- 1Department of Hematology, Affiliated Hospital of Guizhou Medical University, Guiyang, Guizhou, China
- 2Department of Hematology, Hematological Institute of Guizhou Province, Guiyang, Guizhou, China
- 3Key Laboratory of Hematological Disease Diagnostic and Treatment Centre, Guizhou Province Hematopoietic Stem Cell Transplantation Centre, Guiyang, Guizhou, China
The co evolution of tumor cells and microenvironmental matrix components almost determines the series of processes involved in cancer occurrence and progression. However, many anti-cancer treatments are designed around tumor cells, neglecting the supportive role of stromal cells. Cancer-associated fibroblasts (CAFs), as the main stromal cells in tumor microenvironment, are currently considered as a key component promoting tumorigenesis, development, and regulating the transfer of tumor cells to distant locations through secretion of different growth factors, cytokines, chemokines, and the degradation of extracellular matrix. Therefore, the strategy of targeting both cancer cells and CAFs shows great potential in cancer treatment. In hematological malignancies, the role of CAFs in the progression of tumors has gradually been recently tapped. This review describes the role and functional characteristics of CAFs in tumors, mainly concentrates on the potential role of CAFs in the disease progression of hematological malignancies according to recent findings, and emphasizes the importance of CAFs as a key target to overcome tumor progression and improve treatment efficacy.
1 Introduction
The tumor microenvironment (TME) is a dynamic network which is composed of stromal cells (fibroblasts, endothelial cells, immune cells, adipocytes, pericytes and bone marrow-derived cells, etc.), extracellular matrix (ECM), soluble cytokines and growth factors. The occurrence and development of stromal component provide a supportive environment for a variety of cancers cells (1–3). During the occurrence and development of tumors, tumor cells and their surrounding stromal microenvironment are in close proximity, and the extensive and multi-layered “cross-talk” between them adapts TME to support tumor survival, growth and metastasis (4, 5). Targeting the TME is currently one of the directions to improve the efficacy of tumor therapy (6–9). In the treatment of hematological malignancies, part of the reason for the poor efficacy may be that the tumor cells are protected by the “educated” microenvironment, which offers a natural refuge for tumor cells to escape the killing of chemotherapy drugs and thus becomes a possible root cause of tumor progression (10–12).
Cancer-associated fibroblasts (CAFs), a major component of TME, reside in symbiotic relationship with cancer cells, supporting them to survive from cancer drugs (13–17). In the TME, CAFs are in a continuously activated state, which not only promotes the growth of tumor cells, but also secretes various cytokines, chemokines and inflammatory mediators in a paracrine manner through cell and cell interactions, initiates the proteolysis and structural modification of the ECM, provides convenient conditions for tumor cells to escape chemotherapeutics, initiates metabolic reprogramming, and finally results in tumor progression and tumor cell migration to distant locations (18–26). It is of note that CAFs have been shown to have different origins, phenotypes, and functions, while most of them contribute to tumor progression. Considering its critical role in promoting tumor progression, CAFs have recently become a therapeutic target for a variety of tumors (25, 27). In the study of hematological malignancies, recent studies have indicated that CAFs are also a vital component in promoting tumor progression (28, 29). In addition, they may become a potential target for the treatment of hematological malignancies. This review introduces the origin, activation mechanism and role of CAFs in tumors, mainly elaborates on the possible roles played by CAFs in hematological malignancies, and potential therapeutic strategies targeting CAFs, as well as elucidates the heterogeneity of CAFs based on current research status.
2 Origins of CAFs
CAFs, as important stromal cells in the TME, usually originate from stromal cells in the microenvironment. These interstitial cells mainly include resident tissue fibroblasts, adipocytes, pericytes and bone marrow-derived mesenchymal stem cells (BM-MSCs). In addition, both epithelial cells and endothelial cells can be activated into CAFs through interstitial transformation. Among them, normal fibroblasts (NFs) are an important source of CAFs (30, 31). NFs typically moderately express α-smooth muscle actin (α-SMA), fibroblast activation protein (FAP), fibroblast specific protein-1 (FSP-1), and vimentin, while CAFs often highly express these proteins (32). Fibroblasts are in a quiescent state under normal physiological conditions and can be activated in tissue repair and TME. During tissue repair, the transient moderate activation of myofibroblasts is beneficial for the repair of tissue integrity. However, activated CAFs in the TME can maintain a sustained state of activation and become important factors affecting tumor occurrence and progression.
BM-MSCs are another important source of CAFs (33–35). Quante et al. (33) considered that at least 25% of activated CAFs were derived from BM-MSCs. The morphological characteristics of CAFs are similar to BM-MSCs, being adherent and growing in a spindle shape. Research has shown that the two have a high degree of consistency in immune phenotype and function (35, 36). On the one hand, CAFs expresses cell surface markers of MSCs and has a certain degree of multipotent differentiation potential. On the other hand, MSCs also express certain surface marker proteins of CAFs, such as α-SMA, nestin, and vimentin, but the expression levels of CAFs surface markers, cytokines, growth factors, and chemokines are higher.
In addition, adipocytes are also one of the sources of CAFs (37, 38). The study by Simiczyjew et al. (39) demonstrated that adipocytes co-cultured with melanoma cells could exhibit fibroblast characteristics and secrete higher levels of IL-6 and serpine1, while producing less C-C motif chemokine ligand 2 (CCL2), chemokine (C-X-C motif) ligand 1 (CXCL1), and angiogenic molecules. Lactic acidosis is a characteristic of the TME, research has shown that human subcutaneous adipose-derived stem cells committed to adipocytes can acquire myofibroblast, pro-fibrotic, and pro-inflammatory phenotypes when cultured in an acidic environment (40). In the study of gastric cancer (GC), adipocytes, when co-cultured with GC cells, significantly increased the expression levels of CAFs markers FSP-1, inflammatory cytokines, PAI-1, and IL-6, while the invasiveness of GC cells was enhanced, suggesting that adipocytes can acquire the CAFs phenotype under the stimulation of GC cells, which further promotes the invasion process of GC cells (41). Moreover, epithelial cells, endothelial cells, stellate cells, and pericytes in the TME are also possible sources of CAFs (42–44), and transformed CAFs may play an important role in the occurrence and development of various tumors.
3 The markers and characteristics of CAFs
For the markers of CAFs, a number of intracellular, extracellular and cell surface proteins with increased expression have been used to isolate or identify CAFs, including α-SMA, FAP, FSP-1, vimentin, periostein, platelet derived growth factor receptor-α/β, and neuron glial antigen 2 (NG2), etc. In addition, there are some negative indicators which can be used to help exclude CAFs. For instance, the antibody against CD31 is employed to demonstrate a lack of endothelial cell contamination, while cytokeratin is used to exclude epithelial components. Among these markers, α-SMA and FAP have been known as the specific markers for myofibroblasts, and their high expression often indicates a poor tumor prognosis (33, 45–52). However, till the present, no unique marker can be identified to investigate the existence of CAFs. Dzobo et al. (53) conducted a database analysis and found that CAFs markers exhibit differential expression in different tumors, and the expression patterns of CAFs markers in different tumor types are also variable. CAFs are mostly characterized based on a combination of the above markers and different tumor types.
It is worth noting that due to the different sources of CAFs, they have various phenotypes, including the myofibrotic CAFs subtype (myCAFs) with α-SMA+FAP+ and lacking the expression of inflammatory cytokines, the inflammatory CAFs subtype with low expression of α-SMA and secretion of interleukin-6 (IL-6) and other inflammatory mediators, and the antigen-presenting CAFs subtype (apCAFs) with high expression of major histocompatibility complex (MHC) class II (MHCII)-related genes H2-Ab1, CD74 and other regulators of immune activity (27, 54–56) (Figure 1). Recently, reports by Cords et al. (57), Xiao et al. (58), and Zhang et al. (59) have provided a more detailed classification of the different CAF subtypes found in various tumors. Additionally, Lavie et al. (44) summarized the main CAF subtypes and characteristics of various organs in the body based on single-cell sequencing data. Because of these different subtypes, CAFs exert a variety of biological roles in tumorigenesis, progression, immune regulation, tumor metabolism and metastasis. Sahai et al. (60) suggested that a new naming framework could be constructed based on the functions of CAFs, and standardized methods could be established to detect and identify CAFs. This might lead to a more detailed and accurate classification and understanding of the characteristics of CAFs.
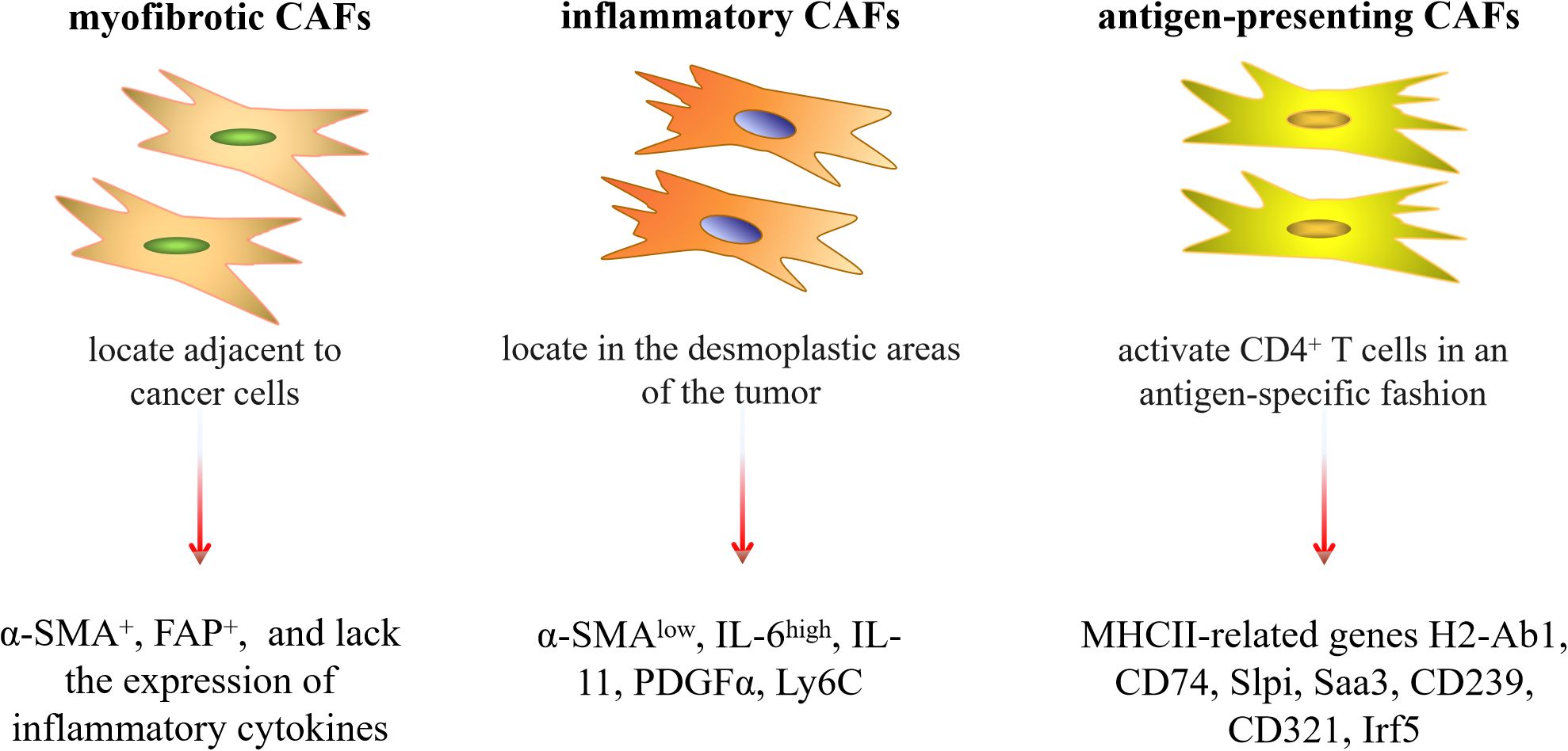
Figure 1. Three common subtypes of CAFs and their characteristics. Slpi, secretory Leukocyte Peptidase Inhibitor; Saa3, serum Amyloid A3; Irf5, interferon regulatory factor 5.
4 The activation mechanism of CAFs in tumor microenvironment
Since the activation of CAFs in the TME is a vital factor leading to malignant progression of tumors, understanding the activation mechanism of CAFs in the TME may be a key measure to find effective intervention methods to block the activation of CAFs in the microenvironment and enhance the efficacy of tumor treatment.
In the TME, to obtain invasive and tumor-promoting phenotypes, NFs and other stromal cells undergo continuous activation via different mechanisms. Cancer cells secrete cytokines and soluble components into the surrounding microenvironment, and then stimulate the recruitment and activation of fibroblasts. During this process, transforming growth factor β (TGF-β) is currently recognized as the key factor to stimulate the activation of CAFs (33, 47, 61–65), which is a cytokine essential for inducing the fibrotic response and activating the cancer stroma and can be expressed by both tumor cells and CAFs. Under the action of TGF-β, NFs or potential stromal cells undergo morphological changes and transform into CAFs, while the latter exert an important role in the occurrence, development and progression of tumors.
In addition to the classic TGF-β pathway stimulating CAFs activation, there are also some other potential factors that can stimulate CAFs activation. The study conducted by Weber et al. (66) showed that tumor cell-derived osteopontin (OPN) mediated the transformation of MSCs to CAFs and therefore increased tumor cell growth and metastasis, while the process was still dependent on myeloid zinc finger 1 and TGF-β1. In the study of GC, it was found that helicobacter pylori (Hp) infection might induce the transformation of MSCs into CAFs, contributing to the occurrence and development of GC (67). The reduced extracellular pH value may also be an important factor for promoting the conversion of MSCs into CAFs (68). Additionally, progranulin (PGRN) can directly or indirectly activate CAFs through the epithelial-mesenchymal transition (EMT) program to promote the invasiveness of ovarian cancer cells (69). A recent study indicated that overexpression of galectin-1 could induce the transformation of NFs into CAFs (70). These results suggest that the activation mechanisms of CAFs may vary in different TME, and we need to conduct categorical research on different tumors to better comprehend the activation factors of CAFs in tumors.
5 CAFs in solid tumors - the tumor-promoting function
CAFs have been widely studied in promoting tumor angiogenesis, facilitating tumor invasion and metastasis, enhancing tumor resistance and tumor immune escape in a variety of solid tumors. For tumor angiogenesis, tumor growth depends on the formation of new blood vessels, and CAFs can promote tumor angiogenesis through secretion or high expression of various pro-angiogenic and other factors, including vascular endothelial growth factor (VEGF) (71–73), interleukin-6 (IL-6) (72), fibroblast growth factor (FGF) (71), CCL2 (74), galectin-1 (75), milk fat globule-EGF factor 8 (MFGE8) (76), Wingless-type MMTV integration site family member 2 (WNT2) (77) and FOS-like 2 (FOSL2) (78). Meanwhile, the exosome (79), exosome microRNA (80) and extracellular vesicles (EVs) (81) derived from CAFs are also key components that promote tumor angiogenesis. These angiogenic factors derived from CAFs provide convenient conditions for the growth and metastasis of tumors. In terms of tumor invasion and metastasis, CAFs stimulate the invasion and metastasis of cancer cells by promoting epithelial mesenchymal transformation (82, 83), overexpressing its own markers (24, 48), or secreting of growth factors (23, 84), cytokines such as IL1β/IL-1R (85–87), IL-6 (88), IL-11 (89), IL-17a (90), IL-22 (91) and leukemia inhibitory factor (92), chemokine receptors (93), adhesion factors (94, 95), exosomes (96–102), and various metalloproteinases (MMPs) (23, 71, 88). In addition, CAFs secrete a large amount of ECM proteins to promote ECM synthesis and reshape tumor matrix, which is also one of the vital reasons for the formation of tumor aggressive microenvironment. These proteins mainly include collagen type I, collagen type III, fibronectin (FN) and vimentin. Among them, FN is the main ECM protein, which promotes tumor targeted migration and invasion through interacting with its integrin receptors (103, 104). Recent studies have also indicated that CAFs that overexpression of FN1 and periosteal protein can significantly promote the wound healing and invasion ability of tumor cells (105). For tumor resistance, CAFs can secrete various factors making cancer cells develop drug resistance. Among them, IL-6 secreted by CAFs can reduce the response of cancer cells to chemotherapeutics (106, 107), and stromal cell-derived factor-1 (SDF-1) secreted by CAFs (108) can stimulate the malignant progression of pancreatic cancer and mediate the development of gemcitabine resistance. CAFs can also secrete CCL5, which promotes up-regulation of androgen receptor expression in prostate cancer cells, resulting in resistance to enzalutamide treatment, and improves the expression of tumor programmed death ligand 1 (PD-L1), causing immune escape (109). In addition, CAFs inhibit ferroptosis and reduce cisplatin sensitivity in nasopharyngeal carcinoma by secreting FGF5 and activating downstream FGFR2/Nrf2 signaling pathways (110). In the study of esophageal squamous cell carcinoma, CAFs promote tumor cell growth by secreting plasminogen activator inhibitor-1 (PAI-1) and attenuate the therapeutic sensitivity of cisplatin (111). Meanwhile, the secretion of exosomes by CAFs is also one of the important factors which can mediate tumor drug resistance. The exosomes secreted by CAFs promote tumor cell metastasis and drug resistance to chemotherapy drugs by maintaining tumor cell stemness and promoting epithelial mesenchymal transformation (101). Moreover, CAFs may enhance drug resistance through inhibiting drug accumulation and combating drug-induced oxidative stress (112). For tumor immune escape, CAFs can induce immunosuppressive cell infiltration and create immunosuppressive TME, contributing to poor prognosis (105, 113–115). A high proportion of CAFs is more likely to cause distant metastasis of the tumor and present a higher level of immune invasion (116, 117). Additionally, The interaction between CAFs and immune cells in TME is also an important factor in promoting tumor progression (118). CAFs can enhance the infiltration and function of myeloid suppressor cells, T cells and other immune cells, reduce the number and activity of tumor-infiltrated cytotoxic T cells in tumor tissues, and make tumors insensitive to PD-1 treatment. Depletion of CAFs can improve the efficacy of tumor immunotherapy (119).
In addition to the above-mentioned aspects, CAFs have been shown to alter the architecture and physical properties of the ECM, influencing tumor cells growth, migration and invasion (120–122). Meanwhile, the age-related secretion phenotype (SASP) of CAFs is also an important factor influencing tumor progression (123). Fan et al. (124) identified a new myCAFs subpopulation of aging like tetraspanning protein-8 (TSPAN8) (+) in breast cancer. TSPAN8 (+) myCAFs can resist chemotherapy by secreting SASP related factors IL-6 and IL-8, thus enhancing the stemness of surrounding breast cancer cells. Therefore, the combination of traditional tumor therapy and anti-aging drugs may also be one of the strategies to improve the effectiveness of tumor treatment. These findings demonstrate that CAFs exert an important role in the progression of solid tumors (Figure 2), and targeting CAFs may be an effective strategy to improve the therapeutic efficacy of multiple solid tumors.
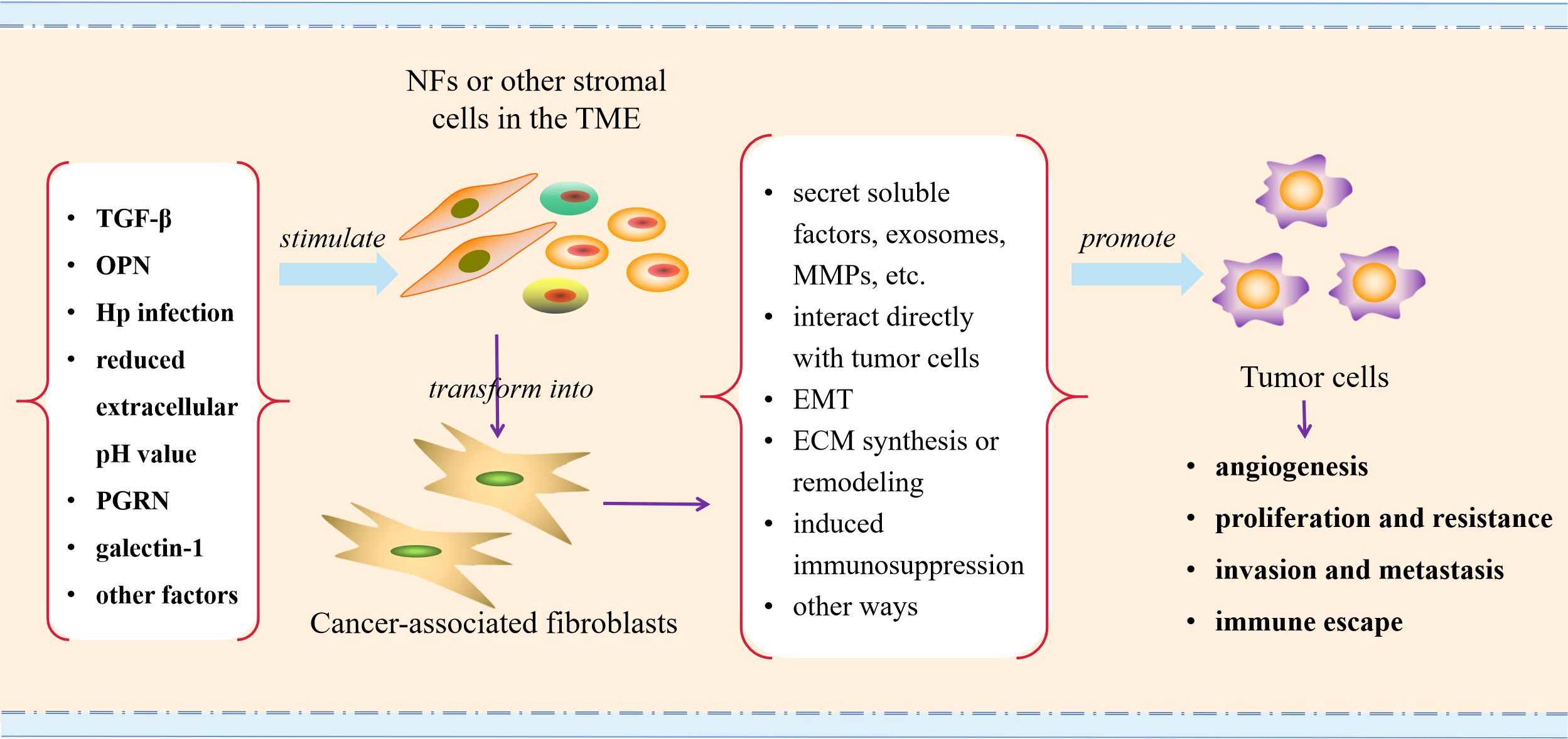
Figure 2. Schematic illustration of CAFs activating in TME and promoting tumor progression. TGF-β, transforming growth factor β; OPN, osteopontin; Hp, helicobacter pylori; PGRN, progranulin; MMPs, metalloproteinases; EMT, epithelial-mesenchymal transition; ECM, extracellular matrix.
6 CAFs in solid tumors - the tumor-restraining function
Although most studies have confirmed that CAFs are closely related to tumor progression, some research has also shown that CAFs have a tumor-suppressing effect. The cell surface and secretory protein meflin is expressed in cultured MSCs, fibroblasts, and pericytes. meflin-positive CAFs are related to a better prognosis in pancreatic ductal cell carcinoma. Overexpression of meflin inhibits tumor growth, while lack of meflin results in significant tumor progression and poor histological differentiation (125). In the research on lung cancer, CD200+ CAFs can increase the sensitivity of epidermal growth factor receptor (EGFR) gene mutation-positive lung cancer cells to gefitinib (126). Similarly, the IL-8 produced by CAFs can inhibit the proliferation of biliary tract cancers cell line OCUCh-LM1 (127). Yes-associated protein 1 (YAP1) is a protein with multiple functional domains and belongs to the Yes-related protein family. Song et al. (128) recently discovered that YAP1 can regulate the phenotype of CAFs, which can transform CAFs from the tumor-promoting subtype that promotes ECM deposition to the tumor-suppressing subtype that stimulates anti-tumor immunity, thereby increasing the treatment sensitivity of immune-checkpoint blockade. myCAF is one of the important subtypes that promote tumor progression. Bhattacharjee et al. (129) found that the collagen type I expressed by myCAF can inhibit tumor growth by collagen physically restricting tumor spread, and the absence of collagen type I can promote the growth of metastatic tumors. In the research on rectal cancer, Qin et al. (130) showed that neoadjuvant chemotherapy can significantly reshape the CAF subtypes, and the reshaped CAF subtypes regulate the TME through spatial recruitment and crosstalk, activate immunity through multiple cytokines, and inhibit tumor progression. In addition, CD143+ CAFs can predict better survival outcomes for colorectal cancer patients (131). In the research on malignant melanoma, it was found that CD9+ exosomes derived from CAFs have a significant inhibitory effect on the proliferation of malignant melanoma cells, and compared with CD9- patients, CD9+ patients have better disease-free survival rates (132). These research results suggest that in addition to the well-known tumor-promoting functions of CAFs, they also possess potential anti-tumor functions and subtypes. These functions and subtypes should be given due attention.
7 CAFs in hematological malignancies
Hematological malignancies mainly include various kinds of leukemia, multiple myeloma (MM) and malignant lymphoma. With the continuous improvement of treatment programs, the five-year survival of hematologic malignancies has increased in recent years. However, whether this has translated into greater long-term survival is unknown (133). TME is an important factor affecting blood tumor survival, drug resistance and disease progression. CAFs in TME have been shown to exert a key role in promoting tumor progression in studies of acute myeloid leukemia (AML), acute lymphoblastic leukemia (ALL), chronic lymphoblastic leukemia (CLL), MM, lymphoma and myeloproliferative neoplasm (MPN) (Table 1). In the following content, we focus on elaborating on the biological functions of CAFs in the above-mentioned diseases based on current research progress, hoping to provide reference for the search for potential therapeutic targets.
7.1 CAFs promote the progression of acute leukemia
Acute leukemia (AL) is a common malignant tumor type in hematological system. Most patients with AL achieve complete remission after induction chemotherapy. However, the prognosis of quite a few high-risk patients is still poor, and the long-term survival is not optimistic. Therefore, the new treatment strategy provides great hope for further improving the efficacy of leukemia. During the past few years, the significant rise of immunotherapy has revolutionized the treatment of leukemia.
Recently, studies have shown that the TME has played a key role in leukemia progression (10–12, 156–159). The TME is a contributing factor to the failure or success of leukemia treatment, which may lead to a shift in treatment methods and concepts. CAFs are important stromal cells in TME, and their role in AL has been gradually revealed, which is expected to become a potential therapeutic target for leukemia.
Research on AML has shown that the interaction between leukemia cells and bone marrow niche can affect hematopoietic function in AML, promote leukemia cell survival, and lead to chemotherapy resistance (157, 160–162). For CAFs in AML microenvironment, Zhai et al. (134) found that CAFs co-cultured with AML cell lines could significantly prolong the survival of leukemia cells, reduce apoptosis, and lower the sensitivity of leukemia cells to chemotherapy drugs. Growth differentiation factor 15 (GDF15) secreted by CAFs may be an important factor for the protective effect of CAFs-mediated chemotherapy. Targeting or down-regulating GDF15 can significantly improve the sensitivity of leukemia cells to chemotherapy drugs. Another study demonstrated that the high expression of the CAFs marker FAPα in bone marrow stromal cells reduced the sensitivity of leukemia cells to cytarabine by stimulating the β-catenin signaling pathway (135). Suggesting that CAFs and its marker FAPα in AML microenvironment may be an important factor for the poor prognosis of AML patients.
ALL is one of the common types of leukemia. Research has shown that there are abnormally activated stromal cells in the bone marrow of patients with ALL, which can form specific stromal niches to protect leukemia cells from chemotherapy drug damage (163). Targeting TME is an important direction in the treatment of ALL (156). The study performed by Burt et al. (136) on ALL found that MSCs could be activated into CAFs under the stimulation of daunorubicin or cytarabine to promote the progression of leukemia. Moreover, co-culturing leukemia cells with human choroid plexus fibroblasts could enable the latter to obtain CAFs phenotype (137). Meanwhile, our previous research found that MSCs in the ALL microenvironment acquired CAFs phenotype, which played an essential role in promoting ALL cell migration and invasion (138). The SDF-1/C-X-C chemokine receptor type 4 (CXCR4) axis, as a signaling axis facilitating the interaction between tumor cells and stromal cells, promotes the integration α5β1 on leukemia cells to bind to FN in MSCs with CAFs phenotype, thus stimulating the interaction between ALL cells and MSCs with CAFs phenotype, which may be a potential molecular mechanism that fosters the progression of ALL. Application of CXCR4 inhibitor AMD3100 or targeting down-regulation of integrin β1 can weaken the promoting effect of MSCs with CAFs phenotype on leukemia cell migration and invasion (139).
Adult T-cell leukemia/lymphoma (ATLL) refers to a rare aggressive T-cell malignant tumor caused by human T-cell leukemia virus type 1 infection. Joo et al. (140) employed single-cell RNA sequencing and T-cell receptor clone analysis to dissect different cell types, and identified a new subset of CAFs showing abundant EGFR-related transcripts, including early growth response 1 and 2 (EGR1 and EGR2). Further study showed that CAFs in ATLL exerted an essential role in CD4 T cell proliferation through FGF7-FGF1 and PDGFA-PDGFRA/B signaling.
Based on the above research, CAFs promote leukemia progression to varying degrees. To this end, Li et al. (164) constructed a leukemia-associated fibroblastic tumor cell line HXWMF-1. This provides a convenient way to further explore the role of CAFs in leukemia.
7.2 CAFs promote the progression of chronic lymphoblastic leukemia
Chronic leukemia is a type of malignant tumor which is originated from bone marrow hematopoietic stem cells, including CLL and chronic myeloid leukemia. Studies on CAFs in chronic leukemia mainly concentrate on CLL, which is a clonal malignant disease of lymphocytes, and the survival and proliferation of tumor cells cannot be separated from the surrounding stromal microenvironment. In the TME of CLL, the activation of CAFs is mainly dependent on CLL-derived factors (165). Co-culturing CLL cells with MSCs can promote the acquisition of CAFs phenotype in MSCs (166). Yang et al. (141) showed that exosomes secreted by leukemia cells could transport miR-146a to MSCs, and miR-146a transported to MSCs promoted the transformation of MSCs into CAFs by targeting the down-regulation of ubiquitin-specific peptidase 16. In Paggetti’s study (142), CLL-derived exosomes facilitated the acquisition of CAFs phenotype by fusing with BM-MSCs and endothelial cells, and further accelerated the disease progression of CLL. LYN protein is a non-receptor tyrosine protein kinase, belonging to the Src family of kinases. A recent study showed that it was overexpressed in fibroblasts of lymph nodes in CLL patients, which could regulate the polarization of fibroblasts towards inflammatory cancer-associated phenotype, therefore promoting leukemia survival (143). These results suggest that stromal cells in the CLL microenvironment can be activated to CAFs in response to the stimulation of leukemia cells, which may be a key component in the study of the pathogenesis of CLL.
7.3 CAFs promote the progression of multiple myeloma
CAFs exerts a vital role in the biobehavior of MM, and myeloma cells can induce MSCs differentiate into CAFs in a dose-dependent manner (167). Ge et al. (144) found that the FAP, a marker of CAFs, was highly expressed in MM bone marrow and could promote the growth of myeloma cells. Zi et al. (145) also showed similar results, and demonstrated that FAP could protect MM cells from apoptosis induced by bortezomib via β-catenin signaling pathway. In another study, Frassanito et al (146) showed that the expression levels of CAFs markers (FSP-1, α-SMA, FAP) in bone marrow of patients with active MM were obviously higher than those in patients with MM remission, patients with monoclonal gammopathy of undetermined significance, and patients suffering from iron deficiency anemia. In the MM microenvironment, activated CAFs promote the chemotaxis, adhesion, proliferation and reduce apoptosis of MM cells through cytokine signaling and intercellular contact. In further studies, the research team found that CAFs were insensitive to bortezomib treatment and protected MM cells from bortezomib-induced apoptosis. Bortezomib can trigger CAFs to secrete high levels of IL-6, IL-8, insulin-like growth factor (IGF)-1 and TGF-β, induce reactive oxygen species and activate autophagy in bortezomib resistant CAFs to reduce treatment sensitivity (147). Ciavarella et al. (148) also suggested that CAFs promoted the proliferation and invasion potential of MM cells, and inhibition of urokinase plasminogen activator receptor (u-PAR) gene expression in CAFs significantly weakened the above biological effects. CAFs-derived exosomes are also a key component promoting the progression of MM, and CAF-derived exosome miR-21 can enter MM endothelial cells in a time-dependent manner and initiate angiogenesis by promoting proliferation, migration, and tubule formation (80).
Considering role of CAFs in the progression of MM, the use of recombinant human erythropoietin as a potential therapeutic strategy can inhibit cell proliferation of MM patient-derived CAFs while increasing CAFs apoptosis (168). Recently, study has also shown that CAFs can hinder the anti-tumor activity of CAR-T cells and promote MM progression, further demonstrating that dual target CAR-T cell therapy targeting both MM cells and CAFs can significantly improve the therapeutic efficacy of CAR-T cell therapy (169). These results indicate that CAFs exert a vital role in the MM microenvironment, and targeting CAFs may be an effective intervention to improve the therapeutic efficacy of MM patients.
7.4 CAFs promote the progression of lymphoma
Lymphoma is one of the most common malignancies in blood system, including non-Hodgkin lymphoma (NHL) and Hodgkin lymphoma (HL). In lymphoma TME, CAFs can support lymphoma cell survival by increasing glycolysis and induce drug resistance in lymphoma cells by secreting exosomes (149). CAFs also promote the survival of lymphoma cells by secreting pyruvate (150). Hodgkin and Reed Sternberg (HRS) cells are characteristic cells of HL. Studies have demonstrated that HRS cells adhered to fibroblasts are usually protected from damage induced by the therapeutic drug Brentuximab Vedotin (151). FAP is one of the common markers of CAFs. Jin et al. (170) found through 68Ga-FAPI PET/CT that the uptake of FAP in HL lesions increased and was correlated with the intensity of immunostaining. In aggressive NHL lesions, FAP has moderate to severe immunostaining, while in indolent NHL lesions, FAP staining is weaker. Fungal granuloma (MF) is a primary cutaneous T-cell lymphoma (CTCL). Mehdi et al.’s (171) study also showed that the expression of CAFs marker FAP α increased with disease staging. Co-culture of MF fibroblasts with CTCL cell line MyLa cells could increase the expression of IL16 and IL4 in MyLa cells, and inhibit the expression of Th1 markers IFNG and TBX21. Additionally compared with MF fibroblasts, normal fibroblasts inhibited MKI67 expression in MyLa cells. Another study revealed that CAFs protected MF cells from doxorubicin-induced cell death and increased their migration by secreting CXCL12 (152). It is suggested that monitoring FAP expression may be a way to characterize lymphoma. Diffuse large B-cell lymphoma is the most common type of NHL. Apollonio et al. (153) found that co-culturing primary human lymphoid fibroblasts (HLF) with DLBCL cell lines induced the expression of CAFs markers FAP α and α-SMA in HLF, and exhibited significant changes in the cytoskeleton and matrix remodeling ability, while up-regulating PD-L1 expression and driving immune suppression. In further research, the research team found that DLBCL tumor cells also converted fibroblast reticular cells (FRCs) into immunosuppressive CAFs. Lymphoma FRCs can exhibit CAFs like immunophenotypes, including FAP, α-SMA, and upregulation of immune regulatory MHC class I, PD-L1, and PD-L2 molecules (172). For therapeutic interventions, the study performed by Aoki et al. (173) showed that the compound emetine hindered the potential of CAFs to support tumor cell viability in vitro and significantly inhibit tumor growth in vivo. Additionally, emetine induced cell death in primary refractory lymphoma cells with MYC rearrangement. Collectively, these findings suggest that CAFs may be a key factor in the progression of lymphoma.
7.5 CAFs in myeloproliferative neoplasm
MPN is a clonal and chronic disease characterized by myeloid cell proliferation. Regarding the study of CAFs in MPN, Schmitt-Graeff et al. (174) showed that the expression of CAFs marker α-SMA was up-regulated in MPN, and its expression increased with the aggravation of fibrosis degree. In previous studies, our research group also found that CAFs markers were expressed to varying degrees in MPN, among which, the expression of α-SMA, FAP, and lysyl-oxidase-like 2 (LOXL2) was related to the degree of bone marrow fibrosis. In vitro cell experiments demonstrated that the use of recombinant human LOXL2 significantly increased the expression of α-SMA and FAP in MSCs, suggesting that LOXL2 might be capable of stimulating MSCs to obtain the CAFs phenotype (154). Insulin like growth factor binding proteins (IGFBPs) are a family of six highly homologous protein members with high affinity for IGF. Longhitano et al. (155) found that IGFBP-6 expression significantly increased in primary myelofibrosis (PMF), and IGFBP-6 stimulated the up-regulation of CAFs markers α-SMA, FAP, and TGF-β expression in human bone marrow stromal cells HS5, suggesting that IGFBP-6 could stimulate the transformation of MSCs into CAFs and might be correlated with the progression of myelofibrosis. However, there is currently no detailed report on the mechanism by which CAFs exert a role in MPN.
In the aforementioned study, we found that CAFs mainly play a tumor-promoting role in hematological malignancies. Whether they possess certain tumor-suppressing subtypes or tumor-inhibitory biological functions remains to be further explored and demonstrated. In light of this, we have summarized the activation of CAFs and the main molecular pathways through which they promote the progression of hematological malignancies (Figure 3), and compared the different roles played by CAFs in solid tumors and hematological malignancies (Figure 4) to make the research situation of CAFs in tumors more visually clear.
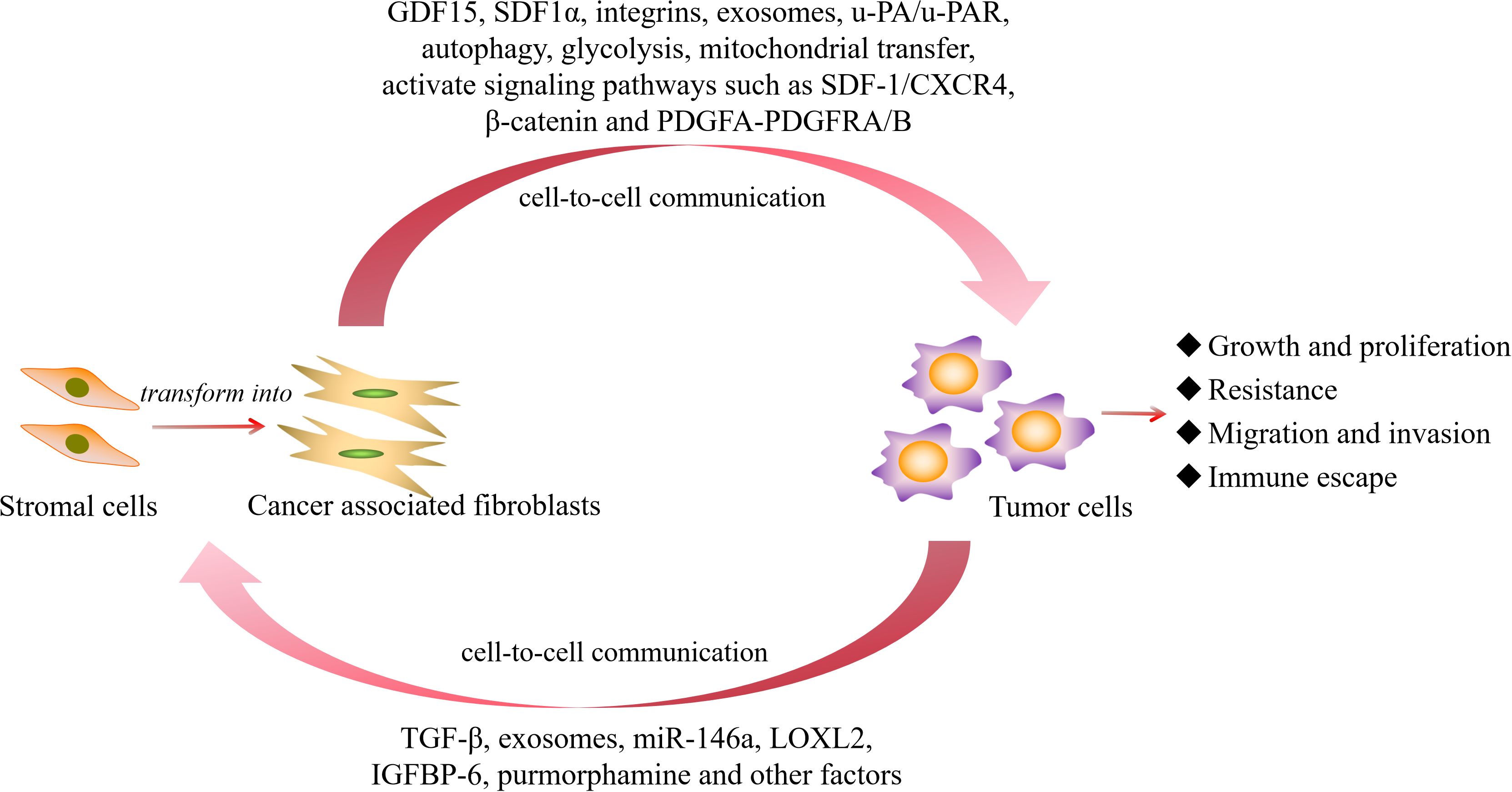
Figure 3. The activation of CAFs and the main molecular pathways that promote the progression of hematological malignancies. GDF15, growth differentiation factor 15; SDF1α, stromal cell-derived factor-1 α; u-PA/u-PAR, urokinase plasminogen activator/urokinase plasminogen activator receptor; PDGF, platelet-derived growth factor; TGF-β, transforming growth factor β; LOXL2, lysyl-oxidase-like 2; IGFBP-6, insulin like growth factor binding protein 6.
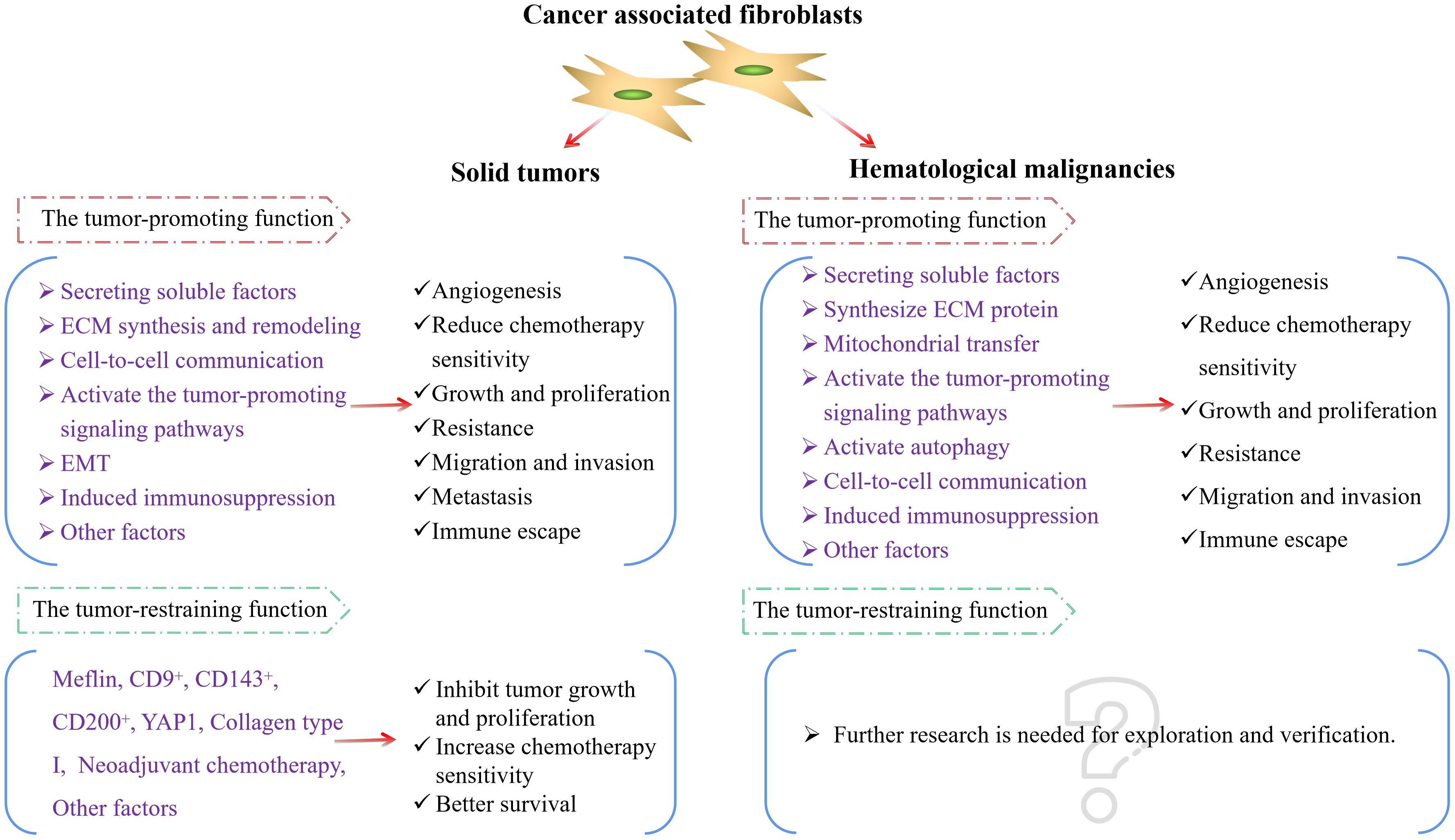
Figure 4. CAFs in solid tumors and hematological malignancies. ECM, extracellular matrix; EMT, epithelial-mesenchymal transition; YAP1, Yes-associated protein 1.
8 Strategies for targeting CAFs
For the therapeutic strategies targeting CAFs, many studies have conducted corresponding explorations. Through exploring the various related published research works, a series of studies and explorations are currently focused on inhibiting the poor prognostic markers of CAFs, inactivating CAFs, targeting the signaling pathway activated by CAFs and its downstream factors, or preventing the interaction between tumor cells and CAFs (42, 59, 175).
FAP is an important marker of CAFs. The study performed by Akai et al. (176) showed that near-infrared light immunotherapy (NIR-PIT) targeting FAP effectively induced CAFs-specific cell death without damaging adjacent normal cells. At the same time, the use of FAP inhibitor Talabostat (PT100) might also be one of the options to improve the efficacy of tumor treatment (177). The CXCR4/SDF-1 axis exerts an important role in coordinating tumor cells and CAFs (32). The use of CXCR4 inhibitor AMD3100 may be one of the strategies to reduce the interaction between CAFs and tumor cells and then improve therapeutic efficacy (139). Bao et al. ‘s (178) study revealed that the combination of FAP-targeted radiopharmaceutics [177Lu] Lu-DOTAGA.(SA.FAPi)2 and AMD3100 significantly inhibited cell proliferation, migration and colony formation in triple-negative breast cancer cells, and showed synergistic effects on the 4T1 tumor models, while reducing the number of bone marrow-derived suppressor cells. It is suggested that [177Lu] Lu-DOTAGA.(SA.FAPi)2 combined with AMD3100 may be an effective treatment for tumor. Ripretinib is a potent receptor tyrosine kinase inhibitor. Mori et al. (179) showed that in the presence of the chemotherapy drug carboplatin, ripretinib could prevent CAFs survival and inhibit the proliferation of ovarian clear cell carcinoma. The use of the flavonoids Oroxylin A (OA) can also inactivate CAFs and hinder the proliferation and invasion of tumor cells (180). Meanwhile, appropriate digoxin can inhibit the sub-population of tumor stem cells and the production of CAFs cytokines in the CAFs-tumor cell co-culture system, and digoxin combined with chemotherapy can improve the therapeutic effect of tumor (181). Low dose digoxin can inhibit the expression of TGF-β-induced CAFs marker fibronectin expression without producing adverse cytotoxicity (30). In addition, 1,25 (OH) 2D3 can inhibit the activation of CAFs in tumors (182). It is indicated that the combination of conventional chemotherapy and CAFs targeting drugs may be a potential strategy to enhance the therapeutic effect of tumor treatment. In addition, The use of dual targeting strategy to simultaneously target tumor cells and CAFs may be an effective treatment option (169, 183).
Moreover, multiple clinical trials have been conducted to explore the potential impact of intervention and targeting CAFs on the therapeutic efficacy of tumor treatment. Among them, the clinical trials of the therapy targeting CAFs marker FAP (NCT05442151, NCT05641896, NCT04621435, etc.) are currently underway. Some clinical experiments have been completed, but no research results have been posted (NCT05547321, NCT04857138, NCT05043714). As CXCR4/SDF-1 is a key signaling axis mediating the interaction between CAFs and tumor cells, several clinical studies targeting CXCR4 have also been carried out. A phase 2 clinical trial using CXCR4 antagonist BL-8040 and pembrolizumab in metastatic pancreatic patients (NCT02907099) showed that the application of BL-8040 and pembrolizumab could increase the quantity of T cell infiltration in tumor tissues, but was accompanied by 26.67% severe adverse events and 33.33% non-severe adverse events. In hematological malignancies, several clinical trials using CXCR4 antagonists or targeting CXCR4 have also been conducted, but some completed clinical trials (NCT01120457, NCT04274738, NCT01236144, NCT01010880) have not posted experimental results. A recent phase 1 clinical trial of CXCR4 modified B-cell maturation antigen CAR-T for relapsed/refractory MM is currently recruiting, but no preliminary results have been presented (NCT04727008). Moreover, several clinical trials using targeting downstream signaling pathways of CAFs (NCT01333475, NCT02392572) and intervention of their adverse prognostic secretory factors (TGF-β, PDGFR, VEGF, MMP, etc.) have also been conducted (NCT02423343, NCT02146222, NCT02202746, NCT00033215, NCT00001683), expecting to directly or indirectly consume CAFs, reduce or eliminate their tumor-promoting characteristics to improve the therapeutic efficacy of tumor treatment. However, the results of some clinical trials are not satisfactory and are accompanied by varying degrees of adverse events. At present, there are still some clinical trials underway, with the expectation that the therapeutic efficacy based on the intervention of CAFs will be further verified in more types of tumors.
Overall, although a number of basic and clinical trial studies on the treatment of CAFs have been carried out, due to the various subtypes and mechanisms of CAFs in different tumors, there is currently no unified treatment target. Additionally, although multiple clinical trials have been conducted, some results are not satisfactory and are accompanied by varying degrees of adverse events, which may be a challenge for current treatment. This requires further exploration and argumentation in future research.
9 The heterogeneity of CAFs needs attention
In the aforementioned content, we mentioned that CAFs can originate from various stromal cells in the TME, and can promote tumor progression through multiple mechanisms and signaling pathways. However, they also possess the biological function of inhibiting tumor progression. Simultaneously, CAFs may present different subtypes in different tumor or different stages of the tumor (43, 175, 184–186). These results reveal the heterogeneity of CAFs in the TME.
To explore the composition of bone marrow stromal cells, Baryawno et al. (187) performed single-cell RNA sequencing on non-hematopoietic bone marrow cells of C57Bl/6 mice and found 17 subtypes of stromal subsets, including fibroblast subpopulations consisting of 5 clusters. Fibroblasts-1 and 2 expressed progenitor cell markers CD34 and MSCs markers, but did not express endothelial and pericyte genes. Fibroblasts-3, 4, and 5 correlated with tendon/ligament cells. They jointly express Sox9 and TF Scleraxis. This indicates the existence of different subtypes of fibroblasts in the bone marrow microenvironment. In addition, numerous studies on single-cell sequencing analysis have revealed that CAFs can exhibit distinct subtypes, markers, and biological functions in tumors and different organs (44, 188–191). In recent years, an increasing number of CAFs subtypes have been discovered and defined, which makes CAFs more heterogeneous.
Meanwhile, CAF markers may exert differential biological functions in tumors, and undergo subtype changes during the tumor progression process. α-SMA is an important marker of CAFs, and studies have suggested that its high expression can promote tumor progression (33, 52). However, there were also study showing that depletion of α-SMA+ myofibroblasts leads to enhanced EMT and cancer stem cells, and low myofibroblasts in tumors is associated with poor survival (192). In the study of cervical cancer, recent research performed by Bueno-Urquiza et al. (46) showed that CAFs exhibited a myofibroblast like phenotype (CAF α-SMA+FAP+) in the early stages of cervical cancer, while in the late stages, they exhibited a primitive phenotype (CAF α-SMA- FAP+). In the study of pancreatic ductal adenocarcinoma, Tao et al. (193) identified NFs and nine different subtypes of CAFs, and found that the CAFs subtypes exhibited plasticity in differentiation, transitioning from early normal-like CAFs (nCAFs) to iCAFs and myCAFs, ultimately leading to more invasive proliferative CAFs (pCAFs). In addition, in breast cancer, Kashyap et al. (194) found that when CAFs conditioned medium from different subtypes of breast cancer and different breast cancer cell lines were cultured and treated with chemotherapy drugs, there exited differences in the abundance of CAFs secreted proteins in each group, suggesting that there were heterogeneous CAFs populations in the microenvironment of different cancer subtypes. In the study of intrahepatic cholangiocarcinoma, Hu et al. (195) showed that CAFs could be categorized into cancer suppressive or cancer promoting types (rCAFs or pCAFs). Among them, polycomb group ring finger 4 (PCGF4) promoted cell migration, drug resistance activity and stem cell characteristics. rCAFs triggered proteasome-dependent degradation of PCGF4, while pCAFs enhanced the stability of PCGF4 by activating the IL-6/p-STAT3 pathway.
Apart from the fact that different CAFs subtypes may lead to the heterogeneity of CAFs, the role that CAFs play in promoting or inhibiting tumor growth in different tumors is also one of the characteristics that reflect their heterogeneity. Just as we mentioned in the previous content. These findings suggest that CAFs from different tumor types or different CAFs sources may have variable typical markers and present different phenotypes and functions. Researchers need to focus on these issues in the study process to more accurately explore the possible role of CAFs in different tumors and contribute to finding potential treatment strategies.
10 Discussion and conclusion
TME is a complex and dynamic microenvironment that supports the survival and proliferation of tumor cells. The occurrence, development and metastasis of tumors are closely related to the internal and external environments in which tumor cells are located. This includes not only the structure, function and metabolism of the tissue where the tumor is located, but also the intrinsic environment surrounding the tumor cells. The interaction between the two enables TME to adapt to support the survival, growth and metastasis of tumors.
CAFs, as an important stromal cell component in the TME, play a significant role in the occurrence and development of tumors. In this review, we summarize and elaborate on the origin, markers, characteristics, and research progress and treatment strategies of CAFs in solid tumors and hematological malignancies, and emphasize the heterogeneity of CAFs. Based on literature reports, we found that the activation of CAFs in the TME can originate from multiple stromal cells and has various markers and biological subtypes. In the research of solid tumors, most studies have found that they play an important role in promoting tumor angiogenesis, growth, proliferation, and mediating tumor resistance, invasion, and immune escape. However, some studies have also reported that CAFs can have biological subtypes and functional characteristics that inhibit tumor progression. Recently, based on single-cell sequencing studies, more biological subtypes of CAFs have been revealed, and these different subtypes of CAFs further reveal the heterogeneity and diversity of the functions of CAFs. In the research of hematological malignancies, the possible roles of CAFs have been revealed in diseases such as AML, ALL, CLL, MM, lymphoma and MPN. Most of them have exerted tumor-promoting biological functions. Whether CAFs have more biological subtypes and whether they have the function of inhibiting tumor progression require further exploration and verification in the future.
Regarding the therapeutic strategies targeting CAFs, studies have achieved satisfactory results in basic research. However, in clinical trials, the intervention treatments targeting CAFs still face many issues that need to be addressed, including long-term efficacy, drug safety, and adverse events.
In conclusion, CAFs are not only considered to be a vital factor for promoting tumor progression and leading to tumor immune escape in solid tumors, but also a key component in promoting tumor progression in hematological malignancies. It interacts with tumor cells, secretes cytokines, promotes tumor EMT, promotes ECM remodeling, and directly interacts with tumor cells, thereby promoting tumor cell growth and proliferation, and mediating the process of tumor drug resistance and invasion. Blocking the interaction between CAFs and tumor cells may enhance the sensitivity of tumor cells to chemotherapeutic drugs, and reduce the proliferation and invasion of tumor cells.
Based on this, targeting CAFs might be an important therapeutic measure to improve TME and reduce tumor progression. However, during the research process, we need to pay attention to the heterogeneity of CAFs, as well as the inhibitory subtypes and functions of CAFs on tumors. Precisely targeting the tumor-promoting subtypes of CAFs or inhibiting CAFs activation and inhibiting the interaction between CAFs and tumor cells might be one of the effective options for improving tumor treatment strategies in the future. Overall, an increasing number of studies are still needed to demonstrate the therapeutic efficacy of targeted CAFs to accurately achieve the purpose of improving the long-term survival of tumors.
Author contributions
CP: Writing – original draft, Writing – review & editing. LZ: Formal analysis, Investigation, Supervision, Writing – review & editing. JW: Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by National Natural Science Foundation of China (82060026 and 82370168), Science and Technology Projects in Guizhou Province (Qian Ke He Foundation-ZK(2024) general 223) and the Doctoral research start-up Fund of Affiliated Hospital of Guizhou Medical University (gyfybsky-2024-35).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bussard KM, Mutkus L, Stumpf K, Gomez-Manzano C, and Marini FC. Tumor-associated stromal cells as key contributors to the tumor microenvironment. Breast Cancer research: BCR. (2016) 18:84. doi: 10.1186/s13058-016-0740-2
2. Oya Y, Hayakawa Y, and Koike K. Tumor microenvironment in gastric cancers. Cancer Sci. (2020) 111:2696–707. doi: 10.1111/cas.14521
3. Siemann DW and Siemann DW. Tumor microenvironment. Chichester, West Sussex: Wiley-Blackwell (2011).
4. Xiao Y and Yu D. Tumor microenvironment as a therapeutic target in cancer. Pharmacol Ther. (2021) 221:107753. doi: 10.1016/j.pharmthera.2020.107753
5. Anderson NM and Simon MC. The tumor microenvironment. Curr biology: CB. (2020) 30:R921–5. doi: 10.1016/j.cub.2020.06.081
6. Bejarano L, Jordāo M, and Joyce JA. Therapeutic targeting of the tumor microenvironment. Cancer Discov. (2021) 11:933–59. doi: 10.1158/2159-8290.CD-20-1808
7. Thakkar S, Sharma D, Kalia K, and Tekade RK. Tumor microenvironment targeted nanotherapeutics for cancer therapy and diagnosis: a review. Acta Biomater. (2020) 101:43–68. doi: 10.1016/j.actbio.2019.09.009
8. Fang B, Lu Y, Li X, Wei Y, Ye D, Wei G, et al. Targeting the tumor microenvironment, a new therapeutic approach for prostate cancer. Prostate Cancer Prostatic Dis. (2024) 28:260–9. doi: 10.1038/s41391-024-00825-z
9. Kumari S, Advani D, Sharma S, Ambasta RK, and Kumar P. Combinatorial therapy in tumor microenvironment: where do we stand? Biochimica et biophysica acta. Rev cancer. (2021) 1876:188585. doi: 10.1016/j.bbcan.2021.188585
10. Shafat MS, Gnaneswaran B, Bowles KM, and Rushworth SA. The bone marrow microenvironment – home of the leukemic blasts. Blood Rev. (2017) 31:277–86. doi: 10.1016/j.blre.2017.03.004
11. Chiarini F, Lonetti A, Evangelisti C, Buontempo F, Orsini E, Evangelisti C, et al. Advances in understanding the acute lymphoblastic leukemia bone marrow microenvironment: from biology to therapeutic targeting. Biochim Biophys Acta (BBA) - Mol Cell Res. (2016) 1863:449–63. doi: 10.1016/j.bbamcr.2015.08.015
12. Zhou J, Mauerer K, Farina L, and Gribben JG. The role of the tumor microenvironment in hematological Malignancies and implication for therapy. Front Bioscience A J Virtual Library. (2005) 10:1581–96. doi: 10.2741/1642
13. Oleynikova NA, Danilova NV, Mikhailov IA, Semina EV, and Malkov PG. Cancer-associated fibroblasts and their significance in tumor progression. Arkhiv patologii. (2020) 82:68–77. doi: 10.17116/patol20208201168
14. Bonollo F, Thalmann GN, Kruithof-de Julio M, and Karkampouna S. The role of cancer-associated fibroblasts in prostate cancer tumorigenesis. Cancers (Basel). (2020) 12:1887. doi: 10.3390/cancers12071887
15. Joshi RS, Kanugula SS, Sudhir S, Pereira MP, Jain S, and Aghi MK. The role of cancer-associated fibroblasts in tumor progression. Cancers (Basel). (2021) 13:1399. doi: 10.3390/cancers13061399
16. Ozmen E, Demir TD, and Ozcan G. Cancer-associated fibroblasts: protagonists of the tumor microenvironment in gastric cancer. Front Mol Biosci. (2024) 11:1340124. doi: 10.3389/fmolb.2024.1340124
17. Dzobo K, Senthebane DA, and Dandara C. The tumor microenvironment in tumorigenesis and therapy resistance revisited. Cancers (Basel). (2023) 15:376. doi: 10.3390/cancers15020376
18. Jung JG and Le A. Targeting metabolic cross talk between cancer cells and cancer-associated fibroblasts. Adv Exp Med Biol. (2021) 1311:205–14. doi: 10.1007/978-3-030-65768-0_15
19. Errarte P, Larrinaga G, and López JI. The role of cancer-associated fibroblasts in renal cell carcinoma. An example of tumor modulation through tumor/non-tumor cell interactions. J Adv Res. (2020) 21:103–8. doi: 10.1016/j.jare.2019.09.004
20. Alexander J and Cukierman E. Cancer associated fibroblast: mediators of tumorigenesis. Matrix biology: J Int Soc Matrix Biol. (2020) 91–92:19–34. doi: 10.1016/j.matbio.2020.05.004
21. Linxweiler J, Hajili T, Körbel C, Berchem C, Zeuschner P, Müller A, et al. Cancer-associated fibroblasts stimulate primary tumor growth and metastatic spread in an orthotopic prostate cancer xenograft model. Sci Rep. (2020) 10:1–13. doi: 10.1038/s41598-020-69424-x
22. Meng Q, Luo X, Chen J, Wang D, Chen E, Zhang W, et al. Unmasking carcinoma-associated fibroblasts: key transformation player within the tumor microenvironment. Biochim Biophys Acta Rev cancer. (2020) 1874:188443. doi: 10.1016/j.bbcan.2020.188443
23. Pape J, Magdeldin T, Stamati K, Nyga A, Loizidou M, Emberton M, et al. Cancer-associated fibroblasts mediate cancer progression and remodel the tumouroid stroma. Br J Cancer. (2020) 123:1178–90. doi: 10.1038/s41416-020-0973-9
24. Knuutila JS, Riihilä P, Nissinen L, Heiskanen L, Kallionpää RE, Pellinen T, et al. Cancer-associated fibroblast activation predicts progression, metastasis, and prognosis of cutaneous squamous cell carcinoma. Int J Cancer. (2024) 155:1112–27. doi: 10.1002/ijc.34957
25. Liu Q, Yao F, Wu L, Xu T, Na J, Shen Z, et al. Heterogeneity and interplay: the multifaceted role of cancer-associated fibroblasts in the tumor and therapeutic strategies. Clin Transl Oncol. (2024) 26:2395–417. doi: 10.1007/s12094-024-03492-7
26. Li Z, Sun C, and Qin Z. Metabolic reprogramming of cancer-associated fibroblasts and its effect on cancer cell reprogramming. Theranostics. (2021) 11:8322–36. doi: 10.7150/thno.62378
27. Guo T and Xu J. Cancer-associated fibroblasts: a versatile mediator in tumor progression, metastasis, and targeted therapy. Cancer Metastasis Rev. (2024) 43:1095–116:. doi: 10.1007/s10555-024-10186-7
28. Gu L, Liao P, and Liu H. Cancer-associated fibroblasts in acute leukemia. Front Oncol. (2022) 12:1022979. doi: 10.3389/fonc.2022.1022979
29. Ding Z, Shi R, Hu W, Tian L, Sun R, Wu Y, et al. Cancer-associated fibroblasts in hematologic Malignancies: elucidating roles and spotlighting therapeutic targets. Front Oncol. (2023) 13:1193978. doi: 10.3389/fonc.2023.1193978
30. Coleman DT, Gray AL, Stephens CA, Scott ML, and Cardelli JA. Repurposed drug screen identifies cardiac glycosides as inhibitors of tgf-beta-induced cancer-associated fibroblast differentiation. Oncotarget. (2016) 7:32200–9. doi: 10.18632/oncotarget.8609
31. Alshehri JH, Al-Nasrallah HK, Al-Ansari MM, and Aboussekhra A. Activation of mammary epithelial and stromal fibroblasts upon exposure to escherichia coli metabolites. Cells. (2024) 13:1723. doi: 10.3390/cells13201723
32. Teng F, Tian W, Wang Y, Zhang Y, Guo F, Zhao J, et al. Cancer-associated fibroblasts promote the progression of endometrial cancer via the sdf-1/cxcr4 axis. J Hematol Oncol. (2016) 9:8. doi: 10.1186/s13045-015-0231-4
33. Quante M, Tu SP, Tomita H, Gonda T, Wang SSW, Takashi S, et al. Bone marrow-derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer Cell. (2011) 19:257–72. doi: 10.1016/j.ccr.2011.01.020
34. Mishra PJ, Mishra PJ, Humeniuk R, Medina DJ, Alexe G, Mesirov JP, et al. Carcinoma-associated fibroblast-like differentiation of human mesenchymal stem cells. Cancer Res. (2008) 68:4331–9. doi: 10.1158/0008-5472.CAN-08-0943
35. Hematti P. Mesenchymal stromal cells and fibroblasts: a case of mistaken identity? Cytotherapy. (2012) 14:516–21. doi: 10.3109/14653249.2012.677822
36. Paunescu V, Bojin FM, Tatu CA, Gavriliuc OI, Rosca A, Gruia AT, et al. Tumour-associated fibroblasts and mesenchymal stem cells: more similarities than differences. J Cell Mol Med. (2011) 15:635–46. doi: 10.1111/j.1582-4934.2010.01044.x
37. Sato S, Hiruma T, Koizumi M, Yoshihara M, Nakamura Y, Tadokoro H, et al. Bone marrow adipocytes induce cancer-associated fibroblasts and immune evasion, enhancing invasion and drug resistance. Cancer Sci. (2023) 114:2674–88. doi: 10.1111/cas.15786
38. Iyoshi S, Yoshihara M, Nakamura K, Sugiyama M, Koya Y, Kitami K, et al. Pro-tumoral behavior of omental adipocyte-derived fibroblasts in tumor microenvironment at the metastatic site of ovarian cancer. Int J Cancer. (2021) 149:1961–72. doi: 10.1002/ijc.33770
39. Simiczyjew A, Wądzyńska J, Pietraszek-Gremplewicz K, Kot M, Ziętek M, Matkowski R, et al. Melanoma cells induce dedifferentiation and metabolic changes in adipocytes present in the tumor niche. Cell Mol Biol Lett. (2023) 28:58. doi: 10.1186/s11658-023-00476-3
40. Andreucci E, Fioretto BS, Rosa I, Matucci-Cerinic M, Biagioni A, Romano E, et al. Extracellular lactic acidosis of the tumor microenvironment drives adipocyte-to-myofibroblast transition fueling the generation of cancer-associated fibroblasts. Cells. (2023) 12:939. doi: 10.3390/cells12060939
41. Hamabe-Horiike T, Harada SI, Yoshida K, Kinoshita J, Yamaguchi T, and Fushida S. Adipocytes contribute to tumor progression and invasion of peritoneal metastasis by interacting with gastric cancer cells as cancer associated fibroblasts. Cancer Rep (Hoboken). (2023) 6:e1647. doi: 10.1002/cnr2.1647
42. Dzobo K and Dandara C. Architecture of cancer-associated fibroblasts in tumor microenvironment: mapping their origins, heterogeneity, and role in cancer therapy resistance. OMICS. (2020) 24:314–39. doi: 10.1089/omi.2020.0023
43. Biffi G and Tuveson DA. Diversity and biology of cancer-associated fibroblasts. Physiol Rev. (2021) 101:147–76. doi: 10.1152/physrev.00048.2019
44. Lavie D, Ben-Shmuel A, Erez N, and Scherz-Shouval R. Cancer-associated fibroblasts in the single-cell era. Nat Cancer. (2022) 3:793–807. doi: 10.1038/s43018-022-00411-z
45. Mathieson L, Koppensteiner L, Dorward DA, O’Connor RA, and Akram AR. Cancer-associated fibroblasts expressing fibroblast activation protein and podoplanin in non-small cell lung cancer predict poor clinical outcome. Br J Cancer. (2024) 130:1758–69. doi: 10.1038/s41416-024-02671-1
46. Bueno-Urquiza LJ, Godínez-Rubí M, Villegas-Pineda JC, Vega-Magaña AN, Jave-Suárez LF, Puebla-Mora AG, et al. Phenotypic heterogeneity of cancer associated fibroblasts in cervical cancer progression: fap as a central activation marker. Cells. (2024) 13:560. doi: 10.3390/cells13070560
47. Huang M, Fu M, Wang J, Xia C, Zhang H, Xiong Y, et al. TGF-β1-activated cancer-associated fibroblasts promote breast cancer invasion, metastasis and epithelial-mesenchymal transition by autophagy or overexpression of fap-α. Biochem Pharmacol. (2021) 188:114527. doi: 10.1016/j.bcp.2021.114527
48. Jia J, Martin T, Ye L, Meng L, Xia N, Jiang W, et al. Fibroblast activation protein-α promotes the growth and migration of lung cancer cells via the pi3k and sonic hedgehog pathways. Int J Mol Med. (2017) 41:275–83. doi: 10.3892/ijmm.2017.3224
49. Sandberg TP, Stuart MPME, Oosting J, Tollenaar RAEM, Sier CFM, and Mesker WE. Increased expression of cancer-associated fibroblast markers at the invasive front and its association with tumor-stroma ratio in colorectal cancer. BMC Cancer. (2019) 19:284. doi: 10.1186/s12885-019-5462-2
50. Solano-Iturri JD, Beitia M, Errarte P, Calvete-Candenas J, Etxezarraga MC, Loizate A, et al. Altered expression of fibroblast activation protein-α (FAP) in colorectal adenoma-carcinoma sequence and in lymph node and liver metastases. Aging. (2020) 12:10337–58. doi: 10.18632/aging.103261
51. Fitzgerald AA and Weiner LM. The role of fibroblast activation protein in health and Malignancy. Cancer Metastasis Rev. (2020) 39:783–803. doi: 10.1007/s10555-020-09909-3
52. Zhan S, Liu Z, Zhang M, Guo T, Quan Q, Huang L, et al. Overexpression of b7-h3 in α-sma-positive fibroblasts is associated with cancer progression and survival in gastric adenocarcinomas. Front Oncol. (2019) 9:1466. doi: 10.3389/fonc.2019.01466
53. Dzobo K and Dandara C. Broadening drug design and targets to tumor microenvironment? Cancer-associated fibroblast marker expression in cancers and relevance for survival outcomes. OMICS. (2020) 24:340–51. doi: 10.1089/omi.2020.0042
54. öhlund D, Handly-Santana A, Biffi G, Elyada E, Almeida AS, Ponz-Sarvise M, et al. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J Exp Med. (2017) 214:579–96. doi: 10.1084/jem.20162024
55. Huang H, Wang Z, Zhang Y, Pradhan RN, Ganguly D, Chandra R, et al. Mesothelial cell-derived antigen-presenting cancer-associated fibroblasts induce expansion of regulatory t cells in pancreatic cancer. Cancer Cell. (2022) 40:656–73. doi: 10.1016/j.ccell.2022.04.011
56. Elyada E, Bolisetty M, Laise P, Flynn WF, Courtois ET, Burkhart RA, et al. Cross-species single-cell analysis of pancreatic ductal adenocarcinoma reveals antigen-presenting cancer-associated fibroblasts. Cancer Discov. (2019) 9:1102–23. doi: 10.1158/2159-8290.CD-19-0094
57. Cords L, de Souza N, and Bodenmiller B. Classifying cancer-associated fibroblasts-the good, the bad, and the target. Cancer Cell. (2024) 42:1480–5. doi: 10.1016/j.ccell.2024.08.011
58. Xiao Y, Wang Z, Gu M, Wei P, Wang X, and Li W. Cancer-associated fibroblasts: heterogeneity and their role in the tumor immune response. Clin Exp Med. (2024) 24:126. doi: 10.1007/s10238-024-01375-3
59. Zhang H, Yue X, Chen Z, Liu C, Wu W, Zhang N, et al. Define cancer-associated fibroblasts (cafs) in the tumor microenvironment: new opportunities in cancer immunotherapy and advances in clinical trials. Mol Cancer. (2023) 22:159. doi: 10.1186/s12943-023-01860-5
60. Sahai E, Astsaturov I, Cukierman E, Denardo DG, Egeblad M, Evans RM, et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat Rev Cancer. (2020) 20:174–86. doi: 10.1038/s41568-019-0238-1
61. De Boeck A, Hendrix A, Maynard D, Van Bockstal M, Daniëls A, Pauwels P, et al. Differential secretome analysis of cancer-associated fibroblasts and bone marrow-derived precursors to identify microenvironmental regulators of colon cancer progression. Proteomics. (2013) 13:379–88. doi: 10.1002/pmic.201200179
62. Tan H, Gong W, Zhou K, Xiao Z, Hou F, Huang T, et al. Cxcr4/tgf-β1 mediated hepatic stellate cells differentiation into carcinoma-associated fibroblasts and promoted liver metastasis of colon cancer. Cancer Biol Ther. (2020) 21:258–68. doi: 10.1080/15384047.2019.1685157
63. Caja L, Dituri F, Mancarella S, Caballero-Diaz D, Moustakas A, Giannelli G, et al. Tgf-β and the tissue microenvironment: relevance in fibrosis and cancer. Int J Mol Sci. (2018) 19:1294. doi: 10.3390/ijms19051294
64. Yegodayev KM, Novoplansky O, Golden A, Prasad M, Levin L, Jagadeeshan S, et al. Tgf-beta-activated cancer-associated fibroblasts limit cetuximab efficacy in preclinical models of head and neck cancer. Cancers (Basel). (2020) 12:339. doi: 10.3390/cancers12020339
65. Tan HX, Cao ZB, He TT, Huang T, Xiang CL, and Liu Y. Tgfβ1 is essential for mscs-cafs differentiation and promotes hct116 cells migration and invasion via jak/stat3 signaling. Onco Targets Ther. (2019) 12:5323–34. doi: 10.2147/OTT.S178618
66. Weber CE, Kothari AN, Wai PY, Li NY, Driver J, Zapf MA, et al. Osteopontin mediates an mzf1-tgf-β1-dependent transformation of mesenchymal stem cells into cancer-associated fibroblasts in breast cancer. Oncogene. (2015) 34:4821–33. doi: 10.1038/onc.2014.410
67. Zhang Q, Wang M, Huang F, Yang T, Cai J, Zhang X, et al. H. Pylori infection-induced msc differentiation into cafs promotes epithelial-mesenchymal transition in gastric epithelial cells. Int J Mol Med. (2013) 32:1465–73. doi: 10.3892/ijmm.2013.1532
68. Zhu H, Guo S, Zhang Y, Yin J, Yin W, Tao S, et al. Proton-sensing gpcr-yap signalling promotes cancer-associated fibroblast activation of mesenchymal stem cells. Int J Biol Sci. (2016) 12:389–96. doi: 10.7150/ijbs.13688
69. Dong T, Yang D, Li R, Zhang L, Zhao H, Shen Y, et al. Pgrn promotes migration and invasion of epithelial ovarian cancer cells through an epithelial mesenchymal transition program and the activation of cancer associated fibroblasts. Exp Mol Pathol. (2016) 100:17–25. doi: 10.1016/j.yexmp.2015.11.021
70. Zhang L, Chen W, Li X, Wang G, Xing F, and Zhu X. Galectin-1 overexpression induces normal fibroblasts translate into cancer-associated fibroblasts and attenuates the sensitivity of anlotinib in lung cancer. Cell Adh Migr. (2024) 18:1–11. doi: 10.1080/19336918.2024.2335881
71. Ben Baruch B, Mantsur E, Franco-Barraza J, Blacher E, Cukierman E, and Stein R. Cd38 in cancer-associated fibroblasts promotes pro-tumoral activity. Lab investigation; J Tech Methods pathology. (2020) 100:1517–31. doi: 10.1038/s41374-020-0458-8
72. Ando N, Hara M, Shiga K, Yanagita T, Takasu K, Nakai N, et al. Eicosapentaenoic acid suppresses angiogenesis via reducing secretion of il−6 and vegf from colon cancer−associated fibroblasts. Oncol Rep. (2019) 42:339–49. doi: 10.3892/or.2019.7141
73. Huang B, Huang M, and Li Q. Cancer-associated fibroblasts promote angiogenesis of hepatocellular carcinoma by vegf-mediated ezh2/vash1 pathway. Technol Cancer Res Treat. (2019) 18:1078147553. doi: 10.1177/1533033819879905
74. Yu D, Xu H, Zhou J, Fang K, Zhao Z, and Xu K. Pdpn/ccl2/stat3 feedback loop alter caf heterogeneity to promote angiogenesis in colorectal cancer. Angiogenesis. (2024) 27:809–25. doi: 10.1007/s10456-024-09941-9
75. Tang D, Gao J, Wang S, Ye N, Chong Y, Huang Y, et al. Cancer-associated fibroblasts promote angiogenesis in gastric cancer through galectin-1 expression. Tumour Biol. (2016) 37:1889–99. doi: 10.1007/s13277-015-3942-9
76. Liu B, Zhang B, Qi J, Zhou H, Tan L, Huang J, et al. Targeting mfge8 secreted by cancer-associated fibroblasts blocks angiogenesis and metastasis in esophageal squamous cell carcinoma. Proc Natl Acad Sci U.S.A. (2023) 120:e1987053176. doi: 10.1073/pnas.2307914120
77. Unterleuthner D, Neuhold P, Schwarz K, Janker L, Neuditschko B, Nivarthi H, et al. Cancer-associated fibroblast-derived wnt2 increases tumor angiogenesis in colon cancer. Angiogenesis. (2020) 23:159–77. doi: 10.1007/s10456-019-09688-8
78. Wan X, Guan S, Hou Y, Qin Y, Zeng H, Yang L, et al. Fosl2 promotes vegf-independent angiogenesis by transcriptionnally activating wnt5a in breast cancer-associated fibroblasts. Theranostics. (2021) 11:4975–91. doi: 10.7150/thno.55074
79. Shi Y, Zhu H, Jiang H, Yue H, Yuan F, and Wang F. Cancer-associated fibroblasts-derived exosomes from chemoresistant patients regulate cisplatin resistance and angiogenesis by delivering vegfa in colorectal cancer. Anticancer Drugs. (2023) 34:422–30. doi: 10.1097/CAD.0000000000001445
80. Miaomiao S, Xiaoqian W, Yuwei S, Chao C, Chenbo Y, Yinghao L, et al. Cancer-associated fibroblast-derived exosome microrna-21 promotes angiogenesis in multiple myeloma. Sci Rep. (2023) 13:9671. doi: 10.1038/s41598-023-36092-6
81. Dai X, Xie Y, and Dong M. Cancer-associated fibroblasts derived extracellular vesicles promote angiogenesis of colorectal adenocarcinoma cells through mir-135b-5p/foxo1 axis. Cancer Biol Ther. (2022) 23:76–88. doi: 10.1080/15384047.2021.2017222
82. Wang X, Sun X, Mu L, and Chen W. Cancer-associated fibroblasts induce epithelial-mesenchymal transition in endometrial cancer cells by regulating pituitary tumor transforming gene. Cancer Invest. (2019) 37:1–10. doi: 10.1080/07357907.2019.1575969
83. Xuefeng X, Hou M, Yang Z, Agudamu A, Wang F, Su X, et al. Epithelial–mesenchymal transition and metastasis of colon cancer cells induced by the fak pathway in cancer-associated fibroblasts. J Int Med Res. (2020) 48:1410563268. doi: 10.1177/0300060520931242
84. Ding X, Ji J, Jiang J, Cai Q, Wang C, Shi M, et al. Hgf-mediated crosstalk between cancer-associated fibroblasts and met-unamplified gastric cancer cells activates coordinated tumorigenesis and metastasis. Cell Death Dis. (2018) 9:867. doi: 10.1038/s41419-018-0922-1
85. Lappano R, Talia M, Cirillo F, Rigiracciolo DC, Scordamaglia D, Guzzi R, et al. The il1β-il1r signaling is involved in the stimulatory effects triggered by hypoxia in breast cancer cells and cancer-associated fibroblasts (cafs). J Exp Clin Cancer Res. (2020) 39:153. doi: 10.1186/s13046-020-01667-y
86. Yang F, Guo Z, He C, Qing L, Wang H, Wu J, et al. Cancer-associated fibroblasts promote cell proliferation and invasion via paracrine wnt/il1β signaling pathway in human bladder cancer. Neoplasma. (2020) 68:79–86. doi: 10.4149/neo_2020_200202N101
87. Zhang X and Hwang YS. Cancer-associated fibroblast stimulates cancer cell invasion in an interleukin-1 receptor (il-1r)-dependent manner. Oncol Lett. (2019) 18:4645–50. doi: 10.3892/ol.2019.10784
88. Zhang Y, Cong X, Li Z, and Xue Y. Estrogen facilitates gastric cancer cell proliferation and invasion through promoting the secretion of interleukin-6 by cancer-associated fibroblasts. Int Immunopharmacol. (2020) 78:105937. doi: 10.1016/j.intimp.2019.105937
89. Wang X, Che X, Liu C, Fan Y, Bai M, Hou K, et al. Cancer-associated fibroblasts-stimulated interleukin-11 promotes metastasis of gastric cancer cells mediated by upregulation of muc1. Exp Cell Res. (2018) 368:184–93. doi: 10.1016/j.yexcr.2018.04.028
90. Zhang J, Li S, Zhao Y, Ma P, Cao Y, Liu C, et al. Cancer-associated fibroblasts promote the migration and invasion of gastric cancer cells via activating il-17a/jak2/stat3 signaling. Ann Transl Med. (2020) 8:877. doi: 10.21037/atm-20-4843
91. Li H, Zhang Q, Wu Q, Cui Y, Zhu H, Fang M, et al. Interleukin-22 secreted by cancer-associated fibroblasts regulates the proliferation and metastasis of lung cancer cells via the pi3k-akt-mtor signaling pathway. Am J Transl Res. (2019) 11:4077–88.
92. Ohata Y, Tsuchiya M, Hirai H, Yamaguchi S, Akashi T, Sakamoto K, et al. Leukemia inhibitory factor produced by fibroblasts within tumor stroma participates in invasion of oral squamous cell carcinoma. PloS One. (2018) 13:e191865. doi: 10.1371/journal.pone.0191865
93. Jung Y, Kim JK, Shiozawa Y, Wang J, Mishra A, Joseph J, et al. Recruitment of mesenchymal stem cells into prostate tumours promotes metastasis. Nat Commun. (2013) 4:1795. doi: 10.1038/ncomms2766
94. Zhou Z, Zhou Q, Wu X, Xu S, Hu X, Tao X, et al. Vcam-1 secreted from cancer-associated fibroblasts enhances the growth and invasion of lung cancer cells through akt and mapk signaling. Cancer Lett. (2020) 473:62–73. doi: 10.1016/j.canlet.2019.12.039
95. Shen J, Zhai J, You Q, Zhang G, He M, Yao X, et al. Cancer-associated fibroblasts-derived vcam1 induced by h. Pylori infection facilitates tumor invasion in gastric cancer. Oncogene. (2020) 39:2961–74. doi: 10.1038/s41388-020-1197-4
96. Fan Y and Yu Y. Cancer-associated fibroblasts-derived exosomal mettl3 promotes the proliferation, invasion, stemness and glutaminolysis in non-small cell lung cancer cells by eliciting slc7a5 m6a modification. Hum Cell. (2024) 37:1120–31. doi: 10.1007/s13577-024-01056-z
97. Lee S, Hong JH, Kim JS, Yoon JS, Chun SH, Hong SA, et al. Cancer-associated fibroblasts activated by mir-196a promote the migration and invasion of lung cancer cells. Cancer Lett. (2021) 508:92–103. doi: 10.1016/j.canlet.2021.03.021
98. Yang F, Ning Z, Ma L, Liu W, Shao C, Shu Y, et al. Exosomal mirnas and mirna dysregulation in cancer-associated fibroblasts. Mol Cancer. (2017) 16:148. doi: 10.1186/s12943-017-0718-4
99. Yan L, Wang P, Fang W, and Liang C. Cancer-associated fibroblasts-derived exosomes-mediated transfer of linc00355 regulates bladder cancer cell proliferation and invasion. Cell Biochem Funct. (2020) 38:257–65. doi: 10.1002/cbf.3462
100. Sun LP, Xu K, Cui J, Yuan DY, Zou B, Li J, et al. Cancer−associated fibroblast−derived exosomal mir−382−5p promotes the migration and invasion of oral squamous cell carcinoma. Oncol Rep. (2019) 42:1319–28. doi: 10.3892/or.2019.7255
101. Hu JL, Wang W, Lan XL, Zeng ZC, Liang YS, Yan YR, et al. Cafs secreted exosomes promote metastasis and chemotherapy resistance by enhancing cell stemness and epithelial-mesenchymal transition in colorectal cancer. Mol Cancer. (2019) 18:91. doi: 10.1186/s12943-019-1019-x
102. Ghofrani-Shahpar M, Pakravan K, Razmara E, Amooie F, Mahmoudian M, Heshmati M, et al. Cancer-associated fibroblasts drive colorectal cancer cell progression through exosomal mir-20a-5p-mediated targeting of pten and stimulating interleukin-6 production. BMC Cancer. (2024) 24:400. doi: 10.1186/s12885-024-12190-0
103. Erdogan B, Ao M, White LM, Means AL, Brewer BM, Yang L, et al. Cancer-associated fibroblasts promote directional cancer cell migration by aligning fibronectin. J Cell Biol. (2017) 216:3799–816. doi: 10.1083/jcb.201704053
104. Miyazaki K, Togo S, Okamoto R, Idiris A, Kumagai H, and Miyagi Y. Collective cancer cell invasion in contact with fibroblasts through integrin-α5β1/fibronectin interaction in collagen matrix. Cancer Sci. (2020) 111:4381–92. doi: 10.1111/cas.14664
105. Song J, Liao H, Li H, Chen H, Si H, Wang J, et al. Identification of a novel cancer-associated fibroblasts gene signature based on bioinformatics analysis to predict prognosis and therapeutic responses in breast cancer. Heliyon. (2024) 10:e29216. doi: 10.1016/j.heliyon.2024.e29216
106. Cheteh EH, Sarne V, Ceder S, Bianchi J, Augsten M, Rundqvist H, et al. Interleukin-6 derived from cancer-associated fibroblasts attenuates the p53 response to doxorubicin in prostate cancer cells. Cell Death Discov. (2020) 6:42. doi: 10.1038/s41420-020-0272-5
107. Louault K, Bonneaud TL, Séveno C, Gomez-Bougie P, Nguyen F, Gautier F, et al. Interactions between cancer-associated fibroblasts and tumor cells promote mcl-1 dependency in estrogen receptor-positive breast cancers. Oncogene. (2019) 38:3261–73. doi: 10.1038/s41388-018-0635-z
108. Wei L, Ye H, Li G, Lu Y, Zhou Q, Zheng S, et al. Cancer-associated fibroblasts promote progression and gemcitabine resistance via the sdf-1/satb-1 pathway in pancreatic cancer. Cell Death Dis. (2018) 9:1065. doi: 10.1038/s41419-018-1104-x
109. Xiong Z, Yu SL, Xie ZX, Zhuang RL, Peng SR, Wang Q, et al. Cancer-associated fibroblasts promote enzalutamide resistance and pd-l1 expression in prostate cancer through ccl5-ccr5 paracrine axis. iScience. (2024) 27:109674. doi: 10.1016/j.isci.2024.109674
110. Liu F, Tang L, Liu H, Chen Y, Xiao T, Gu W, et al. Cancer-associated fibroblasts secrete fgf5 to inhibit ferroptosis to decrease cisplatin sensitivity in nasopharyngeal carcinoma through binding to fgfr2. Cell Death Dis. (2024) 15:279. doi: 10.1038/s41419-024-06671-0
111. Che Y, Wang J, Li Y, Lu Z, Huang J, Sun S, et al. Cisplatin-activated pai-1 secretion in the cancer-associated fibroblasts with paracrine effects promoting esophageal squamous cell carcinoma progression and causing chemoresistance. Cell Death Dis. (2018) 9:759. doi: 10.1038/s41419-018-0808-2
112. Cheteh EH, Augsten M, Rundqvist H, Bianchi J, Sarne V, Egevad L, et al. Human cancer-associated fibroblasts enhance glutathione levels and antagonize drug-induced prostate cancer cell death. Cell Death Dis. (2017) 8:e2848. doi: 10.1038/cddis.2017.225
113. Pacheco-Torres J, Sharma RK, Mironchik Y, Wildes F, Brennen WN, Artemov D, et al. Prostate fibroblasts and prostate cancer associated fibroblasts exhibit different metabolic, matrix degradation and pd-l1 expression responses to hypoxia. Front Mol Biosci. (2024) 11:1354076. doi: 10.3389/fmolb.2024.1354076
114. Barrett R and Puré E. Cancer-associated fibroblasts: key determinants of tumor immunity and immunotherapy. Curr Opin Immunol. (2020) 64:80–7. doi: 10.1016/j.coi.2020.03.004
115. Hilmi M, Nicolle R, Bousquet C, and Neuzillet C. Cancer-associated fibroblasts: accomplices in the tumor immune evasion. Cancers (Basel). (2020) 12:2969. doi: 10.3390/cancers12102969
116. Cai H, Lin Y, Wu Y, Wang Y, Li S, Zhang Y, et al. The prognostic model and immune landscape based on cancer-associated fibroblast features for patients with locally advanced rectal cancer. Heliyon. (2024) 10:e28673. doi: 10.1016/j.heliyon.2024.e28673
117. Chen Q, Wang X, Zheng Y, Ye T, Liu J, Wang JQ, et al. Cancer-associated fibroblasts contribute to the immunosuppressive landscape and influence the efficacy of the combination therapy of pd-1 inhibitors and antiangiogenic agents in hepatocellular carcinoma. Cancer. (2023) 129:3405–16. doi: 10.1002/cncr.34935
118. Mao X, Xu J, Wang W, Liang C, Hua J, Liu J, et al. Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: new findings and future perspectives. Mol Cancer. (2021) 20:131. doi: 10.1186/s12943-021-01428-1
119. Yu L, Liu Q, Huo J, Wei F, and Guo W. Cancer-associated fibroblasts induce immunotherapy resistance in hepatocellular carcinoma animal model. Cell Mol Biol (Noisy-le-Grand France). (2020) 66:36–40. doi: 10.14715/cmb/2020.66.2.5
120. Zhang T, Li X, He Y, Wang Y, Shen J, Wang S, et al. Cancer-associated fibroblasts-derived hapln1 promotes tumour invasion through extracellular matrix remodeling in gastric cancer. Gastric cancer: Off J Int Gastric Cancer Assoc Japanese Gastric Cancer Assoc. (2022) 25:346–59. doi: 10.1007/s10120-021-01259-5
121. Du X, Xu Q, Pan D, Xu D, Niu B, Hong W, et al. Hic-5 in cancer-associated fibroblasts contributes to esophageal squamous cell carcinoma progression. Cell Death Dis. (2019) 10:873. doi: 10.1038/s41419-019-2114-z
122. Neri S, Hashimoto H, Kii H, Watanabe H, Masutomi K, Kuwata T, et al. Cancer cell invasion driven by extracellular matrix remodeling is dependent on the properties of cancer-associated fibroblasts. J Cancer Res Clin Oncol. (2016) 142:437–46. doi: 10.1007/s00432-015-2046-7
123. Zhang Q, Lou Y, Fang H, Sun S, Jin R, Ji Y, et al. Cancer−associated fibroblasts under therapy−induced senescence in the tumor microenvironment (review). Exp Ther Med. (2024) 27:150. doi: 10.3892/etm.2024.12438
124. Fan G, Yu B, Tang L, Zhu R, Chen J, Zhu Y, et al. Tspan8(+) myofibroblastic cancer-associated fibroblasts promote chemoresistance in patients with breast cancer. Sci Transl Med. (2024) 16:eadj5705. doi: 10.1126/scitranslmed.adj5705
125. Mizutani Y, Kobayashi H, Iida T, Asai N, Masamune A, Hara A, et al. Meflin-positive cancer-associated fibroblasts inhibit pancreatic carcinogenesis. Cancer Res. (2019) 79:5367–81. doi: 10.1158/0008-5472.CAN-19-0454
126. Ishibashi M, Neri S, Hashimoto H, Miyashita T, Yoshida T, Nakamura Y, et al. Cd200-positive cancer associated fibroblasts augment the sensitivity of epidermal growth factor receptor mutation-positive lung adenocarcinomas to egfr tyrosine kinase inhibitors. Sci Rep. (2017) 7:46662. doi: 10.1038/srep46662
127. Tanaka R, Kimura K, Eguchi S, Ohira G, Tanaka S, Amano R, et al. Interleukin-8 produced from cancer-associated fibroblasts suppresses proliferation of the ocuch-lm1 cancer cell line. BMC Cancer. (2022) 22:748. doi: 10.1186/s12885-022-09847-z
128. Song H, Lu T, Han D, Zhang J, Gan L, Xu C, et al. Yap1 inhibition induces phenotype switching of cancer-associated fibroblasts to tumor suppressive in prostate cancer. Cancer Res. (2024) 84:3728–42. doi: 10.1158/0008-5472.CAN-24-0932
129. Bhattacharjee S, Hamberger F, Ravichandra A, Miller M, Nair A, Affo S, et al. Tumor restriction by type i collagen opposes tumor-promoting effects of cancer-associated fibroblasts. J Clin Invest. (2021) 131:e146987. doi: 10.1172/JCI146987
130. Qin P, Chen H, Wang Y, Huang L, Huang K, Xiao G, et al. Cancer-associated fibroblasts undergoing neoadjuvant chemotherapy suppress rectal cancer revealed by single-cell and spatial transcriptomics. Cell Rep Med. (2023) 4:101231. doi: 10.1016/j.xcrm.2023.101231
131. Chen S, Fan L, Lin Y, Qi Y, Xu C, Ge Q, et al. Bifidobacterium adolescentis orchestrates cd143(+) cancer-associated fibroblasts to suppress colorectal tumorigenesis by wnt signaling-regulated gas1. Cancer Commun (Lond). (2023) 43:1027–47. doi: 10.1002/cac2.12469
132. Fujii N, Yashiro M, Hatano T, Fujikawa H, and Motomura H. Cd9-positive exosomes derived from cancer-associated fibroblasts might inhibit the proliferation of Malignant melanoma cells. Anticancer Res. (2023) 43:25–33. doi: 10.21873/anticanres.16130
133. Pulte D, Jansen L, and Brenner H. Changes in long term survival after diagnosis with common hematologic Malignancies in the early 21st century. Blood Cancer J. (2020) 10:56. doi: 10.1038/s41408-020-0323-4
134. Zhai Y, Zhang J, Wang H, Lu W, Liu S, Yu Y, et al. Growth differentiation factor 15 contributes to cancer-associated fibroblasts-mediated chemo-protection of aml cells. J Exp Clin Cancer Res. (2016) 35:147. doi: 10.1186/s13046-016-0405-0
135. Mei S, Zhang Y, Yu L, Chen G, and Zi F. Expression and role of fibroblast activation protein α in acute myeloid leukemia. Oncol Rep. (2021) 45:641–51. doi: 10.3892/or.2020.7874
136. Burt R, Dey A, Aref S, Aguiar M, Akarca A, Bailey K, et al. Activated stromal cells transfer mitochondria to rescue acute lymphoblastic leukemia cells from oxidative stress. Blood. (2019) 134:1415–29. doi: 10.1182/blood.2019001398
137. Fernández-Sevilla LM, Valencia J, Flores-Villalobos MA, Gonzalez-Murillo Á, Sacedón R, Jiménez E, et al. The choroid plexus stroma constitutes a sanctuary for paediatric b-cell precursor acute lymphoblastic leukaemia in the central nervous system. J Pathol. (2020) 252:189–200. doi: 10.1002/path.5510
138. Pan C, Liu P, Ma D, Zhang S, Ni M, Fang Q, et al. Bone marrow mesenchymal stem cells in microenvironment transform into cancer-associated fibroblasts to promote the progression of b-cell acute lymphoblastic leukemia. BioMed Pharmacother. (2020) 130:110610. doi: 10.1016/j.biopha.2020.110610
139. Pan C, Fang Q, Liu P, Ma D, Cao S, Zhang L, et al. Mesenchymal stem cells with cancer-associated fibroblast-like phenotype stimulate sdf-1/cxcr4 axis to enhance the growth and invasion of b-cell acute lymphoblastic leukemia cells through cell-to-cell communication. Front Cell Dev Biol. (2021) 9:708513. doi: 10.3389/fcell.2021.708513
140. Joo EH, Bae JH, Park J, Bang YJ, Han J, Gulati N, et al. Deconvolution of adult t-cell leukemia/lymphoma with single-cell rna-seq using frozen archived skin tissue reveals new subset of cancer-associated fibroblast. Front Immunol. (2022) 13:856363. doi: 10.3389/fimmu.2022.856363
141. Yang Y, Li J, and Geng Y. Exosomes derived from chronic lymphocytic leukaemia cells transfer mir-146a to induce the transition of mesenchymal stromal cells into cancer-associated fibroblasts. J Biochem. (2020) 168:491–8. doi: 10.1093/jb/mvaa064
142. Paggetti J, Haderk F, Seiffert M, Janji B, Distler U, Ammerlaan W, et al. Exosomes released by chronic lymphocytic leukemia cells induce the transition of stromal cells into cancer-associated fibroblasts. Blood. (2015) 126:1106–17. doi: 10.1182/blood-2014-12-618025
143. Vom SA, Rebollido-Rios R, Lukas A, Koch M, von Lom A, Reinartz S, et al. Lyn kinase programs stromal fibroblasts to facilitate leukemic survival via regulation of c-jun and thbs1. Nat Commun. (2023) 14:1330. doi: 10.1038/s41467-023-36824-2
144. Ge Y, Zhan F, Barlogie B, Epstein J, Shaughnessy J Jr, and Yaccoby S. Fibroblast activation protein (fap) is upregulated in myelomatous bone and supports myeloma cell survival. Br J Haematol. (2006) 133:83–92. doi: 10.1111/j.1365-2141.2006.05976.x
145. Zi FM, He JS, Li Y, Wu C, Wu WJ, Yang Y, et al. Fibroblast activation protein protects bortezomib-induced apoptosis in multiple myeloma cells through beta-catenin signaling pathway. Cancer Biol Ther. (2014) 15:1413–22. doi: 10.4161/cbt.29924
146. Frassanito MA, Rao L, Moschetta M, Ria R, Di Marzo L, De Luisi A, et al. Bone marrow fibroblasts parallel multiple myeloma progression in patients and mice: in vitro and in vivo studies. Leukemia. (2014) 28:904–16. doi: 10.1038/leu.2013.254
147. Frassanito MA, De Veirman K, Desantis V, Marzo LD, Vergara D, Ruggieri S, et al. Halting pro-survival autophagy by tgfbeta inhibition in bone marrow fibroblasts overcomes bortezomib resistance in multiple myeloma patients. Leukemia. (2016) 30:640–8. doi: 10.1038/leu.2015.289
148. Ciavarella S, Laurenzana A, De Summa S, Pilato B, Chilla A, Lacalamita R, et al. U-par expression in cancer associated fibroblast: new acquisitions in multiple myeloma progression. BMC Cancer. (2017) 17:215. doi: 10.1186/s12885-017-3183-y
149. Kunou S, Shimada K, Takai M, Sakamoto A, Aoki T, Hikita T, et al. Exosomes secreted from cancer-associated fibroblasts elicit anti-pyrimidine drug resistance through modulation of its transporter in Malignant lymphoma. Oncogene. (2021) 40:3989–4003. doi: 10.1038/s41388-021-01829-y
150. Sakamoto A, Kunou S, Shimada K, Tsunoda M, Aoki T, Iriyama C, et al. Pyruvate secreted from patient-derived cancer-associated fibroblasts supports survival of primary lymphoma cells. Cancer Sci. (2019) 110:269–78. doi: 10.1111/cas.13873
151. Bankov K, Döring C, Ustaszewski A, Giefing M, Herling M, Cencioni C, et al. Fibroblasts in nodular sclerosing classical hodgkin lymphoma are defined by a specific phenotype and protect tumor cells from brentuximab-vedotin induced injury. Cancers (Basel). (2019) 11:1687. doi: 10.3390/cancers11111687
152. Aronovich A, Moyal L, Gorovitz B, Amitay-Laish I, Naveh HP, Forer Y, et al. Cancer-associated fibroblasts in mycosis fungoides promote tumor cell migration and drug resistance through cxcl12/cxcr4. J Invest Dermatol. (2021) 141:619–27. doi: 10.1016/j.jid.2020.06.034
153. Apollonio B, Nicholas NS, Sutton LA, Salisbury J, Patten PE, Kassam S, et al. Diffuse large b-cell lymphoma (dlbcl) tumor cells reprogram lymphatic fibroblasts into cancer-associated fibroblasts (cafs) that contribute to tumor microenvironment (tme)-driven immune privilege. Blood. (2015) 126:1474. doi: 10.1182/blood.V126.23.1474.1474
154. Liu X, Xu N, Pan C, Zhou X, Liu Q, Feng R, et al. Emerging potential roles of lysyl-oxidase-like 2 stimulates cancer associated fibroblasts promoting myelofibrosis of myeloproliferative neoplasms. Blood. (2016) 128:5495. doi: 10.1182/blood.V128.22.5495.5495
155. Longhitano L, Tibullo D, Vicario N, Giallongo C, La Spina E, Romano A, et al. Igfbp-6/sonic hedgehog/tlr4 signalling axis drives bone marrow fibrotic transformation in primary myelofibrosis. Aging (Albany NY). (2021) 13:25055–71. doi: 10.18632/aging.203779
156. Kotecha RS and Cheung LC. Targeting the bone marrow microenvironment: a novel therapeutic strategy for pre-b acute lymphoblastic leukemia. Oncotarget. (2019) 10:1756–7. doi: 10.18632/oncotarget.26720
157. Wang A and Zhong H. Roles of the bone marrow niche in hematopoiesis, leukemogenesis, and chemotherapy resistance in acute myeloid leukemia. Hematology. (2018) 23:729–39. doi: 10.1080/10245332.2018.1486064
158. Tabe Y and Konopleva M. Advances in understanding the leukaemia microenvironment. Br J Haematol. (2014) 164:767–78. doi: 10.1111/bjh.12725
159. Dander E, Palmi C, D’Amico G, and Cazzaniga G. The bone marrow niche in b-cell acute lymphoblastic leukemia: the role of microenvironment from pre-leukemia to overt leukemia. Int J Mol Sci. (2021) 22:4426. doi: 10.3390/ijms22094426
160. Ganesan S, Mathews V, and Vyas N. Microenvironment and drug resistance in acute myeloid leukemia: do we know enough? Int J Cancer. (2022) 150:1401–11. doi: 10.1002/ijc.33908
161. Lewuillon C, Laguillaumie MO, Quesnel B, Idziorek T, Touil Y, and Lemonnier L. Put in a “Ca2+ll” to acute myeloid leukemia. Cells. (2022) 11:543. doi: 10.3390/cells11030543
162. Menter T and Tzankov A. Tumor microenvironment in acute myeloid leukemia: adjusting niches. Front Immunol. (2022) 13:811144. doi: 10.3389/fimmu.2022.811144
163. Duan CW, Shi J, Chen J, Wang B, Yu YH, Qin X, et al. Leukemia propagating cells rebuild an evolving niche in response to therapy. Cancer Cell. (2014) 25:778–93. doi: 10.1016/j.ccr.2014.04.015
164. Li Y and Gu L. Establishment and characterization of hxwmf-1: the first mouse fibroblastic tumor cell line derived from leukemia-associated fibroblasts. Cancer Cell Int. (2021) 21:177. doi: 10.1186/s12935-021-01870-7
165. van Attekum MHA, Eldering E, and Kater AP. Chronic lymphocytic leukemia cells are active participants in microenvironmental cross-talk. Haematologica (Roma). (2017) 102:1469–76. doi: 10.3324/haematol.2016.142679
166. Schulze-Edinghausen L, Dürr C, öztürk S, Zucknick M, Benner A, Kalter V, et al. Dissecting the prognostic significance and functional role of progranulin in chronic lymphocytic leukemia. Cancers (Basel). (2019) 11:822. doi: 10.3390/cancers11060822
167. Urdeitx P, Mousavi SJ, Avril S, and Doweidar MH. Computational modeling of multiple myeloma interactions with resident bone marrow cells. Comput Biol Med. (2023) 153:106458. doi: 10.1016/j.compbiomed.2022.106458
168. Desantis V, Frassanito MA, Tamma R, Saltarella I, Di Marzo L, Lamanuzzi A, et al. Rhu-epo down-regulates pro-tumorigenic activity of cancer-associated fibroblasts in multiple myeloma. Ann Hematol. (2018) 97:1251–8. doi: 10.1007/s00277-018-3293-x
169. Sakemura R, Hefazi M, Siegler EL, Cox MJ, Larson DP, Hansen MJ, et al. Targeting cancer-associated fibroblasts in the bone marrow prevents resistance to cart-cell therapy in multiple myeloma. Blood. (2022) 139:3708–21. doi: 10.1182/blood.2021012811
170. Jin X, Wei M, Wang S, Wang G, Lai Y, Shi Y, et al. Detecting fibroblast activation proteins in lymphoma using 68ga-fapi pet/ct. J Nucl Med. (2021) 63:212–7. doi: 10.2967/jnumed.121.262134
171. Mehdi SJ, Moerman-Herzog A, and Wong HK. Normal and cancer fibroblasts differentially regulate twist1, tox and cytokine gene expression in cutaneous t-cell lymphoma. BMC Cancer. (2021) 21:492. doi: 10.1186/s12885-021-08142-7
172. Apollonio B, Jarvis P, Phillips B, Kühnl A, Salisbury J, Zacharioudakis G, et al. Diffuse large b-cell lymphoma remodels the fibroblastic reticular network that acquires aberrant immunosuppressive capabilities; Implications for the regulation of anti-tumor immunity in the immuno-oncology era. Blood. (2018) 132:675. doi: 10.1182/blood-2018-99-116409
173. Aoki T, Shimada K, Sakamoto A, Sugimoto K, Morishita T, Kojima Y, et al. Emetine elicits apoptosis of intractable b-cell lymphoma cells with myc rearrangement through inhibition of glycolytic metabolism. Oncotarget. (2017) 8:13085–98. doi: 10.18632/oncotarget.14393
174. Schmitt-Graeff AH, Nitschke R, and Zeiser R. The hematopoietic niche in myeloproliferative neoplasms. Mediators Inflamm. (2015) 2015:1–11. doi: 10.1155/2015/347270
175. Chen X and Song E. Turning foes to friends: targeting cancer-associated fibroblasts. Nat Rev Drug Discov. (2019) 18:99–115. doi: 10.1038/s41573-018-0004-1
176. Akai M, Noma K, Kato T, Nishimura S, Matsumoto H, Kawasaki K, et al. Fibroblast activation protein-targeted near-infrared photoimmunotherapy depletes immunosuppressive cancer-associated fibroblasts and remodels local tumor immunity. Br J Cancer. (2024) 130:1647–58. doi: 10.1038/s41416-024-02639-1
177. Wen Z, Liu Q, Wu J, Xu B, Wang J, Liang L, et al. Fibroblast activation protein α-positive pancreatic stellate cells promote the migration and invasion of pancreatic cancer by cxcl1-mediated akt phosphorylation. Ann Transl Med. (2019) 7:532. doi: 10.21037/atm.2019.09.164
178. Bao G, Wang Z, Liu L, Zhang B, Song S, Wang D, et al. Targeting cxcr4/cxcl12 axis via [(177)lu]lu-dotaga.(Sa.fapi)(2) with cxcr4 antagonist in triple-negative breast cancer. Eur J Nucl Med Mol Imaging. (2024) 51:2744–57. doi: 10.1007/s00259-024-06704-y
179. Mori Y, Okimoto Y, Sakai H, Kanda Y, Ohata H, Shiokawa D, et al. Targeting pdgf signaling of cancer-associated fibroblasts blocks feedback activation of hif-1α and tumor progression of clear cell ovarian cancer. Cell Rep Med. (2024) 5:101532. doi: 10.1016/j.xcrm.2024.101532
180. Cao Y, Cao W, Qiu Y, Zhou Y, Guo Q, Gao Y, et al. Oroxylin a suppresses actn1 expression to inactivate cancer-associated fibroblasts and restrain breast cancer metastasis. Pharmacol Res. (2020), 159:104981. doi: 10.1016/j.phrs.2020.104981
181. Lee PJ, Ho CC, Ho H, Chen WJ, Lin CH, Lai YH, et al. Tumor microenvironment-based screening repurposes drugs targeting cancer stem cells and cancer-associated fibroblasts. Theranostics. (2021) 11:9667–86. doi: 10.7150/thno.62676
182. Ferrer-Mayorga G, Gomez-Lopez G, Barbachano A, Fernandez-Barral A, Pena C, Pisano DG, et al. Vitamin d receptor expression and associated gene signature in tumour stromal fibroblasts predict clinical outcome in colorectal cancer. Gut. (2017) 66:1449–62. doi: 10.1136/gutjnl-2015-310977
183. Wang H, Liu H, Sun C, Liu C, Jiang T, Yin Y, et al. Nanoparticles dual targeting both myeloma cells and cancer-associated fibroblasts simultaneously to improve multiple myeloma treatment. Pharmaceutics. (2021) 13:274. doi: 10.3390/pharmaceutics13020274
184. Patel AK and Singh S. Cancer associated fibroblasts: phenotypic and functional heterogeneity. Front bioscience (Landmark edition). (2020) 25:961–78. doi: 10.2741/4843.
185. Miyai Y, Esaki N, Takahashi M, and Enomoto A. Cancer-associated fibroblasts that restrain cancer progression (rcafs): hypotheses and perspectives. Cancer Sci. (2020) 111:1047–57. doi: 10.1111/cas.14346
186. Ma C, Yang C, Peng A, Sun T, Ji X, Mi J, et al. Pan-cancer spatially resolved single-cell analysis reveals the crosstalk between cancer-associated fibroblasts and tumor microenvironment. Mol Cancer. (2023) 22:170. doi: 10.1186/s12943-023-01876-x
187. Baryawno N, Przybylski D, Kowalczyk MS, Kfoury Y, Severe N, Gustafsson K, et al. A cellular taxonomy of the bone marrow stroma in homeostasis and leukemia. Cell. (2019) 177:1915–32. doi: 10.1016/j.cell.2019.04.040
188. Wan Y, Hu Q, Sun K, Shi J, Liu L, Zhang X, et al. Heterogenous cancer-associated fibroblasts related tumor microenvironment marked by cd10/klf4/tiam1 were identified in pancreatic adenocarcinoma by integrated transcriptomics. Front Immunol. (2025) 16:1557698. doi: 10.3389/fimmu.2025.1557698
189. Cumming J, Maneshi P, Dongre M, Alsaed T, Dehghan-Nayeri MJ, Ling A, et al. Dissecting fap+ cell diversity in pancreatic cancer uncovers an interferon-response subtype of cancer-associated fibroblasts with tumor-restraining properties. Cancer Res. (2025). doi: 10.1158/0008-5472.CAN-23-3252
190. Liu Y, Sinjab A, Min J, Han G, Paradiso F, Zhang Y, et al. Conserved spatial subtypes and cellular neighborhoods of cancer-associated fibroblasts revealed by single-cell spatial multi-omics. Cancer Cell. (2025) 43:905–24. doi: 10.1016/j.ccell.2025.03.004
191. Zhang X, Ren B, Liu B, Wang R, Li S, Zhao Y, et al. Single-cell rna sequencing and spatial transcriptomics reveal the heterogeneity and intercellular communication of cancer-associated fibroblasts in gastric cancer. J Transl Med. (2025) 23:344. doi: 10.1186/s12967-025-06376-8
192. Özdemir BC, Pentcheva-Hoang T, Carstens JL, Zheng X, Wu CC, Simpson TR, et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell. (2014) 25:719–34. doi: 10.1016/j.ccr.2014.04.005
193. Tao M, Liu W, Chen J, Liu R, Zou J, Yu B, et al. Transcriptome landscape of cancer-associated fibroblasts in human pdac. Adv Sci (Weinh). (2025) 12:e2415196. doi: 10.1002/advs.202415196
194. Kashyap D, Bhattacharya S, Irinike S, Khare S, Das A, Singh G, et al. Cancer associated fibroblasts modulate the cytotoxicity of anti-cancer drugs in breast cancer: an in vitro study. Breast Dis. (2024) 43:25–36. doi: 10.3233/BD-230011
Keywords: cancer associated fibroblasts, microenvironment, tumor progression, hematological malignancies, heterogeneity
Citation: Pan C, Zheng L and Wang J (2025) Cancer associated fibroblasts in tumors: focusing on solid tumors and hematological malignancies. Front. Oncol. 15:1596947. doi: 10.3389/fonc.2025.1596947
Received: 20 March 2025; Accepted: 05 June 2025;
Published: 23 June 2025.
Edited by:
Kevin Dzobo, University of Cape Town, South AfricaReviewed by:
Mzwandile Mbele, University of Cape Town, South AfricaHarry Chiririwa, Vaal University of Technology, South Africa
Copyright © 2025 Pan, Zheng and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jishi Wang, d2FuZ2ppc2hpOTY0NkAxNjMuY29t
†These authors have contributed equally to this work
 Chengyun Pan
Chengyun Pan Lin Zheng1†
Lin Zheng1† Jishi Wang
Jishi Wang