- 1Mount Gambier & Districts Health Service, Limestone Coast Local Health Network Inc, Mount Gambier, SA, Australia
- 2School of Pharmacy and Medical Sciences, Griffith University, Southport, QLD, Australia
- 3Faculty of Medicine, The University of Melbourne, Parkville, VIC, Australia
- 4Genesis Care, John Flynn Hospital, Tugun, QLD, Australia
- 5School of Medicine, Griffith University, Southport, QLD, Australia
Introduction: Hypo-fractionated radiotherapy (HFRT) regimens can induce immune system activation and help to identify a therapeutic window after RT by measuring cytotoxic T-cell concentration. Here, we summarise previous preclinical and clinical studies on the effects of HFRT on the immune system, both locally and systemically. We also investigate the existing data on the optimal dose and fractionation scheme of HFRT to enhance local and distant anti-tumour immunity.
Methods: A search was conducted using the PubMed, ScienceDirect, and Google Scholar databases. The systematic review was conducted in accordance with the PRISMA-DTA guidelines. Quality was assessed utilising the Prediction model Risk Of Bias ASsessment Tool (PROBAST). Data from publications that met quality criteria were grouped via (1) hypo-fractionated radiotherapy, (2) CD8+ T-cells infiltration, (3), immune stimulation, and (4) abscopal effect.
Results: After eligibility consideration, 12 studies (7 = preclinical and 5 = clinical) were selected for this systematic review article. Ten of the 12 studies observed T-cell infiltration into the tumour environment following HFRT. Moreover, six of 12 preclinical and clinical studies tested the HFRT schemes with several-day intervals to control tumour growth. To assess the possible immunogenic impact of HFRT on the immune system both locally and systemically, eight previous studies examined the abscopal effect (AE) and response rates following optimal HFRT schedules.
Conclusions: Existing literature suggests that HFRT with an optimal regimen can induce the activation of T lymphocytes and break tumour tolerance while simultaneously reducing the frequency of Tregs. The collected studies also suggested that optimal dosages and fractions of HFRT induce an immune response. However, it should be further explored to provide clinicians with information that would be valuable when making decisions regarding patient care. This strategy may simplify protocols, increase cancer patients’ response rate to treatment, lower costs, and lower their chance of toxicity and developing immune-related side effects after receiving chemotherapy and immunotherapy.
1 Introduction
Radiation therapy (RT) is usually considered a “local” treatment modality in cancer therapy because radiation can only directly eradicate cancer cells within the radiation field. Because of recent developments in image guidance and RT delivery methods, single ablative high doses can be safely delivered to many tumour sites by using stereotactic radiosurgery (SRS), stereotactic body RT (SBRT), or stereotactic ablative body irradiation (SABR) (1–4). High doses of radiation can be achieved by a single treatment (extreme oligo-fractionation) or by 2 to 5 high-dose treatments (oligo-fractionation or hypo-fractionation), serving as an alternative to conventional daily low-dose fractionated treatments (<3 Gy) over several weeks (5). Limited results showed improved efficacy compared with traditional fractionated RT in managing advanced or metastatic colorectal, liver, and non-small cell lung tumours (2). The outcome can be comparable to surgery for resectable tumours, and SRS can be applied to unresectable tumours (2, 6).
Hypo-fractionated RT (HFRT) is a modern radiation technique that provides targeted high-dose irradiation to a tumour while limiting damage to surrounding normal tissues (7–9). HFRT directly kills tumour cells via DNA double-strand breaks and propagates dose-dependent vascular damage and destruction of the tumour microenvironment, causing secondary tumour cell death (10–12). Massive tumour cell death because of DNA damage and vascular injury functions can produce strong anti-tumour immunity. Therefore, it has been reported that the anti-tumour immune response plays a significant role in the outcome of SABR (10–12). However, RT may result in poor outcomes in patients with a weakened immune system, whereas it may effectively eradicate tumours in patients with a more robust immune system (13, 14).
It has been shown that RT may contribute to making tumours visible to the immune system (15–19). After RT treatment, MHC-I molecules display an increased pool of peptides for antigen presentation (20). Dendritic cells (DCs) can capture tumour-associated antigens (TAAs) released to the tumour periphery (21). These DCs become active via toll-like receptor (TLRs) recognition, in which endogenous danger signals emitted by dying tumour cells are identified (21). The activation of DCs is characterised by the upregulation of cell surface molecules involved in antigen presentation and co-stimulation (e.g., CD80 and CD86) and the release of pro-inflammatory cytokines (21). Thus, activated DCs migrate to secondary lymphoid organs, where TAAs are presented to CD4+ Th cells in the MHC-II context (21). Active, effector T-cells may recirculate through the body and generate a tumour-specific immune response in distant areas (21). Using this mechanism, adaptive immune responses may help to eradicate metastasis of tumours that do not express MHC-II. CD4+ T-cells may help kill tumour cells through several mechanisms (21). One such mechanism enables the development of tumour-specific CD8+ T-cells that recognise tumour peptides by MHC-I (21).
A growing body of evidence suggests that the systemic anti-tumour effect in metastatic disease in response to high-dose local radiation results in the regression of non-irradiated distant tumour sites (22). This phenomenon, known as the abscopal effect (AE) of radiation, was first described by RH Mole in 1953 (23). Multiple mechanisms have been proposed to cause the AE (16, 24), such as the systemic secretion of specific cytokines and chemokines, a systemic immune response against local tumour antigens released, or local inflammation that can lead to a distant effect (25). In any case, the hypothesis that the AE is immune-mediated is becoming stronger. If the radiation dose is sufficient to generate cell death, this can lead to the induction of the adaptive immune response. RT directly elicits an innate immune recognition of tumour by releasing danger signals”. Thus, these signals can increase immune-mediated cell death, which promotes the uptake of circulating tumour antigens by DCs via cross-priming and ultimately leads to the activation of tumour-specific T-cell response (26). The tumour-specific T-cells are capable of recirculating throughout the body, detecting any tumour cells (across multiple antigens) and eradicating them (24, 27). Therefore, tumours that are even at a distance from the irradiated field can be immunologically killed (24, 27). This is described as an AE (24, 27, 28).
Here, we summarise previous preclinical and clinical studies on the effects of hypo-fractionated RT (HFRT) on the immune system locally and systemically. We also investigate the existing data on the optimal dose and fractionation scheme of HFRT to enhance local and distant anti-tumour immunity.
2 Materials and methods
2.1 Search strategy and study selection
This systematic review followed the PRISMA statement for reporting systematic reviews and meta-analyses (29). A comprehensive electronic search was conducted between March and October 2024 using PubMed (https://pubmed.ncbi.nlm.nih.gov/), ScienceDirect (https://www.sciencedirect.com/), and Google Scholar (https://scholar.google.com.au/) databases for articles published between 2010 and 2024. The studies investigated RT-induced immune stimulation at optimal HFRT improves AE and clinical outcomes. The systematic search for relevant studies was carried out using the following keywords: RT, hypo-fractionation RT, immune system, anti-tumour CD8+ T, Infiltration of CD8+ cytotoxic T-cells, tumour-specific, monocytic myeloid-derived suppressor cells (M-MDSCs), immune stimulation, RT-schedule, RT-dose, RT-fraction, and clinical outcome. Similarly, we performed a manual review of references to select additional studies. Table 1 summarises the search strategy of this systematic review.
2.2 Selection (inclusion and exclusion) criteria
The titles and abstracts of relevant studies were evaluated for their contents, ensuring adherence to this systematic review article’s inclusion and exclusion criteria. Inclusion criteria were (I) the studies investigating RT-induced immune stimulation; (II) the studies investigating immune cells such as CD8+ cytotoxic T-cells, regulatory T-cells (Tregs), and M-MDSCs after using HFRT; (III) the studies monitoring optimal RT-type, RT-dose, RT-fraction, RT-schedule, (IV) the studies investigating AE after using HFRT, time to AE, and site of AE; (IV) the studies recorded patient’s characteristics and association with the clinical outcome; and (V) the studies analysed the association of HFRT-induced immune stimulation and improved clinical outcomes including complete response (CR), partial response (PR) and stable disease (SD).
The exclusion criteria for this systematic review were (I) editorials, (II) case reports, (III) studies did not have primary data, (IV) studies did not report bystander effect (BE) and AE following HFRT in metastatic disease, (V) studies monitoring RT with Immunotherapy/Chemotherapy combination, (VI) studies which were not written in English, and (VII) did not have full text available. The articles that fulfilled the inclusion criteria were shortlisted, and the primary characteristics are summarised in Table 2.
2.3 Data extraction and quality assessment method
The data were extracted from selected studies by two authors. The extracted data included (I) general information (first author, publication’s year, method of patient recruitment, and sampling methods); (II) clinical characteristics (T-stage, age, treatment option, RT-type, RT-dose, RT-fraction, and RT-schedule); (III) T-cell response following HFRT and (IV) clinical outcomes (time to AE, site of AE, biochemical recurrence, side effects of RT or RT-induced toxicity, treatment response (CR and PR), tumour control, PFS and OS).
The study’s quality was assessed using the PROBAST (Prediction Model Risk of Bias Assessment Tool), which evaluates the applicability and risk of bias in diagnostic tests (30). To address discrepancies in interpretation, two assessors jointly assessed one article first. Articles were then scored for each study, and section deficiencies were noted for further discussion. The relevant published articles were retrieved and imported into an Endnote X21 database (31). Analogous articles were identified and deleted using the Endnote’s duplicate function. We considered studies only describing multivariable-adjusted hazard ratios (aHR). Moreover, we excluded studies that reported crude or unadjusted outcome measures between patients treated with HFRT.
3 Results
3.1 Systematic review analysis
The literature search identified 1431 preclinical and clinical studies: 140 from PubMed, 550 from Science Direct, and 741 from Google Scholar, respectively. Of these 1431 studies, 832 were excluded after reviewing the titles and abstracts, and 599 were selected at the first screening stage. At the second screening stage, 550 studies were removed after full-text examination, and 14 were selected. Furthermore, 49 studies were assessed for eligibility, and 34 studies were removed for the following reasons: (1) case report = 5; (2) editorial = 5; (3) lack of present primary data = 4; (4) lack of bystander and abscopal information = 3; (5) no full text available = 7; (6) studies investigating RT effect in combination with Immunotherapy/Chemotherapy = 13. After eligibility consideration, 12 (7 = preclinical and 5 = clinical) studies were selected. Figure 1 shows our literature search and selection strategy as a flowchart.
3.2 T-cell response following HFRT
To evaluate the potential immunogenic modulation of HFRT at the optimal schedule, 10 of the 12 selected studies have observed T-cell infiltration to the tumour environment following HFRT (Table 3) (5, 32–40).
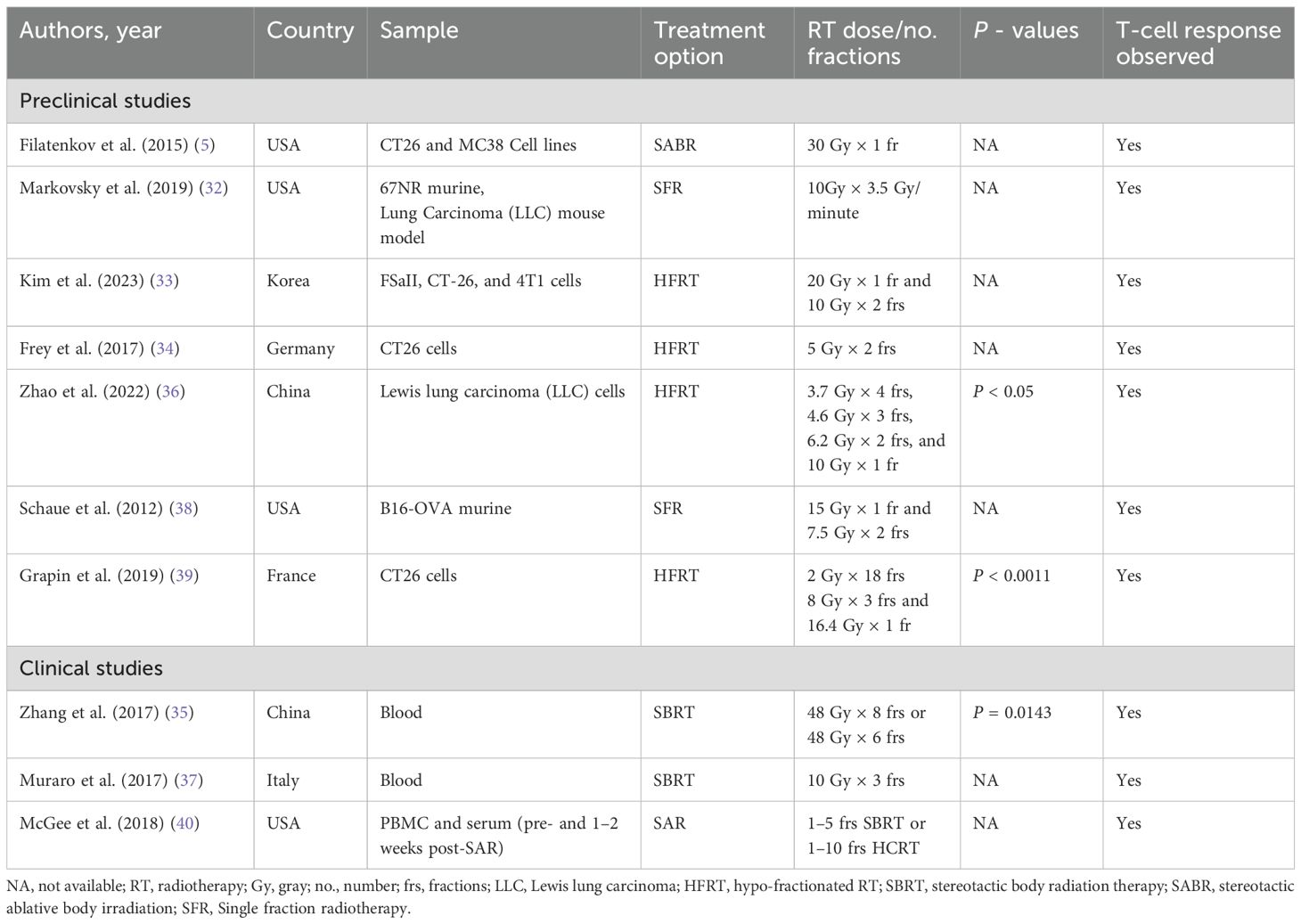
Table 3. Studies reported tumour-infiltrating CD8+ T-cell response in cancer patients following HFRT.
Out of 10, 7 preclinical studies reported an increased infiltration of T-cells to the tumour microenvironment after HFRT (5, 32–34, 36, 38, 39). For example, Filatenkov et al. reported that the unirradiated tumour with HFRT contained approximately 19% CD8+ T-cells, the irradiated tumour contained approximately 70% at day 35, and the percentage of MDSCs decreased after day 24 (5). In addition, there was a trend toward an increase in CD8+ T-cells in both irradiated and non-irradiated parts of the tumour seven days post-10 Gy RT (32). Kim et al. reported that five-day spacing was more effective than a one-day interval in enhancing anti-tumour immunity via activating the CD8+ T-cells and suppressing the M-MDSCs (33).
Some studies have demonstrated that optimal RT dose and fraction can cause immunologic effects and increased CD8+ T-cell infiltration in the tumour microenvironment (34, 36, 38, 39). Fray et al. stated that on day 8, more cytotoxic T-cells and a lower percentage of Tregs (CD4+/CD25+/FoxP3+) were identified in the irradiated tumours using irradiation two fractions × 5 Gy (34). In addition, an increase in CD8+ T-cells concentration was observed from 48 h to 3 weeks after HFRT in 4.6 Gy × 3 fractions and 6.2 Gy × 2 fractions (p < 0.05), but not in 3.7 Gy × 4 fractions and 10 Gy × 1 fraction (36). A single HFRT dose of 15 Gy increased CD8+ T-cell responses and decreased Tregs (38). The increased proportion of CD8+ T-cells was noticed on day seven after the first HFRT session in the 1 Fraction × 16.4 Gy group (p = 0.002), 3 fractions × 8 Gy group (p < 0.001), and in the 18 fractions × 2 Gy group (p < 0.001); versus 1.4% ± 0.3% in the control group (39).
In the clinical study group, three studies also stated the effect of HFRT on T-cell infiltration in cancer patients (35, 37, 40). Zhang et al. demonstrated that HFRT increased the frequency of CD8+ T-cell infiltration but decreased the frequency of inhibitory Tregs (35). Moreover, Muraro et al. also identified that half of the patients showed increased numbers of activated natural killer (NK) cells and T-cells (CD4+ and CD8+) immediately after the first dose of SBRT (37). Additionally, activated CD25+ CD4+ memory T-cells and CD25+ CD8+ memory T-cells increased following SAR to parenchymal sites, not bone or brain (40).
3.3 Tumour control following HFRT
To evaluate the efficacy of HFRT delivered in various schedules, 6 out of 12 selected preclinical and clinical studies tested the HFRT schemes with several-day intervals to control tumour growth (Table 4) (33, 34, 36, 38, 39, 41).
In the preclinical studies group, 5 out of 6 reported tumour control after HFRT (33, 34, 36, 38, 39). Kim et al. reported that tumour growth delays by a five-day interval RT (p = 0.0293) or a seven-day interval RT (p = 0.0434) were more significant than those by a one-day interval (p = 0.6413) (33). Moreover, tumour growth was significantly delayed in the mice irradiated with 2 fractions × 5 Gy in a 4-day interval (34).
To evaluate the tumour control at different RT schedules, Zhao et al. reported that tumour growth was considerably delayed in the 6.2 Gy × 2 fractions group compared with the control group (p < 0.01) (36). Furthermore, the group receiving local single-dose HFRT at 7.5 and 15 Gy showed significant tumour control, whereas the group receiving 5 Gy had a minimal effect (38). In addition, Grapin et al. monitored the tumour’s growth with 18 fractions × 2 Gy and 3 fractions × 8 Gy regimens and found the most extended tumour growth delay compared to 1 fraction × 16.4 Gy (39).
On the other hand, only one clinical study reported that the bulky tumour control rate was 95% for the SBRT groups compared with 20% in the other two groups (41).
3.4 Consequent vaccine-like effect following HFRT
BE, or AE effect of HFRT, is a rare and unpredictable outcome encountered during the metastatic treatment, where tumour regression is observed to be distant from the irradiated volume. Eight previous preclinical and clinical studies have reported AE and clinical outcomes at optimal HFRT schedules (Table 5) (5, 32, 35–37, 40–42).
In the preclinical studies group, three studies have observed immunological effects and response rates following the use of HFRT. For example, 13 of the 14 mice achieved complete remissions when treated with 30 Gy, while 3 of 5 developed complete tumour remissions when the HFRT dose was specified at 20 Gy (5). Eight 67NR models (35%) experienced a significant AE after partial irradiation with a single dose of 10 Gy (32). Another preclinical study also stated that those treated with 6.2 Gy × 2 fractions showed a noteworthy improvement in OS compared to the control group (36).
In the clinical studies group, Kim et al. observed a better OS in patients treated with HFRT regimens of 48 Gy × 6 fractions or 48 Gy × 8 fractions, which activate the immune system three weeks after treatment (35). The patients showed increased numbers of activated natural killer (NK) cells immediately after the first SBRT dose, showing better PFS (37). Authors from another study have identified an AE in lung and liver cancer patients treated with 1–5 fractions of SBRT or 1–10 fractions of HCRT, but it was not observed in bone and brain (40).
Moreover, Tubin et al. observed AE in 45% (9/20) of patients treated with SBRT (41). They also observed that SBRT was more likely to improve survival OS rates (p = 0.099), cancer-specific survival (CSS) (p = 0.049) and PFS rates (p = 0.003) (41). Another Tubin study reported significant BE and AE response rates of 96% (22/23 patients) and 52% (12/23 patients), and improved OS 70% (16/23) and PFS 87% (20/23) rates, respectively, in patients whose bulky tumours were partially irradiated (42).
4 Discussion
Though RT has long been used in cancer therapy, it has a history of immunosuppressive side effects. Researchers believe that lymphopenia can result from localised RT, which includes radiation to the chest or central nervous system (43, 44). The leading causes of this are the radiation exposure of the bloodstream and the inherent radiation sensitivity of immune cells, even at low radiation doses (<1 Gy) (43–45). Although radiation has long been believed to suppress the immune system, there is a bunch of evidence showing that radiation can, under certain conditions, actually increase the immune system’s ability to fight cancer (5, 27, 32–41, 46–48).
Established tumour cells often lose their capacity to present antigens through various genetic and epigenetic mechanisms, enabling them to avoid the immune system. Radiation’s direct cytotoxic effects may cause the release of tumour-specific antigens, which can then prompt antigen-presenting cells to trigger a T-cell immune response (49). Although dendritic cells can present tumour antigens to T cells, the successful activation of tumour antigen-specific T-cell immunity requires additional danger signals to enhance T-cell activation (49). Therefore, during radiation-induced cell death, both tumour antigen release and presentation are improved, helping to activate an immune response (50). These specific events following radiation-induced tumour cell killing have led to the concept of utilising RT as a method of in situ vaccination” (51, 52).
Considering the increasing evidence that underlying anti-immune responses may be essential in eradicating certain tumours with SBRT, investigations have been conducted to delineate optimal radiation schedules for maximising anti-tumoural immunity in animal models (53, 54). Marciscano et al. extensively reviewed past studies on the optimal dose and fractionation schedule for increasing anti-tumoural immunity (55). Bae et al. reported that three days of fraction intervals significantly decreased gastrointestinal complications without impairing the tumour control rate of SABR in hepatocellular carcinoma (14). Moreover, using immunological hot and cold tumours, researchers also compared anti-tumoural immunity exposed to two fractions of irradiation administered on consecutive days or at intervals of 5 days in the mouse model (33).
Furthermore, when radiation is administered at moderate or higher dose fractions, local RT can activate CD8+ cytotoxic T-cells involved in both local and systemic tumour control (abscopal) (24, 46, 56). Therefore, in previous studies, RT with 3 to 5 doses of <10 to 12 Gy appears particularly immunogenic (11, 38, 57–59). Some earlier studies revealed that hRT elevates CD8+ concentration between days 5 and 8 after hRT (34, 60, 61). Filatenkov et al. reported that irradiation with 1 Fraction × 30 Gy was curative and induced protective CD8+ T-cell-mediated immunity (5). A similar protracted schedule (4 fractions × 5 Gy over 14 days) failed to locally control B16 melanoma tumours expressing a model antigen with a low total dose of RT and large inter-fraction intervals; however, a single 20 Gy fraction did so (46). Moreover, SRS with a single dose of at least 30 Gy has been suggested to be more effective than daily fractionated radiation (2, 6).
Several researchers have previously reported substantial immune effects and tumour reduction/cure through selective and time-dependent RT, which targets the immune system instead of the tumour (62–64). The effectiveness of these methods depended on the ability to determine when Tregs were dividing synchronously and periodically during cell division (65). At this brief window in time (mitosis), the Tregs were highly sensitive to selective ablation, thus mitigating or removing their homeostatic immunosuppressive effects on other tumour-specific immune cells not in mitosis at that specific time point (64). Due to the tumour’s underlying immunology, RT may evolve towards more “immunologically relevant” schedules to break tumour tolerance locally and systemically (66, 67).
Contrary to the results of RT studies, some studies, in combination with immunotherapy, found no evidence of AE and response rate after using HFRT (68, 69). For example, McBride’s and Kim et al. studies found no evidence of AE and improved clinical outcomes by adding SBRT to nivolumab and Nivolumab plus ipilimumab in patients with metastatic head and neck squamous cell carcinoma (HNSCC) and Advanced Merkel Cell Carcinoma, respectively (68, 69). The small sample size may have contributed to the lack of evidence of an additional benefit or support for AE with the addition of SBRT, as mentioned in these clinical trials. Some previous studies have shown potential therapeutic benefits with systemic therapies given at the right time to selectively ablate synchronously dividing suppressor T cells (now called Regulatory T Cells) while sparing the effector T cells (63, 70, 71). Therefore, it suggests that the timing of immunotherapy and RT may play a role in treatment efficacy via immune modulation. We believe that additional investigation is warranted to determine the optimal RT dose and timing, immunotherapeutic agent, and large patient cohort to fully evaluate the potential of the AE on the response rate.
5 Conclusions
Our systematic review data revealed that HFRT with an optimal regimen can induce the activation of T lymphocytes while simultaneously reducing the frequency of Tregs. These studies also suggested that optimal dosages and fractions of HFRT induce immune response. However, it should be further explored to provide clinicians with information that would be valuable when making decisions regarding patient care. This strategy may increase cancer patients’ response rate to treatment, lower the cost and length of treatment and lower thir chance of developing immune-related side effects and general toxicity after receiving chemotherapy and immunotherapy.
Author contributions
JS: Conceptualization, Data curation, Methodology, Writing – original draft, Writing – review & editing. MA: Conceptualization, Data curation, Methodology, Supervision, Writing – original draft, Writing – review & editing. SB: Conceptualization, Data curation, Methodology, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. Genesis Cancer Care covered the cost of publication for this work; however, no funding was received for the preparation of this manuscript.
Acknowledgments
We acknowledge Genesis Cancer Care Research for Open-access publishing facilitation.
Conflict of interest
Author JS was employed by Limestone Coast Local Health Network Inc.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Durante M, Reppingen N, and Held KD. Immunologically augmented cancer treatment using modern radiotherapy. Trends Mol Med. (2013) 19:565–82. doi: 10.1016/j.molmed.2013.05.007
2. Goodman KA, Wiegner EA, Maturen KE, Zhang Z, Mo Q, Yang G, et al. Dose-escalation study of single-fraction stereotactic body radiotherapy for liver Malignancies. Int J Radiat oncology biology physics. (2010) 78:486–93. doi: 10.1016/j.ijrobp.2009.08.020
3. Favaudon V, Caplier L, Monceau V, Pouzoulet F, Sayarath M, Fouillade C, et al. Ultrahigh dose-rate FLASH irradiation increases the differential response between normal and tumor tissue in mice. Sci Trans Med. (2014) 6:245ra93. doi: 10.1126/scitranslmed.3008973
4. Park C, Papiez L, Zhang S, Story M, and Timmerman RD. Universal survival curve and single fraction equivalent dose: useful tools in understanding potency of ablative radiotherapy. Int J Radiat oncology biology physics. (2008) 70:847–52. doi: 10.1016/j.ijrobp.2007.10.059
5. Filatenkov A, Baker J, Mueller AM, Kenkel J, Ahn GO, Dutt S, et al. Ablative tumor radiation can change the tumor immune cell microenvironment to induce durable complete remissions. Clin Cancer Res. (2015) 21:3727–39. doi: 10.1158/1078-0432.CCR-14-2824
6. Loo BW Jr. Stereotactic ablative radiotherapy (SABR) for lung cancer: What does the future hold? J Thorac Dis. (2011) 3:150–2. doi: 10.3978/j.issn.2072-1439.2011.06.08
7. Jaffray DA. Image-guided radiotherapy: from current concept to future perspectives. Nat Rev Clin Oncol. (2012) 9:688–99. doi: 10.1038/nrclinonc.2012.194
8. Bae SH, Kim MS, Jang WI, Kay CS, Kim W, Kim ES, et al. A survey of stereotactic body radiotherapy in Korea. Cancer Res Treat. (2015) 47:379–86. doi: 10.4143/crt.2014.021
9. Lund CR, Cao JQ, Liu M, Olson R, Halperin R, and Schellenberg D. The distribution and patterns of practice of stereotactic ablative body radiotherapy in Canada. J Med Imaging Radiat Sci. (2014) 45:8–15. doi: 10.1016/j.jmir.2013.09.001
10. Rodriguez-Ruiz ME, Vitale I, Harrington KJ, Melero I, and Galluzzi L. Immunological impact of cell death signaling driven by radiation on the tumor microenvironment. Nat Immunol. (2020) 21:120–34. doi: 10.1038/s41590-019-0561-4
11. Vanpouille-Box C, Alard A, Aryankalayil MJ, Sarfraz Y, Diamond JM, Schneider RJ, et al. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat Commun. (2017) 8:15618. doi: 10.1038/ncomms15618
12. McLaughlin M, Patin EC, Pedersen M, Wilkins A, Dillon MT, Melcher AA, et al. Inflammatory microenvironment remodelling by tumour cells after radiotherapy. Nat Rev Cancer. (2020) 20:203–17. doi: 10.1038/s41568-020-0246-1
13. Kang JK, Kim MS, Cho CK, Yang KM, Yoo HJ, Kim JH, et al. Stereotactic body radiation therapy for inoperable hepatocellular carcinoma as a local salvage treatment after incomplete transarterial chemoembolization. Cancer. (2012) 118:5424–31. doi: 10.1002/cncr.27533
14. Bae SH, Kim MS, Kim SY, Jang WI, Cho CK, Yoo HJ, et al. Severe intestinal toxicity after stereotactic ablative radiotherapy for abdominopelvic Malignancies. Int J colorectal disease. (2013) 28:1707–13. doi: 10.1007/s00384-013-1717-6
15. Kaminski JM, Shinohara E, Summers JB, Niermann KJ, Morimoto A, and Brousal J. The controversial abscopal effect. Cancer Treat Rev. (2005) 31:159–72. doi: 10.1016/j.ctrv.2005.03.004
16. Kaur P and Asea A. Radiation-induced effects and the immune system in cancer. Front Oncol. (2012) 2:191. doi: 10.3389/fonc.2012.00191
17. Ganss R, Ryschich E, Klar E, Arnold B, and Hämmerling GJ. Combination of T-cell therapy and trigger of inflammation induces remodeling of the vasculature and tumor eradication. Cancer Res. (2002) 62:1462–70.
18. Desai S, Kumar A, Laskar S, and Pandey BN. Cytokine profile of conditioned medium from human tumor cell lines after acute and fractionated doses of gamma radiation and its effect on survival of bystander tumor cells. Cytokine. (2013) 61:54–62. doi: 10.1016/j.cyto.2012.08.022
19. Beatty G and Paterson Y. IFN-gamma-dependent inhibition of tumor angiogenesis by tumor-infiltrating CD4+ T cells requires tumor responsiveness to IFN-gamma. J Immunol (Baltimore Md: 1950). (2001) 166:2276–82. doi: 10.4049/jimmunol.166.4.2276
20. Zeng J, Harris TJ, Lim M, Drake CG, and Tran PT. Immune modulation and stereotactic radiation: improving local and abscopal responses. BioMed Res Int. (2013) 2013:658126. doi: 10.1155/2013/658126
21. Sologuren I, Rodríguez-Gallego C, and Lara PC. Immune effects of high dose radiation treatment: implications of ionizing radiation on the development of bystander and abscopal effects. Trans Cancer Res. (2014) 3:18–31.
22. Dagoglu N, Karaman S, Caglar HB, and Oral EN. Abscopal effect of radiotherapy in the immunotherapy era: systematic review of reported cases. Cureus. (2019) 11:e4103. doi: 10.7759/cureus.4103
23. Mole RH. Whole body irradiation; radiobiology or medicine? Br J Radiol. (1953) 26:234–41. doi: 10.1259/0007-1285-26-305-234
24. Demaria S, Ng B, Devitt ML, Babb JS, Kawashima N, Liebes L, et al. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat oncology biology physics. (2004) 58:862–70. doi: 10.1016/j.ijrobp.2003.09.012
25. Camphausen K, Moses MA, Ménard C, Sproull M, Beecken WD, Folkman J, et al. Radiation abscopal antitumor effect is mediated through p53. Cancer Res. (2003) 63:1990–3.
26. Del Prete A, Salvi V, Soriani A, Laffranchi M, Sozio F, Bosisio D, et al. Dendritic cell subsets in cancer immunity and tumor antigen sensing. Cell Mol Immunol. (2023) 20:432–47. doi: 10.1038/s41423-023-00990-6
27. Frey B, Rubner Y, Wunderlich R, Weiss EM, Pockley AG, Fietkau R, et al. Induction of abscopal anti-tumor immunity and immunogenic tumor cell death by ionizing irradiation - implications for cancer therapies. Curr medicinal Chem. (2012) 19:1751–64. doi: 10.2174/092986712800099811
28. Siva S, MacManus MP, Martin RF, and Martin OA. Abscopal effects of radiation therapy: a clinical review for the radiobiologist. Cancer letters. (2015) 356:82–90. doi: 10.1016/j.canlet.2013.09.018
29. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (Clinical Res ed). (2021) 372:n71. doi: 10.1136/bmj.n71
30. Wolff RF, Moons KGM, Riley RD, Whiting PF, Westwood M, Collins GS, et al. PROBAST: A tool to assess the risk of bias and applicability of prediction model studies. Ann Internal Med. (2019) 170:51–8. doi: 10.7326/M18-1376
31. Oliveira MA. BJCVS/RBCCV and endnote. Rev Bras cirurgia cardiovascular: orgao oficial da Sociedade Bras Cirurgia Cardiovascular. (2015) 30:127. doi: 10.5935/1678-9741.20150013
32. Markovsky E, Budhu S, Samstein RM, Li H, Russell J, Zhang Z, et al. An antitumor immune response is evoked by partial-volume single-dose radiation in 2 murine models. Int J Radiat oncology biology physics. (2019) 103:697–708. doi: 10.1016/j.ijrobp.2018.10.009
33. Kim H, Lee E, Cho H, Kim E, Jang WI, Yang K, et al. Five-day spacing of two fractionated ablative radiotherapies enhances antitumor immunity. Int J Radiat oncology biology Phys. (2023) 2:498–511. doi: 10.1016/j.ijrobp.2023.09.014
34. Frey B, Rückert M, Weber J, Mayr X, Derer A, Lotter M, et al. Hypofractionated irradiation has immune stimulatory potential and induces a timely restricted infiltration of immune cells in colon cancer tumors. Front Immunol. (2017) 8:231. doi: 10.3389/fimmu.2017.00231
35. Zhang T, Yu H, Ni C, Zhang T, Liu L, Lv Q, et al. Hypofractionated stereotactic radiation therapy activates the peripheral immune response in operable stage I non-small-cell lung cancer. Sci Rep. (2017) 7:4866. doi: 10.1038/s41598-017-04978-x
36. Zhao X, Li J, Zheng L, Yang Q, Chen X, Chen X, et al. Immune response on optimal timing and fractionation dose for hypofractionated radiotherapy in non-small-cell lung cancer. Front Mol biosciences. (2022) 9:786864. doi: 10.3389/fmolb.2022.786864
37. Muraro E, Furlan C, Avanzo M, Martorelli D, Comaro E, Rizzo A, et al. Local high-dose radiotherapy induces systemic immunomodulating effects of potential therapeutic relevance in oligometastatic breast cancer. Front Immunol. (2017) 8:1476. doi: 10.3389/fimmu.2017.01476
38. Schaue D, Ratikan JA, Iwamoto KS, and McBride WH. Maximizing tumor immunity with fractionated radiation. Int J Radiat oncology biology physics. (2012) 83:1306–10. doi: 10.1016/j.ijrobp.2011.09.049
39. Grapin M, Richard C, Limagne E, Boidot R, Morgand V, Bertaut A, et al. Optimized fractionated radiotherapy with anti-PD-L1 and anti-TIGIT: a promising new combination. J immunotherapy cancer. (2019) 7:160. doi: 10.1186/s40425-019-0634-9
40. McGee HM, Daly ME, Azghadi S, Stewart SL, Oesterich L, Schlom J, et al. Stereotactic ablative radiation therapy induces systemic differences in peripheral blood immunophenotype dependent on irradiated site. Int J Radiat oncology biology physics. (2018) 101:1259–70. doi: 10.1016/j.ijrobp.2018.04.038
41. Tubin S, Khan MK, Salerno G, Mourad WF, Yan W, and Jeremic B. Mono-institutional phase 2 study of innovative Stereotactic Body RadioTherapy targeting PArtial Tumor HYpoxic (SBRT-PATHY) clonogenic cells in unresectable bulky non-small cell lung cancer: profound non-targeted effects by sparing peri-tumoral immune microenvironment. Radiat Oncol (London England). (2019) 14:212. doi: 10.1186/s13014-019-1410-1
42. Tubin S, Popper HH, and Brcic L. Novel stereotactic body radiation therapy (SBRT)-based partial tumor irradiation targeting hypoxic segment of bulky tumors (SBRT-PATHY): improvement of the radiotherapy outcome by exploiting the bystander and abscopal effects. Radiat Oncol (London England). (2019) 14:21. doi: 10.1186/s13014-019-1227-y
43. Campian JL, Ye X, Brock M, and Grossman SA. Treatment-related lymphopenia in patients with stage III non-small-cell lung cancer. Cancer Invest. (2013) 31:183–8. doi: 10.3109/07357907.2013.767342
44. Harisiadis L, Kopelson G, and Chang CH. Lymphopenia caused by cranial irradiation in children receiving craniospinal radiotherapy. Cancer. (1977) 40:1102–8. doi: 10.1002/1097-0142(197709)40:3<1102::AID-CNCR2820400319>3.0.CO;2-0
45. Yovino S, Kleinberg L, Grossman SA, Narayanan M, and Ford E. The etiology of treatment-related lymphopenia in patients with Malignant gliomas: modeling radiation dose to circulating lymphocytes explains clinical observations and suggests methods of modifying the impact of radiation on immune cells. Cancer Invest. (2013) 31:140–4. doi: 10.3109/07357907.2012.762780
46. Lee Y, Auh SL, Wang Y, Burnette B, Wang Y, Meng Y, et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood. (2009) 114:589–95. doi: 10.1182/blood-2009-02-206870
47. Trovo M, Furlan C, Polesel J, Fiorica F, Arcangeli S, Giaj-Levra N, et al. Radical radiation therapy for oligometastatic breast cancer: Results of a prospective phase II trial. Radiotherapy Oncol J Eur Soc Ther Radiol Oncol. (2018) 126:177–80. doi: 10.1016/j.radonc.2017.08.032
48. Zhang X and Niedermann G. Abscopal effects with hypofractionated schedules extending into the effector phase of the tumor-specific T-cell response. Int J Radiat oncology biology physics. (2018) 101:63–73. doi: 10.1016/j.ijrobp.2018.01.094
49. Demaria S and Formenti SC. Sensors of ionizing radiation effects on the immunological microenvironment of cancer. Int J Radiat Biol. (2007) 83:819–25. doi: 10.1080/09553000701481816
50. Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. (2007) 13:1050–9. doi: 10.1038/nm1622
51. Rong Y and Welsh J. Basics of particle therapy II biologic and dosimetric aspects of clinical hadron therapy. Am J Clin Oncol. (2010) 33:646–9. doi: 10.1097/COC.0b013e3181cdf0fe
52. Formenti SC and Demaria S. Radiation therapy to convert the tumor into an in situ vaccine. Int J Radiat oncology biology physics. (2012) 84:879–80. doi: 10.1016/j.ijrobp.2012.06.020
53. Lasky CE, Pratt CL, Hilliard KA, Jones JL, and Brown CR. T cells exacerbate lyme borreliosis in TLR2-deficient mice. Front Immunol. (2016) 7:468. doi: 10.3389/fimmu.2016.00468
54. Ai W, Li H, Song N, Li L, and Chen H. Optimal method to stimulate cytokine production and its use in immunotoxicity assessment. Int J Environ Res Public Health. (2013) 10:3834–42. doi: 10.3390/ijerph10093834
55. Marciscano AE, Haimovitz-Friedman A, Lee P, Tran PT, Tomé WA, Guha C, et al. Immunomodulatory effects of stereotactic body radiation therapy: preclinical insights and clinical opportunities. Int J Radiat oncology biology physics. (2021) 110:35–52. doi: 10.1016/j.ijrobp.2019.02.046
56. Lugade AA, Moran JP, Gerber SA, Rose RC, Frelinger JG, and Lord EM. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J Immunol (Baltimore Md: 1950). (2005) 174:7516–23. doi: 10.4049/jimmunol.174.12.7516
57. Vanpouille-Box C, Formenti SC, and Demaria S. Toward precision radiotherapy for use with immune checkpoint blockers. Clin Cancer Res. (2018) 24:259–65. doi: 10.1158/1078-0432.CCR-16-0037
58. Dewan MZ, Galloway AE, Kawashima N, Dewyngaert JK, Babb JS, Formenti SC, et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res. (2009) 15:5379–88. doi: 10.1158/1078-0432.CCR-09-0265
59. Formenti SC. Optimizing dose per fraction: A new chapter in the story of the abscopal effect? Int J Radiat oncology biology Phys. (2017) 99:677–9. doi: 10.1016/j.ijrobp.2017.07.028
60. Hettich M, Lahoti J, Prasad S, and Niedermann G. Checkpoint Antibodies but not T Cell-Recruiting Diabodies Effectively Synergize with TIL-Inducing γ-Irradiation. Cancer Res. (2016) 76:4673–83. doi: 10.1158/0008-5472.CAN-15-3451
61. Dovedi SJ, Cheadle EJ, Popple AL, Poon E, Morrow M, Stewart R, et al. Fractionated radiation therapy stimulates antitumor immunity mediated by both resident and infiltrating polyclonal T-cell populations when combined with PD-1 blockade. Clin Cancer Res. (2017) 23:5514–26. doi: 10.1158/1078-0432.CCR-16-1673
62. Hellström KE and Hellström I. Evidence that tumor antigens enhance tumor growth in vivo by interacting with a radiosensitive (suppressor)? cell population. Proc Natl Acad Sci United States America. (1978) 75:436–40. doi: 10.1073/pnas.75.1.436
63. Awwad M and North RJ. Sublethal, whole-body ionizing irradiation can be tumor promotive or tumor destructive depending on the stage of development of underlying antitumor immunity. Cancer immunology immunotherapy: CII. (1988) 26:55–60. doi: 10.1007/BF00199848
64. North RJ and Awwad M. Elimination of cycling CD4+ suppressor T cells with an anti-mitotic drug releases non-cycling CD8+ T cells to cause regression of an advanced lymphoma. Immunology. (1990) 71:90–5.
65. Awwad M and North RJ. Cyclophosphamide-induced immunologically mediated regression of a cyclophosphamide-resistant murine tumor: a consequence of eliminating precursor L3T4+ suppressor T-cells. Cancer Res. (1989) 49:1649–54.
66. Schreiber RD, Old LJ, and Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Sci (New York NY). (2011) 331:1565–70. doi: 10.1126/science.1203486
67. Schaue D, Kachikwu EL, and McBride WH. Cytokines in radiobiological responses: a review. Radiat Res. (2012) 178:505–23. doi: 10.1667/RR3031.1
68. McBride S, Sherman E, Tsai CJ, Baxi S, Aghalar J, Eng J, et al. Randomized phase II trial of nivolumab with stereotactic body radiotherapy versus nivolumab alone in metastatic head and neck squamous cell carcinoma. J Clin Oncol. (2021) 39:30–7. doi: 10.1200/JCO.20.00290
69. Kim S, Wuthrick E, Blakaj D, Eroglu Z, Verschraegen C, Thapa R, et al. Combined nivolumab and ipilimumab with or without stereotactic body radiation therapy for advanced Merkel cell carcinoma: a randomised, open label, phase 2 trial. Lancet (London England). (2022) 400:1008–19. doi: 10.1016/S0140-6736(22)01659-2
70. Quevedo F, Ashdown ML, Suman VJ, Robinson A, KottsChade LA, Kaur JS, et al. Possible therapeutic reversal of immune suppression in patients with metastatic melanoma by timed delivery of temozolomide chemotherapy: A pilot study. J Clin Oncol. (2009) 27:e20013–e. doi: 10.1200/jco.2009.27.15_suppl.e20013
Keywords: hypo-fractionated radiotherapy, CD8+ T-cells infiltration, immune stimulation, abscopal effect, clinical outcomes
Citation: Singh J, Ashdown M and Baxi S (2025) Potential immunogenic modulation of hypo-fractionated radiotherapy at optimal schedules and the subsequent vaccine-like effect of local irradiation - a systematic review. Front. Oncol. 15:1546875. doi: 10.3389/fonc.2025.1546875
Received: 17 December 2024; Accepted: 22 September 2025;
Published: 03 October 2025.
Edited by:
Ning Ji, China Academy of Chinese Medical Sciences, ChinaReviewed by:
Maurizio Valeriani, Sapienza University of Rome, ItalySushma Agrawal, Sanjay Gandhi Post Graduate Institute of Medical Sciences (SGPGI), India
Yingying Wang, City of Hope National Medical Center, United States
Copyright © 2025 Singh, Ashdown and Baxi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jagtar Singh, amFndGFyLnNpbmdoQHNhLmdvdi5hdQ==
†ORCID: Jagtar Singh, orcid.org/0000-0002-0457-2650
 Jagtar Singh
Jagtar Singh Martin Ashdown3
Martin Ashdown3



