- 1Department of Respiratory Medicine, North China University of Science and Technology Affiliated Hospital, Tangshan, China
- 2Department of Respiratory Medicine, Tangshan Gongren Hospital, Tangshan, China
Mixed squamous cell and glandular papilloma (MSCGP) is a rare benign lung tumor, which mostly occurs in the central airway and primarily affects middle-aged and elderly individuals. Due to the limited understanding of the clinical characteristics of elderly patients, clinicians often lack a comprehensive grasp of MSCGP, leading to misdiagnosis. Therefore, in this article, we report a case of an elderly patient with central MSCGP and provide a comprehensive review of the relevant literature on elderly patients with MSCGP, aiming to improve the diagnostic rate of this disease.
1 Introduction
Mixed squamous cell and glandular papilloma (MSCGP) of the lung is an extremely rare benign tumor that mainly affects the elderly. Currently, there is a lack of review studies on the clinical characteristics of MSCGP in the elderly population. Clinicians have an insufficient understanding of the clinical features of this tumor, which often leads to misdiagnosis as mucoepidermoid carcinoma, adenocarcinoma, or other diseases. This has serious consequences for public health. This study reports a case of an elderly male patient with MSCGP of the lung and provides a literature review focused on the elderly population.
2 Case report
A 62-year-old man presented with an unexplained cough and was ultimately diagnosed with MSCGP of the lung based on chest computed tomography and bronchoscopy. One month earlier, the patient developed an irritating dry cough with no other relevant symptoms. To further clarify the cause of the cough, the patient was admitted to the respiratory department of a local hospital. Chest radiography revealed no abnormalities. Lung function tests suggested reductions in the volume of 1 s and maximum mid-expiratory flow rate, and the bronchodilation test was negative; thus, the cough was attributed to bronchitis. Although the patient was given antibacterial and antitussive treatment for bronchitis, the symptom of irritating dry cough did not improve significantly. Subsequently, he was admitted to the respiratory department of North China University of Science and Technology Affiliated Hospital for further evaluation.
There was no other past history of disease except for type 2 diabetes. However, the patient had 50 years of smoking history. Physical examination revealed lower respiratory sounds in the left lung, and no obvious abnormalities were found in the rest of the the physical examination. Chest computed tomography performed at our hospital indicated the growth of a mass in the left main bronchus (Figure 1).
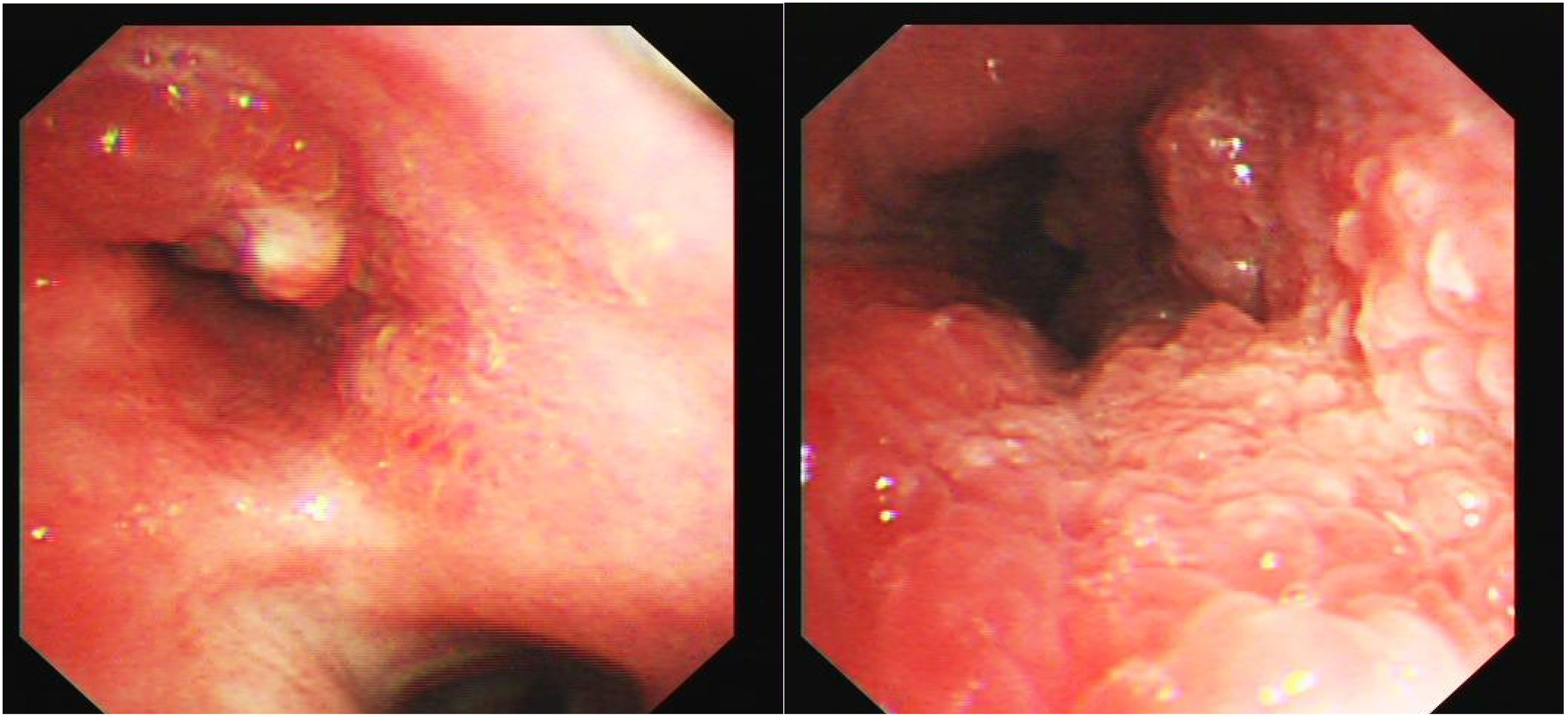
Bronchoscopy revealed a cauliflower-like neoplasm invading the carina of the left main bronchus (Figure 2). Consequently, we performed bronchoscopy biopsy, and the pathological results of bronchoscopy forceps were analyzed. Histological findings revealed papillary structures in the tumor area against a mucinous background. The center of the papillae had a fibrovascular core, in which a large number of lymphocytes and eosinophils were found. The surface was covered with squamous and glandular epithelium, including pseudostratified ciliated or non-ciliated columnar epithelium and mucinous columnar epithelium, with no cellular dysplasia or necrosis observed (Figure 3).

Figure 3. Pathological result of bronchoscope forceps showing mixed squamous cell and glandular papilloma of the lung. Hematoxylin and eosin staining, original magnification: ×100.
Immunohistochemistry results indicated that squamous epithelial cells were positive for cytokeratin 5/6 (CK5/6) and p40 expression, whereas glandular epithelial cells were positive for CK7 and thyroid transcription factor 1 (TTF-1) expression. The Ki-67 proliferation index of the tumor was 3%. Laboratory findings revealed normal serum carcinoembryonic antigen (CEA) levels, blood routine test results, C-reactive protein concentration, and erythrocyte sedimentation rate. Therefore, the patient was diagnosed with MSCGP of the lung. Because the MSCGP of the lung invaded the carina, radical resection was not performed. The patient underwent bronchoscopic interventional treatment, which included argon high-frequency electroknife resection and CO2 cryoablation. The patient refused further treatment. No evidence of recurrence was observed after 6 months of follow-up.
3 Discussion
The lung MSCGP is an extremely rare benign tumor composed of bidirectionally differentiated squamous and glandular epithelial cells (1). It was first identified by Flieder in 1998 (2). The distribution of MSCGP is from the third to the sixth decade of life (3).
In 2015, the World Health Organization classified solitary endobronchial papillomas into three categories according to different epithelial components: squamous epithelial papilloma, glandular papilloma, and MSCGP; among them, MSCGP is the rarest type (4). The clinical manifestations of pulmonary MSCGP are diverse and not specific, and the corresponding symptoms (e.g.,irritating dry cough, sputum, hemoptysis, and chest pain) are often caused by mass obstruction and oppression (5).
A comprehensive search of PubMed, CNKI, and Wanfang databases case reports and case series published between 2010 and 2024.The language limit was English and Chinese.The search used keywords including “MSCGP” or “Solitary endobronchial papilloma.” Our search focused exclusively on patients aged ≥60 years who met the diagnostic criteria for MSCGP.
The characteristics of MSCGP described in previous reports are shown in Table 1 (6–33). Most elderly patients with MSCGP included in the studies were from Asia and Europe. This indicates that clinicians in these countries attach importance to the diagnosis of MSCGP. It has been previously suggested that tumors are more commonly found in men (male-to-female ratio: 16:5), and most patients have a history of smoking.

Table 1. . Summary of published cases of mixed squamous cell and glandular papilloma of the lungs in elderly patients.
However, our literature review summarizing the clinical features of MSCGP in elderly patients in the past 14 years revealed a male-to-female ratio of 1:1 (i.e., 17 males and 17 females), indicating that there is no sex difference in the incidence of the disease. It also confirmed that smoking history is related to MSCGP in the elderly, and the level of the smoking index may affect the incidence of MSCGP in this population. Moreover, we found that MSCGP in the elderly not only occurs more frequently in the central airway, but also more often in the peripheral airway. However, previous studies have shown that MSCGP in elderly individuals is more prevalent in the central airways,the reason for this inconsistency remains unclear.
Among the 34 elderly patients with MSCGP, 19 had a variety of symptoms, among which cough (42%) was the most common,followed by hemoptysis or bloody phlegm (19%), chest pain (15%), chest distress (12%), wheezing (4%), weight loss (4%), and left limb weakness (4%). Cough, hemoptysis, and chest pain are the main symptoms in elderly patients with MSCGP. Previous studies have also demonstrated that cough, hemoptysis, and dyspnea are common symptoms of MSCGP in adults (3); nevertheless, the main symptoms in the elderly were not different from those observed in adult patients. Furthermore, 15 patients diagnosed with MSCGP through physical examination did not develop any symptoms. This shows that the onset of MSCGP is insidious and not characterized by typical clinical manifestations; hence, it is easily overlooked by clinicians.
Furthermore, we found that the maximum diameter of MSCGP lesions mostly ranged from 1 cm to 5 cm in elderly patients. Among the 34 elderly patients with MSCGP, only one patient showed diffuse growth in the central airway. The patient reported in this study also showed diffuse growth in the central airway. For tumor tissues that diffuse along the central airway, radical resection of the lobe or segment of the lung is not possible. Therefore, only interventional treatments under bronchoscopy such as argon plasma coagulation, cryotherapy, and laser therapy can be performed. According to our literature review, radical resection of the lobe or segment of the lung has been adopted in most cases reported to date. Regarding prognosis after resection, the postoperative follow-up time exceeded 2 months, with the longest reaching 61 months, indicating a longer survival period in elderly patients.
Typically, the histological morphology of MSCGP is mainly composed of a mixture of papillae with vascular cores, which consist of glandular epithelial cells covering the surface and squamous epithelial cells and basal cells beneath them (34). In most of the included elderly MSCGP cases, histological features such as mucous components, ciliated columnar cells, and papillary structures were observed. MSCGP needs to be differentiated from malignant tumors such as mucinous adenocarcinoma and adenosquamous carcinoma. When the background of MSCGP is rich in mucous components, with glandular epithelium as the main component and relatively few squamous epithelial components, it can easily be misdiagnosed as mucinous adenocarcinoma.
Pulmonary mucinous adenocarcinoma shows invasive growth, obvious stromal reactions, significant cellular dysplasia, and a high Ki-67 proliferation index. When MSCGP has a similar mixed ratio of glandular epithelium and squamous epithelium, it is highly likely to be misdiagnosed as a pulmonary adenosquamous carcinoma, particularly under low-power microscopy. Under high-power microscopy, cellular and nuclear dysplasia of the squamous epithelium and glandular epithelium was observed, keratin pearls and intercellular bridges in the squamous epithelial areas were identified, and an accurate diagnosis was eventually made based on the immunohistochemistry results. Immunohistochemical detection can improve the diagnostic accuracy of MSCGP. Among the 34 elderly patients with MSCGP, 29 underwent immunohistochemical detection. The results showed that CK5/6, CK7, TTF-1, p63, and p40 were positively expressed in both glandular and squamous epithelial cells of MSCGP. Among these markers, TTF-1 and CK5/6 were expressed in glandular epithelial cells and squamous epithelial cells, respectively. Moreover, Ki-67 levels were detected in most cases; the analysis showed that the maximum proliferation index of tumor cells did not exceed 5%. Therefore, Ki-67 detection helps distinguish between benign and malignant tumors, underscoring the importance of immunohistochemistry in the diagnosis of MSCGP.
It has been reported that 71.4% of patients with MSCGP have elevated levels of blood tumor markers (35). However, some reports indicated that the serum levels of CEA and squamous cell carcinoma antigen (SCC) were normal, and the number of tumor cells secreting these factors was too low to elevate their serum levels (36, 37). Among the 34 elderly patients with MSCGP included in our review, only 14 underwent blood tumor marker testing. Three patients had simultaneous elevation of CEA and SCC antigen levels, three patients had elevated CEA levels alone, and the remaining eight patients had normal levels of blood tumor markers. The results revealed that CEA and SCC antigen levels increased in elderly patients with MSCGP. Therefore, blood tumor marker tests should be routinely performed in such patients. The increase in blood CEA and SCC antigen levels has a certain reference value for diagnosis. In clinical practice, attention should be paid to the combined increase in CEA and SCC antigen levels.
In summary, through our literature review, we conducted a comprehensive and thorough examination of the clinical characteristics of MSCGP in elderly patients. This analysis may enable the medical staff to recognize that MSCGP rarely occurs and can imitate malignant lesions. This information should be considered in the differential diagnosis of elderly patients in the future to improve the diagnostic rate of MSCGP.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Medical Ethics Committee of the Affiliated Hospital of North China University of Science and Technology. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the patient for the publication of this case report.
Author contributions
PZ: Conceptualization, Writing – original draft. YW: Writing – review & editing, Data curation. CH: Writing – original draft. YJ: Writing – original draft, Investigation. XH: Conceptualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Wang XX, Li R, Feng X, Ma HH, Lu ZF, Xia C, et al. Clinicopathological analysis of pulmonary mixed squamous cell and glandular papilloma. Chin J Pathol. (2019) 48:318–21. doi: 10.3760/cma.j.issn.0529-5807.2019.04.012
2. Flieder DB, Koss MN, Nicholson A, Sesterhenn IA, Petras RE, Travis WD, et al. Solitary pulmonary papillomas in adults: a clinicopathologic and in situ hybridization study of 14 cases combined with 27 cases in the literature. Am J Surg Pathol. (1998) 22:1328–42. doi: 10.1097/00000478-199811000-00003
3. Tryfon S, Dramba V, Zoglopitis F, Iakovidis D, Sakkas L, Kontakiotis T, et al. Solitary papillomas of the lower airways: epidemiological, clinical, and therapeutic data during a 22-year period and review of the literature. J Thorac Oncol. (2012) 7:643–8. doi: 10.1097/JTO.0b013e3182468d06
4. Travis WD. The 2015 WHO classification of lung tumors. Pathologe. (2014) 2:188. doi: 10.1007/s00292-014-1974-3
5. Huang YL, Chang YL, Chen KC, and Wu CT. Mixed squamous cell and glandular papilloma of the lung: a case report of a novel mutation in the BRAF gene and coexistent HPV infection, possible relationship to ciliated muconodular papillary tumor. Pathol Int. (2019) 69:104–9. doi: 10.1111/pin.12747
6. Lu CL, Xu X, Zhang SF, Zhang WY, Li FY, and Liao DY. Solitary bronchial papilloma: a clinicopathological study of four cases and review of literature. J Clin Exp Pathol. (2010) 26:67–72. doi: 10.3969/j.issn.1001-7399.2010.01.017
7. Yabuki H, Tabata T, Sugawara T, Fukaya K, Murakami K, and Fujimura S. Mixed squamous and glandular papilloma. Kyobu Geka. (2013) 66:541–4.
8. Lin D, Jiang Y, Wang J, Ding L, Xin FJ, Zhao H, et al. Pulmonary mixed squamous cell and glandular papilloma mimicking adenocarcinoma: a case study and literature review. J Thorac Dis. (2013) 5:E129–32. doi: 10.3978/j.issn.2072-1439.2013.07.38
9. Kozu Y, Maniwa T, Ohde Y, and Nakajima T. A solitary mixed squamous cell and glandular papilloma of the lung. Ann Thorac Cardiovasc Surg. (2014) 20 Suppl:625–8. doi: 10.5761/atcs.cr.13-00029
10. Yun JS, Kim DW, Choi YD, Choi YD, Na KJ, and Song SY. Mixed squamous cell and glandular papilloma of the lung in a 64-year-old woman. Korean J Thorac Cardiovasc Surg. (2014) 47:55–8. doi: 10.5090/kjtcs.2014.47.1.55
11. Chen SM, Qu LJ, and Zheng ZY. A case of mixed pulmonary squamous cell carcinoma and papillary adenoma. J Clin Exp Pathol. (2014) 30:1193–4. doi: 10.13315/j.cnki.cjcep.2014.10.035
12. Feng AN, Wu HY, Zhou Q, Sun Q, Fan XS, Zhang YF, et al. Solitary endobronchial papillomas with false impression of Malignant transformation: report of two cases and review of the literature. Int J Clin Exp Pathol. (2015) 8:8607–12.
13. Abe J, Ito S, Takahashi S, Sato I, Tanaka R, Sato T, et al. Mixed squamous cell and glandular papilloma of the lung resembling early adenocarcinoma: a case report. Ann Med Surg (Lond). (2016) 7:61–4. doi: 10.1016/j.amsu.2016.03.025
14. Ryo M, Toshi M, Akihiko Y, and Date H. Expression of p16Ink4a in mixed squamous cell and glandular papilloma of the lung. Pathol Int. (2017) 67:306–10. doi: 10.1111/pin.12531
15. Yabuki K, Matsuyama A, Obara K, Takenaka M, Tanaka F, Nakatani Y, et al. A unique case of a huge mixed squamous cell and glandular papilloma of non-endobronchial origin with a peripheral growth. Respir Med Case Rep. (2018) 24:108–12. doi: 10.1016/j.rmcr.2018.05.001
16. Liu YM and Meng QD. Three cases of mixed squamous cell and adenomatoid papilloma of the lung: a report. J Diag Pathol. (2018) 25:533–4. doi: 10.3969/j.issn.1007-8096.2018.07.013
17. Feng L, Li GS, Zhang PX, and Zhang LZ. Analysis of clinicopathological features in pulmonary mixed squamous cell and glandular papilloma. J Dalian Med Univ. (2018) 40:257–61. doi: 10.11724/jdmu.2018.03.15
18. Li F, He M, Li F, Li Y, and Song Y. Histologic characteristics and prognosis of lung mixed squamous cell and glandular papilloma: six case reports. Int J Clin Exp Pathol. (2019) 12:3542–8.
19. Lin DL, Xing XM, Ran WW, Zhao H, Li GQ, Xu J, et al. Pulmonary peripheral glandular papilloma and mixed squamous cell and glandular papilloma frequently harbour the BRAF V600E mutation. Histopathology. (2020) 76:997–1004. doi: 10.1111/his.14098
20. Jie C, Si-bai S, and Shi-lan Li. Clinicopathological analysis of pulmonary mixed squamous cell and glandular papilloma. J Anhui Vocational Coll Health. (2020) 19:89–91. doi: 10.3969/j.issn.1671-8054.2020.05.042
21. Chen S, Liu FY, Yang GL, and Cheng XZ. A rare case of mixed squamous cell and papillary adenoma of the lung. J Diag Pathol. (2020) 27:244. doi: 10.3969/j.issn.1007-8096.2020.04.007
22. Wang B, Yang L, Lin J, Wang Y, Wang SM, and Zhong DR. Clinicopathological features of bronchiolar adenoma versus mixed squamous cell and glandular papilloma: a comparative analysis. Chin J Pathol. (2021) 50:458–64. doi: 10.3760/cma.j.cn112151-20201006-00761
23. Wang X, Liu H, Zhai D, Qin YN, Fan CF, and Zhang D. Multiple primary lung tumors of different pathological types including squamous cell carcinoma, adenocarcinoma, and mixed squamous cell and glandular papilloma: a case report. Onco Targets Ther. (2022) 15:13–9. doi: 10.2147/OTT.S344086
24. Konaka Y, Endoh M, Sasage T, Nakahashi K, Suzuki H, Ogata S, et al. Growing mixed squamous cell and glandular papilloma difficult to differentiate from primary lung cancer: report of a case. Kyobu Geka. (2022) 75:731–4.
25. Arora I, Gupta N, Angeles Montero M, Montero M, and Viola P. Pulmonary mixed squamous and glandular papilloma: diagnostic challenges of a rare lesion when the clock is ticking. How to avoid interpretation mistakes. Pathologica. (2022) 114:391–4. doi: 10.32074/1591-951X-809
26. Che W, Liu JS, Chen XY, Wang CF, Yuan F, and Wang X. Pulmonary mixed squamous cell and glandular papilloma clinicopathological characteristics of 2 cases and misdiagnosis analysis of frozen section. J Diagn Concepts Pract. (2022) 21:476–81. doi: 10.16150/j.1671-2870.2022.04.010
27. Haga N, Ito K, Kasai Y, and Masuya D. Solitary mixed squamous and glandular papilloma of the peripheral lung:report of a case. Kyobu Geka. (2022) 75:652–5.
28. Sato K, Otsuki Y, Kato A, Misaki N, Go T, and Yokomise H. Mixed squamous cell and glandular papilloma in the peripheral lung:report of a case. Kyobu Geka. (2023) 76:251–4.
29. Wang QZ, Li JS, Guo YL, Guo YF, Li ZQ, and Zhou YH. Clinical pathological analysis and literature review of pulmonary mixed squamous cell and glandular papilloma. J Clin Pathol Res. (2023) 43:1907–12. doi: 10.11817/j.issn.2095-6959.2023.220085
30. Cao Q, Li B, Lian H, and Li J. Mixed squamous cell and glandular papilloma of the bronchus. Pulmonology. (2024) 30:96–9. doi: 10.1016/j.pulmoe.2023.07.005
31. Nitanda H, Homma T, Taguchi R, Umesaki T, Ichiki Y, Sakaguchi H, et al. Pulmonary pleomorphic carcinoma arising in mixed squamous and glandular papilloma: a case report. Thorac Cancer. (2024) 15:1385–9. doi: 10.1111/1759-7714.15322
32. Zhang J, Dong A, and Wang Y. FDG PET/CT in a case of solitary mixed squamous cell and glandular papilloma of the lung with high CEA level. Clin Nucl Med. (2024) 49:e288–9. doi: 10.1097/RLU.0000000000005025
33. Huo XS, Zhang Y, Dong YY, Li L, Zhou H, An P, et al. Bronchoscopic interventional treatment of mixed squamous cell and glandular papilloma of diffuse trachea: a case report and literature review. Chin J Lung Cancer. (2024) 27:711–6. doi: 10.3779/j.issn.1009-3419.2024.102.31
34. Hu X, Zhao W, Li F, Wang P, and Cai J. Clinical and imaging features of pulmonary mixed squamous cell and glandular papilloma: a case report and literature review. Front Med (Lausanne). (2024) 11:1437597. doi: 10.3389/fmed.2024.1437597
35. Iijima Y, Nakajima Y, Kinoshita H, Akiyama H, Nishimura Y, and Hirata T. Mixed squamous cell and glandular papilloma of the lung-A case report and literature review in Japan. Int J Surg Case Rep. (2020) 68:39–42. doi: 10.1016/j.ijscr.2020.02.021
36. Inamura K, Kumasaka T, Furuta R, Shimada K, Hiyama N, Furuhata Y, et al. Mixed squamous cell and glandular papilloma of the lung: a case study and literature review. Pathol Int. (2011) 61:252–8. doi: 10.1111/j.1440-1827.2011.02659.x
Keywords: papilloma, squamous cell, lung neoplasms, aged, bronchi, case reports
Citation: Zhang P, Wang Y, Huang C, Ji Y and Han X (2025) Pulmonary mixed squamous cell and glandular papilloma in the elderly: a case report and literature review. Front. Oncol. 15:1606426. doi: 10.3389/fonc.2025.1606426
Received: 05 April 2025; Accepted: 13 November 2025; Revised: 11 November 2025;
Published: 16 December 2025.
Edited by:
Lizza E. L. Hendriks, Maastricht University Medical Centre, NetherlandsReviewed by:
Ran Wang, Anhui Medical University, ChinaDalibor Jovanovic, University of Kragujevac, Serbia
Copyright © 2025 Zhang, Wang, Huang, Ji and Han. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoqing Han, dHNteXpwcEAxMjYuY29t
 Panpan Zhang
Panpan Zhang Yuan Wang1
Yuan Wang1