- 1Department of Clinical Laboratory, WeiFang People’s Hospital, Shandong Second Medical University, Weifang, China
- 2School of Medical Laboratory, Shandong Second Medical University, Weifang, China
As a malignant tumor with high morbidity and mortality, lung cancer is associated with a variety of risk factors, including smoking, exposure to occupational carcinogens, familial inheritance, and chronic lung disease. Lung cancer is often detected late and has a complex pathogenesis, so early diagnosis and intervention of lung cancer are essential. Finding effective targets is important to develop new treatments for lung cancer. As a member of Group 4A of the nuclear receptor subfamily, Nuclear Receptor Subfamily 4 Group A Member 1 (NR4A1) is an immediate early gene that encodes a transcription factor that plays a regulatory role when the cell and tissue microenvironment changes. NR4A1 plays a pro-cancer role in solid tumors including lung cancer, but a tumor suppressor role in hematological malignancies. NR4A1 palys a role through multiple mechanisms in lung cancer, including promoting cell proliferation by forming a complex with p300/specific protein 1 (Sp1) and acting on the survivin and AMP-activated protein kinase (AMPK)/mechanistic Target of Rapamycin Complex 1 (mTORC1) pathways, promoting metastasis and invasion by inducing the occurrence of transforming growth factor-β (TGF-β) dependent epithelial-mesenchymal transition (EMT), promoting vascular remodeling by acting on vascular endothelial growth factor A (VEGF-A), promoting immune escape by acting on programmed cell death-1 (PD-1) dependent T cell exhaustion, promoting cell apoptosis interacted with B-cell lymphoma-2 (Bcl-2) and promoting metabolic reprogramming by increasing fatty acid oxidation. In recent years, several studies on NR4A1-related agonists and inhibitors in lung cancer have been reported. These compounds are expected to become drugs for targeted tumor therapy, but current research is limited to cellular and animal experiments. It still takes time to verify and evaluate clinical applications, other biological effects and potential side effects. This review summarizes the biological role of NR4A1 in lung cancer and describes the molecular mechanisms and signaling pathways regulated by NR4A1. This paper will provide a theoretical basis for the early treatment of lung cancer by using NR4A1-related compounds in the clinic.
1 Introduction
According to global cancer statistics, in 2022, the number of lung cancer cases reached 2.481 million cases, accounting for 12.4% of cancers worldwide. Lung cancer accounts for 18.7% of all cancer deaths, ranking first among all types of cancer (1). The American Cancer Society 2025 model estimates 226,650 new cases of lung cancer and 124,730 deaths in the United States in 2025 (2). Its high incidence and mortality are important issues in the field of global public health. From a histologic point of view, lung cancer is mainly classified into two major types: small cell lung carcinoma (SCLC) and non-small cell lung carcinoma (NSCLC). NSCLC accounts for about 80%–85% of lung cancer cases and can be further subdivided into three main subtypes: adenocarcinoma, squamous cell carcinoma, and large cell carcinoma (3). Lung cancer is highly insidious, so most of the patients are in the middle to late stage when they are diagnosed, and the primary tumor is often accompanied by localized or distant metastasis. The treatment strategies for lung cancer include surgical resection (4), chemotherapy (5), radiotherapy (6), targeted therapy (7, 8), immunotherapy (9, 10), nano drug delivery therapy system (11), molecular targeted treatment system (12), photothermal treatment strategy (13). Despite the various treatment modalities, the prognosis of lung cancer is still poor and the five-year survival rate of patients is relatively low. The search for new treatment methods for lung cancer and the identification of key target genes have become important tasks that need to be solved urgently.
Recent studies have found that nuclear receptor NR4A1 is widely expressed in a variety of tumors, including lung cancer. NR4A1, a transcription factor, regulates related signaling pathways and participates in tumor cell proliferation (14), migration (15), invasion (16), apoptosis (17) and immunoregulation (18). The aim of this article is to summarize the regulatory role and mechanism of NR4A1 in lung cancer to provide a sound theoretical basis for the clinical treatment of lung cancer.
2 Structure and role in oncology of NR4A1
NR4A1 (Nuclear Receptor subfamily 4 group A member 1, also known as Nur77/TR3) is an early stress gene that acts as an important transcription factor to regulate the expression of multiple target genes (19). NR4A1 is also thought to mediate tumor metabolism (20). In addition to NR4A1, the NR4A nuclear receptor subfamily also includes NR4A2 and NR4A3. The three exhibits similar structures, including a DNA-binding domain (DBD), C-terminal ligand binding domain (LBD) and N-terminal trans-activation domain (TAD) (Figure 1). The intermediate DBD can interact specifically with the DNA sequence of NBRE and NurRE. The TAD contains the ligand-independent activation function 1 (AF-1) region, which regulates the activity of transcription factors. The DBD can form a response element with NBRE (AAAGGTCA) or interact with NurRE (TGATATTTX6AAATGCCA) DNA sequences as a homodimer or heterodimer (21). NR4A1 forms a heterodimer with the retinoid X receptor (RXR), which then binds to the DR5 response element to produce transcriptional activation (sequence: AGGTCA-NNNAA-AGGTCA) (22, 23). In addition to the three binding modes mentioned above, NR4A1 forms DNA-binding complexes with Sp1 and p300 to exert transcriptional activation in lung and pancreatic cancer cells (24, 25). The LBD contains the ligand-dependent activation function 2 (AF-2) region, which recognizes the corresponding ligand to ensure transcriptional activity (26, 27).
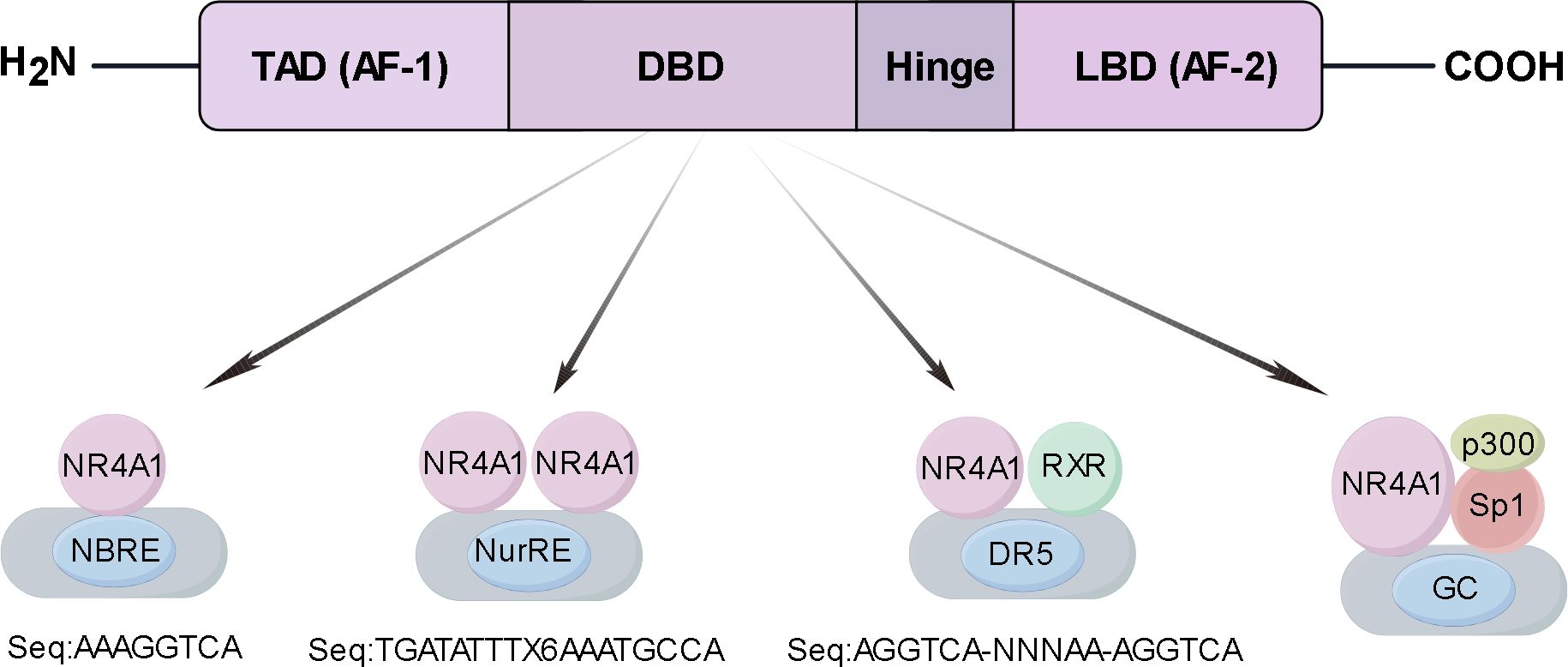
Figure 1. A NR4A1 structure and functional domains schematic. Structure of NR4A1 including DNA-binding domain (DBD), C-terminal ligand-binding domain (LBD), hinge region (Hinge), and N-terminal trans-activating domain (TAD). The DBD region interacts specifically with the DNA sequences of the NBRE and the NurRE. NR4A1 forms a heterodimer with the retinoid X receptor (RXR), which then binds to the DR5 response element to produce transcriptional activation. NR4A1 forms DNA-binding complexes with Sp1 and p300 to exert transcriptional.
NR4A1 was initially characterized as a gene inducible by serum growth factors, and its overexpression has been found in a variety of solid tumors (28–30). In breast cancer, inflammatory factors can induce NR4A1 expression both in vivo and in vitro. Whole-genome cDNA screening results showed that nuclear receptor NR4A1 is a strong activator of transforming growth factor-β (TGF-β) signaling, which can enhance the migration, invasion, and metastasis of breast cancer cells (31). The long non-coding RNA MALAT1 modulates NR4A1 expression through a downstream regulatory element in breast cancer cells (32). NR4A1 is highly expressed in high-grade serous ovarian cancer samples with poor progression-free survival and is mainly localized in the cytoplasm and nucleus (33). NR4A1 regulates endoplasmic reticulum stress and Reactive oxygen species (ROS) levels in pancreatic cancer cells to promote cell proliferation and survival (34). Immunohistochemical staining of 20 colon tumors and 20 normal colon tissues showed that the proportion of colon tumors with high NR4A1 staining was as high as 60% (12/20), while that of normal colon tissues was only 10% (2/20) (35). NR4A1 promotes invasion and metastasis of colorectal cancer cells by up-regulating matrix metalloproteinase-9 and subsequently down-regulating E-cadherin (36). Cheng et al. findings suggest that targeting NR4A1 with OSI-930 may be a promising therapeutic strategy for COAD patients with high levels of immune infiltration (37). Although NR4A1 expression promotes the growth of these tumors, NR4A1 regulated by long non-coding RNAs activates the apoptosis signaling pathway and inhibits the progression of endometrioid endometrial carcinoma (38). NR4A1 is also required for melanoma growth (39). Phosphoserine phosphatase reduces 2-hydroxyglutarate levels and inhibits histone demethylases in melanoma cells, upregulates NR4A1 expression and promotes tumor growth and metastasis (40). NR4A1 also plays an important role in the developmental process of bladder cancer (41). In addition, NR4A1 showed opposite effects in hematologic tumors compared with solid tumors. Low expression of NR4A1 has been found in acute myeloid leukemia (42–44) and chronic myelodysplastic/myeloproliferative diseases (45), where it exhibits tumor-suppressive effects (Figure 2A). These conflicting roles of NR4A1 that NR4A1 may play different roles depending on the type and location of the cancer.
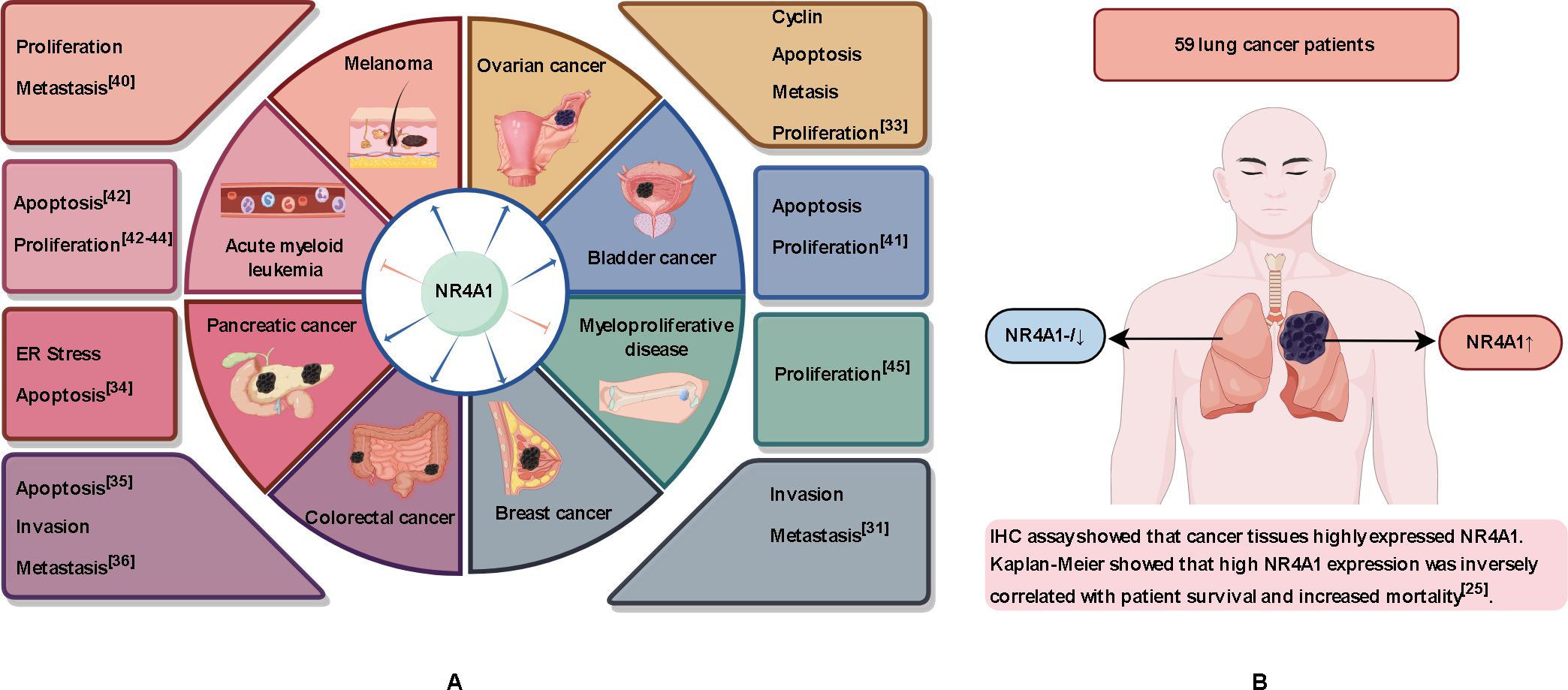
Figure 2. Role of NR4A1 in different tissues. (A) NR4A1 plays a tumor-promoting role in lung, breast, colorectal, pancreatic, melanoma, and bladder cancers. NR4A1 exerts an inhibitory effect in acute myeloid leukemia and myeloproliferative diseases. (B) NR4A1 expression is normal or low in tissues of healthy individuals and in tissue adjacent to the tumor in lung cancer patients, but NR4A1 is overexpressed in cancerous tissues.
3 Correlation between NR4A1 expression and lung cancer
The expression level of NR4A1 varies with the subcellular localization. NR4A1 plays a multi-effect regulatory role and although it is generally accepted that NR4A1 has a pro-cancer effect, the correlation between high expression and adverse clinical outcomes is controversial. Seong et al. collected RNA-seq data from 1013 lung cancer cases and 397 normal tissue samples from the TCGA and GTEx databases and found that NR4A1 expression was lower in lung adenocarcinoma (LUAD) and lung squamous cell carcinoma (LUSC) than in normal tissue (46). Huang et al. similarly performed RNA-seq using tumor and tumor-adjacent tissues from four LUAD patients and found low expression of NR4A1 in the cancer tissues (47). We searched the GEPIA and TissGDB databases and found that the expression of NR4A1 was significantly reduced in LUAD and LUSC. However, Lee et al. collected tissue from 59 patients with NSCLC and adjacent normal lung tissue and, by immunohistochemical analysis, showed that NR4A1 was expressed in lung cancer but there was low or no expression in normal lung tissue (Figure 2B). Overexpression of NR4A1 is associated with reduced survival and poor clinical outcomes in patients with NSCLC (25). Yang et al. used the STRING database to show that NR4A1 expression correlates with RNA polymerase I subunit B (POLR1B) activity, and POLR1B is an important modulator of lung cancer cell proliferation (48). Although the role of NR4A1 in lung cancer remains to be verified, a growing number of studies have found that NR4A1 plays a pro-cancer role in lung cancer (49–51).
4 Role and molecular mechanism of NR4A1 in the pathogenesis of lung cancer
The role of NR4A1 in lung cancer involves multiple aspects, including transcriptional regulation, protein-protein interactions, and post-translational modifications. NR4A1 is regulated at both the transcriptional and post-transcriptional levels and regulates downstream signaling pathways involved in lung cancer processes, including angiogenesis, cell proliferation, migration, invasion, apoptosis, and immune regulation (Figure 3). The role of NR4A1 also depends on the subcellular localization, expression level, and co-activator/co-repressor factors. NR4A1 interferes with intracellular regulation at different levels through various signaling pathways associated with many cancers, and understanding these interactions may elucidate the role of this family member in tumorigenesis and tumor suppression.
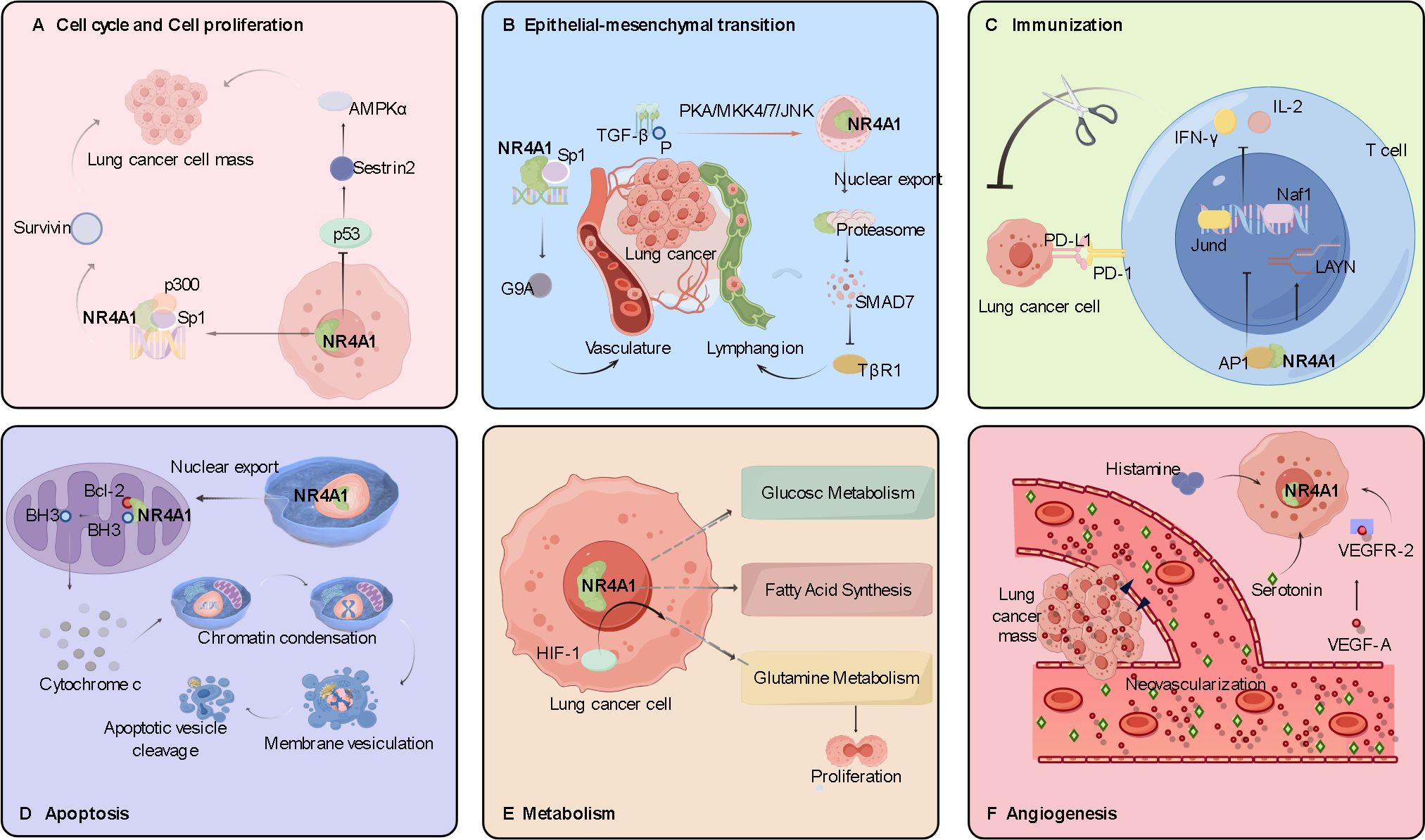
Figure 3. Role and molecular mechanism of NR4A1 in the pathogenesis of lung cancer. (A) The role of NR4A1 in lung cancer cell proliferation. The NR4A1/Sp1/p300 complex upregulates survivin; NR4A1 inhibits p53 acetylation, which in turn induces activation of the AMPK/mTORC1 pathway. (B) The role of NR4A1 in lung cancer cell migration and invasion. The NR4A1/Sp1 complex promotes G9A expression; NR4A1 export from the nucleus is induced by phosphorylation of the TGF-β/TGF-β receptor. (C) The role of NR4A1 in immunomodulation. The NR4A1/AP-1 complex decreases tumor cell killing by T cells by reducing IFN-γ and IL-2 secretion. (D) The role of NR4A1 in apoptosis of lung cancer cells. Nuclear export of NR4A1 indirectly induces apoptosis by NR4A1 binding to mitochondrial Bcl-2. (E) The role of NR4A1 in lung cancer cell metabolism. (F) The pro-angiogenic role of NR4A1 in lung cancer cells. VEGF-A binding to VEGF-A receptor 2 upregulates NR4A1 expression and participates in angiogenesis.
4.1 Cell cycle and cell proliferation
Molecules that inhibit tumor cell proliferation or cell cycle checkpoints play an important role in alleviating tumor progression. Stimulation by serum and epidermal growth factor induces trans-activation of NR4A1 in H460 and Calu-6 lung cancer cells, and high expression of NR4A1 promotes cell cycle progression, exerting a positive effect on mitosis in lung cancer cells (49). Zhang et al. found that NR4A1 promotes cell mitosis and survival via its transcriptional activity in the nucleus (50). Lee’s group found that NR4A1 promotes the proliferation of lung cancer cells in two ways. The first involves the expression of p300, which has histone acetyltransferase activity. In A549 and H460 cells, p300 enhances NR4A1 acetylation and protein stability. NR4A1 interacts with specific protein 1 (Sp1) or specific protein 4 (Sp4) to form the NR4A1-Sp1/Sp4-p300 DNA-binding complex. This complex binds to GC-rich promoters and upregulates survivin expression, thereby promoting lung cancer cell survival. The second way that NR4A1 promotes lung cancer cell proliferation is by inhibiting p53 expression to induce Adenosine 5’-monophosphate (AMP)-activated protein kinase (AMPK)/mechanistic Target of Rapamycin Complex 1 (mTORC1) pathway activation (25).
4.2 Epithelial-mesenchymal transition
Tumor cell metastasis is the main cause of lung cancer patient mortality. Tumor cells can acquire invasive ability through epithelial-mesenchymal transition (EMT), after which they invade vascular or lymphatic vessels and ultimately distant organs. The high metastasis and mortality rates of lung cancer are related to EMT in lung tumors (51–53). TGF-β acts as a multifunctional regulator of migration and upregulates the expression of key EMT regulators, such as Snail and δEF1/SIP1 (51, 54, 55). Erik’s group found that TGF-β-induced migration depends on the nuclear export of NR4A1 in cell lines such as A549, H460, and H1299, and the migration could be blocked by the NR4A1 inhibitor DIM-C-pPhOH (56). Paraspeckle Component 1 (PSPC1) is an activator of TGF-β-dependent EMT. Safe et al. found that NR4A1, by acting as a transcription factor, enhances the promoter sequence activation of the PSPC1 gene, thereby promoting EMT (57). Fan et al. similarly concluded that the nuclear long non-coding RNA (lncRNA) LETS1 inhibits SMAD7-induced TGF-β type I receptor polyubiquitylation through activation of NR4A1 expression in A549 cells, thereby promoting TGF-β-induced EMT migration and extravasation of cancer cells (58).
4.3 Angiogenesis
Tumor growth requires the formation of new blood vessels to deliver oxygen and nutrients. Vascular endothelial growth factor (VEGF) is a highly specific pro-vascular endothelial cell growth factor, which plays a key role in tumorigenesis and development (59). Using a DNA microarray assay, Zeng et al. found that NR4A1 gene expression was upregulated in human umbilical vein endothelial cells (HUVECs), and knockdown of NR4A1 limited the effect of VEGF-A on HUVECs and inhibited tumor angiogenesis (60). It has also been reported that histamine and serotonin play positive roles in angiogenesis Previously, it was also reported that histamine and serotonin play positive roles in angiogenesis (61). Qin et al. implanted histamine or serotonin pellets subcutaneously in wild-type mice and found that both induced angiogenesis in a dose-dependent manner, but little angiogenesis occurred in NR4A1-/- mice (62).
4.4 Immunomodulation
Immunomodulation is involved in the entire process of tumorigenesis and development. Tumor cells can evade immune surveillance as well as resist immune defense in various ways, such as through gene mutation or tumor antigen defects. In recent years, more and more studies have shown that NR4A1 can help tumor cells achieve immune escape by affecting the function of immune cells in the tumor microenvironment (TME), and then regulate tumor development (63–66).
In the immune system, NR4A1 regulates the immune response mainly by inhibiting the recognition and proliferation of T cells, thus reducing the body’s ability to monitor and attack lung cancer cell. Liu et al. identified NR4A1 as a key molecule in T cell dysfunction by genome-wide analysis, and NR4A1 was stably highly expressed in tolerant T cells (Ttol). In T cells, overexpression of NR4A1 in combination with activating protein-1 (AP-1) inhibits the expression of effector genes, such as Jund and Naf1, which reduces their secretion of interferon-gamma (IFN-γ) and Interleukin-2 (IL-2), leading to a significant decrease in the tumor-killing effect of T cells (67). Yang et al. also found that NR4A1 upregulates the LAYN gene at the transcriptional level, thereby inhibiting the killing function of CD8+ T cells against LUAC (68). Sana et al. found that tumor growth was significantly inhibited in Mice lacking NR4A1 and NR4A2 genes specifically in Tregs subcutaneously inoculated with Lewis lung carcinoma cells, and inhibition of NR4A1 in tumor-infiltrating regulatory T cells (TI-Tregs) breaks down the immune tolerance to tumor cells and promotes the antitumor activity of tumor-infiltrating CD8+ T cells (69).
In recent years, chimeric antigen receptor (CAR) T cell therapy has been widely used in leukemia and lymphoma but is less effective in solid tumors, such as lung cancer. NR4A1 promotes the expression of inhibitory receptors, such as programmed cell death-1 (PD-1), leading to the depletion or dysfunction of CAR T cells, which ultimately allows lung cancer cells to evade the immune response (70, 71). Kensuke et al. transferred NR4A1/2/3 gene knockout CAR T-cells into A549 tumor-bearing immunodeficient mice and reached a similar conclusion (72). The above studies indicate that NR4A1 depletes T cells, inhibiting the proliferation and killing function of T cells, and is thus a promising tumor immunotherapy target for mediating T cells.
4.5 Apoptosis
NR4A1 plays a pro-apoptotic role in a variety of cancers, in part because of its localization in the nucleus. When NR4A1 translocates from the nucleus to the mitochondria, it interacts directly with the Bcl-2 protein and change the Bcl-2 conformation, exposing the pro-apoptotic BH3 domain, which triggers the release of cytochrome c and indirectly induces apoptosis (73–81). TIAM1, a small GTPase RAC1 activator, interacts with NR4A1 in the nucleus of SCLC cells to reduce their cell viability and tumorigenicity. Malayoside, an extract from Antiaris toxicaria Lesch, activates ERK1/2 and p38 and phosphorylates NR4A1 in H460 cells, prompting NR4A1 to translocate to the mitochondria (82). NR4A1 also binds to the promoter of the anti-apoptotic protein BRE, exerting a pro-apoptotic effect by interfering with BRE function (83). Martin et al. found that NR4A1-derived peptides can induce apoptosis in paclitaxel-resistant cancer cells by acting on Bcl-2 (84). Liu et al. Found that quinoline derivative 10E modulates the pro-apoptotic nuclear export of NR4A1 in A549 and H460 cells (85). These findings suggest that the effect of NR4A1 on Bcl-2 provides a theoretical basis for targeted cancer therapy.
4.6 Metabolic reprogramming
Tumor cells provide substrates and energy for themselves through metabolic reprogramming activities, such as glycolysis, glutamine metabolism, fatty acid metabolism, and nucleic acid and amino acid metabolism, to promote tumor cell activity. NR4A family receptors are considered mediators of metabolic markers in tumors. Holla et al. found that NR4A1 plays a pro-oncogenic role in the regulation of fatty acid oxidation pathways in colon cancer (86). In breast cancer and melanoma, Poirot et al. found that the cholesterol metabolite dendrogenin A activates NR4A1 expression and exhibits tumor suppressor effects (87). Dysregulation of glutamine metabolism occurs in a variety of solid tumor cells and is essential for cancer cell proliferation (88). Hypoxia-inducible factor 1 (HIF-1) is an important regulator of glutamine metabolism, and Christoph et al. found that the expression of HIF-1 and NR4A1 was upregulated in A549 cells (89). The expression of NR4A1 in metabolic pathways is expected to inhibit the abnormal metabolism of tumor cells. NR4A1 potentially leads to targets for the control of tumor development.
5 Research progress of NR4A1 inhibitors or activators
Targeted therapies can attack tumor cells more precisely than chemotherapy and can reduce the incidental killing of normal cells. In recent years, studies have shown that targeted inhibition of NR4A1 can play an important role in preventing the development of lung cancer. In future clinical applications, in addition to the use of cisplatin, Nimotuzumab, and Bevacizumab alone in the treatment of lung cancer, the combination of NR4A1-targeted drugs with monoclonal antibody chemotherapy may provide a better treatment option for patients.
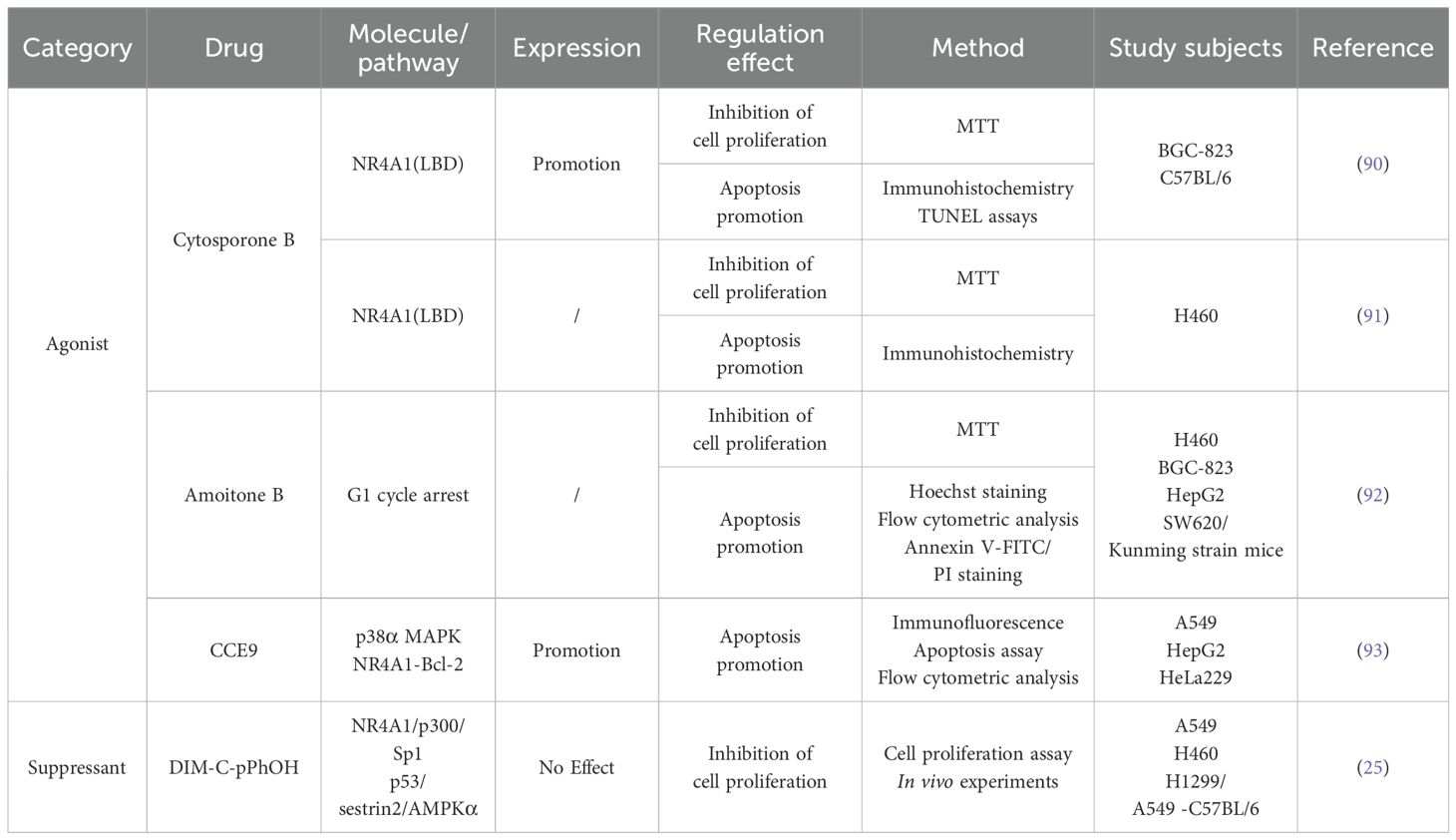
Cytosporone B (Csn-B), a natural agonist of NR4A1, specifically binds to the LBD region of NR4A1 and enhances the trans-activation of NR4A1. Wu’s laboratory found that Csn-B promotes the translocation of NR4A1 from the nucleus to the mitochondria, mediating the onset of apoptosis. They also measured the proliferative effects of Csn-B on several tumor cell lines and found that Csn-B inhibited the proliferation of BGC-823 human gastric cancer cells and SW620 human colon cancer cells by >70%, but inhibited H1299 human lung cancer cells and HepG2 human liver cancer cells by ≥40% (90). Dawson et al. showed that the interaction of Csn-B with the NR4A1 LBD region inhibited the viability of H460 lung cancer cells with a weaker efficiency than the positive control, DIM-Ph-4-CF3 (91). Amoitone B, a Csn-B analog nanocrystal, preferentially targets lung tissue and may be a potentially effective antitumor agent, although it has only a moderate inhibitory effect on H460 cells (92). The compound CCE9, which is extracted from Chinese herbal plants, can induce NR4A1 expression and Bcl-2 phosphorylation, leading to NR4A1 cytoplasmic localization and induction of the NR4A1-Bcl-2 apoptosis pathway in a p38α MAPK-dependent manner (93). The above results suggest that Csn-B and its derivatives have pro-apoptotic effects in a variety of tumor cells, but their antitumor effects are selective. Therefore, the inhibitory effect of Csn-B and its derivatives on lung cancer cells needs to be further studied.
DIM-C-pPhOH (C-DIM-8), a cruciferous plant-derived indole compound, and its derivatives are commonly employed in research exploring cancer cell proliferation and apoptotic pathways (94, 95). Lee et al. found that in lung cancer cells (A549, H460, and H1299), DIM-C-pPhOH reduced NR4A1 trans-activation and inactivated the NR4A1/p300/Sp1 complex, which in turn exhibited antitumor activity and low toxicity (25). Two C-DIM analogs, DIM-C-pPhOCH3 (C-DIM-5) and C-DIM-8, induced apoptosis in A549 cells, leading to a G0/G1-to-S phase block and tumor growth inhibition (96). Kumaravel found that C-DIM reduced PSPC1-mediated TGFβ cancer-promoting activity by inhibiting NR4A1, the upstream regulator of PSPC1 (57) Summary of the main targeted drugs is shown in Table 1.
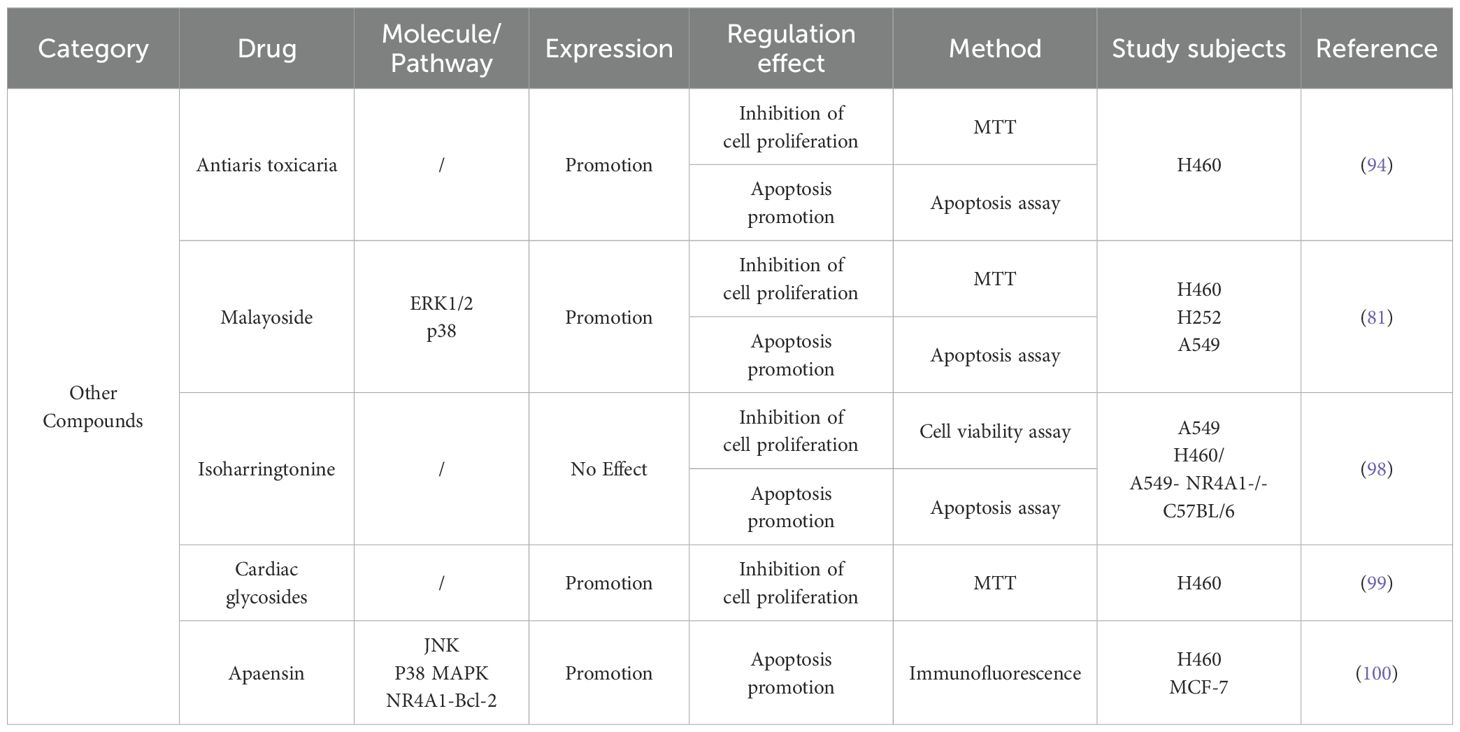
In addition to C-DIM, coumarin derivative apaensin has been found to be an inducer targeting NR4A1, which has anticancer effects by regulating the NR4A1-Bcl-2 apoptotic pathway (97). A coumarin derivative extracted from Arrowwood Antiaris toxicaria (98), resveratrol extracted from fruits and vegetables (99), cardiac glycosides (100), malayoside (82), isoharringtonine (IHT) (101) and other compounds have similar effects on lung cancer cell and exert anticancer effects by inhibiting lung cancer cell growth, inducing NR4A1 nuclear export, and activating NR4A1-Bcl-2 apoptosis pathway. Other compounds are shown in Table 2.
The above natural and synthetic compounds can affect the expression and action of NR4A1 and have a good prospect of becoming the basis of tumor-targeted therapy. However, the effect is limited to molecular, cellular, and animal experiments, and it will take time to verify and evaluate the clinical applications, other biological effects, and potential side effects.
6 Summary and prospect
According to our current understanding, NR4A1 plays an important role in the occurrence and development of lung cancer. NR4A1 is a potential antitumor drug target, and precise targeting of NR4A1 can induce tumor cell apoptosis and inhibit cell growth. However, there is still a long way to go in researching NR4A1 as a target for chemoprevention of lung cancer. By using single-cell RNA sequencing technology combined with spatial transcriptomics, the dynamic changes of heterogeneity within tumors can be analyzed, especially in the constantly evolving tumor microenvironment. Future research directions can be more inclined to search for molecular targets that address intratumoral heterogeneity. In addition, the role of NR4A1 in cancer depends on the degree of expression and the subcellular site and is characterized by tissue selectivity. The development of subcellular specific NR4A1 modulators may be a direction for future precision therapy.
Author contributions
MJ: Writing – original draft, Conceptualization. YW: Supervision, Writing – review & editing. MS: Software, Writing – original draft. WG: Data curation, Writing – original draft. SL: Writing – review & editing, Project administration. ZP: Funding acquisition, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was funding by project ZR202111160201 supported by Shandong Provincial Natural Science Foundation (ZP).
Acknowledgments
Figures were created with Figdraw.com by our team. We appreciate the graphic design materials and platform provided by Figdraw.com (https://www.figdraw.com/static/index.html). We thank Dr. Xiang-dong Wang for his suggestions and help with this manuscript. We thank Matthew Grimshaw, PhD, from Liwen Bianji (Edanz) (www.liwenbianji.cn), for modifying the English text of a draft of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
2. Siegel RL, Kratzer TB, Giaquinto AN, Sung H, and Jemal A. Cancer statistics, 2025. CA: A Cancer J Clin. (2025) 75:10–45. doi: 10.3322/caac.21871
3. Popper H. Pathologic diagnosis of lung cancer – recent developments. Curr Opin Oncol. (2024) 36:57. doi: 10.1097/CCO.0000000000001011
4. Niu Z, Cao Y, Du M, Sun S, Yan Y, Zheng Y, et al. Robotic-assisted versus video-assisted lobectomy for resecta ble non-small-cell lung cancer: the RVlob randomized controlled trial. eClinicalMedicine. (2024) 74:102707. doi: 10.1016/j.eclinm.2024.102707
5. Forde PM, Spicer J, Lu S, Provencio M, Mitsudomi T, Awad MM, et al. Neoadjuvant Nivolumab plus Chemotherapy in Resectab le Lung Cancer. New Engl J Med. (2022) 386:1973–85. doi: 10.1056/NEJMoa2202170
6. Levy A, Adebahr S, Hurkmans C, Ahmed M, Ahmad S, Guckenberger M, et al. Stereotactic body radiotherapy for centrally located inoperable early-stage NSCLC: EORTC 22113–08113 lungTech phase II trial results. J Thorac Oncol. (2024) 19:1297–309. doi: 10.1016/j.jtho.2024.05.366
7. Wathoni N, Puluhulawa LE, Joni IM, Muchtaridi M, Mohammed AFA, Elamin KM, et al. Monoclonal antibody as a targeting mediator for nanoparticle targeted delivery system for lung cancer. Drug Delivery. (2022) 29:2959–70. doi: 10.1080/10717544.2022.2120566
8. Bouchard N and Daaboul N. Lung cancer: targeted therapy in 2025. Curr Oncol. (2025) 32:146. doi: 10.3390/curroncol32030146
9. Huang S, Huang Z, Huang X, Luo R, Liang W, and Qin T. Comparative long-term outcomes of pembrolizumab plus chemotherapy versus pembrolizumab monotherapy as first-line therapy for metastatic non-small-cell lung cancer: a systematic review and network meta-analysis. Front Immunol. (2024) 15:1375136. doi: 10.3389/fimmu.2024.1375136
10. Dennehy C, Conroy MR, and Forde PM. Immunotherapy for resectable lung cancer. Cancer. (2025) 131:e35849. doi: 10.1002/cncr.35849
11. Pi C, Zhao W, Zeng M, Yuan J, Shen H, Li K, et al. Anti-lung cancer effect of paclitaxel solid lipid nanoparticles delivery system with curcumin as co-loading partner in vitro and in vivo. Drug Delivery. (2022) 29:1878–91. doi: 10.1080/10717544.2022.2086938
12. Araghi M, Mannani R, Heidarnejad maleki A, Hamidi A, Rostami S, Safa SH, et al. Recent advances in non-small cell lung cancer targeted therapy; an update review. Cancer Cell Int. (2023) 23:162. doi: 10.1186/s12935-023-02990-y
13. Saleem HM, Ramaiah P, Gupta J, Jalil AT, Kadhim NA, Alsaikhan F, et al. Nanotechnology-empowered lung cancer therapy: From EMT role in cancer metastasis to application of nanoengineered structures for modulating growth and metastasis. Environ Res. (2023) 232:115942. doi: 10.1016/j.envres.2023.115942
14. Jiang C, He J, Xu S, Wang Q, and Cheng J. NR4A1 promotes LEF1 expression in the pathogenesis of papillary thyroid cancer. Cell Death Discov. (2022) 8:1–8. doi: 10.1038/s41420-022-00843-7
15. Li W, Shi Y, Guo Y, and Tian S. Nur77 promotes invasion and migration of gastric cancer cells through the NF-κB/IL-6 pathway. J South Med Univ. (2022) 42:1410–7. doi: 10.12122/j.issn.1673-4254.2022.09.19
16. Liu Z, Gu Y, Cheng X, Jiang H, Huang Y, Zhang Y, et al. Upregulation lnc-NEAT1 contributes to colorectal cancer progression through sponging miR-486-5p and activating NR4A1/Wnt/β-catenin pathway. Cancer Biomark. (2021) 30:309–19. doi: 10.3233/CBM-201733
17. Chen X, Gao M, Xia Y, Wang X, Qin J, He H, et al. Phase separation of Nur77 mediates XS561-induced apoptosis by promoting the formation of Nur77/Bcl-2 condensates. Acta Pharm Sin B. (2024) 14:1204–21. doi: 10.1016/j.apsb.2023.11.017
18. Mohankumar K, Wright G, Kumaravel S, Shrestha R, Zhang L, Abdelrahim M, et al. Bis-indole-derived NR4A1 antagonists inhibit colon tumor and splenic growth and T-cell exhaustion. Cancer Immunol Immunother. (2023) 72:3985–99. doi: 10.1007/s00262-023-03530-3
19. Deng S, Chen B, Huo J, and Liu X. Therapeutic potential of NR4A1 in cancer: Focus on metabolism. Front Oncol. (2022) 12:972984. doi: 10.3389/fonc.2022.972984
20. Wu L and Chen L. Characteristics of Nur77 and its ligands as potential anticancer compounds (Review). Mol Med Rep. (2018) 18:4793–801. doi: 10.3892/mmr.2018.9515
21. Jiang L, Dai S, Li J, Liang X, Qu L, Chen X, et al. Structural basis of binding of homodimers of the nuclear receptor NR4A2 to selective Nur-responsive DNA elements. J Biol Chem. (2019) 294:19795–803. doi: 10.1074/jbc.RA119.010730
22. Cao X, Liu W, Lin F, Li H, Kolluri SK, Lin B, et al. Retinoid X receptor regulates nur77/thyroid hormone receptor 3-dependent apoptosis by modulating its nuclear export and mitochondrial targeting. Mol Cell Biol. (2004) 24:9705–25. doi: 10.1128/MCB.24.22.9705-9725.2004
23. Lévesque D and Rouillard C. Nur77 and retinoid X receptors: crucial factors in dopamine-related neuroadaptation. Trends Neurosci. (2007) 30:22–30. doi: 10.1016/j.tins.2006.11.006
24. Lee S-O, Abdelrahim M, Yoon K, Chintharlapalli S, Papineni S, Kim K, et al. Inactivation of the orphan nuclear receptor TR3/nur77 inhibits pancreatic cancer cell and tumor growth. Cancer Res. (2010) 70:6824–36. doi: 10.1158/0008-5472.CAN-10-1992
25. Lee S-O, Andey T, Jin U-H, Kim K, Sachdeva M, and Safe S. The Nuclear Receptor TR3 Regulates mTORC1 Signaling in Lung Cancer Cells Expressing Wild-type p53. Oncogene. (2012) 31:3265–76. doi: 10.1038/onc.2011.504
26. Kumar R and Thompson EB. The structure of the nuclear hormone receptors. Steroids. (1999) 64:310–9. doi: 10.1016/S0039-128X(99)00014-8
27. To SK, Zeng J-Z, and Wong AS. Nur77: a potential therapeutic target in cancer. Expert Opin Ther Targets. (2012) 16:573–85. doi: 10.1517/14728222.2012.680958
28. Safe S and Karki K. The paradoxical roles of orphan nuclear receptor 4A (NR4A) in cancer. Mol Cancer Res. (2021) 19:180–91. doi: 10.1158/1541-7786.MCR-20-0707
29. Hailemariam A, Upadhyay S, Srivastava V, Hafiz Z, Zhang L, Tsui WNT, et al. Perfluorooctane sulfonate (PFOS) and related compounds induce nuclear receptor 4A1 (NR4A1)-dependent carcinogenesis. Chem Res Toxicol. (2025) 38:705–16. doi: 10.1021/acs.chemrestox.4c00528
30. Zhang S, Miao H, Han T, Wu X, Liang C, Qian J, et al. MCM8 promotes NR4A1-mediated E2F1 transcription and facilitates renal cell carcinoma through enhancing aerobic glycolysis. Cell Biol Toxicol. (2025) 41:51. doi: 10.1007/s10565-025-10002-0
31. Zhou F, Drabsch Y, Dekker TJA, de Vinuesa AG, Li Y, Hawinkels LJAC, et al. Nuclear receptor NR4A1 promotes breast cancer invasion and metastasis by activating TGF-β signalling. Nat Commun. (2014) 5:3388. doi: 10.1038/ncomms4388
32. Wernig-Zorc S, Schwartz U, Martínez-Rodríguez P, Inalef J, Pavicic F, Ehrenfeld P, et al. The long non-coding RNA MALAT1 modulates NR4A1 expression through a downstream regulatory element in specific cancer cell types. Int J Mol Sci. (2024) 25:5515. doi: 10.3390/ijms25105515
33. Delgado E, Boisen MM, Laskey R, Chen R, Song C, Sallit J, et al. High expression of orphan nuclear receptor NR4A1 in a subset of ovarian tumors with worse outcome. Gynecologic Oncol. (2016) 141:348–56. doi: 10.1016/j.ygyno.2016.02.030
34. Lee S-O, Jin U-H, Kang JH, Kim SB, Guthrie AS, Sreevalsan S, et al. The orphan nuclear receptor NR4A1 (Nur77) regulates oxidative and endoplasmic reticulum stress in pancreatic cancer cells. Mol Cancer Res. (2014) 12:527–38. doi: 10.1158/1541-7786.MCR-13-0567
35. Cho SD, Yoon K, Chintharlapalli S, Abdelrahim M, Lei P, Hamilton S, et al. Nur77 agonists induce proapoptotic genes and responses in colon cancer cells through nuclear receptor–dependent and nuclear receptor–independent pathways. Cancer Res. (2007) 67:674–83. doi: 10.1158/0008-5472.CAN-06-2907
36. Wang J-R, Gan W-J, Li X-M, Zhao Y-Y, Li Y, Lu X-X, et al. Orphan nuclear receptor Nur77 promotes colorectal cancer invasion and metastasis by regulating MMP-9 and E-cadherin. Carcinogenesis. (2014) 35:2474–84. doi: 10.1093/carcin/bgu157
37. Li M, Zhang Z, Li M, Chen Z, Tang W, and Cheng X. NR4A1 as a potential therapeutic target in colon adenocarcinoma: a computational analysis of immune infiltration and drug response. Front Genet. (2023) 14:1181320. doi: 10.3389/fgene.2023.1181320
38. Sun L, Zhou R, Dong J, Liu S, Jiao Y, Wang L, et al. Lnc-NA inhibits proliferation and metastasis in endometrioid endometrial carcinoma through regulation of NR4A1. J Cell Mol Med. (2019) 23:4699–710. doi: 10.1111/jcmm.14345
39. Li X, Wang Z, Zheng Y, Guan Y, Yang P, Chen X, et al. Nuclear receptor nur77 facilitates melanoma cell survival under metabolic stress by protecting fatty acid oxidation. Mol Cell. (2018) 69:480–492.e7. doi: 10.1016/j.molcel.2018.01.001
40. Rawat V, Malvi P, Della Manna D, Yang ES, Bugide S, Zhang X, et al. PSPH promotes melanoma growth and metastasis by metabolic deregulation-mediated transcriptional activation of NR4A1. Oncogene. (2021) 40:2448–62. doi: 10.1038/s41388-021-01683-y
41. Dae Cho S, Lee S-O, Chintharlapalli S, Abdelrahim M, Khan S, Yoon K, et al. Activation of nerve growth factor-induced Bα by methylene-substituted diindolylmethanes in bladder cancer cells induces apoptosis and inhibits tumor growth. Mol Pharmacol. (2010) 77:396–404. doi: 10.1124/mol.109.061143
42. Mullican SE, Zhang S, Konopleva M, Ruvolo V, Andreeff M, Milbrandt J, et al. Abrogation of nuclear receptors Nr4a3 and Nr4a1 leads to development of acute myeloid leukemia. Nat Med. (2007) 13:730–5. doi: 10.1038/nm1579
43. Duren RP, Boudreaux SP, and Conneely OM. Genome wide mapping of NR4A binding reveals cooperativity with ETS factors to promote epigenetic activation of distal enhancers in acute myeloid leukemia cells. PloS One. (2016) 11:e0150450. doi: 10.1371/journal.pone.0150450
44. Rahmani B, Rostami S, Mortazavi Y, and Soleiman Soltanpour M. Investigation methylation status of tumor suppressor gene NR4A1 and NR4A3 and frequency of rs1569686 polymorphism of DNMT3B gene in patients with acute myeloid leukemia. Mol Biol Res Commun. (2025) 14:149–56. doi: 10.22099/mbrc.2024.51563.2058
45. Ramirez-Herrick AM, Mullican SE, Sheehan AM, and Conneely OM. Reduced NR4A gene dosage leads to mixed myelodysplastic/myeloproliferative neoplasms in mice. Blood. (2011) 117:2681–90. doi: 10.1182/blood-2010-02-267906
46. Park S-W and Han M-R. A pan-cancer analysis unveiling the function of NR4A family genes in tumor immune microenvironment, prognosis, and drug response. Genes Genomics. (2024) 46:977–90. doi: 10.1007/s13258-024-01539-1
47. Huang F, Xue F, Wang Q, Huang Y, Wan Z, Cao X, et al. Transcription factor-target gene regulatory network analysis in human lung adenocarcinoma. J Thorac Dis. (2023) 15:6996–7012. doi: 10.21037/jtd-23-1688
48. Yang F, Liu H, Zhao J, Ma X, and Qi W. POLR1B is upregulated and promotes cell proliferation in non−small cell lung cancer. Oncol Lett. (2020) 19:671–80. doi: 10.3892/ol.2019.11136
49. Kolluri SK, Bruey-Sedano N, Cao X, Lin B, Lin F, Han Y-H, et al. Mitogenic effect of orphan receptor TR3 and its regulation by MEKK1 in lung cancer cells. Mol Cell Biol. (2003) 23:8651–67. doi: 10.1128/MCB.23.23.8651-8667.2003
50. Zhang X. Targeting nur77 translocation. Expert Opin Ther Targets. (2007) 11:69–79. doi: 10.1517/14728222.11.1.69
51. Shi Y, Wu H, Zhang M, Ding L, Meng F, and Fan X. Expression of the epithelial-mesenchymal transition-related proteins and their clinical significance in lung adenocarcinoma. Diagn Pathol. (2013) 8:89. doi: 10.1186/1746-1596-8-89
52. Lafuente-Sanchis A, Olmo A, Carretero J, Alcacer Fernandez-Coronado J, Estors-Guerrero M, Martínez-Hernández NJ, et al. Clinical significance of epithelial–mesenchymal transition-related markers expression in the micrometastatic sentinel lymph node of NSCLC. Clin Trans Oncol. (2020) 22:381–91. doi: 10.1007/s12094-019-02138-3
53. Li F, Song Q-Z, Zhang Y-F, Wang X-R, Cao L-M, Li N, et al. Identifying the EMT-related signature to stratify prognosis and evaluate the tumor microenvironment in lung adenocarcinoma. Front Genet. (2022) 13:1008416. doi: 10.3389/fgene.2022.1008416
54. Huang L, Liu X, Chen Q, Yang J, Zhang D, Zhao Y, et al. TGF-β-induced lncRNA TBUR1 promotes EMT and metastasis in lung adenocarcinoma via hnRNPC-mediated GRB2 mRNA stabilization. Cancer Lett. (2024) 600:217153. doi: 10.1016/j.canlet.2024.217153
55. Lee JH, Sánchez-Rivera FJ, He L, Basnet H, Chen FX, Spina E, et al. TGF-β and RAS jointly unmask primed enhancers to drive metastasis. Cell. (2024) 187:6182–6199.e29. doi: 10.1016/j.cell.2024.08.014
56. Hedrick E, Mohankumar K, and Safe S. TGFβ-induced lung cancer cell migration is NR4A1-dependent. Mol Cancer Res. (2018) 16:1991–2002. doi: 10.1158/1541-7786.MCR-18-0366
57. Mohankumar K, Shrestha R, and Safe S. Nuclear receptor 4A1 (NR4A1) antagonists target paraspeckle component 1 (PSPC1) in cancer cells. Mol Carcinogenesis. (2022) 61:73–84. doi: 10.1002/mc.23362
58. Fan C, González-Prieto R, Kuipers TB, Vertegaal ACO, van Veelen PA, Mei H, et al. The lncRNA LETS1 promotes TGF-β–induced EMT and cancer cell migration by transcriptionally activating a TβR1-stabilizing mechanism. Sci Signaling. (2023) 16:eadf1947. doi: 10.1126/scisignal.adf1947
59. Li Q-Q, Guo M, He G-H, Xi K-H, Zhou M-Y, Shi R-Y, et al. VEGF-induced Nrdp1 deficiency in vascular endothelial cells promotes cancer metastasis by degrading vascular basement membrane. Oncogene. (2024) 43:1836–51. doi: 10.1038/s41388-024-03038-9
60. Zeng H, Qin L, Zhao D, Tan X, Manseau EJ, Van Hoang M, et al. Orphan nuclear receptor TR3/Nur77 regulates VEGF-A–induced angiogenesis through its transcriptional activity. J Exp Med. (2006) 203:719–29. doi: 10.1084/jem.20051523
61. Zauberman H, Michaelson IC, Bergmann F, and Maurice DM. Stimulation of neovascularization of the cornea by biogenic amines. Exp Eye Res. (1969) 8:77–83. doi: 10.1016/S0014-4835(69)80083-7
62. Qin L, Zhao D, Xu J, Ren X, Terwilliger EF, Parangi S, et al. The vascular permeabilizing factors histamine and serotonin induce angiogenesis through TR3/Nur77 and subsequently truncate it through thrombospondin-1. Blood. (2013) 121:2154–64. doi: 10.1182/blood-2012-07-443903
63. Myers DR, Lau T, Markegard E, Lim HW, Kasler H, Zhu M, et al. Tonic LAT-HDAC7 signals sustain nur77 and irf4 expression to tune naive CD4 T cells. Cell Rep. (2017) 19:1558–71. doi: 10.1016/j.celrep.2017.04.076
64. Karki K, Wright GA, Mohankumar K, Jin U-H, Zhang X-H, and Safe S. A bis-indole–derived NR4A1 antagonist induces PD-L1 degradation and enhances antitumor immunity. Cancer Res. (2020) 80:1011–23. doi: 10.1158/0008-5472.CAN-19-2314
65. Srirat T, Hayakawa T, Mise-Omata S, Nakagawara K, Ando M, Shichino S, et al. NR4a1/2 deletion promotes accumulation of TCF1+ stem-like precursors of exhausted CD8+ T cells in the tumor microenvironment. Cell Rep. (2024) 43:113898. doi: 10.1016/j.celrep.2024.113898
66. Wang L, Xiao Y, Luo Y, Master RP, Mo J, Kim M-C, et al. PROTAC-mediated NR4A1 degradation as a novel strategy for cancer immunotherapy. J Exp Med. (2024) 221:e20231519. doi: 10.1084/jem.20231519
67. Liu X, Wang Y, Lu H, Li J, Yan X, Xiao M, et al. Genome-wide analysis identifies NR4A1 as a key mediator of T cell dysfunction. Nature. (2019) 567:525–9. doi: 10.1038/s41586-019-0979-8
68. Yang B, Deng B, Jiao X-D, Qin B-D, Lu Y, Zhang W, et al. Low-dose anti-VEGFR2 therapy promotes anti-tumor immunity in lung adenocarcinoma by down-regulating the expression of layilin on tumor-infiltrating CD8+T cells. Cell Oncol. (2022) 45:1297–309. doi: 10.1007/s13402-022-00718-0
69. Hibino S, Chikuma S, Kondo T, Ito M, Nakatsukasa H, Omata-Mise S, et al. Inhibition of nr4a receptors enhances antitumor immunity by breaking treg-mediated immune tolerance. Cancer Res. (2018) 78:3027–40. doi: 10.1158/0008-5472.CAN-17-3102
70. Chen J, López-Moyado IF, Seo H, Lio C-WJ, Hempleman LJ, Sekiya T, et al. NR4A transcription factors limit CAR T cell function in solid tumours. Nature. (2019) 567:530–4. doi: 10.1038/s41586-019-0985-x
71. Kouro T, Himuro H, and Sasada T. Exhaustion of CAR T cells: potential causes and solutions. J Trans Med. (2022) 20:239. doi: 10.1186/s12967-022-03442-3
72. Nakagawara K, Ando M, Srirat T, Mise-Omata S, Hayakawa T, Ito M, et al. NR4A ablation improves mitochondrial fitness for long persistence in human CAR-T cells against solid tumors. J Immunotherapy Cancer. (2024) 12:e008665. doi: 10.1136/jitc-2023-008665
73. Li H, Kolluri SK, Gu J, Dawson MI, Cao X, Hobbs PD, et al. Cytochrome c release and apoptosis induced by mitochondrial targeting of nuclear orphan receptor TR3. Science. (2000) 289:1159–64. doi: 10.1126/science.289.5482.1159
74. Lin B, Kolluri SK, Lin F, Liu W, Han Y-H, Cao X, et al. Conversion of bcl-2 from protector to killer by interaction with nuclear orphan receptor nur77/TR3. Cell. (2004) 116:527–40. doi: 10.1016/S0092-8674(04)00162-X
75. Maddika S, Booy EP, Johar D, Gibson SB, Ghavami S, and Los M. Cancer-specific toxicity of apoptin is independent of death receptors but involves the loss of mitochondrial membrane potential and the release of mitochondrial cell-death mediators by a Nur77-dependent pathway. J Cell Sci. (2005) 118:4485–93. doi: 10.1242/jcs.02580
76. Thompson J and Winoto A. During negative selection, Nur77 family proteins translocate to mitochondria where they associate with Bcl-2 and expose its proapoptotic BH3 domain. J Exp Med. (2008) 205:1029–36. doi: 10.1084/jem.20080101
77. Kolluri SK, Zhu X, Zhou X, Lin B, Chen Y, Sun K, et al. A short nur77-derived peptide converts bcl-2 from a protector to a killer. Cancer Cell. (2008) 14:285–98. doi: 10.1016/j.ccr.2008.09.002
78. Liu J, Zhou W, Li S-S, Sun Z, Lin B, Lang Y-Y, et al. Modulation of orphan nuclear receptor nur77-mediated apoptotic pathway by acetylshikonin and analogues. Cancer Res. (2008) 68:8871–80. doi: 10.1158/0008-5472.CAN-08-1972
79. Wang A, Rud J, Olson CM Jr, Anguita J, and Osborne BA. Phosphorylation of nur77 by the MEK-ERK-RSK cascade induces mitochondrial translocation and apoptosis in T cells1. J Immunol. (2009) 183:3268–77. doi: 10.4049/jimmunol.0900894
80. Kim H-J, Kim J-Y, Lee SJ, Kim H-J, Oh CJ, Choi Y-K, et al. α-lipoic acid prevents neointimal hyperplasia via induction of p38 mitogen-activated protein kinase/nur77-mediated apoptosis of vascular smooth muscle cells and accelerates postinjury reendothelialization. Arteriosclerosis Thrombosis Vasc Biol. (2010) 30:2164–72. doi: 10.1161/ATVBAHA.110.212308
81. Niu G, Lu L, Gan J, Zhang D, Liu J, and Huang G. Dual roles of orphan nuclear receptor TR3/nur77/NGFI-B in mediating cell survival and apoptosis. Int Rev Cell Mol Biol. (2014) 313:219–58. doi: 10.1016/B978-0-12-800177-6.00007-4
82. Hu Q-Y, Zhang X-K, Wang J-N, Chen H-X, He L-P, Tang J-S, et al. Malayoside, a cardenolide glycoside extracted from Antiaris toxicaria Lesch, induces apoptosis in human non-small lung cancer cells via MAPK-Nur77 signaling pathway. Biochem Pharmacol. (2021) 190:114622. doi: 10.1016/j.bcp.2021.114622
83. Liu J, Zeng H, Zhang L, Zhan Y, Chen Y, Wang Y, et al. A Unique Pharmacophore for Activation of the Nuclear Orphan Receptor Nur77 In vivo and In vitro. Cancer Res. (2010) 70:3628–37. doi: 10.1158/0008-5472.CAN-09-3160
84. Pearce MC, Gamble JT, Kopparapu PR, O’Donnell EF, Mueller MJ, Jang HS, et al. Induction of apoptosis and suppression of tumor growth by Nur77-derived Bcl-2 converting peptide in chemoresistant lung cancer cells. Oncotarget. (2018) 9:26072–85. doi: 10.18632/oncotarget.25437
85. Liu H, Wang Q, Lan W, Liu D, Huang J, and Yao J. Radiosensitization effect of quinoline-indole-schiff base derivative 10E on non-small cell lung cancer cells in vitro and in tumor xenografts. Investigational New Drugs. (2024) 42:405–17. doi: 10.1007/s10637-024-01451-1
86. Holla VR, Wu H, Shi Q, Menter DG, and DuBois RN. Nuclear Orphan Receptor NR4A2 modulates fatty acid oxidation pathways in colorectal cancer. J Biol Chem. (2011) 286:30003–9. doi: 10.1074/jbc.M110.184697
87. Silvente-Poirot S and Poirot M. The tumor-suppressor cholesterol metabolite is a new class of LXR modulator activating lethal autophagy in cancers. Biochem Pharmacol. (2018) 153:75. doi: 10.1016/j.bcp.2018.01.046
88. Xiang L, Mou J, Shao B, Wei Y, Liang H, Takano N, et al. Glutaminase 1 expression in colorectal cancer cells is induced by hypoxia and required for tumor growth, invasion, and metastatic colonization. Cell Death Dis. (2019) 10:1–15. doi: 10.1038/s41419-018-1291-5
89. Wohlkoenig C, Leithner K, Olschewski A, Olschewski H, and Hrzenjak A. TR3 is involved in hypoxia-induced apoptosis resistance in lung cancer cells downstream of HIF-1α. Lung Cancer. (2017) 111:15–22. doi: 10.1016/j.lungcan.2017.06.013
90. Zhan Y, Du X, Chen H, Liu J, Zhao B, Huang D, et al. Cytosporone B is an agonist for nuclear orphan receptor Nur77. Nat Chem Biol. (2008) 4:548–56. doi: 10.1038/nchembio.106
91. Xia Z, Cao X, Rico-Bautista E, Yu J, Chen L, Chen J, et al. Relative impact of 3- and 5-hydroxyl groups of cytosporone B on cancer cell viability. MedChemComm. (2013) 4:332–9. doi: 10.1039/C2MD20243C
92. Hao L, Luan J, Zhang D, Li C, Guo H, Qi L, et al. Research on the in vitro anticancer activity and in vivo tissue distribution of Amoitone B nanocrystals. Colloids Surffaces B: Biointerfaces. (2014) 117:258–66. doi: 10.1016/j.colsurfb.2014.02.042
93. Liu J, Wang G-H, Duan Y-H, Dai Y, Bao Y, Hu M, et al. Modulation of the Nur77-Bcl-2 apoptotic pathway by p38α MAPK. Oncotarget. (2017) 8:69731–45. doi: 10.18632/oncotarget.19227
94. Upadhyay S, Hailemariam AE, Mariyam F, Hafiz Z, Martin G, Kothari J, et al. Bis-indole derivatives as dual nuclear receptor 4A1 (NR4A1) and NR4A2 ligands. Biomolecules. (2024) 14:284. doi: 10.3390/biom14030284
95. Upadhyay S, Lee M, Zhang L, Oany AR, Mikheeva SA, Mikheev AM, et al. Dual nuclear receptor 4A1 (NR4A1/NR4A2) ligands inhibit glioblastoma growth and target TWIST1. Mol Pharmacol. (2025) 107:100009. doi: 10.1016/j.molpha.2024.100009
96. Andey T, Patel A, Jackson T, Safe S, and Singh M. 1,1-Bis (3′-indolyl)-1-(p-substitutedphenyl)methane compounds inhibit lung cancer cell and tumor growth in a metastasis model. Eur J Pharm Sci. (2013) 50:227–41. doi: 10.1016/j.ejps.2013.07.007
97. Zhou Y, Zhao W, Xie G, Huang M, Hu M, Jiang X, et al. Induction of Nur77-dependent apoptotic pathway by a coumarin derivative through activation of JNK and p38 MAPK. Carcinogenesis. (2014) 35:2660–9. doi: 10.1093/carcin/bgu186
98. Liu Q, Tang J-S, Hu M-J, Liu J, Chen H-F, Gao H, et al. Antiproliferative cardiac glycosides from the latex of antiaris toxicaria. J Natural Products. (2013) 76:1771–80. doi: 10.1021/np4005147
99. Zhang L, Martin G, Mohankumar K, Hampton JT, Liu WR, and Safe S. Resveratrol binds nuclear receptor 4A1 (NR4A1) and acts as an NR4A1 antagonist in lung cancer cells. Mol Pharmacol. (2022) 102:80–91. doi: 10.1124/molpharm.121.000481
100. Li X-S, Hu M-J, Liu J, Liu Q, Huang Z-X, Li S-L, et al. Cardiac glycosides from the bark of Antiaris toxicaria. Fitoterapia. (2014) 97:71–7. doi: 10.1016/j.fitote.2014.05.013
Keywords: NR4A1, lung cancer, molecular mechanism, DIM-C-pPhOH, cytosporone B, signaling pathways
Citation: Jin M, Wang Y, Song M, Guo W, Li S and Pu Z (2025) Targeting the nuclear orphan receptor NR4A1: a key target in lung cancer progression and therapeutic resistance. Front. Oncol. 15:1566598. doi: 10.3389/fonc.2025.1566598
Received: 25 January 2025; Accepted: 15 June 2025;
Published: 01 July 2025.
Edited by:
Hugo de Jonge, University of Pavia, ItalyReviewed by:
Surya Kant Tripathi, University of North Carolina at Chapel Hill, United StatesHongchun Wang, Shandong University, China
Copyright © 2025 Jin, Wang, Song, Guo, Li and Pu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zeqing Pu, MTc4NjI5OTUzNzVAMTYzLmNvbQ==; Shirong Li, bHNyMjI3MEAxNjMuY29t
 Minhan Jin
Minhan Jin Yuhui Wang
Yuhui Wang Mingze Song
Mingze Song Wenfei Guo
Wenfei Guo Shirong Li
Shirong Li Zeqing Pu
Zeqing Pu