- 1Southern University of Science and Technology Yantian Hospital, Shenzhen, Guangdong, China
- 2Shenzhen Peking University-The Hong Kong University of Science and Technology Medical Center, Shenzhen, China
- 3Peking University Shenzhen Hospital, Shenzhen, China
Forkhead box Q1 (FOXQ1) is a member of the Forkhead box gene family and an important transcription factor closely associated with several human diseases, especially tumorigenesis and tumor progression. This review aims to explore advances in the study of the biological functions of FOXQ1 in several tumors, such as colorectal cancer, breast cancer, esophageal cancer, nasopharyngeal cancer, lung cancer, hepatocellular cancer, pancreatic cancer, gastric cancer, melanoma, bone-related disease, immune and inflammatory disease, regulatory factors of FOXQ1 expression, and mechanism of tissue-specific function. FOXQ1 influences the pathological progression of these diseases through different targets genes and signaling pathways, which we also review in detail. In conclusion, more and more FOXQ1 applications and different pathologic mechanisms are bound to be reported in future studies.
Introduction
The forkhead box (FOX) family is present in wide-ranging organisms, from yeast to humans. Based on the homology of DNA−binding domains, more than 100 FOX family members have been reported and been divided into 19 subfamilies, denoted as FOXA-S, and more than fifty types of FOX proteins were encoded by the human genome (1–4). The FOX family is characterized by a conserved winged-helix DNA-binding domain. As transcriptional regulators, FOX proteins participate in diverse biological processes and are closely linked to tumor initiation and progression (5–7).
The FOX family not only has numerous members and extensive functions involving multiple biological processes (8–10). FOXQ1 as one of the main members of the FOX family that has also been studied by numbers scholars. The human FOXQ1 gene is located at 6p25.3 and comprises 2661 base pairs, encoding 403 amino acids (aas). The FOXQ1 protein is divided into three domains: The alanine and glycine enrichment region, the proline-enrichment region and the forkhead box domain (FHD) or winged-helix domain. The N-terminal alanine/glycine-rich domain (at aa 13-103) and the C-terminal proline-rich domain (at aa 221-397) were associated with the transcriptional regulatory activity of the protein (2) (Figure 1). It is related to the occurrence and development of various human diseases, especially various tumors (1, 5, 11). This article provides a review of the research progress on the role, pathogenic mechanism, and prevention and treatment of FOXQ1 in various tumors and immune diseases.

Figure 1. Location and structure of the gene coordinate of human FOXQ1: Scale bar (blue ruler at top). The prediction of possible transcription activation domains was done in ADpred (104). FOXQ1 (ENSG 00000164379): the green box indicates the gene’s Ensembl ID and coordinate location. ENSTO0000296839: the purple box indicates the gene transcript ID and coordinate position. The DNA-binding domain (DBD), the nuclear localization sequence (NLS), Alanine/glycine-rich region and proline-rich region.
Disease pathogenesis roles of FOXQ1
FOXQ1 is a transcription factor that plays a key pathological role in various diseases. This section reviews its pathological mechanisms in different cancers, bone-related diseases, immune and inflammatory diseases.
FOXQ1 and colorectal cancer
Colorectal cancer (CRC) is a major health problem, with multiple tissue pathological processes and the pathological mechanisms that are currently not fully clear (12–14). Jia Yun Liu et al. (15) found that FOXQ1 is markedly overexpressed in CRC cells and CRC samples from clinical patients. FOXQ1 promotes cancer aggressiveness, such as cell proliferation, migration, and invasion in vitro, as well as growth in vivo, through the activation of the FAK/PI3K/AKT signaling pathway. FOXQ1 can promote the invasion and metastasis of CRC through the heparin-binding epidermal growth factor (HB-EGF)/EGFR pathway (16). TGF-β induces the expression of FOXQ1, inducing the tumor characteristic in CRC cells by modulating the Wnt/β-catenin pathway (17). Mediated sirtuin 1(SIRT1) expression and promoting β-catenin nuclear translocation are the key functions of FOXQ1in the therapy of CRC (18). Zhihu Liu et al. (19) demonstrated that miR-106a can improve the sensitivity of CRC cells to oxaliplatin through direct downregulation of FOXQ1expression. miR-378a acts as an inhibitor by preventing the abnormal activation of FOXQ1-cMYC axis signaling to improve the tumor characteristics of CRC (20). miR-342 is a tumor suppressive miRNA targeting the regulation of FOXQ1 biological function and thus affecting CRC development and clarified FOXQ1 as a valuable prognostic marker for CRC. FOXQ1 expression was validated in two large screening cohorts (n=550) and an independent clinical validation cohort (n=134), demonstrating that high FOXQ1 expression is an independent prognostic factor in colorectal cancer patients (21).
Based on the above reports, FOXQ1 plays a crucial role in the occurrence and development of CRC, it is also regulated by other factors. The long non-coding RNA (lncRNA) RP9 pseudogene (RP9P), by mediating the activity of miR-133a-3p/FOXQ1 axis regulates CRC cell tumorigenesis (22). LINC00543 remolds the tumor-microenvironment in CRC and enhances the epithelial-mesenchymal transition (EMT) of CRC cells through the pre-miR-506-3p/FOXQ1 axis (23). Similarly, the JAK2/STAT3/miR-506-3p/FOXQ1 signaling pathway plays a key role in the interaction between immune cells and cancer cells in the CRC pathological microenvironment (24). Hui Tang, et al. found that FOXQ1 can inhibit angiogenesis and reduce macrophage recruitment and may thus serve as a therapeutic target in CRC (25).
FOXQ1 and breast cancer
Breast cancer is the most common global malignancy and the leading cause of cancer deaths, especially in female patients. Despite some progress in its treatment, metastatic tumors remain incurable and require new therapeutic targets and strategies (26, 27). Some issues of concern include the regular supervision of survivors of cancer, the management of treatment side effects, the implementation of a normal lifestyle and the regulation of psychological factors (28, 29). Fahed A Elian et al. (30) found that FOXQ1 is differentially expressed in breast cancer subtypes and indicates a lower overall survival rate in breast cancer patients with low expression of FOXQ1. FOXQ1 protein expression is significantly higher in triple-negative breast cancer samples than in normal mammary tissues, so it is a novel target of breast cancer stem cells’ inhibition by diallyl trisulfide (31).
The RNA-binding protein Hu antigen R (HuR) is upregulated and is a direct target that regulates FOXQ1 in human breast cancer. These two factors’ interactions regulate the invasion and metastasis of breast cancer (32). By activating of the FGFR1-MEK-ERK2-c-FOS signaling axis, FOXQ1 expression is upregulated, FOXQ1 is an essential factor mediating the FGFR1 signaling and promotes breast cancer worsening (33). Other studies have found that FOXQ1 increases complex I-linked oxidative phosphorylation and contributes to the oncogenic role in human breast cancer cells through the direct transcriptional regulation of NDUFS1 and NDUFV1 (34). FOXQ1 is a therapy target of a small- molecule drug, Withaferin A (WA), derived from a medicinal plant (Withania somnifer). It inhibits breast cancer cell proliferation and cell migration (35). Additionally, miR-937 acts as an inhibitor to directly target and regulate FOXQ1, thereby limiting breast cancer cell expansion and cancer progression (36).
Haijun Zhang et al. (37) found that FOXQ1 expression is regulated by TGF-β1, and its repressed expression of E-cadherin mediates EMT by recruiting the mixed-lineage leukemia/histone methyltransferase 2 (MLL/KMT2) histone methyltransferase complex as a transcriptional coactivator (38). Additionally, the HuR inhibitors KH-3 can break the mRNA interaction of HuR-FOXQ1 axles, leading to the inhibition of breast cancer invasion (32). FOXQ1 directly transcriptionally regulates interleukin (IL)-8, IL-1α, and vascular endothelial growth factor (VEGF) to affect the pathological mechanism of human breast cancer cells (31, 39). Similarly, Nuclear isoform of RAPH1 (named RAPH1-i3) interacts with FOXQ1 and can promote breast cancer progression and radio-resistance (40). FOXQ1, as a transcription regulator, mediates the biological function of multiple genes in breast cancer, so it is a key target for chemical and biological targeted therapy (41–43). However, benzyl isothiocyanate as a breast cancer chemoprevention agent regulates FOXQ1 expression and mediates the inhibition of EMT in human breast cancer cells (44).
FOXQ1 and esophageal cancer
Esophageal squamous cell carcinoma (ESCC) is one of the most malignant cancers and lacks unified standards for diagnosis and treatment (45, 46). FOXQ1’s abnormal upregulation of expression in human ESCC cells and knockdown of FOXQ1 can restrain the tumor characteristics of different ESCC cells in a mouse xenograft model in vivo (47). However, FOXQ1 acts an oncogene in human ESCC cells by negatively regulating CDH1 to promote ESCC cell proliferation and metastasis (48). The biological function of FOXQ1 is still being explored in ESCC, and current research is not very extensive.
FOXQ1 and nasopharyngeal carcinoma
FOXQ1 promotes EGFR expression at mRNA and protein levels, mediates the EGFR signaling activity, and increases the metastasis of nasopharyngeal carcinoma (NPC) by inducing vasculogenic mimicry (49). miR-342-3p and miR-124 targets the regulation of FOXQ1 expression by mediating NPC cell growth and invasion (50, 51). Moreover, lncRNA 00667 acts an oncogene by promoting NPC cell growth through competitive targeting and binding to miR-4319 to upregulate FOXQ1 expression (52). Circular RNA CRIM1 inhibits the suppressive effects of miR-422a on its target gene FOXQ1 and leads to its expression upregulation, resulting in NPC metastasis, EMT, and docetaxel chemoresistance (53).
FOXQ1 and lung cancer
FOXQ1 is highly expressed in non-small cell lung cancer (NSCLC) tissue samples from patients and can be potentially used as an EMT marker in NSCLC (54, 55). Further survival analysis surfaced high FOXQ1 expression as an independent prognostic factor (54). Likewise, FOXQ1 is over-expressed in NSCLC cancer tissue compared with adjacent tissue. FOXQ1 expression was increased in tumor tissue (61.3% high expression and 38.7% low expression) compared with paired adjacent tissue (37.8% high expression and 62.2% low expression) (P < 0.001). It is associated with the malignant features of tumors. High FOXQ1 expression is an independent risk factor for disease-free survival and overall survival in patients with NSCLC, indicating the potential prognostic value of FOXQ1 for NSCLC (56). miR-133 also downregulates FOXQ1 expression to mediate the EMT and antagonizes lung cancer tumorigenesis (57).
FOXQ1 and hepatocellular carcinoma
The high expression of FOXQ1 in hepatocellular carcinoma (HCC) cells may be related to its biological function as a therapeutic target for HCC. Liposomal clodronate combined with cisplatin or sorafenib inhibits FOXQ1 expression in HCC cells and restrains their proliferation, migration, and invasion (58, 59). Certainly, these studies have demonstrated that high FOXQ1 expression is an independent factor in the prognosis of HCC (59). miR-4319 is a post-transcriptional regulator that directly dampens the expression of FOXQ1 to decrease cell proliferation, inhibit EMT, accelerate apoptosis, and prevent cancer stemness in HCC (60). Additionally, FOXQ1/NDRG1 (N-myc downstream-regulated gene 1) axis plays a key role between HCC and cancer-associated fibroblast (CAF) crosstalk (61). FOXQ1 further induces sex determining region Y-box 12 (Sox12) overexpression and promotes HCC invasion and metastasis through the transcriptional activation of Twost1 and fibroblast growth factor binding protein 1 (FGFBP1). Further results showed that patients with positive FoxQ1/Sox12 expression had a poorer prognosis (62).
FOXQ1 and pancreatic cancer
Zhan HX, et al. (63) found that FOXQ1 expression is negatively associated with the overall survival of PC patients. FOXQ1 overexpression in PC stem-like cells and inhibition of FOXQ1 attenuates tumor formation, growth, and so on. Thus, it may be a novel therapy for achieving a better treatment outcome of PC (64). FOXQ1 via promotes the transcription of Lactate dehydrogenase A (LDHA) and increases its expression in pancreatic cancer (PC). It then activates aerobic glycolysis to stimulate PC cell proliferation, tumor stemness, invasion, metastasis, and so on (65).
FOXQ1 and gastric cancer
A high expression of FOXQ1 is involved in the acquisition of the mesenchymal phenotype of GC cells. The subsequent activation of the expression of the Snail signaling pathway is essential for EMT induction. Thus, FOXQ1 is a potential prognostic marker and is therapeutic for patients with GC (66, 67). miR-96-5p inhibits the protein expression of FOXQ1 suppresses the proliferation, migration, and EMT of gastric cancer cells (68). miR-345 inhibits metastasis in vitro and in vivo, as well as the EMT of GC cells, via the targeted regulation of FOXQ1 expression (69). miR-519 was abnormally low expression and involve to GC tumor characteristics and regulation the biological behavior of GC cells via direct targeting FOXQ1 (70). Accordingly, miR-1271 acts as a novel tumor suppressor that inhibits the proliferation, invasion, and EMT of the GC cells by downregulating the expression of FOXQ1 (71). Similarly, tumor-associated macrophages are play important roles in the tumor microenvironment, such as promoting EMT, invasion, and migration in GC cells. These biological processes may be mediated by FOXQ1 (72).
FOXQ1 and melanoma
Despite rapid advances in tumor diagnostics and pathogenic molecular mechanisms in recent years, the early clinical diagnosis and histopathology of melanoma remain inadequate (73, 74). Archis Bagati, et al. (75) found a novel mechanism involving the opposite roles of FOXQ1 in the regulation of N-cadherin (CDH2) gene, invasion and metastasis in melanoma versus carcinoma cells, FOXQ1 through interacts with nuclear β-catenin and TLE and altering the levels of these two proteins suppressor melanomas. FOXQ1 also directly regulates the transcriptional activation of the MITF gene (a melanocytic lineage-specific regulator of differentiation) and thus promotes the differentiation of normal and transformed melanocytic cells (76).
FOXQ1 and bone-related diseases
Abnormal mesenchymal stem cells (MSCs) osteogenic differentiation abnormal is play very important role in multiple bone diseases (77, 78). Clinical findings show a higher fracture risk of osteoporosis (OP) patients complicated with type II diabetes mellitus (T2DM) compared with the non-T2DM patients. MSCs from T2DM patients also show a weaker osteogenic potent. Upon investigation, FOXQ1 downregulation causes a decline in osteogenic potential of T2DM-BSMC. This mechanism may be the key to research on the treatment of diabetic OP (79). FOXQ1 acts as a mediator that regulates the activities of Wnt/beta-catenin signaling by binding with ANXA2 to promote osteogenic differentiation of MSCs (80). FOXQ1 reportedly inhibits Osteoarthritis (OA) progression by downregulating pyroptosis induced by NLR family pyrin domain containing 3 (NLRP3) (81).
FOXQ1 and immune and inflammatory diseases
As one of the early and extensively studied forkhead genes, the biological structure and function of FOXQ1 have been relatively thoroughly researched (82, 83). We have reviewed the biological functions and pathogenic mechanisms of FOXQ1 in multiple malignant tumors, so exploring it’s necessary to explore its precise function and mechanism in the immune and inflammation system is necessary. High FOXQ1 expression in natural killer/T-cell lymphoma is correlated with cell proliferation, growth, and apoptosis induction. These functions affect multiple pathological processes, such as high Ann Arbor stage, bone marrow involvement, poor prognosis, and inhibition of the shh signaling pathway (84). Similarly, FOXQ1 is highly expressed and directly regulates the inhibition of neurexins 3 (NRXN3) expression, thereby promoting to proliferation and migration in glioma cells (85).
Recent research reports USP10 deubiquitinating regulates FOXQ1, which plays a protective role in sepsis-induced acute kidney injury (S-AKI) by inducing inflammation and apoptosis (86). FOXQ1 also reportedly binds directly to inhibit prostaglandin-endoperoxide synthase 2 (PTGS2), and cyclin-dependent kinase 5 (CDK5) to promote apoptosis and inflammation while inhibiting neurite outgrowth in Alzheimer Disease (87). Xiaohui Ma et al. found that miR-125b directly targets FOXQ1 by regulating the neuronal cell apoptosis and phosphorylation of Tau (88). In chronic inflammatory atopic dermatitis pathology, FOXQ1 stimulates monocyte motility and increases pro-inflammatory potential. It can also induce monocyte migration toward MCP-1, which is essential for monocyte influx into inflammatory sites (89). Another study has found that FOXQ1 may influence the NK-cell and alloimmune cytotoxic T-cell functions in the differentiation and development of hair shaft (90).
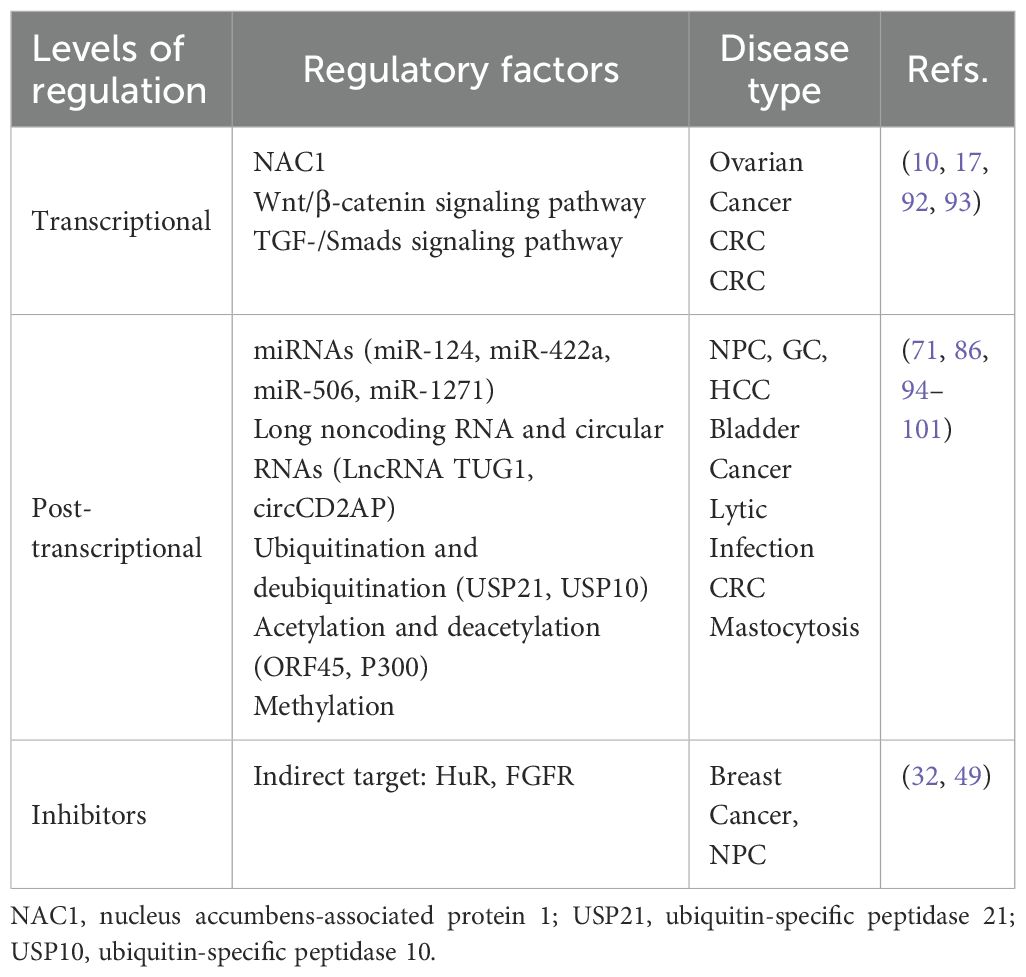
Based on the above insights into FOXQ1’s role across various diseases, we propose distinct approaches for the clinical translation of FOXQ1. Indirect Suppression of FOXQ1: Indirect inhibition strategies downregulate FOXQ1 expression through upstream pathway inhibitors (such as those targeting Wnt/β-catenin or TGF-β signaling pathways) or miRNA mimics (37). Hypothetical Direct FOXQ1 Targeting: Directly targeting the transcription factor FOXQ1 itself remains a medium-to-long-term research direction, with the core challenge being the widespread “druggability” issue inherent to transcription factors (91). Additionally, we summarized the expression patterns of FOXQ1 in the aforementioned diseases, along with its corresponding direct or indirect targets and various biological functions (Table 1).
Regulatory factors of FOXQ1 expression
Many factors have been shown to regulate FOXQ1 expression in different human diseases. Here we focus on the regulation at the transcriptional levels, post-transcriptional levels and inhibitors (Table 2).
Transcriptional regulation of FOXQ1: A study reported that nucleus accumbens-associated protein 1 (NAC1) transcriptionally regulates FOXQ1 expression (92). Additionally, FOXQ1 is regulated as a downstream gene of some classical signaling pathways by members of this pathway, such as glycogen synthase kinase 3, an inhibitor of Wnt/β-catenin, activates FOXQ1 expression in solid tumors (10). Similarly, several studies have also found that FOXQ1 expression is regulated by the TGF-/Smads pathway. The mechanism remains unclear, but CRC cells treated with TGF for 3 days significantly upregulate both mRNA and protein levels of FOXQ1. Interestingly, further investigation of these cells indicated alterations in the Wnt pathway, including vascular endothelial growth factor (VEGF)-A, matrix metalloproteinase 2, vimentin, N-cadherin and E-cadherin, these results indicate that FOXQ1 provides crosstalk between the Wnt and TGF-β signaling pathways (17, 93).
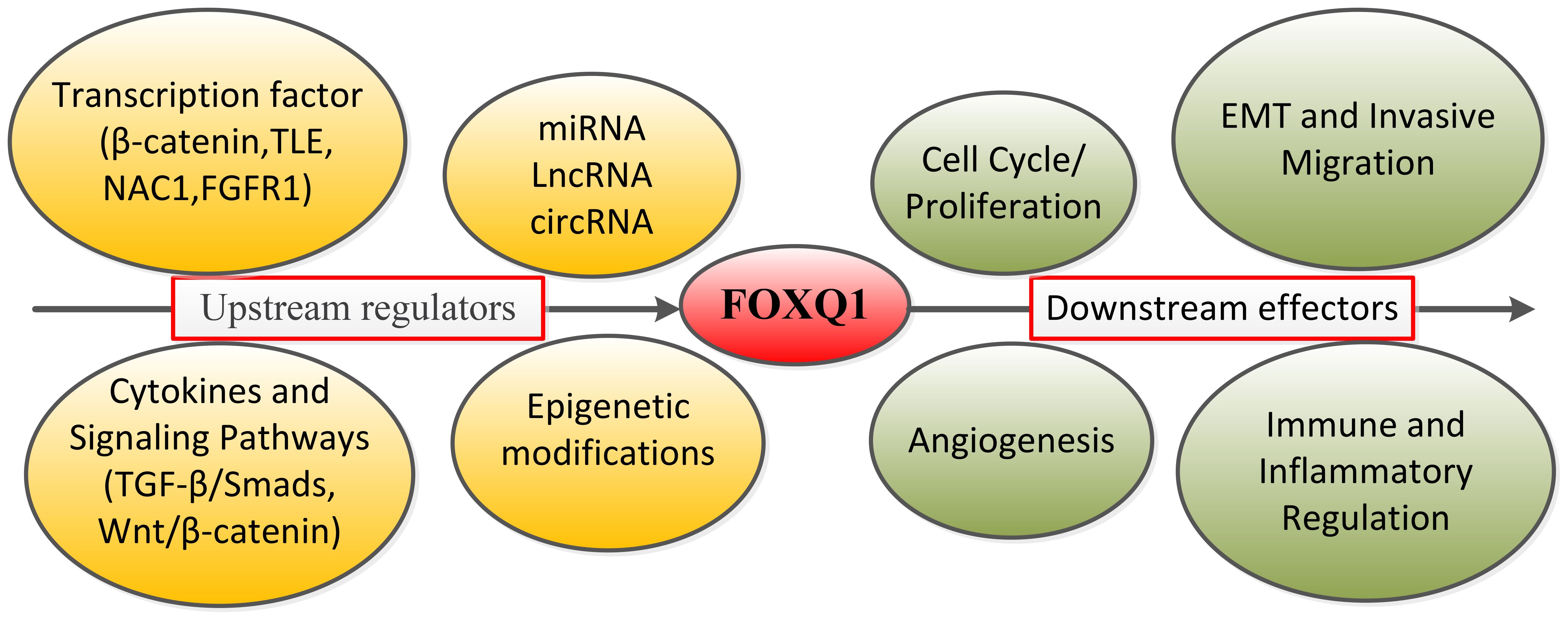
Post-transcriptional regulation of FOXQ1: In the above review of multiple cancers, we have described multiple miRNAs targeting FOXQ1. miR-124 inhibits NPC amplification, migration and invasion by regulating FOXQ1 expression through targeted binding to 3’-untranslated region (3’UTR) (94). Similarly, members of miR-422a, miR-506 and miR-1271 have been found to regulate the expression and biological functions of FOXQ1 (71, 95, 96). Similarly, long noncoding RNA-activated FOXQ1 epitopes promote bladder cancer cell expansion and migration (97). Qi Zhao et al. found that FOXQ1 expression was regulated by Ubiquitin-specific protease10 deubiquitylation, which was effective in alleviating inflammation and apoptosis in acute kidney injury through the CREB5/NF-κB signaling axis (86). In contrast, Ubiquitin-specific protease21 ubiquitination inhibits FOXQ1 expression promoting Bladder cancer EMT and stemness (98). Additionally, FOXQ1 exerts a protective effect against S-AKI-induced inflammation and apoptosis by targeting the CREB5/NF-κB pathway through deubiquitination mediated by USP10 (86). Natalie Atyeo et al. (99) reported that histone acetylation regulating FOXQ1 plays a key role in oral epithelial cell infection. Additionally, recent studies have reported that P300-mediated acetylation of the FOXQ1 complex activates super-enhancers, thereby promoting proliferation and metastasis in CRC (100). FOXQ1 expression is subject to promoter region methylation which plays a key role in the pathological progression of mastocytosis patients (101). Therefore, based on the content of the above review, we summarize the core upstream regulatory factors and downstream effects of FOXQ1 (Figure 2).
Inhibitors: We searched the literature and found no direct inhibitors of FOXQ1, but indirect inhibitors have been reported. KH-3 is an effective inhibitor of the RNA-binding protein HuR. By interfering with HuR-FOXQ1 mRNA interactions, KH-3 inhibits breast cancer cell invasion and delays lung colony formation (32). Yunfan Luo et al. (49) reported that FOXQ1 promotes EGFR expression at both mRNA and protein levels. EGFR inhibitor drugs can suppress FOXQ1-induced vasculogenic mimicry formation, thereby inhibiting the growth and metastasis of NPC. Therefore, depending on the underlying pathological mechanism, FOXQ1 can serve as a direct or indirect therapeutic target.
Mechanism of tissue-specific function
Transcription of FOXQ1 is differentially regulated across different cell types, potentially yielding protein isoforms with distinct or even opposing functions. Kaneda et al. found that FOXQ1 is highly expressed in CRC, promoting tumor formation, growth, angiogenesis, and anti-apoptosis, suggesting that FOXQ1 may play a positive role in certain cancer types (102). FOXQ1 expression was detected in various tumor cells, where it promotes cell growth/proliferation, migration/invasion, angiogenesis, tumorigenicity, and metastasis by upregulating axonal protein 3, zinc finger protein E-box binding homolog 1/2, vascular endothelial growth factor, Wnt proteins, and Bcl proteins. These tumors include breast cancer, liver cancer, glioma, CRC, and ovarian cancer (37, 85, 102, 103).
Mikhail Nikiforov et al. (75, 76) investigated the key role played by the protein FOXQ1 in the development of melanoma, a distinct type of cancer that originates from different cell types compared to other cancers. The transcription factor FOXQ1 suppresses the progression of the same process in melanoma cells that induce carcinogenesis, a process dependent on the balance between two types of proteins: β-catenin and members of the TLE family. When interacting with FOXQ1, these proteins can convert each other into either transcription activators or repressors, thereby inducing or suppressing the expression of N-cadherin (CDH2 gene), a key regulator of tumor invasion and metastasis.
Conclusion
FOXQ1 has been demonstrated to be closely associated with the development of various of tumors. It orchestrates a wide range of biological functions in human diseases, such as cell invasion and migration, proliferation, differentiation, apoptosis, inflammation production and vascular proliferation, induction of fibrosis and aggressive behavior. Further in-depth studies are required to elucidate FOXQ1 gene expression, protein function, and its mechanisms of action in human disease. Although FOXQ1 has been validated as an oncogene, its downstream targets and the signaling pathways it modulates are governed in a cancer-type-specific manner. Its specific mechanism in tumor infiltration and migration is also not fully understood. Thus, future in-depth studies on FOXQ1 functions may confirm its potential as a key marker of cancer diagnosis and therapy in the future. Future research should focus on developing targeted therapeutic strategies for FOXQ1, such as designing small-molecule inhibitors or RNA therapeutics to precisely regulate its activity. Concurrently, leveraging emerging disease models like organoids and single-cell sequencing will enable systematic analysis of FOXQ1’s spatiotemporal expression patterns and regulatory networks across diverse tissue contexts. This approach will provide actionable biomarkers and therapeutic options for clinical translation.
Author contributions
XF: Resources, Writing – original draft. LZ: Software, Writing – original draft. PS: Resources, Supervision, Writing – review & editing. YH: Supervision, Writing – original draft, Funding acquisition, Investigation.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by Guangdong Basic and Applied Basic Research Foundation (No.2024A1515013128), Shenzhen Science and Technology Program (No. JCYJ20230807095113026). Shenzhen Yantian District Healthcare Science and Technology Program Projects (No. YTWS20240205).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Katoh M and Katoh M. Human FOX gene family (Review). Int J Oncol. (2004) 25:1495–500. doi: 10.3892/ijo.25.5.1495
2. Tang H, Zhang J, and Guo Q. Research progress on the regulation of tumor initiation and development by the forkhead box Q1 gene. J Cancer Res Ther. (2018) 14:6–11. doi: 10.4103/jcrt.JCRT_701_17
3. Herman L, Todeschini AL, and Veitia RA. Forkhead transcription factors in health and disease. Trends Genet. (2021) 37:460–75. doi: 10.1016/j.tig.2020.11.003
4. Hu W, Li M, Wang Y, Zhong C, Si X, Shi X, et al. Comprehensive bioinformatics analysis reveals the significance of forkhead box family members in pancreatic adenocarcinoma. Aging (Albany NY). (2023) 15:92–107. doi: 10.18632/aging.204455
5. Weigel D, Jurgens G, Kuttner F, Seifert E, and Jackle H. The homeotic gene fork head encodes a nuclear protein and is expressed in the terminal regions of the Drosophila embryo. Cell. (1989) 57:645–58. doi: 10.1016/0092-8674(89)90133-5
6. Carlsson P and Mahlapuu M. Forkhead transcription factors: key players in development and metabolism. Dev Biol. (2002) 250:1–23. doi: 10.1006/dbio.2002.0780
7. Xu J, Liu H, Lan Y, and Jiang R. Cis-repression of foxq1 expression affects foxf2-mediated gene expression in palate development. Front Cell Dev Biol. (2021) 9:665109. doi: 10.3389/fcell.2021.665109
8. Newman JA, Aitkenhead H, Gavard AE, Rota IA, Handel AE, Hollander GA, et al. The crystal structure of human forkhead box N1 in complex with DNA reveals the structural basis for forkhead box family specificity. J Biol Chem. (2020) 295:2948–58. doi: 10.1074/jbc.RA119.010365
9. Qiao Y, Jiang X, Lee ST, Karuturi RK, Hooi SC, and Yu Q. FOXQ1 regulates epithelial-mesenchymal transition in human cancers. Cancer Res. (2011) 71:3076–86. doi: 10.1158/0008-5472.CAN-10-2787
10. Christensen J, Bentz S, Sengstag T, Shastri VP, and Anderle P. FOXQ1, a novel target of the Wnt pathway and a new marker for activation of Wnt signaling in solid tumors. PloS One. (2013) 8:e60051. doi: 10.1371/journal.pone.0060051
11. Zhu X, Hua E, Tu Q, Liu M, Xu L, and Feng J. Foxq1 promotes alveolar epithelial cell death through tle1-mediated inhibition of the NF-kappaB signaling pathway. Am J Respir Cell Mol Biol. (2024) 71:53–65. doi: 10.1165/rcmb.2023-0317OC
12. Shin AE, Giancotti FG, and Rustgi AK. Metastatic colorectal cancer: mechanisms and emerging therapeutics. Trends Pharmacol Sci. (2023) 44:222–36. doi: 10.1016/j.tips.2023.01.003
13. Mahmoud NN. Colorectal cancer: preoperative evaluation and staging. Surg Oncol Clin N Am. (2022) 31:127–41. doi: 10.1016/j.soc.2021.12.001
14. Wong SH and Yu J. Gut microbiota in colorectal cancer: mechanisms of action and clinical applications. Nat Rev Gastroenterol Hepatol. (2019) 16:690–704. doi: 10.1038/s41575-019-0209-8
15. Liu JY, Wu XY, Wu GN, Liu FK, and Yao XQ. FOXQ1 promotes cancer metastasis by PI3K/AKT signaling regulation in colorectal carcinoma. Am J Transl Res. (2017) 9:2207–18.
16. Zhang JJ, Cao CX, Wan LL, Zhang W, Liu ZJ, Wang JL, et al. Forkhead Box q1 promotes invasion and metastasis in colorectal cancer by activating the epidermal growth factor receptor pathway. World J Gastroenterol. (2022) 28:1781–97. doi: 10.3748/wjg.v28.i17.1781
17. Peng X, Luo Z, Kang Q, Deng D, Wang Q, Peng H, et al. FOXQ1 mediates the crosstalk between TGF-beta and Wnt signaling pathways in the progression of colorectal cancer. Cancer Biol Ther. (2015) 16:1099–109. doi: 10.1080/15384047.2015.1047568
18. Yang M, Liu Q, Dai M, Peng R, Li X, Zuo W, et al. FOXQ1-mediated SIRT1 upregulation enhances stemness and radio-resistance of colorectal cancer cells and restores intestinal microbiota function by promoting beta-catenin nuclear translocation. J Exp Clin Cancer Res. (2022) 41:70. doi: 10.1186/s13046-021-02239-4
19. Liu Z, Qin Y, Dong S, Chen X, Huo Z, and Zhen Z. Overexpression of miR-106a enhances oxaliplatin sensitivity of colorectal cancer through regulation of FOXQ1. Oncol Lett. (2020) 19:663–70. doi: 10.3892/ol.2019.11151
20. Liu N, Zhang T, Steer CJ, and Song G. MicroRNA-378a-3p prevents initiation and growth of colorectal cancer by fine tuning polyamine synthesis. Cell Biosci. (2022) 12:192. doi: 10.1186/s13578-022-00930-3
21. Weng W, Okugawa Y, Toden S, Toiyama Y, Kusunoki M, and Goel A. FOXM1 and FOXQ1 are promising prognostic biomarkers and novel targets of tumor-suppressive miR-342 in human colorectal cancer. Clin Cancer Res. (2016) 22:4947–57. doi: 10.1158/1078-0432.CCR-16-0360
22. Jin Z, Liu B, Lin B, Yang R, Wu C, Xue W, et al. The Novel lncRNA RP9P Promotes Colorectal Cancer Progression by Modulating miR-133a-3p/FOXQ1 Axis. Front Oncol. (2022) 12:843064. doi: 10.3389/fonc.2022.843064
23. Zheng J, Dou R, Zhang X, Zhong B, Fang C, Xu Q, et al. LINC00543 promotes colorectal cancer metastasis by driving EMT and inducing the M2 polarization of tumor associated macrophages. J Transl Med. (2023) 21:153. doi: 10.1186/s12967-023-04009-6
24. Wei C, Yang C, Wang S, Shi D, Zhang C, Lin X, et al. Crosstalk between cancer cells and tumor associated macrophages is required for mesenchymal circulating tumor cell-mediated colorectal cancer metastasis. Mol Cancer. (2019) 18:64. doi: 10.1186/s12943-019-0976-4
25. Tang H, Zheng J, Bai X, Yue KL, Liang JH, Li DY, et al. Forkhead box Q1 is critical to angiogenesis and macrophage recruitment of colorectal cancer. Front Oncol. (2020) 10:564298. doi: 10.3389/fonc.2020.564298
26. Katsura C, Ogunmwonyi I, Kankam HK, and Saha S. Breast cancer: presentation, investigation and management. Br J Hosp Med (Lond). (2022) 83:1–7. doi: 10.12968/hmed.2021.0459
27. Criscitiello C and Corti C. Breast cancer genetics: diagnostics and treatment. Genes (Basel). (2022) 13:1593. doi: 10.3390/genes13091593
28. Kandi LA, Hostler AC, Howard M, and Teven CM. Breast cancer survivorship: understanding breast tissues’ Potentiating role in precipitating deviations from baseline sexual function post oncologic resection. Plast Reconstr Surg Glob Open. (2024) 12:e5573. doi: 10.1097/GOX.0000000000005573
29. Valente S and Roesch E. Breast cancer survivorship. J Surg Oncol. (2024) 130:8–15. doi: 10.1002/jso.27627
30. Elian FA, Are U, Ghosh S, Nuin P, Footz T, McMullen TPW, et al. FOXQ1 is differentially expressed across breast cancer subtypes with low expression associated with poor overall survival. Breast Cancer (Dove Med Press). (2021) 13:171–88. doi: 10.2147/BCTT.S282860
31. Kim SH, Kaschula CH, Priedigkeit N, Lee AV, and Singh SV. Forkhead box Q1 is a novel target of breast cancer stem cell inhibition by diallyl trisulfide. J Biol Chem. (2016) 291:13495–508. doi: 10.1074/jbc.M116.715219
32. Wu X, Gardashova G, Lan L, Han S, Zhong C, Marquez RT, et al. Targeting the interaction between RNA-binding protein HuR and FOXQ1 suppresses breast cancer invasion and metastasis. Commun Biol. (2020) 3:193. doi: 10.1038/s42003-020-0933-1
33. Lin Y, Lin F, Zhang Z, Peng L, Yang W, Yang M, et al. The FGFR1 signaling pathway upregulates the oncogenic transcription factor FOXQ1 to promote breast cancer cell growth. Int J Biol Sci. (2023) 19:744–59. doi: 10.7150/ijbs.74574
34. Kim SH and Singh SV. The FoxQ1 transcription factor is a novel regulator of electron transport chain complex I subunits in human breast cancer cells. Mol Carcinog. (2022) 61:372–81. doi: 10.1002/mc.23381
35. Kim SH, Singh KB, Hahm ER, and Singh SV. The role of forkhead box Q1 transcription factor in anticancer effects of withaferin A in breast cancer. Cancer Prev Res (Phila). (2021) 14:421–32. doi: 10.1158/1940-6207.CAPR-20-0590
36. Han X, Guo X, Zhang W, and Cong Q. MicroRNA-937 inhibits the Malignant phenotypes of breast cancer by directly targeting and downregulating forkhead box Q1. Onco Targets Ther. (2019) 12:4813–24. doi: 10.2147/OTT.S207593
37. Zhang H, Meng F, Liu G, Zhang B, Zhu J, Wu F, et al. Forkhead transcription factor foxq1 promotes epithelial-mesenchymal transition and breast cancer metastasis. Cancer Res. (2011) 71:1292–301. doi: 10.1158/0008-5472.CAN-10-2825
38. Mitchell AV, Wu L, James Block C, Zhang M, Hackett J, Craig DB, et al. FOXQ1 recruits the MLL complex to activate transcription of EMT and promote breast cancer metastasis. Nat Commun. (2022) 13:6548. doi: 10.1038/s41467-022-34239-z
39. Kim SH, Hahm ER, Singh KB, and Singh SV. Novel mechanistic targets of forkhead box Q1 transcription factor in human breast cancer cells. Mol Carcinog. (2020) 59:1116–28. doi: 10.1002/mc.23241
40. Liu Q, Cao Y, Wei X, Dong H, Cui M, Guan S, et al. Nuclear isoform of RAPH1 interacts with FOXQ1 to promote aggressiveness and radioresistance in breast cancer. Cell Death Dis. (2023) 14:803. doi: 10.1038/s41419-023-06331-9
41. Meng F, Speyer CL, Zhang B, Zhao Y, Chen W, Gorski DH, et al. PDGFRalpha and beta play critical roles in mediating Foxq1-driven breast cancer stemness and chemoresistance. Cancer Res. (2015) 75:584–93. doi: 10.1158/0008-5472.CAN-13-3029
42. Huang X, Wu J, Wang Y, Xian Z, Li J, Qiu N, et al. FOXQ1 inhibits breast cancer ferroptosis and progression via the circ_0000643/miR-153/SLC7A11 axis. Exp Cell Res. (2023) 431:113737. doi: 10.1016/j.yexcr.2023.113737
43. Kim SH, Hahm ER, and Singh SV. Forkhead Box Q1 is a novel regulator of autophagy in breast cancer cells. Mol Carcinog. (2023) 62:1449–59. doi: 10.1002/mc.23588
44. Sehrawat A, Kim SH, Vogt A, and Singh SV. Suppression of FOXQ1 in benzyl isothiocyanate-mediated inhibition of epithelial-mesenchymal transition in human breast cancer cells. Carcinogenesis. (2013) 34:864–73. doi: 10.1093/carcin/bgs397
45. Cui Y, Ren W, Du X, Yang L, and Tan B. Research progress of multiple primary Malignancies associated with esophageal cancer. Cancer Control. (2023) 30:10732748231176641. doi: 10.1177/10732748231176641
46. Zhu H, Ma X, Ye T, Wang H, Wang Z, Liu Q, et al. Esophageal cancer in China: Practice and research in the new era. Int J Cancer. (2023) 152:1741–51. doi: 10.1002/ijc.34301
47. Wang P, Lv C, Zhang T, Liu J, Yang J, Guan F, et al. FOXQ1 regulates senescence-associated inflammation via activation of SIRT1 expression. Cell Death Dis. (2017) 8:e2946. doi: 10.1038/cddis.2017.340
48. Pei Y, Wang P, Liu H, He F, and Ming L. FOXQ1 promotes esophageal cancer proliferation and metastasis by negatively modulating CDH1. BioMed Pharmacother. (2015) 74:89–94. doi: 10.1016/j.biopha.2015.07.010
49. Luo Y, Wang J, Wang F, Liu X, Lu J, Yu X, et al. Foxq1 promotes metastasis of nasopharyngeal carcinoma by inducing vasculogenic mimicry via the EGFR signaling pathway. Cell Death Dis. (2021) 12:411. doi: 10.1038/s41419-021-03674-z
50. Cui Z and Zhao Y. microRNA-342-3p targets FOXQ1 to suppress the aggressive phenotype of nasopharyngeal carcinoma cells. BMC Cancer. (2019) 19:104. doi: 10.1186/s12885-018-5225-5
51. Peng XH, Huang HR, Lu J, Liu X, Zhao FP, Zhang B, et al. MiR-124 suppresses tumor growth and metastasis by targeting Foxq1 in nasopharyngeal carcinoma. Mol Cancer. (2014) 13:186. doi: 10.1186/1476-4598-13-186
52. Liao B, Yi Y, Zeng L, Wang Z, Zhu X, Liu J, et al. LINC00667 sponges miR-4319 to promote the development of nasopharyngeal carcinoma by increasing FOXQ1 expression. Front Oncol. (2020) 10:632813. doi: 10.3389/fonc.2020.632813
53. Hong X, Liu N, Liang Y, He Q, Yang X, Lei Y, et al. Circular RNA CRIM1 functions as a ceRNA to promote nasopharyngeal carcinoma metastasis and docetaxel chemoresistance through upregulating FOXQ1. Mol Cancer. (2020) 19:33. doi: 10.1186/s12943-020-01149-x
54. Feng J, Zhang X, Zhu H, Wang X, Ni S, and Huang J. FoxQ1 overexpression influences poor prognosis in non-small cell lung cancer, associates with the phenomenon of EMT. PloS One. (2012) 7:e39937. doi: 10.1371/journal.pone.0039937
55. Feng J, Xu L, Ni S, Gu J, Zhu H, Wang H, et al. Involvement of FoxQ1 in NSCLC through regulating EMT and increasing chemosensitivity. Oncotarget. (2014) 5:9689–702. doi: 10.18632/oncotarget.2103
56. Li L, Xu B, Zhang H, Wu J, Song Q, and Yu J. Potentiality of forkhead box Q1 as a biomarker for monitoring tumor features and predicting prognosis in non-small cell lung cancer. J Clin Lab Anal. (2020) 34:e23031. doi: 10.1002/jcla.23031
57. Xiao B, Liu H, Gu Z, and Ji C. Expression of microRNA-133 inhibits epithelial-mesenchymal transition in lung cancer cells by directly targeting FOXQ1. Arch Bronconeumol. (2016) 52:505–11. doi: 10.1016/j.arbres.2015.10.016
58. Zhou Y, Chen Y, Ma C, Shao B, and Zhang F. Liposomal clodronate combined with Cisplatin or Sorafenib inhibits hepatocellular carcinoma cell proliferation, migration and invasion by suppressing FOXQ1 expression. Cell Mol Biol (Noisy-le-grand). (2020) 66:49–54. doi: 10.14715/cmb/2019.66.1.8
59. Wang W, He S, Ji J, Huang J, Zhang S, and Zhang Y. The prognostic significance of FOXQ1 oncogene overexpression in human hepatocellular carcinoma. Pathol Res Pract. (2013) 209:353–8. doi: 10.1016/j.prp.2013.03.005
60. Han S, Shi Y, Sun L, Liu Z, Song T, and Liu Q. MiR-4319 induced an inhibition of epithelial-mesenchymal transition and prevented cancer stemness of HCC through targeting FOXQ1. Int J Biol Sci. (2019) 15:2936–47. doi: 10.7150/ijbs.38000
61. Luo Q, Wang CQ, Yang LY, Gao XM, Sun HT, Zhang Y, et al. FOXQ1/NDRG1 axis exacerbates hepatocellular carcinoma initiation via enhancing crosstalk between fibroblasts and tumor cells. Cancer Lett. (2018) 417:21–34. doi: 10.1016/j.canlet.2017.12.021
62. Huang W, Chen Z, Shang X, Tian D, Wang D, Wu K, et al. Sox12, a direct target of FoxQ1, promotes hepatocellular carcinoma metastasis through up-regulating Twist1 and FGFBP1. Hepatology. (2015) 61:1920–33. doi: 10.1002/hep.27756
63. Zhan HX, Xu JW, Wang L, Wu D, Zhang GY, and Hu SY. FoxQ1 is a novel molecular target for pancreatic cancer and is associated with poor prognosis. Curr Mol Med. (2015) 15:469–77. doi: 10.2174/1566524015666150630125247
64. Bao B, Azmi AS, Aboukameel A, Ahmad A, Bolling-Fischer A, Sethi S, et al. Pancreatic cancer stem-like cells display aggressive behavior mediated via activation of FoxQ1. J Biol Chem. (2014) 289:14520–33. doi: 10.1074/jbc.M113.532887
65. Wu C, Zheng C, Chen S, He Z, Hua H, Sun C, et al. FOXQ1 promotes pancreatic cancer cell proliferation, tumor stemness, invasion and metastasis through regulation of LDHA-mediated aerobic glycolysis. Cell Death Dis. (2023) 14:699. doi: 10.1038/s41419-023-06207-y
66. Zhang J, Liu Y, Zhang J, Cui X, Li G, Wang J, et al. FOXQ1 promotes gastric cancer metastasis through upregulation of Snail. Oncol Rep. (2016) 35:3607–13. doi: 10.3892/or.2016.4736
67. Liang SH, Yan XZ, Wang BL, Jin HF, Yao LP, Li YN, et al. Increased expression of FOXQ1 is a prognostic marker for patients with gastric cancer. Tumour Biol. (2013) 34:2605–9. doi: 10.1007/s13277-013-0808-x
68. Yang XY, Li N, Deng WY, Ma YJ, Han XL, Zhang ZY, et al. miRNA-96-5p inhibits the proliferation and migration of gastric cancer cells by targeting FoxQ1. Zhonghua Zhong Liu Za Zhi. (2019) 41:193–9. doi: 10.3760/cma.j.issn.0253-3766.2019.03.008
69. Feng A, Yuan X, and Li X. MicroRNA-345 inhibits metastasis and epithelial-mesenchymal transition of gastric cancer by targeting FOXQ1. Oncol Rep. (2017) 38:2752–60. doi: 10.3892/or.2017.6001
70. Xu J, You Q, Wei Z, Fu H, Zhang Y, Hu Z, et al. miR-519 inhibits epithelial-mesenchymal transition and biologic behavior of gastric cancer cells by down-regulating FOXQ1. Int J Clin Exp Pathol. (2020) 13:425–36.
71. Xiang XJ, Deng J, Liu YW, Wan LY, Feng M, Chen J, et al. MiR-1271 inhibits cell proliferation, invasion and EMT in gastric cancer by targeting FOXQ1. Cell Physiol Biochem. (2015) 36:1382–94. doi: 10.1159/000430304
72. Guo J, Yan Y, Yan Y, Guo Q, Zhang M, Zhang J, et al. Tumor-associated macrophages induce the expression of FOXQ1 to promote epithelial-mesenchymal transition and metastasis in gastric cancer cells. Oncol Rep. (2017) 38:2003–10. doi: 10.3892/or.2017.5877
73. Bobos M. Histopathologic classification and prognostic factors of melanoma: a 2021 update. Ital J Dermatol Venerol. (2021) 156:300–21. doi: 10.23736/S2784-8671.21.06958-3
74. Koscielecka K, Kubik-Machura D, Kuc A, Furmanek F, and Mecik-Kronenberg T. Melanoma during pregnancy as a complicated medical problem. Obstet Gynecol Surv. (2023) 78:115–23. doi: 10.1097/OGX.0000000000001109
75. Bagati A, Bianchi-Smiraglia A, Moparthy S, Kolesnikova K, Fink EE, Lipchick BC, et al. Melanoma suppressor functions of the carcinoma oncogene FOXQ1. Cell Rep. (2017) 20:2820–32. doi: 10.1016/j.celrep.2017.08.057
76. Bagati A, Bianchi-Smiraglia A, Moparthy S, Kolesnikova K, Fink EE, Kolesnikova M, et al. FOXQ1 controls the induced differentiation of melanocytic cells. Cell Death Differ. (2018) 25:1040–9. doi: 10.1038/s41418-018-0066-y
77. Mahajan A and Bhattacharyya S. Immunomodulation by mesenchymal stem cells during osteogenic differentiation: Clinical implications during bone regeneration. Mol Immunol. (2023) 164:143–52. doi: 10.1016/j.molimm.2023.11.006
78. Roszkowski S. Therapeutic potential of mesenchymal stem cell-derived exosomes for regenerative medicine applications. Clin Exp Med. (2024) 24:46. doi: 10.1007/s10238-023-01282-z
79. Xia SL, Ma ZY, Wang B, Gao F, Guo SY, and Chen XH. A gene expression profile for the lower osteogenic potent of bone-derived MSCs from osteoporosis with T2DM and the potential mechanism. J Orthop Surg Res. (2022) 17:402. doi: 10.1186/s13018-022-03291-2
80. Xiang L, Zheng J, Zhang M, Ai T, and Cai B. FOXQ1 promotes the osteogenic differentiation of bone mesenchymal stem cells via Wnt/beta-catenin signaling by binding with ANXA2. Stem Cell Res Ther. (2020) 11:403. doi: 10.1186/s13287-020-01928-9
81. Luo Z, Zeng H, Yang K, and Wang Y. FOXQ1 inhibits the progression of osteoarthritis by regulating pyroptosis. Aging (Albany NY). (2024) 16:5077–90. doi: 10.18632/aging.205600
82. Jonsson H and Peng SL. Forkhead transcription factors in immunology. Cell Mol Life Sci. (2005) 62:397–409. doi: 10.1007/s00018-004-4365-8
83. Earley AM, Dixon CT, and Shiau CE. Genetic analysis of zebrafish homologs of human FOXQ1, foxq1a and foxq1b, in innate immune cell development and bacterial host response. PloS One. (2018) 13:e0194207. doi: 10.1371/journal.pone.0194207
84. Liu P and Chen L. Inhibition of sonic hedgehog signaling blocks cell migration and growth but induces apoptosis via suppression of FOXQ1 in natural killer/T-cell lymphoma. Leuk Res. (2018) 64:1–9. doi: 10.1016/j.leukres.2017.11.001
85. Sun HT, Cheng SX, Tu Y, Li XH, and Zhang S. FoxQ1 promotes glioma cells proliferation and migration by regulating NRXN3 expression. PloS One. (2013) 8:e55693. doi: 10.1371/journal.pone.0055693
86. Zhao Q, Zhang R, Wang Y, Li T, Xue J, and Chen Z. FOXQ1, deubiquitinated by USP10, alleviates sepsis-induced acute kidney injury by targeting the CREB5/NF-kappaB signaling axis. Biochim Biophys Acta Mol Basis Dis. (2024) 1870:167331. doi: 10.1016/j.bbadis.2024.167331
87. Zhuang J, Chen Z, Cai P, Wang R, Yang Q, Li L, et al. Targeting MicroRNA-125b Promotes Neurite Outgrowth but Represses Cell Apoptosis and Inflammation via Blocking PTGS2 and CDK5 in a FOXQ1-Dependent Way in Alzheimer Disease. Front Cell Neurosci. (2020) 14:587747. doi: 10.3389/fncel.2020.587747
88. Ma X, Liu L, and Meng J. MicroRNA-125b promotes neurons cell apoptosis and Tau phosphorylation in Alzheimer’s disease. Neurosci Lett. (2017) 661:57–62. doi: 10.1016/j.neulet.2017.09.043
89. Ovsiy I, Riabov V, Manousaridis I, Michel J, Moganti K, Yin S, et al. IL-4 driven transcription factor FoxQ1 is expressed by monocytes in atopic dermatitis and stimulates monocyte migration. Sci Rep. (2017) 7:16847. doi: 10.1038/s41598-017-17307-z
90. Hong HK, Noveroske JK, Headon DJ, Liu T, Sy MS, Justice MJ, et al. The winged helix/forkhead transcription factor Foxq1 regulates differentiation of hair in satin mice. Genesis. (2001) 29:163–71. doi: 10.1002/gene.1020
91. Bushweller JH. Targeting transcription factors in cancer - from undruggable to reality. Nat Rev Cancer. (2019) 19:611–24. doi: 10.1038/s41568-019-0196-7
92. Gao M, Shih Ie M, and Wang TL. The role of forkhead box Q1 transcription factor in ovarian epithelial carcinomas. Int J Mol Sci. (2012) 13:13881–93. doi: 10.3390/ijms131113881
93. Jdeed S, Lengyel M, and Uray IP. Redistribution of the SWI/SNF Complex Dictates Coordinated Transcriptional Control over Epithelial-Mesenchymal Transition of Normal Breast Cells through TGF-beta Signaling. Cells. (2022) 11:2633. doi: 10.3390/cells11172633
94. Valencia-Sanchez MA, Liu J, Hannon GJ, and Parker R. Control of translation and mRNA degradation by miRNAs and siRNAs. Genes Dev. (2006) 20:515–24. doi: 10.1101/gad.1399806
95. Zhang J, Yang Y, Yang T, Yuan S, Wang R, Pan Z, et al. Double-negative feedback loop between microRNA-422a and forkhead box (FOX)G1/Q1/E1 regulates hepatocellular carcinoma tumor growth and metastasis. Hepatology. (2015) 61:561–73. doi: 10.1002/hep.27491
96. Zhang Z, Ma J, Luan G, Kang L, Su Y, He Y, et al. MiR-506 suppresses tumor proliferation and invasion by targeting FOXQ1 in nasopharyngeal carcinoma. PloS One. (2015) 10:e0122851. doi: 10.1371/journal.pone.0122851
97. Tan J, Liu B, Zhou L, Gao J, Wang XK, Liu Y, et al. LncRNA TUG1 promotes bladder cancer Malignant behaviors by regulating the miR-320a/FOXQ1 axis. Cell Signal. (2022) 91:110216. doi: 10.1016/j.cellsig.2021.110216
98. Wang J, Tan J, Zhang Y, Zhou L, and Liu Y. circCD2AP promotes epithelial mesenchymal transition and stemness in bladder cancer by regulating FOXQ1/USP21 axis. iScience. (2024) 27:108447. doi: 10.1016/j.isci.2023.108447
99. Atyeo N, Chae MY, Toth Z, Sharma A, and Papp B. Kaposi’s sarcoma-associated herpesvirus immediate early proteins trigger FOXQ1 expression in oral epithelial cells, engaging in a novel lytic cycle-sustaining positive feedback loop. J Virol. (2023) 97:e0169622. doi: 10.1128/jvi.01696-22
100. Yang WD, Zhang ZH, Zhao MY, Shao K, Ma YF, Shen Q, et al. P300-dependent acetylation of the FOXQ1 complex activates super-enhancers to promote colorectal cancer proliferation and metastasis. Commun Biol. (2025) 8:1016. doi: 10.1038/s42003-025-08430-z
101. Gorska A, Urbanowicz M, Grochowalski L, Seweryn M, Sobalska-Kwapis M, Wojdacz T, et al. Genome-wide DNA methylation and gene expression in patients with indolent systemic mastocytosis. Int J Mol Sci. (2023) 24:13910. doi: 10.3390/ijms241813910
102. Kaneda H, Arao T, Tanaka K, Tamura D, Aomatsu K, Kudo K, et al. FOXQ1 is overexpressed in colorectal cancer and enhances tumorigenicity and tumor growth. Cancer Res. (2010) 70:2053–63. doi: 10.1158/0008-5472.CAN-09-2161
103. Xia L, Huang W, Tian D, Zhang L, Qi X, Chen Z, et al. Forkhead box Q1 promotes hepatocellular carcinoma metastasis by transactivating ZEB2 and VersicanV1 expression. Hepatology. (2014) 59:958–73. doi: 10.1002/hep.26735
Keywords: FOXQ1, forkhead box, epithelial-mesenchymal transition, cancer, metastasis
Citation: Feng X, Zhang L, Shi P and Hu Y (2025) Advances in the study of FOXQ1: biological functions and mechanisms. Front. Oncol. 15:1650022. doi: 10.3389/fonc.2025.1650022
Received: 19 June 2025; Accepted: 30 October 2025;
Published: 19 November 2025.
Edited by:
Sherine Elsawa, University of New Hampshire, United StatesReviewed by:
Hui Tang, The First People’s Hospital of Yunnan Province, ChinaNaresh Sah, Texas Tech University Health Sciences Center, United States
Copyright © 2025 Feng, Zhang, Shi and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yiping Hu, aHV5aXBpbmcxMjZAMTI2LmNvbQ==; Peiyao Shi, c2hpcGVpeWFvMjAyNEAxNjMuY29t
 Xiaojian Feng1
Xiaojian Feng1 Yiping Hu
Yiping Hu