- 1Department of Nuclear Medicine, Mianyang Central Hospital, School of Medicine, University of Electronic Science and Technology of China, Mianyang, China
- 2National Health Commission (NHC) Key Laboratory of Nuclear Technology Medical Transformation (Mianyang Central Hospital), Mianyang, China
- 3Department of Pathology, Mianyang Central Hospital, School of Medicine, University of Electronic Science and Technology of China, Mianyang, China
- 4Department of Ultrasound, Mianyang Central Hospital, School of Medicine, University of Electronic Science and Technology of China, Mianyang, China
- 5Department of Hematology, Mianyang Central Hospital, School of Medicine, University of Electronic Science and Technology of China, Mianyang, China
Primary thyroid lymphoma is a rare hematologic malignancy of the thyroid gland, accounting for approximately 5% of all malignant thyroid tumors. The most common pathological type is B-cell-derived non-Hodgkin’s lymphoma, mainly diffuse large B-cell lymphoma, followed by mucosa-associated lymphoid tissue lymphoma and mixed types. The clinical and radiographic characteristics of primary thyroid lymphoma are non-specific, often leading to misdiagnosis as thyroiditis and a delay in treatment. A 60-year-old woman was referred to our hospital with neck swelling that had persisted for a week. Histopathological findings of a thyroid biopsy revealed mucosa-associated lymphoid tissue lymphoma. Bone marrow examination revealed atypical lymphocytes on myelograms. [18F]FDG PET/CT images showed increased [18F]FDG uptake in both lobes of the thyroid gland and the cervical lymph nodes. The patient was diagnosed with stage IV primary thyroid mucosa-associated lymphoid tissue lymphoma. The patient subsequently received four cycles of R-CEOP chemotherapy and remained under follow-up. Due to the rarity of this case, we conducted a systematic literature review to better understand the disease and improve timely diagnosis and treatment.
Introduction
Primary thyroid lymphoma (PTL) is a rare hematologic malignancy of the thyroid gland, accounting for approximately 5% of all malignant thyroid tumors (1). The most common pathological type is B-cell-derived non-Hodgkin’s lymphoma, mainly diffuse large B-cell lymphoma (DLBCL) (70%), followed by mucosa-associated lymphoid tissue (MALT) lymphoma (15–30%) and mixed types (2, 3). PTL is more prevalent in older women (4). Moreover, its clinical and radiographic characteristics are non-specific. The most common clinical manifestation is a rapidly enlarging (<6 months) neck mass, often combined with cervical lymph node enlargement or compression symptoms, such as dyspnea and dysphagia (2, 5), especially among patients with a background of Hashimoto’s thyroiditis (HT) (6). We report a case of primary MALT lymphoma of the thyroid gland. Due to the rarity of this case, we conducted a systematic review of the literature on PTL to enhance clinicians’ understanding of the disease and improve diagnosis and treatment strategies.
Case presentation
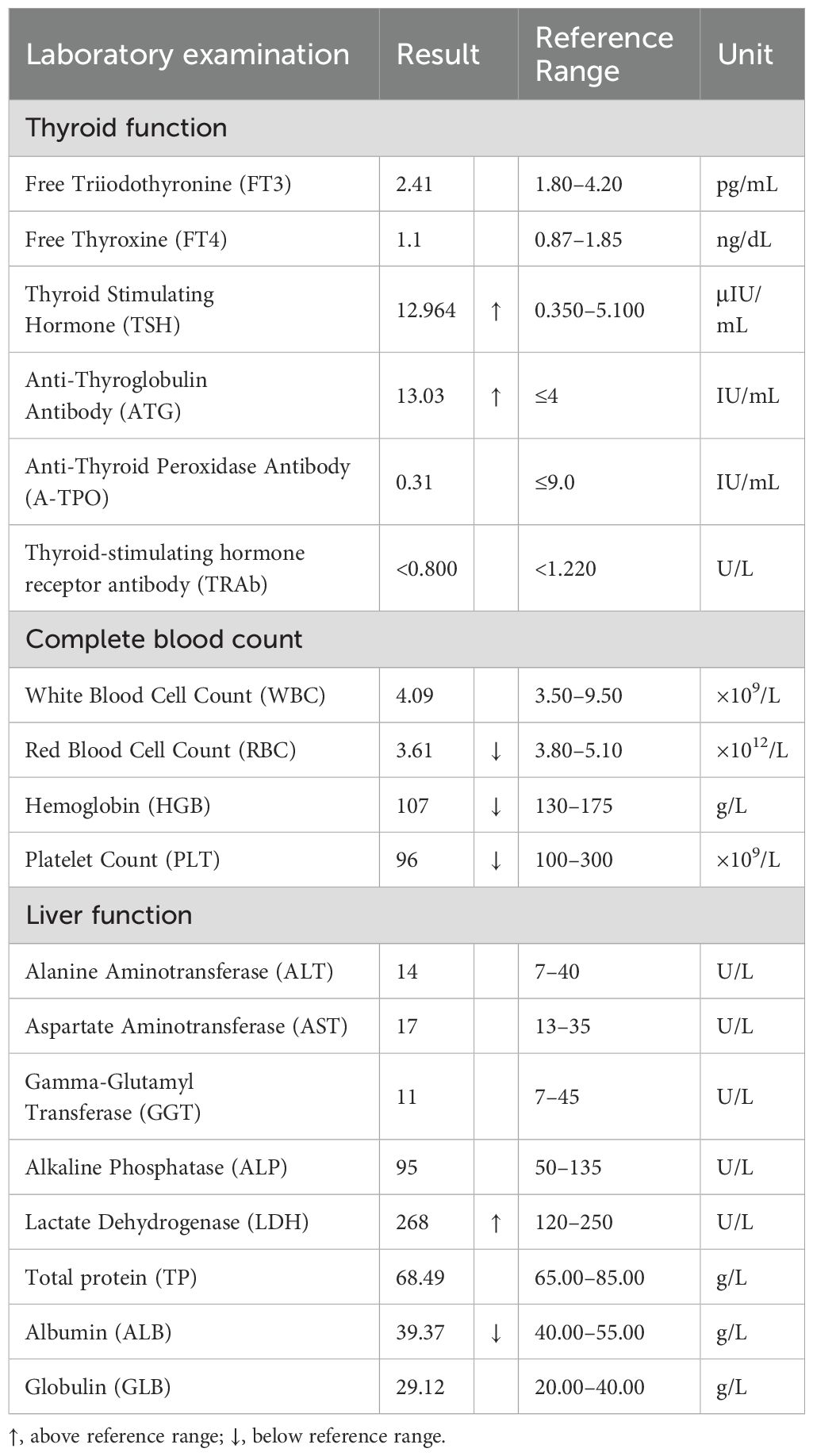
A 60-year-old woman presented with neck swelling that had persisted for 1 week. The patient was previously in good health and had no family history of thyroid disease. Physical examination revealed neck swelling and bilateral thyroid enlargement. Enlarged superficial lymph nodes were palpable in the neck, exhibiting a hard texture and difficult to move. Laboratory examinations revealed that the anti-thyroglobulin antibody levels were above the normal range. The patient had mild anemia and normal tumor markers, renal function, and coagulation function. Key laboratory results are presented in Table 1. Figure 1 illustrates the ultrasound images. Ultrasound images revealed a thyroid gland with abnormal morphology, an unsmooth capsule, and uneven thickening of parenchymal echoes. Color doppler flow imaging (CDFI) showed increased blood flow signals. Hypoechoic lesions were evident in both thyroid gland lobes, with a clear boundary, a regular shape, and an uneven internal echo. The hypoechoic lesion in the left lobe was about 4.3×3.1×2.8 cm in size, and that in the right lobe was about 2.8×2.6×1.8 cm in size. CDFI revealed abundant blood flow signals in and around the lesions (Figures 1A, B, the left lobe, yellow arrow; Figures 1C, D, the right lobe, blue arrow). According to the ACR Thyroid Imaging, Reporting and Data System (TI-RADS) (7), the hypoechoic lesions were awarded four points; hence, the risk levels were characterized as TR4. Multiple lymph nodes were apparent in the bilateral neck (level IV), and the boundary between the cortex and medulla of the lymph nodes was unclear.
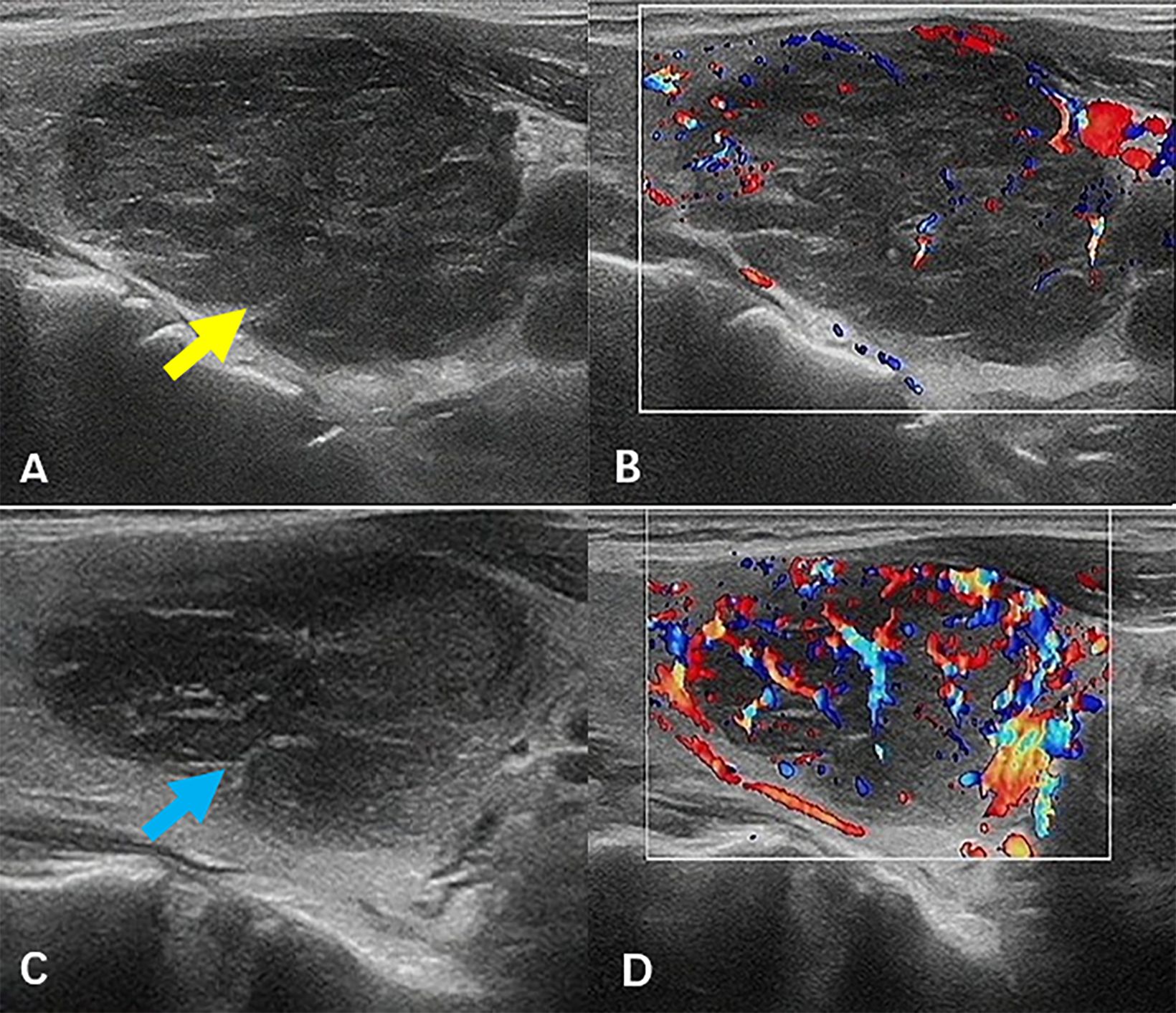
Figure 1. Ultrasound images. Hypoechoic lesions were evident in both thyroid gland lobes (A, B, the left lobe, yellow arrow; C, D, the right lobe, blue arrow), with a clear boundary, a regular shape, and an uneven internal echo. CDFI revealed abundant blood flow signals in and around the lesions. CDFI, color doppler flow imaging.
Subsequently, the patient underwent an ultrasound-guided thyroid core needle biopsy (CNB). Pathological findings revealed that the thyroid tissue was infiltrated by numerous lymphocytes and plasma cells [Figure 2A, haematoxylin and eosin (HE) staining, magnification ×100]. Immunohistochemical analysis showed negative staining for CD5 (Figure 2B, magnification ×200) and positive staining for CD20, BcL-2 (approximately 80%), and Pan-Cytokeratin (P-CK) (Figures 2C–E, magnification ×200). Moreover, immunoglobulin gene rearrangement demonstrated immunoglobulin kappa light chain allele B (IGKB) positivity (Figure 2F). Eventually, the patient was diagnosed with MALT lymphoma through a combination of histology, immunohistochemistry, and immunoglobulin gene rearrangement. Bone marrow morphology revealed atypical lymphocytes on myelograms.
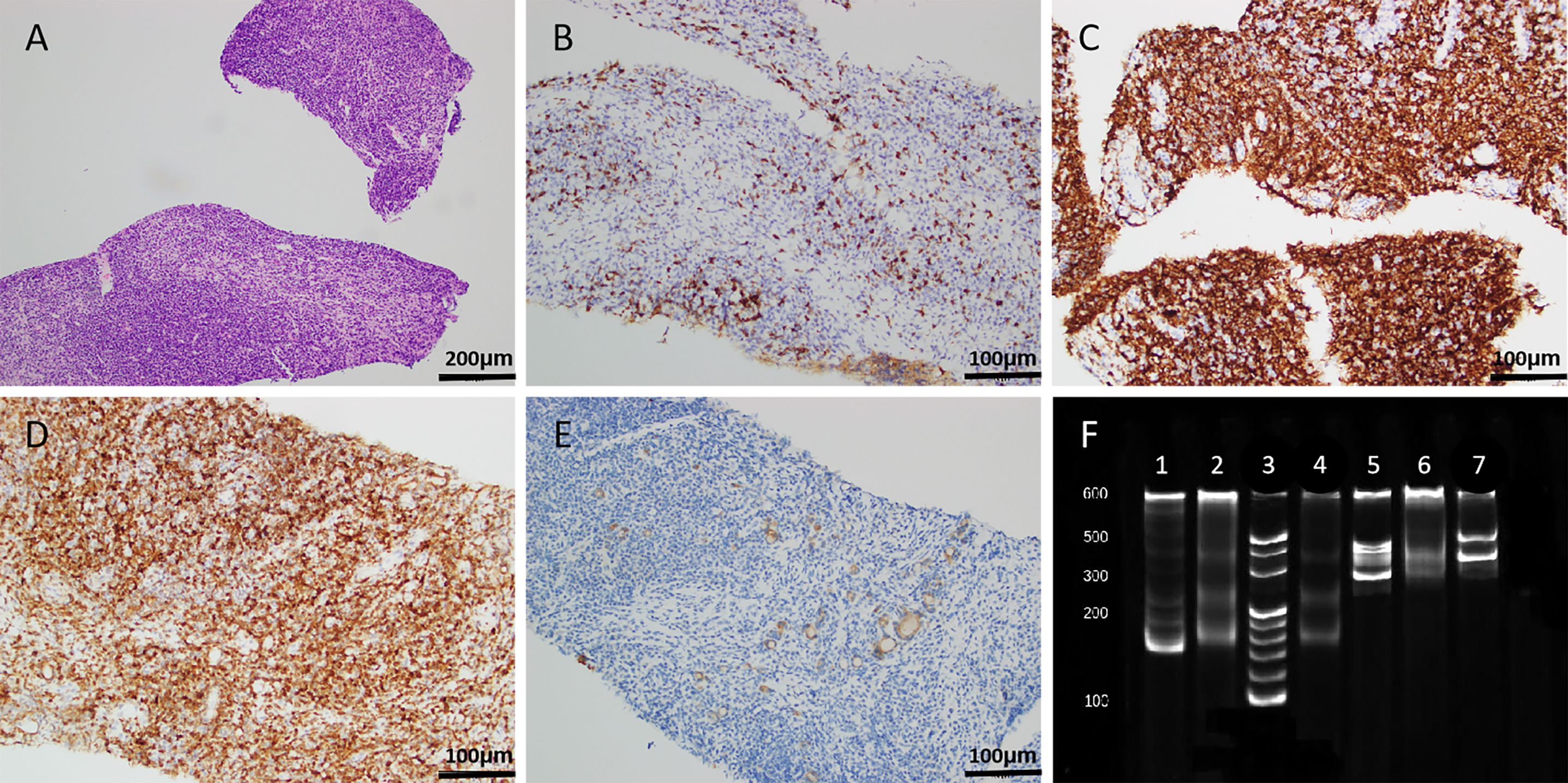
Figure 2. Histopathological results. (A, HE staining, magnification ×100) The thyroid tissue was infiltrated by numerous lymphocytes and plasma cells. (B–E, magnification ×200) Immunohistochemical analysis showed negative staining for CD5 (B) and positive staining for CD20 (C), BcL-2 (approximately 80%, D), and P-CK (E). (F) Immunoglobulin gene rearrangement demonstrated IGKB positivity (lane 1, positive control for IGKA; lane 2, negative control for IGKA; lane 3, marker; lane 4, IGKA; lane 5, positive control for IGKB; lane 6, negative control for IGKB; lane 7, IGKB). HE, haematoxylin and eosin; P-CK, Pan-Cytokeratin; IGKA, immunoglobulin kappa light chain allele A; IGKB, immunoglobulin kappa light chain allele B.
2-Deoxy-2-[18F]fluoro-D-glucose ([18F]FDG) positron emission tomography/computed tomography (PET/CT) was performed for pre-treatment staging. Maximum density projections of the anteroposterior positions (Figure 3A) showed intense [18F]FDG uptake in both thyroid gland lobes. Localized [18F]FDG uptake could also be observed in the left level IIa, level VI, and level VII of the neck. Axial images (Figure 3B, PET images; Figure 3C, CT images; Figure 3D, fusion images) revealed intense [18F]FDG uptake with a standardized uptake value maximum (SUVmax) of 19.6 in both thyroid gland lobes, with diffuse enlargement and reduced density of both lobes on a corresponding CT scan. Transverse PET (Figures 3E, H, K), CT (Figures 3F, I, L), and fusion images (Figures 3G, J, M) showed lymph nodes in the left level IIa, level VI, and level VII of the neck, with short diameters of approximately 0.5 cm, 0.8 cm, and 1.0 cm, respectively; moderate [18F]FDG uptake with an SUVmax of 5.3, 7.0, and 6.1, respectively; and suspected lymphoma involvement. No abnormal increase in [18F]FDG uptake was detected at other sites.
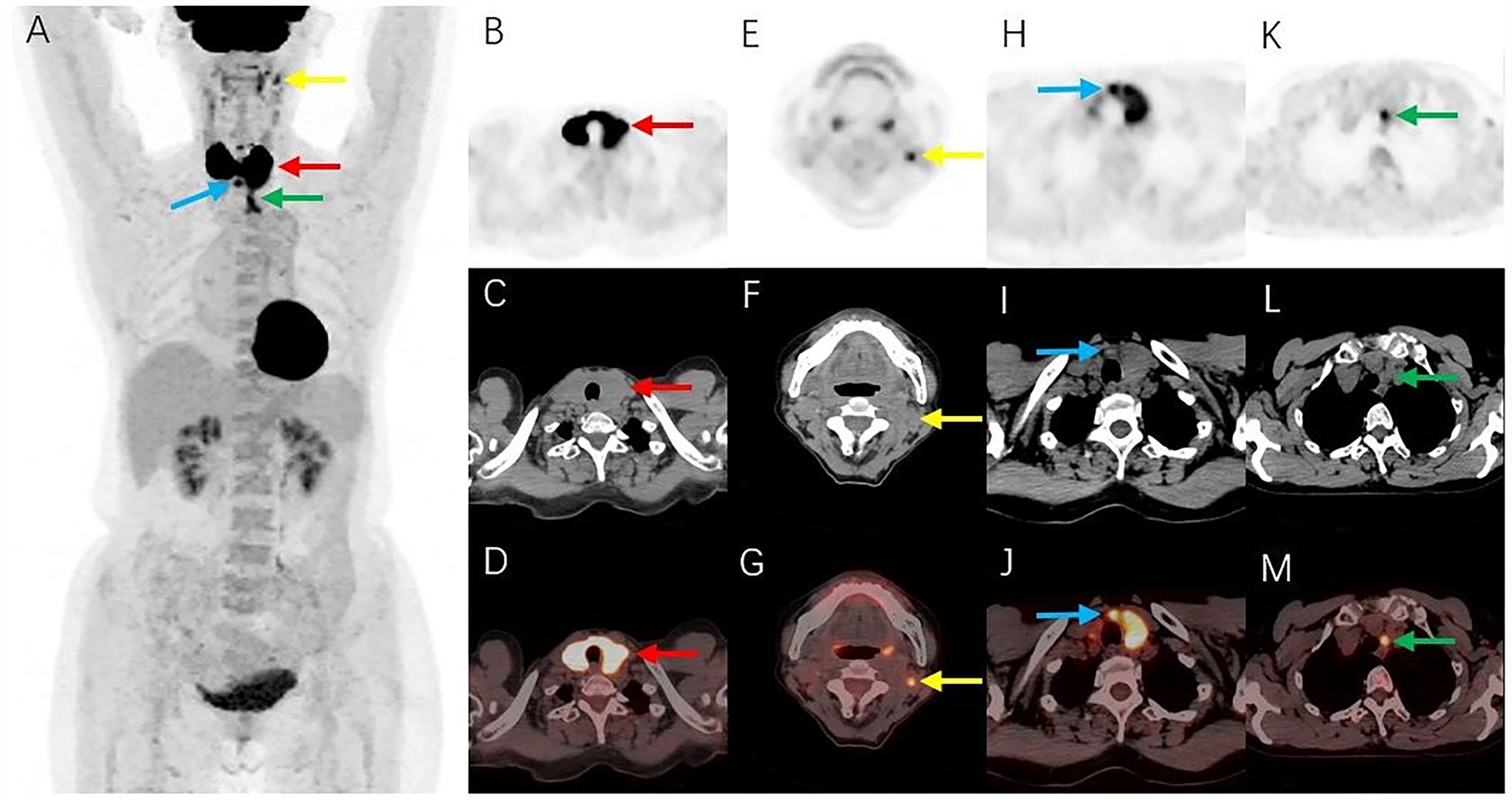
Figure 3. [18F]FDG PET/CT images. (A) Maximum intensity projections showed intense FDG uptake in both thyroid gland lobes (red arrow). Localized FDG uptake was also observed in the left level IIa (yellow arrow), level VI (blue arrow), and level VII (green arrow) of the neck. (B–D) Axial images (B, PET images; C, CT images; D, fusion images) showed intense FDG uptake in both thyroid gland lobes (red arrows), with diffuse enlargement and reduced density of both lobes on a corresponding CT scan. (E–M) Transverse PET (E, H, K), CT (F, I, L), and fusion images (G, J, M) showed lymph nodes with moderate FDG uptake in the left level IIa (yellow arrows), level VI (blue arrows), and level VII (green arrows) of the neck. [18F]FDG, 2-Deoxy-2-[18F]fluoro-D-glucose; PET/CT, positron emission tomography/computed tomography.
The patient subsequently received four cycles of R-CEOP immunochemotherapy: Rituximab 600 mg day 0, cyclophosphamide 1.2 g day 1, vincristine 2 mg day 1, epirubicin 110 mg day 1, and prednisone acetate 60 mg qd day 1–5. After therapy, the neck swelling disappeared, and the patient was in good condition. Figure 4 shows the post-treatment ultrasound images. Ultrasonography revealed that the size and shape of the thyroid gland were restored to normal, the capsule was smooth, and the parenchymal echoes were slightly increased. CDFI revealed no abnormal blood flow signal. A hypoechoic lesion was observed in the lower pole of the left lobe of the thyroid gland, with an unclear boundary, a regular shape, and an uneven internal echo (Figure 4A, yellow arrow). The size range was about 1.5×1.1×0.6 cm. CDFI revealed blood flow signals around the lesion (Figure 4B). A similar echo pattern was seen in the middle pole of the right lobe of the thyroid gland, with a range of about 0.8×0.6×0.4 cm (Figure 4C, blue arrow). The patient remains under close follow-up and continues to be in remission.
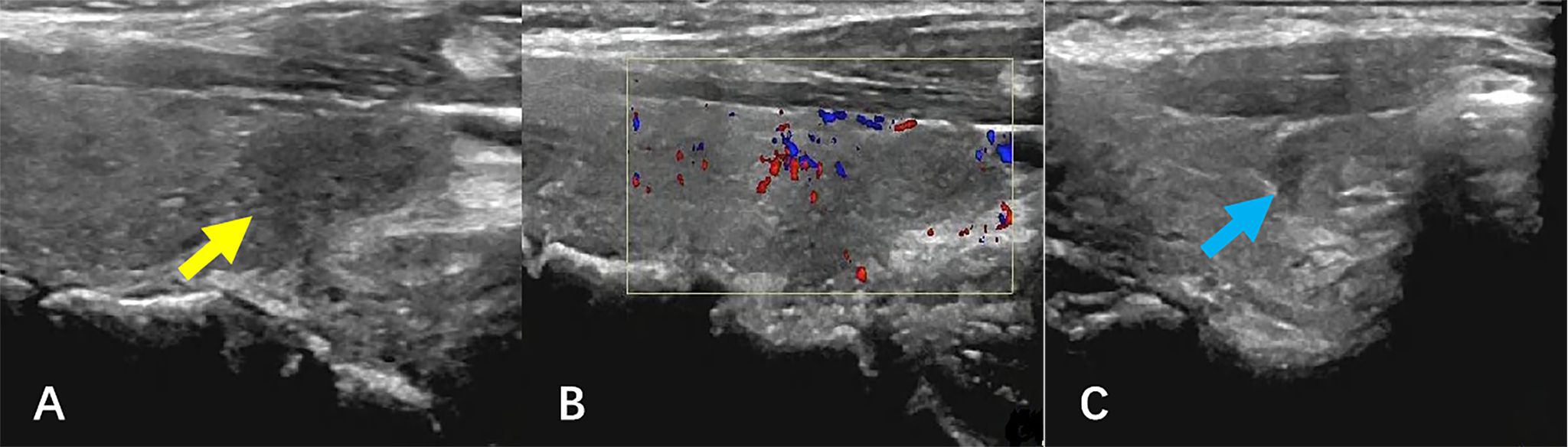
Figure 4. Ultrasound images after therapy. (A, B) The hypoechoic lesion was observed in the lower pole of the left lobe of the thyroid gland (yellow arrow), with an unclear boundary, a regular shape, and an uneven internal echo. CDFI revealed blood flow signals around the lesion. (C) A similar echo was seen in the middle pole of the right lobe of the thyroid gland (blue arrow), and CDFI showed no blood flow signal. CDFI, Color doppler flow imaging.
Systematic literature review
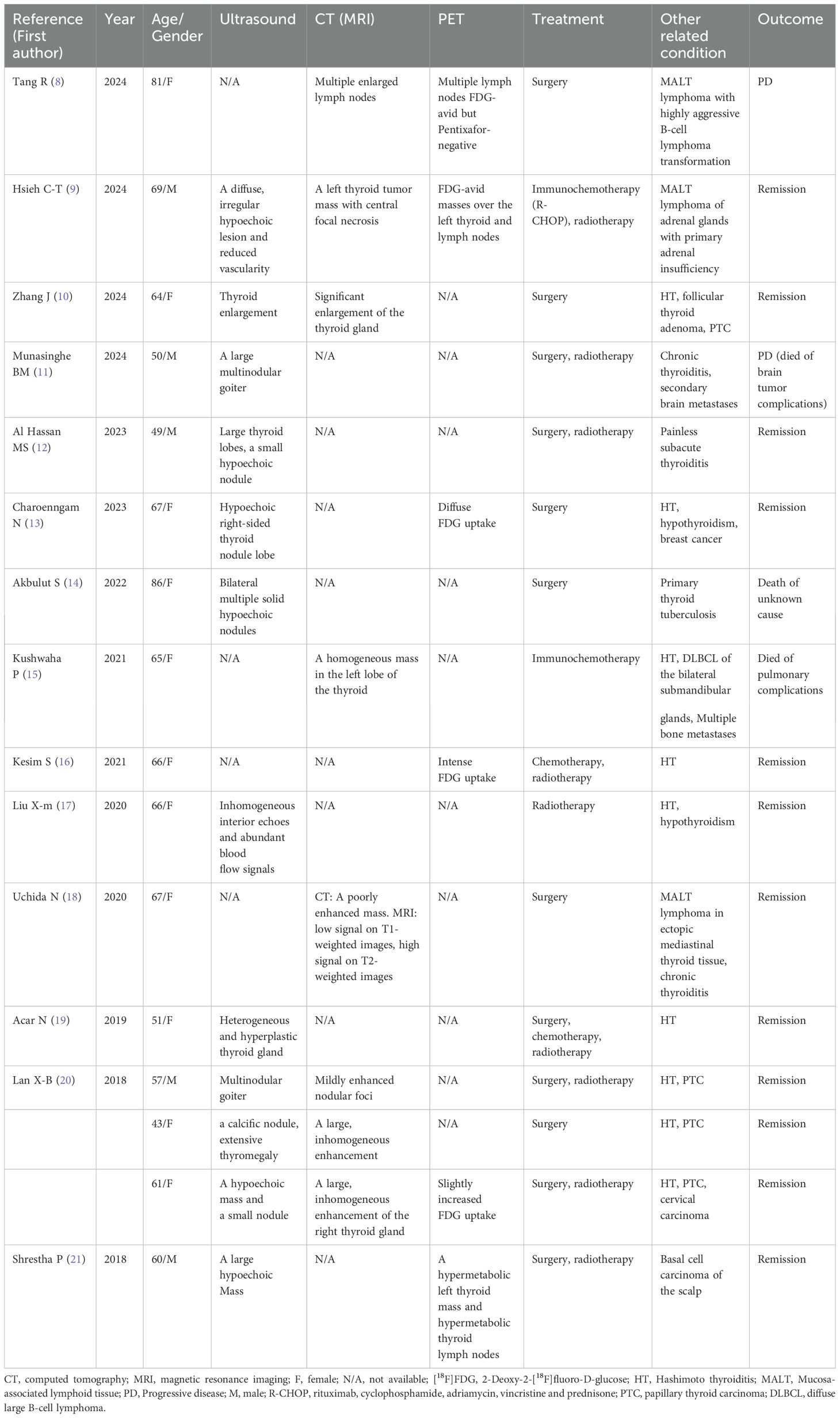
We searched the latest reports on this condition published in the PubMed database using the keyword combinations “thyroid lymphoma AND MALT” and “thyroid lymphoma AND mucosa-associated lymphoid tissue” from 2018 till June 2024. Initially, the reports were filtered and selected based on their titles and abstracts, followed by a comprehensive evaluation of their full text. We included only reports in the English language, focusing on imaging results, treatments, and outcomes. We excluded reviews, original articles, and reports without imaging results and complete medical history. Finally, we included 14 papers (8–21) in our analysis, which described 16 patients ranging in age from 43 to 86 years, with the majority being female (11/16).
The main symptom was neck swelling, and some patients also had cervical lymph node enlargement or compression symptoms, such as dyspnea and dysphagia. Ultrasound and CT findings were mainly characterized by thyroid enlargement and solid space-occupying lesions. Ultrasound showed hypoechoic lesions, and enhanced CT showed uneven enhancement. MRI of one patient showed a mass with low signal intensity on T1-weighted images and high signal intensity on T2-weighted images. All six patients had positive [18F]FDG uptake on [18F]FDG PET, and the SUVmax ranged from 6.0 to 26.82. Among the 16 patients with thyroid MALT lymphoma, 12 had thyroiditis, four had papillary thyroid carcinoma (PTC), one had thyroid tuberculosis, and five had other lymphomas or primary malignant tumors. The therapies included surgery, radiotherapy, chemotherapy, and immunochemotherapy, with surgery being the main treatment (12/16). Twelve patients responded well to treatment and showed remission changes. Table 2 summarizes the details including imaging findings, treatments, and outcomes.
Discussion
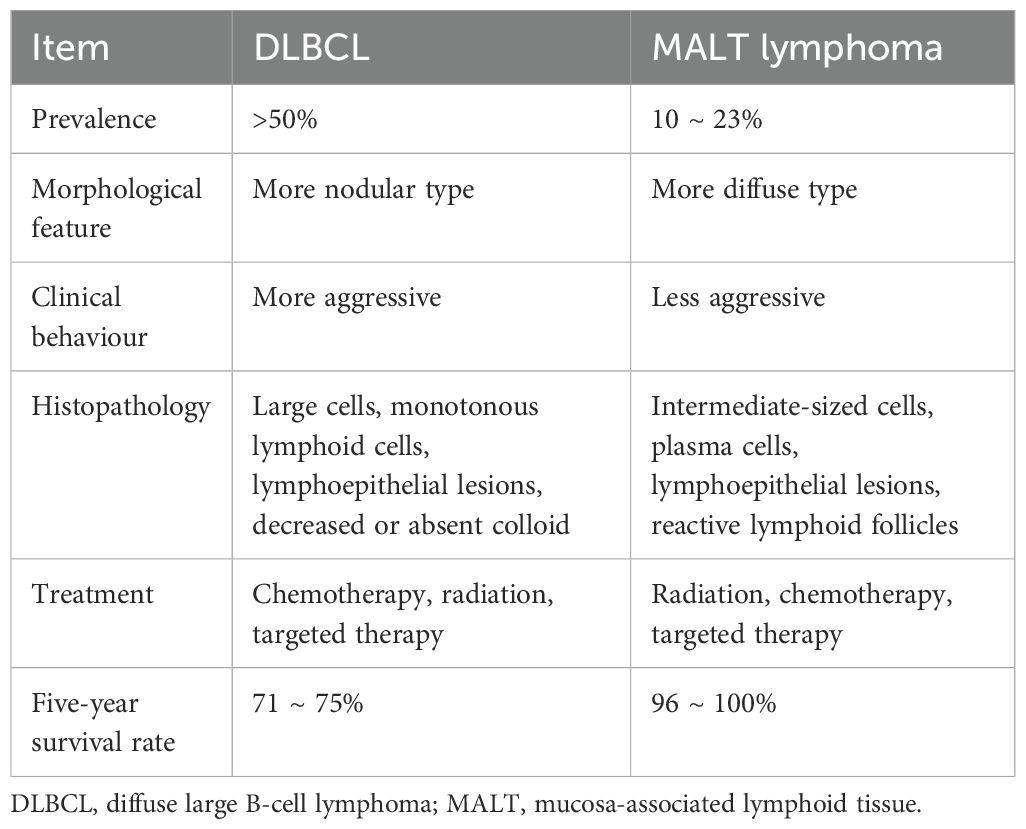
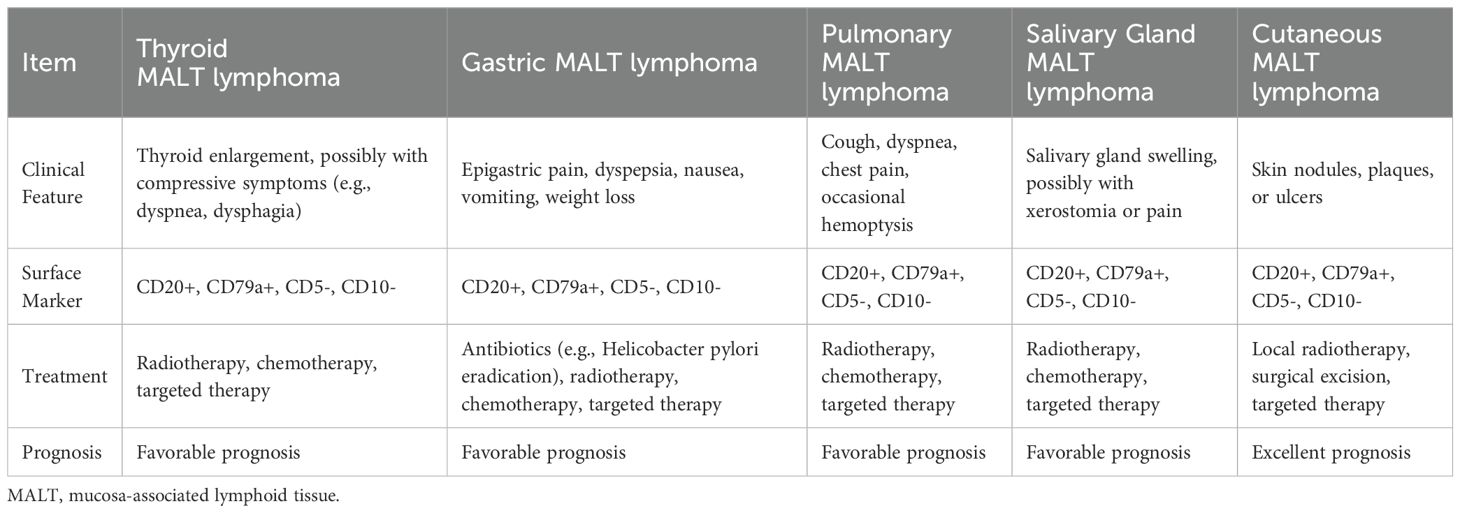
PTL is rare, accounting for approximately 5% of all malignant thyroid tumors (1). Most PTLs are B-cell-derived non-Hodgkin’s lymphoma, and the treatment and prognosis depend on the pathological type and stage of the tumor (2). The pathological types are mainly DLBCL, followed by MALT lymphoma and mixed types (3). Table 3 provides an overview of thyroid DLBCL and MALT lymphoma. Table 4 provides the differences between thyroid MALT lymphoma and other MALT lymphomas.
PTL is more prevalent in older females (4). Its clinical characteristics are non-specific, and the most common clinical manifestation is a rapidly enlarging (<6 months) neck mass, often combined with cervical lymph node enlargement or compression symptoms, such as dyspnea and dysphagia (2, 5), especially among patients with a background of HT (6). HT is a common autoimmune inflammatory disease of the thyroid gland and is widely recognized as a risk factor for PTL (22). Antonio et al. (6) reported that the prevalence of PTL combined with HT was 78.9%, and HT prevalence was significantly higher in patients with MALT lymphoma than in those with DLBCL. The ultrasound images of this patient indicated that HT could not be excluded and was speculated to be an important contributing factor to PTL.
Thyroid function in patients with thyroid MALT lymphoma varies, typically presenting as hypothyroidism, but it may also appear normal or hyperthyroid in the early stages. Changes in thyroid function may be closely related to HT and tumor infiltration. Thyroid MALT lymphoma exhibits a complex relationship with thyroid function. Hypothyroidism may lead to immunosuppression, increasing the risk of tumor development (23). Conversely, prolonged elevation of thyroid stimulating hormone levels may persistently stimulate thyroid cell proliferation, elevating the risk of malignant transformation (24). Thyroid function should be closely monitored and timely intervention should be implemented in the management of thyroid MALT lymphoma.
Ultrasonography is the primary imaging method for assessing the thyroid gland, playing a significant role in the diagnosis and differential diagnosis of thyroid-associated diseases. The ultrasonographic features of PTL include significantly hypoechoic lesions, linear or striped hyperechoic patterns, posterior echo enhancement, and slightly increased rich blood flow (25, 26). Additionally, some scholars have reported that the ultrasound manifestations of PTL vary by pathological types (27). MALT lymphoma typically presents as the diffuse type, while DLBCL tends to be more nodular. The diffuse type of MALT lymphoma in this case was consistent with the above conclusions. Table 5 summarizes the imaging differences between MALT lymphoma and other primary thyroid malignancies.
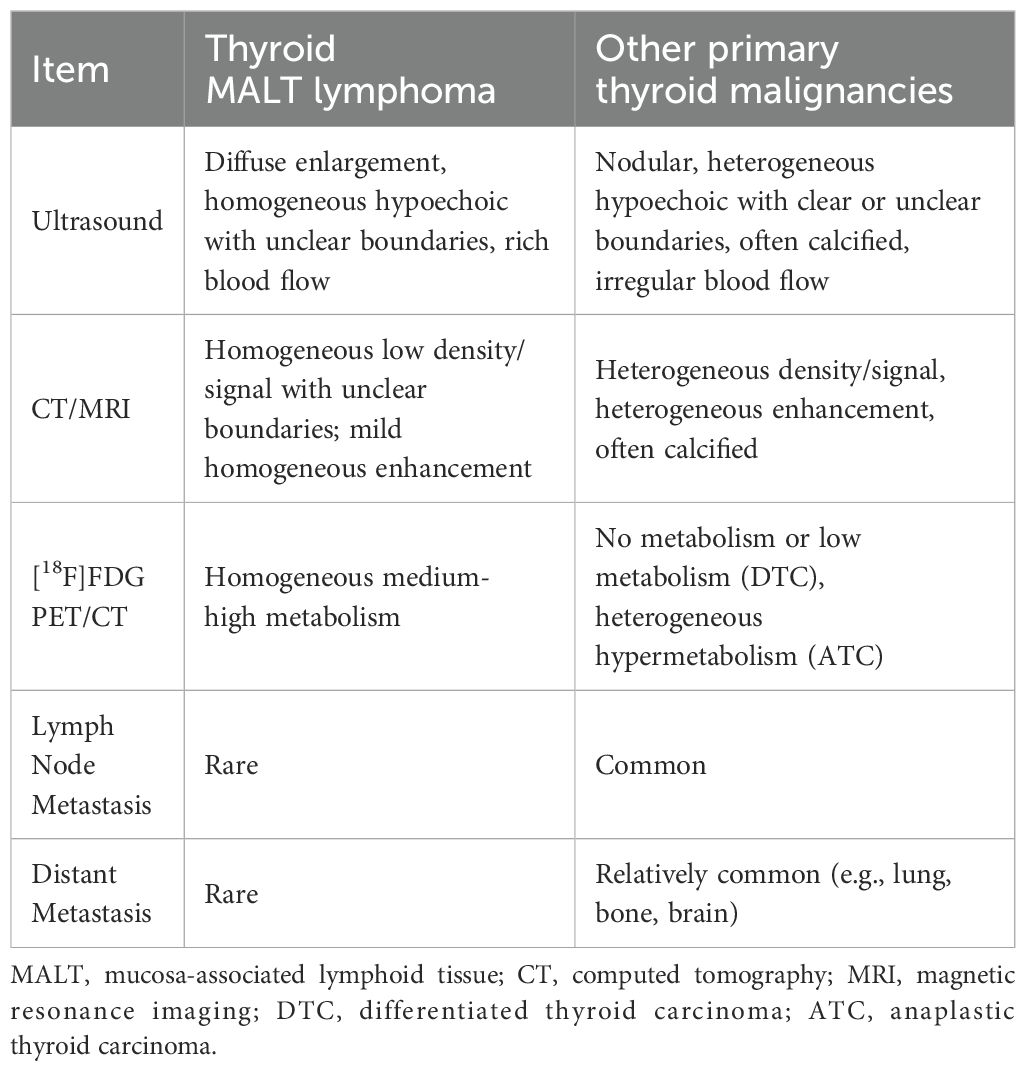
Table 5. Differences of imaging characteristics between MALT lymphoma and other primary thyroid malignancies.
Ultrasound-guided CNB and fine needle aspiration (FNA) are widely used in the diagnosis of thyroid cancer; however, both methods make it relatively difficult to determine the pathologic subtype of PTL (28). The combination of immunochemical staining, flow cytometry, and polymerase chain reaction detection of immunoglobulin heavy chain gene rearrangement enhances the diagnostic value of CNB and FNA (29). In immunohistochemical staining, CD5, CD10, and CD23 are negative in MALT cases, whereas CD19, CD20, and CD45 are usually positive in DLBCL cases. Commonly used immunohistochemical markers are listed in Table 6. In this case, the patient underwent ultrasound-guided CNB combined with immunohistochemistry and gene rearrangement to accurately diagnose MALT lymphoma.
PTL typically shows hypermetabolic nodules or diffuse hypermetabolism in both lobes on [18F]FDG PET/CT (30, 31), with or without hypermetabolic lymph nodes. On CT, a diffuse mass is common. The long axis of the unilateral lesion is usually consistent with the long axis of the thyroid gland, and bilateral lesions usually grow along the contour of the gland. The internal density is often reduced, and cystic degeneration, necrosis, and calcification are rare (30). In this case, [18F]FDG PET/CT before treatment showed decreased thyroid density uniformity, expansive growth, and diffuse hypermetabolism, consistent with previous reports. However, the patient did not undergo PET/CT for post-treatment evaluation due to economic reasons. Our case demonstrates that in addition to primary thyroid cancer and chronic thyroiditis, PTL should also be considered when PET/CT imaging shows thyroid lesions with diffuse elevated [18F]FDG uptake (30, 32). Nakadate et al. reported that [18F]FDG PET/CT may be useful in distinguishing between PTL and chronic thyroiditis, as SUVmax values are significantly higher, and CT density is significantly lower in patients with PTL compared to those with chronic thyroiditis (30). This emphasizes that our case of primary thyroid MALT lymphoma displayed intense [18F]FDG accumulation on [18F]FDG PET/CT, whereas MALT lymphoma elsewhere in the body was typically only mildly or moderately metabolized (33–36). We believe this may be due to the partial overlap of the [18F]FDG uptake between MALT lymphoma and chronic thyroiditis. In addition, high [18F]FDG uptake implies that primary thyroid MALT lymphoma may have the potential to transform into aggressive lymphoma. [18F]FDG PET/CT imaging may aid in staging and evaluating the efficacy of thyroid lymphoma treatment.
At present, radiotherapy and chemotherapy are the main treatment strategies for PTL (4). Surgery is primarily used for biopsy or relief of compression symptoms. Blind surgical resection may result in permanent loss of thyroid function and increase the incidence of surgical complications. Rituximab is also a treatment option (37). The treatment flowchart for extranodal MALT lymphoma (non-gastric) is illustrated in Figure 5. In our case, the patient received R-CEOP chemotherapy, which demonstrated significant efficacy, and the patient achieved normal thyroid function. However, the side effects of chemotherapy, including myelosuppression, cardiotoxicity, and gastrointestinal reactions, limit its application in certain patients (38). Emerging alternatives such as targeted therapy and immunotherapy provide more options for patients with MALT lymphoma (39, 40). In the future, advancements in precision medicine, immunotherapy, and combination treatment strategies are expected to further enhance therapeutic outcomes and improve patients’ quality of life.
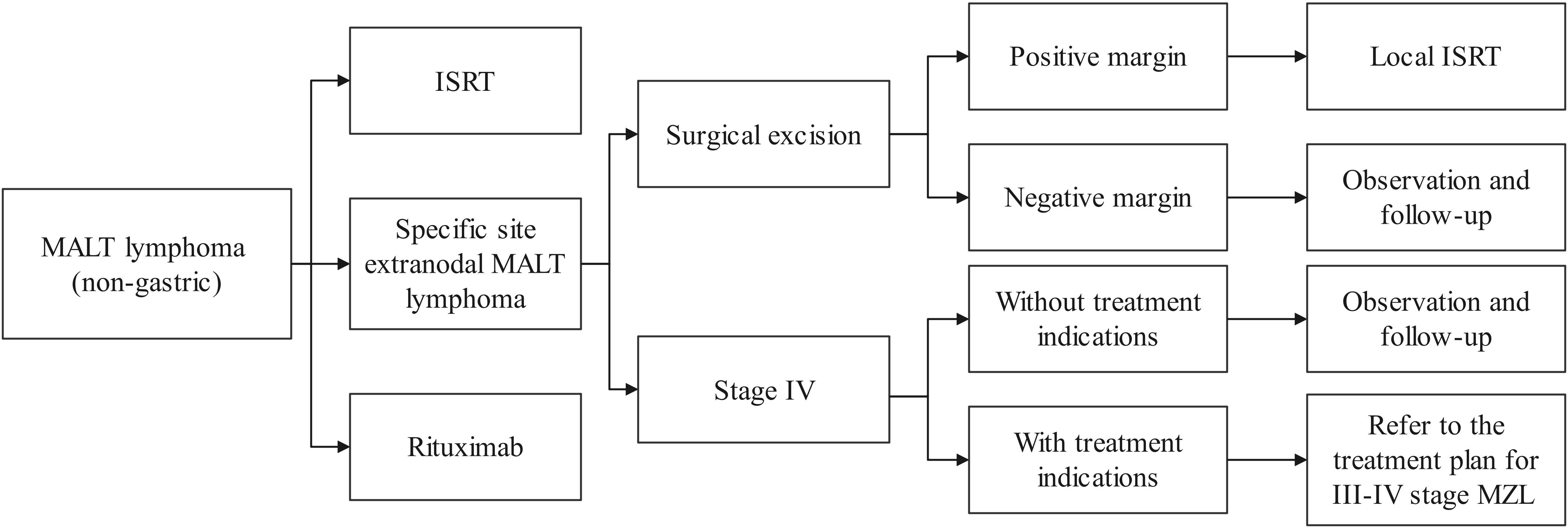
Figure 5. Treatment flowchart for extranodal MALT lymphoma (non-gastric). MALT, mucosa-associated lymphoid tissue; ISRT, involved site radiation therapy; MZL, marginal zone lymphoma.
Conclusion
In conclusion, it is important to consider the diagnosis of PTL in patients presenting with an enlarged neck mass and a history of HT, especially when accompanied by ‘B symptoms’ of lymphoma (fever, night sweats, weight loss, etc.). Ultrasonography and [18F]FDG PET/CT are helpful in the diagnosis and staging of PTL. Ultrasound-guided biopsy with pathological examination enables accurate subtype identification. Clinicians should avoid unnecessary thyroidectomy, which may lead to permanent thyroid function loss, and minimize the risk of surgical damage to adjacent organs and tissues, including the parathyroid gland. Future research should focus on optimizing patient management and treatment strategies to improve outcomes.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by ethics committee of Mianyang Central Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
LX: Data curation, Writing – original draft, Writing – review & editing. ZZ: Data curation, Methodology, Writing – review & editing. LZ: Data curation, Methodology, Writing – review & editing. JY: Resources, Writing – review & editing. DL: Resources, Writing – review & editing. FL: Resources, Writing – review & editing. QZ: Investigation, Writing – review & editing. DH: Conceptualization, Funding acquisition, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We thank the patient for granting permission to publish this information.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Florindez JA, Alderuccio JP, Reis IM, Lossos IS. Primary thyroid lymphoma: survival analysis of seer database (1995-2016). Leuk Lymph. (2021) 62:2796–9. doi: 10.1080/10428194.2021.1933479
2. Stein SA, Wartofsky L. Primary thyroid lymphoma: A clinical review. J Clin Endocrinol Metab. (2013) 98:3131–8. doi: 10.1210/jc.2013-1428
3. Karvounis E, Kappas I, Angelousi A, Makris GM, Kassi E. Mucosa-associated lymphoid tissue lymphoma of the thyroid gland: A systematic review of the literature. Eur Thyroid J. (2020) 9:11–8. doi: 10.1159/000502204
4. Pavlidis ET, Pavlidis TE. A review of primary thyroid lymphoma: molecular factors, diagnosis and management. J Invest Surg. (2019) 32:137–42. doi: 10.1080/08941939.2017.1383536
5. Wang Y, Wang S, Liu Z, Liao Q, Chen G, Xu X. Diagnosis methods and treatment strategy for primary thyroid lymphoma: A retrospective analysis of 41 cases in China. Eur J Cancer Prev. (2021) 30:108–12. doi: 10.1097/CEJ.0000000000000599
6. Travaglino A, Pace M, Varricchio S, Insabato L, Giordano C, Picardi M, et al. Hashimoto thyroiditis in primary thyroid non-hodgkin lymphoma. Am J Clin Pathol. (2020) 153:156–64. doi: 10.1093/ajcp/aqz145
7. Tessler FN, Middleton WD, Grant EG, Hoang JK, Berland LL, Teefey SA, et al. Acr thyroid imaging, reporting and data system (Ti-rads): white paper of the acr ti-rads committee. J Am Coll Radiol. (2017) 14:587–95. doi: 10.1016/j.jacr.2017.01.046
8. Tang R, Chen X, Liu M, Shu Q, Cai L. Malt lymphoma in histologic transformation. Clin Nuclear Med. (2024) 49:276–7. doi: 10.1097/rlu.0000000000005057
9. Hsieh C-T, Yu J-T, Tsao T-Y, Tseng YH. Mucosa-associated lymphoid tissue lymphoma of thyroid and adrenal glands with primary adrenal insufficiency. Endocrinol Diabetes Metab Case Rep. (2024) 2024:23–0019. doi: 10.1530/edm-23-0019
10. Zhang J, Wu J, Sun L, Chen Y, Yu Z. Simultaneous primary thyroid malt lymphoma and papillary thyroid cancer. Front Endocrinol. (2024) 15:1372661. doi: 10.3389/fendo.2024.1372661
11. Munasinghe BM, Karunatileke CT, Prashanthan J, Ranathunga ND. Primary thyroid malt lymphoma with a probable brain secondary in a male patient: A case report. Int J Surg Case Rep. (2024) 114:109126. doi: 10.1016/j.ijscr.2023.109126
12. Al Hassan MS, El Ansari W, Darweesh A, Sharaf Eldeen MZ, Obiedat S, Abdelaal A. Mucosa-associated lymphoid tissue lymphoma (Malt lymphoma) in the thyroid mimicking a painless subacute (De quervain’s) thyroiditis on presentation, fine needle aspiration and cytology, and ultrasound findings: A rare case report. Int J Surg Case Rep. (2023) 106:108147. doi: 10.1016/j.ijscr.2023.108147
13. Charoenngam N, Marangoz MS, Lamothe S, James BC. Thyroid mucosa-associated lymphoid tissue lymphoma presenting as intermediate-risk thyroid nodule with positive kras mutation. AACE Clin Case Rep. (2023) 9:44–7. doi: 10.1016/j.aace.2023.01.004
14. Akbulut S, Demyati K, Yavuz R, Sogutcu N, Samdanci ET, Yagmur Y. Coexistence of tuberculosis and extranodal marginal zone lymphoma of the thyroid gland: case report and literature review. Ann Med Surg. (2022) 78:103861. doi: 10.1016/j.amsu.2022.103861
15. Kushwaha P, Singh M, Mandal S, Dhingra S, Jain S. Dlbcl of bilateral submandibular glands and maltoma of thyroid—a rare coexistence. Cytopathology. (2021) 32:523–6. doi: 10.1111/cyt.12960
16. Kesim S, Ozguven S. Primary thyroid malt lymphoma and treatment response demonstrated on fdg pet/ct. Rev Española Med Nuclear e Imag Mol (English Edition). (2021) 40:372–3. doi: 10.1016/j.remnie.2020.12.001
17. Liu X-m, Ma D-l, Yuan G, Xie J-H. Progressively enlarging goiter: case reports of primary thyroid lymphoma and literature review. Curr Med Sci. (2020) 40:518–22. doi: 10.1007/s11596-020-2204-2
18. Uchida N, Yoshida M. Mucosa-associated lymphoid tissue (Malt) lymphoma developing in ectopic mediastinal thyroid tissue: A case report. Surg Case Rep. (2020) 6:97. doi: 10.1186/s40792-020-00857-2
19. Acar N, Acar T, Avcı A, Haciyanlı M. Approach to primary thyroid lymphoma: case series. Turkish J Surg. (2019) 35:142–5. doi: 10.5578/turkjsurg.4132
20. Lan XB, Cao J, Zhu XH, Han Z, Huang YQ, Ge MH, et al. Concomitant papillary thyroid carcinoma and mucosa-associated lymphoid tissue thyroid lymphoma in the setting of hashimoto thyroiditis. Int J Clin Exp Pathol (2018) 11:3076–83.
21. Shrestha P, Aderhold K, Swierczynski S, Lin C, Herb R. Primary thyroid maltoma– a rare diagnosis of an unassuming thyroid nodule. J Community Hosp Internal Med Perspect. (2018) 8:42–5. doi: 10.1080/20009666.2018.1424487
22. Watanabe N, Noh JY, Narimatsu H, Takeuchi K, Yamaguchi T, Kameyama K, et al. Clinicopathological features of 171 cases of primary thyroid lymphoma: A long-term study involving 24553 patients with hashimoto’s disease. Br J Haematol. (2011) 153:236–43. doi: 10.1111/j.1365-2141.2011.08606.x
23. De Vito P, Incerpi S, Pedersen JZ, Luly P, Davis FB, Davis PJ. Thyroid hormones as modulators of immune activities at the cellular level. Thyroid. (2011) 21:879–90. doi: 10.1089/thy.2010.0429
24. Boelaert K, Horacek J, Holder RL, Watkinson JC, Sheppard MC, Franklyn JA. Serum thyrotropin concentration as a novel predictor of Malignancy in thyroid nodules investigated by fine-needle aspiration. J Clin Endocrinol Metab. (2006) 91:4295–301. doi: 10.1210/jc.2006-0527
25. Ota H, Ito Y, Matsuzuka F, Kuma S, Fukata S, Morita S, et al. Usefulness of ultrasonography for diagnosis of Malignant lymphoma of the thyroid. Thyroid. (2006) 16:983–7. doi: 10.1089/thy.2006.16.983
26. Zhang X, Wei B, Nong L, Zhang H, Gao Y, Ou J. The usefulness of serial ultrasound in thyroid mucosa-associated lymphoid tissue lymphoma. Front Endocrinol (Lausanne). (2022) 13:1054584. doi: 10.3389/fendo.2022.1054584
27. Gu LS, Cui NY, Wang Y, Che SN, Zou SM, He W, et al. Comparison of sonographic characteristics of primary thyroid lymphoma and anaplastic thyroid carcinoma. J Thorac Dis. (2017) 9:4774–84. doi: 10.21037/jtd.2017.09.48
28. Czopnik P, Aporowicz M, Niepokoj-Czopnik A, Wojtczak B, Domoslawski P, Bolanowski M. Primary thyroid lymphoma: A rare but challenging diagnosis. Pol Arch Intern Med. (2017) 127:361–4. doi: 10.20452/pamw.4037
29. Hirokawa M, Kudo T, Ota H, Suzuki A, Kobayashi K, Miyauchi A. Preoperative diagnostic algorithm of primary thyroid lymphoma using ultrasound, aspiration cytology, and flow cytometry. Endocr J. (2017) 64:859–65. doi: 10.1507/endocrj.EJ17-0111
30. Nakadate M, Yoshida K, Ishii A, Koizumi M, Tochigi N, Suzuki Y, et al. Is 18F-FDG PET/CT useful for distinguishing between primary thyroid lymphoma and chronic thyroiditis? Clin Nuclear Med. (2013) 38:709–14. doi: 10.1097/RLU.0b013e31829b2686
31. Agrawal R, Agrawal A. Imaging features in a rare case of maltoma of the thyroid. Clin Nuclear Med. (2010) 35:620–1. doi: 10.1097/RLU.0b013e3181e4df53
32. Tsubaki F, Kurata S, Tani J, Sumi A, Fujimoto K, Abe T. Clinical significance of patterns of increased [18F]-FDG uptake in the thyroid gland: A pictorial review. Jpn J Radiol. (2018) 36:181–93. doi: 10.1007/s11604-017-0715-y
33. Kim D-H, Jeong JY, Lee S-W, Lee J, Ahn B-C. 18F-FDG PET/CT finding of bilateral primary breast mucosa-associated lymphoid tissue lymphoma. Clin Nuclear Med. (2015) 40:e148–e9. doi: 10.1097/rlu.0000000000000545
34. Albano D, Borghesi A, Bosio G, Bertoli M, Maroldi R, Giubbini R, et al. Pulmonary mucosa-associated lymphoid tissue lymphoma: 18F-FDG PET/CT and CT findings in 28 patients. Br J Radiol. (2017) 90:20170311. doi: 10.1259/bjr.20170311
35. Wang W, Ni X, Tang T, Wang J, Li Y, Song X. The role of 18F-FDG PET/CT in diagnosis and treatment evaluation for ocular adnexal mucosa-associated lymphoid tissue lymphoma. Br J Radiol. (2022) 95:20210635. doi: 10.1259/bjr.20210635
36. Albano D, Bertoli M, Ferro P, Fallanca F, Gianolli L, Picchio M, et al. 18F-FDG PET/CT in gastric malt lymphoma: A bicentric experience. Eur J Nucl Med Mol Imaging. (2017) 44:589–97. doi: 10.1007/s00259-016-3518-y
37. Walsh S, Lowery AJ, Evoy D, McDermott EW, Prichard RS. Thyroid lymphoma: recent advances in diagnosis and optimal management strategies. Oncologist. (2013) 18:994–1003. doi: 10.1634/theoncologist.2013-0036
38. Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, et al. Chop chemotherapy plus rituximab compared with chop alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. (2002) 346:235–42. doi: 10.1056/NEJMoa011795
39. Zucca E, Arcaini L, Buske C, Johnson PW, Ponzoni M, Raderer M, et al. Marginal zone lymphomas: esmo clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. (2020) 31:17–29. doi: 10.1016/j.annonc.2019.10.010
Keywords: primary thyroid lymphoma, mucosa-associated lymphoid tissue lymphoma, case report, system review, literature review
Citation: Xiao L, Zhao Z, Zhou L, Yan J, Luo D, Liu F, Zhou Q and Huang D (2025) Clinical and imaging features of primary thyroid MALT lymphoma. Front. Oncol. 15:1498609. doi: 10.3389/fonc.2025.1498609
Received: 21 September 2024; Accepted: 16 April 2025;
Published: 08 May 2025.
Edited by:
Sharon R. Pine, University of Colorado Anschutz Medical Campus, United StatesReviewed by:
Ran Zhu, Soochow University, ChinaYuting Ke, Massachusetts Institute of Technology, United States
Jongmyung Kim, Moffitt Cancer Center, United States
Copyright © 2025 Xiao, Zhao, Zhou, Yan, Luo, Liu, Zhou and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dan Huang, aHVhbmdkYW4yMzQ2QDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
 Liming Xiao
Liming Xiao Ziyi Zhao1,2†
Ziyi Zhao1,2† Li Zhou
Li Zhou Fucen Liu
Fucen Liu