- 1Department of Biomedical and Biotechnological Sciences, University of Catania, Catania, Italy
- 2IRCCS Centro Neurolesi Bonino Pulejo, C.da Casazza, Messina, Italy
- 3Department of Ophthalmology, University of Catania, Catania, Italy
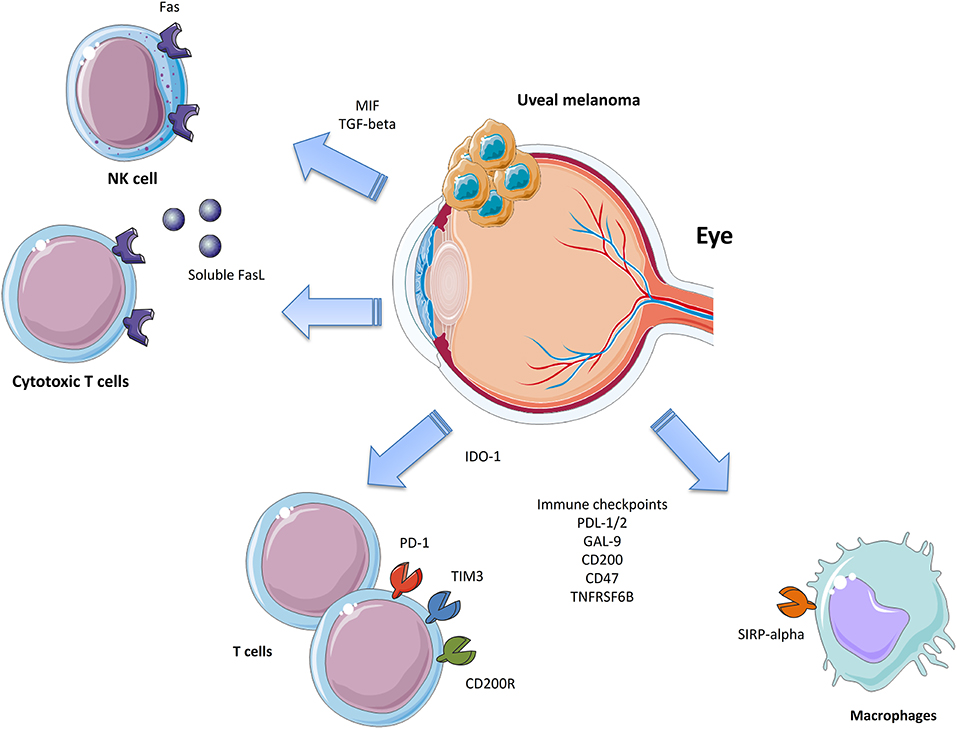
Uveal Melanoma (UM) represents the most common primary intraocular malignant tumor in adults. Although it originates from melanocytes as cutaneous melanoma, it shows significant clinical and biological differences with the latter, including high resistance to immune therapy. Indeed, UM can evade immune surveillance via multiple mechanisms, such as the expression of inhibitory checkpoints (e.g., PD-L1, CD47, CD200) and the production of IDO-1 and soluble FasL, among others. More in-depth understanding of these mechanisms will suggest potential targets for the design of novel and more effective management strategies for UM patients.
Introduction
Uveal melanoma (UM) is a malignant cancer of the eye that is thought to arise from the melanocytes within the uveal tract of the eye. It differs from cutaneous melanoma (CM), which arises from skin melanocytes, and has distinct clinical and biological features. UM, with an annual incidence of six cases per million, is the most common primary intraocular malignant tumor in adults. It mainly originates from the choroid (~85%), while the remaining cases arise from the ciliary body (5–8%) and the iris (3–5%) (1).
Cutaneous and uveal melanocytes have the same embryonic origin and cellular function, however, they undergo different tumoral transformation processes (2). The majority of CMs (~80%) present mutations in BRAF, NRAS, and NF1 genes (2). Instead, in UM, the most common mutations involve GNAQ/11 (83% of the cases) and recurrent alterations can be found on the BAP1 gene (~40%) (2). CM shows several cytogenetic alterations, involving loss of chromosomes 4, 5, 6q, 8p, 9p, 10q, 11q, 12q, 14, 15, 16, 21, and 22 (3), and gain of 1q, 6p, 7, 8q, 18, and 20q (4, 5). In UM, chromosomal aberrations mainly include monosomy 3 (50%) and 6p and 8q gain. UM tumors with monosomy 3 and polysomy 8q have high metastatic risk and a poor prognosis (6, 7). Ludmil and collaborators have shown that CM has the highest somatic mutation prevalence (8), while UM has low somatic mutation rates (9). It is believed that a high mutational burden is predictive of the response to immunotherapy (10), as the neoantigens that derive from tumor-specific mutations can be targets for anti-tumor immune responses. Therefore, the reduced number of neoantigens on UM cells may explain why immune-checkpoint inhibitors are insufficient in UM but can be effective in CM. However, as a low mutational load may also bring the activation of neoantigen-specific T cells (11, 12), it is reasonable to believe that the tumor microenvironment and intrinsic cancer cell phenotypic patterns may be pivotal in the regulation of the ability of T cells to respond to cancer-specific antigens.
In this review, we will discuss key aspects of the immunobiology of UM and potential novel immunotherapeutic targets.
The Eye: An Immune-privileged Site for Uveal Melanoma?
The eye has been proposed to be an immunologically privileged site, possibly providing UM with a protective niche. This protection has been attributed to cell surface molecules and soluble factors able to impair, weaken, or disturb the immune system. The immune privilege of the eye is instrumental to protecting ocular tissues and preserving vision from damage that may occur following inflammatory reactions (13, 14). Both physical and biochemical mechanisms maintains the immune privilege of the eye (13, 15, 16). The intraocular compartments are separated from the blood circulation by the blood-ocular-barrier, which comprises the blood-aqueous barrier and the blood-retinal barrier (15). The blood-aqueous barrier is made up of tight junctions between the endothelial cells of the ciliary blood vessels and between the lining epithelial cells (15). The aqueous humor is a transparent and colorless medium that is present in the anterior and posterior chambers of the eye. The aqueous humor is secreted by the ciliary epithelium and enters the posterior chamber. Afterwards, it flows around the lens and the pupil into the anterior chamber. Finally, the aqueous humor leaves the eye by passive flow at the anterior chamber angle, in the supraciliary and suprachoroidal space, through the choroidal vessels or through scleral pores (17, 18). In the early seminal work by Taylor and colleagues (19), it was found that primed T cells, activated in vitro in the presence of the aqueous humor, produced lower levels of IFN-γ and IL-4 with generation of TGF-β-producing regulatory T cells. TGF-β is an immunomodulatory cytokine primarily produced by Th3 cells that exhibits multiple immunosuppressive properties and has been shown to counteract immunoinflammatory and autoimmune responses both in vitro and in vivo (20, 21). Recent studies have indicated that, through its immunosuppressive properties exerted in the tumor microenvironment, TGF-β may play a pathogenic role in oncogenesis by suppressing anti-cancer cell-mediated immune responses. On this basis, much attention has recently been focused on the possibility that specific inhibitors of TGF-β, such as antibodies, antisense molecules, and small-molecule tyrosine kinase inhibitors, may represent novel therapeutic approaches for the treatment of certain forms of cancers, possibly including UM (22, 23). In addition, apart from being rich in TGF-β, other studies have demonstrated that the aqueous humor contains large amounts of the pleiotropic cytokine Macrophage Migration Inhibitory Factor (MIF), which promotes immune privilege by inhibiting NK cell activity (24), though MIF possesses proinflammatory properties that qualify it as an important mediator of several autoimmune diseases such as multiple sclerosis and Guillain Barrè syndrome (25, 26). Recent data also highlight that MIF can activate multiple oncogenic pathways, including the inhibition of p53, production of HIF-1α (Hypoxia-inducible factor 1-alpha), and activation of the PI3K/Akt/mTOR pathway. These observations have attracted much attention to the role of MIF in the pathogenesis of several types of cancer, including glioblastoma, melanoma, and head and neck cancer, among others, and on the possible use of specific MIF inhibitors in these diseases (27–30).
Other molecules that have been detected in the aqueous humor and could dampen anti-tumor immune responses include α-melanocyte-stimulating hormone (α-MSH), calcitonin gene-related peptide (CGRP), vasoactive intestinal peptide (VIP), and somatostatin, by which delayed-type hypersensitivity reactions are suppressed and Treg cell activity is induced (13, 31, 32).
Finally, iris and ciliary body epithelial cells are able to prevent T cell activation and proliferation via direct cell-to-cell contact (33).
The absence of afferent lymphatics also limits the homing of immune cells to and from the secondary lymphoid organs. However, studies by Camelo and colleagues have shown that, after intracameral and subconjunctival injection, antigens reach the ipsilateral head and neck lymph nodes via the conjunctival lymphatics, and that antigen administration into the anterior chamber is internalized by ocular Antigen Presenting Cells (APCs) and presented in a tolerogenic fashion in the spleen (34, 35). This is referred to as anterior chamber-associated immune deviation (ACAID). In ACAID, eye-derived APCs promote the expansion of tolerogenic B cells in order to induce invariant natural killer T cells and antigen-specific Tregs. In particular, afferent CD4+ Tregs act in the secondary lymphoid organs to suppress the initial activation and differentiation of naïve T cells into Th1 effector cells, while efferent CD8+ Tregs act in the eye, inhibiting the delayed hypersensitivity responses [reviewed by (36)].
Apparently, this condition of immune privilege should promote the incidence of intraocular tumors; however, as reported in the American Cancer Society 2008 statistics, UM is about 15 times less frequent than CM. Despite this, it is likely the UM may receive an advantage from the ocular immune privilege that, coupled to the acquisition of immune-regulatory properties, could eventually result in clinically relevant tumors.
After leaving the eye, the ability of UM cells to express pro-oncogenic molecules such as indoleamine dioxygenase-1 (IDO-1), MIF, and PD-L1 (37–39) re-establish their immune privilege and provide the possibility to set up metastatic disease.
Also, in contradiction with the immune surveillance hypothesis, in the case of UM, the immune system seems to promote cancer development, maintenance, and progression. Indeed, the presence of Tumor-Associated Lymphocytes (TILs) and Tumor-Associated Macrophages (TAMs) correlates with a poor prognosis (40–42).
It is of note that the choroid is located outside of the above-mentioned outer blood-retina barrier. Choroidal capillaries are fenestrated and very leaky (43, 44); therefore, the choroidal space is considered to be exposed to the systemic immune surveillance. It is possible that, once the primary choroidal melanoma grows and breaks the outer blood-retina-barrier, the tumor could utilize the immune suppressive mechanisms of the affected eye to tolerize the immune attacks against melanoma cells.
Immunobiology of Primary UM
UM cells express tumor-specific antigens, including the Melanoma Antigen Gene (MAGE) family proteins, premelanosome protein gp100, and tyrosinase (45–47), that are recognized by elements of the immune system. Accordingly, in vitro data show that circulating CD8+ CTL from UM patients or from primary UMs are able to lyse UM cells (48–50). NK cells are able to induce cytotoxicity in UM cell lines, such as OCM-3 (32). However, both the innate and adaptive effector immune responses can be circumvented by UM cells. Some of these strategies are common to those that provide the immune privilege to the eye. Indeed, the immune privilege is not absolute nor permanent, and it can be overcome as is shown by the development of uveitis and the rejection of corneal transplant.
On the other hand, preclinical data have demonstrated that the intraocular transplantation of ultraviolet light (UV)-induced tumors in syngeneic mice subjected to CTL-mediated rejection and that the adoptive transfer of CD8+ TILs in immune-deficient mice challenged with intraocular UV-induced tumors exhibited anti-cancer actions (51). Altogether, these data provide evidence that UM cells put in place specific immune escape mechanisms responsible for its progressive course and bad prognosis.
Resistance to Cell-Mediated Immune Responses
Natural killer (NK) cells have been shown to control the growth of liver metastases (52). Decreased tumor expression of Class I MHC molecules, ligands for NK inhibitor receptors, is associated with longer metastasis-free survival (53), while the loss of NK activator receptors (i.e., MIC-A and MIC-B) is associated with tumor progression (54). Cytotoxic T lymphocytes (CTL) and Natural Killer (NK) cells exert anti-tumor functions by inducing apoptosis via the activation of the death receptors of the TNF superfamily, including TNF-α, TRAIL, and FasL. However, UM cells are resistant to FasL-induced apoptosis (55). Indeed, the production of a soluble form of FasL from UM cells protects UM cells from apoptosis as, by acting in an autocrine manner, it binds Fas expressed by UM cells themselves, blocking the engagement of Fas expressed on CTL and NK cells, which, given its trimeric structure, is more than 1,000 times more efficient in inducing apoptosis (55).
Moreover, as previously stated, the aqueous humor contains TGF-β and MIF, which have profound inhibitory effects on NK cells (24). In particular, TGF-β and MIF act sequentially to dampen NK function as MIF provides immediate inhibition (24), while TGF-β exerts long-term inhibitory function (56).
The presence of TILs and TAMs in UMs correlates with a poor prognosis (40–42). Although this observation is unexpected and contrasts with data from other cancer types, there may be multiple reasons for this peculiar UM feature. One possible explanation is that the production of pro-inflammatory cytokines, and in particular IFN-γ, is able to induce the upregulation of MHC class I molecules, which help UM cells to escape from NK cytolysis and promote the expression of IDO-1 and of inhibitory immune checkpoints, e.g., PD-L1. Another and not mutually exclusive explanation is that the IL-2 secreted by infiltrating lymphocytes may have a proliferative effect on UM cells as well. It has been found that UM cells express the receptor for IL-2 and IL-15, which may promote their survival and growth and deplete essential factors for the action and proliferation of both T and NK cells (57). Moreover, IFN-γ can sustain cancer growth by inducing a downregulation of tumor antigens (58, 59).
However, the mechanisms regulating the cancer-microenvironment crosstalk remain elusive. In a recent study by Rothermel et al. (60), analysis of TILs cultures from cutaneous and UM showed that UM TILs were predominantly CD4+, while in CM were mainly composed of CD8+ T cells. Also, the absence of melanin pigmentation in the primary tumor was strongly correlated with highly reactive UM TILs. It is believed that UM cells interact with infiltrating cells and skew their phenotype to an immune-regulatory type. Recent studies have identified the presence of CD4+ and Forkhead box P3 (FoxP3)+ Treg cells within primary UMs, and their frequency has been found to correlate with metastatic dissemination (61, 62). In patients with primary UM, while circulating anti-tumor CD3–CD56dim NK cells and CD8+ and double-negative CD3+CD56+ NK-T cells decrease, pro-tumoral ICOS+CD4+FoxP3+ Treg cells increase (63), further supporting a role for Treg in tumor progression. The striking correlation between tumor size and high metastatic risk primary UMs infiltrated by CD8+ T cells seems to suggest that UM may promote the generation of CD8+ Tregs (41, 64). Accordingly, Streilein and Niederkorn showed that elimination of CD8+ Treg in a murine model of UM was sufficient to induce tumor rejection (65). It has also been found that patients with primary UMs and liver metastases bear increased frequencies of circulating CD11b+CD15+ cells, which could represent immunosuppressive myeloid-derived suppressor cells (63, 66). Interestingly, untreated metastatic UM (and breast cancer patients, as well) have an increased percentage of circulating CD127–CD25–CD4+ T cells in the blood, as compared to healthy people. This cell population, considered to be “chronically stimulated” CD4+ T cells, shares features observed in anergic cells from tumor-bearing mice, i.e., reduced proliferation ability and diminished cytokine production. Accordingly, these cells have significant transcriptome overlapping that mirrors that of mouse anergic cells (67).
An increased body of data is accumulating for IDO-1 as an evading mechanism put in place by cancer to elude the immune surveillance (68). A potential role for IDO-1 has already been described in several tumor types, including colorectal cancer (69), hepatocarcinoma (70), endometrial cancer (71), and CM (72). T lymphocytes require the amino acid tryptophan for survival and clonal expansion. The enzyme IDO-1 catalyzes the rate-limiting step in tryptophan catabolism, which leads to the oxidation of L-tryptophan to N-formylkynurenine. IDO-1 is expressed by the retina, iris/ciliary body, lens, and cornea (73, 74). Although, Chen and colleagues failed to observe the expression of IDO-1 in both primary UM samples and in liver UM metastases (75), UM cell lines exposed in vitro to IFN-γ, significantly upregulate IDO-1 expression (75). These data suggest a potential role for IDO-1 as an immune escape mechanism. Despite these data, the role of IDO-1 in metastatic UM remains questionable, as specific anti-IDO-1 strategies have yet to prove efficacy in UM patients.
Closely related to IDO-1, tryptophan 2,3-dioxygenase (TDO) is a heme-containing enzyme, encoded by the TDO2 gene. Terai et al. (76) have recently reported that TDO2 mRNA is expressed by 62% of primary UM and correlates with a poor prognosis. Also, the Authors show that TDO expression is upregulated by 3.5-fold upon in vitro stimulation of UM cells with recombinant TNF-alpha. These observations point to a complementary and, possibly, overlapping role of TDO and IDO-1 in the immune-evading strategies of advanced UM, and, therefore, novel pharmacological interventions aimed at inhibiting the kynurenine pathway, targeting both enzymes simultaneously, are strongly warranted.
Inhibitory Immune Checkpoints in Primary Uveal Melanoma
The immune system uses a diverse set of antigens to distinguish tumor cells from their healthy cells. The amplitude of the T cell response is regulated by both co-stimulatory and inhibitory molecules, known as “immune checkpoints,” which are essential for the maintenance of self-tolerance. In cancer, multiple inhibitory checkpoints may be modulated, including programmed death ligand-1/2 (PD-L1/2), CD47, Galectin 9, and TNFRSF6B, for which ligands expressed on T cells or APCs may act synchronously or sequentially to promote overall suppression of the immune responses (77). Robertson et al. (78), by performing a multiplatform analysis of 80 primary UM samples from the TGCA dataset, identified four distinct UM subtypes, two with poor prognosis monosomy of chromosome 3 (named M3) and two with better prognosis disomy of chromosome 3 (named D3). Deconvolution analysis of both DNA methylation and RNA-seq data revealed that a CD8+ T cell infiltrate was present in ~30% of M3 samples, whereas it was almost absent in D3 samples. Also, they found that genes co-expressed with CD8A were associated with immunosuppression (IDO1, TIGIT, IL6, IL10, and FOXP3), T cell migration (CXCL9 and CXCL13), cell-mediated cytotoxicity (PRF1 and GZMA), and interferon-γ signaling (IFNG, IFNGR1, and IRF1). Moreover, HLA expression was higher in M3 samples as compared to D3 samples and correlated with CD8A expression (78). Accordingly, Maat and colleagues, in a comparative immunohistochemical analysis of M3 and D3 samples, observed that M3 tumors have a significantly higher number of infiltrating macrophages and express higher levels of MHC class I and II (79).
Elucidation of the complex network of stimulatory and inhibitory signals that contribute to immune regulation and its dysregulation in cancer may lead to more effective therapeutic opportunities to enhance anti-tumor immune responses.
PD-1/PD-L1
PD-1 is expressed on T lymphocytes and has the two ligands, PD-L1 (also known as B7-H1 or CD274) and PD-L2, that belong to the B7 superfamily. Both are expressed on APCs and cancer cells. A lack of expression of PD-L1 has been observed in primary UM. Yang and collaborators (80) found that PD-L1 was not expressed by primary UM in situ, and, similarly, Kaunitz et al. (81) observed that only 10% of UM samples expressed PD-L1. Interestingly, when present, the expression of PD-L1 on tumor cells was mainly associated with the presence of CD8+ T-lymphocytes, consistent with an adaptive mechanism of expression. This is in line with the observation that, in UM cell lines, derived from primary tumors PD-L1 and PD-L2, expression significantly increased under inflammatory conditions. Also, PD-L1 was found to inversely correlate with OS, PFS, and thickness of the tumor (82).
CD47
CD47 is an immunoglobulin-like domain containing protein expressed by the tumor cell surface that inhibits macrophage phagocytosis by binding the signal regulatory protein α (SIRPα) on APCs. CD47 downregulation is associated with macrophage phagocytosis of senescent or damaged cells. On the contrary, upregulation of CD47 inhibits phagocytosis. The interaction between CD47 and SIRPα activates the tyrosine phosphorylation of the cytoplasmic region of SIRPα, thus recruiting the tyrosine phosphatase, SHP-2, which acts by dephosphorylating its substrates, and functions as a negative signaling regulator. CD47 is overexpressed in many different cancer cell types and represents an independent negative prognostic factor (83, 84). We have shown that UM cells lines dramatically upregulate CD47 expression after incubation with an activated T cell supernatant, and that higher levels of CD47 were associated with significantly lower disease-free survival time. Accordingly, the expression of CD47 in primary UM samples was an independent predictor of recurrence disease (82). In UM, we also found that CD47 levels did not significantly change in the different stages of the disease, and that patients with the lowest expression of CD47 had improved progression-free survival (PFS), even after correcting for the presence of BAP1, GNAQ, and GNA11 mutations (85). Interestingly, deconvolution analysis of infiltrating immune cell populations showed a significantly higher proportion of CD4+ and CD8+ T cells in patients with high CD47 levels, with the most represented populations being the Th2, Treg, and CD8+ TCM cells (85). Finally, we demonstrated that a large number of transcripts are differentially expressed between tumors expressing high and low levels of CD47, with a significant enrichment of interferon IFN-alpha regulated genes (85).
CD200
CD200 (also known as OX-2) is a type 1a glycoprotein, capable of modulating the immune system via its inhibitory receptor CD200R, which is expressed on both myeloid and lymphoid cells. It contains two extracellular immunoglobulin domains and a small intracellular domain with no known signaling motif. CD200R expression, the cognate ligand for CD200, is mainly restricted to the myeloid lineage of cells (86). Accordingly, CD200-deficient mice show hyperactivation of macrophages and enhanced inflammation in autoimmune disease models (87). CD200 has been found to be a good predictor of recurrent disease in UM (82).
Gal9 and TNFRSF6B
A potential prognostic role for GAL9 and TNFRSF6B has also been recently evaluated (82). Higher levels of expression of these proteins have been associated with a better PFS. Galectin 9 is protein encoded by the gene GAL9 that, interacting with its cognate receptor, TIM-3, is able to inhibit Th1 responses, triggering the apoptosis of CTLs and increasing Tregs suppressive activity. Conversely, it was shown in a preclinical model of melanoma that GAL9 increased the NK-mediated cytolysis of cancer cells. Accordingly, a recent meta-analysis on solid cancer patients showed that higher levels of GAL9 correlated with improved OS, reduced depth of invasion, and negative distal tumor dissemination (88).
TNFRSF6B belongs to the tumor necrosis factor receptor superfamily and functions as a decoy receptor for FasL, tumor necrosis factor-like ligand 1A (TL1A), and lymphotoxin analogs (LIGHT). TNFRSF6B expression correlated with reduced OS in patients with solid tumors, but it did not influence recurrence-free survival (89). Along the same lines, higher levels of TNFRSF6B were associated with longer PFS in UM (82).
Nitric Oxide
Nitric oxide (NO) is an endogenous gas produced from neural, constitutive, or inducible nitric oxide synthases (NOS) from L-arginine. Together with Hydrogen Sulfide and Carbon Monoxide, NO represents the main gaseous endogenous system in the body. It is of interest that recent data indicate that these gas-signaling molecules play critical roles in regulating signal transduction and cellular homeostasis. Interestingly, through various administrations, these molecules also exhibit potential in cancer treatment (90, 91). As, out of the three gases, the role of NO in cancer and UM is the most widely known, we will briefly review the literature of NO in UM. NO plays pleiotropic biological functions ranging from blood pressure homoeostasis to the regulation of responses to infectious agents and modulation of immune responses and oncogenesis. Depending on the concentration and location of the effects, NO may often exert dichotomic roles in the regulation of the same process (91). In the setting of cancer, depending on the type of tumor and doses and location of its action, NO has been shown to exert both anti- and pro-oncogenic properties (92, 93). As a matter of fact, expression of iNOS has been shown to represent a negative prognostic factors for multiple types of cancer, including primary UM (94, 95). In particular, recent evidence seems to indicate that NO may act as an addition local immune checkpoint inhibitor, favoring immune evasion of the tumor, by modulation of the acquisition of stem cell-like capacities, the metabolic reprogramming of tumor-infiltrating immune cells, and the induction of myeloid-derived suppressor cells that deplete arginine, via the iNOS pathway, and consequently inhibit T cell function (96, 97).
However, despite these above-mentioned data that strongly support the concept that endogenous NO represents a powerful oncogenic mediator in the maintenance and progression of UM, data by ourselves and others indicate that exogenous NO-derivatives of parental drugs possess enhanced anticancer properties in preclinical models of blood cancer, bladder and prostate cancer, and cutaneous melanoma (98–102).
Dual strategies, therefore, could be envisaged aimed at targeting the NO-producing enzymes and the signaling pathway mediated by NO in UM. Further studies are needed to highlight this concept, along with an evaluation of the other endogenous gases and their donors, H2S in particular, in UM.
Immunobiology of UM Liver Metastasis
The principal organ for UM metastasis is the liver, which is involved in up to 87% of patients with metastatic disease. The liver is often the first metastatic site in UM, and in almost 40% of patients it is the only site of systemic metastasis. Unlike CM, where metastasis to the central nervous system (CNS) occurs in 40–60% of cases, only 4–15% of UM spread to the CNS. Holfort et al. (103) found that UM patients with CNS metastasis either had multiple organ metastasis, which included the CNS, or showed selective CNS metastasis, and, interestingly, a longer interval from primary tumor to CNS metastasis was observed in patients with selective CNS metastasis as compared with the multiple organ metastatic group (103). The peculiar metastatic pattern of UM cannot be explained only by circulation, as the lungs are the first organ that UM cells encounter during their hematogenous spreading. Other factors should therefore be involved, although the exact mechanisms that guide the establishment of liver metastasis in UM remain speculative.
It is believed that the homing of UM cells to the liver is dependent on the expression of the CXCR4, the chemokine receptor for CXCL12, which is highly expressed in the liver (104). Recent data have also demonstrated that exosomes from UM cells expressing integrin αvβ5 are taken up by liver cells, inducing the establishment of a pre-metastatic niche that promote liver tropism (105, 106). However, it is likely that the immunological microenvironment of the liver may favor UM metastatic growth, protecting cancer cells from cytotoxic immune responses [reviewed by (107)]. The Liver must be considered an immuno-modulatory organ as it is continually exposed to exogenous antigens, such as food allergens and low levels of lipopolysaccharide, from the gut. The peculiar anatomy of the liver promotes both direct and indirect priming of lymphocytes, and it can modulate the immune response to pathogens and tumor cells through its ability to induce antigen-specific tolerance. Several highly specialized cellular types are located within the sinusoidal structure and in the parenchyma of the liver, including liver sinusoidal endothelial cells (LSECs), Kupffer cells (KCs), NK cells, and NKT cells. LSECs are capable of receptor-mediated phagocytosis and can present blood-derived antigens to both CD4+ T and CD8+ T cells. Upon stimulation, LSECs also produce the chemokines, CXCL9 and CXCL10, that recruit T lymphocytes. On the other hand, LSECs may express the inhibitory immune checkpoint PD-L1, thus controlling T cell activation (108–112).
KCs, the most abundant tissue macrophages in the body, reside within the sinusoidal vascular space and are able to recognize microorganisms and tumor cells via the C-type lectin receptor Dectin-2 (113). However, KCs may also produce soluble factors, such as IL-10 and prostaglandin E2 (PGE2), that induce a downregulation of MHC class II expression and of the costimulatory molecules, CD80 and CD86, on LSECs, dampening antigen presentation to Helper T cells (114).
The liver also hosts diverse populations of both resident and transiting lymphocytes that are strikingly different from those observed in other tissues and in the circulation. Approximately half of the population of hepatic lymphocytes are represented by NK cells. Liver-resident NK cells are compose of CD49+ NK cells and Eomeshi NK cells, the latter located in the sinusoidal space and accounting for 50% of human liver NK cells (115). NK cells respond to a variety of cell-surface ligands expressed by damaged, tumoral, or infected cells, and exert direct cytotoxicity by releasing cytotoxic granules containing perforin and granzymes (116). NKT cells represent an important immunomodulatory population of the liver. These cells have a restricted TCR repertoire and are able to respond to lipid antigens. However, NKT cells may sustain both inflammatory and anti-inflammatory responses, producing cytokines, such as IFN-γ, IL-4, and IL-17, based on the type of the activating signal (116). NK cells are thought to control metastases growth of UM (117), while NK T cells are able to suppress the cytotoxicity of NK cells via bone marrow-derived cells (118).
Despite the increasing understanding of the immune-phenotypic architecture of the liver, the immune suppressive pathways involved in metastatic UM and the liver tumor microenvironment remain largely elusive. Krishna et al. (119) have recently characterized the immune cell infiltrates in liver metastatic UM and found that CD4+ TILs were located within the tumor, whereas CD8+ TILs tended to be peritumoral. Also, CD68+ and CD163+ TAMs of “indeterminate” morphology were observed, suggesting the presence of protumorigenic M2 macrophages (119). It is worth noting that a meta-analysis of the transcriptomic features of metastatic UM samples (120) found no differences in the expression of genes involved in immune evasion (including HLA molecules, immune checkpoints, cytokines, and anti-inflammatory factors). Hence, we may speculate that intrinsic transcriptomic features of UM cells allow the development and progression of hepatic metastatic disease.
Considering the pre-existing immune tolerance against UM cells, the low mutational burden, and the hepatic immune-modulating microenvironment, it is reasonable that the combination of these factors may promote the more frequent establishment of metastasis in the liver as compared to other organs.
Immunotherapy in Advanced/Metastatic Uveal Melanoma
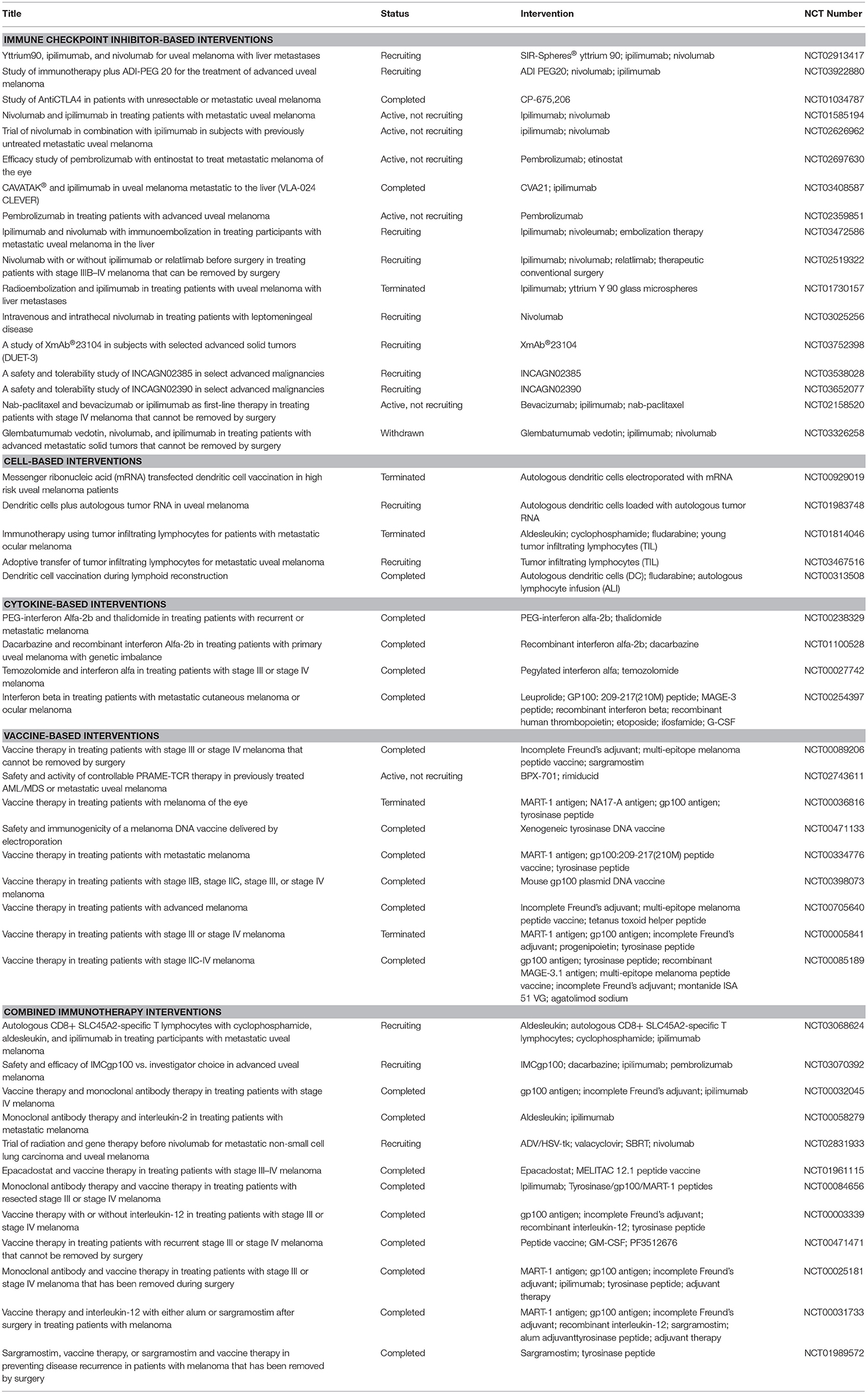
Cancer immunotherapy differs from conventional chemotherapeutic agents in that it enhances the immune responses toward tumor cells rather than affecting cancer cell survival and proliferation via radio- or chemical-induced toxicity. Immunotherapy encompasses several subtypes of treatment modality, including vaccination, cell-based therapies using patients' immune cells, and immunomodulatory agents, among which anti-checkpoint inhibitor therapies have been successful in some solid tumors. A list of the current immunotherapy trials enlisted in ClinicalTrials.gov is presented in Table 1.
The immune-based therapies that have improved the overall survival (OS) of CM patients have not yet led to significant clinical benefits in unresectable/metastatic UM patients (121).
For example, while the immunomodulatory antibodies against the antigen associated with cytotoxic T lymphocytes 4 (CTLA-4) and PD-1/PD-L1 have significantly ameliorated the course of metastatic CM, they have failed when translated to UM patients (121). A multicenter retrospective study on UM patients treated with anti-CTLA-4 or anti-PD-1 mAbs revealed that the adjusted OS of patients with immunotherapy was not significantly different from that of patients treated with chemotherapy, with an unadjusted median OS of 13.38 and 11.02 months, respectively (122). Despite this, the increasing understanding of the immunology of cancer may in the future suggest the possibility of novel pharmacological strategies. Since both CM and UM originate from the melanocyte as same precursor, there might be subsequent factors of differentiation or local factors that are responsible for the different responses to immunomodulatory approaches. On the other hand, the first prospective study of ipilimumab in high-risk primary UM in an adjuvant setting showed that DMFS at 36 months was 80%, as compared to a historical DMFS of 50%; in two of 10 patients, however, treatment discontinuation was required due to grade 3–4 toxicity (123).
The PD-1/PD-L1 pathway is responsible for inhibiting T cell activation in the periphery. To date, the largest clinical trial using anti-PD-1 receptor monoclonal antibodies was conducted on 58 metastatic UM patients treated with either pembrolizumab, nivolumab, or atezolizumab. Of the 56 evaluable patients, only 3.6% obtained partial responses and 8.9% presented a stable disease (124). Also, in a prospective observational cohort single-arm study investigating the efficacy and safety of pembrolizumab as first-line therapy for metastatic UM patients, Rossi et al. found that the efficacy of pembrolizumab does not seem particularly different when compared to other agents for metastatic UM, although responding patients had a remarkable disease control (125). This is in sharp contrast to the response observed in patients with CM, where pembrolizumab significantly increased recurrence-free survival as compared to placebo (75.4% vs. 61.0% for the 1-year rate of recurrence-free survival, respectively) (126).
Prospective studies on anti-PD-1 therapy, alone or in combination with other agents, are currently ongoing (127). Disappointing results have also been obtained with ipilimumab, the anti-CTLA-4 antibody. In a retrospective study on 82 Stage 4 UM patients who received ipilimumab, only 5% had an objective response and 29% had stable disease exceeding 3 months. Median OS was 6.0 months and median PFS was 3.6 months, with a 31 and 11% 1-year OS and PFS, respectively (128). Again, this is in strong contrast with data from patients with stage III CM, where ipilimumab was associated with a 5-year rate of recurrence-free survival of 40.8%, as compared with 30.3% in the placebo group, and to a rate of OS at 5 years of 65.4%, as compared with 54.4% in the placebo group (129).
Tremelimumab, an anti-CTLA-4 antibody, has also been tested in a Phase II study on 11 advanced UM patients who had not previously received other immunotherapy drugs. None of them showed clinical benefit (130).
In a Phase II multicenter single-arm open-label study of nivolumab in combination with ipilimumab (NIVO+IPI) in untreated patients with metastatic UM (Clinical trial identification EudraCT:201500442915), ORR was 12%, with disease stabilization in 52% of patients and a Disease Control Rate of 64% (95% CI 50.7–77.3). With a median follow-up of 7.06 months, PFS was 3.27 months and the median OS was 12.7 months, showing that the combination of NIVO+IPI is a feasible option for UM patients (131). Also, in another single-center trial, sequential/concomitant immune-checkpoint inhibitor treatment produced a longer median OS than single-agent ipilimumab or anti-PD1, with a median OS of 23.7 months (sequential ipilimumab and anti-PD-1) vs. single-agent ipilimumab (13.8 months) and single-agent PD-1 (14.7 months) (132).
Accordingly, in a retrospective case series of eight patients treated with ipilimumab and nivolumab combination along with transarterial chemoembolization (TACE) followed by nivolumab maintenance and monthly TACE procedures, two patients showed a partial response, four had stable disease, and the remaining two patient had disease progression (133). Along the same lines, in a preliminary retrospective case series using Yttrium-90 transarterial radioembolization (TARE) and immunotherapy (either ipilimumab, nivolumab, or pembrolizumab) for UM hepatic metastases, it was found that TARE in addition to immunotherapy is safe and effective (134).
TILs treatment has given promising results in metastatic UM, but no definite results have been yet achieved. In a Phase II clinical trial on 21 patients treated with TILs therapy (NCT01814046), seven of 20 patients showed objective tumor regression. On the other hand, when fewer than 3% of tumor-reactive T cells, fewer than 2 × 109 tumor-reactive T cells, or low tumor-induced IFN-γ release were observed, patients underwent poor clinical responses (135). This study suggests that adoptive transfer of TILs with threshold production of IFN-gamma could promote objective tumor regression, but more effort is needed to increase the percentage of responding patients.
Another promising immunotherapeutic approach is represented by the use of the bispecific antibody IMCgp100. IMCgp100 binds the melanocyte protein gp100 on one end and is constituted by an anti-CD3 single-chain variable fragment on the other end. Therefore, IMCgp100 is able to recognize melanoma cells and contextually activate T cells responses, leading to tumor cytolysis. In two Phase I trials, i.e., NCT01211262 and NCT2570308, IMCgp100 treatment was associated with prolonged disease stabilization with a 1-year OS of 73%. Interestingly, IMCgp100 treatment induced an increase in the percentage of infiltrating PD-1+/CD8+ lymphocytes and an upregulation in PD-L1 expression, suggesting the utility of a combinatorial/sequential treatment regime with immune checkpoint inhibitors (136).
The potential therapeutic value of current available immunotherapeutics will be dissected in the near future, following the results of the several ongoing clinical trials. However, the relatively low number of patients with UM and the extremely aggressive nature of this cancer hinders the possibility of easily deciphering the actual potential of immune-based therapies. Also, we should avoid the misconception that the failure of a target-specific approach is synonymous with absence of biological relevance of the selected targets. The failure of a treatment may be due to intrinsic properties of the drug used (e.g., issues with its pharmacokinetics and pharmacodynamics) as well as to the presence of overlapping and/or redundant pathways that may function as a compensatory mechanism to allow tumor growth and progression, hence the need for combining pre-existing therapies. The potential advantages of a combinatory treatment are 2-fold. On one hand, it may have higher efficacy and overcome resistance coming from potential compensatory mechanisms; on the other hand, it will allow the downscaling of the doses of the drugs used, with a possible reduction of the associated toxic adverse effects. Notably, in a case report by Afzal and colleagues, a patient with metastatic UM treated with a combination of ipilimumab and nivolumab, following the progression with the single-agent, nivolumab, demonstrated a durable response without recurrence for more than 22 months after treatment (137).
Conclusions
Metastatic UM still represents an unmet medical need as there is no current approved treatment able to significantly increase the OS in patients. Chemotherapy has not proven successful and current immune-based therapies, despite the encouraging results coming from CM, have had unsatisfactory results. UM cells evade immune responses via several mechanisms that inhibit both the innate and adaptive immune system (Figure 1). It is therefore of the utmost importance to increase the understanding of the mechanisms put in place by UM to evade the immune surveillance in order to develop novel therapeutic strategies. It is likely that simultaneously targeting multiple immune-escape mechanisms may give an opportunity for the treatment of these patients. This, in fact, would allow them to overcome the unfavorable effects of boosting the immune responses, which in turn induce the establishment of additional immune-evading strategies, such as the upregulation of IDO-1, CD47, PD-L1, and MHC molecules. Promising results may be obtained, for instance, by the combination of TILs in association to anti-CD47 treatment or IDO-1 inhibition. Indeed, TILs-based therapies are currently ongoing and promising, but only partial responses are being observed. Monoclonal antibodies targeting CD47 are also under investigation in two Phase I trials on advanced solid and hematologic cancers (NCT02678338 and NCT02367196). The successful completion of these trials will provide more paths to follow in the search for novel and more effective management options for UM patients. Though not even preclinical proof of concept efficacy has so far been generated, with other “pathogenic”-tailored therapeutic approaches it can be expected that the emerging families of specific inhibitors of TGF-β (138) and MIF (139) also have the potential to be effective in some cases of UM.
Author Contributions
MB, PF, FN, and MR designed and wrote the manuscript. EM, AL, AR, MF, VB, and TA reviewed and approved the final manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by current research funds 2019 of IRCCS Centro Neurolesi Bonino Pulejo, Messina-Italy.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Berus T, Halon A, Markiewicz A, Orlowska-Heitzman J, Romanowska-Dixon B, Donizy P. Clinical, histopathological and cytogenetic prognosticators in uveal melanoma - A comprehensive review. Anticancer Res. (2017) 37:6541–9. doi: 10.21873/anticanres.12110
2. Pandiani C, Béranger GE, Leclerc J, Ballotti R, Bertolotto C. Focus on cutaneous and uveal melanoma specificities. Genes Dev. (2017) 31:724–43. doi: 10.1101/gad.296962.117
3. van den Bosch T, Kilic E, Paridaens D, de Klein A. Genetics of uveal melanoma and cutaneous melanoma: two of a kind? Dermatol Res Pract. (2010) 2010:360136. doi: 10.1155/2010/360136
4. Bastian BC, LeBoit PE, Hamm H, Bröcker EB, Pinkel D. Chromosomal gains and losses in primary cutaneous melanomas detected by comparative genomic hybridization. Cancer Res. (1998) 58:2170–5. doi: 10.1016/S0923-1811(98)83846-2
5. Pirker C, Holzmann K, Spiegl-Kreinecker S, Elbling L, Thallinger C, Pehamberger H, et al. Chromosomal imbalances in primary and metastatic melanomas: over-representation of essential telomerase genes. Melanoma Res. (2003) 13:483–92. doi: 10.1097/00008390-200310000-00007
6. de Lange MJ, Razzaq L, Versluis M, Verlinde S, Dogrusöz M, Böhringer S, et al. Distribution of GNAQ and GNA11 mutation signatures in uveal melanoma points to a light dependent mutation mechanism. PLoS ONE. (2015) 10:e0138002. doi: 10.1371/journal.pone.0138002
7. Versluis M, de Lange MJ, van Pelt SI, Ruivenkamp CAL, Kroes WGM, Cao J, et al. Digital PCR validates 8q dosage as prognostic tool in uveal melanoma. PLoS ONE. (2015) 10:e0116371. doi: 10.1371/journal.pone.0116371
8. Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SAJR, Behjati S, Biankin AV, et al. Signatures of mutational processes in human cancer. Nature. (2013) 500:415–21. doi: 10.1038/nature12477
9. Johnson CP, Kim IK, Esmaeli B, Amin-Mansour A, Treacy DJ, Carter SL, et al. Systematic genomic and translational efficiency studies of uveal melanoma. PLoS ONE. (2017) 12:e0178189. doi: 10.1371/journal.pone.0178189
10. Samstein RM, Lee C-H, Shoushtari AN, Hellmann MD, Shen R, Janjigian YY, et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat Genet. (2019) 51:202–6. doi: 10.1038/s41588-018-0312-8
11. Kalaora S, Wolf Y, Feferman T, Barnea E, Greenstein E, Reshef D, et al. Combined analysis of antigen presentation and T-cell recognition reveals restricted immune responses in melanoma. Cancer Discov. (2018) 8:1366–75. doi: 10.1158/2159-8290.CD-17-1418
12. Tran E, Ahmadzadeh M, Lu Y-C, Gros A, Turcotte S, Robbins PF, et al. Immunogenicity of somatic mutations in human gastrointestinal cancers. Science. (2015) 350:1387–90. doi: 10.1126/science.aad1253
13. Niederkorn JY. Ocular immune privilege and ocular melanoma: parallel universes or immunological plagiarism? Front Immunol. (2012) 3:148. doi: 10.3389/fimmu.2012.00148
14. McMenamin PG, Saban DR, Dando SJ. Immune cells in the retina and choroid: two different tissue environments that require different defenses and surveillance. Prog Retin Eye Res. (2019) 70:85–98. doi: 10.1016/j.preteyeres.2018.12.002
15. Forrester JV, Xu H. Good news-bad news: the Yin and Yang of immune privilege in the eye. Front Immunol. (2012) 3:338. doi: 10.3389/fimmu.2012.00338
16. McKenna KC, Previte DM. Influence of CD8+ T regulatory cells on intraocular tumor development. Front Immunol. (2012) 3:303. doi: 10.3389/fimmu.2012.00303
17. Goel M, Picciani RG, Lee RK, Bhattacharya SK. Aqueous humor dynamics: a review. Open Ophthalmol J. (2010) 4:52. doi: 10.2174/1874364101004010052
18. Alm A, Nilsson SFE. Uveoscleral outflow – A review. Exp Eye Res. (2009) 88:760–8. doi: 10.1016/j.exer.2008.12.012
19. Taylor AW, Alard P, Yee DG, Streilein JW. Aqueous humor induces transforming growth factor-beta (TGF-beta)-producing regulatory T-cells. Curr Eye Res. (1997) 16:900–8. doi: 10.1076/ceyr.16.9.900.5043
20. Nicoletti F, Di Marco R, Patti F, Reggio E, Nicoletti A, Zaccone P, et al. Blood levels of transforming growth factor-beta 1 (TGF-beta1) are elevated in both relapsing remitting and chronic progressive multiple sclerosis (MS) patients and are further augmented by treatment with interferon-beta 1b (IFN-beta1b). Clin Exp Immunol. (1998) 113:96–9. doi: 10.1046/j.1365-2249.1998.00604.x
21. Daneshmandi S, Karimi MH, Pourfathollah AA. TGF-β engineered mesenchymal stem cells (TGF-β/MSCs) for treatment of Type 1 diabetes (T1D) mice model. Int Immunopharmacol. (2017) 44:191–6. doi: 10.1016/j.intimp.2017.01.019
22. Batlle E, Massagué J. Transforming growth factor-β signaling in immunity and cancer. Immunity. (2019) 50:924–40. doi: 10.1016/j.immuni.2019.03.024
23. Chen Y, Di C, Zhang X, Wang J, Wang F, Yan J, et al. Transforming growth factor β signaling pathway: a promising therapeutic target for cancer. J Cell Physiol. (2019). doi: 10.1002/jcp.29108. [Epub ahead of print].
24. Apte RS, Sinha D, Mayhew E, Wistow GJ, Niederkorn JY. Cutting edge: role of macrophage migration inhibitory factor in inhibiting NK cell activity and preserving immune privilege. J Immunol. (1998) 160:5693–6.
25. Fagone P, Mazzon E, Cavalli E, Bramanti A, Petralia MC, Mangano K, et al. Contribution of the macrophage migration inhibitory factor superfamily of cytokines in the pathogenesis of preclinical and human multiple sclerosis: in silico and in vivo evidences. J Neuroimmunol. (2018) 322:46–56. doi: 10.1016/j.jneuroim.2018.06.009
26. Nicoletti F, Créange A, Orlikowski D, Bolgert F, Mangano K, Metz C, et al. Macrophage migration inhibitory factor (MIF) seems crucially involved in Guillain-Barré syndrome and experimental allergic neuritis. J Neuroimmunol. (2005) 168:168–74. doi: 10.1016/j.jneuroim.2005.07.019
27. Presti M, Mazzon E, Basile M, Petralia M, Bramanti A, Colletti G, et al. Overexpression of macrophage migration inhibitory factor and functionally-related genes, D-DT, CD74, CD44, CXCR2 and CXCR4, in glioblastoma. Oncol Lett. (2018) 16:2881–6. doi: 10.3892/ol.2018.8990
28. Soumoy L, Kindt N, Ghanem G, Saussez S, Journe F. Role of macrophage migration inhibitory factor (MIF) in melanoma. Cancers. (2019) 11:529. doi: 10.3390/cancers11040529
29. Lechien JR, Nassri A, Kindt N, Brown DN, Journe F, Saussez S. Role of macrophage migration inhibitory factor in head and neck cancer and novel therapeutic targets: a systematic review. Head Neck. (2017) 39:2573–84. doi: 10.1002/hed.24939
30. Oliveira CS, de Bock CE, Molloy TJ, Sadeqzadeh E, Geng XY, Hersey P, et al. Macrophage migration inhibitory factor engages PI3K/Akt signalling and is a prognostic factor in metastatic melanoma. BMC Cancer. (2014) 14:630. doi: 10.1186/1471-2407-14-630
31. Apte RS, Niederkorn JY. Isolation and characterization of a unique natural killer cell inhibitory factor present in the anterior chamber of the eye. J Immunol. (1996) 156:2667–73.
32. Apte RS, Mayhew E, Niederkorn JY. Local inhibition of natural killer cell activity promotes the progressive growth of intraocular tumors. Invest Ophthalmol Vis Sci. (1997) 38:1277–82. doi: 10.1016/S0002-9394(14)70856-6
33. Yoshida M, Takeuchi M, Streilein JW. Participation of pigment epithelium of iris and ciliary body in ocular immune privilege. 1. Inhibition of T-cell activation in vitro by direct cell-to-cell contact. Invest Ophthalmol Vis Sci. (2000) 41:811–21.
34. Camelo S, Kezic J, Shanley A, Rigby P, McMenamin PG. Antigen from the anterior chamber of the eye travels in a soluble form to secondary lymphoid organs via lymphatic and vascular routes. Invest Opthalmol Vis Sci. (2006) 47:1039–46. doi: 10.1167/iovs.05-1041
35. Camelo S, Shanley A, Voon ASP, McMenamin PG. The distribution of antigen in lymphoid tissues following its injection into the anterior chamber of the rat eye. J Immunol. (2004) 172:5388–95. doi: 10.4049/jimmunol.172.9.5388
36. Keino H, Horie S, Sugita S. Immune privilege and eye-derived T-regulatory cells. J Immunol Res. (2018) 2018:1679197. doi: 10.1155/2018/1679197
37. Liu M, Wang X, Wang L, Ma X, Gong Z, Zhang S, et al. Targeting the IDO1 pathway in cancer: from bench to bedside. J Hematol Oncol. (2018) 11:100. doi: 10.1186/s13045-018-0644-y
38. Mangano K, Mazzon E, Basile MS, Di Marco R, Bramanti P, Mammana S, et al. Pathogenic role for macrophage migration inhibitory factor in glioblastoma and its targeting with specific inhibitors as novel tailored therapeutic approach. Oncotarget. (2018) 9:17951–70. doi: 10.18632/oncotarget.24885
39. Borch TH, Donia M, Andersen MH, Svane IM. Reorienting the immune system in the treatment of cancer by using anti-PD-1 and anti-PD-L1 antibodies. Drug Discov Today. (2015) 20:1127–34. doi: 10.1016/j.drudis.2015.07.003
40. Anastassiou G, Coupland SE, Stang A, Boeloeni R, Schilling H, Bornfeld N. Expression of Fas and Fas ligand in uveal melanoma: biological implication and prognostic value. J Pathol. (2001) 194:466–72. doi: 10.1002/path.926
41. de la Cruz PO, Specht CS, McLean IW. Lymphocytic infiltration in uveal malignant melanoma. Cancer. (1990) 65:112–5. doi: 10.1002/1097-0142(19900101)65:1<112::AID-CNCR2820650123>3.0.CO;2-X
42. Mäkitie T, Summanen P, Tarkkanen A, Kivelä T. Tumor-infiltrating macrophages (CD68(+) cells) and prognosis in malignant uveal melanoma. Invest Ophthalmol Vis Sci. (2001) 42:1414–21.
43. Törnquist P, Alm A, Bill A. Permeability of ocular vessels and transport across the blood-retinal-barrier. Eye. (1990) 4:303–9. doi: 10.1038/eye.1990.41
44. Campbell M, Humphries P. The blood-retina barrier: tight junctions and barrier modulation. Adv Exp Med Biol. (2012) 763:70–84. doi: 10.1007/978-1-4614-4711-5_3
45. Chen PW, Murray TG, Salgaller ML, Ksander BR. Expression of MAGE genes in ocular melanoma cell lines. J Immunother. (1997) 20:265–75. doi: 10.1097/00002371-199707000-00003
46. Luyten GP, van der Spek CW, Brand I, Sintnicolaas K, de Waard-Siebinga I, Jager MJ, et al. Expression of MAGE, gp100 and tyrosinase genes in uveal melanoma cell lines. Melanoma Res. (1998) 8:11–6. doi: 10.1097/00008390-199802000-00003
47. Mulcahy KA, Rimoldi D, Brasseur F, Rodgers S, Liénard D, Marchand M, et al. Infrequent expression of the MAGE gene family in uveal melanomas. Int J Cancer. (1996) 66:738–42. doi: 10.1002/(SICI)1097-0215(19960611)66:6<738::AID-IJC5>3.0.CO;2-0
48. Kan-Mitchell J, Liggett PE, Harel W, Steinman L, Nitta T, Oksenberg JR, et al. Lymphocytes cytotoxic to uveal and skin melanoma cells from peripheral blood of ocular melanoma patients. Cancer Immunol Immunother. (1991) 33:333–40. doi: 10.1007/BF01756599
49. Ksander BR, Geer DC, Chen PW, Salgaller ML, Rubsamen P, Murray TG. Uveal melanomas contain antigenically specific and non-specific infiltrating lymphocytes. Curr Eye Res. (1998) 17:165–73. doi: 10.1076/ceyr.17.2.165.5607
50. Ksander BR, Rubsamen PE, Olsen KR, Cousins SW, Streilein JW. Studies of tumor-infiltrating lymphocytes from a human choroidal melanoma. Invest Ophthalmol Vis Sci. (1991) 32:3198–208.
51. Knisely TL, Niederkorn JY. Emergence of a dominant cytotoxic T lymphocyte antitumor effector from tumor-infiltrating cells in the anterior chamber of the eye. Cancer Immunol Immunother. (1990) 30:323–30. doi: 10.1007/BF01786881
52. Ly LV, Bronkhorst IHG, van Beelen E, Vrolijk J, Taylor AW, Versluis M, et al. Inflammatory cytokines in eyes with uveal melanoma and relation with macrophage infiltration. Invest Ophthalmol Vis Sci. (2010) 51:5445–51. doi: 10.1167/iovs.10-5526
53. Maat W, van der Slik AR, Verhoeven DHJ, Alizadeh BZ, Ly LV, Verduijn W, et al. Evidence for natural killer cell–mediated protection from metastasis formation in uveal melanoma patients. Invest Opthalmol Vis Sci. (2009) 50:2888–95. doi: 10.1167/iovs.08-2733
54. Vetter CS, Lieb W, Bröcker E-B, Becker JC. Loss of nonclassical MHC molecules MIC-A/B expression during progression of uveal melanoma. Br J Cancer. (2004) 91:1495–9. doi: 10.1038/sj.bjc.6602123
55. Hallermalm K, De Geer A, Kiessling R, Levitsky V, Levitskaya J. Autocrine secretion of Fas ligand shields tumor cells from Fas-mediated killing by cytotoxic lymphocytes. Cancer Res. (2004) 64:6775–82. doi: 10.1158/0008-5472.CAN-04-0508
56. Rook AH, Kehrl JH, Wakefield LM, Roberts AB, Sporn MB, Burlington DB, et al. Effects of transforming growth factor beta on the functions of natural killer cells: depressed cytolytic activity and blunting of interferon responsiveness. J Immunol. (1986) 136:3916–20.
57. He Y-G, Mayhew E, Mellon J, Niederkorn JY. Expression and possible function of IL-2 and IL-15 receptors on human uveal melanoma cells. Invest Opthalmol Vis Sci. (2004) 45:4240–6. doi: 10.1167/iovs.04-0599
58. Beatty GL, Paterson Y. IFN-gamma can promote tumor evasion of the immune system in vivo by down-regulating cellular levels of an endogenous tumor antigen. J Immunol. (2000) 165:5502–8. doi: 10.4049/jimmunol.165.10.5502
59. Le Poole IC, Riker AI, Quevedo ME, Stennett LS, Wang E, Marincola FM, et al. Interferon-gamma reduces melanosomal antigen expression and recognition of melanoma cells by cytotoxic T cells. Am J Pathol. (2002) 160:521–8. doi: 10.1016/S0002-9440(10)64871-7
60. Rothermel LD, Sabesan AC, Stephens DJ, Chandran SS, Paria BC, Srivastava AK, et al. Identification of an immunogenic subset of metastatic uveal melanoma. Clin Cancer Res. (2016) 22:2237–49. doi: 10.1158/1078-0432.CCR-15-2294
61. Lagouros E, Salomao D, Thorland E, Hodge DO, Vile R, Pulido JS. Infiltrative T regulatory cells in enucleated uveal melanomas. Trans Am Ophthalmol Soc. (2009) 107:223–8.
62. Mougiakakos D, Johansson CC, Trocme E, All-Ericsson C, Economou MA, Larsson O, et al. Intratumoral forkhead box P3-positive regulatory T cells predict poor survival in cyclooxygenase-2-positive uveal melanoma. Cancer. (2010) 116:2224–33. doi: 10.1002/cncr.24999
63. Achberger S, Aldrich W, Tubbs R, Crabb JW, Singh AD, Triozzi PL. Circulating immune cell and microRNA in patients with uveal melanoma developing metastatic disease. Mol Immunol. (2014) 58:182–6. doi: 10.1016/j.molimm.2013.11.018
64. Durie FH, Campbell AM, Lee WR, Damato BE. Analysis of lymphocytic infiltration in uveal melanoma. Invest Ophthalmol Vis Sci. (1990) 31:2106–10.
65. Streilein JW, Niederkorn JY. Characterization of the suppressor cell(s) responsible for anterior chamber-associated immune deviation (ACAID) induced in BALB/c mice by P815 cells. J Immunol. (1985) 134:1381–7.
66. McKenna KC, Kapp JA. Accumulation of immunosuppressive CD11b+ myeloid cells correlates with the failure to prevent tumor growth in the anterior chamber of the eye. J Immunol. (2006) 177:1599–608. doi: 10.4049/jimmunol.177.3.1599
67. Alonso R, Flament H, Lemoine S, Sedlik C, Bottasso E, Péguillet I, et al. Induction of anergic or regulatory tumor-specific CD4+ T cells in the tumor-draining lymph node. Nat Commun. (2018) 9:2113. doi: 10.1038/s41467-018-04524-x
68. Uyttenhove C, Pilotte L, Théate I, Stroobant V, Colau D, Parmentier N, et al. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med. (2003) 9:1269–74. doi: 10.1038/nm934
69. Brandacher G, Perathoner A, Ladurner R, Schneeberger S, Obrist P, Winkler C, et al. Prognostic value of indoleamine 2,3-dioxygenase expression in colorectal cancer: effect on tumor-infiltrating T cells. Clin Cancer Res. (2006) 12:1144–51. doi: 10.1158/1078-0432.CCR-05-1966
70. Ishio T, Goto S, Tahara K, Tone S, Kawano K, Kitano S. Immunoactivative role of indoleamine 2,3-dioxygenase in human hepatocellular carcinoma. J Gastroenterol Hepatol. (2004) 19:319–26. doi: 10.1111/j.1440-1746.2003.03259.x
71. Ino K, Yoshida N, Kajiyama H, Shibata K, Yamamoto E, Kidokoro K, et al. Indoleamine 2,3-dioxygenase is a novel prognostic indicator for endometrial cancer. Br J Cancer. (2006) 95:1555–61. doi: 10.1038/sj.bjc.6603477
72. Weinlich G, Murr C, Richardsen L, Winkler C, Fuchs D. Decreased serum tryptophan concentration predicts poor prognosis in malignant melanoma patients. Dermatology. (2007) 214:8–14. doi: 10.1159/000096906
73. Beutelspacher SC, Pillai R, Watson MP, Tan PH, Tsang J, McClure MO, et al. Function of indoleamine 2,3-dioxygenase in corneal allograft rejection and prolongation of allograft survival by over-expression. Eur J Immunol. (2006) 36:690–700. doi: 10.1002/eji.200535238
74. Ryu Y-H, Kim J-C. Expression of indoleamine 2,3-dioxygenase in human corneal cells as a local immunosuppressive factor. Invest Ophthalmol Vis Sci. (2007) 48:4148–52. doi: 10.1167/iovs.05-1336
75. Chen PW, Mellon JK, Mayhew E, Wang S, He YG, Hogan N, et al. Uveal melanoma expression of indoleamine 2,3-deoxygenase: establishment of an immune privileged environment by tryptophan depletion. Exp Eye Res. (2007) 85:617–25. doi: 10.1016/j.exer.2007.07.014
76. Terai M, Link E, Link E, Lam B, Orloff M, Sato T. Abstract 3805: expression of tryptophan−2, 3-dioxygense (TDO) in metastatic uveal melanoma. Cancer Res. (2018) 78:3805. doi: 10.1158/1538-7445.AM2018-3805
77. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. (2012) 12:252–64. doi: 10.1038/nrc3239
78. Robertson AG, Shih J, Yau C, Gibb EA, Oba J, Mungall KL, et al. Integrative analysis identifies four molecular and clinical subsets in uveal melanoma. Cancer Cell. (2017) 32:204–20.e15. doi: 10.1016/j.ccell.2017.07.003
79. Maat W, Ly LV, Jordanova ES, de Wolff-Rouendaal D, Schalij-Delfos NE, Jager MJ. Monosomy of chromosome 3 and an inflammatory phenotype occur together in uveal melanoma. Invest Ophthalmol Vis Sci. (2008) 49:505–10. doi: 10.1167/iovs.07-0786
80. Yang W, Chen PW, Li H, Alizadeh H, Niederkorn JY. PD-L1 expression by human uveal melanoma inhibits T-cell function. Invest Ophthalmol Vis Sci. (2008) 49:2398. doi: 10.1167/iovs.07-1606
81. Kaunitz GJ, Cottrell TR, Lilo M, Muthappan V, Esandrio J, Berry S, et al. Melanoma subtypes demonstrate distinct PD-L1 expression profiles. Lab Invest. (2017) 97:1063–71. doi: 10.1038/labinvest.2017.64
82. Basile MS, Mazzon E, Russo A, Mammana S, Longo A, Bonfiglio V, et al. Differential modulation and prognostic values of immune-escape genes in uveal melanoma. PLoS ONE. (2019) 14:e0210276. doi: 10.1371/journal.pone.0210276
83. Zhao H-J, Pa F, Shi Y-C, Luo X, Ren R-R, Peng L-H, et al. Prognostic significance of CD47 in human malignancies: a systematic review and meta-analysis. Transl Cancer Res. (2018) 7:609–21. doi: 10.21037/tcr.2018.05.31
84. Matlung HL, Szilagyi K, Barclay NA, van den Berg TK. The CD47-SIRPα signaling axis as an innate immune checkpoint in cancer. Immunol Rev. (2017) 276:145–64. doi: 10.1111/imr.12527
85. Petralia MC, Mazzon E, Fagone P, Russo A, Longo A, Avitabile T, et al. Characterization of the pathophysiological role of CD47 in uveal melanoma. Molecules. (2019) 24:2450. doi: 10.3390/molecules24132450
86. Barclay AN, Wright GJ, Brooke G, Brown MH. CD200 and membrane protein interactions in the control of myeloid cells. Trends Immunol. (2002) 23:285–90. doi: 10.1016/S1471-4906(02)02223-8
87. Hoek RM, Ruuls SR, Murphy CA, Wright GJ, Goddard R, Zurawski SM, et al. Down-regulation of the macrophage lineage through interaction with OX2 (CD200). Science. (2000) 290:1768–71. doi: 10.1126/science.290.5497.1768
88. Zhou X, Sun L, Jing D, Xu G, Zhang J, Lin L, et al. Galectin-9 expression predicts favorable clinical outcome in solid tumors: a systematic review and meta-analysis. Front Physiol. (2018) 9:452. doi: 10.3389/fphys.2018.00452
89. Ge H, Liang C, Ren S, Yue C, Wu J. Prognostic value of DcR3 in solid tumors: a meta-analysis. Clin Chim Acta. (2018) 481:126–31. doi: 10.1016/j.cca.2018.02.038
90. Li S, Liao R, Sheng X, Luo X, Zhang X, Wen X, et al. Hydrogen gas in cancer treatment. Front Oncol. (2019) 9:696. doi: 10.3389/fonc.2019.00696
91. Fagone P, Mazzon E, Bramanti P, Bendtzen K, Nicoletti F. Gasotransmitters and the immune system: mode of action and novel therapeutic targets. Eur J Pharmacol. (2018) 834:92–102. doi: 10.1016/j.ejphar.2018.07.026
92. Hussain SP, Trivers GE, Hofseth LJ, He P, Shaikh I, Mechanic LE, et al. Nitric oxide, a mediator of inflammation, suppresses tumorigenesis. Cancer Res. (2004) 64:6849–53. doi: 10.1158/0008-5472.CAN-04-2201
93. El-Sehemy A, Postovit L-M, Fu Y. Nitric oxide signaling in human ovarian cancer: a potential therapeutic target. Nitric Oxide Biol Chem. (2016) 54:30–7. doi: 10.1016/j.niox.2016.02.002
94. Liao W, Ye T, Liu H. Prognostic value of inducible nitric oxide synthase (iNOS) in human cancer: a systematic review and meta-analysis. Biomed Res Int. (2019) 2019:6304851. doi: 10.1155/2019/6304851
95. Johansson CC, Mougiakakos D, Trocme E, All-Ericsson C, Economou MA, Larsson O, et al. Expression and prognostic significance of iNOS in uveal melanoma. Int J Cancer. (2010) 126:2682–9. doi: 10.1002/ijc.24984
96. PeÑarando J, Aranda E, RodrÍguez-Ariza A. Immunomodulatory roles of nitric oxide in cancer: tumor microenvironment says “NO” to antitumor immune response. Transl Res. (2019) 210:99–108. doi: 10.1016/j.trsl.2019.03.003
97. Newton JM, Hanoteau A, Liu H-C, Gaspero A, Parikh F, Gartrell-Corrado RD, et al. Immune microenvironment modulation unmasks therapeutic benefit of radiotherapy and checkpoint inhibition. J Immunother Cancer. (2019) 7:216. doi: 10.1186/s40425-019-0698-6
98. Paskas S, Mazzon E, Basile MS, Cavalli E, Al-Abed Y, He M, et al. Lopinavir-NO, a nitric oxide-releasing HIV protease inhibitor, suppresses the growth of melanoma cells in vitro and in vivo. Invest New Drugs. (2019) 37:1014–28. doi: 10.1007/s10637-019-00733-3
99. Basile M, Mazzon E, Krajnovic T, Draca D, Cavalli E, Al-Abed Y, et al. Anticancer and differentiation properties of the nitric oxide derivative of lopinavir in human glioblastoma cells. Molecules. (2018) 23:2463. doi: 10.3390/molecules23102463
100. Maksimovic-Ivanic D, Mojic M, Bulatovic M, Radojkovic M, Kuzmanovic M, Ristic S, et al. The NO-modified HIV protease inhibitor as a valuable drug for hematological malignancies: role of p70S6K. Leuk Res. (2015) 39:1088–95. doi: 10.1016/j.leukres.2015.06.013
101. Paskaš S, Krajnović T, Basile MS, Dunderović D, Cavalli E, Mangano K, et al. Senescence as a main mechanism of Ritonavir and Ritonavir-NO action against melanoma. Mol Carcinog. (2019) 58:1362–75. doi: 10.1002/mc.23020
102. Seabra AB, Durán N. Nitric oxide donors for prostate and bladder cancers: current state and challenges. Eur J Pharmacol. (2018) 826:158–68. doi: 10.1016/j.ejphar.2018.02.040
103. Holfort SK, Lindegaard J, Isager P, Prause JU, Heegaard S. CNS metastasis from malignant uveal melanoma: a clinical and histopathological characterisation. Br J Ophthalmol. (2009) 93:641–4. doi: 10.1136/bjo.2008.145664
104. Li H, Alizadeh H, Niederkorn JY. Differential expression of chemokine receptors on uveal melanoma cells and their metastases. Invest Opthalmol Vis Sci. (2008) 49:636–43. doi: 10.1167/iovs.07-1035
105. Hoshino A, Costa-Silva B, Shen T-L, Rodrigues G, Hashimoto A, Mark MT, et al. Tumour exosome integrins determine organotropic metastasis. Nature. (2015) 527:329–35. doi: 10.1038/nature15756
106. Angi M, Kalirai H, Prendergast S, Simpson D, Hammond DE, Madigan MC, et al. In-depth proteomic profiling of the uveal melanoma secretome. Oncotarget. (2016) 7:49623–35. doi: 10.18632/oncotarget.10418
107. Bakalian S, Marshall J-C, Logan P, Faingold D, Maloney S, Di Cesare S, et al. Molecular pathways mediating liver metastasis in patients with uveal melanoma. Clin Cancer Res. (2008) 14:951–6. doi: 10.1158/1078-0432.CCR-06-2630
108. Knolle PA, Schmitt E, Jin S, Germann T, Duchmann R, Hegenbarth S, et al. Induction of cytokine production in naive CD4(+) T cells by antigen-presenting murine liver sinusoidal endothelial cells but failure to induce differentiation toward Th1 cells. Gastroenterology. (1999) 116:1428–40. doi: 10.1016/S0016-5085(99)70508-1
109. Lohse A, Knolle P, Bilo K, Uhrig A, Waldmann C, Ibe M, et al. Antigen-presenting function and B7 expression of murine sinusoidal endothelial cells and Kupffer cells. Gastroenterology. (1996) 110:1175–81. doi: 10.1053/gast.1996.v110.pm8613007
110. Steffan AM, Gendrault JL, McCuskey RS, McCuskey PA, Kirn A. Phagocytosis, an unrecognized property of murine endothelial liver cells. Hepatology. (1986) 6:830–6. doi: 10.1002/hep.1840060505
111. Höchst B, Schildberg FA, Böttcher J, Metzger C, Huss S, Türler A, et al. Liver sinusoidal endothelial cells contribute to CD8 T cell tolerance toward circulating carcinoembryonic antigen in mice. Hepatology. (2012) 56:1924–33. doi: 10.1002/hep.25844
112. Diehl L, Schurich A, Grochtmann R, Hegenbarth S, Chen L, Knolle PA. Tolerogenic maturation of liver sinusoidal endothelial cells promotes B7-homolog 1-dependent CD8+ T cell tolerance. Hepatology. (2007) 47:296–305. doi: 10.1002/hep.21965
113. Kimura Y, Inoue A, Hangai S, Saijo S, Negishi H, Nishio J, et al. The innate immune receptor Dectin-2 mediates the phagocytosis of cancer cells by Kupffer cells for the suppression of liver metastasis. Proc Natl Acad Sci USA. (2016) 113:14097–102. doi: 10.1073/pnas.1617903113
114. Knolle PA, Uhrig A, Hegenbarth S, Löser E, Schmitt E, Gerken G, et al. IL-10 down-regulates T cell activation by antigen-presenting liver sinusoidal endothelial cells through decreased antigen uptake via the mannose receptor and lowered surface expression of accessory molecules. Clin Exp Immunol. (1998) 114:427–33. doi: 10.1046/j.1365-2249.1998.00713.x
115. Male V. Liver-resident NK cells: the human factor. Trends Immunol. (2017) 38:307–9. doi: 10.1016/j.it.2017.02.008
116. Jenne CN, Kubes P. Immune surveillance by the liver. Nat Immunol. (2013) 14:996–1006. doi: 10.1038/ni.2691
117. Jones NM, Yang H, Zhang Q, Morales-Tirado VM, Grossniklaus HE. Natural killer cells and pigment epithelial-derived factor control the infiltrative and nodular growth of hepatic metastases in an Orthotopic murine model of ocular melanoma. BMC Cancer. (2019) 19:484. doi: 10.1186/s12885-019-5712-3
118. Sadegh L, Chen PW, Brown JR, Han Z, Niederkorn JY. NKT cells act through third party bone marrow-derived cells to suppress NK cell activity in the liver and exacerbate hepatic melanoma metastases. Int J Cancer. (2015) 137:1085–94. doi: 10.1002/ijc.29480
119. Krishna Y, McCarthy C, Kalirai H, Coupland SE. Inflammatory cell infiltrates in advanced metastatic uveal melanoma. Hum Pathol. (2017) 66:159–66. doi: 10.1016/j.humpath.2017.06.005
120. Fagone P, Caltabiano R, Russo A, Lupo G, Anfuso CD, Basile MS, et al. Identification of novel chemotherapeutic strategies for metastatic uveal melanoma. Sci Rep. (2017) 7:44564. doi: 10.1038/srep44564
121. Oliva M, Rullan AJ, Piulats JM. Uveal melanoma as a target for immune-therapy. Ann Transl Med. (2016) 4:172. doi: 10.21037/atm.2016.05.04
122. Mignard C, Deschamps Huvier A, Gillibert A, Duval Modeste AB, Dutriaux C, Khammari A, et al. Efficacy of immunotherapy in patients with metastatic mucosal or uveal melanoma. J Oncol. (2018) 2018:1908065. doi: 10.1155/2018/1908065
123. Fountain E, Bassett R, Cain S, Posada L, Gombos D, Hwu P, et al. Adjuvant ipilimumab in high-risk uveal melanoma. Cancers. (2019) 11:152. doi: 10.3390/cancers11020152
124. Algazi AP, Tsai KK, Shoushtari AN, Munhoz RR, Eroglu Z, Piulats JM, et al. Clinical outcomes in metastatic uveal melanoma treated with PD-1 and PD-L1 antibodies. Cancer. (2016) 122:3344–53. doi: 10.1002/cncr.30258
125. Rossi E, Pagliara MM, Orteschi D, Dosa T, Sammarco MG, Caputo CG, et al. Pembrolizumab as first-line treatment for metastatic uveal melanoma. Cancer Immunol Immunother. (2019) 68:1179–85. doi: 10.1007/s00262-019-02352-6
126. Eggermont AMM, Blank CU, Mandala M, Long GV, Atkinson V, Dalle S, et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma. N Engl J Med. (2018) 378:1789–801. doi: 10.1056/NEJMoa1802357
127. Yang J, Manson DK, Marr BP, Carvajal RD. Treatment of uveal melanoma: where are we now? Ther Adv Med Oncol. (2018) 10:175883401875717. doi: 10.1177/1758834018757175
128. Maio M, Danielli R, Chiarion-Sileni V, Pigozzo J, Parmiani G, Ridolfi R, et al. Efficacy and safety of ipilimumab in patients with pre-treated, uveal melanoma. Ann Oncol. (2013) 24:2911–5. doi: 10.1093/annonc/mdt376
129. Eggermont AMM, Chiarion-Sileni V, Grob J-J, Dummer R, Wolchok JD, Schmidt H, et al. Prolonged survival in stage III melanoma with ipilimumab adjuvant therapy. N Engl J Med. (2016) 375:1845–55. doi: 10.1056/NEJMoa1611299
130. Joshua AM, Monzon JG, Mihalcioiu C, Hogg D, Smylie M, Cheng T. A phase 2 study of tremelimumab in patients with advanced uveal melanoma. Melanoma Res. (2015) 25:342–7. doi: 10.1097/CMR.0000000000000175
131. Piulats Rodriguez JM, De La Cruz Merino L, Espinosa E, Alonso Carrión L, Martin Algarra S, López-Castro R, et al. Phase II multicenter, single arm, open label study of nivolumab in combination with ipilimumab in untreated patients with metastatic uveal melanoma (GEM1402.NCT02626962). Ann Oncol. (2018) 29:mdy289.003. doi: 10.1093/annonc/mdy289.003
132. Hernandez M, Neninger E, Santiesteban E, Camacho K, Hernandez N, Amador R, et al. Efficacy of racotumomab or nimotuzumab vs docetaxel as second-line therapy for advanced non-small cell lung cancer patients. Ann Oncol. (2018) 29 (suppl_8):viii400–41. doi: 10.1093/annonc/mdy288
133. Karivedu V, Eldessouki I, Taftaf A, Zhu Z, Makramalla A, Karim NA. Nivolumab and ipilimumab in the treatment of metastatic uveal melanoma: a single-center experience. Case Rep Oncol Med. (2019) 2019:3560640. doi: 10.1155/2019/3560640
134. Zheng J, Irani Z, Lawrence D, Flaherty K, Arellano RS. Combined effects of yttrium-90 transarterial radioembolization around immunotherapy for hepatic metastases from uveal melanoma: a preliminary retrospective case series. J Vasc Interv Radiol. (2018) 29:1369–75. doi: 10.1016/j.jvir.2018.04.030
135. Chandran SS, Somerville RPT, Yang JC, Sherry RM, Klebanoff CA, Goff SL, et al. Treatment of metastatic uveal melanoma with adoptive transfer of tumour-infiltrating lymphocytes: a single-centre, two-stage, single-arm, phase 2 study. Lancet Oncol. (2017) 18:792–802. doi: 10.1016/S1470-2045(17)30251-6
136. Sacco JJ, Kalirai H, Kenyani J, Figueiredo CR, Coulson JM, Coupland SE. Recent breakthroughs in metastatic uveal melanoma: a cause for optimism? Future Oncol. (2018) 14:1335–8. doi: 10.2217/fon-2018-0116
137. Afzal MZ, Mabaera R, Shirai K. Metastatic uveal melanoma showing durable response to anti-CTLA-4 and anti-PD-1 combination therapy after experiencing progression on anti-PD-1 therapy alone. J Immunother Cancer. (2018) 6:13. doi: 10.1186/s40425-018-0322-1
138. Colak S, ten Dijke P. Targeting TGF-β signaling in cancer. Trends Cancer. (2017) 3:56–71. doi: 10.1016/j.trecan.2016.11.008
Keywords: uveal melanoma, inhibitory checkpoints, immunotherapy, immune-escape, immune-privilege
Citation: Basile MS, Mazzon E, Fagone P, Longo A, Russo A, Fallico M, Bonfiglio V, Nicoletti F, Avitabile T and Reibaldi M (2019) Immunobiology of Uveal Melanoma: State of the Art and Therapeutic Targets. Front. Oncol. 9:1145. doi: 10.3389/fonc.2019.01145
Received: 04 May 2019; Accepted: 15 October 2019;
Published: 05 November 2019.
Edited by:
Jason Roszik, University of Texas MD Anderson Cancer Center, United StatesReviewed by:
Sarah Coupland, University of Liverpool, United KingdomChandrani Chattopadhyay, University of Texas MD Anderson Cancer Center, United States
Takami Sato, Thomas Jefferson University, United States
Copyright © 2019 Basile, Mazzon, Fagone, Longo, Russo, Fallico, Bonfiglio, Nicoletti, Avitabile and Reibaldi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Paolo Fagone, cGFvbG9mYWdvbmVAeWFob28uaXQ=
 Maria Sofia Basile1
Maria Sofia Basile1 Emanuela Mazzon
Emanuela Mazzon Paolo Fagone
Paolo Fagone Antonio Longo
Antonio Longo Andrea Russo
Andrea Russo Matteo Fallico
Matteo Fallico Vincenza Bonfiglio
Vincenza Bonfiglio Ferdinando Nicoletti
Ferdinando Nicoletti Michele Reibaldi
Michele Reibaldi