- 1Affiliated Hospital of Nantong University, Medical School of Nantong University, Nantong, China
- 2Department of Otolaryngology, Affiliated Hospital of Nantong University, Medical School of Nantong University, Nantong, China
- 3Department of Otolaryngology Head and Neck Surgery, Affiliated Huai’an Hospital of Xuzhou Medical University, Huai’an, China
- 4Department of Oncology, Nantong Tumor Hospital, Nantong, China
Laryngeal squamous cell carcinoma (LSCC) is a prevalent malignancy with high mortality and recurrence rates, necessitating novel therapeutic strategies. Recent research highlights the pivotal role of metabolic reprogramming and immune microenvironment alterations in LSCC pathogenesis, providing promising avenues for targeted therapy. This review summarizes the metabolic characteristics of LSCC, including glycolysis, lipid metabolism, and amino acid biosynthesis, and their implications for tumor progression and therapeutic resistance. Additionally, this review further describes the tumor microenvironment’s immunosuppressive landscape, including immune checkpoint regulation, tumor-associated macrophages, and T-cell dysfunction. The integration of metabolic and immune-targeted strategies represents a promising frontier in LSCC treatment, warranting further investigation.
1 Introduction
Laryngeal squamous cell carcinoma (LSCC) is a prevalent and aggressive malignancy, representing approximately one-third of head and neck cancers (1, 2). Recent research highlights the pivotal roles of metabolic reprogramming and the tumor immune microenvironment (TME) in cancer progression (3–6). Metabolic reprogramming, involving alterations in glucose, lipid, and amino acid metabolism, supports tumor growth, survival, and therapy resistance (7–12). Simultaneously, the TME, comprising immune cells, stromal components, and extracellular matrix elements, facilitates immune evasion and tumor progression (13–15). Immunotherapy, especially immune checkpoint inhibitors (ICIs) like pembrolizumab and nivolumab, has transformed the treatment of recurrent or metastatic LSCC, improving survival and quality of life (16). However, variability in treatment response and resistance mechanisms necessitate a deeper understanding of the metabolic and immune landscape of LSCC to optimize therapeutic outcomes.
This review summarizes the metabolic characteristics of LSCC, including glycolysis, lipid metabolism, and amino acid biosynthesis, and their implications for tumor progression. We also examine the immunosuppressive features of the TME, such as immune checkpoint regulation and T-cell dysfunction, and their impact on immunotherapy. Finally, we provide the advances in immunotherapy and the potential of integrating metabolic and immune-targeted strategies to enhance precision medicine in LSCC management. By synthesizing current knowledge, this review aims to guide the development of more effective treatments for LSCC.
2 The tumor microenvironment in laryngeal squamous cell carcinoma
2.1 Composition of the tumor microenvironment in laryngeal squamous cell carcinoma
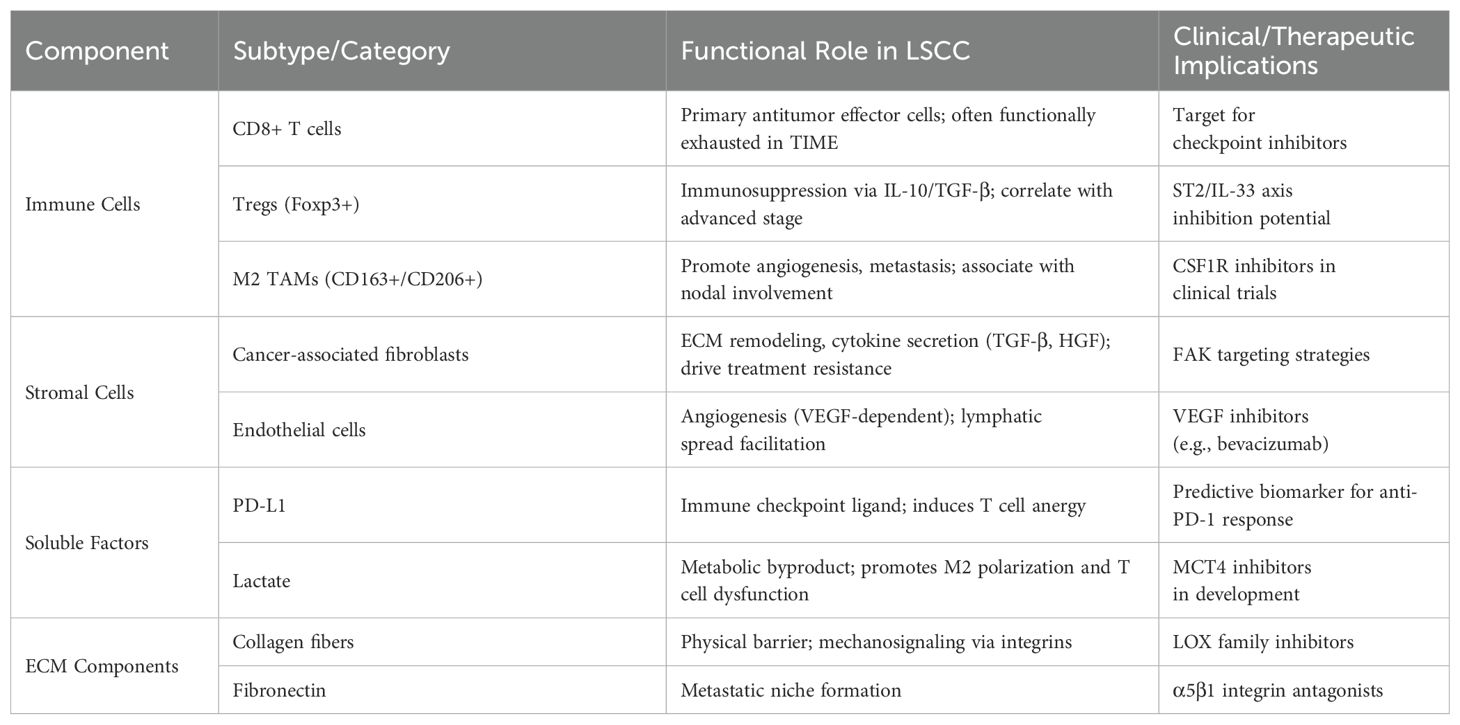
The tumor microenvironment is a complex ecosystem shaped by interactions among malignant cells, cancer stem cells (CSCs), and stromal components, including vascular-associated cells and extracellular matrix (ECM) elements, during tumorigenesis and progression (17–20). This environment undergoes metabolic reprogramming, influencing gene expression, cellular differentiation, and tumor cell functionality. CSCs, a rare but critical subpopulation with self-renewal capacity (3, 7), play a key role in tumor recurrence and metastasis. The stromal compartment of the TME includes non-immune and immune cells. Non-immune stromal cells, such as fibroblasts, endothelial cells, and pericytes, provide structural and metabolic support. Immune cells, including lymphocytes and macrophages, facilitate immune evasion and promote immune tolerance (13). Cancer-associated fibroblasts (CAFs) are particularly significant due to their role in ECM remodeling and supporting LSCC proliferation. Key immune subsets, such as dendritic cells, tumor-infiltrating lymphocytes (TILs), and tumor-associated macrophages (TAMs), exert diverse immunomodulatory effects (16).
Non-cellular TME components include ECM proteins, cytokines, chemokines, growth factors, proteases, and non-coding RNAs (21–29). ECM proteins regulate biomechanical properties, influencing cancer cell adhesion, survival, differentiation, and invasion. Secreted factors create a pro-angiogenic and immunosuppressive landscape, promoting tumor progression. Non-coding RNAs, implicated in LSCC radio-resistance (30), show potential as diagnostic and prognostic biomarkers, with their downregulation linked to reduced tumor proliferation and metastasis.
2.2 Tumor microenvironment and the development of laryngeal squamous cell carcinoma
Cancer progression involves genetic alterations (31, 32), such as oncogene overexpression and tumor suppressor gene silencing, leading to epithelial cell changes and precancerous lesions (33–35). TME promotes tissue invasion, metastasis, and immune evasion, facilitating malignant transformation (36–40). Recent studies highlight key TME components in laryngeal squamous cell carcinoma (LSCC) progression, identifying potential therapeutic targets (Table 1). Genomic analyses link LSCC risk to overexpression of SRY-box transcription factor 2 (SOX2), cortactin (CTTN), and focal adhesion kinase (FAK) (41, 42). SU (43) identified CD163+ TAMs and Ki-67 proliferation as dysplasia severity indicators, with Ki-67 facilitating spheroid formation, offering new predictors for LSCC risk stratification. LSCC invasion and metastasis involve complex mechanisms. KLOBUCAR (44) linked ladinin-1 to MIF-CD44-β1 integrin signaling, increasing LSCC cell motility. TOPF (45) demonstrated LSCC-derived factors in lymph nodes promote CD163+ TAMs, raising nodal metastasis risk. Immune evasion is critical in LSCC. Elevated Tregs in LSCC patients suppress CD4+ and CD25+ T cell proliferation (46). WEN (47) found IL-33 increases Foxp3+ GATA3+ Tregs and suppresses T cell proliferation via IL-10 and TGF-β1, with ST2 inhibition reversing this effect. PD-L1 overexpression in LSCC binds PD-1, inducing T cell anergy or apoptosis. Yu et al. (48) noted higher PD-L1 levels in LSCC, negatively correlating with CD8+ TILs and CD16+ M1 TAMs but positively with CD206+ M2 TAMs.
These findings illustrate the multifaceted mechanisms by which LSCC invades surrounding tissues and metastasizes. Building upon this mechanistic understanding, recent studies have identified biomarkers that may assist in risk stratification and prognosis of LSCC. Prognostic biomarkers in LSCC include CD3, CD8, CD57, and S100, correlating with better outcomes, while CTLA-4 predicts poor prognosis (49). Tumor-stroma interactions, including stroma-rich tumors and fibroblastic patterns, indicate aggressive disease (50). Inflammatory factors regulate disease progression and significantly influence the efficacy of therapies (51–56). Vassil et al. (57) found PD-L1 expression correlates with TIL density and survival, which was also observed in IL-12Rβ2+ TILs (58). Further, Schulter et al. (59) identified CD31-positive vasculature and VEGF as markers for recurrence risk.
3 Metabolic characteristics of the microenvironment in laryngeal squamous cell carcinoma
The tumor microenvironment (TME) in cancer undergoes dynamic alterations in cellular and extracellular components, driving metabolic reprogramming—a hallmark of malignancy (60–63). Metabolomics, utilizing mass spectrometry (MS) or nuclear magnetic resonance (NMR) spectroscopy, enables comprehensive profiling of metabolites in biological specimens, aiding in biomarker identification and elucidating disease mechanisms (64). NMR was used to analyze LSCC tissues, revealing elevated lactate, amino acids, choline compounds, creatine, taurine, and glutathione, alongside reduced triglycerides (64). Fei et al. (65) employed GC-TOF-MS and UPLC-TOF-MS to identify 41 differentially expressed metabolites in LSCC tissues, including upregulated TCA cycle intermediates, lactate, purine, and pyrimidine metabolites, and downregulated fatty acid derivatives. Urine metabolomics further distinguished LSCC patients from controls, highlighting pantothenic acid, palmitic acid, myristic acid, oleamide, sphingosine, and phytoglycine as potential diagnostic biomarkers (66). These findings underscore the role of lipid metabolism in bio-membrane biosynthesis and cancer cell proliferation. Current studies emphasize glycolysis, lipid metabolism, and amino acid biosynthesis as key metabolic features of LSCC, aligning with genomic and proteomic insights (67). These findings highlight the importance of metabolic reprogramming in LSCC pathogenesis and the potential of metabolomics for identifying therapeutic targets and biomarkers.
3.1 Glucose metabolism
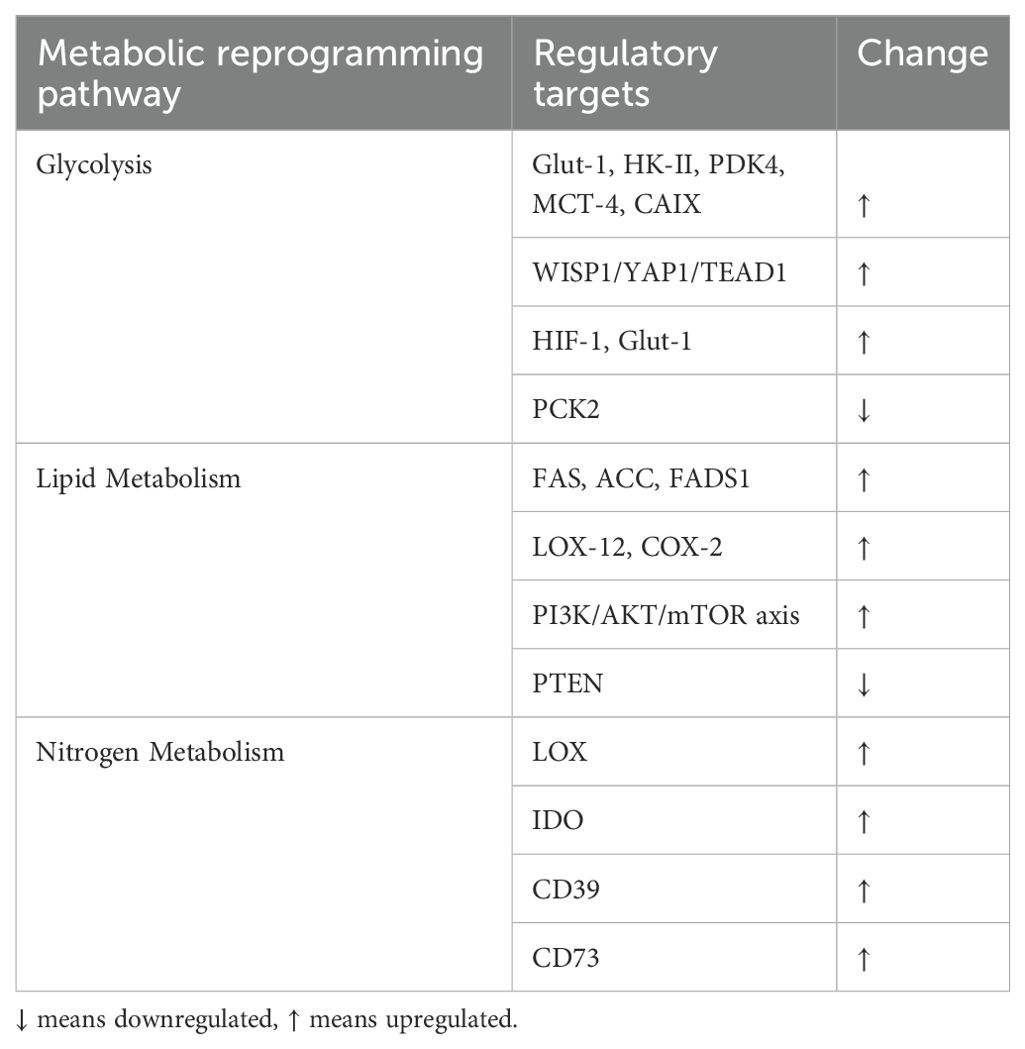
The Warburg effect, as a hallmark of cancer metabolism, involves a preference for glycolysis over oxidative phosphorylation for ATP production, even under aerobic conditions (68). This metabolic shift promotes radiation resistance and malignant progression in cancers like LSCC. Glycolysis is initiated by glucose uptake via glucose transporter-1 (Glut-1) and phosphorylation by hexokinase-II (HK-II), with pyruvate converted to lactate by lactate-dehydrogenase (LDH) and exported via monocarboxylate transporter-4 (MCT-4) to prevent intracellular acidification (69). Glut-1, a key driver of the Warburg effect, was studied by WANG (70) in Hep-2 cells, revealing that aberrant WISP1 expression enhanced glucose uptake, lactate production, and cisplatin resistance by upregulating YAP1 and TEAD1. LU (71) demonstrated that CRISPR/Cas9-mediated knockout of HIF-1 and Glut-1 impaired glucose uptake and LSCC progression. HU (72) linked PCK2 downregulation to suppressed LSCC progression and Glut-1 interaction.
HK-II, regulated by lncRNA loc285194 (73), miR-125a (74), and miR-125b-5p (75), is central to glycolysis. XU (76) found that lncRNA PCAT19 suppresses PDK4 via the miR-182/PDK4 axis, promoting glycolysis. LIU (77) showed that FOXJ1 knockdown attenuates glycolysis by inhibiting the Wnt/β-catenin pathway. MCT-4 facilitates lactate efflux, while MCT-1 mediates uptake, creating metabolic coupling (78). CURRY (79) observed high MCT-4 and low MCT-1 expression in tumor cells, promoting glycolysis. Targeting MCT-4 offers therapeutic potential. WANG (69) linked Glut-1, MCT-4, and CAIX expression to LSCC grade, with combined inhibition suppressing glycolysis. LIU (67) identified RASSF1, PGK1, CAII, and CAXII as key metabolic regulators in LSCC.
3.2 Lipid metabolism
Lipid-mediated signaling pathways are crucial in LSCC pathogenesis. Cancer cells upregulate fatty acid and phospholipid biosynthesis, producing metabolites that modify membrane components and act as signaling molecules. Fatty acid biosynthesis begins with citrate export from mitochondria, converted to acetyl-CoA by ATP citrate lyase. Acetyl-CoA carboxylase (ACC) then generates malonyl-CoA, which fatty acid synthase (FAS) converts to palmitic acid, a precursor for other fatty acids. FAS (80) and ACC (81) are overexpressed in LSCC, highlighting their role in fatty acid biosynthesis and potential as prognostic markers. Fatty acid desaturase 1 (FADS1), a key enzyme in polyunsaturated fatty acid biosynthesis, converts linoleic acid to arachidonic acid (AA). Zhao (82) found elevated FADS1 in LSCC tissues, with knockdown impairing cell proliferation, migration, and invasion, suggesting FADS1 promotes LSCC via AKT/mTOR signaling.
Lipid metabolism plays a crucial role in laryngeal squamous cell carcinoma (LSCC) progression through its interplay with inflammation and oncogenic signaling pathways. Arachidonic acid (AA), released from membrane phospholipids by phospholipase A2 (PLA2), serves as a substrate for cyclooxygenase (COX) and lipoxygenase (LOX) to generate pro-inflammatory eicosanoids (83, 84). Studies have demonstrated elevated expression of PLCγ-2 and LOX-12 in LSCC, which correlates with advanced clinical stage, poor differentiation, and metastatic potential, while COX-2 overexpression has been associated with tumor recurrence (85). Early-stage LSCC exhibits increased levels of linoleic acid (LA), AA, and saturated fatty acids that enhance LOX and COX-2 activity, driving oxidative stress, inflammatory responses, angiogenesis, and immune evasion through upregulation of NF-κB and Bcl-2 (86, 87). Notably, reduced PTEN expression in LSCC tissues serves as a prognostic indicator, and DJ-1 silencing has been shown to restore PTEN expression, thereby inhibiting tumor cell proliferation and invasion (88–90). These findings collectively identify FAS, ACC, LOX-12, COX-2, and PTEN as critical regulators in LSCC lipid metabolism, highlighting the therapeutic potential of targeting fatty acid biosynthesis, AA metabolism, and the PTEN/PI3K/AKT/mTOR axis in LSCC treatment (86).
3.3 Nitrogen metabolism
Nitrogen is essential for proteins, DNA, and RNA. In humans, it is primarily used for urea biosynthesis in the liver, with disruptions in the urea cycle common in tumors, leading to upregulated pyrimidine biosynthesis and amino acid metabolism (91). The lysyl oxidase (LOX) family stabilizes collagen and elastic fibers, regulating EMT and tumor progression (92). Elevated LOX expression correlates with poor prognosis and metastasis in LSCC (93). Tryptophan metabolism via indoleamine 2,3-dioxygenase (IDO) produces kynurenine, an immunosuppressive metabolite. ENGIN (94) found higher IDO activity in advanced LSCC, with elevated serum neopterin post-resection indicating poor outcomes. Adenosine, an extracellular signaling molecule, activates tumor cell receptors, promoting growth. WILKAT (95) showed A2B receptor inhibition reduces tumor growth and vascularization. Hypoxia and inflammation in the TME increase adenosine production, enhancing immunosuppression. CD39 and CD73, elevated in head and neck cancer, accelerate ATP hydrolysis, increasing adenosine and reinforcing immunosuppression (96).
Moreover, metabolic reprogramming shapes the immune microenvironment by modulating nutrient availability, altering metabolite composition, and producing immunosuppressive byproducts. For instance, lactate accumulation due to enhanced glycolysis impairs CD8+ T cell and NK cell cytotoxicity while promoting regulatory T cell (Treg) differentiation. Lipid accumulation in the TME compromises dendritic cell function and fosters M2-like macrophage polarization, promoting immune evasion. Elevated IDO activity in tryptophan metabolism generates kynurenine, suppressing T cell proliferation and driving Treg expansion. These alterations jointly contribute to immune dysfunction and resistance to immune checkpoint inhibitors in LSCC (Table 2).
4 Advances in immunotherapy for laryngeal squamous cell carcinoma
4.1 Cytotoxic agent
Cytotoxic agents such as cisplatin, 5-fluorouracil (5-Fu), and docetaxel play a central role in laryngeal preservation strategies, exerting antineoplastic effects through mechanisms involving DNA damage or inhibition of protein synthesis (97). Cisplatin, a cornerstone in the treatment of LSCC, demonstrates substantial efficacy but is limited by dose-dependent nephrotoxicity. Similarly, 5-Fu, a widely utilized chemotherapeutic for solid tumors, carries a cardiotoxicity risk ranging from 0% to 35% (98). Docetaxel, effective against various metastatic malignancies, is frequently associated with peripheral neuropathy, alopecia, and neutropenia—adverse effects that often necessitate dose modifications (99). Clinical studies have provided supportive evidence for laryngeal preservation. Urba et al. Urba et al. (100) reported a 3-year laryngeal preservation rate of 70% using induction chemotherapy, exceeding the 64% rate observed in the Veterans Affairs trial (101). The RTOG 91–11 trial and its follow-up corroborated these findings (102, 103). Notably, incorporation of docetaxel into the cisplatin and 5-Fu induction regimen—forming the TPF regimen—has demonstrated superior outcomes. The TPF regimen yielded improved laryngeal preservation rates at both 5 years (74% vs. 58%) and 10 years (70% vs. 47%) compared to PF alone (104), establishing TPF as a more effective induction strategy (105, 106).
However, concerns regarding patient selection and generalizability have emerged. Olsen (107) emphasized that participants in these trials were generally younger and presented with limited nodal disease, potentially limiting the applicability of findings to broader patient populations. Furthermore, conflicting evidence has challenged the universal adoption of laryngeal preservation protocols. For instance, Nocon (108) and Bates (109) reported improved survival with total laryngectomy. Dyckhoff (110) further noted that patients with T4-stage LSCC treated with chemoradiotherapy exhibited a twofold increase in mortality risk compared to those receiving total laryngectomy followed by adjuvant radiotherapy. Collectively, these findings underscore the importance of individualized treatment planning, as the heterogeneity in tumor staging, patient characteristics, and treatment response continues to constrain the universal implementation of induction chemotherapy or concurrent chemoradiotherapy as a standard approach.
4.2 Epidermal growth factor receptor monoclonal antibodies
Monoclonal antibodies targeting the EGFR, such as cetuximab and nimotuzumab, are the only clinically approved targeted therapies for head and HNSCC (111). EGFR is overexpressed in approximately 90% of HNSCC cases, and its aberrant activation in laryngeal carcinoma promotes uncontrolled proliferation, radiotherapy resistance, and poor prognosis (112, 113). Physiologically, EGFR regulates epidermal cell development, with expression limited to undifferentiated basal keratinocytes and diminishing as cells migrate to the epithelial surface. Activation by ligands like epidermal growth factor (EGF) and TGF-α triggers tyrosine kinase signaling, driving growth-associated transcription in normal and malignant cells. Cetuximab and nimotuzumab competitively inhibit EGFR activation, disrupting downstream signaling, reducing cellular survival, and enhancing tumor-targeting efficacy compared to conventional chemotherapy.
EGFR inhibition enhances radiosensitivity in laryngeal carcinoma, improving radiotherapy outcomes (114). Cetuximab combined with radiotherapy extended locoregional control to 24.4 months versus 14.9 months with radiotherapy alone, likely due to enhanced apoptosis without increased toxicity (115). Bonner et al. (116) reported a 3-year laryngeal preservation rate of 87.9% with cetuximab plus radiotherapy, compared to 76.8% with radiotherapy alone. Similarly, Noronha et al. (117) observed a 74.1% laryngeal preservation rate with nimotuzumab combined with cisplatin and 5-fluorouracil (5-FU), supporting anti-EGFR antibodies’ role in organ preservation (118). However, the RTOG 10–16 trial found lower 5-year overall survival with cetuximab plus radiotherapy (77.9%) versus cisplatin plus radiotherapy (84.6%) in HPV-positive laryngeal carcinoma (119). While cetuximab is less effective than cisplatin in cisplatin-tolerant patients, it remains a viable alternative for cisplatin-resistant or intolerant individuals. Despite its clinical utility, cetuximab benefits only a subset of patients, underscoring the importance of patient selection in optimizing EGFR-targeted therapy.
4.3 Immune checkpoint inhibitors
Immune checkpoint inhibitors have significantly advanced the treatment of cancer (120–123), particularly for recurrent or metastatic cases. In 2016, the FDA approved PD-1 inhibitors nivolumab and pembrolizumab for platinum-refractory recurrent/metastatic HNSCC (124). These inhibitors counteract tumor immune evasion by restoring T-cell-mediated cytotoxicity, targeting the PD-1/PD-L1 axis, a key regulator of T-cell activation. PD-1, expressed on immune cells, interacts with PD-L1 to suppress T-cell activity via the PI3K-AKT pathway, promoting immune tolerance (125, 126). Nivolumab and pembrolizumab block PD-1, preventing immune suppression and enhancing antitumor immunity.
Pembrolizumab’s efficacy was first shown in the KEYNOTE-012 trial (127), with the phase III KEYNOTE-048 trial (128) confirming its role as a first-line treatment. Recently, a randomized, double-blind, phase 3 trial evaluated the efficacy and safety of the PD-1 monoclonal antibody finotonlimab (SCT-I10A) combined with cisplatin plus 5-fluorouracil (C5F) as first-line treatment for recurrent HNSCC (NCT04146402). In the finotonlimab plus C5F group, the median OS was 14.1 months, compared with 10.5 months in the placebo plus C5F group. This study highlights the effectiveness of immunotherapy combined with chemotherapy in recurrent or metastatic head and HNSCC (129). Furthermore, clinical studies have shown that PD-L1-high HNSCC patients treated with a PD-L1 inhibitor combined with 5-azacytidine (5-aza) experienced a significant extension in overall survival (OS) (NCT03019003) (130). To date, numerous clinical trials are still underway, evaluating the efficacy and optimal dosage of immune checkpoint inhibitors, exploring other immune targets, and providing new therapeutic targets for the treatment of laryngeal cancer. In addition to PD-1, other immune checkpoints such as cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and lymphocyte activation gene-3 (LAG-3) are being investigated (131, 132).
5 Conclusion
Laryngeal squamous cell carcinoma (LSCC) is characterized by profound metabolic reprogramming and an immunosuppressive tumor microenvironment, both of which contribute to disease progression and therapeutic resistance. Immunotherapy, particularly immune checkpoint inhibitors (ICIs), has demonstrated substantial promise in treating recurrent or metastatic LSCC. However, limitations in response rates and the development of resistance necessitate combinatorial strategies.
Recent preclinical and early-phase clinical studies have highlighted the potential of combining metabolic inhibitors with ICIs to enhance antitumor immunity. For instance, co-administration of PD-1 inhibitors with glycolytic inhibitors such as 2-deoxy-D-glucose (2-DG) has been shown to restore CD8+ T cell function and reduce tumor burden in murine models of head and HNSCC, including LSCC. Similarly, targeting lipid metabolism using fatty acid oxidation (FAO) inhibitors like etomoxir in combination with PD-1 blockade has led to augmented T cell infiltration and enhanced antitumor efficacy in preclinical studies. In clinical contexts, a phase I trial combining pembrolizumab with the glutaminase inhibitor CB-839 (telaglenastat) in solid tumors demonstrated favorable safety and preliminary antitumor activity, supporting the translational relevance of metabolic-immune co-targeting strategies. These findings underscore the therapeutic potential of dual modulation of tumor metabolism and immune checkpoints, offering a promising avenue for overcoming immune resistance and improving clinical outcomes in LSCC. Future research should aim to delineate optimal combinations, identify predictive biomarkers, and validate efficacy through large-scale clinical trials. Integrating metabolic and immune-targeted therapies represents a rational and potentially transformative approach for precision medicine in LSCC treatment.
Author contributions
KM: Conceptualization, Data curation, Formal Analysis, Methodology, Project administration, Writing – original draft. QM: Investigation, Methodology, Software, Supervision, Validation, Writing – original draft. BF: Funding acquisition, Project administration, Validation, Writing – original draft. TN: Data curation, Formal Analysis, Methodology, Writing – original draft. ZZ: Conceptualization, Funding acquisition, Investigation, Software, Writing – original draft. HN: Funding acquisition, Writing – original draft, Writing – review & editing, Resources.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by National Natural Science Foundation of China (Grant No. 82371130), Basic Research Project of Huai’an (HAB2024015), Special Research Project of Nantong Municipal Health Commission (QN2024027), and Jiangsu Provincial Research Hospital (YJXYY202204-2-YSB02).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Patterson RH, Fischman VG, Wasserman I, Siu J, Shrime MG, Fagan JJ, et al. Global burden of head and neck cancer: economic consequences, health, and the role of surgery. Otolaryngology–Head Neck Surg. (2020) 162:296–303. doi: 10.1177/0194599819897265
2. Piotrowski I, Zhu X, Saccon TD, Ashiqueali S, Schneider A, de Carvalho Nunes AD, et al. miRNAs as Biomarkers for Diagnosing and Predicting Survival of Head and Neck Squamous Cell Carcinoma Patients. Cancers (Basel). (2021) 13:3980. doi: 10.3390/cancers13163980
3. Xia Z, Chen S, He M, Li B, Deng Y, Yi L, et al. Editorial: Targeting metabolism to activate T cells and enhance the efficacy of checkpoint blockade immunotherapy in solid tumors. Front Immunol. (2023) 14:1247178. doi: 10.3389/fimmu.2023.1247178
4. Zhang P, Pei S, Wu L, Xia Z, Wang Q, Huang X, et al. Integrating multiple machine learning methods to construct glutamine metabolism-related signatures in lung adenocarcinoma. Front Endocrinol (Lausanne). (2023) 14:1196372. doi: 10.3389/fendo.2023.1196372
5. Zhang X, Zhuge J, Liu J, Xia Z, Wang H, Gao Q, et al. : Prognostic signatures of sphingolipids: Understanding the immune landscape and predictive role in immunotherapy response and outcomes of hepatocellular carcinoma. Front Immunol. (2023) 14:1153423. doi: 10.3389/fimmu.2023.1153423
6. Liu J, Zhang P, Yang F, Jiang K, Sun S, Xia Z, et al. Integrating single-cell analysis and machine learning to create glycosylation-based gene signature for prognostic prediction of uveal melanoma. Front Endocrinol (Lausanne). (2023) 14:1163046. doi: 10.3389/fendo.2023.1163046
7. Greco A, Rizzo MI, De Virgilio A, Gallo A, Fusconi M, Pagliuca G, et al. Cancer stem cells in laryngeal cancer: what we know. Eur Arch Otorhinolaryngol. (2016) 273:3487–95. doi: 10.1007/s00405-015-3837-9
8. Soltani M, Zhao Y, Xia Z, Ganjalikhani Hakemi M, Bazhin AV. The importance of cellular metabolic pathways in pathogenesis and selective treatments of hematological Malignancies. Front Oncol. (2021) 11:767026. doi: 10.3389/fonc.2021.767026
9. Zhao S, Zhang X, Gao F, Chi H, Zhang J, Xia Z, et al. Identification of copper metabolism-related subtypes and establishment of the prognostic model in ovarian cancer. Front Endocrinol (Lausanne). (2023) 14:1145797. doi: 10.3389/fendo.2023.1145797
10. Wang X, Zhao Y, Strohmer DF, Yang W, Xia Z, Yu C. The prognostic value of MicroRNAs associated with fatty acid metabolism in head and neck squamous cell carcinoma. Front Genet. (2022) 13:983672. doi: 10.3389/fgene.2022.983672
11. Zuo D, Li C, Liu T, Yue M, Zhang J, Ning G. Construction and validation of a metabolic risk model predicting prognosis of colon cancer. Sci Rep. (2021) 11:6837. doi: 10.1038/s41598-021-86286-z
12. Zhong Y, Zhang Y, Wei S, Chen J, Zhong C, Cai W, et al. Dissecting the effect of sphingolipid metabolism gene in progression and microenvironment of osteosarcoma to develop a prognostic signature. Front Endocrinol (Lausanne). (2022) 13:1030655. doi: 10.3389/fendo.2022.1030655
13. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. (2011) 144:646–74. doi: 10.1016/j.cell.2011.02.013
14. Xie H, Xi X, Lei T, Liu H, Xia Z. CD8(+) T cell exhaustion in the tumor microenvironment of breast cancer. Front Immunol. (2024) 15:1507283. doi: 10.3389/fimmu.2024.1507283
15. Deng Y, Shi M, Yi L, Naveed Khan M, Xia Z, Li X. Eliminating a barrier: Aiming at VISTA, reversing MDSC-mediated T cell suppression in the tumor microenvironment. Heliyon. (2024) 10:e37060. doi: 10.1016/j.heliyon.2024.e37060
16. Bai S, Yang X, Zhang N, Zhang F, Shen Z, Yang N, et al. Function of tumor infiltrating lymphocytes in solid tumors - a review. Sheng Wu Gong Cheng Xue Bao. (2019) 35:2308–25. doi: 10.13345/j.cjb.190300
17. Zhang C, Sun D, Li C, Liu Y, Zhou Y, Zhang J. Development of cancer-associated fibroblasts subtype and prognostic model in gastric cancer and the landscape of tumor microenvironment. Int J Biochem Cell Biol. (2022) 152:106309. doi: 10.1016/j.biocel.2022.106309
18. Liu T, Li C, Zhang J, Hu H, Li C. Unveiling efferocytosis-related signatures through the integration of single-cell analysis and machine learning: a predictive framework for prognosis and immunotherapy response in hepatocellular carcinoma. Front Immunol. (2023) 14:1237350. doi: 10.3389/fimmu.2023.1237350
19. Huang L, Sun F, Liu Z, Jin W, Zhang Y, Chen J, et al. Probing the potential of defense response-associated genes for predicting the progression, prognosis, and immune microenvironment of osteosarcoma. Cancers (Basel). (2023) 15:2405. doi: 10.3390/cancers15082405
20. Liu G, Xiong D, Che Z, Chen H, Jin W. A novel inflammation-associated prognostic signature for clear cell renal cell carcinoma. Oncol Lett. (2022) 24:307. doi: 10.3892/ol.2022.13427
21. Huang L, Jin W, Bao Y, Zeng X, Zhang Y, Zhou J, et al. Identification and validation of long noncoding RNA AC083900.1 and RP11-283C24.1 for prediction of progression of osteosarcoma. Mutat Res. (2023) 827:111828. doi: 10.1016/j.mrfmmm.2023.111828
22. Wang Z, Yan S, Liao S, Zhang Y, Wu S, Zhou M, et al. Dysregulated lncSNHG12 suppresses the invasion and migration of trophoblasts by regulating Dio2/Snail axis to involve in recurrent spontaneous abortion. Biochem Pharmacol. (2024) 229:116459. doi: 10.1016/j.bcp.2024.116459
23. Zhao Y, Wei K, Chi H, Xia Z, Li X. IL-7: A promising adjuvant ensuring effective T cell responses and memory in combination with cancer vaccines? Front Immunol. (2022) 13:1022808. doi: 10.3389/fimmu.2022.1022808
24. Gong X, Chi H, Strohmer DF, Teichmann AT, Xia Z, Wang Q. Exosomes: A potential tool for immunotherapy of ovarian cancer. Front Immunol. (2022) 13:1089410. doi: 10.3389/fimmu.2022.1089410
25. Balkwill FR, Capasso M, Hagemann T. The tumor microenvironment at a glance. J Cell Sci. (2012) 125:5591–6. doi: 10.1242/jcs.116392
26. Gong X, Chi H, Xia Z, Yang G, Tian G. Advances in HPV-associated tumor management: Therapeutic strategies and emerging insights. J Med Virol. (2023) 95:e28950. doi: 10.1002/jmv.28950
27. Xiong J, Chi H, Yang G, Zhao S, Zhang J, Tran LJ, et al. Revolutionizing anti-tumor therapy: unleashing the potential of B cell-derived exosomes. Front Immunol. (2023) 14:1188760. doi: 10.3389/fimmu.2023.1188760
28. Zhou W, Li S, Zhang X, Li C, Zhang J. miR-143-3p shuttled by M2 macrophage-derived extracellular vesicles induces progression of colorectal cancer through a ZC3H12A/C/EBPbeta axis-dependent mechanism. Int Immunopharmacol. (2023) 119:110137. doi: 10.1016/j.intimp.2023.110137
29. Liu T, Yin L, Yan G, Li C, Wang L. A meta-analysis of microRNA-17 as a potential biomarker in diagnosis of colorectal cancer. Cell Mol Biol (Noisy-le-grand). (2018) 64:86–93. doi: 10.14715/cmb/2018.64.6.15
30. Wang K, Tang J, Liu X, Wang Y, Chen W, Zheng R. UBR5 regulates proliferation and radiosensitivity in human laryngeal carcinoma via the p38/MAPK signaling pathway. Oncol Rep. (2020) 44:685–97. doi: 10.3892/or.2020.7620
31. Gu J, Wang Y, Zhang H, Gu H, Zhu H. SIGLEC1 has the potential to be an immune-related prognostic indicator in colon adenocarcinoma: a study based on transcriptomic data and Mendelian randomization analysis. Discov Oncol. (2025) 16:324. doi: 10.1007/s12672-025-02093-2
32. Liu Y, Cheng L, Song X, Li C, Zhang J, Wang L. A TP53-associated immune prognostic signature for the prediction of the overall survival and therapeutic responses in pancreatic cancer. Math Biosci Eng. (2022) 19:191–208. doi: 10.3934/mbe.2022010
33. Li Z, Zhou H, Xia Z, Xia T, Du G, Franziska SD, et al. HMGA1 augments palbociclib efficacy via PI3K/mTOR signaling in intrahepatic cholangiocarcinoma. Biomark Res. (2023) 11:33. doi: 10.1186/s40364-023-00473-w
34. Zhai X, Xia Z, Du G, Zhang X, Xia T, Ma D, et al. LRP1B suppresses HCC progression through the NCSTN/PI3K/AKT signaling axis and affects doxorubicin resistance. Genes Dis. (2023) 10:2082–96. doi: 10.1016/j.gendis.2022.10.021
35. Zhang H, Zhai X, Liu Y, Xia Z, Xia T, Du G, et al. : NOP2-mediated m5C Modification of c-Myc in an EIF3A-Dependent Manner to Reprogram Glucose Metabolism and Promote Hepatocellular Carcinoma Progression. Res (Wash D C). (2023) 6:0184. doi: 10.34133/research.0184
36. Chi H, Zhao S, Yang J, Gao X, Peng G, Zhang J, et al. : T-cell exhaustion signatures characterize the immune landscape and predict HCC prognosis via integrating single-cell RNA-seq and bulk RNA-sequencing. Front Immunol. (2023) 14:1137025. doi: 10.3389/fimmu.2023.1137025
37. Chi H, Jiang P, Xu K, Zhao Y, Song B, Peng G, et al. A novel anoikis-related gene signature predicts prognosis in patients with head and neck squamous cell carcinoma and reveals immune infiltration. Front Genet. (2022) 13:984273. doi: 10.3389/fgene.2022.984273
38. Zhu H, Zhao Y, Wang Y, Wei G, Liu J. Understanding the relationship between cuproptosis and the development of hepatocellular carcinoma: implications for targeted therapies. Front Immunol. (2025) 16:1557223. doi: 10.3389/fimmu.2025.1557223
39. Jiang S, Yang X, Lin Y, Liu Y, Tran LJ, Zhang J, et al. Unveiling Anoikis-related genes: A breakthrough in the prognosis of bladder cancer. J Gene Med. (2024) 26:e3651. doi: 10.1002/jgm.v26.1
40. Yang H, Li Z, Zhu S, Wang W, Zhang J, Zhao D, et al. Molecular mechanisms of pancreatic cancer liver metastasis: the role of PAK2. Front Immunol. (2024) 15:1347683. doi: 10.3389/fimmu.2024.1347683
41. Granda-Diaz R, Menendez ST, Pedregal Mallo D, Hermida-Prado F, Rodriguez R, Suarez-Fernandez L, et al. : the novel role of SOX2 as an early predictor of cancer risk in patients with laryngeal precancerous lesions. Cancers (Basel). (2019) 11:286. doi: 10.3390/cancers11030286
42. Villaronga MA, Hermida-Prado F, Granda-Diaz R, Menendez ST, Alvarez-Teijeiro S, Quer M, et al. : immunohistochemical expression of cortactin and focal adhesion kinase predicts recurrence risk and laryngeal cancer risk beyond histologic grading. Cancer Epidemiol Biomarkers Prev. (2018) 27:805–13. doi: 10.1158/1055-9965.EPI-17-1082
43. Su C, Jia S, Liu H. Immunolocalization of CD163+ Tumor-associated macrophages and symmetric proliferation of ki-67 as biomarkers to differentiate new different grades of laryngeal dysplasia. Am J Clin Pathol. (2018) 149:8–16. doi: 10.1093/ajcp/aqx107
44. Klobucar M, Sedic M, Gehrig P, Grossmann J, Bilic M, Kovac-Bilic L, et al. Basement membrane protein ladinin-1 and the MIF-CD44-beta1 integrin signaling axis are implicated in laryngeal cancer metastasis. Biochim Biophys Acta. (2016) 1862:1938–54. doi: 10.1016/j.bbadis.2016.07.014
45. Topf MC, Tuluc M, Harshyne LA, Luginbuhl A. Macrophage type 2 differentiation in a patient with laryngeal squamous cell carcinoma and metastatic prostate adenocarcinoma to the cervical lymph nodes. J Immunother Cancer. (2017) 5:60. doi: 10.1186/s40425-017-0264-z
46. Sun W, Li WJ, Fu QL, Wu CY, Lin JZ, Zhu XL, et al. Functionally distinct subsets of CD4(+) regulatory T cells in patients with laryngeal squamous cell carcinoma are indicative of immune deregulation and disease progression. Oncol Rep. (2015) 33:354–62. doi: 10.3892/or.2014.3553
47. Wen YH, Lin HQ, Li H, Zhao Y, Lui VWY, Chen L, et al. Stromal interleukin-33 promotes regulatory T cell-mediated immunosuppression in head and neck squamous cell carcinoma and correlates with poor prognosis. Cancer Immunol Immunother. (2019) 68:221–32. doi: 10.1007/s00262-018-2265-2
48. Yu D, Cheng J, Xue K, Zhao X, Wen L, Xu C. Expression of programmed death-ligand 1 in laryngeal carcinoma and its effects on immune cell subgroup infiltration. Pathol Oncol Res. (2019) 25:1437–43. doi: 10.1007/s12253-018-0501-x
49. Karpathiou G, Casteillo F, Giroult JB, Forest F, Fournel P, Monaya A, et al. Prognostic impact of immune microenvironment in laryngeal and pharyngeal squamous cell carcinoma: Immune cell subtypes, immuno-suppressive pathways and clinicopathologic characteristics. Oncotarget. (2017) 8:19310–22. doi: 10.18632/oncotarget.14242
50. Karpathiou G, Vieville M, Gavid M, Camy F, Dumollard JM, Magne N, et al. Prognostic significance of tumor budding, tumor-stroma ratio, cell nests size, and stroma type in laryngeal and pharyngeal squamous cell carcinomas. Head Neck. (2019) 41:1918–27. doi: 10.1002/hed.25629
51. Wang JF, Wang JS, Liu Y, Ji B, Ding BC, Wang YX, et al. Knockdown of integrin beta1 inhibits proliferation and promotes apoptosis in bladder cancer cells. Biofactors. (2025) 51:e2150. doi: 10.1002/biof.2150
52. Zhai X, Zhang H, Xia Z, Liu M, Du G, Jiang Z, et al. : Oxytocin alleviates liver fibrosis via hepatic macrophages. JHEP Rep. (2024) 6:101032. doi: 10.1016/j.jhepr.2024.101032
53. Xiao J, Lin H, Liu B, Xia Z, Zhang J, Jin J. Decreased S1P and SPHK2 are involved in pancreatic acinar cell injury. Biomark Med. (2019) 13:627–37. doi: 10.2217/bmm-2018-0404
54. Xiao J, Huang K, Lin H, Xia Z, Zhang J, Li D, et al. Mogroside II(E) inhibits digestive enzymes via suppression of interleukin 9/interleukin 9 receptor signalling in acute pancreatitis. Front Pharmacol. (2020) 11:859. doi: 10.3389/fphar.2020.00859
55. Zhang H, Xia T, Xia Z, Zhou H, Li Z, Wang W, et al. KIF18A inactivates hepatic stellate cells and alleviates liver fibrosis through the TTC3/Akt/mTOR pathway. Cell Mol Life Sci. (2024) 81:96. doi: 10.1007/s00018-024-05114-5
56. Zhang H, Ni M, Wang H, Zhang J, Jin D, Busuttil RW, et al. Gsk3beta regulates the resolution of liver ischemia/reperfusion injury via MerTK. JCI Insight. (2023) 8:e151819. doi: 10.1172/jci.insight.151819
57. Vassilakopoulou M, Avgeris M, Velcheti V, Kotoula V, Rampias T, Chatzopoulos K, et al. : evaluation of PD-L1 expression and associated tumor-infiltrating lymphocytes in laryngeal squamous cell carcinoma. Clin Cancer Res. (2016) 22:704–13. doi: 10.1158/1078-0432.CCR-15-1543
58. Tao Y, Gross N, Liu Y, Zhang L, Li G, Huang Z, et al. A high ratio of IL-12Rbeta2-positive tumor-infiltrating lymphocytes indicates favorable prognosis in laryngeal cancer. Oncol. (2017) 74:148–56. doi: 10.1016/j.oraloncology.2017.10.006
59. Schluter A, Weller P, Kanaan O, Nel I, Heusgen L, Hoing B, et al. l: CD31 and VEGF are prognostic biomarkers in early-stage, but not in late-stage, laryngeal squamous cell carcinoma. BMC Cancer. (2018) 18:272. doi: 10.1186/s12885-018-4180-5
60. Wilkie MD, Lau AS, Vlatkovic N, Jones TM, Boyd MT. Metabolic signature of squamous cell carcinoma of the head and neck: Consequences of TP53 mutation and therapeutic perspectives. Oncol. (2018) 83:1–10. doi: 10.1016/j.oraloncology.2018.05.018
61. Zhang J, Peng G, Chi H, Yang J, Xie X, Song G, et al. CD8 + T-cell marker genes reveal different immune subtypes of oral lichen planus by integrating single-cell RNA-seq and bulk RNA-sequencing. BMC Health. (2023) 23:464. doi: 10.1186/s12903-023-03138-0
62. Zhang X, Zhang P, Cong A, Feng Y, Chi H, Xia Z, et al. Unraveling molecular networks in thymic epithelial tumors: deciphering the unique signatures. Front Immunol. (2023) 14:1264325. doi: 10.3389/fimmu.2023.1264325
63. Wang Y, Wang J, Liu J, Zhu H. Immune-related diagnostic markers for benign prostatic hyperplasia and their potential as drug targets. Front Immunol. (2024) 15:1516362. doi: 10.3389/fimmu.2024.1516362
64. Somashekar BS, Kamarajan P, Danciu T, Kapila YL, Chinnaiyan AM, Rajendiran TM, et al. Magic angle spinning NMR-based metabolic profiling of head and neck squamous cell carcinoma tissues. J Proteome Res. (2011) 10:5232–41. doi: 10.1021/pr200800w
65. Fei MJ, Xu YN, Wang JD. Preliminary findings for metabolite profiles of papillary thyroid carcinoma and laryngeal squamous cell carcinoma. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. (2017) 31:1561–5. doi: 10.13201/j.issn.1001-1781.2017.20.005
66. Chen J, Hou H, Chen H, Luo Y, Zhang L, Zhang Y, et al. Urinary metabolomics for discovering metabolic biomarkers of laryngeal cancer using UPLC-QTOF/MS. J Pharm BioMed Anal. (2019) 167:83–9. doi: 10.1016/j.jpba.2019.01.035
67. Liu F, Du J, Liu J, Wen B. Identification of key target genes and pathways in laryngeal carcinoma. Oncol Lett. (2016) 12:1279–86. doi: 10.3892/ol.2016.4750
68. Tripathi P, Kamarajan P, Somashekar BS, MacKinnon N, Chinnaiyan AM, Kapila YL, et al. Delineating metabolic signatures of head and neck squamous cell carcinoma: phospholipase A2, a potential therapeutic target. Int J Biochem Cell Biol. (2012) 44:1852–61. doi: 10.1016/j.biocel.2012.06.025
69. Wang YB, Xu O, Zhang RJ, Shan CG. A preliminary study on the relationship between GLUT-1, MCT-4 and CA in laryngeal squamous cell carcinoma. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. (2017) 31:510–514;519. doi: 10.13201/j.issn.1001-1781.2017.07.005
70. Wang L, Sun J, Gao P, Su K, Wu H, Li J, et al. Wnt1-inducible signaling protein 1 regulates laryngeal squamous cell carcinoma glycolysis and chemoresistance via the YAP1/TEAD1/GLUT1 pathway. J Cell Physiol. (2019) 234:15941–50. doi: 10.1002/jcp.v234.9
71. Lu ZJ, Yu Q, Zhou SH, Fan J, Shen LF, Bao YY, et al. Construction of a GLUT-1 and HIF-1alpha gene knockout cell model in HEp-2 cells using the CRISPR/Cas9 technique. Cancer Manag Res. (2019) 11:2087–96. doi: 10.2147/CMAR.S183859
72. Hu Y, Deng K, Pan M, Liu S, Li W, Huang J, et al. Down-regulation of PCK2 inhibits the invasion and metastasis of laryngeal carcinoma cells. Am J Transl Res. (2020) 12:3842–57.
73. Gao Y, Wang Z, Tong J, Zheng Y. LncRNA loc285194 inhibits tumor growth of laryngeal squamous cell carcinoma cells by downregulating hexokinase 2. Exp Ther Med. (2019) 18:2378–84. doi: 10.3892/etm.2019.7761
74. Sun Z, Zhang W, Li Q. miR-125a suppresses viability and glycolysis and induces apoptosis by targeting Hexokinase 2 in laryngeal squamous cell carcinoma. Cell Biosci. (2017) 7:51. doi: 10.1186/s13578-017-0178-y
75. Hui L, Zhang J, Guo X. MiR-125b-5p suppressed the glycolysis of laryngeal squamous cell carcinoma by down-regulating hexokinase-2. BioMed Pharmacother. (2018) 103:1194–201. doi: 10.1016/j.biopha.2018.04.098
76. Xu S, Guo J, Zhang W. lncRNA PCAT19 promotes the proliferation of laryngocarcinoma cells via modulation of the miR-182/PDK4 axis. J Cell Biochem. (2019) 120:12810–21. doi: 10.1002/jcb.v120.8
77. Liu L, Zhang P, Shao Y, Quan F, Li H. Knockdown of FOXJ1 inhibits the proliferation, migration, invasion, and glycolysis in laryngeal squamous cell carcinoma cells. J Cell Biochem. (2019) 120:15874–82. doi: 10.1002/jcb.v120.9
78. Li F, Simon MC. Cancer cells don’t live alone: metabolic communication within tumor microenvironments. Dev Cell. (2020) 54:183–95. doi: 10.1016/j.devcel.2020.06.018
79. Curry JM, Tuluc M, Whitaker-Menezes D, Ames JA, Anantharaman A, Butera A, et al. Cancer metabolism, stemness and tumor recurrence: MCT1 and MCT4 are functional biomarkers of metabolic symbiosis in head and neck cancer. Cell Cycle. (2013) 12:1371–84. doi: 10.4161/cc.24092
80. Villaronga M, Hermida-Prado F, Granda-Díaz R, Menéndez ST, Álvarez-Teijeiro S, Quer M, et al. immunohistochemical expression of cortactin and focal adhesion kinase predicts recurrence risk and laryngeal cancer risk beyond histologic grading. Cancer Epidemiol Biomarkers Prev. (2018) 27:805–13. doi: 10.1158/1055-9965.EPI-17-1082
81. Li K, Zhang C, Chen L, Wang P, Fang Y, Zhu J, et al. The role of acetyl-coA carboxylase2 in head and neck squamous cell carcinoma. PeerJ. (2019) 7:e7037. doi: 10.7717/peerj.7037
82. Zhao R, Tian L, Zhao B, Sun Y, Cao J, Chen K, et al. FADS1 promotes the progression of laryngeal squamous cell carcinoma through activating AKT/mTOR signaling. Cell Death Dis. (2020) 11:272. doi: 10.1038/s41419-020-2457-5
83. Louw L, Claassen J. Rationale for adjuvant fatty acid therapy to prevent radiotherapy failure and tumor recurrence during early laryngeal squamous cell carcinoma. Prostaglandins Leukotrienes Essential Fatty Acids. (2008) 78:21–6. doi: 10.1016/j.plefa.2007.10.007
84. Lin Q, Jin W, Shan D. Concerns regarding the AtTEnd trial in advanced endometrial carcinoma. Lancet Oncol. (2024) 25:e534. doi: 10.1016/S1470-2045(24)00486-8
85. Wang J, Li X, Xu O, Shan C. The role and clinical significance of 12-LOX passway in arachidonic acid metabolism induced by phospholipase Cgamma-2 in laryngeal squamous cell carcinoma. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. (2013) 27:1355–9.
86. Kourelis K, Vandoros G, Kourelis T, Papadas T, Goumas P, Sotiropoulou-Bonikou G. Low COX2 in tumor and upregulation in stroma mark laryngeal squamous cell carcinoma progression. Laryngoscope. (2009) 119:1723–9. doi: 10.1002/lary.v119:9
87. Kourelis K, Sotiropoulou-Bonikou G, Vandoros G, Repanti M, Varakis I, Goumas P. Coordinated upregulation of COX-2 and NF-kappaB is a steady feature of laryngeal carcinogenesis. ORL J Otorhinolaryngol Relat Spec. (2007) 69:181–9. doi: 10.1159/000099229
88. Mastronikolis NS, Tsiambas E, Papadas TA, Karameris A, Ragos V, Peschos D, et al. Deregulation of PTEN expression in laryngeal squamous cell carcinoma based on tissue microarray digital analysis. Anticancer Res. (2017) 37:5521–4. doi: 10.21873/anticanres.11983
89. Sullu Y, Gun S, Atmaca S, Karagoz F, Kandemir B. Poor prognostic clinicopathologic features correlate with VEGF expression but not with PTEN expression in squamous cell carcinoma of the larynx. Diagn Pathol. (2010) 5:35. doi: 10.1186/1746-1596-5-35
90. Zhu XL, Sun W, Lei WB, Zhuang HW, Hou WJ, Wen WP. DJ-1-induced phosphatase and tensin homologue downregulation is associated with proliferative and invasive activity of laryngeal cancer cells. Mol Med Rep. (2015) 12:2003–8. doi: 10.3892/mmr.2015.3617
91. Lee JS, Adler L, Karathia H, Carmel N, Rabinovich S, Auslander N, et al. : urea cycle dysregulation generates clinically relevant genomic and biochemical signatures. Cell. (2018) 174:1559–1570.e1522. doi: 10.1016/j.cell.2018.07.019
92. Cano A, Santamaría PG, Moreno-Bueno G. LOXL2 in epithelial cell plasticity and tumor progression. Future Oncol. (2012) 8:1095–108. doi: 10.2217/fon.12.105
93. Peinado H, Moreno-Bueno G, Hardisson D, Peírez-Goímez E, Santos V, Mendiola M, et al. : lysyl oxidase–like 2 as a new poor prognosis marker of squamous cell carcinomas. Cancer Res. (2008) 68:4541–50. doi: 10.1158/0008-5472.CAN-07-6345
94. Engin AB, Gunaydin RO, Kesikli SA, Fuchs D, Hosal AS. Serum neopterin concentrations and tryptophan degradation pattern in patients with late stage larynx carcinoma. Pteridines. (2017) 28:91–5. doi: 10.1515/pterid-2017-0004
95. Wilkat M, Bast H, Drees R, Dünser J, Mahr A, Azoitei N, et al. : Adenosine receptor 2B activity promotes autonomous growth, migration as well as vascularization of head and neck squamous cell carcinoma cells. Int J Cancer. (2020) 147:202–17. doi: 10.1002/ijc.v147.1
96. Mandapathil M, Szczepanski MJ, Szajnik M, Ren J, Lenzner DE, Jackson EK, et al. Increased ectonucleotidase expression and activity in regulatory T cells of patients with head and neck cancer. Clin Cancer Res. (2009) 15:6348–57. doi: 10.1158/1078-0432.CCR-09-1143
97. Mesía R, Vázquez S, Grau JJ, García-Sáenz JA, Lozano A, García C, et al. : A phase 2 open label, single-arm trial to evaluate the combination of cetuximab plus taxotere, cisplatin, and 5-flurouracil as an induction regimen in patients with unresectabl e squamous cell carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. (2016) 94:289–96. doi: 10.1016/j.ijrobp.2015.10.019
98. More LA, Lane S, Asnani A. 5-FU cardiotoxicity: vasospasm, myocarditis, and sudden death. Curr Cardiol Rep. (2021) 23:17. doi: 10.1007/s11886-021-01441-2
99. Amaya C, Smith ER, Xu XX. Low intensity ultrasound as an antidote to taxane/paclitaxel-induced cytotoxicity. J Cancer. (2022) 13:2362–73. doi: 10.7150/jca.71263
100. Urba S, Wolf G, Eisbruch A, Worden F, Lee J, Bradford C, et al. Single-cycle induction chemotherapy selects patients with advanced laryngeal cancer for combined chemoradiation: a new treatment paradigm. J Clin Oncol. (2006) 24:593–8. doi: 10.1200/JCO.2005.01.2047
101. Ji Z, Wang X, Xin J, Ma L, Zuo D, Li H, et al. : Multiomics reveals tumor microenvironment remodeling in locally advanced gastric and gastroesophageal junction cancer following neoadjuvant immunotherapy and chemotherapy. J Immunother Cancer. (2024) 12:e010041. doi: 10.1136/jitc-2024-010041
102. Forastiere AA, Zhang Q, Weber RS, Maor MH, Goepfert H, Pajak TF, et al. : Long-term results of RTOG 91-11: a comparison of three nonsurgical treatment strategies to preserve the larynx in patients with locally advanced larynx cancer. J Clin Oncol. (2013) 31:845–52. doi: 10.1200/JCO.2012.43.6097
103. Forastiere AA, Goepfert H, Maor M, Pajak TF, Weber R, Morrison W, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. (2003) 349:2091–8. doi: 10.1056/NEJMoa031317
104. Janoray G, Pointreau Y, Garaud P, Chapet S, Alfonsi M, Sire C, et al. Long-term results of a multicenter randomized phase III trial of induction chemotherapy with cisplatin, 5-fluorouracil, +/- docetaxel for larynx preservation. J Natl Cancer Inst. (2016) 108:djv368. doi: 10.1093/jnci/djv368
105. Takácsi-Nagy Z, Hitre E, Remenár É, Oberna F, Polgár C, Major T, et al. Docetaxel, cisplatin and 5-fluorouracil induction chemotherapy followed by chemoradiotherapy or chemoradiotherapy alone in stage III-IV unresectabl e head and neck cancer: Results of a randomized phase II study. Strahlenther Onkol. (2015) 191:635–41. doi: 10.1007/s00066-015-0829-z
106. Lorch JH, Goloubeva O, Haddad RI, Cullen K, Sarlis N, Tishler R, et al. Induction chemotherapy with cisplatin and fluorouracil alone or in combination with docetaxel in locally advanced squamous-cell cancer of the head and neck: long-term results of the TAX 324 randomised phase 3 trial. Lancet Oncol. (2011) 12:153–9. doi: 10.1016/S1470-2045(10)70279-5
107. Olsen KD. Reexamining the treatment of advanced laryngeal cancer. Head Neck. (2010) 32:1–7. doi: 10.1002/hed.21294
108. Nocon CC, Yesensky J, Ajmani GS, Bhayani MK. Failed larynx preservation and survival in patients with advanced larynx cancer. Am J Otolaryngol. (2019) 40:542–6. doi: 10.1016/j.amjoto.2019.04.014
109. Bates JE, Amdur RJ, Morris CM, Hitchcock KE, Dziegielewski PT, Boyce BJ, et al. Curative-dose chemoradiotherapy versus total laryngectomy for stage T3-T4 squamous cell carcinoma of the larynx: an “Apples-to-apples” Analysis of the national cancer database. Am J Clin Oncol. (2019) 42:527–33. doi: 10.1097/COC.0000000000000550
110. Dyckhoff G, Plinkert PK, Ramroth H. A change in the study evaluation paradigm reveals that larynx preservation compromises survival in T4 laryngeal cancer patients. BMC Cancer. (2017) 17:609. doi: 10.1186/s12885-017-3608-7
111. Taberna M, Oliva M, Mesia R. Cetuximab-containing combinations in locally advanced and recurrent or metastatic head and neck squamous cell carcinoma. Front Oncol. (2019) 9:383. doi: 10.3389/fonc.2019.00383
112. Liang K, Ang KK, Milas L, Hunter N, Fan Z. The epidermal growth factor receptor mediates radioresistance. Int J Radiat Oncol Biol Phys. (2003) 57:246–54. doi: 10.1016/S0360-3016(03)00511-X
113. Kalyankrishna S, Grandis JR. Epidermal growth factor receptor biology in head and neck cancer. J Clin Oncol. (2006) 24:2666–72. doi: 10.1200/JCO.2005.04.8306
114. Harari PM, Huang SM. Head and neck cancer as a clinical model for molecular targeting of therapy: combining EGFR blockade with radiation. Int J Radiat Oncol Biol Phys. (2001) 49:427–33. doi: 10.1016/S0360-3016(00)01488-7
115. Egloff AM, Lee JW, Langer CJ, Quon H, Vaezi A, Grandis JR, et al. : Phase II study of cetuximab in combination with cisplatin and radiation in unresectabl e, locally advanced head and neck squamous cell carcinoma: Eastern cooperative oncology group trial E3303. Clin Cancer Res. (2014) 20:5041–51. doi: 10.1158/1078-0432.CCR-14-0051
116. Bonner J, Giralt J, Harari P, Spencer S, Schulten J, Hossain A, et al. Cetuximab and radiotherapy in laryngeal preservation for cancers of the larynx and hypopharynx: A secondary analysis of a randomized clinical trial. JAMA Otolaryngol Head Neck Surg. (2016) 142:842–9. doi: 10.1001/jamaoto.2016.1228
117. Patil VM, Noronha V, Joshi A, Agarwal J, Ghosh-Laskar S, Budrukkar A, et al. A randomized phase 3 trial comparing nimotuzumab plus cisplatin chemoradiotherapy versus cisplatin chemoradiotherapy alone in locally advanced head and neck cancer. Cancer. (2019) 125:3184–97. doi: 10.1002/cncr.v125.18
118. Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. (2006) 354:567–78. doi: 10.1056/NEJMoa053422
119. Gillison ML, Trotti AM, Harris J, Eisbruch A, Harari PM, Adelstein DJ, et al. Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG Oncology RTOG 1016): a randomised, multicentre, non-inferiority trial. Lancet. (2019) 393:40–50. doi: 10.1016/S0140-6736(18)32779-X
120. Chi H, Xie X, Yan Y, Peng G, Strohmer DF, Lai G, et al. Natural killer cell-related prognosis signature characterizes immune landscape and predicts prognosis of HNSCC. Front Immunol. (2022) 13:1018685. doi: 10.3389/fimmu.2022.1018685
121. Chi H, Yang J, Peng G, Zhang J, Song G, Xie X, et al. Circadian rhythm-related genes index: A predictor for HNSCC prognosis, immunotherapy efficacy, and chemosensitivity. Front Immunol. (2023) 14:1091218. doi: 10.3389/fimmu.2023.1091218
122. Ren Q, Zhang P, Lin H, Feng Y, Chi H, Zhang X, et al. A novel signature predicts prognosis and immunotherapy in lung adenocarcinoma based on cancer-associated fibroblasts. Front Immunol. (2023) 14:1201573. doi: 10.3389/fimmu.2023.1201573
123. Xue M, Jin W. Editorial: Immunological precision therapeutics: integrating multi-omics technologies and comprehensive approaches for personalized immune intervention. Front Immunol. (2025) 16:1581238. doi: 10.3389/fimmu.2025.1581238
124. Cohen EEW, Bell RB, Bifulco CB, Burtness B, Gillison ML, Harrington KJ, et al. The Society for Immunotherapy of Cancer consensus statement on immunotherapy for the treatment of squamous cell carcinoma of the head and neck (HNSCC). J Immunother Cancer. (2019) 7:184. doi: 10.1186/s40425-019-0662-5
125. Patsoukis N, Brown J, Petkova V, Liu F, Li L, Boussiotis VA. Selective effects of PD-1 on Akt and Ras pathways regulate molecular components of the cell cycle and inhibit T cell proliferation. Sci Signal. (2012) 5:ra46. doi: 10.1126/scisignal.2002796
126. Jin W, Yang Q, Chi H, Wei K, Zhang P, Zhao G, et al. Ensemble deep learning enhanced with self-attention for predicting immunotherapeutic responses to cancers. Front Immunol. (2022) 13:1025330. doi: 10.3389/fimmu.2022.1025330
127. Okada T, Fushimi C, Matsuki T, Okamoto I, Sato H, Kondo T, et al. Comparison of dosage of nivolumab in efficacy and safety for recurrent metastatic squamous cell carcinoma. Anticancer Res. (2022) 42:1607–13. doi: 10.21873/anticanres.15635
128. Burtness B, Harrington KJ, Greil R, Soulières D, Tahara M, de Castro G Jr., et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. (2019) 394:1915–28. doi: 10.1016/S0140-6736(19)32591-7
129. Shi Y, Guo W, Wang W, Wu Y, Fang M, Huang X, et al. : Finotonlimab with chemotherapy in recurrent or metastatic head and neck cancer: a randomized phase 3 trial. Nat Med. (2024) 30:2568–75. doi: 10.1038/s41591-024-03110-7
130. Qin T, Mattox AK, Campbell JS, Park JC, Shin KY, Li S, et al. Epigenetic therapy sensitizes anti-PD-1 refractory head and neck cancers to immunotherapy rechallenge. J Clin Invest. (2025) 135:e181671. doi: 10.1172/JCI181671.71
131. Bayrak AF, Eliyatkin NO, Islek A, Ozkul Y, Kilic HS, Aktas S. Association of immune response with overall and disease-free survival in laryngeal squamous cell carcinomas. Am J Otolaryngol. (2022) 43:103477. doi: 10.1016/j.amjoto.2022.103477
132. Li W, You J, Xue H, Liu Y, Chen J, Zheng X, et al. Unlocking the potential of HHLA2: identifying functional immune infiltrating cells in the tumor microenvironment and predicting clinical outcomes in laryngeal squamous cell carcinoma. Cancer Immunol Immunother. (2024) 73:207. doi: 10.1007/s00262-024-03791-6
Keywords: laryngeal squamous cell carcinoma, metabolic reprogramming, tumor microenvironment, immune checkpoint inhibitors, glycolysis, precision medicine, immunotherapy
Citation: Ma K, Mao Q, Fei B, Ni T, Zhang Z and Ni H (2025) Metabolic reprogramming and immune microenvironment characteristics in laryngeal carcinoma: advances in immunotherapy. Front. Immunol. 16:1589243. doi: 10.3389/fimmu.2025.1589243
Received: 07 March 2025; Accepted: 08 April 2025;
Published: 30 April 2025.
Edited by:
Lilong Zhang, Renmin Hospital of Wuhan University, ChinaReviewed by:
Binggang Liu, The Central Hospital of Yongzhou, ChinaCopyright © 2025 Ma, Mao, Fei, Ni, Zhang and Ni. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haosheng Ni, ZW50bmhzQG50dS5lZHUuY24=; Zhenxin Zhang, enp4emgzNjlAc2luYS5jb20=
†These authors have contributed equally to this work
 Kexin Ma1†
Kexin Ma1† Tingting Ni
Tingting Ni Haosheng Ni
Haosheng Ni