Abstract
Introduction:
More than half of patients with tracheal carcinoma (TC) do not receive radical treatment, but the clinical characteristics, palliative treatment options, and prognosis of this group remain unclear.
Methods:
This retrospective study analyzed 94 single primary TC patients (42 with tracheal squamous cell carcinoma [TSCC] and 52 with tracheal adenoid cystic carcinoma [TACC]) admitted to the Emergency General Hospital and Dongzhimen Hospital, Beijing University of Chinese Medicine. Kaplan-Meier survival curves, Log-rank tests, univariate and multivariate Cox and AFT models were used to assess overall survival (OS).
Results:
Among 89 patients without radical treatment, the median survival was 57 months, with 5-year and 10-year survival rates of 46.33% and 13.43%, respectively. Univariate analysis identified pathological type, smoking history, initial tumor extension (ITE), and targeted therapy as significant prognostic factors. The AFT model revealed that the median OS for TSCC patients was significantly shorter than for TACC patients, with a time ratio (TR) of 0.243 (95% CI: 0.153-0.386; P < 0.01), while targeted therapy was associated with a 1.790-fold increase in OS (TR: 1.790, 95% CI: 1.061-3.020; P = 0.029). Patients with extensive ITE had worse outcomes, with a TR of 0.628 (95% CI: 0.406-0.971; P = 0.037). Smokers had a TR of 0.601 (95% CI: 0.397-0.912; P = 0.017) compared with non-smokers. Subgroup analysis showed that smoking history was strongly associated with shorter OS in TSCC but not in TACC.
Conclusions:
Pathological type, ITE, targeted therapy and smoking history are important factors for evaluating the prognosis of TC patients receiving palliative treatment.
1 Introduction
Tracheal carcinoma(TC) is a rare malignancy with an annual incidence of 0.075 to 1 case per 100,000 individuals (1–3). Since 1980, clinical studies have demonstrated a substantial increase in the 5-year survival rate of TC, rising from 5.2% to 31.7% (3). The two primary pathological types, tracheal squamous cell carcinoma (TSCC) and tracheal adenoid cystic carcinoma (TACC), together account for more than 75% of cases (4). Most clinical studies on TC are based on data from large public databases, such as the Surveillance, Epidemiology, and End Results (SEER) program and the National Cancer Database (NCDB) (5, 6). Surgical resection is the preferred treatment for localized TC, and previous research has mainly focused on evaluating outcomes of surgery alone or in combination with other therapies (7, 8). However, due to the complex anatomical location and the high incidence of delayed or incorrect diagnosis, 60% to 80% of patients are not eligible for radical treatment (7, 9).
Palliative treatment is a crucial option for patients with TC who are not eligible for radical therapies (6, 10, 11). The main palliative treatment strategies for TC include radiotherapy, chemotherapy (platinum-based or other chemotherapeutic agents administered systemically or locally), targeted therapy (molecular targeted agents or vascular targeted agents such as Endostar delivered systemically or locally), immunotherapy, and bronchoscopic interventions, such as photodynamic therapy (PDT) and endobronchial stenting (12–15). Although palliative treatment has been extensively studied in various solid tumors due to its important role (16), research on its use in TC remains limited. This is primarily due to a lack of relevant variables in public databases and the predominance of single-center studies that focus on individual palliative approaches, leaving comprehensive evaluations of its overall effectiveness insufficiently explored (5, 9, 17–19).
This study retrospectively analyzed patients with single primary TC from two centers, detailing the clinical characteristics, evaluating palliative treatment options, and investigating potential factors influencing long-term prognosis.
2 Materials and methods
2.1 Study design and patients
This retrospective cohort study included 113 patients diagnosed with single primary TC at Beijing Emergency General Hospital (EG) and Dongzhimen Hospital, Beijing University of Chinese Medicine (DZM) between January 2010 and January 2023. Exclusion criteria were as follows: 1) prior radical treatment, including surgery or radiotherapy (radical surgery was defined as a procedure aiming for curative intent with complete resection of the tumor along with potentially involved surrounding tissues and lymph nodes, achieving negative surgical margins (6); radical radiotherapy was defined as radiation therapy with curative intent, delivered at an average dose exceeding 60 Gy (17)); and 2) incomplete tumor characteristic data. Ultimately, 94 patients who received palliative treatment were included in the final analysis (Supplementary Figure S1).
The study was conducted in accordance with the Declaration of Helsinki (2013 revision) and was approved by the ethics committees of DZM (No. 2024DZMEC-039-02) and EG (No. K24-24). Informed consent was waived by the ethics committees.
2.2 Data and definitions
Pathological subtype, age, sex, initial symptoms, smoking history, family history of cancer, and treatment history (including surgery, radiotherapy, chemotherapy, immunotherapy, targeted therapy, bronchoscopic interventional therapy, and PDT) were collected from medical records, interventional procedure reports, and pathological examination reports in the electronic medical record system.
Given the particular anatomical location of TC, we did not use the T stage in TNM staging but instead referred to the scheme proposed by Jin et al. to assess central airway stenosis (20).We believe this protocol offers a more suitable approach for the comprehensive evaluation of airway tumors in palliative treatment. Initial Tumor Extension(ITE) was categorized as I Zone, II Zone, and III Zone, based on invasion into the upper, middle, and lower thirds of the trachea. Initial Wall Invasion(IWI) was classified into 4 types: simply located in the lumen, outside the lumen, lumen wall, and mixed type. Initial Airway Narrowing(IAN) was defined based on the degree (%) of stenosis in the diameter of the trachea. Stenosis ≤25% was defined as Grade 1, 26%-50% as Grade 2, 51-75% as Grade 3, 76%-90% as Grade 4, and 91%-100% as Grade 5. We also collected data on regional lymph node metastasis and distant tumor metastasis. All patients underwent radiological and bronchoscopic examinations at the time of diagnosis. To ensure data consistency between the two participating centers, all original bronchoscopic images and CT scans were independently reviewed by two experienced radiologists and two experienced bronchoscopists. Any discrepancies were resolved through consensus.
The follow-up endpoint was overall survival (OS), defined as the time from pathological diagnosis to death from any cause or the last follow-up. Due to data limitations, cancer-specific mortality could not be distinguished. Survival data were obtained from the Chinese Center for Disease Control and Prevention or through telephone follow-up. The follow-up cutoff date was December 31, 2023. Patients with missing survival data were excluded from the survival analysis.
2.3 Statistical analysis
Quantitative data were expressed as the mean and standard deviation (SD). Significance levels were calculated using the equal variance t-test, Wilcoxon signed-rank test, or Mann-Whitney U test, depending on pathological grouping. Categorical data were presented as frequencies and percentages, and comparisons were made using the chi-square test or Fisher’s exact test.
Kaplan-Meier survival curves were constructed, and 5 -, 10 -, and 15-year survival rates, as well as median survival times, were calculated. The Log-rank test and univariate Cox regression analysis were initially performed to identify key prognostic variables. However, the proportional-hazards assumption was tested and found to be violated for pathological type and smoking history (Supplementary Table S1). Since multivariate Cox regression analysis relies on this assumption, it may not be suitable for our data. To address this limitation, we employed the accelerated failure time (AFT) model, which does not require the proportional-hazards assumption and allows for direct modeling of survival time. In the AFT model, covariate effects are expressed in terms of a Time Ratio (TR), where TR > 1 indicates prolonged survival time, while TR < 1 suggests a shortened survival time. The optimal distribution for the AFT model was determined based on the Akaike Information Criterion (AIC), with the model yielding the lowest AIC value selected for final analysis (21). In the subgroup analysis, univariate Cox regression analysis was initially performed by pathological group to identify variables with a significance level of P < 0.1. These selected variables were then included in a multivariate Cox regression model to adjust for other potential confounders, allowing for the identification of independent prognostic factors in each pathological subgroup.
All statistical analyses were performed using R software, version 4.4.0 (https://www.r-project.org/), and Rstudio 2024.04.1 + 748 (https://posit.co/). The following R packages were utilized: “arsenal”, “gtsummary”, “car”, “tidyverse”, “survival”, “ggplot2”, “dplyr”, “survival”, “survminer”, “readr”, “gridExtra”, “arsenal”, “forestplot”. All tests were two-sided, and P < 0.05 was considered statistically significant.
3 Results
3.1 Demographic and baseline characteristics
The demographic, baseline and therapeutic characteristics of the 94 patients are summarized in Table 1. TACC was the most common subtype(52/94, 55.3%), followed by TSCC (42/94, 44.7%). A total of 52.1% of the patients were former or current smokers. The most common initial symptoms were cough with expectoration (41.5%) and dyspnea (22.3%). Additionally, 69.1% of the patients had an ITE limited to one zone.
Table 1
| Characteristics | TACC, N = 52 | TSCC, N = 42 | P Value |
|---|---|---|---|
| Sex | 0.003 | ||
| Female | 25 (48.1%) | 8 (19.0%) | |
| Male | 27 (51.9%) | 34 (81.0%) | |
| Age (years) | 47.0 ± 13.0 | 61.1 ± 12.5 | <0.001 |
| Initial Symptoms | <0.001 | ||
| Cough & Sputum | 27 (51.9%) | 12 (28.6%) | |
| Dyspnea | 16 (30.8%) | 5 (11.9%) | |
| Hemoptysis | 5 (9.6%) | 18 (42.9%) | |
| Other Symptoms | 4 (7.7%) | 7 (16.7%) | |
| Smoking History | 0.011 | ||
| No | 31 (59.6%) | 14 (33.3%) | |
| Yes | 21 (40.4%) | 28 (66.7%) | |
| Family Cancer History | 0.200 | ||
| No | 39 (75.0%) | 36 (85.7%) | |
| Yes | 13 (25.0%) | 6 (14.3%) | |
| Initial Tumor Extensiona | 0.400 | ||
| 1 | 34 (65.4%) | 31 (73.8%) | |
| 2 | 13 (25.0%) | 10 (23.8%) | |
| 3 | 5 (9.6%) | 1 (2.4%) | |
| Initial Airway Narrowing | 0.800 | ||
| 1 | 6 (11.5%) | 5 (11.9%) | |
| 2 | 10 (19.2%) | 10 (23.8%) | |
| 3 | 17 (32.7%) | 9 (21.4%) | |
| 4 | 16 (30.8%) | 16 (38.1%) | |
| 5 | 3 (5.8%) | 2 (4.8%) | |
| Initial Wall Invasionb | 0.300 | ||
| W1 | 17 (32.7%) | 18 (42.9%) | |
| W2 | 35 (67.3%) | 24 (57.1%) | |
| Tumor Metastasis | <0.001 | ||
| Both | 5 (9.6%) | 1 (2.4%) | |
| Extra-pulm | 2 (3.8%) | 5 (11.9%) | |
| Intra-pulm | 22 (42.3%) | 3 (7.1%) | |
| None | 23 (44.2%) | 33 (78.6%) | |
| Lymph Node Status | 0.089 | ||
| No | 35 (67.3%) | 21 (50.0%) | |
| Yes | 17 (32.7%) | 21 (50.0%) | |
| Radiotherapy | 0.110 | ||
| No | 20 (38.5%) | 23 (54.8%) | |
| Yes | 32 (61.5%) | 19 (45.2%) | |
| Chemotherapyc | 0.200 | ||
| No | 32 (61.5%) | 20 (47.6%) | |
| Yes | 20 (38.5%) | 22 (52.4%) | |
| Immunotherapyd | 0.130 | ||
| No | 48 (92.3%) | 42 (100.0%) | |
| Yes | 4 (7.7%) | 0 (0.0%) | |
| Targeted Therapye | 0.140 | ||
| No | 38 (73.1%) | 36 (85.7%) | |
| Yes | 14 (26.9%) | 6 (14.3%) | |
| PDT | 0.071 | ||
| No | 45 (86.5%) | 41 (97.6%) | |
| Yes | 7 (13.5%) | 1 (2.4%) | |
| Tracheoscopic Tx | 0.600 | ||
| Standard | 30 (57.7%) | 22 (52.4%) | |
| Standard&Stent | 22 (42.3%) | 20 (47.6%) |
Clinical features of the 94 patients with TC.
a ITE violation of any 1 of Zone I, Zone II, and Zone III was marked as 1, violation of 2 zones was marked as 2, and violation of 3 zones was marked as 3. When the invasion range was 1 Zone, it was defined as E1, and when the invasion range was more than 1 Zone, it was marked as E2. b In IWI, simply located in the lumen, outside the lumen, lumen wall, mixed type. Among the four types of invasion, the tumor with only one invasion type was defined as W1, and the tumor with no one invasion type was defined as W2. c Chemotherapy involved both bronchoscopic injections of gemcitabine and cisplatin, and systemic chemotherapy with platinum-based or other chemotherapeutic agents. d Immunotherapy: All four patients who received immunotherapy were diagnosed with TACC, and the immunotherapeutic agent used was IL-2. e Targeted therapy here refers to bronchoscopic administration of vascular-targeted drugs, including Endostar and Anlotinib.
Compared with TACC patients, TSCC patients were older(61.1 ± 12.5 vs 47.0 ± 13.0,P <0.001), had a higher proportion of males (81.0% vs 51.9%,P =0.003), and a greater percentage of smokers (66.7% vs 40.4%, P =0.011). The proportion of patients with tumor metastasis at the initial diagnosis was lower in TSCC than in TACC (21.4% vs 55.8%, P <0.001). The proportion of patients with lymph node metastasis in the initial diagnosis was higher in TSCC than in TACC, though the difference was not statistically significant (50.0% vs 32.7%, P =0.089).
3.2 Therapeutic characteristics
Compared with immunotherapy and targeted therapy, radiotherapy and chemotherapy were more commonly used as palliative treatments for TC patients, with 54.3% and 44.5% of patients undergoing radiotherapy and chemotherapy, respectively. A small number of patients received PDT, with the proportion of TSCC patients undergoing PDT being lower than that of TACC patients (2.4% vs 13.5%, P =0.071).
All patients received bronchoscopic interventions. Overall, we did not observe any statistically significant differences in the choice of treatment between TSCC and TACC patients. Due to the limited documentation of specific adverse reactions to various palliative treatments in the medical records, our study did not report on the adverse effects of palliative therapies.
3.3 Patient survival analysis
We performed a survival analysis on 89 (37 TSCC and 52 TACC) TC patients with complete survival data, with a median follow-up duration of 53 months, during which 67 patients had died. The overall median survival time was 57 months, with TSCC patients having a median survival time of 13 months, compared to 87 months for TACC patients. The 5- and 10-year OS rates for the entire cohort were 13.4% and 46.3%, respectively. For TSCC, the 5- and 10-year survival rates were 13.9% and 3.8%, respectively, while for TACC, they were 65.8% and 21.1% (Figures 1A, B).
Figure 1
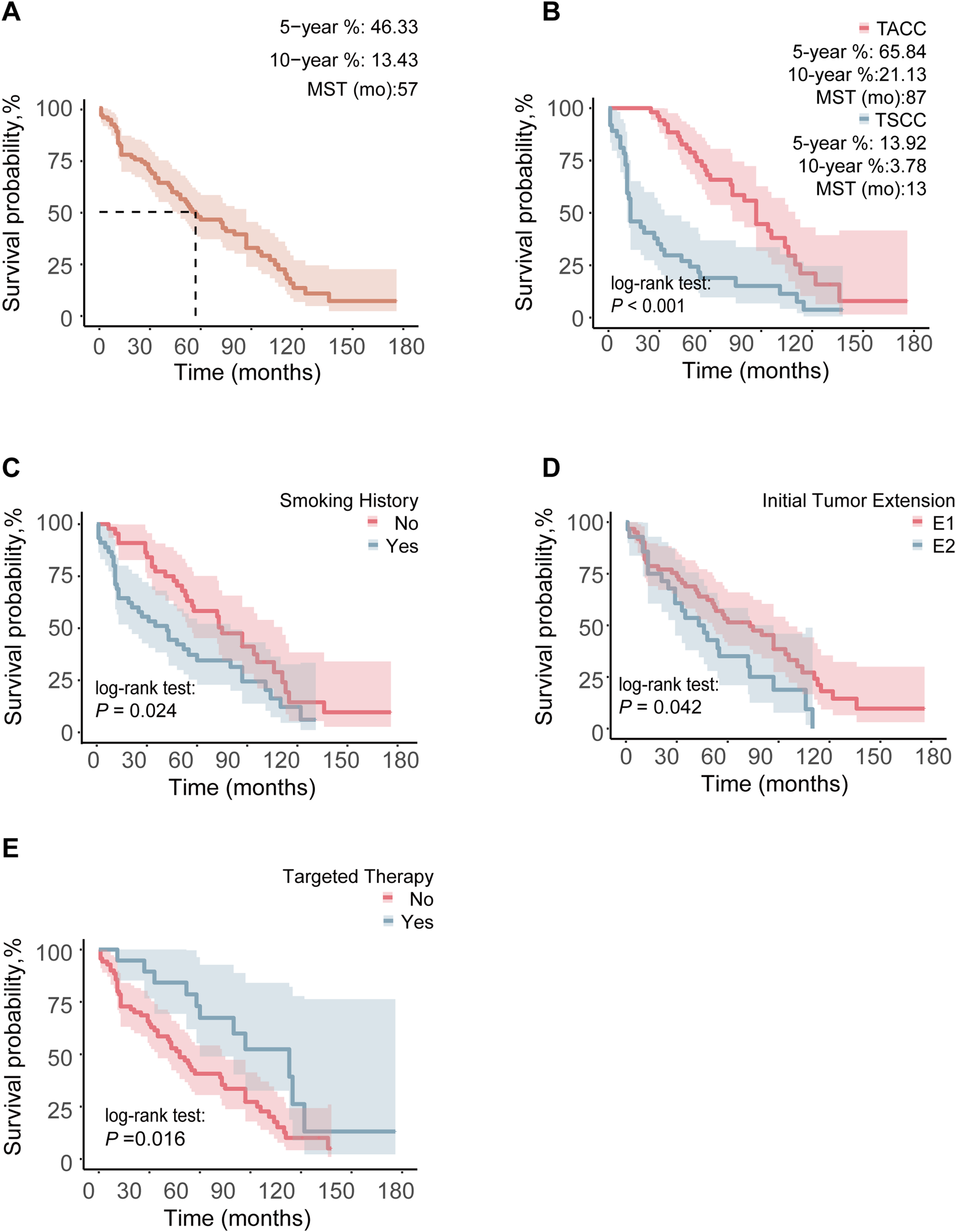
Comparison of OS between different groups in 89 TC patients: (A) OS curve of the whole population, (B) pathological type, (C) smoking history, (D) ITE, (E) targeted therapy. Any 1 of Zone I, Zone II, and Zone III invaded by ITE was marked as E1, and when the invasion area was more than 1 Zone, it was marked as E2.
3.4 Kaplan-Meier and Cox regression analyses
Prognostic analysis was conducted on 89 patients. In the Kaplan-Meier analysis, factors significantly associated with longer OS included TACC (P < 0.001), absence of smoking history (P = 0.024), limited ITE (P = 0.042), targeted therapy (P = 0.016), and PDT (P = 0.043) (Figures 1B–E, Supplementary Figure S2).
Subsequently, univariate Cox regression analysis was performed for 89 patients (Table 2), revealing that TACC (P < 0.001), absence of smoking history (P = 0.025), limited ITE (P = 0.044), and targeted therapy (P = 0.018) were significantly associated with prolonged OS. We also conducted tests for the proportional-hazards assumption (Supplementary Table S1), which indicated that the proportional hazards assumption was violated for pathological type and smoking history. As a result, we opted not to proceed with multivariate Cox regression analysis.
Table 2
| Variable | HR (95%CI) | P Value |
|---|---|---|
| Pathology | <0.001 | |
| TACC | Reference | |
| TSCC | 3.175 (1.950,5.168) | |
| Sex | 0.061 | |
| Female | Reference | |
| Male | 1.643 (0.978,2.759) | |
| Age | 1.015 (0.997,1.034) | 0.110 |
| Symptoms | 0.156 | |
| Hemoptysis | Reference | |
| Non-Hemoptysis | 0.673 (0.390,1.163) | |
| Smoking History | 0.025 | |
| No | Reference | |
| Yes | 1.741 (1.071,2.831) | |
| Family Cancer History | 0.857 | |
| No | Reference | |
| Yes | 1.058 (0.571,1.962) | |
| Initial Tumor Extensiona | 0.044 | |
| E1 | Reference | |
| E2 | 1.708 (1.014,2.876) | |
| Initial Airway Narrowing | 0.603 | |
| I-III | Reference | |
| IV-V | 0.875 (0.530,1.446) | |
| Initial Wall Invasionb | 0.223 | |
| W1 | Reference | |
| W2 | 1.372 (0.825,2.283) | |
| Tumor Metastasis | 0 | 0.077 |
| No | Reference | |
| Yes | 0.641 (0.391,1.049) | |
| Lymph Node Status | 0.859 | |
| No | Reference | |
| Yes | 1.045 (0.640,1.707) | |
| Radiation | 0.270 | |
| No | Reference | |
| Yes | 0.759 (0.465,1.239) | |
| Chemotherapy | 0.791 | |
| No | Reference | |
| Yes | 1.067 (0.660,1.726) | |
| Immunotherapy | 0.135 | |
| No | Reference | |
| Yes | 0.408 (0.126,1.323) | |
| Targeted Therapy | 0.018 | |
| No | Reference | |
| Yes | 0.458 (0.239,0.877) | |
| PDT | 0.055 | |
| No | Reference | |
| Yes | 0.320 (0.100,1.027) | |
| Tracheoscopic Tx | 0.132 | |
| Standard | Reference | |
| Standard & Stent | 1.448 (0.894,2.346) |
Univariate Cox regression analysis of OS in 89 TC patients.
a ITE violation of any 1 of Zone I, Zone II, and Zone III was marked as 1, violation of 2 zones was marked as 2, and violation of 3 zones was marked as 3. When the invasion range was 1 Zone, it was defined as E1, and when the invasion range was more than 1 Zone, it was marked as E2. b In IWI, simply located in the lumen, outside the lumen, lumen wall, mixed type. Among the four types of invasion, the tumor with only one invasion type was defined as W1, and the tumor with no one invasion type was defined as W2.
Notably, in our cohort, targeted therapy referred to the bronchoscopic administration of vascular-targeting agents to facilitate tumor debulking (13, 15). Anlotinib and Endostar were the most commonly used agents, and no molecular targeted therapies were employed in this study.
3.5 AFT analysis
Covariates potentially related to survival were incorporated into the multivariate AFT analysis, based on clinical experience, Kaplan-Meier analysis, and univariate Cox regression results. The multivariate AFT analysis revealed that non-smoking (TR: 0.601, 95% CI:0.397-0.912; P = 0.017), TSCC(TR: 0.243, 95% CI:0.153-0.386; P < 0.01), limited ITE (TR: 0.628, 95% CI: 0.406-0.971; P = 0.037), and targeted therapy (TR: 1.790, 95% CI: 1.061-3.020; P = 0.029) were significantly associated with longer OS (Figure 2).
Figure 2
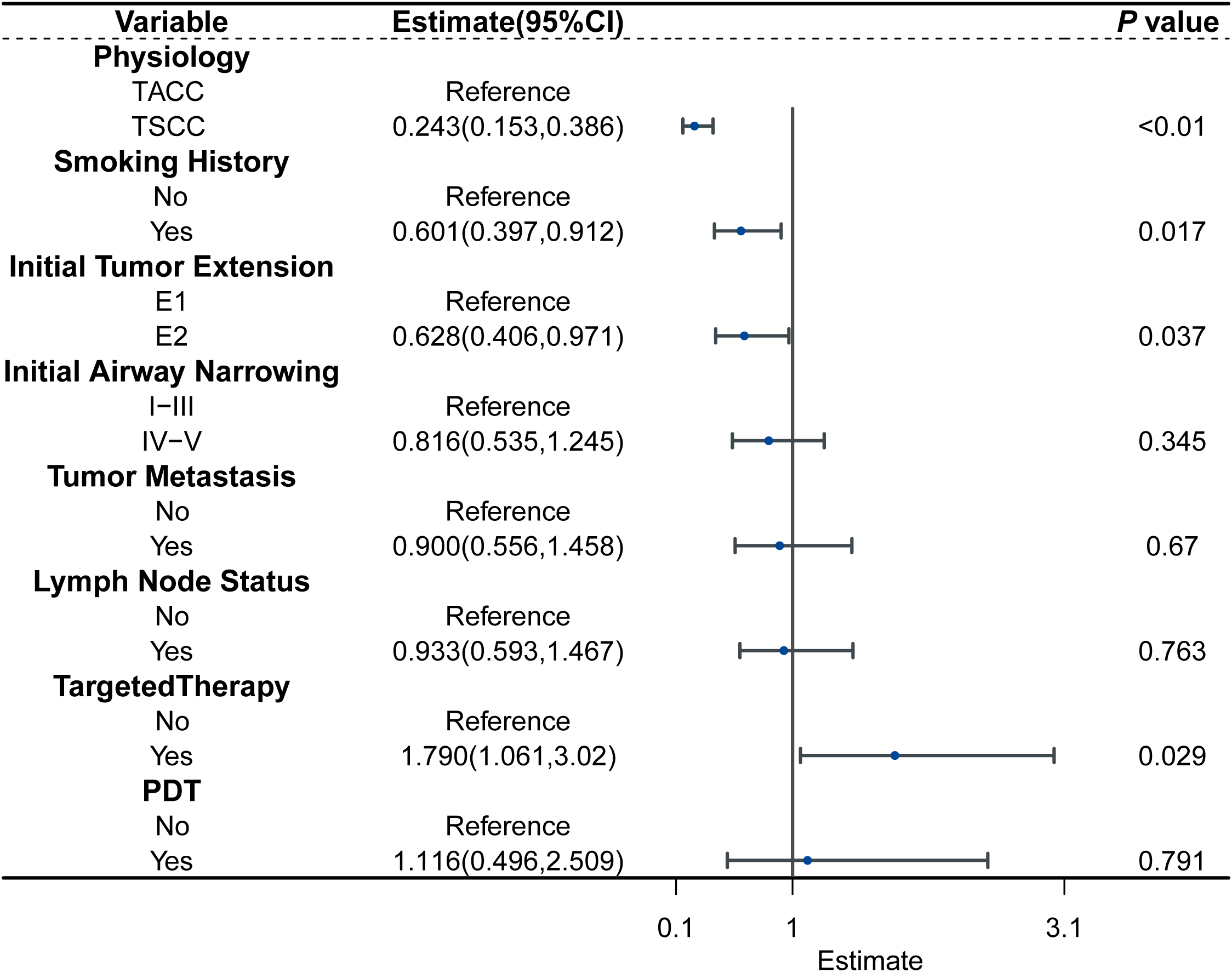
Forest plot for the AFT model characterizing the association between the variable and survival.
To ensure the robustness of the model, multicollinearity was assessed using the variance inflation factor (VIF). All covariates exhibited VIF values well below the commonly accepted threshold of 5, indicating that multicollinearity was not a concern in this analysis (Supplementary Table S2).
3.6 Subgroup analysis
Subgroup analyses were conducted based on pathology. In the univariate Cox regression analysis of TSCC, non-smoking history (HR: 3.082, 95% CI:1.388-6.843; P =0.006), limited ITE(HR: 1.919, 95% CI: 0.884-4.164; P =0.099), and tracheoscopic debridement (HR: 2.393, 95% CI: 1.182-4.846; P =0.015) were significantly associated with longer OS. Variables with a P -value of less than 0.1 in the univariate Cox analysis were included in the multivariate analysis. In the multivariate Cox regression analysis of TSCC, smoking history (HR: 3.328, 95% CI: 1.469-7.539; P =0.004) was significantly associated with shorter OS. In the univariate Cox regression analysis of TACC, ITE (HR: 2.158, 95%CI:1.041-4.474; P = 0.039), targeted therapy (HR:0.612, 95%CI: 0.212-1.138; P = 0.097), and PDT(HR: 0.284, 95%CI:0.066-1.219; P = 0.090) were analyzed. The above variables were included in the multivariate analysis, but no variable in the multivariate Cox regression analysis for TACC had a statistically significant association with patient survival (Figure 3, Supplementary Figures S3, S4).
Figure 3
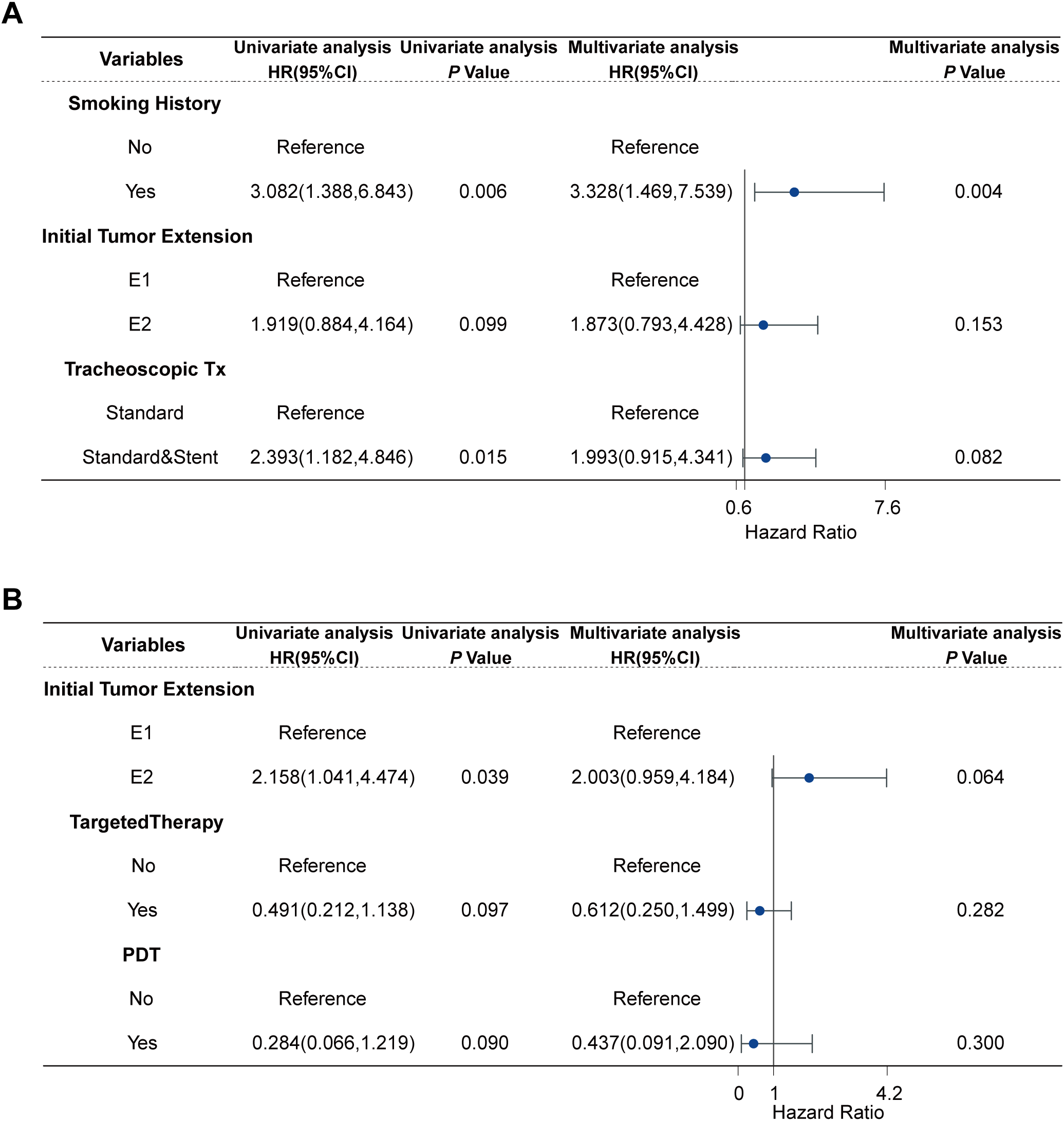
Cox regression analysis of TC patients: (A) Cox regression analysis of 37 TSCC, (B) Cox regression analysis of 52 TACC.
4 Discussion
This study enrolled 94 patients with TC who received palliative treatment at two centers. To our knowledge, this represents the largest two-center retrospective study on palliative treatment for TC, offering updated epidemiological insights into this condition. Furthermore, we analyzed prognostic factors for TC patients undergoing palliative treatment, identifying pathological type, smoking history, ITE, and targeted therapy as key determinants influencing the outcomes of palliative treatment.
Previous studies have indicated a gender disparity in the prevalence of TSCC, with a 2-4 times higher occurrence in males compared to females, while no such gender difference exists in TACC (12, 22). Our study identified a significant age difference in onset between TSCC and TACC, with the mean age of onset for TSCC being over 60 years, consistent with prior research showing a higher prevalence of TSCC in individuals over this age (12, 23). Consistent with previous findings, TSCC prevalence was strongly associated with smoking history, whereas TACC showed no significant correlation with smoking history (12, 23). Significant differences were also observed in the distribution of initial symptoms between TSCC and TACC; hemoptysis was frequently the first symptom in TSCC patients, potentially linked to ulcer formation caused by TSCC (12, 24). ITE was identified as an independent predictor of OS in TC, in line with prior studies that assessed ITE via CT or surgery (8). Although no significant differences were observed between TSCC and TACC in IAN and IWI, these parameters have introduced innovations in assessing tumors within the palliative treatment dimension and may hold potential for predicting recurrence or short-term prognosis in TC (25). Our study also revealed a statistically significant difference in tumor metastasis between TSCC and TACC, with TACC showing a higher rate of metastasis, contrasting with previous findings (12). However, no significant difference in lymph node metastasis was found between TSCC and TACC. Given that most patients in this study had lost the opportunity for radical treatment, resulting in a higher likelihood of lymphatic and distant metastasis, and some metastasis data were unknown, this result should be interpreted with caution (12, 26).
Previous studies have reported that only about 28% of TSCC patients undergo surgical treatment (6). One primary reason is the contraindications to airway surgery, including severe medical complications, extensive ITE, or a history of previous tracheal surgery, which may render patients ineligible for surgery. Additionally, the complexity of airway surgery means that only a limited number of medical institutions can perform these procedures (6, 27). Furthermore, the high risk of complications and mortality associated with surgery in TSCC patients further reduces the likelihood of surgical intervention (6). One study reported a 30-day mortality rate of 4.7% and a 90-day mortality rate of 10.5% in patients undergoing radical surgery (9). Postoperative complications, such as tracheoesophageal fistula, anastomotic dehiscence, airway stenosis, and recurrent laryngeal nerve injury, vary among institutions, with some studies reporting rates as high as 44.6% (6, 28). Most complications, except airway stenosis, typically occur within eight days post-surgery (29). Even at Massachusetts General Hospital, a leading institution in tracheal resection, the postoperative complication rate is 18.2% (27). Our study reports the survival outcomes of TSCC patients receiving palliative treatment, which, to our knowledge, has not been previously documented despite the substantial number of TSCC patients receiving such care. Palliative treatment has been widely employed in various solid tumors, particularly lung cancer (16, 30). Numerous studies have demonstrated that palliative treatment plays a crucial role in prolonging survival and alleviating symptoms in lung cancer patients (31–33). Importantly, key palliative treatments, such as radiotherapy and chemotherapy, carry relatively low risks (17). Common interventions like bronchoscopic interventions for TC also present lower rates of postoperative complications and are not limited by TC growth patterns (34, 35). It is noteworthy that although not statistically significant, stent placement appeared to be a potential risk factor for OS (P =0.082, Figure 3A). We believe that this is because patients requiring stent placement are usually those with severe tumor invasion, the disease itself may lead to poor prognosis, and stent placement can damage the airway, leading to complications such as airway stenosis above and below the stent and tracheoesophageal fistula (36). Nevertheless, the role of stent implantation should not be underestimated. In patients with severe tumor invasion, stents provide rapid and essential support for survival and significantly improve the quality of life (37). Other studies have also demonstrated a survival benefit associated with stenting (38).
The 5- and 10-year survival rates for TACC patients receiving palliative treatment were 65.84% and 21.13%, respectively, with a median survival time of 87 months. A recent study similarly reported 5- and 10-year survival rates of 63.7% and 46.4% in 28 TACC patients treated with non-surgical approaches (39). The discrepancy in 10-year survival rates between the studies may be attributed to the small sample size of 28 patients and the inclusion of patients receiving both radical radiotherapy and palliative treatment in the non-surgical cohort (39). Our study found that extensive ITE tended to be a risk factor for OS (P=0.060, Figure 3B), while other factors showed no significant impact on prognosis. TACC is a slow-growing, low-grade malignancy that often progresses very slowly, sometimes taking many years to worsen even without treatment. This indolent nature may partly explain why multivariate Cox regression did not show a significant palliative effect (12, 22, 39, 40). TACC is also characterized by a high recurrence rate, with positive surgical margins being a major risk factor (4, 39, 41). Previous studies have suggested that 59.8% of TACC surgery may have positive surgical margins, mainly because of the infiltrative growth characteristics of TACC that spreads in the submucosa and around the nerve (42). We do not deny that surgery remains the treatment of choice for localized TACC (12). However, considering the characteristics of easy recurrence and high metastasis, as well as the high difficulty and risk of operation, the applicability of surgical treatment has been reduced to a certain extent (43, 44). In this study, multiple palliative treatment regimens did not significantly prolong OS in TACC patients, which may be due to the indolent nature of TACC and the unclear palliative treatment status of some patients; therefore, these findings should be interpreted cautiously. The role of palliative treatment in TACC treatment has gained increasing recognition. A retrospective analysis by Lee et al. reported that none of the patients experienced disease progression within three months after receiving low-dose palliative radiotherapy (56.3–69.3 Gy) (18). The short-term efficacy of palliative treatment in TACC has also been demonstrated in previous cases (45). Recent research highlights the advantages of palliative treatment in preserving the trachea and delaying recurrence (46). Given its low risk, rapid symptom relief, and potential to maintain quality of life, palliative treatment remains the optimal choice when radical treatment is not feasible (11).
This study has several limitations. First, as a two-center retrospective study, the data were obtained from historical medical records, which may introduce selection and information biases. Second, due to the rarity of tracheal carcinoma, the study spanned a long period, potentially affecting the consistency of treatment strategies. Third, the retrospective nature of the data limited our ability to evaluate outcomes such as short-term prognosis, progression-free survival, quality of life, and psychological stress. In addition, we were unable to obtain data for mutation analysis. Despite these limitations, our findings provide valuable insights into the palliative treatment of TC. Future research should focus on defining the optimal timing, protocols, and duration of palliative interventions. Additionally, the individualization and complexity of palliative care regimens necessitate attention to treatment frequency, medication dosage, and the economic status of patients, as these factors may influence prognosis (9, 11).
5 Conclusions
This study summarizes the characteristics of palliative treatment for TC, highlighting that TACC, non-smoking history, limited invasion, and targeted therapy have a positive impact on prolonging OS in TC patients. The role of palliative treatment in TC needs to be further explored and verified, and more attention should be paid to non-clinical factors such as the initiation time, specific dosage, short-term prognosis, economic factors, and mental and emotional factors involved in palliative treatment programs to clarify the optimal treatment options for TC.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committees of Dongzhimen Hospital, Beijing University of Chinese Medicine, the Ethics Committees of Emergency General Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because the study is a retrospective study.
Author contributions
QH: Data curation, Formal Analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. JT: Data curation, Formal Analysis, Methodology, Writing – original draft, Writing – review & editing. YL: Data curation, Investigation, Visualization, Writing – review & editing. ZW: Methodology, Project administration, Resources, Writing – review & editing. LL: Resources, Writing – review & editing, Data curation. HZ: Resources, Writing – review & editing, Project administration. NZ: Conceptualization, Project administration, Writing – review & editing. HW: Conceptualization, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the subject of Dongzhimen Hospital, Beijing University of Chinese Medicine (HX-DZM-202308, HX-DZM-202222) and the Innovative Development Project for Young Physicians of the Professional Committee of Respiratory Disease Drug Research of China Association of Traditional Chinese Medicine (No. HXQNJJ-2023-014).
Acknowledgments
We would like to thank Ms. Liu Yunning from the Chinese Center for Disease Control and Prevention for her assistance in querying survival data, and Dr. Lu Jinyu from Beijing Haidian District Maternal and Child Health Care Hospital for her contributions to the preparation of the figures.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. During the preparation of this work, the authors used ChatGPT-4o by OpenAI to enhance language quality. After using this tool, the authors reviewed and edited the content as needed and took full responsibility for the content of the publication.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1532005/full#supplementary-material
Supplementary Figure S1Flow diagram for the exclusion procedure of patients.
Supplementary Figure S2Comparison of overall survival between different groups in 89 SPTC patients: (A) Sex, (B) Age, (C) Initial Symptoms, (D) Family Cancer History, (E) Initial Airway Narrowing, (F) Initial Wall Invasion, (G) Tumor Metastasis, (H) Lymph Node Status, (I) Radiation, (J) Chemotherapy, (K) Tracheoscopic Tx.
Supplementary Figure S3Cox regression analysis of 37 SCC patients.
Supplementary Figure S4Cox regression analysis of 52 ACC patients.
References
1
Webb BD Walsh GL Roberts DB Sturgis EM . Primary tracheal Malignant neoplasms: the University of Texas MD Anderson Cancer Center experience. J Am Coll Surg. (2006) 202:237–46. doi: 10.1016/j.jamcollsurg.2005.09.016
2
Junker K . Pathology of tracheal tumors. Thorac Surg Clin. (2014) 24:7–11. doi: 10.1016/j.thorsurg.2013.09.008
3
Nilssen Y Solberg S Brustugun OT Møller B Sundset A Wahl SGF et al . Tracheal cancer: a rare and deadly but potentially curable disease that also affects younger people. Eur J Cardiothorac Surg. (2023) 64. doi: 10.1093/ejcts/ezad244
4
Desai N Zambetti BR Wong DL Schachter AE Judge NP Valaulikar GS et al . Outcomes and predictors of survival for tracheal cancer. J Surg Oncol. (2023) 128:1251–8. doi: 10.1002/jso.v128.8
5
Zheng Z Du Z Fang Z Shi Y Chen X Jin M et al . Survival benefit of radiotherapy and nomogram for patients with primary tracheal Malignant tumors: a propensity score-matched SEER database analysis. J Cancer Res Clin Oncol. (2023) 149:9919–26. doi: 10.1007/s00432-023-04896-8
6
Benissan-Messan DZ Merritt RE Bazan JG D’Souza DM Abdel-Rasoul M Moffatt-Bruce SD et al . National utilization of surgery and outcomes for primary tracheal cancer in the United States. Ann Thorac Surg. (2020) 110:1012–22. doi: 10.1016/j.athoracsur.2020.03.048
7
Agrawal S Jackson C Celie KB Dodhia C Monie D Monzon J et al . Survival trends in patients with tracheal carcinoma from 1973 to 2011. Am J Otolaryngol. (2017) 38:673–7. doi: 10.1016/j.amjoto.2017.08.005
8
Li J Tan F Wang Y Xue Q Gao Y Mu J et al . Clinical characteristics, surgical treatments, prognosis, and prognostic factors of primary tracheal cancer patients: 20-year data of the National Cancer Center, China. Transl Lung Cancer Res. (2022) 11:735–43. doi: 10.21037/tlcr-22-258
9
Hararah MK Stokes WA Oweida A Patil T Amini A Goddard J et al . Epidemiology and treatment trends for primary tracheal squamous cell carcinoma. Laryngoscope. (2020) 130:405–12. doi: 10.1002/lary.v130.2
10
Gaissert HA Grillo HC Shadmehr MB Wright CD Gokhale M Wain JC et al . Long-term survival after resection of primary adenoid cystic and squamous cell carcinoma of the trachea and carina. Ann Thorac Surg. (2004) 78:1889–96; discussion 96-7. doi: 10.1016/j.athoracsur.2004.05.064
11
Bischoff KE Patel K Boscardin WJ O’Riordan DL Pantilat SZ Smith AK . Prognoses associated with palliative performance scale scores in modern palliative care practice. JAMA Netw Open. (2024) 7:e2420472. doi: 10.1001/jamanetworkopen.2024.20472
12
Macchiarini P . Primary tracheal tumours. Lancet Oncol. (2006) 7:83–91. doi: 10.1016/S1470-2045(05)70541-6
13
Jiang W Yang X Wang X Li Y Yang X Wang N et al . Bronchoscopic intratumoral injections of cisplatin and endostar as concomitants of standard chemotherapy to treat Malignant central airway obstruction. Postgrad Med J. (2022) 98:104–12. doi: 10.1136/postgradmedj-2020-138823
14
Piórek A Płużański A Knetki-Wróblewska M Winiarczyk K Tabor S Kowalski DM et al . Tracheal tumors: clinical practice guidelines for palliative treatment and follow-up. Oncol Rev. (2024) 18:1451247. doi: 10.3389/or.2024.1451247
15
Wang Y Li Y Yin B Yang X Wang F Wang H et al . Papillary squamous cell carcinoma successfully treated with bronchoscopic intratumoral injections of cisplatin and Endostar: a case report. J Int Med Res. (2021) 49:3000605211047077. doi: 10.1177/03000605211047077
16
Osagiede O Colibaseanu DT Spaulding AC Frank RD Merchea A Kelley SR et al . Palliative care use among patients with solid cancer tumors: A national cancer data base study. J Palliat Care. (2018) 33:149–58. doi: 10.1177/0825859718777320
17
Zeng R Wang H Cai X Guo X Ping Y Yang Q . Radiotherapy for primary tracheal carcinoma: experience at a single institution. Technol Cancer Res Treat. (2021) 20:15330338211034273. doi: 10.1177/15330338211034273
18
Lee JH Jang JY Noh JM Yang K Pyo H . Dose-escalated radiotherapy for primary tracheobronchial adenoid cystic carcinoma. Cancers (Basel). (2024) 16. doi: 10.3390/cancers16112127
19
Koul R Alomrann R Rathod S Kim J Leylek A Ahmed N et al . Clinical characteristics and prognosis of primary tracheal cancer: A single institution experience. Int J Hematol Oncol Stem Cell Res. (2018) 12:298–302. doi: 10.18502/ijhoscr.v12i4.108
20
Jin F Li Q Li S Wang H Bai C Zeng Y et al . Interventional bronchoscopy for the treatment of Malignant central airway stenosis: an expert recommendation for China. Respiration. (2019) 97:484–94. doi: 10.1159/000497213
21
Kc M Fan J Hyslop T Hassan S Cecchini M Wang SY et al . Relative burden of cancer and noncancer mortality among long-term survivors of breast, prostate, and colorectal cancer in the US. JAMA Netw Open. (2023) 6:e2323115. doi: 10.1001/jamanetworkopen.2023.23115
22
Albers E Lawrie T Harrell JH Yi ES . Tracheobronchial adenoid cystic carcinoma: a clinicopathologic study of 14 cases. Chest. (2004) 125:1160–5. doi: 10.1378/chest.125.3.1160
23
Piórek A Płużański A Teterycz P Tabor S Winiarczyk K Knetki-Wróblewska M et al . Clinicopathological characteristics of patients with primary tracheal tumors: Analysis of eighty-nine cases. Thorac Cancer. (2024) 15:878–83. doi: 10.1111/1759-7714.15231
24
Zhengjaiang L Pingzhang T Dechao Z Reddy-Kolanu G Ilankovan V . Primary tracheal tumours: 21 years of experience at Peking Union Medical College, Beijing, China. J Laryngol Otol. (2008) 122:1235–40. doi: 10.1017/S0022215108001710
25
Wang SC Yin LK Zhang Y Xue LM Ye JD Tao GY et al . CT diagnosis and prognosis prediction of tracheal adenoid cystic carcinoma. Eur J Radiol. (2021) 140:109746. doi: 10.1016/j.ejrad.2021.109746
26
Wo Y Li S Wang Y Lu T Qin Y Sun X et al . Predictors of nodal metastasis and prognostic significance of lymph node ratio and total lymph node count in tracheobronchial adenoid cystic carcinoma. Cancer Manag Res. (2018) 10:5919–25. doi: 10.2147/CMAR.S182069
27
Tapias LF Mathisen DJ . Prevention and management of complications following tracheal resections-lessons learned at the Massachusetts General Hospital. Ann Cardiothorac Surg. (2018) 7:237–43. doi: 10.21037/acs.2018.01.20
28
Bibas BJ Terra RM Oliveira Junior AL Tamagno MF Minamoto H Cardoso PF et al . Predictors for postoperative complications after tracheal resection. Ann Thorac Surg. (2014) 98:277–82. doi: 10.1016/j.athoracsur.2014.03.019
29
Shuman EA Kim YJ Rodman J O’Dell K . Timing of complications in open airway reconstruction. Laryngoscope. (2024) 134:3527–31. doi: 10.1002/lary.v134.8
30
Rodrigues G Higgins KA Rimner A Amini A Chang JY Chun SG et al . American radium society appropriate use criteria for unresectable locally advanced non-small cell lung cancer. JAMA Oncol. (2024) 10:799–806. doi: 10.1001/jamaoncol.2024.0294
31
Støchkel Frank M Schou Nørøxe D Nygård L Fredberg Persson G . Fractionated palliative thoracic radiotherapy in non-small cell lung cancer - futile or worth-while? BMC Palliat Care. (2018) 17:15. doi: 10.1186/s12904-017-0270-4
32
Chen M Yang L Yu H Yu H Wang S Tian L et al . Early palliative care in patients with non-small-cell lung cancer: A randomized controlled trial in southwest China. Am J Hosp Palliat Care. (2022) 39:1304–11. doi: 10.1177/10499091211072502
33
Brule SY Al-Baimani K Jonker H Zhang T Nicholas G Goss G et al . Palliative systemic therapy for advanced non-small cell lung cancer: Investigating disparities between patients who are treated versus those who are not. Lung Cancer. (2016) 97:15–21. doi: 10.1016/j.lungcan.2016.04.007
34
Cavaliere S Foccoli P Farina PL . Nd: YAG laser bronchoscopy. A five-year experience with 1,396 applications in 1,000 patients. Chest. (1988) 94:15–21. doi: 10.1378/chest.94.1.15
35
Ost DE Ernst A Grosu HB Lei X Diaz-Mendoza J Slade M et al . Complications following therapeutic bronchoscopy for Malignant central airway obstruction: results of the AQuIRE registry. Chest. (2015) 148:450–71. doi: 10.1378/chest.14-1530
36
Madariaga MLL Gaissert HA . Overview of Malignant tracheal tumors. Ann Cardiothorac Surg. (2018) 7:244–54. doi: 10.21037/acs.2018.03.04
37
Saji H Furukawa K Tsutsui H Tsuboi M Ichinose S Usuda J et al . Outcomes of airway stenting for advanced lung cancer with central airway obstruction. Interact Cardiovasc Thorac Surg. (2010) 11:425–8. doi: 10.1510/icvts.2010.238196
38
Razi SS Lebovics RS Schwartz G Sancheti M Belsley S Connery CP et al . Timely airway stenting improves survival in patients with Malignant central airway obstruction. Ann Thorac Surg. (2010) 90:1088–93. doi: 10.1016/j.athoracsur.2010.06.093
39
Wang Y Cai S Gao S Xue Q Mu J Gao Y et al . Tracheobronchial adenoid cystic carcinoma: 50-year experience at the national cancer center, China. Ann Thorac Surg. (2019) 108:873–82. doi: 10.1016/j.athoracsur.2019.03.065
40
van Weert S Bloemena E van der Waal I de Bree R Rietveld DH Kuik JD et al . Adenoid cystic carcinoma of the head and neck: a single-center analysis of 105 consecutive cases over a 30-year period. Oral Oncol. (2013) 49:824–9. doi: 10.1016/j.oraloncology.2013.05.004
41
Dantas AN Morais EF Macedo RA Tinôco JM Morais Mde L . Clinicopathological characteristics and perineural invasion in adenoid cystic carcinoma: a systematic review. Braz J Otorhinolaryngol. (2015) 81:329–35. doi: 10.1016/j.bjorl.2014.07.016
42
Yang CJ Shah SA Ramakrishnan D Raman V Diao K Wang H et al . Impact of positive margins and radiation after tracheal adenoid cystic carcinoma resection on survival. Ann Thorac Surg. (2020) 109:1026–32. doi: 10.1016/j.athoracsur.2019.08.094
43
Khaitan PG . Endoscopic resection for tracheal adenoid cystic carcinoma? Is it time to change how we practice? Ann Thorac Surg. (2021) 111:1093–4. doi: 10.1016/j.athoracsur.2020.05.136
44
Yang H Yao F Tantai J Zhao Y Tan Q Zhao H . Resected tracheal adenoid cystic carcinoma: improvements in outcome at a single institution. Ann Thorac Surg. (2016) 101:294–300. doi: 10.1016/j.athoracsur.2015.06.073
45
Teng J Zou H Wang H . Primary tracheal adenoid cystic carcinoma: therapeutic challenges posed by unresectable. Arch Bronconeumol. (2024) 60:50–2. doi: 10.1016/j.arbres.2023.10.003
46
Rajeev-Kumar G Juloori A . To test and preserve the trachea. Int J Radiat Oncol Biol Phys. (2024) 119:1333. doi: 10.1016/j.ijrobp.2024.04.018
Summary
Keywords
primary tracheal carcinoma, palliative treatment, initial tumor extension, long-term outcomes, prognostic analysis
Citation
Hong Q, Teng J, Luo Y, Wang Z, Zou H, Li L, Zhang N and Wang H (2025) Prognosis of palliative treatment for primary tracheal carcinoma: a two-center retrospective study. Front. Oncol. 15:1532005. doi: 10.3389/fonc.2025.1532005
Received
21 November 2024
Accepted
25 February 2025
Published
13 March 2025
Volume
15 - 2025
Edited by
Antonio D’Andrilli, Sapienza University of Rome, Italy
Reviewed by
Giovanni Bocchialini, University Hospital of Parma, Italy
Giuseppe Giaccone, Cornell University, United States
Updates
Copyright
© 2025 Hong, Teng, Luo, Wang, Zou, Li, Zhang and Wang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongwu Wang, wanghongwu2015@126.com; Nan Zhang, Zhangnan20191217@sina.com
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.