- 1Doctoral School of Biomedical Sciences, “Dunarea de Jos” University, Galati, Romania
- 2Hematology Department, “Sf. Apostol Andrei” Emergency County Hospital, Galati, Romania
- 3Clinical Surgical Department, “Dunarea de Jos” University from Galati, Galati, Romania
- 4Clinical Medical Department, Faculty of Medicine and Pharmacy, “Dunarea de Jos” University of Galati, Galati, Romania
- 5Neurology Department, Fundeni Clinical Institute, Bucharest, Romania
- 6Multidisciplinary Integrated Center for Dermatological Interface Research, “Dunărea de Jos” University, Galati, Romania
- 7Morphological and Functional Sciences Department, “Dunarea de Jos University”, Galati, Romania
- 8Pathology Department, “Sf. Apostol Andrei” Emergency County Hospital, Galati, Romania
- 9Clinical Medical Department, “Dunarea de Jos University”, Galati, Romania
- 10Clinical Hospital for Infectious Diseases, “Sf. Cuv. Parascheva”, Galati, Romania
Introduction: Lymphoma is a significant cause of mortality among people living with human immunodeficiency virus (PLWH). The objective of our study was to assess the characteristics of lymphomas in PLWH in a single center from the southeast of Romania.
Methods: We retrospectively analyzed the prevalence and clinical and demographic characteristics of patients with lymphoma associated with HIV/AIDS monitored over a period of 15 years. Kaplan–Meier analysis was used to estimate survival rates and evaluate the risk of mortality in lymphoma patients.
Results: Among the 476 new cases of HIV/AIDS registered, 9 cases of lymphoma were identified, representing a prevalence of 1.89%. Overall mortality was 13.6%, with lymphoma contributing to 10.76% of HIV/AIDS-related deaths. The average age at lymphoma diagnosis was 37 years, with most patients being men and smokers with sexually transmitted HIV. Common coinfections included hepatitis B virus (HBV) and tuberculosis. Advanced-stage disease (Ann Arbor stage IV) and type B clinical symptoms were present in half of the cases. Oncological treatment was provided in 5 cases, achieving a survival rate of 30%.
Conclusions: The high mortality highlights the need for early diagnosis and an integrated therapeutic approach to improve the prognosis of patients with HIV-associated lymphomas.
1 Introduction
Globally, the epidemic of the human immunodeficiency virus (HIV) is ongoing, with an estimated 39.9 million people living with HIV (PLWH) (1).
Nowadays, due to the huge scientific advances in the understanding of the viral pathways, as well as their diagnosis, treatment, and prevention, HIV has become a chronic, manageable disease, with a longer life expectancy. However, despite the advancements in public health strategies and treatment, a definitive cure for HIV is not yet available. The persistent viral inflammation in PLWH is related to frailty, in terms of precocious aging and frequent additional chronic conditions, such as cardiovascular, kidney, and bone diseases and various cancers (2).
HIV-positive people have a higher risk of developing various diseases, including cancer. Progressive immunosuppression secondary to HIV infection is a risk factor for the development of a variety of malignancies (3, 4).
The prolonged suppression of HIV replication with undetectable blood viral load under effective antiretroviral therapies (ARTs) has decreased the opportunistic infections and neoplasms associated with severe acquired immunodeficiency syndrome (AIDS).
The pathogenesis of HIV/AIDS-associated lymphoma is multifactorial, involving immune dysregulation, genetic mutations, viral coinfections, and chronic activation of B lymphocytes (2). These lymphomas predominantly originate from B cells and exhibit clonal immunoglobulin rearrangements (5).
Co-infections with HTLV-1, EBV, and HHV8 are oncogenic viruses that increase the susceptibility of PLWH to developing HIV/AIDS-associated lymphomas (5–7). An elevated risk of lymphomas was reported in HIV-positive individuals with HBV and HCV coinfections (8).
As an AIDS indicator, non-Hodgkin lymphoma (NHL) has continued to be the most common type of cancer and a leading cause of mortality in PLWH (9). Furthermore, Hodgkin lymphoma (HL) is not classified as an AIDS-defining illness, but studies have reported an increased incidence of HL in PLWH compared to the general population (10).
NHL is the 12th most common cancer worldwide, with a global incidence of 5.6 cases per 100,000 people in 2022, while HL ranks 26th and has a much lower incidence of 0.95 cases per 100,000 people (11). The incidence of NHL and HL in people living with HIV (PLWH) was 10- to 20-fold higher compared to the general population, but the clinical features and prognostic factors remain poorly differentiated from non-HIV lymphoma (12, 13).
Some types of lymphomas, such as primary diffuse large B-cell lymphoma or primary cerebral lymphoma, are opportunistic diseases considered as indicators for the classification in the AIDS stage (14, 15).
Regarding AIDS-associated lymphomas, it is estimated that more than 40% of patients in the advanced stages of immunosuppression could be diagnosed with one of these hematologic malignancies (16).
In developed countries, NHL is the most common cause of death associated with HIV infection, accounting for 23% to 30% of all AIDS-related deaths (16). HIV-related NHLs occur in patients with advanced HIV infection with a T-lymphocyte (CD4+) count of less than 100 cells/μL and a high HIV viral load (17).
HL is the most common type of cancer in HIV-positive patients, not associated with AIDS. HL in non-HIV patients had a bimodal age distribution with an initial peak at 20–30 years and a second peak at 50–65 years, while the mean age of presentation of HL in HIV-positive patients was 41 years in European countries and 34 years in African countries (18, 19). Globally, 0.4% of new cancer cases and 0.2% of cancer-related deaths were due to HL in 2020 (20). The incidence of HL varied by sex, age, and geographic location. People at a higher risk for HL included men, adolescents, young adults, and those with a history of Epstein–Barr virus infection, HIV/AIDS, autoimmune diseases, exposure to pollution, and smoking as well as family history (21–23).
The most common histologic types of HIV-associated lymphomas were diffuse large B-cell lymphoma (DLBCL; 37%), HL (26%), and Burkitt lymphoma (BL; 20%). Low CD4+ T-cell counts and HIV-1 viral replication (VL) are independent risk factors for DLBCL in people living with HIV (24). Other types, such as primary effusion lymphoma (PEL), primary central nervous system lymphoma (PCNSL), and plasmablastic lymphoma (PBL), were less common (24, 25).
The GLOBOCAN database, designed by the International Agency for Research on Cancer (IARC), contains projected national cancer estimates up to 2024 derived from the best available recorded data from national (or subnational) cancer registries and national centralized registry systems in 185 countries or territories around the world (11, 26, 27).
The GLOBOCAN database provides comprehensive global data on cancer epidemiology, including lymphomas (12). However, while significant data exist on HIV-associated lymphomas globally (28), their epidemiology and clinical profiles in Romania remain poorly understood, necessitating localized studies.
Therefore, the present study provides a comprehensive analysis of the frequency, clinical features, and mortality associated with HIV/AIDS-related lymphomas in a single-center cohort from Romania. By focusing on a population characterized by unique epidemiological and clinical features, including pediatric HIV cohort survivors, the study highlights challenges such as advanced disease stages at diagnosis, high mortality rates, and limited access to innovative therapies like CAR-T. These findings emphasize the urgent need for personalized management strategies and underline the importance of addressing regional challenges in the care of patients with HIV and lymphoma.
2 Materials and methods
2.1 Study design and statistical analysis
This study is an observational, descriptive, and retrospective analysis, which aims to evaluate the survival outcomes of patients diagnosed with lymphoma in the context of HIV/AIDS. We performed a comparative analysis using Kaplan–Meier (K-M) survival curves to assess the prognosis of these patients over a period of 15 years (2008–2022). To conduct the statistical survival analysis, we divided the patients into two groups: one group consisting of patients with HIV/AIDS and lymphoma versus another group with HIV/AIDS only.
Patients with incomplete or missing medical records, especially regarding HIV diagnosis or histological confirmation of lymphoma, were excluded from the study to ensure the accuracy of the data.
The HIV/AIDS database was obtained from the Infectious Diseases Clinic Hospital Galati electronic database, which is a single center for PLWH in the Galati district (29–33). Our center was located on the southeast border of Romania, providing healthcare for PLWH, with an estimated general population of 600,000 people.
We selected the diagnostic-related group codes B.20–24, from 1 January 2008 to 31 December 2022 (34). Additionally, we have identified the HIV/AIDS patients with lymphomas, by the diagnostic code B.21.1, B.21.2, or B.21.3, specifying HIV disease resulting in Burkitt lymphoma, non-Hodgkin lymphoma, or other malignant neoplasms of the lymphoid, hematopoietic, and related tissue (34) All the cases were revised in December 2023, covering at least 1 year of HIV/AIDS follow-up. The cases were categorized as follows: retained in care (patients continuing to be monitored and treated in the center), deaths, and lost to follow-up (patients with no updated information). The endpoint of the study was either death or 31 December 2022. The frequency of lymphomas in PLWH was calculated by dividing the number of HIV/AIDS and lymphoma cases by the total number of HIV/AIDS diagnosed cases during the study time. Mortality was calculated among patients with HIV/AIDS and lymphomas and was compared with mortality among the cases with HIV/AIDS only.
To achieve the second objective, we have selected cases with HIV/AIDS and lymphoma from our institution’s archived records, and we have studied their detailed medical files.
2.2 HIV and lymphoma patient overview
We analyzed demographic data, HIV history, and lymphoma history. We examined whether the lymphoma diagnosis was an indicator of immunosuppression and a reason for HIV testing. HIV history comprised the pattern of transmission, age and year of diagnosis, staging of HIV diagnosis, coinfections, nadir of CD4 count, and antiretroviral treatment (ART) experience. The stages of HIV infection were evaluated using the CDC classification system, considering the immunological level based on CD4 count and the clinical criteria for AIDS-related conditions (35). The nadir of CD4 count means the lowest ever count in an individual with HIV. Antiretroviral therapy was used according to the timeline versions of European guidelines, combining nucleoside(tide) reverse transcriptase inhibitor (NRTI), non-nucleoside reverse transcriptase inhibitor (NNRTI), protease inhibitor (PI), or integrase inhibitor class (II) (36).
2.3 Lymphoma staging and clinical features
In the characterization of lymphoma, the year of diagnosis, concomitant CD4 count, staging of lymphoma, histopathology, oncologic treatment, major complications, and comorbidities were considered. The diagnosis of lymphoma was based on an anatomical-pathological examination supplemented by immunohistochemistry, performed on various relevant tissues, including lymph nodes, skin or mucous membranes, and bone marrow. The lymphomas were staged according to the Ann Arbor classification system. The Ann Arbor staging system classifies HL and NHL by disease extent: stage I (single lymph node region), stage II (multiple lymph node regions on the same side of the diaphragm), stage III (lymph nodes on both sides of the diaphragm), and stage IV (diffuse or disseminated involvement of one or more extra lymphatic organs, or either involvement of the liver, bone marrow, or lungs). Each stage is subdivided into A (no systemic symptoms), B (systemic symptoms—fever exceeding 38°C without a known cause, severe night sweats, or weight loss greater than 10% of body weight within 6 months before diagnosis), E (spread of lymphoma to organs outside the lymphatic system, such as the liver, bone marrow, or lungs), S (spleen involvement), and bulky disease [for HL, bulk is defined as any single node or nodal mass with a diameter ≥10 cm or mediastinal mass ratio (maximum width of mediastinal mass/maximum intrathoracic diameter >0.33); for DLBCL, bulky disease means any nodal or extra nodal tumor mass with a diameter of ≥7.5 cm] (37–39).
2.4 Statistical analysis and survival assessment
To analyze data, the K-M survival analysis was employed. To conduct the univariate approach of K-M, we divided the patients into two groups: one group consisting of patients with HIV/AIDS and lymphoma and another group with HIV/AIDS only. Incomplete observations of patients such as those lost to follow-up or without an event occurring by the end of the study were censored, meaning their data were considered incomplete in the survival analysis. The log-rank test was used to compare the survival estimates between groups, considering a p-value lower than 0.05 as significant. We used the SPSS software package version 26 for the Kaplan–Meier survival curve analysis of both groups. We analyzed the characteristics of patients with HIV/AIDS and HL or NHL using the two-sample test due to a small sample size.
3 Results
3.1 The frequency and mortality of HIV/AIDS-associated lymphomas
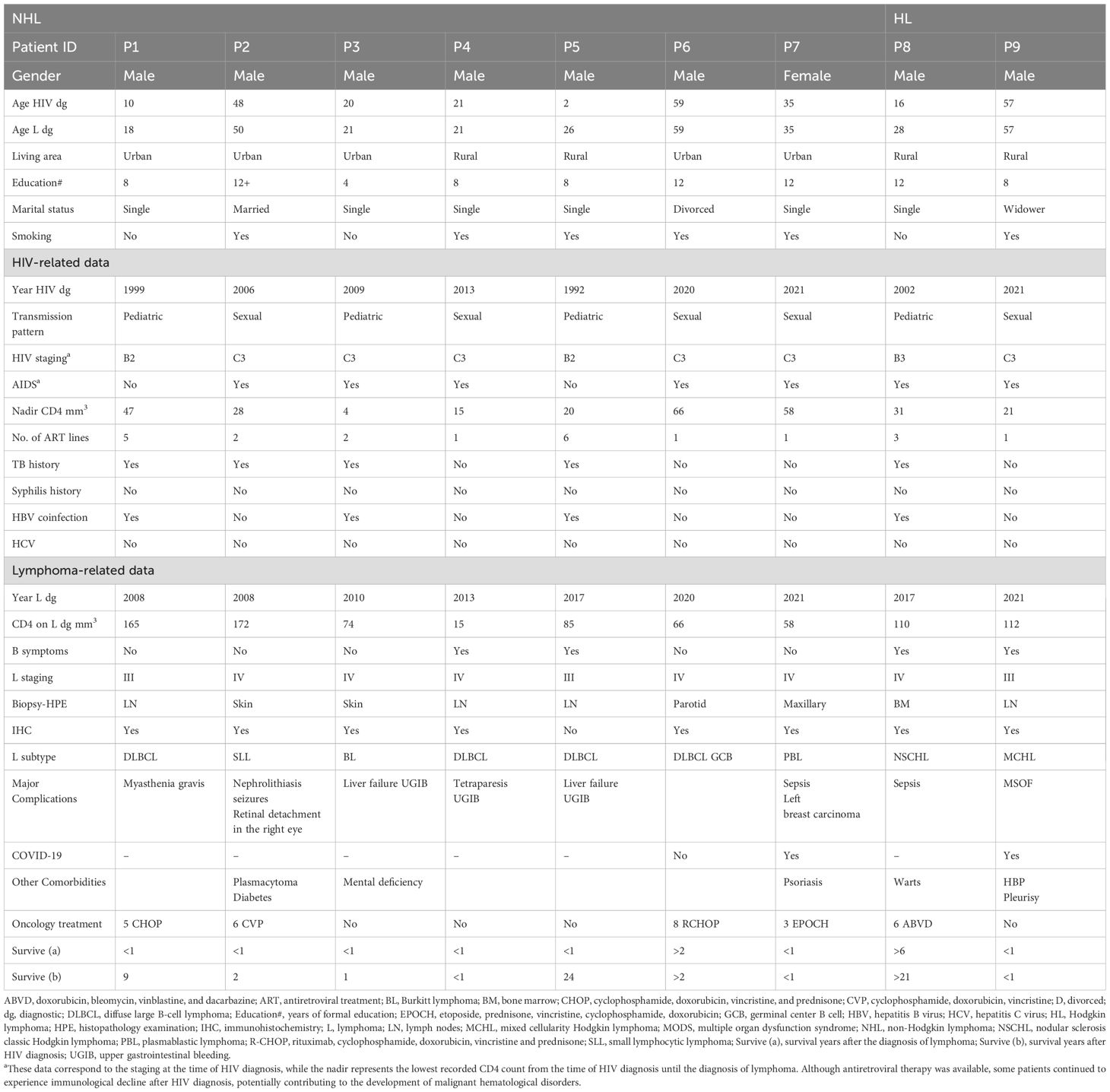
From 2008 to 2022, 476 PLWH were monitored in our center. These 476 cases were divided into two groups: a group of 9 PLWH diagnosed with lymphoma (7 NHL and 2 HL) (Table 1) and a group of 467 PLWH with HIV/AIDS only, who did not develop lymphoma.
Of the total 476 PLWH, 366 were alive, 65 died, and 45 were lost to follow-up. Thus, the overall death rate was 15.08%. The 45 cases lost to follow-up were not excluded from the analysis.
Among the 476 cases of PLWH, we have identified 9 cases with lymphomas, specified as 7 NHL and 2 HL, meaning a 1.89% (9/476 × 100) prevalence of lymphoma comorbidity. Of the 65 total deaths reported in the study, 7 were PLWH diagnosed with lymphomas, accounting for 10.76% of all deaths. The mortality rate of HIV/AIDS-associated lymphomas was 77.7% (7/9 × 100), compared with 12.41% (58/467 × 100) deaths in the HIV/AIDS-only group.
The analysis of K-M curves identified a chi-square value of 13,456 (df = 1), and an associated p-value (Sig.) <0.001, indicating that PLWH diagnosed with lymphoma had a significantly shorter life expectancy compared to those with HIV/AIDS only.
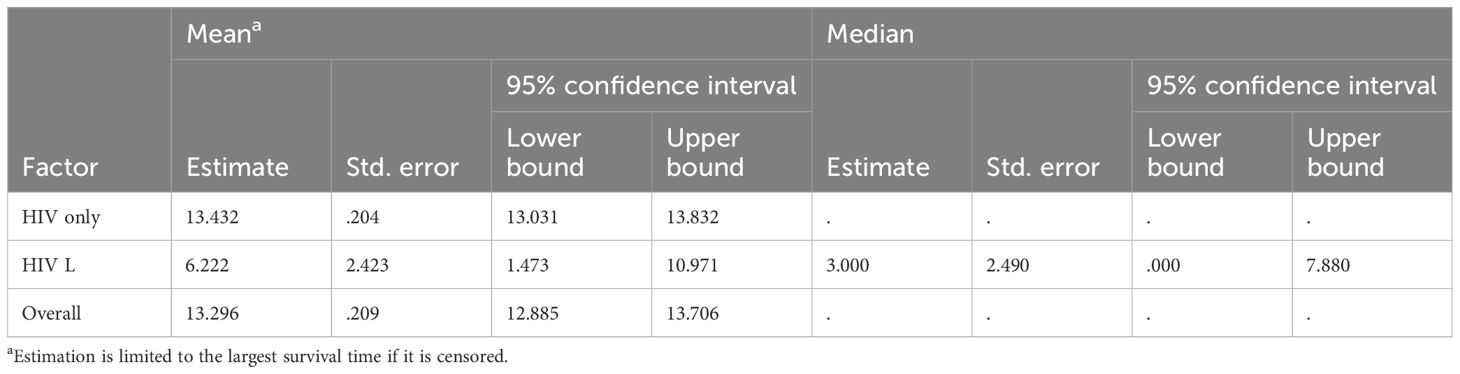
The analysis of Kaplan–Meier curves identified a statistically significant difference in survival distributions between the two groups (chi-square = 13.456, df = 1, p < 0.001), indicating that PLWH diagnosed with lymphoma had a significantly shorter life expectancy compared to those with HIV/AIDS only (Figure 1, Table 2).
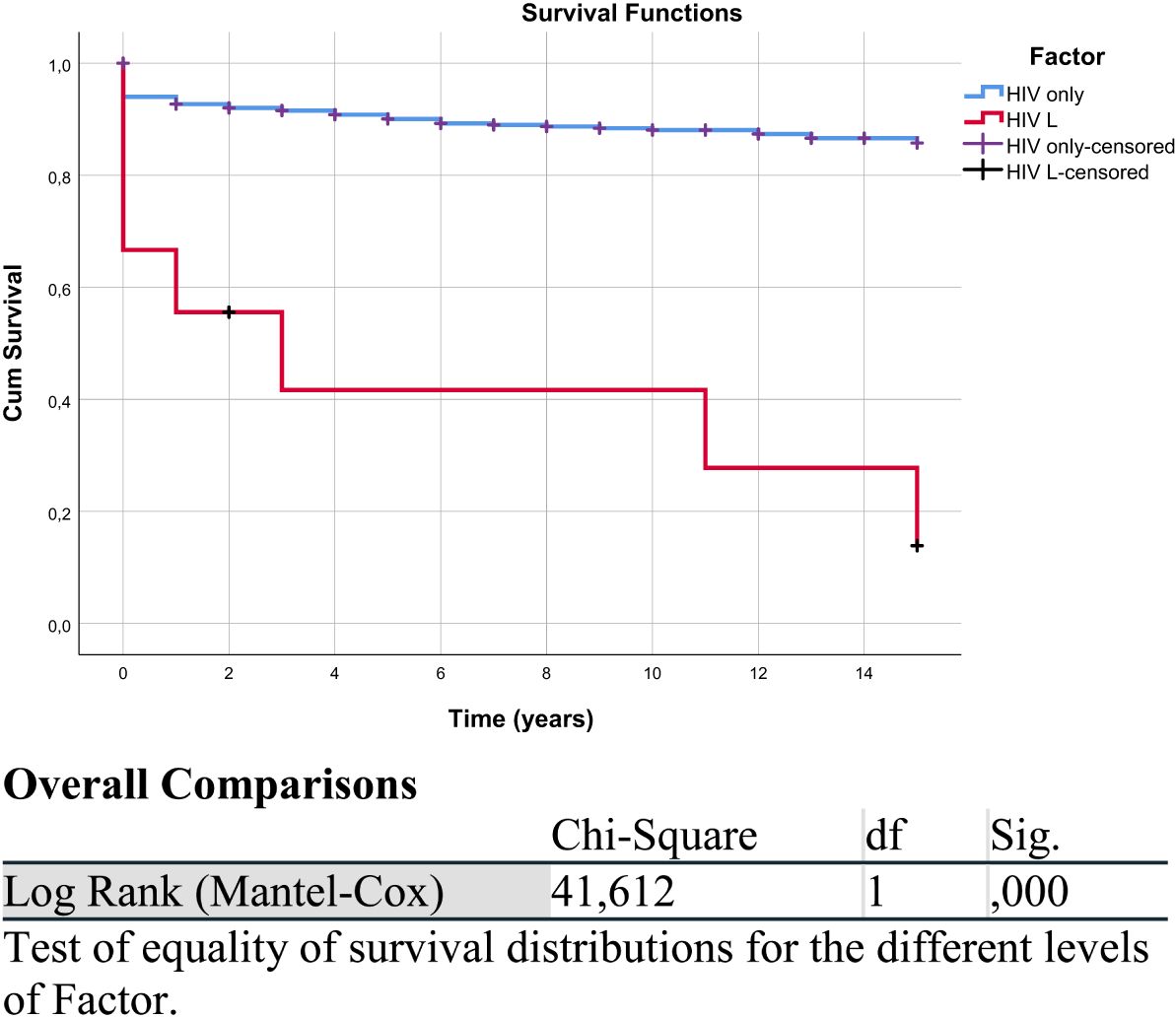
Figure 1. Kaplan–Meier survival curve of the HIV/AIDS and lymphoma (HIV L) group compared with the HIV/AIDS-only group. “HIV only-censored” and “HIV L-censored” refer to patients in the respective groups (HIV without lymphoma and HIV with lymphoma) whose data were censored, meaning they did not experience the event of interest, defined here as overall survival (death from any cause), or were lost to follow-up. The overall comparison table corresponds to this figure.
3.2 Characteristics of PLWH diagnosed with lymphomas
3.2.1 Demographic characteristics
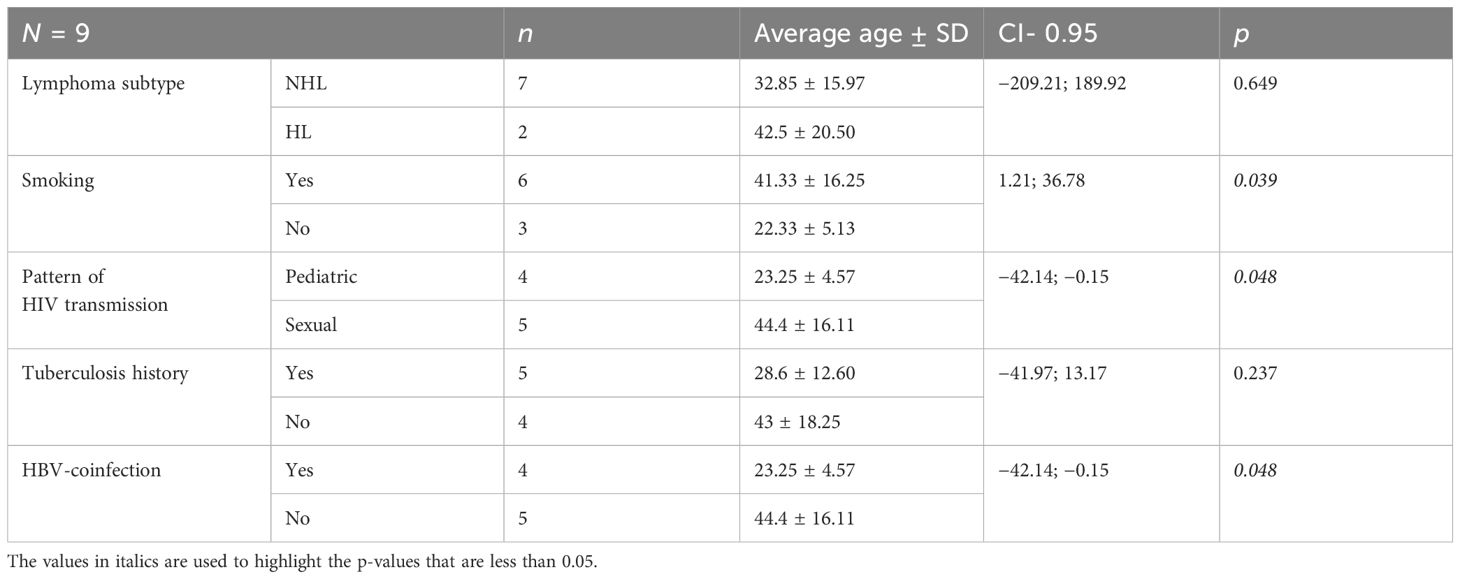
The demographic characteristics described in this section apply to the PLWH cases diagnosed with lymphoma. Among the nine cases, seven were diagnosed with NHL and two with Hodgkin lymphoma (HL). All patients were Caucasian, most of them were men (eight out of nine) living in an urban area (five out of nine), and all but one had completed secondary education. According to marital status, most were single (six out of nine), one case was divorced, one was married, and one was widowed. The age at HIV diagnosis for the nine PLWH diagnosed with lymphoma ranged between 2 and 59 years, including four patients with a pediatric pattern of HIV infection and five sexually transmitted cases (Table 1). None of the patients were intravenous drug users. The average age of lymphoma diagnosis was 35 ± 16.18, varying between 18 and 60 years. The diagnostic age of lymphomas was lower in smokers and patients with a pediatric pattern of transmission, history of tuberculosis, and hepatitis B virus (HBV) coinfection (Table 3).
3.2.2 HIV history characteristics
On HIV diagnosis, seven out of nine patients were late presenters (AIDS stage), while immunosuppression had already progressed in the remaining two patients. As a result, all of them experienced a very low average nadir CD4 count of 32.22 ± 20.65, ranging between 4 and 66/mm3 (Table 1). A history of tuberculosis was found in five out of the nine cases, and four out of the nine cases had both HBV coinfection and tuberculosis. There was no coinfection with hepatitis C virus (HCV) or syphilis. All patients have experienced at least one line of ART after HIV diagnosis, but three of them had multiple ART treatments. Baseline HIV viral load was variable, and undetectable levels under ART were temporarily achieved in only two cases (P2, P3).
3.2.3 Characteristics of HIV/AIDS lymphoma cases
Lymphoma was an indicator of immunosuppression, as it is frequently observed in individuals with advanced HIV infection, where the immune system is significantly compromised. It was identified as an indicator for HIV diagnosis in four out of nine cases, all of them being “very late presenters” (patients P4, P6, P7, P9) (Table 1). The average CD4 count during the diagnosis of lymphoma was 95.22 ± 50.61, ranging from 15 to 172/mm3. In patient P3, the small lymphocytic lymphoma was interpreted as part of the immune reconstitution inflammatory syndrome (IRIS) following the diagnosis and treatment of tuberculosis coinfection and HIV. Specifically, the ART regimen of patient P3 included zidovudine + lamivudine + efavirenz and enfuvirtide. In the other cases (patients P1, P2, P5, and P8), the interval after HIV diagnosis to lymphoma occurrence ranged between 2 and 24 years (Table 1).
The diagnosis of lymphoma was confirmed by anatomopathological examinations of biopsy specimens of lymph node (4/9), skin or mucosa (4/9), and bone marrow (1/9). Immunohistochemistry was unavailable in one single case (P5).
The histological subtype of NHL was mostly DLBCL (44.44%). Other subtypes included small lymphocytic lymphoma, BL, and PBL, with one case for each. HL represented 22.22%. The subtypes were determined by microscopic evaluation and confirmation with specific immunohistochemical markers. The staging of lymphomas revealed invasion of extralymphatic tissues in six out of nine patients (P2, P3, P4, P6, P7, P8), which means stage IV according to Ann Arbor staging (11, 34). The other three patients (P1, P5, P9) were categorized as stage III. The signs of B category (33) were present in four out of nine patients: fever (P4, P5, P8, P9), over 10% of weight loss in the last 6 months (P4), and night sweats (P4) (Table 1).
Chemotherapy was provided to five patients (P1, P2, P6, P7, P8), and two of them had survived (P6, P8). The other three patients (P1, P2, P7) were non-responders to chemotherapy, and the lymphomas had progressed to a fatal outcome. P1 and P6 received cyclophosphamide, doxorubicin, vincristine, and prednisone treatment (CHOP), but P6 also received immunotherapy with rituximab (R), which may be an important factor in the patient’s profound response and survival (Table 1). The 1-year survival rate was 22.22%, and there were only two survivors: one case each of NHL and HL. The inability to administer chemotherapy in some cases was due to late presentation and the presence of active infections, such as COVID-19 (P9), as well as delays in obtaining immunohistochemical results.
One particular case was a female patient (P7) who had two types of cancers. The first one was an oral PBL, an indicator of HIV diagnosis, and the second was a tumor of the left breast that was detected 3 months later, during the chemotherapy for lymphoma. The breast carcinoma diagnosis was supported by the anatomopathological exam, but the result was available after the patient died.
4 Discussion
4.1 Pathogenesis of HIV/AIDS-associated lymphoma: mechanisms and risk factors
HIV-related chronic inflammation is associated with the disruption of cytokines and chemokines that play a role in the tumor microenvironment (40).
The mechanisms of enhanced death in HIV-infected cells versus uninfected ones are increased apoptosis, pyroptosis, and ferroptosis (41). The HIV-related CD4 T-cell death involves approximately a 95% caspase-1-mediated pyroptosis pattern, while the proportion of cell apoptosis is less than 5% (42). Significant differences in microenvironments were found between sporadic DLBCL and HIV-associated DLBCL, which was highly angiogenic with a higher density of microvessels (43). The type of lymphoma was significantly influenced by the degree of CD4 cell depletion and immune dysfunction (44).Patients with CD4+ T-cell depletion are more likely to develop aggressive subtypes such as DLBCL, PEL, or PBL. In contrast, those with higher CD4+ counts are prone to centroblastic DLBCL and BL (24).
However, HIV could influence oncogenesis independent of immunosuppression using a direct pro-oncogenic mechanism (45). The dysregulation of the cell cycle influences the non-immune microenvironment by increasing the extracellular matrix, profibrogenic factors, and aberrant lymphangiogenesis (46).
Chronic activation of B lymphocytes during HIV immune dysfunction leads to hypergammaglobulinemia, impaired humoral immunity, and germinal center hyperplasia, which can ultimately result in lymphoma development (47).
The degree of immune dysfunction, particularly reflected by CD4+ T-cell counts, plays a pivotal role in the clinical presentation, treatment response, and prognosis of HIV-associated lymphomas. Patients with severe immunosuppression, indicated by nadir CD4 counts below 50 cells/mm³, more frequently present with advanced-stage lymphomas (stages III/IV) and systemic symptoms, complicating management and worsening outcomes. Conversely, higher CD4 counts at lymphoma diagnosis correlate with better chemotherapy tolerance and longer survival, as observed in our cohort (48). For example, patient 1, with a CD4 count of 165 cells/mm³, exhibited a favorable response and prolonged survival, while patient 4, with a CD4 count of 15 cells/mm³, faced treatment-limiting complications.
Chronic immune activation driven by persistent HIV replication exacerbates immune dysfunction, depleting CD4+ T cells, disrupting immune surveillance, and fostering oncogenesis (49, 50).
These findings underline the necessity of integrating immune and virological monitoring into the diagnostic and therapeutic planning for HIV-associated lymphoma patients.
4.2 Epidemiology of HIV/AIDS and lymphoma co-occurrence
Recent updates from the United States (US) on HIV-associated lymphomas during the antiretroviral therapy indicate increasing incidence trends, with DLBCL being the most common subtype, followed by HL, concordant with the results of our study (13).
Some prospective cohort studies on different populations, conducted in Switzerland, the USA, or China, have reported the incidence of lymphomas in PLWH between 2% and 2.14% (51–53). We found an incidence of 1.89%, with a slight difference. It could possibly be explained by the younger HIV age demographics of patients in this study (HIV diagnosis age 2–57 years old). However, it is important to note that the prevalence of lymphomas increases significantly in patients diagnosed with AIDS, where more than 40% are affected by these malignancies (54). This highlights the critical need for early HIV diagnosis and management to prevent progression to advanced stages associated with a higher lymphoma burden (10).
Globally, malignancies, including lymphomas, are more common in men (55). The underlying reasons for this gender difference remain unclear but may involve extrinsic factors such as exposure to environmental carcinogens and intrinsic factors, comprising different immune and hormonal profiles, body size, and tumor biology in men and women (56). Our study confirms the predominance of lymphomas in men, most of them smokers, with a sexually transmitted pattern and severe immunosuppression. These demographic factors are commonly observed in other cohorts, and several studies suggest that smoking and advanced immunosuppression may contribute to a higher incidence and poor prognosis of lymphoma in HIV-infected individuals (46).
Lymphomas can develop across all age groups. Our study found that the average age at lymphoma diagnosis was 35 years, younger than that of other reports, such as 42 years in a Brazilian study, 48 years in a US study, or 43.6 years in a Chinese Study (18, 57, 58). The comparison may be influenced by the differences in sample size and age distribution across the studies.
Studies from Europe and the USA indicate that lymphomas are frequently diagnosed in patients with advanced immunosuppression, often as a late-stage complication in individuals with poorly controlled HIV (57). Furthermore, all our patients presented with advanced lymphomas (stage III/IV) and severe HIV immunosuppression (CD4 counts < 200/mm³). Although antiretroviral therapy was available, some patients continued to experience immunological decline after HIV diagnosis, potentially contributing to the development of malignant hematological disorders.
Studies in some European countries have identified a lower mortality rate of lymphoma among HIV patients. A French study for 10 years reported a mortality rate of 8.8% among 82,000 HIV patients (59). Another study conducted in the USA found a significantly higher mortality rate for HIV-associated lymphoma than for other HIV-related diseases (60). In Botswana, mortality in HIV HL was comparable to that of non-HIV HL, due to an equal increased access to oncologic care for PLWH and those without HIV (61).
The mortality rate of PLWH with lymphoma in our study was 77.7%, notably higher than the mortality rate of 13.65% in the HIV-only group. This stark difference underscores the severe impact of lymphoma on the survival of HIV Romanian patients.
4.3 Particularities of the Romanian HIV/AIDS epidemic and oncologic risk
Romania is a Central-Eastern European country with 18,359 alive PLWH reported by the Ministry of Health in June 2024 (62, 63). More than half of the cases are survivors of the “Romanian HIV pediatric cohort,” which means that patients were born mainly between 1988 and 1989. Most of them were infected with HIV during early childhood by a small amount of blood transfusions or inappropriate use of syringes and needles in healthcare institutions during the communist era. After the fall of communism in December 1989, the availability of HIV diagnosis tests revealed that Romania accounted for 60% of pediatric AIDS cases in Europe at that time (64).
The “HIV pediatric cohort” is characterized by its homogeneous Caucasian race, with many cases of long-term survival from early childhood to adulthood, predominantly of the HIV-1 subtype F, with a high rate of coinfection with hepatitis B virus and multiple antiretroviral drug experience (65). Growing up with HIV is a complex individual burden, involving physical, functional, psychological, and sociobehavioral development, putting one at high risk of increasing comorbidities. Three subjects with NHL (P1, P4, P5) and one with HL (P8) from our case series were part of this pediatric HIV cohort. They were diagnosed with HIV at the ages of 10 years, 19 years, 2 years, and 14 years, respectively, and developed lymphoma diagnosed at the ages of 18 years, 21 years, 26 years, and 29 years, respectively. Beyond the pediatric cohort (born in the 1990s), the “adult” HIV epidemic has progressed through increased sexual transmission or by intravenous drug use (66). A multicenter national database of oncologic HIV/AIDS-associated malignancies should be developed in Romania to better understand these rare comorbidities in our specific population and improve management strategies.
4.4 Immune reconstitution inflammatory syndrome
IRIS is a hyperinflammatory reaction that occurs when the immune system is recovering under antiretroviral therapy, mostly in PLWH with low CD4 counts and opportunistic infections with tuberculosis or cryptococcosis (67, 68). IRIS occurs in two forms: “paradoxical” IRIS is characterized by the exacerbation of a previously treated infection after ART is started, and “unmasking” IRIS is defined by the activation of a previously undiagnosed infection soon after antiretroviral therapy is started (69).
The “unmasking” IRIS was applied to one of our case series (P3). A study involving a cohort of 482 patients with HIV/AIDS-associated lymphoma from 1996 to 2011 found that 48 patients (10%) met the criteria for unmasking lymphoma. Among these cases, 10 (21%) were classified as HL, 19 (40%) as DLBCL, 4 (8%) as BL, 9 (19%) as PCNSL, and 6 (12%) as other NHL (70).
4.5 Real-life treatment, available options, prognosis, and future perspectives
The treatment of HIV infection and lymphoma requires an integrated approach, given the complex interplay between these two conditions. ARV is essential for the suppression of HIV replication and the recovery of immune function, while chemotherapy is the usual treatment of lymphoma with immunosuppressive adverse events (44).
Since 2018, the National Comprehensive Cancer Network (NCCN) guidelines have included dedicated recommendations for treating HIV-associated B-cell lymphomas (20, 71), highlighting the evolving approach to managing these co-occurring conditions in clinical practice.
The current standard practice is to continue or to initiate antiretrovirals during chemotherapy (66). PLWH who have well-controlled onco-hematologic disease have a life expectancy comparable to the general population. This group remains underrepresented in clinical trials, as seropositive status continues to be an exclusion criterion for most lymphoma trials (72, 73).
Therapeutic protocols such as R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone), R-CDE (rituximab, cyclophosphamide, doxorubicin, etoposide), R-EPOCH, and (DA)-EPOCH-R (dose-adjusted etoposide, prednisone, vincristine, doxorubicin, and cyclophosphamide based on CD4 count plus rituximab) achieved a complete response rate of 69%–91%, with a 2-year survival rate of 62%–75% and low mortality from infectious causes. The addition of immunotherapy proved beneficial for patients with HIV-associated lymphomas (74).
The relationship between the number of chemotherapy cycles and prognosis in HIV-associated lymphomas offers valuable insights into treatment outcomes. In our cohort, patients underwent a range of regimens, including five cycles of CHOP, six cycles of CVP, eight cycles of R-CHOP, three cycles of EPOCH, and six cycles of ABVD. These data underline the importance of treatment intensity and duration in influencing overall survival (OS) and progression-free survival (PFS). Existing literature emphasizes that completing the full prescribed chemotherapy regimen is typically associated with higher rates of complete response and a reduced likelihood of recurrence. However, in HIV-positive patients, severe immunosuppression and associated complications can compromise treatment tolerance, often leading to premature discontinuation of chemotherapy. This underscores the need for personalized approaches to optimize both tolerability and efficacy in this unique patient population (75). The degree of immunosuppression, reflected in nadir CD4 counts and HIV staging, expresses the resources of immune recovery under ART and influences not only lymphoma aggressiveness but also the ability to complete treatment. Advanced immunodepression characterized all patients in our study with HIV-associated lymphomas, suggesting late presentation and delayed diagnosis of both HIV infection and lymphoma. This may reflect systemic deficiencies in screening and early detection within the Romanian healthcare system. Immune function markers such as CD4 counts are valuable prognostic indicators, as they reflect both the host’s capacity to tolerate chemotherapy and the biological aggressiveness of lymphoma in HIV-infected patients (76).
Autologous stem cell transplantation (ASCT) is provided equally for both HIV-negative and HIV-positive lymphoma patients from some European countries (77). In recent years, this therapeutic option has also been used in Romania for a few patients with HIV and lymphoma. The first two cases of HIV-associated DLBCL treated with the chimeric antigen receptor T-cell therapy (CAR-T) axicabtagene ciloleucel were reported in 2019. This report demonstrated that CAR-T cells can be successfully produced in HIV patients undergoing ART, even with CD4 counts as low as 52 cells/mm³ (78, 79). In Romania, CAR-T-cell therapy has been available since 2022 for adult patients with relapsed or refractory diffuse large B-cell lymphoma and for acute lymphoblastic leukemia who have failed at least two lines of systemic chemotherapy. However, this revolutionary treatment has not yet been administered to HIV-positive patients in our country.
4.6 Limitations of the study
The first limitation of our study is the retrospective design and the inconsistent availability of data. The evaluation and treatment decisions were not unitary because the guidelines and protocols for both HIV and lymphomas have been changed, considering the extended duration of the study. The HIV viral load was not systematically available due to either the temporary lack of reagents or the limited procedures caused by the COVID-19 pandemic. The limited population size diminishes the statistical power, and a study involving a larger patient cohort could yield more robust and precise conclusions in future research. Immunohistochemistry and other procedures for cancer diagnosis are missing because these are not covered by the health insurance and patients could not afford them. Serological evaluation of EBV and HHV-8 with oncogenic potential was not available.
5 Conclusions
Lymphoma is a rare comorbidity in PLWH from the South-East of Romania; however, it has a serious impact on life expectancy, due to the high mortality rate. The median age of PLWH with lymphoma was 35 years, ranging from 18 to 59 years old. The most frequent subtype of lymphoma was DLBCL. A low CD4 count is associated with more aggressive forms of lymphomas, with a low survival on 1-year follow-up. Smoking, HBV coinfection, and chronic infection with HIV of patients from “the pediatric cohort” are predictors for the younger age of lymphoma diagnosis. Most cases of lymphomas occurred in men with HIV/AIDS. Delay of the oncohematological diagnostic, the limited access to screening and treatment, and poor education for earlier medical visits are the main difficulties in the management of lymphomas in PLWH in Romania. These challenges highlight the need for sustained health strategies dedicated to this specific population.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Liliana Baroiu Clinical Medical Department, Dunarea de Jos University, Galati, Romania. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
M-DP-C: Conceptualization, Formal Analysis, Investigation, Writing – original draft. IC: Investigation, Validation, Visualization, Writing – original draft. CG: Data curation, Investigation, Validation, Writing – original draft. AA: Investigation, Methodology, Software, Writing – review & editing. EN: Investigation, Methodology, Validation, Writing – original draft. MA: Conceptualization, Investigation, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The publication fee for this article was supported by the Doctoral School of the “Dunarea de Jos” University of Galati, Romania.
Acknowledgments
The paper was academically supported by “Dunărea de Jos” University, Galaţi, Romania.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ABVD, doxorubicin, bleomycin, vinblastine, and dacarbazine; AIDS, acquired immunodeficiency syndrome; ART, antiretroviral therapy; BL, Burkitt lymphoma; BM, bone marrow; CAR-T, chimeric antigen receptor T-cell therapy; CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisone; CVP, cyclophosphamide, doxorubicin, vincristine; dg, diagnostic; DLBCL, diffuse large B-cell lymphoma; EBV, Epstein–Barr virus; Education#, years of formal education; EPOCH, etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin; GCB, germinal center B cell; HBV, hepatitis B virus; HCV, hepatitis C virus; HHV8, human herpesvirus-8; HL, Hodgkin lymphoma; HPE, histology examination; HTLV1, human T-lymphotropic virus type 1; IHC, immunohistochemistry; II, integrase inhibitor; L, lymphoma; LN, lymph nodes; MCHL, mixed cellularity Hodgkin lymphoma; MODS, multiple organ dysfunction syndrome; NHL, non-Hodgkin lymphoma; NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; NCSHL, nodular sclerosis classic Hodgkin lymphoma; PL, plasmablastic lymphoma; PCL, primary cerebral lymphoma; PI, protease inhibitor; RCDVP, rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone; Survive (a), small lymphocytic lymphoma; Survive (b), survival years after the diagnostic of lymphoma; UGIB, upper gastrointestinal bleeding; US, United States.
References
1. WHO. The urgency of now: AIDS at a crossroads. Geneva: Joint United Nations Programme on HIV/AIDS (2024). Available at: https://www.unaids.org/sites/default/files/media_asset/2024-unaids-global-aids-update_en.pdf.
2. Funderburg NT, Huang SSY, Cohen C, Ailstock K, Cummings M, Lee JC, et al. Changes to inflammatory markers during 5 years of viral suppression and during viral blips in people with HIV initiating different integrase inhibitor based regimens. Front Immunol. (2024) 15:1488799. doi: 10.3389/fimmu.2024.1488799
3. Landgren O, Goedert JJ, Rabkin CS, Wilson WH, Dunleavy K, Kyle RA, et al. Circulating serum free light chains as predictive markers of AIDS-related lymphoma. J Clin Oncol. (2010) 28:773–9. doi: 10.1200/JCO.2009.25.1322
4. Borgia M, Dal Bo M, and Toffoli G. Role of virus-related chronic inflammation and mechanisms of cancer immune-suppression in pathogenesis and progression of hepatocellular carcinoma. Cancers. (2021) 13:4387. doi: 10.3390/cancers13174387
5. Carbone A, Volpi CC, Gualeni AV, and Gloghini A. Epstein–Barr virus associated lymphomas in people with HIV. Curr Opin HIV AIDS. (2017) 12:39–46. doi: 10.1097/COH.0000000000000333
6. Noy A. Optimizing treatment of HIV-associated lymphoma. Blood. (2019) 134:1385–94. doi: 10.1182/blood-2018-01-791400
7. Rana I, Dahlberg S, Steinmaus C, and Zhang L. Benzene exposure and non-Hodgkin lymphoma: a systematic review and meta-analysis of human studies. Lancet Planetary Health. (2021) 5:e633–43. doi: 10.1016/S2542-5196(21)00149-2
8. Wang Q, De Luca A, Smith C, Zangerle R, Sambatakou H, Bonnet F, et al. Chronic hepatitis B and C virus infection and risk for non-Hodgkin lymphoma in HIV-infected patients: a cohort study. Ann Internal Med. (2017) 166:9–17. doi: 10.7326/M16-0240
9. Berhan A, Bayleyegn B, and Getaneh Z. HIV/AIDS associated lymphoma: review. Blood Lymphatic Cancer Targets Ther. (2022) 12:31–45. doi: 10.2147/BLCTT.S36132
10. Poizot-Martin I, Lions C, Allavena C, Huleux T, Bani-Sadr F, Cheret A, et al. Spectrum and incidence trends of AIDS- and non–AIDS-defining cancers between 2010 and 2015 in the french dat'AIDS cohort. Cancer Epidemiol Biomarkers Prev. (2021) 30:554–63. doi: 10.1158/1055-9965.EPI-20-1045
11. Ferlay J, Ervik M, Lam F, Laversanne M, Colombet M, Mery L, et al. Global Cancer Observatory: Cancer Today. Lyon, France: International Agency for Research on Cancer (2024). Available at: https://gco.iarc.who.int/today.
12. Kimani SM, Painschab MS, Horner MJ, Muchengeti M, Fedoriw Y, Shiels MS, et al. Epidemiology of haematological Malignancies in people living with HIV. Lancet HIV. (2020) 7:e641–651. doi: 10.1016/S2352-3018(20)30118-1
13. Wang C, Xiao Q, Zhang X, and Liu Y. HIV associated lymphoma: latest updates from 2023 ASH annual meeting. Exp Hematol Oncol. (2024) 13:65. doi: 10.1186/s40164-024-00530-6
14. Kaplan JE, Benson C, Holmes KK, Brooks JT, Pau A, Masur H, et al. Panel on Opportunistic Infections in Adults and Adolescents with HIV. In: Guidelines for the prevention and treatment of opportunistic infections in adults and adolescents with HIV: recommendations from the Centers for Disease Control and Prevention, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. (United States: NIH, CDC, IDSA). (2019). Available at: http://aidsinfo.nih.gov/contentfiles/lvguidelines/adult_oi.pdf.
15. Horberg M, Thompson M, Agwu A, Colasanti J, Haddad M, Jain M, et al. Primary care guidance for providers of care for persons with human immunodeficiency virus: 2024 update by the HIV medicine association of the infectious diseases society of America. Clin Infect Dis. (2024) ciaa479. doi: 10.1093/cid/ciae479
16. Re A, Cattaneo C, and Rossi G. HIV and lymphoma: from epidemiology to clinical management. Mediterr J Hematol Infect Dis. (2019) 11:e2019004. doi: 10.4084/mjhid.2019.004
17. Chu Y, Liu Y, Fang X, Jiang Y, Ding M, Ge X, et al. The epidemiological patterns of non-Hodgkin lymphoma: global estimates of disease burden, risk factors, and temporal trends. Front Oncol. (2023) 13:1059914. doi: 10.3389/fonc.2023.1059914
18. Hleyhel M, Bouvier AM, Belot A, Tattevin P, Pacanowski J, Genet P, et al. Risk of non-AIDS-defining cancers among HIV-1-infected individuals in France between 1997 and 2009: results from a french cohort. AIDS. (2014) 28:2109–18. doi: 10.1097/QAD.0000000000000382
19. Naidoo N, Abayomi A, Locketz C, Musaigwa F, and Greiwal R. Incidence of Hodgkin lymphoma in HIV-positive and HIV-negative patients at a tertiary hospital in South Africa (2005-2016) and comparison with other African countries. S Afr Med J. (2018) 108:563–7. doi: 10.7196/SAMJ.2018.v108i7.12844
20. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
21. Huang J, Pang WS, Lok V, Zhang L, Lucero-Prisno DE III, Xu W, et al. Incidence, mortality, risk factors, and trends for Hodgkin lymphoma: a global data analysis. J Hematol Oncol. (2022) 15:57. doi: 10.1186/s13045-022-01281-9
22. Hu J, Zhang X, Tao H, and Jia Y. The prognostic value of Epstein–Barr virus infection in Hodgkin lymphoma: A systematic review and meta-analysis. Front Oncol. (2022) 12:1034398. doi: 10.3389/fonc.2022.1034398
23. Taj T, Poulsen AH, Ketzel M, Geels C, Brandt J, Christensen JH, et al. Long-term residential exposure to air pollution and Hodgkin lymphoma risk among adults in Denmark: a population-based case–control study. Cancer Causes Control. (2021) 32:935–42. doi: 10.1007/s10552-021-01446-w
24. Hübel K, Bower M, Aurer I, Bastos-Oreiro M, Besson C, Brunnberg U, et al. Human immunodeficiency virus-associated Lymphomas: EHA–ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. HemaSphere. (2024) 8:e150. doi: 10.1002/hem3.150
25. Castelli R, Schiavon R, Preti C, and Ferraris L. HIV-related lymphoproliferative diseases in the era of combination antiretroviral therapy. Cardiovasc Hematol Disord Drug Targets. (2020) 20:175–80. doi: 10.2174/1871529X20666200415121009
26. Ferlay J, Colombet M, Soerjomataram I, Parkin DM, Pineros M, Znaor A, et al. Cancer statistics for the year 2020: an overview. Int J Cancer. (2021) 149:778–89. doi: 10.1002/ijc.33588
27. Singh D, Vacarella S, Gini A, Silva NDP, Steliarova-Foucher E, and Bray F. Global patterns of Hodgkin lymphoma incidence and mortality in 2020 and a prediction of the future burden in 2040. Int J Cancer. (2022) 150:1941–7. doi: 10.1002/ijc.33948
28. Pongas GN and Ramos JC. HIV-associated lymphomas: progress and new challenges. J Clin Med. (2022) 11:1447. doi: 10.3390/jcm11051447
29. Marinescu AG, Mardarescu M, Vintila S, Batan-Gherghe A, and Iosif I. Compartimentul pentru Monitorizarea şi Evaluarea Infecţiei HIV/SIDA în România – INBI “Prof.Dr.M.Balş”. In: Evoluția HIV în România – 31 Decembrie 2024. (Bucharest, Romania: Compartment for Monitoring and Evaluation of HIV/AIDS Infection in Romania)(2024). Available at: https://www.cnlas.ro/index.php/date-statistice.
30. Arbune M, Georgescu C, Tutunaru D, Dobre M, and Nechita A. Epidemiological aspects of new HIV/AIDS diagnoses in south-east Romania. BMC Infect Dis. (2014) 14:P53. doi: 10.1186/1471-2334-14-S2-P53
31. Arbune M and Debita M. Current issues on HIV adults in South East of Romania. Ro J Infect Dis. (2018) 21:1. doi: 10.37897/RJID.2018.1.2
32. EACSociety. Available online at: https://www.eacsociety.org/media/2_neuropsychological_assessment_in_hivaids_and_its_challenges_in_galati:-_dr.marbune-_galati_Romania.pdf (Accessed December 14, 2024).
33. Arbune M, Padurariu-Covit MD, Rebegea LF, Lupasteanu G, Arbune AA, Stefanescu V, et al. AIDS related kaposi's sarcoma: A 20-year experience in a clinic from the South-East of Romania. J Clin Med. (2021) 10:5346. doi: 10.3390/jcm10225346
34. World Health Organization. International Statistical Classification of Diseases and Related Health Problems 10th Revision. (Geneva, Switzerland: World Health Organization) (2019).
35. Centers for Disease Control. 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults, (Atlanta: Morbidity and Mortality Weekly Report Recommendations and Reports (MMWR Recomm Rep)). Vol. 41. (1992). pp. 1–19.
36. European AIDS Clinical Society Guidelines . Available online at: https://www.eacsociety.org/guidelines/guidelines-archive (Accessed October 10, 2024).
37. Zelenetz AD, Gordon LI, Abramson JS, Advani RH, Andreadis B, Bartlett NL, et al. NCCN Guidelines® insights: B-cell lymphomas, version 6.2023: featured updates to the NCCN guidelines. J Natl Compr Canc Netw. (2023) 21:1118–31. doi: 10.6004/jnccn.2023.0057
38. Gobbi PG, Cavalli C, Gendarini A, Crema A, Ricevuti G, Federico M, et al. Reevaluation of prognostic significance of symptoms in Hodgkin's disease. Cancer. (1985) 56:2874–80. doi: 10.1002/1097-0142(19851215)56:12<2874::AID-CNCR2820561227>3.0.CO;2-2
39. Hoppe R, Advani RH, Ambinder RF, Armand P, Bello CM, Beniez CM, et al. Hodgkin Lymphoma Version 4.2024 — October 22, 2024. NCCN Clinical Practice Guidelines in Oncology (2024). Available online at: https://www.nccn.org/professionals/physician_gls/pdf/hodgkins.pdf (Accessed December 20, 2024).
40. Leal VNC, Reis EC, and Pontillo A. Inflammasome in HIV infection: lights and shadows. Mol Immunol. (2020) 118:9–18. doi: 10.1016/j.molimm.2019.12.001
41. Cao D, Khanal S, Wang L, Li Z, Zhao J, Nguyen LN, et al. A matter of life or death: productively infected and bystander CD4 T cells in early HIV infection. Front Immunol. (2021) 11:626431. doi: 10.3389/fimmu.2020.626431
42. Lv T, Cao W, and Li T. HIV-related immune activation and inflammation: current understanding and strategies. J Immunol Res. (2021) 1:7316456. doi: 10.1155/2021/7316456
43. Rice AP. The HIV-1 Tat protein: mechanism of action and target for HIV-1 cure strategies. Curr Pharm Design. (2017) 23:4098–102. doi: 10.2174/1381612823666170704130635
44. Isaguliants M, Bayurova E, Avdoshina D, Kondrashova A, Chiodi F, and Palefsky JM. Oncogenic effects of HIV-1 proteins, mechanisms behind. Cancers. (2021) 13:305. doi: 10.3390/cancers13020305
45. Okuma A and Idahor C. Mechanism of increased cancer risk in HIV. Eur J Health Sci. (2020) 5:42–50. doi: 10.47672/ejhs.522
46. Zhang F and Bieniasz PD. HIV-1 Vpr induces cell cycle arrest and enhances viral gene expression by depleting CCDC137. eLife. (2020) 9:e55806. doi: 10.7554/eLife.55806
47. Bertuzzi C, Sabattini E, Bacci F, Agostinelli C, and Ferri GG. Two different extranodal lymphomas in an HIV+ patient: a case report and review of the literature. Case Rep Hematol. (2019) 1:8959145. doi: 10.1155/2019/8959145
48. Ma W-L, Liu W-D, Sun H-Y, Sheng W-H, Hsieh S-M, Wu S-J, et al. Complete response to front-line therapies is associated with long-term survival in HIV-related lymphomas in Taiwan. J Microbiol Immunol Infect. (2024) 57:426–36. doi: 10.1016/j.jmii.2024.04.001
49. Omar A, Marques N, and Crawford N. Cancer and HIV: the molecular mechanisms of the deadly duo. Cancers (Basel). (2024) 16:546. doi: 10.3390/cancers16030546
50. Lurain K, Ramaswami R, and Yarchoan R. The role of viruses in HIV-associated lymphomas. Semin Hematol. (2022) 59:183–91. doi: 10.1053/j.seminhematol.2022.11.002
51. Franceschi S, Lise M, Clifford G, Rickenbach M, Levi F, Maspoli M, et al. Changing patterns of cancer incidence in the early- and late-HAART periods: the Swiss HIV Cohort Study. Br J Cancer. (2010) 103:416–22. doi: 10.1038/sj.bjc.6605756
52. Long JL, Engels EA, Moore RD, and Gebo KA. Incidence and outcomes of Malignancy in the HAART era in an urban cohort of HIV-infected individuals. AIDS. (2008) 22:489–96. doi: 10.1097/QAD.0b013e3282f47082
53. Li CB, Zhou Y, Wang Y, Liu S, Wang W, Lu X, et al. In-hospital mortality and causes of death in people diagnosed with HIV in a general hospital in Shenyang, China: a cross-sectional study. Front Public Health. (2021) 9:774614. doi: 10.3389/fpubh.2021.774614
54. Phillips AA and Smith DA. Health disparities and the global landscape of lymphoma care today. Am Soc Clin Oncol Educ Book. (2017) 37:226–33. doi: 10.1200/EDBK_175444
55. Radkiewicz C, Bruchfeld JB, Weibull CE, Jeppesen ML, Frederiksen H, Lambe M, et al. Sex differences in lymphoma incidence and mortality by subtype: a population-based study. Am J Hematol. (2022) 98:23–30. doi: 10.1002/ajh.26744
56. Pfreundschuh M. Age and sex in non-Hodgkin lymphoma therapy: it's not all created equal, or is it? Am Soc Clin Oncol Educ Book. (2017) 37:505–11. doi: 10.1200/EDBK_175447
57. Vargas JC, Marques MO, Pereira J, Braga WMT, Hamerschlak N, Tabacof J, et al. Factors associated with survival in patients with lymphoma and HIV. AIDS. (2023) 37:1217–26. doi: 10.1097/QAD.0000000000003549
58. Wu D, Chen C, Zhang M, Li Z, Wang S, Shi J, et al. The clinical features and prognosis of 100 AIDS-related lymphoma cases. Sci Rep. (2019) 9:5381. doi: 10.1038/s41598-019-41869-9
59. Vandenhende MA, Roussillon C, Henard S, Morlat P, Oksenhendler E, Aumaitre H, et al. Cancer-related causes of death among HIV-infected patients in France in 2010: evolution since 2000. PloS One. (2015) 10:e0129550. doi: 10.1371/journal.pone.0129550
60. Shiels MS, Pfeiffer RM, Gail MH, Hall HI, Li J, Chaturvedi AK, et al. Cancer burden in the HIV-infected population in the United States. J Natl Cancer Institute. (2011) 103:753–62. doi: 10.1093/jnci/djr076
61. Moahi K, Ralefala T, Nkele I, Triedman S, Sohani A, Musimar Z, et al. HIV and hodgkin lymphoma survival: A prospective study in Botswana. JCO Global Oncol. (2022) 8:e2100163. doi: 10.1200/GO.21.00163
62. WHO Regional Office for Europe and European Centre for Disease Prevention and Control. HIV/AIDS surveillance in Europe 2024–2023 data. Copenhagen: WHO Regional Office for Europe (2024). Available at: https://www.ecdc.europa.eu/en/publications-data/hiv-aids-surveillance-europe-2024-2023-data (Accessed January 3, 2025).
63. Mardarescu M. Future directions in HIV in relation to the four”95”. 2024 ECDC Network meeting, 15–17 April 2024. (Stockholm, Sweden: European Centre for Disease Prevention and Control (ECDC)) (2024). Available at: https://www.cnlas.ro/images/doc/2024/HIV%20Future%20Directions.pdf.
64. Ministry of Health. National Institute of Infectious Diseases “Prof. Dr. Matei Balş” Compartment for Monitoring and Evaluation of HIV/AIDS Infection in Romania. Statistic data (2003, 2012, 2024). Available online at: https://www.cnlas.ro/index.php/date-statistice (Accessed December 24, 2024).
65. Preda M and Manolescu LC. Romania, a harbour of HIV-1 subtype F1: where are we after 33 years of HIV-1 infection? Viruses. (2022) 14:2081. doi: 10.3390/v14092081
66. Arbune M, Georgescu CV, and Voinescu DC. AIDS-related lymphoma in a young HIV late presenter patient. Romanian J Morphology Embryology. (2016) 57:273–6.
67. Oseso LN, Chiao EY, and Ignacio RAB. Evaluating antiretroviral therapy initiation in HIV-associated Malignancy: Is there enough evidence to inform clinical guidelines? J Natl Compr Canc Netw. (2018) 16:927–32. doi: 10.6004/jnccn.2018.7057
68. Sereti I. Immune reconstruction inflammatory syndrome in HIV infection: Beyond what meets the eye. Top Antivir Med. (2020) 27:106–11.
69. Vargas JC, Cecyn KZ, Oliveira Marques M, Pereira J, Tobias Braga WM, Hamerschlak N, et al. Immune reconstitution inflammatory syndrome-associated lymphoma: A retrospective Brazilian cohort. EJHaem. (2023) 5:147–52. doi: 10.1002/jha2.835
70. Gopal S, Patel MR, Achenbach CJ, Yanik EL, Cole SR, Napravnik S, et al. Lymphoma immune reconstitution inflammatory syndrome in the center for AIDS research network of integrated clinical systems cohort. Clin Infect Dis. (2014) 59:279–86. doi: 10.1093/cid/ciu270
71. McNally GA. HIV and cancer: An overview of AIDS-defining and non–AIDS-defining cancers in patients with HIV. Clin J Oncol Nurs. (2019) 23:327–31. doi: 10.1188/19.CJON.327-331
72. Uldrick TS, Ison G, Rudek MA, Noy A, Schwartz K, Bruinooge S, et al. Modernizing clinical trial eligibility criteria: Recommendations of the American Society of Clinical Oncology-Friends of Cancer Research HIV Working Group. J Clin Oncol. (2017) 35:3774–80. doi: 10.1200/JCO.2017.73.7338
73. Vora KB, Ricciuti B, and Awad MM. Exclusion of patients living with HIV from cancer immune checkpoint inhibitor trials. Sci Rep. (2021) 11:6637. doi: 10.1038/s41598-021-86081-w
74. Carbone A, Vaccher E, and Gloghini A. Hematologic cancers in individuals infected by HIV. Blood. (2022) 139:995–1012. doi: 10.1182/blood.2020005469
75. Wang C, Wu Y, Liu J, Min H, Huang Y, Wei G, et al. Impact of initial chemotherapy cycles and clinical characteristics on outcomes for HIV-associated diffuse large B cell lymphoma patients: The Central and Western China AIDS Lymphoma League 001 study (CALL-001 study). Front Immunol. (2023) 14:1153790. doi: 10.3389/fimmu.2023.1153790
76. Hernández-Ramírez RU, Qin L, Lin H, Leyden W, Neugebauer RS, Althoff KN, et al. North American AIDS Cohort Collaboration on Research and Design of the International Epidemiologic Databases to Evaluate AIDS Collaborators. Association of immunosuppression and HIV viraemia with non-Hodgkin lymphoma risk overall and by subtype in people living with HIV in Canada and the USA: a multicentre cohort study. Lancet HIV. (2019) 6:e240–9. doi: 10.1016/S2352-3018(18)30360-6
77. Re A, Cattaneo C, and Montoto S. Treatment management of haematological Malignancies in people living with HIV. Lancet Haematol. (2020) 7:e679–89. doi: 10.1016/S2352-3026(20)30115-0
78. Bertoli D, Re A, Chiarini M, Sottini A, Serana F, Giustini V, et al. B-and T-lymphocyte number and function in HIV+/HIV– lymphoma patients treated with high-dose chemotherapy and autologous bone marrow transplantation. Sci Rep. (2016) 6:37995. doi: 10.1038/srep37995
79. Abbasi A, Peeke S, Shah N, Mustafa J, Khatun F, Lombardo A, et al. Axicabtagene ciloleucel CD19 CAR-T cell therapy results in high rates of systemic and neurologic remissions in ten patients with refractory large B-cell lymphoma including two with HIV and viral hepatitis. J Hematol Oncol. (2020) 13:1–4. doi: 10.1186/s13045-019-0838-y
Keywords: HIV, AIDS, lymphoma, antiretroviral treatment, opportunistic infections, non-AIDS co-morbidities
Citation: Padurariu-Covit M-D, Chiscop I, Gutu C, Arbune A-A, Niculet E and Arbune M (2025) Retrospective analysis of HIV-associated lymphomas: insights from a single Romanian center over 15 years. Front. Oncol. 15:1569433. doi: 10.3389/fonc.2025.1569433
Received: 31 January 2025; Accepted: 21 May 2025;
Published: 18 June 2025.
Edited by:
Nicola Sgherza, AOU Policlinico Consorziale di Bari, ItalyReviewed by:
Chaoyu Wang, Chongqing University, ChinaLaura E. Martinez, University of California, Los Angeles, United States
Copyright © 2025 Padurariu-Covit, Chiscop, Gutu, Arbune, Niculet and Arbune. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anca-Adriana Arbune, YW5jYS5hcmJ1bmVAaWNmdW5kZW5pLnJv
†These authors have contributed equally to this work
 Monica-Daniela Padurariu-Covit
Monica-Daniela Padurariu-Covit Iulia Chiscop3†
Iulia Chiscop3† Anca-Adriana Arbune
Anca-Adriana Arbune Elena Niculet
Elena Niculet Manuela Arbune
Manuela Arbune