- 1Laboratory of Biological Chemistry, School of Medicine, Faculty of Health Sciences, Aristotle University of Thessaloniki, Thessaloniki, Greece
- 21st Propedeutic Department of Surgery, American Hellenic Educational Progressive Association (AHEPA) University Hospital, Aristotle University of Thessaloniki, Thessaloniki, Greece
- 3Laboratory of Medical Biology, School of Medicine, Faculty of Health Science, Aristotle University of Thessaloniki, Thessaloniki, Greece
- 4Department of Surgery, Faculty of Medicine, The Hashemite University, Zarqa, Jordan
Introduction: Papillary thyroid cancer (PTC) accounts for up to 80% of thyroid malignancies. New diagnostic and therapeutic options are suggested including innovative molecular methods. MicroRNAs (miRNAs) are nonprotein coding single-stranded RNAs that regulate many cell processes. The aim of the present study is to review the deregulated miRNAs associated with PTCs.
Methods: A bibliographic research was conducted, resulting in 272 articles referred to miRNAs and PTC. Regarding our exclusion criteria, 183 articles were finally included in our review.
Results: A remarkably large number of miRNAs have been found to be deregulated during PTC manifestation in the literature. The deregulated miRNAs are detected in tissue samples, serum/plasma, and FNA samples of patients with PTC. These miRNAs are related to several molecular pathways, involving genes and proteins responsible for important biological processes. MiRNA deregulation is associated with tumor aggressiveness, including larger tumor size, multifocality, extrathyroidal extension, lymphovascular invasion, lymph node and distant metastasis, and advanced tumor node metastasis stage.
Conclusion: MiRNAs are proposed as new diagnostic and therapeutic tools regarding PTC. They could be essential biomarkers for PTC diagnosis applied in serum and FNA samples, while their contribution to prognosis is of great importance.
Introduction
Thyroid cancer is the fifth most frequent cancer in women and gains the forefront among endocrine gland malignancies. Papillary thyroid cancer (PTC) is the most common type of thyroid cancer, and its global incidence has increased rapidly in the last decades (1). The diagnostic and therapeutic process for PTC is determined by Thyroid Associations Guidelines, suggesting ultrasound and Fine-Needle Aspiration (FNA) as diagnostic tools until now and surgery as the treatment option (2). Nevertheless, FNA cytology may be inconclusive. Furthermore, guidelines have proposed specific clinicopathological features, such as age, gender, cancer subtype, tumor size >2 cm, extrathyroidal extension, multicentricity, and BRAF V600E mutation, to be indicative of recurrence and poorer prognosis (2). Novel molecular biomarkers should be discovered and applied as useful tools for the diagnostic and therapeutic management of PTC, so that the lesions are better characterized regarding their malignancy and aggressiveness.
MicroRNAs (MiRNAs) are endogenous, nonprotein coding single-stranded RNAs containing between 19 and 24 nucleotides. These molecules have been found in the genome of the majority of complex organisms (3). The expression of miRNAs is initiated in cell nuclei by the transcription of miRNA genes. The first product, named pri-miRNA, is cleaved by the Drosha DGCR8 (DiGeorge critical region 8) complex to become pre-miRNA. Pre-miRNA is then exported to the cytoplasm by exportin-5 and matures to miRNA-duplex by the Dicer enzyme. One strand is the guide strand, which enters the RNA-induced silencing complex (RISC), and the other one is the passenger strand, which is degraded. Mature miRNA binds to the 3’-UTR end of mRNA, and the stability or translation of mRNA is determined by the complementarity between the two molecules (4). Due to the range of complementary binding between miRNA and mRNA, miRNA can bind with several mRNAs and to regulate a wide variety of protein-coding gene transcripts. MiRNA plays an essential role in post-transcriptional processes, being involved in the regulation of a large number of proteins responsible for many cell functions (5). Taking into account the binding facility and regulatory effects of miRNAs, their deregulation, due to their abnormal expression, may be involved in many biological processes, whose deregulation may be associated with cancer development (4). MiRNA database counts 38,589 entries until now, and these molecules and their regulatory networks are being explored as new diagnostic and therapeutic targets for many diseases (6, 7).
The aim of the present systematic review is to examine the most important deregulated miRNAs in PTCs, defined as the most frequently detected molecules in the majority of international genetic studies.
Methods
A bibliographic research was conducted using PubMed, Scopus, and Embase from 2010 until January 2021. The search terms employed were “microRNAs” OR “miRNAs” AND ‘‘ papillary thyroid cancer” OR “papillary thyroid carcinoma”. There were 263 articles found in these databases and 9 in the literature, from which only 211 articles were associated with our subject. Only well-conducted genetic studies aiming to explore the association between miRNAs and their diagnostic and therapeutic application in PTC were included in our systematic review, so 28 articles were excluded as they were literature reviews. Finally, our article database included 183 articles (Figure 1).
Results
MicroRNAs Upregulated in Thyroid Specimen
A large number of upregulated miRNAs are detected in PTC specimens as summarized several studies internationally (4, 8, 9).
MiRNA 146a and 146b are widely the most well-studied deregulated miRNA in tumorous thyroid tissues. They are upregulated in thyroid specimens compared with normal tissue and benign thyroid pathology, as summarized in the Chou et al. review (10). The overexpression of mir146a was found to be due to the decreased ubiquitin-proteasome mediated degradation of HIFα, owning to increased activity of LSD1. Mir-146a inhibited the expression of GABPA and in this way contributed to the inhibition of apoptosis and stimulation of PTC cell malignancy (11). MiR146b targeted molecular pathways such as the MAPK/ERK pathway and TGF-β and was found to be involved in several cellular functions such as actin cytoskeleton formation affecting thyroid cell migration and invasion (10, 12). In particular, miR-146b targets the IL-1 receptor-associated kinase 1 (IRAK1), in which its inhibition is correlated with increased PTC cell proliferation, tumor invasiveness, and aggressiveness, probably due to the deregulation of the E-cadherin-mediated EMT (13, 14). MiR-146b-5p plays a significant role in PTC development, affecting cell proliferation and invasion, which is enhanced during the TGF-β1-induced EMT (epithelial–mesenchymal transition) signaling pathway (15). Another negative regulator of the Wnt/β-catenin pathway involved in the EMT is ZNRF3, which is also downregulated by miR-146b-5p (10, 16). Moreover, MiR146b-5p targets both the gene expression of RARβ and CCDC6 and promotes tumor development (17, 18). Another family member is MiR146b-3p, which is highly expressed in metastatic cell lines and enhances cell invasion and metastasis by suppressing the NF2 gene expression (19). The reduced expression of the THRβ is mediated by miR-146a (20). The increased expression of MiR146b can be due to DNA hypomethylation and therefore increased expression. The upregulation of MiR146b can be a sensitive (91–96%) and specific (96–97%) marker for the discretion of benign and malignant thyroid lesions and a prognostic marker for recurrence (21–23). Moreover, the expression of the miR146 family is associated with the tumor stage according to TNM, tumor aggressiveness, classical tall cell variant of PTC (in particular miR-146b-5p), and increased lymph node metastasis risk (14, 20, 22, 24–26). Interestingly the expression of mir-146b-5p was higher in PTC specimens without marked lymphocytic infiltration, compared to specimens from lymphocytic thyroiditis, indicating that mir-146b-5p could contribute to the inhibition of NKG2D and to the escape of the immune response (27). MiR146a overexpression in tissue was correlated with female gender, greater size, central lymph node metastasis, multifocality, extrathyroidal extension, and advanced tumor stage in TNM according to statistical analysis results of a cohort study by Sun et al. (28).
MiRNA 221 and 222 were also found frequently upregulated in PTC specimens due to the interaction of the high-mobility group box 1 protein (HMGB1) with the receptor for advanced glycation end-products (RAGE). The activation of the HMGB1/RAGE pathway beside the contribution to the chronic inflammation and the inhibition of the phosphatase and tensin homolog (PTEN), a regulator of the cell, induces the expression of miR-221 and -222 and contributes to PTC development (29, 30). MiR-221 binds to the TIMP3 mRNA. As result of the induced TIMP3 gene suppression, the cell proliferation and PTC growth and aggressiveness are increased (31). Moreover, a negative correlation was found among the expression of miR-221, -222, -146b, and p27(kip1) mRNA levels in PTC cells (32). Upregulation of miR-221 possibly through interleukin 17 and miR-222 is correlated with the advanced TNM stage, capsular invasion, extrathyroidal extension, and lymph node metastasis, a fact that is also included in American Thyroid Association risk factors guidelines (22, 33, 34). A cohort study conducted by Dai et al. showed that both miR-221 and -222 are predictive biomarkers of PTC recurrence. However, only miR-221 was the independent factor (35). The downregulation of the THRβ gene was also inversely correlated with the overexpression of miR-221 and was associated with the aggressiveness of PTC (20). MiR-222 directly targets the 3′-UTR of PPP2R2A, which expresses the Protein Phosphatase 2 Regulatory Subunit B Alpha (PPP2R2A), a tumor suppressor, altering the Akt signaling pathway, and therefore enhancing the tumor metastasis in nude mice (36, 37). Suresh et al. imply that racial disparity plays a role in miR-221 overexpression, However, there is no race-specific difference in miR-222 upregulation in PTC patients (38).
MiR-595 expression was found to be upregulated in PTC cells resulting to the downregulation of the sex-determining region Y-box 17 (Sox17) and consequently the overexpression of the inflammatory cytokine interleukin (IL22), which promoted cell migration (39). Increased cell proliferation, migration, and in vivo transplantation were also seen due to the miR-1270 binding to SCAI and its inverse regulation (40). Interestingly, the expression of MiR-21 was found to be increased in PTC cell lines under hypoxic conditions and promoted angiogenesis through direct targeting and inhibition of TGFB1 and COL4A1 gene expression. Therefore, the increased endothelial tube formation and angiogenesis could be associated with the increased PTC recurrence (41, 42). Another possible oncogenic pathway for miR-21 action implied PDCD4 downregulation and enhancement of cell proliferation and invasion (43). According to Rosignolo et al., in addition to the correlation of the overexpression of miR-21 with increased risk of recurrence, there is a statistically significant correlation with tall cell variant in PTC specimens (22). MiR-183 is another oncogene described, which, by inhibiting the expression of the PDCD4 gene, enhanced the PTC progression and inhibited apoptosis (44). MiR-182 was also shown to promote tumor growth and invasion by inhibiting the CHL1 gene expression (45). Overexpression of miR-92a-3p was found to be associated with PTC nodal metastases, whereas a negative correlation between the expression of the VHL and miR-92a was observed in PTC specimens with vascular invasion (46). Fang et al. found that miR-625-3p overexpression promoted the expression of the astrocyte elevated gene 1 (AEG1) and induced the activation of Wnt/β-catenin and JNK signaling cascade, which promoted the proliferation, migration, and invasion of thyroid cancer cells (47). The oncogene function of miR-96 was shown to be mediated by suppressing the expression of FOXO1, affecting the signaling pathway of the Akt/FOXO1/Bim axis, and therefore mediated the proliferation and the survival of PTC cells (48). The reduced expression of the THRβ gene was also found to be negatively correlated with the increased expression of miR181a in PTC specimens (20). CYLD, a member of the Wnt/β catenin pathway and a negative regulator of NF-κB, was found to be underregulated by MiR-181b, which was overexpressed in PTC specimens (49). Therefore, both miR-181a and miR-181b are suggested to be possible therapeutic targets in PTC therapy.
MiR-155 is referred to as a biomarker for the discrimination between benign and malignant thyroid lesions and for greater size, extrathyroid extension, central lymph node metastasis, advanced TNM stage, and poor prognosis (50). Upregulated miR-34a and -424 are essential markers for tumor aggressiveness, as indicated by bioinformatics analysis of data derived from the Cancer Genome Atlas (51). The has-miR-200a-5p as an overexpressed molecule is a sensitive biomarker and could be combined with immunohistochemical markers such as TPO, CD56, Galectin 3, MC, CK19, and BRAF both to detect and to distinguish between PTC and benign thyroid tumor with papillary hyperplasia (52).
The Let-7c microRNA family is known to have an important role in PTC tumorigenesis mainly by reducing RAS levels and acting as a tumor suppressor gene, as summarized in the Perdas et al. review (53). However, let-7b and let-7c were found to be overexpressed in PTC samples.
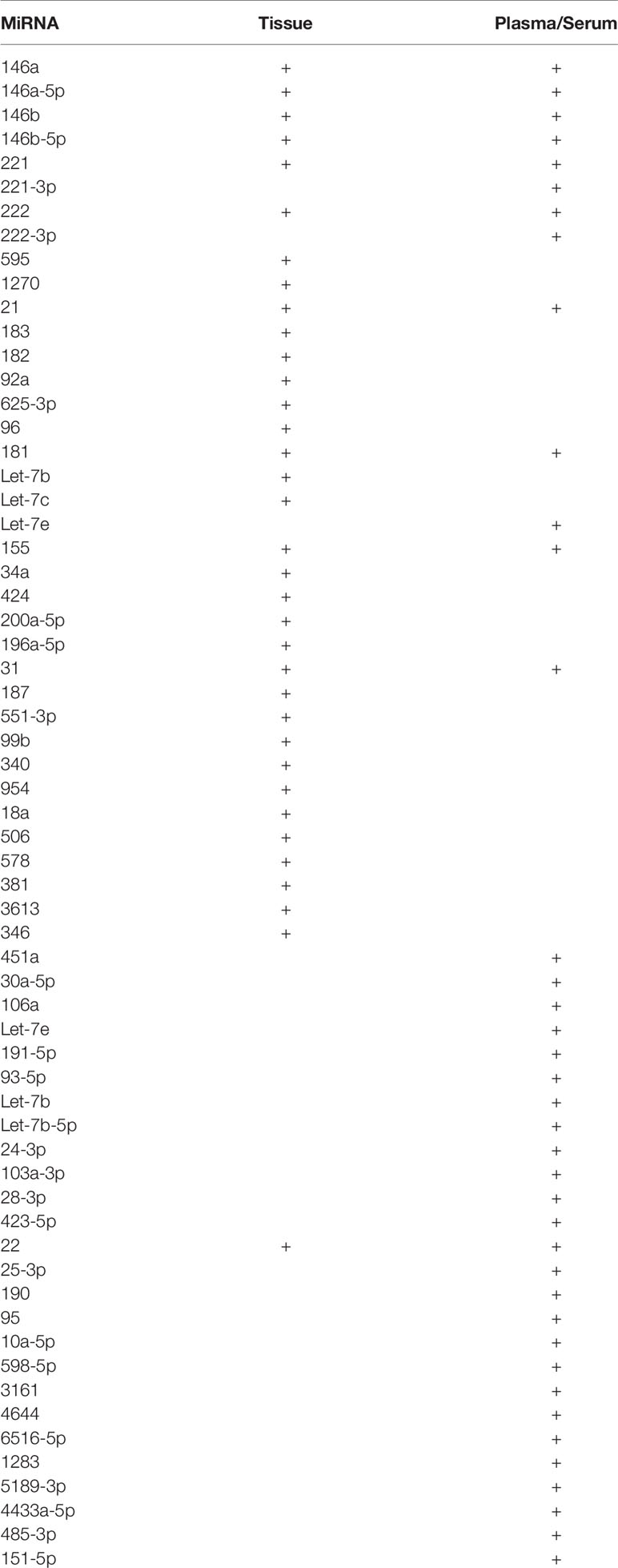
Other microRNAs that are found in international studies to be upregulated are miR- 196a-5p, -31, -187, -551-3p, -99b, -340, -954, -18a, -506, -578, -381, -3613, and -346 (4, 24, 38, 54, 55) (Table 1). The effects of upregulated miRNA in papillary thyroid cancer cell lines and tissue are summarized in Supplementary Table 1.
MicroRNAs Downregulated in Thyroid Specimen
A notable group of downregulated miRNAs, influencing genes’ expression and cellular processes, has been also found in both in PTC cell lines and specimens. They regulate cell cycle and proliferation, migration, and invasion, such as miR-7, which was found to be downregulated in PTC samples. However, upon the overexpression of miR-7, the expression of the oncogene CKS2 (cyclin-dependent kinase regulatory subunit 2) is suppressed, affecting downstream cell cycle regulation and resulting to cell cycle arrest in the G0/G1 phase (56). Interestingly, the cotreatment of PTC cells with mir-7-5p and chemotherapeutics loaded on cubosomes statistically significantly inhibited the proliferation and spheroid formation (57). Also, the overexpression of miR-791, found to be underexpressed in PTC specimens, induced cell cycle in the G0/G1 phase by inhibiting the expression of cyclin D1, CDK6, and CDK4 (58). The downregulation of miR-144 expression in PTC is associated with a larger tumor size. MiR-144 inhibited the cell proliferation by targeting the WW domain−containing transcription regulator 1 (WWTR1) (59). Moreover, miR-144 targeted the transcription factor E2F8 and induced cell cycle arrest in G1-phase arrest by downregulating Cyclin D1 (60). Mir144-3p was found to be suppressed by BAG5, and therefore the expression of fibronectin 1 (FN1) was maintained to be increased, promoting the invasion in PTC cells (61). The direct interaction of miR-144-3p with FN1 was also found to mediate the oncogenic function of SphK1 in PTC cells (62).
The downregulation of miR-1266 in PTC could be responsible for the overexpression of FGFR2 (63). MiR-335 acted also as a tumor suppressor by targeting the zinc finger E-box binding homeobox 2 (ZEB2) (64). Another form, the miR-335-5p, could target intercellular adhesion molecule 1 (ICAM-1) and accelerates cell migration and invasion (65). Through the Akt/mTOR pathway, miR-718 targeted phosphoinositide-dependent protein kinase 1 (PDPK1) and inhibited cell glucose metabolism and PTC progression (66). MiR-148a increased Bax expression and caspase−3/9 levels and enhanced apoptosis. The inhibition of lymphatic metastasis of PTC was due to suppressed phosphorylation of STAT3 and the inhibition of PI3K/Akt signaling. Inhibition of CDK8 expression was found to be another mechanism of miR-148a-mediated inhibition of PTC cell growth, migration, and invasiveness (67, 68). One of the most well-studied oncogenes for thyroid malignancies is BRAF, in which its 3’-untranslated region was found to be directly targeted by miR-9-5p, and therefore was negatively regulated in papillary cell lines (69). MiR-449 and miR-202-3p was found to be negative regulators of PTC through inhibiting the Wnt/β-catenin signaling pathway (70, 71). MiR-126 was found to be underexpressed both in PTC specimens and cells, whereas its functional role lies on the inhibition of VEGF-A-mediated angiogenesis (72). Moreover, miR-126 was found to regulate the Wnt/β-catenin signaling pathway via regulating the low-density lipoprotein receptor−related protein 6 (LRP6). The aberrant expression of miR-126 was found to be related to lymph node metastasis and advanced TNM stage (73). VEGF was found to be inhibited by miR-622 too. In PTC samples, the reduced expression of miR-622 was found to correlate with lymph node metastasis and advanced TNM stage (74). Also, the underexpression of miR-150 in PTC samples was found to be negatively correlated with TNM stage and lymph node metastasis. Mir-150 directly targeted the endogenous Rho−associated protein kinase 1 (ROCK1) (75). MiR-205 is another tumor suppressive molecule that targeted the action of both YAP1 and VEGFA and inhibited cell proliferation, cell cycle progression, and angiogenesis (76, 77).
MiR-204-5p and miR-7-2 were also described as tumor suppressors. Their underexpression in the PTC specimen indicated their possible role as tumor stage biomarkers. Mir-204-5p was found to be capable of inhibiting cancer progression by regulating the expression of TNRRSF12A, associated with angiogenesis, and IGFBP5, associated with cell cycle progression and proliferation (14, 78). Furthermore, the downregulation of miR-204 was found to be strongly related to follicular and tall cell variant type, extrathyroidal extension and metastasis of PTC, and the presence of BRAF-V600E mutation (22, 78, 79). Decreased Dicer gene expression in malignant tissues correlated greatly with aggressive features: extrathyroidal extension, angiolymphatic invasion, multifocality, lymph node and distant metastasis, and recurrence. The occurrence of BRAF-V600E mutation and the aggressive characteristics of PTC were found to be related with the decreased expression of DICER-mRNA (80). However, these results are controversial since Penha et al. found that DICER1 mRNA was overexpressed in 70% of the PTC samples. However, Dicer1 protein levels were downregulated and affected PTC proliferation and differentiation (81). MiR-451a, one of the most reported miRs, was found to be underexpressed in PTC tissue and associated with aggressive clinicopathological features, tall cell variant of PTC, extrathyroidal extension, and advanced tumor stage. Mir-451a targeted the expression of MIF, c-MYC, and AKT1 and thereby inhibited the Akt/mTOR signaling (82). Via the Akt pathway and by targeting the expression of ARFGEF1 and IRS2 genes, respectively, miR-215 and miR-766 detected in lower levels promoted lymph node metastasis, while miR-431 was suppressed in lymph node metastasis, accounting for an important biomarker for metastatic disease, regulating cytoskeleton formation by E-cadherin and Vimentin, and inhibiting the Hedgehog pathway (83–85). MiR-486-5p underexpression, KIAA1199 overexpression, and EMT formation were described to be connected to lymph node metastasis, TNM stage, recurrence, and survival (86, 87). MiR-486-5p targets the FBN1 gene (88). MiR-940, -16, -15a, -126, and -199a-3p, miR-26a-5p, and miR-564 were found to be negatively regulated in PTC specimens and cell lines and were associated with bilateral tumor, multicentricity, extrathyroidal extension, cervical lymph node metastasis, distant metastasis, advanced TNM stage, and recurrence (89–93). A genetic study applying small RNA deep sequencing demonstrated that miR-30c-2-3p, -876-5p, -138-1-3p and -138-5p, -139-3p and -139-5p, -504, -152, -873-5p, and -199b-5p were downregulated in PTC specimens and mainly underexpressed in PTC with lymph node metastasis, concluding that these may be useful biomarkers for metastatic cancer (94). The induced overexpression of miR-139 in PTC cell lines inhibited the expression of FN1 (95). Furthermore, miR- 152 and -20b (by impairing the MAPK/ERK signaling pathway) were found to be related to more aggressive types of papillary thyroid cancer, advanced TNM stage, and lymph node metastasis (51, 96). Mir-326 was found to be downregulated in PTC specimens and cells, whereas there was a significant correlation with tumor stage and metastatic properties. Mir-326 could inhibit the progression of PTC by impairing Ki-67, N-cadherin, MAPK1, and ERBB4 (97). Through the MAPK signaling pathway, miR-TG participates in PTC progression. Interestingly, miR-TG was found to be encoded within the thyroglobulin (TG) gene (98). MiR-369-3p was detected as negatively regulated in classical, follicular, and tall cell variant of PTC, whereas the expression of the TSPAN13 gene was enhanced (99). Another poorly expressed miR both in PTC tissue and cells was miR-448, which reduced expression was correlated with lymph node metastasis, and TNM stage (100). Mechanistically, the expression of the miR-448 gene is inhibited by the binding of KDM5B, a specific lysine demethylase, resulting to the overexpression of TGIF1.
The Let miRNAs family, as already mentioned, was found to be involved in PTC development. Besides the upregulated family members, let-7f, -7d, and -7g were found to be downregulated in PTC samples, whereas let-7f suppressed the MAPK/ERK signaling pathway (53). Another family member, Let-7a, was found to negatively regulate the expression of lin28 and AKT2 genes and via the c-myc pathway is associated with TNM stage, lymph node metastasis, and recurrence (36, 101). Let-7e was found to be capable to prevent PCT progression by directly inhibiting the translation of HMGB1 mRNA (102).
Other miRNAs found to be downregulated in PTC but with unknown molecular mechanism are miR-140-3p, miR-99a, miR-374a, miR-372, miR-363, miR-299-3p, miR-135b, miR-107, miR-103, miR-122-5p, and miR-10a-5p (22, 54, 55, 103). It is notable that miR-122-5p is expressed in both malignant and benign thyroid tumors in comparison to adjacent normal tissue (103). MiR-654-3p, miR-361-5p (via ROCK1), miR-497 (via Akt3), miR-744 (via NOB1), miR-613 (via SphK2), miR-4500 (via PLXNC1), miR-577 (via SphK2), miR-29a-3p (via OTUB2), miR-101 (via RAC1), miR-195 (via CCND1 and FGF2), miR-329 (via WNT1), miR-4728 (via SOS1 and MAPK signaling pathway), miR-199a-5p (via SNAI1), miR-758-3p (via TAB1), miR-219-5p (via ERα), miR-206 (via MAP4K3), miR-128 (via SphK1), and miR143-3p (via MSI2) were found in lower levels in PTC specimens and cells with enhanced cell proliferation, migration, and invasion (104–120) (Table 2). The effects of upregulated miRNA in papillary thyroid cancer cell lines and tissue are summarized in Supplementary Table 2.
MicroRNAs Deregulated in PTC Serum
A remarkable amount of genetic studies reported deregulated miRNAs in patients’ serum and plasma, indicating their potential role as valuable biomarkers for PTC development, metastasis, and recurrence risk, with great sensitivity and specificity (121). MiR-25-3p, -451a, -146b, -30a-5p, -106a, -155, and let-7e upregulation in serum and plasma in PTC account for an important tool for the diagnosis of PTC with sensitivity and specificity of more than 68% (122–125). MiR-190 and -95 could also be useful biomarkers for malignancy, since both of them have been found overexpressed in serum (126). According to the Graham et al. study, serum miRNAs 146a-5p and -199-3p were found to be downregulated and miR-10a-5p and let-7b-5p upregulated in PTC versus benign thyroid pathologies, while miR-150-5p, -146a-5p, and 342-3p were downregulated and miR-191-5p, -93-5p, and let-7b-5p were overexpressed in PTC versus normal thyroid (127). The next-generation sequencing was applied in order to compare the expression of PTC exosomes with nodular goiter (NG). MiR-598-5p, miR-3161, miR-4644, miR-6516-5p, and miR-1283 were found only in the PTC serum. However, mir-5189-3p, found to be highly expressed in the PTC serum, was characterized as the optimum biomarker for the distinction between PTC and NG. On the other hand, the concentration of mir-5010-3p was significantly low detected in the serum of PTC patients (128). Using small RNA sequencing, Dai et al. found that miR-376a-3p, miR-4306, miR-4433a-5p, and miR-485-3p were significantly upregulated in patients’ serum with PTC compared to samples from patients with benign thyroid nodules or healthy control (129). However, the authors suggest only miR-485-3p and miR-4433a-5p as putative biomarkers due to their diagnostic accuracy, whereas overexpression of 485-3p is indicative for higher risk. In another study, miR-221-3p, -146a-5p, -222-3p, 24-3p, 146b-5p, -191-5p, 103a-3p, and -28-3p were found in high levels in patient’s serum prior to thyroidectomy compared with control samples, suggesting them as possible biomarkers. On the other hand, the serum levels of miR-95-3p and -190a-5p were very low, almost undetectable (130). It is of great importance that post-operatively the miR levels were found lower, even more significant for miR-221-3p and 146a-5p (128). MiR-222 was found to be increased in the serum of patients with PTC who carried the BRAF V600E mutation. MiR-181a and -146a differ between cancerous and benign lesions and pre- and post-operatively (131). Similar results were reported by Yorurker et al. who demonstrated that the levels of miR-21, -151-5p, -221, -222, and -31 in the serum were indicative of TNM stage, metastasis, and tumor size. After thyroidectomy, miR-222, -221, and -146b levels were decreased and associated with advanced tumor stage, tumor size, and recurrence (132, 133). MiR-22 was found to be upregulated in both the tissue and serum of PTC patients and was believed to be a potential biomarker for metastatic disease (134). The exosomal miR-423-5p was found to be elevated in the serum of patients with PTC according to the study of Ye et al. (135).
The differential diagnosis between follicular and PTC versus benign tumors could be based on changes in plasma miRNAs. MiR-31 was found to be upregulated in PTC, while miR-21 was exceeded in follicular TC. MiR-181a was inversely expressed in both PTC and FTC and could find application as a helpful biomarker to distinguish them (121). The discrimination between benign and malignant thyroid tumors was assisted by the detection of miR-221, -222, -146b, and -21 in the serum, which were increased in PTC and reduced after surgery. Nevertheless, the increased serum levels postoperative was found to correlate with poor prognosis (136, 137) (Tables 1, 2).
MicroRNAs Detected in FNA
Fine-needle aspiration (FNA) is widely established as an ultrasound-guided minimal invasive method, useful for the differential diagnosis between benign and malignant thyroid lesions. As miRNAs take the forefront in diagnostic process, their aberrant expression was also examined in the FNA specimen in order to contribute to accurate diagnosis. All aforementioned deregulated miRNAs could be also examined in FNA. The molecules already demonstrated in international literature will be mentioned subsequently. MiR-30a-5p may be a diagnostic marker in FNA tissues, as it was found to be elevated also in the serum (123). MiR-222, -214, and -181b were found upregulated in FNA samples in cases suspected for PTC and associated with tumor aggressiveness (138). MiR-146b, -31, -551b, -221, and -375 were reported to give accurate results in FNA samples tested for PTC vs. FTC vs. Hurtle carcinoma vs. benign goiter (139) (Table 3).
Debatable Deregulated MicroRNAs in PTC
In the international literature, there are a few significant differences among the studies regarding miRNA deregulation in PTC development.
Let -7d,-7g, -7e, and -7f miRNAs were reported to be deregulated in PTC cell lines. Let- 7e and -7g were found to be positively regulated according to Liu et al., while Perdas et al. demonstrated different regulatory directions (let -7f downregulated in TPC cell line, -7d and 7b upregulated in TPC and IHH4 cell line, respectively) (53, 54). By targeting the cell cycle, miR-214 downregulation caused PSMD10 overexpression, which through metallopeptidase MMP2 and MMP9 action and GSK−3β/β−catenin and AKT signaling accelerated cell proliferation and migration. Furthermore, miR-214 is associated with lymph node metastasis, tumor size, and advanced TNM stage (140). Contrariwise, Zarkesh et al. reported that miR-214 is elevated in PTC tissue (138). MiR-30a is downregulated according to Morani et al. and upregulated according to Peng et al. (103, 141). MiR-15a was found to be negatively regulated, acting via the Akt pathway, and associated with bilateral tumor, multicentricity, extrathyroidal extension, metastatic disease, and advanced TNM stage, while it was included in the upregulated group of miRNAs by Liu et al. (54, 89, 142). MiR-524-5p targeted FOXE1 and ITGA3 gene expression and was found to be involved in cell autophagy process and demonstrated as inconsistently regulated by two different studies (54, 143). MiR-31, negatively regulated, was described to target HuR gene expression and to induce malignant progression of PTC, while Suresh et al. reported miR-31 as an elevated molecule in the PTC specimen (38, 144). MiR-375 downregulation was found to enhance ERBB2 gene expression according to Wang et al.’s study, whereas it was positively regulated in PTC according to Saiselet et al.’s study (94, 145). MiR-509 inhibited the PAX6 gene expression and acted as a tumor suppressor, reported as downregulated in PTC by Zhang et al., while it was found to be upregulated in another study (94, 146). MiR-98 was found to be positively expressed in Liu et al.’s study and negatively expressed in Suresh et al.’s study (38, 54). MiR-137 was found to be downregulated, affecting CXCL12 gene expression and was associated with TNM stage and nodal metastasis, whereas Zarkesh et al. described it to be overexpressed in PTC tissues (138, 147).
Furthermore, many polymorphisms have been detected in miRNAs, increasing the risk for PTC development. MiR-146a, let-7 and miR-181 polymorphisms are correlated with higher risk for PTC (148, 149). Let-7 rs10877887 polymorphism seems to increase the risk for PTC, and rs13293512 is related to advanced risk for lymph metastasis (150). MiR-608 rs4919510, miR-149 rs2292832, and miR-34b/c rs4938723 polymorphisms were found to be associated with susceptibility for PTC progression (151–153).
Let-7e, miR-181b, -135a, -15b, -320, and -484 were described to be related to familial PTC (154).
Other Noncoding RNAs Deregulated in PTC—LncRNA and CircRNA
As genome-sequencing methods developed, new molecules have been discovered preliminarily. Long noncoding RNAs (lncRNAs) are a large (with a length of more than 200 nucleotides) and diverse group of transcribed RNA molecules that do not encode proteins. They account for the major part of the noncoding transcriptome and are important regulators of gene expression and have a wide range of functions in cellular and developmental processes (155). Circular RNA (circRNA) consists a class of one-stripe RNA whose 3’ and 5’ ends are covalently linked. These are produced by the alternative splicing, a process during which different proteins are encoded by one single gene, when different exons may be included or not in the final mRNA (156).
Regarding PTC, there are a few studies published about lncRNA and circRNA and their role on PTC development. They combine lncRNA with microRNA and related genes to create the genetic pathway responsible for PTC. Zhao et al. reported the results of their study on the regulatory network of lncRNAs and suggested several possible functional combinations of miRNAs and lncRNAs such as lncRNA AC108463.1 with miR-221, miR-222, miR-876, miR-150, and miR-205 (157). LncRNA TUG1 functions as an oncogene, as it targeted the mir-145/ZEB1 pathway and, by inhibiting TUG1 gene expression, promoted cell proliferation, migration, and EMT formation (158). When LncRNA LINC00313 is upregulated, miR-4429 is downregulated and cell proliferation is induced in PTC, while the prognosis is worsening (158). Overexpression of LncRNA BISPR was associated with miR-21-5p, which was downregulated in PTC and both influenced cell invasiveness (159). LncRNA RMRP collaborated with miR-675 and was found to be downregulated in PTC cell lines, targeting MAPK1 gene expression, and was found to be related to advanced TNM stage and lymph node metastasis (160). LncRNA UCA1 and LINC00514 were found to be overexpressed in PTC cells and acted as an endogenous RNA competitor for miR-204 and promoted PTC (161, 162). Lnc PTCSC3, a member of the Wnt/β-catenin pathway, was found to be downregulated sponging miR-574-5p (163). Another molecular pathway, PI3K/Akt is involved in the lncRNA/miRNA axis, as lncRNA ABHD11-AS1 sponging for miR-1301-3p targeting STAT3, as it was found to be upregulated in PTC (164). LncRNA TTN-AS1 seems to target the same pathway PI3K/Akt through miR-153-3p/ZNRF2 regulation (165). LncRNA HOTTIP was found to upregulate the miR-637/Akt1 axis, enhancing PTC development (166). LncRNA GAS8-AS1 was detected to be downregulated in PTC cell lines and inhibited cell proliferation. It targeted CCND2, mediated by miR-135-5p (167). LncRNA DGCR5 acted as another tumor suppressor. However, it was downregulated in PTC and interacted with miR-2861, which was upregulated (168). LncRNA HOXA-AS2, being upregulated in PTC cell lines and by repressing miR-520c-3p and its target gene S100A4, seems to promote PTC progression (169). LncRNA TNRC6C-AS1 was found to be positively regulated in PTC and by repressing miR-129-5p, suggesting another PTC development axis (170).
LncRNAs have been correlated to TNM stage, prognosis, extrathyroidal extension, lymph node, or distant metastasis of PTC. Regarding these, lncRNA NEAT1_2 overexpression, sponging mir-106b-5p and enhancing ATAD2 protein expression, plays an important role in TNM stage and tumor size (171). Through lncRNA NEAT1 upregulation and miR-129-5p inhibition, another pathway is suggested for PTC (172). miR-129 through MAL2 gene is downregulated in PTC as proved by another study (173). LncRNA PVT1 was found to be upregulated in PTC—combined with IGF1R overexpression and miR-30a downregulation—and is related to TNM stage, lymph node metastasis, and tumor infiltration (174).
Regarding circular RNAs, circRNA ZFR plays an oncogenic role in PTC by regulating the miR-1261/C8orf4 axis (175). CircNUP214 acted as an oncogene by regulating ZEB2 through binding to miR-145 (176). Circ0004458 was found to be associated with miR-885-5p, negatively regulated in PTC and targeting RAC1 gene expression (177). Circ- ITCH regulated miR-22-3p, and they moderated together the PTC progression via the Wnt/β-catenin signaling pathway (178). Interestingly, exosomes from the PTC cancer stem cell (PTC-CSC) model were transferred beside the transcription factors SLUG and SOX2, the lncRNA MALAT1, and the linc-ROR resulting to the induction of EMT (179). Recently, Dai et al. found DOCK9-AS2, another exosomal lncRNA derived from CSC-like cells, to be upregulated in PTC and detectable in the plasma exosomes of PTC patients (180). Exosomal DOCK9 was transmitted from PTC-CSC and promoted the stemness upon the interaction with SP1, resulting to sponging miR-1972, upregulating CTNNB1, and finally activating the Wnt/β-catenin signaling pathway. In another study, Li et al. found that estrogen receptor β (ERβ) and lncRNA H19 are overexpressed in PTC-CSC suggesting a positive regulatory interaction of both factors in order to induce and maintain the cancer stem-like features (181). Also, lncRNA LINC00311 seems to have a key role in promoting the stem-like features by regulating the miR‐330‐5p/TLR4 pathway (182). Moreover, BANCR (BRAF-activated noncoding RNA) regulates the expression of CSC markers LGR5 and EpCAM via the c-Raf/MEK/ERK signaling pathway (183). More studies are necessary in order to clarify the role of lncRNA in the induction and maintenance of the stem cell-like features of the PTC cells. The new insights will contribute to the development of better therapy strategies by directly inhibiting the induction of stemness.
Conclusion
Deregulated miRNAs linked to PTC form a large group of molecules with complex regulatory networks, including protein coding genes, mRNAs, proteins, and regulatory enzymes (Figure 2). The scientific study on these molecules has not been completed yet, as many molecular pathways should be explored in the future. MiRNAs stand for potential biomarkers, useful both in diagnosis (through FNA samples and serum tests) and in treatment management of PTC, characterizing tumor stage and aggressiveness and therefore guiding the therapeutic process.
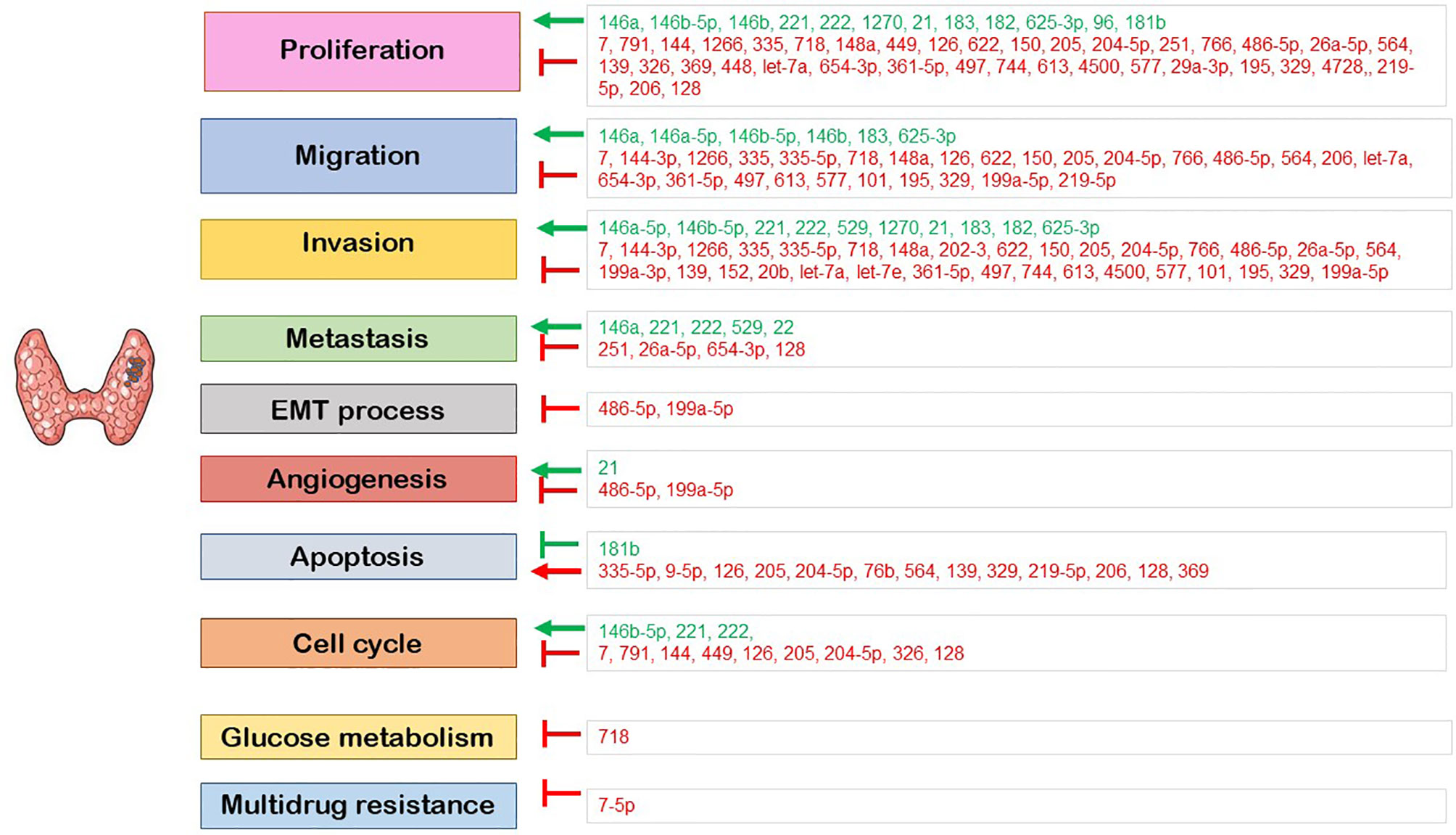
Figure 2 The role of the deregulated miRNA in the regulation of cellular processes. Red: downregulated miRNA; green: upregulated miRNA; ⟶: induction; ⊣: inhibition.
Author Contributions
MP and AGC conducted the bibliographic search and the writing of the manuscript. SB and AC contributed to the final version of the manuscript. AC, MP, and KG provided critical feedback on the review. TP designed and supervised the review. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.755097/full#supplementary-material
Abbreviations
AEG1, astrocyte elevated gene 1; AKT1; ARFGEF1, ADP Ribosylation Factor Guanine Nucleotide Exchange Factor 1; ATAD2, ATPase Family AAA Domain Containing 2; CCDC6, Coiled-Coil Domain Containing 6; CCND1, Cyclin D1 gene; CCND2, Cyclin G2 gene; CDK8, Cyclin Dependent Kinase 8; CDKN1C, Cyclin Dependent Kinase Inhibitor 1C; CHL1, Cell Adhesion Molecule L1 Like; CKD6, Cell division protein kinase 6; CKS2, cyclin-dependent kinase regulatory subunit 2; c-MYC; COL4A1, Collagen alpha-1(IV); CPK4, calcium-dependent protein kinase 4; CSC, Cancer Stem Cell; CXCL12, C-X-C Motif Chemokine Ligand 12; CYLD, Ubiquitin carboxyl-terminal hydrolase; EMT, epithelial–mesenchymal transition; Erα, Estrogen receptor α; Erβ, Estrogen Receptor β; ERBB2, Erb-B2 Receptor Tyrosine Kinase 2; ERBB4, Erb-B2 Receptor Tyrosine Kinase 4; FBN1, Fibrillin 1; FGF2, Fibroblast Growth Factor 2; FGFR2, Fibroblast growth factor receptor 2; FN1, Fibronectin 1; FNA, Fine Needle Aspiration; FOXE1, Forkhead Box E1; FOXO1, Forkhead Box O1; GA-binding protein; GABPA, GA-binding protein transcription factor alpha; GSK, glycogen synthase kinase; GSK-3β, glycogen synthase kinase; HMGA2, High Mobility Group AT-Hook 2; HMGB1, High Mobility Group Box 1; HOTAIR, HOX transcript antisense intergenic RNA; HuR, ELAV-like protein 1; ICAM1, Intercellular Adhesion Molecule 1; IGF1R, Insulin Like Growth Factor 1 Receptor; IGFBP5, Insulin Like Growth Factor Binding Protein 5; IRAK1, Interleukin 1 Receptor Associated Kinase 1; IRS2, Insulin receptor substrate 2; ITGA3, Integrin Subunit Alpha 3; KDM5B, Lysine specific demethylase 5B; KIAA1199, Cell Migration Inducing Hyaluronidase 1; LRP6, Low-density lipoprotein receptor−related protein 6; LSD1, Lysine-specific demethylase 1; MAL2, T-cell differentiation protein 2; MAP4K3, Mitogen-Activated Protein Kinase Kinase Kinase Kinase 3; MAPK, Mitogen-Activated protein kinase; MIF, Macrophage migration inhibitory factor; miRNA, microRNA; MMP2, Matrix Metallopeptidase 2; MMP9, Matrix Metallopeptidase 9; MSH2, DNA mismatch repair protein Msh2/MutS homolog 2; MSH3, DNA mismatch repair protein Msh3/MutS homolog 3; NF2, Neurofibromin 2; NKG2D, receptor the natural killer group 2 member D; NOB1, RNA-binding protein NOB1; OTUB2, Ubiquitin thioesterase OTUB2; PAX6, Paired box protein Pax-6; PDCD4, Programmed Cell Death Protein 4; PDPK1, 3-Phosphoinositide Dependent Protein Kinase 1; PLXNC1, Plexin C1; POLD3, DNA Polymerase Delta 3, Accessory Subunit; PPP2R2A, Protein Phosphatase 2 Regulatory Subunit Balpha; PRRX1, Paired Related Homeobox 1; PSMD10, proteasome 26S subunit non−ATPase 10; PSMD10, Proteasome 26S Subunit, Non-ATPase 10; PTC, Papillary Thyroid Cancer; PTEN, Phosphatase and tensin homolog; RAC1, Rac Family Small GTPase 1; RAC1, Ras-related C3 botulinum toxin substrate 1; RAGE, receptor for advanced glycation endproducts; RARβ, Retinoic acid receptor beta; RMPR, lncRNA mitochondrial RNA processing endoribonuclease; ROCK1, Endogenous Rho−associated protein kinase 1; ROR, Regulator of reprogramming; S100A4, S100 Calcium Binding Protein A4; SCAI, Suppressor Of Cancer Cell Invasion; SLC5A5, Solute Carrier Family 5 Member 5; SMAD4, Mothers against decapentaplegic homolog 4; SNAI1, Snail Family Transcriptional Repressor 1; SOS1, SOS Ras/Rac Guanine Nucleotide Exchange Factor 1; SOX17, SRY-Box Transcription Factor 17; SphK1, Sphingosine Kinase 1; SphK2, Sphingosine Kinase 2; TAB1, TGF-Beta Activated Kinase 1 (MAP3K7) Binding Protein 1; TGFB1, Transforming Growth Factor Beta 1; TGF-β, transforming growth factor β; TGIF1, transforming growth factor β-induced factor 1; THRβ, thyroid hormone receptor β; TIMP3, TIMP Metallopeptidase Inhibitor 3; TNFRSF12A, Tumor necrosis factor receptor superfamily member 12A; TNM, Tumor Node Metastasis; TNRRSF12A, TNF Receptor Superfamily Member 12A; TRPM3, Transient Receptor Potential Cation Channel Subfamily M Member 3; TSPAN13, Tetraspanin 13; UCA1, urothelial carcinoma−associated 1; VEGF-A, Vascular endothelial growth factor A; VHL, Von Hippel-Lindau; WNT1, Wnt Family Member 1; WWTR1, WW Domain Containing Transcription Regulator 1; YAP1, Yes Associated Protein 1; ZEB1, Zinc Finger E-Box Binding Homeobox 1; ZEB2, Zinc Finger E-Box Binding Homeobox 2; ZNRF2, Zinc And Ring Finger 2; ZNRF3, Zinc And Ring Finger 3; ATA, American Thyroid Association
Glossary
AEG1: astrocyte elevated gene 1
AKT1, ARFGEF1: ADP Ribosylation Factor Guanine Nucleotide Exchange Factor 1
ATA: American Thyroid Association
ATAD2: ATPase Family AAA Domain Containing 2
CCDC6: Coiled-Coil Domain Containing 6
CCND1: Cyclin D1 gene
CCND2: Cyclin G2 gene
CDK8: Cyclin Dependent Kinase 8
CDKN1C: Cyclin Dependent Kinase Inhibitor 1C
CHL1: Cell Adhesion Molecule L1 Like
CKD6: Cell division protein kinase 6
CKS2: cyclin-dependent kinase regulatory subunit 2
c-MYC: COL4A1, Collagen alpha-1(IV)
CPK4: calcium-dependent protein kinase 4
CSC: Cancer Stem Cell
CXCL12: C-X-C Motif Chemokine Ligand 12
CYLD: Ubiquitin carboxyl-terminal hydrolase
EMT: epithelial–mesenchymal transition
Erα: Estrogen receptor α
Erβ: Estrogen Receptor β
ERBB2: Erb-B2 Receptor Tyrosine Kinase 2
ERBB4: Erb-B2 Receptor Tyrosine Kinase 4
FBN1: Fibrillin 1
FGF2: Fibroblast Growth Factor 2
FGFR2: Fibroblast growth factor receptor 2
FN1: Fibronectin 1
FNA: Fine Needle Aspiration
FOXE1: Forkhead Box E1
FOXO1: Forkhead Box O1
GA-binding protein, GABPA: GA-binding protein transcription factor alpha
GSK: glycogen synthase kinase
GSK-3β: glycogen synthase kinase
HMGA2: High Mobility Group AT-Hook 2
HMGB1: High Mobility Group Box 1
HOTAIR: HOX transcript antisense intergenic RNA
HuR: ELAV-like protein 1
ICAM1: Intercellular Adhesion Molecule 1
IGF1R: Insulin Like Growth Factor 1 Receptor
IGFBP5: Insulin Like Growth Factor Binding Protein 5
IRAK1: Interleukin 1 Receptor Associated Kinase 1
IRS2: Insulin receptor substrate 2
ITGA3: Integrin Subunit Alpha 3
KDM5B: Lysine specific demethylase 5B
KIAA1199: Cell Migration Inducing Hyaluronidase 1
LRP6: Low-density lipoprotein receptor−related protein 6
LSD1: Lysine-specific demethylase 1
MAL2: T-cell differentiation protein 2
MAP4K3: Mitogen-Activated Protein Kinase Kinase Kinase Kinase 3
MAPK: Mitogen-Activated protein kinase
MIF: Macrophage migration inhibitory factor
miRNA: microRNA
MMP2: Matrix Metallopeptidase 2
MMP9: Matrix Metallopeptidase 9
MSH2: DNA mismatch repair protein Msh2/MutS homolog 2
MSH3: DNA mismatch repair protein Msh3/MutS homolog 3
NF2: Neurofibromin 2
NKG2D: receptor the natural killer group 2 member D
NOB1: RNA-binding protein NOB1
OTUB2: Ubiquitin thioesterase OTUB2
PAX6: Paired box protein Pax-6
PDCD4: Programmed Cell Death Protein 4
PDPK1: 3-Phosphoinositide Dependent Protein Kinase 1
PLXNC1: Plexin C1
POLD3: DNA Polymerase Delta 3, Accessory Subunit
PPP2R2A: Protein Phosphatase 2 Regulatory Subunit Balpha
PRRX1: Paired Related Homeobox 1
PSMD10: proteasome 26S subunit non−ATPase 10
PSMD10: Proteasome 26S Subunit, Non-ATPase 10
PTC: Papillary Thyroid Cancer
PTEN: Phosphatase and tensin homolog
RAC1: Rac Family Small GTPase 1
RAC1: Ras-related C3 botulinum toxin substrate 1
RAGE: receptor for advanced glycation endproducts
RARβ: Retinoic acid receptor beta
RMPR: lncRNA mitochondrial RNA processing endoribonuclease
ROCK1: Endogenous Rho−associated protein kinase 1
ROR: Regulator of reprogramming
S100A4: S100 Calcium Binding Protein A4
SCAI: Suppressor Of Cancer Cell Invasion
SLC5A5: Solute Carrier Family 5 Member 5
SMAD4: Mothers against decapentaplegic homolog 4
SNAI1: Snail Family Transcriptional Repressor 1
SOS1: SOS Ras/Rac Guanine Nucleotide Exchange Factor 1
SOX17: SRY-Box Transcription Factor 17
SphK1: Sphingosine Kinase 1
SphK2: Sphingosine Kinase 2
TAB1: TGF-Beta Activated Kinase 1 (MAP3K7) Binding Protein 1
TGFB1: Transforming Growth Factor Beta 1
TGF-β: transforming growth factor β
TGIF1: transforming growth factor β-induced factor 1
THRβ: thyroid hormone receptor β
TIMP3: TIMP Metallopeptidase Inhibitor 3
TNFRSF12A: Tumor necrosis factor receptor superfamily member 12A
TNM: Tumor Node Metastasis
TNRRSF12A: TNF Receptor Superfamily Member 12A
TRPM3: Transient Receptor Potential Cation Channel Subfamily M Member 3
TSPAN13: Tetraspanin 13
UCA1: urothelial carcinoma−associated 1
VEGF-A: Vascular endothelial growth factor A
VHL: Von Hippel-Lindau
WNT1: Wnt Family Member 1
WWTR1: WW Domain Containing Transcription Regulator 1
YAP1: Yes Associated Protein 1
ZEB1: Zinc Finger E-Box Binding Homeobox 1
ZEB2: Zinc Finger E-Box Binding Homeobox 2
ZNRF2: Zinc And Ring Finger 2
ZNRF3: Zinc And Ring Finger 3
References
1. Lim H, Devesa S, Sosa J, Check D, Kitahara C. Trends in Thyroid Cancer Incidence and Mortality in the United States, 1974-2013. JAMA (2017) 317:1338–48. doi: 10.1001/jama.2017.2719
2. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients With Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid: Off J Am Thyroid Assoc (2016) 26:1–133. doi: 10.1089/thy.2015.0020
3. Bartel D. MicroRNAs: Target Recognition and Regulatory Functions. Cell (2009) 136:215–33. doi: 10.1016/j.cell.2009.01.002
4. Lee JC, Gundara JS, Glover A, Serpell J, Sidhu SB. MicroRNA Expression Profiles in the Management of Papillary Thyroid Cancer. Oncologist (2014) 19:1141–7. doi: 10.1634/theoncologist.2014-0135
5. Hussain M. Micro-RNAs (miRNAs): Genomic Organisation, Biogenesis and Mode of Action. Cell Tissue Res (2012) 349:405–13. doi: 10.1007/s00441-012-1438-0
6. miRBase: the microRNA database. Available at: https://www.mirbase.org/.
7. Alles J, Fehlmann T, Fischer U, Backes C, Galata V, Minet M, et al. An Estimate of the Total Number of True Human miRNAs. Nucleic Acids Res (2019) 47:3353–64. doi: 10.1093/nar/gkz097
8. Mohamad Yusof A, Jamal R, Muhammad R, Abdullah Suhaimi SN, Mohamed Rose I, Saidin S, et al. Integrated Characterization of MicroRNA and mRNA Transcriptome in Papillary Thyroid Carcinoma. Front Endocrinol (2018) 9:158. doi: 10.3389/fendo.2018.00158
9. Chengfeng X, Gengming C, Junjia Z, Yunxia L. MicroRNA Signature Predicts Survival in Papillary Thyroid Carcinoma. J Cell Biochem (2019) 120:17050–8. doi: 10.1002/jcb.28966
10. Chou CK, Liu RT, Kang HY. MicroRNA-146b: A Novel Biomarker and Therapeutic Target for Human Papillary Thyroid Cancer. Int J Mol Sci (2017) 18(3):636. doi: 10.3390/ijms18030636
11. Long M, Zhu Y, Chen Z, Lin S, Peng X, Luo D, et al. Lysine-Specific Demethylase 1 Affects the Progression of Papillary Thyroid Carcinoma via HIF1α and microRNA-146a. J Clin Endocrinol Metab (2020) 105(7):dgaa182. doi: 10.1210/clinem/dgaa182
12. Lima CR, Geraldo MV, Fuziwara CS, Kimura ET, Santos MF. MiRNA-146b-5p Upregulates Migration and Invasion of Different Papillary Thyroid Carcinoma Cells. BMC Cancer (2016) 16:108. doi: 10.1186/s12885-016-2146-z
13. Chou CK, Chi SY, Huang CH, Chou FF, Huang CC, Liu RT, et al. IRAK1, a Target of miR-146b, Reduces Cell Aggressiveness of Human Papillary Thyroid Carcinoma. J Clin Endocrinol Metab (2016) 101:4357–66. doi: 10.1210/jc.2016-2276
14. Qiu Z, Li H, Wang J, Sun C. miR-146a and miR-146b in the Diagnosis and Prognosis of Papillary Thyroid Carcinoma. Oncol Rep (2017) 38:2735–40. doi: 10.3892/or.2017.5994
15. Hardin H, Guo Z, Shan W, Montemayor-Garcia C, Asioli S, Yu XM, et al. The Roles of the Epithelial-Mesenchymal Transition Marker PRRX1 and miR-146b-5p in Papillary Thyroid Carcinoma Progression. Am J Pathol (2014) 184:2342–54. doi: 10.1016/j.ajpath.2014.04.011
16. Deng X, Wu B, Xiao K, Kang J, Xie J, Zhang X, et al. MiR-146b-5p Promotes Metastasis and Induces Epithelial-Mesenchymal Transition in Thyroid Cancer by Targeting ZNRF3. Cell Physiol Biochem (2015) 35:71–82. doi: 10.1159/000369676
17. Czajka AA, Wojcicka A, Kubiak A, Kotlarek M, Bakula-Zalewska E, Koperski L, et al. Family of microRNA-146 Regulates RARbeta in Papillary Thyroid Carcinoma. PloS One (2016) 11:e0151968. doi: 10.1371/journal.pone.0151968
18. Jia M, Shi Y, Li Z, Lu X, Wang J. MicroRNA-146b-5p as an oncomiR Promotes Papillary Thyroid Carcinoma Development by Targeting CCDC6. Cancer Lett (2019) 443:145–56. doi: 10.1016/j.canlet.2018.11.026
19. Yu C, Zhang L, Luo D, Yan F, Liu J, Shao S, et al. MicroRNA-146b-3p Promotes Cell Metastasis by Directly Targeting NF2 in Human Papillary Thyroid Cancer. Thyroid (2018) 28(12):1627–41. doi: 10.1089/thy.2017.0626
20. Rosignolo F, Maggisano V, Sponziello M, Celano M, Di Gioia C, D’Agostino M, et al. Reduced Expression of Thrβ in Papillary Thyroid Carcinomas: Relationship With BRAF Mutation, Aggressiveness and miR Expression. J Endocrinol Invest (2015) 38:1283–9. doi: 10.1007/s40618-015-0309-4
21. Ortiz I, Barros-Filho MC, Dos Reis MB, Beltrami CM, Marchi FA, Kuasne H, et al. Loss of DNA Methylation Is Related to Increased Expression of miR-21 and miR-146b in Papillary Thyroid Carcinoma. Clin Epigenet (2018) 10:144. doi: 10.1186/s13148-018-0579-8
22. Rosignolo F, Memeo L, Monzani F, Colarossi C, Pecce V, Verrienti A, et al. MicroRNA-Based Molecular Classification of Papillary Thyroid Carcinoma. Int J Oncol (2017) 50:1767–77. doi: 10.3892/ijo.2017.3960
23. Chruścik A, Lam A. Clinical Pathological Impacts of microRNAs in Papillary Thyroid Carcinoma: A Crucial Review. Exp Mol Pathol (2015) 99:393–8. doi: 10.1016/j.yexmp.2015.08.013
24. Ab Mutalib NS, Othman SN, Mohamad Yusof A, Abdullah Suhaimi SN, Muhammad R, Jamal R. Integrated microRNA, Gene Expression and Transcription Factors Signature in Papillary Thyroid Cancer With Lymph Node Metastasis. PeerJ (2016) 4:e2119. doi: 10.7717/peerj.2119
25. Qiu J, Zhang W, Zang C, Liu X, Liu F, Ge R, et al. Identification of Key Genes and miRNAs Markers of Papillary Thyroid Cancer. Biol Res (2018) 51:45. doi: 10.1186/s40659-018-0188-1
26. Han PA, Kim HS, Cho S, Fazeli R, Najafian A, Khawaja H, et al. Association of BRAF V600E Mutation and MicroRNA Expression With Central Lymph Node Metastases in Papillary Thyroid Cancer: A Prospective Study From Four Endocrine Surgery Centers. Thyroid (2016) 26:532–42. doi: 10.1089/thy.2015.0378
27. Al-Abdallah A, Jahanbani I, Mehdawi H, Ali RH, Al-Brahim N, Mojiminiyi O, et al. Down-Regulation of the Human Major Histocompatibility Complex Class I Chain-Related Gene A (MICA) and Its Receptor Is Mediated by microRNA-146b-5p and Is a Potential Mechanism of Immunoediting in Papillary Thyroid Carcinoma. Exp Mol Pathol (2020) 113:104379. doi: 10.1016/j.yexmp.2020.104379
28. Sun M, Fang S, Li W, Li C, Wang L, Wang F, et al. Associations of miR-146a and miR-146b Expression and Clinical Characteristics in Papillary Thyroid Carcinoma. Cancer Biomark (2015) 15:33–40. doi: 10.3233/CBM-140431
29. Mardente S, Mari E, Consorti F, Di Gioia C, Negri R, Etna M, et al. HMGB1 Induces the Overexpression of miR-222 and miR-221 and Increases Growth and Motility in Papillary Thyroid Cancer Cells. Oncol Rep (2012) 28:2285–9. doi: 10.3892/or.2012.2058
30. Mardente S, Mari E, Massimi I, Fico F, Faggioni A, Pulcinelli F, et al. HMGB1-Induced Cross Talk Between PTEN and miRs 221/222 in Thyroid Cancer. BioMed Res Int (2015) 2015:512027. doi: 10.1155/2015/512027
31. Diao Y, Fu H, Wang Q. MiR-221 Exacerbate Cell Proliferation and Invasion by Targeting TIMP3 in Papillary Thyroid Carcinoma. Am J Ther (2017) 24:e317. doi: 10.1097/MJT.0000000000000420
32. Acibucu F, Dökmetaş H, Tutar Y, Elagoz S, Kilicli F. Correlations Between the Expression Levels of Micro-RNA146b, 221, 222 and p27Kip1 Protein mRNA and the Clinicopathologic Parameters in Papillary Thyroid Cancers. Exp Clin Endocrinol Diabetes (2014) 122:137–43. doi: 10.1055/s-0034-1367025
33. Xiang D, Tian B, Yang T, Li Z. miR-222 Expression Is Correlated With the ATA Risk Stratifications in Papillary Thyroid Carcinomas. Medicine (2019) 98:e16050. doi: 10.1097/MD.0000000000016050
34. Jiang X, Zhang H, Chen Y, Peng L. Expression of microRNA-221 and IL-17 in Papillary Thyroid Carcinoma and Correlation With Clinicopathologic Features. Zhonghua Bing Li Xue Za Zhi (2017) 46:160–5. doi: 10.3760/cma.j.issn.0529-5807.2017.03.004
35. Dai L, Wang Y, Chen L, Zheng J, Li J, Wu X. MiR-221, a Potential Prognostic Biomarker for Recurrence in Papillary Thyroid Cancer. World J Surg Oncol (2017) 15:11. doi: 10.1186/s12957-016-1086-z
36. Huang J, Lin H, Zhong M, Huang J, Sun S, Lin L, et al. Role of Lin28A/let-7a/C-Myc Pathway in Growth and Malignant Behavior of Papillary Thyroid Carcinoma. Med Sci Mon (2018) 24:8899–909. doi: 10.12659/MSM.908628
37. Huang Y, Yu S, Cao S, Yin Y, Hong S, Guan H, et al. MicroRNA-222 Promotes Invasion and Metastasis of Papillary Thyroid Cancer Through Targeting Protein Phosphatase 2 Regulatory Subunit B Alpha Expression. Thyroid (2018) 28:1162–73. doi: 10.1089/thy.2017.0665
38. Suresh R, Sethi S, Ali S, Giorgadze T, Sarkar FH. Differential Expression of MicroRNAs in Papillary Thyroid Carcinoma and Their Role in Racial Disparity. J Canc Sci Ther (2015) 7:145–54. doi: 10.4172/1948-5956.1000340
39. Mei Z, Zhou L, Zhu Y, Jie K, Fan D, Chen J, et al. Interleukin-22 Promotes Papillary Thyroid Cancer Cell Migration and Invasion Through microRNA-595/Sox17 Axis. Tumor Biol (2016) 37:11753–62. doi: 10.1007/s13277-016-5030-1
40. Yi T, Zhou X, Sang K, Zhou J, Ge L. MicroRNA-1270 Modulates Papillary Thyroid Cancer Cell Development by Regulating SCAI. BioMed Pharmacother (2019) 109:2357–64. doi: 10.1016/j.biopha.2018.08.150
41. Wu F, Li F, Lin X, Xu F, Cui R, Zhong J, et al. Exosomes Increased Angiogenesis in Papillary Thyroid Cancer Microenvironment. Endocr Relat Cancer (2019) 26(5):525–38. doi: 10.1530/ERC-19-0008
42. Sondermann A, Andreghetto F, Moulatlet A, da Silva Victor E, de Castro M, Nunes F, et al. MiR-9 and miR-21 as Prognostic Biomarkers for Recurrence in Papillary Thyroid Cancer. Clin Exp Metastasis (2015) 32:521–30. doi: 10.1007/s10585-015-9724-3
43. Zhang J, Yang Y, Liu Y, Fan Y, Liu Z, Wang X, et al. MicroRNA-21 Regulates Biological Behaviors in Papillary Thyroid Carcinoma by Targeting Programmed Cell Death 4. J Surg Res (2014) 189:68–74. doi: 10.1016/j.jss.2014.02.012
44. Wei C, Song H, Sun X, Li D, Song J, Hua K, et al. miR-183 Regulates Biological Behavior in Papillary Thyroid Carcinoma by Targeting the Programmed Cell Death 4. Oncol Rep (2015) 34:211–20. doi: 10.3892/or.2015.3971
45. Zhu H, Fang J, Zhang J, Zhao Z, Liu L, Wang J, et al. miR-182 Targets CHL1 and Controls Tumor Growth and Invasion in Papillary Thyroid Carcinoma. Biochem Biophys Res Commun (2014) 450:857–62. doi: 10.1016/j.bbrc.2014.06.073
46. Todorović L, Stanojević B, Mandušić V, Petrović N, Živaljević V, Paunović I, et al. Expression of VHL Tumor Suppressor mRNA and miR-92a in Papillary Thyroid Carcinoma and Their Correlation With Clinical and Pathological Parameters. Med Oncol (2018) 35:17. doi: 10.1007/s12032-017-1066-3
47. Fang L, Kong D, Xu W. MicroRNA-625-3p Promotes the Proliferation, Migration and Invasion of Thyroid Cancer Cells by Up-Regulating Astrocyte Elevated Gene 1. BioMed Pharmacother (2018) 120:203–11. doi: 10.1016/j.biopha.2018.03.043
48. Song H, Luo Y, Li DF, Wei C, Hua K, Song J, et al. MicroRNA-96 Plays an Oncogenic Role by Targeting FOXO1 and Regulating AKT/FOXO1/Bim Pathway in Papillary Thyroid Carcinoma Cells. Int J Clin Exp Pathol (2015) 8:9889–900.
49. Li D, Jian W, Wei C, Song H, Gu Y, Luo Y, et al. Down-Regulation of miR-181b Promotes Apoptosis by Targeting CYLD in Thyroid Papillary Cancer. Int J Clin Exp Pathol (2014) 7:7672–80.
50. Zhu Y, Zheng K, Zhang H, Chen L, Wu K, Ren C, et al. Expression of microRNA-155 in Papillary Thyroid Carcinoma and Its Clinical Significance. [Article in Chinese]. Nan Fang Yi Ke Da Xue Xue Bao (2016) 36:1364.
51. Cong D, He M, Chen S, Liu X, Liu X, Sun H. Expression Profiles of Pivotal microRNAs and Targets in Thyroid Papillary Carcinoma: An Analysis of The Cancer Genome Atlas. OncoTargets Ther (2015) 8:2271–7. doi: 10.2147/OTT.S85753
52. Wang X, Huang S, Li X, Jiang D, Yu H, Wu Q, et al. A Potential Biomarker hsa-miR-200a-5p Distinguishing Between Benign Thyroid Tumors With Papillary Hyperplasia and Papillary Thyroid Carcinoma. PloS One (2018) 13:e0200290. doi: 10.1371/journal.pone.0200290
53. Perdas E, Stawski R, Nowak D, Zubrzycka M. The Role of miRNA in Papillary Thyroid Cancer in the Context of miRNA Let-7 Family. Int J Mol Sci (2016) 17(6):909. doi: 10.3390/ijms17060909
54. Liu X, He M, Hou Y, Liang B, Zhao L, Ma S, et al. Expression Profiles of microRNAs and Their Target Genes in Papillary Thyroid Carcinoma. Oncol Rep (2013) 29:1415–20. doi: 10.3892/or.2013.2263
55. Wang Z, Lv J, Zou X, Huang Z, Zhang H, Liu Q, et al. A Three Plasma microRNA Signature for Papillary Thyroid Carcinoma Diagnosis in Chinese Patients. Gene (2019) 693:37–45. doi: 10.1016/j.gene.2019.01.016
56. Hua K, Jin J, Zhang H, Zhao B, Wu C, Xu H, et al. MicroRNA-7 Inhibits Proliferation, Migration and Invasion of Thyroid Papillary Cancer Cells via Targeting CKS2. Int J Oncol (2016) 49:1531–40. doi: 10.3892/ijo.2016.3660
57. Gajda E, Godlewska M, Mariak Z, Nazaruk E, Gawel D. Combinatory Treatment With miR-7-5p and Drug-Loaded Cubosomes Effectively Impairs Cancer Cells. Int J Mol Sci (2020) 21(14):5039. doi: 10.3390/ijms21145039
58. Gao X, Chen C, Tian Z, Yuan F, Jia G. MicroRNA-791 Is an Independent Prognostic Factor of Papillary Thyroid Carcinoma and Inhibits the Proliferation of PTC Cells. Eur Rev Med Pharmacol Sci (2018) 22:5562–8. doi: 10.26355/eurrev_201809_15819
59. Sun W, Lan X, Wang Z, Dong W, He L, Zhang T, et al. MicroRNA-144 Inhibits Proliferation by Targeting WW Domain-Containing Transcription Regulator Protein 1 in Papillary Thyroid Cancer. Oncol Lett (2018) 15:1007–13. doi: 10.3892/ol.2017.7440
60. Sun J, Shi R, Zhao S, Li X, Lu S, Bu H, et al. E2F8, a Direct Target of miR-144, Promotes Papillary Thyroid Cancer Progression via Regulating Cell Cycle. J Exp Clin Cancer Res (2017) 36(1):40. doi: 10.1186/s13046-017-0504-6
61. Zhang DL, Wang JM, Wu T, Du X, Yan J, Du ZX, et al. BAG5 Promotes Invasion of Papillary Thyroid Cancer Cells via Upregulation of Fibronectin 1 at the Translational Level. Biochim Biophys Acta Mol Cell Res (2020) 1867(9):118715. doi: 10.1016/j.bbamcr.2020.118715
62. Liang W, Xie Z, Cui W, Guo Y, Xu L, Wu J, et al. Comprehensive Gene and microRNA Expression Profiling Reveals a Role for miRNAs in the Oncogenic Roles of SphK1 in Papillary Thyroid Cancer. J Cancer Res Clin Oncol (2017) 143:601–11. doi: 10.1007/s00432-016-2315-0
63. Fu Y, Zheng H, Zhang D, Zhou L, Sun H. MicroRNA-1266 Suppresses Papillary Thyroid Carcinoma Cell Metastasis and Growth via Targeting FGFR2. Eur Rev Med Pharmacol Sci (2018) 22:3430–8. doi: 10.26355/eurrev_201806_15166
64. Kan Q, Su Y, Yang H. MicroRNA-335 Is Downregulated in Papillary Thyroid Cancer and Suppresses Cancer Cell Growth, Migration and Invasion by Directly Targeting ZEB2. Oncol Lett (2017) 14:7622–8. doi: 10.3892/ol.2017.7126
65. Luo L, Xia L, Zha B, Zuo C, Deng D, Chen M, et al. miR-335-5p Targeting ICAM-1 Inhibits Invasion and Metastasis of Thyroid Cancer Cells. BioMed Pharmacother (2018) 106:983–90. doi: 10.1016/j.biopha.2018.07.046
66. Wang X, Qi M. miR-718 Is Involved in Malignancy of Papillary Thyroid Cancer Through Repression of PDPK1. Pathol Res Pract (2018) 214:1787–93. doi: 10.1016/j.prp.2018.08.022
67. Xu Y, Han YF, Zhu SJ, Dong JD, Ye B. Mirna148a Inhibits Cell Growth of Papillary Thyroid Cancer Through STAT3 and PI3K/AKT Signaling Pathways. Oncol Rep (2017) 38:3085–93. doi: 10.3892/or.2017.5947
68. Han C, Zheng W, Ge M, Wang K, Xiang Y, Wang P. Downregulation of Cyclin-Dependent Kinase 8 by microRNA-148a Suppresses Proliferation and Invasiveness of Papillary Thyroid Carcinomas. Am J Cancer Res (2017) 7:2081–90.
69. Guo F, Hou X, Sun Q. MicroRNA-9-5p Functions as a Tumor Suppressor in Papillary Thyroid Cancer via Targeting BRAF. Oncol Let (2018) 16:6815–21. doi: 10.3892/ol.2018.9423
70. Li Z, Huang X, Xu J, Su Q, Zhao J, Ma J. miR-449 Overexpression Inhibits Papillary Thyroid Carcinoma Cell Growth by Targeting RET Kinase-Beta-Catenin Signaling Pathway. Inter J Oncol (2016) 49:1629–37. doi: 10.3892/ijo.2016.3659
71. Chen B, Yin J, Liu J, Zhu R, Zheng Y, Wang X. MiR-202-3p Functions as a Tumor Suppressor and Reduces Cell Migration and Invasion in Papillary Thyroid Carcinoma. Eur Rev Med Pharmacol Sci (2019) 23:1145–50. doi: 10.26355/eurrev_201902_17005
72. Salajegheh A, Vosgha H, Rahman M, Amin M, Smith R, Lam A. Interactive Role of miR-126 on VEGF-A and Progression of Papillary and Undifferentiated Thyroid Carcinoma. Hum Pathol (2016) 51:75–85. doi: 10.1016/j.humpath.2015.12.018
73. Wen Q, Zhao J, Bai L, Wang T, Zhang H, Ma Q. miR-126 Inhibits Papillary Thyroid Carcinoma Growth by Targeting LRP6. Oncol Rep (2015) 34:2202–10. doi: 10.3892/or.2015.4165
74. Wang R, Ma Q, Ji L, Yao Y, Ma M, Wen Q. miR-622 Suppresses Tumor Formation by Directly Targeting VEGFA in Papillary Thyroid Carcinoma. OncoTargets Ther (2018) 11:1501–9. doi: 10.2147/OTT.S156810
75. Cheng L, Zhou R, Chen M, Feng L, Li H. MicroRNA-150 Targets Rho-Associated Protein Kinase 1 to Inhibit Cell Proliferation, Migration and Invasion in Papillary Thyroid Carcinoma. Mol Med Rep (2017) 16:2217–24. doi: 10.3892/mmr.2017.6842
76. Salajegheh A, Vosgha H, Md Rahman A, Amin M, Smith RA, Lam AK. Modulatory Role of miR-205 in Angiogenesis and Progression of Thyroid Cancer. J Mol Endocrinol (2015) 55:183–96. doi: 10.1530/JME-15-0182
77. Li D, Wang Q, Li N, Zhang S. Mir205 Targets YAP1 and Inhibits Proliferation and Invasion in Thyroid Cancer Cells. Mol Med Rep (2018) 18:1674–81. doi: 10.3892/mmr.2018.9074
78. Liu L, Wang J, Li X, Ma J, Shi C, Zhu H, et al. MiR-204-5p Suppresses Cell Proliferation by Inhibiting IGFBP5 in Papillary Thyroid Carcinoma. Biochem Biophys Res Commun (2015) 457:621–6. doi: 10.1016/j.bbrc.2015.01.037
79. Xia F, Wang W, Jiang B, Chen Y, Li X. DNA Methylation-Mediated Silencing of miR-204 Is a Potential Prognostic Marker for Papillary Thyroid Carcinoma. Cancer Manag Res (2019) 11:1249–62. doi: 10.2147/CMAR.S184566
80. Erler P, Keutgen X, Crowley M, Zetoune T, Kundel A, Kleiman D, et al. Dicer Expression and microRNA Dysregulation Associate With Aggressive Features in Thyroid Cancer. Surgery (2014) 156:1342–50. doi: 10.1016/j.surg.2014.08.007
81. Penha RCC, Sepe R, De Martino M, Esposito F, Pellecchia S, Raia M, et al. Role of Dicer1in Thyroid Cell Proliferation and Differentiation. Cell Cycle (2017) 16:2282–9. doi: 10.1080/15384101.2017.1380127
82. Minna E, Romeo P, Dugo M, De Cecco L, Todoerti K, Pilotti S, et al. miR-451a Is Underexpressed and Targets AKT/mTOR Pathway in Papillary Thyroid Carcinoma. Oncotarget (2016) 7:12731–47. doi: 10.18632/oncotarget.7262
83. Han J, Zhang M, Nie C, Jia J, Wang F, Yu J, et al. miR-215 Suppresses Papillary Thyroid Cancer Proliferation, Migration, and Invasion Through the AKT/GSK-3beta/Snail Signaling by Targeting ARFGEF1. Cell Death Dis (2019) 10:195. doi: 10.1038/s41419-019-1444-1
84. Zhao J, Li Z, Chen Y, Zhang S, Guo L, Gao B, et al. MicroRNA−766 Inhibits Papillary Thyroid Cancer Progression by Directly Targeting Insulin Receptor Substrate 2 and Regulating the PI3K/Akt Pathway. Int J Oncol (2019) 54:315–25. doi: 10.3892/ijo.2018.4615
85. Liu Y, Li L, Liu Z, Yuan Q, Lu X. Downregulation of MiR-431 Expression Associated With Lymph Node Metastasis and Promotes Cell Invasion in Papillary Thyroid Carcinoma. Cancer Biomark (2018) 22:727–32. doi: 10.3233/CBM-181253
86. Jiao X, Ye J, Wang X, Yin X, Zhang G, Cheng X. KIAA1199, a Target of MicoRNA-486-5p, Promotes Papillary Thyroid Cancer Invasion by Influencing Epithelial-Mesenchymal Transition (EMT). Med Sci Mon (2019) 25:6788–96. doi: 10.12659/MSM.918682
87. Wen DY, Pan DH, Lin P, Mo QY, Wei YP, Luo YH, et al. Downregulation of Mir4865p in Papillary Thyroid Carcinoma Tissue: A Study Based on Microarray and miRNA Sequencing. Mol Med Rep (2018) 18:2631–42. doi: 10.3892/mmr.2018.9247
88. Ma X, Wei J, Zhang L, Deng D, Liu L, Mei X, et al. miR-486-5p Inhibits Cell Growth of Papillary Thyroid Carcinoma by Targeting Fibrillin-1. BioMed Pharmacother (2016) 80:220–6. doi: 10.1016/j.biopha.2016.03.020
89. Hu J, Li C, Liu C, Zhao S, Wang Y, Fu Z. Expressions of miRNAs in Papillary Thyroid Carcinoma and Their Associations With the Clinical Characteristics of PTC. Cancer Biomark (2017) 18:87–94. doi: 10.3233/CBM-161723
90. Xiong Y, Kotian S, Zeiger MA, Zhang L, Kebebew E. miR-126-3p Inhibits Thyroid Cancer Cell Growth and Metastasis, and Is Associated With Aggressive Thyroid Cancer. PloS One (2015) 10:e0130496. doi: 10.1371/journal.pone.0130496
91. Shi D, Wang H, Ding M, Yang M, Li C, Yang W, et al. MicroRNA-26a-5p Inhibits Proliferation, Invasion and Metastasis by Repressing the Expression of Wnt5a in Papillary Thyroid Carcinoma. OncoTargets Ther (2019) 12:6605–16. doi: 10.2147/OTT.S205994
92. Song Z, Yang H, Wu X, Kong C, Xu C. microRNA-564 Inhibits the Aggressive Phenotypes of Papillary Thyroid Cancer by Directly Targeting Astrocyte-Elevated Gene-1. OncoTargets Ther (2019) 12:4869–81. doi: 10.2147/OTT.S201282
93. Liu C, Xing M, Wang L, Zhang K. miR-199a-3p Downregulation in Thyroid Tissues Is Associated With Invasion and Metastasis of Papillary Thyroid Carcinoma. Br J BioMed Sci (2017) 74:90–4. doi: 10.1080/09674845.2016.1264705
94. Saiselet M, Gacquer D, Spinette A, Craciun L, Decaussin-Petrucci M, Andry G, et al. New Global Analysis of the microRNA Transcriptome of Primary Tumors and Lymph Node Metastases of Papillary Thyroid Cancer. BMC Genomics (2015) 16:828. doi: 10.1186/s12864-015-2082-3
95. Ye Y, Zhuang J, Wang G, He S, Ni J, Xia W. MicroRNA-139 Targets Fibronectin 1 to Inhibit Papillary Thyroid Carcinoma Progression. Oncol Lett (2017) 14:7799–806. doi: 10.3892/ol.2017.7201
96. Hong S, Yu S, Li J, Yin Y, Liu Y, Zhang Q, et al. MiR-20b Displays Tumor-Suppressor Functions in Papillary Thyroid Carcinoma by Regulating the MAPK/ERK Signaling Pathway. Thyroid (2016) 26:1733–43. doi: 10.1089/thy.2015.0578
97. Nie FR, Li QX, Wei HF, Ma Y. miR-326 Inhibits the Progression of Papillary Thyroid Carcinoma by Targeting MAPK1 and ERBB4. Neoplasma (2020) 67(3):604–13. doi: 10.4149/neo_2020_190731N696
98. Kolanowska M, Wojcicka A, Kubiak A, Swierniak M, Kotlarek M, Maciag M, et al. Functional Analysis of a Novel, Thyroglobulin-Embedded microRNA Gene Deregulated in Papillary Thyroid Carcinoma. Sci Rep (2017) 7:9942. doi: 10.1038/s41598-017-10318-w
99. Li P, Dong M, Wang Z. Downregulation of TSPAN13 by miR-369-3p Inhibits Cell Proliferation in Papillary Thyroid Cancer (PTC). Bosnian J Basic Med Sci (2019) 19:146–54. doi: 10.17305/bjbms.2018.2865
100. Pu Y, Xiang J, Zhang J. KDM5B-Mediated microRNA-448 Up-Regulation Restrains Papillary Thyroid Cancer Cell Progression and Slows Down Tumor Growth via TGIF1 Repression. Life Sci (2020) 250:117519. doi: 10.1016/j.lfs.2020.117519
101. Zhou B, Shan H, Su Y, Xia K, Zou R, Shao Q. Let-7a Inhibits Migration, Invasion and Tumor Growth by Targeting AKT2 in Papillary Thyroid Carcinoma. Oncotarget (2017) 8(41):69746–55. doi: 10.18632/oncotarget.19261
102. Ding C, Yu H, Shi C, Shi T, Qin H, Cui Y. MiR-Let-7e Inhibits Invasion and Magration and Regulates HMGB1 Expression in Papillary Thyroid Carcinoma. Biom Pharmacother (2019) 110:528–36. doi: 10.1016/j.biopha.2018.11.057
103. Peng Y, Li C, Luo DC, Ding JW, Zhang W, Pan G. Expression Profile and Clinical Significance of microRNAs in Papillary Thyroid Carcinoma. Molecules (2014) 19:11586–99. doi: 10.3390/molecules190811586
104. Geraldo M, Nakaya H, Kimura E. Down-Regulation of 14q32-Encoded miRNAs and Tumor Suppressor Role for miR-654-3p in Papillary Thyroid Cancer. Oncotarget (2017) 8:9597–607. doi: 10.18632/oncotarget.14162
105. Li R, Dong B, Wang Z, Jiang T, Chen G. MicroRNA-361-5p Inhibits Papillary Thyroid Carcinoma Progression by Targeting ROCK1. BioMed Pharmacother (2018) 102:988–95. doi: 10.1016/j.biopha.2018.03.122
106. Zhuang J, Ye Y, Wang G, Ni J, He S, Hu C, et al. MicroRNA497 Inhibits Cellular Proliferation, Migration and Invasion of Papillary Thyroid Cancer by Directly Targeting AKT3. Mol Med Rep (2017) 16:5815–22. doi: 10.3892/mmr.2017.7345
107. Liu H, Guo J, Chai H, Meng X. MicroRNA−744 Suppresses Cell Proliferation and Invasion of Papillary Thyroid Cancer by Directly Targeting NOB1. Mol Med Rep (2019) 19:1903–10. doi: 10.3892/mmr.2019.9826
108. Qiu W, Yang Z, Fan Y, Zheng Q. MicroRNA-613 Inhibits Cell Growth, Migration and Invasion of Papillary Thyroid Carcinoma by Regulating Sphk2. Oncotarget (2016) 7:39907–15. doi: 10.18632/oncotarget.9530
109. Li R, Teng X, Zhu H, Han T, Liu Q. MiR-4500 Regulates PLXNC1 and Inhibits Papillary Thyroid Cancer Progression. Horm Cancer (2019) 10:150–60. doi: 10.1007/s12672-019-00366-1
110. Xue K, Hu D, Zhao L, Li N, Shen H. MiR-577 Inhibits Papillary Thyroid Carcinoma Cell Proliferation, Migration and Invasion by Targeting Sphk2. Eur Rev Med Pharmacol Sci (2017) 21:3794–800.
111. Ma Y, Sun Y. miR-29a-3p Inhibits Growth, Proliferation, and Invasion of Papillary Thyroid Carcinoma by Suppressing NF-kappaB Signaling via Direct Targeting of OTUB2. Cancer Man Res (2019) 11:13–23. doi: 10.2147/CMAR.S184781
112. Wang C, Lu S, Jiang J, Jia X, Dong X, Bu P. Hsa-microRNA-101 Suppresses Migration and Invasion by Targeting Rac1 in Thyroid Cancer Cells. Oncol Lett (2014) 8:1815–21. doi: 10.3892/ol.2014.2361
113. Yin Y, Hong S, Yu S, Huang Y, Chen S, Liu Y, et al. MiR-195 Inhibits Tumor Growth and Metastasis in Papillary Thyroid Carcinoma Cell Lines by Targeting CCND1 and FGF2. Int J Endocrinol (2017) 2017:6180425. doi: 10.1155/2017/6180425
114. Wu L, Pei F, Men X, Wang K, Ma D. miR-329 Inhibits Papillary Thyroid Cancer Progression via Direct Targeting WNT1. Oncol Lett (2018) 16:3561–8. doi: 10.3892/ol.2018.9102
115. Liu Z, Zhang J, Gao J, Li Y. MicroRNA-4728 Mediated Regulation of MAPK Oncogenic Signaling in Papillary Thyroid Carcinoma. Saudi J Biol Sci (2018) 25:986–90. doi: 10.1016/j.sjbs.2018.05.014
116. Ma S, Jia W, Ni S. miR-199a-5p Inhibits the Progression of Papillary Thyroid Carcinoma by Targeting SNAI1. Biochem Biophys Res Commun (2018) 497:181–6. doi: 10.1016/j.bbrc.2018.02.051
117. Chen J, Xu Z, Yu C, Wu Z, Yin Z, Fang F, et al. MiR-758-3p Regulates Papillary Thyroid Cancer Cell Proliferation and Migration by Targeting TAB1. Pharmazie (2019) 74:235–8. doi: 10.1691/ph.2019.8933
118. Huang C, Cai Z, Huang M, Mao C, Zhang Q, Lin Y, et al. miR-219-5p Modulates Cell Growth of Papillary Thyroid Carcinoma by Targeting Estrogen Receptor α. J Clin Endocrinol Metab (2015) 100:E204–213. doi: 10.1210/jc.2014-2883
119. Liu F, Yin R, Chen X, Chen W, Qian Y, Zhao Y, et al. Over-Expression of miR-206 Decreases the Euthyrox-Resistance by Targeting MAP4K3 in Papillary Thyroid Carcinoma. BioMed Pharmacother (2019) 114:108605. doi: 10.1016/j.biopha.2019.108605
120. Cao XZ, Bin H, Zang ZN. MiR-128 Suppresses the Growth of Thyroid Carcinoma by Negatively Regulating SPHK1. BioMed Pharmacother (2019) 109:1960–6. doi: 10.1016/j.biopha.2018.08.052
121. Samsonov R, Burdakov V, Shtam T, Radzhabovа Z, Vasilyev D, Tsyrlina E, et al. Plasma Exosomal miR-21 and miR-181a Differentiates Follicular From Papillary Thyroid Cancer. Tumour Biol (2016) 37:12011–21. doi: 10.1007/s13277-016-5065-3
122. Li M, Song Q, Li H, Lou Y, Wang L. Circulating miR-25-3p and miR-451a May Be Potential Biomarkers for the Diagnosis of Papillary Thyroid Carcinoma. PloS One (2015) 10:e0132403. doi: 10.1371/journal.pone.0132403
123. Igci Y, Ozkaya M, Korkmaz H, Bozgeyik E, Bayraktar R, Ulasli M, et al. Expression Levels of miR-30a-5p in Papillary Thyroid Carcinoma: A Comparison Between Serum and Fine Needle Aspiration Biopsy Samples. Genet Test Mol Biomark (2015) 19:418–23. doi: 10.1089/gtmb.2015.0062
124. Lee Y, Lim Y, Lee J, Wang S, Park H, Kim S, et al. Differential Expression Levels of Plasma-Derived miR-146b and miR-155 in Papillary Thyroid Cancer. Oral Oncol (2015) 51:77–83. doi: 10.1016/j.oraloncology.2014.10.006
125. Shen C, Qiu Z, Song H, Wei W, Luo Q. miRNA-106a Directly Targeting RARB Associates With the Expression of Na(+)/I(-) Symporter in Thyroid Cancer by Regulating MAPK Signaling Pathway. J Exp Clin Cancer Res (2016) 35:101. doi: 10.1186/s13046-016-0377-0
126. Pilli T, Cantara S, Marzocchi C, Cardinale S, Santini C, Cevenini G, et al. Diagnostic Value of Circulating microRNA-95 and -190 in the Differential Diagnosis of Thyroid Nodules: A Validation Study in 1000 Consecutive Patients. Thyroid (2017) 27:1053–7. doi: 10.1089/thy.2017.0035
127. Graham ME, Hart RD, Douglas S, Makki FM, Pinto D, Butler AL, et al. Serum microRNA Profiling to Distinguish Papillary Thyroid Cancer From Benign Thyroid Masses. J Otolaryngol Head Neck Surg (2015) 44:33. doi: 10.1186/s40463-015-0083-5
128. Pan Q, Zhao J, Li M, Liu X, Xu Y, Li W, et al. Exosomal miRNAs Are Potential Diagnostic Biomarkers Between Malignant and Benign Thyroid Nodules Based on Next-Generation Sequencing. Carcinogenesis (2020) 41(1):18–24. doi: 10.1093/carcin/bgz160
129. Dai D, Tan Y, Guo L, Tang A, Zhao Y. Identification of Exosomal miRNA Biomarkers for Diagnosis of Papillary Thyroid Cancer by Small RNA Sequencing. Eur J Endocrinol (2020) 182(1):111–21. doi: 10.1530/EJE-19-0524
130. Rosignolo F, Sponziello M, Giacomelli L, Russo D, Pecce V, Biffoni M, et al. Identification of Thyroid-Associated Serum microRNA Profiles and Their Potential Use in Thyroid Cancer Follow-Up. J Endocr Soc (2017) 1:3–13. doi: 10.1210/js.2016-1032
131. Rezaei M, Khamaneh A, Zarghami N, Vosoughi A, Hashemzadeh S. Evaluating Pre- and Post-Operation Plasma miRNAs of Papillary Thyroid Carcinoma (PTC) Patients in Comparison to Benign Nodules. BMC Cancer (2019) 19:690. doi: 10.1186/s12885-019-5849-0
132. Zhang Y, Xu D, Pan J, Yang Z, Chen M, Han J, et al. Dynamic Monitoring of Circulating microRNAs as a Predictive Biomarker for the Diagnosis and Recurrence of Papillary Thyroid Carcinoma. Oncol Lett (2017) 13:4252–66. doi: 10.3892/ol.2017.6028
133. Yoruker EE, Terzioglu D, Teksoz S, Uslu FE, Gezer U, Dalay N. MicroRNA Expression Profiles in Papillary Thyroid Carcinoma, Benign Thyroid Nodules and Healthy Controls. J Cancer (2016) 7:803–9. doi: 10.7150/jca.13898
134. Wang D, Guo C, Kong T, Mi G, Li J, Sun Y. Serum miR-22 May Be a Biomarker for Papillary Thyroid Cancer. Oncol Lett (2019) 17:3355–61. doi: 10.3892/ol.2019.10011
135. Ye W, Deng X, Fan Y. Exosomal Mirna423-5p Mediated Oncogene Activity in Papillary Thyroid Carcinoma: A Potential Diagnostic and Biological Target for Cancer Therapy. Neoplasma (2019) 66:516–23. doi: 10.4149/neo_2018_180824N643
136. Zhang Y, Pan J, Xu D, Yang Z, Sun J, Sun L, et al. Combination of Serum microRNAs and Ultrasound Profile as Predictive Biomarkers of Diagnosis and Prognosis for Papillary Thyroid Microcarcinoma. Oncol Rep (2018) 40:3611–24. doi: 10.3892/or.2018.6776
137. Lee JC, Zhao JT, Clifton-Bligh RJ, Gill A, Gundara JS, Ip JC, et al. MicroRNA-222 and microRNA-146b Are Tissue and Circulating Biomarkers of Recurrent Papillary Thyroid Cancer. Cancer (2013) 119:4358–65. doi: 10.1002/cncr.28254
138. Zarkesh M, Zadeh-Vakili A, Akbarzadeh M, Nozhat Z, Fanaei S, Hedayati M, et al. BRAF V600E Mutation and microRNAs Are Helpful in Distinguishing Papillary Thyroid Malignant Lesions: Tissues and Fine Needle Aspiration Cytology Cases. Life Sci (2019) 223:166–73. doi: 10.1016/j.lfs.2019.03.034
139. Titov SE, Ivanov MK, Demenkov PS, Katanyan GA, Kozorezova ES, Malek AV, et al. Combined Quantitation of HMGA2 mRNA, microRNAs, and Mitochondrial-DNA Content Enables the Identification and Typing of Thyroid Tumors in Fine-Needle Aspiration Smears. BMC Cancer (2019) 19:1010. doi: 10.1186/s12885-019-6154-7
140. Liu F, Lou K, Zhao X, Zhang J, Chen W, Qian Y, et al. miR-214 Regulates Papillary Thyroid Carcinoma Cell Proliferation and Metastasis by Targeting PSMD10. Int J Mol Med (2018) 42:3027–36. doi: 10.3892/ijmm.2018.3902
141. Morani F, Titone R, Pagano L, Galetto A, Alabiso O, Aimaretti G, et al. Autophagy and Thyroid Carcinogenesis: Genetic and Epigenetic Links. Endocrine Rel Cancer (2014) 21:R13–29. doi: 10.1530/ERC-13-0271
142. Jin J, Zhang J, Xue Y, Luo L, Wang S, Tian H. miRNA-15a Regulates the Proliferation and Apoptosis of Papillary Thyroid Carcinoma via Regulating AKT Pathway. OncoTargets Ther (2019) 12:6217–26. doi: 10.2147/OTT.S213210
143. Liu H, Chen X, Lin T, Chen X, Yan J, Jiang S. MicroRNA-524-5p Suppresses the Progression of Papillary Thyroid Carcinoma Cells via Targeting on FOXE1 and ITGA3 in Cell Autophagy and Cycling Pathways. J Cell Physiol (2019) 234:18382–91. doi: 10.1002/jcp.28472
144. Wu D, Wang B, Shang J, Song J, Zhang H. miR-31 Reduces Cell Growth of Papillary Thyroid Carcinoma by RNA-Binding Protein HuR. Clin Lab (2015) 61:1625–34. doi: 10.7754/Clin.Lab.2015.150404
145. Wang XZ, Hang YK, Liu JB, Hou YQ, Wang N, Wang MJ. Over-Expression of microRNA-375 Inhibits Papillary Thyroid Carcinoma Cell Proliferation and Induces Cell Apoptosis by Targeting ERBB2. J Pharmacol Sci (2016) 130:78–84. doi: 10.1016/j.jphs.2015.12.001
146. Zhang S, Wang Q, Li D, Huang B, Hou X, Wang D. MicroRNA−509 Targets PAX6 to Inhibit Cell Proliferation and Invasion in Papillary Thyroid Carcinoma. Mol Med Rep (2019) 19:1403–9. doi: 10.3892/mmr.2018.9750
147. Dong S, Jin M, Li Y, Ren P, Liu J. MiR-137 Acts as a Tumor Suppressor in Papillary Thyroid Carcinoma by Targeting CXCL12. Oncol Rep (2016) 35:2151–8. doi: 10.3892/or.2016.4604
148. Dong G, Zhang R, Xu J, Guo Y. Association Between microRNA Polymorphisms and Papillary Thyroid Cancer Susceptibility. Int J Clin Exp Pathol (2015) 8:13450–7.
149. Jin H, Liang Y, Wang X, Zhu J, Sun R, Chen P, et al. Association Between a Functional Polymorphism Rs712 Within Let-7-Binding Site and Risk of Papillary Thyroid Cancer. Med Oncol (2014) 31:221. doi: 10.1007/s12032-014-0221-3
150. Wang Y, Wei T, Xiong J, Chen P, Wang X, Zhang L, et al. Association Between Genetic Polymorphisms in the Promoter Regions of Let-7 and Risk of Papillary Thyroid Carcinoma: A Case-Control Study. Medicine (2015) 94:e1879. doi: 10.1097/MD.0000000000001879
151. Wei WJ, Lu ZW, Li DS, Wang Y, Zhu YX, Wang ZY, et al. Association of the miR-149 Rs2292832 Polymorphism With Papillary Thyroid Cancer Risk and Clinicopathologic Characteristics in a Chinese Population. Int J Mol Sci (2014) 15:20968–81. doi: 10.3390/ijms151120968
152. Chen P, Sun R, Pu Y, Bai P, Yuan F, Liang Y, et al. Pri-Mir-34b/C and Tp-53 Polymorphisms Are Associated With The Susceptibility of Papillary Thyroid Carcinoma: A Case-Control Study. Medicine (2015) 94:e1536. doi: 10.1097/MD.0000000000001536
153. Dai Z, Lv J, Liu K, Lei X, Li W, Wu G, et al. The Role of microRNA-608 Polymorphism on the Susceptibility and Survival of Cancer: A Meta-Analysis. Aging (Albany NY) (2018) 10:1402–14. doi: 10.18632/aging.101476
154. Tomsic J, Fultz R, Liyanarachchi S, Genutis L, Wang Y, Li W, et al. Variants in microRNA Genes in Familial Papillary Thyroid Carcinoma. Oncotarget (2017) 8:6475–82. doi: 10.18632/oncotarget.14129
155. Jathar S, Kumar V, Srivastava J, Tripathi V. Technological Developments in lncRNA Biology. Adv Exp Med Biol (2017) 1008:283–323. doi: 10.1007/978-981-10-5203-3_10
156. Barrett SP, Salzman J. Circular RNAs: Analysis, Expression and Potential Functions. Development (2016) 143:1838–47. doi: 10.1242/dev.128074
157. Zhao Y, Wang H, Wu C, Yan M, Wu H, Wang J, et al. Construction and Investigation of lncRNA-Associated ceRNA Regulatory Network in Papillary Thyroid Cancer. Oncol Rep (2018) 39:1197–206. doi: 10.3892/or.2018.6207
158. Lei H, Gao Y, Xu X. LncRNA TUG1 Influences Papillary Thyroid Cancer Cell Proliferation, Migration and EMT Formation Through Targeting miR-145. Acta Biochim Biophys Sin (2017) 49:588–97. doi: 10.1093/abbs/gmx047
159. Zhang H, Cai Y, Zheng L, Zhang Z, Lin X, Jiang N. LncRNA BISPR Promotes the Progression of Thyroid Papillary Carcinoma by Regulating miR-21-5p. Int J Immunopathol Pharmacol (2018) 32:2058738418772652. doi: 10.1177/2058738418772652
160. Wang J, Xiao T, Zhao M. MicroRNA-675 Directly Targets MAPK1 to Suppress the Oncogenicity of Papillary Thyroid Cancer and Is Sponged by Long Non-Coding RNA RMRP. OncoTargets Ther (2019) 12:7307–21. doi: 10.2147/OTT.S213371
161. Li D, Cui C, Chen J, Hu Z, Wang Y, Hu D. Long Noncoding RNA UCA1 Promotes Papillary Thyroid Cancer Cell Proliferation via Mir204mediated BRD4 Activation. Mol Med Rep (2018) 18:3059–67. doi: 10.3892/mmr.2018.9246
162. Li X, Zhong W, Xu Y, Yu B, Liu H. Silencing of lncRNA LINC00514 Inhibits the Malignant Behaviors of Papillary Thyroid Cancer Through miR-204-3p/CDC23 Axis. Biochem Biophys Res Commun (2019) 508:1145–8. doi: 10.1016/j.bbrc.2018.12.051
163. Wang X, Lu X, Geng Z, Yang G, Shi Y. LncRNA PTCSC3/miR-574-5p Governs Cell Proliferation and Migration of Papillary Thyroid Carcinoma via Wnt/β-Catenin Signaling. J Cell Biochem (2017) 118:4745–52. doi: 10.1002/jcb.26142
164. Wen J, Wang H, Dong T, Gan P, Fang H, Wu S, et al. STAT3-Induced Upregulation of lncRNA ABHD11-AS1 Promotes Tumour Progression in Papillary Thyroid Carcinoma by Regulating miR-1301-3p/STAT3 Axis and PI3K/AKT Signalling Pathway. Cell Prolif (2019) 52:e12569. doi: 10.1111/cpr.12569
165. Cui M, Luo Z, Lin Z, Shi L, Hong Y, Yan C. Long Non-Coding RNA TTN-AS1 Facilitates Tumorigenesis of Papillary Thyroid Cancer Through Modulating the miR-153-3p/ZNRF2 Axis. J Gene Med (2019) 21(5):e3083. doi: 10.1002/jgm.3083
166. Yuan Q, Liu Y, Fan Y, Liu Z, Wang X, Jia M, et al. LncRNA HOTTIP Promotes Papillary Thyroid Carcinoma Cell Proliferation, Invasion and Migration by Regulating miR-637. Int J Biochem Cell Biol (2018) 98:1–9. doi: 10.1016/j.biocel.2018.02.013
167. Chen N, Yin D, Lun B, Guo X. LncRNA GAS8-AS1 Suppresses Papillary Thyroid Carcinoma Cell Growth Through the miR-135b-5p/CCND2 Axis. Biosci Rep (2019) 39(1):BSR20181440. doi: 10.1042/BSR20181440
168. Chen F, Yin S, Zhu J, Liu P, Yang C, Feng Z, et al. lncRNA DGCR5 Acts as a Tumor Suppressor in Papillary Thyroid Carcinoma via Sequestering miR-2861. Exp Ther Med (2019) 17:895–900. doi: 10.3892/etm.2018.7012
169. Xia F, Chen Y, Jiang B, Du X, Peng Y, Wang W, et al. Long Noncoding RNA HOXA-AS2 Promotes Papillary Thyroid Cancer Progression by Regulating miR-520c-3p/S100A4 Pathway. Cell Physiol Biochem (2018) 50:1659–72. doi: 10.1159/000494786
170. Hou S, Lin Q, Guan F, Lin C. LncRNA TNRC6C-AS1 Regulates UNC5B in Thyroid Cancer to Influence Cell Proliferation, Migration, and Invasion as a Competing Endogenous RNA of miR-129-5p. J Cell Biochem (2018) 119:8304–16. doi: 10.1002/jcb.26868
171. Sun W, Lan X, Zhang H, Wang Z, Dong W, He L, et al. NEAT1_2 Functions as a Competing Endogenous RNA to Regulate ATAD2 Expression by Sponging microRNA-106b-5p in Papillary Thyroid Cancer. Cell Death Dis (2018) 9:380. doi: 10.1038/s41419-018-0418-z
172. Zhang H, Cai Y, Zheng L, Zhang Z, Lin X, Jiang N. Long Noncoding RNA NEAT1 Regulate Papillary Thyroid Cancer Progression by Modulating miR-129-5p/KLK7 Expression. J Cell Physiol (2018) 233:6638–48. doi: 10.1002/jcp.26425
173. Gao X, Chen Z, Li A, Zhang X, Cai X. MiR-129 Regulates Growth and Invasion by Targeting MAL2 in Papillary Thyroid Carcinoma. BioMed Pharmacother (2018) 105:1072–8. doi: 10.1016/j.biopha.2018.06.050
174. Feng K, Liu Y, Xu L, Zhao L, Jia C, Xu M. Long Noncoding RNA PVT1 Enhances the Viability and Invasion of Papillary Thyroid Carcinoma Cells by Functioning as ceRNA of microRNA-30a Through Mediating Expression of Insulin Like Growth Factor 1 Receptor. BioMed Pharmacother (2018) 104:686–98. doi: 10.1016/j.biopha.2018.05.078
175. Wei H, Pan L, Tao D, Li R. Circular RNA circZFR Contributes to Papillary Thyroid Cancer Cell Proliferation and Invasion by Sponging miR-1261 and Facilitating C8orf4 Expression. Biochem Biophys Res Commun (2018) 503:56–61. doi: 10.1016/j.bbrc.2018.05.174
176. Li X, Tian Y, Hu Y, Yang Z, Zhang L, Luo J. CircNUP214 Sponges miR-145 to Promote the Expression of ZEB2 in Thyroid Cancer Cells. Biochem Biophys Res Commun (2018) 507:168–72. doi: 10.1016/j.bbrc.2018.10.200
177. Jin X, Wang Z, Pang W, Zhou J, Liang Y, Yang J, et al. Upregulated Hsa_Circ_0004458 Contributes to Progression of Papillary Thyroid Carcinoma by Inhibition of miR-885-5p and Activation of RAC1. Med Sci Monit (2018) 24:5488–500. doi: 10.12659/MSM.911095
178. Wang M, Chen B, Ru Z, Cong L. CircRNA Circ-ITCH Suppresses Papillary Thyroid Cancer Progression Through miR-22-3p/CBL/β-Catenin Pathway. Biochem Biophys Res Commun (2018) 504:283–8. doi: 10.1016/j.bbrc.2018.08.175
179. Hardin H, Helein H, Meyer K, Robertson S, Zhang R, Zhong W, et al. Thyroid Cancer Stem-Like Cell Exosomes: Regulation of EMT via Transfer of lncRNAs. Lab Invest (2018) 98(9):1133–42. doi: 10.1038/s41374-018-0065-0
180. Dai W, Jin X, Han L, Huang H, Ji Z, Xu X, et al. Exosomal lncRNA DOCK9-AS2 Derived From Cancer Stem Cell-Like Cells Activated Wnt/β-Catenin Pathway to Aggravate Stemness, Proliferation, Migration, and Invasion in Papillary Thyroid Carcinoma. Cell Death Dis (2020) 11(9):743. doi: 10.1038/s41419-020-02827-w
181. Li M, Chai HF, Peng F, Meng YT, Zhang LZ, Zhang L, et al. Estrogen Receptor β Upregulated by lncRNA-H19 to Promote Cancer Stem-Like Properties in Papillary Thyroid Carcinoma. Cell Death Dis (2018) 9(11):1120. doi: 10.1038/s41419-018-1077-9
182. Gao Y, Wang F, Zhang L, Kang M, Zhu L, Xu L, et al. LINC00311 Promotes Cancer Stem-Like Properties by Targeting miR-330-5p/TLR4 Pathway in Human Papillary Thyroid Cancer. Cancer Med (2020) 9(4):1515–28. doi: 10.1002/cam4.2815
Keywords: microRNAs (miRNAs), papillary thyroid cancer, thyroid cancer, microRNA thyroid, diagnostic test
Citation: Papaioannou M, Chorti AG, Chatzikyriakidou A, Giannoulis K, Bakkar S and Papavramidis TS (2022) MicroRNAs in Papillary Thyroid Cancer: What Is New in Diagnosis and Treatment. Front. Oncol. 11:755097. doi: 10.3389/fonc.2021.755097
Received: 07 August 2021; Accepted: 08 December 2021;
Published: 03 February 2022.
Edited by:
Anton F. Engelsman, Academic Medical Center, NetherlandsReviewed by:
Weiren Luo, The Second Affiliated Hospital of Southern University of Science and Technology, ChinaHao Zhang, Dalian Medical University, China
Copyright © 2022 Papaioannou, Chorti, Chatzikyriakidou, Giannoulis, Bakkar and Papavramidis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Theodosios S. Papavramidis, cGFwYXZyYW1pZGlzQGhvdG1haWwuY29t
 Maria Papaioannou
Maria Papaioannou Angeliki G. Chorti
Angeliki G. Chorti Anthoula Chatzikyriakidou
Anthoula Chatzikyriakidou Kleanthis Giannoulis2
Kleanthis Giannoulis2 Sohail Bakkar
Sohail Bakkar Theodosios S. Papavramidis
Theodosios S. Papavramidis