- 1Neurology Department, Nanjing Hospital of Chinese Medicine Affiliated to Nanjing University of Chinese Medicine, Nanjing, China
- 2Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 3Department of Health Research Methods, Evidence, and Impact (HEI), McMaster University, Hamilton, ON, Canada
- 4Gynecology Department, World Health Organization Collaborating Centre (WHOCC), Chengdu, China
Objective: Assisted reproductive technology (ART) has contributed to the birth of over 10 million children worldwide; however, its long-term health impacts, especially the potential risk of childhood cancer, continue to be a subject of debate. This study aims to examine the most current risk associations between ART and childhood cancer.
Methods: We conducted a comprehensive search across PubMed, Embase, Web of Science, and Cochrane Library databases up to August 1, 2024. These studies aimed to explore the association between ART and childhood cancer risk, covering overall cancers, haematological malignancies, neural tumors, other solid tumors, and 11 specific cancers. Pooled analyses of risk estimates and 95% confidence intervals were conducted using random effects models, while cumulative meta-analyses were conducted to provide a time-based summary of the evidence. The study was prospectively registered on PROSPERO (CRD42024547262).
Results: Sixteen large sample observational studies were included. Our findings showed a 21% increased risk of overall cancer in children conceived via ART (relative risk [RR] = 1.21, 95% CI, 1.11–1.33), with elevated risks also noted for haematological malignancies (RR = 1.16, 95% CI, 1.05–1.28), neural tumors (RR = 1.19, 95% CI, 1.07–1.32), and other solid tumors (RR = 1.48, 95% CI, 1.26–1.73). Six specific cancer types also demonstrated higher risks. The direction and magnitude of the effects remained relatively constant over time, while the degree of precision increased as data from newer studies were incorporated. Sensitivity analyses confirmed the robustness of these findings, and no publication bias was found.
Conclusions: Our findings suggest a potential risk association between ART and childhood cancer, raising concerns regarding the future application of ART. These findings are critical in informing infertile couples considering ART about the potential risks involved.
Clinical trial registration: https://www.crd.york.ac.uk/prospero/, identifier CRD42024547262.
Introduction
Reports indicate that 48 million couples and 186 million individuals worldwide suffer from infertility (1, 2). The social consequences of infertility are significant, as the problem can lead to psychological distress, social stigmatization, economic strain, and even divorce, thereby positioning it as a public health priority (2, 3). This situation has improved with ongoing advancements in medical treatments and technology, enabling millions of infertile couples to conceive through ART (4). Currently, more than 10 million children have been born through ART globally, accounting for 5.1% of all U.S. births (5). Previously, this technology was considered safe; however, the short-term outcomes of children conceived via ART, including fetal birth defects and growth abnormalities, have been well-documented (6–8). However, the medium- and long-term effects of ART on child health remain largely unknown (9). In particular, the association between ART and the risk of childhood cancer has been a subject of ongoing controversy (5). Childhood cancers constitute the second most common cause of childhood mortality in developed countries; however, their etiology remains elusive, and their disease burden is projected to rise significantly, with ART recognized as a possible risk factor (10, 11). Several studies have indicated that certain childhood cancers originate in the early stages of fetal development and that events surrounding conception may play a crucial role in these cancers (9, 12). Given that each stage of ART implementation differs fundamentally from natural conception, it may impact the early developmental stages of the embryo (13, 14). Consequently, several researchers have proposed that ART could be a potential risk factor for childhood cancer (15, 16).
In the 1990s, studies suggested that ART might increase the risk of childhood cancer (17); however, subsequent research revealed no significant risk association between ART and childhood cancer (18), and it may even offer protective benefits (19). The conclusions of these studies lack persuasiveness due to limited sample sizes and variability in research methodologies. Recent large cohort studies investigating the link between ART and childhood cancer have yielded inconsistent and controversial conclusions (20–22). Currently, six published systematic reviews and meta-analyses on this topic have not reached a unanimous conclusion regarding the risk association between ART and childhood cancer, and the opinions are evenly divided into those in favor and those against. In 2005, Raimondi et al. (23) reported that there is no evidence supporting an increased risk of childhood cancer associated with ART. In 2019, a meta-analysis of cohort studies by Gilboa et al. (24) concluded that ART, particularly IVF, is not associated with an overall increased risk of childhood cancer. In 2020, Zhang et al. (25) demonstrated that IVF, intracytoplasmic sperm injection (ICSI), and fertility drugs are not linked to the risk of offspring cancer; however, contrasting results were observed with frozen embryo transfer methods. Conversely, in 2013, Hargreave et al. (15) concluded that fertility treatments might increase the risk of overall childhood cancers, haematological malignancies, neural tumors, and other solid tumors. In 2019, Wang et al. (16) further concluded that ART increases the risk of childhood cancers. Additionally, in 2019, Chiavarini et al. (26) reported a positive association between ART and the risk of childhood cancer. These studies have found some evidence for a link between ART and childhood cancer risk. However, a careful review has revealed methodological flaws, including design limitations and inadequate analytical approaches. For instance, studies with smaller sample sizes may increase the risk of biased results, necessitating revalidation of these findings.
In addition, larger cohort studies conducted over the past four years have introduced new and robust evidence into the field. Notably, two cohort studies (20, 27) encompassing nearly 10 million participants have significantly enriched our knowledge base, enhancing the persuasiveness of our findings. Therefore, the aim of this study was to further assess the impact of ART on childhood cancer and to examine future research trends. This study also aims to provide couples considering ART with the latest information on cancer risk and to assess whether ART should be included as a risk factor for childhood cancer.
Methods
Registration of review protocol
This study adhered strictly to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2020 (PRISMA) (28) guidelines for a systematic review and meta-analysis of studies examining the relationship between ART and childhood cancer risk. The study protocol was registered and published in the Prospective Registry for International Systematic Reviews (PROSPERO), Registration No. CRD42024547262.
Search strategy
We conducted searches in the PubMed, Embase, Web of Science, and Cochrane Library databases from inception to August 1, 2024, for relevant publications. The language of the publications was limited to English. The search strategies included terms related to exposure (“Assisted reproductive technology”, “Intracytoplasmic sperm injection”, “In vitro fertilization”, “ART”, “ICSI”, “IVF”), specific populations (“infant”, “pediatric”, “childhood”), and outcomes (“Neoplasms”, “Cancer”, “Tumor”) via both MeSH terms and free-text keywords. The reference lists of related articles were manually reviewed to identify any relevant studies missed in the initial search. More detailed information on the search process is available in the Search Strategies for the Supplementary Material.
Eligibility criteria
As recommended, the PECO-S framework was employed to delineate the research questions (29, 30). P-population: children from any country or region; E-exposure: number of children conceived via ART > 4000; C-comparison: unexposed populations, including naturally conceived children, the general population, children not conceived via ART, and naturally conceived children of mothers with low fertility; O-outcome: the outcome of interest was the risk of childhood cancer; S-study design: our focus was on observational study reports, including cohort studies, case-control studies, and cross-sectional studies.
We used the following exclusion criteria (1): duplicates of literature or reports on the same cohort (2); studies with unavailable data (3); studies on the offspring of childhood cancer survivors (4); nonrelevant exposures, such as fertility medications (5); nonrelevant outcomes, such as birth defects and cardiovascular disease in children conceived via ART (6); nonrelevant study designs, such as intervention studies, randomized controlled trials, study protocols, reviews, commentaries, and case reports.
It is noteworthy that, as childhood cancer is a rare disease, several studies have indicated that a sample size of at least 20,000 children is required to observe a doubling of cancer risk within a cohort (31–33). In contrast, most existing large-sample studies (e.g., the Nordic ART registry) have typically included ≥4,000 children conceived via ART. A previous meta-analysis on this topic recommended a minimum sample size of 5,000 children conceived through ART; however, considering both statistical validity and data availability, a threshold of 4,000 was selected (24). This approach encompasses most high-quality studies while mitigating the confounding effects of heterogeneity due to small sample sizes.
Definition
ART is defined to include all interventions where human oocytes, sperm, or embryos are treated in vitro for reproductive purposes (34, 35), including artificial insemination, conventional IVF, ICSI, oocyte and embryo donation, and other forms of treatment; fertility drugs alone are not included in this study.
Childhood cancers are defined with reference to the International Classification of Childhood Cancer (ICCC-3) (36), and classified on the basis of the results of previous studies. The classification encompasses overall cancers, haematological malignancies, neural tumors, other solid tumors, and 11 specific cancers: leukemia [including acute lymphoblastic leukemia (ALL) and acute myelocytic leukemia (AML)], lymphoma, central nervous system tumors (CNS tumors), peripheral nervous cell tumors (including neuroblastoma and other peripheral nervous cell tumors), retinoblastoma, hepatic tumors, renal tumors, bone tumors and extraosseous sarcomas, germ cell tumors, embryonal tumors, and epithelial tumors and melanoma.
Study selection
Literature screening was conducted in two stages. Initially, two authors (JYJ and ZSP) imported the search results into EndNote X9, removed duplicates, and preliminarily selected studies by reviewing titles and abstracts on the basis of predefined inclusion and exclusion criteria. The second stage involved a detailed review of the full texts to confirm their suitability for the meta-analysis. Any disagreements during the screening process were resolved through discussions with a third author (WHY) until a consensus was reached.
Data extraction
Two researchers (JYJ and ZSP) independently extracted the data. Disputes were resolved by consulting a third researcher (WHY) or, if necessary, by contacting the study authors for clarification of unclear or missing information. Data on the following study characteristics were extracted: lead author, publication date, study design, geographic location, duration of study, years of follow-up, maternal age at conceiving, type of ART exposure, unexposed population, type of outcome cancer, number of cancers in exposed children/total number of exposed children, number of cancers in unexposed children/total number of unexposed children, RR, 95% confidence intervals, and a list of adjusted covariates. In cases of multiple publications on the same cohort, priority was given to selecting the most comprehensive and up-to-date combined study (with the longest follow-up or analysis covering the most participants).
Data synthesis and analysis
RR and 95% CI were used as summary indicators of associations. The hazard ratio (HR), odds ratio (OR), and standardized incidence ratio (SIR) are assumed to approximate the same RR metrics because of the lower incidence of childhood cancer. This study analyzed the combined RR via the DerSimonian–Laird random effects model. In addition, according to Wang et al. (16), for treatments that reported data on the risk of different types of cancer within the same category, the RR for the combined cancer category was calculated in pooled analyses using a fixed-effects model in the same study (for details, see Research Data Harmonization). We used the Cochran Q test and the I² statistic to evaluate heterogeneity among studies, with an I² value greater than 50% and a p-value less than 0.05 indicating substantial heterogeneity (37, 38). To assess the extent of heterogeneity, we estimated the 95% prediction interval, which is expected to contain the true effects of 95% of future studies (39, 40). In addition, when the number of studies reporting on a cancer outcome exceeded 10, a cumulative meta-analysis was conducted for that cancer to evaluate the accumulation of evidence over time (41, 42). Additionally, subgroup analyses were conducted using prespecified study-level characteristics, including geographic location, maternal age at conceiving, and various unexposed populations (naturally conceived children, the general population, children not conceived via ART, and children from mothers with low fertility who were naturally conceived), to identify significant factors contributing to the heterogeneity of the primary outcome for this cancer. To ensure the reliability of the naturally conceived population, the general population and children not conceived via ART were combined in the subgroup analyses. In addition, given that childhood cancers encompass various cancer types and ART comprises multiple therapy forms, these factors may also contribute to heterogeneity. Therefore, pooled analyses were performed for individual cancer types, and the associations between different ART types and childhood cancer risk were discussed (the number of studies reporting on some ART exceeded 5). We performed ‘leave-one-out’ sensitivity analyses by removing one study per iteration to examine the impact of individual studies on the overall effect (cancer outcomes with ≥ 5 studies). Funnel plots were visually inspected for publication bias, and Egger regression was used to detect potential publication bias (43); adjustments were made via the trim and fill method if significant bias was identified (44). A two-tailed P value of 0.05 was considered statistically significant.
Evaluation of study quality
The quality of the studies included in this meta-analysis was assessed via the Newcastle–Ottawa Scale (NOS) (45). The NOS comprises eight items across three dimensions—selection, comparability, and outcome—with each item scoring one point. Specifically, items concerning the control of confounders could receive up to two points. Studies were categorized as low (0–3), medium (4–6), or high (7–9) quality on the basis of their scores. In addition, based on the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) (46), this study assessed the quality of evidence for each outcome and categorized it as “high”, “moderate”, “low”, or “very low” quality to support the conclusions.
Software, data, and code availability
Statistical analyses were performed via Stata 16 (Stata Corp, College Station, Texas), and 95% prediction intervals were calculated via the R package “metamisc” in R Studio version (version 4.4.1).
Results
Literature search
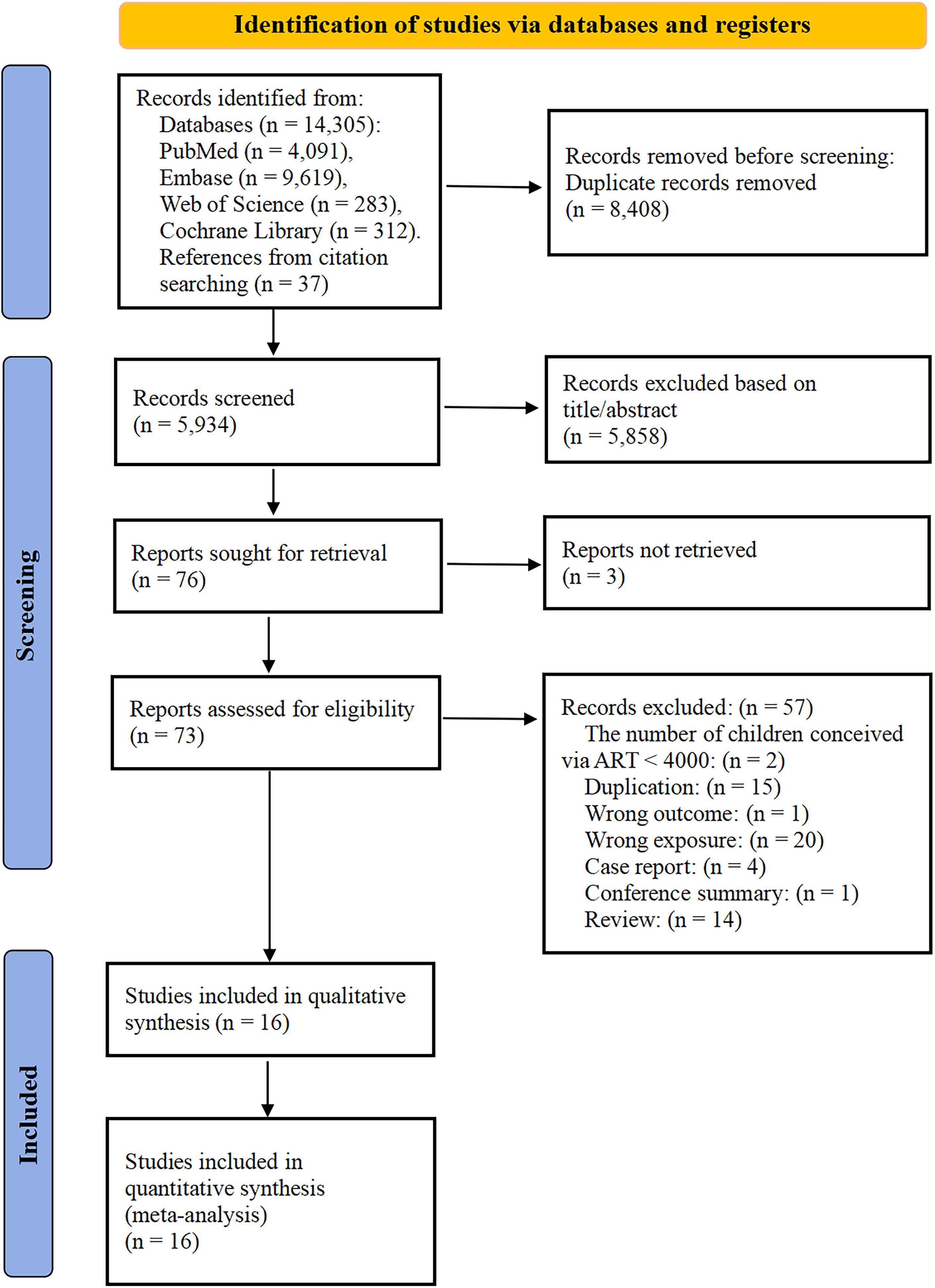
Figure 1 displayed the PRISMA flowchart for this study. A total of 14,342 potentially relevant articles were identified through comprehensive database and citation searches. After removing 8,408 duplicates, a total of 73 documents were deemed eligible for full-text review after screening the titles and abstracts. After further review, 57 records were excluded for the following reasons (eTable 1). Ultimately, 16 studies that reported on ART and childhood cancer risk were included in this meta-analysis.
Characteristics and quality of the included studies
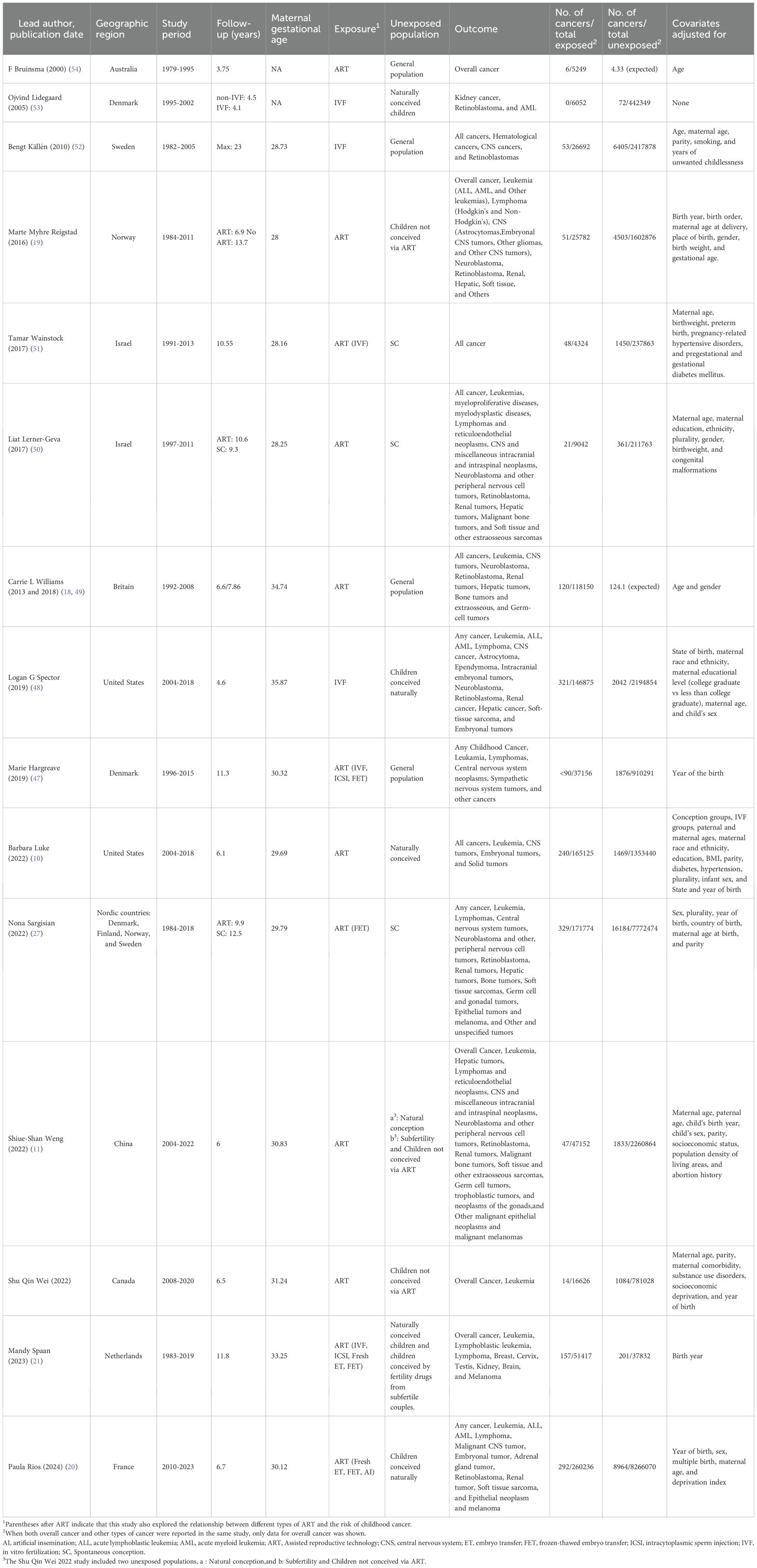
Table 1 summarized the study designs and demographic characteristics of the included articles, covering 16 large-scale observational studies, which were all classified as cohort studies (10, 11, 18–22, 27, 47–54).
This meta-analysis imposed no stringent publication date restrictions; however, included studies were published between 2000 and 2024. Nine studies originated from Europe (France, the Netherlands, the United Kingdom, Denmark, Finland, Norway, Sweden) (18–21, 27, 47, 49, 52, 53), three from Asia (China, Israel) (11, 50, 51), three from North America (United States, Canada) (10, 22, 48), and one from Oceania (Australia) (54). The average follow-up duration was 7.5 years, and the maternal age at conceiving ranged from 28 to 36 years. In this study, 1,091,652 children were born through ART, of whom 1,789 were diagnosed with cancer. Moreover, in the control group, 28,612,981 children were born, of whom 46,573 were diagnosed with cancer. These groups included children aged 0–18 years. Additionally, except for the studies by Wainstock and Bruinsma et al. (51, 54), the other studies provided detailed reports on the associations between ART and three types of cancers in children: haematological malignancies, neural tumors, and other solid tumors. Eight additional studies (10, 20, 21, 27, 47, 48, 51, 52) investigated the associations between various types of ART and the overall risk of cancer in children. Among the 16 cohort studies, the unexposed people in 8 studies were naturally conceived children (10, 11, 20, 27, 48, 50, 51, 53), 5 studies had general populations (18, 47, 49, 52, 54), 2 studies had children not conceived via ART (19, 22), and 2 studies had mixed populations (11, 21), including low-fertility but naturally reproducing individuals and children not conceived via ART. There were two groups of unexposed populations in the study by Weng et al. (11): one naturally conceiving population and one mixed population. All studies adjusted or matched for potential confounders when estimating the association between ART and childhood cancer risk, with the exception of six studies (18, 21, 47, 49, 53, 54) that differed in the extent of adjustment, as they adjusted only for confounders such as year of birth, age, and sex.
Among the cohort studies included in this study, 15 reported overall cancers, 14 reported haematological malignancies, 12 reported neural tumors, and 13 reported other solid tumors (eTable 2).
Additionally, the number of studies reporting on each specific cancer type was as follows: 12 studies reported on leukemia (3 on ALL and 4 on AML), 8 on lymphoma, 12 on CNS tumors, 8 on peripheral nervous cell tumors, 10 on retinoblastoma, 5 on hepatic tumors, 10 on renal tumors, 7 on bone tumors and extraosseous sarcomas, 2 on germ cell tumors, 3 on embryonal tumors, and 4 on epithelial tumors and melanoma (eTable 2).
Among the various types of ART, 5 studies have explored the association between IVF and the risk of childhood cancer compared to the general population and unexposed populations conceived naturally.
The 16 studies included in this meta-analysis had NOS scores ranging from 7 to 9. Therefore, the overall quality of this study was rated as high (eTable 3).
ART and overall risk of cancer
In the present study, we observed a 21% increase in overall cancer risk (RR = 1.21, 95% CI, 1.11–1.33) in children conceived via ART (Figure 2a). Heterogeneity was observed among the studies (I² = 56.08%, p = 0.004). We excluded five studies (18, 21, 47, 49, 54) that varied in the degree of adjustment for confounders, resulting in a pooled effect size of (RR = 1.26, 95% CI, 1.14–1.41) (eFigure Forest Plot 1). Despite this, heterogeneity among the included studies persisted (I² = 61.87%, p < 0.001). Excluding four studies, two (10, 20) that combined risk estimates for several types of ART and two (48, 52) that employed a single ART risk estimate instead of any ART risk estimate resulted in a pooled effect size of (RR = 1.22, 95% CI, 1.06–1.40) (eFigure Forest Plot 2), while significant heterogeneity persisted (I² = 65.77%, p < 0.001). A visual inspection of the funnel plots indicated that the distribution of studies was generally symmetrical, and Egger’s regression test revealed no potential publication bias (t = 1.18, p = 0.258) (eFigure Publication bias 1–3). The 95% prediction interval for the pooled analysis ranged from 1.09–1.75, indicating that the true RR for any given study typically lies within this range.
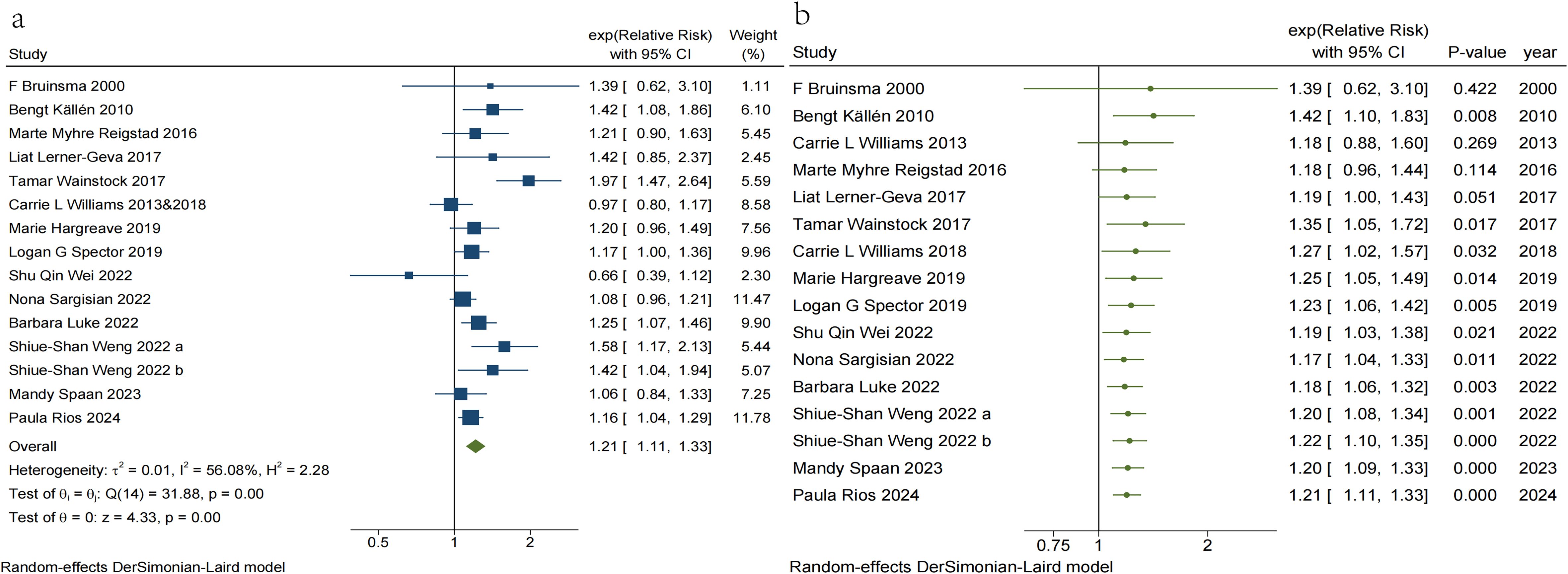
Figure 2. (a) Traditional meta-analysis of Risk of Overall cancer in children conceived via ART. (b) Cumulative meta-analysis of Risk of Overall cancer in children conceived via ART.
In addition, a cumulative meta-analysis based on year of publication demonstrated (Figure 2b) that the first evidence of an increased risk of overall childhood cancer with ART emerged in 2010, with combined RR values ranging from 1.17 to 1.42. However, in 2013, expanded data indicated that the risk association lacked statistical significance. Subsequent research published in 2017 reaffirmed the strong association between ART and overall childhood cancer risk. Although subsequent studies have enhanced the precision of point estimates, there has been no substantial change in the direction or magnitude of the risk association, which has largely stabilized.
ART and the risk of haematological malignancies, neural tumors and other solid tumors
Figure 3 illustrates the risk associations for haematological malignancies, neural tumors, and other solid tumors associated with ART, respectively. The pooled results indicated that the risk for haematological malignancies (RR = 1.16, 95% CI, 1.05–1.28), neural tumors (RR = 1.19, 95% CI, 1.07–1.32), and other solid tumors (RR = 1.48, 95% CI, 1.26–1.73) significantly increased in children conceived via ART. Heterogeneity was observed only in studies reporting other solid tumors (I² = 64.83%, p < 0.05), and cancer type may significantly influence heterogeneity. The visual funnel plots were symmetrical, and none of the Egger regression tests indicated potential publication bias in any category (haematological malignancies: t = 2.16, p = 0.052; neural tumors: t = -0.14, p = 0.891; other solid tumors: t = 1.54, p = 0.151) (see eTable 2, eFigure Publication bias 4–6 for details).
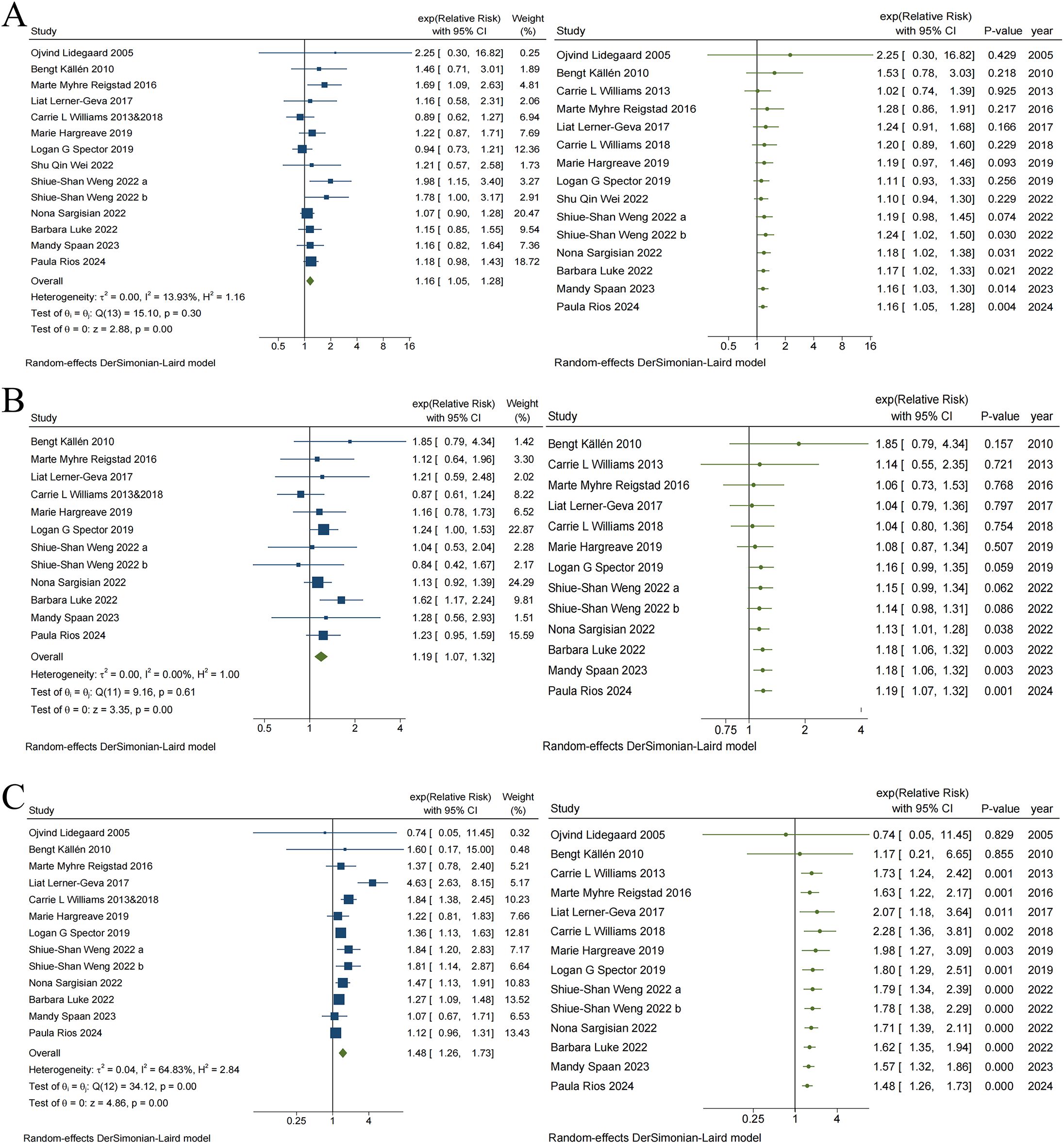
Figure 3. Traditional and Cumulative meta-analysis of Risk of Haematological malignancies (A), Neural tumors (B) and Other solid tumors (C) in children conceived via ART.
In addition, the cumulative meta-analysis indicated that evidence supporting an increased risk of haematological malignancies (Figure 3A) and neural tumors (Figure 3B) among children conceived via ART emerged in 2022, with combined RR values fluctuating between 1.02 to 2.25 and 1.04 to 1.85, respectively. Since 2022, there have been no substantial changes in the direction or magnitude of these risk associations, which have remained stable. In contrast, evidence of risk associations between ART and other solid tumors (Figure 3C), which emerged in 2013, ranged from 0.74 to 2.28 for the combined RR. Data from 2018 further reinforced the strength of this risk association. Subsequent studies increased the precision of the point estimates but did not alter the direction or magnitude of the association.
ART and the risk of specific cancers
eTable 2 summarizes the associations of risk between ART and specific childhood cancers. Six specific cancers exhibited a significantly increased risk, including leukemia (RR = 1.16, 95% CI, 1.03–1.32) (Figure 4A), CNS tumors (RR = 1.22, 95% CI, 1.09–1.38) (Figure 4B), retinoblastoma (RR = 1.74, 95% CI, 1.15–2.63) (Figure 4C), hepatic tumors (RR = 2.73, 95% CI, 1.90–3.91), bone tumors and extraosseous sarcomas (RR = 1.62, 95% CI, 1.26–2.07), and epithelial tumors and melanoma (RR = 1.67, 95% CI, 1.22–2.29). Additionally, there were also seven specific cancers for which the risk was not significantly associated with ART, including lymphoma (RR = 1.12, 95% CI, 0.90–1.39), ALL (RR = 1.14, 95% CI, 0.95–1.36), AML (RR = 1.25, 95% CI, 0.72–2.18), peripheral nervous cell tumors (RR = 1.01, 95% CI, 0.79–1.29), renal tumors (RR = 1.27, 95% CI, 0.98–1.65) (Figure 4D), germ cell tumors (RR = 0.60, 95% CI, 0.25–1.42), and embryonal tumors (RR = 1.14, 95% CI, 0.99–1.32). Heterogeneity was observed solely in the study of retinoblastoma (I² = 52.86%, p = 0.02), suggesting that different types of cancer might significantly influence heterogeneity. Visual inspection of the funnel plot revealed symmetry, and no publication bias was detected via Egger’s test (p > 0.05), with the exception of germ cell tumors (t = -5.23, p = 0.035) (eFigure Forest Plot 3–11, eFigure Publication bias 7–19).
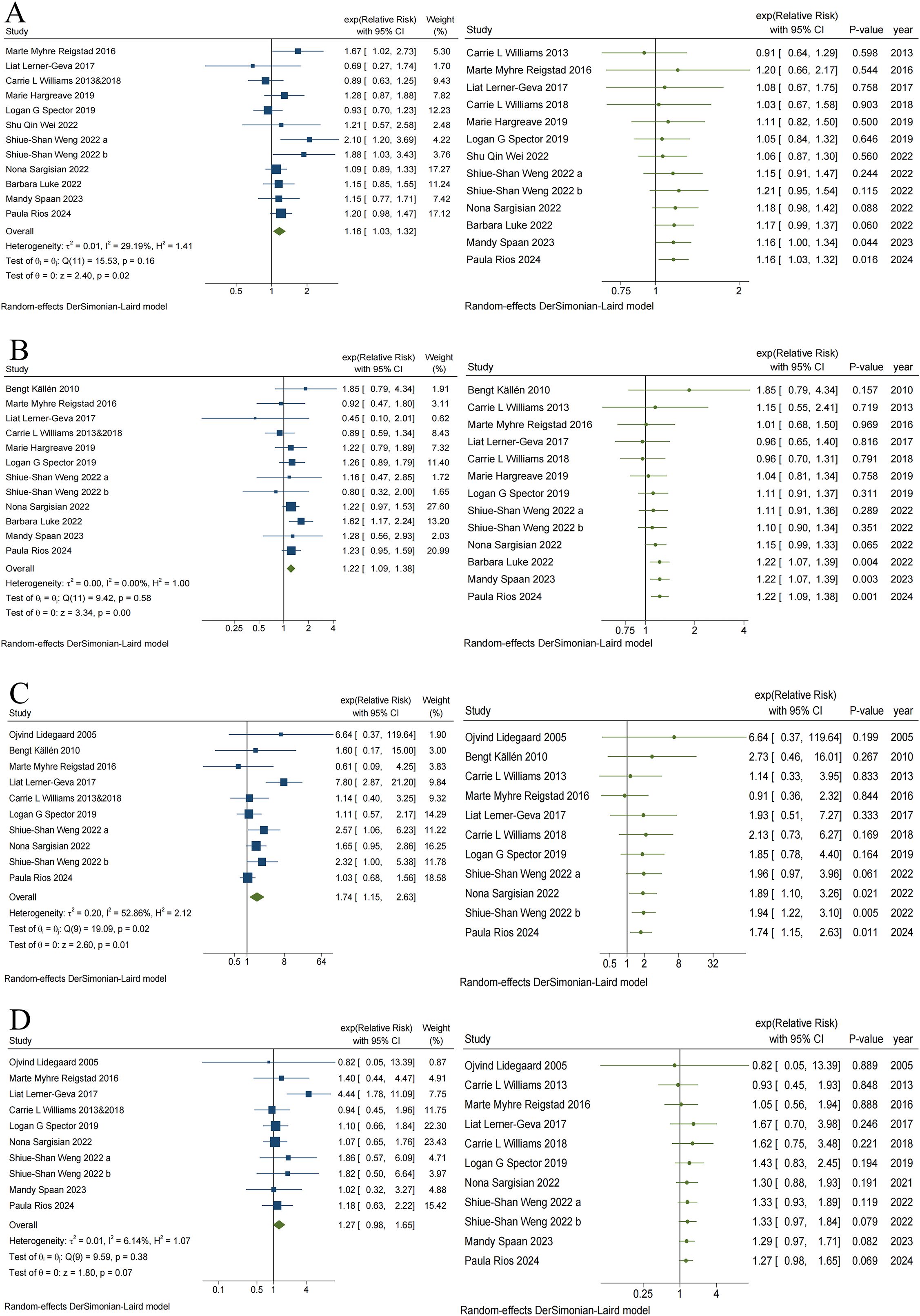
Figure 4. Traditional and Cumulative of Risk of Leukemia (A), CNS tumors (B), Retinoblastoma (C) and Renal tumors (D) in children conceived via ART.
The results of the cumulative meta-analysis on ART and leukemia are presented in Figure 4A. Evidence indicating a risk of leukemia in children conceived via ART first emerged in 2024, with combined RR values ranging from 0.91 to 1.21. Additionally, evidence indicating the risk of CNS tumors (Figure 4B) and retinoblastoma (Figure 4C) in children conceived via ART first emerged in 2022, with combined RR values ranging from 0.96 to 1.85 and 0.91 to 6.64, respectively. No significant changes in direction, estimation, or precision were observed in the cumulative meta-analysis of these three cancer outcomes with subsequent additions to the study data. However, the cumulative meta-analysis of ART and renal tumor risk (Figure 4D) indicates that the effect of ART on renal tumors continues to be insignificant, despite the addition of new data over time.
Subgroup analyses
In this study, subgroup stratification was carried out for overall cancers, haematological malignancies, neural tumors, other solid tumors, leukemia, CNS tumors, retinoblastoma, and renal tumors among children conceived via ART (eTable 4, Figure 5, and eFigure Subgroup Analysis Forest Plot 1–24).
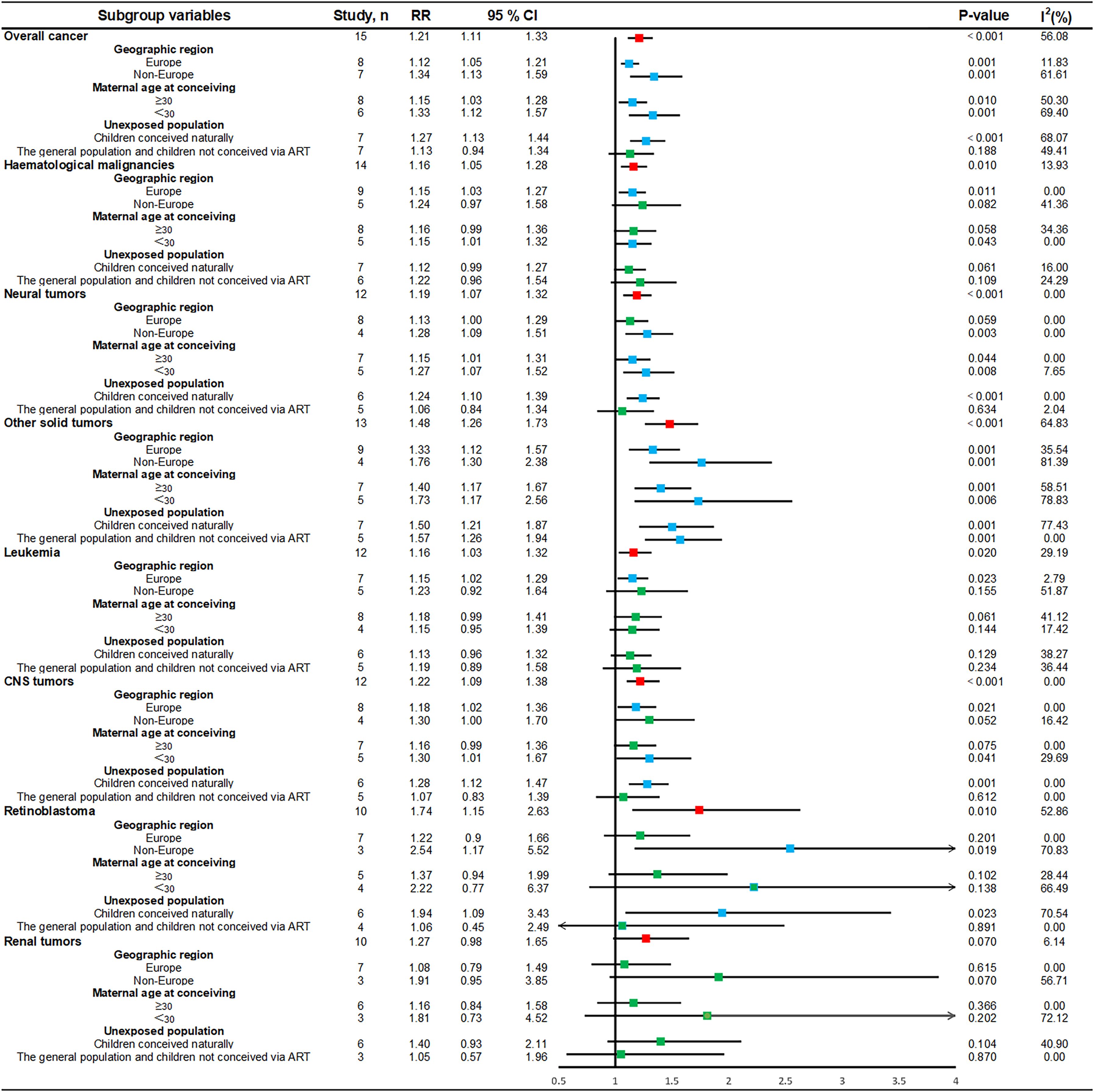
Figure 5. Subgroup forest plot of the association between ART and childhood cancer risk. CI, confidence interval; rr, relative risk; parallel lines, represent confidence intervals for individual effect sizes; vertical black line, invalid line of binary classification variable (1.00); red box, summary value of effect sizes for each type of cancer; green box, estimates of effect sizes that are currently not statistically significant; blue box, estimates of effect sizes that are statistically significant; I2, heterogeneity between studies within subgroups; p-value: p < 0.05 indicates statistical significance.
Stratified analyses by geographic region revealed a trend toward a significantly lower cancer risk for studies conducted in European countries, whereas non-European countries presented a significantly higher cancer risk ratio for overall cancers (RR = 1.34, 95% CI, 1.13–1.59), neural tumors (RR = 1.28, 95% CI, 1.09–1.51), other solid tumors (RR = 1.76, 95% CI, 1.30–2.38), and retinoblastoma (RR = 2.54, 95% CI, 1.17–5.52). Stratified analysis by maternal age at conceiving indicated a trend towards a reduced risk association between ART and childhood cancer when maternal age was ≥30 years, such as overall cancer (RR = 1.15, 95% CI, 1.03–1.28), neural tumors (RR = 1.15, 95% CI, 1.01–1.31), and other solid tumors (RR = 1.40, 95% CI, 1.17–1.67). In contrast, the risk associations tended to increase at maternal age at conceiving less than 30 years, as was observed for overall cancers (RR = 1.33, 95% CI, 1.12–1.57), neural tumors (RR = 1.27, 95% CI, 1.07–1.52), other solid tumors (RR = 1.73, 95% CI, 1.17–2.56), and CNS tumors (RR = 1.30, 95% CI, 1.01–1.67). Stratified by the unexposed population, there was a significantly increased risk ratio of cancer, such as overall cancer (RR = 1.27, 95% CI, 1.13–1.44), neural tumors (RR = 1.24, 95% CI, 1.10–1.39), other solid tumors (RR = 1.50, 95% CI, 1.21–1.87), CNS tumors (RR = 1.28, 95% CI, 1.12–1.47), and retinoblastoma (RR = 1.94, 95% CI, 1.09–3.43), and an increased risk of other solid tumors (RR = 1.57, 95% CI, 1.26–1.94), among children conceived via ART compared with the general population or children not conceived via ART.
Specific cancers often show a potentially positive associated risk in various types of stratified analyses; however, the rarity of these cancers makes conducting actual studies more challenging. In this regard, we cannot exclude the possibility that the elevated risk significance may not be detectable due to limited statistical power and an insufficient number of studies.
IVF and overall risk of cancer
This meta-analysis did not find conclusive evidence for an association between IVF and childhood cancer risk (RR = 1.28, 95% CI, 0.98–1.66) (eFigure Specific types of ART Forest Plot 1). Importantly, owing to high heterogeneity (I² = 81.14%, p < 0.001), which was significantly reduced (I² = 45.49%, p = 0.14) after we considered the confounding of small-sample studies and excluded the study with the lowest number of exposures with an IVF exposure of 4,324 (51), the combined effect sizes were (RR = 1.13, 95% CI, 0.96–1.32) (eFigure Specific types of ART Forest Plot 2), and conclusions remained unchanged. Both before and after the exclusion of studies, visual funnel plots remained symmetrical, and Egger’s test did not reveal publication bias (all p > 0.05) (eFigure Publication bias 20–21).
Sensitivity analysis
In a sensitivity analysis, this study evaluated the impact of various exclusion criteria on overall cancer risk estimates for children conceived via ART (eFigure sensitivity analysis 2–3). The results remained largely unchanged by the exclusion of either the five studies with differing confounder adjustments or the four studies that adjusted for ART exposure. Additionally, not a single study influenced the risk outcomes for overall cancer, haematological malignancies, neural tumors, other solid tumors, or specific cancers among children conceived via ART, confirming the robustness of the results (eFigure sensitivity analysis 4-14).
Evidence appraisal using GRADE
In contrast to randomized controlled studies, observational studies are usually graded up or down from Grade C due to deficiencies in study design. For detailed evaluation procedures, see etable 5. Most of the results of the current study had an evidence grade of C, especially retinoblastoma, hepatic tumors, bone tumors and extraosseous sarcomas, which had a grade of B. Overall, the GRADE scores indicate that the level of evidence for the results of the current study is of high quality among observational studies and can effectively support the risk association between ART and childhood cancer.
Discussion
To our knowledge, this is the largest and most comprehensive meta-analysis to explore the association between ART and childhood cancer risk, utilizing a large sample from observational studies. This meta-analysis, which involved 30 million children, demonstrated that ART was associated with an increased risk of overall cancer in children (RR = 1.21, 95% CI, 1.11–1.33) compared with natural conception, the general population, children not conceived via ART, or the unexposed population with low fertility but natural conception. Specifically, the risks for hematological malignancies (RR = 1.16, 95% CI, 1.05–1.28), neural tumors (RR = 1.19, 95% CI, 1.07–1.32), other solid tumors (RR = 1.48, 95% CI, 1.26–1.73), and certain specific cancers including leukemia, CNS tumors, retinoblastoma, hepatic tumors, bone tumors and extraosseous sarcomas, and epithelial tumors and melanomas, were significantly elevated. In addition, the findings suggested that different types of specific cancers might significantly influence the heterogeneity of the primary outcome. A further outcome of our research, a cumulative meta-analysis based on time lapses, revealed initial associations between ART and other solid tumors as early as 2013, with new cancer type risks emerging in 2022. Although subsequent studies have refined our estimates, no significant shifts in the direction or magnitude of risk associations have been observed, indicating stable outcomes. The data consistently support a robust link between ART and childhood cancer risk. However, we cannot rule out potential confounders such as the effects of ART and underlying parental infertility, which may contribute to increased cancer risk in offspring. Information on procedures such as IVF and ICSI (55), along with pertinent time-varying covariates during treatment (56), will aid in further clarifying the mechanisms underlying this association. Furthermore, this association remained consistent across subgroup analyses involving geographic regions, maternal age at conceiving, and populations of unexposed groups. Notably, women younger than 30 years who used ART might have had a higher risk ratio of cancer in their offspring (RR age<30 = 1.33, RR age≥30 = 1.15) highlighting the importance of promoting natural conception among younger women. These findings provide valuable insights for future research, clinical practice, and advice for couples considering ART.
Our results contradict those of a 2019 meta-analysis by Gilboa et al. (24), which reported no significant association between ART and childhood cancer risk (RR = 0.99; 95% CI, 0.85–1.15). This discrepancy may stem from Gilboa’s inclusion of data from 2018 case-control studies (57), potentially introducing bias into the pooled results. Conversely, Wang et al.’s (16) meta-analysis of observational studies from the same year, which explored fertility treatments and childhood cancer risk, aligned closely with our findings, with RRs for overall cancer, haematological malignancies, and other solid tumors in offspring of 1.16, 1.39, and 1.57, respectively. Nevertheless, their study concluded that the association between ART and the risk of neural tumors was not significant (RR = 1.15, 95% CI, 0.89–1.47), a finding that contradicts our conclusions. This discrepancy could be attributed to the inclusion of inconsistent study types and a greater number of small cohort studies (17, 31, 58, 59). In contrast, our study utilized more comprehensive and representative data, comprising 1,091,652 children conceived via ART and 1,789 childhood cancer cases, nearly threefold the number included in the 29 observational studies, which featured 327,884 children conceived through fertility treatments and 578 childhood cancer cases. In the 2020 study by Zhang et al. (25), which explored various fertility treatments and their risk for offspring cancer, our IVF findings aligned with their conclusions, revealing no evidence of an increased childhood cancer risk associated with IVF. Considering the scarcity of research on various ART types and their associations with childhood cancer risk, along with the very low incidence of cancer, the inclusion of numerous studies with small sample sizes and zero cancer cases in the exposure group in meta-analyses can exacerbate confounding issues, potentially leading to unreliable outcomes (60). Therefore, we advocate the inclusion of large studies to more accurately determine the actual risk association between ART and childhood cancer.
Despite pooling and analyzing data from a large sample of studies, we encountered the same issues as those reported by Wang et al. It remains unclear whether factors related to the ART implementation process or underlying parental fertility issues contribute to the increased risk of childhood cancers. The mechanisms underlying ART’s increased risk of childhood cancers remain largely unproven, with current hypotheses suggesting epigenetic disorders as a potentially influential pathway (61–63). Dynamic epigenetic regulation of gene imprinting, governed by both DNA methylation-dependent and DNA methylation-independent mechanisms, dictates the expression of specific genes on the basis of parental origin (64). Recent studies (65, 66) have established a connection between abnormal gene imprinting and the onset of childhood cancers, including retinoblastoma and neuroblastoma. Studies (67, 68) indicate that ART encompasses various stages of conception, ranging from gamete production stimulation to embryo in vitro culture, which includes processes such as ovarian stimulation, in vitro oocyte maturation, and gamete or embryo cryopreservation. Each step of the ART process has the potential to disrupt the normal genetic imprinting process, thereby increasing the risk of adverse pregnancy and neonatal outcomes (7, 69). Research conducted by Luke et al. (10) identified an increased cancer risk in children conceived via ART as well as their siblings who were not, suggesting that shared genetic or environmental factors may influence cancer susceptibility. In addition, infertility itself might increase epigenetic risk in children (70). Research (71) suggested that children born to mothers facing fertility challenges are at increased risk of developing cancer during childhood and early adulthood. Moreover, the largest cohort study in Asia, conducted by Weng et al. (11), indicated that this increased cancer risk may stem more from ART than from underlying fertility issues.
Existing studies have demonstrated heterogeneous results for the association between ART and childhood cancer risk. Both this study and the meta-analysis by Wang et al. indicated that the association of some cancer types with ART failed to reach statistical significance (72), potentially due to the low incidence of rare tumors leading to insufficient statistical power. However, several cohort studies have identified potential trends in risk for specific tumor types. A large cohort study in the United Kingdom, with a follow-up period of up to 17 years, observed no increase in overall cancer risk in ART-conceived children, but noted an increased risk of hepatoblastoma and rhabdomyosarcoma (18). This result is in alignment with the observed trend of increased risk of liver tumors in the US cohort (48). Conversely, the study across four Nordic countries highlighted an increased risk of central nervous system tumors and malignant epithelial tumors (73). Importantly, data from the Danish 10 million cohort revealed an elevated risk of leukemia, sympathetic nervous system tumors, and epithelial tumors and melanoma in FET-conceived children compared to naturally-conceived children, although these associations must be interpreted with caution due to the limited number of cases (n<5) (27, 47). Notably, the association of leukemia, the most common malignancy in children, with ART has demonstrated a relatively stable trend of elevated risk in multicenter studies (19, 20). The variability of the available evidence may be attributed to (1) confounding effects of underlying infertility and its associated pathologic states (2), biological differences in various ART technology options (e.g., IVF, FET), and (3) disparities in the completeness of cancer registry systems across geographic regions. Future studies should establish mega-sample cohorts through international multicenter collaborations and implement uniform exposure classification criteria to elucidate the causal associations and potential biological mechanisms between specific cancer types and ART.
We recommend cross-linking future international (non-national) registries that report outcomes after ART treatment with cancer registries. Additionally, these registries should record a specific set of variables associated with increased cancer risk, such as type of infertility, duration of infertility, mother’s age at conception, pregnancy cycle, primary versus secondary infertility, male infertility, parental smoking status, mother’s BMI, fetal growth/birth weight, and other relevant factors. To draw definitive conclusions, reliable databases are needed to avoid the risk of false or premature alarms, which can have detrimental consequences in couples’ counseling.
Strengths and limitations
The key strengths of this evaluation include the following (1): Given the low prevalence of childhood cancer, the inclusion of a large dataset comprising 30 million childhood participants, 50,000 of whom had childhood cancer, enhances the statistical testing capabilities. This approach reduces potential confounders and minimizes small-sample bias, enabling more precise assessment of risk associations between ART and childhood cancer (2). All included studies are high-quality cohort studies rigorously assessed for their methodology, with risk of bias assessments and sensitivity analyses ensuring the credibility and robustness of findings (3). Time-cumulative meta-analysis was used to dynamically assess the trend between ART and childhood cancer risk, with consistent results confirming the positive risk association (4). Childhood cancers often develop before age 4 (74). A key strength of this study is the average follow-up period of 7.5 years, which enhances our ability to observe trends in overall and specific childhood cancer risks (5). The study explored various subgroup levels and ART types. Interestingly, the association with cancer risk in children conceived via ART was more pronounced when the mother was under 30 years of age at delivery, highlighting the importance of hierarchical analysis to complement the results. Overall, the results of this study are more comprehensive and robust than those of previous studies with smaller samples.
While the current meta-analysis has certain limitations, these limitations are primarily intrinsic to the individual studies included rather than to the meta-analytical methodology itself (1). The absence of specific ART-related details such as ovarian stimulation regimens and the limited number of unexposed people with low fertility but natural reproduction in the studies included in the current meta-analysis may interfere with an accurate assessment of the association between ART and the risk of cancer outcomes (2). The included studies were all cohort designs, reducing selection bias and categorization errors, but were also prone to missing-follow-up bias. The absence of case-control and cross-sectional studies might affect the completeness and certainty of the results (3). Additionally, the observational study design has deficiencies and requires further improvement. For example, controlling for concordance in fertility levels between exposed and non-exposed populations is essential to fully explore the risk association between ART and childhood cancer (4). We acknowledged that although all the studies included in this analysis were large cohort studies, the number of cases involving certain specific rare cancers was still small. This limitation affected the statistical precision of these estimates, and therefore caution needs to be exercised in interpreting the results, particularly with data on specific types of cancers (5). The associations between specific ART and various childhood cancers could not be thoroughly investigated due to the scarcity of extensive studies that uniformly reported on individual ART and particular types of cancer.
Conclusions
Our meta-analysis, which involved high-quality observational studies with large samples, revealed a significant association between ART and increased risks of childhood cancer overall, haematological malignancies, neural tumors, other solid tumors, and six specific cancers. The results of the cumulative meta-analysis indicated that the direction and magnitude of the effects on these outcomes were largely consistent over time. These findings suggest that infertile couples considering ART should be aware of the potential increased cancer risk to their children.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author/s.
Author contributions
YJ: Data curation, Investigation, Methodology, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. QD: Data curation, Methodology, Validation, Visualization, Writing – review & editing. SZ: Conceptualization, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. HW: Conceptualization, Formal Analysis, Methodology, Software, Visualization, Writing – original draft, Writing – review & editing. XH: Conceptualization, Project administration, Resources, Validation, Writing – original draft, Writing – review & editing. RF: Data curation, Investigation, Resources, Software, Supervision, Writing – original draft. JT: Conceptualization, Supervision, Visualization, Writing – original draft, Writing – review & editing. QF: Formal Analysis, Supervision, Visualization, Writing – original draft, Writing – review & editing. QZ: Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. YL: Conceptualization, Funding acquisition, Resources, Software, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by National Natural Science Foundation of China (81804022).
Acknowledgments
Thank you for the great contribution to the field included in the article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1555420/full#supplementary-material
Abbreviations
AI, artificial insemination; ALL, Acute lymphoblastic leukemia; AML, Acute myelocytic leukemia; ART, Assisted reproductive technology; CI, Confidence interval; CNS tumors, central nervous system tumors; ET, embryo transfer; FET, frozen ET; HR, hazard ratio; ICSI, Intracytoplasmic sperm injection; IVF, In vitro fertilization; NOS, Newcastle Ottawa Scale; OR, Odds ratio; RR, Relative risk; SIR, Standardized incidence ratios.
References
1. Smeenk J, Wyns C, De Geyter C, Kupka M, Bergh C, Cuevas Saiz I, et al. ART in Europe, 2019: results generated from European registries by ESHRE†. Hum Reprod (Oxford England). (2023) 38:2321–38. doi: 10.1093/humrep/dead197
2. Inhorn MC and Patrizio P. Infertility around the globe: new thinking on gender, reproductive technologies and global movements in the 21st century. Hum Reprod update. (2015) 21:411–26. doi: 10.1093/humupd/dmv016
3. Ofosu-Budu D and Hanninen V. Correction to: Living as an infertile woman: the case of southern and northern Ghana. Reprod Health. (2020) 17:198. doi: 10.1186/s12978-020-01053-z
4. Calhaz-Jorge C, De Geyter CH, Kupka MS, Wyns C, Mocanu E, Motrenko T, et al. Survey on ART and IUI: legislation, regulation, funding and registries in European countries: The European IVF-monitoring Consortium (EIM) for the European Society of Human Reproduction and Embryology (ESHRE). Hum Reprod Open. (2020) 2020:hoz044. doi: 10.1093/hropen/hoz044
5. Levi-Setti PE and Patrizio P. Assisted reproductive technologies (ART) and childhood cancer: is the risk real? J assisted Reprod Genet. (2018) 35:1773–5. doi: 10.1007/s10815-018-1274-6
6. Kissin DM, Jamieson DJ, and Barfield WD. Monitoring health outcomes of assisted reproductive technology. New Engl J Med. (2014) 371:91–3. doi: 10.1056/NEJMc1404371
7. Hansen M, Kurinczuk JJ, Milne E, de Klerk N, and Bower C. Assisted reproductive technology and birth defects: a systematic review and meta-analysis. Hum Reprod update. (2013) 19:330–53. doi: 10.1093/humupd/dmt006
8. Qin J, Liu X, Sheng X, Wang H, and Gao S. Assisted reproductive technology and the risk of pregnancy-related complications and adverse pregnancy outcomes in singleton pregnancies: a meta-analysis of cohort studies. Fertility sterility. (2016) 105:73–85.e1-6. doi: 10.1016/j.fertnstert.2015.09.007
9. Pinborg A, Wennerholm UB, and Bergh C. Long-term outcomes for children conceived by assisted reproductive technology. Fertility sterility. (2023) 120:449–56. doi: 10.1016/j.fertnstert.2023.04.022
10. Luke B, Brown MB, Wantman E, Schymura MJ, Browne ML, Fisher SC, et al. The risks of birth defects and childhood cancer with conception by assisted reproductive technology. Hum Reprod (Oxford England). (2022) 37:2672–89. doi: 10.1093/humrep/deac196
11. Weng SS, Huang YT, Huang YT, Li YP, and Chien LY. Assisted reproductive technology and risk of childhood cancers. JAMA network Open. (2022) 5:e2230157. doi: 10.1001/jamanetworkopen.2022.30157
12. Bhattarai KR, Mobley RJ, Barnett KR, Ferguson DC, Hansen BS, Diedrich JD, et al. Investigation of inherited noncoding genetic variation impacting the pharmacogenomics of childhood acute lymphoblastic leukemia treatment. Nat Commun. (2024) 15:3681. doi: 10.1038/s41467-024-48124-4
13. Okun N and Sierra S. Pregnancy outcomes after assisted human reproduction. J obstetrics gynaecology Canada: JOGC = J d’obstetrique gynecologie du Canada: JOGC. (2014) 36:64–83. doi: 10.1016/S1701-2163(15)30685-X
14. Zhang S, Luo Q, Meng R, Yan J, Wu Y, and Huang H. Long-term health risk of offspring born from assisted reproductive technologies. J assisted Reprod Genet. (2024) 41:527–50. doi: 10.1007/s10815-023-02988-5
15. Hargreave M, Jensen A, Toender A, Andersen KK, and Kjaer SK. Fertility treatment and childhood cancer risk: a systematic meta-analysis. Fertility sterility. (2013) 100:150–61. doi: 10.1016/j.fertnstert.2013.03.017
16. Wang T, Chen L, Yang T, Wang L, Zhao L, Zhang S, et al. Cancer risk among children conceived by fertility treatment. Int J cancer. (2019) 144:3001–13. doi: 10.1002/ijc.32062
17. Doyle P, Bunch KJ, Beral V, and Draper GJ. Cancer incidence in children conceived with assisted reproduction technology. Lancet (London England). (1998) 352:452–3. doi: 10.1016/S0140-6736(05)79186-8
18. Williams CL, Bunch KJ, Stiller CA, Murphy MF, Botting BJ, Wallace WH, et al. Cancer risk among children born after assisted conception. New Engl J Med. (2013) 369:1819–27. doi: 10.1056/NEJMoa1301675
19. Reigstad MM, Larsen IK, Myklebust T, Robsahm TE, Oldereid NB, Brinton LA, et al. Risk of cancer in children conceived by assisted reproductive technology. Pediatrics. (2016) 137:e20152061. doi: 10.1542/peds.2015-2061
20. Rios P, Herlemont P, Fauque P, Lacour B, Jouannet P, Weill A, et al. Medically assisted reproduction and risk of cancer among offspring. JAMA network Open. (2024) 7:e249429. doi: 10.1001/jamanetworkopen.2024.9429
21. Spaan M, Pontesilli M, van den Belt-Dusebout AW, Burger CW, van den Heuvel-Eibrink MM, Ravelli ACJ, et al. Cancer risk in children, adolescents, and young adults conceived by ART in 1983-2011. Hum Reprod Open. (2023) 2023:hoad027. doi: 10.1093/hropen/hoad027
22. Wei SQ, Luu TM, Bilodeau-Bertrand M, and Auger N. Assisted reproductive technology and childhood morbidity: a longitudinal cohort study. Fertility sterility. (2022) 118:360–8. doi: 10.1016/j.fertnstert.2022.04.025
23. Raimondi S, Pedotti P, and Taioli E. Meta-analysis of cancer incidence in children born after assisted reproductive technologies. Br J cancer. (2005) 93:1053–6. doi: 10.1038/sj.bjc.6602838
24. Gilboa D, Koren G, Barer Y, Katz R, Rotem R, Lunenfeld E, et al. Assisted reproductive technology and the risk of pediatric cancer: A population based study and a systematic review and meta analysis. Cancer Epidemiol. (2019) 63:101613. doi: 10.1016/j.canep.2019.101613
25. Zhang Y, Gao R, Chen H, Xu W, Yang Y, Zeng X, et al. The association between fertility treatments and the incidence of paediatric cancer: A systematic review and meta-analysis. Eur J Cancer (Oxford England: 1990). (2020) 138:133–48. doi: 10.1016/j.ejca.2020.08.001
26. Chiavarini M, Ostorero A, Naldini G, and Fabiani R. Cancer risk in children and young adults (Offspring) born after medically assisted reproduction: A systematic review and meta-analysis. J (2019) 2:430–48. doi: 10.3390/j2040028
27. Sargisian N, Lannering B, Petzold M, Opdahl S, Gissler M, Pinborg A, et al. Cancer in children born after frozen-thawed embryo transfer: A cohort study. PloS Med. (2022) 19:e1004078. doi: 10.1371/journal.pmed.1004078
28. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (Clinical Res ed). (2021) 372:n71. doi: 10.1136/bmj.n71
29. Dekkers OM, Vandenbroucke JP, Cevallos M, Renehan AG, Altman DG, and Egger M. COSMOS-E: Guidance on conducting systematic reviews and meta-analyses of observational studies of etiology. PloS Med. (2019) 16:e1002742. doi: 10.1371/journal.pmed.1002742
30. Morgan RL, Whaley P, Thayer KA, and Schünemann HJ. Identifying the PECO: A framework for formulating good questions to explore the association of environmental and other exposures with health outcomes. Environ Int. (2018) 121:1027–31. doi: 10.1016/j.envint.2018.07.015
31. Lerner-Geva L, Toren A, Chetrit A, Modan B, Mandel M, Rechavi G, et al. The risk for cancer among children of women who underwent in vitro fertilization. Cancer. (2000) 88:2845–7. doi: 10.1002/1097-0142(20000615)88:12<2845::AID-CNCR26>3.0.CO;2-E
32. Bal MH, Harlev A, Sergienko R, Levitas E, Har-Vardi I, Zeadna A, et al. Possible association between in vitro fertilization technologies and offspring neoplasm. Fertility sterility. (2021) 116:105–13. doi: 10.1016/j.fertnstert.2020.12.013
33. Kaatsch P. Epidemiology of childhood cancer. Cancer Treat Rev. (2010) 36:277–85. doi: 10.1016/j.ctrv.2010.02.003
34. Zegers-Hochschild F, Adamson GD, de Mouzon J, Ishihara O, Mansour R, Nygren K, et al. International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary of ART terminology, 2009. Fertility sterility. (2009) 92:1520–4. doi: 10.1016/j.fertnstert.2009.09.009
35. Zegers-Hochschild F, Adamson GD, Dyer S, Racowsky C, de Mouzon J, Sokol R, et al. The international glossary on infertility and fertility care, 2017. Fertility sterility. (2017) 108:393–406. doi: 10.1093/humrep/dex234
36. Steliarova-Foucher E, Stiller C, Lacour B, and Kaatsch P. International Classification of Childhood Cancer, third edition. Cancer. (2005) 103:1457–67. doi: 10.1002/cncr.v103:7
37. Higgins JP and Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21:1539–58. doi: 10.1002/sim.v21:11
38. Higgins JP, Thompson SG, Deeks JJ, and Altman DG. Measuring inconsistency in meta-analyses. BMJ (Clinical Res ed). (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
39. Riley RD, Higgins JP, and Deeks JJ. Interpretation of random effects meta-analyses. BMJ (Clinical Res ed). (2011) 342:d549. doi: 10.1136/bmj.d549
40. Higgins JP, Thompson SG, and Spiegelhalter DJ. A re-evaluation of random-effects meta-analysis. J R Stat Soc Ser A (Statistics Society). (2009) 172:137–59. doi: 10.1111/j.1467-985X.2008.00552.x
41. Kulinskaya E and Koricheva J. Use of quality control charts for detection of outliers and temporal trends in cumulative meta-analysis. Res synthesis Methods. (2010) 1:297–307. doi: 10.1002/jrsm.v1.3/4
42. Muellerleile P and Mullen B. Sufficiency and stability of evidence for public health interventions using cumulative meta-analysis. Am J Public Health. (2006) 96:515–22. doi: 10.2105/AJPH.2003.036343
43. Egger M, Davey Smith G, Schneider M, and Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical Res ed). (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
44. Mavridis D and Salanti G. How to assess publication bias: funnel plot, trim-and-fill method and selection models. Evidence-Based Ment Health. (2014) 17:30. doi: 10.1136/eb-2013-101699
45. Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available online at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (Accessed September 3, 2024).
46. Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. (2011) 64:383–94. doi: 10.1016/j.jclinepi.2010.04.026
47. Hargreave M, Jensen A, Hansen MK, Dehlendorff C, Winther JF, Schmiegelow K, et al. Association between fertility treatment and cancer risk in children. Jama. (2019) 322:2203–10. doi: 10.1001/jama.2019.18037
48. Spector LG, Brown MB, Wantman E, Letterie GS, Toner JP, Doody K, et al. Association of in vitro fertilization with childhood cancer in the United States. JAMA pediatrics. (2019) 173:e190392. doi: 10.1001/jamapediatrics.2019.0392
49. Williams CL, Bunch KJ, Murphy MFG, Stiller CA, Botting BJ, Wallace WH, et al. Cancer risk in children born after donor ART. Hum Reprod (Oxford England). (2018) 33:140–6. doi: 10.1093/humrep/dex333
50. Lerner-Geva L, Boyko V, Ehrlich S, Mashiach S, Hourvitz A, Haas J, et al. Possible risk for cancer among children born following assisted reproductive technology in Israel. Pediatr Blood Cancer. (2017) 64. doi: 10.1002/pbc.26292
51. Wainstock T, Walfisch A, Shoham-Vardi I, Segal I, Harlev A, Sergienko R, et al. Fertility treatments and pediatric neoplasms of the offspring: results of a population-based cohort with a median follow-up of 10 years. Am J obstetrics gynecology. (2017) 216:314.e1–.e14. doi: 10.1016/j.ajog.2017.01.015
52. Källén B, Finnström O, Lindam A, Nilsson E, Nygren KG, and Olausson PO. Cancer risk in children and young adults conceived by in vitro fertilization. Pediatrics. (2010) 126:270–6. doi: 10.1542/peds.2009-3225
53. Lidegaard O, Pinborg A, and Andersen AN. Imprinting diseases and IVF: Danish National IVF cohort study. Hum Reprod (Oxford England). (2005) 20:950–4. doi: 10.1093/humrep/deh714
54. Bruinsma F, Venn A, Lancaster P, Speirs A, and Healy D. Incidence of cancer in children born after in-vitro fertilization. Hum Reprod (Oxford England). (2000) 15:604–7. doi: 10.1093/humrep/15.3.604
55. Hargreave M. Cancer risk among children born after fertility treatment. JAMA network Open. (2024) 7:e249435. doi: 10.1001/jamanetworkopen.2024.9435
56. Buck Louis GM, Schisterman EF, Dukic VM, and Schieve LA. Research hurdles complicating the analysis of infertility treatment and child health. Hum Reprod (Oxford England). (2005) 20:12–8. doi: 10.1093/humrep/deh542
57. Gilboa D, Koren G, Katz R, Rotem R, and Lunenfeld EJF. Assisted reproductive technology is not associated with an increased risk of pediatric cancer. Sterilit. (2018) 110:e16–e7. doi: 10.1016/j.fertnstert.2018.07.064
58. Bradbury BD and Jick H. In vitro fertilization and childhood retinoblastoma. Br J Clin Pharmacol. (2004) 58:209–11. doi: 10.1111/j.1365-2125.2004.02109.x
59. Hargreave M, Jensen A, Nielsen TS, Colov EP, Andersen KK, Pinborg A, et al. Maternal use of fertility drugs and risk of cancer in children–a nationwide population-based cohort study in Denmark. Int J cancer. (2015) 136:1931–9. doi: 10.1002/ijc.29235
60. Lin L. Bias caused by sampling error in meta-analysis with small sample sizes. PloS One. (2018) 13:e0204056. doi: 10.1371/journal.pone.0204056
61. Chen W, Peng Y, Ma X, Kong S, Tan S, Wei Y, et al. Integrated multi-omics reveal epigenomic disturbance of assisted reproductive technologies in human offspring. EBioMedicine. (2020) 61:103076. doi: 10.1016/j.ebiom.2020.103076
62. Neelanjana M and Sabaratnam A. Malignant conditions in children born after assisted reproductive technology. Obstetrical gynecological survey. (2008) 63:669–76. doi: 10.1097/OGX.0b013e318181a9f0
63. Whitelaw N, Bhattacharya S, Hoad G, Horgan GW, Hamilton M, and Haggarty P. Epigenetic status in the offspring of spontaneous and assisted conception. Hum Reprod (Oxford England). (2014) 29:1452–8. doi: 10.1093/humrep/deu094
64. Zhang G, Mao Y, Zhang Y, Huang H, and Pan J. Assisted reproductive technology and imprinting errors: analyzing underlying mechanisms from epigenetic regulation. Hum fertility (Cambridge England). (2023) 26:864–78. doi: 10.1080/14647273.2023.2261628
65. Manipalviratn S, DeCherney A, and Segars J. Imprinting disorders and assisted reproductive technology. Fertility sterility. (2009) 91:305–15. doi: 10.1016/j.fertnstert.2009.01.002
66. Paulsen M and Ferguson-Smith AC. DNA methylation in genomic imprinting, development, and disease. J pathology. (2001) 195:97–110. doi: 10.1002/path.890
67. Barberet J, Romain G, Binquet C, Guilleman M, Bruno C, Ginod P, et al. Do frozen embryo transfers modify the epigenetic control of imprinted genes and transposable elements in newborns compared with fresh embryo transfers and natural conceptions? Fertility sterility. (2021) 116:1468–80. doi: 10.1016/j.fertnstert.2021.08.014
68. Jiang Z, Wang Y, Lin J, Xu J, Ding G, and Huang H. Genetic and epigenetic risks of assisted reproduction. Best Pract Res Clin obstetrics gynaecology. (2017) 44:90–104. doi: 10.1016/j.bpobgyn.2017.07.004
69. Buckett WM, Chian RC, Holzer H, Dean N, Usher R, and Tan SL. Obstetric outcomes and congenital abnormalities after in vitro maturation, in vitro fertilization, and intracytoplasmic sperm injection. Obstetrics gynecology. (2007) 110:885–91. doi: 10.1097/01.AOG.0000284627.38540.80
70. Barberet J, Binquet C, Guilleman M, Romain G, Bruno C, Martinaud A, et al. Does underlying infertility in natural conception modify the epigenetic control of imprinted genes and transposable elements in newborns? Reprod biomedicine Online. (2022) 44:706–15. doi: 10.1016/j.rbmo.2022.01.004
71. Hargreave M, Jensen A, Deltour I, Brinton LA, Andersen KK, and Kjaer SK. Increased risk for cancer among offspring of women with fertility problems. Int J cancer. (2013) 133:1180–6. doi: 10.1002/ijc.28110
72. Abbas-Aghababazadeh F, Mo Q, and Fridley BL. Statistical genomics in rare cancer. Semin Cancer Biol. (2020) 61:1–10. doi: 10.1016/j.semcancer.2019.08.021
73. Sundh KJ, Henningsen AK, Källen K, Bergh C, Romundstad LB, Gissler M, et al. Cancer in children and young adults born after assisted reproductive technology: a Nordic cohort study from the Committee of Nordic ART and Safety (CoNARTaS). Hum Reprod (Oxford England). (2014) 29:2050–7. doi: 10.1093/humrep/deu143
Keywords: assisted reproductive technology, childhood cancer, relative risk, systematic review, meta-analysis, preventive health
Citation: Jiang Y, Dai Q, Zhang S, Wang H, He X, Fu R, Tan J, Fu Q, Zhang Q and Lu Y (2025) Cumulative temporal association between assisted reproductive technology and childhood cancer: a systematic review and meta-analysis of observational studies. Front. Oncol. 15:1555420. doi: 10.3389/fonc.2025.1555420
Received: 04 January 2025; Accepted: 28 April 2025;
Published: 20 May 2025.
Edited by:
Luca Giacomelli, Polistudium srl, ItalyReviewed by:
Felix M. Onyije, International Agency For Research On Cancer (IARC), FranceGustavo Dib Dangoni, University of São Paulo, Brazil
Copyright © 2025 Jiang, Dai, Zhang, Wang, He, Fu, Tan, Fu, Zhang and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qinxiu Zhang, WmhxaW54aXVAMTYzLmNvbQ==; Yan Lu, U29waGllbHVvODk2QG5qdWNtLmVkdS5jbg==
†These authors share first authorship
 Yanjie Jiang
Yanjie Jiang Qiangqiang Dai2†
Qiangqiang Dai2† Shipeng Zhang
Shipeng Zhang Hanyu Wang
Hanyu Wang Xingyi He
Xingyi He Rui Fu
Rui Fu Junwen Tan
Junwen Tan Qinwei Fu
Qinwei Fu Qinxiu Zhang
Qinxiu Zhang Yan Lu
Yan Lu