and Coll RC. NLRP3 inflammasome priming: A riddle wrapped in a mystery inside an enigma. J Leukocyte Biol. (2020) 108:937–52. doi: 10.1002/JLB.3MR0720-513R
128. Hendrayani S-F, Al-Harbi B, Al-Ansari MM, Silva G, and Aboussekhra A. The inflammatory/cancer-related IL-6/STAT3/NF-κB positive feedback loop includes AUF1 and maintains the active state of breast myofibroblasts. Oncotarget. (2016) 7:41974–85.
129. An Y, Zhang H, Wang C, Jiao F, Xu H, Wang X, et al. Activation of ROS/MAPKs/NF-κB/NLRP3 and inhibition of efferocytosis in osteoclast-mediated diabetic osteoporosis. FASEB Journal: Off Publ Fed Am Societies For Exp Biol. (2019) 33:12515–27. doi: 10.1096/fj.201802805RR
130. Fan Y, Zhao L, Lai Y, Lu K, and Huang J. CRISPR-Cas9-mediated loss of function of β-catenin attenuates intervertebral disc degeneration. Mol Ther Nucleic Acids. (2022) 28:387–96. doi: 10.1016/j.omtn.2022.03.024
131. Yang S, Zhang F, Ma J, and Ding W. Intervertebral disc ageing and degeneration: The antiapoptotic effect of oestrogen. Ageing Res Rev. (2020) 57:100978. doi: 10.1016/j.arr.2019.100978
132. Sun K, Jing X, Guo J, Yao X, and Guo F. Mitophagy in degenerative joint diseases. Autophagy. (2021) 17:2082–92. doi: 10.1080/15548627.2020.1822097
133. Li HR, Cui Q, Dong ZY, Zhang JH, Li HQ, and Zhao L. Downregulation of miR-27b is involved in loss of type II collagen by directly targeting matrix metalloproteinase 13 (MMP13) in human intervertebral disc degeneration. Spine (Phila Pa 1976). (2016) 41:E116–23. doi: 10.1097/BRS.0000000000001139
134. Zhou M, He SJ, Liu W, Yang MJ, Hou ZY, Meng Q, et al. EZH2 upregulates the expression of MAPK1 to promote intervertebral disc degeneration via suppression of miR-129-5p. J Gene Med. (2022) 24:e3395. doi: 10.1002/jgm.v24.3
135. Cheng X, Zhang G, Zhang L, Hu Y, Zhang K, Sun X, et al. Mesenchymal stem cells deliver exogenous miR-21 via exosomes to inhibit nucleus pulposus cell apoptosis and reduce intervertebral disc degeneration. J Cell Mol Med. (2018) 22:261–76. doi: 10.1111/jcmm.2018.22.issue-1
136. Zhang C, Qiu Y, and Yuan F. The long non-coding RNA maternally expressed 3-micorRNA-15a-5p axis is modulated by melatonin and prevents nucleus pulposus cell inflammation and apoptosis. Basic Clin Pharmacol Toxicol. (2023) 133:603–19. doi: 10.1111/bcpt.v133.5
137. Tang N, Dong Y, Xiao T, and Zhao H. LncRNA TUG1 promotes the intervertebral disc degeneration and nucleus pulposus cell apoptosis though modulating miR-26a/HMGB1 axis and regulating NF-κB activation. Am J Transl Res. (2020) 12:5449–64.
138. Yang H, Tian W, Wang S, Liu X, Wang Z, Hou L, et al. TSG-6 secreted by bone marrow mesenchymal stem cells attenuates intervertebral disc degeneration by inhibiting the TLR2/NF-κB signaling pathway. Lab Invest. (2018) 98:755–72. doi: 10.1038/s41374-018-0036-5
139. Liu MC, Chen WH, Wu LC, Hsu WC, Lo WC, Yeh SD, et al. Establishment of a promising human nucleus pulposus cell line for intervertebral disc tissue engineering. Tissue Eng Part C Methods. (2014) 20:1–10. doi: 10.1089/ten.tec.2013.0048
140. van der Gaag WH, Roelofs PD, Enthoven WT, van Tulder MW, and Koes BW. Non-steroidal anti-inflammatory drugs for acute low back pain. Cochrane Database Syst Rev. (2020) 4:Cd013581. doi: 10.1002/14651858.CD013581
141. Tobinick E. Perispinal etanercept for neuroinflammatory disorders. Drug Discov Today. (2009) 14:168–77. doi: 10.1016/j.drudis.2008.10.005
142. Li H, Wang X, Pan H, Xiao C, Wang C, Guo S, et al. The mechanisms and functions of IL-1β in intervertebral disc degeneration. Exp Gerontol. (2023) 177:112181. doi: 10.1016/j.exger.2023.112181
143. Sainoh T, Orita S, Miyagi M, Inoue G, Yamauchi K, Suzuki M, et al. Single intradiscal injection of the interleukin-6 receptor antibody tocilizumab provides short-term relief of discogenic low back pain; prospective comparative cohort study. J Orthop Sci. (2016) 21:2–6. doi: 10.1016/j.jos.2015.10.005
144. Richardson SM, Kalamegam G, Pushparaj PN, Matta C, Memic A, Khademhosseini A, et al. Mesenchymal stem cells in regenerative medicine: Focus on articular cartilage and intervertebral disc regeneration. Methods. (2016) 99:69–80. doi: 10.1016/j.ymeth.2015.09.015
145. Kim Y, An SB, Lee SH, Lee JJ, Kim SB, Ahn JC, et al. Enhanced intervertebral disc repair via genetically engineered mesenchymal stem cells with tetracycline regulatory system. Int J Mol Sci. (2023) 24. doi: 10.3390/ijms242216024
146. Hu S, Xing H, Zhang J, Zhu Z, Yin Y, Zhang N, et al. Mesenchymal stem cell-derived extracellular vesicles: immunomodulatory effects and potential applications in intervertebral disc degeneration. Stem Cells Int. (2022) 2022:7538025. doi: 10.1155/2022/7538025
147. Munda M and Velnar T. Stem cell therapy for degenerative disc disease: Bridging the gap between preclinical promise and clinical potential. Biomol Biomed. (2024) 24:210–8. doi: 10.17305/bb.2023.9518
148. Wu J, Yu L, Liu Y, Xiao B, Ye X, Zhao H, et al. Hypoxia regulates adipose mesenchymal stem cells proliferation, migration, and nucleus pulposus-like differentiation by regulating endoplasmic reticulum stress via the HIF-1α pathway. J Orthop Surg Res. (2023) 18:339. doi: 10.1186/s13018-023-03818-1
149. Jin Y, Wu O, Chen Q, Chen L, Zhang Z, Tian H, et al. Hypoxia-preconditioned BMSC-derived exosomes induce mitophagy via the BNIP3-ANAX2 axis to alleviate intervertebral disc degeneration. Adv Sci (Weinheim Baden Wurttemberg Germany). (2024) 11:e2404275. doi: 10.1002/advs.202404275
150. Zhang Y, Zhang J, Sun Z, Wang H, Ning R, Xu L, et al. MAPK8 and CAPN1 as potential biomarkers of intervertebral disc degeneration overlapping immune infiltration, autophagy, and ceRNA. Front Immunol. (2023) 14:1188774. doi: 10.3389/fimmu.2023.1188774
151. Xu C, Zhang M, Li K, Ni M, Bai Y, Zhang J, et al. CD24(hi)CD38(hi) B regulatory cells from patients with end plate inflammation presented reduced functional potency. Int Immunopharmacol. (2019) 70:295–301. doi: 10.1016/j.intimp.2019.02.034
152. Zhang Y, Zheng L, Fang J, Ni K, Hu X, Ye L, et al. Macrophage migration inhibitory factor (MIF) promotes intervertebral disc degeneration through the NF-κB pathway, and the MIF inhibitor CPSI-1306 alleviates intervertebral disc degeneration in a mouse model. FASEB J. (2023) 37:e23303.
153. Nakazawa KR, Walter BA, Laudier DM, Krishnamoorthy D, Mosley GE, Spiller KL, et al. Accumulation and localization of macrophage phenotypes with human intervertebral disc degeneration. Spine J. (2018) 18:343–56. doi: 10.1016/j.spinee.2017.09.018
154. Wang C, Yu X, Yan Y, Yang W, Zhang S, Xiang Y, et al. Tumor necrosis factor-α: a key contributor to intervertebral disc degeneration. Acta Biochim Biophys Sin (Shanghai). (2017) 49:1–13. doi: 10.1093/abbs/gmw112
155. Zhou X, Chen L, Grad S, Alini M, Pan H, Yang D, et al. The roles and perspectives of microRNAs as biomarkers for intervertebral disc degeneration. J Tissue Eng Regener Med. (2017) 11:3481–7. doi: 10.1002/term.2261
156. Xi Y, Jiang T, Wang W, Yu J, Wang Y, Wu X, et al. Long non-coding HCG18 promotes intervertebral disc degeneration by sponging miR-146a-5p and regulating TRAF6 expression. Sci Rep. (2017) 7:13234. doi: 10.1038/s41598-017-13364-6
157. Wang Z, Zhang J, Zheng W, and He Y. Long non-coding RNAs H19 and HOTAIR implicated in intervertebral disc degeneration. Front Genet. (2022) 13:843599. doi: 10.3389/fgene.2022.843599
158. Zhang Y, Han S, Kong M, Tu Q, Zhang L, and Ma X. Single-cell RNA-seq analysis identifies unique chondrocyte subsets and reveals involvement of ferroptosis in human intervertebral disc degeneration. Osteoarthritis Cartilage. (2021) 29:1324–34. doi: 10.1016/j.joca.2021.06.010
159. Tan J, Shi M, Li B, Liu Y, Luo S, and Cheng X. Role of arachidonic acid metabolism in intervertebral disc degeneration: identification of potential biomarkers and therapeutic targets via multi-omics analysis and artificial intelligence strategies. Lipids Health Dis. (2023) 22:204. doi: 10.1186/s12944-023-01962-5
160. Atluri S, Murphy MB, Dragella R, Herrera J, Boachie-Adjei K, Bhati S, et al. Evaluation of the effectiveness of autologous bone marrow mesenchymal stem cells in the treatment of chronic low back pain due to severe lumbar spinal degeneration: A 12-month, open-label, prospective controlled trial. Pain Physician. (2022) 25:193–207.
161. Kämpe A, Knebel A, Carlson R, Rohn K, and Tipold A. Evaluation of the involvement of Th17-cells in the pathogenesis of canine spinal cord injury. PloS One. (2021) 16:e0257442. doi: 10.1371/journal.pone.0257442
162. Hu B, Lv X, Wei L, Wang Y, Zheng G, Yang C, et al. Sensory nerve maintains intervertebral disc extracellular matrix homeostasis via CGRP/CHSY1 axis. Adv Sci (Weinh). (2022) 9:e2202620. doi: 10.1002/advs.202202620
163. Liu Z, Wang H, Yuan Z, Wei Q, Han F, Chen S, et al. High-resolution 3D printing of angle-ply annulus fibrosus scaffolds for intervertebral disc regeneration. Biofabrication. (2022) 15.
164. Li ZY, Zhou AF, Li G, Zhou LY, Pu PM, Zhu K, et al. Chronic spinal cord compression associated with intervertebral disc degeneration in SPARC-null mice. Neural Regener Res. (2023) 18:634–42. doi: 10.4103/1673-5374.350210
165. Zhang W, Nie L, Guo YJ, Han LX, Wang X, Zhao H, et al. Th17 cell frequency and IL-17 concentration correlate with pre- and postoperative pain sensation in patients with intervertebral disk degeneration. Orthopedics. (2014) 37:e685–91. doi: 10.3928/01477447-20140626-62
166. Wang Y, Kang J, Guo X, Zhu D, Liu M, Yang L, et al. Intervertebral disc degeneration models for pathophysiology and regenerative therapy -benefits and limitations. J Invest Surg. (2022) 35:935–52. doi: 10.1080/08941939.2021.1953640
167. Kleinstiver BP, Pattanayak V, Prew MS, Tsai SQ, Nguyen NT, Zheng Z, et al. High-fidelity CRISPR-Cas9 nucleases with no detectable genome-wide off-target effects. Nature. (2016) 529:490–5. doi: 10.1038/nature16526
168. Lu Y, Godbout K, Lamothe G, and Tremblay JP. CRISPR-Cas9 delivery strategies with engineered extracellular vesicles. Mol Ther Nucleic Acids. (2023) 34:102040. doi: 10.1016/j.omtn.2023.102040
169. Zhang S, Shen J, Li D, and Cheng Y. Strategies in the delivery of Cas9 ribonucleoprotein for CRISPR/Cas9 genome editing. Theranostics. (2021) 11:614–48. doi: 10.7150/thno.47007
170. Ariss MM, Islam A, Critcher M, Zappia MP, and Frolov MV. Single cell RNA-sequencing identifies a metabolic aspect of apoptosis in Rbf mutant. Nat Commun. (2018) 9:5024. doi: 10.1038/s41467-018-07540-z
171. Tu J, Li W, Yang S, Yang P, Yan Q, Wang S, et al. Single-cell transcriptome profiling reveals multicellular ecosystem of nucleus pulposus during degeneration progression. Adv Sci (Weinh). (2022) 9:e2103631. doi: 10.1002/advs.202103631
172. Son HG, Hwang MH, Lee S, Kim AG, Kim TW, Kim JH, et al. Intervertebral disc organ-on-a-chip: an innovative model to study monocyte extravasation during nucleus pulposus degeneration. Lab Chip. (2023) 23:2819–28. doi: 10.1039/D3LC00032J
173. Zheng HD, Sun YL, Kong DW, Yin MC, Chen J, Lin YP, et al. Deep learning-based high-accuracy quantitation for lumbar intervertebral disc degeneration from MRI. Nat Commun. (2022) 13:841. doi: 10.1038/s41467-022-28387-5
174. Lin Z, Wang H, Song J, Xu G, Lu F, Ma X, et al. The role of mitochondrial fission in intervertebral disc degeneration. Osteoarthritis Cartilage. (2023) 31:158–66. doi: 10.1016/j.joca.2022.10.020
175. Gruber HE, Hoelscher GL, Bethea S, and Hanley EN Jr. Mitochondrial membrane potential and nuclear and gene expression changes during human disc cell apoptosis: in vitro and in vivo annulus findings. Spine (Phila Pa 1976). (2015) 40:876–82. doi: 10.1097/BRS.0000000000000936
176. Zorov DB, Juhaszova M, and Sollott SJ. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol Rev. (2014) 94:909–50. doi: 10.1152/physrev.00026.2013
177. Kaarniranta K, Pawlowska E, Szczepanska J, Jablkowska A, and Blasiak J. Role of mitochondrial DNA damage in ROS-mediated pathogenesis of age-related macular degeneration (AMD). Int J Mol Sci. (2019) 20. doi: 10.3390/ijms20102374
178. Liu MX, Jin L, Sun SJ, Liu P, Feng X, Cheng ZL, et al. Metabolic reprogramming by PCK1 promotes TCA cataplerosis, oxidative stress and apoptosis in liver cancer cells and suppresses hepatocellular carcinoma. Oncogene. (2018) 37:1637–53. doi: 10.1038/s41388-017-0070-6
179. Shao Z, Wang B, Shi Y, Xie C, Huang C, Chen B, et al. Senolytic agent Quercetin ameliorates intervertebral disc degeneration via the Nrf2/NF-κB axis. Osteoarthritis Cartilage. (2021) 29:413–22. doi: 10.1016/j.joca.2020.11.006
180. Lluis JM, Buricchi F, Chiarugi P, Morales A, and Fernandez-Checa JC. Dual role of mitochondrial reactive oxygen species in hypoxia signaling: activation of nuclear factor-{kappa}B via c-SRC and oxidant-dependent cell death. Cancer Res. (2007) 67:7368–77. doi: 10.1158/0008-5472.CAN-07-0515
181. Murphy MP and Hartley RC. Mitochondria as a therapeutic target for common pathologies. Nat Rev Drug Discov. (2018) 17:865–86. doi: 10.1038/nrd.2018.174
182. Heggli I, Teixeira GQ, Iatridis JC, Neidlinger-Wilke C, and Dudli S. The role of the complement system in disc degeneration and Modic changes. JOR Spine. (2024) 7:e1312. doi: 10.1002/jsp2.1312
183. Klopf J, Brostjan C, Eilenberg W, and Neumayer C. Neutrophil extracellular traps and their implications in cardiovascular and inflammatory disease. Int J Mol Sci. (2021) 22. doi: 10.3390/ijms22020559
184. Suyama K, Sakai D, and Watanabe M. The role of IL-17-mediated inflammatory processes in the pathogenesis of intervertebral disc degeneration and herniation: A comprehensive review. Front Cell Dev Biol. (2022) 10:857164. doi: 10.3389/fcell.2022.857164
185. Xiang H, Yan F, and Liu H. The genetic association identified between intervertebral disc degeneration and associated risk factors based on a systems biology approach. Spine (Phila Pa 1976). (2022) 47:E370–e384. doi: 10.1097/BRS.0000000000004312
186. Guo W, Mu K, Li WS, Gao SX, Wang LF, Li XM, et al. Identification of mitochondria-related key gene and association with immune cells infiltration in intervertebral disc degeneration. Front Genet. (2023) 14:1135767. doi: 10.3389/fgene.2023.1135767
187. Deng YJ, Wang XG, Li Z, Wang B, Li J, Ma J, et al. Comprehensive analysis of senescence-related genes and immune infiltration in intervertebral disc degeneration: a meta-data approach utilizing bulk and single-cell RNA sequencing data. Front Mol Biosci. (2023) 10:1296782. doi: 10.3389/fmolb.2023.1296782
188. Zhou Z, Qin W, Zhang P, He J, Cheng Z, Gong Y, et al. Potential molecular targets and drugs for basement membranes-related intervertebral disk degeneration through bioinformatics analysis and molecular docking. BMC Musculoskelet Disord. (2023) 24:772. doi: 10.1186/s12891-023-06891-z
189. Zhou T, Lin H, Cheng Z, Ji C, Zhang C, and Tian J. Mechanism of microRNA-146a-mediated IL-6/STAT3 signaling in lumbar intervertebral disc degeneration. Exp Ther Med. (2017) 14:1131–5. doi: 10.3892/etm.2017.4611
190. Karli P, MARTLé V, Bossens K, Summerfield A, Doherr MG, Turner P, et al. Dominance of chemokine ligand 2 and matrix metalloproteinase-2 and -9 and suppression of pro-inflammatory cytokines in the epidural compartment after intervertebral disc extrusion in a canine model. Spine J. (2014) 14:2976–84. doi: 10.1016/j.spinee.2014.05.021
191. Gadjradj PS, Harhangi BS, Amelink J, Van Susante J, Kamper S, Van Tulder M, et al. Percutaneous transforaminal endoscopic discectomy versus open microdiscectomy for lumbar disc herniation: A systematic review and meta-analysis. Spine (Phila Pa 1976). (2021) 46:538–49. doi: 10.1097/BRS.0000000000003843
192. Dinarello CA. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol Rev. (2018) 281:8–27. doi: 10.1111/imr.2018.281.issue-1
193. Zhou Y, Chen Z, Yang X, Cao X, Liang Z, Ma H, et al. Morin attenuates pyroptosis of nucleus pulposus cells and ameliorates intervertebral disc degeneration via inhibition of the TXNIP/NLRP3/Caspase-1/IL-1β signaling pathway. Biochem Biophys Res Commun. (2021) 559:106–12. doi: 10.1016/j.bbrc.2021.04.090
194. Wang X, Tan Y, Liu F, Wang J, Liu F, Zhang Q, et al. Pharmacological network analysis of the functions and mechanism of kaempferol from Du Zhong in intervertebral disc degeneration (IDD). J Orthop Translat. (2023) 39:135–46. doi: 10.1016/j.jot.2023.01.002
195. Gong Y, Qiu J, Jiang T, Li Z, Zhang W, Zheng X, et al. Maltol ameliorates intervertebral disc degeneration through inhibiting PI3K/AKT/NF-κB pathway and regulating NLRP3 inflammasome-mediated pyroptosis. Inflammopharmacology. (2023) 31:369–84. doi: 10.1007/s10787-022-01098-5
196. Das UN. Bioactive lipids in intervertebral disc degeneration and its therapeutic implications. Biosci Rep. (2019) 39. doi: 10.1042/BSR20192117
197. Du X, Wang X, Cui K, Chen Y, Zhang C, Yao K, et al. Tanshinone IIA and astragaloside IV inhibit miR-223/JAK2/STAT1 signalling pathway to alleviate lipopolysaccharide-induced damage in nucleus pulposus cells. Dis Markers. (2021) 2021:6554480. doi: 10.1155/2021/6554480
198. Flores RR, Carbo L, Kim E, Van Meter M, De Padilla CML, Zhao J, et al. Adenoviral gene transfer of a single-chain IL-23 induces psoriatic arthritis-like symptoms in NOD mice. FASEB J. (2019) 33:9505–15. doi: 10.1096/fj.201900420R
199. Hu B, Wang J, Wu X, Chen Y, Yuan W, and Chen H. Interleukin-17 upregulates vascular endothelial growth factor by activating the JAK/STAT pathway in nucleus pulposus cells. Joint Bone Spine. (2017) 84:327–34. doi: 10.1016/j.jbspin.2016.05.014
200. Elshamly M, Kinslechner K, Grohs JG, Weinmann D, Walzer SM, Windhager R, et al. Galectins-1 and -3 in human intervertebral disc degeneration: non-uniform distribution profiles and activation of disease markers involving NF-κB by galectin-1. J Orthop Res. (2019) 37:2204–16. doi: 10.1002/jor.v37.10
201. Phillips KL, Cullen K, Chiverton N, Michael AL, Cole AA, Breakwell LM, et al. Potential roles of cytokines and chemokines in human intervertebral disc degeneration: interleukin-1 is a master regulator of catabolic processes. Osteoarthritis Cartilage. (2015) 23:1165–77. doi: 10.1016/j.joca.2015.02.017
202. Shi ZW, Zhu L, Song ZR, Liu TJ, and Hao DJ. Roles of p38 MAPK signalling in intervertebral disc degeneration. Cell Prolif. (2023) 56:e13438. doi: 10.1111/cpr.13438
203. Francisco V, Ait Eldjoudi D, González-Rodríguez M, Ruiz-Fernández C, Cordero-Barreal A, Marques P, et al. Metabolomic signature and molecular profile of normal and degenerated human intervertebral disc cells. Spine J. (2023) 23:1549–62. doi: 10.1016/j.spinee.2023.06.005
204. Nasto LA, Seo HY, Robinson AR, Tilstra JS, Clauson CL, Sowa GA, et al. ISSLS prize winner: inhibition of NF-κB activity ameliorates age-associated disc degeneration in a mouse model of accelerated aging. Spine (Phila Pa 1976). (2012) 37:1819–25. doi: 10.1097/BRS.0b013e31824ee8f7
205. Zhang X, Chen J, Huang B, Wang J, Shan Z, Liu J, et al. Obesity mediates apoptosis and extracellular matrix metabolic imbalances via MAPK pathway activation in intervertebral disk degeneration. Front Physiol. (2019) 10:1284. doi: 10.3389/fphys.2019.01284
206. Ge J, Cheng X, Yuan C, Qian J, Wu C, Cao C, et al. Syndecan-4 is a novel therapeutic target for intervertebral disc degeneration via suppressing JNK/p53 pathway. Int J Biol Sci. (2020) 16:766–76. doi: 10.7150/ijbs.40189
207. Mahmoud M, Kokozidou M, GöGELE C, Werner C, Auffarth A, Kohl B, et al. Does vitamin K2 influence the interplay between diabetes mellitus and intervertebral disc degeneration in a rat model? Nutrients. (2023) 15.
208. Yan S, Xie LY, Duan XX, Tan JX, Yang S, Meng L, et al. Electroacupuncture improves apoptosis of nucleus pulposus cells via the IL-22/JAK2-STAT3 signaling pathway in a rat model of cervical intervertebral disk degeneration. Acupunct Med. (2024) 42:146–54. doi: 10.1177/09645284241248465
209. Chi S, Li S, Xu Z, Yang G, Song Y, Liao Z, et al. The involvement of DDX3X in compression-induced nucleus pulposus pyroptosis. Biochem Biophys Res Commun. (2023) 655:1–10. doi: 10.1016/j.bbrc.2023.02.074
210. Chao-Yang G, Peng C, and Hai-Hong Z. Roles of NLRP3 inflammasome in intervertebral disc degeneration. Osteoarthritis Cartilage. (2021) 29:793–801. doi: 10.1016/j.joca.2021.02.204
211. Bai J, Zhang Y, Fan Q, Xu J, Shan H, Gao X, et al. Reactive oxygen species-scavenging scaffold with rapamycin for treatment of intervertebral disk degeneration. Adv Healthc Mater. (2020) 9:e1901186. doi: 10.1002/adhm.201901186
212. Zhang S, Wang P, Hu B, Liu W, Lv X, Chen S, et al. HSP90 inhibitor 17-AAG attenuates nucleus pulposus inflammation and catabolism induced by M1-polarized macrophages. Front Cell Dev Biol. (2021) 9:796974. doi: 10.3389/fcell.2021.796974
213. Yamamoto Y, Kokubo Y, Nakajima H, Honjoh K, Watanabe S, and Matsumine A. Distribution and polarization of hematogenous macrophages associated with the progression of intervertebral disc degeneration. Spine (Phila Pa 1976). (2022) 47:E149–e158. doi: 10.1097/BRS.0000000000004222
214. Zhang P, Rong K, Guo J, Cui L, Kong K, Zhao C, et al. Cynarin alleviates intervertebral disc degeneration via protecting nucleus pulposus cells from ferroptosis. BioMed Pharmacother. (2023) 165:115252. doi: 10.1016/j.biopha.2023.115252
215. Shi S, Kang XJ, Zhou Z, He ZM, Zheng S, and He SS. Excessive mechanical stress-induced intervertebral disc degeneration is related to Piezo1 overexpression triggering the imbalance of autophagy/apoptosis in human nucleus pulpous. Arthritis Res Ther. (2022) 24:119. doi: 10.1186/s13075-022-02804-y
216. Pan H, Li H, Guo S, Wang C, Long L, Wang X, et al. The mechanisms and functions of TNF-α in intervertebral disc degeneration. Exp Gerontol. (2023) 174:112119. doi: 10.1016/j.exger.2023.112119
217. Ge J, Yan Q, Wang Y, Cheng X, Song D, Wu C, et al. IL-10 delays the degeneration of intervertebral discs by suppressing the p38 MAPK signaling pathway. Free Radic Biol Med. (2020) 147:262–70. doi: 10.1016/j.freeradbiomed.2019.12.040
218. Wang S, Wei J, Fan Y, Ding H, Tian H, Zhou X, et al. Progranulin is positively associated with intervertebral disc degeneration by interaction with IL-10 and IL-17 through TNF pathways. Inflammation. (2018) 41:1852–63. doi: 10.1007/s10753-018-0828-1
219. Hanaei S, Abdollahzade S, Sadr M, Mirbolouk MH, Fattahi E, Khoshnevisan A, et al. Association of interleukin 2, interleukin 12, and interferon-γ with intervertebral disc degeneration in Iranian population. BMC Med Genet. (2020) 21:143. doi: 10.1186/s12881-020-01081-3
220. Zhang K, Gao L, Wang HX, Ye L, Shi YY, Yang WY, et al. Interleukin-18 inhibition protects against intervertebral disc degeneration via the inactivation of caspase-3/9 dependent apoptotic pathways. Immunol Invest. (2022) 51:1895–907. doi: 10.1080/08820139.2022.2077113
221. Ye S, Ju B, Wang H, and Lee KB. Bone morphogenetic protein-2 provokes interleukin-18-induced human intervertebral disc degeneration. Bone Joint Res. (2016) 5:412–8. doi: 10.1302/2046-3758.59.BJR-2016-0032.R1
222. Chen Y, Cao X, Pan B, Du H, Li B, Yang X, et al. Verapamil attenuates intervertebral disc degeneration by suppressing ROS overproduction and pyroptosis via targeting the Nrf2/TXNIP/NLRP3 axis in four-week puncture-induced rat models both in vivo and in vitro. Int Immunopharmacol. (2023) 123:110789. doi: 10.1016/j.intimp.2023.110789
223. Hu YC, Zhang XB, Lin MQ, Zhou HY, Cong MX, Chen XY, et al. Nanoscale treatment of intervertebral disc degeneration: mesenchymal stem cell exosome transplantation. Curr Stem Cell Res Ther. (2023) 18:163–73. doi: 10.2174/1574888X17666220422093103
Keywords: intervertebral disc degeneration, immune microenvironment, immune modulation, immune cells, therapeutic strategies
Citation: Ren Q, Chen L, Ma Y, Huang Y and Wang S (2025) Immune microenvironment in intervertebral disc degeneration: pathophysiology and therapeutic potential. Front. Immunol. 16:1563635. doi: 10.3389/fimmu.2025.1563635
Received: 21 January 2025; Accepted: 09 June 2025;
Published: 04 July 2025.
Edited by:
Ashish Diwan, University of New South Wales, AustraliaReviewed by:
Qizhao Huang, Chongqing Medical University, ChinaLei Ma, Third Hospital of Hebei Medical University, China
Copyright © 2025 Ren, Chen, Ma, Huang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yansheng Huang, eXNoZzE5OTFAMTYzLmNvbQ==; Sibo Wang, d2FuZ3NpYm93YW5nZmVpQDE2My5jb20=
 Qian Ren
Qian Ren Ling Chen2
Ling Chen2 Yansheng Huang
Yansheng Huang Sibo Wang
Sibo Wang