- 1Center for Endocrinology and Metabolic Diseases, The People’s Hospital of Liaoning Province, Shenyang, China
- 2Department of Endocrine and Metabolism, Zhuhai People’s Hospital (Zhuhai Hospital Affiliated with Jinan University), Zhuhai, China
Background: The global incidence of papillary thyroid carcinoma (PTC) is increasing significantly. In response, active surveillance (AS) has been promoted for Low-risk PTC owing to the absence of associated mortality. However, the association between age, sex, and risk of tumor progression remains unclear. This study aimed to assess the age- and sex-specific Impact on the progression of low-risk PTC.
Methods: PubMed and Web of Science Core Collection were searched for articles published up to 1 January 2024. Articles reporting patients with PTC undergoing AS were included. Studies involving patients who underwent total or partial thyroidectomy or radiofrequency ablation were excluded. Random- and fixed-effect models were applied to obtain pooled proportions and 95% CIs.
Results: A total of 972 unique citations were screened and 39 full-text articles were reviewed, including eight cohorts. The mean or median age ranged from 41.5 to 53.1 years, with a predominant inclusion of female patients (76.39%–87.80%). The pooled risk ratio for tumor progression (a growth of 3 mm or more in maximal diameter or lymph node metastasis) in older adults (aged over 30–50 years) compared with younger individuals was 0.58 (95% CI, 0.47–0.71; 4,725 patients, six studies). However, for male patients, the pooled risk ratio for tumor progression compared with female individuals was 1.11 (95% CI, 0.64–1.93; 4,916 patients, six studies).
Conclusion: This meta-analysis suggests that advanced age may be associated with a lower risk of progression of papillary thyroid microcarcinomas during active surveillance. No significant differences were observed between sexes.
Introduction
Over the past two decades, the global incidence of thyroid cancer has increased rapidly (1), with PTC being the most common subtype, accounting for approximately 85% of cases (2). The World Health Organization defines PTC with a maximum diameter of ≤10 mm as papillary thyroid microcarcinomas (PTMC) (3), and autopsy studies have detected PTMC in 5%–36% of the population (4). This increase is partly attributed to advancements in diagnostic techniques such as high-resolution ultrasonography (US) and fine-needle aspiration biopsy (FNAB).
PTC, particularly in its microcarcinoma form (PTMC), generally has excellent prognosis. Patients with PTC exhibit a 10-year overall survival rate exceeding 95% (5), whereas those with PTMC have a 10-year disease-specific survival rate of >99% (6). Although most guidelines recommend total or partial thyroidectomy with therapeutic central and lateral lymph node dissection as the first-line treatment for PTMC (7, 8), these surgical interventions are associated with significant costs and a reduced quality of life (9, 10).
AS and thermal ablation have been proposed as alternative management strategies to mitigate overdiagnosis and overtreatment. AS, initially developed for low-risk prostate cancer (11), was introduced for low-risk PTC by Professor Ito and his team at Kuma Hospital, Japan (12). Over time, the concept of observing very low-risk thyroid cancers under AS has gained acceptance among both patients and clinicians.
Since 2010, numerous medical institutions have established cohorts to study AS in low-risk PTC patients. By 2020, outcomes from these cohorts with follow-up periods ranging from 5 to 10 years have been progressively published (13–20). However, the influence of aging and gender on the risk of PTC progression during AS remains unclear, with conflicting findings reported in literature (21, 22).
This study aimed to evaluate the age- and sex-specific impact of AS on tumor progression in low-risk PTC through a comprehensive meta-analysis.
Materials and methods
This systematic review and meta-analysis was performed according to an updated version of The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement published in 2020 (23).
Search strategy
A comprehensive search of the PubMed and Web of Science Core Collection (WOSCC) databases was conducted to identify original literature reporting on patients undergoing AS for PTC.
The following search terms were used: ((“active surveillance”) OR (observation)) AND ((papillary thyroid carcinoma) OR (papillary thyroid microcarcinoma) OR (papillary microcarcinoma of the thyroid) OR (thyroid microcarcinoma)). No starting date was specified, and the literature search was updated on 1 January 2024. Only English language publications were included in this study. Relevant article bibliographies were scrutinized for any additional suitable articles. The exclusion criteria included case reports, letters, conference abstracts, reviews, meta-analyses, guidelines, study protocols, and statements. Patients who underwent total or partial thyroidectomy and radiofrequency ablation were also excluded from the study. Three independent reviewers screened all the unique citations for relevance, reviewed the full-text articles, and reached a consensus.
Data extraction
Two reviewers independently extracted data using standardized forms: (1) Characteristics of the included studies: institution, country of origin, authors, publication year, study design, follow-up duration, and funding information. (2) Characteristics of study participants: patient numbers, mean age, male-to-female ratio, largest primary tumor dimension, prevalence of thyroid hormone used during AS, inclusion of multifocal papillary thyroid cancer, and larger PTCs (>10 mm). (3) Clinical outcomes of patients undergoing AS: increase in maximal nodule diameter, development of new cervical lymph node metastasis (LNM) on ultrasound, and suspected distant metastasis. A consensus on the extracted data was achieved through discussion.
Statistical analysis
Continuous variables were described using mean and standard deviation or median and interquartile range (IQR), whereas categorical variables were presented as frequencies with percentages. The meta-analysis results, expressed as risk ratios (RR) with 95% confidence intervals, were used. Heterogeneity between studies was assessed using I² statistics. If no statistical proof of heterogeneity (I² <50%) was found, a fixed- effects model was applied; otherwise, a random- effects model was used. A meta-analysis was conducted using Review Manager (RevMan) version 5.3.
Results
Systematic literature search
A total of 972 publications were identified in the search updated until 1 January 2024, comprising 390 from PubMed and 582 from the Web of Science Core Collection (WOSCC). After removing 306 duplicates and excluding 192 non-original studies based on titles and abstracts, 39 full-text articles were assessed for eligibility. After careful selection, 21 publications were excluded owing to potential overlapping patient cohorts, considering author names, time periods of patient inclusion, and affiliations. In this process, priority is given to the most recent and abundant studies. Additionally, six studies were excluded due to unavailable outcomes, and four were excluded due to the absence of age or sex subtypes. Finally, seven studies were deemed eligible for the meta-analysis (13–20). Among these, six studies were included in the age-specific analysis (14–19) and six were included in the sex-specific analysis (13–15, 18–20) (Figure 1).
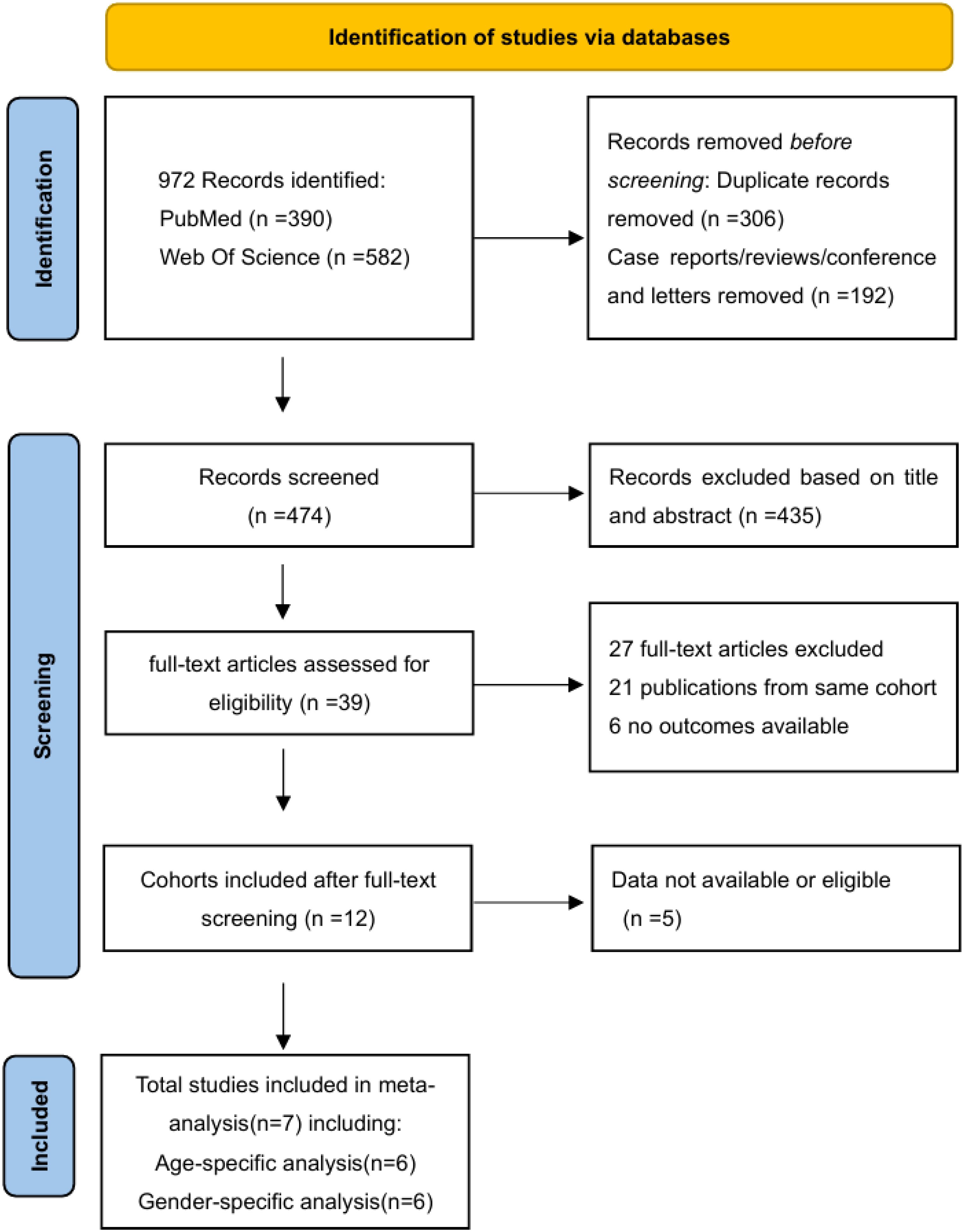
Figure 1. PRISMA flow diagram of literature search and study selection. A total of 972 records were identified from databases, with 306 duplicates removed. After screening titles/abstracts, 39 full-text articles were assessed for eligibility, and seven studies met inclusion criteria for quantitative synthesis.
Description of the included original studies
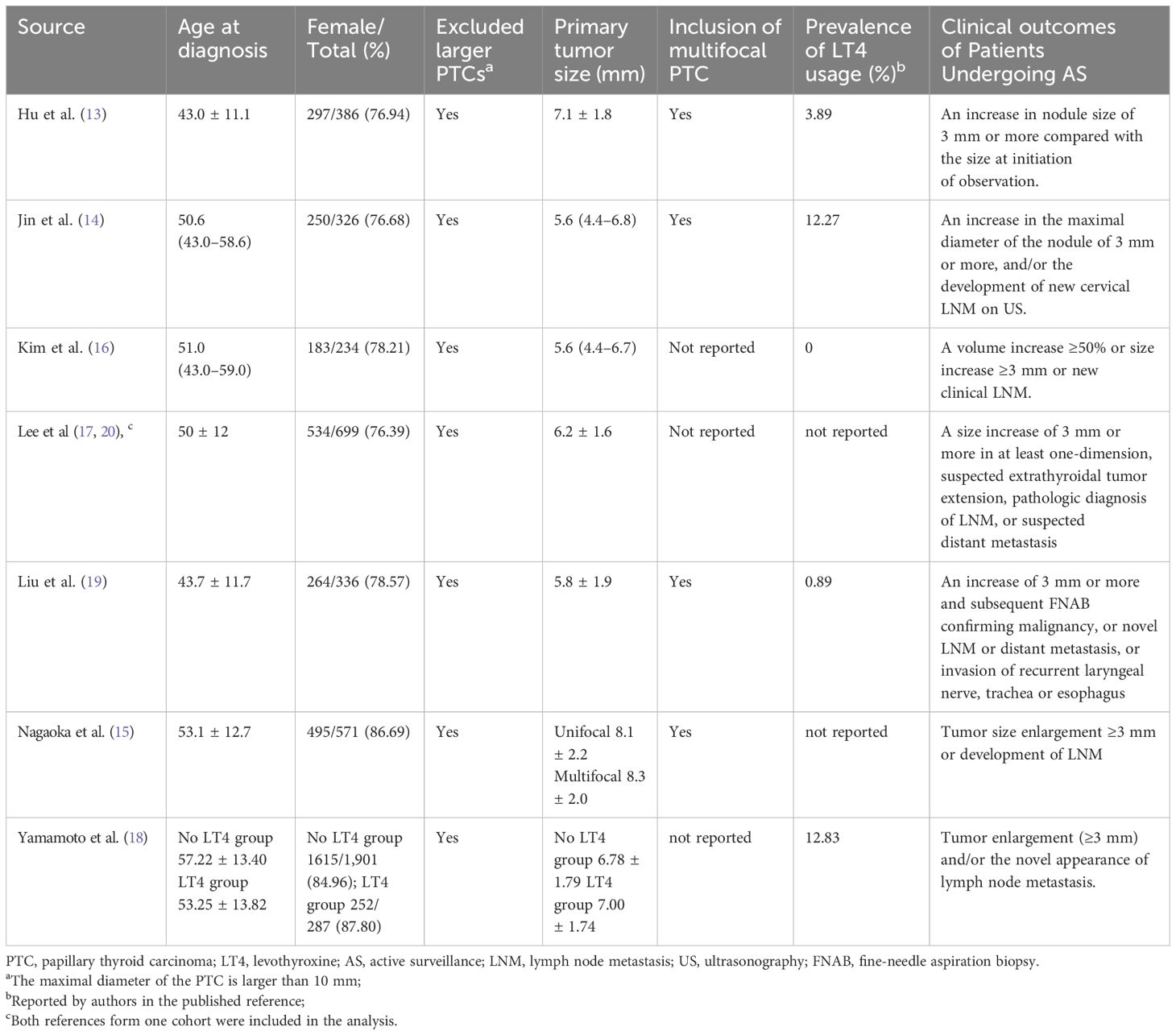
Table 1 presents the characteristics of the studies included. All studies originating in Japan, Korea, China, and Argentina were published after 2020. Two studies were conducted in systematically well-managed cohorts, including the earliest and longest (1993–2019) cohort initiated by Ito et al. at Kuma Hospital (18) (Japan) and the Multicenter Prospective Cohort Study of Active Surveillance on Papillary Thyroid Microcarcinoma (MAeSTro) (NCT02938702) from Korea (17, 20). Notably, these two studies included cohorts with more than 1,000 participants. Studies in China had shorter median follow-up periods (12 and 28.5 months) (13, 19), while others had mean or median follow-up periods ranging from 41.4 months to 7.6 years (14–18, 20).
Clinical characteristics
Table 2 presents the characteristics of the study participants. The mean or median age ranged from 41.5 to 53.1 years, with a predominant inclusion of female patients (76.39%–87.80%). All the studies included patients with papillary microcarcinomas (<1.0 cm). Most studies included multifocal PTC, though three studies did not report this feature. Levothyroxine use varied significantly among studies. Excluding two studies that did not report this information, reports of TSH-suppressive therapy ranged from 0% to 46.3%. Most studies defined tumor progression as a diameter increase ≥3 mm or lymph node metastasis, with one study including a volume increase of ≥50% (16).
Results of the meta-analyses
The fixed-effects meta-analysis examined the impact of aging on tumor progression under AS by incorporating data from six studies, totaling 4,725 patients. The pooled Risk Ratio (RR) for tumor growth of ≥3 mm in maximal diameter or lymph node metastasis in older adults (aged over 30–50 years) compared with younger individuals was 0.58 (95% CI, 0.47–0.71) (Figure 2). No statistically significant heterogeneity was observed in this study (I² = 0, df = 5, P = 0.74).
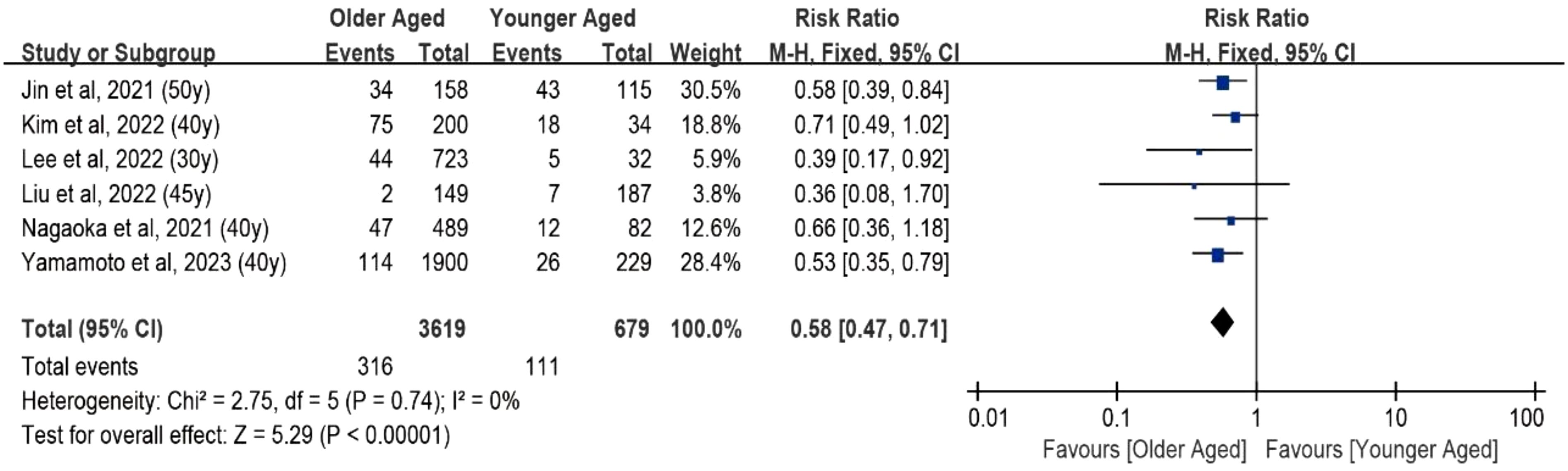
Figure 2. Forest plot of pooled risk ratios of tumor enlargement relative to age. Forest plot of the age-specific risk of a tumor progression (a size increase of 3 mm or more in at least one dimension, lymph node metastasis, or distant metastasis). Age was the cut point used in the references included in the analysis.
In the meta-analysis assessing the impact of sex on tumor progression in AS, data from six studies with a total of 4,916 patients were included. However, a relatively high level of heterogeneity was identified among the studies (I² = 69%; df = 5; P = 0.007). Consequently, a random effects meta-analysis was conducted. As illustrated in Figure 3, for male patients, the pooled risk ratio for tumor progression compared with female individuals was 1.11 (95% CI, 0.64–1.93; 4,916 patients, six studies).
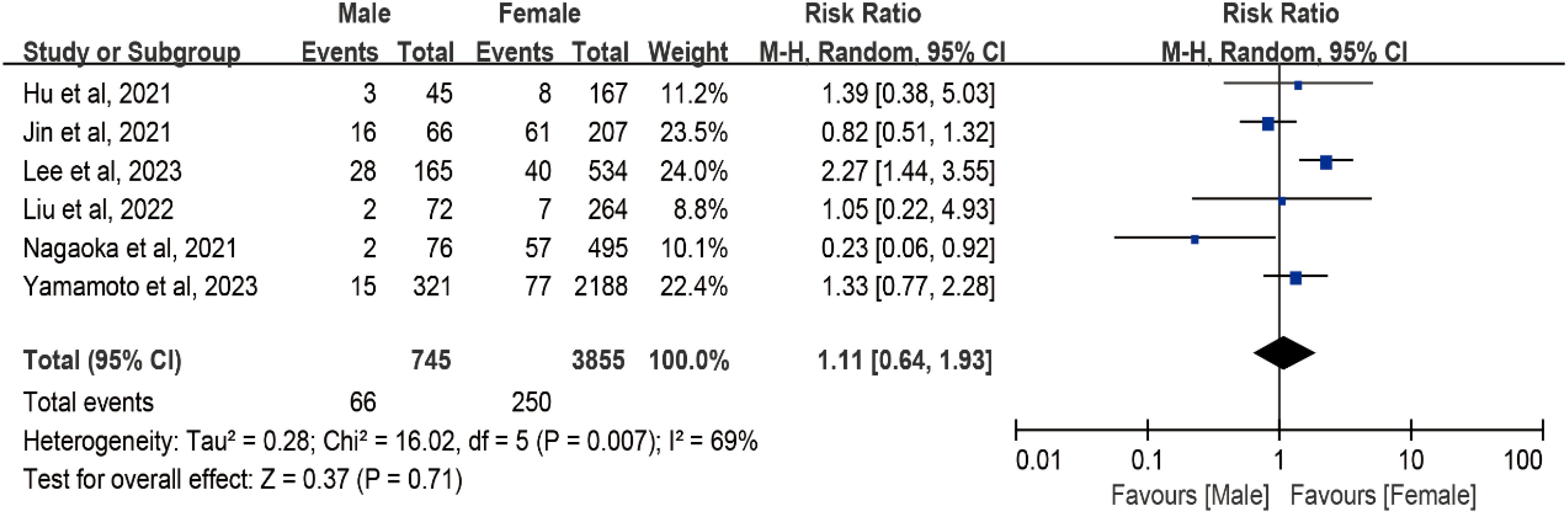
Figure 3. Forest plot of pooled risk ratios of tumor enlargement relative to sex. Forest plot of the gender-specific risk of a tumor progression (a size increase of 3 mm or more in at least one dimension, lymph node metastasis, or distant metastasis).
Considering the limited number of included studies, further sensitivity analyses exploring subgroup effects were not conducted. Furthermore, due to the inclusion of fewer than 10 studies, an analysis of publication bias could not be meaningfully interpreted and was therefore not performed.
Discussion
In the 1990s, advancements in detection techniques revealed that most low-risk PTMC exhibited latent, slow, or no progression (24–26). Kuma Hospital (Kobe, Japan) pioneered AS as an alternative approach for low-risk PTMC in 1993 (12), followed by the Cancer Institute Hospital (Tokyo, Japan), adopting a similar concept in 1995 (27). Over time, these two institutions have contributed significantly to our knowledge of this subject.
By 2010, after a decade of follow-up, the safety and benefits of AS had been substantiated. This pivotal evidence led to the Japanese Association of Endocrine Surgeons and the Japanese Society of Thyroid Surgeons publishing the first edition of the guidelines, endorsing AS as a viable management option for low-risk PTMC (28). Five years later, the American Thyroid Association (ATA) also recommended AS as a strategy for managing low-risk PTMC (29). However, it is essential to note that the prevalence of disease progression under AS varies significantly during follow-up across different cohorts, ranging from 6.5% to 39.7% (16, 17). The variation in the risk of tumor progression under AS prompted the exploration of influencing risk factors. The roles of aging and sex remain debatable.
Our study incorporated data from eight cohorts of PTC patients undergoing AS, revealing a reduced risk of tumor enlargement (defined as both a maximal diameter increase of ≥3 mm, a tumor volume increase of ≥50%, and/or the development of new lymph node metastasis from baseline) with advancing age. The older the low-risk PTC was diagnosed, the lower the risk of tumor progression. However, the risk of surgical complications of thyroidectomy significantly increases with age. Considering both the economic burden and impact on the quality of life of patients caused by surgery and postoperative complications, this dual consideration becomes a compelling factor for doctors when making decisions regarding the management of low-risk PTMC in elderly individuals.
Thyroid cancer (TC) incidence shows a strong sex difference, with most populations having an incidence that is about three times higher in female than in male; the same trend was also observed in PTC (30). I Interestingly, the mortality rate of TC shows less disparity between sexes (1). Some studies have identified male sex as a factor positively associated with poor outcomes in patients with surgically treated PTC (31), while others have different perspectives (32, 33). The results varied among the AS groups. In our study, although a higher prevalence was found in the female group, no difference in tumor progression under AS was observed between sexes. This provides solid evidence for decision-making regarding low-risk PTC in both males and females, indicating that sex should not be a significant factor. Neither male nor female patients exhibited a higher risk of tumor progression during AS.
Strengths and limitations
The strengths of this study include a thorough electronic database search conducted by an experienced specialist, independent duplicate reviews for study selection, and meticulous appraisal of the data from the included studies.
However, limitations include the small number of available studies and patients, limited follow-up periods in some studies, challenges in evaluating publication bias or subgroup effects due to scarce data, constrained statistical power for detecting heterogeneity, and limited exploration of gray literature.
Conclusion
In this systematic review and meta-analysis, we found that older adults (aged 30–50 years) may have a reduced risk of progression in papillary thyroid microcarcinoma (PTMC) under active surveillance (AS). No significant differences in progression risk were observed between sexes. This study focused on PTMC and provided robust evidence to support clinical decision-making in the management of low-risk PTMC. These findings underscore the importance of individualized treatment strategies to achieve optimal outcomes in each patient.
Author contributions
YW: Data curation, Methodology, Investigation, Software, Writing – original draft. LW: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the China Postdoctoral Science Foundation (Grant #2022YFC3602300).
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Pizzato M, Li M, Vignat J, Laversanne M, Singh D, La Vecchia C, et al. The epidemiological landscape of thyroid cancer worldwide: GLOBOCAN estimates for incidence and mortality rates in 2020. Lancet Diabetes Endocrinol. (2022) 10:264–72. doi: 10.1016/S2213-8587(22)00035-3
2. LiVolsi VA. Papillary thyroid carcinoma: an update. Modern pathology: an Off J United States Can Acad Pathology Inc. (2011) 24 Suppl 2:S1–9. doi: 10.1038/modpathol.2010.129
3. Hedinger C, Williams ED, and Sobin LH. The WHO histological classification of thyroid tumors: a commentary on the second edition. Cancer. (1989) 63:908–11. doi: 10.1002/1097-0142(19890301)63:5<908::AID-CNCR2820630520>3.0.CO;2-I
4. Furuya-Kanamori L, Bell KJL, Clark J, Glasziou P, and Doi SAR. Prevalence of differentiated thyroid cancer in autopsy studies over six decades: A meta-analysis. J Clin oncology: Off J Am Soc Clin Oncol. (2016) 34:3672–9. doi: 10.1200/JCO.2016.67.7419
5. Al-Ibraheem A, Al-Rasheed U, Mashhadani N, Abdlkadir AS, Al-Adhami DA, Ruzzeh S, et al. Long-Term Survival Analysis and Prognostic Factors of Arabic Patients with Differentiated Thyroid Carcinoma: A 20-Year Observational Study at the King Hussein Cancer Center (KHCC) Involving 528 Patients. Cancers. (2023) 15(16):4102. doi: 10.3390/cancers15164102
6. van Velsen EFS, Stegenga MT, van Kemenade FJ, Kam BLR, van Ginhoven TM, Visser WE, et al. Comparing the prognostic value of the eighth edition of the American joint committee on cancer/tumor node metastasis staging system between papillary and follicular thyroid cancer. Thyroid: Off J Am Thyroid Assoc. (2018) 28:976–81. doi: 10.1089/thy.2018.0066
7. Francis GL, Waguespack SG, Bauer AJ, Angelos P, Benvenga S, Cerutti JM, et al. Management guidelines for children with thyroid nodules and differentiated thyroid cancer. Thyroid. (2015) 25:716–59. doi: 10.1089/thy.2014.0460
8. Miyauchi A, Ito Y, and Oda H. Insights into the management of papillary microcarcinoma of the thyroid. Thyroid. (2018) 28:23–31. doi: 10.1089/thy.2017.0227
9. Lang BH and Wong CK. A cost-effectiveness comparison between early surgery and non-surgical approach for incidental papillary thyroid microcarcinoma. Eur J Endocrinol. (2015) 173:367–75. doi: 10.1530/EJE-15-0454
10. Oda H, Miyauchi A, Ito Y, Sasai H, Masuoka H, Yabuta T, et al. Comparison of the costs of active surveillance and immediate surgery in the management of low-risk papillary microcarcinoma of the thyroid. Endocr J. (2017) 64:59–64. doi: 10.1507/endocrj.EJ16-0381
11. Smith DP and Wittert GA. Low risk prostate cancer and an opportunity lost: more activity required in active surveillance. Med J Aust. (2018) 208:430–1. doi: 10.5694/mja2.2018.208.issue-10
12. Ito Y, Uruno T, Nakano K, Takamura Y, Miya A, Kobayashi K, et al. An observation trial without surgical treatment in patients with papillary microcarcinoma of the thyroid. Thyroid. (2003) 13:381–7. doi: 10.1089/105072503321669875
13. Hu YL, Cao XY, Zhou YR, Ye XH, Wang JX, Li X, et al. Management of sonographically suspicious thyroid nodules 1 cm or smaller and candidacy for active surveillance: experience of a tertiary center in China. Endocrine practice: Off J Am Coll Endocrinol Am Assoc Clin Endocrinologists. (2021) 27:903–11. doi: 10.1016/j.eprac.2021.02.006
14. Jin M, Kim HI, Ha J, Jeon MJ, Kim WG, Lim DJ, et al. Tumor volume doubling time in active surveillance of papillary thyroid microcarcinoma: A multicenter cohort study in korea. Thyroid: Off J Am Thyroid Assoc. (2021) 31:1494–501. doi: 10.1089/thy.2021.0094
15. Nagaoka R, Ebina A, Toda K, Jikuzono T, Saitou M, Sen M, et al. Multifocality and progression of papillary thyroid microcarcinoma during active surveillance. World J Surg. (2021) 45:2769–76. doi: 10.1007/s00268-021-06185-2
16. Kim HI, Jin M, Ko NG, Oh YL, Shin JH, Kim JH, et al. Effect of TSH levels during active surveillance of PTMC according to age. Endocrine-related Cancer. (2022) 29:191–200. doi: 10.1530/ERC-21-0403
17. Won H-R, Jeon E, Heo DB, Chang JW, Shong M, Kim JR, et al. Age-Dependent clinicopathological characteristics of patients with T1b papillary thyroid carcinoma: implications for the possibility of active surveillance. Ann Surg Oncol. (2022) 30(4):2246–2253. doi: 10.1245/s10434-022-13011-z.
18. Yamamoto M, Miyauchi A, Ito Y, Fujishima M, Sasaki T, and Kudo T. Active surveillance outcomes of patients with low-risk papillary thyroid microcarcinoma according to levothyroxine treatment status. Thyroid: Off J Am Thyroid Assoc. (2023) 33:1182–9. doi: 10.1089/thy.2023.0046
19. Liu C, Zhao H, Xia Y, Cao Y, Zhang L, Zhao Y, et al. Active surveillance of highly suspicious thyroid nodules cohort in China shows a worse psychological status in younger patients. Front Oncol. (2022) 12:981495. doi: 10.3389/fonc.2022.981495
20. Lee JY, Kim JH, Kim YK, Lee CY, Lee EK, Moon JH, et al. US predictors of papillary thyroid microcarcinoma progression at active surveillance. Radiology. (2023) 309:e230006. doi: 10.1148/radiol.230006
21. Alhashemi A, Goldstein DP, and Sawka AM. A systematic review of primary active surveillance management of low-risk papillary carcinoma. Curr Opin Oncol. (2016) 28:11–7. doi: 10.1097/CCO.0000000000000244
22. Griffin A, Brito JP, Bahl M, and Hoang JK. Applying criteria of active surveillance to low-risk papillary thyroid cancer over a decade: how many surgeries and complications can be avoided? Thyroid: Off J Am Thyroid Assoc. (2017) 27:518–23. doi: 10.1089/thy.2016.0568
23. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (Clinical Res ed). (2021) 372:n71. doi: 10.1136/bmj.n71
24. Iida F, Sugenoya A, and Muramatsu A. Clinical and pathologic properties of small differentiated carcinomas of the thyroid gland. World J Surg. (1991) 15:511–5. doi: 10.1007/BF01675648
25. Rodriguez JM, Moreno A, Parrilla P, Sola J, Soria T, Tebar FJ, et al. Papillary thyroid microcarcinoma: clinical study and prognosis. Eur J Surg = Acta chirurgica. (1997) 163:255–9.
26. Hay ID, Grant CS, van Heerden JA, Goellner JR, Ebersold JR, and Bergstralh EJ. Papillary thyroid microcarcinoma: a study of 535 cases observed in a 50-year period. Surgery. (1992) 112:1139–1146; discussion 1146-1137.
27. Sugitani I, Toda K, Yamada K, Yamamoto N, Ikenaga M, and Fujimoto Y. Three distinctly different kinds of papillary thyroid microcarcinoma should be recognized: our treatment strategies and outcomes. World J Surg. (2010) 34:1222–31. doi: 10.1007/s00268-009-0359-x
28. Takami H, Ito Y, Okamoto T, and Yoshida A. Therapeutic strategy for differentiated thyroid carcinoma in Japan based on a newly established guideline managed by Japanese Society of Thyroid Surgeons and Japanese Association of Endocrine Surgeons. World J Surg. (2011) 35:111–21. doi: 10.1007/s00268-010-0832-6
29. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 american thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the american thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid: Off J Am Thyroid Assoc. (2016) 26:1–133. doi: 10.1089/thy.2015.0020
30. Kocarnik JM, Compton K, Dean FE, Fu W, Gaw BL, Harvey JD, et al. Cancer incidence, mortality, years of life lost, years lived with disability, and disability-Adjusted life years for 29 cancer groups from 2010 to 2019: A systematic analysis for the global burden of disease study 2019. JAMA Oncol. (2022) 8:420–44. doi: 10.1001/jamaoncol.2021.6987
31. Oh HS, Park S, Kim M, Kwon H, Song E, Sung TY, et al. Young age and male sex are predictors of large-Volume central neck lymph node metastasis in clinical N0 papillary thyroid microcarcinomas. Thyroid: Off J Am Thyroid Assoc. (2017) 27:1285–90. doi: 10.1089/thy.2017.0250
32. Yan Z, Gang LW, Yan GS, and Zhou P. Prediction of the invasiveness of PTMC by a combination of ultrasound and the WNT10A gene. Front Endocrinol. (2022) 13:1026059. doi: 10.3389/fendo.2022.1026059
Keywords: active surveillance, papillary thyroid cancer, oncologic outcomes, aging, meta-analysis
Citation: Wang Y and Wang L (2025) Age- and sex-specific impact on the progression of low-risk papillary thyroid carcinoma under active surveillance: a meta-analysis. Front. Oncol. 15:1547345. doi: 10.3389/fonc.2025.1547345
Received: 18 December 2024; Accepted: 19 May 2025;
Published: 18 June 2025.
Edited by:
Lee Peng Karen-Ng, University of Malaya, MalaysiaReviewed by:
Rana Salman Anjum, University at Buffalo, United StatesCopyright © 2025 Wang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Wang, d2x6aHBobWdAMTYzLmNvbQ==
 Yang Wang1
Yang Wang1 Li Wang
Li Wang