- 1University Institute of Biochemistry and Biotechnology, PMAS-Arid Agriculture University Rawalpindi, Rawalpindi, Pakistan
- 2National Reference Laboratory for Tuberculosis, National TB Control Program, Islamabad, Pakistan
- 3Department of Medicinal Chemistry, College of Pharmacy, University of Minnesota, Minneapolis, MN, United States
- 4Department of Microbiology & Immunology, University of Minnesota Medical School, Minneapolis, MN, United States
- 5Department of Microbiology, Quaid-i-Azam University, Islamabad, Pakistan
- 6Institute of Molecular Biology and Biotechnology, The University of Lahore, Lahore, Pakistan
- 7Masonic Cancer Center, University of Minnesota, Minneapolis, MN, United States
Datura innoxia is a medicinal plant from the Solanaceae family, having medicinal properties and some toxic effects. It is widely distributed across Asia, Africa, Europe, the Americas, and other tropical and subtropical regions, where it is utilized by local pharmaceutical industries. In this study, bioassay-guided fractionation and LC-MS/MS analysis were used for the identification of secondary metabolites with anti-tuberculosis activity in methanolic leaf extracts of D. innoxia. Bioassay-guided fractionation was conducted using normal and reverse phase column chromatography, and the fractions were assayed for antituberculosis activity in vitro by serial dilution in Mycobacterium tuberculosis H37Ra cultures. The structures of known secondary metabolites in the purified extracts were identified using LC-ESI-MS/MS mass spectroscopy. A purified fraction of the methanolic extract of D. innoxia leaves inhibited M. tuberculosis growth at concentrations as low as 25 μg/mL. Metabolic profiling with LC-ESI-MS/MS enabled the identification of the purified extract of 16 known metabolites, including loliolide, scopolamine, kuromanin, isoquercitrin, moupinamide, methyl isoquinoline-3-carboxylate, trans-3-Indoleacrylic acid, tyramine, (3β,5ξ,9ξ)-3,6,19-trihydroxyurs-12-en-28-oic acid, milbemycin A3 oxime, methyl jasmonate, nicotinamide, methyl ferulate, trifolin, 2-[(1S,2S,4aR,8aS)-1-hydroxy-4a-methyl-8-methylidene-decahydronaphthalen-2-yl]prop-2-enoic acid, and methyl 4-hydroxycinnamate. These results indicate that D. innoxia is a rich natural source of potential antitubercular secondary metabolites.
1 Introduction
Mycobacterium tuberculosis (Mtb) is a Bacillus bacterium that causes the infectious disease tuberculosis (TB). TB ranks among the top 10 diseases in the world in terms of both mortality and morbidity (Rodriguez-Takeuchi et al., 2019). According to the WHO global report, an estimated 10.6 million individuals have active Mtb infections, resulting in approximately 1.30 million reported deaths (Chunrong et al., 2023), whereas approximately one-fourth of the world population (≈ 2 billion people) is estimated to be latently infected with Mtb (Chin et al., 2023). Despite significant treatment advances, TB remains a serious global health concern (Sharifi-Rad et al., 2020). Approximately 40 years ago, a standardized 6-month treatment regimen for tuberculosis was established, based on the use of four first-line drugs: isoniazid, rifampicin, ethambutol, and pyrazinamide. This regimen is widely recommended and has been shown to cure approximately 85% of patients with drug-sensitive tuberculosis (WHO, 2013). One of the biggest obstacles to TB management worldwide is the rapid spread of drug-resistant TB strains. These strains are currently present in most nations and are growing alarmingly. Multidrug-resistant (MDR) TB isolates that are resistant to isoniazid and rifampicin, the two first-line medications for TB therapy, have been found in every country surveyed (Cazzaniga et al., 2021).
Natural products have played a vital role in the discovery of new drugs; today, more than 25% of conventional drugs on the market are either directly or indirectly derived from plant secondary metabolites (Marealle et al., 2023). Similarly, medicinal plants and their extracts have served as valuable resources for the discovery and development of alternative treatments for TB (Mpeirwe et al., 2023; Tuyiringire et al., 2020; Karimi, 2023). According to floral research, there are approximately 500,000 plant species on the planet, and 120,000 of those species have biologically active compounds that can be used to treat illnesses (Kallassy, 2017; Houghton, 2001), particularly in developing countries, where the World Health Organization estimated that 70%−80% of the population depends on traditional medicines for their primary source of medication (Akinyemi et al., 2005; Maluleka and Ngoepe, 2018).
Datura is a genus of medicinal herbs in the nightshade family (Solanaceae), commonly known as jimsonweed or thornapple, which have both toxic and medicinal properties (Sharma et al., 2021). Datura species are widely cultivated in Asia, Africa, Europe, America, and other tropical and subtropical regions for use in herbal medicine preparations (Gaire and Subedi, 2013; Lakusic et al., 2017). Datura species have been reported to possess antidiabetic, antimicrobial, anti-cancer, anti-asthmatic, anti-inflammatory, analgesic, antioxidant, cytotoxic, insecticidal, and neurological activities, and wound healing (Alam et al., 2021; Al-Snafi, 2017).
2 Materials and methods
2.1 Plant collection and identification
Leaves of Datura innoxia (Figure 1) were collected between March and June 2020, near Islamabad, Pakistan. The plant material was identified and authenticated by Professor Rahmatullah Quraishi, Department of Botany, PMAS Arid Agriculture University, Rawalpindi, Pakistan. A voucher specimen was submitted to the Herbarium of Medicinal Plants and assigned a unique herbarium number (PMAS-177).
2.2 Extraction of plant material
The leaves of D. innoxia were washed with tap water to eliminate impurities and dried at room temperature. The dried leaves were crushed into a powder. To obtain crude extracts of the dried powder, successive extraction with maceration was conducted as previously used (Ahmed et al., 2023). Briefly, powdered plant material (200 g) was suspended in 1,000 mL of methanol (plant biomass to solvent ratio of 1:5 w/v) in an Erlenmeyer flask and shaken for 48 h at room temperature. The initial filtration was performed using a muslin cloth, followed by fine filtration with Whatman No. 1 filter paper. To increase the extract yield, an additional aliquot of methanol (at a plant biomass to solvent ratio of 1:3 w/v) was added to the extraction residue, which was then subjected to an additional 72 h extraction and filtered. The combined filtrates were evaporated using a rotary evaporator at lower pressure to yield a crude methanolic extract (CME). This extract was subsequently fractionated into the three samples: non-polar fraction (CME extracted with n-hexane), moderately polar fraction (residue from n-hexane extraction then extracted with ethyl acetate), and highly polar fraction (residue from ethyl acetate extraction then extracted with distilled water). A rotary evaporator was used to evaporate the solvents at reduced pressure, and all fractions were tested for anti-mycobacterial activity. The most active crude fraction (ethyl acetate) was stored at 2–4°C for further analysis (Ahmed et al., 2023; Jabeen et al., 2022). The D. innoxia extracts and their fractions demonstrated sufficient stability, with no detectable changes in anti-TB activity observed in repeated experiments.
2.3 Fractionation of ethyl acetate extract
Normal-phase column chromatography was used to fractionate 6.30 g of ethyl acetate extract using silica gel (60–120 mesh) (Ahmed et al., 2023). The fractionation was carried out using a stepwise gradient of n-hexane:ethyl acetate in the following volume ratios: 100:0, 80:20, 60:40, 40:60, 20:80, and 0:100 (v/v). This was followed by elution with ethyl acetate:methanol in ratios of 100:0, 80:20, 60:40, 40:60, 20:80, and 0:100 (v/v). The collected fractions were evaporated under reduced pressure. The yield (%) of each fraction was calculated using the following formula:
2.4 Bacterial culture conditions
Mycobacterium tuberculosis H37Ra was grown in Middlebrook 7H9 broth medium supplemented with 0.2% (v/v) glycerol (Sigma Chemical Co.), 10% (w/v) oleic acid, albumin, dextrose, catalase (OADC; Difco), and 0.05% (w/v) tyloxapol (Sigma). Minimum bactericidal concentration (MBC) measurements were performed on Middlebrook 7H10 agar media supplemented with 0.2% glycerol and 10% (w/v) OADC (Martin et al., 2005).
2.5 Minimum inhibitory concentration and minimum bactericidal concentration determinations
3-[4,5-Dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) assay was used to determine the MICs of the crude plant extracts, with minor modifications to the method described by Martin et al. (2005). Briefly, the fractions were evaporated to dryness, and the residues were accurately weighed before being dissolved in DMSO to prepare stock solutions of known concentrations. Two-fold serial dilutions of the samples were prepared to achieve final concentrations ranging from 200 μg/mL to 12.5 μg/mL. Subsequently, 5 μL of the diluted samples was added to a 96-well plate, followed by 95 μL of the H37Ra culture (final OD600 = 0.01). The cultures were incubated for 7 days at 37°C, and MICs were recorded by visual observations. Each concentration above the visually observed MICs was serially diluted, and 10 μL of each dilution was plated on Middlebrook 7H10 agar plates. The agar plates were incubated at 37°C for 3 weeks. The MBC was recorded as the lowest concentration that resulted in a 99% reduction in colony-forming units (CFUs) in the initial inoculum. MTT solution (10 μL of 5 mg/mL) was added to all the wells of the 96-well plate, followed by overnight incubation. Then, 50 μL of formazan solubilization buffer (Abate et al., 1998) was added, and incubation was continued for at least 4 h at 37°C. A color change from yellow to violet indicated bacterial growth, and MICs were interpreted accordingly (Vilchèze et al., 2011).
2.6 Preparative thin layer chromatography
Preparative TLC was carried out on the most active fraction (F10) of the crude extract of D. innoxia leaves (ethyl acetate) on 250-micron silica gel layers developed with chloroform: ethanol (75:25). Distinct bands were individually scraped from the plate, transferred to mini-columns, and eluted with methanol. The eluate was filtered through a paper and concentrated under reduced pressure (Nimbeshaho et al., 2020).
2.7 Reverse phase column chromatography
Sample material eluted from the prep-TLC band (F10B5), measuring 4 mg, was dissolved in the minimum amount of MeOH and applied to the column with 5 g of octadecyl-functionalized silica gel to collect the effluent (Choudhari et al., 2009). Columns were eluted sequentially with 10 mL of the following percentages of MeOH in water: 10%, 20%, 30%, 40%, 50%, 60%, 70%, 80%, 90%, and 100% MeOH, and the effluents were collected as fractions. The collected fractions were evaporated under reduced pressure, and the residues were assayed for antituberculosis activity using the MTT assay described above.
2.8 LC-ESI-MS/MS data acquisition and analysis
LC-MS/MS phytochemical profiling of the extracted fraction RC08 was carried out using an Orbitrap Fusion mass spectrometer (Thermo Scientific, Waltham, MA, USA) connected to a Dionex UltiMate 3000 RSLCnano UPLC system. The sample (5 μl) injections were subjected to chromatographic separations using a mobile phase of water with 0.1% formic acid (A) and acetonitrile (B) on an Acquity HSS (Waters, Milford, MA) C18 reversed-phase column (100 × 2.1 mm, 1.8 μm particle size). The initial conditions were 2% B for 3 min, followed by a linear gradient to 95% B over 49 min (long) and 12 min (short) with a 2-min hold at 95% B. The column was then re-equilibrated with 2% B for the next run. Electrospray ionization was used to acquire MS data, with full scan Orbitrap detection (m/z 100–1,000, resolution 120,000) and data-dependent HCD fragmentation (stepped 20%, 35%, and 60%) with a 1-s cycle time, 6 s dynamic exclusion, 1.6 Da quadrupole isolation width, exclusion mass width ±10 ppm, and 15,000 resolution Orbitrap detection. Each sample was performed independently in the negative (M – H+) and positive (M + H+) modes. Compound Discoverer 3.3 (Thermo Scientific, Waltham, MA) was used for data processing, and analyte identification was done by searching the Thermo Scientific mzCloud and NIST 2020 high-resolution mass spectral databases (Flamini, 2013).
3 Results
3.1 Bioassay-guided fractionation of the D. innoxia leaf ethyl acetate extract
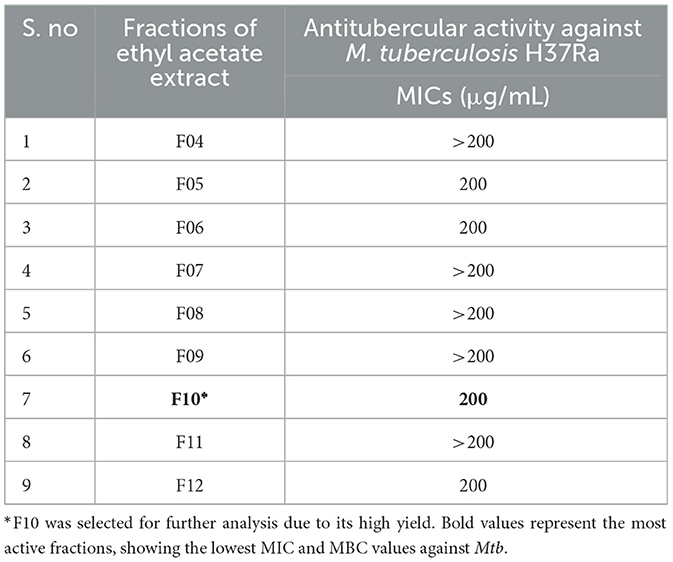
A total of 12 fractions were obtained from the column chromatography of the ethyl acetate extract of Datura innoxia leaves. The highest yield was obtained for fraction #F9 (28.26%), followed by F10 (20.06%), F6 (16.15%), and others (Figure 2).
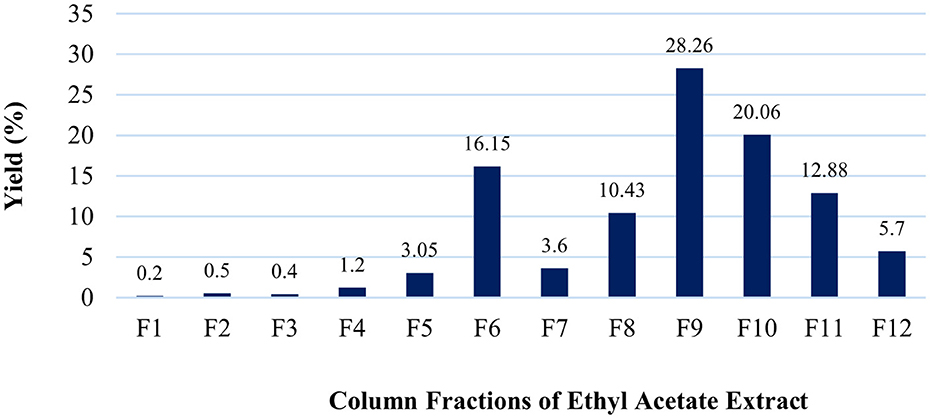
Figure 2. Fractionation yield (%) was obtained from the ethyl acetate extract of the D. innoxia leaves.
Fractions of extracts with a yield ≥1.23% (77.5 mg) were assayed for antitubercular activity in cultures of M. tuberculosis H37Ra over a range of concentrations (200 μg/mL to 3.15 μg/mL). Fractions F05, F06, F10, and F12 significantly inhibited Mtb growth at 200 μg/mL (Table 1).
3.1.1 Preparative TLC fractionation
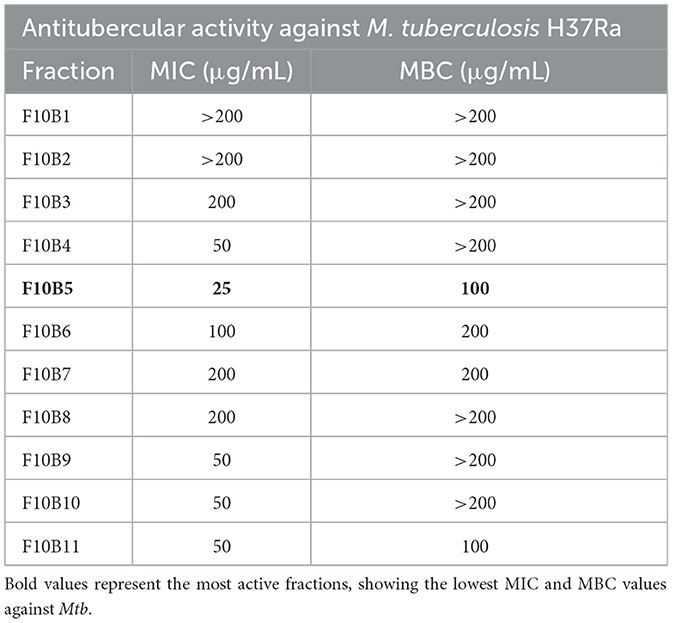
Fraction F10, which had the highest total activity, was further purified by preparative TLC, yielding 11 distant bands under UV visualization (Supplementary Figure 15). All bands were individually scraped from the plates, extracted with methanol, the solutions were evaporated, and the residues were assayed for antitubercular activity in M. tuberculosis H37Ra cultures. Fraction F10B5 exhibited the highest antitubercular activity (MIC = 25 μg/mL; MBC = 100 μg/mL) (Table 2).
3.1.2 Reverse-phase column fractionation
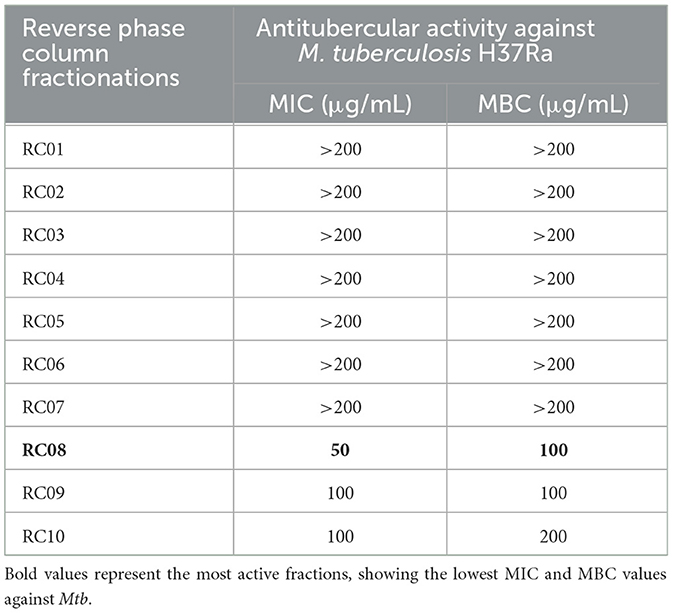
Fraction F10B5 was purified on a reverse-phase column, resulting in 10 sub-fractions, which were assayed for antitubercular activity. Fraction RC08 exhibited the most active MIC and MBC values at 50 μg/mL and 100 μg/mL, respectively (Table 3). The lower specific activity of RC08 relative to F10B5 may represent assay variability or removal of a co-activator or other active components.
3.2 Characterization and metabolomic profiling of the RC08 fraction of D. innoxia leaf extract
Fraction RC08 was subjected to metabolic profiling using LC-MS/MS. The reverse-phase LC column and gradient conditions used in LC-MS/MS resulted in much better separation and resolution than the step gradient open column used in the previous fractionation step. An example of a chromatogram obtained in positive ion mode is given in Figure 3, and one in negative ion mode is given in Figure 4. Additional chromatograms are provided in Supplementary Figures 1–14. The data analysis in this study identified 16 known compounds, including 12 compounds in the positive ion mode spectrum (Table 4) and four compounds in the negative ion spectrum (Table 5) based on the high-resolution mass spectrum (which identifies the molecular weight and elemental composition) and the electron impact spectra by comparison with available databases. The structures of the identified metabolites are given in Figure 5.
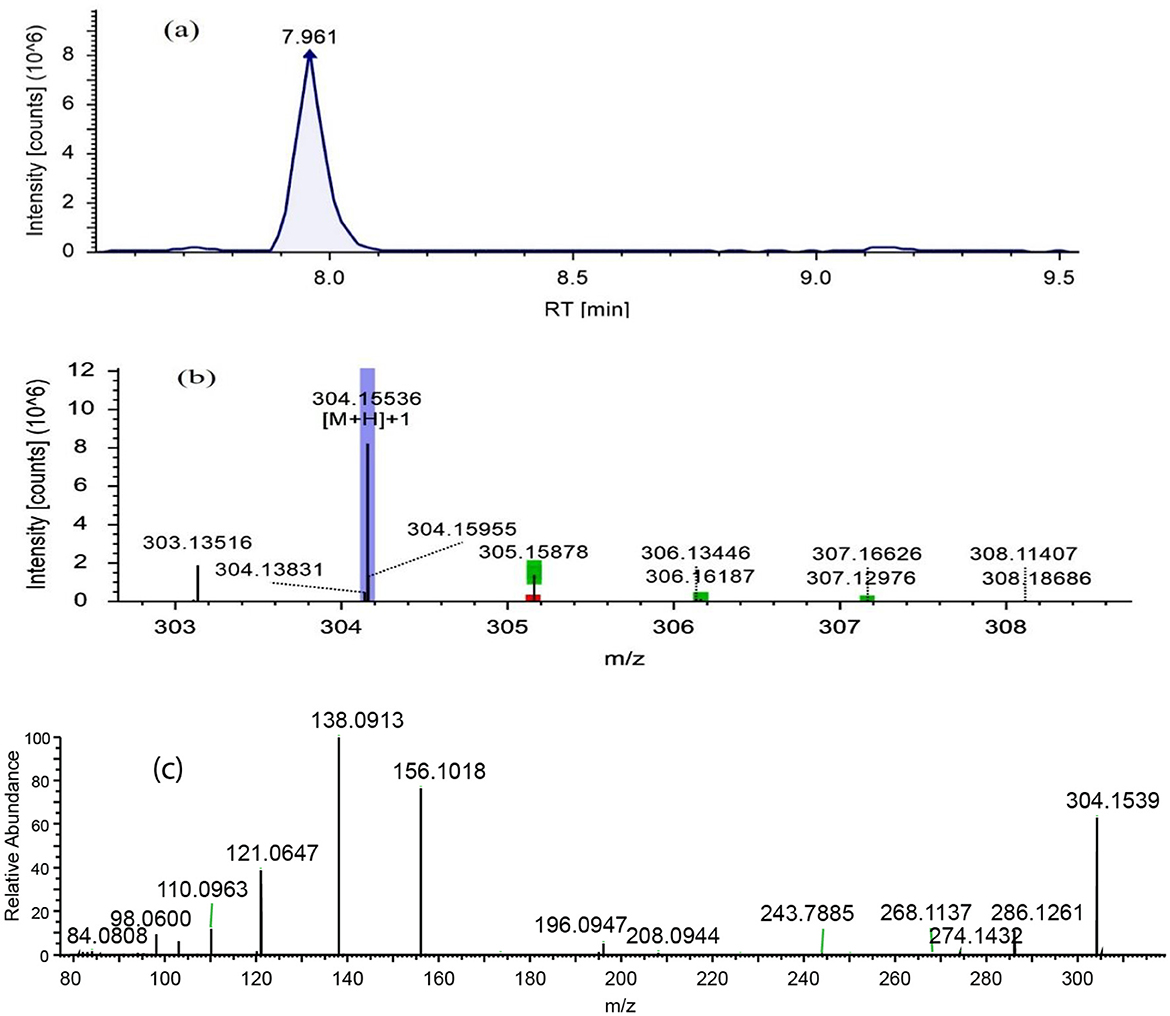
Figure 3. LC-ESI-MS/MS chromatograms of fraction RC08 in positive ion mode were used for the detection of scopolamine. (a) Extracted ion chromatogram, (b) high-resolution mass spectrum (MS1), and (c) fragmentation mass spectrum for the mass ion at m/z 304.15536 (MS2). RT, retention time.
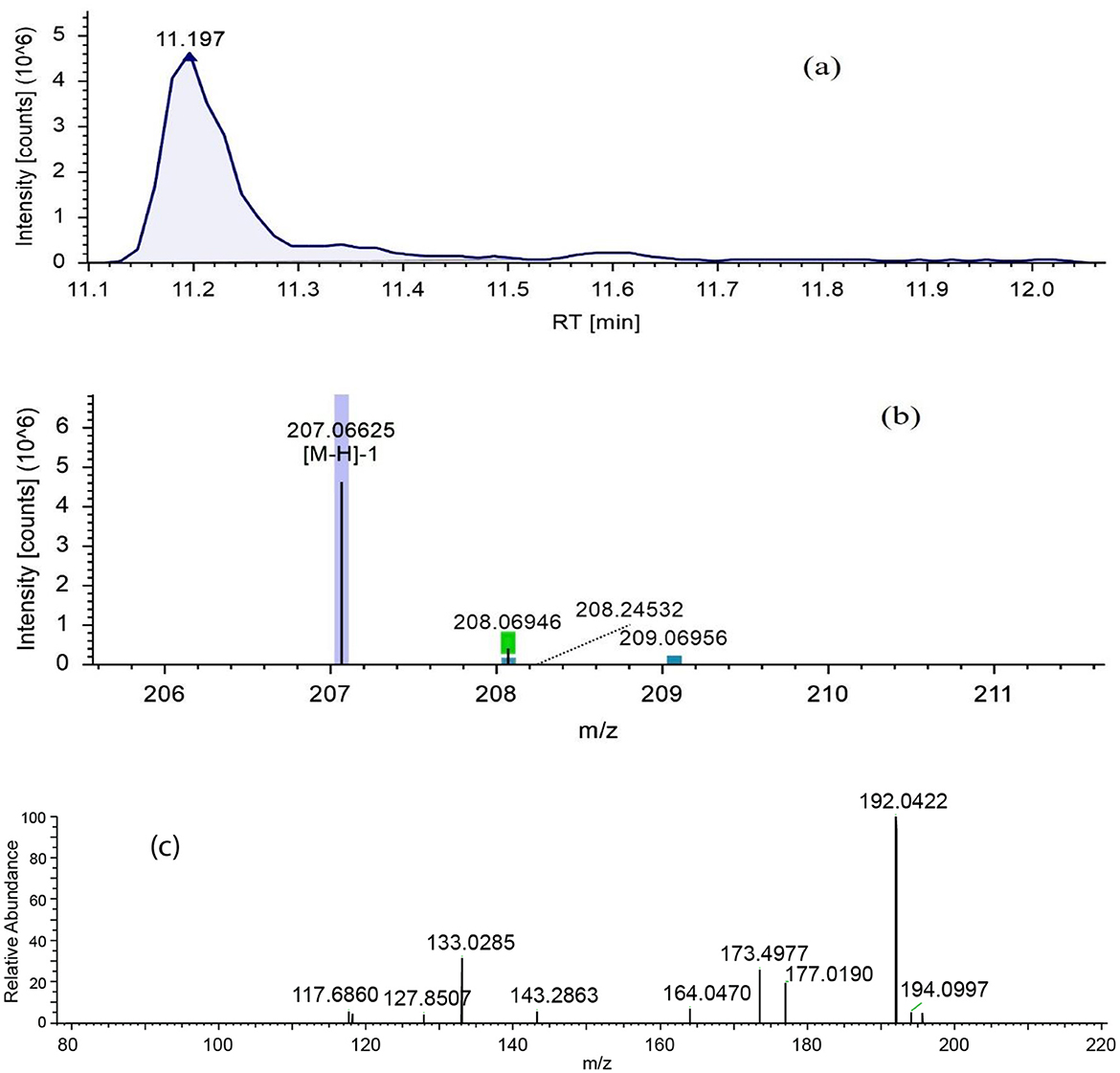
Figure 4. LC-ESI-MS/MS chromatograms of fraction RC08 in negative ion mode for the detection of methyl ferulate. (a) Extracted ion chromatogram, (b) high resolution mass spectrum (MS1), and (c) fragmentation mass spectrum for the mass ion at m/z 207.06625 (MS2). RT, retention time.
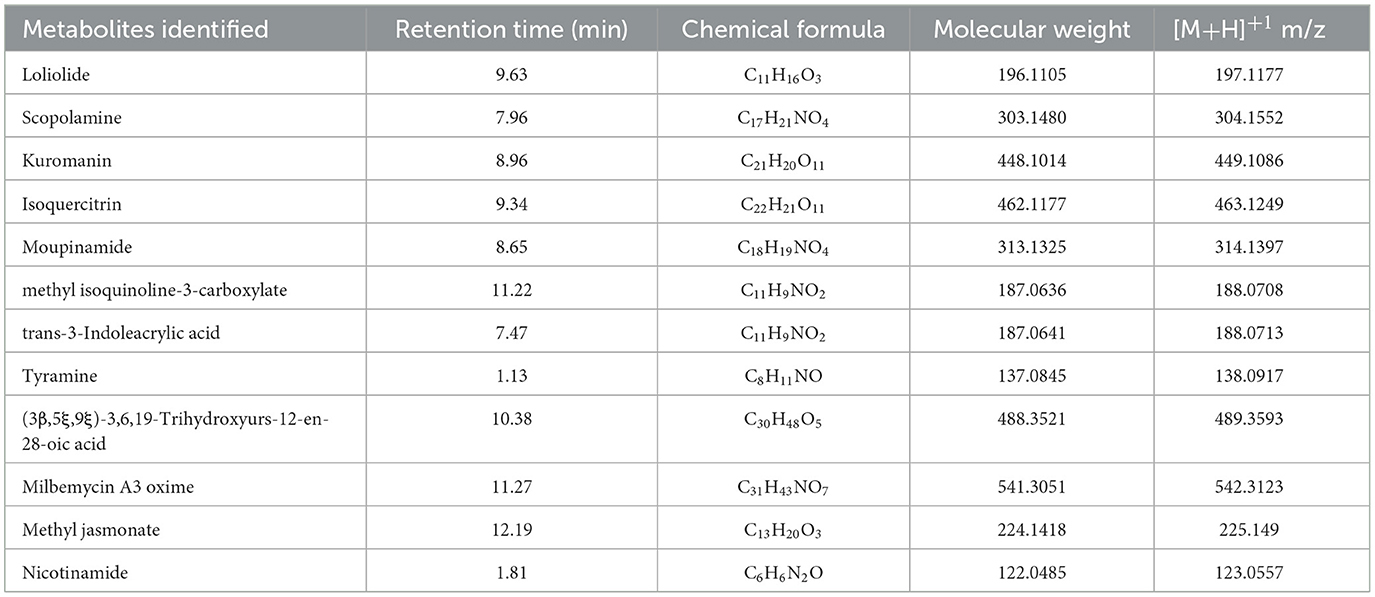
Table 4. Secondary metabolites identified in the RC08 fraction of Datura innoxia leaves by positive ion mode LC-MS/MS analysis.
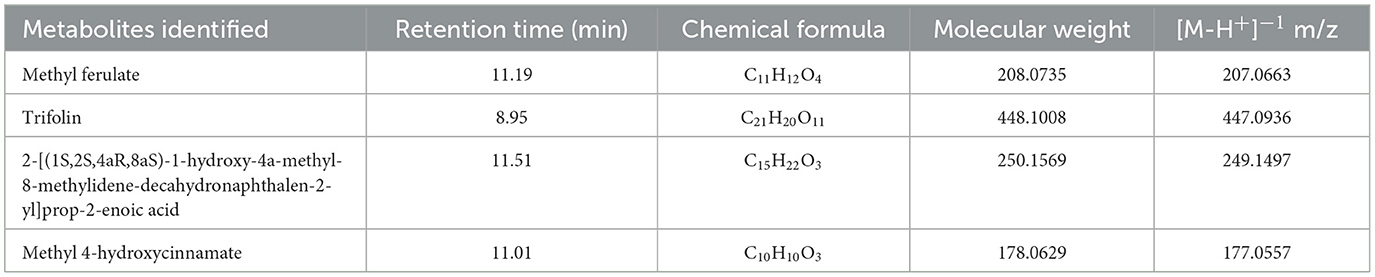
Table 5. Secondary metabolites identified in the RC08 fraction of Datura innoxia leaves by negative ion mode LC-MS/MS analysis.
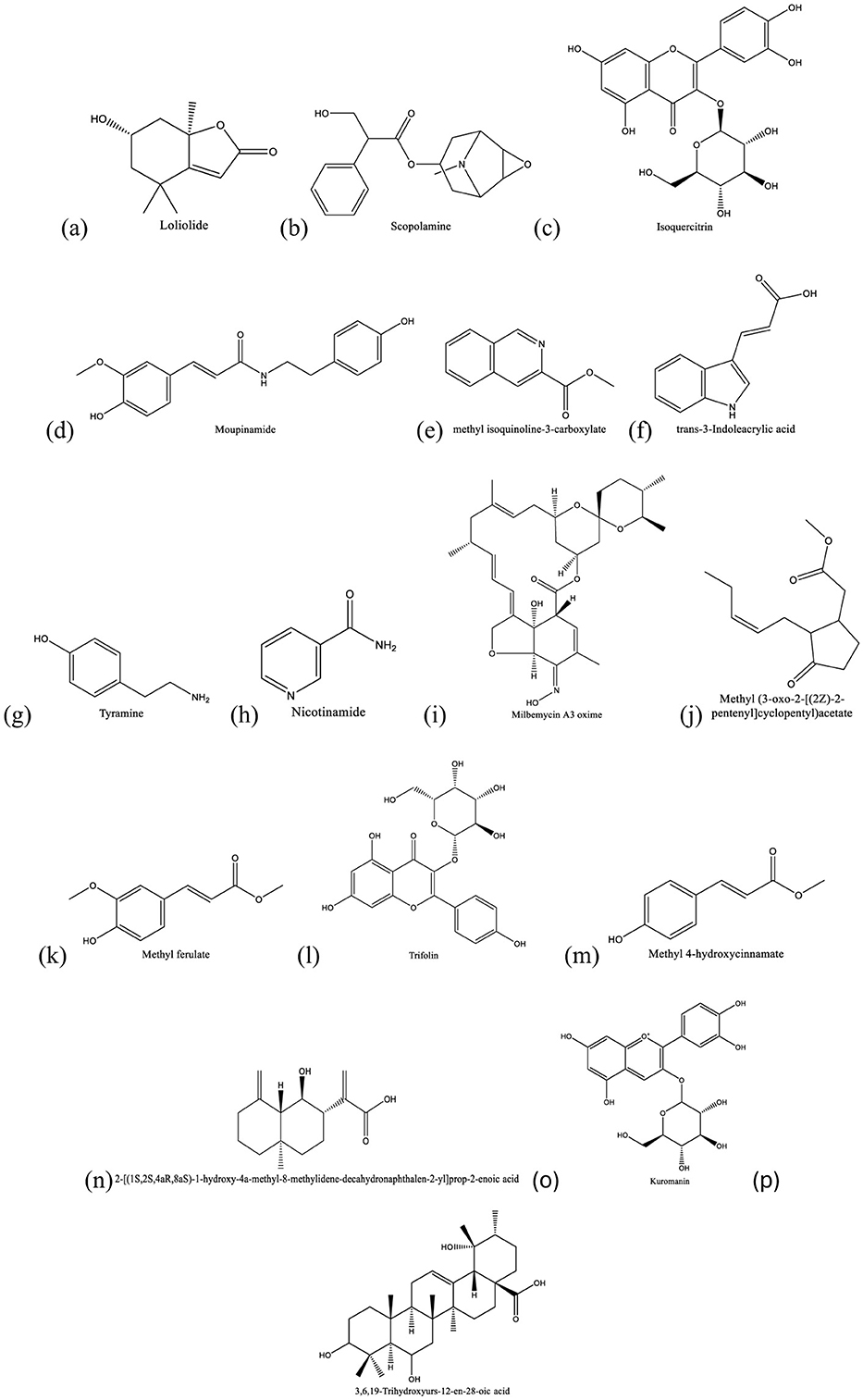
Figure 5. Structures of secondary metabolites identified by LC-ESI-MS/MS. (a) Loliolide, (b) scopolamine, (c) isoquercitrin, (d) moupinamide, (e) methyl isoquinoline-3-carboxylate, (f) trans-3-Indoleacrylic acid, (g) tyramine, (h) nicotinamide (i) milbemycin A3 oxime, (j) methyl jasmonate, (k) methyl ferulate, (l) trifolin, (m) methyl 4-hydroxycinnamate and (n) 2-[(1S,2S,4aR,8aS)-1-hydroxy-4a-methyl-8-methylidene-decahydronaphthalen-2-yl]prop-2-enoic acid, (o) kuromanin, and (p) (3β,5ξ,9ξ)-3,6,19-trihydroxyurs-12-en-28-oic acid.
4 Discussion
Natural products have a proven record for the development of new drugs, including potential anti-TB agents (Oosthuizen et al., 2019). In the present research, Datura species were screened for antimycobacterial potential in cultures of Mycobacterium tuberculosis H37Ra (Vilchèze et al., 2011). Numerous secondary metabolites with pharmaceutical potential have been found in Datura species (Alam et al., 2021; Al-Snafi, 2017). The most active of the Datura species extracts included in the study, that of D. innoxia, was subjected to bioassay-guided fractionation using solvent extraction and chromatographic techniques to reduce the number of inactive components and to reduce the potential for masking biological activity with impurities (Sytar and Smetanska, 2023).
Among the known compounds identified as secondary metabolites in D. innoxia was loliolide, a monoterpene lactone and benzofuran found in many plants, which exhibits various biological activities, including antifungal, antitumor, cytoprotective, antibiotic, antioxidant, antimalarial, and anticancer properties (Silva et al., 2021; Yang et al., 2011; Grabarczyk et al., 2015). Scopolamine is a tropane alkaloid belonging to the Solanaceae family of plants, including angel's trumpet, devil's trumpet, henbane, mandrake, deadly nightshade, and corkwood (Isopencu et al., 2023). Scopolamine, first approved by the U.S. Food and Drug Administration in 1979, is used to prevent motion sickness and postoperative nausea, acting by an anticholinergic mechanism (Swaminathan et al., 2020; Palazón et al., 2008). Moupinamide (N-trans-feruloyltyramine), which has been found in a variety of plants, including eggplant (Song et al., 2021), has some potential therapeutic activities, including inhibition of COX 1 and COX 2 (Park, 2009), stimulation of lipophagy by dihydroceramides (Lee et al., 2021) making it a possible non-alcoholic fatty liver disease therapeutic and cytotoxicity with SW480 cells (Villada Ramos et al., 2023). Kaempferol 3-O-galactoside (trifolin), a member of the flavonol group, has been extracted from medicinal plants and reported to have anticancer effects against promyelocytic leukemia, histiocytic lymphoma, skin melanoma, and lung cancer (Imran et al., 2019). Tyramine has peripheral cardiovascular effects when orally ingested, making it potentially useful for treating hypotension (Blob et al., 2007). Methyl 4-hydroxycinnamate is found in a variety of plants and has potential therapeutic applications as a melanin synthesis inhibitor, anti-inflammatory agent, and antifungal agent (Roulier et al., 2020). Trans-3-indoleacrylic acid is found in a wide variety of plant sources, such as canola straw, and is of interest as an algaecide (Effiong et al., 2022). Trans-3-indoleacrylic acid is also produced by gut bacteria, which facilitates the development of colorectal cancer (Cui et al., 2024). Other metabolites identified in the D. innoxia extract were primary plant metabolites, including nicotinamide, methyl jasmonate, and methyl ferulate. Primary plant metabolites that co-purify with the antitubercular activity in D. innoxia extract would be expected to be detected and identified.
The only secondary metabolite identified in the D. innoxia extract with reported antitubercular activity is milbemycin oxime (Muñoz-Muñoz et al., 2021). Milbemycin oxime has been reported to be more active against M. tuberculosis and other Mycobacterium species than other milbemycins or closely related avermectins, with an MIC lower than 8 μg/mL (Lim et al., 2013). Milbemycin oxime is produced by Streptomyces hygroscopicus subspecies aureolacrimosus (Takiguchi et al., 1983) and has not been reported to be produced by D. innoxia. Examination of the total ion flow in the chromatogram at the milbemycin oxime peak indicated that it was present only in trace amounts in the D. innoxia extracts. Assessment of the antitubercular activity of a series of pure, commercially available authentic standards for components identified in the D. innoxia extract and their amounts in the various purification fractions indicated that antitubercular activity was co-purified primarily with trans-3-indoleacrylic acid (manuscript in preparation). The origin of milbemycin oxime in D. innoxia extracts is unknown. Antibiotic production by endophytes has been widely observed (Martinez-Klimova et al., 2017), and milbemycin has been reported to be produced by the endophytic fungus Penicillium citrinum in the Indian medicinal plant, Azadirachta indica (Kumari et al., 2021). Extensive additional studies would be required to determine if milbemycin oxime could have been produced in sufficient amounts by an endophytic microbe with the required biosynthetic gene cluster; by the D. innoxia plant (if its genome includes the required biosynthetic gene cluster); or as a contaminant on the leaf surface before collection or during drying.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
SK: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Visualization, Writing – original draft, Writing – review & editing. MR: Formal analysis, Investigation, Methodology, Resources, Supervision, Validation, Writing – review & editing. MS: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. ZJ: Formal analysis, Investigation, Methodology, Software, Writing – review & editing, Resources. AB: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. SI: Writing – review & editing, Formal analysis, Methodology, Validation. SQ: Writing – review & editing, Conceptualization, Data curation, Investigation, Methodology. ST: Supervision, Writing – review & editing, Formal analysis, Resources, Validation. MK: Writing – review & editing, Data curation, Formal analysis, Investigation, Methodology, Resources, Software. PV: Writing – review & editing, Data curation, Investigation, Resources, Software. WS: Formal analysis, Resources, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors are grateful to Prof. Dr. Rehmatullah Qureshi for his valuable assistance in plant identification. The authors also acknowledge the financial support from the Higher Education Commission of Pakistan under the International Research Support Initiative Program (IRSIP) and NRPU Projects 10448 and 10406 for this research. The authors appreciate the technical support provided by the National Reference Laboratory for Tuberculosis, the National TB Control Program, Islamabad, Pakistan, and the National Center for Industrial Biotechnology (NCIB), PMAS-AAUR, for this project.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2025.1553282/full#supplementary-material
References
Abate, G., Mshana, R. N., and Miörner, H. (1998). Evaluation of a colorimetric assay based on 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) for rapid detection of rifampicin resistance in Mycobacterium tuberculosis. Int. J. Tuberc. Lung Dis. 2, 1011–1016.
Ahmed, W., Mansoor, Q., Ahmad, M. S., Zainab, T., and Shah, M. A. (2023). TRAIL. mediated apoptosis ruling and anticancer trigger by fine-tuned nano spheres of Fagonia cretica methanolic extracts as novel cancer regime. Sci. Rep. 13:671. doi: 10.1038/s41598-023-27441-6
Akinyemi, K. O., Oladapo, O., Okwara, C. E., Ibe, C. C., and Fasure, K. A. (2005). Screening of crude extracts of six medicinal plants used in South-West Nigerian unorthodox medicine for anti-methicillin resistant Staphylococcus aureus activity. BMC Complement. Altern. Med. 5:6. doi: 10.1186/1472-6882-5-6
Alam, W., Khan, H., Khan, S. A., Nazir, S., and Akkol, E. K. (2021). Datura metel: a review on chemical constituents, traditional uses and pharmacological activities. Curr. Pharm. Des. 27, 2545–2557. doi: 10.2174/1381612826666200519113752
Al-Snafi, A. (2017). Medical importance of Datura fastuosa (syn: Datura metel) and Datura stramonium -a review. IOSR J. Pharm. 7, 43–58. doi: 10.9790/3013-0702014358
Blob, L. F., Sharoky, M., Campbell, B. J., Kemper, E. M., Gilmor, M. G., VanDenberg, C. M., et al. (2007). Effects of a tyramine-enriched meal on blood pressure response in healthy male volunteers treated with selegiline transdermal system 6 mg/24 hour. CNS Spectr. 12, 25–34. doi: 10.1017/S1092852900020496
Cazzaniga, G., Mori, M., Chiarelli, L. R., Gelain, A., Meneghetti, F., Villa, S., et al. (2021). Natural products against key Mycobacterium tuberculosis enzymatic targets: Emerging opportunities for drug discovery. Eur. J. Med. Chem. 224, 113732. doi: 10.1016/j.ejmech.2021.113732
Chin, K. L., Anibarro, L., Sarmiento, M. E., and Acosta, A. (2023). Challenges and the way forward in diagnosis and treatment of tuberculosis infection. Trop. Med. Infect. Dis. 8:89. doi: 10.3390/tropicalmed8020089
Choudhari, S. M., Ananthanarayan, L., and Singhal, R. S. (2009). Purification of lycopene by reverse phase chromatography. Food Bioproc. Technol. 2, 391–399. doi: 10.1007/s11947-008-0054-1
Chunrong, L., Weiguo, T., Puxuan, L., and Haopeng, W. (2023). 2023 WHO Tuberculosis Report: key data analysis for China and the global world. Electr. J. Emer. Infect. Dis. 8:73. doi: 10.19871/j.cnki.xfcrbzz.2023.06.014
Cui, W., Guo, M., Liu, D., Xiao, P., Yang, C., Huang, H., et al. (2024). Gut microbial metabolite facilitates colorectal cancer development via ferroptosis inhibition. Nat. Cell Biol. 26, 124–137. doi: 10.1038/s41556-023-01314-6
Effiong, K., Hu, J., Xu, C., Zhang, Y., Yu, S., Tang, T., et al. (2022). 3-Indoleacrylic acid from canola straw as a promising antialgal agent - Inhibition effect and mechanism on bloom-forming Prorocentrum donghaiense. Mar. Pollut. Bull. 178:113657. doi: 10.1016/j.marpolbul.2022.113657
Flamini, R. (2013). Recent applications of mass spectrometry in the study of grape and wine polyphenols. ISRN Spectrosc. 2013:813563. doi: 10.1155/2013/813563
Gaire, B. P., and Subedi, L. (2013). A review on the pharmacological and toxicological aspects of Datura stramonium L. J. Integr. Med. 11, 73–79. doi: 10.3736/jintegrmed2013016
Grabarczyk, M., Wińska, K., Maczka, W., Potaniec, B., and Anio,ł, M. (2015). Loliolide - the most ubiquitous lactone. Acta Univ. Lodziensis Folia Biol. Oecol. 11, 1–8. doi: 10.1515/fobio-2015-0001
Houghton, P. J. (2001). Old yet new—pharmaceuticals from plants. J. Chem. Educ. 78, 175. doi: 10.1021/ed078p175
Imran, M., Salehi, B., Sharifi-Rad, J., Aslam Gondal, T., Saeed, F., Imran, A., et al. (2019). Kaempferol: a key emphasis to its anticancer potential. Molecules 24:2277. doi: 10.3390/molecules24122277
Isopencu, G. O., Covaliu-Mierlă, C. I., and Deleanu, I-, M. (2023). From plants to wound dressing and transdermal delivery of bioactive compounds. Plants 12:2661. doi: 10.3390/plants12142661
Jabeen, A., Mansoor, Q., Zainab, T., Farooqi, A. A., Qayyum, M., Mansha, A., et al. (2022). Natural plant extracts mediated expression regulation of TGF-β receptors and SMAD genes in human cancer cell lines. Mol. Biol. Rep. 49, 4171–4178. doi: 10.1007/s11033-022-07250-2
Kallassy, H. (2017). Phytochemistry and biological activities of selected Lebanese plant species (Crataegus azarolus L. and Ephedra campylopoda). Human health and pathology. Université de Limoges; Université Libanaise.
Karimi, S. M. (2023). An evidence-based review of medicinal plants for tuberculosis management cited by Avicenna. CABI 2023, 313–331. doi: 10.1079/9781800621671.0009
Kumari, P., Singh, A., Singh, D. K., Sharma, V. K., Kumar, J., Gupta, V. K., et al. (2021). Isolation and purification of bioactive metabolites from an endophytic fungus Penicillium citrinum of Azadirachta indica. South African J. Botany 139, 449–457. doi: 10.1016/j.sajb.2021.02.020
Lakusic, D., Rat, M., Anačkov, G., and Jovanovi,ć, S. (2017). Datura inoxia Mill. (Solanaceae), a new alien species in Serbia. Biol. Nyssana. 8, 47–51.
Lee, S.-H., Veeriah, V., and Levine, F. (2021). Liver fat storage is controlled by HNF4α through induction of lipophagy and is reversed by a potent HNF4α agonist. Cell Death Dis. 12:603. doi: 10.1038/s41419-021-03862-x
Lim, L. E., Vilchèze, C., Ng, C., Jacobs, W. R., Ramón-García, S., Thompson, C. J., et al. (2013). Anthelmintic avermectins kill Mycobacterium tuberculosis, including multidrug-resistant clinical strains. Antimicrob. Agents Chemother. 57, 1040–1046. doi: 10.1128/AAC.01696-12
Maluleka, J., and Ngoepe, M. (2018). Integrating traditional medical knowledge into mainstream healthcare in Limpopo Province. Inf. Dev. 35:026666691878594. doi: 10.1177/0266666918785940
Marealle, A., Qwarse, M., Innocent, E., Nondo, R., Machumi, F., Andrae-Marobela, K., et al. (2023). Bioassay-guided isolation of antimycobacterial compounds from Aphloia theiformis (Vahl) Benn root ethanolic extract. Phytomed. Plus 3:100406. doi: 10.1016/j.phyplu.2023.100406
Martin, A., Morcillo, N., Lemus, D., Montoro, E., Telles, M. A., Simboli, N., et al. (2005). Multicenter study of MTT and resazurin assays for testing susceptibility to first-line anti-tuberculosis drugs. Int. J. Tuberc. Lung Dis. 9, 901–906.
Martinez-Klimova, E., Rodríguez-Peña, K., and Sánchez, S. (2017). Endophytes as sources of antibiotics. Biochem. Pharmacol. 134, 1–17. doi: 10.1016/j.bcp.2016.10.010
Mpeirwe, M., Mugisha, T., Orikiriza, P., Ogwang, P., Ssesazi, D., Bazira, J., et al. (2023). Anti-mycobacterial activity of medicinal plant extracts used in the treatment of tuberculosis by traditional medicine practitioners in Uganda. Open Access. 01, 33–42. doi: 10.4236/pp.2023.142003
Muñoz-Muñoz, L., Shoen, C., Sweet, G., Vitoria, A., Bull, T. J., Cynamon, M., et al. (2021). Repurposing avermectins and milbemycins against mycobacteroides abscessus and other nontuberculous mycobacteria. Antibiotics 10:381. doi: 10.3390/antibiotics10040381
Nimbeshaho, F., Mwangi, C., Orina, F., Meryl, C., Adipo, N., Jones, O., et al. (2020). Antimycobacterial activities, cytotoxicity and phytochemical screening of extracts for three medicinal plants growing in Kenya. J. Med Plants Res. 14, 129–143. doi: 10.5897/JMPR2020.6905
Oosthuizen, C. B., Gasa, N., Hamilton, C. J., and Lall, N. (2019). Inhibition of mycothione disulphide reductase and mycobacterial biofilm by selected South African plants. South African J. Botany. 120, 291–297. doi: 10.1016/j.sajb.2018.09.015
Palazón, J., Navarro-Ocaña, A., Hernandez-Vazquez, L., and Mirjalili, M. H. (2008). Application of metabolic engineering to the production of scopolamine. Molecules 13, 1722–1742. doi: 10.3390/molecules13081722
Park, J. B. (2009). Isolation and characterization of N-feruloyltyramine as the P-selectin expression suppressor from garlic (Allium sativum). J. Agric. Food Chem. 57, 8868–8872. doi: 10.1021/jf9018382
Rodriguez-Takeuchi, S. Y., Renjifo, M. E., and Medina, F. J. (2019). Extrapulmonary tuberculosis: pathophysiology and imaging findings. Radiographics 39, 2023–2037. doi: 10.1148/rg.2019190109
Roulier, B., Pérès, B., and Haudecoeur, R. (2020). Advances in the design of genuine human tyrosinase inhibitors for targeting melanogenesis and related pigmentations. J. Med. Chem. 63, 13428–13443. doi: 10.1021/acs.jmedchem.0c00994
Sharifi-Rad, J., Salehi, B., Stojanović-Radi,ć, Z. Z., Fokou, P. V. T., Sharifi-Rad, M., Mahady, G. B., et al. (2020). Medicinal plants used in the treatment of tuberculosis - Ethnobotanical and ethnopharmacological approaches. Biotechnol. Adv. 44:107629. doi: 10.1016/j.biotechadv.2020.107629
Sharma, M., Dhaliwal, I., Rana, K., Delta, A. K., and Kaushik, P. (2021). Phytochemistry, pharmacology, and toxicology of datura species-a review. Antioxidant 10:1291. doi: 10.3390/antiox10081291
Silva, J., Alves, C., Martins, A., Susano, P., Simões, M., Guedes, M., et al. (2021). Loliolide, a New Therapeutic Option for Neurological Diseases? In vitro Neuroprotective and Anti-Inflammatory Activities of a Monoterpenoid Lactone Isolated from Codium tomentosum. Int. J. Mol. Sci. 22:1888. doi: 10.3390/ijms22041888
Song, Y., Mei, T., Liu, Y., Kong, S., Zhang, J., Xie, M., et al. (2021). Metabolites identification of chemical constituents from the eggplant (Solanum melongena L.) Calyx in Rats by UPLC/ESI/qTOF-MS Analysis and Their Cytotoxic Activities. Front. Pharmacol. 12:655008. doi: 10.3389/fphar.2021.655008
Swaminathan, S. K., Strasinger, C., Kelchen, M., Carr, J., Ye, W., Wokovich, A., et al. (2020). Determination of rate and extent of scopolamine release from transderm scop® transdermal drug delivery systems in healthy human adults. AAPS PharmSciTech. 21:117. doi: 10.1208/s12249-020-01658-4
Sytar, O., and Smetanska, I. (2023). Special Issue—“Bioactive Compounds from Natural Sources II”. Molecules 28:4450. doi: 10.3390/molecules28114450
Takiguchi, Y., Ono, M., Muramatsu, S., Ide, J., Mishima, H., and Terao, M. (1983). Milbemycins, a new family of macrolide antibiotics. Fermentation, isolation and physico-chemical properties of milbemycins D, E, F, G, and H. J. Antibiot. 36, 502–508. doi: 10.7164/antibiotics.36.502
Tuyiringire, N., Deyno, S., Weisheit, A., Tolo, C. U., Tusubira, D., Munyampundu, J. P., et al. (2020). Three promising antimycobacterial medicinal plants reviewed as potential sources of drug hit candidates against multidrug-resistant tuberculosis. Tuberculosis 124:101987. doi: 10.1016/j.tube.2020.101987
Vilchèze, C., Baughn, A. D., Tufariello, J., Leung, L. W., Kuo, M., Basler, C. F., et al. (2011). Novel inhibitors of InhA efficiently kill Mycobacterium tuberculosis under aerobic and anaerobic conditions. Antimicrob. Agents Chemother. 55, 3889–3898. doi: 10.1128/AAC.00266-11
Villada Ramos, J. A., Aguillón Osma, J., Restrepo Cortes, B., Loango Chamarro, N., and Maldonado Celis, M. E. (2023). Identification of potential bioactive compounds of Passiflora edulis leaf extract against colon adenocarcinoma cells. Biochem. Biophy. Rep. 34:101453. doi: 10.1016/j.bbrep.2023.101453
Keywords: Mycobacterium tuberculosis, Datura innoxia, antitubercular activity, LC-MS/MS, scopolamine, milbemycin A3 oxime
Citation: Khan SA, Rather MA, Sheeraz Ahmad M, Jia Z, Baughn AD, Iqbal S, Qadir SM, Tahseen S, Khan MU, Villalta PW and Shier WT (2025) Identification of Datura innoxia as a potential source of antimycobacterial components. Front. Microbiol. 16:1553282. doi: 10.3389/fmicb.2025.1553282
Received: 30 December 2024; Accepted: 15 May 2025;
Published: 01 July 2025.
Edited by:
Jichan Jang, Gyeongsang National University, Republic of KoreaReviewed by:
Laurent Roberto Chiarelli, University of Pavia, ItalySatya Deo Pandey, University of Louisville, United States
Copyright © 2025 Khan, Rather, Sheeraz Ahmad, Jia, Baughn, Iqbal, Qadir, Tahseen, Khan, Villalta and Shier. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Muhammad Sheeraz Ahmad, ZHIuc2hlZXJhekB1YWFyLmVkdS5waw==; W. Thomas Shier, c2hpZXIwMDFAdW1uLmVkdQ==; Anthony D. Baughn, YWJhdWdobkB1bW4uZWR1
 Sajjad Ahmed Khan
Sajjad Ahmed Khan Muzafar Ahmad Rather
Muzafar Ahmad Rather Muhammad Sheeraz Ahmad
Muhammad Sheeraz Ahmad Ziyi Jia
Ziyi Jia Anthony D. Baughn
Anthony D. Baughn Sajid Iqbal
Sajid Iqbal Syed Mehmood Qadir
Syed Mehmood Qadir Sabira Tahseen
Sabira Tahseen Muhammad Umer Khan
Muhammad Umer Khan Peter W. Villalta
Peter W. Villalta W. Thomas Shier
W. Thomas Shier