- 1Department of Laboratory Medicine, The Affiliated Hospital, Southwest Medical University, Luzhou, China
- 2The Laboratory Department of the Second People’s Hospital of Neijiang, Neijiang, China
- 3Neijiang Vocational College of Health and Wellness, Neijiang, China
- 4Department of Medical Laboratory, Dongxing District People’s Hospital of Neijiang, Neijiang, China
Lung cancer remains the leading cause of cancer-related mortality worldwide, with its progression shaped not only by tumor-intrinsic factors but also by a complex and immunosuppressive tumor microenvironment (TME). Within this niche, diverse immune populations—including CD8+ cytotoxic T cells, CD4+ helper T cell subsets (Th1, Th17, Tregs), B cells, natural killer (NK) cells, tumor-associated macrophages (TAMs), and myeloid-derived suppressor cells (MDSCs)—collectively regulate immune surveillance and tumor escape. While effector lymphocytes mediate antitumor responses, their function is often attenuated by TAM- and MDSC-driven immunosuppression via cytokines (IL-10, TGF-β), metabolic disruption, and immune checkpoint expression. High densities of M2-polarized TAMs and MDSCs correlate with poor prognosis and resistance to therapy. Immune checkpoint inhibitors targeting PD-1/PD-L1 and CTLA-4 have improved outcomes in lung cancer, yet therapeutic efficacy remains limited by the immunosuppressive TME. This review outlines the functional roles of key immune cell subsets in lung cancer and highlights emerging strategies to reprogram the TME and enhance immunotherapeutic responsiveness.
1 Introduction
Lung cancer remains the foremost contributor to global cancer-related mortality (1). Beyond intrinsic tumor cell behavior, its pathogenesis and progression are orchestrated by the surrounding tumor microenvironment (TME), a dynamic and multifaceted ecosystem that governs neoplastic proliferation, immune evasion, and metastatic competence (2, 3). The pulmonary TME comprises not only transformed epithelial cells but also a diverse milieu of stromal and immune components, including fibroblasts, vascular and lymphatic elements, extracellular matrix molecules, and a repertoire of cytokines and chemokines (4). Among these, immune constituents such as tumor-associated macrophages (TAMs) (5), dendritic cell populations (6), myeloid-derived suppressor cells (MDSCs) (7), and tumor-infiltrating lymphocytes (TILs) (8) play central roles. Their functional plasticity enables either antitumor cytotoxicity or tumor-promoting activities, including suppression of effector immunity, support for tumor growth, and facilitation of metastatic spread (9).
The therapeutic modulation of immune cells within the TME has redefined treatment paradigms for advanced pulmonary malignancies. Immune checkpoint inhibitors, particularly those targeting PD-1/PD-L1 and CTLA-4, have yielded durable responses and improved survival outcomes in subsets of patients (10). Meanwhile, emerging strategies, such as cytokine-based therapies and tumor vaccines, are under active investigation, offering new prospects for immune reprogramming (11). This review synthesizes current insights into biological functions of principal immune cell populations within lung cancer, highlighting their potential for translation into immunotherapeutic applications.
2 Tumor-infiltrating lymphocytes in the lung cancer
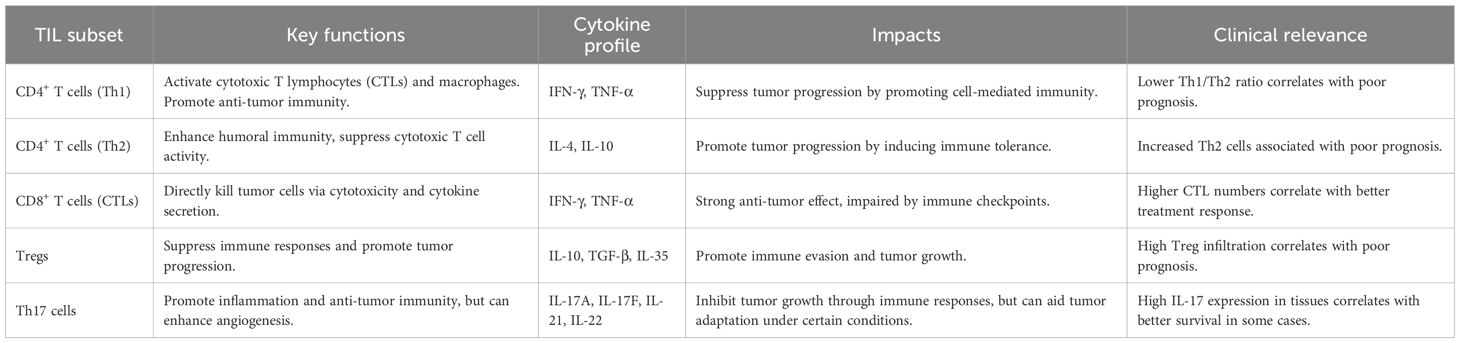
Tumor-infiltrating lymphocytes (TILs) represent a heterogeneous population of adaptive immune cells predominantly localized within the stromal regions of lung tumors, mobilized via antigen-specific responses to tumor-derived neoantigens. These cells are central to antitumor immunity, contributing to both immune surveillance and cytolytic elimination of malignant epithelial cells. Key subsets include cytotoxic CD8+ T cells, CD4+ helper T cells, regulatory T cells (Tregs), and tumor-infiltrating B cells (TIL-Bs), each exerting distinct immunoregulatory functions within the dynamic tumor microenvironment (12, 13) (Table 1).
2.1 Roles of CD4+ T cells in lung cancer progression
2.1.1 Th1 cells in the lung cancer
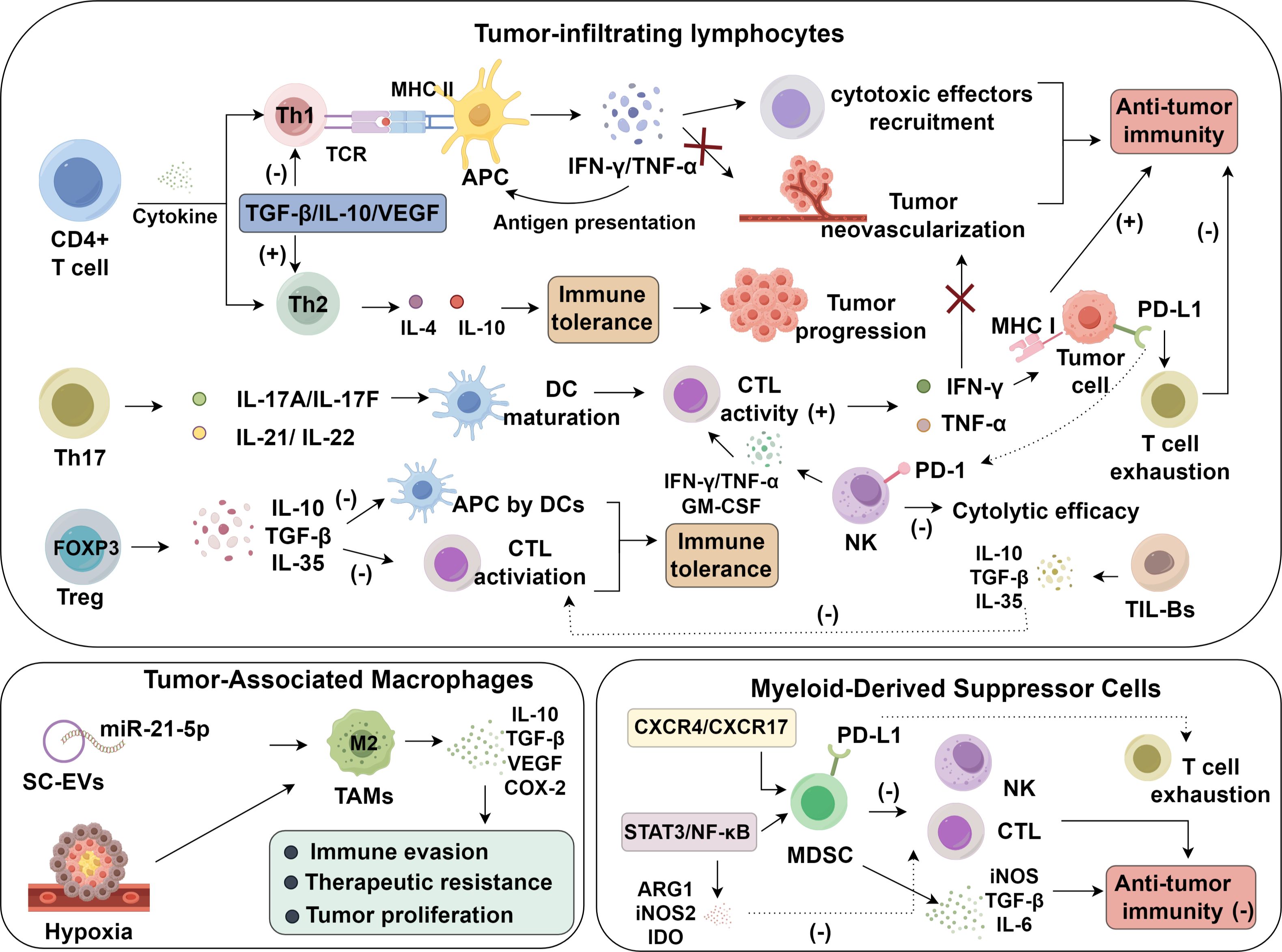
Th1 cells, a subset of CD4+ T lymphocytes, are activated upon MHC class II–restricted antigen recognition by professional antigen-presenting cells, initiating transcriptional programs that culminate in pro-inflammatory cytokine production (14). Within the TME, Th1 cells diverge into classical and Th1-like subsets with distinct effector profiles, and their balance is essential for sustaining antitumor immunity (15). The canonical Th1–Th2 paradigm delineates opposing immune trajectories. Th1 cells orchestrating cytotoxic responses and Th2 cells mediating humoral immunity, yet this dichotomy is frequently disrupted in lung cancer due to tumor-induced immune reprogramming (12). Crosstalk between neoplastic epithelial cells and helper T cell subsets often skews this balance toward a Th2-dominant state, thereby fostering a permissive TME (12). Upon activation, Th1 cells secrete IFN-γ and TNF-α, which augment antigen presentation, recruit cytotoxic cells, suppress angiogenesis, and trigger tumor cell apoptosis. Conversely, Th2 cytokines such as IL-4 and IL-10 dampen cell-mediated responses and promote tolerance (16). In lung cancer, Th2 cells are enriched within the TME compared to adjacent lung tissue, correlating with tumor grade and invasiveness. In contrast, decreased Th1 infiltration and a reduced Th1/Th2 ratio associate with immune dysfunction and disease progression (17). Notably, the immunosuppressive cytokine IL-10 not only favors Th2 polarization but also activates STAT3 signaling in dendritic cells, leading to downregulation of MHC class II expression and impaired costimulatory signaling. This diminishes antigen presentation capacity and directly attenuates Th1 priming and expansion. As a result, the IL-10/STAT3 axis plays a pivotal role in disabling effective Th1-driven antitumor responses and contributes to an immunoevasive tumor phenotype. Additionally, Tumor-derived factors such as TGF-β, IL-10, and VEGF further reinforce this immunosuppressive axis, subverting effector T cell function and establishing an immune-privileged niche conducive to lung cancer recurrence and metastasis (18). These dynamic shifts in CD4+ T cell plasticity represent critical determinants of lung cancer immunobiology and suggest promising avenues for therapeutic intervention.
2.1.2 Th17 cells and regulatory T cells in lung carcinogenesis
Within the immune ecosystem of lung cancer, T helper 17 (Th17) cells and regulatory T cells (Tregs) exert immunologically antagonistic functions. Th17 cells orchestrate pro-inflammatory responses through the secretion of IL-17A, IL-17F, IL-21, and IL-22, which bolster local immunity and potentiate antitumor surveillance (19). Mechanistic studies indicate that IL-17 enhances dendritic cell (DC) maturation and activates CD8+ cytotoxic T lymphocytes (CTLs), facilitating tumor antigen clearance (20–22). Clinically, high intratumoral IL-17 expression correlates with improved progression-free and overall survival in lung cancer patients, highlighting its prognostic value (23). However, IL-17 also exhibits tumor-promoting properties in certain contexts by inducing VEGF expression and promoting neovascularization, thus fostering tumor growth and metastatic spread (24, 25). This pro-angiogenic function of IL-17 partially explains its tumor-promoting effects observed in specific microenvironmental settings. Thus, the dichotomous nature of Th17 activity reflects its context-specific function in the dynamic TME.
In contrast, FOXP3+ Tregs critically suppress antitumor immunity by dampening CTL function and impairing antigen presentation through the secretion of IL-10, TGF-β, and IL-35 (26–29). These suppressive factors attenuate antigen presentation by DCs, downregulate costimulatory signaling, and blunt effector T cell priming, leading to an immunologically inert environment (30). In addition to cytokine secretion, FOXP3+ Tregs suppress antitumor responses through CTLA-4–dependent mechanisms, whereby CTLA-4 engagement downregulates CD80/CD86 expression on dendritic cells, thereby diminishing their capacity to deliver essential costimulatory signals to naïve T cells. Clinically, increased intratumoral Treg infiltration is inversely associated with patient survival outcomes (31). Thus, the opposing roles of Th17 and Treg cells highlight a delicate immunological balance in lung carcinogenesis. Their functional dichotomy provides a compelling rationale for therapeutic interventions aimed at restoring immune equilibrium, either by amplifying Th17-mediated responses or selectively targeting Treg-mediated suppression (32) (Figure 1).
2.2 Roles of CD8+ cytotoxic T lymphocytes in lung cancer
Cytotoxic CD8+ T lymphocytes (CTLs) are pivotal mediators of adaptive immunity, executing antigen-specific killing of tumor cells via recognition of tumor-associated antigens (TAAs) presented by MHC class I molecules. Upon activation, CTLs release perforin and granzymes to induce apoptosis in target cells (33, 34), and secrete pro-inflammatory cytokines such as IFN-γ and TNF-α, which collectively reinforce antitumor immunity and suppress neovascularization (35–37). IFN-γ further upregulates MHC-I expression on tumor cells, enhancing immune visibility. Notably, clinical studies have established a positive correlation between the density of tumor-infiltrating CTLs and favorable outcomes in patients with non-small cell lung cancer (38, 39), underscoring their prognostic and therapeutic significance. Nonetheless, the cytotoxic potential of CTLs is frequently compromised by immune evasion strategies within the TME. Inhibitory checkpoint molecules such as PD-1 and CTLA-4, upregulated upon T cell activation, are engaged by ligands like PD-L1 expressed on tumor and stromal cells, inducing T cell exhaustion and diminishing effector function (40, 41). Additionally, suppressive elements in the TME, including regulatory T cells, TGF-β, and IL-10, attenuate CTL proliferation and survival. To circumvent these immunosuppressive pathways, current immunotherapies, particularly immune checkpoint inhibitors targeting PD-1/PD-L1 and CTLA-4, aim to restore CTL activity in lung cancer (42, 43). Moreover, combination strategies involving checkpoint blockade, cancer vaccines, cytokine supplementation, or costimulatory agonists are under investigation to potentiate CTL responses in lung cancer treatment (44).
2.3 Natural killer cells: mechanisms of action and dysregulation in lung cancer
Natural killer (NK) cells are pivotal effectors of the innate immune system, acting as frontline defenders against oncogenic transformation through antigen-independent cytotoxicity. Residing predominantly in peripheral blood and secondary lymphoid tissues such as the spleen, NK cells execute immunosurveillance by detecting stress-induced ligands, such as MICA/B and ULBPs, via activating receptors including NKG2D, NKp30, and NKp46 (45). Upon engagement, NK cells unleash cytotoxic granules loaded with perforin and granzymes, perforating tumor cell membranes and initiating caspase-dependent apoptosis (46, 47). Beyond granule-mediated killing, NK cells execute apoptosis via death receptor pathways. By expressing ligands such as FasL and TRAIL, NK cells activate extrinsic apoptosis through Fas and TRAIL receptors on target cells, thereby contributing to immune clearance of transformed epithelium (48). In addition to direct cytolysis, NK cells secrete IFN-γ, TNF-α, and granulocyte-macrophage colony-stimulating factor (GM-CSF), which synergistically enhance dendritic cell maturation and potentiating CD8+ T cell–mediated cytotoxicity (49, 50). Moreover, NK cells facilitate antibody-dependent cellular cytotoxicity (ADCC) through CD16 (FcγRIII)-mediated recognition of antibody-opsonized tumor cells, further amplifying antitumor immunity (51).
Despite their inherent cytotoxic capabilities, NK cells are functionally compromised within the lung cancer microenvironment due to a constellation of immunosuppressive cues. Tumor-secreted factors, including TGF-β, IL-10, and prostaglandin E2 (PGE2), interfere with NK cell activation, suppress the synthesis and release of key effector molecules such as IFN-γ and perforin, and downregulate expression of activating receptors like NKG2D, collectively diminishing antitumor functionality (52, 53). Additionally, lung tumors elevate the expression of immunoinhibitory ligands, such as PD-L1 and HLA-G, which engage suppressive receptors including PD-1, NKG2A, and various KIR family members on NK cells, thereby attenuating their cytolytic efficacy (54–56). Hypoxic conditions within the tumor core further impair NK cell function by upregulating hypoxia-inducible factor 1α (HIF-1α), which alters NK cell metabolism and reduces granule-mediated killing efficiency (57–59). Moreover, tumors exploit ectonucleotidases CD39 and CD73 to generate extracellular adenosine, which accumulates in the hypoxic TME and binds to A2A adenosine receptors on NK cells. This adenosinergic signaling pathway profoundly suppresses NK cell cytotoxicity by downregulating perforin and granzyme production, impairing cytokine secretion, and inhibiting target cell lysis. These suppressive signals transform the TME into a hostile landscape for NK cell activity that facilitates immune escape and tumor progression. To restore NK cell function, therapeutic strategies targeting immunosuppressive axes, such as blockade of TGF-β and IL-10 or stimulation with IL-15/IL-21, have shown promise in preclinical models (60). Immune checkpoint inhibitors targeting NK-specific pathways, including anti-NKG2A antibody Monalizumab, have demonstrated robust potential to reinvigorate NK responses (61). Meanwhile, adoptive cellular therapy utilizing chimeric antigen receptor-engineered NK cells (CAR-NK) has emerged as a transformative modality. CAR-NK cells engineered to express tumor-targeting constructs—such as HER2-directed chimeric receptors—demonstrate enhanced tumor recognition and cytotoxicity against lung cancer cells, representing a novel frontier in innate immune-based immunotherapy (62).
2.4 Functional dichotomy of tumor-infiltrating B cells in lung cancer
Tumor-infiltrating B cells (TIL-Bs) constitute a heterogeneous immune population within the lung cancer microenvironment, exhibiting both immunostimulatory and immunosuppressive activities. On the one hand, TIL-Bs can enhance antitumor immunity by producing tumor-specific immunoglobulins that mediate antibody-dependent cellular cytotoxicity (ADCC), presenting tumor-derived antigens to CD4+ T helper cells, and facilitating the activation of CD8+ cytotoxic T lymphocytes (63, 64). These functions potentiate adaptive immune responses and are associated with favorable clinical outcomes, including prolonged disease-free survival and reduced recurrence in lung cancer patients (12, 65, 66). However, not all B cell subsets are protective. A specialized subpopulation termed regulatory B cells (Bregs) has been identified as a driver of immunosuppression and tumor progression. Bregs exert their immunoregulatory effects primarily through the secretion of anti-inflammatory cytokines, including IL-10, TGF-β, and IL-35, thereby impairing T cell–mediated cytotoxicity and promoting immune evasion (67). Notably, IL-35 has been shown to not only suppress CD8+ T cell effector functions but also actively promote the expansion and stability of Tregs, establishing a feedforward loop that reinforces the immunosuppressive tumor microenvironment. In addition to their immunoregulatory secretome, Bregs may directly enhance tumorigenesis through contact-dependent mechanisms that support tumor cell survival and proliferation (68). This IL-35–Breg–Treg axis is a critical driver of immune evasion in lung cancer. The immunological balance between effector TIL-Bs and immunosuppressive Bregs is therefore critical in shaping the trajectory of tumor–immune dynamics in lung cancer. Immunomodulatory therapies that selectively augment TIL-B activity or neutralize Breg-derived cytokines represent promising avenues for future development in lung cancer immunotherapy (69).
3 Tumor-associated macrophages in lung cancer invasion and immunosuppression
Tumor-associated macrophages (TAMs) represent a predominant immune subset within the lung tumor microenvironment and act as pivotal orchestrators linking chronic inflammation to oncogenesis. These cells display remarkable phenotypic plasticity, transitioning between classically activated M1-like states—associated with tumor suppression—and alternatively activated M2-like phenotypes that facilitate tumor progression. In lung cancer, TAMs are predominantly polarized toward the M2 phenotype, which promotes immune evasion, invasion, and therapeutic resistance through secretion of key immunoregulatory factors including IL-10, TGF-β, VEGF, and COX–2 (70). The polarization toward an M2 phenotype is chiefly driven by IL-4 and IL-13 which engage the IL-4Rα chain and activate STAT6. Upon activation, STAT6 translocates to the nucleus to induce expression of M2-associated genes including Arg1, Mrc1 (CD206), and Ym1, thereby establishing a transcriptional program that promotes tissue remodeling, immune suppression, and tumor tolerance. This IL-4/IL-13/STAT6 axis is a central pathway in the immunosuppressive reprogramming of macrophages within the lung tumor microenvironment.
Under hypoxic stress, macrophages increase the expression of HIF-1α, IL-10, and VEGF, thereby creating an immune-refractory, pro-angiogenic microenvironment that enhances tumor adaptability and resistance (71, 72). Notably, mesenchymal stem cell–derived extracellular vesicles (MSC-EVs) have been implicated in promoting M2 polarization through delivery of miR-21-5p. This miRNA-mediated signaling cascade enhances angiogenesis and accelerates tumor proliferation, contributing to the aggressive phenotype of lung carcinoma (73). Clinically, M2 TAM abundance correlates with unfavorable prognosis in lung cancer, whereas reduced M2 infiltration is linked to improved survival and enhanced therapeutic responsiveness (74). These findings support the development of macrophage-targeted interventions, including agents that reprogram M2 TAMs into M1-like phenotypes or disrupt key immunosuppressive axes such as IL-10 or VEGF signaling.
4 Myeloid-derived suppressor cells and immune tolerance in lung cancer
MDSCs, a phenotypically and functionally diverse group of immature myeloid progenitors, expand dramatically in response to chronic inflammation and tumor-derived cues within the lung cancer microenvironment. These cells are key enforcers of immunosuppression, dismantling anti-tumor immune surveillance and promoting malignant progression. MDSCs compromise cytotoxic T cell function through several coordinated mechanisms: they inhibit T cell proliferation, induce apoptosis via arginase-1 (ARG1), inducible nitric oxide synthase (iNOS), and ROS, and suppress the activation of effector T lymphocytes through multiple checkpoints (75, 76). In addition to dampening T cell responses, MDSCs foster the expansion and stabilization of regulatory T cells (Tregs) by producing immunosuppressive cytokines, particularly IL-10 and TGF-β. They also deprive T cells of L-arginine by overexpressing ARG1, which leads to downregulation of CD3ζ chain and cyclin D3, both essential for T cell receptor signaling and cell cycle progression. Furthermore, the release of nitric oxide (NO) interferes with JAK3/STAT5 signaling, while peroxynitrite (ONOO-), a reactive nitrogen species, modifies amino acids on TCRs, blunting T cell-mediated recognition of tumor antigens (77).
Beyond T cells, MDSCs suppress the cytolytic capacity of natural killer (NK) cells and impair B lymphocyte functions, including antibody production and clonal expansion. These suppressive effects are largely mediated by iNOS activity and prostaglandin E2 (PGE2), both of which modulate cellular activation thresholds and cytokine output (78). Clinically, elevated MDSC frequencies in tumor tissue or peripheral blood have been associated with diminished responses to chemotherapy and shorter overall survival in patients with lung malignancies (79, 80). Their recruitment is governed by chemotactic axes such as CCL2 and CXCR4, while sustained STAT3 and NF-κB activation reinforces their immunosuppressive phenotype (81, 82), upregulating enzymes including ARG1, iNOS, and indoleamine 2,3-dioxygenase (IDO), alongside anti-inflammatory cytokines that collectively suppress CTL function (83). Given their pivotal role in immune evasion, MDSCs represent compelling targets for immunomodulatory therapy. Strategies aimed at simultaneously inhibiting MDSC function and restoring T cell activity have shown promising preclinical results. Notably, dual inhibition of the C5aR1 and the PD-1/PD-L1 axis has been shown to enhance anti-tumor immune responses, leading to reduced tumor burden and decreased metastatic dissemination (84, 85). Likewise, co-administration of MEK inhibitors with immune checkpoint inhibitors augments infiltration of CD8+ and CD4+ T cells, enhances antigen-specific responses, and prolongs survival in murine models of lung cancer (85).
An abundance of MDSCs in the lung cancer setting inversely correlates with therapeutic efficacy, particularly in the context of chemotherapy and immunotherapy (86). Their suppressive activity directly interferes with the ability of T and NK cells to mount effective anti-tumor responses, thereby compromising clinical outcomes (87). This immunosuppressive program is mediated through upregulation of iNOS, TGF-β, and IL-6, alongside Treg induction and expansion (88–91). The trafficking of MDSCs into tumor tissues is driven by gradients of chemokines and corresponding receptors—including CXCR4 and CXCR17—which direct their accumulation in the tumor bed (81, 82, 92). Persistent activation of STAT3 and NF-κB transcription factors reinforces the MDSC phenotype, promoting the expression of immunosuppressive mediators such as ARG1, iNOS2, IDO, and cytokines that suppress T cell effector function (93). Notably, PD-L1 expression on MDSCs plays a central role in mediating T cell exhaustion and immune escape in lung cancer, highlighting their intersection with immune checkpoint pathways (94, 95). Dual targeting of PD-1 and either the C5a/C5aR1 or MEK pathway has shown superior efficacy in preclinical models, leading to enhanced T cell recruitment and sustained suppression of tumor growth. These findings provide a strong rationale for integrated treatment regimens that concurrently target immune checkpoints and myeloid-derived suppressors to optimize outcomes in lung cancer therapy.
5 Conclusion
Lung cancer progression is shaped not only by intrinsic tumor biology but also by a profoundly immunosuppressive microenvironment, wherein tumor-associated macrophages, myeloid-derived suppressor cells, regulatory T cells, and dysfunctional NK and B cells coordinate to dampen antitumor immunity. Although immune checkpoint blockade has improved survival in subsets of patients, its efficacy remains limited by persistent immunosuppressive signals, metabolic dysfunction, and cellular exhaustion within the tumor niche. Emerging strategies—such as dual inhibition of PD-1/PD-L1 and myeloid-derived pathways (C5aR1, STAT3), reprogramming of TAM and Treg phenotypes, and adoptive cell therapies—offer a promising path toward immune reinvigoration and durable clinical response.
However, several limitations remain. Most preclinical models fail to fully recapitulate the complexity and heterogeneity of the human TME, which limits translational predictability. Moreover, the dynamic plasticity of immune cell subsets and inter-patient variability in immune landscapes pose challenges for biomarker development and therapy stratification. Future research should focus on spatially resolved, single-cell, and multi-omics profiling of the TME to decipher patient-specific immunological architecture. Integrating these insights with longitudinal clinical data will be critical for developing precision immunotherapies that can circumvent resistance, restore immune surveillance, and ultimately transform lung cancer management.
Author contributions
KC: Writing – original draft. LL: Writing – original draft. YL: Writing – original draft. GY: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted without any commercial or financial relationships that could be construed as potential conflicts of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Lim JU, Ryu WK, Park N, Choi J, Lee E, Lee SY, et al. Current and future perspectives in extensive-stage small-cell lung cancer. Ther Adv Med Oncol. (2025) 17:17588359251326705. doi: 10.1177/17588359251326705
2. Ge Y, Zhou Q, Pan F, and Wang R. Utilizing nanoparticles to overcome anti-PD-1/PD-L1 immunotherapy resistance in non-small cell lung cancer: A potential strategy. Int J Nanomedicine. (2025) 20:2371–94. doi: 10.2147/IJN.S505539
3. Rolver MG, Camacho-Roda J, Dai Y, Flinck M, IalChina R, Hindkaer J, et al. Tumor microenvironment acidosis favors pancreatic cancer stem cell properties and in vivo metastasis. iScience. (2025) 28:111956. doi: 10.1016/j.isci.2025.111956
4. Liu Y, Liu H, and Xiong Y. Metabolic pathway activation and immune microenvironment features in non-small cell lung cancer: insights from single-cell transcriptomics. Front Immunol. (2025) 16:1546764. doi: 10.3389/fimmu.2025.1546764
5. Yang Q, Zhang H, Wei T, Lin A, Sun Y, Luo P, et al. Single-cell RNA sequencing reveals the heterogeneity of tumor-associated macrophage in non-small cell lung cancer and differences between sexes. Front Immunol. (2021) 12:756722. doi: 10.3389/fimmu.2021.756722
6. Lin M, Chen D, Shao Z, Liu Q, Hao Z, Xin Z, et al. Inflammatory dendritic cells restrain CD11b(+)CD4(+) CTLs via CD200R in human NSCLC. Cell Rep. (2024) 43:113767. doi: 10.1016/j.celrep.2024.113767
7. Jeong H, Koh J, Kim S, Yim J, Song SG, Kim H, et al. Cell-intrinsic PD-L1 signaling drives immunosuppression by myeloid-derived suppressor cells through IL-6/Jak/Stat3 in PD-L1-high lung cancer. J Immunother Cancer. (2025) 13. doi: 10.1136/jitc-2024-010612
8. Creelan BC, Wang C, Teer JK, Toloza EM, Yao J, Kim S, et al. Tumor-infiltrating lymphocyte treatment for anti-PD-1-resistant metastatic lung cancer: a phase 1 trial. Nat Med. (2021) 27:1410–8. doi: 10.1038/s41591-021-01462-y
9. Chen H, Zhang T, Zhang Y, Wu H, Fang Z, Liu Y, et al. Deciphering the tumor microenvironment cell-infiltrating landscape reveals microenvironment subtypes and therapeutic potentials for nonsquamous NSCLC. JCI Insight. (2022) 7. doi: 10.1172/jci.insight.152815
10. Sun D, Liu J, Zhou H, Shi M, Sun J, Zhao S, et al. Classification of tumor immune microenvironment according to programmed death-ligand 1 expression and immune infiltration predicts response to immunotherapy plus chemotherapy in advanced patients with NSCLC. J Thorac Oncol. (2023) 18:869–81. doi: 10.1016/j.jtho.2023.03.012
11. Koyama S, Akbay EA, Li YY, Aref AR, Skoulidis F, Herter-Sprie GS, et al. Wong KK: STK11/LKB1 deficiency promotes neutrophil recruitment and proinflammatory cytokine production to suppress T-cell activity in the lung tumor microenvironment. Cancer Res. (2016) 76:999–1008. doi: 10.1158/0008-5472.CAN-15-1439
12. Bremnes RM, Busund LT, Kilvaer TL, Andersen S, Richardsen E, Paulsen EE, et al. The role of tumor-infiltrating lymphocytes in development, progression, and prognosis of non-small cell lung cancer. J Thorac Oncol. (2016) 11:789–800. doi: 10.1016/j.jtho.2016.01.015
13. Lopez de Rodas M, Nagineni V, Ravi A, Datar IJ, Mino-Kenudson M, Corredor G, et al. Role of tumor infiltrating lymphocytes and spatial immune heterogeneity in sensitivity to PD-1 axis blockers in non-small cell lung cancer. J Immunother Cancer. (2022) 10. doi: 10.1136/jitc-2021-004440
14. Forsyth KS and Eisenlohr LC. Giving CD4+ T cells the slip: viral interference with MHC class II-restricted antigen processing and presentation. Curr Opin Immunol. (2016) 40:123–9. doi: 10.1016/j.coi.2016.03.003
15. Zheng X, Hu Y, and Yao C. The paradoxical role of tumor-infiltrating immune cells in lung cancer. Intract able Rare Dis Res. (2017) 6:234–41. doi: 10.5582/irdr.2017.01059
16. Mateu-Jimenez M, Curull V, Pijuan L, Sanchez-Font A, Rivera-Ramos H, Rodriguez-Fuster A, et al. Systemic and tumor th1 and th2 inflammatory profile and macrophages in lung cancer: influence of underlying chronic respiratory disease. J Thorac Oncol. (2017) 12:235–48. doi: 10.1016/j.jtho.2016.09.137
17. Ma J, Liu H, and Wang X. Effect of ginseng polysaccharides and dendritic cells on the balance of Th1/Th2 T helper cells in patients with non-small cell lung cancer. J Tradit Chin Med. (2014) 34:641–5. doi: 10.1016/S0254-6272(15)30076-5
18. Domagala-Kulawik J, Osinska I, and Hoser G. Mechanisms of immune response regulation in lung cancer. Transl Lung Cancer Res. (2014) 3:15–22.
19. Wang R, Yang L, Zhang C, Wang R, Zhang Z, He Q, et al. Th17 cell-derived IL-17A promoted tumor progression via STAT3/NF-kappaB/Notch1 signaling in non-small cell lung cancer. Oncoimmunology. (2018) 7:e1461303.
20. Armstrong D, Chang CY, Lazarus DR, Corry D, and Kheradmand F. Lung cancer heterogeneity in modulation of th17/IL17A responses. Front Oncol. (2019) 9:1384. doi: 10.3389/fonc.2019.01384
21. Qianmei Y, Zehong S, Guang W, Hui L, and Lian G. Recent advances in the role of Th17/Treg cells in tumor immunity and tumor therapy. Immunol Res. (2021) 69:398–414. doi: 10.1007/s12026-021-09211-6
22. Gamal W, Sahakian E, and Pinilla-Ibarz J. The role of Th17 cells in chronic lymphocytic leukemia: friend or foe? Blood Adv. (2023) 7:2401–17. doi: 10.1182/bloodadvances.2022008985
23. Chen X, Wan J, Liu J, Xie W, Diao X, Xu J, et al. Increased IL-17-producing cells correlate with poor survival and lymphangiogenesis in NSCLC patients. Lung Cancer. (2010) 69:348–54. doi: 10.1016/j.lungcan.2009.11.013
24. Nicola S, Ridolfi I, Rolla G, Filosso P, Giobbe R, Boita M, et al. IL-17 promotes nitric oxide production in non-small-cell lung cancer. J Clin Med. (2021) 10. doi: 10.3390/jcm10194572
25. Laszczych D, Czernicka A, Gostomczyk K, Szylberg L, and Borowczak J. The role of IL-17 in the pathogenesis and treatment of glioblastoma-an update on the state of the art and future perspectives. Med Oncol. (2024) 41:187.
26. Wang Y, Li J, Nakahata S, and Iha H. Complex role of regulatory T cells (Tregs) in the tumor microenvironment: their molecular mechanisms and bidirectional effects on cancer progression. Int J Mol Sci. (2024) 25. doi: 10.3390/ijms25137346
27. Liu J, Zhang B, Zhang G, and Shang D. Reprogramming of regulatory T cells in inflammatory tumor microenvironment: can it become immunotherapy turning point? Front Immunol. (2024) 15:1345838. doi: 10.3389/fimmu.2024.1345838
28. Wang J, Gong R, Zhao C, Lei K, Sun X, and Ren H. Human FOXP3 and tumour microenvironment. Immunology. (2023) 168:248–55. doi: 10.1111/imm.13520
29. Zong Y, Deng K, and Chong WP. Regulation of Treg cells by cytokine signaling and co-stimulatory molecules. Front Immunol. (2024) 15:1387975. doi: 10.3389/fimmu.2024.1387975
30. Moreno Ayala MA, Campbell TF, Zhang C, Dahan N, Bockman A, Prakash V, et al. CXCR3 expression in regulatory T cells drives interactions with type I dendritic cells in tumors to restrict CD8(+) T cell antitumor immunity. Immunity. (2023) 56:1613–1630.e1615.
31. Devi-Marulkar P, Fastenackels S, Karapentiantz P, Goc J, Germain C, Kaplon H, et al. Regulatory T cells infiltrate the tumor-induced tertiary lymphoid structures and are associated with poor clinical outcome in NSCLC. Commun Biol. (2022) 5:1416. doi: 10.1038/s42003-022-04356-y
32. Duan MC, Han W, Jin PW, Wei YP, Wei Q, Zhang LM, et al. Disturbed th17/treg balance in patients with non-small cell lung cancer. Inflammation. (2015) 38:2156–65. doi: 10.1007/s10753-015-0198-x
33. Chen W, Hua Y, Shan C, Wei J, Zhou Y, and Pan S. PD-1(+) IFN-gamma(+) subset of CD8(+) T cell in circulation predicts response to anti-PD-1 therapy in NSCLC. Front Oncol. (2023) 13:1182301. doi: 10.3389/fonc.2023.1182301
34. Hannani D, Leplus E, Laurin D, Caulier B, Aspord C, Madelon N, et al. A new plasmacytoid dendritic cell-based vaccine in combination with anti-PD-1 expands the tumor-specific CD8+ T cells of lung cancer patients. Int J Mol Sci. (2023) 24. doi: 10.3390/ijms24031897
35. Ao YQ, Gao J, Zhang LX, Deng J, Wang S, Lin M, et al. Tumor-infiltrating CD36(+)CD8(+)T cells determine exhausted tumor microenvironment and correlate with inferior response to chemotherapy in non-small cell lung cancer. BMC Cancer. (2023) 23:367. doi: 10.1186/s12885-023-10836-z
36. Besse B, Charrier M, Lapierre V, Dansin E, Lantz O, Planchard D, et al. Dendritic cell-derived exosomes as maintenance immunotherapy after first line chemotherapy in NSCLC. Oncoimmunology. (2016) 5:e1071008. doi: 10.1080/2162402X.2015.1071008
37. Schafer H, Subbarayan K, Massa C, Vaxevanis C, Mueller A, and Seliger B. Correlation of the tumor escape phenotype with loss of PRELP expression in melanoma. J Transl Med. (2023) 21:643. doi: 10.1186/s12967-023-04476-x
38. Ricciuti B, Wang X, Alessi JV, Rizvi H, Mahadevan NR, Li YY, et al. Association of high tumor mutation burden in non-small cell lung cancers with increased immune infiltration and improved clinical outcomes of PD-L1 blockade across PD-L1 expression levels. JAMA Oncol. (2022) 8:1160–8. doi: 10.1001/jamaoncol.2022.1981
39. Freitas-Dias C, Goncalves F, Martins F, Lemos I, Goncalves LG, and Serpa J. Interaction between NSCLC cells, CD8(+) T-cells and immune checkpoint inhibitors potentiates coagulation and promotes metabolic remodeling-new cues on CAT-VTE. Cells. (2024) 13. doi: 10.3390/cells13040305
40. Perez-Ruiz E, Minute L, Otano I, Alvarez M, Ochoa MC, Belsue V, et al. Prophylactic TNF blockade uncouples efficacy and toxicity in dual CTLA-4 and PD-1 immunotherapy. Nature. (2019) 569:428–32. doi: 10.1038/s41586-019-1162-y
41. Skoulidis F, Araujo HA, Do MT, Qian Y, Sun X, Cobo AG, et al. Heymach JV: CTLA4 blockade abrogates KEAP1/STK11-related resistance to PD-(L)1 inhibitors. Nature. (2024) 635:462–71. doi: 10.1038/s41586-024-07943-7
42. Hiltbrunner S, Cords L, Kasser S, Freiberger SN, Kreutzer S, Toussaint NC, et al. Acquired resistance to anti-PD1 therapy in patients with NSCLC associates with immunosuppressive T cell phenotype. Nat Commun. (2023) 14:5154. doi: 10.1038/s41467-023-40745-5
43. Liu Z, Wang T, She Y, Wu K, Gu S, Li L, et al. N(6)-methyladenosine-modified circIGF2BP3 inhibits CD8(+) T-cell responses to facilitate tumor immune evasion by promoting the deubiquitination of PD-L1 in non-small cell lung cancer. Mol Cancer. (2021) 20:105. doi: 10.1186/s12943-021-01398-4
44. Chen SW, Zhu SQ, Pei X, Qiu BQ, Xiong D, Long X, et al. Cancer cell-derived exosomal circUSP7 induces CD8(+) T cell dysfunction and anti-PD1 resistance by regulating the miR-934/SHP2 axis in NSCLC. Mol Cancer. (2021) 20:144. doi: 10.1186/s12943-021-01448-x
45. Liu X, Song J, Zhang H, Liu X, Zuo F, Zhao Y, et al. Immune checkpoint HLA-E:CD94-NKG2A mediates evasion of circulating tumor cells from NK cell surveillance. Cancer Cell. (2023) 41:272–287 e279. doi: 10.1016/j.ccell.2023.01.001
46. Guo C, Dong M, Wang X, Yu J, Jin X, Cheng S, et al. A novel MICA/B-targeted chimeric antigen receptor augments the cytotoxicity of NK cells against tumor cells. Biochem Biophys Res Commun. (2024) 710:149918. doi: 10.1016/j.bbrc.2024.149918
47. Vulpis E, Loconte L, Cassone C, Antonangeli F, Caracciolo G, Masuelli L, et al. Cross-dressing of multiple myeloma cells mediated by extracellular vesicles conveying MIC and ULBP ligands promotes NK cell killing. Int J Mol Sci. (2023) 24. doi: 10.3390/ijms24119467
48. Pang Z, Wang Z, Li F, Feng C, and Mu X. Current progress of CAR-NK therapy in cancer treatment. Cancers (Basel). (2022) 14. doi: 10.3390/cancers14174318
49. Enomoto Y, Li P, Jenkins LM, Anastasakis D, Lyons GC, Hafner M, et al. Cytokine-enhanced cytolytic activity of exosomes from NK Cells. Cancer Gene Ther. (2022) 29:734–49. doi: 10.1038/s41417-021-00352-2
50. Paul S, Chhatar S, Mishra A, and Lal G. Natural killer T cell activation increases iNOS(+)CD206(-) M1 macrophage and controls the growth of solid tumor. J Immunother Cancer. (2019) 7:208. doi: 10.1186/s40425-019-0697-7
51. Veneziani I, Alicata C, Pelosi A, Landolina N, Ricci B, D’Oria V, et al. Toll-like receptor 8 agonists improve NK-cell function primarily targeting CD56(bright)CD16(-) subset. J Immunother Cancer. (2022) 10.
52. Zhao W, Wang H, Zhang X, Zhang L, Pu W, Ma Y, et al. Effects of IFN-gamma on the immunological microenvironment and TAM polarity in stage IA non-small cell lung cancer and its mechanisms. BMC Pulm Med. (2024) 24:46. doi: 10.1186/s12890-023-02809-6
53. Herault A, Mak J, de la Cruz-Chuh J, Dillon MA, Ellerman D, Go M, et al. NKG2D-bispecific enhances NK and CD8+ T cell antitumor immunity. Cancer Immunol Immunother. (2024) 73:209. doi: 10.1007/s00262-024-03795-2
54. Patel SA, Nilsson MB, Yang Y, Le X, Tran HT, Elamin YY, et al. IL6 mediates suppression of T- and NK-cell function in EMT-associated TKI-resistant EGFR-mutant NSCLC. Clin Cancer Res. (2023) 29:1292–304. doi: 10.1158/1078-0432.CCR-22-3379
55. Yu DP, Han Y, Zhao QY, and Liu ZD. CD3+ CD4+ and CD3+ CD8+ lymphocyte subgroups and their surface receptors NKG2D and NKG2A in patients with non-small cell lung cancer. Asian Pac J Cancer Prev. (2014) 15:2685–8. doi: 10.7314/APJCP.2014.15.6.2685
56. Beelen NA, Valckx VTC, Bos GMJ, and Wieten L. Interfering with KIR and NKG2A immune checkpoint axes to unleash NK cell immunotherapy. Best Pract Res Clin Haematol. (2024) 37:101568. doi: 10.1016/j.beha.2024.101568
57. Li J, Wang L, Chen X, Li L, Li Y, Ping Y, et al. CD39/CD73 upregulation on myeloid-derived suppressor cells via TGF-beta-mTOR-HIF-1 signaling in patients with non-small cell lung cancer. Oncoimmunology. (2017) 6:e1320011.
58. Ni J, Wang X, Stojanovic A, Zhang Q, Wincher M, Buhler L, et al. Single-cell RNA sequencing of tumor-infiltrating NK cells reveals that inhibition of transcription factor HIF-1alpha unleashes NK cell activity. Immunity. (2020) 52:1075–1087 e1078.
59. Pelletier A, Nelius E, Fan Z, Khatchatourova E, Alvarado-Diaz A, He J, et al. Resting natural killer cell homeostasis relies on tryptophan/NAD(+) metabolism and HIF-1alpha. EMBO Rep. (2023) 24:e56156.
60. Du F, Qi X, Zhang A, Sui F, Wang X, Proud CG, et al. MRTF-A-NF-kappaB/p65 axis-mediated PDL1 transcription and expression contributes to immune evasion of non-small-cell lung cancer via TGF-beta. Exp Mol Med. (2021) 53:1366–78. doi: 10.1038/s12276-021-00670-3
61. Patel SP, Alonso-Gordoa T, Banerjee S, Wang D, Naidoo J, Standifer NE, et al. Phase 1/2 study of monalizumab plus durvalumab in patients with advanced solid tumors. J Immunother Cancer. (2024) 12. doi: 10.1136/jitc-2023-007340
62. Zhang X, Guo Y, Ji Y, Gao Y, Zhang M, Liu Y, et al. Cytokine release syndrome after modified CAR-NK therapy in an advanced non-small cell lung cancer patient: A case report. Cell Transplant. (2022) 31:9636897221094244. doi: 10.1177/09636897221094244
63. Bao J, Betzler AC, Hess J, and Brunner C. Exploring the dual role of B cells in solid tumors: implications for head and neck squamous cell carcinoma. Front Immunol. (2023) 14:1233085. doi: 10.3389/fimmu.2023.1233085
64. Rastogi I, Jeon D, Moseman JE, Muralidhar A, Potluri HK, and McNeel DG. Role of B cells as antigen presenting cells. Front Immunol. (2022) 13:954936. doi: 10.3389/fimmu.2022.954936
65. Lo Tartaro D, Aramini B, Masciale V, Paschalidis N, Lofaro FD, Neroni A, et al. Metabolically activated and highly polyfunctional intratumoral VISTA(+) regulatory B cells are associated with tumor recurrence in early-stage NSCLC. Mol Cancer. (2025) 24:16. doi: 10.1186/s12943-024-02209-2
66. Federico L, McGrail DJ, Bentebibel SE, Haymaker C, Ravelli A, Forget MA, et al. Distinct tumor-infiltrating lymphocyte landscapes are associated with clinical outcomes in localized non-small-cell lung cancer. Ann Oncol. (2022) 33:42–56. doi: 10.1016/j.annonc.2021.09.021
67. Patel AJ, Willsmore ZN, Khan N, Richter A, Naidu B, Drayson MT, et al. Regulatory B cell repertoire defects predispose lung cancer patients to immune-related toxicity following checkpoint blockade. Nat Commun. (2022) 13:3148. doi: 10.1038/s41467-022-30863-x
68. Jansen K, Cevhertas L, Ma S, Satitsuksanoa P, Akdis M, and van de Veen W. Regulatory B cells, A to Z. Allergy. (2021) 76:2699–715. doi: 10.1111/all.14763
69. Catalan D, Mansilla MA, Ferrier A, Soto L, Oleinika K, Aguillon JC, et al. Immunosuppressive mechanisms of regulatory B cells. Front Immunol. (2021) 12:611795. doi: 10.3389/fimmu.2021.611795
70. Che D, Zhang S, Jing Z, Shang L, Jin S, Liu F, et al. Corrigendum to “Macrophages induce EMT to promote invasion of lung cancer cells through the IL-6-mediated COX-2/PGE(2)/beta-catenin signalling pathway” [Mol. Immunol 90 (2017) 197-210]. Mol Immunol. (2020) 126:165–6.
71. Zhang J, Cao J, Ma S, Dong R, Meng W, Ying M, et al. Tumor hypoxia enhances Non-Small Cell Lung Cancer metastasis by selectively promoting macrophage M2 polarization through the activation of ERK signaling. Oncotarget. (2014) 5:9664–77. doi: 10.18632/oncotarget.1856
72. Aktar T, Modak S, Majumder D, and Maiti D. A detailed insight into macrophages’ role in shaping lung carcinogenesis. Life Sci. (2024) 352:122896. doi: 10.1016/j.lfs.2024.122896
73. Ren W, Hou J, Yang C, Wang H, Wu S, Wu Y, et al. Extracellular vesicles secreted by hypoxia pre-challenged mesenchymal stem cells promote non-small cell lung cancer cell growth and mobility as well as macrophage M2 polarization via miR-21-5p delivery. J Exp Clin Cancer Res. (2019) 38:62. doi: 10.1186/s13046-019-1027-0
74. Jackute J, Zemaitis M, Pranys D, Sitkauskiene B, Miliauskas S, Vaitkiene S, et al. Distribution of M1 and M2 macrophages in tumor islets and stroma in relation to prognosis of non-small cell lung cancer. BMC Immunol. (2018) 19:3. doi: 10.1186/s12865-018-0241-4
75. Raber PL, Thevenot P, Sierra R, Wyczechowska D, Halle D, Ramirez ME, et al. Subpopulations of myeloid-derived suppressor cells impair T cell responses through independent nitric oxide-related pathways. Int J Cancer. (2014) 134:2853–64. doi: 10.1002/ijc.28622
76. Luan Y, Mosheir E, Menon MC, Wilson D, Woytovich C, Ochando J, et al. Monocytic myeloid-derived suppressor cells accumulate in renal transplant patients and mediate CD4(+) Foxp3(+) Treg expansion. Am J Transplant. (2013) 13:3123–31. doi: 10.1111/ajt.12461
77. Crook KR, Jin M, Weeks MF, Rampersad RR, Baldi RM, Glekas AS, et al. Myeloid-derived suppressor cells regulate T cell and B cell responses during autoimmune disease. J Leukoc Biol. (2015) 97:573–82. doi: 10.1189/jlb.4A0314-139R
78. Liu W, Wu TC, Hong DM, Hu Y, Fan T, Guo WJ, et al. Carnosic acid enhances the anti-lung cancer effect of cisplatin by inhibiting myeloid-derived suppressor cells. Chin J Nat Med. (2018) 16:907–15. doi: 10.1016/S1875-5364(18)30132-8
79. Wang Y, Fan X, and Wu X. Ganoderma lucidum polysaccharide (GLP) enhances antitumor immune response by regulating differentiation and inhibition of MDSCs via a CARD9-NF-kappaB-IDO pathway. Biosci Rep. (2020) 40.
80. Feng PH, Lee KY, Chang YL, Chan YF, Kuo LW, Lin TY, et al. CD14(+)S100A9(+) monocytic myeloid-derived suppressor cells and their clinical relevance in non-small cell lung cancer. Am J Respir Crit Care Med. (2012) 186:1025–36. doi: 10.1164/rccm.201204-0636OC
81. Sawanobori Y, Ueha S, Kurachi M, Shimaoka T, Talmadge JE, Abe J, et al. Chemokine-mediated rapid turnover of myeloid-derived suppressor cells in tumor-bearing mice. Blood. (2008) 111:5457–66. doi: 10.1182/blood-2008-01-136895
82. Takahashi R, Amano H, Ito Y, Eshima K, Satoh T, Iwamura M, et al. Microsomal prostaglandin E synthase-1 promotes lung metastasis via SDF-1/CXCR4-mediated recruitment of CD11b(+)Gr1(+)MDSCs from bone marrow. BioMed Pharmacother. (2020) 121:109581. doi: 10.1016/j.biopha.2019.109581
83. Qu J, Liu L, Xu Q, Ren J, Xu Z, Dou H, et al. CARD9 prevents lung cancer development by suppressing the expansion of myeloid-derived suppressor cells and IDO production. Int J Cancer. (2019) 145:2225–37. doi: 10.1002/ijc.32355
84. Ajona D, Ortiz-Espinosa S, Moreno H, Lozano T, Pajares MJ, Agorreta J, et al. A combined PD-1/C5a blockade synergistically protects against lung cancer growth and metastasis. Cancer Discov. (2017) 7:694–703. doi: 10.1158/2159-8290.CD-16-1184
85. Lee JW, Zhang Y, Eoh KJ, Sharma R, Sanmamed MF, Wu J, et al. The combination of MEK inhibitor with immunomodulatory antibodies targeting programmed death 1 and programmed death ligand 1 results in prolonged survival in kras/p53-driven lung cancer. J Thorac Oncol. (2019) 14:1046–60. doi: 10.1016/j.jtho.2019.02.004
86. Liu NN, Yi CX, Wei LQ, Zhou JA, Jiang T, Hu CC, et al. The intratumor mycobiome promotes lung cancer progression via myeloid-derived suppressor cells. Cancer Cell. (2023) 41:1927–1944 e1929. doi: 10.1016/j.ccell.2023.08.012
87. Liang M, Sun Z, Chen X, Wang L, Wang H, Qin L, et al. E3 ligase TRIM28 promotes anti-PD-1 resistance in non-small cell lung cancer by enhancing the recruitment of myeloid-derived suppressor cells. J Exp Clin Cancer Res. (2023) 42:275. doi: 10.1186/s13046-023-02862-3
88. Li X, Ke Y, Hernandez AL, Yu J, Bian L, Hall SC, et al. Inducible nitric oxide synthase (iNOS)-activated Cxcr2 signaling in myeloid cells promotes TGFbeta-dependent squamous cell carcinoma lung metastasis. Cancer Lett. (2023) 570:216330. doi: 10.1016/j.canlet.2023.216330
89. Wang Y, Schafer CC, Hough KP, Tousif S, Duncan SR, Kearney JF, et al. Myeloid-derived suppressor cells impair B cell responses in lung cancer through IL-7 and STAT5. J Immunol. (2018) 201:278–95. doi: 10.4049/jimmunol.1701069
90. Zhu X, Zhang X, Shen J, Zheng S, Li H, Han B, et al. Gut microbiota-dependent modulation of pre-metastatic niches by Jianpi Yangzheng decoction in the prevention of lung metastasis of gastric cancer. Phytomedicine. (2024) 128:155413. doi: 10.1016/j.phymed.2024.155413
91. Wan Y, Mu X, Zhao J, Li L, Xu W, and Zhang M. Myeloid−derived suppressor cell accumulation induces Treg expansion and modulates lung Malignancy progression. BioMed Rep. (2024) 20:68. doi: 10.3892/br.2024.1754
92. Hsu YL, Yen MC, Chang WA, Tsai PH, Pan YC, Liao SH, et al. CXCL17-derived CD11b(+)Gr-1(+) myeloid-derived suppressor cells contribute to lung metastasis of breast cancer through platelet-derived growth factor-BB. Breast Cancer Res. (2019) 21:23. doi: 10.1186/s13058-019-1114-3
93. Ren J, Ying J, Liu H, Hu S, Li J, and Zhou D. Stimulator of interferon genes signal in lung cancer regulates differentiation of myeloid-derived suppressor cells in the tumor microenvironment via the interferon regulatory factor 3/NF-kappaB pathway. J Interferon Cytokine Res. (2025) 45:29–37. doi: 10.1089/jir.2024.0150
94. Sheida F, Razi S, Keshavarz-Fathi M, and Rezaei N. The role of myeloid-derived suppressor cells in lung cancer and targeted immunotherapies. Expert Rev Anticancer Ther. (2022) 22:65–81. doi: 10.1080/14737140.2022.2011224
Keywords: TAM, MDSC, immune evasion, tumor microenvironment, lung cancer, immunotherapy
Citation: Chen K, Luo L, Li Y and Yang G (2025) Reprogramming the immune microenvironment in lung cancer. Front. Immunol. 16:1684889. doi: 10.3389/fimmu.2025.1684889
Received: 13 August 2025; Accepted: 16 September 2025;
Published: 29 September 2025.
Edited by:
Lilong Zhang, Renmin Hospital of Wuhan University, ChinaReviewed by:
Lexin Wang, Ningxia Medical University, ChinaCopyright © 2025 Chen, Luo, Li and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ge Yang, WUcyMDA4MDU0MzBAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Kai Chen
Kai Chen Linqi Luo4†
Linqi Luo4†