- Geriatric Department of the First Affiliated Hospital of China Medical University, Shenyang, Liaoning, China
The global incidence and mortality rates of liver cancer remain persistently high, attributable to its multifaceted etiology. Recent research has increasingly focused on the interaction between gut microbiota and the liver’s immune system. The gut-liver axis, which connects the gut and liver via the portal vein system and biliary tract, is crucial in maintaining homeostasis; however, dysbiosis can compromise the gut’s barrier function. The gut microbiota exerts influence over hepatic immune cells through various mechanisms, including alterations in microbial composition, production of metabolic products, and the presence of pathogen-associated molecular patterns. These interactions contribute to immune evasion, inflammation, and remodeling of the tumor microenvironment in liver cancer, thereby affecting the efficacy of immunotherapy. Despite these insights, the precise mechanisms underlying these interactions and their potential clinical applications remain inadequately understood. This article aims to review the mechanisms of interaction between the gut microbiota and the local immune response in liver cancer, integrating the latest research advancements. Additionally, it will explore the impact of these interactions on the tumor immune microenvironment, with the objective of providing a theoretical foundation and potential strategies for prevention and therapeutic intervention.
1 Introduction
The global incidence and mortality rates of hepatocellular carcinoma (HCC) are on the rise, presenting a significant public health challenge. The pathogenesis of HCC is multifaceted, involving a range of factors such as viral infections, metabolic disorders, and chronic inflammation. Typically, HCC develops through a series of pathological processes, including chronic hepatitis, liver fibrosis, and cirrhosis. Recent studies have identified metabolic diseases, particularly non-alcoholic fatty liver disease (NAFLD), as major contributors to cirrhosis and HCC among younger populations (1). Additionally, emerging research indicates that the gut microbiome can influence liver metabolism and immune responses via the gut-liver axis, thereby playing a role in the onset and progression of this malignancy (2, 3). This finding offers a novel perspective on HCC treatment, as the involvement of gut microbiota and their metabolites in modulating tumor immunity is increasingly recognized, making it an emerging area of interest in HCC therapeutics.
An increasing body of evidence indicates that the development of HCC is intricately linked to significant gut microbiota imbalances. Research demonstrates that the gut microbiota of individuals diagnosed with this cancer type frequently exhibits substantial alterations, characterized by a pronounced reduction in microbial diversity (4). In patients with HCC, gut microbiota disruption is marked by a significant drop in the Bacteroidetes to Firmicutes ratio, from 0.92 ± 0.15 to 0.48 ± 0.08 (p<0.01), and a 120-fold increase in Fusobacterium nucleatum. This is closely linked to the progression of liver cirrhosis and cancer (5). Such microbial imbalances are hypothesized to activate hepatic immune cells, thereby enhancing liver inflammation and fostering the formation of a tumor microenvironment through various mechanisms, including the production of carcinogenic substances, disruption of immune system equilibrium, modification of metabolic processes, and increased intestinal permeability. These factors collectively position dysbiosis as a potential catalyst in the pathogenesis of HCC (3, 6). For instance, metabolites generated by gut microbial imbalances, such as short-chain fatty acids (SCFAs), may influence liver immune surveillance by modulating T cell function, potentially either promoting or inhibiting the progression of this disease. This interaction may represent a critical mechanism underlying the advancement of HCC (7). Furthermore, the composition and functionality of gut microbiota are not only associated with the development of HCC, but also with the diversity and compositional alterations of the microbiota, which may serve as predictors of patient responses to immunotherapy and are closely linked to the prognosis of liver cancer (8). In certain instances, modulating the composition of gut microbiota has the potential to enhance the efficacy of immunotherapeutic interventions (9). For example, modifications to the gut microbiota structure can improve the effectiveness of immune checkpoint inhibitors (10), thereby increasing patient survival rates and providing novel treatment strategies and insights.
In conclusion, liver cancer is not solely associated with traditional risk factors. It is also intricately connected to the complex interactions with gut microbiota. This article seeks to examine recent research on the microbiota-immune dialogue in liver cancer, with a comprehensive analysis of its molecular mechanisms and potential clinical applications. Such an exploration not only elucidates the pathological mechanisms underlying liver cancer but also identifies novel targets and directions for future Precision Therapeutics.
2 The liver: a unique nexus of immunity and microbial crosstalk
2.1 Distinct characteristics of the liver microenvironment
2.1.1 Mechanism of immune tolerance
The liver’s immune tolerance is primarily attributed to its unique cellular composition. Kupffer cells, the liver’s resident macrophages, play a dual role in maintaining immune tolerance by both inducing Treg differentiation through PD-L1 expression (MFI = 285 ± 35) and IL-10 secretion (1200 ± 150 pg/mL), and clearing gut-derived antigens (11, 12). Notably, intestinal microbiota critically modulates this Treg-inducing capacity of Kupffer cells. Clinical studies show that gut microbiota perturbations (e.g., reduced diversity with Shannon index dropping from 3.8 to 2.9 in HCC patients) correlate with impaired Treg function and disrupted hepatic tolerance (13). Liver sinusoidal endothelial cells (LSECs) possess a unique fenestrated structure that enables them to efficiently capture antigens from the bloodstream, exhibiting an antigen-presenting capability that is 8–10 times greater than that of conventional endothelial cells (14). Through the cross-presentation of antigens, LSECs induce tolerance in CD8+ T cells (15). Furthermore, the gut microbiota plays a pivotal role in the maintenance of liver immune tolerance. It achieves this by regulating the gut-liver axis, modulating Treg function, preserving the tolerant state of Kupffer cells, and preventing the excessive activation of natural killer T (NKT) cells (16). Disruption of the intestinal barrier may permit the translocation of gut microbiota and their metabolites into the liver, thereby directly initiating liver immune tolerance.
Clinical evidence reveals three key microbiota-derived metabolites regulating liver tolerance. 1.Secondary bile acids: Elevated DCA (2.8-fold increase in HCC) activates TGR5 on Kupffer cells, increasing IL-10 production by 40% but simultaneously inducing PD-L1 expression (17). 2.Tryptophan metabolites: Kynurenine/AhR signaling upregulates hepatic PD-L1 while suppressing CD8+ T cell IFN-γ production by 65 ± 8% (18). 3.SCFAs: Butyrate (optimal 2-4mM) maintains Treg function via GPR43, but concentrations <1mM (as in HCC) reduce Foxp3+ cells by 35 ± 5% (19).
2.1.2 Bidirectional communication in the gut-liver axis
The gut-liver axis functions as a bidirectional regulatory system that connects the intestine and liver, playing a crucial role in maintaining the body’s immune homeostasis through various mechanisms. The primary anatomical foundation of this axis is formed by the portal vein and bile acid circulation, while metabolite diffusion serves as a key communication pathway. Bacterial translocation and immune surveillance contribute to establishing a defense barrier. The integrity of the intestinal barrier is essential for preserving this balance. Clinical studies demonstrate intestinal barrier dysfunction as a pivotal event in HCC progression. The diminution of intestinal tight junction integrity results in the formation of leaky gut, facilitating the translocation of various microbial metabolites from the intestine into the bloodstream, which subsequently elicits an immune response. For instance, a 60% reduction in occludin, a tight junction protein, results in the detection of 10^3 copies/mL of bacterial 16S rRNA in portal vein blood (20). Elevated portal SCFA levels (butyrate +220%) paradoxically indicate barrier breach (21), while reduction in occludin correlates with increase in hepatic IL-6.
Kupffer cells efficiently eliminate these foreign substances in a CD14-dependent manner; however, persistent activation of the Toll-like receptor 4 (TLR4)/MyD88 pathway may lead to pathological fibrosis (22). In patients with chronic liver diseases, such as liver cancer, alterations in the gut microbiota are closely associated with the severity of liver disease, with reduced microbial diversity being linked to increased liver inflammation (23).
2.2 The evolution of microbiome-immune dynamics in liver cancer development
In the initial phases of liver cancer, dysbiosis of the gut microbiome plays a pivotal role in inducing liver inflammation and fibrosis via the lipopolysaccharide (LPS)-TLR4 axis. Empirical studies have demonstrated that the concentration of LPS in the portal vein blood of patients with liver cirrhosis is markedly elevated compared to that in healthy individuals. These LPS molecules activate TLR4 receptors on Kupffer cells, thereby initiating the NF-κB signaling pathway. This activation leads to the secretion of inflammatory mediators, such as interleukin-6 (IL-6) and senescence-associated secretory phenotype (SASP) factors, which collectively contribute to liver fibrosis and the progression to HCC (24). As liver cancer advances, the tumor microenvironment increasingly suppresses immune responses. This suppression facilitates the proliferation of immunosuppressive cells, including myeloid-derived suppressor cells (MDSCs) (25), and results in the upregulation of T cell exhaustion markers such as programmed cell death protein 1 (PD-1), along with increased co-expression of TIM-3 and LAG-3. These changes collectively establish an immunosuppressive microenvironment that enables tumors to evade immune surveillance. During the metastatic phase of liver cancer, Fusobacterium nucleatum activates the β-catenin signaling pathway in hepatic cells through the FadA adhesin, thereby facilitating tumor cell invasion (26). Concurrently, microbial metabolites, including trimethylamine N-oxide, can stimulate the MAPK pathway, leading to increased VEGF secretion and promoting tumor proliferation and migration (27).
3 Mechanisms of gut microbiota-driven immune dysregulation
3.1 Primary mechanisms of gut microbiota-driven immune regulation
3.1.1 Microbial metabolites as immune modulators
The primary metabolic products of the gut microbiota encompass SCFAs, secondary bile acids (including deoxycholic acid [DCA] and lithocholic acid [LCA]), trimethylamine N-oxide, tryptophan metabolites, and LPS, among others. These metabolites not only contribute to energy metabolism but also serve as pivotal regulators of the immune response within the gut-liver axis. They modulate immune responses through various pathways, exhibiting both pro-carcinogenic and anti-carcinogenic properties (28, 29). Collectively, these metabolites interact in multifaceted ways to maintain immune homeostasis, thereby influencing the onset and progression of diseases such as liver cancer. The mechanisms through which gut microbiota metabolites exert their effects on the gut-liver axis are intricate and varied, suggesting potential novel targets for future liver disease treatments. Further investigation is warranted to elucidate the specific mechanisms by which these metabolites impact liver health and disease, with the objective of developing innovative therapeutic strategies centered on gut microbiota metabolites.
3.1.2 Pathogen-mimicry: pathogen-associated molecular patterns and immune activation
Pathogen mimicry refers to the phenomenon whereby pathogens emulate host biomolecules through their molecular structures, thereby evading the host’s immune response. This mechanism is pivotal in the pathogenesis of various infectious diseases and is particularly significant in the progression of malignancies such as liver cancer, with substantial clinical implications (30). PAMPs are integral to immune activation, as they are recognized by pattern recognition receptors (PRRs), which subsequently initiate intracellular signaling pathways. These pathways lead to the production of pro-inflammatory molecules, thereby instigating the host’s initial response to infection and facilitating the subsequent activation of adaptive immunity (31). Nevertheless, pathogens can modulate the host’s immune response by mimicking host molecules, enabling their survival and replication within the host. A comprehensive understanding of the interplay between PAMPs, pathogen mimicry, and immune activation not only enhances our insight into the mechanisms underlying infectious diseases and tumorigenesis but also identifies potential targets for the development of novel vaccines and therapeutic interventions.
3.1.3 Direct microbial-immune cell crosstalk
Recent studies on liver cancer have increasingly elucidated a direct communication process between microbes and immune cells, which is essential for the maintenance of immune homeostasis. Gut microbes can modulate immune cell function through direct interactions or by releasing metabolic products (32). Furthermore, these microbes influence immune responses by regulating the differentiation and functionality of immune cells. The interactions between microbes and immune cells also encompass the regulation of various cytokines and signaling pathways (33). Cytokines, as critical mediators of intercellular communication, play a pivotal role in the interaction between microbes and immune cells; they are instrumental in regulating the proliferation, differentiation, and function of immune cells, thereby shaping the intensity and nature of immune responses.
3.2 Multifaceted interactions between gut microbiota and HCC pathogenesis
3.2.1 Disruption of the gut barrier and immune activation in liver cancer
The integrity of the gut barrier is essential for maintaining intestinal homeostasis and immune equilibrium. Its disruption is intricately linked to immune activation in liver cancer (34). A compromised gut barrier allows for the translocation of gut microbes and their metabolites into the bloodstream, facilitating the movement of PAMPs to the liver. This process modulates hepatic immune responses through mechanisms such as immune cell activation and systemic inflammation. The gut-liver immune axis maintains tolerance through balanced microbiota composition and intact intestinal barrier. Increased intestinal permeability (evidenced by 60% occludin reduction) allows microbial translocation (10^3 copies/mL bacterial 16S rRNA in portal blood), which dysregulates hepatic immune tolerance via two mechanisms: 1.Direct modulation of Kupffer cell function: LPS from translocated bacteria activates TLR4/NF-κB pathway in Kupffer cells, shifting their phenotype from IL-10-producing tolerogenic to pro-inflammatory. 2.Treg modulation: Microbial metabolites like butyrate (at physiological 2-4mM) maintain Treg suppressive function via HDAC inhibition, while dysbiosis-induced reduction (to <1mM in HCC) impairs Foxp3+ Treg stability. A reduction in the expression of the tight junction protein occludin in the gut permits bacterial products, such as LPS, to access the liver via the portal vein. The LPS-TLR4 axis is crucial in the pathogenesis of leaky gut syndrome. The translocation of LPS, resulting from increased intestinal permeability, activates TLR4 on hepatic Kupffer cells, thereby initiating the NF-κB signaling pathway and promoting the release of inflammatory mediators (35). LPS, in conjunction with signaling molecules such as adenosine triphosphate (ATP), can activate the NLRP3 inflammasome, thereby facilitating the maturation of interleukin-1β (IL-1β) and exacerbating hepatic inflammation (36). Antigens originating from the gut are transported to the liver by dendritic cells, which subsequently activate CD4+ T cells, including T helper 17 (Th17) cells, and CD8+ T cells, thereby enhancing specific immune responses (37). Pro-inflammatory factors derived from the gut, such as transforming growth factor-beta (TGF-β), can activate hepatic stellate cells, resulting in collagen deposition and fibrosis.
A randomized double-blind controlled trial (RCT) focusing on irritable bowel syndrome (IBS) demonstrated that a composite probiotic formulation containing Lactobacillus and Bifidobacterium significantly decreased intestinal permeability in IBS patients (38). Additional studies have suggested that supplementation with probiotics can reduce serum LPS levels in patients with NAFLD, thereby mitigating intestinal leakage (39). Nonetheless, the impact of these probiotics on gut barrier function in patients with liver cancer remains unclear, necessitating further investigation.
The initial activation of Kupffer cells in the liver is mediated through TLRs recognizing LPS. Kupffer cells, the primary resident macrophages in the liver, are adept at recognizing and responding to gut-derived bacterial components via various PRRs, with a particular emphasis on TLRs. Empirical studies have demonstrated a significant correlation between the activation of Kupffer cells and the pathogenesis of liver cancer, particularly under conditions of chronic inflammation. Upon recognition of LPS via TLRs, Kupffer cells trigger a cascade of signaling pathways, notably the NF-κB and MAPK pathways, which culminate in the secretion of pro-inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α) and IL-6, thereby intensifying hepatic inflammation. Furthermore, LPS stimulation results in an elevated expression of hypoxia-inducible factor 1-alpha (HIF-1α) in Kupffer cells, indicating its potential role in facilitating adaptation to hypoxic conditions and modulating metabolic processes (40). In the pathophysiological development of liver cancer, Kupffer cells play a dual role by not only participating in the inflammatory response but also modulating hepatocyte proliferation and fibrosis through the secretion of various cytokines and chemokines, thereby contributing to the progression of liver cancer to a certain degree (41). The initial immune activation mechanism of Kupffer cells, which involves the recognition of LPS through TLRs, constitutes a fundamental aspect of the liver’s immune response. This mechanism also provides a critical framework for understanding liver inflammation and regeneration. Future research should focus on elucidating strategies to enhance the prognosis of liver-related diseases by regulating the functionality of Kupffer cells, thereby offering novel insights for precision treatment approaches.
3.2.2 Dual immunoregulatory role of microbial metabolites
The involvement of SCFAs in the pathogenesis and progression of HCC constitutes a complex and multifaceted area of research. This field encompasses various mechanisms, including immune regulation, metabolic intervention, and the protection of the intestinal barrier. In the context of hepatic innate immune cells, SCFAs, particularly butyrate, exert regulatory effects on the polarization and functionality of liver macrophages through multiple mechanisms. Hu et al. demonstrated that gut-derived SCFAs can attenuate hepatic inflammatory responses by promoting the polarization of macrophages towards the M2 phenotype, thereby mitigating liver damage. Within the milieu of liver cancer, an optimal concentration of SCFAs is crucial for maintaining macrophage activity against tumors; however, a reduction in SCFA levels is associated with an increase in pro-tumorigenic M2 macrophages (42). Furthermore, SCFAs modulate inflammatory gene expression at the epigenetic level by inhibiting histone deacetylase (HDAC) activity, which influences their function within the liver cancer microenvironment (43). Recent investigations have revealed that butyrate modulates the activation of the NLRP3 inflammasome in liver macrophages via G protein-coupled receptors GPR43 and GPR109A. This modulation plays a dual role in the progression of liver cancer: initially suppressing excessive inflammatory responses and subsequently potentially facilitating immune suppression (44). Furthermore, research conducted by Ma et al. indicates that dysbiosis results in diminished SCFA levels, which are associated with a decrease in liver NKT cell populations and compromised immune surveillance. SCFAs have the capacity to modulate NKT cells through the CXCL16-CXCR6 signaling pathway, thereby facilitating the progression of liver cancer (4). Additionally, Hu et al. identified that gut-derived SCFAs augment the anti-liver cancer efficacy of type 3 innate lymphoid cells (ILC3s) by enhancing IFN-γ production. SCFAs not only exert a direct influence on the functionality of ILC3s but also regulate their interaction with NKT cells, collectively contributing to immune surveillance in HCC. Moreover, SCFAs impact the maturation and functionality of liver DCs. Zheng et al. demonstrated that butyrate induces the transformation of liver DCs into a tolerogenic phenotype via the GPR109A signaling pathway, thereby reducing their secretion of pro-inflammatory cytokines and altering their antigen presentation capabilities (45). Within the liver cancer microenvironment, fluctuations in SCFA levels can modulate the capacity of DCs to activate T cells, consequently affecting the efficacy of anti-tumor immune responses.
Regarding hepatic adaptive immune cells, SCFAs particularly butyrate, can enhance the anti-tumor activity of CD8+ T cells through various mechanisms, including metabolic reprogramming, epigenetic regulation, and immune checkpoint modulation. Specifically, butyrate facilitates the transition of CD8+ T cells from glycolysis to oxidative phosphorylation, thereby improving mitochondrial function and promoting T cell persistence and memory formation (46). Additionally, SCFAs can upregulate the expression of effector and memory-associated genes, such as IFN-γ, Granzyme B, and T-bet, by inhibiting HDACs, which in turn enhances the cytotoxic function of CD8+ T cells (47). Furthermore, butyrate has been shown to inhibit HDAC3 activity by 78 ± 5% at a concentration of 10 μM, while propionate reduces IL-1β secretion from Kupffer cells from 1250 ± 150 to 380 ± 50 pg/mL via the GPR43 receptor (48). SCFAs have been shown to downregulate the expression of PD-1 in CD8+ T cells within the liver cancer microenvironment, thereby mitigating T cell exhaustion and enhancing the efficacy of anti-PD-1/PD-L1 immunotherapy. Furthermore, SCFAs play a significant role in the differentiation and functional modulation of CD4+ T cell subsets. Notably, SCFAs, particularly butyrate, have been found to enhance the expression of Foxp3 and facilitate the differentiation and stability of Tregs by promoting histone acetylation in the promoter region of the Foxp3 gene (49). In the context of the liver cancer microenvironment, an optimal level of Tregs is crucial for regulating excessive inflammatory responses; however, an overabundance of Tregs may lead to immune suppression and contribute to tumor progression. The effects of SCFAs on Th17 cells are more complex; while SCFAs can inhibit Th17 cell differentiation and thereby reduce inflammatory responses, under certain conditions, they may also enhance Th17 cell functionality, thereby participating in anti-tumor immunity (50).
In the context of immune-suppressive cells, SCFAs have been shown to attenuate the recruitment of MDSCs by downregulating the expression of chemokines such as CCL2 within the liver cancer microenvironment (51). Additionally, SCFAs diminish the capacity of MDSCs to produce immunosuppressive molecules, including arginase 1 (Arg1) and inducible nitric oxide synthase (iNOS), via the GPR43 signaling pathway, thereby mitigating their suppression of T cells and NK cells. SCFAs also facilitate the differentiation of MDSCs into M1 macrophages, thereby converting them into tumor-fighting cells. Research conducted by Bi et al. has demonstrated that SCFAs modulate the function of neutrophils in the liver cancer microenvironment by influencing their recruitment and polarization (52). Specifically, SCFAs decrease the proportion of N2 (pro-tumor) neutrophils while increasing N1 (anti-tumor) neutrophils, which is advantageous for the immune-mediated control of liver cancer. Given the dual regulatory role of SCFAs, future investigations should aim to optimize their dosage and administration strategies to enhance their anticancer efficacy while minimizing potential adverse effects.
Secondary bile acids can promote the establishment of an immunosuppressive microenvironment in the liver through the activation of the TGR5 and FXR receptor pathways, consequently influencing tumor immune surveillance and evasion. Secondary bile acids, particularly elevated levels of DCA, have been shown to induce the polarization of Kupffer cells towards the M2 phenotype, which is immunosuppressive, via the TGR5 and FXR receptor signaling pathways. This polarization results in the secretion of inhibitory cytokines, including IL-10 and TGF-β, thereby suppressing anti-tumor immune responses (53). Furthermore, DCA modulates the inflammatory response of Kupffer cells through the NF-κB and STAT3 signaling pathways, promoting inflammation in the early stages of liver cancer and potentially facilitating immune suppression in later stages. The regulation of NK and NKT cells by secondary bile acids is crucial within the liver cancer immune microenvironment. Research conducted by Ma et al. demonstrated that dysregulated bile acid metabolism results in increased DCA levels, which inhibit the expression of CXCL16 in the liver, thereby reducing the recruitment of CXCR6+ NKT cells and diminishing the liver’s immune surveillance against tumors (43). Additionally, DCA impairs the cytotoxic function of NK cells against liver cancer cells by altering the expression of activation receptors and the release of cytotoxic molecules, such as perforin and granzyme B (54). Elevated concentrations of secondary bile acids have been shown to inhibit the secretion of IFN-γ by CD8+ T cells, while simultaneously promoting the proliferation of MDSCs, inducing apoptosis and functional exhaustion in CD8+ T cells, and upregulating inhibitory receptors such as PD-1 and TIM-3. These effects collectively diminish the anti-tumor efficacy of CD8+ T cells. Furthermore, secondary bile acids facilitate the differentiation of Tregs and suppress the function of Th17 cells via the FXR and TGR5 signaling pathways, thereby establishing an immunosuppressive milieu within the liver cancer microenvironment. Elevated levels of DCA have been implicated in the increased accumulation of MDSCs in the liver through the COX2-PGE2 pathway, thereby augmenting their immunosuppressive capabilities (55). Some studies propose that the strategic use of secondary bile acids in conjunction with liver cancer immunotherapy could represent a promising therapeutic avenue. Specifically, modulating the metabolism of secondary bile acids may restore the anti-tumor function of CD8+ T cells, thereby enhancing the efficacy of immunotherapeutic interventions.
Various metabolites, including TMAO, have been implicated in exacerbating liver inflammatory responses. TMAO achieves this by activating the NLRP3 inflammasome, which in turn stimulates Kupffer cells to secrete pro-inflammatory cytokines such as IL-1β and IL-18. Additionally, TMAO facilitates macrophage polarization towards the M1 phenotype through the NF-κB signaling pathway, potentially exerting anti-tumor effects during the early stages of liver cancer. However, prolonged inflammation may contribute to the progression of liver cancer (56). TMAO also influences T cell differentiation and function by promoting Th17 cell differentiation while inhibiting Treg cell function, thereby intensifying liver inflammatory responses. Furthermore, TMAO can promote the expansion and activation of MDSCs, enhancing their immunosuppressive capabilities and facilitating liver cancer progression. In parallel, tryptophan metabolites significantly impact T cell differentiation via the aryl hydrocarbon receptor (AhR) signaling pathway (57). Specifically, kynurenine has been shown to promote tumor cell expression of PD-L1 through the AhR-NF-κB pathway.
3.2.3 Microbiota-mediated metabolic-immune crosstalk
In the pathogenesis of liver cancer, the gut microbiota is pivotal in facilitating the interaction between metabolic processes and immune responses. While there is presently insufficient research to establish a direct causal link between metabolic disorders induced by abnormal microbiota and the development of HCC, it is well-established that an imbalance in gut microbiota can result in metabolic abnormalities. These abnormalities are associated with the onset and progression of NAFLD, which is a significant risk factor for HCC. Furthermore, small intestinal bacterial overgrowth and dysbiosis of gut microbiota are recognized as critical factors in the progression of NAFLD to non-alcoholic steatohepatitis (NASH) and decompensated liver disease (58). Dysbiosis can influence tryptophan metabolism, which may promote liver cancer through the upregulation of sterol regulatory element-binding protein 2 (SREBP2) (59). Tryptophan metabolites are capable of modulating the expression of PD-1 via the AhR pathway, consequently impacting T cell functionality. As previously discussed, metabolites derived from the gut microbiota, including short-chain fatty acids, bile acids, and indole, exert significant effects on hepatic immune and metabolic functions through the gut-liver axis. For example, succinate can enhance the infiltration of cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) positive T cells by interacting with its receptor SUCNR1, thus modifying the immune milieu of the tumor microenvironment (60). The potential implications of this research for immunotherapy in liver cancer are significant. Alterations in the liver microbiota composition may reduce the liver’s metabolic capacity for drugs, thereby influencing the effectiveness of liver cancer treatments. Empirical evidence suggests that modulating the gut microbiota can enhance the efficacy of immunotherapy for liver cancer. Interventions such as probiotics, prebiotics, antibiotics, and fecal microbiota transplantation may emerge as crucial adjuncts to liver cancer immunotherapy. These strategies enhance immunotherapy by modulating the host immune system, minimizing adverse effects, and improving survival rates among liver cancer patients.
3.2.4 Microbiota-driven mechanisms of immune evasion
Recent investigations have elucidated the role of gut microbiota in modulating immune evasion mechanisms in liver cancer through multiple pathways. Primarily, gut microbiota can facilitate immune evasion by modulating the host immune system. Dysbiosis within the gut microbiota can precipitate immune system disorders, thereby impairing the efficacy of anti-tumor immune responses. For instance, metabolites produced by specific gut bacteria have been shown to inhibit immune cell activity, consequently diminishing their capacity to identify and eliminate tumor cells. Furthermore, the gut microbiota can influence immune evasion by modulating immune cells within the liver cancer microenvironment, such as by altering the activity of T cells and natural killer cells, which in turn promotes tumor growth and metastasis. Additionally, the gut microbiota may contribute to immune evasion in liver cancer by regulating the expression of immune checkpoint molecules. Empirical evidence suggests that gut microbiota can modulate the expression of PD-L1 via its metabolites, thereby suppressing anti-tumor immune responses and facilitating tumor immune evasion (61). The mechanisms of immune evasion facilitated by microbiota present significant challenges in the treatment of liver cancer.
4 Therapeutic potential of microbiota modulation in HCC
4.1 Dysbiosis in HCC patients
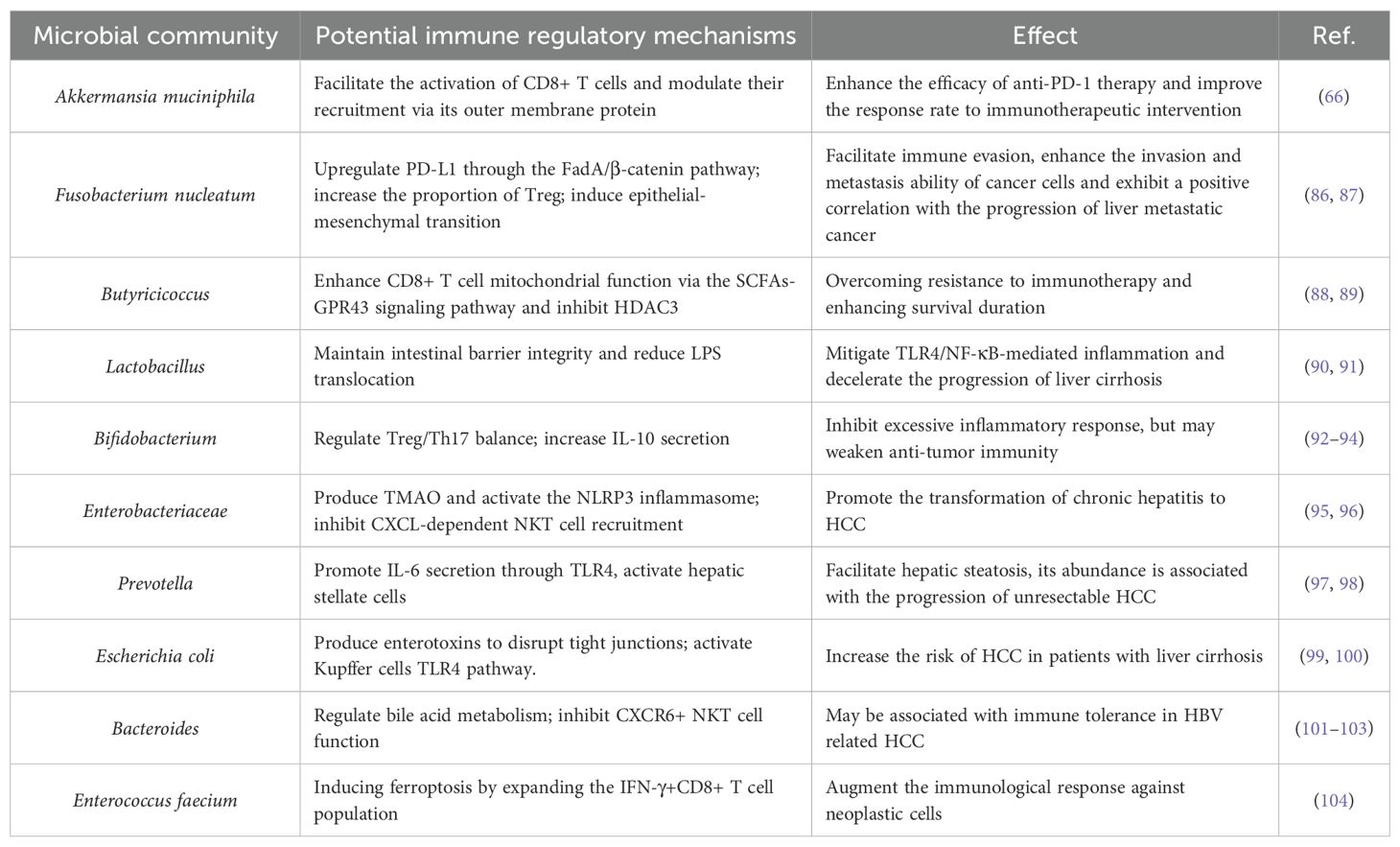
Dysbiosis is frequently observed in individuals with HCC. A comparative study involving HCC patients, individuals with cirrhosis, and healthy controls identified two bacterial species, Odoribacter splanchnicus and Ruminococcus bicirculans, as being significantly associated with HCC. Additionally, the study highlighted five metabolites—ouabain, taurochenodeoxycholic acid, glycochenodeoxycholate, theophylline, and xanthine—as key biomarkers (62). Research into the gut microbiota of HCC patients reveals a marked reduction in microbial diversity. Beneficial bacteria, such as Lactobacilli and Bifidobacteria, are present at diminished levels, whereas pathogenic bacteria, including Proteus and Escherichia coli, are found in increased abundance. Clinical data indicate a decrease in the Bacteroidetes to Firmicutes ratio from 0.92 in healthy individuals to 0.48 in HCC patients, alongside a notable increase in the abundance of Fusobacterium nucleatum from 0.01% in healthy subjects to 1.2% in HCC patients (63). Further investigations suggest that an elevated Firmicutes population coupled with reduced Bacteroidetes may contribute to immune suppression within the hepatic microenvironment. The abundance of Fusobacterium nucleatum is strongly correlated with the prevalence of Tregs, as evidenced by a correlation coefficient of r=0.62 (p<0.001) (64). Fusobacterium nucleatum facilitates the proliferation of Tregs and attenuates the activity of effector T cells by modulating cytokine levels within the tumor microenvironment and enhancing the expression of PD-L1 on the surfaces of tumor cells (65). This interaction consequently confers a survival advantage to hepatocellular carcinoma cells.
4.2 Microbiota typing-guided immunotherapy stratification
Microbiota typing is emerging as a novel biomarker with significant implications for immunotherapy, paving the way for the advancement of personalized medicine. Akkermansia muciniphila, a prevalent Gram-negative bacterium within the gut microbiome, has been identified in recent studies as having a positive correlation with patient responses to anti-PD-1 therapy (66). Patients exhibiting higher levels of Akkermansia muciniphila tend to experience improved survival rates and extended periods without disease progression, indicating its potential utility as a predictive biomarker for immunotherapy efficacy. Notably, there was a significant association between decreased level of gut microbial metabolite butyrate and increased resistance to sorafenib (67). Recent research has initiated an investigation into dietary interventions designed to restore butyrate levels, to assess the potential efficacy of this intervention in improving drug resistance (68). The lack of butyrate-producing bacteria (such as Butyricicoccus pullicaecorum and Roseburia intestinalis) may serve as an indicator of resistance in patients with HCC undergoing sorafenib therapy. However, further conclusive research is necessary to substantiate this hypothesis.
Resistance remains a significant challenge in the treatment of liver cancer, prompting researchers to investigate various strategies to overcome this obstacle and enhance therapeutic outcomes. Recent studies have substantiated that a reduction in gut microbiota diversity is significantly correlated with resistance to anti-PD-1 therapy. The underlying mechanism may involve a decrease in butyrate-producing bacteria, which impairs CD8+ T cell function. Concurrently, an increase in pathogenic bacteria, such as Escherichia coli, may contribute to T cell exhaustion via the LPS-TLR4 signaling pathway (69, 70). Numerous tumor cells circumvent immune system attacks by downregulating the expression of Major Histocompatibility Complex Class I (MHC-I). Studies have demonstrated that specific interventions, including Fhit gene transfection, can restore or augment MHC-I expression, thereby enhancing the efficacy of immunotherapy (71). There is a paucity of research to substantiate the use of microbiota as a supplementary intervention to enhance the efficacy of immunotherapy. The potential for probiotics to indirectly modulate MHC-I expression in tumor cells through the regulation of interactions between gut microbiota and the immune system warrants further investigation, as this may offer a potential approach to improving resistance to sorafenib. Further clinical studies are required to validate the impact of gut microbiota interventions, such as butyrate supplementation, on drug resistance in patients with HCC.
4.3 Synergistic treatment model targeting microbiota
Recent advancements in liver cancer treatment research have increasingly focused on the interplay between the microbiome and local immune responses, marking a shift towards precision medicine. Microbiota-targeted therapies have demonstrated significant potential, as the gut microbiota composition in liver cancer patients is closely associated with their clinical characteristics. Personalized interventions may therefore enhance therapeutic efficacy. Current research methodologies include the use of probiotics, prebiotics, synbiotics, and fecal microbiota transplantation (FMT). It is recommended to integrate microbiota modulation strategies to enhance treatment efficacy and minimize adverse effects. Despite the promising prospects, challenges such as individual variability, long-term stability, and tolerance persist, highlighting the need for standardized and personalized approaches in clinical trials.
Inulin, a prebiotic, demonstrates significant potential in tumor immunotherapy, particularly in conjunction with the oncolytic virus T-VEC (72). T-VEC, derived from the herpes simplex virus, has received FDA approval for the treatment of unresectable melanoma. Research indicates that inulin may promote the proliferation of beneficial microbiota, enhance anti-tumor immune responses, and potentially improve gut barrier function, thereby reducing chronic inflammation and creating a more conducive microenvironment for T-VEC. Alterations in the gut microbiota of liver cancer patients have been associated with liver tumor development, and optimizing this microbiota is anticipated to augment the therapeutic efficacy of T-VEC. However, excessive consumption of inulin may elevate the risk of NAFLD and potentially hepatic carcinoma (73). As a prebiotic, inulin plays a role in modulating the gut microbiota and enhancing the production of SCFAs. Nonetheless, an overabundance of SCFAs may disrupt hepatic lipid metabolism, potentially leading to the development of fatty liver. The risks associated with excessive inulin intake warrant further investigation and validation. It is imperative for clinicians and nutritionists to carefully consider individual variability and consumption levels when recommending inulin as a dietary supplement to mitigate potential health risks. Future research should aim to elucidate the dose-response relationship between inulin intake and liver health, thereby providing more robust scientific guidance.
FMT can enhance the effectiveness of immune checkpoint inhibitors and oncolytic virus therapy in treating tumors. Studies indicate that FMT boosts beneficial microbiota, improving patient responses to these treatments and leading to better outcomes. When combined with anti-CTLA-4 antibodies, FMT can synergistically modulate the microbiota and immune system, achieving an objective response rate of 54% (74). FMT can be timed with immunotherapy to optimize gut microbiota, boost the immune system, and improve treatment outcomes. It also enhances oncolytic virus replication in tumors, increasing anti-tumor effects. Studies in mice show that FMT leads to greater tumor shrinkage and longer survival after oncolytic virus treatment, supporting its use in liver cancer. Additionally, FMT combined with oncolytic virus therapy alters gut microbiota, increases beneficial bacteria, and enhances immune status, potentially affecting tumor growth via the gut-brain or gut-immune axis.
Engineered bacteria are gaining attention in liver cancer treatment by using genetic engineering to release immune molecules at tumor sites, enhancing immune responses. For instance, Escherichia coli Nissle 1917 (EcN) can secrete CXCL16 (75), activating the p38 MAPK pathway to recruit and enhance NK cell activity, showing promise in therapy. Similarly, attenuated Salmonella typhimurium can deliver IL-12 to activate CD103+ dendritic cells, boosting immune responses (76). IL-12 enhances T cell and NK cell activation, boosting tumor cell destruction. As liver cancer treatment moves towards immunotherapy, CAR-T cell therapy offers new hope. Researchers are using engineered bacteria to deliver IL-15 and FLT3L at tumor sites, improving CAR-T cell infiltration and local immune response.
However, challenges like microbiota differences, FMT standardization, and safety concerns remain. For example, IL-15 engineered bacteria therapy has a 28% incidence of cytokine release syndrome (77), and severe infections after FMT occur in about 3.5% of cases. Future research must address these issues to optimize FMT and immunotherapy.
5 Insights from microbiota-immune crosstalk in HCC associated hepatic disorders
5.1 Gut microbiota’s role in liver cancer in NAFLD
NAFLD, a prevalent liver disease globally, is strongly associated with HCC, with gut microbiota imbalance being a major factor. Research indicates notable shifts in NAFLD patients’ gut microbiota, including reduced diversity (Shannon index from 3.8 to 2.9) and increased levels of Proteobacteria, Escherichia Shigella and Erysipelotrichaceae. The abundance of Prevotella in the NAFLD group, as well as the abundance of Bacteroidetes, is lower compared to the healthy control group (78). Moreover, elevated fecal counts of Escherichia coli, have been correlated with the occurrence of HCC in patients with cirrhosis (79). In NAFLD patients with liver cancer, fluctuations in gut bacteria are linked to systemic inflammation, indicating a joint role in liver cancer development. Alterations in gut microbiota metabolites like TMAO and the gut virome are also tied to NAFLD severity and may affect its progression to HCC. Modifying gut microbiota could potentially improve NAFLD and lower HCC risk, though the exact mechanisms remain unclear. Further research is essential to uncover liver cancer pathogenesis and enhance early diagnosis and intervention.
5.2 Microbial regulation in chronic viral hepatitis
Individuals with chronic viral hepatitis, such as hepatitis B and C, exhibit reduced gut microbial diversity compared to healthy individuals. Specific gut bacterial taxa are associated with liver inflammation, potentially leading to increased fibrosis and a heightened risk of liver cancer. A notable reduction in the Bifidobacteria/Enterobacteriaceae (B/E) ratio was observed across various stages of liver disease progression, with the most pronounced decrease occurring in patients with decompensated HBV cirrhosis (80). These findings are supported by another study, which demonstrated that HBV infection is associated with an increase in potentially pathogenic bacteria, such as Enterobacteriaceae, potentially contributing to the progression of liver disease (81). Additionally, abnormal serum bile acid profiles, characterized by a 2.8-fold increase in glycochenodeoxycholic acid (GCDCA), correlate with reduced hepatic expression of CXCL16 (r = -0.71) (81). In the context of chronic hepatitis B infection, dysbiosis of the gut microbiota may influence viral replication and immune responses. Studies suggest that patients who have achieved a functional cure exhibit a greater abundance of SCFA-producing bacteria in their gut. Notably, butyrate, a specific SCFA, has been shown to inhibit HBV production, indicating that gut microbiota may modulate HBV replication through SCFA-mediated mechanisms. In patients with chronic hepatitis C, microbial translocation and T cell activation are linked to the progression of the disease. Antiviral therapy has been shown to decrease the levels of microbial translocation markers and ameliorate liver damage. Additionally, the association between chronic viral hepatitis and metabolic syndrome underscores the potential involvement of gut microbiota in this process. Research indicates that individuals with chronic hepatitis C frequently exhibit fatty liver, and alterations in the gut microbiota composition may be associated with hepatic fat accumulation (82). This accumulation of fat exacerbates inflammation and fibrosis within the liver, thereby perpetuating a detrimental cycle.
Gut microbiota interventions have potential in enhancing liver function and reducing inflammation in chronic viral hepatitis patients. These therapies can rebalance gut bacteria and boost antiviral treatment effectiveness by strengthening the immune response. Understanding gut bacteria’s role is crucial for preventing and treating liver cancer.
5.3 Gut microbiota and liver cancer in AIH patients
AIH patients often experience gut microbiota dysbiosis, potentially linked to liver cancer. In AIH mouse models, impaired gut barriers allow bacteria to reach the liver, worsening inflammation and possibly leading to cancer (83). Both AIH patients and models show gut microbiota changes affecting T follicular helper and regulatory cells (84), disrupting immune detection of liver cancer. Metabolic issues like fatty liver and fibrosis, linked to dysbiosis, are also cancer risk factors. Thus, targeting gut microbiota with probiotics or dietary changes might reduce liver cancer risk in AIH patients.
5.4 The oral-gut-liver axis and its immune regulatory role
The oral-gut-liver axis plays a crucial role in metabolism and immune regulation. Alterations in oral microbiota can increase gut permeability, leading to systemic inflammation, metabolic disorders, and liver damage. Harmful oral bacteria can reach the liver via the gut, potentially causing conditions like NAFLD, liver fibrosis (85), and increasing liver cancer risk. Balancing oral microbiota with probiotics and mouthwash, along with dietary changes and supplements, may enhance liver function by regulating this axis.
This review examines the interactions between gut microbiota and the immune environment in liver cancer, highlighting clinical translation prospects. Research shows that gut microbiota influences liver immune balance through metabolic products and molecular patterns via the portal vein, with TLR4/NF-κB and bile acid-FXR/TGR5 pathways being crucial (Figure 1). Dysbiosis, like increased Fusobacterium nucleatum and decreased butyrate-producing bacteria, can promote immune evasion in liver cancer by affecting Kupffer cell polarization, T cell balance, and CD8+ T cell exhaustion. Additionally, specific microbiota traits, such as Akkermansia muciniphila abundance, may indicate immune therapy response (Table 1). Microbiota interventions, including fecal transplants, engineered bacteria, and metabolite supplements, could improve current therapies but are challenged by individual variability and safety concerns. Microbial metabolites like butyrate have concentration-dependent effects, boosting antitumor immunity at low levels and causing tolerance at high levels. More evidence is needed to establish causation, requiring further animal model studies for precise control.
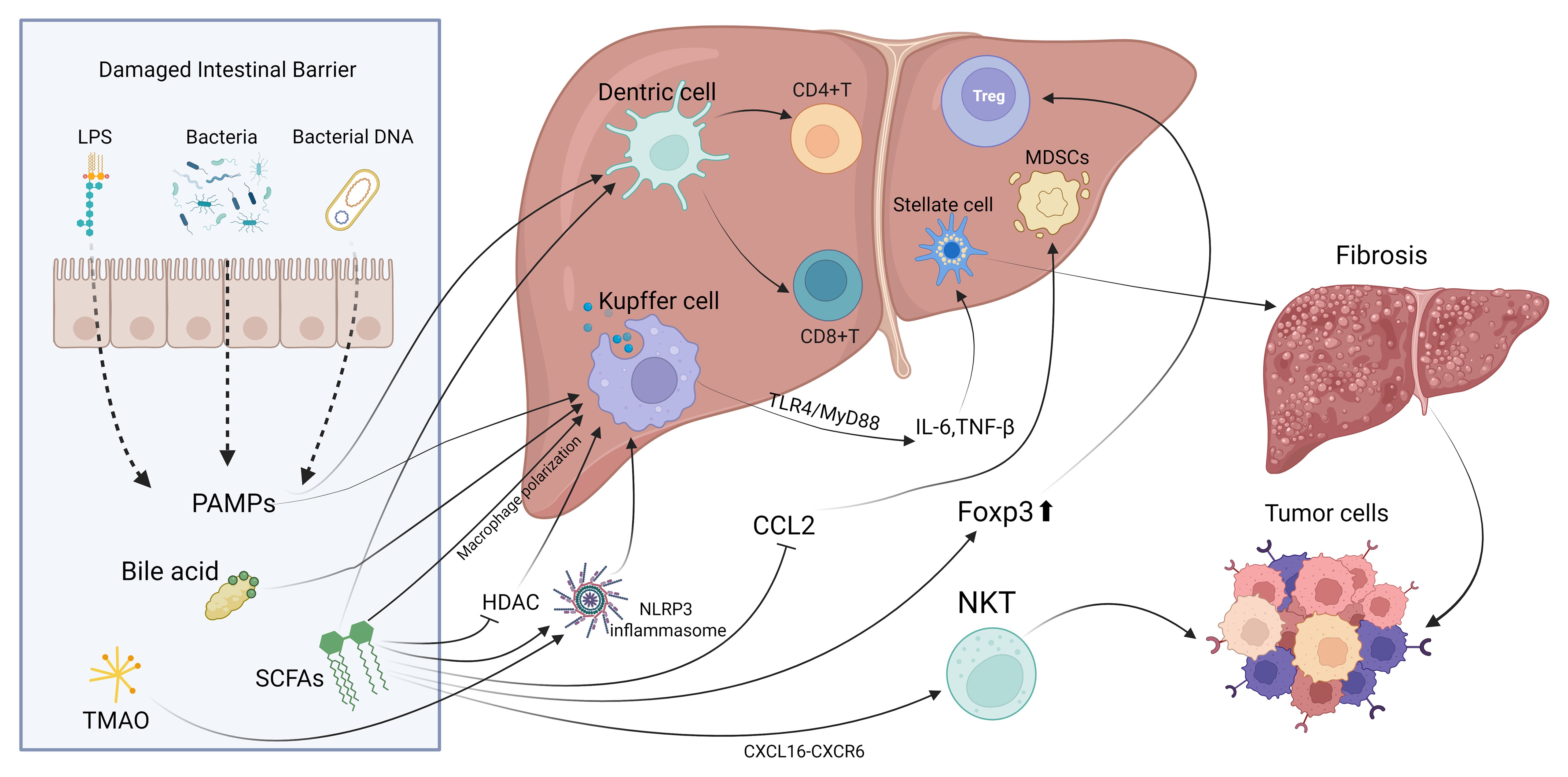
Figure 1. The principal mechanisms and pathways through which gut microbiota interact with local immune cells in the liver.
Future research should aim to identify crucial strains, metabolic products, and signaling pathways, understand the origins and dynamics of tumor-associated microorganisms, validate microbial biomarkers, and create personalized interventions like engineered bacteria or metabolic antagonists to boost synergy with current therapies. Technological advances, such as humanized organoid models and AI-driven multi-omics integration, will address microbial diversity and translational challenges, facilitating the transition from mechanistic studies to precise clinical applications and offering new strategies to overcome immune therapy resistance in liver cancer.
6 Conclusion
In conclusion, the gut microbiota and its metabolites are vital in liver cancer development, affecting the local immune environment through complex mechanisms. Understanding their interaction with liver cancer enhances our knowledge of its pathogenesis and offers new precision treatment options. Probiotics and microbial management strategies show promise in improving immune responses and influencing disease progression by regulating gut microbiota. Future multi-omics approaches will further advance personalized immunotherapy for liver cancer. Ongoing research and clinical implementation are crucial for the early diagnosis and accurate treatment of HCC, thereby enhancing patient outcomes.
Author contributions
SL: Formal analysis, Investigation, Supervision, Writing – review & editing. YX: Investigation, Methodology, Resources, Writing – original draft. BW: Investigation, Resources, Writing – original draft. NG: Formal analysis, Investigation, Resources, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Wen J, Xia M, Luo H, Zhu L, Li M, and Hou Y. Global, regional, and national burden of liver cancer in adolescents and young adults from 1990 to 2021: an analysis of the global burden of disease study 2021 and forecast to 2040. Front Public Health. (2025) 13:1547106. doi: 10.3389/fpubh.2025.1547106
2. Fianchi F, Liguori A, Gasbarrini A, Grieco A, and Miele L. Nonalcoholic fatty liver disease (NAFLD) as model of gut-liver axis interaction: from pathophysiology to potential target of treatment for personalized therapy. Int J Mol Sci. (2021) 22(12):6485. doi: 10.3390/ijms22126485
3. Scarpellini E, Scarlata GGM, Santori V, Scarcella M, Kobyliak N, and Abenavoli L. Gut microbiota, deranged immunity, and hepatocellular carcinoma. Biomedicines. (2024) 12(8):1797. doi: 10.3390/biomedicines12081797
4. Zhang N, Gou Y, Liang S, Chen N, Liu Y, He Q, et al. Dysbiosis of gut microbiota promotes hepatocellular carcinoma progression by regulating the immune response. J Immunol Res. (2021) 2021:4973589. doi: 10.1155/2021/4973589
5. Sole C, Guilly S, Da Silva K, Llopis M, Le-Chatelier E, Huelin P, et al. Alterations in gut microbiome in cirrhosis as assessed by quantitative metagenomics: relationship with acute-on-chronic liver failure and prognosis. Gastroenterology. (2021) 160:206–18 e13. doi: 10.1053/j.gastro.2020.08.054
6. Park J, Kim M, Kang SG, Jannasch AH, Cooper B, Patterson J, et al. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway. Mucosal Immunol. (2015) 8:80–93. doi: 10.1038/mi.2014.44
7. Jia H, Lyu W, Hirota K, Saito E, Miyoshi M, Hohjoh H, et al. Eggshell membrane modulates gut microbiota to prevent murine pre-cachexia through suppression of T helper cell differentiation. J Cachexia Sarcopenia Muscle. (2022) 13:2088–101. doi: 10.1002/jcsm.13019
8. Sardar P, Beresford-Jones BS, Xia W, Shabana O, Suyama S, Ramos RJF, et al. Gut microbiota-derived hexa-acylated lipopolysaccharides enhance cancer immunotherapy responses. Nat Microbiol. (2025) 10:795–807. doi: 10.1038/s41564-025-01930-y
9. Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews MC, Karpinets TV, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. (2018) 359:97–103. doi: 10.1126/science.aan4236
10. Mao J, Wang D, Long J, Yang X, Lin J, Song Y, et al. Gut microbiome is associated with the clinical response to anti-PD-1 based immunotherapy in hepatobiliary cancers. J Immunother Cancer. (2021) 9(12):e003334. doi: 10.1136/jitc-2021-003334
11. Heymann F, Peusquens J, Ludwig-Portugall I, Kohlhepp M, Ergen C, Niemietz P, et al. Liver inflammation abrogates immunological tolerance induced by Kupffer cells. Hepatology. (2015) 62:279–91. doi: 10.1002/hep.27793
12. Gong J, Cao D, Chen Y, Li J, Gong J, and Zeng Z. Role of programmed death ligand 1 and Kupffer cell in immune regulation after orthotopic liver transplantation in rats. Int Immunopharmacol. (2017) 48:8–16. doi: 10.1016/j.intimp.2017.04.009
13. Deng Z, Mei S, Ouyang Z, Wang R, Wang L, Zou B, et al. Dysregulation of gut microbiota stimulates NETs-driven HCC intrahepatic metastasis: therapeutic implications of healthy faecal microbiota transplantation. Gut Microbes. (2025) 17:2476561. doi: 10.1080/19490976.2025.2476561
14. von Oppen N, Schurich A, Hegenbarth S, Stabenow D, Tolba R, Weiskirchen R, et al. Systemic antigen cross-presented by liver sinusoidal endothelial cells induces liver-specific CD8 T-cell retention and tolerization. Hepatology. (2009) 49:1664–72. doi: 10.1002/hep.22795
15. Kremer KN, Khammash HA, Miranda AM, Rutt LN, Twardy SM, Anton PE, et al. Liver sinusoidal endothelial cells regulate the balance between hepatic immunosuppression and immunosurveillance. Front Immunol. (2024) 15:1497788. doi: 10.3389/fimmu.2024.1497788
16. Lee SK, Kwon JH, Jang JW, Bae SH, Yoon SK, Jung ES, et al. The critical role of regulatory T cells in immune tolerance and rejection following liver transplantation: interactions with the gut microbiome. Transplantation. (2025) 109:784–93. doi: 10.1097/TP.0000000000005220
17. Lou G, Ma X, Fu X, Meng Z, Zhang W, Wang YD, et al. GPBAR1/TGR5 mediates bile acid-induced cytokine expression in murine Kupffer cells. PloS One. (2014) 9:e93567. doi: 10.1371/journal.pone.0093567
18. Liu Y, Liang X, Dong W, Fang Y, Lv J, Zhang T, et al. Tumor-repopulating cells induce PD-1 expression in CD8(+) T cells by transferring kynurenine and ahR activation. Cancer Cell. (2018) 33:480–94 e7. doi: 10.1016/j.ccell.2018.02.005
19. Choi G, Na H, Kuen DS, Kim BS, and Chung Y. Autocrine TGF-beta1 maintains the stability of foxp3(+) regulatory T cells via IL-12Rbeta2 downregulation. Biomolecules. (2020) 10(6):819. doi: 10.3390/biom10060819
20. Ellis RJ, Iudicello JE, Heaton RK, Isnard S, Lin J, Routy JP, et al. Markers of gut barrier function and microbial translocation associate with lower gut microbial diversity in people with HIV. Viruses. (2021) 13(10):1891. doi: 10.3390/v13101891
21. Sakamoto Y, Sasaki K, Omatsu M, Hamada K, Nakanishi Y, Itatani Y, et al. Differential involvement of LUBAC-mediated linear ubiquitination in intestinal epithelial cells and macrophages during intestinal inflammation. J Pathol. (2023) 259:304–17. doi: 10.1002/path.6042
22. Mandal P, Roychowdhury S, Park PH, Pratt BT, Roger T, and Nagy LE. Adiponectin and heme oxygenase-1 suppress TLR4/MyD88-independent signaling in rat Kupffer cells and in mice after chronic ethanol exposure. J Immunol. (2010) 185:4928–37. doi: 10.4049/jimmunol.1002060
23. Ponziani FR, Bhoori S, Castelli C, Putignani L, Rivoltini L, Del Chierico F, et al. Hepatocellular carcinoma is associated with gut microbiota profile and inflammation in nonalcoholic fatty liver disease. Hepatology. (2019) 69:107–20. doi: 10.1002/hep.30036
24. Dapito DH, Mencin A, Gwak GY, Pradere JP, Jang MK, Mederacke I, et al. Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4. Cancer Cell. (2012) 21:504–16. doi: 10.1016/j.ccr.2012.02.007
25. Hsieh CC, Hung CH, Chiang M, Tsai YC, and He JT. Hepatic stellate cells enhance liver cancer progression by inducing myeloid-derived suppressor cells through interleukin-6 signaling. Int J Mol Sci. (2019) 20(20):5079. doi: 10.3390/ijms20205079
26. Rubinstein MR, Wang X, Liu W, Hao Y, Cai G, and Han YW. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/beta-catenin signaling via its FadA adhesin. Cell Host Microbe. (2013) 14:195–206. doi: 10.1016/j.chom.2013.07.012
27. Zhou C, Basnet R, Zhen C, Ma S, Guo X, Wang Z, et al. Trimethylamine N-oxide promotes the proliferation and migration of hepatocellular carcinoma cell through the MAPK pathway. Discov Oncol. (2024) 15:346. doi: 10.1007/s12672-024-01178-8
28. Gagliani N, Hu B, Huber S, Elinav E, and Flavell RA. The fire within: microbes inflame tumors. Cell. (2014) 157:776–83. doi: 10.1016/j.cell.2014.03.006
29. Yao Y, Cai X, Fei W, Ye Y, Zhao M, and Zheng C. The role of short-chain fatty acids in immunity, inflammation and metabolism. Crit Rev Food Sci Nutr. (2022) 62:1–12. doi: 10.1080/10408398.2020.1854675
30. Maguire C, Wang C, Ramasamy A, Fonken C, Morse B, Lopez N, et al. Molecular mimicry as a mechanism of viral immune evasion and autoimmunity. Nat Commun. (2024) 15:9403. doi: 10.1038/s41467-024-53658-8
31. Zeromski J, Kierepa A, Brzezicha B, Kowala-Piaskowska A, and Mozer-Lisewska I. Pattern recognition receptors: significance of expression in the liver. Arch Immunol Ther Exp (Warsz). (2020) 68:29. doi: 10.1007/s00005-020-00595-1
32. He Y, Fu L, Li Y, Wang W, Gong M, Zhang J, et al. Gut microbial metabolites facilitate anticancer therapy efficacy by modulating cytotoxic CD8(+) T cell immunity. Cell Metab. (2021) 33:988–1000 e7. doi: 10.1016/j.cmet.2021.03.002
33. Barrow F, Khan S, Fredrickson G, Wang H, Dietsche K, Parthiban P, et al. Microbiota-driven activation of intrahepatic B cells aggravates NASH through innate and adaptive signaling. Hepatology. (2021) 74:704–22. doi: 10.1002/hep.31755
34. Grgurevic I, Bozin T, Mikus M, Kukla M, and O’Beirne J. Hepatocellular carcinoma in non-alcoholic fatty liver disease: from epidemiology to diagnostic approach. Cancers (Basel). (2021) 13(22):5844. doi: 10.3390/cancers13225844
35. Ma W, Liu K, He Y, Deng S, Liu Y, and Wang D. Sodium humate ameliorates LPS-induced liver injury in mice by inhibiting TLR4/NF-kappaB and activating NRF2/HO-1 signaling pathways. Mol Biol Rep. (2024) 51:204. doi: 10.1007/s11033-023-09083-z
36. Gombault A, Baron L, and Couillin I. ATP release and purinergic signaling in NLRP3 inflammasome activation. Front Immunol. (2012) 3:414. doi: 10.3389/fimmu.2012.00414
37. Edwards M and Brockmann L. Microbiota-dependent modulation of intestinal anti-inflammatory CD4(+) T cell responses. Semin Immunopathol. (2025) 47:23. doi: 10.1007/s00281-025-01049-6
38. Skrzydlo-Radomanska B, Prozorow-Krol B, Cichoz-Lach H, Majsiak E, Bierla JB, Kanarek E, et al. The effectiveness and safety of multi-strain probiotic preparation in patients with diarrhea-predominant irritable bowel syndrome: A randomized controlled study. Nutrients. (2021) 13(3):756. doi: 10.3390/nu13030756
39. Xue L, He J, Gao N, Lu X, Li M, Wu X, et al. Probiotics may delay the progression of nonalcoholic fatty liver disease by restoring the gut microbiota structure and improving intestinal endotoxemia. Sci Rep. (2017) 7:45176. doi: 10.1038/srep45176
40. Ting KKY, Yu P, Dow R, Ibrahim H, Karim S, Polenz CK, et al. Cholesterol accumulation impairs HIF-1alpha-dependent immunometabolic reprogramming of LPS-stimulated macrophages by upregulating the NRF2 pathway. Sci Rep. (2024) 14:11162. doi: 10.1038/s41598-024-61493-6
41. Slevin E, Baiocchi L, Wu N, Ekser B, Sato K, Lin E, et al. Kupffer cells: inflammation pathways and cell-cell interactions in alcohol-associated liver disease. Am J Pathol. (2020) 190:2185–93. doi: 10.1016/j.ajpath.2020.08.014
42. Hu C, Xu B, Wang X, Wan WH, Lu J, Kong D, et al. Gut microbiota-derived short-chain fatty acids regulate group 3 innate lymphoid cells in HCC. Hepatology. (2023) 77:48–64. doi: 10.1002/hep.32449
43. Ma C, Han M, Heinrich B, Fu Q, Zhang Q, Sandhu M, et al. Gut microbiome-mediated bile acid metabolism regulates liver cancer via NKT cells. Science. (2018) 360(6391):eaan5931. doi: 10.1126/science.aan5931
44. Sun T, Xie R, He H, Xie Q, Zhao X, Kang G, et al. Kynurenic acid ameliorates NLRP3 inflammasome activation by blocking calcium mobilization via GPR35. Front Immunol. (2022) 13:1019365. doi: 10.3389/fimmu.2022.1019365
45. Zheng Y, Wang T, Tu X, Huang Y, Zhang H, Tan D, et al. Gut microbiome affects the response to anti-PD-1 immunotherapy in patients with hepatocellular carcinoma. J Immunother Cancer. (2019) 7:193. doi: 10.1186/s40425-019-0650-9
46. Luu M, Riester Z, Baldrich A, Reichardt N, Yuille S, Busetti A, et al. Microbial short-chain fatty acids modulate CD8(+) T cell responses and improve adoptive immunotherapy for cancer. Nat Commun. (2021) 12:4077. doi: 10.1038/s41467-021-24331-1
47. Lisiero DN, Soto H, Everson RG, Liau LM, and Prins RM. The histone deacetylase inhibitor, LBH589, promotes the systemic cytokine and effector responses of adoptively transferred CD8+ T cells. J Immunother Cancer. (2014) 2:8. doi: 10.1186/2051-1426-2-8
48. Maruta H and Yamashita H. Acetic acid stimulates G-protein-coupled receptor GPR43 and induces intracellular calcium influx in L6 myotube cells. PloS One. (2020) 15:e0239428. doi: 10.1371/journal.pone.0239428
49. Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. (2013) 504:446–50. doi: 10.1038/nature12721
50. McBride DA, Dorn NC, Yao M, Johnson WT, Wang W, Bottini N, et al. Short-chain fatty acid-mediated epigenetic modulation of inflammatory T cells in vitro. Drug Delivery Transl Res. (2023) 13:1912–24. doi: 10.1007/s13346-022-01284-6
51. Zhang W, Ling J, Xu B, Wang J, Chen Z, and Li G. Gut microbiome-mediated monocytes promote liver metastasis. Int Immunopharmacol. (2024) 133:111877. doi: 10.1016/j.intimp.2024.111877
52. Bi W, Li X, Jiang Y, Gao T, Zhao H, Han Q, et al. Tumor-derived exosomes induce neutrophil infiltration and reprogramming to promote T-cell exhaustion in hepatocellular carcinoma. Theranostics. (2025) 15:2852–69. doi: 10.7150/thno.104557
53. Hu J, Wang C, Huang X, Yi S, Pan S, Zhang Y, et al. Gut microbiota-mediated secondary bile acids regulate dendritic cells to attenuate autoimmune uveitis through TGR5 signaling. Cell Rep. (2021) 36:109726. doi: 10.1016/j.celrep.2021.109726
54. Huang Y, Liao H, Luo J, Wei H, Li A, Lu Y, et al. Reversing NK cell exhaustion: a novel strategy combining immune checkpoint blockade with drug sensitivity enhancement in the treatment of hepatocellular carcinoma. Front Oncol. (2024) 14:1502270. doi: 10.3389/fonc.2024.1502270
55. Xia S, Wu J, Zhou W, Zhang M, Zhao K, Liu J, et al. SLC7A2 deficiency promotes hepatocellular carcinoma progression by enhancing recruitment of myeloid-derived suppressors cells. Cell Death Dis. (2021) 12:570. doi: 10.1038/s41419-021-03853-y
56. Deng L, Xie W, Lin M, Xiong D, Huang L, Zhang X, et al. Taraxerone inhibits M1 polarization and alleviates sepsis-induced acute lung injury by activating SIRT1. Chin Med. (2024) 19:159. doi: 10.1186/s13020-024-01002-z
57. Kou Z, Yang R, Lee E, Cuddapah S, Choi BH, and Dai W. Oxidative stress modulates expression of immune checkpoint genes via activation of AhR signaling. Toxicol Appl Pharmacol. (2022) 457:116314. doi: 10.1016/j.taap.2022.116314
58. Ghoshal UC, Goel A, and Quigley EMM. Gut microbiota abnormalities, small intestinal bacterial overgrowth, and non-alcoholic fatty liver disease: An emerging paradigm. Indian J Gastroenterol. (2020) 39:9–21. doi: 10.1007/s12664-020-01027-w
59. Chen W, Wen L, Bao Y, Tang Z, Zhao J, Zhang X, et al. Gut flora disequilibrium promotes the initiation of liver cancer by modulating tryptophan metabolism and up-regulating SREBP2. Proc Natl Acad Sci U S A. (2022) 119:e2203894119. doi: 10.1073/pnas.2203894119
60. Li H, Tan H, Liu Z, Pan S, Tan S, Zhu Y, et al. Succinic acid exacerbates experimental autoimmune uveitis by stimulating neutrophil extracellular traps formation via SUCNR1 receptor. Br J Ophthalmol. (2023) 107:1744–9. doi: 10.1136/bjophthalmol-2021-320880
61. Lin Q, Wang H, Chen W, Wei X, Chen J, Deng Y, et al. Isobutyric acid promotes immune evasion in colorectal cancer via increased PD-L1 expression. Cancer Med. (2024) 13:e70397. doi: 10.1002/cam4.70397
62. Li X, Yi Y, Wu T, Chen N, Gu X, Xiang L, et al. Integrated microbiome and metabolome analysis reveals the interaction between intestinal flora and serum metabolites as potential biomarkers in hepatocellular carcinoma patients. Front Cell Infect Microbiol. (2023) 13:1170748. doi: 10.3389/fcimb.2023.1170748
63. Huo R, Chen Y, Li J, Xu Q, Guo J, Xu H, et al. Altered gut microbiota composition and its potential association in patients with advanced hepatocellular carcinoma. Curr Oncol. (2023) 30:1818–30. doi: 10.3390/curroncol30020141
64. Cao P, Chen Q, Shi C, Wang L, and Gong Z. Fusobacterium nucleatum promotes the development of acute liver failure by inhibiting the NAD(+) salvage metabolic pathway. Gut Pathog. (2022) 14:29. doi: 10.1186/s13099-022-00503-2
65. Wu J, Li Q, and Fu X. Fusobacterium nucleatum contributes to the carcinogenesis of colorectal cancer by inducing inflammation and suppressing host immunity. Transl Oncol. (2019) 12:846–51. doi: 10.1016/j.tranon.2019.03.003
66. Zhang Z, Shi X, Ji J, Guo Y, Peng Q, Hao L, et al. Dihydroartemisinin increased the abundance of Akkermansia muciniphila by YAP1 depression that sensitizes hepatocellular carcinoma to anti-PD-1 immunotherapy. Front Med. (2023) 17:729–46. doi: 10.1007/s11684-022-0978-2
67. Kumar M, Kaur R, Kanthaje S, Dhiman RK, and Chakraborti A. Bacterial metabolite butyrate in modulating sorafenib-targeted microRNAs to curtail its resistance in hepatocellular carcinoma. J Cancer Res Clin Oncol. (2023) 149:5823–39. doi: 10.1007/s00432-022-04544-7
68. Che Y, Chen G, Guo Q, Duan Y, Feng H, and Xia Q. Gut microbial metabolite butyrate improves anticancer therapy by regulating intracellular calcium homeostasis. Hepatology. (2023) 78:88–102. doi: 10.1097/HEP.0000000000000047
69. Gopalakrishnan V, Helmink BA, Spencer CN, Reuben A, and Wargo JA. The influence of the gut microbiome on cancer, immunity, and cancer immunotherapy. Cancer Cell. (2018) 33:570–80. doi: 10.1016/j.ccell.2018.03.015
70. Kang X, Lau HC, and Yu J. Modulating gut microbiome in cancer immunotherapy: Harnessing microbes to enhance treatment efficacy. Cell Rep Med. (2024) 5:101478. doi: 10.1016/j.xcrm.2024.101478
71. Pulido M, Chamorro V, Romero I, Algarra I, SM A, Collado A, et al. Restoration of MHC-I on tumor cells by fhit transfection promotes immune rejection and acts as an individualized immunotherapeutic vaccine. Cancers (Basel). (2020) 12(6):1563. doi: 10.3390/cancers12061563
72. Boucher E, Plazy C, Richard ML, Suau A, Mangin I, Cornet M, et al. Inulin prebiotic reinforces host cancer immunosurveillance via γdelta T cell activation. Front Immunol. (2023) 14:1104224. doi: 10.3389/fimmu.2023.1104224
73. Singh V, Yeoh BS, Chassaing B, Xiao X, Saha P, Aguilera Olvera R, et al. Dysregulated microbial fermentation of soluble fiber induces cholestatic liver cancer. Cell. (2018) 175:679–94 e22. doi: 10.1016/j.cell.2018.09.004
74. Lin A, Jiang A, Huang L, Li Y, Zhang C, Zhu L, et al. From chaos to order: optimizing fecal microbiota transplantation for enhanced immune checkpoint inhibitors efficacy. Gut Microbes. (2025) 17:2452277. doi: 10.1080/19490976.2025.2452277
75. Helmy YA, Kassem II, and Rajashekara G. Immuno-modulatory effect of probiotic E. coli Nissle 1917 in polarized human colonic cells against Campylobacter jejuni infection. Gut Microbes. (2021) 13:1–16. doi: 10.1080/19490976.2020.1857514
76. Hyun J, Jun S, Lim H, Cho H, You SH, Ha SJ, et al. Engineered attenuated salmonella typhimurium expressing neoantigen has anticancer effects. ACS Synth Biol. (2021) 10:2478–87. doi: 10.1021/acssynbio.1c00097
77. Liu B, Wang K, Li Q, Xiao Z, Chen Z, Zhang Y, et al. Engineered VNP20009 expressing IL-15&15Ralpha augments anti-tumor immunity for bladder cancer treatment. Biomaterials. (2025) 315:122951. doi: 10.1016/j.biomaterials.2024.122951
78. Shen F, Zheng RD, Sun XQ, Ding WJ, Wang XY, and Fan JG. Gut microbiota dysbiosis in patients with non-alcoholic fatty liver disease. Hepatobiliary Pancreat Dis Int. (2017) 16:375–81. doi: 10.1016/S1499-3872(17)60019-5
79. Grat M, Wronka KM, Krasnodebski M, Masior L, Lewandowski Z, Kosinska I, et al. Profile of gut microbiota associated with the presence of hepatocellular cancer in patients with liver cirrhosis. Transplant Proc. (2016) 48:1687–91. doi: 10.1016/j.transproceed.2016.01.077
80. Lu H, Wu Z, Xu W, Yang J, Chen Y, and Li L. Intestinal microbiota was assessed in cirrhotic patients with hepatitis B virus infection. Intestinal microbiota of HBV cirrhotic patients. Microb Ecol. (2011) 61:693–703. doi: 10.1007/s00248-010-9801-8
81. Padilha MDM, Melo FTV, Laurentino RV, da Silva A, and Feitosa RNM. Dysregulation in the microbiota by HBV and HCV infection induces an altered cytokine profile in the pathobiome of infection. Braz J Infect Dis. (2025) 29:104468. doi: 10.1016/j.bjid.2024.104468
82. Arendt BM, Mohammed SS, Aghdassi E, Prayitno NR, Ma DW, Nguyen A, et al. Hepatic fatty acid composition differs between chronic hepatitis C patients with and without steatosis. J Nutr. (2009) 139:691–5. doi: 10.3945/jn.108.101782
83. Zhang H, Liu M, Zhong W, Zheng Y, Li Y, Guo L, et al. Leaky gut driven by dysbiosis augments activation and accumulation of liver macrophages via RIP3 signaling pathway in autoimmune hepatitis. Front Immunol. (2021) 12:624360. doi: 10.3389/fimmu.2021.624360
84. Oja AE, Brasser G, Slot E, van Lier RAW, Pascutti MF, and Nolte MA. GITR shapes humoral immunity by controlling the balance between follicular T helper cells and regulatory T follicular cells. Immunol Lett. (2020) 222:73–9. doi: 10.1016/j.imlet.2020.03.008
85. Lei Y, Li S, He M, Ao Z, Wang J, Wu Q, et al. Oral pathogenic bacteria and the oral-gut-liver axis: A new understanding of chronic liver diseases. Diagn (Basel). (2023) 13(21):3324. doi: 10.3390/diagnostics13213324
86. Yu Y, Yin H, Wu B, Zhao W, Wang Y, Aili A, et al. Fusobacterium nucleatum promotes colorectal cancer liver metastasis via miR-5692a/IL-8 axis by inducing epithelial-mesenchymal transition. J BioMed Sci. (2025) 32:5. doi: 10.1186/s12929-024-01097-4
87. Duizer C, Salomons M, van Gogh M, Grave S, Schaafsma FA, Stok MJ, et al. Fusobacterium nucleatum upregulates the immune inhibitory receptor PD-L1 in colorectal cancer cells via the activation of ALPK1. Gut Microbes. (2025) 17:2458203. doi: 10.1080/19490976.2025.2458203
88. Sivan A, Corrales L, Hubert N, Williams JB, Aquino-Michaels K, Earley ZM, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. (2015) 350:1084–9. doi: 10.1126/science.aac4255
89. Luu M, Weigand K, Wedi F, Breidenbend C, Leister H, Pautz S, et al. Regulation of the effector function of CD8(+) T cells by gut microbiota-derived metabolite butyrate. Sci Rep. (2018) 8:14430. doi: 10.1038/s41598-018-32860-x
90. Liu H, Kang X, Yang X, Yang H, Kuang X, Ren P, et al. Compound probiotic ameliorates acute alcoholic liver disease in mice by modulating gut microbiota and maintaining intestinal barrier. Probiot Antimicrob Proteins. (2023) 15:185–201. doi: 10.1007/s12602-022-10005-x
91. Xu H, Xiong J, Xu J, Li S, Zhou Y, Chen D, et al. Mosapride stabilizes intestinal microbiota to reduce bacterial translocation and endotoxemia in CCl(4)-induced cirrhotic rats. Dig Dis Sci. (2017) 62:2801–11. doi: 10.1007/s10620-017-4704-x
92. Loo TM, Kamachi F, Watanabe Y, Yoshimoto S, Kanda H, Arai Y, et al. Gut microbiota promotes obesity-associated liver cancer through PGE(2)-mediated suppression of antitumor immunity. Cancer Discov. (2017) 7:522–38. doi: 10.1158/2159-8290.CD-16-0932
93. Zhang Q, Ma C, Duan Y, Heinrich B, Rosato U, Diggs LP, et al. Gut microbiome directs hepatocytes to recruit MDSCs and promote cholangiocarcinoma. Cancer Discov. (2021) 11:1248–67. doi: 10.1158/2159-8290.CD-20-0304
94. Omenetti S and Pizarro TT. The treg/th17 axis: A dynamic balance regulated by the gut microbiome. Front Immunol. (2015) 6:639. doi: 10.3389/fimmu.2015.00639
95. Dai S, Liu F, Qin Z, Zhang J, Chen J, Ding WX, et al. Kupffer cells promote T-cell hepatitis by producing CXCL10 and limiting liver sinusoidal endothelial cell permeability. Theranostics. (2020) 10:7163–77. doi: 10.7150/thno.44960
96. Laivacuma S, Oblate O, and Derovs A. Gut microbiota and the gut-liver axis in liver disease: from chronic viral hepatitis to cirrhosis, hepatocellular carcinoma, and microbiome-based therapies. Microorganisms. (2025) 13(5):1053. doi: 10.3390/microorganisms13051053
97. Xu QY, Ren TY, Zhou YC, Xu J, Du LD, Hong DY, et al. Prevotella copri-produced 5-aminopentanoic acid promotes pediatric metabolic dysfunction-associated steatotic liver disease. Hepatobiliary Pancreat Dis Int. (2025) 24:303–15. doi: 10.1016/j.hbpd.2025.02.004
98. Lee PC, Wu CJ, Hung YW, Lee CJ, Chi CT, Lee IC, et al. Gut microbiota and metabolites associate with outcomes of immune checkpoint inhibitor-treated unresectable hepatocellular carcinoma. J Immunother Cancer. (2022) 10(6):e004779. doi: 10.1136/jitc-2022-004779
99. Zhao J, Yang W, Gao B, Wang H, Chen L, Shan C, et al. Escherichia coli HPI-induced duodenitis through ubiquitin regulation of the TLR4/NF-kappaB pathway. BMC Vet Res. (2025) 21:66. doi: 10.1186/s12917-025-04515-3
100. Sanduzzi Zamparelli M, Rocco A, Compare D, and Nardone G. The gut microbiota: A new potential driving force in liver cirrhosis and hepatocellular carcinoma. United Eur Gastroenterol J. (2017) 5:944–53. doi: 10.1177/2050640617705576
101. Wang L, Wang Z, Zhao Y, Yang B, Huang G, Li J, et al. Gut microbiota-mediated bile acid metabolism aggravates biliary injury after liver transplantation through mitochondrial apoptosis. Int Immunopharmacol. (2024) 143:113413. doi: 10.1016/j.intimp.2024.113413
102. Wu X, Sun R, Chen Y, Zheng X, Bai L, Lian Z, et al. Oral ampicillin inhibits liver regeneration by breaking hepatic innate immune tolerance normally maintained by gut commensal bacteria. Hepatology. (2015) 62:253–64. doi: 10.1002/hep.27791
103. Song G, Shi Y, Zhang M, Goswami S, Afridi S, Meng L, et al. Global immune characterization of HBV/HCV-related hepatocellular carcinoma identifies macrophage and T-cell subsets associated with disease progression. Cell Discov. (2020) 6:90. doi: 10.1038/s41421-020-00214-5
104. Yu H, Lin G, Jiang J, Yao J, Pan Z, Xie H, et al. Synergistic activity of Enterococcus Faecium-induced ferroptosis via expansion of IFN-gamma(+)CD8(+) T cell population in advanced hepatocellular carcinoma treated with sorafenib. Gut Microbes. (2024) 16:2410474. doi: 10.1080/19490976.2024.2410474
Keywords: hepatocellular carcinoma, gut microbiota, the gut-liver axis, local immune response, the immune microenvironment, immune modulation
Citation: Xu Y, Wen B, Gao N and Liu S (2025) Decoding the microbial-local immune dialogue in liver cancer: from ecological drivers to precision therapeutics. Front. Oncol. 15:1629479. doi: 10.3389/fonc.2025.1629479
Received: 15 May 2025; Accepted: 29 August 2025;
Published: 19 September 2025.
Edited by:
Lili Sheng, Shanghai University of Traditional Chinese Medicine, ChinaReviewed by:
Prasant Kumar Jena, Cedars Sinai Medical Center, United StatesNaim Abu-Freha, Soroka Medical Center, Israel
Copyright © 2025 Xu, Wen, Gao and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shu Liu, bGl1c2h1QGNtdS5lZHUuY24=
 Yichi Xu
Yichi Xu Bo Wen
Bo Wen Nan Gao
Nan Gao Shu Liu
Shu Liu