- 1The First Clinical Medical College, Lanzhou University, Lanzhou, China
- 2Department of Endocrinology, First Hospital of Lanzhou University, Lanzhou, China
- 3Gansu Provincial Endocrine Disease Clinical Medicine Research Center, Lanzhou, China
Thyroid cancer represents the most prevalent malignant neoplasm within the endocrine system, exhibiting a steadily increasing global incidence. Non-coding RNAs (ncRNAs) have emerged as a pivotal focus in thyroid cancer research, demonstrating significant involvement in tumor progression through epigenetic regulation. Contemporary studies reveal widespread dysregulation of ncRNA expression profiles in thyroid malignancies, where differentially expressed ncRNAs exert either tumor-suppressive or oncogenic functions by modulating epithelial-mesenchymal transition (EMT) mechanisms. This review synthesizes current knowledge on EMT-related ncRNA mechanisms driving thyroid carcinogenesis and evaluates their diagnostic and therapeutic potential. By elucidating these molecular interactions, we aim to catalyze discovery of novel ncRNA-mediated pathways, advance targeted treatment strategies, and ultimately enhance clinical outcomes for thyroid cancer patients.
1 Introduction
Thyroid cancer, a malignancy arising from follicular epithelial or parafollicular C-cells of the thyroid gland, constitutes the most prevalent endocrine malignancy in head and neck oncology. Recent epidemiological data from the United States (2024) document 44,020 new thyroid cancer diagnoses (2.2% of total cancer incidence) with 2,170 mortality events (0.355% of cancer-related deaths) (1). Histopathological classification delineates three principal subtypes: Differentiated Thyroid Carcinoma [DTC; encompassing papillary (PTC), follicular (FTC), and Hürthle cell variants], Medullary Thyroid Carcinoma (MTC), and Anaplastic Thyroid Carcinoma (ATC). DTC predominates, representing 84% of thyroid malignancies (2, 3). While most DTC cases exhibit favorable prognoses, clinical challenges persist for patients with advanced differentiation grades and those presenting with poorly differentiated/undifferentiated histotypes, who demonstrate elevated risks of recurrence and metastasis (4). Emerging evidence identifies multifactorial etiology involving ionizing radiation exposure, dietary iodine levels, metabolic disorders (obesity/diabetes), environmental factors (food additives), and tobacco use alongside diagnostic surveillance biases (5, 6).
Epithelial-Mesenchymal Transition (EMT), an evolutionarily conserved biological process, serves as a critical driver of tumor cell invasiveness and metastatic dissemination, substantially complicating therapeutic interventions and prognostic predictions. This multistep phenotypic plasticity is orchestrated through synergistic molecular crosstalk among multiple signaling pathways (7), enabling epithelial cells to adopt mesenchymal characteristics. Characteristic transformations include loss of apical-basal polarity, dissolution of adherens junctions, and augmented migratory capacity. While physiologically essential during embryogenesis, EMT becomes pathologically activated in disease states, particularly facilitating cancer progression through enhanced tissue invasion and distant metastasis (8).
Concurrently, non-coding RNAs (ncRNAs) have emerged as a focus of intense investigation in oncological research, with particular relevance to thyroid cancer pathogenesis and therapeutic innovation. Defined as functionally active RNA species lacking protein-coding potential, ncRNAs exert regulatory control over gene expression networks, intracellular signaling cascades, and oncogenic transformation (9). Mechanistic studies demonstrate that EMT induction in thyroid malignancies is critically modulated by specific ncRNA signatures, establishing direct links between ncRNA dysregulation and metastatic progression (10). Consequently, EMT-associated ncRNAs present dual diagnostic and therapeutic potential. Elucidating the precise molecular circuitry through which ncRNAs govern EMT dynamics promises to reveal novel targets for metastasis suppression and recurrence prevention. Such advancements hold translational significance for optimizing clinical management strategies, potentially elevating both survival outcomes and quality-of-life metrics in thyroid cancer patients.
2 EMT
EMT describes a biological process wherein epithelial cells progressively acquire mesenchymal characteristics through coordinated loss of apical-basal polarity and intercellular adhesion properties (11). Originally conceptualized by Greenberg and Hay in 1982 (12), this fundamental mechanism has subsequently been shown to mediate both physiological processes (e.g., embryonic morphogenesis and wound healing) and pathological tumor dissemination. EMT activation triggers dissolution of epithelial tight junction complexes coupled with cytoskeletal reorganization and upregulation of motility-associated proteins (13). These molecular alterations induce characteristic morphological changes from cuboidal to spindle-shaped cells with pseudopodia formation and front-rear polarity establishment, enabling chemotaxis-driven tumor cell migration that facilitates metastatic spread (14).
2.1 Characteristics of the EMT process
2.1.1 Progressivity
Studies demonstrate that EMT progresses through multiple intermediate states, displaying gradations of epithelial and mesenchymal markers between polarized epithelial and fully mesenchymal phenotypes (11, 13, 15). Single-cell analyses reveal that hybrid epithelial/mesenchymal states predominate in human tissues, whereas complete mesenchymal conversion rarely occurs in both physiological and neoplastic contexts (16). Notably, epithelial-derived malignancies acquire migratory competence through tumor-associated dedifferentiation mechanisms, directly facilitating metastatic dissemination (17). The partial EMT state—characterized by incomplete mesenchymal transformation—confers three critical oncogenic properties: (i) enhanced motility, (ii) bidirectional phenotypic plasticity, and (iii) lineage reprogramming capacity (18). Intratumoral heterogeneity analyses further suggest that epithelial-dominant subclones exhibit elevated malignant potential and preferential metastatic tropism compared to mesenchymal counterparts (19, 20).
2.1.2 Reversibility
Epithelial-mesenchymal transition demonstrates bidirectional plasticity, wherein epithelial and mesenchymal phenotypes undergo reciprocal conversion mediated by microenvironmental cues (13). Notably, clinical evidence documents that circulating tumor cells in distant metastases frequently retain epithelial markers (11), contradicting the classical EMT paradigm predicting complete mesenchymal transformation. This apparent paradox may be reconciled through mesenchymal-epithelial transition (MET) mechanisms, whereby metastasized mesenchymal-like cells regain epithelial characteristics to facilitate metastatic niche formation. While EMT activation is essential for initiating metastatic dissemination, its maintenance proves dispensable during subsequent colonization phases.
2.2 Mechanisms
The regulatory network governing epithelial-mesenchymal transition involves a dynamic, hierarchical system driven by coordinated integration of extracellular microenvironmental cues, intracellular signaling pathway activation, transcription factor cascades, and epigenetic modifications across multiple biological scales (21–23).
2.2.1 External stimuli and tumor microenvironment
The precise temporal dynamics underlying metastatic initiation in primary tumors remain incompletely understood. Notably, EMT activation is primarily mediated through TME-derived stress signaling, with key drivers encompassing hypoxia, TGF-β pathway activation, pro-inflammatory cytokines (e.g., IL-6), growth factors (e.g., EGF), and extracellular matrix remodeling (24). These factors coordinately promote tumor cell dissociation from primary sites and subsequent migration via interlinked molecular cascades (25).
2.2.1.1 Hypoxia/hypoxia-inducible factor-1α
Hypoxia-Inducible Factor-1α (HIF-1α) is one of the key microenvironmental factors that induce cancer metastasis. Under hypoxic conditions, HIF-1α is up-regulated and regulates the expression of EMT markers and a variety of EMT transcription factors (26), including Snail, Twist1, ZEB1, ZEB2, and SIP1. HIF-1α up-regulation is significantly associated with lymph node metastasis and distant metastasis, and is involved in tumor growth, metabolism, angiogenesis, and metastasis by regulating gene expression (27). At the chromatin level, HIF-1α regulates the expression of EMT markers through histone modifications. For example, H3K4 acetylation (H3K4Ac) marks the promoters of EMT-related genes or transcription factors and promotes the repression of epithelial genes and activation of mesenchymal genes, such as E-cadherin and poikilodulin. It has been demonstrated that HIF-1α overexpression induces EMT, down-regulates the epithelial marker E-cadherin and up-regulates the mesenchymal marker waveform proteins, and correlates with a highly aggressive and metastatic phenotype (28). In addition, IL-11 promotes hypoxia-induced EMT through HIF-1α induction and enhances the invasive and migratory capacity of undifferentiated thyroid cancer (ATC) cells (29). In the thyroid cancer cell line FTC133, HIF-1α overexpression increased Twist gene expression, whereas inhibition of HIF-1α activity abrogated the increase in Twist gene expression under hypoxic conditions (30). In addition, hypoxia mediates immunosuppression, and HIF-1α inhibits the cGAS-STING pathway through activation of anti-apoptotic genes, up-regulation of PD-L1, activation of the CD39/CD73 pathway, and up-regulation of miR25/93, thereby conferring the ability of tumor cells to evade immune surveillance (24).
2.2.1.2 TGF-β family proteins: inducers of EMT
Transforming growth factor-β1 (TGF-β1) serves as a master regulator of EMT, orchestrating tumor invasion and metastasis through three molecular tiers (1): transcriptional reprogramming, (2) post-transcriptional modulation, and (3) translational control (31, 32).
Within Smad-dependent signaling, ligand-bound TGF-β receptors phosphorylate Smad2/3 and Smad1/5/8, which subsequently form heteromeric complexes with Smad4 that undergo nuclear translocation. These nuclear complexes coordinate with EMT master regulators (Snail1/2, ZEB1/2, Twist) to suppress epithelial markers (e.g., E-cadherin) and drive cellular plasticity. Notably, Smad3 specifically governs the Snail1/2-ZEB1/2-Twist transcriptional axis through promoter binding affinity modulation, establishing an auto-amplification circuit for sustained EMT activation.
Non-canonical pathways engage PI3K-Akt-mTOR and MAPK cascades, executing chromatin topology reorganization, epigenetic landscape reshaping, alternative mRNA splicing, and miRNA-mediated gene silencing to fine-tune EMT progression (24, 33, 34). Exogenous TGF-β triggers exosome-mediated intercellular communication, significantly suppressing papillary thyroid carcinoma (TPC1) proliferation via EMT- and stemness-related marker induction. Mechanistically, platelet-derived TGF-β coordinates Smad/NF-κB crosstalk to potentiate EMT-driven metastatic dissemination.
2.2.2 Core signaling networks
2.2.2.1 Wnt/β-catenin pathway
The Wnt protein family comprises evolutionarily conserved, multidomain glycoproteins encoded by polycistronic genes, forming intricate receptor-mediated signaling networks critical for embryonic morphogenesis and tissue homeostasis (35). Central to stem cell maintenance and regenerative processes, the Wnt/β-catenin pathway operates through spatiotemporally coordinated ligand-receptor interactions.
Nineteen identified Wnt ligands exhibit spatiotemporal-specific expression patterns mediated by autocrine/paracrine mechanisms (36). Their biogenesis initiates with endoplasmic reticulum (ER)-lumenal palmitoylation catalyzed by membrane-bound O-acyltransferase PORCN, enabling hydrophobic modification essential for secretory trafficking. Mature Wnt proteins complex with the cargo receptor Wntless (WLS), subsequently mediating intercellular communication via vesicular transport systems and regulated exocytosis.
The Wnt signaling network bifurcates into two principal transduction cascades: (1) Canonical Pathway (β-catenin-dependent): Ligand-receptor engagement inhibits β-catenin degradation complexes, enabling cytoplasmic β-catenin stabilization and subsequent nuclear import. Nuclear β-catenin forms transcriptional complexes with T-cell factor/lymphoid enhancer factor (TCF/LEF) to activate metastasis-associated gene programs (37); (2) Non-canonical Pathway (β-catenin-independent): Frizzled (FZD) co-receptors with LRP5/6 activate Dishevelled phosphoprotein clusters, orchestrating planar cell polarity (PCP) signaling and mechanotransduction-regulated migratory phenotypes.
2.2.2.2 PI3K/AKT/mTOR pathway
Phosphatidylinositol 3-kinase (PI3K), a lipid-modifying enzyme in the phosphoinositide 3-kinase family, orchestrates oncogenic progression through dual regulation of mitogenic signaling and metabolic reprogramming. Receptor tyrosine kinase (RTK)-initiated extracellular cues undergo RAS-mediated activation, facilitating membrane recruitment of class IA PI3K catalytic subunit p110α. This enzymatic subunit converts phosphatidylinositol-4,5-bisphosphate (PIP2) to phosphatidylinositol-3,4,5-trisphosphate (PIP3), thereby initiating AKT/mTOR signaling cascades critical for tumor survival.
The mammalian target of rapamycin (mTOR), a serine/threonine kinase, functions as a metabolic gatekeeper by integrating growth factor inputs, nutrient availability, and bioenergetic states. Mechanistically, mTOR complex 2 (mTORC2) phosphorylates AKT at Ser473 and protein kinase C (PKC) isoforms, thereby coordinating cytoskeletal reorganization essential for EMT-driven cell motility (38). TGF-β receptors engage PI3K regulatory subunit p85 to activate AKT signaling, which subsequently triggers mTORC1-dependent translational control mechanisms that potentiate EMT progression (39, 40).
2.2.3 Transcription factor network
The EMT is coordinately regulated by core transcription factors, primarily comprising the SNAIL, TWIST, and zinc-finger E-box binding (ZEB) families (41). These transcriptional regulators are activated through canonical signaling pathways including Wnt and TGF-β, subsequently initiating signaling cascades that drive EMT progression.
Key transcriptional regulators such as TWIST1/2, inhibitor of differentiation (ID) proteins, and E12/E47 can induce EMT through individual or synergistic mechanisms. TWIST1, a basic helix-loop-helix (bHLH) transcription factor, mediates both embryonic morphogenesis and pathological EMT by dual regulation of cadherin switching: suppressing E-cadherin while promoting N-cadherin expression (42).
The SNAIL zinc-finger family (SNAI1/SLUG) exerts transcriptional repression of E-cadherin through specific binding to E-box motifs in its promoter. During embryogenesis, SNAIL signaling facilitates epithelial cell delamination and subsequent mesenchymal differentiation. Clinically relevant, this pathway’s dysregulation correlates with metastatic progression in malignancies such as papillary thyroid carcinoma and melanoma. Experimental models demonstrate that in BRAF-mutant thyroid carcinomas undergoing dedifferentiation, SNAI1/ZEB1/ZEB2 overexpression induces: (1) marked suppression of tight junction/desmosome-related genes; and (2) concurrent upregulation of intermediate filament and basement membrane components - a molecular signature validated across multiple studies (43–45).
ZEB1 is a pleiotropic transcription factor belonging to a family of zinc finger and homology frame transcription factors located on chromosome 10p11.2.ZEB1 coordinates gene transcription through the RAS/ERK, TGF-β, PI3K/Akt, and NF-κB signaling pathways, regulates key developmental processes and drives tumorigenesis and metastasis.
2.2.4 Epigenetic and post-transcriptional regulation
Epigenetic modifications dynamically modulate access to EMT-associated genes via multiple pathways, including DNA methylation (e.g., hypermethylation of the E-cadherin promoter) and histone acetylation/deacetylation (e.g., H3K4Ac). Many studies have focused on investigating heritable molecular factors independent of DNA sequence changes, including DNA methylation, histone modifications, noncoding RNAs, and chromatin remodeling (46). DNA methylation markers linked to colorectal cancer (CRC) (e.g., SFRP1, SFRP2, SDC2) can regulate transcriptional elements and non-coding RNA production, playing a key role in EMT (47). For example, deacetylated TWIST1 (K73/76) interacts with the NuRD deacetylase complex, recruiting it to repress epithelial gene transcription, while acetylated TWIST1 (acK73/76) interacts with BRD8 and the TIP60 complex to activate MYC transcription (48). In metastatic cancers, GRHL1 expression is downregulated, which may reduce epithelial cell adhesion and promote a migratory phenotype (49). The epigenetic state of EMT intermediates is synergistically maintained across multiple regulatory levels, exhibiting the typical components and limitations of complex networks (50). For instance, the miR-200 family inhibits EMT by targeting ZEB1/2, and long noncoding RNAs (e.g., MUF) enhance EMT via Wnt/β-catenin or TGF-β signaling. Additionally, epigenetic feedback in ZEB1 loops and random biomolecule allocation during cell division contribute to solid tumor cells’ EMT resistance (51).
2.2.5 Cellular phenotype remodeling
The activation of EMT induces profound morphological alterations and molecular reprogramming in cancer cells. This process is characterized by upregulated expression of mesenchymal markers including N-cadherin, vimentin, fibroblast-specific protein 1 (FSP1), and fibronectin, concomitant with downregulation of epithelial markers such as E-cadherin and occludin (11, 14, 52). E-cadherin, a transmembrane protein localized at intercellular adherens junctions and basolateral membranes, serves as a critical regulator for maintaining epithelial integrity. Its downregulation disrupts adherens junction assembly, while reduced occludin expression compromises tight junction integrity, ultimately resulting in cell junction complex disassembly. Notably, N-cadherin exhibits weaker homotypic binding affinity compared to E-cadherin, conferring enhanced cellular motility and invasive properties. Cytoskeletal reorganization manifests through diminished cytokeratin expression and elevated vimentin levels, which coordinately regulate intracellular trafficking and membrane protein dynamics (14). The dissolution of apical-basal polarity enables establishment of front-rear polarity, facilitating integrin-mediated matrix engagement during invasive progression. This polarity transition promotes pseudopodia formation and directional migration through Rho GTPase-dependent mechanisms. Collectively, these coordinated molecular and architectural adaptations substantially augment cancer cell metastatic competence.
3 EMT and TC
Thyroid surgery, radioactive iodine therapy, and thyroid-stimulating hormone (TSH) suppression remain the primary treatment modalities for thyroid cancer (53). Research indicates that TC displays significant heterogeneity in EMT-related regulatory factors and cell signaling pathways. This heterogeneity is characterized by altered cell morphology, decreased cell adhesion, and enhanced migratory capacity (54). These alterations facilitate tumor invasion and metastasis. The metastatic cascade involves three key steps: invasion of surrounding tissues by tumor cells, intravasation of tumor cells into the circulatory system through endothelial cells, and colonization of distal tissues with subsequent formation of metastatic foci.
3.1 EMT and the regulation of invasiveness in TC
Tumor invasion represents a pathological progression wherein malignant cells breach endothelial barriers through active migration or passive dissemination, infiltrating adjacent normal tissues and establishing distant metastases. This process is defined by cellular polarity loss, architectural disorganization, and acquisition of migratory/invasive capabilities (55). Significantly, within primary tumor microenvironments, the epithelial-mesenchymal transition program is preferentially activated in discrete neoplastic subpopulations. Histopathological evaluations reveal pronounced EMT phenotypes at tumor-stroma interfaces adjacent to desmoplastic connective tissue. These regions exhibit characteristic molecular signatures including downregulation of E-cadherin coupled with aberrant activation of transcriptional repressors (Snail, Slug, ZEB1, ZEB2) and the Twist1 basic helix-loop-helix transcription factor. Mechanistically, Twist1 drives invasive pseudopod formation through coordinated induction of platelet-derived growth factor receptor α (PDGFRα) expression and Src kinase activation, thereby potentiating tumor cell invasion (11) (Figure 1).
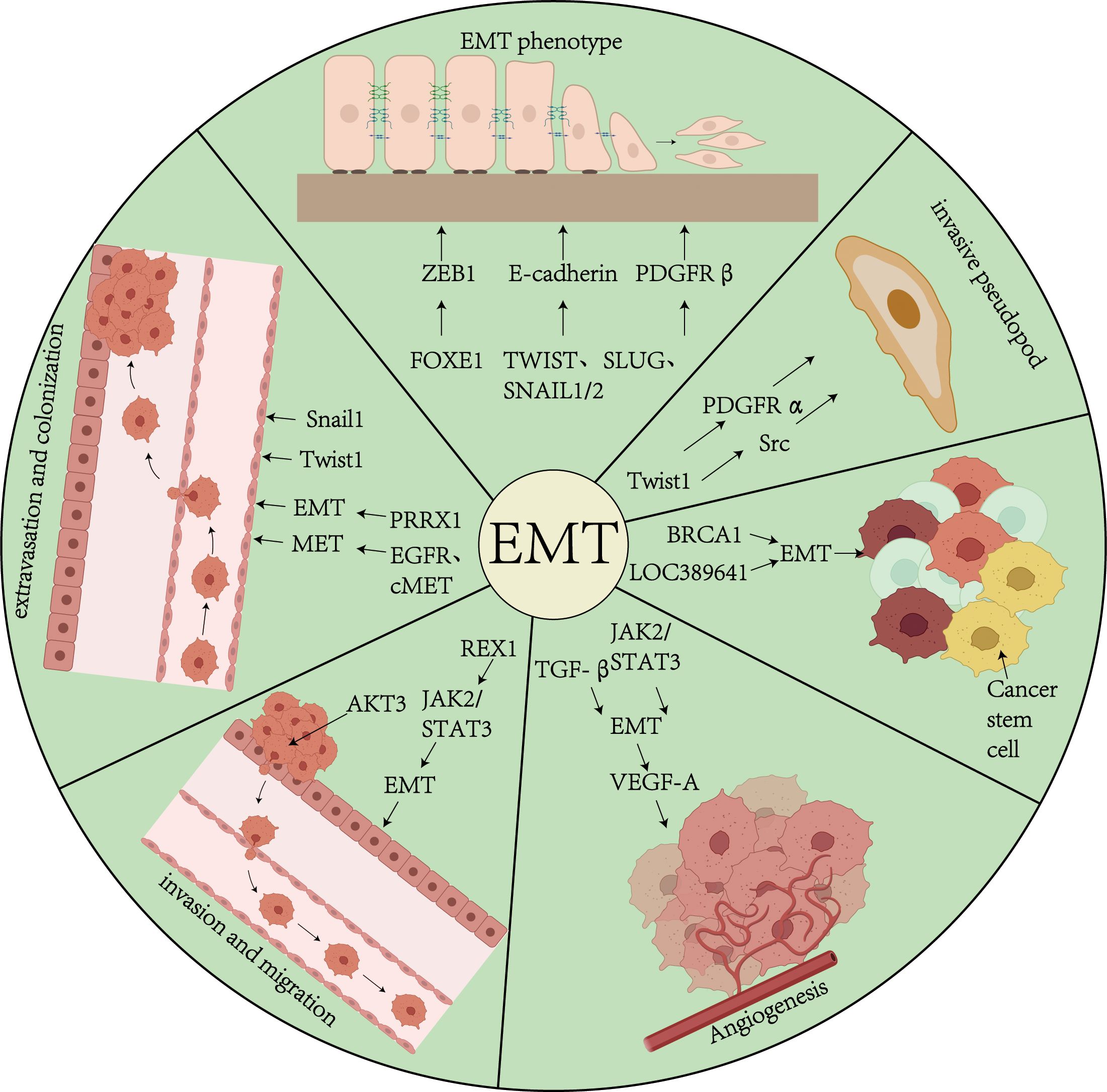
Figure 1. Overview of EMT and Tumors. This figure demonstrates the role of biological processes of EMT in tumor progression in thyroid cancer. EMT affects tumor progression through a variety of pathways and mechanisms, including the TGF-β signaling pathway, transcription factors (Snail, Twist, ZEB1/2), etc., which drive the transformation of epithelial cells to a mesenchymal phenotype, and promote the formation of cellular pseudopods, proliferation of tumor stem cells, angiogenesis, invasion and colonization.
Emerging insights into thyroid carcinogenesis highlight pivotal EMT regulatory networks. FOXE1 has been demonstrated to directly bind the ZEB1 promoter, enhancing its transcriptional activation to drive EMT-mediated migratory and invasive phenotypes (56). This FOXE1-ZEB1 signaling axis constitutes a critical regulatory pathway in thyroid cancer EMT. Conversely, BRCA1 exerts tumor-suppressive effects via dual mechanisms: (1) upregulating E-cadherin to preserve epithelial integrity, and (2) concurrently suppressing mesenchymal markers (vimentin, PDGFRβ, p-PKCα, TWIST, ZEB1), effectively inhibiting dedifferentiation and reducing invasive potential (57).
Recent investigations further elucidate microRNA-mediated EMT modulation. miR-200c post-transcriptionally targets the 3’ untranslated region (UTR) of parathyroid hormone-like hormone (PTHLH) mRNA, attenuating proliferation, invasion, and EMT progression in anaplastic thyroid carcinoma (ATC) models (58).
3.2 EMT and migration of TC
Tumor cell migration is the process by which cancer cells disseminate from the primary tumor to other organs via the vascular or lymphatic systems (55). Cancer stem cells (CSCs) and metastatic cells possess the ability to promote angiogenesis, which supports their tumorigenicity and metastatic potential. EMT-mediated angiogenesis is considered a mechanism that facilitates the entry of tumor cells into the systemic circulation. The upregulation of vascular endothelial growth factor A (VEGF-A), a protein that promotes neoangiogenesis, enhances tumor angiogenesis, thereby providing additional nutrients and oxygen to support tumor growth. EMT not only activates genes associated with cell differentiation, motility, and adhesion but also induces the expression of genes involved in angiogenesis regulation, including VEGF-A. Additionally, ZEB1, through its zinc finger domain, directly binds to E-box elements, thereby initiating the EMT process, enhancing cancer cell stemness and migration, and promoting chemoresistance (59) (Figure 1).
Studies have shown that TGF-β transiently upregulates VEGF-A expression (60). In thyroid cancer, the JAK2/STAT3 signaling pathway not only directly induces the EMT process and enhances cancer cell migration but also synergistically promotes VEGF-A secretion, thereby regulating tumor metastasis and angiogenesis. Dong quai methylin downregulates the JAK2/STAT3 signaling pathway, thereby inhibiting VEGF-A expression to limit angiogenesis (61) and directly reversing the EMT phenotype, which in turn suppresses thyroid cancer migration. Conversely, REX1 significantly enhances EMT-dependent migration by activating the JAK2/STAT3 signaling pathway and may indirectly promote the formation of a pro-angiogenic microenvironment (62), further confirming the synergistic role of EMT and angiogenesis in thyroid cancer.
Follicular thyroid cancer (FTC) is associated with a poor prognosis due to its high tumor proliferative activity. In FTC, vimentin expression is low. Single-cell RNA sequencing of FTC samples revealed that UBE2C is significantly upregulated, paradoxically enhancing cell proliferation. UBE2C mediates K29-linked ubiquitination, leading to vimentin degradation, but inhibits cell migration and invasion through EMT regulation.
3.3 EMT and colonization of TC
Colonization refers to the process by which circulating tumor cells (CTCs) reside and proliferate in a distal organ. Specifically, after dislodging from the primary tumor and entering the circulation, tumor cells adhere to the vascular endothelium of the distal organ and subsequently extravasate into the parenchymal tissues for dissemination (11). Through the reversal of epithelial-mesenchymal transition, known as mesenchymal-epithelial transition (MET), tumor cells ultimately colonize the distal organ and develop into metastatic foci. CTCs extravasate and migrate into the organ parenchyma through cellular and molecular mechanisms, a process that may involve EMT. For example, the expression of Twist1 or Snail1 promotes tumor cell extravasation, suggesting that EMT may facilitate both extravasation and initial colonization (63). PRRX1, a paired homoeobox factor 1 transcription factor, acts as a regulator of EMT and modulates migration and invasive properties during EMT. During the reversal from EMT to MET, the expression of Twist and PRRX1 decreases, while the expression of other biomarkers (e.g., EGFR and cMET) increases, thereby regulating cancer cell colonization (4) (Figure 1).
In the cascade of thyroid cancer metastasis, the distal colonization capacity of tumor cells is closely associated with the dynamic regulation of EMT. Notably, despite follicular thyroid cancer exhibiting high proliferative activity and poor prognostic features, its metastatic colonization ability is subject to specific regulation. Single-cell RNA sequencing has revealed that aberrant upregulation of UBE2C in FTC leads to vimentin degradation via K29-linked ubiquitination. This unique molecular mechanism enhances tumor cell proliferation while impairing cell migration and invasion by inhibiting the EMT process (64). This finding indicates that metastatic colonization in thyroid cancer depends not only on EMT activation but also on a delicate balance between EMT and proliferative activity: while strong EMT inhibition can limit initial invasion, it may paradoxically promote the final colonization of metastatic foci by maintaining the epithelial phenotype. This bidirectional role of EMT regulation offers novel insights into understanding the organ-specific colonization patterns of thyroid cancer metastasis.
In the cascade of tumor metastasis, ncRNAs are closely associated with the EMT in thyroid cancer. MicroRNAs and long non-coding RNAs have been identified as key regulators of the EMT process, and some of these molecules are also implicated in the proliferation of cancer stem cells (CSCs) (65). For instance, the downregulation of LOC389641, a long non-coding RNA (lncRNA) located in the p21 region of chromosome 8, promotes proliferation, wound healing, clonal formation, migration, and invasion of papillary thyroid carcinoma (PTC) cells by modulating the EMT process (66).
4 Non-coding RNAs
Non-coding RNAs are RNA molecules that do not code for proteins. They perform diverse functions in cells and play crucial roles in various biological processes, including gene expression regulation, cell signaling, and disease development. Based on their size and function, ncRNAs can be classified into several categories, such as long non-coding RNAs (lncRNAs), microRNAs (miRNAs), and circular RNAs (circRNAs).
4.1 MiRNAs and TC
MicroRNAs (miRNAs) are a class of highly conserved endogenous small noncoding RNA molecules, typically comprising approximately 22 nucleotides (67, 68). miRNAs are transcribed from DNA by RNA polymerase II or III to generate primary miRNAs (pri-miRNAs) (69). In the nucleus, pri-miRNAs are processed by a complex containing RNase III enzyme, forming precursor miRNAs (pre-miRNAs) with a stem-loop hairpin structure (approximately 60–70 nucleotides). Subsequently, pre-miRNAs are transported to the cytoplasm and further processed by another complex to form mature miRNA duplexes (70). Mature miRNAs bind to target mRNAs through the miRNA-induced silencing complex (miRISC), inhibiting translation or promoting mRNA degradation, thereby regulating gene expression. In malignant tumors, miRNA expression dysregulation plays a critical role in cancer development. For instance, overexpression of miR-221 and miR-222 in gastrointestinal, hepatocellular, and thyroid cancers can target and suppress tumor suppressor genes. Additionally, miRNAs are widely involved in regulating various biological processes, including the cell cycle, cell proliferation, differentiation, migration, apoptosis, and immune response (71) (Figure 2).
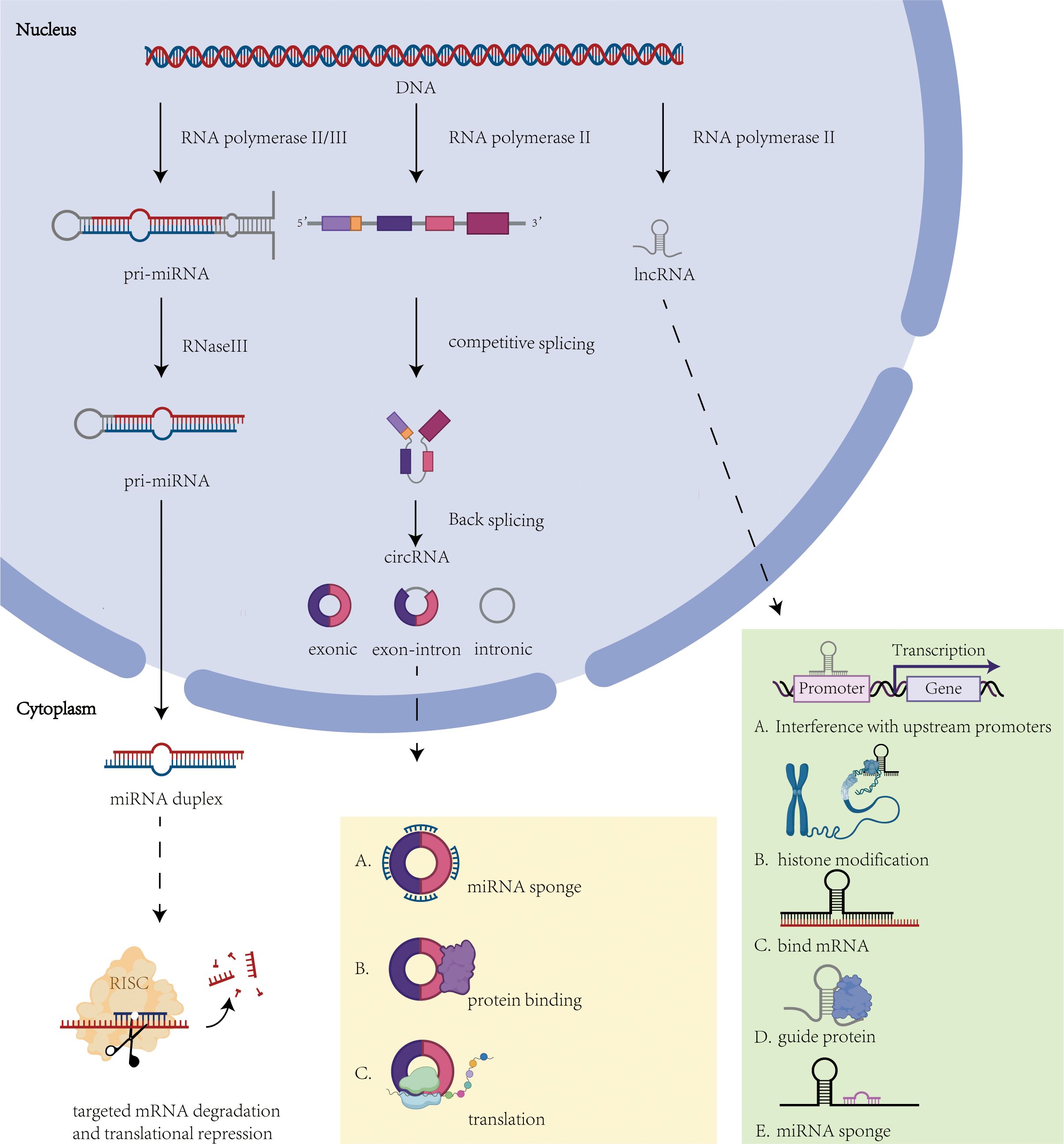
Figure 2. Overview of ncRNA biogenesis and function. This figure illustrates the molecular mechanisms underlying the biogenesis and function of non-coding RNAs. Solid arrows indicate the biogenesis pathways of ncRNAs, while dashed arrows indicate the roles played by ncRNAs. (1) miRNA mechanism of action: regulation of gene expression through miRISC. (2) CircRNA mechanism of action: act as miRNA sponges, regulate protein interactions or signaling pathways. (3) lncRNA mechanism: interferes with upstream promoter transcription, histone modification, interferes with mRNA, binds proteins and acts as miRNA sponge.
Increasing evidence indicates that miRNAs serve as key biomarkers in the occurrence, progression, detection, and prognosis of TC. miR-493-5p targets METTL3, whose expression level correlates positively with the degree of thyroid cancer differentiation and whose low expression is closely associated with tumor progression and poor prognosis. METTL3 regulates PAX8 expression via m6A modification, thereby affecting the differentiation and chemosensitivity of thyroid cancer cells (72). miR-31-5p is significantly upregulated in papillary thyroid cancer tissues and in K1 cells harboring the BRAF p.V600E mutation. This miRNA promotes cell proliferation by inducing the expression of EMT markers and upregulating the YAP/β-catenin signaling axis, and is closely associated with cell adhesion, migration, and invasion (73). miRNAs regulate key signaling pathways and target genes (e.g., SNAI1, PSMD10) to exert dual roles (cancer-promoting or cancer-suppressing) in thyroid cancer development (74). Abnormal changes in their expression profiles provide potential markers for the early diagnosis, prognostic assessment, and targeted therapy of thyroid cancer. For example, developing small molecule inhibitors targeting SNAI1 or KIAA1199 based on the regulatory networks of miR-199a-5p, miR-486-5p, and miR-654-3p may represent a novel therapeutic strategy (75–77). These findings offer new targets for thyroid cancer treatment.
4.2 CircRNA and TC
Circular RNAs (circRNAs) are covalently closed single-stranded RNA molecules transcribed by RNA polymerase II (Pol II). These non-coding RNAs originate from precursor mRNAs that undergo exon/intron sequence rearrangement via spliceosome-mediated reverse splicing, forming characteristic closed-loop structures (78, 79). CircRNAs exhibit pleiotropic regulatory functions within tumor microenvironments, including modulation of cellular invasiveness, angiogenesis, EMT, and chemoresistance (80, 81) (Figure 2).
The molecular mechanisms of circRNAs in thyroid carcinogenesis are classified into four principal categories: miRNA Sponge Effect:CircRNAs competitively sequester miRNAs through complementary base pairing, thereby attenuating miRNA-mediated post-transcriptional repression of target mRNAs and upregulating oncogene expression. Key examples include: circPVT1 aberrantly overexpressed in medullary thyroid carcinoma, circPVT1 promotes tumor proliferation and metastasis by sponging miR-455-5p to activate the CXCL12 signaling axis (82).circ_0003747 demonstrates elevated expression in thyroid carcinoma cells, functioning as a molecular sponge for miR-338-3p. Mechanistically, miR-338-3p suppresses tumor progression by directly targeting the 3’UTR of PLCD3 – an oncogene validated in thyroid cancer cell lines (83). circFAT1(e2) specifically upregulated in papillary thyroid carcinoma (PTC), circFAT1(e2) enhances ZEB1 expression via miR-873 sequestration, driving PTC cell proliferation, migration, and invasion (84); Protein Trafficking Regulation:CircRNAs facilitate proper protein folding and structural stabilization through spatial conformational modifications while competitively inhibiting physiological protein-ligand interactions.; RNA-Protein Complex Assembly:CircRNAs dynamically interact with specific proteins to form functional ribonucleoprotein complexes, critically modulating signaling networks during thyroid oncogenesis.;Translational Control: Certain circRNAs recruit translational machinery to regulate protein synthesis. For instance, hsa_circ_0006943 binds CSNK2A1, enhancing its interaction with Akt to activate the PI3K-Akt pathway and induce EMT, thereby accelerating thyroid cancer progression (85).
Collectively, circRNAs orchestrate multidimensional regulatory circuits governing thyroid cancer initiation, progression, and microenvironmental adaptation. Their mechanistic diversity highlights substantial potential as diagnostic biomarkers and therapeutic targets.
4.3 LncRNAs and TC
Long noncoding RNAs (lncRNAs) are a class of noncoding RNAs exceeding 200 nucleotides in length (86). The majority of lncRNAs are transcribed by RNA polymerase II from intergenic or exonic regions (71). These transcripts typically undergo splicing and are modified with a 7-methylguanosine cap at the 5’ end and polyadenylation at the 3’ end (87). lncRNAs exert a pivotal role in thyroid cancer development, progression, and microenvironmental remodeling by modulating gene expression through multiple mechanisms, including epigenetic modification, signaling pathway regulation, and molecular sponge action (Figure 2).
Long non-coding RNAs orchestrate core cellular processes including differentiation, autophagy, cell cycle regulation, proliferation-apoptosis balance, invasion-migration capacity, and stemness maintenance through epigenetic networks. In thyroid carcinogenesis, lncRNAs drive malignancy via five principal mechanisms: Cis-Regulatory Modulation: LncRNAs transcribed from upstream promoter regions of protein-coding genes spatially impede downstream transcriptional machinery. Notable examples: LINC00891 activates the EZH2/SMAD2/3 axis to induce epithelial-mesenchymal transition in thyroid carcinoma. Mechanistically, EZH2 overexpression rescues EMT suppression caused by LINC00891 knockdown (88). LINC02454 demonstrates oncogenic activity in thyroid carcinoma through CREB1 phosphorylation-mediated HMGA2 transcriptional activation (89); Chromatin Reprogramming: LncRNAs mediate chromatin topology reorganization and histone modification. Bisulfite sequencing revealed hypermethylation at CpG islands within the MEG3 differentially methylated region (DMR), correlating with its tumor-suppressive silencing in thyroid malignancies (90); RNA Interference Cascade: LncRNA-mRNA duplex formation disrupts canonical splicing. Aberrant splice variants generate endogenous siRNAs via Dicer processing, triggering RNA interference-mediated mRNA degradation. ZNF674-AS1 functions as a ceRNA to suppress thyroid cancer progression by sequestering miR-181a and restoring SOCS4 expression (91); Protein Interaction Networks: LncRNAs scaffold functional ribonucleoprotein complexes. LINC00887 drives papillary thyroid carcinoma (PTC) progression by inducing G1/S phase arrest to promote proliferative advantage and apoptotic resistance; miRNA Sponge Activity: LncRNAs competitively bind miRNAs to derepress oncogenic targets. lncRNA n384546/TUG1 elevated in PTC, these lncRNAs adsorb miR-145-5p to upregulate AKT3 and ZEB1, synergistically enhancing tumor invasiveness (92, 93).
Collectively, lncRNA networks constitute promising diagnostic biomarkers and therapeutic targets in thyroid cancer management.
5 Role of EMT-related ncRNAs in TC
5.1 EMT-related miRNAs and their association with TC
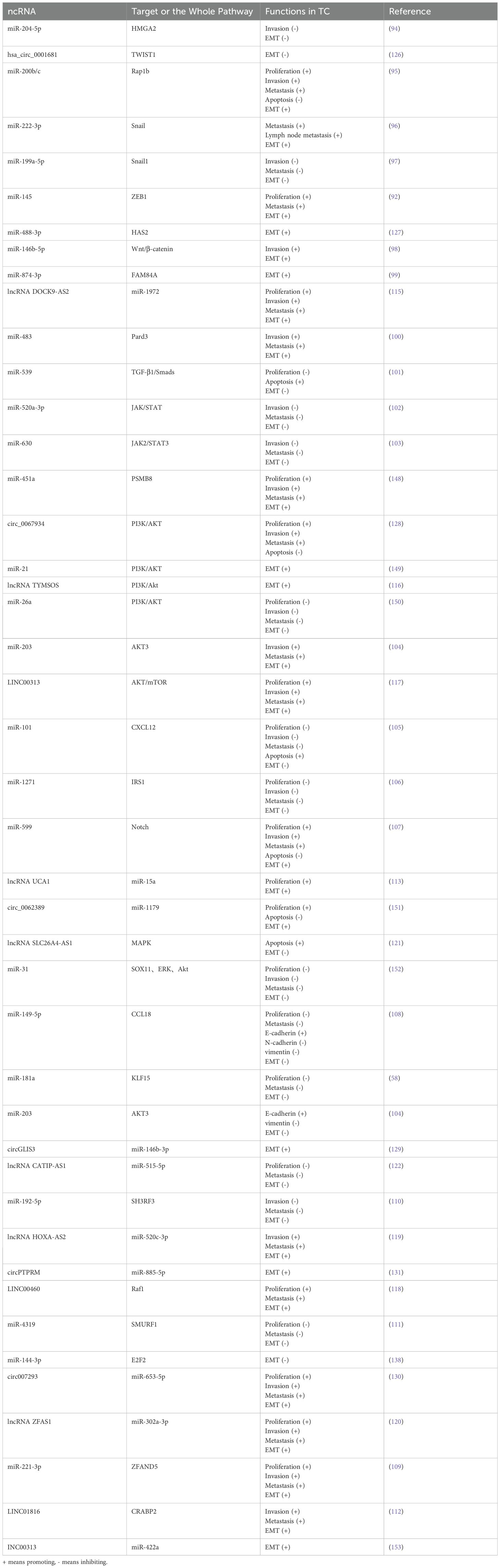
Epithelial-mesenchymal transition is a critical process in TC invasion and metastasis. miRNAs exert significant regulatory effects by targeting EMT-associated transcription factors or signaling pathway molecules. miR-204-5p is downregulated in TC and directly targets the 3’-UTR of high-mobility group protein 2 (HMGA2), thereby inhibiting its expression and suppressing the MAPK signaling pathway. This action reduces the invasiveness of TPC-1 and BCPAP cells (94). The silencing of HMGA2 significantly inhibits the EMT process, suggesting that miR-204-5p may serve as a potential therapeutic target for TC. Additionally, miR-200b/c targets Rap1b to inhibit the NF-κB/Twist1 signaling pathway, thereby suppressing the proliferation, invasion, migration, and EMT of papillary thyroid carcinoma (PTC) cells (95). Overexpression of Rap1b is closely associated with the malignant progression of TC The BRAFV600E mutation upregulates miR-222-3p, which induces EMT by targeting Snail and is significantly associated with lymph node metastasis in PTC patients (96). miR-199a-5p is significantly downregulated in thyroid undifferentiated carcinoma (ATC), and its overexpression inhibits EMT by targeting Snail, thereby suppressing the migration and invasion of ATC cells both in vitro and in vivo (97).
miRNAs can also affect EMT by modulating signaling pathways. For example, miR-146b-5p enhances Wnt/β-catenin signaling through downregulation of ZNRF3, inducing an EMT phenotype that correlates with extra-thyroidal infiltration and advanced staging in TC (98). In PTC, miR-874-3p is downregulated, and overexpression of its target gene FAM84A activates the Wnt/β-catenin pathway, driving the EMT phenotype (99). miR-483 regulates TGF-β1/Smads signaling by targeting Pard3 (100), and miR-539 activates the TGF-β1/Smads pathway by targeting SLPI (101), thereby regulating EMT in TC. miRNAs also regulate EMT-related markers (e.g., vimentin, E-cadherin) through pathways such as JAK2/STAT3, PI3K/AKT, and Notch, including miR-520a-3p, miR-630, miR-203, miR-101, miR-1271, miR-599, miR-221/222, miR-149-5p, miR-21, etc. (102–108). Additionally, miR-15a, miR-192-5p, miR-4319etc., inhibit EMT by targeting elements such as the Hippo/JNK pathway, SH3RF3, SMURF1, etc., while miR-221-3p and miR-34c-5ppromote EMT and TC metastasis by targeting ZFAND5 and CRABP2 (109–113) (Figure 3).
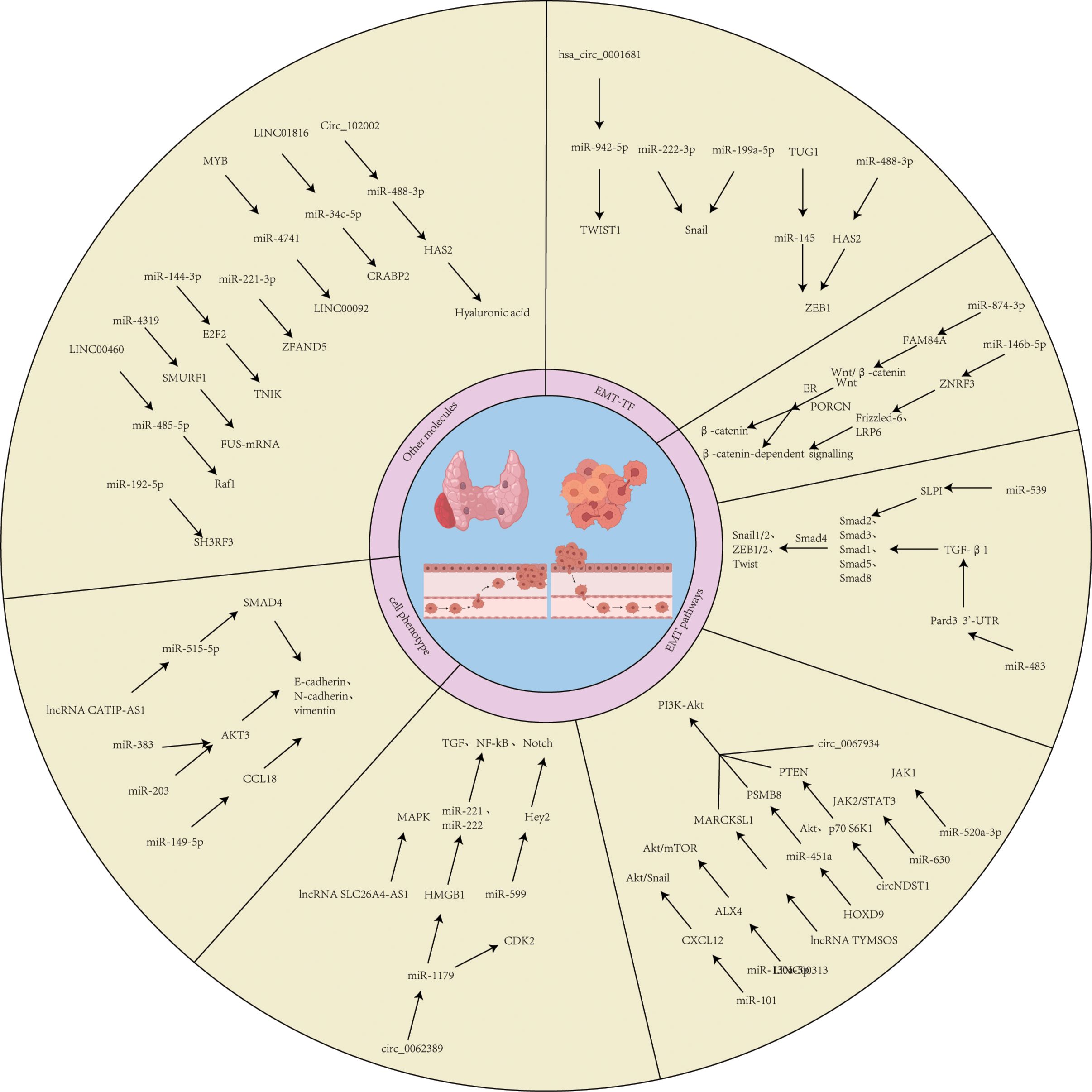
Figure 3. Common regular pathways of ncRNAs. This figure depicts the cross-cutting regular pathways of miRNAs, circRNAs and lncRNAs in EMT transcription factors, EMT signaling pathways, cellular phenotypes and epigenetics.
In thyroid cancer, miR-146b-5p significantly affects tumor progression by regulating the EMT process. Studies have shown that miR-146b-5p is overexpressed in papillary thyroid carcinoma (PTC) with lymph node metastasis and downregulates the protein expression of zinc RING finger protein 3 (ZNRF3) by directly targeting its 3′-UTR region (98). Bioinformatics analysis and luciferase reporter assays confirmed that miR-146b-5p inhibits ZNRF3 protein expression by directly binding to its 3′-UTR region. The inhibition of ZNRF3 activates the Wnt/β-catenin signaling pathway, as evidenced by the upregulation of Frizzled-6 and LRP6 receptor expression, which in turn promotes PTC cell migration, invasion, and EMT characteristics (downregulation of the epithelial marker E-cadherin and upregulation of the mesenchymal markers N-cadherin and vimentin). Similarly, TGF-β1 treatment results in altered PTC cell morphology, downregulation of E-cadherin, and upregulation of mesenchymal markers such as Slug, Snail, and Twist. Notably, overexpression of ZNRF3 or the use of Wnt/β-catenin signaling pathway inhibitors can reverse the pro-oncogenic effects of miR-146b-5p, suggesting its potential as a therapeutic target. Although TGF-β1 transiently upregulates miR-146b-5p, its long-term inhibition enhances the proliferation and invasiveness of PTC cells, indicating that miR-146b-5p may balance tumor progression through dynamic regulation (114).
These studies suggest that EMT-related miRNAs influence the aggressiveness of thyroid cancer through a complex regulatory network, providing an important basis for the development of targeted therapies and prognostic markers. Future studies are needed to further validate their clinical potential and explore combination therapy strategies.
5.2 EMT-related lncRNAs and their association with TC
During TC progression, lncRNAs)play a key role by regulating EMT-related signaling pathways and target gene expression. lncRNAs influence the EMT process by competitively binding miRNAs or directly regulating signaling pathway molecules. For example, lncRNA TUG1 is significantly upregulated in TC tissues by competitively binding to miR-145 and deregulating its inhibition of ZEB1, thereby promoting ZEB1-mediated EMT process and enhancing the proliferation and migration of thyroid cancer cells (92). DOCK9-AS2 is upregulated in papillary thyroid carcinoma (PTC) by sponge adsorption of miR-1972 CTNNB1 (β-catenin), activating the Wnt/β-catenin signaling pathway, which in turn induces an EMT phenotype and promotes invasion and metastasis of PTC cells (115). TYMSOS, as an oncogenic lncRNA, up-regulates the expression of MARCKSL1 and activates the PI3K/Akt pathway by binding to miR-130a-5p and accelerating the proliferation and EMT process of TC cells (116). LINC00313 inhibits its expression by binding to the ALX4 promoter, activates the AKT/mTOR signaling axis, and promotes TC proliferation, migration, and EMT (117). In addition, oncogenic lncRNA UCA1 targets miR-15a through Hippo and JNK signaling pathways and accelerates the EMT process of TC (113). LINC00460 upregulates Raf1 through sponge adsorption of miR-485-5p, which significantly enhances the EMT phenotype of PTC (118). LINCRNAs can also affect EMT by regulating the expression of oncogenes, such as HOXA-AS2 upregulates S100A4 and ZFAS1 deregulates its repression of downstream oncogenes. HOXA-AS2 enhances cancer cell proliferation, migration, and EMT through sponge adsorption of miR-520c-3p (119). LncRNA ZFAS1 enhances cancer cell proliferation, migration, and EMT by targeting miR-302a-3p (120). LINC01816 is highly expressed in TC tissues and may promote CRABP2 overexpression through sponge adsorption of miR-34c-5p, inducing EMT and cancer cell metastasis (112) (Figure 3).
Some long non-coding RNAs function to inhibit EMT. For example, SLC26A4-AS1 is lowly expressed in papillary thyroid carcinoma (PTC), and its overexpression inhibits the expression of EMT-related genes by activating TP53 and inactivating the MAPK pathway, thereby reducing cell migration and invasion (121). Additionally, CATIP-AS1 inhibits TC metastasis by reversing the expression of EMT markers (e.g., E-cadherin and N-cadherin) through the upregulation of SMAD4 by inhibiting miR-515-5p (122). These studies suggest that lncRNAs have dual regulatory roles in thyroid cancer, acting as both tumor suppressors and oncogenic factors, and their clinical translational potential needs to be further verified.
LncRNA RMST expression is significantly reduced in ATC and only slightly reduced in DTC. RMST may inhibit the expression of EMT markers (e.g., SLUG, TWIST1, NES1) by regulating the Wnt/β-catenin, Notch, and Hedgehog signaling pathways and significantly reduce the expression of stem cell markers (e.g., OCT4, SOX2, NANOG) (123), thereby suppressing the aggressive phenotype of ATC cells. SOX2 is an important transcription factor associated with stem cell properties and tumor aggressiveness. RMST may function by directing translational and post-translational modifications, including DNA methylation and SUMOylation (124) and its expression is negatively correlated with SOX2 and acts by helping Sox2 transcription factors bind to their target promoters (125). LncRNAs play a key role in thyroid cancer development, metastasis, and drug resistance through multidimensional regulation of EMT-related pathways and target genes. Future studies are needed to further validate their clinical potential and develop precise lncRNA-based therapeutic strategies.
5.3 EMT-related circRNAs and their association with TC
In recent years, the regulatory role of circRNAs in the EMT of TC has garnered increasing attention. circRNAs influence the EMT process in TC by acting as molecular sponges for miRNAs, modulating key signaling pathways (e.g., PI3K/AKT, HAS2/ZEB1), or regulating EMT-related transcription factors (e.g., TWIST1, ZEB1). For instance, hsa_circ_0001681 is upregulated in TC and functions as a sponge for miR-942-5p, thereby relieving the inhibitory effect of miR-942-5p on TWIST1 (126). As a core transcription factor of EMT, TWIST1 upregulation suppresses E-cadherin expression and induces mesenchymal marker expression, ultimately promoting the EMT process and tumor progression in TC (25). Circ_102002 negatively regulates miR-488-3p expression in papillary thyroid carcinoma (PTC) by acting as a sponge. The target gene of miR-488-3p, HAS2, accelerates the EMT process by synthesizing hyaluronic acid to activate ZEB1. Aberrant expression of circ_102002 enhances HAS2/ZEB1 axis activity, thereby promoting the migration and invasion of PTC cells (127). Additionally, circ_0067934 is significantly overexpressed in thyroid tumors and induces an EMT phenotype by activating the PI3K/AKT signaling pathway. This action promotes cancer cell proliferation, migration, and invasion while inhibiting apoptosis. Its effects are closely related to EMT markers, such as upregulation of N-cadherin and downregulation of E-cadherin (128). In contrast, circGLIS3 expression is reduced in TC and upregulates AIF1L expression by binding to miR-146b-3p, thereby reducing its inhibition of AIF1L mRNA. AIF1L may affect cellular phenotypic transition and inhibit the malignant progression of TC by regulating EMT-related genes (129). Some circRNAs are associated with treatment resistance. For example, circ007293, delivered to PTC cells via exosomes, competitively binds miR-653-5p and relieves the inhibition of PAX6 by miR-653-5p. Activation of PAX6 promotes the EMT process and enhances cell invasion and migration. Silencing circ007293 significantly inhibits its pro-oncogenic effects (130). circPTPRM acts as a sponge for miR-885-5p and significantly enhances the malignant phenotype of TC (131) (Figure 3). These studies suggest that targeting circRNAs may be a novel strategy to reverse TC resistance (Tables 1, 2).
6 Potential clinical applications of ncRNA in thyroid cancer
Thyroid surgery, radioactive iodine therapy, and TSH suppression remain the mainstay treatments for thyroid cancer (42). Preliminary studies suggest that total thyroidectomy may be more cost-effective and efficacious than hemithyroidectomy (93, 94). For patients with high-risk thyroid cancer, total thyroidectomy combined with adjuvant radioactive iodine therapy is typically required. In contrast, for low-risk thyroid cancers measuring 1–4 cm in diameter, hemithyroidectomy may be considered (132, 133). Radioactive iodine (RAI) is the first-line treatment for metastatic thyroid cancer. Although surgery and RAI therapy are effective for most patients with DTC, approximately 60% of patients with aggressive metastatic DTC are resistant to RAI therapy and have a poor prognosis (134). Mechanisms of RAI resistance include genetic mutations (e.g., BRAF V600E mutation, TERT promoter mutation), dysfunction of iodine-transporting proteins (e.g., NIS), and disturbances in the tumor microenvironment (TME).
Strategies to overcome RAI resistance include (1): tyrosine kinase inhibitors (TKIs) that improve RAI uptake by inhibiting multiple receptors (e.g., VEGFR, c-KIT, PDGFRα/β; (2) drugs that restore RAI affinity (e.g., BRAF inhibitors, HDAC inhibitors, MEK inhibitors (135); (3) drugs that induce redifferentiation of DTC cells and restore NIS expression and iodine uptake capacity (e.g., retinoic acid [RA], all-trans retinoic acid [ATRA]); and (4) the use of nanoparticles as drug carriers to increase RAI concentration in tumor tissues (136). Although TKI treatment improves progression-free survival (PFS) in some patients, the complete remission rate remains low, and patients may discontinue treatment due to adverse effects. Therefore, further research into the molecular mechanisms of RAI resistance and the development of more effective therapeutic strategies, such as drugs targeting specific gene mutations or fusions and improved drug delivery through nanotechnology, are needed.
ncRNAs play a significant role in the clinical management of thyroid cancer patients and can serve as biomarkers for diagnosis, prognosis, and treatment. For example, downregulation of PAR5 lncRNA in undifferentiated ATC serves as a marker of aggressiveness, whereas overexpression of miR-146b in PTC is associated with a poorer prognosis (137). Additionally, ncRNA assessment may help predict therapeutic response and optimize treatment strategies.
Non-coding RNAs play a significant role as potential biomarkers and therapeutic targets in TC therapy. For example, Yi et al. found that miR-144-3p significantly inhibits the proliferation, migration, invasion, and EMT of thyroid cancer cells by downregulating the expression of E2F2 and TNIK, as detected by quantitative reverse transcription-polymerase chain reaction (qRT-PCR) (138). Additionally, overexpression of ciRS-7 promotes the progression of papillary thyroid carcinoma (PTC) by regulating the miR-7/EGFR axis and is closely associated with aggressive clinicopathological features, such as tumor size and lymph node metastasis (139). Orlandella FM et al. found, through real-time quantitative polymerase chain reaction (qPCR) and immunohistochemistry analyses, that restoring JAM-A expression can inhibit the malignant features of thyroid undifferentiated carcinoma (ATC) cell lines, including cell proliferation, motility, and trans-endothelial migration, suggesting that JAM-A could be a potential therapeutic target for thyroid cancer (140). JAM-A expression can be increased and its function restored by modulating gene expression (e.g., via transcription factors or miRNAs) or by activating the PI3K/Akt and MAPK signaling pathways.
Moreover, ZEB1 plays a crucial role at the forefront of tumor invasion, and CD73 is involved in regulating ZEB1 non-coding RNA through its 3’UTR, which in turn affects the progression of PTC. Studies have shown that siRNA molecules targeting CD73 and ZEB1, in combination with RGD-coupled chitosan lactate nanoparticles, can effectively treat thyroid cancer and improve prognosis (141). Metformin shows potential therapeutic value by inhibiting the mTOR signaling pathway and targeting JAK2 in the JAK2/STAT3 signaling pathway, thereby effectively inhibiting the proliferation, migration, and EMT of thyroid cancer cell lines (142, 143). Meanwhile, Tnnt1 significantly promotes the proliferation, colony formation, migration, invasion, and EMT of PTC cells by activating the p38/JNK signaling pathway (144). Silymarin inhibits the proliferation, metastasis, and invasion of PTC cells by modulating the FN1/AKT signaling pathway and inhibiting the EMT process. Additionally, researchers are exploring immunotherapies for thyroid cancer; for example, silymarin reverses the immunosuppressive state of thyroid cancer by regulating the expression levels of immune checkpoint genes (145).
An in-depth investigation of the regulatory mechanisms of ncRNAs in the EMT process of thyroid cancer is expected to lead to breakthroughs in the discovery of early screening biomarkers and precise therapeutic targets, thereby improving the prognosis, prevention, and treatment of thyroid cancer.
7 Summary
TC is one of the major malignancies of the endocrine system. In recent years, research has primarily focused on the effects of growth factors, transcription factors, non-coding RNA (ncRNA), DNA methylation, and other regulatory factors on the EMT, as well as the mechanisms underlying EMT in thyroid cancer invasion and metastasis. In cancer therapy, targeting ncRNAs involved in the EMT process has emerged as a promising intervention strategy, potentially preventing or inhibiting the deleterious effects of tumors.
Targeting ncRNAs exerts a crucial regulatory role in cancer progression and may represent a novel therapeutic strategy against thyroid cancer in the future. There are two main approaches to targeting ncRNAs: the first involves inhibiting tumor progression by suppressing oncogene-associated overexpressed ncRNAs, while the second involves inhibiting cancer development by activating or upregulating tumor suppressor gene-associated ncRNAs. For example, downregulation of miR-146b-5p or upregulation of miR-874-3p inhibits the Wnt/β-catenin signaling pathway, thereby blocking EMT activation and inhibiting thyroid cancer progression (99). Additionally, miR-99a-3p is downregulated in PTC tissues and cells, whereas upregulation of miR-99a-3p inhibits EMT, resistance to anoikis, and the migratory and invasive capabilities of PTC cells. ITGA2 has been identified as a downstream effector of GRP94. Overexpression of miR-99a-3p suppresses ITGA2 expression, while overexpression of GRP94 reverses the inhibitory effect of miR-99a-3p on PTC metastasis (146). Different types of ncRNAs can inhibit the EMT process by regulating various transcription factors, signaling pathways, or cellular markers. Therapeutic strategies targeting EMT-associated ncRNAs can be developed by designing specific small molecules or drugs to target these ncRNAs, thereby inhibiting the EMT process and reducing tumor invasion and metastasis (68, 147).
Author contributions
RZ: Investigation, Methodology, Writing – original draft. JT: Investigation, Methodology, Writing – review & editing. JP: Methodology, Writing – review & editing. RM: Methodology, Resources, Writing – review & editing. SF: Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by 1.National Key R&D Program of China (No. 2023YFC2508300); 2.National Key R&D Program of China (2023YFC3503400); 3.Gansu Province Natural Science Foundation (22JR5RA914); 4.Gansu Province Joint Research Foundation (23JRRA1490); 5.Lanzhou Municipal Science and Technology Development Guidance Project (2019-ZD-38); 6.Lanzhou University Innovation and Entrepreneurship training program (20240060079); 7.Lanzhou University Innovation and Entrepreneurship training program (20240060062).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Siegel RL, Giaquinto AN, and Jemal A. Cancer statistics, 2024. CA Cancer J Clin. (2024) 74:12–49. doi: 10.3322/caac.21820
2. Boucai L, Zafereo M, and Cabanillas ME. Thyroid cancer: A review. Jama. (2024) 331:425–35. doi: 10.1001/jama.2023.26348
4. Guo Z, Hardin H, and Lloyd RV. Cancer stem-like cells and thyroid cancer. Endocr Relat Cancer. (2014) 21:T285–300. doi: 10.1530/ERC-14-0002
5. Liu Y, Su L, and Xiao H. Review of factors related to the thyroid cancer epidemic. Int J Endocrinol. (2017) 2017:5308635. doi: 10.1155/2017/5308635
6. Nikiforov YE and Nikiforova MN. Molecular genetics and diagnosis of thyroid cancer. Nat Rev Endocrinol. (2011) 7:569–80. doi: 10.1038/nrendo.2011.142
7. Bracken CP and Goodall GJ. The many regulators of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. (2022) 23:89–90. doi: 10.1038/s41580-021-00442-x
8. Manfioletti G and Fedele M. Epithelial-mesenchymal transition (EMT). Int J Mol Sci. (2023) 24(14):11386. doi: 10.3390/ijms241411386
9. Liu B, Sun L, and Song E. Non-coding RNAs regulate tumor cell plasticity. Sci China Life Sci. (2013) 56:886–90. doi: 10.1007/s11427-013-4554-5
10. Chan SH and Wang LH. Regulation of cancer metastasis by microRNAs. J BioMed Sci. (2015) 22:9. doi: 10.1186/s12929-015-0113-7
11. Mittal V. Epithelial mesenchymal transition in tumor metastasis. Annu Rev Pathol. (2018) 13:395–412. doi: 10.1146/annurev-pathol-020117-043854
12. Greenburg G and Hay ED. Epithelia suspended in collagen gels can lose polarity and express characteristics of migrating mesenchymal cells. J Cell Biol. (1982) 95:333–9. doi: 10.1083/jcb.95.1.333
13. Dongre A and Weinberg RA. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat Rev Mol Cell Biol. (2019) 20:69–84. doi: 10.1038/s41580-018-0080-4
14. Lamouille S, Xu J, and Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. (2014) 15:178–96. doi: 10.1038/nrm3758
15. Pastushenko I and Blanpain C. EMT transition states during tumor progression and metastasis. Trends Cell Biol. (2019) 29:212–26. doi: 10.1016/j.tcb.2018.12.001
16. Derynck R and Weinberg RA. EMT and cancer: more than meets the eye. Dev Cell. (2019) 49:313–6. doi: 10.1016/j.devcel.2019.04.026
17. Tiwari N, Gheldof A, Tatari M, and Christofori G. EMT as the ultimate survival mechanism of cancer cells. Semin Cancer Biol. (2012) 22:194–207. doi: 10.1016/j.semcancer.2012.02.013
18. Aiello NM and Kang Y. Context-dependent EMT programs in cancer metastasis. J Exp Med. (2019) 216:1016–26. doi: 10.1084/jem.20181827
19. Zhang N, Ng AS, Cai S, Li Q, Yang L, and Kerr D. Novel therapeutic strategies: targeting epithelial-mesenchymal transition in colorectal cancer. Lancet Oncol. (2021) 22:e358–e68. doi: 10.1016/S1470-2045(21)00343-0
20. Morin C, Moyret-Lalle C, Mertani HC, Diaz JJ, and Marcel V. Heterogeneity and dynamic of EMT through the plasticity of ribosome and mRNA translation. Biochim Biophys Acta Rev Cancer. (2022) 1877:188718. doi: 10.1016/j.bbcan.2022.188718
21. Mukherjee A, Ha P, Wai KC, and Naara S. The role of ECM remodeling, EMT, and adhesion molecules in cancerous neural invasion: changing perspectives. Adv Biol (Weinh). (2022) 6:e2200039. doi: 10.1002/adbi.202200039
22. Mardente S, Romeo MA, Asquino A, Po A, Gilardini Montani MS, and Cirone M. HHV-6A infection of papillary thyroid cancer cells induces several effects related to cancer progression. Viruses. (2023) 15(10):2122. doi: 10.3390/v15102122
23. Xu X, Zhang J, Tian Y, Gao Y, Dong X, Chen W, et al. CircRNA inhibits DNA damage repair by interacting with host gene. Mol Cancer. (2020) 19:128. doi: 10.1186/s12943-020-01246-x
24. Lin YT and Wu KJ. Epigenetic regulation of epithelial-mesenchymal transition: focusing on hypoxia and TGF-β signaling. J BioMed Sci. (2020) 27:39. doi: 10.1186/s12929-020-00632-3
25. Castaneda M, den Hollander P, Kuburich NA, Rosen JM, and Mani SA. Mechanisms of cancer metastasis. Semin Cancer Biol. (2022) 87:17–31. doi: 10.1016/j.semcancer.2022.10.006
26. Klaus A, Fathi O, Tatjana TW, Bruno N, and Oskar K. Expression of hypoxia-associated protein HIF-1α in follicular thyroid cancer is associated with distant metastasis. Pathol Oncol Res. (2018) 24:289–96. doi: 10.1007/s12253-017-0232-4
27. Wang N, Dong CR, Jiang R, Tang C, Yang L, Jiang QF, et al. Overexpression of HIF-1α, metallothionein and SLUG is associated with high TNM stage and lymph node metastasis in papillary thyroid carcinoma. Int J Clin Exp Pathol. (2014) 7:322–30.
28. Lan L, Luo Y, Cui D, Shi BY, Deng W, Huo LL, et al. Epithelial-mesenchymal transition triggers cancer stem cell generation in human thyroid cancer cells. Int J Oncol. (2013) 43:113–20. doi: 10.3892/ijo.2013.1913
29. Zhong Z, Hu Z, Jiang Y, Sun R, Chen X, Chu H, et al. Interleukin-11 promotes epithelial-mesenchymal transition in anaplastic thyroid carcinoma cells through PI3K/Akt/GSK3β signaling pathway activation. Oncotarget. (2016) 7:59652–63. doi: 10.18632/oncotarget.10831
30. Yang YJ, Na HJ, Suh MJ, Ban MJ, Byeon HK, Kim WS, et al. Hypoxia induces epithelial-mesenchymal transition in follicular thyroid cancer: involvement of regulation of twist by hypoxia inducible factor-1α. Yonsei Med J. (2015) 56:1503–14. doi: 10.3349/ymj.2015.56.6.1503
31. Zhong J, Liu C, Zhang QH, Chen L, Shen YY, Chen YJ, et al. TGF-β1 induces HMGA1 expression: The role of HMGA1 in thyroid cancer proliferation and invasion. Int J Oncol. (2017) 50:1567–78. doi: 10.3892/ijo.2017.3958
32. Wang X, Eichhorn PJA, and Thiery JP. TGF-β, EMT, and resistance to anti-cancer treatment. Semin Cancer Biol. (2023) 97:1–11. doi: 10.1016/j.semcancer.2023.10.004
33. Jayachandran J, Srinivasan H, and Mani KP. Molecular mechanism involved in epithelial to mesenchymal transition. Arch Biochem Biophys. (2021) 710:108984. doi: 10.1016/j.abb.2021.108984
34. Katsuno Y and Derynck R. Epithelial plasticity, epithelial-mesenchymal transition, and the TGF-β family. Dev Cell. (2021) 56:726–46. doi: 10.1016/j.devcel.2021.02.028
35. Piera-Velazquez S and Jimenez SA. Endothelial to mesenchymal transition: role in physiology and in the pathogenesis of human diseases. Physiol Rev. (2019) 99:1281–324. doi: 10.1152/physrev.00021.2018
36. Jung YS and Park JI. Wnt signaling in cancer: therapeutic targeting of Wnt signaling beyond β-catenin and the destruction complex. Exp Mol Med. (2020) 52:183–91. doi: 10.1038/s12276-020-0380-6
37. Niehrs C. The complex world of WNT receptor signalling. Nat Rev Mol Cell Biol. (2012) 13:767–79. doi: 10.1038/nrm3470
38. Annunziata MC, Parisi M, Esposito G, Fabbrocini G, Ammendola R, and Cattaneo F. Phosphorylation sites in protein kinases and phosphatases regulated by formyl peptide receptor 2 signaling. Int J Mol Sci. (2020) 21(11):3818. doi: 10.3390/ijms21113818
39. Karimi Roshan M, Soltani A, Soleimani A, Rezaie Kahkhaie K, Afshari AR, and Soukhtanloo M. Role of AKT and mTOR signaling pathways in the induction of epithelial-mesenchymal transition (EMT) process. Biochimie. (2019) 165:229–34. doi: 10.1016/j.biochi.2019.08.003
40. Lamouille S, Connolly E, Smyth JW, Akhurst RJ, and Derynck R. TGF-β-induced activation of mTOR complex 2 drives epithelial-mesenchymal transition and cell invasion. J Cell Sci. (2012) 125:1259–73. doi: 10.1242/jcs.095299
41. Wu J, Zhang Y, Cheng R, Gong W, Ding T, Zhai Q, et al. Expression of epithelial-mesenchymal transition regulators TWIST, SLUG and SNAIL in follicular thyroid tumours may relate to widely invasive, poorly differentiated and distant metastasis. Histopathology. (2019) 74:780–91. doi: 10.1111/his.2019.74.issue-5
42. Lv N, Shan Z, Gao Y, Guan H, Fan C, Wang H, et al. Twist1 regulates the epithelial-mesenchymal transition via the NF-κB pathway in papillary thyroid carcinoma. Endocrine. (2016) 51:469–77. doi: 10.1007/s12020-015-0714-7
43. Mitchell B, Dhingra JK, and Mahalingam M. BRAF and epithelial-mesenchymal transition: lessons from papillary thyroid carcinoma and primary cutaneous melanoma. Adv Anat Pathol. (2016) 23:244–71. doi: 10.1097/PAP.0000000000000113
44. Knauf JA, Sartor MA, Medvedovic M, Lundsmith E, Ryder M, Salzano M, et al. Progression of BRAF-induced thyroid cancer is associated with epithelial-mesenchymal transition requiring concomitant MAP kinase and TGFβ signaling. Oncogene. (2011) 30:3153–62. doi: 10.1038/onc.2011.44
45. Buehler D, Hardin H, Shan W, Montemayor-Garcia C, Rush PS, Asioli S, et al. Expression of epithelial-mesenchymal transition regulators SNAI2 and TWIST1 in thyroid carcinomas. Mod Pathol. (2013) 26:54–61. doi: 10.1038/modpathol.2012.137
46. Tan T, Shi P, Abbas MN, Wang Y, Xu J, Chen Y, et al. Epigenetic modification regulates tumor progression and metastasis through EMT (Review). Int J Oncol. (2022) 60(6):70. doi: 10.3892/ijo.2022.5360
47. Yang M, Sun M, and Zhang H. The interaction between epigenetic changes, EMT, and exosomes in predicting metastasis of colorectal cancers (CRC). Front Oncol. (2022) 12:879848. doi: 10.3389/fonc.2022.879848
48. Yu X, He T, Tong Z, Liao L, Huang S, Fakhouri WD, et al. Molecular mechanisms of TWIST1-regulated transcription in EMT and cancer metastasis. EMBO Rep. (2023) 24:e56902. doi: 10.15252/embr.202356902
49. Kiri S and Ryba T. Cancer, metastasis, and the epigenome. Mol Cancer. (2024) 23:154. doi: 10.1186/s12943-024-02069-w
50. Voon DC, Huang RY, Jackson RA, and Thiery JP. The EMT spectrum and therapeutic opportunities. Mol Oncol. (2017) 11:878–91. doi: 10.1002/mol2.2017.11.issue-7
51. Jia W, Tripathi S, Chakraborty P, Chedere A, Rangarajan A, Levine H, et al. Epigenetic feedback and stochastic partitioning during cell division can drive resistance to EMT. Oncotarget. (2020) 11:2611–24. doi: 10.18632/oncotarget.v11i27
52. Manfioletti G and Fedele M. Epithelial-mesenchymal transition (EMT) 2021. Int J Mol Sci. (2022) 23(10):5848. doi: 10.3390/ijms23105848
53. Nabhan F, Dedhia PH, and Ringel MD. Thyroid cancer, recent advances in diagnosis and therapy. Int J Cancer. (2021) 149:984–92. doi: 10.1002/ijc.v149.5
54. Yasui K, Shimamura M, Mitsutake N, and Nagayama Y. SNAIL induces epithelial-to-mesenchymal transition and cancer stem cell-like properties in aldehyde dehydroghenase-negative thyroid cancer cells. Thyroid. (2013) 23:989–96. doi: 10.1089/thy.2012.0319
55. Huang Y, Hong W, and Wei X. The molecular mechanisms and therapeutic strategies of EMT in tumor progression and metastasis. J Hematol Oncol. (2022) 15:129. doi: 10.1186/s13045-022-01347-8
56. Bai F, Liu X, Zhang X, Mao Z, Wen H, Ma J, et al. p18INK4C and BRCA1 inhibit follicular cell proliferation and dedifferentiation in thyroid cancer. Cell Cycle. (2023) 22:1637–53. doi: 10.1080/15384101.2023.2225938
57. Gugnoni M, Sancisi V, Gandolfi G, Manzotti G, Ragazzi M, Giordano D, et al. Cadherin-6 promotes EMT and cancer metastasis by restraining autophagy. Oncogene. (2017) 36:667–77. doi: 10.1038/onc.2016.237
58. Sun CX, Liu BJ, Su Y, Shi GW, Wang Y, and Chi JF. MiR-181a promotes cell proliferation and migration through targeting KLF15 in papillary thyroid cancer. Clin Transl Oncol. (2022) 24:66–75. doi: 10.1007/s12094-021-02670-1
59. Yu Q, Chen J, Zhong C, Yu L, Zhu Y, Xi X, et al. Polyphyllin VII as a potential medication for targeting epithelial mesenchymal transitionin in thyroid cancer. J Pharmacol Sci. (2024) 156:49–56. doi: 10.1016/j.jphs.2024.07.002
60. Fantozzi A, Gruber DC, Pisarsky L, Heck C, Kunita A, Yilmaz M, et al. VEGF-mediated angiogenesis links EMT-induced cancer stemness to tumor initiation. Cancer Res. (2014) 74:1566–75. doi: 10.1158/0008-5472.CAN-13-1641
61. Vosgha H, Ariana A, Smith RA, and Lam AK. miR-205 targets angiogenesis and EMT concurrently in anaplastic thyroid carcinoma. Endocr Relat Cancer. (2018) 25:323–37. doi: 10.1530/ERC-17-0497
62. Deng Z, Xu M, Ding Z, Kong J, Liu J, Zhang Z, et al. ID2 promotes tumor progression and metastasis in thyroid cancer. Endocrine. (2024) 84:1051–63. doi: 10.1007/s12020-023-03674-3
63. Zhang Y and Weinberg RA. Epithelial-to-mesenchymal transition in cancer: complexity and opportunities. Front Med. (2018) 12:361–73. doi: 10.1007/s11684-018-0656-6
64. Xu L, Dai B, Zhang L, Chen W, Rong S, Chen J, et al. UBE2C mediates follicular thyroid carcinoma invasion and metastasis via K29-Specific vimentin ubiquitination. Cancer Lett. (2025) 617:217624. doi: 10.1016/j.canlet.2025.217624
65. Chen Y, Jia L, Zhao K, Chen Z, Han Y, and He X. CTHRC1 promotes anaplastic thyroid cancer progression by upregulating the proliferation, migration, and invasion of tumor cells. PeerJ. (2023) 11:e15458. doi: 10.7717/peerj.15458
66. Jensen K, Bikas A, Patel A, Kushchayeva Y, Costello J, McDaniel D, et al. Nelfinavir inhibits proliferation and induces DNA damage in thyroid cancer cells. Endocr Relat Cancer. (2017) 24:147–56. doi: 10.1530/ERC-16-0568
67. Babashah S and Soleimani M. The oncogenic and tumour suppressive roles of microRNAs in cancer and apoptosis. Eur J Cancer. (2011) 47:1127–37. doi: 10.1016/j.ejca.2011.02.008
68. Lima CR, Gomes CC, and Santos MF. Role of microRNAs in endocrine cancer metastasis. Mol Cell Endocrinol. (2017) 456:62–75. doi: 10.1016/j.mce.2017.03.015
69. Lee Y, Kim M, Han J, Yeom KH, Lee S, Baek SH, et al. MicroRNA genes are transcribed by RNA polymerase II. EMBO J. (2004) 23:4051–60. doi: 10.1038/sj.emboj.7600385
70. Lin S and Gregory RI. MicroRNA biogenesis pathways in cancer. Nat Rev Cancer. (2015) 15:321–33. doi: 10.1038/nrc3932
71. Liu Y, Khan S, Li L, Ten Hagen TLM, and Falahati M. Molecular mechanisms of thyroid cancer: A competing endogenous RNA (ceRNA) point of view. BioMed Pharmacother. (2022) 146:112251. doi: 10.1016/j.biopha.2021.112251
72. Kang N, Zhao Z, Wang Z, Ning J, Wang H, Zhang W, et al. METTL3 regulates thyroid cancer differentiation and chemosensitivity by modulating PAX8. Int J Biol Sci. (2024) 20:3426–41. doi: 10.7150/ijbs.84797
73. Maggisano V, Capriglione F, Verrienti A, Celano M, Sponziello M, Pecce V, et al. Expression of miR-31-5p affects growth, migration and invasiveness of papillary thyroid cancer cells. Endocrine. (2023) 79:517–26. doi: 10.1007/s12020-022-03267-6
74. Liu F, Lou K, Zhao X, Zhang J, Chen W, Qian Y, et al. miR-214 regulates papillary thyroid carcinoma cell proliferation and metastasis by targeting PSMD10. Int J Mol Med. (2018) 42:3027–36. doi: 10.3892/ijmm.2018.3902
75. Geraldo MV, Nakaya HI, and Kimura ET. Down-regulation of 14q32-encoded miRNAs and tumor suppressor role for miR-654-3p in papillary thyroid cancer. Oncotarget. (2017) 8:9597–607. doi: 10.18632/oncotarget.14162
76. Ma S, Jia W, and Ni S. miR-199a-5p inhibits the progression of papillary thyroid carcinoma by targeting SNAI1. Biochem Biophys Res Commun. (2018) 497:181–6. doi: 10.1016/j.bbrc.2018.02.051
77. Jiao X, Ye J, Wang X, Yin X, Zhang G, and Cheng X. KIAA1199, a target of micoRNA-486-5p, promotes papillary thyroid cancer invasion by influencing epithelial-mesenchymal transition (EMT). Med Sci Monit. (2019) 25:6788–96. doi: 10.12659/MSM.918682
78. Zhou WY, Cai ZR, Liu J, Wang DS, Ju HQ, and Xu RH. Circular RNA: metabolism, functions and interactions with proteins. Mol Cancer. (2020) 19:172. doi: 10.1186/s12943-020-01286-3
79. Zhu G, Chang X, Kang Y, Zhao X, Tang X, Ma C, et al. CircRNA: A novel potential strategy to treat thyroid cancer (Review). Int J Mol Med. (2021) 48(5):201. doi: 10.3892/ijmm.2021.5034
80. Viralippurath Ashraf J, Sasidharan Nair V, Saleh R, and Elkord E. Role of circular RNAs in colorectal tumor microenvironment. BioMed Pharmacother. (2021) 137:111351. doi: 10.1016/j.biopha.2021.111351
81. Natua S, Dhamdhere SG, Mutnuru SA, and Shukla S. Interplay within tumor microenvironment orchestrates neoplastic RNA metabolism and transcriptome diversity. Wiley Interdiscip Rev RNA. (2022) 13:e1676. doi: 10.1002/wrna.v13.2
82. Zheng X, Rui S, Wang XF, Zou XH, Gong YP, and Li ZH. circPVT1 regulates medullary thyroid cancer growth and metastasis by targeting miR-455-5p to activate CXCL12/CXCR4 signaling. J Exp Clin Cancer Res. (2021) 40:157. doi: 10.1186/s13046-021-01964-0
83. Dou XL, Xia FD, and Li XY. Circ_0003747 promotes thyroid cancer progression by sponging miR-338-3p to upregulate PLCD3 expression. Epigenetics. (2023) 18:2210339. doi: 10.1080/15592294.2023.2210339
84. Liu J, Li H, Wei C, Ding J, Lu J, Pan G, et al. circFAT1(e2) Promotes Papillary Thyroid Cancer Proliferation, Migration, and Invasion via the miRNA-873/ZEB1 Axis. Comput Math Methods Med. (2020) 2020:1459368. doi: 10.1155/2020/1459368
85. Shu C, Wang S, Hu J, Xu M, Deng H, Maimaiti Y, et al. CircNDST1 promotes papillary thyroid cancer progression via its interaction with CSNK2A1 to activate the PI3K-Akt pathway and epithelial-mesenchymal transition. J Endocrinol Invest. (2023) 46:545–57. doi: 10.1007/s40618-022-01928-x
86. Chan JJ and Tay Y. Noncoding RNA: RNA regulatory networks in cancer. Int J Mol Sci. (2018) 19(5):1310. doi: 10.3390/ijms19051310
87. TaNiue K and Akimitsu N. The functions and unique features of lncRNAs in cancer development and tumorigenesis. Int J Mol Sci. (2021) 22(2):632. doi: 10.3390/ijms22020632
88. Si Y, Wen J, Hu C, Chen H, Lin L, Xu Y, et al. LINC00891 promotes tumorigenesis and metastasis of thyroid cancer by regulating SMAD2/3 via EZH2. Curr Med Chem. (2024) 31:3818–33. doi: 10.2174/0929867330666230522115945
89. Cao Y, Li J, Du Y, Sun Y, Liu L, Fang H, et al. LINC02454 promotes thyroid carcinoma progression via upregulating HMGA2 through CREB1. FASEB J. (2023) 37:e23288. doi: 10.1096/fj.202301070RR
90. Alves LF, da Silva IN, de Mello DC, Fuziwara CS, Guil S, Esteller M, et al. Epigenetic regulation of DLK1-DIO3 region in thyroid carcinoma. Cells. (2024) 13(12):1001. doi: 10.3390/cells13121001
91. Tous C, Muñoz-Redondo C, Gavilán A, Bravo-Gil N, Baco-Antón F, Navarro-González E, et al. Delving into the Role of lncRNAs in Papillary Thyroid Cancer: Upregulation of LINC00887 Promotes Cell Proliferation, Growth and Invasion. Int J Mol Sci. (2024) 25(3):1587. doi: 10.3390/ijms25031587
92. Lei H, Gao Y, and Xu X. LncRNA TUG1 influences papillary thyroid cancer cell proliferation, migration and EMT formation through targeting miR-145. Acta Biochim Biophys Sin (Shanghai). (2017) 49:588–97. doi: 10.1093/abbs/gmx047
93. Feng J, Zhou Q, Yi H, Ma S, Li D, Xu Y, et al. A novel lncRNA n384546 promotes thyroid papillary cancer progression and metastasis by acting as a competing endogenous RNA of miR-145-5p to regulate AKT3. Cell Death Dis. (2019) 10:433. doi: 10.1038/s41419-019-1637-7
94. Van Branteghem C, Augenlicht A, Demetter P, Craciun L, and Maenhaut C. Unraveling the roles of miR-204-5p and HMGA2 in papillary thyroid cancer tumorigenesis. Int J Mol Sci. (2023) 24(13):10764. doi: 10.3390/ijms241310764
95. Zhou B, Xu J, Chen Y, Gao S, Feng X, and Lu X. miR-200b/c-RAP1B axis represses tumorigenesis and Malignant progression of papillary thyroid carcinoma through inhibiting the NF-κB/Twist1 pathway. Exp Cell Res. (2020) 387:111785. doi: 10.1016/j.yexcr.2019.111785
96. Gao Y, Xiang D, Li W, Zheng X, Wang L, Li Z, et al. BRAF(V600E) Mutation-Responsive miRNA-222-3p Promotes Metastasis of Papillary Thyroid Cancer Cells via Snail-Induced EMT. Front Endocrinol (Lausanne). (2022) 13:843334. doi: 10.3389/fendo.2022.843334
97. Hao F, Bi YN, Wang L, Wang Y, Ma J, Cui P, et alRetraction to: miR-199a-5p suppresses epithelial-mesenchymal-transition in anaplastic thyroid carcinoma cells via targeting Snail signals. Cancer Biomark. (2022) 34:697. doi: 10.3233/CBM-229002
98. Deng X, Wu B, Xiao K, Kang J, Xie J, Zhang X, et al. MiR-146b-5p promotes metastasis and induces epithelial-mesenchymal transition in thyroid cancer by targeting ZNRF3. Cell Physiol Biochem. (2015) 35:71–82. doi: 10.1159/000369676
99. Ding Y, Wu L, Zhuang X, Cai J, Tong H, Si Y, et al. The direct miR-874-3p-target FAM84A promotes tumor development in papillary thyroid cancer. Mol Oncol. (2021) 15:1597–614. doi: 10.1002/1878-0261.12941
100. Zhang X, Liu L, Deng X, Li D, Cai H, Ma Y, et al. MicroRNA 483-3p targets Pard3 to potentiate TGF-β1-induced cell migration, invasion, and epithelial-mesenchymal transition in anaplastic thyroid cancer cells. Oncogene. (2019) 38:699–715. doi: 10.1038/s41388-018-0447-1
101. Xu CB, Liu XS, Li JQ, Zhao X, Xin D, and Yu D. microRNA-539 functions as a tumor suppressor in papillary thyroid carcinoma via the transforming growth factor β1/Smads signaling pathway by targeting secretory leukocyte protease inhibitor. J Cell Biochem. (2019) 120:10830–46. doi: 10.1002/jcb.v120.6
102. Bi CL, Zhang YQ, Li B, Guo M, and Fu YL. MicroRNA-520a-3p suppresses epithelial-mesenchymal transition, invasion, and migration of papillary thyroid carcinoma cells via the JAK1-mediated JAK/STAT signaling pathway. J Cell Physiol. (2019) 234:4054–67. doi: 10.1002/jcp.v234.4
103. Pan XM, He XY, Yang YL, Jia WJ, Yang ZQ, Yan D, et al. MiR-630 inhibits papillary thyroid carcinoma cell growth, metastasis, and epithelial-mesenchymal transition by suppressing JAK2/STAT3 signaling pathway. Eur Rev Med Pharmacol Sci. (2019) 23:2453–60. doi: 10.26355/eurrev_201903_17392
104. You A, Fu L, Li Y, Li X, and You B. MicroRNA-203 restrains epithelial-mesenchymal transition, invasion and migration of papillary thyroid cancer by downregulating AKT3. Cell Cycle. (2020) 19:1105–21. doi: 10.1080/15384101.2020.1746490
105. Chen F, Yang D, Ru Y, Cao S, and Gao A. MicroRNA-101 targets CXCL12-mediated akt and snail signaling pathways to inhibit cellular proliferation and invasion in papillary thyroid carcinoma. Oncol Res. (2019) 27:691–701. doi: 10.3727/096504018X15426763753594
106. Chen Y, Hao SA, Jiang Y, Gao B, Tian WG, Zhang S, et al. MicroRNA-1271 inhibits the progression of papillary thyroid carcinoma by targeting IRS1 and inactivating AKT pathway. Eur Rev Med Pharmacol Sci. (2019) 23:7989–99. doi: 10.26355/eurrev_201909_19015
107. Wang DP, Tang XZ, Liang QK, Zeng XJ, Yang JB, and Xu J. microRNA-599 promotes apoptosis and represses proliferation and epithelial-mesenchymal transition of papillary thyroid carcinoma cells via downregulation of Hey2-depentent Notch signaling pathway. J Cell Physiol. (2020) 235:2492–505. doi: 10.1002/jcp.v235.3
108. Sun C, Wang P, Gao T, and Chi J. CCL18 knockdown suppresses cell growth and migration in thyroid cancer. J Healthc Eng. (2022) 2022:1548155. doi: 10.1155/2022/1548155
109. Yu F, Deng X, Zhong Y, Guo B, Zhang X, and Wu B. Hypoxic papillary thyroid carcinoma cells-secreted exosomes deliver miR-221-3p to normoxic tumor cells to elicit a pro-tumoral effect by regulating the ZFAND5. Exp Cell Res. (2023) 431:113716. doi: 10.1016/j.yexcr.2023.113716
110. Fu S, Ma C, Tang X, Ma X, Jing G, Zhao N, et al. MiR-192-5p inhibits proliferation, migration, and invasion in papillary thyroid carcinoma cells by regulation of SH3RF3. Biosci Rep. (2021) 41(9):BSR20210342. doi: 10.1042/BSR20210342
111. Bian S. miR-4319 inhibited the development of thyroid cancer by modulating FUS-stabilized SMURF1. J Cell Biochem. (2020) 121:174–82. doi: 10.1002/jcb.v121.1
112. Zhao H, Zhu X, Luo Y, Liu S, Wu W, Zhang L, et al. LINC01816 promotes the migration, invasion and epithelial−mesenchymal transition of thyroid carcinoma cells by sponging miR−34c−5p and regulating CRABP2 expression levels. Oncol Rep. (2021) 45(5):81. doi: 10.3892/or.2021.8032
113. Li D, Hao S, and Zhang J. Long non-coding RNA UCA1 exerts growth modulation by miR-15a in human thyroid cancer TPC-1 cells. Artif Cells Nanomed Biotechnol. (2019) 47:1815–22. doi: 10.1080/21691401.2019.1606007
114. Hardin H, Guo Z, Shan W, Montemayor-Garcia C, Asioli S, Yu XM, et al. The roles of the epithelial-mesenchymal transition marker PRRX1 and miR-146b-5p in papillary thyroid carcinoma progression. Am J Pathol. (2014) 184:2342–54. doi: 10.1016/j.ajpath.2014.04.011
115. Dai W, Jin X, Han L, Huang H, Ji Z, Xu X, et al. Exosomal lncRNA DOCK9-AS2 derived from cancer stem cell-like cells activated Wnt/β-catenin pathway to aggravate stemness, proliferation, migration, and invasion in papillary thyroid carcinoma. Cell Death Dis. (2020) 11:743. doi: 10.1038/s41419-020-02827-w
116. Jia J, Liu Y, Yang X, Wu Z, Xu X, Kang F, et al. IncRNA TYMSOS promotes epithelial-mesenchymal transition and metastasis in thyroid carcinoma through regulating MARCKSL1 and activating the PI3K/akt signaling pathway. Crit Rev Eukaryot Gene Expr. (2022) 33:1–14. doi: 10.1615/CritRevEukaryotGeneExpr.2022043838
117. Zhao X and Hu X. Downregulated long noncoding RNA LINC00313 inhibits the epithelial-mesenchymal transition, invasion, and migration of thyroid cancer cells through inhibiting the methylation of ALX4. J Cell Physiol. (2019) 234:20992–1004. doi: 10.1002/jcp.v234.11
118. Li G and Kong Q. LncRNA LINC00460 promotes the papillary thyroid cancer progression by regulating the LINC00460/miR-485-5p/Raf1 axis. Biol Res. (2019) 52:61. doi: 10.1186/s40659-019-0269-9
119. Xia F, Chen Y, Jiang B, Du X, Peng Y, Wang W, et al. Long noncoding RNA HOXA-AS2 promotes papillary thyroid cancer progression by regulating miR-520c-3p/S100A4 pathway. Cell Physiol Biochem. (2018) 50:1659–72. doi: 10.1159/000494786
120. Chen W, Zhai L, Liu H, Li Y, Zhang Q, Xu D, et al. Downregulation of lncRNA ZFAS1 inhibits the hallmarks of thyroid carcinoma via the regulation of miR−302−3p on cyclin D1. Mol Med Rep. (2021) 23(1):2. doi: 10.3892/mmr.2020.11640
121. Wang DP, Tang XZ, Liang QK, Zeng XJ, Yang JB, and Xu J. Overexpression of long noncoding RNA SLC26A4-AS1 inhibits the epithelial-mesenchymal transition via the MAPK pathway in papillary thyroid carcinoma. J Cell Physiol. (2020) 235:2403–13. doi: 10.1002/jcp.v235.3
122. Qi F, Tang J, Cai Z, Wang G, and Wang Z. Long non-coding RNA CATIP antisense RNA 1 (lncRNA CATIP-AS1) downregulation contributes to the progression and metastasis of thyroid cancer via epithelial-mesenchymal transition (EMT) pathway. Bioengineered. (2022) 13:7592–606. doi: 10.1080/21655979.2022.2047400
123. Yakushina VD, Strelnikov VV, Tanas AS, and Lavrov AV. Long noncoding RNA landscapes specific to benign and Malignant thyroid neoplasms of distinct histological subtypes. Sci Rep. (2021) 11:16728. doi: 10.1038/s41598-021-96149-2
124. Chen X, Liu K, Xu W, Zhou G, and Yuan C. Tumor-related molecular regulatory mechanisms of long non-coding RNA RMST: recent evidence. Mini Rev Med Chem. (2022) 22:1374–9. doi: 10.2174/1389557521666211202150646
125. De Martino M, Pellecchia S, Esposito F, Liotti F, Credendino SC, Prevete N, et al. The lncRNA RMST is drastically downregulated in anaplastic thyroid carcinomas where exerts a tumor suppressor activity impairing epithelial-mesenchymal transition and stemness. Cell Death Discov. (2023) 9:216. doi: 10.1038/s41420-023-01514-x
126. Wang T and Huang Y. Downregulation of hsa_circ_0001681 suppresses epithelial-mesenchymal transition in thyroid carcinoma via targeting to miR-942-5p/TWIST1 signaling pathway. J Bioenerg Biomembr. (2021) 53:609–20. doi: 10.1007/s10863-021-09907-2
127. Zhang W, Liu T, Li T, and Zhao X. Hsa_circRNA_102002 facilitates metastasis of papillary thyroid cancer through regulating miR-488-3p/HAS2 axis. Cancer Gene Ther. (2021) 28:279–93. doi: 10.1038/s41417-020-00218-z
128. Wang H, Yan X, Zhang H, and Zhan X. CircRNA circ_0067934 overexpression correlates with poor prognosis and promotes thyroid carcinoma progression. Med Sci Monit. (2019) 25:1342–9. doi: 10.12659/MSM.913463
129. Cao S, Yin Y, Hu H, Hong S, He W, Lv W, et al. CircGLIS3 inhibits thyroid cancer invasion and metastasis through miR-146b-3p/AIF1L axis. Cell Oncol (Dordr). (2023) 46:1777–89. doi: 10.1007/s13402-023-00845-2
130. Lin Q, Qi Q, Hou S, Chen Z, Jiang N, Zhang L, et al. Exosomal circular RNA hsa_circ_007293 promotes proliferation, migration, invasion, and epithelial-mesenchymal transition of papillary thyroid carcinoma cells through regulation of the microRNA-653-5p/paired box 6 axis. Bioengineered. (2021) 12:10136–49. doi: 10.1080/21655979.2021.2000745
131. Chen D, Jiang X, Luo H, Hua Q, and Zhang F. CircPTPRM accelerates Malignancy of papillary thyroid cancer via miR-885-5p/DNMT3A axis. J Clin Lab Anal. (2022) 36:e24688. doi: 10.1002/jcla.v36.10
132. Ciarallo A and Rivera J. Radioactive iodine therapy in differentiated thyroid cancer: 2020 update. AJR Am J Roentgenol. (2020) 215:285–91. doi: 10.2214/AJR.19.22626
133. Ullmann TM, Papaleontiou M, and Sosa JA. Current controversies in low-risk differentiated thyroid cancer: reducing overtreatment in an era of overdiagnosis. J Clin Endocrinol Metab. (2023) 108:271–80. doi: 10.1210/clinem/dgac646
134. Volpe F, Nappi C, Zampella E, Di Donna E, Maurea S, Cuocolo A, et al. Current advances in radioactive iodine-refractory differentiated thyroid cancer. Curr Oncol. (2024) 31:3870–84. doi: 10.3390/curroncol31070286
135. Oh JM and Ahn BC. Molecular mechanisms of radioactive iodine refractoriness in differentiated thyroid cancer: Impaired sodium iodide symporter (NIS) expression owing to altered signaling pathway activity and intracellular localization of NIS. Theranostics. (2021) 11:6251–77. doi: 10.7150/thno.57689
136. Liu Y, Wang J, Hu X, Pan Z, Xu T, Xu J, et al. Radioiodine therapy in advanced differentiated thyroid cancer: Resistance and overcoming strategy. Drug Resist Updat. (2023) 68:100939. doi: 10.1016/j.drup.2023.100939
137. De Martino M, Esposito F, Capone M, Pallante P, and Fusco A. Noncoding RNAs in thyroid-follicular-cell-derived carcinomas. Cancers (Basel). (2022) 14(13):3079. doi: 10.3390/cancers14133079
138. Yi D, Zhang D, Zeng Z, Zhang S, Li M, and Zhang Y. MicroRNA-144-3p represses the growth and EMT of thyroid cancer via the E2F2/TNIK axis in cells and male BALB/c nude mice. Endocrinology. (2022) 163(7):BQAC071. doi: 10.1210/endocr/bqac071
139. Han JY, Guo S, Wei N, Xue R, Li W, Dong G, et al. ciRS-7 promotes the proliferation and migration of papillary thyroid cancer by negatively regulating the miR-7/epidermal growth factor receptor axis. BioMed Res Int. (2020) 2020:9875636. doi: 10.1155/2020/9875636
140. Orlandella FM, Mariniello RM, Iervolino PLC, Auletta L, De Stefano AE, Ugolini C, et al. Junctional adhesion molecule-A is down-regulated in anaplastic thyroid carcinomas and reduces cancer cell aggressiveness by modulating p53 and GSK3 α/β pathways. Mol Carcinog. (2019) 58:1181–93. doi: 10.1002/mc.23001
141. Vedovatto S, Oliveira FD, Pereira LC, Scheffel TB, Beckenkamp LR, Bertoni APS, et al. CD73 mitigates ZEB1 expression in papillary thyroid carcinoma. Cell Commun Signal. (2024) 22:145. doi: 10.1186/s12964-024-01522-z
142. Han B, Cui H, Kang L, Zhang X, Jin Z, Lu L, et al. Metformin inhibits thyroid cancer cell growth, migration, and EMT through the mTOR pathway. Tumour Biol. (2015) 36:6295–304. doi: 10.1007/s13277-015-3315-4
143. Liu W, Wang X, Wang L, Mei Y, Yun Y, Yao X, et al. Oridonin represses epithelial-mesenchymal transition and angiogenesis of thyroid cancer via downregulating JAK2/STAT3 signaling. Int J Med Sci. (2022) 19:965–74. doi: 10.7150/ijms.70733
144. Jiang C, Xu F, Yi D, Jiang B, Wang R, Wu L, et al. Testosterone promotes the migration, invasion and EMT process of papillary thyroid carcinoma by up-regulating Tnnt1. J Endocrinol Invest. (2024) 47:149–66. doi: 10.1007/s40618-023-02132-1
145. Xie W, Li H, Lin Q, and Ke N. Network pharmacological analysis and experimental validation of the effects of silybin on proliferation, migration, and immunotherapy of papillary thyroid cancer. Endocr Metab Immune Disord Drug Targets. (2024) 24:672–90. doi: 10.2174/0118715303248000230922185110
146. Gao Y, Pan Y, Wang T, Yao Y, Yuan W, Zhu X, et al. MicroRNA-99a-3p/GRP94 axis affects metastatic progression of human papillary thyroid carcinoma by regulating ITGA2 expression and localization. Acta Biochim Biophys Sin (Shanghai). (2021) 53:1650–61. doi: 10.1093/abbs/gmab147
147. Slack FJ and Chinnaiyan AM. The role of non-coding RNAs in oncology. Cell. (2019) 179:1033–55. doi: 10.1016/j.cell.2019.10.017
148. Zhong Y, Yu F, Yang L, Wang Y, Liu L, Jia C, et al. HOXD9/miR-451a/PSMB8 axis is implicated in the regulation of cell proliferation and metastasis via PI3K/AKT signaling pathway in human anaplastic thyroid carcinoma. J Transl Med. (2023) 21:817. doi: 10.1186/s12967-023-04538-0
149. Zang C, Sun J, Liu W, Chu C, Jiang L, and Ge R. miRNA-21 promotes cell proliferation and invasion via VHL/PI3K/AKT in papillary thyroid carcinoma. Hum Cell. (2019) 32:428–36. doi: 10.1007/s13577-019-00254-4
150. Wu YC, Li SY, and Jia YF. MicroRNA-26a suppresses the Malignant biological behaviors of papillary thyroid carcinoma by targeting ROCK1 and regulating PI3K/AKT signaling pathway. Eur Rev Med Pharmacol Sci. (2019) 23:8940–9. doi: 10.26355/eurrev_201910_19292
151. Wang Y, Zong H, and Zhou H. Circular RNA circ_0062389 modulates papillary thyroid carcinoma progression via the miR-1179/high mobility group box 1 axis. Bioengineered. (2021) 12:1484–94. doi: 10.1080/21655979.2021.1914470
152. Wang Y, Liu BG, and Zhou CX. MicroRNA-31 inhibits papillary thyroid carcinoma cell biological progression by directly targeting SOX11 and regulating epithelial-to-mesenchymal transition, ERK and Akt signaling pathways. Eur Rev Med Pharmacol Sci. (2019) 23:5863–73. doi: 10.26355/eurrev_201907_18329
153. Yan DG, Liu N, Chao M, Tu YY, and Liu WS. SP1-induced upregulation of long noncoding RNA LINC00313 contributes to papillary thyroid cancer progression via the miR-422a. Eur Rev Med Pharmacol Sci. (2019) 23:1134–44. doi: 10.26355/eurrev_201902_17004
154. Xu E, Zhao J, Ma J, Wang C, Zhang C, Jiang H, et al. miR-146b-5p promotes invasion and metastasis contributing to chemoresistance in osteosarcoma by targeting zinc and ring finger 3. Oncol Rep. (2016) 35:275–83. doi: 10.3892/or.2015.4393
155. Zhang Z, Liu ZB, Ren WM, Ye XG, and Zhang YY. The miR-200 family regulates the epithelial-mesenchymal transition induced by EGF/EGFR in anaplastic thyroid cancer cells. Int J Mol Med. (2012) 30:856–62. doi: 10.3892/ijmm.2012.1059
156. Liu M, Deng H, Zhao Y, Li C, and Liu H. Impact of microRNA-21-5p on the growth of thyroid cancer cells via targeting the recombinant sclerostin domain containing protein 1. Zhong Nan Da Xue Xue Bao Yi Xue Ban. (2021) 46:1054–62. doi: 10.11817/j.issn.1672-7347.2021.200764
157. Zeng L, Yuan S, Zhou P, Gong J, Kong X, and Wu M. Circular RNA Pvt1 oncogene (CircPVT1) promotes the progression of papillary thyroid carcinoma by activating the Wnt/β-catenin signaling pathway and modulating the ratio of microRNA-195 (miR-195) to vascular endothelial growth factor A (VEGFA) expression. Bioengineered. (2021) 12:11795–810. doi: 10.1080/21655979.2021.2008639
Keywords: thyroid cancer, epithelial-mesenchymal transition, non-coding RNAs, miRNAs, circRNAs, lncRNAs, diagnostic biomarkers
Citation: Zhao R, Tian J, Peng J, Ma R and Fu S (2025) The regulatory network of epithelial-mesenchymal transition-associated non-coding RNAs in thyroid cancer: molecular mechanisms, clinical implications, and therapeutic strategies. Front. Oncol. 15:1592467. doi: 10.3389/fonc.2025.1592467
Received: 14 March 2025; Accepted: 13 May 2025;
Published: 02 June 2025.
Edited by:
Marco De Martino, National Research Council (CNR), ItalyReviewed by:
Francesco Esposito, National Research Council (CNR), ItalyAntonella Napolitano, Federico II University Hospital, Italy
Copyright © 2025 Zhao, Tian, Peng, Ma and Fu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Songbo Fu, ZnVzYkBsenUuZWR1LmNu
 Rui Zhao
Rui Zhao Jiaju Tian
Jiaju Tian Jing Peng
Jing Peng Ruiting Ma
Ruiting Ma Songbo Fu1,2,3*
Songbo Fu1,2,3*