Abstract
Background/Aim:
This study develops a machine learning-based predictive model to evaluate the survival outcomes of elderly patients with unresectable hepatocellular carcinoma (HCC) undergoing radiotherapy.
Methods:
The 2377 patients from SEER database were divided into training and internal validation cohorts. Additionally, 99 patients from our hospital were used for an external validation cohort. In the training cohort, 101 machine learning-based radiomics models were developed, and the optimal model’s performance was subsequently evaluated in both the internal and external validation cohorts.
Results:
The StepCox + GBM model demonstrated the highest C-index of 0.7 in the training cohort. The model was further evaluated using area under the receiver operating characteristic (AUC-ROC) curves, with AUC values ranging from 0.736 to 0.783, indicating strong predictive performance. Furthermore, the calibration curve and decision curves confirmed that the model had good predictive performance.
Conclusions:
The StepCox + GBM model could help optimize the use of radiotherapy for elderly HCC patients, improving survival outcomes and guiding personalized treatment strategies.
Introduction
Hepatocellular carcinoma (HCC) is one of the most prevalent cancers globally, with high incidence and mortality rates, particularly in regions with a high prevalence of hepatitis B virus (HBV) and hepatitis C virus (HCV) (1, 2). While surgical resection remains the preferred treatment for localized HCC, a significant number of patients are diagnosed at an advanced stage, where surgery is not feasible. For these patients, treatment options include transarterial chemoembolization (TACE), systemic therapy, and radiotherapy (3–5). Radiotherapy, especially for unresectable tumors, offers a non-invasive alternative to relieve symptoms, control tumor growth, and improve quality of life (6).
The prognosis for elderly HCC patients is relatively poor due to both their advanced age and the challenges associated with managing comorbidities (7). Elderly patients often present with more advanced stages of HCC and are less likely to tolerate aggressive treatments such as surgery or systemic therapies (8, 9). Radiotherapy has emerged as a promising modality for improving survival outcomes in elderly patients with unresectable HCC (10). However, there is a lack of reliable predictive models that can accurately identify elderly patients who are most likely to benefit from radiotherapy, thereby limiting the ability to tailor treatment strategies effectively.
Machine learning (ML) offers a powerful approach to constructing predictive models from complex datasets (11). ML algorithms can identify patterns and relationships within large datasets that may not be immediately apparent through traditional statistical methods (12). By leveraging the capabilities of machine learning, researchers have the potential to develop more precise and individualized treatment strategies for elderly patients with unresectable HCC (13–15). These models could help identify high-risk patients, thus assisting clinicians in making more informed treatment decisions.
To develop a robust predictive model for elderly patients with unresectable HCC undergoing radiotherapy, we will integrate data from the SEER database with data from our hospital. Through the application of machine learning techniques, we aim to construct a model that predicts the treatment outcomes for elderly patients with unresectable HCC undergoing radiotherapy, identifies high-risk patients, and assists clinicians in making more informed treatment decisions.
Materials and methods
Eligibility criteria
This study included 2377 unresectable HCC patients who received beam radiation from the SEER-17 registries (2000–2020) (Supplementary Figure S1), and 99 unresectable HCC patients treated with Gamma knife radiosurgery (GKR) across 363 hospitals (16). The inclusion criteria were: (a) no surgical treatment recommended, (b) received external beam radiotherapy, (c) complete information available, and (d) age ≥ 60 years. Patients with incomplete follow-up data or those who underwent surgery were excluded. The study was approved by the institutional review boards.
GKR
GKR was executed using a Treatment Planning System (TPS), where radiation oncologists delineated the treatment area based on contrast-enhanced CT images. The gross target volume (GTV), including the primary liver tumor, was defined using these imaging techniques, and a 5–10 mm margin was added around the GTV to create the planning target volume (PTV) via TPS. The median tumor margin dose was 42 Gy (ranging from 39 to 42 Gy).
Machine learning models
The 2377 patients were randomly divided into a training cohort (n = 1661) and an internal validation cohort (n = 716) at a 7:3 ratio. Additionally, data from our hospital were used for an external validation cohort (n = 99). Univariate Cox regression analysis was used to identify clinical factors affecting overall survival (OS) in the training cohort. Using these factors, 101 machine learning-based radiomics models were developed. The model’s performance was then assessed in both the internal and external validation cohorts using Harrell’s C-index, decision curve analysis (DCA), calibration curves, and receiver operating characteristic (ROC) curves.
Statistical analysis
Categorical variables between the training, internal validation, and external validation cohorts were compared using the chi-square test. All statistical analyses were conducted using R software (version 3.3.2). A p-value of < 0.05 was regarded as statistically significant.
Results
Clinical features
In the training cohort, the majority of patients were male (78.5%), White (74.9%), and had stage T1 tumors (37.8%). The internal validation cohort also had a high proportion of male patients (77.0%) and White patients (76.4%), with T1 tumors being the most common (34.2%). The external validation cohort consisted entirely of patients from the “Other” race group (100%) and had a higher proportion of T2 tumors (31.3%). In terms of AFP status, the majority of patients in the training and internal validation cohorts were AFP positive (45.4% and 42.0%, respectively), while the external validation cohort had an even split between positive and negative AFP. The three patient groups showed differences in race, grade, T, N, M, tumor stage, AFP levels, and tumor size (Table 1).
Table 1
| Training cohort | Internal validation | External validation | p | |
|---|---|---|---|---|
| Patient | 1661 | 716 | 99 | |
| Sex | 0.645 | |||
| Female | 357 (21.5) | 165 (23.0) | 20 (20.2) | |
| Male | 1304 (78.5) | 551 (77.0) | 79 (79.8) | |
| Race | <0.001 | |||
| White | 1244 (74.9) | 547 (76.4) | 0 | |
| Black | 185 (11.1) | 68 (9.5) | 0 | |
| Other | 232 (14.0) | 101 (14.1) | 99 (100.0) | |
| Grade | <0.001 | |||
| 1 | 172 (10.4) | 82 (11.5) | 0 | |
| 2 | 181 (10.9) | 76 (10.6) | 0 | |
| 3 | 112 (6.7) | 58 (8.1) | 0 | |
| 4 | 8 (0.5) | 5 (0.7) | 0 | |
| Unknown | 1188 (71.5) | 495 (69.1) | 99 (100.0) | |
| T | ||||
| T1 | 628 (37.8) | 245 (34.2) | 29 (29.3) | <0.001 |
| T2 | 251 (15.1) | 106 (14.8) | 31 (31.3) | |
| T3 | 329 (19.8) | 162 (22.6) | 14 (14.1) | |
| T4 | 116 (7.0) | 52 (7.3) | 25 (25.3) | |
| Tx | 337 (20.3) | 151 (21.1) | 0 | |
| N | ||||
| N0 | 1189 (71.6) | 509 (71.1) | 72 (72.7) | <0.001 |
| N1 | 152 (9.2) | 71 (9.9) | 27 (27.3) | |
| Unknown | 320 (19.3) | 136 (19.0) | 0 | |
| M | ||||
| M0 | 800 (48.2) | 336 (46.9) | 79 (79.8) | <0.001 |
| M1 | 739 (44.5) | 330 (46.1) | 20 (20.2) | |
| Unknown | 122 (7.3) | 50 (7.0) | 0 | |
| Stage | <0.001 | |||
| I | 412 (24.8) | 158 (22.1) | 4 (4.0) | |
| II | 137 (8.2) | 62 (8.7) | 23 (23.2) | |
| III | 162 (9.8) | 67 (9.4) | 29 (29.3) | |
| IV | 765 (46.1) | 347 (48.5) | 43 (43.4) | |
| Unknown | 185 (11.1) | 82 (11.5) | 0 | |
| AFP | <0.001 | |||
| Negative | 269 (16.2) | 129 (18.0) | 49 (49.5) | |
| Positive | 754 (45.4) | 301 (42.0) | 50 (50.5) | |
| Unknown | 638 (38.4) | 286 (39.9) | 0 | |
| Size, cm | ||||
| < 5 | 643 (38.7) | 271 (37.8) | 64 (64.6) | <0.001 |
| ≥ 5 | 704 (42.4) | 311 (43.4) | 35 (35.4) | |
| Unknown | 314 (18.9) | 134 (18.7) | 0 |
Clinical characteristics of HCC patients.
HCC, hepatocellular carcinoma; AFP, alpha fetoprotein.
Machine learning models construction
In the univariate COX analysis, Sex, Grade, T, N, M, Stage, and Size were confirmed as prognostic indicators for OS. Subsequently, 101 machine learning models were constructed in the training set, and StepCox[forward] + GBM exhibited the highest concordance index (C-index) of 0.7 (Figure 1). In the internal validation set and external validation set, the C-indices were 0.68 and 0.59, respectively (Figure 2). Based on the StepCox[forward] + GBM model, the risk score for each patient was calculated and divided into high-risk and low-risk groups based on the median. The high-risk group showed a shorter OS compared to the low-risk group in both the training set (Figure 3A) and the internal (Figure 3B) and external validation sets (Figure 3C).
Figure 1

Compare the C-index of 101 machine learning algorithms in the training set, internal validation set, and external validation set.
Figure 2
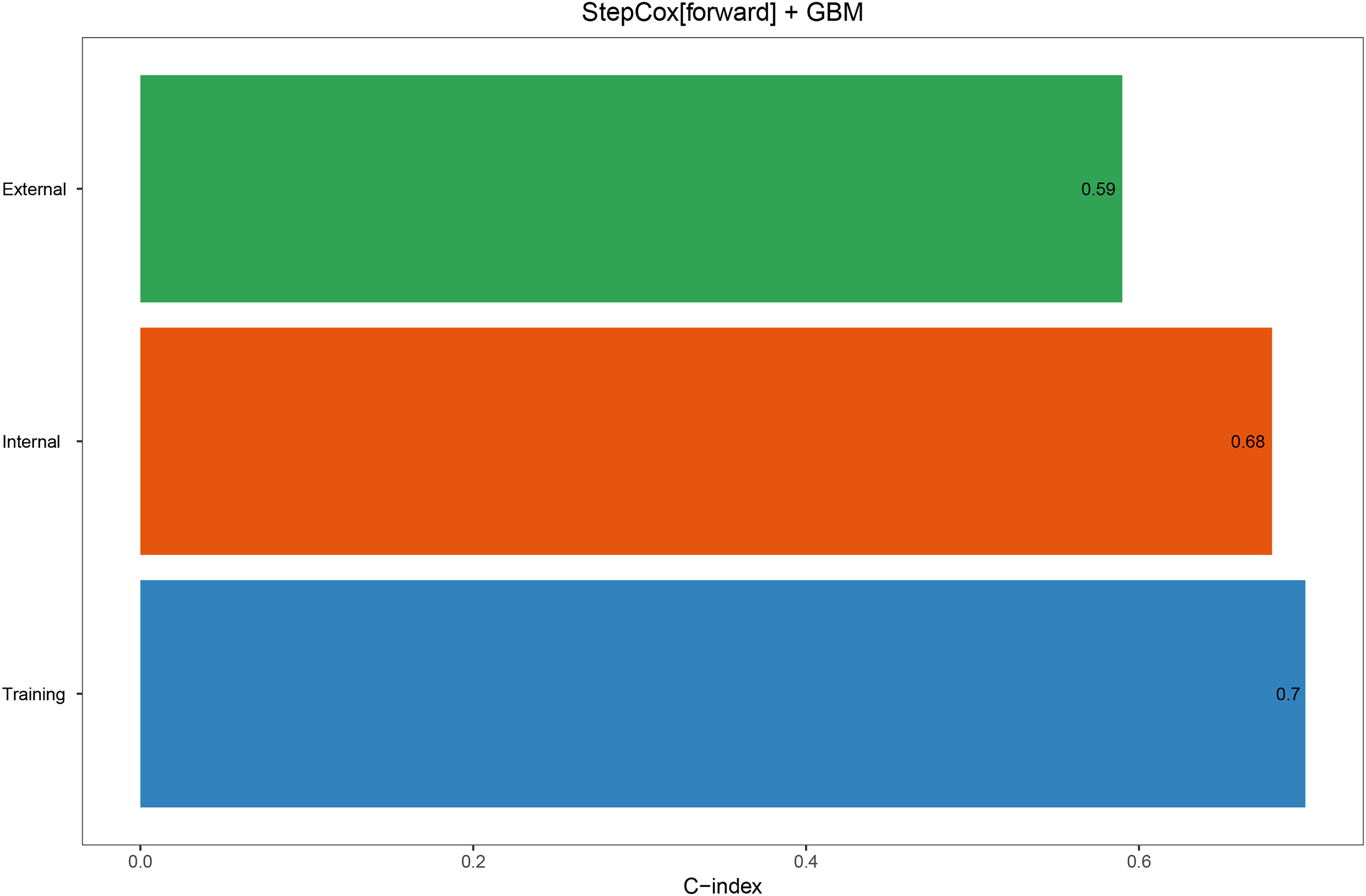
The C-index of the StepCox + GBM model in the training set, internal validation set, and external validation set.
Figure 3

The risk scores calculated using the StepCox + GBM model were divided into two groups (high and low) based on the median cutoff value. The OS of the two groups was compared in the training set (A), internal validation set (B), and external validation set (C).
Model evaluation
Next, we calculated the Area Under the ROC (AUC-ROC) values for the 1, 2, and 3-year OS based on the risk score. In the internal validation set, the AUC values were 0.769, 0.777, and 0.760, respectively (Figure 4A), and in the external validation set, the values were 0.744, 0.736, and 0.783, respectively (Figure 4B). Furthermore, the calibration curves (Figures 5A, B) and decision curves (Figures 6A, B) confirmed that the model had good predictive performance.
Figure 4

In the internal validation set (A) and external validation set (B), the StepCox + GBM model was used to evaluate the Area Under the Curve - Receiver Operating Characteristic for 1-, 2-, and 3- year overall survival.
Figure 5
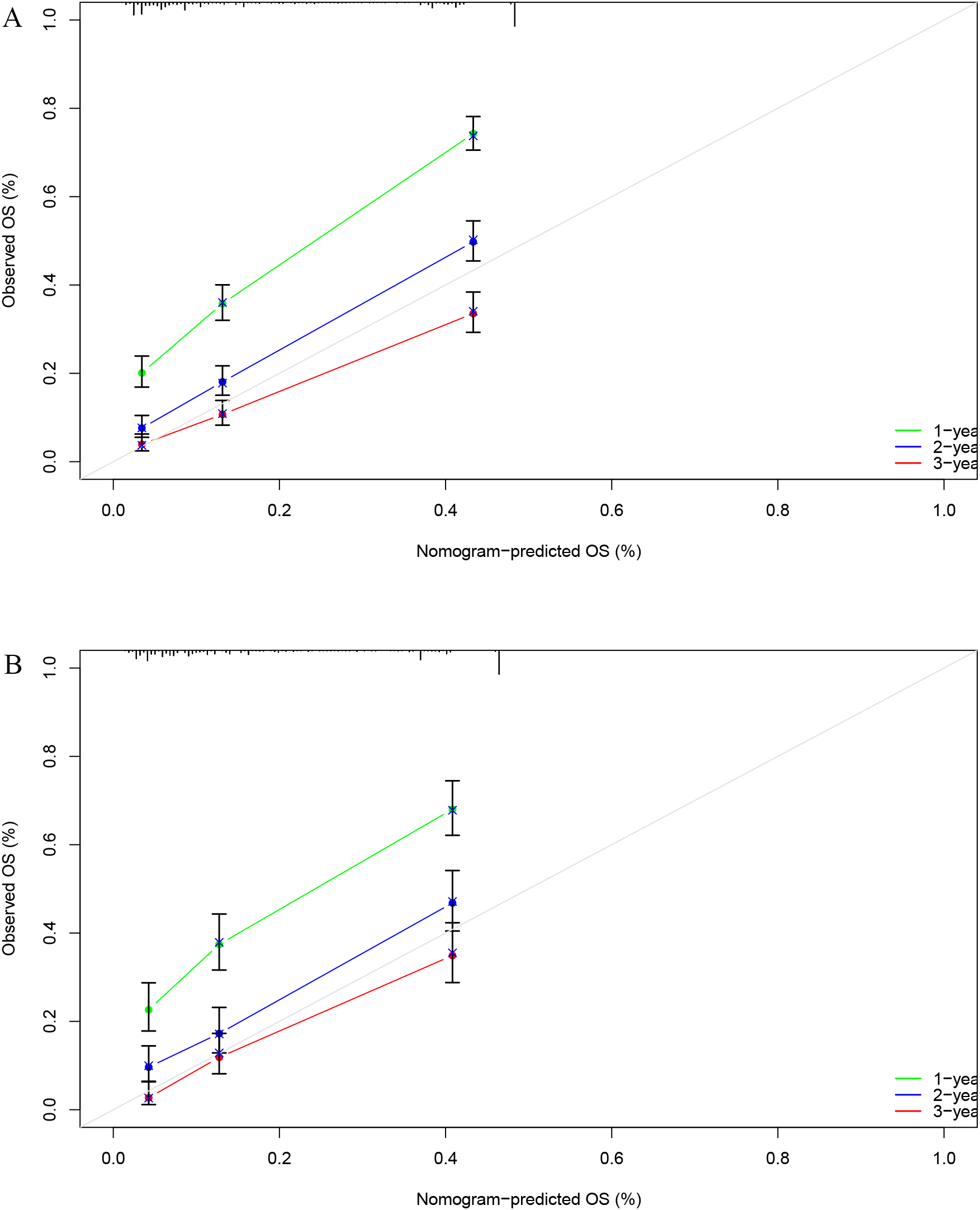
In the internal validation set (A) and external validation set (B), the calibration curve confirmed that the StepCox + GBM model has good predictive performance.
Figure 6
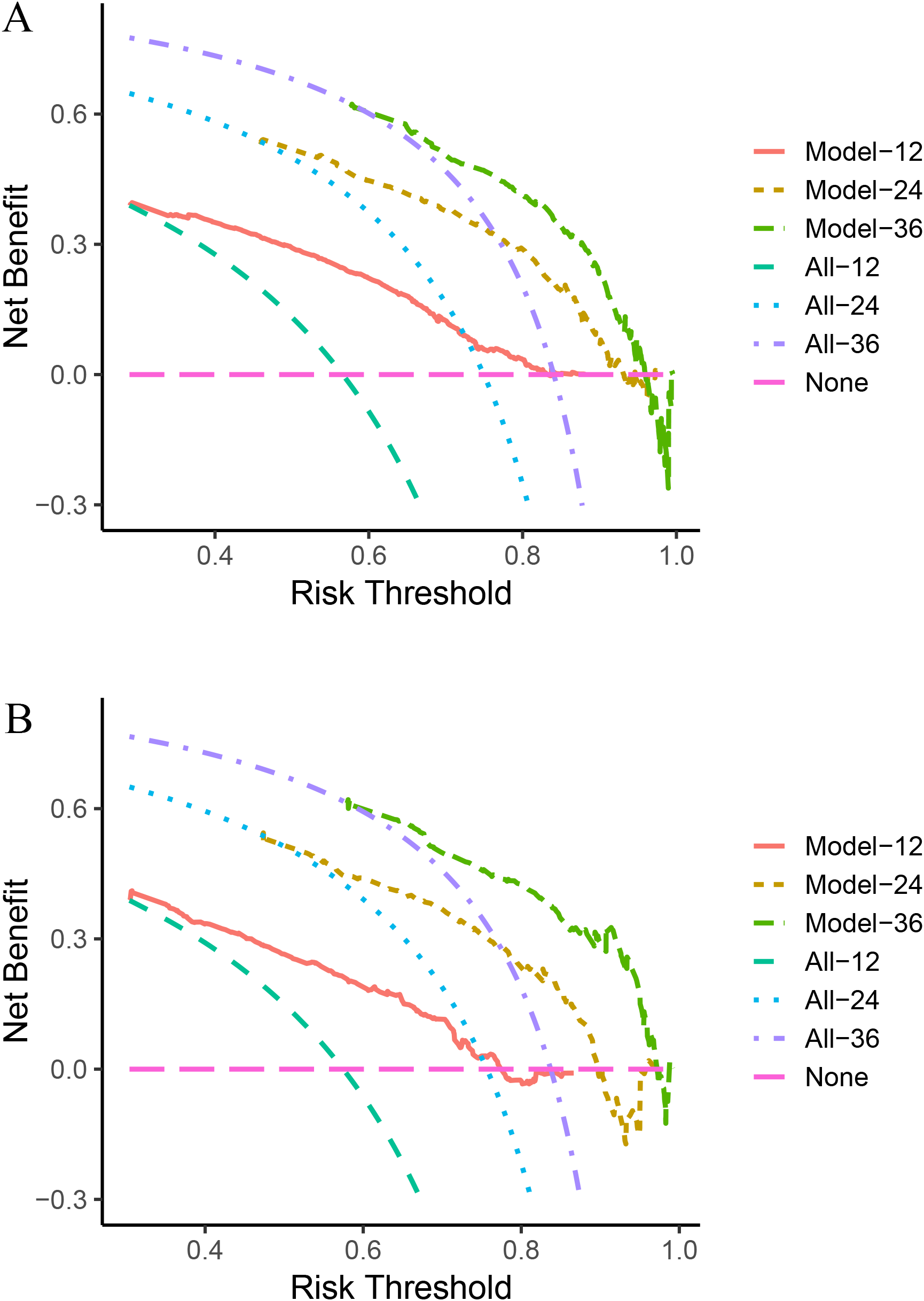
In the internal validation set (A) and external validation set (B), the decision curve analysis confirmed that the StepCox + GBM model has good predictive performance.
Discussion
HCC is a major health burden worldwide, with high mortality and incidence rates. The prognosis for patients with unresectable HCC is generally poor, and the treatment options are limited (17). This study is clinically significant as it aims to address a critical gap in personalized treatment for elderly patients with unresectable HCC. By leveraging data from both the SEER database and our hospital, this research builds a predictive model to help clinicians make more informed treatment decisions. By predicting the outcomes of radiotherapy for these patients, the model may guide the selection of appropriate treatments, ensuring that high-risk patients are identified and provided with the necessary care. The ability to predict treatment outcomes and tailor interventions accordingly could potentially improve OS and quality of life in elderly HCC patients, who are often more vulnerable due to the challenges posed by comorbidities and the advanced stage of their disease (18).
The treatment of elderly patients with unresectable HCC remains one of the most challenging aspects of managing liver cancer (19). These patients often present with more advanced stages of the disease, complicating treatment strategies (20). Additionally, their advanced age, frailty, and multiple comorbidities reduce their ability to tolerate aggressive treatments such as surgical resection or systemic therapies like chemotherapy (21). As a result, treatment options are limited, and survival outcomes for elderly patients are typically poor. In this context, radiotherapy has emerged as an essential non-invasive treatment option for elderly patients with unresectable tumors. Radiotherapy can help control tumor growth, alleviate symptoms such as pain or bleeding, and improve the quality of life. It is particularly valuable for those who are not candidates for surgery or other invasive treatments (22, 23). Despite its benefits, the selection of patients who will most benefit from radiotherapy remains a clinical challenge. Therefore, predictive models, such as the one presented in this study, could greatly aid in the identification of patients who are most likely to respond to radiotherapy, optimizing treatment strategies.
The machine learning-based predictive model developed in this study demonstrates strong predictive performance. The model incorporates clinical factors identified through univariate Cox regression analysis, such as sex, grade, T stage, N stage, M stage, tumor size, and others, to predict overall survival outcomes for elderly patients with unresectable HCC undergoing radiotherapy. The model’s performance was validated using both internal and external validation cohorts, showing a concordance index (C-index) of 0.7 in the training cohort, 0.68 in the internal validation cohort, and 0.59 in the external validation cohort, indicating a good predictive capability. Furthermore, the AUC-ROC values for 1, 2, and 3-year OS, ranging from 0.736 to 0.783 in both the internal and external validation sets, further underscore the model’s robustness. By calculating individual risk scores and categorizing patients into high-risk and low-risk groups, clinicians can better tailor their treatment strategies for elderly HCC patients. This risk stratification could potentially guide the use of radiotherapy, ensuring that high-risk patients receive appropriate interventions while avoiding unnecessary treatments for low-risk patients.
Recent studies on radiotherapy for HCC have highlighted several promising developments. Advances in techniques such as stereotactic body radiotherapy (SBRT) and proton therapy have shown significant improvements in treatment efficacy, especially for patients with unresectable tumors (24, 25). SBRT, with its precision in targeting tumors while sparing surrounding healthy tissues, has demonstrated high rates of tumor control and promising survival outcomes in both early and advanced stages of HCC (26, 27). These innovations in radiotherapy techniques and their integration with machine learning models make this study particularly relevant in the evolving landscape of liver cancer treatment.
We evaluated 101 different machine learning algorithms and identified the StepCox + GBM model as the one with the best predictive performance. This combined model demonstrates superior predictive performance compared to single-model approaches, offering significant clinical value, especially in the context of personalized medicine (28). By integrating these two powerful algorithms, the study leverages their complementary strengths—StepCox’s ability to identify significant clinical factors and GBM’s capability to handle complex non-linear relationships in the data (29, 30). This synergy enhances the model’s overall accuracy and robustness, providing a more reliable tool for predicting survival outcomes in elderly patients with unresectable HCC undergoing radiotherapy. The ability to identify high-risk patients and predict their treatment response before therapy begins would help clinicians optimize resources and improve patient outcomes. Moreover, the combined model has the potential to continuously evolve as more data becomes available, further refining its accuracy and predictive power, making it a valuable asset for clinical decision-making and individualized treatment plans (31).
The predictive model offers several clinical benefits: it enables personalized treatment decisions by identifying high-risk patients who may benefit from more aggressive or additional therapies, while allowing low-risk patients to avoid overtreatment and its associated toxicities. Moreover, by tailoring treatment based on predicted outcomes, the model facilitates optimized resource allocation, ensuring that the patients most likely to benefit receive appropriate interventions. Finally, by providing an objective, data-driven estimation of survival outcomes, the model enhances prognostic accuracy, thereby supporting clinical decision-making and potentially improving overall survival and quality of life for this vulnerable patient population.
Despite the promising results, there are several limitations to this study. First, the model was based on retrospective data from the SEER database and our hospital, which may introduce selection bias. The external validation cohort, while adding strength to the study, may still differ in terms of patient demographics and treatment protocols, potentially affecting the model’s generalizability. Second, the model does not account for potential changes in treatment regimens or advancements in radiotherapy techniques that may have occurred after the data collection period. Additionally, the model’s reliance on clinical factors such as tumor stage and size may not fully capture the complexity of individual patients’ responses to radiotherapy. Future studies could incorporate genetic, molecular, and radiomic data to further refine the model and improve its predictive accuracy.
Conclusion
This study presents a machine learning-based predictive model that shows strong performance in predicting the survival outcomes of elderly patients with unresectable HCC undergoing radiotherapy. The model’s ability to stratify patients into high-risk and low-risk groups based on clinical factors provides valuable insights that can aid in clinical decision-making.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The study was approved by 363 Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
XL: Conceptualization, Methodology, Project administration, Resources, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. YS: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by The Beijing Medical Award Foundation (YXI-2023-0227-0131) and the Health Commission of Chengdu (2023591).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1585125/full#supplementary-material
References
1
Wang Y Deng B . Hepatocellular carcinoma: molecular mechanism, targeted therapy, and biomarkers. Cancer Metastasis Rev. (2023) 42:629–52. doi: 10.1007/s10555-023-10084-4
2
Gilles H Garbutt T Landrum J . Hepatocellular carcinoma. Crit Care Nurs Clin North Am. (2022) 34:289–301. doi: 10.1016/j.cnc.2022.04.004
3
Shanker MD Moodaley P Soon W Liu HY Lee YY Pryor DI . Stereotactic ablative radiotherapy for hepatocellular carcinoma: A systematic review and meta-analysis of local control, survival and toxicity outcomes. J Med Imaging Radiat Oncol. (2021) 65:956–68. doi: 10.1111/1754-9485.13309
4
Su K Gu T Xu K Wang J Liao H Li X et al . Gamma knife radiosurgery versus transcatheter arterial chemoembolization for hepatocellular carcinoma with portal vein tumor thrombus: a propensity score matching study. Hepatol Int. (2022) 16:858–67. doi: 10.1007/s12072-022-10339-2
5
Liu J Wang P Shang L Zhang Z Tian Y Chen X et al . TACE plus tyrosine kinase inhibitors and immune checkpoint inhibitors versus TACE plus tyrosine kinase inhibitors for the treatment of patients with hepatocellular carcinoma: a meta-analysis and trial sequential analysis. Hepatol Int. (2024) 18:595–609. doi: 10.1007/s12072-023-10591-0
6
Rim CH Park S Yoon WS Shin IS Park HC . Radiotherapy for bone metastases of hepatocellular carcinoma: a hybrid systematic review with meta-analyses. Int J Radiat Biol. (2023) 99:419–30. doi: 10.1080/09553002.2022.2094020
7
Amato B Aprea G De Rosa D Milone M di Domenico L Amato M et al . Laparoscopic hepatectomy for HCC in elderly patients: risks and feasibility. Aging Clin Exp Res. (2017) 29:179–83. doi: 10.1007/s40520-016-0675-6
8
Liu YW Yong CC Lin CC Wang CC Chen CL Cheng YF et al . Liver resection in elderly patients with hepatocellular carcinoma: age does matter. Updates Surg. (2021) 73:1371–80. doi: 10.1007/s13304-021-01021-7
9
Antwi SO Li Z Mody K Roberts LR Patel T . Independent and joint use of statins and metformin by elderly patients with diabetes and overall survival following HCC diagnosis. J Clin Gastroenterol. (2020) 54:468–76. doi: 10.1097/MCG.0000000000001182
10
Qiu X Cai J Chen H Yao J Xiao C Li R et al . Chemotherapy combined with radiotherapy can benefit more unresectable HCC patients with portal and/or hepatic vein invasion: a retrospective analysis of the SEER database. Front Oncol. (2023) 13:1098686. doi: 10.3389/fonc.2023.1098686
11
Chen D Liu J Zang L Xiao T Zhang X Li Z et al . Integrated machine learning and bioinformatic analyses constructed a novel stemness-related classifier to predict prognosis and immunotherapy responses for hepatocellular carcinoma patients. Int J Biol Sci. (2022) 18:360–73. doi: 10.7150/ijbs.66913
12
Dong B Zhang H Duan Y Yao S Chen Y Zhang C . Development of a machine learning-based model to predict prognosis of alpha-fetoprotein-positive hepatocellular carcinoma. J Transl Med. (2024) 22:455. doi: 10.1186/s12967-024-05203-w
13
Lee T Rawding PA Bu J Hyun S Rou W Jeon H et al . Machine-learning-based clinical biomarker using cell-free DNA for hepatocellular carcinoma (HCC). Cancers (Basel). (2022) 14(9):2061. doi: 10.3390/cancers14092061
14
Hsieh C Laguna A Ikeda I Maxwell AWP Chapiro J Nadolski G et al . Using machine learning to predict response to image-guided therapies for hepatocellular carcinoma. Radiology. (2023) 309:e222891. doi: 10.1148/radiol.222891
15
Raman AG Jones C Weiss CR . Machine learning for hepatocellular carcinoma segmentation at MRI: radiology in training. Radiology. (2022) 304:509–15. doi: 10.1148/radiol.212386
16
Chidambaranathan-Reghupaty S Fisher PB Sarkar D . Hepatocellular carcinoma (HCC): Epidemiology, etiology and molecular classification. Adv Cancer Res. (2021) 149:1–61. doi: 10.1016/bs.acr.2020.10.001
17
Kirchner T Marquardt S Werncke T Kirstein MM Brunkhorst T Wacker F et al . Comparison of health-related quality of life after transarterial chemoembolization and transarterial radioembolization in patients with unresectable hepatocellular carcinoma. Abdominal Radiol (New York). (2019) 44:1554–61. doi: 10.1007/s00261-018-1802-y
18
Fan C Jin G Sun C Ma D Chen C Qiao P et al . Clinical characteristics, treatment patterns and survival outcome of hepatocellular carcinoma patients aged 70 years or older: a single-center retrospective study from China. Aging Clin Exp Res. (2014) 26:123–30. doi: 10.1007/s40520-013-0142-6
19
Jiang Y Guo L Han L Li H Li C Wen L et al . Thymidine kinase 1 appears to be a marker for the prognosis of hepatocellular carcinoma based on a large-scale, multicenter study. J Cancer Res Clin Oncol. (2023) 149:14271–82. doi: 10.1007/s00432-023-05089-z
20
Sasaki R Fukushima M Haraguchi M Miuma S Miyaaki H Hidaka M et al . Liver function in older patients with unresectable hepatocellular carcinoma after administration of lenvatinib. Anticancer Res. (2021) 41:2025–32. doi: 10.21873/anticanres.14970
21
Kanno H Hashimoto K Sakai H Ogata T Fukutomi S Akashi M et al . Safety and feasibility of liver resection including major hepatectomy for geriatric patients with hepatocellular carcinoma: a retrospective observational study. BMC Cancer. (2024) 24:765. doi: 10.1186/s12885-024-12514-0
22
Liu CM Huang BS Yen YH Wang YM Huang EY Hsu HC et al . Concurrent sorafenib and radiotherapy versus radiotherapy alone for locally advanced hepatocellular carcinoma: A propensity-matched analysis. J Hepatocell Carcinoma. (2021) 8:963–73. doi: 10.2147/JHC.S323302
23
Jo IY Park HC Kim ES Yeo SG Kim M Seong J et al . Stereotactic ablative radiotherapy for pulmonary oligometastases from primary hepatocellular carcinoma: a multicenter and retrospective analysis (KROG 17-08). Japanese J Clin Oncol. (2022) 52:616–22. doi: 10.1093/jjco/hyac028
24
Kumar R Yadav HP Thaper D Kamal R Gupta A Kirti S . Efficacy and toxicity of SBRT in advanced hepatocellular carcinoma with portal vein tumor thrombosis - a retrospective study. Rep Pract Oncol Radiother: J GreatPoland Cancer Center Poznan Polish Soc Radiat Oncol. (2021) 26:573–81. doi: 10.1016/j.ijrobp.2020.07.1883
25
Komiyama S Takeda A Tateishi Y Tsurugai Y Eriguchi T Horita N . Comparison of stereotactic body radiotherapy and transcatheter arterial chemoembolization for hepatocellular carcinoma: Systematic review and meta-analysis. Radiother Oncol: J Eur Soc Ther Radiol Oncol. (2025) 202:110614. doi: 10.1016/j.radonc.2024.110614
26
Sun J Zhang A Li W Wang Q Li D Zhang D et al . Biologically effective dose (BED) escalation of stereotactic body radiotherapy (SBRT) for hepatocellular carcinoma patients (≤5 cm) with CyberKnife: protocol of study. Radiat Oncol (London England). (2020) 15:20. doi: 10.1186/s13014-020-1471-1
27
den Toom W Negenman EM Willemssen F van Werkhoven E Porte RJ de Wilde RF et al . Long-term outcomes of more than a decade treating patients with stereotactic body radiation therapy for hepatocellular carcinoma. Clin Trans Radiat Oncol. (2024) 49:100878. doi: 10.1016/j.ctro.2024.100878
28
Angelis I Exarchos T . Hepatocellular carcinoma detection using machine learning techniques. Adv Exp Med Biol. (2021) 1338:21–9. doi: 10.1007/978-3-030-78775-2_4
29
Wang T Han L Xu J Guo B . Identification of vitamin D-related signature for predicting the clinical outcome and immunotherapy response in hepatocellular carcinoma. Medicine. (2024) 103:e37998. doi: 10.1097/MD.0000000000037998
30
Kim HY Lampertico P Nam JY Lee HC Kim SU Sinn DH et al . An artificial intelligence model to predict hepatocellular carcinoma risk in Korean and Caucasian patients with chronic hepatitis B. J Hepato. (2022) 76:311–8. doi: 10.1016/j.jhep.2021.09.025
31
Chen C Peng R Jin S Tang Y Liu H Tu D et al . Identification of potential biomarkers for hepatocellular carcinoma based on machine learning and bioinformatics analysis. Discov Oncol. (2024) 15:808. doi: 10.1007/s12672-024-01667-w
Summary
Keywords
hepatocellular carcinoma, radiotherapy, survival prognosis, machine learning, survival
Citation
Shi Y and Liu X (2025) Machine learning for prognostic impact in elderly unresectable hepatocellular carcinoma undergoing radiotherapy. Front. Oncol. 15:1585125. doi: 10.3389/fonc.2025.1585125
Received
28 February 2025
Accepted
31 March 2025
Published
16 April 2025
Volume
15 - 2025
Edited by
Yunwei Han, The Affiliated Hospital of Southwest Medical University, China
Reviewed by
Ke Xu, Chongqing General Hospital, China
Susu Xiao, Sichuan University, China
Updates
Copyright
© 2025 Shi and Liu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xianguo Liu, 363.lxg@163.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.