- 1Department of Neurosurgery, The First Hospital of Jilin University, Changchun, China
- 2Cancer Center, The First Hospital of Jilin University, Changchun, China
Early growth response factor 1 (EGR1) is a transcription factor that is mainly involved in the processes of tissue injury, immune responses, and fibrosis. Recent studies have shown that EGR1 is closely related to the initiation and progression of cancer and may participate in tumor cell proliferation, invasion, and metastasis and in tumor angiogenesis. Nonetheless, the specific mechanism whereby EGR1 modulates these processes remains to be elucidated. This review article summarizes possible mechanisms of action of EGR1 in tumorigenesis and tumor progression and may serve as a reference for clinical efficacy predictions and for the discovery of new therapeutic targets.
Introduction
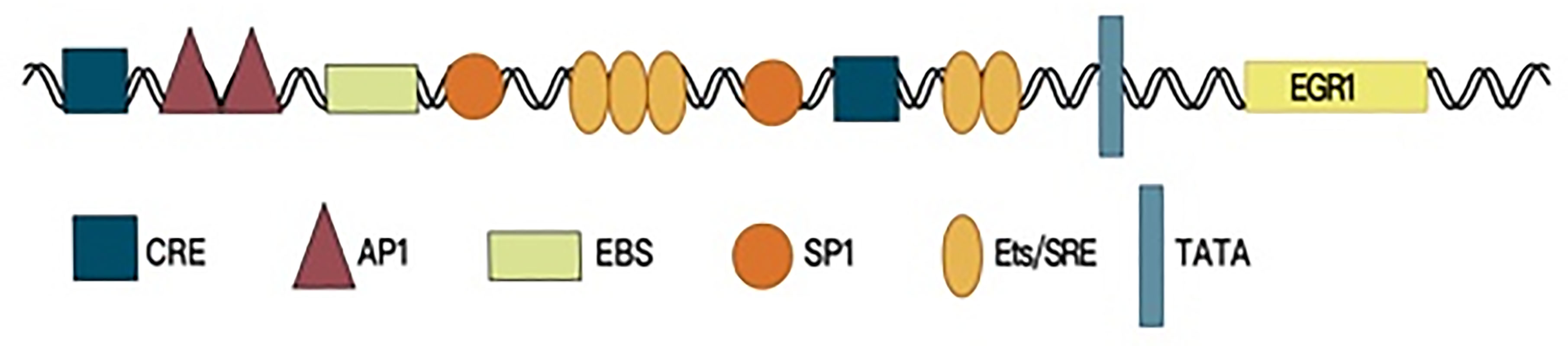
Early growth response factor 1 (EGR1) is a member of the EGR family and is also known as EGR-1, NEFI-A, Zif268, Krox-24, and TIS85. The EGR1 gene is located in human chromosomal region 5q23-31, and the protein is an important transcription factor (1), which contains an activation regulatory region, repressive regulatory region, and three Cys2-His2 subclass zinc finger structures, which specifically recognize and bind target genes and regulate their transcription. The EGR1 promoter region contains serum response elements. A variety of growth factors can start EGR1 gene expression by interacting with this sequence, which contains the characteristic motif CC(A/T)6GG, namely, the CArG box (2, 3). Furthermore, the EGR1 promoter contains an EGR1-binding sequence (EBS), which forms a negative feedback loop to control EGR1 expression (4, 5) (Figure 1).
EGR1 is widely expressed in many cell types and participates in important physiological processes, such as cell proliferation, differentiation, invasion, and apoptosis. When cells are stimulated by growth factors, tumor necrosis factor, inflammatory factors, ionizing radiation, reactive oxygen species, or other factors (6–9), EGR1 can be activated through the MAPK signaling pathway. EGR1 transcription depends on the RAS–RAF–MEK1/2–ERK1/2 signal transduction pathway, and activated EGR1 can either cause or inhibit the expression of its target genes, thus playing a part in transcriptional regulation (10).
As a transcription factor, EGR1 performs a regulatory function in cell growth, but the roles of EGR1 are different in different tumors. For example, EGR1 expression is higher in a prostate tumor than in surrounding prostate tissue (11), and the expression of EGR1 in the prostate tumor positively correlates with malignancy (12). Similar clinical associations have been found in gastric cancer (13). The expression of EGR1 is significantly higher in a primary gastric tumor and metastases than in normal gastric tissues, and EGR1 expression correlates with tumor size, depth of invasion, tumor stage, and prognosis (14). Therefore, EGR1 plays an oncogenic part in prostate and gastric cancers. Nonetheless, EGR1 upregulates tumor suppressor gene p21Waf1/Cip1 and leads to tumor cell apoptosis in gliomas and melanocytomas (15, 16), and consequently, EGR1 serves as a tumor suppressor in these cancers.
EGR1 also plays a dual role in different signaling pathways. EGR1 suppresses transformation and counteracts apoptosis via coordinated activation of TGF-β1, FN, p21Waf1/Cip1, and FAK, thereby leading to enhanced cell attachment and reduced caspase activity (17). On the other hand, in cells with decreased adhesion, EGR1 enhances PTEN-mediated downregulation of AKT expression, thus increasing apoptosis (18). EGR1 regulates the attachment and survival of normal cells but induces apoptosis in abnormal cells with decreased adhesion.
Therefore, EGR1 performs important functions in tumor cell proliferation, angiogenesis, invasion, and immune responses (19–21). Research on the mechanism of action of EGR1 in cancer is expected to point to a new cancer treatment strategy and/or a new marker for theranostics. The purposes of this review are to summarize the roles of EGR1 in tumor cell proliferation, apoptosis, and metastasis and in the tumor microenvironment as well as to discuss the possible signaling pathways in which EGR1 is involved to provide a new perspective on cancer treatment.
The Oncogenic Effects of EGR1
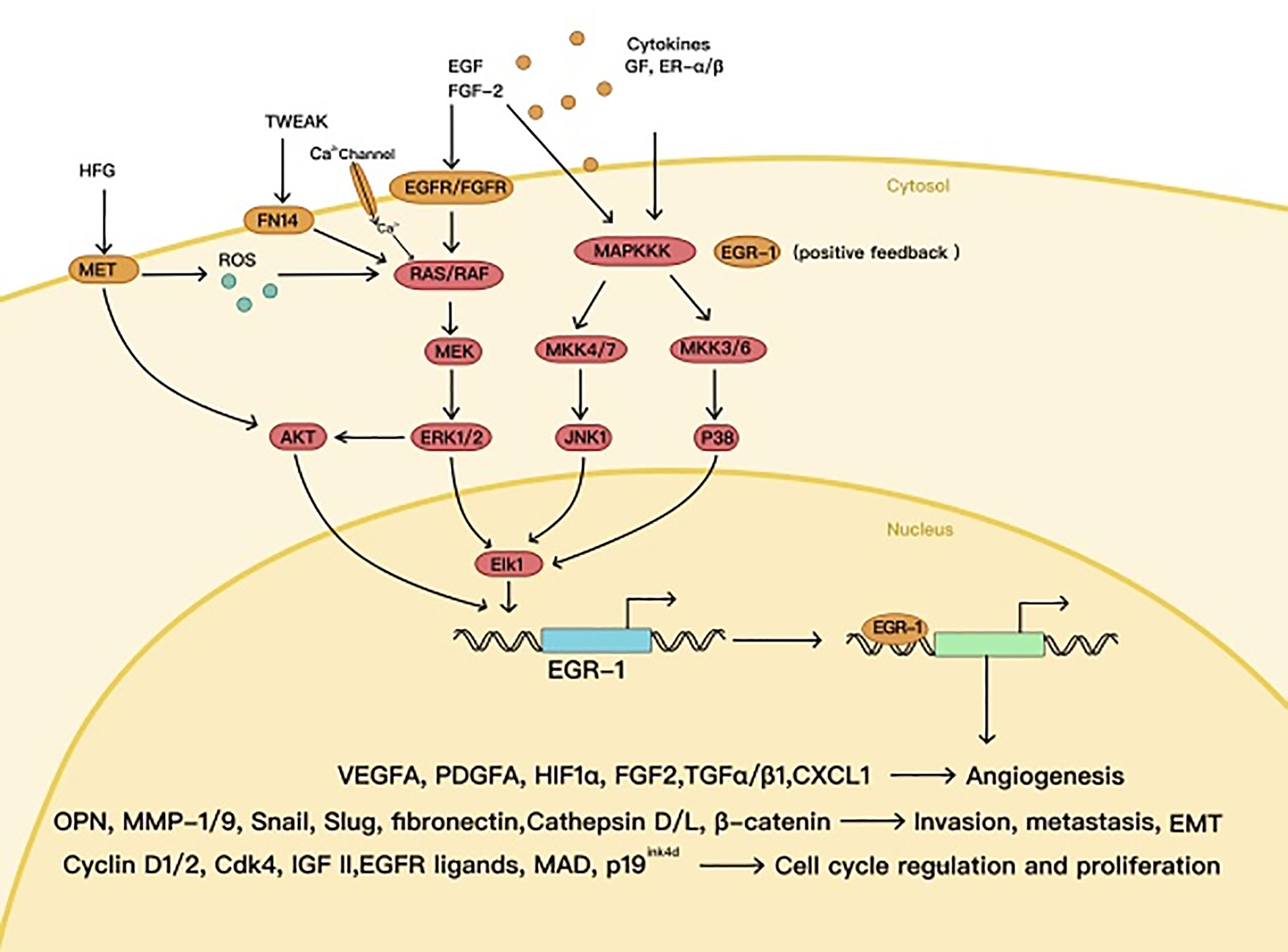
High expression of EGR1 has been observed in various tumors, in which EGR1 can play an oncogenic role, e.g., glioma, lung cancer, gastrointestinal tumors, and melanoma (22–25). As a downstream protein of the MAPK signaling pathway (26), EGR1 can promote transcriptional activation of cyclin D1 in many tumor types and maintain the mitosis of tumor cells (27). Meanwhile, EGR1 contributes to tumor metastasis as well, by starting SLUG and SNAIL expression (28, 29). Under hypoxic conditions, EGR1 increases VEGFA expression, mediated by HIF1α, and directly activates VEGFA transcription (30), thus promoting the formation of blood and lymphatic vessels in the tumor (31) (Figure 2).
The Roles of EGR1 in Cell Cycle Regulation and Tumor Cell Proliferation
Various authors have shown that high EGR1 expression may increase the proliferation of specific types of tumor cells by affecting the cell cycle. For example, the high expression of EGR1 in prostate cancer is a potential precancerous event. The level of the EGR1 protein in prostate cancer tissue positively correlates with the Gleason score, which predicts the tumor proliferation ability, and negatively correlates with the differentiation degree of prostate cancer cells (32). These data indicate that EGR1 participates in prostate cancer progression (33). There has been a similar finding in gastric cancer: patients whose tumor is histologically classified as malignant exhibit higher tumor EGR1 levels and a stronger ability of tumor cells to proliferate (24).
The MAPK–ERK pathway is a classic proliferation signaling cascade that is triggered by growth factors. In C6 cells, estrogen receptor β (ERβ) can launch the RAF–MEK1–ERK–ELK1 signaling pathway and upregulate EGR1 (10). EGR1 expression decreases significantly after treatment with PD98059, a MAPK–ERK pathway inhibitor, suggesting that EGR1 is a downstream gene of the MAPK–ERK pathway (26). Through inhibition of MAPK phosphorylation, the nuclear concentration of EGR1 can be reduced while the proliferation of breast cancer cells can be significantly suppressed (34). Cyclin D1 is necessary for the progression of the G1 cell cycle phase and can shorten this phase, whereas the RAS–RAF–MEK–ERK pathway plays an important role in the initiation of cyclin D1 gene expression (35, 36). EGR1 activated by the MAPK cascade can directly bind to the cyclin D1 promoter, strengthen the expression of cyclin D1, and then may result in cell proliferation by advancing cells from the G1 phase to S phase (27). EGR1 is also able to induce tumor cell proliferation by upregulating other cell cycle–related proteins such as cyclin D2 and CDK4 (28). Overexpressed EGR1 may directly trigger the p38 MAPK signaling pathway too (24). In summary, the mechanism behind the interaction between EGR1 and the MAPK–ERK signaling pathway is a positive feedback process. EGR1 overexpression not only promotes tumor growth but also launches the p38 MAPK signaling pathway. The EGR1-activated MAPK–ERK signaling pathway further enhances EGR1 expression, and the upregulated EGR1 accelerates cell proliferation by controlling the expression of cyclin-dependent kinases (CDKs) (24, 27, 28, 33).
The Participation of EGR1 in Tumor Invasion and Metastasis
Epithelial–mesenchymal transition (EMT) means a morphological change of epithelial cells to mesenchymal cells, which is an important mechanism of tumor cell invasion and metastasis. EGR1 contributes to tumor invasion and metastasis mainly by starting the expression of E-cadherin transcriptional inhibitors (SNAIL and SLUG). In other words, SNAIL and SLUG can inhibit the expression of E-cadherin, and EGR1 plays an important role in tumor EMT by regulating SNAIL and SLUG. In hormone-independent prostate cancer, CXCL5 (also known as ENA78) enhances EGR1 transcription via the RAF–MEK–ERK pathway, thus increasing SNAIL expression, tumor cell metastasis, and EMT (28). In hepatocellular carcinoma cells, EGR1 induced by hepatocyte growth factor (HGF) can directly bind to the promoter region of SNAIL, increase its expression, and lead to tumor cell metastasis (37). In ovarian cancer cells, epidermal growth factor (EGF) has been shown to induce EGR1 and upregulate SLUG, which can decrease the expression of E-cadherin and then enhance tumor metastasis (29). In hepatocellular carcinoma, EGR1 induces SLUG through the ERK–AKT–EGR1–SLUG signaling cascade and stimulates EMT of cancer cells (38).
Additionally, interstitial-space–related genes, such as matrix metalloproteinase 1 (MMP1), MMP9, cathepsin, and zinc finger-binding homeobox 1 (ZEB1), play an important part in tumor metastasis. EGR1 can directly bind to the promoter region of MMP1 and trigger its expression. SNAIL may enhance the expression of interstitial-space–related genes, such as MMP9 and ZEB1. A variety of MAPK pathways, such as ERK1 and -2, JNK, and p38 kinase cascades, participate in the expression of MMP1 caused by tumor necrosis factor alpha (TNF-α) by upregulating EGR1 and promote tumor invasion and metastasis (39). EGR1 can synergistically act with SNAIL on the promoter regions of MMP9 and ZEB1, thereby enhancing their transcription and initiating tumor cell invasion and metastasis (40). In oral squamous cell carcinoma, overexpression of hTERT activates tumor invasiveness by raising the expression of cathepsin D via EGR1 (41). EGR1 is also known to stimulate EMT of non–small cell lung cancer (NSCLC) cells through the mut-p53–EGR1–cathepsin L signaling cascade (42). EGR1 can directly bind to the promoter sequences of a variety of interstitial-space–related genes to start their expression and therefore performs an important function in tumor invasion and metastasis.
In gastric cancer, EGR1 has been reported to enhance tumor cell proliferation and invasion by increasing β-catenin expression (43). In breast cancer, S100A4 drives the nuclear localization of EGR1 by promoting the binding of EGR1 to importin 7, and EGR1 next enhances tumor invasion and metastasis by downregulating β-catenin through the PTEN–AKT–GSK3β signal transduction (44). Consequently, EGR1 may control β-catenin expression and enhance tumor invasion and metastasis, but the specific mechanism is yet to be revealed.
The Roles of EGR1 in Tumor Angiogenesis
Tumor angiogenesis is an important mechanism of tumor growth and metastasis, and many achievements in the treatment of cancers have been made through inhibition of tumor angiogenesis. Due to the rapid growth of a tumor, its central area is often in a state of hypoxia. The latter is a potent angiogenesis-stimulating factor. Hypoxia-inducible factors (HIFs) are upregulated by hypoxic conditions and increase the expression of vascular endothelial growth factor (VEGF) family proteins. Furthermore, HIFs stimulate angiogenesis and support tumor cell survival. Under hypoxic conditions, EGR1 expression in prostate cancer cells increases, and EGR1 directly binds to the HIF1 promoter, causes HIF1 expression, and contributes to tumor angiogenesis (45). EGR1 is reported to directly initiate VEGFA expression in lung cancer cells by binding to the VEGFA promoter and to increase angiogenesis by enhancing HIF1α-mediated VEGFA expression (30).
EGR1 is important for the initiation of the growth and migration of vascular endothelial cells and associated angiogenesis because EGR1 acts via fibroblast growth factor 2 (FGF2) (46–48), which can launch EGR1 expression (49). Furthermore, EGR1 may lead to angiogenesis through the NT1–DCC–VEGF pathway (50). Many types of cancer cells secrete extracellular vesicles, which are known to stimulate the migration of vascular endothelial cells by upregulating EGR1. This is an important angiogenesis-enhancing mechanism (51).
The proliferation and migration of lymphatic endothelial cells is another important adaptive response of tumor cells to hypoxia. EGR1 can participate in hypoxia-induced lymphangiogenesis through the VEGF signal transduction pathway (31), but the underlying molecular mechanism needs further investigation.
Antitumor Effects of EGR1
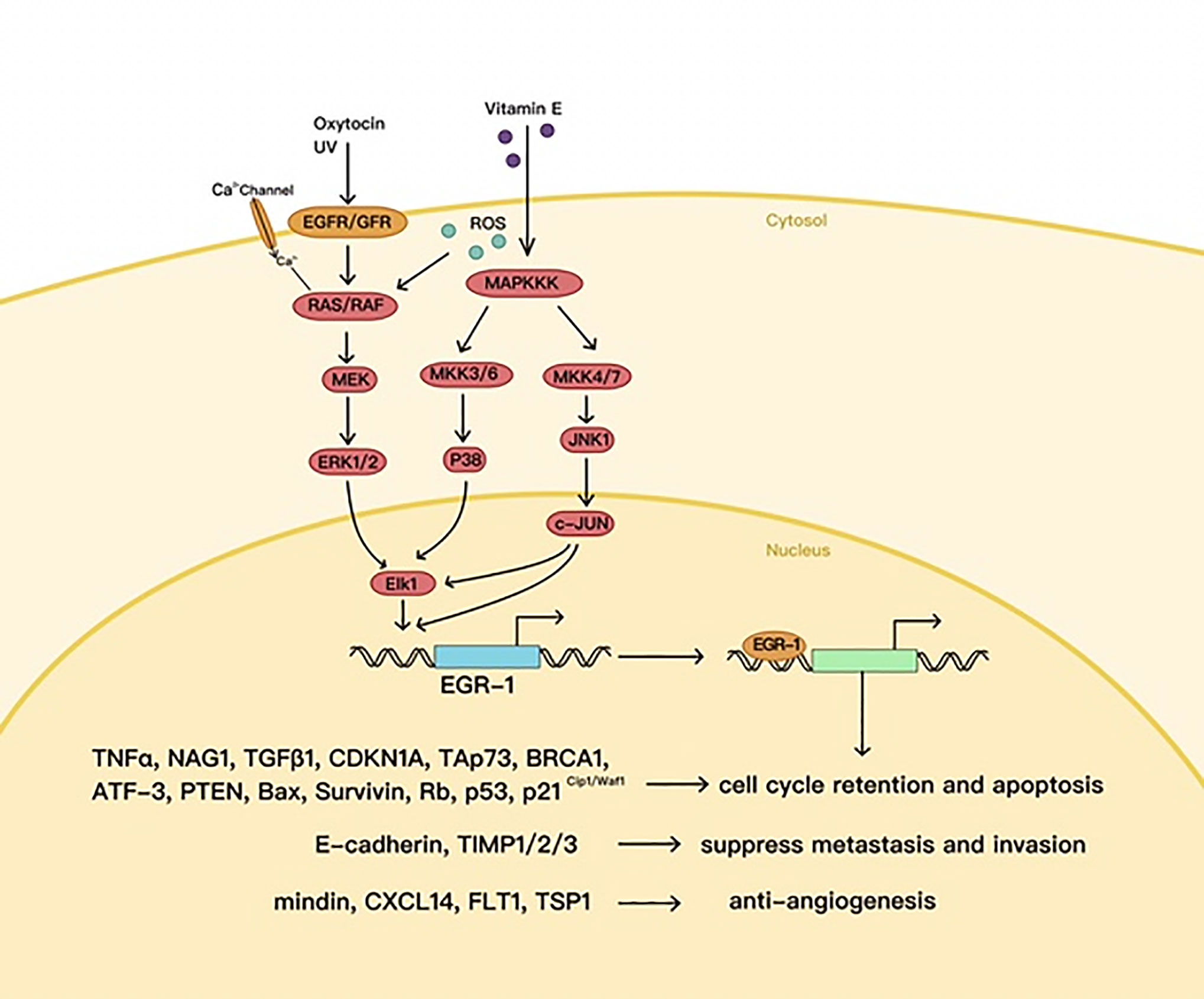
In certain cases, EGR1 plays an antitumor part, e.g., in p53 (TP53)-deficient prostate cells, where EGR1 is believed to promote apoptosis by activating TNF-α (52). EGR1 also increases tumor cell apoptosis by directly upregulating tumor suppressors called non-steroidal anti-inflammatory drug (NSAID)-activated gene 1 (NAG1) and PTEN (53–55) (Figure 3).
Mechanisms Whereby EGR1 Induces Tumor Cell Apoptosis
Apoptosis is a process of programmed cell death under physiological conditions, and EGR1 causes tumor cell apoptosis through a variety of mechanisms. One such mechanism is direct binding to promoters of various apoptosis-inducing factors, such as BAX, NAG1, and PTEN, and stimulation of their expression (8, 56, 57). NAG1 belongs to the transforming growth factor β (TGF-β) superfamily and inhibits the growth of tumor cells. Recently, it was found that an EBS is present in the NAG1 promoter, and EGR1 expression significantly increases NAG1-mediated apoptosis in colon, lung, and liver cancers (53, 54). NSAIDs lead to apoptosis in a COX2-dependent manner by launching the PPARγ–EGR1–NAG1 signaling pathway. In addition, NSAIDs can directly strengthen NAG1 expression that is mediated by EGR1 and promote apoptosis in a COX2-independent manner (8). In pancreatic cancer cells, δ-tocotrienol triggers EGR1 expression via the JNK–c-Jun pathway, and the upregulated EGR1 binds to the BAX promoter to initiate the expression of BAX, which causes apoptosis of pancreatic cancer cells (56). JUN is the upstream transcription factor of EGR1 and is known to directly bind to the promoter region of EGR1 and to start its expression. In multiple myeloma, EGR1 that is induced by JUN triggers the EGR1–survivin–caspase signaling cascade and drives tumor cell apoptosis (58). PTEN is an important tumor suppressor gene, and there is an EBS in the PTEN promoter. EGR1 may regulate PTEN expression by targeting the PTEN promoter, thus resulting in tumor cell apoptosis (55). Research suggests that vitamin D receptor, EGR1, and p300 synergistically initiate PTEN expression and apoptosis in cancer cells (57).
EGR1 may play a key role in the regulation of DNA repair mechanisms by controlling key effectors, including p53, p21, and BRCA1. As a tumor suppressor, p53 is mainly involved in the monitoring of DNA damage and maintenance of genomic stability and is highly implicated in human cancers. EGR1 can act on the TP53 promoter and initiate the expression of p53, which in turn further activates EGR1, thus forming a feedback loop (59, 60). In vitro studies indicate that EGR1-deficient cells are characterized by p53 inactivation and loss of responsiveness to DNA damage, thereby revealing an important function of EGR1 in upstream transcriptional regulation of p53 (61). In p53-deficient prostate cancer cells, EGR1 is thought to stimulate apoptosis by initiating TNF-α expression (52). P21Waf1/Cip1 is the main target of p53 and controls the cell cycle. On the other hand, EGR1 can induce p21Waf1/Cip1 expression independently of p53 through ERK and JNK MAPK–ELK1–EGR1 pathways to launch DNA repair and enhance apoptosis, suggesting that EGR1 is a good therapeutic target in p53-mutant tumors (62). As a tumor suppressor, the product of breast cancer susceptibility gene BRCA1 participates in gene transcription and DNA repair. Three EBSs have been identified in the enhancer region of BRCA1, and EGR1 is able to directly bind to the EBSs and to start BRCA1 gene expression thus initiating DNA repair and inhibiting cancer progression (63).
Mechanisms Whereby EGR1 Suppresses Tumor Invasion and Metastasis
EGR1 has been reported to inhibit tumor invasion and metastasis in many tumor types. TGF-β1 can downregulate EGR1-induced NSCLC cell EMT, and high expression of EGR1 significantly reduces EMT (64). The mechanism may be related to the regulation of SNAIL, SLUG, and E-cadherin expression by EGR1. In head and neck squamous cell carcinoma, oxytocin is thought to inhibit tumor invasion and metastasis by upregulating EGR1 through an EGFR-and-ERK–dependent pathway (65). The mechanism in question may be related to E-cadherin overexpression. In hepatocellular carcinoma, it has been demonstrated that β-lapachone induces the expression of EGR1, and the latter may suppress invasion and metastasis by affecting the expression of TSP1, SNAIL, and E-cadherin (66). Liu et al. have reported that the use of thalidomide against leukemic cells may inhibit their metastasis, and this phenomenon was attributed to upregulated EGR1 (67). In subsequent studies, it has been revealed that LY294002 inhibits the invasiveness and metastasis of leukemic cells by upregulating EGR1, and this mechanism is independent of the PI3K–AKT pathway (68). Overexpressed EGR1 can significantly repress tumor cell invasion in fibrosarcoma, and the underlying mechanism may be related to increased expression of tissue inhibitor of metalloproteinase 2 (TIMP2), which is regulated by EGR1 (69). Nasopharyngeal carcinoma–associated gene 6 (NGX6) is expressed in diverse tumors and is considered a tumor suppressor. EGR1 can directly increase NGX6 expression by binding to its promoter region and in this way inhibit tumor invasion and metastasis (70). Although EGR1 is known to reduce invasion and metastasis of many types of tumors, the specific mechanism needs to be further researched.
Mechanisms Underlying the Antiangiogenic Action of EGR1
Angiogenesis is the formation of new blood vessels from the existing vascular system and is necessary for many physiological and pathological processes. Neovascularization provides nutrition and oxygen and removes carbon dioxide and other metabolic waste. When EGR1 is continuously expressed, a variety of antiangiogenic genes are overexpressed, such as CXCL14, TIMP1, TIMP3, and FLT1, which inhibit tumor angiogenesis (71). EGR1 can also diminish tumor angiogenesis by upregulating TIMP2 (69). According to colon cancer studies, EGR1 controls mindin expression at the transcriptional level by binding to its promoter. Overexpression of mindin both inhibits the expression of HIF1α and VEGFA in colon cancer cells and reduces VEGFR2 phosphorylation in endothelial cells, resulting in antiangiogenic changes (72). NGX6 is a metastasis suppressor gene whose functions are related to cell proliferation, cell cycle, and tumor angiogenesis. Recent research suggests that there are overlapping binding sites for Sp-1 and EGR1 in a NGX6 promoter region and that EGR1 increases NGX6 expression and decreases tumor angiogenesis (70, 73). Fluorouracil is widely used in many cancer therapies and is believed to upregulate EGR1 through the p38 MAPK pathway; this drug inhibits tumor angiogenesis. Overexpressed EGR1 binds to the thrombospondin 1 (TSP1) promoter, enhances TSP1 expression, and diminishes tumor angiogenesis (74, 75). EGR1 can interact with a variety of antiangiogenic factors, such as mindin, NGX6, and TSP1, and may be used as a major target of antivascular therapy to develop relevant drugs.
Applications of EGR1 in Cancer Treatments
The Mechanism of Action of the EGR1-and-Radiotherapy Combination
The promoter of EGR1 contains the characteristic CArG box sequence for SRF binding, which is activated by radiation (76, 77). EGR1 is reported to regulate target genes—after ionizing radiation induces it—e.g., TNF-α, TP53, RB, and BAX, and to cause tumor cell growth arrest or cell death (52, 78). Because EGR1 can lead to the activation of apoptosis-associated factors and downregulate survival-related factors to reduce radiation resistance, EGR1 expression in primary tumors is related to the tumor radiation response, and EGR1 expression in irradiated tissues also correlates with residual tumor size and tumor recurrence (79). The CArG box in the promoter of EGR1 is key to the initiation of the EGR1-related antitumor effect by radiotherapy. Bickenbach K.A. et al. have ligated the CArG box to the transcription start site of TNF cDNA—to construct a vector combining the EGR1 promoter and the tumor-killing gene—and then transfected it into tumor cell lines (2). This vector effectively enhances the killing of radiotherapy-resistant tumor cells by radiation (2). The CArG box in the EGR1 promoter is an effective anticancer tool for suicide gene therapy combined with radiotherapy and has a great potential to improve the efficiency of antitumor therapies.
The Mechanism of Action of EGR1 in Combination With Various Antineoplastic Drugs
One of the key mechanisms of action of CD20-targeting drugs is direct stimulation of cell death, but the signal transduction cascade at work here remains unclear. It has been found that CD20-targeting drugs, such as rituximab and obinutuzumab, strengthen EGR1 expression by increasing calcium influx via different mechanisms and subsequently lead to cell death. Inhibition of calcium influx by calcium channel blockers (CCBs) can prevent EGR1 induction by CD20-targeting drugs and weaken these drugs’ effects in vivo and in vitro. By analyzing the actions of CCBs in patients treated with CD20-targeting drugs in the GOYA and REMARC clinical trials, investigators found that patients who received both a CCB and a CD20-targeting drug showed shorter progression-free survival and overall survival (80). These results mean that EGR1 is the key mediator of cell death that is directly caused by CD20-targeting drugs and offer a rationale for EGR1 use as a new predictive quantitative biomarker of therapeutic responses to CD20-targeting drugs (80).
Certain natural compounds affect the regulation of EGR1 expression too. Quercetin initiates the expression of NAG1 by upregulating EGR1 and mediates apoptosis of colon cancer cells (81). The EGR1-binding site in the promoter region of NAG1 is the key point in the underlying mechanism. Resveratrol stimulates cancer cell apoptosis by upregulating EGR1 (82). Shi et al. have constructed a suicide-causing gene therapy vector by inserting the EGR1 promoter upstream of the GADD45A cDNA gene, and a combination of this vector and resveratrol inhibited the proliferation of lung cancer cells in vitro (83). Studies have shown that resveratrol effectively triggers a suicide-causing gene therapy vector constructed by means of the EGR1 promoter, and the CArG box in the EGR1 promoter may be the target site of resveratrol (84). The CArG box in the promoter of EGR1 can be activated by cisplatin too. Wang et al. have constructed an adenoviral vector that contains the CArG box and human wild-type TP53 gene; this vector enhances the therapeutic response to the antitumor treatment with cisplatin in NSCLC cell-transplanted mice (85). The suicide-causing gene therapy vector constructed by means of the EGR1 promoter sequence is known to be activated by many compounds, such as resveratrol and cisplatin; this knowledge may point to a new strategy for targeted cancer treatment. The investigation into the mechanism of action and molecular interactions of EGR1 in tumors should lead to new breakthroughs in antitumor therapies.
Prognostic Value of EGR1
In lung cancer, the downregulation of PTEN and EGR1 expression is related to tumor drug resistance (86, 87). The expression of EGR1 predetermines PTEN levels, which may predict treatment resistance resulting from PTEN pathway loss. Accordingly, low EGR1 levels are associated with poor prognosis (86). Besides, the EGR1 expression level is closely related to the pathological features and prognosis of patients with nasopharyngeal carcinoma, and high EGR1 expression correlates with a low histopathological grade and good clinical prognosis (88). EGR1 expression is higher in pituitary adenomas than in healthy control tissue samples, and EGR1 levels in invasive pituitary adenomas are even higher (89). EGR1 can serve as a prognostic factor in pituitary adenomas (89). In gastric cancer, EGR1 status is associated with malignancy, tumor stage, and prognosis (13, 14). EGR1 is of great value in the prognosis of the above tumor types, but the specific mechanism of action here and the prognostic value of EGR1 in other cancers need to be further explored.
Discussion
In summary, EGR1 is a key molecule implicated in many signaling pathways. In some cases, EGR1 is a tumor suppressor that helps to monitor DNA damage, promotes tumor cell apoptosis, and enhances the anticancer effects of radiotherapy and chemotherapy. By contrast, in certain tumor microenvironments, such as hypoxic ones, EGR1 expression increases to maintain tumor cell survival, proliferation, and metastasis and tumor angiogenesis. Abnormal EGR1 expression is common among diverse human tumors, but its role requires further research. At present, the specific mechanism underlying the EGR1 “duality” is still unclear but may be linked with the following: Sp-1 expression (70), the expression of other EGR family members (90), the EGR1 self-inhibition zone, and other factors (91). Several of the EGR1 downstream target genes have multiple overlapping SP1 and EGR1 binding sites in their proximal promoter regions. The regulatory relationship in this overlapping area is complex, in some genes the two factors are synergistic, and antagonistic in others (70, 92, 93). The duality of EGR1 is related to the regulatory relationship between the two factors. EGR transcription factor family includes EGR1, EGR2, EGR3, and EGR4. All of them have similar zinc finger structures and can bind to the same EBS. Wang et al. have reported that the EGR family genes can be verified after EGR4 knockdown in pGCs (94). Tourtellotte et al. showed that EGR4 loss upregulated EGR1 and slightly altered EGR2 and EGR3 (95). When any one of the coding genes in the EGR family is knockdown, there will be a competitive effect on the expression of the other three. NAB1 and NAB2 are repressor proteins of EGR1, which can bind to the zinc finger structures of EGR1. EGR1 can bind to the promoter region of NAB1 and NAB2 to induce their expression. When EGR1 was overexpressed, EGR1 upregulated the expression of NAB1 and NAB2, and negative feedback regulated its own expression. Thus the expression of downstream genes is regulated by EGR1 itself (91, 96). Thus, the mechanisms of action of EGR1 in various cancers require additional exploration. Determining and exploiting the involvement of EGR1 in cancer will help develop new therapies for patients with malignant tumors.
Author Contributions
BW carried out the primary literature search, drafted and revised the manuscript, and participated in discussions. HG, HY, HX, and GZ helped modify the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by National Nature and Science Foundation of China (81772684 and 81672505), Scientific Research Foundation of Jilin province (20200201469JC, 20200404101YY, 20200201613JC, 20200201388JC, 20190701042GH, 20180101152JC), Health and Wellness Technology Enhancement Project of Jilin Province (2019J004 and 2017J045), Research and Planning Project of the 13th Five-Year Science and Technology Project of Jilin Provincial Department of Education (JJKH20201077KJ and JJKH20180191KJ) and Interdisciplinary Innovation Project of First Hospital of Jilin University (JDYYJC001).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Sukhatme V, Cao X, Chang L, Tsai-Morris C, Stamenkovich D, Ferreira P, et al. A zinc finger-encoding gene coregulated with c-fos during growth and differentiation, and after cellular depolarization. Cell (1988) 53(1):37–43. doi: 10.1016/0092-8674(88)90485-0
2. Bickenbach KA, Veerapong J, Shao MY, Mauceri HJ, Posner MC, Kron SJ, et al. Resveratrol is an effective inducer of CArG-driven TNF-alpha gene therapy. Cancer Gene Ther (2008) 15(3):133–9. doi: 10.1038/sj.cgt.7701103
3. Marignol L, Coffey M, Hollywood D, Lawler M. Radiation to control transgene expression in tumors. Cancer Biol Ther (2007) 6(7):1005–12. doi: 10.4161/cbt.6.7.4477
4. Pagel J, Deindl E. Early growth response 1–a transcription factor in the crossfire of signal transduction cascades. Indian J Biochem Biophys (2011) 48(4):226–35.
5. Cao X, Mahendran R, Guy G, Tan Y. Detection and characterization of cellular EGR-1 binding to its recognition site. J Biol Chem (1993) 268(23):16949–57. doi: 10.1016/S0021-9258(19)85286-9
6. Jeong S, Kim H, Ryu H, Ryu W, Park Y, Bae H, et al. ZnO nanoparticles induce TNF-α expression via ROS-ERK-Egr-1 pathway in human keratinocytes. J Dermatol Sci (2013) 72(3):263–73. doi: 10.1016/j.jdermsci.2013.08.002
7. Rockel J, Bernier S, Leask A. Egr-1 inhibits the expression of extracellular matrix genes in chondrocytes by TNFalpha-induced MEK/ERK signalling. Arthritis Res Ther (2009) 11(1):R8. doi: 10.1186/ar2595
8. Vaish V, Piplani H, Rana C, Vaiphei K, Sanyal S. NSAIDs may regulate EGR-1-mediated induction of reactive oxygen species and non-steroidal anti-inflammatory drug-induced gene (NAG)-1 to initiate intrinsic pathway of apoptosis for the chemoprevention of colorectal cancer. Mol Cell Biochem (2013) 378:47–64. doi: 10.1007/s11010-013-1593-y
9. Shin S, Kim H, Jang H, Hwang Y, Choe J, Kim J, et al. γ-Oryzanol suppresses COX-2 expression by inhibiting reactive oxygen species-mediated Erk1/2 and Egr-1 signaling in LPS-stimulated RAW264.7 macrophages. Biochem Biophys Res Commun (2017) 491(2):486–92. doi: 10.1016/j.bbrc.2017.07.016
10. Kim JH, Jeong IY, Lim Y, Lee YH, Shin SY. Estrogen receptor beta stimulates Egr-1 transcription via MEK1/Erk/Elk-1 cascade in C6 glioma cells. BMB Rep (2011) 44(7):452–7. doi: 10.5483/BMBRep.2011.44.7.452
11. Gabriel KN, Jones AC, Nguyen JP, Antillon KS, Janos SN, Overton HN, et al. Association and regulation of protein factors of field effect in prostate tissues. Int J Oncol (2016) 49(4):1541–52. doi: 10.3892/ijo.2016.3666
12. Li L, Ameri A, Wang S, Jansson K, Casey O, Yang Q, et al. EGR1 regulates angiogenic and osteoclastogenic factors in prostate cancer and promotes metastasis. Oncogene (2019) 38(35):6241–55. doi: 10.1038/s41388-019-0873-8
13. Ma Z, Gao X, Shuai Y, Wu X, Yan Y, Xing X, et al. EGR1-mediated linc01503 promotes cell cycle progression and tumorigenesis in gastric cancer. Cell Proliferation (2020) 54(1):e12922. doi: 10.1111/cpr.12922
14. Myung E, Park YL, Kim N, Chung CY, Park HB, Park HC, et al. Expression of early growth response-1 in human gastric cancer and its relationship with tumor cell behaviors and prognosis. Pathol Res Pract (2013) 209(11):692–9. doi: 10.1016/j.prp.2013.08.001
15. Calogero A, Arcella A, De Gregorio G, Porcellini A, Mercola D, Liu C, et al. The early growth response gene EGR-1 behaves as a suppressor gene that is down-regulated independent of ARF/Mdm2 but not p53 alterations in fresh human gliomas. Clin Cancer Res (2001) 7(9):2788–96.
16. Schmidt K, Carroll JS, Yee E, Thomas DD, Wert-Lamas L, Neier SC, et al. The lncRNA SLNCR Recruits the Androgen Receptor to EGR1-Bound Genes in Melanoma and Inhibits Expression of Tumor Suppressor p21. Cell Rep (2019) 27(8):2493–507.e4. doi: 10.1016/j.celrep.2019.04.101
17. de Belle I, Huang RP, Fan Y, Liu C, Mercola D, Adamson ED. p53 and Egr-1 additively suppress transformed growth in HT1080 cells but Egr-1 counteracts p53-dependent apoptosis. Oncogene (1999) 18(24):3633–42. doi: 10.1038/sj.onc.1202696
18. Ryu J, Choe S, Ryu S, Park E, Lee B, Kim T, et al. Paradoxical induction of growth arrest and apoptosis by EGF via the up-regulation of PTEN by activating Redox factor-1/Egr-1 in human lung cancer cells. Oncotarget (2017) 8(3):4181–95. doi: 10.18632/oncotarget.13809
19. Du K, Wu X, Ji X, Liang N, Li Z. Early growth response 1 promoted the invasion of glioblastoma multiforme by elevating HMGB1. J Neurosurg Sci (2020). doi: 10.23736/S0390-5616.20.05107-3
20. Chen J, Zhan Y, Xu J, Wang Y, Gao Q. EGR1 Overexpression Inhibits the Occurrence of Preeclampsia by Binding to MicroRNA-574 Promoter and Upregulating GAB1. Reprod Sci (Thousand Oaks Calif) (2020) 28:1112–21. doi: 10.1007/s43032-020-00392-3
21. Wu Y, Li D, Wang Y, Liu X, Zhang Y, Qu W, et al. Pseudomonas aeruginosaBeta-Defensin 2 and 3 Promote Bacterial Clearance of by Inhibiting Macrophage Autophagy through Downregulation of Early Growth Response Gene-1 and c-FOS. Front Immunol (2018) 9:211. doi: 10.3389/fimmu.2018.00211
22. Knudsen AM, Eilertsen I, Kielland S, Pedersen MW, Sørensen MD, Dahlrot RH, et al. Expression and prognostic value of the transcription factors EGR1 and EGR3 in gliomas. Sci Rep (2020) 10(1):9285. doi: 10.1038/s41598-020-66236-x
23. Feng YH, Su YC, Lin SF, Lin PR, Wu CL, Tung CL, et al. Oct4 upregulates osteopontin via Egr1 and is associated with poor outcome in human lung cancer. BMC Cancer (2019) 19(1):791. doi: 10.1186/s12885-019-6014-5
24. Park SY, Kim JY, Lee SM, Chung JO, Lee KH, Jun CH, et al. Expression of early growth response gene-1 in precancerous lesions of gastric cancer. Oncol Lett (2016) 12(4):2710–5. doi: 10.3892/ol.2016.4962
25. Liu J, Grogan L, Nau MM, Allegra CJ, Chu E, Wright JJ. Physical interaction between p53 and primary response gene Egr-1. Int J Oncol (2001) 18(4):863–70. doi: 10.3892/ijo.18.4.863
26. Schwachtgen JL, Houston P, Campbell C, Sukhatme V, Braddock M. Fluid shear stress activation of egr-1 transcription in cultured human endothelial and epithelial cells is mediated via the extracellular signal-related kinase 1/2 mitogen-activated protein kinase pathway. J Clin Invest (1998) 101(11):2540–9. doi: 10.1172/JCI1404
27. Xiao D, Chinnappan D, Pestell R, Albanese C, Weber HC. Bombesin regulates cyclin D1 expression through the early growth response protein Egr-1 in prostate cancer cells. Cancer Res (2005) 65(21):9934–42. doi: 10.1158/0008-5472.CAN-05-1830
28. Kuo PL, Chen YH, Chen TC, Shen KH, Hsu YL. CXCL5/ENA78 increased cell migration and epithelial-to-mesenchymal transition of hormone-independent prostate cancer by early growth response-1/snail signaling pathway. J Cell Physiol (2011) 226(5):1224–31. doi: 10.1002/jcp.22445
29. Cheng JC, Chang HM, Leung PC. Egr-1 mediates epidermal growth factor-induced downregulation of E-cadherin expression via Slug in human ovarian cancer cells. Oncogene (2013) 32(8):1041–9. doi: 10.1038/onc.2012.127
30. Shimoyamada H, Yazawa T, Sato H, Okudela K, Ishii J, Sakaeda M, et al. Early growth response-1 induces and enhances vascular endothelial growth factor-A expression in lung cancer cells. Am J Pathol (2010) 177(1):70–83. doi: 10.2353/ajpath.2010.091164
31. Ji RC. Hypoxia and lymphangiogenesis in tumor microenvironment and metastasis. Cancer Lett (2014) 346(1):6–16. doi: 10.1016/j.canlet.2013.12.001
32. Gregg J, Fraizer G. Transcriptional Regulation of EGR1 by EGF and the ERK Signaling Pathway in Prostate Cancer Cells. Genes Cancer (2011) 2(9):900–9. doi: 10.1177/1947601911431885
33. Gitenay D, Baron VT. Is EGR1 a potential target for prostate cancer therapy? Future Oncol (2009) 5(7):993–1003. doi: 10.2217/fon.09.67
34. Johnson AE, Le IP, Buchwalter A, Burnatowska-Hledin MA. Estrogen-dependent growth and estrogen receptor (ER)-alpha concentration in T47D breast cancer cells are inhibited by VACM-1, a cul 5 gene. Mol Cell Biochem (2007) 301:13–20. doi: 10.1007/s11010-006-9392-3
35. Yaari S, Jacob-Hirsch J, Amariglio N, Haklai R, Rechavi G, Kloog Y. Disruption of cooperation between Ras and MycN in human neuroblastoma cells promotes growth arrest. Clin Cancer Res (2005) 11(12):4321–30. doi: 10.1158/1078-0432.CCR-04-2071
36. Wan L, Chen M, Cao J, Dai X, Yin Q, Zhang J, et al. The APC/C E3 Ligase Complex Activator FZR1 Restricts BRAF Oncogenic Function. Cancer Discovery (2017) 7(4):424–41. doi: 10.1158/2159-8290.CD-16-0647
37. Grotegut S, von Schweinitz D, Christofori G, Lehembre F. Hepatocyte growth factor induces cell scattering through MAPK/Egr-1-mediated upregulation of Snail. EMBO J (2006) 25(15):3534–45. doi: 10.1038/sj.emboj.7601213
38. Chen HA, Kuo TC, Tseng CF, Ma JT, Yang ST, Yen CJ, et al. Angiopoietin-like protein 1 antagonizes MET receptor activity to repress sorafenib resistance and cancer stemness in hepatocellular carcinoma. Hepatol (Baltimore Md) (2016) 64(5):1637–51. doi: 10.1002/hep.28773
39. Yeo H, Lee J, Kim J, Ahn S, Jeong J, Choi J, et al. Transcription factor EGR-1 transactivates the MMP1 gene promoter in response to TNFα in HaCaT keratinocytes. BMB Rep (2020) 53(6):323–8. doi: 10.5483/BMBRep.2020.53.6.290
40. Wu W, You R, Cheng C, Lee M, Lin T, Hu C. Snail collaborates with EGR-1 and SP-1 to directly activate transcription of MMP 9 and ZEB1. Sci Rep (2017) 7(1):17753. doi: 10.1038/s41598-017-18101-7
41. Park YJ, Kim EK, Bae JY, Moon S, Kim J. Human telomerase reverse transcriptase (hTERT) promotes cancer invasion by modulating cathepsin D via early growth response (EGR)-1. Cancer Lett (2016) 370(2):222–31. doi: 10.1016/j.canlet.2015.10.021
42. Wang W, Xiong Y, Ding X, Wang L, Zhao Y, Fei Y, et al. Cathepsin L activated by mutant p53 and Egr-1 promotes ionizing radiation-induced EMT in human NSCLC. J Exp Clin Cancer Res CR (2019) 38(1):61. doi: 10.1186/s13046-019-1054-x
43. Sun T, Tian H, Feng Y, Zhu Y, Zhang W. Egr-1 promotes cell proliferation and invasion by increasing β-catenin expression in gastric cancer. Dig Dis Sci (2013) 58(2):423–30. doi: 10.1007/s10620-012-2356-4
44. Yang W, Nam K, Ju J, Lee K, Oh S, Shin I. S100A4 negatively regulates β-catenin by inducing the Egr-1-PTEN-Akt-GSK3β degradation pathway. Cell Signal (2014) 26(10):2096–106. doi: 10.1016/j.cellsig.2014.06.007
45. Sperandio S, Fortin J, Sasik R, Robitaille L, Corbeil J, de Belle I. The transcription factor Egr1 regulates the HIF-1alpha gene during hypoxia. Mol Carcinog (2009) 48(1):38–44. doi: 10.1002/mc.20454
46. Kundumani-Sridharan V, Niu J, Wang D, Van Quyen D, Zhang Q, Singh N, et al. 15(S)-hydroxyeicosatetraenoic acid-induced angiogenesis requires Src-mediated Egr-1-dependent rapid induction of FGF-2 expression. Blood (2010) 115(10):2105–16. doi: 10.1182/blood-2009-09-241802
47. Brown K, Lau J, Dom A, Witte T, Luo H, Crabtree C, et al. MG624, an α7-nAChR antagonist, inhibits angiogenesis via the Egr-1/FGF2 pathway. Angiogenesis (2012) 15(1):99–114. doi: 10.1007/s10456-011-9246-9
48. Fahmy R, Dass C, Sun L, Chesterman C, Khachigian L. Transcription factor Egr-1 supports FGF-dependent angiogenesis during neovascularization and tumor growth. Nat Med (2003) 9(8):1026–32. doi: 10.1038/nm905
49. Suda M, Shimizu I, Yoshida Y, Hayashi Y, Ikegami R, Katsuumi G, et al. Inhibition of dipeptidyl peptidase-4 ameliorates cardiac ischemia and systolic dysfunction by up-regulating the FGF-2/EGR-1 pathway. PloS One (2017) 12(8):e0182422. doi: 10.1371/journal.pone.0182422
50. Sheng J, Liu D, Kang X, Chen Y, Jiang K, Zheng W. Egr-1 increases angiogenesis in cartilage via binding Netrin-1 receptor DCC promoter. J Orthop Surg Res (2018) 13(1):125. doi: 10.1186/s13018-018-0826-x
51. Yoon Y, Kim D, Yoon C, Park J, Kim Y, Roh T, et al. Egr-1 activation by cancer-derived extracellular vesicles promotes endothelial cell migration via ERK1/2 and JNK signaling pathways. PloS One (2014) 9(12):e115170. doi: 10.1371/journal.pone.0115170
52. Ahmed MM, Sells SF, Venkatasubbarao K, Fruitwala SM, Muthukkumar S, Harp C, et al. Ionizing radiation-inducible apoptosis in the absence of p53 linked to transcription factor EGR-1. J Biol Chem (1997) 272(52):33056–61. doi: 10.1074/jbc.272.52.33056
53. Shin DY, Kim GY, Kim ND, Jung JH, Kim SK, Kang HS, et al. Induction of apoptosis by pectenotoxin-2 is mediated with the induction of DR4/DR5, Egr-1 and NAG-1, activation of caspases and modulation of the Bcl-2 family in p53-deficient Hep3B hepatocellular carcinoma cells. Oncol Rep (2008) 19(2):517–26. doi: 10.3892/or.19.2.517
54. Chen YL, Lin PC, Chen SP, Lin CC, Tsai NM, Cheng YL, et al. Activation of nonsteroidal anti-inflammatory drug-activated gene-1 via extracellular signal-regulated kinase 1/2 mitogen-activated protein kinase revealed a isochaihulactone-triggered apoptotic pathway in human lung cancer A549 cells. J Pharmacol Exp Ther (2007) 323(2):746–56. doi: 10.1124/jpet.107.126193
55. Virolle T, Adamson ED, Baron V, Birle D, Mercola D, Mustelin T, et al. The Egr-1 transcription factor directly activates PTEN during irradiation-induced signalling. Nat Cell Biol (2001) 3(12):1124–8. doi: 10.1038/ncb1201-1124
56. Wang C, Husain K, Zhang A, Centeno BA, Chen DT, Tong Z, et al. EGR-1/Bax pathway plays a role in vitamin E δ-tocotrienol-induced apoptosis in pancreatic cancer cells. J Nutr Biochem (2015) 26(8):797–807. doi: 10.1016/j.jnutbio.2015.02.008
57. Pan L, Matloob AF, Du J, Pan H, Dong Z, Zhao J, et al. Vitamin D stimulates apoptosis in gastric cancer cells in synergy with trichostatin A /sodium butyrate-induced and 5-aza-2’-deoxycytidine-induced PTEN upregulation. FEBS J (2010) 277(4):989–99. doi: 10.1111/j.1742-4658.2009.07542.x
58. Chen L, Wang S, Zhou Y, Wu X, Entin I, Epstein J, et al. Identification of early growth response protein 1 (EGR-1) as a novel target for JUN-induced apoptosis in multiple myeloma. Blood (2010) 115(1):61–70. doi: 10.1182/blood-2009-03-210526
59. Nair P, Muthukkumar S, Sells S, Han S, Sukhatme V, Rangnekar V. Early growth response-1-dependent apoptosis is mediated by p53. J Biol Chem (1997) 272(32):20131–8. doi: 10.1074/jbc.272.32.20131
60. Yu J, Baron V, Mercola D, Mustelin T, Adamson E. A network of p73, p53 and Egr1 is required for efficient apoptosis in tumor cells. Cell Death Differ (2007) 14(3):436–46. doi: 10.1038/sj.cdd.4402029
61. Krones-Herzig A, Adamson E, Mercola D. Early growth response 1 protein, an upstream gatekeeper of the p53 tumor suppressor, controls replicative senescence. Proc Natl Acad Sci U S A (2003) 100(6):3233–8. doi: 10.1073/pnas.2628034100
62. Choi BH, Kim CG, Bae YS, Lim Y, Lee YH, Shin SY. p21 Waf1/Cip1 expression by curcumin in U-87MG human glioma cells: role of early growth response-1 expression. Cancer Res (2008) 68(5):1369–77. doi: 10.1158/0008-5472.CAN-07-5222
63. Shin SY, Chang GK, Lee YH. Egr-1 regulates the transcription of the BRCA1 gene by etoposide. BMB Rep (2013) 46(2):92–6. doi: 10.5483/bmbrep.2013.46.2.202
64. Shan L, Song Y, Su D, Liu Y, Shi X, Lu S. Early Growth Response Protein-1 Involves in Transforming Growth factor-β1 Induced Epithelial-Mesenchymal Transition and Inhibits Migration of Non-Small-Cell Lung Cancer Cells. Asian Pac J Cancer Prev APJCP (2015) 16(9):4137–42. doi: 10.7314/APJCP.2015.16.9.4137
65. Kim J, Kang S, Lee H, Choi S, Hong S. Oxytocin inhibits head and neck squamous cell carcinoma cell migration by early growth response-1 upregulation. Anti-cancer Drugs (2017) 28(6):613–22. doi: 10.1097/CAD.0000000000000501
66. Kim S, Kwon J, Jeong Y, Kim G, Kim N, Choi Y. Induction of Egr-1 is associated with anti-metastatic and anti-invasive ability of beta-lapachone in human hepatocarcinoma cells. Biosci Biotechnol Biochem (2007) 71(9):2169–76. doi: 10.1271/bbb.70103
67. Liu P, Li J, Lu H, Xu B. Thalidomide inhibits leukemia cell invasion and migration by upregulation of early growth response gene 1. Leuk Lymphoma (2009) 50(1):109–13. doi: 10.1080/10428190802588352
68. Liu P, Xu B, Li J, Lu H. LY294002 inhibits leukemia cell invasion and migration through early growth response gene 1 induction independent of phosphatidylinositol 3-kinase-Akt pathway. Biochem Biophys Res Commun (2008) 377(1):187–90. doi: 10.1016/j.bbrc.2008.09.094
69. Huang R, Li S, Yang W, Chen L, Yao C, Huang R. Early Growth Response-1 Suppresses Human Fibrosarcoma Cell Invasion and Angiogenesis. Cancer Genomics Proteomics (2006) 3(2):71–82.
70. Liu M, Wang X, Peng Y, Shen S, Li G. Egr-1 regulates the transcription of NGX6 gene through a Sp1/Egr-1 overlapping site in the promoter. BMC Mol Biol (2014) 15:14. doi: 10.1186/1471-2199-15-14
71. Lucerna M, Pomyje J, Mechtcheriakova D, Kadl A, Gruber F, Bilban M, et al. Sustained expression of early growth response protein-1 blocks angiogenesis and tumor growth. Cancer Res (2006) 66(13):6708–13. doi: 10.1158/0008-5472.CAN-05-2732
72. Wang L, Liu Y, Yang B, Li P, Cheng X, Xiao C, et al. The extracellular matrix protein mindin attenuates colon cancer progression by blocking angiogenesis via Egr-1-mediated regulation. Oncogene (2018) 37(5):601–15. doi: 10.1038/onc.2017.359
73. Xiao J, Mao Y, Zhou Y, Xiang K. Clinicopathological significance of NGX6 expression in breast cancer and its relationship to angiogenesis. Int J Clin Exp Med (2015) 8(12):22198–203.
74. Zhao H, Ooyama A, Yamamoto M, Ikeda R, Haraguchi M, Tabata S, et al. Molecular basis for the induction of an angiogenesis inhibitor, thrombospondin-1, by 5-fluorouracil. Cancer Res (2008) 68(17):7035–41. doi: 10.1158/0008-5472.CAN-07-6496
75. Zhao H, Ooyama A, Yamamoto M, Ikeda R, Haraguchi M, Tabata S, et al. Down regulation of c-Myc and induction of an angiogenesis inhibitor, thrombospondin-1, by 5-FU in human colon cancer KM12C cells. Cancer Lett (2008) 270(1):156–63. doi: 10.1016/j.canlet.2008.04.045
76. Spencer JA, Major ML, Misra RP. Basic fibroblast growth factor activates serum response factor gene expression by multiple distinct signaling mechanisms. Mol Cell Biol (1999) 19(6):3977–88. doi: 10.1128/MCB.19.6.3977
77. Naoum G, Zhu Z, Buchsbaum D, Curiel D, Arafat W. Survivin a radiogenetic promoter for glioblastoma viral gene therapy independently from CArG motifs. Clin Trans Med (2017) 6(1):11. doi: 10.1186/s40169-017-0140-y
78. Das A, Chendil D, Dey S, Mohiuddin M, Mohiuddin M, Milbrandt J, et al. Ionizing radiation down-regulates p53 protein in primary Egr-1-/- mouse embryonic fibroblast cells causing enhanced resistance to apoptosis. J Biol Chem (2001) 276(5):3279–86. doi: 10.1074/jbc.M008454200
79. Ahmed MM. Regulation of radiation-induced apoptosis by early growth response-1 gene in solid tumors. Curr Cancer Drug Targets (2004) 4(1):43–52. doi: 10.2174/1568009043481704
80. Spasevska I, Matera E, Chettab K, Ville J, Potier-Cartereau M, Jordheim L, et al. Calcium Channel Blockers Impair the Antitumor Activity of Anti-CD20 Monoclonal Antibodies by Blocking EGR-1 Induction. Mol Cancer Ther (2020) 19:2371–81. doi: 10.1158/1535-7163.MCT-19-0839
81. Lim JH, Park JW, Min DS, Chang JS, Lee YH, Park YB, et al. NAG-1 up-regulation mediated by EGR-1 and p53 is critical for quercetin-induced apoptosis in HCT116 colon carcinoma cells. Apoptosis (2007) 12(2):411–21. doi: 10.1007/s10495-006-0576-9
82. Thiel G, Rössler OG. Resveratrol regulates gene transcription via activation of stimulus-responsive transcription factors. Pharmacol Res (2017) 117:166–76. doi: 10.1016/j.phrs.2016.12.029
83. Shi Q, Bhatia D. Resveratrol-Responsive CArG Elements from the Egr-1 Promoter for the Induction of GADD45α to Arrest the G2/M Transition. Methods Mol Biol (2019) 1895:111–22. doi: 10.1007/978-1-4939-8922-5_9
84. Shi Q, Sutariya V, Varghese Gupta S, Bhatia D. GADD45α-targeted suicide gene therapy driven by synthetic CArG promoter E9NS sensitizes NSCLC cells to cisplatin, resveratrol, and radiation regardless of p53 status. OncoTargets Ther (2019) 12:3161–70. doi: 10.2147/OTT.S192061
85. Wang WD, Li R, Chen ZT, Li DZ, Duan YZ, Cao ZH. Cisplatin-controlled p53 gene therapy for human non-small cell lung cancer xenografts in athymic nude mice via the CArG elements. Cancer Sci (2005) 96(10):706–12. doi: 10.1111/j.1349-7006.2005.00105.x
86. Ferraro B, Bepler G, Sharma S, Cantor A, Haura EB. EGR1 predicts PTEN and survival in patients with non-small-cell lung cancer. J Clin Oncol (2005) 23(9):1921–6. doi: 10.1200/JCO.2005.08.127
87. Maeda M, Murakami Y, Watari K, Kuwano M, Izumi I, Ono M, et al. CpG hypermethylation contributes to decreased expression of PTEN during acquired resistance to gefitinib in human lung cancer cell lines. Lung Cancer (2015). doi: 10.1016/j.lungcan.2015.01.009
88. Zhong J, Wang H, Yu J, Zhang J, Wang S, Yang X, et al. Correlations of the expressions of c-Jun and Egr-1 proteins with clinicopathological features and prognosis of patients with nasopharyngeal carcinoma. Cancer Biomarkers Section A Dis Markers (2017) 19(2):213–20. doi: 10.3233/CBM-161710
89. Sun S, Fang X, Li Y, Wang Q, Li Y. Expression and clinical significance of EGR-1 and PTEN in the pituitary tumors of elderly patients. Oncol Lett (2017) 14(2):2165–9. doi: 10.3892/ol.2017.6375
90. Mayer S, Rössler O, Endo T, Charnay P, Thiel G. Epidermal-growth-factor-induced proliferation of astrocytes requires Egr transcription factors. J Cell Sci (2009) 122:3340–50. doi: 10.1242/jcs.048272
91. Lawson M, Tsutsumi R, Zhang H, Talukdar I, Butler B, Santos S, et al. Pulse sensitivity of the luteinizing hormone beta promoter is determined by a negative feedback loop Involving early growth response-1 and Ngfi-A binding protein 1 and 2. Mol Endocrinol (Baltimore Md) (2007) 21(5):1175–91. doi: 10.1210/me.2006-0392
92. Snyder R, Thekkumkara T. Interplay between EGR1 and SP1 is critical for 13-cis retinoic acid-mediated transcriptional repression of angiotensin type 1A receptor. J Mol Endocrinol (2013) 50(3):361–74. doi: 10.1530/JME-12-0154
93. Cui M, Penn M, Chisolm G. Native and oxidized low density lipoprotein induction of tissue factor gene expression in smooth muscle cells is mediated by both Egr-1 and Sp1. J Biol Chem (1999) 274(46):32795–802. doi: 10.1074/jbc.274.46.32795
94. Wang J, Zhao Y, Gu K, Yu P, Zhang B, Wang W, et al. The novel porcine gene early growth response 4 (Egr4) is differentially expressed in the ovaries of Erhualian and Pietrain pigs. Reprod Fertil Dev (2014) 26(4):587–98. doi: 10.1071/RD12380
95. O’Donovan K, Tourtellotte W, Millbrandt J, Baraban J. The EGR family of transcription-regulatory factors: progress at the interface of molecular and systems neuroscience. Trends Neurosci (1999) 22(4):167–73. doi: 10.1016/S0166-2236(98)01343-5
Keywords: EGR1, cancer, proliferation, apoptosis, angiogenesis, invasion, migration, metastasis
Citation: Wang B, Guo H, Yu H, Chen Y, Xu H and Zhao G (2021) The Role of the Transcription Factor EGR1 in Cancer. Front. Oncol. 11:642547. doi: 10.3389/fonc.2021.642547
Received: 16 December 2020; Accepted: 25 February 2021;
Published: 24 March 2021.
Edited by:
Isacco Ferrarini, Brown University, United StatesReviewed by:
Yao Tong, The Scripps Research Institute, United StatesXiang Zhao, Huazhong University of Science and Technology, China
Copyright © 2021 Wang, Guo, Yu, Chen, Xu and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gang Zhao, Z3poYW9Aamx1LmVkdS5jbg==; Haiyang Xu, eHVoYWl5QGpsdS5lZHUuY24=
 Bin Wang
Bin Wang Hanfei Guo
Hanfei Guo Hongquan Yu
Hongquan Yu Yong Chen
Yong Chen Haiyang Xu
Haiyang Xu Gang Zhao
Gang Zhao