- 1Department of Hepatobiliary and Pancreatic Surgery, Shaoxing People’s Hospital, Shaoxing, China
- 2Shaoxing Key Laboratory of Minimally Invasive Abdominal Surgery and Precise Treatment of Tumor, Shaoxing, China
- 3Department of Ultrasound, Shaoxing People’s Hospital, Shaoxing, China
- 4Department of Pathology, Shaoxing People’s Hospital, Shaoxing, China
- 5School of Medicine, Shaoxing University, Shaoxing, Zhejiang, China
Background: Microvascular invasion (MVI) is a critical determinant of poor prognosis in hepatocellular carcinoma (HCC). Accurate preoperative prediction of MVI is essential for optimizing surgical and therapeutic strategies. This study aims to develop a combined model integrating intratumoral, peritumoral, and clinical features from ultrasound-based radiomics for MVI prediction.
Methods: Ultrasound images of 119 patients with pathologically confirmed HCC were analyzed. A total of 1,414 radiomics features were extracted from intratumoral and peritumoral regions. Feature selection was performed using intraclass correlation coefficient (ICC) analysis, t-tests, and least absolute shrinkage and selection operator (LASSO) regression. Logistic regression, Random Forest, and other machine learning algorithms were applied to construct predictive models. The best-performing intratumoral, peritumoral, and clinical models were combined using logistic regression. SHapley Additive exPlanations (SHAP) analysis, logistic regression coefficients, and partial dependence analysis were employed to evaluate feature contributions and interactions.
Results: Both intratumoral and peritumoral models achieved high AUCs (0.781 and 0.792, respectively), with no statistically significant difference between them. The combined model, incorporating tumor size, achieved the highest AUC (0.903, 95% CI: 0.780–1.000) and superior performance across all evaluation metrics. Tumor size exhibited the smallest logistic regression coefficient but the highest SHAP contribution, indicating strong interactions with intratumoral and peritumoral features. Interaction analyses revealed that the combined effects of tumor size and radiomics features significantly enhanced predictive performance.
Conclusion: This study demonstrates that combining intratumoral, peritumoral, and clinical features enhances the predictive accuracy for MVI in HCC. The findings underscore the value of feature integration and interactions, providing insights for personalized treatment planning and advancing the clinical utility of ultrasound-based radiomics.
1 Introduction
Hepatocellular carcinoma (HCC) is a leading cause of cancer-related mortality worldwide (1, 2). Microvascular invasion (MVI) is a critical pathological feature of HCC, significantly associated with aggressive tumor behavior, early recurrence, and poor prognosis (3). Accurate preoperative prediction of MVI is crucial for guiding clinical decision-making, including determining surgical margins, the necessity of adjuvant therapies, and personalized treatment strategies (4, 5). However, predicting MVI preoperatively remains challenging with existing imaging modalities such as computed tomography (CT) and magnetic resonance imaging (MRI), which have limitations in detecting microscopic tumor invasion (6–8).
Radiomics, a rapidly evolving field in medical imaging, offers a non-invasive method to extract high-dimensional quantitative features from medical images, enabling deeper insights into tumor heterogeneity (9, 10). While CT- and MRI-based radiomics models have demonstrated promising results in predicting MVI (11, 12), the application of radiomics in ultrasound (US) imaging remains underexplored. Ultrasound imaging, with its real-time capabilities, non-invasive nature, and lower cost, is a particularly appealing modality for radiomics research, especially in regions with limited access to advanced imaging techniques. Integrating both intratumoral and peritumoral regions in radiomics analysis may provide a more comprehensive characterization of MVI-related features, as peritumoral tissue often contains crucial information about tumor invasiveness and interactions with the surrounding microenvironment (13–16).
Despite these advances, several gaps remain. First, ultrasound-based radiomics is underutilized compared to CT or MRI, despite the unique advantages of US imaging. Second, many existing studies focus exclusively on intratumoral features, neglecting the valuable predictive information contained in the peritumoral region. Third, while combining intratumoral, peritumoral, and clinical parameters in a single model shows promise, the individual contributions and interactions of these components within a combined model remain unclear.
Our study aims to address these gaps by developing a radiomics model based on preoperative ultrasound imaging that integrates both intratumoral and peritumoral features. Additionally, a combined model will be constructed by integrating radiomics features with clinical characteristics using logistic regression to improve predictive performance. Furthermore, interpretable analysis methods will be incorporated to visualize and explain the contributions of specific radiomics and clinical features, enhancing the model’s transparency and clinical applicability.
2 Material and methods
2.1 Study population
This retrospective study was conducted at Shaoxing People’s Hospital, following approval from the Institutional Review Board. Written informed consent was obtained from all patients prior to participation.
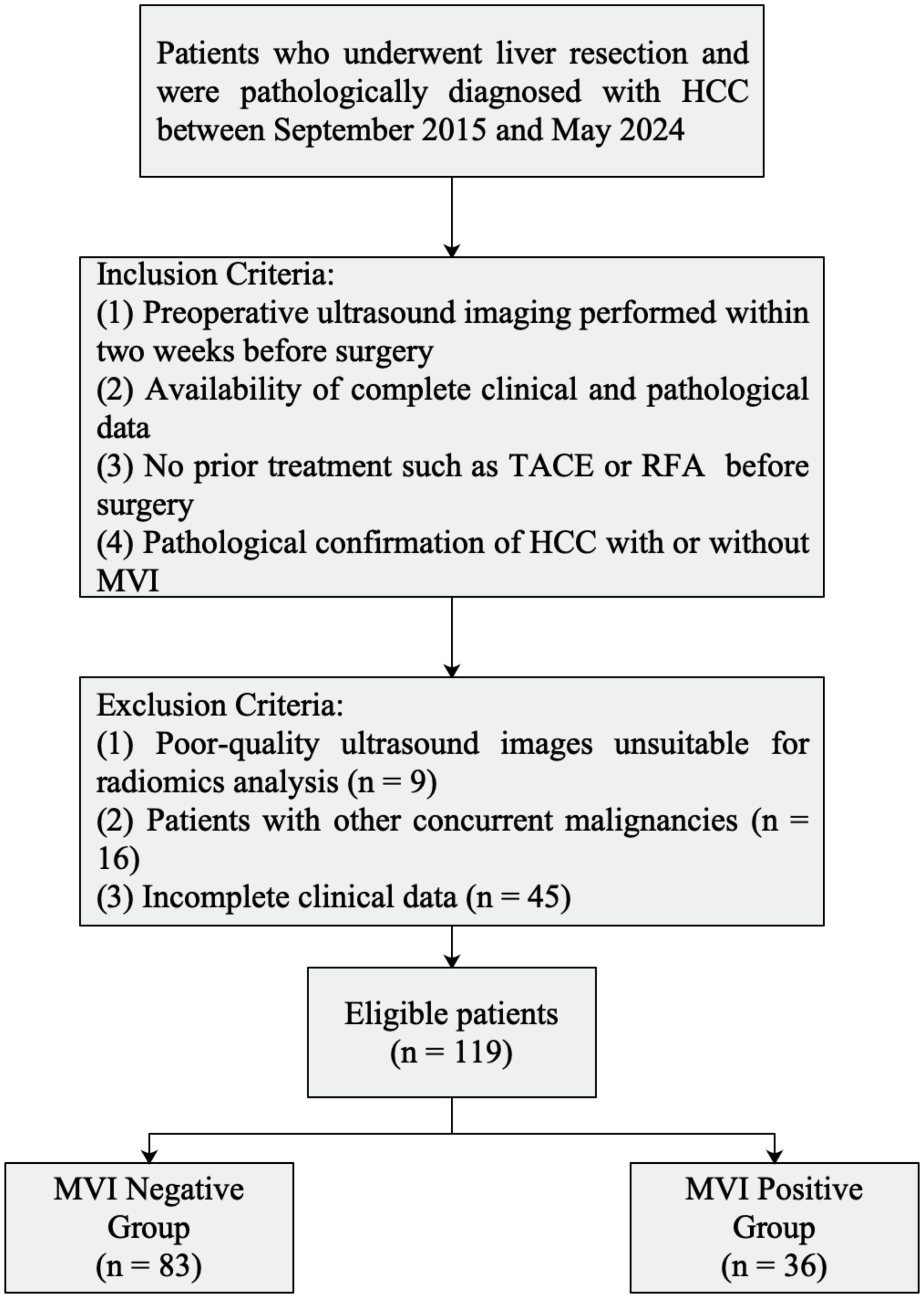
A total of 119 patients with pathologically confirmed HCC who underwent surgical resection at Shaoxing People’s Hospital between September 2019 and May 2024 were enrolled. Inclusion criteria were as follows: (1) preoperative ultrasound imaging performed within two weeks before surgery; (2) availability of complete clinical and pathological data; (3) no prior treatment such as transarterial chemoembolization (TACE) or radiofrequency ablation (RFA) before surgery; and (4) pathological confirmation of HCC with or without MVI. Exclusion criteria included poor-quality ultrasound images unsuitable for radiomics analysis (n = 9), patients with other concurrent malignancies (n = 16), and incomplete clinical data (n = 45).
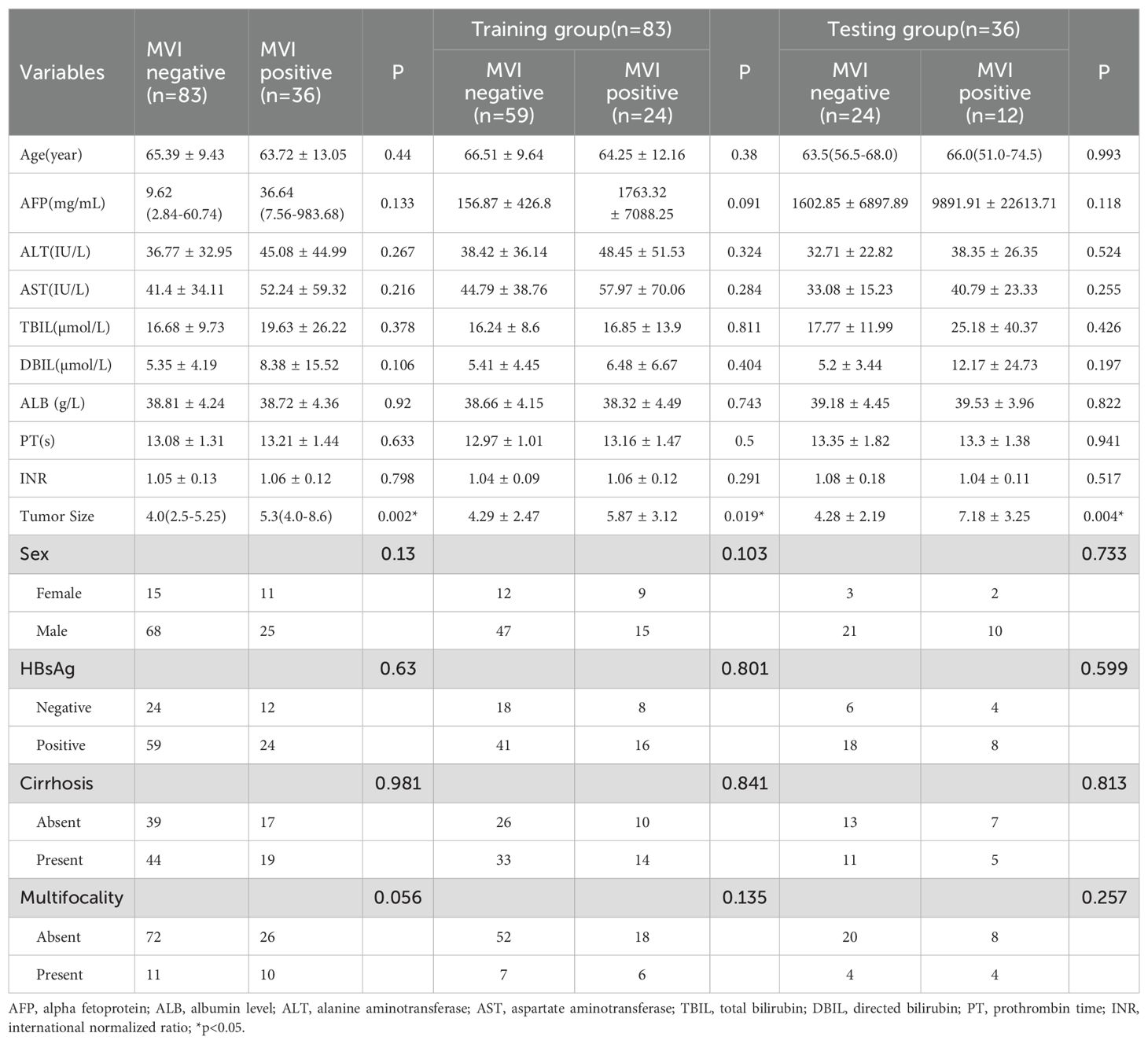
The study cohort was divided into training and validation sets at a 7:3 ratio for model development and evaluation. Baseline characteristics, including demographic, clinical, and pathological data, were collected from medical records (Table 1). Microvascular invasion was defined as the presence of tumor cells within a vascular space lined by endothelial cells beyond the tumor boundary, as confirmed by histopathological examination.
A flowchart illustrating the inclusion and exclusion of patients is presented in Figure 1.
2.2 Ultrasound procedure
Preoperative gray-scale ultrasound imaging was performed using various ultrasound machines (details provided in the Supplementary Materials). All patients underwent standardized ultrasound examination by experienced radiologists two weeks prior to surgery.
During the examination, patients were positioned in a supine or left lateral decubitus position to optimize visualization of the liver and associated structures. The scanning protocol included assessing tumor location, size, echogenicity, and boundary characteristics. Particular attention was given to the peritumoral region to ensure comprehensive imaging data were collected for subsequent radiomics analysis.
Images were stored in Digital Imaging and Communications in Medicine (DICOM) format for consistency and compatibility with radiomics feature extraction workflows.
2.3 Histological and immunohistochemistry
Histopathological examination was performed on surgically resected HCC specimens to confirm the diagnosis and assess MVI. Tissue samples were fixed in 10% formalin, embedded in paraffin, and sectioned at 4-µm thickness. Hematoxylin and eosin staining was used to evaluate tumor differentiation and the presence of MVI. Microvascular invasion was defined as the presence of tumor cells within a vascular lumen lined by endothelial cells beyond the tumor border. Representative HE-stained histological images illustrating MVI-negative (M0), MVI-positive (M1 and M2) are shown in Supplementary Figure S1.
2.4 Region of interest delineation
Region of interest (ROI) delineation was performed using ITK-SNAP software(Version 4.0.0, www.itksnap.org) (17) (Figure 2). Initially, the tumor region was manually delineated by two independent ultrasound radiologists. For peritumoral analysis, the tumor ROI was automatically expanded outward by 1 cm, creating a peritumoral region. This distance was selected based on prior study suggesting that a 1 cm peritumoral zone is most suitable for MVI prediction, as it effectively captures both local tumor invasion and critical tissue interactions near the tumor boundary (18). These automatically generated ROIs were manually adjusted to ensure accuracy and exclude irrelevant structures such as large vessels or adjacent organs. To minimize bias, the two radiologists independently performed ROI delineation with a 1-week interval between assessments and were blinded to the clinical and imaging data.
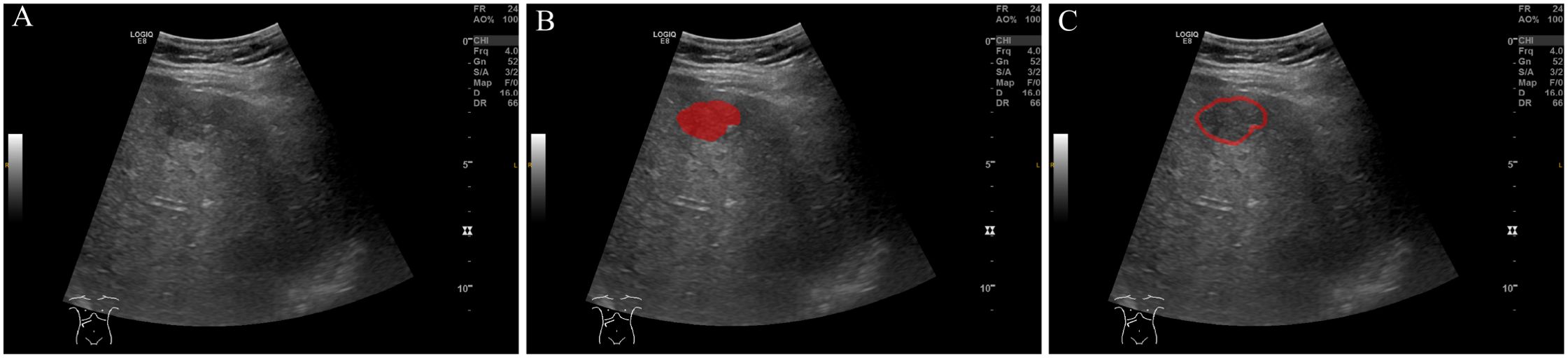
Figure 2. ROI (Region of Interest) delineation on ultrasound images. (A) Original ultrasound image. (B) ROI delineation of the tumor region (in red). (C) ROI delineation including the peritumoral region (in red), encompassing the area surrounding the tumor.
2.5 Radiomics feature extraction and dimension reduction
Radiomics feature extraction was performed on normalized ultrasound images to ensure consistency across imaging systems. The normalization process included resampling images to a uniform spatial resolution of 3 × 3 × 3 mm³, scaling intensity values to 32 gray levels based on a scale of 255, and suppressing machine-specific artifacts or noise. PyRadiomics software was utilized to extract features from both intratumoral and peritumoral regions. After feature extraction, the features were standardized by applying z-score normalization to ensure that all features were on a comparable scale, improving the stability and performance of subsequent analyses. These features encompassed shape characteristics, such as volume and compactness, first-order statistics to describe intensity distributions, texture metrics derived from gray-level co-occurrence and size zone matrices, and higher-order features from wavelet decompositions.
To reduce dimensionality and improve model robustness, a systematic feature selection process was applied. Features with low reproducibility, as assessed by intra- and inter- correlation coefficient (ICC), were excluded (threshold: ICC < 0.75). Highly correlated features (correlation coefficient > 0.8) were removed to eliminate redundancy. Independent samples t-tests were conducted to identify features significantly associated with MVI (p < 0.05), and least absolute shrinkage and selection operator (LASSO) regression was applied to refine the feature set further, prioritizing the most informative predictors for subsequent model development.
2.6 Model construction
Separate models for intratumoral and peritumoral features were developed using various modeling techniques, all trained on the same subset of features selected via LASSO for each region. Optimal hyperparameters were determined using a two-step approach combining Random Search and Grid Search with cross-validation. The best-performing intratumoral and peritumoral models were then combined using logistic regression, incorporating clinical parameters to construct a comprehensive model.
Model performance was assessed using metrics, including AUC, sensitivity, specificity, and accuracy et al. To account for the relatively small sample size, bootstrapping with 1,000 resamples was performed to estimate the confidence intervals of model metrics and to compare the predictive performance of different models statistically. Model evaluation and comparisons were conducted on the validation dataset to ensure robustness and generalizability.
2.7 Analysis of feature importance in the combined model
To analyze the contributions of intratumoral and peritumoral models, along with clinical parameters, within the combined framework, several interpretability techniques were employed. SHAP (Shapley Additive Explanations) analysis quantified both global and local feature importance by perturbing feature values and observing the resulting changes in model predictions. Logistic regression coefficients provided a linear quantification of feature contributions, reflecting the direction and magnitude of each feature’s impact on classification outcomes. Partial dependence analysis (PDA) was conducted to visualize how variations in individual feature values influenced model predictions, highlighting both independent contributions and interactions.
2.8 Statistical analysis
All statistical analyses were performed using Python software (Version 3.11). Continuous variables were expressed as either mean ± standard deviation for normally distributed data or median with the range for non-normally distributed data. Comparative analyses of continuous variables were conducted using the independent samples t-test for normally distributed data or the Mann-Whitney U test for non-normally distributed data. Categorical variables were reported as frequencies or percentages and analyzed using chi-square tests or Fisher’s exact tests, as appropriate. Comparisons of ROC curves were performed using a bootstrapping method with 1,000 resamples to estimate confidence intervals and evaluate the differences in predictive performance. A two-tailed p-value of <0.05 was considered statistically significant throughout the analysis.
3 Results
3.1 Characteristics of the study population
A total of 119 patients were included in this study, comprising 83 MVI-negative and 36 MVI-positive cases. The baseline characteristics of the study population are summarized in Table 1. Tumor size was significantly larger in the MVI-positive group compared to the MVI-negative group across the entire cohort (median [IQR]: 4.0 [2.5–5.25] cm vs. 5.3 [4.0–8.6] cm; p < 0.05). This difference remained significant in both the training cohort (4.29 ± 2.47 cm vs. 5.87 ± 3.12 cm; p = 0.019) and the testing cohort (4.28 ± 2.19 cm vs. 7.18 ± 3.25 cm; p = 0.004).
Serum alpha-fetoprotein (AFP) levels were higher in the MVI-positive group compared to the MVI-negative group, though the difference was not statistically significant (p = 0.133). Other baseline characteristics, including age, liver function markers, and sex, showed no statistically significant differences between the two groups (all p > 0.05). And all the clinical characteristics of the training and testing cohorts showed no significant differences (Supplementary Table S1).
3.2 Feature selection
A total of 1,414 radiomics features were extracted from both intratumoral and peritumoral regions. Due to the limited number of MVI-positive samples, the Synthetic Minority Oversampling Technique (SMOTE) was applied to balance the data within the training set.
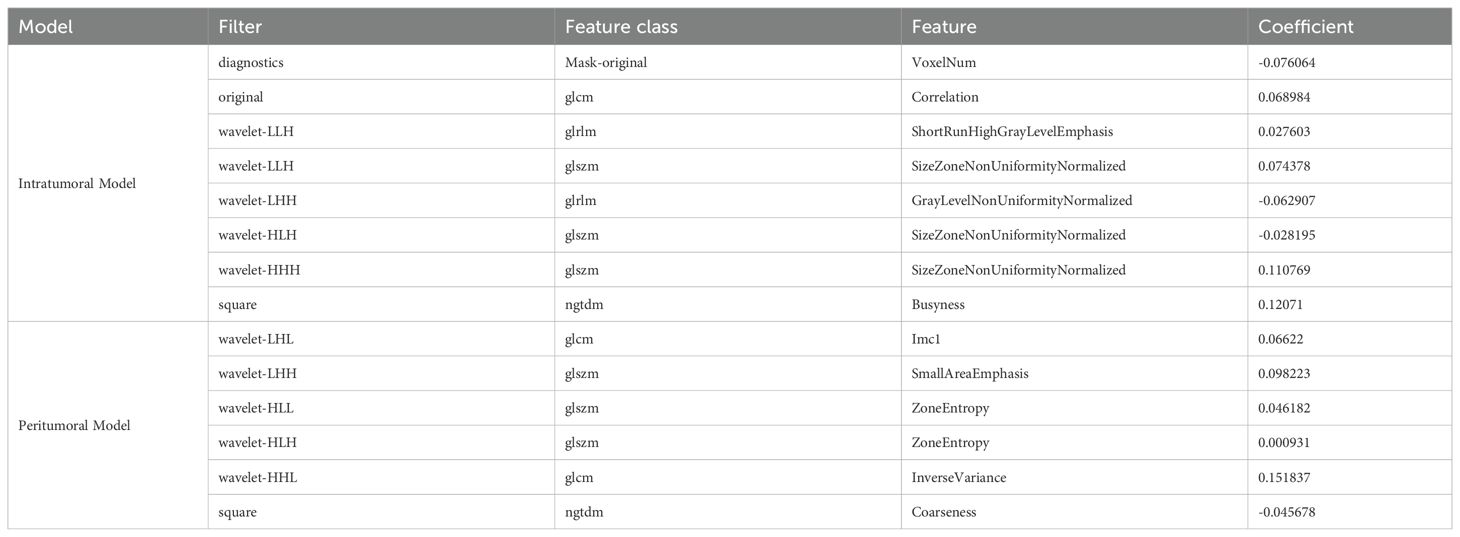
Feature selection involved several steps: ICC analysis to ensure feature reproducibility. Both within-group (intraclass) and between-group (interclass) correlation coefficients were calculated. Features with an ICC value less than 0.75 for either within-group or between-group consistency were excluded to maintain high reproducibility between observers and across groups. In the intratumoral region, 1373 of features were retained after ICC analysis, while 1352 were retained for the peritumoral region. Removal of collinear features addressed redundancy, t-tests were used for univariate analysis, and LASSO regression was applied for dimensionality reduction (details of the LASSO process are provided in Supplementary Figures S2, S3). As a result, 8 radiomics features were selected for the intratumoral model and 6 features for the peritumoral model. The selected features are presented in Table 2.
3.3 Model construction
To construct predictive models, multiple machine learning algorithms were employed, including Support Vector Machine, Random Forest, K Nearest Neighbor, Logistic Regression, Decision Tree, Artificial Neural Network, AdaBoostClassifier, GradientBoostingClassifier, and XGBOOST. The ROC curves for different modeling methods are summarized in Figures 3A, B for the intratumoral and peritumoral models, respectively. Hyperparameter optimization was performed using a two-step approach combining RandomizedSearchCV and GridSearchCV with five-fold cross-validation. The detailed search ranges and the final selected parameters for each model are provided in the Supplementary Materials (Supplementary Tables S2–S4).
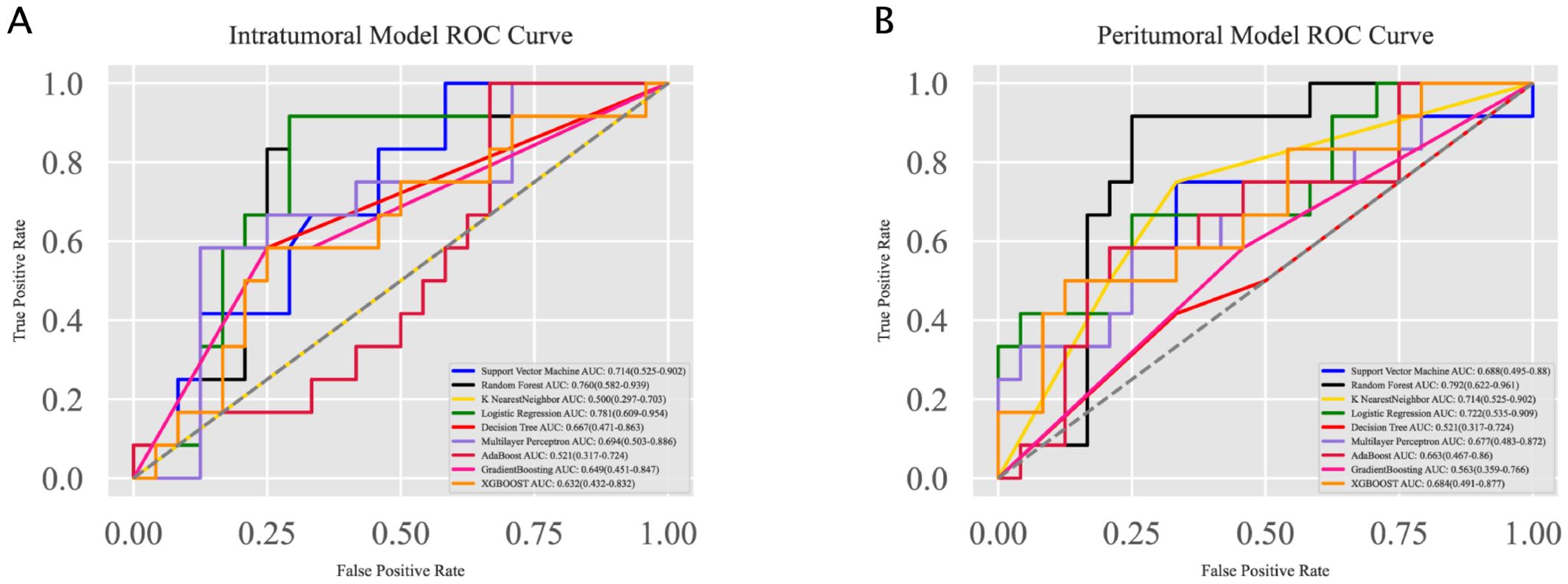
Figure 3. Receiver Operating Characteristic (ROC) curves for the intratumoral and peritumoral models. (A) ROC curves for the intratumoral model constructed using various machine learning algorithms. Logistic Regression achieved the highest AUC of 0.781, followed by Random Forest with an AUC of 0.760. (B) ROC curves for the peritumoral model constructed using the same algorithms. Random Forest achieved the highest AUC of 0.792, followed by Logistic Regression with an AUC of 0.722.
For the intratumoral model, Logistic Regression achieved the highest AUC of 0.781 (95% CI: 0.609–0.954), followed by Random Forest with an AUC of 0.760 (95% CI: 0.582–0.939). For the peritumoral model, Random Forest performed best with an AUC of 0.792 (95% CI: 0.622–0.961), while Logistic Regression also showed strong performance with an AUC of 0.722 (95% CI: 0.535–0.909).
The best-performing intratumoral and peritumoral models (Logistic Regression and Random Forest, respectively) were combined with the clinical parameter tumor size using a logistic regression approach to construct the combined model. The performance evaluation is presented in Figure 4. (A) The ROC curves highlight the discriminatory power of the models for predicting MVI status. The combined model achieved the highest AUC (0.903, 95% CI: 0.780–1.000), surpassing the clinical model (AUC: 0.786, 95% CI: 0.615–0.958), the peritumoral model (AUC: 0.792, 95% CI: 0.622–0.961), and the intratumoral model (AUC: 0.781, 95% CI: 0.609–0.954). (B) The radar chart provides a visual comparison of evaluation metrics, including sensitivity/recall, specificity, precision, accuracy, and F1 score, demonstrating the combined model’s superior overall performance and robustness.
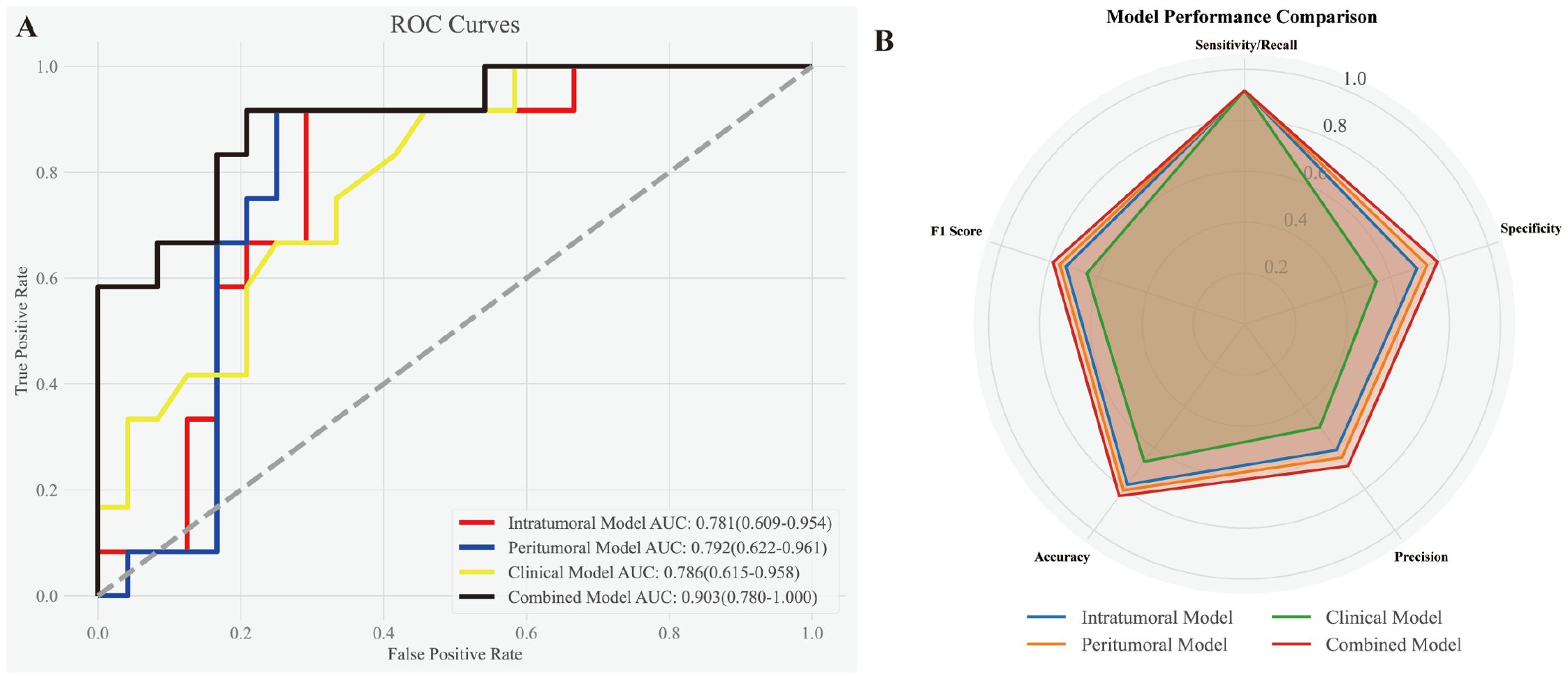
Figure 4. Performance evaluation of the intratumoral, peritumoral, clinical, and combined models. (A) ROC curves demonstrating the discriminatory power of the models for predicting MVI status. The combined model exhibited the highest AUC (0.903, 95% CI: 0.780–1.000), outperforming the clinical model (AUC: 0.786, 95% CI: 0.615–0.958), peritumoral model (AUC: 0.792, 95% CI: 0.622–0.961), and intratumoral model (AUC: 0.781, 95% CI: 0.609–0.954). (B) Radar chart illustrating various evaluation metrics, including sensitivity/recall, specificity, precision, accuracy, and F1 score, for the four models. The combined model consistently achieved the best overall performance across all metrics.
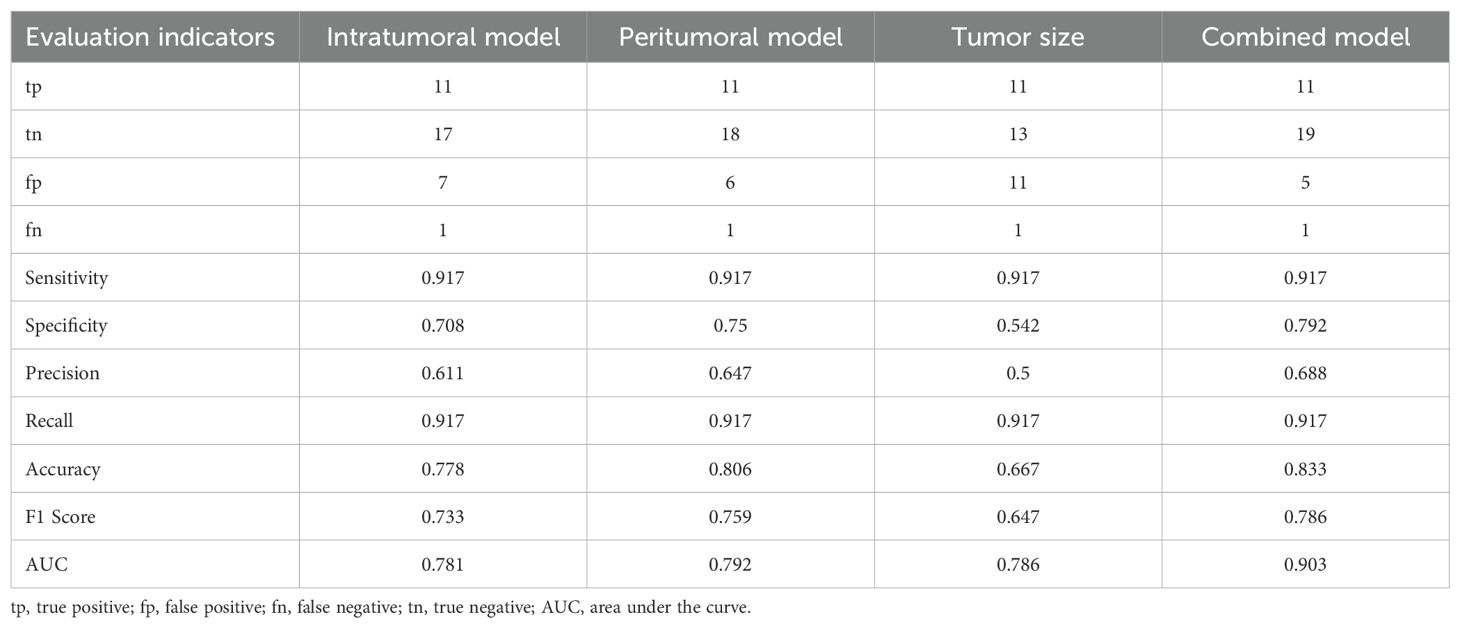
Table 3 summarizes the performance metrics of all four models. While all models achieved the same sensitivity (0.917), the combined model outperformed the others in accuracy (0.833), specificity (0.792), precision (0.688), and F1 score (0.786), reflecting its better balance between precision and recall. The peritumoral model showed slightly better performance than the intratumoral model, with higher specificity (0.75 vs. 0.708), precision (0.647 vs. 0.611), and F1 score (0.759 vs. 0.733). By contrast, the clinical model (tumor size) demonstrated the lowest specificity (0.542) and precision (0.5), indicating its limitations as a standalone predictor. These results further emphasize the strength of the combined model in achieving superior diagnostic performance.
To statistically compare the performance of the models, bootstrapping was performed. The results showed no significant differences between the intratumoral model and the combined model (p = 0.184) or between the peritumoral model and the combined model (p = 0.124). However, a significant difference was observed between the clinical model and the combined model (p = 0.026), highlighting the added predictive value of integrating radiomics features with clinical parameters. Comparisons between the intratumoral and peritumoral models (p = 0.952) and between the clinical model and both the intratumoral (p = 0.978) and peritumoral models (p = 0.982) revealed no significant differences.
In addition to discrimination performance, we evaluated the probabilistic accuracy of the combined model. The calibration curve showed overall good agreement between predicted and observed probabilities, with only mild deviations observed in the mid-probability range (Supplementary Figure S4). The Brier score was 0.151, indicating acceptable probabilistic accuracy. The Hosmer–Lemeshow goodness-of-fit test was non-significant (χ² = 11.45, p = 0.178), suggesting no significant miscalibration.
3.4 Analysis of feature contributions in the combined model
To analyze the contributions of individual features within the combined model, several interpretability techniques were employed. Logistic regression coefficients were analyzed to evaluate the linear contribution of each feature, with results presented in Figure 5A. Tumor size showed the lowest importance in this analysis. SHAP analysis was then used to quantify the global and local importance of each feature, as shown in Figure 5B. In contrast to the logistic regression analysis, tumor size demonstrated the highest contribution in the SHAP analysis, highlighting its critical role in the combined model’s predictions.
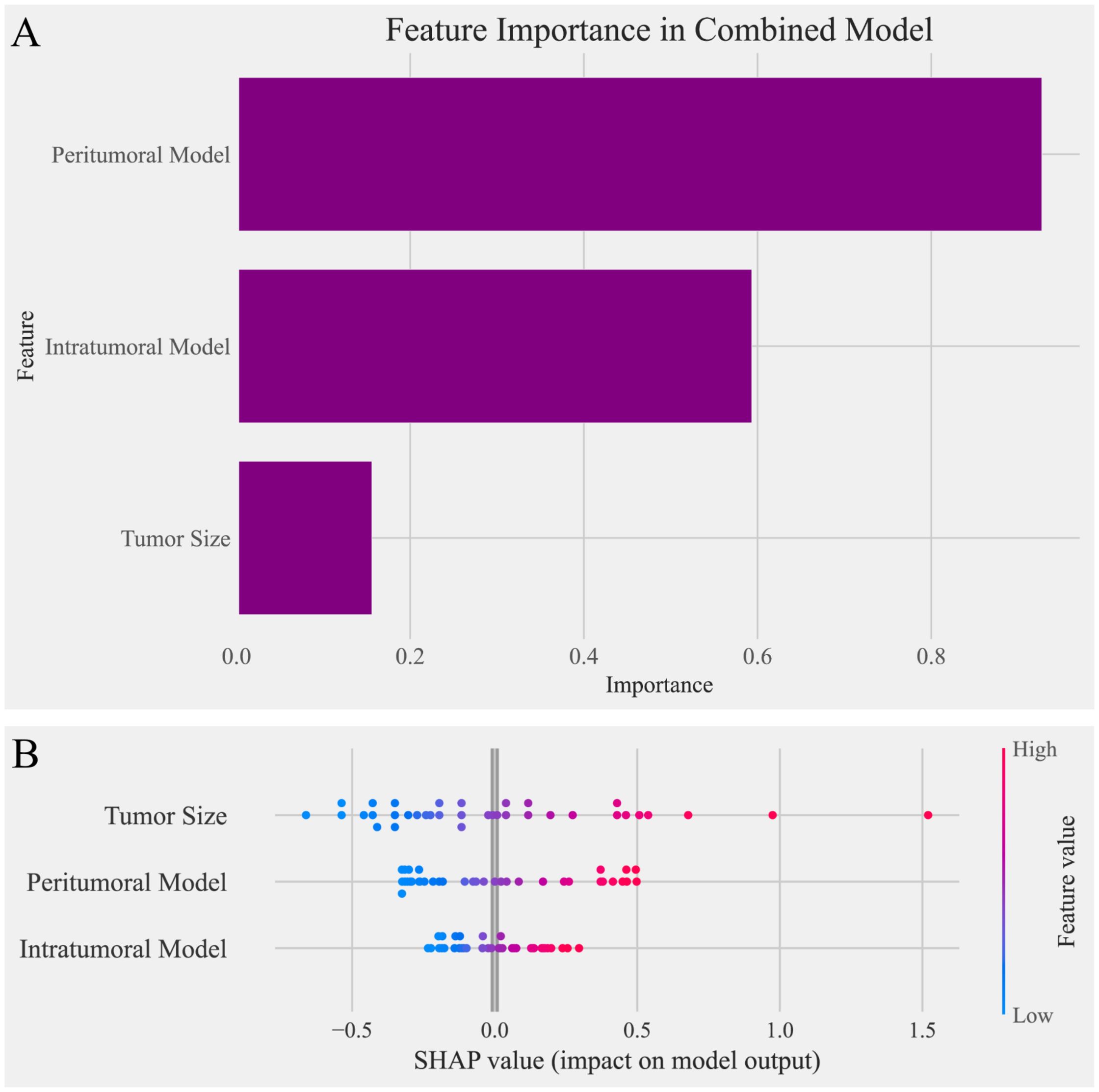
Figure 5. Feature importance analysis in the combined model. (A) Bar chart of feature importance based on logistic regression coefficients in the combined model. The peritumoral model contributed the most to the predictions, followed by the intratumoral model, while tumor size showed the lowest importance in this analysis. (B) SHAP (Shapley Additive Explanations) plot illustrating the impact of individual features on the combined model’s predictions. Tumor size exhibited the highest SHAP values, indicating its dominant contribution to the model’s output, followed by the peritumoral model and intratumoral model. The contrasting results between the two analyses highlight the differences in feature importance interpretations.
PDA was further applied to evaluate the impact of individual features and their interactions on the model’s predictions. Figures 6A–C illustrate the partial dependence plots for the intratumoral model, peritumoral model, and tumor size, respectively. The X-axis represents feature values, and the Y-axis represents partial dependence values, reflecting the positive or negative influence of each feature on the predicted probability. Among these, tumor size (Figure 6C) exhibited the most significant linear increase, emphasizing its critical role in prediction outcomes.
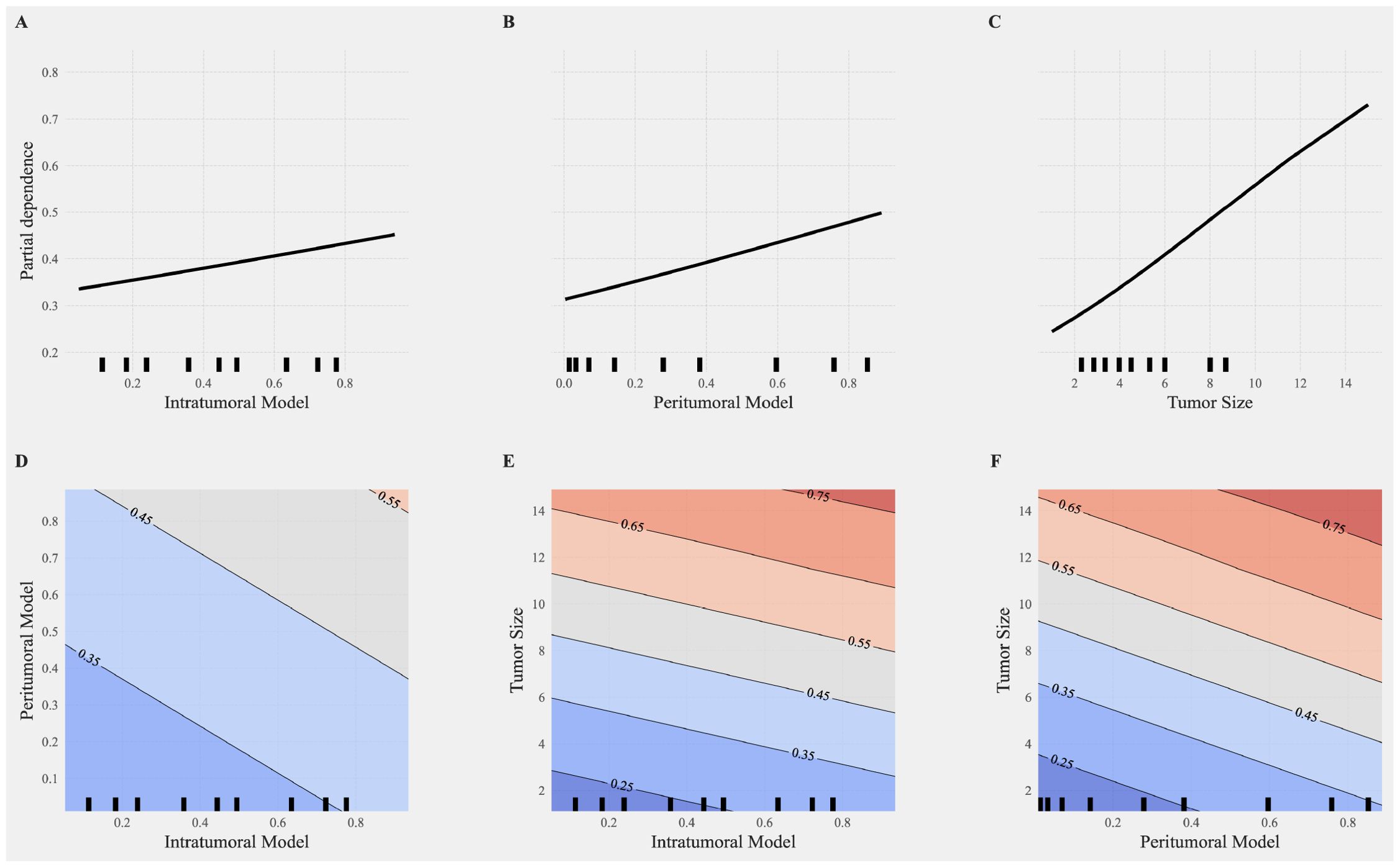
Figure 6. Partial dependence analysis of individual and interactive features in the combined model. (A–C) Partial dependence plots (PDP) for single features, including the intratumoral model (A), peritumoral model (B), and tumor size (C). The X-axis represents feature values, and the Y-axis represents partial dependence values, indicating the positive or negative impact of each feature on the model predictions. Among these, tumor size (C) shows the most significant increase, highlighting its critical importance in the prediction outcomes. (D–F) Interaction effects between features visualized through 2D partial dependence heatmaps. (D) Interaction between intratumoral and peritumoral models. (E) Interaction between tumor size and the intratumoral model. (F) Interaction between tumor size and the peritumoral model. The heatmaps illustrate the contributions of these interactions to the combined model, with more pronounced color gradients indicating stronger interaction effects on the model predictions. High values of tumor size combined with radiomics features (intratumoral or peritumoral) significantly enhance the probability of positive class predictions.
Figures 6D–F depict the interaction effects between features using 2D partial dependence heatmaps. The interactions between the intratumoral and peritumoral models (Figure 6D), tumor size and the intratumoral model (Figure 6E), and tumor size and the peritumoral model (Figure 6F) reveal substantial contributions to the combined model. The pronounced color gradients in these heatmaps indicate that stronger interactions significantly enhance the model’s predictive performance. High values of tumor size combined with radiomics features result in a marked increase in the probability of positive class predictions, underscoring the synergistic effects of these interactions in improving model performance. The PDA results revealed that tumor size and the peritumoral model exhibited stronger independent contributions to positive class predictions, showing significant linear growth trends. In contrast, the intratumoral model’s independent contribution was weaker but still showed a positive effect. The inclusion of interaction features significantly improved model predictions, enhancing diagnostic performance across multiple metrics.
4 Discussion
Our study successfully developed and validated a combined model integrating grayscale ultrasound-based radiomics features from intratumoral and peritumoral regions with clinical parameters, particularly tumor size, to predict MVI in HCC patients. By combining these three components, the model achieved superior diagnostic performance, highlighting the complementary nature of tumor size, intratumoral, and peritumoral radiomics features.
Radiomics features provide a quantitative approach to extracting high-dimensional data from medical images, enabling the characterization of tumor properties that are imperceptible to the human eye (9, 19). In our study, the majority of selected features from both intratumoral and peritumoral regions were wavelet-filtered, underscoring the importance of multi-scale texture analysis in capturing subtle variations associated with MVI. Wavelet filters decompose imaging data into frequency components, allowing the analysis of textures and patterns at different scales and orientations (20, 21). This approach is particularly effective in detecting fine structural details and subtle heterogeneities, which are often linked to biological processes such as tumor invasion and microvascular involvement (22). Previous studies have highlighted the utility of wavelet-based radiomics in enhancing predictive models for various cancers, demonstrating its robustness and versatility in capturing complex imaging features (23). The modeling results in our study demonstrated that LR and RF consistently outperformed other algorithms in both the intratumoral and peritumoral models, achieving the best performance across evaluation metrics. LR is well-suited for linearly separable data, offering simplicity and interpretability (24), while RF excels in handling non-linear relationships and interactions, reducing the risk of overfitting through ensemble learning (25). The strong performance of these algorithms in our models further validates the utility of wavelet-filtered features in predictive modeling and their ability to capture complex imaging patterns associated with MVI.
The intratumoral and peritumoral models both achieved relatively high AUC values, and no statistically significant differences were observed between them. This finding suggests that both intratumoral and peritumoral tissues contain comparable diagnostic information for MVI prediction, consistent with previous studies that have reported similar diagnostic efficacy for tumor and peritumoral features in radiomics-based models (26, 27). It also highlights the significance of peritumoral analysis, which captures interactions between the tumor and its surrounding microenvironment, providing insights complementary to intratumoral characteristics (18, 28, 29). In particular, peritumoral heterogeneity may reflect the presence of microscopic vascular remodeling, immune cell infiltration, and stromal reactions induced by tumor aggressiveness. These peritumoral changes, although not directly visible on conventional imaging, can be captured through radiomic texture analysis. Prior studies have shown that the peritumoral zone is a common site of microvascular invasion and angiogenesis, suggesting that heterogeneity in this region may indirectly indicate vascular infiltration or early metastatic spread (27). Thus, peritumoral features may serve as a surrogate marker of MVI-related microenvironmental alterations, supporting their inclusion in predictive modeling.
The combined model exhibited the highest AUC and the best performance across all evaluation metrics, underscoring its ability to integrate complementary information from tumor size, intratumoral, and peritumoral features. The results suggest that each component provides unique and synergistic contributions, collectively enhancing the model’s predictive performance. Although statistical comparisons between models did not yield significant p-values, the combined model consistently achieved higher AUC, specificity, precision, and F1-score compared to standalone models. These improvements, though not statistically significant, may still hold practical and clinical relevance in settings where improved diagnostic balance and robustness are required. Tumor size emerged as the most critical single predictive factor in the combined model. Its significance aligns with previous studies linking larger tumors to increased aggressiveness and MVI risk (30–32). However, while the clinical model achieved a relatively high AUC, its performance on other metrics was inferior to the radiomics-based models. This disparity likely reflects the limited scope of clinical parameters, which lack the nuanced and multi-dimensional data captured by radiomics features (32). The radiomics models, by integrating high-dimensional features extracted from tumor and peritumoral regions, effectively capture microstructural and textural heterogeneities associated with MVI, providing superior diagnostic performance (33).
Interestingly, tumor size demonstrated contrasting contributions in the combined model; it had the smallest coefficient in logistic regression analysis but the highest importance in SHAP analysis. This discrepancy suggests that tumor size exhibits strong interactions with intratumoral and peritumoral features, and these interactions play a significant role in enhancing the model’s predictive performance (34). Interaction analyses highlighted the synergistic effects among features, revealing complex, non-linear relationships where the combined effects exceeded the sum of individual contributions (35). These interactions enhanced diagnostic precision, model robustness, and biological interpretability (36). In our study, the probability of positive class predictions significantly increased when tumor size, intratumoral features, and peritumoral features simultaneously reached high values. This finding highlights the importance of feature interactions, particularly the interplay between intratumoral and peritumoral features combined with tumor size, in enhancing predictive performance. The combination of tumor size with radiomics features provided deeper insights into the tumor’s biological behavior, suggesting potential links between microenvironmental characteristics and structural changes captured by radiomics (37). Such findings emphasize the need for multi-feature integration to comprehensively characterize the multifaceted nature of MVI, ultimately advancing the diagnostic and prognostic capabilities of predictive models.
Although the combined model demonstrated excellent discrimination (AUC = 0.903) and overall good calibration performance, slight overestimation was observed in a limited mid-range of predicted probabilities. This is reflected in a Brier score of 0.151, indicating modest deviation from ideal probabilistic accuracy. Such discrepancies are not uncommon in binary classification tasks and may be attributed to several factors (38). First, the dataset exhibited class imbalance, with only 36 of 119 cases being MVI-positive, potentially biasing the probability distribution even after SMOTE correction. Second, the relatively small sample size, when combined with high-dimensional radiomics features, increases the risk of overfitting and suboptimal calibration. Third, while SMOTE improves training stability, it may alter the intrinsic feature distribution, affecting the reliability of predicted probabilities (39). Lastly, logistic regression models—while effective for class separation—are not inherently optimized for probabilistic calibration (40). Despite these limitations, the model’s calibration remains clinically acceptable and informative, as evidenced by the close alignment of most points with the ideal curve and a non-significant Hosmer–Lemeshow test (χ² = 11.45, p = 0.1773). Future validation using larger, multicenter datasets is warranted to further improve the model’s probabilistic reliability.
Several limitations should be acknowledged. First, this was a single-center retrospective study with a modest sample size, particularly in the MVI-positive subgroup (n = 36), which may limit the generalizability of the findings. Although SMOTE was employed to balance class distribution, its use in small-sample, high-dimensional radiomics settings may carry a risk of overfitting. To mitigate this, we adopted multiple robustness strategies including cross-validation and an independent internal validation cohort. Additionally, while the model demonstrated strong discrimination, we acknowledge the lack of external validation and prospective confirmation. A multicenter study is currently underway to address these issues and evaluate model performance across diverse clinical environments. Second, ultrasound images were obtained from different machines. Although normalization was applied to reduce variability, we cannot entirely rule out the potential influence of device-related differences on radiomics features. Third, the retrospective design inherently carries a risk of selection bias. Fourth, although MVI can be further stratified into M0, M1, and M2 grades, we treated MVI as a binary classification problem due to limited sample size. Future studies with larger, balanced cohorts are needed to explore fine-grained, multi-class prediction of MVI severity. Finally, the contribution of feature interactions, while significant, requires further validation through molecular biology experiments and independent clinical datasets. Future studies should focus on larger, multicenter cohorts and explore the integration of molecular and multimodal imaging data to enhance model performance and generalizability.
5 Conclusion
This study highlights the feasibility and effectiveness of integrating radiomics features from intratumoral and peritumoral regions with clinical parameters for MVI prediction in HCC. The combined model’s superior performance underscores the potential of this approach in personalized treatment planning, offering valuable insights for advancing radiomics research and its clinical applications in oncology.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Ethics Committee of Shaoxing People’s Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
HF: Data curation, Formal analysis, Funding acquisition, Methodology, Writing – original draft, Writing – review & editing. YH: Conceptualization, Funding acquisition, Investigation, Software, Writing – original draft, Writing – review & editing. BL: Conceptualization, Data curation, Funding acquisition, Resources, Writing – original draft, Writing – review & editing. JY: Data curation, Formal analysis, Supervision, Writing – original draft, Writing – review & editing. DZ: Data curation, Funding acquisition, Validation, Writing – original draft, Writing – review & editing. CH: Data curation, Writing – original draft, Writing – review & editing. LX: Data curation, Writing – original draft, Writing – review & editing. HQ: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. The work was sponsored by Zhejiang Provincial Natural Science Foundation of China (LY22H160008, LBY22H030001), Zhejiang Provincial Medical and Health Science and Technology Project (2023KY1267, 2025KY1663, and 2025KY1680), Science and Technology Program Project of Shaoxing (2022A14020), and Health Science and Technology Program Project of Shaoxing (2022SY001, 2022KY019, and 2023SKY030). The funders did not influence the analysis or publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1566105/full#supplementary-material
Abbreviations
HCC, hepatocellular carcinoma; MVI, microvascular invasion; CT, computer tomography; MRI, magnetic resonance imaging; TACE, transarterial chemoembolization; RFA, radiofrequency ablation; DICOM, digital imaging and communications in medicine; ROI, region of interest; ICC, intra- and inter- correlation coefficient; LASSO, least absolute shrinkage and selection operator; AUC, area under the curve; SHAP, SHapley Additive exPlanations; PDA, Partial dependence analysis; AFP, alpha fetoprotein; SMOTE, Synthetic Minority Oversampling Technique; ALB, albumin level; ALT, alanine aminotransferase; AST, aspartate aminotransferase; TBIL, total bilirubin; DBIL, directed bilirubin; PT, prothrombin time; INR, international normalized ratio; tp, true positive; fp, false positive; fn, false negative; tn, true negative.
References
1. EASL Clinical Practice Guidelines on the management of hepatocellular carcinoma. J Hepatol. (2024). doi: 10.1016/j.jhep.2024.08.028
2. Barcena-Varela M, Monga SP, and Lujambio A. Precision models in hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. (2024). doi: 10.1038/s41575-024-01024-w
3. Hwang YJ, Bae JS, Lee Y, Hur BY, Lee DH, and Kim H. Classification of microvascular invasion of hepatocellular carcinoma: correlation with prognosis and magnetic resonance imaging. Clin Mol Hepatol. (2023) 29:733–46. doi: 10.3350/cmh.2023.0034
4. Kim NR, Bae H, Hwang HS, Han DH, Kim KS, Choi JS, et al. Preoperative prediction of microvascular invasion with gadoxetic acid-enhanced magnetic resonance imaging in patients with single hepatocellular carcinoma: the implication of surgical decision on the extent of liver resection. Liver Cancer. (2024) 13:181–92. doi: 10.1159/000531786
5. Ma L, Zhang C, Wen Y, Xing K, Li S, Geng Z, et al. Imaging-based surrogate classification for risk stratification of hepatocellular carcinoma with microvascular invasion to adjuvant hepatic arterial infusion chemotherapy: a multicenter retrospective study. Int J Surg (London England). (2024). doi: 10.1097/JS9.0000000000001903
6. Ma X, Wei J, Gu D, Zhu Y, Feng B, Liang M, et al. Preoperative radiomics nomogram for microvascular invasion prediction in hepatocellular carcinoma using contrast-enhanced CT. Eur Radiol. (2019) 29:3595–605. doi: 10.1007/s00330-018-5985-y
7. Min JH, Lee MW, Park HS, Lee DH, Park HJ, Lim S, et al. Interobserver variability and diagnostic performance of gadoxetic acid-enhanced MRI for predicting microvascular invasion in hepatocellular carcinoma. Radiology. (2020) 297:573–81. doi: 10.1148/radiol.2020201940
8. Hong SB, Choi SH, Kim SY, Shim JH, Lee SS, Byun JH, et al. MRI features for predicting microvascular invasion of hepatocellular carcinoma: A systematic review and meta-analysis. Liver Cancer. (2021) 10. doi: 10.1159/000513704
9. Lambin P, Rios-Velazquez E, Leijenaar R, Carvalho S, van Stiphout RGPM, Granton P, et al. Radiomics: extracting more information from medical images using advanced feature analysis. Eur J Cancer (Oxford England: 1990). (2012) 48:441–6. doi: 10.1016/j.ejca.2011.11.036
10. Zeng S, Wang X-L, and Yang H. Radiomics and radiogenomics: extracting more information from medical images for the diagnosis and prognostic prediction of ovarian cancer. Military Med Res. (2024) 11:77. doi: 10.1186/s40779-024-00580-1
11. Ma X, Qian X, Wang Q, Zhang Y, Zong R, Zhang J, et al. Radiomics nomogram based on optimal VOI of multi-sequence MRI for predicting microvascular invasion in intrahepatic cholangiocarcinoma. La Radiologia Med. (2023) 128:1296–309. doi: 10.1007/s11547-023-01704-8
12. Zhang X, Ruan S, Xiao W, Shao J, Tian W, Liu W, et al. Contrast-enhanced CT radiomics for preoperative evaluation of microvascular invasion in hepatocellular carcinoma: A two-center study. Clin Trans Med. (2020) 10:e111. doi: 10.1002/ctm2.111
13. Yang J, Dong X, Jin S, Wang S, Wang Y, Zhang L, et al. Radiomics model of dynamic contrast-enhanced MRI for evaluating vessels encapsulating tumor clusters and microvascular invasion in hepatocellular carcinoma. Acad Radiol. (2024). doi: 10.1016/j.acra.2024.07.007
14. Dong Y, Zuo D, Qiu Y-J, Cao J-Y, Wang H-Z, Yu L-Y, et al. Preoperative prediction of microvascular invasion (MVI) in hepatocellular carcinoma based on kupffer phase radiomics features of sonazoid contrast-enhanced ultrasound (SCEUS): A prospective study. Clin Hemorheology Microcirculation. (2022) 81. doi: 10.3233/CH-211363
15. Yang Y, Fan W, Gu T, Yu L, Chen H, Lv Y, et al. Radiomic features of multi-ROI and multi-phase MRI for the prediction of microvascular invasion in solitary hepatocellular carcinoma. Front Oncol. (2021) 11:756216. doi: 10.3389/fonc.2021.756216
16. Gao L, Xiong M, Chen X, Han Z, Yan C, Ye R, et al. Multi-region radiomic analysis based on multi-sequence MRI can preoperatively predict microvascular invasion in hepatocellular carcinoma. Front Oncol. (2022) 12:818681. doi: 10.3389/fonc.2022.818681
17. Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, et al. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. NeuroImage. (2006) 31:1116–28. doi: 10.1016/j.neuroimage.2006.01.015
18. Huang Y and Qian H. Advancing hepatocellular carcinoma management through peritumoral radiomics: enhancing diagnosis, treatment, and prognosis. J Hepatocellular Carcinoma. (2024) 11:2159–68. doi: 10.2147/JHC.S493227
19. Lambin P, Leijenaar RTH, Deist TM, Peerlings J, de Jong EEC, van Timmeren J, et al. Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol. (2017) 14:749–62. doi: 10.1038/nrclinonc.2017.141
20. Zhou J, Lu J, Gao C, Zeng J, Zhou C, Lai X, et al. Predicting the response to neoadjuvant chemotherapy for breast cancer: wavelet transforming radiomics in MRI. BMC Cancer. (2020) 20:100. doi: 10.1186/s12885-020-6523-2
21. Jiang Z, Yin J, Han P, Chen N, Kang Q, Qiu Y, et al. Wavelet transformation can enhance computed tomography texture features: a multicenter radiomics study for grade assessment of COVID-19 pulmonary lesions. Quantitative Imaging In Med Surg. (2022) 12:4758–70. doi: 10.21037/qims-22-252
22. Abd El Kader I, Xu G, Shuai Z, Saminu S, Javaid I, Ahmad IS, et al. Brain tumor detection and classification on MR images by a deep wavelet auto-encoder model. Diagnostics (Basel Switzerland). (2021) 11. doi: 10.3390/diagnostics11091589
23. Ginsburg SB, Algohary A, Pahwa S, Gulani V, Ponsky L, Aronen HJ, et al. Radiomic features for prostate cancer detection on MRI differ between the transition and peripheral zones: Preliminary findings from a multi-institutional study. J Magnetic Resonance Imaging: JMRI. (2017) 46:184–93. doi: 10.1002/jmri.25562
24. Bertsimas D and King A. Logistic regression: from art to science. Stat Sci. (2017) 32:367–84. doi: 10.1214/16-STS602
25. Lever J, Krzywinski M, and Altman N. Points of significance: logistic regression. Nat Methods. (2016) 13:541–2. doi: 10.1038/nmeth.3904
26. Dong Y, Zhou L, Xia W, Zhao X-Y, Zhang Q, Jian J-M, et al. Preoperative prediction of microvascular invasion in hepatocellular carcinoma: initial application of a radiomic algorithm based on grayscale ultrasound images. Front Oncol. (2020) 10:353. doi: 10.3389/fonc.2020.00353
27. Nebbia G, Zhang Q, Arefan D, Zhao X, and Wu S. Pre-operative microvascular invasion prediction using multi-parametric liver MRI radiomics. J Digital Imaging. (2020) 33:1376–86. doi: 10.1007/s10278-020-00353-x
28. Qian H, Shen Z, Zhou D, and Huang Y. Intratumoral and peritumoral radiomics model based on abdominal ultrasound for predicting Ki-67 expression in patients with hepatocellular cancer. Front Oncol. (2023) 13:1209111. doi: 10.3389/fonc.2023.1209111
29. Qian H, Huang Y, Xu L, Fu H, and Lu B. Role of peritumoral tissue analysis in predicting characteristics of hepatocellular carcinoma using ultrasound-based radiomics. Sci Rep. (2024) 14:11538. doi: 10.1038/s41598-024-62457-6
30. Esnaola N, Lauwers G, Mirza N, Nagorney D, Doherty D, Ikai I, et al. Predictors of microvascular invasion in patients with hepatocellular carcinoma who are candidates for orthotopic liver transplantation. J Gastrointestinal Surg. (2002) 6:224–32. doi: 10.1016/S1091-255X(01)00015-4
31. Hu H, Qi S, Zeng S, Zhang P, He L-Y, Wen S, et al. Importance of microvascular invasion risk and tumor size on recurrence and survival of hepatocellular carcinoma after anatomical resection and non-anatomical resection. Front Oncol. (2021) 11:621622. doi: 10.3389/fonc.2021.621622
32. Xu X, Zhang H-L, Liu Q, Sun S, Zhang J, Zhu F, et al. Radiomic analysis of contrast-enhanced CT predicts microvascular invasion and outcome in hepatocellular carcinoma. J Hepatol. (2019) 70:1133–44. doi: 10.1016/j.jhep.2019.02.023
33. Yang L, Gu D, Wei J, Yang C, Rao S, Wang W, et al. A radiomics nomogram for preoperative prediction of microvascular invasion in hepatocellular carcinoma. Liver Cancer. (2019) 8:373–86. doi: 10.1159/000494099
34. Usset J, Staicu A-M, and Maity A. Interaction models for functional regression. Comput Stat Data Anal. (2016) 94:317–29. doi: 10.1016/j.csda.2015.08.020
35. Luo R and Qi X. Interaction model and model selection for function-on-function regression. J Comput Graphical Stat. (2019) 28:309–22. doi: 10.1080/10618600.2018.1514310
36. Grossmann P, Stringfield O, El-Hachem N, Bui MM, Rios Velazquez E, Parmar C, et al. Defining the biological basis of radiomic phenotypes in lung cancer. ELife. (2017) 6. doi: 10.7554/eLife.23421
37. La Greca Saint-Esteven A, Vuong D, Tschanz F, van Timmeren JE, Dal Bello R, Waller V, et al. Systematic review on the association of radiomics with tumor biological endpoints. Cancers. (2021) 13. doi: 10.3390/cancers13123015
38. Niculescu-Mizil A and Caruana R. (2005). Predicting good probabilities with supervised learning, in: Machine Learning, Proceedings of the Twenty-Second International Conference (ICML 2005), Association for Computing Machinery (ACM), New York, NY, USA.
39. Fernández A, García S, Herrera F, and Chawla NV. SMOTE for learning from imbalanced data: progress and challenges, marking the 15-year anniversary. J Artif Int Res. (2018) 61:863–905. doi: 10.1613/jair.1.11192
40. Zadrozny B and Elkan C. Transforming classifier scores into accurate multiclass probability estimates. In: Proceedings of the eighth ACM SIGKDD international conference on Knowledge discovery and data mining. Association for Computing Machinery, Edmonton, Alberta, Canada (2002). doi: 10.1145/775047.775151
Keywords: hepatocellular carcinoma, microvascular invasion, ultrasound radiomics, intratumoral and peritumoral features, logistic regression, predictive modeling
Citation: Fu H, Huang Y, Lu B, Yu J, Zhou D, Hou C, Xu L and Qian H (2025) Integrating intratumoral, peritumoral, and clinical features in an ultrasound-based radiomics model: contributions and synergies for predicting microvascular invasion in hepatocellular carcinoma. Front. Oncol. 15:1566105. doi: 10.3389/fonc.2025.1566105
Received: 27 January 2025; Accepted: 13 August 2025;
Published: 01 September 2025.
Edited by:
Guoliang Qiao, Massachusetts General Hospital and Harvard Medical School, United StatesCopyright © 2025 Fu, Huang, Lu, Yu, Zhou, Hou, Xu and Qian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongwei Qian, cWh3c3hzcm15eUAxNjMuY29t
†These authors have contributed equally to this work
 Hong Fu1,2†
Hong Fu1,2† Yanhua Huang
Yanhua Huang Baochun Lu
Baochun Lu Jianhua Yu
Jianhua Yu Hongwei Qian
Hongwei Qian