- 1Department of Gynaecology, Inner Mongolia People’s Hospital, Hohhot, Inner Mongolia, China
- 2Department of Gynaecology, Qilu Hospital of Shandong University, Jinan, Shandong, China
- 3Department of Clinical Medical Research Center, Affiliated Hospital of Inner Mongolia Medical University, Hohhot, Inner Mongolia, China
- 4Department of Nursing, Inner Mongolia People’s Hospital, Hohhot, Inner Mongolia, China
- 5Department of Nursing, Ordos Traditional Chinese Medicine Hospital, Ordos, Inner Mongolia, China
- 6School of Nursing, Inner Mongolia Medical University, Hohhot, Inner Mongolia, China
Objective: This narrative review aims to synthesize and evaluate existing evidence and practices regarding discharge readiness services for patients undergoing endometrial cancer (EC) surgery, with the goal of providing a reference for clinical practice and future research.
Background: The incidence of EC is rising globally. With the widespread adoption of enhanced recovery after surgery (ERAS) protocols and minimally invasive techniques, hospital stays are shortening, making effective discharge planning crucial for ensuring a safe transition to home and preventing readmissions.
Methods: As a narrative review, this article involved a comprehensive but non-systematic examination of literature from PubMed, Web of Science, CNKI, and Wanfang databases, focusing on key components of discharge readiness. These components include assessment tools, service content development, implementation processes, and outcome evaluation. The synthesis prioritized recent evidence and internationally recognized guidelines.
Results: The review identifies that while generic discharge assessment tools are valuable, they require adaptation to address the specific needs of EC patients (e.g., lymphedema risk, sexual health). Effective service implementation relies on a systematic interdisciplinary collaboration model and nurse-led, personalized education (e.g., using the teach-back method). The integration of digital health platforms shows promise for supporting post-discharge care. Outcome evaluation should encompass both clinical indicators (e.g., 30-day readmission rates) and patient-reported outcomes (e.g., using the Health Education Impact Questionnaire). Current challenges include a lack of standardized pathways and fragmented community resources.
Conclusion: Discharge readiness is a critical determinant of recovery quality for EC surgical patients. This review consolidates core components and processes into a practical framework, highlighting the need for multidisciplinary collaboration, patient-centered education, and technology integration. Future efforts should focus on developing standardized, culturally adapted pathways and conducting robust comparative effectiveness research to establish high-quality, evidence-based service systems.
Relevance to clinical practice: This study focuses on the development of core components and processes for systematic discharge readiness services for postoperative endometrial cancer patients, as well as the identification of practice challenges. The findings advocate for the clinical adoption of standardized frameworks and the enhancement of implementation capacity to optimize discharge transitions, patient recovery, and continuity of care.
Highlights
The identification of key components and processes for systematic discharge readiness services in postoperative endometrial cancer patients is crucial. Establishing a standardized service framework is fundamental for optimizing discharge transitions and ensuring continuity of care. Moreover, enhancing clinical implementation capacity significantly amplifies the framework’s positive impact on patient recovery outcomes.
1 Introduction
Endometrial cancer (EC) remains one of the most common gynecologic malignancies worldwide, with a steadily increasing incidence noted in both global statistics and data from China (1, 2). Surgery remains the primary treatment modality for EC, as recommended by the latest guidelines from the European Society of Gynaecological Oncology(ESGO) (1), often complemented by radiotherapy, chemotherapy, or hormone therapy (3). In recent years, advancements in surgical techniques, particularly the adoption of minimally invasive and robot-assisted procedures, coupled with the implementation of Enhanced Recovery After Surgery (ERAS) protocols, have significantly shortened hospital lengths of stay (4, 5). While this trend enhances healthcare efficiency and is often preferred by patients, it concurrently compresses the timeframe available for postoperative preparation and education, potentially elevating the risk of adverse events after discharge (6).
Postoperative recovery from EC surgery is not without challenges. Patients frequently contend with issues such as pain, urinary retention, lower limb lymphedema, sexual dysfunction, and psychological distress, all of which can profoundly impact their quality of life and long-term recovery (7–9). Therefore, ensuring that patients are adequately prepared for discharge—a concept known as discharge readiness—is paramount. Adequate discharge readiness facilitates a safe and effective transition from hospital to home, helps to reduce preventable readmissions, and supports optimal patient outcomes (10, 11).
The concept of discharge readiness has evolved considerably since its inception. Initially focused on physiological stability, it now encompasses a multidimensional construct including the patient’s knowledge, self-care abilities, psychosocial status, and the adequacy of anticipated support at home (12, 13). Theoretical frameworks, such as the one developed by Galvin et al., posit discharge readiness as the culmination of processes involving assessment, education, and coordination (14). In practice, nursing-led discharge readiness services are pivotal, integrating needs assessment, patient education, and care coordination to bridge the gap between hospital and home (15, 16).
Despite its recognized importance, the standardization of discharge readiness services for EC patients, especially within the Chinese healthcare context, remains in its developmental stages. Challenges persist at multiple levels: a lack of detailed, EC-specific implementation guidelines; inefficient coordination mechanisms between hospitals and community care; and sociocultural barriers that may hinder open communication about sensitive topics like sexual health (17, 18). Conversely, discharge readiness services, particularly for EC patients, are still in an exploratory and developmental stage (19), encountering several challenges. Consequently, there is a pressing need to synthesize existing evidence and international experiences to inform the development of structured, effective, and culturally sensitive discharge readiness services for this patient population.
This narrative review seeks to address this gap by systematically describing the core components of discharge readiness services for EC surgical patients. It will synthesize evidence on assessment tools, the construction of service content, implementation processes, and outcome evaluation. By integrating these elements and proposing an implementation flowchart, this review aims to offer clinicians a practical, evidence-informed reference to enhance the discharge transition experience, improve patient recovery, and lay the groundwork for establishing standardized service pathways in the future.
2 Literature search and screening strategy
This study adopts a “narrative review” approach, aiming to conduct a descriptive synthesis and interpretation of the existing evidence, practical models, and theoretical frameworks in the field of discharge readiness services for patients undergoing EC surgery. To ensure the breadth and representativeness of the content, an extensive literature search and screening were performed; however, the full procedures of a systematic review (e.g., exhaustive search, double independent screening, rigorous risk of bias assessment, or quantitative synthesis) were not followed.
2.1 Literature sources and screening rationale
To construct the content of this review, the authors searched four databases: PubMed, Web of Science, China National Knowledge Infrastructure (CNKI), and Wanfang Data Knowledge Service Platform. The search timeframe primarily focused on from January 2010 to December 2024. The core rationale for literature screening was to identify studies highly relevant to the key components of discharge readiness services for post-EC surgical patients, including assessment tools, service content, implementation processes, and outcome evaluation. During the search, Chinese and English subject terms were used comprehensively, such as “endometrial cancer”, “discharge planning”, “transitional care”, and “discharge readiness”. Literature inclusion was based on the “representativeness and contribution of the studies to the purpose of this review”, rather than conducting a systematic search of all relevant literature.
2.2 Considerations for literature inclusion
In the literature screening process, the following aspects were primarily considered: ① Study population: Patients who underwent surgical treatment for endometrial cancer; ② Intervention/Topic: Studies focusing on the assessment, implementation, or evaluation of discharge readiness services and transitional care; ③ Study type: Priority was given to randomized controlled trials (RCTs), cohort studies, qualitative studies, systematic reviews, clinical guidelines, and expert consensuses. Additionally, other literature that could provide important insights was included as appropriate; ④ Language: Literature published in Chinese or English. The exclusion criteria included: non-research literature (e.g., commentaries, editorials), studies whose population did not consist of post-EC surgical patients, or literature whose content was irrelevant to the core components of discharge readiness services.
3 Discharge readiness assessment tools for EC surgery patients
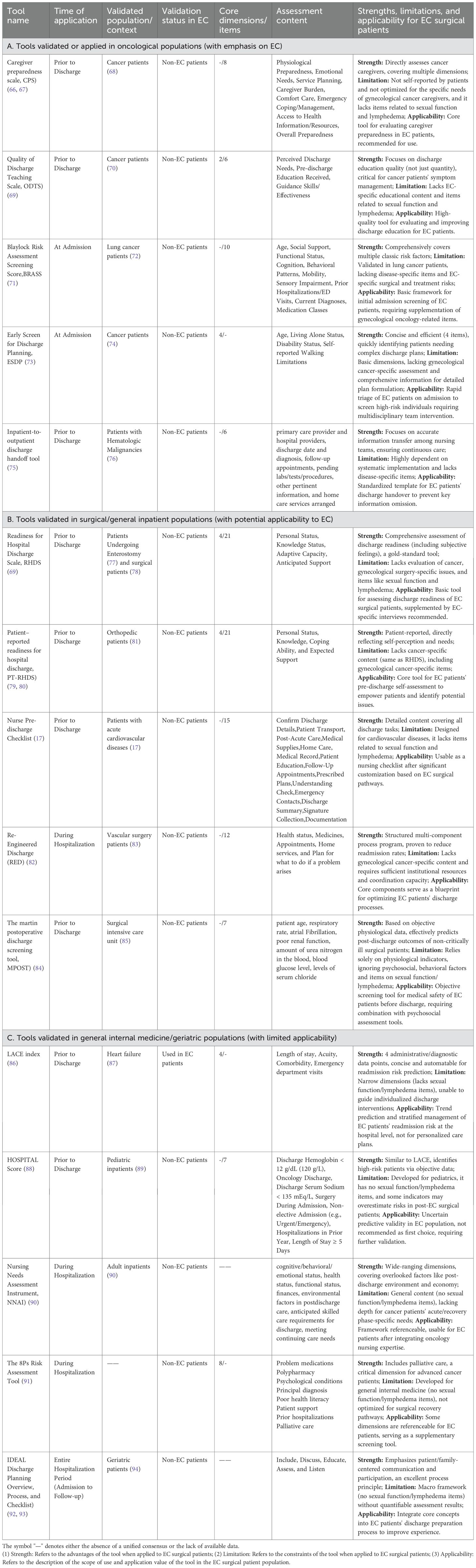
Postoperative recovery in patients undergoing EC surgery exhibits significant variability, necessitating a tailored approach to care. The discharge process represents a critical transition point in the continuum of care, bridging the in-hospital phase and post-discharge recovery. To ensure continuity of care, the utilization of comprehensive discharge readiness assessment tools is indispensable (13). Existing tools, while addressing fundamental dimensions such as health status, psychosocial support, and self-management capabilities, are predominantly generic. These tools are beneficial for general patient recovery but lack specificity to meet the unique needs of EC patients. EC patients present distinct challenges due to the unique characteristics of the disease, treatment modalities (e.g., extent of surgery, adjuvant therapy), and specific rehabilitation requirements, such as the risk of lymphedema, sexual function, and fertility counseling. Current discharge readiness tools require targeted adaptation to effectively address these specialized needs. A critical review of their psychometric properties highlights important gaps in validity and applicability. While several instruments demonstrate varying degrees of psychometric robustness (Table 1), none have been comprehensively validated in EC populations. Consequently, even as ERAS protocols are widely implemented in gynecologic oncology settings (20), the lack of discharge assessment frameworks tailored for EC patients remains a critical gap in ensuring personalized continuity of care.
For example, tools derived from general oncological populations (Part A), such as the Caregiver Preparedness Scale (CPS) and the Quality of Discharge Teaching Scale (ODTS), show reasonable content validity for broad cancer care issues. However, their structural validity within the EC subpopulation remains unverified. Instruments widely recognized as gold standards in surgical settings, such as the Readiness for Hospital Discharge Scale (RHDS) and its patient version (PT-RHDS) (Part B), exhibit strong reliability and construct validity in general surgical cohorts. Nonetheless, their ecological and content validity are limited for EC patients due to the exclusion of disease-specific sequelae, such as those related to gynecological cancer. Predictive tools such as the LACE index (Part C), which excel in criterion and predictive validity for readmission risk, are primarily based on administrative data and lack the ability to address subjective patient readiness or guide individualized discharge planning.
In summary, the current psychometric evidence for discharge readiness tools remains fragmented. Instruments validated for general populations lack EC-specific content validity, while predictive tools fail to provide actionable insights for personalized care planning. Addressing these gaps requires the development of an EC-specific discharge readiness tool that integrates key domains such as sexual health and lymphedema management within a psychometrically sound framework, such as the Readiness for Hospital Discharge Scale (RHDS). Future research should focus on rigorous cross-cultural adaptation and validation to ensure applicability across diverse patient populations.
4 Constructing discharge readiness service content for EC patients
The development of discharge readiness services for EC requires the establishment of a specialized framework that is centered on the characteristics of the disease. This framework should integrate aspects of oncologic treatment, such as the extent of surgery and the need for hormone therapy, as well as patient metabolic profiles, including the high prevalence of comorbidities like obesity, and long-term survivorship management needs. The content construction should adhere to two fundamental mechanisms: a multidimensional needs assessment and an interdisciplinary collaboration model.
4.1 Scientific multidimensional needs assessment mechanism
While traditional needs assessments relying on caregiver reports are limited by subjectivity, evidence demonstrates that structured, objective tools can significantly improve patient outcomes. For instance, the implementation of standardized risk assessment tools has been associated with a 12-30% reduction in avoidable hospital readmissions in general surgical populations by enabling early identification of high-risk patients (21, 22). Furthermore, integrating patient-reported outcome measures (PROMs) into routine assessments has been shown to significantly improve patient-provider communication and identify unmet needs in domains like psychological distress and physical function, leading to more tailored interventions and enhanced quality of life (23). To advance this field in EC care, future research should leverage multi-source data integration, including machine learning models trained on specific clinical datasets (24), to develop predictive, multidimensional assessment systems. Such a system would synthesize physiological status (e.g., recovery biomarkers), psychological status (e.g., anxiety/depression), social support, and environmental factors. Crucially, the transition from theoretical models like QCNN_BaOpt (24) to clinical impact requires validation in EC cohorts, with a focus on quantifying their efficacy in improving concrete endpoints such as readmission rates, patient satisfaction, and symptom burden.
4.2 Systematic interdisciplinary collaboration model
The collaboration within a multidisciplinary team (MDT) is fundamental to delivering high-quality discharge readiness services, with robust evidence supporting its impact on patient outcomes. The discharge team for EC should comprise essential members, including gynecologic oncologists, surgeons, nurses, rehabilitation therapists, nutritionists, psychologists/psychiatrists, and social workers (25, 26). The success of MDT collaboration is contingent upon clearly defined roles, effective communication strategies, and shared decision-making processes. Quantitative analyses demonstrate that structured MDT care can lead to a significant reduction in unplanned 30-day readmissions for complex chronic conditions by improving care coordination and patient education. Specifically, relevant studies (27, 28) have confirmed that the reduction rate can reach 7.4% in direct comparison (from 16.4% to 9.0%) and even exceed 6 percentage points in sustained practice (from 18.9% to 12.6%). Evidence suggests that implementing structured collaboration models, such as the relationship-centered care model, can mitigate team conflicts and further contribute to these positive outcomes (25). Programs like the ASSIST discharge initiative, which extend collaboration to community providers and actively involve patients and caregivers (29, 30), are critical. This collaborative framework, which encompasses the entire continuum from hospitalization to post-discharge follow-up, is essential for ensuring that medical care plans are effectively aligned with patients’ needs at home, ultimately translating into quantifiable improvements in readmission rates and quality of life.
4.3 Nurse-led personalized service implementation
Nurses, as pivotal coordinators and implementers of discharge readiness services, play a crucial role in optimizing postoperative outcomes for surgical patients. Meta-analyses of adult surgical inpatients have demonstrated that nurse-led discharge interventions significantly reduce 30-day readmissions and emergency department visits, while simultaneously improving patients’ activities of daily living, quality of life, and overall satisfaction (31). The effectiveness of these interventions lies in their ability to facilitate more coordinated and comprehensible care transitions, aligning with evidence that nurse-driven strategies outperform conventional approaches in minimizing unplanned healthcare utilization and enhancing patient-reported outcomes within surgical populations.
In the early recovery phase, evidence-based health education has emerged as a cornerstone for empowering patient self-management (32). Specifically, a review focusing on ERAS in minimally invasive gynecologic oncology surgery confirmed that nurse-led discharge education, as a key component of ERAS, increases rates of same-day discharge without elevating readmission risk, while also reducing perioperative and postoperative opioid use without compromising pain control (33). Research underscores the importance of personalized education plans grounded in the best available evidence and tailored to address critical postoperative needs, including activity guidance, treatment adherence, and comorbidity management. To uphold patient-centered care principles, it is essential to adopt structured communication techniques such as the teach-back method, which not only ensures comprehension but also actively engages patients and their caregivers in collaboratively developing individualized discharge plans. Evidence further indicates that inadequate patient engagement is strongly associated with an elevated risk of readmission (34). To ensure continuity of care beyond hospital discharge, implementing a dynamic and iterative cycle of “assessment, education, feedback, and adjustment” is paramount. This framework supports seamless transitions from hospital to home, enabling nurses to provide adaptive, ongoing care that meets patients’ evolving needs during postoperative recovery.
5 Implementation of discharge readiness services for EC patients
The implementation strategy for discharge readiness services exerts a direct and measurable impact on patient outcomes, with variations in effectiveness observed across different care models, such as the case management model and the primary nursing model. Evidence from a study involving total joint arthroplasty patients demonstrated that the perioperative surgical case management model was associated with a significantly reduced 30-day unplanned readmission rate of 2.1%, compared to the typical 5%-10% reported with standard nursing care. Although specific data on length of hospital stay were not provided, refined perioperative interventions under this model may contribute to a slight increase in hospitalization duration (35). This trade-off underscores the necessity for patient-specific strategy optimization, balancing the benefits of reduced readmissions with potential extensions in hospital stay. Patient-specific factors, such as low Activities of Daily Living (ADL) scores (≤60), the need for tube feeding, poorly managed comorbidities, and limited capacity of primary caregivers, have been identified as significant predictors of extended hospital stays (36). These factors highlight the critical importance of tailoring discharge readiness strategies to the individual circumstances and risk profiles of each patient. Systematic reviews evaluating interventions such as care coordination, discharge education, and follow-up provide mixed results (37), further emphasizing the need for personalized approaches that consider both the clinical and psychosocial aspects of postoperative recovery. In light of these findings, developing and implementing discharge readiness models that integrate individualized risk assessments and patient-centered interventions is crucial for improving outcomes. Future research should prioritize identifying predictive factors and refining care strategies to enable a seamless transition from hospital to home, ultimately enhancing recovery while minimizing unnecessary healthcare utilization.
Advancements in contemporary information technology offer innovative opportunities to enhance discharge readiness services, particularly through the integration of digital health solutions. Digital health platforms, encompassing telemedicine applications and online resource portals, have demonstrated their capacity to strengthen communication between healthcare providers and patients, facilitate access to offline services, and support the collection of real-world post-discharge data for service optimization (38, 39). A meta-analysis of 24 randomized controlled trials (RCTs) involving 9,634 patients with heart failure revealed that eHealth self-management interventions—a critical subset of digital health technologies—significantly reduced all-cause and heart failure-related 30-day readmission rates. These interventions also increased medication adherence by 82% and improved self-care behaviors, outcomes primarily driven by enhanced patient engagement (40). These findings underscore the transformative potential of digital health in fostering better health outcomes through patient-centered approaches.
Moreover, machine learning technologies are emerging as powerful tools for analyzing complex clinical datasets, identifying risk patterns, and predicting individual patient needs. Mobile health (mHealth) and AI predictive models are increasingly being integrated into the postoperative transitional care of gynecological cancer patients. Bukke (41) highlighted that AI-driven mobile remote monitoring can continuously track vital signs and preemptively alert medical teams to complications like lymphedema and thrombosis. The “Zhi Kang Fu” WeChat mini-program, developed and implemented in a top-tier hospital in Beijing, China, by Zhang (42), consolidates six core modules, including health education, complication warnings, chemotherapy management, and psychological support. Patients can access personalized care suggestions via intelligent inquiries, and a 1-month trial showed high user acceptance (156.15 ± 30.93 out of a possible 175), significantly enhancing self-management and adherence during home recovery. Delanerolle further confirmed that mHealth platforms powered by machine learning can provide real-time risk assessments of postoperative symptoms and send reminders to both patients and healthcare providers, ensuring a seamless transition from hospital to home care (43). Khudhur also emphasized that the combination of AI predictive models and mobile technology can detect recurrences or treatment-related complications early, offering continuous, precise, and cost-effective support for gynecological cancer patients outside the hospital setting (44).
By harnessing these capabilities, personalized discharge plans and follow-up strategies can be developed to address the unique requirements of diverse patient populations (45). For instance, studies have demonstrated the utility of neural network models in predicting risks associated with discharge delays or optimizing discharge decisions for specific clinical conditions, such as COVID-19 (46, 47). While promising, the application, effectiveness, and generalizability of these technologies within emergency care and oncology-specific discharge readiness services require further investigation and rigorous validation. In summary, digital health platforms and machine learning methodologies hold significant potential to revolutionize discharge readiness strategies. Future research should focus on validating these technologies in diverse clinical settings, including oncology, to ensure their reliability, scalability, and ability to deliver personalized, data-driven care transitions.
Developing a standardized discharge readiness pathway for esophageal cancer patients requires a comprehensive approach that spans the entire care continuum, from hospital admission to post-discharge recovery. Key strategies for pathway implementation include: ① Tiered health education tailored to the disease stage, cognitive function, and self-management capabilities of each patient.② Diverse educational formats, such as in-person instruction, personalized educational materials, audiovisual aids, and digital platforms, to accommodate varying patient preferences and learning needs.③ Structured communication techniques, including the teach-back method, which has proven effective in enhancing patient comprehension, engagement, and knowledge retention (48). A standardized pathway that integrates approaches such as staff training in teach-back techniques, discharge resource folders, and optimized educational documents has demonstrated a synergistic effect. Compared to ad-hoc discharge practices, these strategies significantly improve patients’ satisfaction with discharge-related services. Evidence from satisfaction surveys reveals notable improvements in care transition scores (from 52.41% to 54.49%) and discharge information scores (from 87.38% to 90.12%) (49). These findings highlight the value of a structured, patient-centered discharge readiness pathway in achieving better patient-reported outcomes. Furthermore, the integration of technological tools with humanistic and patient-centered care is essential for optimizing resource allocation and providing continuous support during recovery (13, 50). By facilitating seamless communication and personalized interventions, these tools complement the standardized pathway and contribute to improved postoperative outcomes for EC patients.
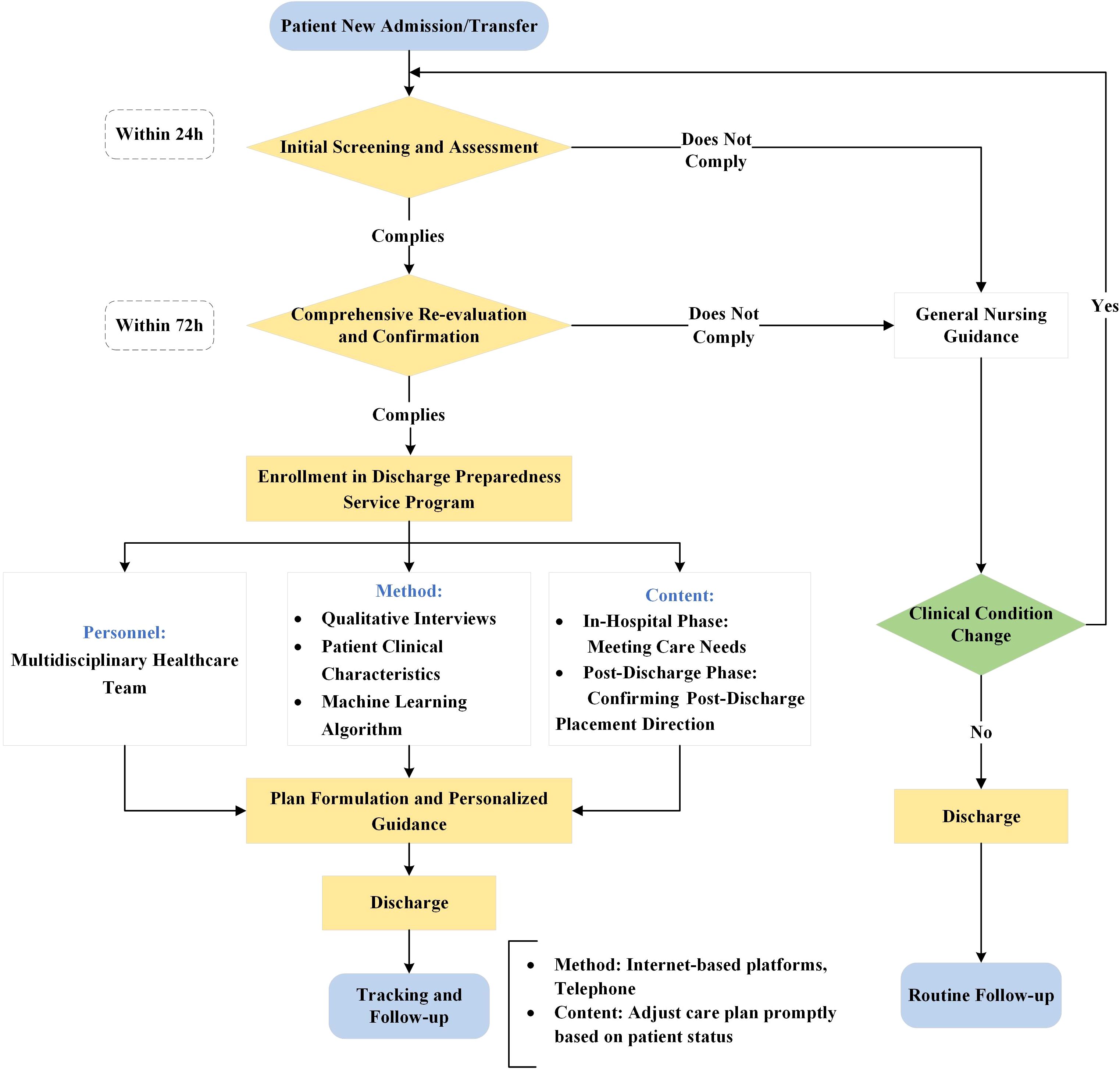
5.1 Core steps of the EC surgery patient discharge readiness service implementation flowchart
The core implementation process of the EC surgery patient discharge readiness service is visualized in a detailed flowchart (Figure 1), which consists of four sequential and interconnected phases as follows.
5.1.1 Screening and initial assessment
Within 24 hours of patient admission or transfer, the healthcare team conducts an initial screening using the Blaylock Risk Assessment Screening Score (BRASS) (51)to identify patients at risk for delayed discharge or those with complex care needs. A total score of 10 or higher indicates the need to proceed to the next step (52, 53).
5.1.2 Comprehensive reassessment and confirmation
For patients who screen positive, a comprehensive assessment should be conducted within 72 hours (50). The assessment should cover domains such as Activities of Daily Living (ADLs), self-care ability and need for assistance, transportation arrangements, home support network, medication management ability, nutritional status and eating ability, and home care service needs (e.g., home visit nursing, hospice care). The use of tools like the Comprehensive Discharge Planning Needs Assessment Form is recommended (54, 55).
5.1.3 Plan development and personalized guidance
Following the reassessment outcomes, MDT collaboratively formulates a personalized discharge plan. The implementation of this plan is structured in phases:①Inpatient Phase: Address in-hospital care requirements and provide training for patients and caregivers on essential post-discharge care skills. ②Discharge Phase: Confirm the discharge destination and coordinate subsequent care arrangements. ③Home Care Phase: Equip primary caregivers with necessary skills and offer information on home nursing services and community resource linkages. ④Transfer to Facility: Contact and coordinate with receiving institutions to ensure a smooth transition.
5.1.4 Tracking and follow-up
Subsequent to discharge, scheduled follow-ups via telephone, home visits, or clinic appointments should be conducted to evaluate recovery progress, care satisfaction, and any unmet needs, allowing for necessary adjustments to the care plan (56). The frequency and mode of follow-up should be tailored to the individual patient’s condition and risk assessment.
6 Outcome evaluation of discharge readiness services for EC patients
Assessing the effectiveness of discharge readiness services is crucial for the ongoing enhancement of healthcare quality. An effective evaluation system should incorporate clinical evidence alongside multidimensional indicators. Research indicates that among patients undergoing minimally invasive hysterectomy, there is no significant difference in the 60-day complication rates between obese and non-obese patients (5.1% vs. 5.4%, P = 0.86), although obese patients experience lower rates of same-day discharge (57). A recent large-scale analysis of the National Cancer Database found that for patients with Type II endometrial cancer undergoing robot-assisted laparoscopy, the 30-day readmission rate was 2.5%, showing no significant difference compared to conventional laparoscopy (2.4%, p=0.819) (58). Factors such as extended hospital stays (>1 day vs. ≤1 day: 40.0% vs. 23.0%, P = 0.04) and postoperative complications (63.3% vs. 13.4%, P<0.01) are significant predictors of readmission (59). The implementation of Enhanced Recovery After Surgery (ERAS) protocols has been shown to effectively reduce hospital stays and associated costs without significantly increasing readmission rates (60). These findings offer a foundation for optimizing discharge strategies tailored to various surgical techniques and patient demographics.
Building upon prognostic evidence and established guideline requirements, such as those outlined in the 2022 ESMO Clinical Practice Guideline for endometrial cancer (32), the evaluation of outcomes necessitates a comprehensive framework that integrates patient outcomes, caregiver experiences, and the utilization of healthcare system resources. The MDT should develop personalized, risk-stratified follow-up plans that consider recurrence risk, treatment side effects, physical and mental health status, and resource accessibility. According to the ESMO guideline, follow-up frequency should be stratified by risk: for low-risk patients, clinical visits are recommended every 6 months for the first 2 years and then annually until 5 years, with telephone follow−up as an acceptable alternative; for high-risk patients, visits are recommended every 3 months for the first 3 years and then every 6 months until 5 years. Patient education on symptom recognition and the option of patient−initiated follow−up (PIFU) are also emphasized (32). Short-term evaluations should concentrate on critical clinical indicators, such as 30-day postoperative complication rates, readmission rates, and the time required for bowel function recovery (61). In contrast, long-term evaluations should incorporate Patient-Reported Outcomes (PROs), employing standardized instruments like the Health Education Impact Questionnaire (HeiQ) to assess self-management domains, including health behaviors, emotional well-being, and social interaction (62). Recent studies have further demonstrated the value of the EORTC QLQ-EN24—an endometrial-cancer-specific PRO module—in capturing post-discharge quality of life. Sobočan et al. applied the Slovenian QLQ-EN24 to 79 EC survivors and found that gastrointestinal symptoms, musculoskeletal pain and sexual/vaginal concerns were significantly linked to poorer multi-domain outcomes, underscoring the scale’s sensitivity to long-term sequelae (63). Likewise, the prospective QLEC cohort (n = 198) administered the QLQ-EN24 together with PROMIS measures and showed that pre-operative obesity predicted worse physical functioning, incident lower-extremity lymphedema and diminished sexual interest, providing baseline risk data for discharge planning (64). In Taiwan, Li et al. validated the Chinese QLQ-EN24 in 105 women ≥ 6 months post-treatment and revealed that those with lower-limb lymphedema reported significantly more severe climacteric symptoms and poorer physical, role, emotional, cognitive and social functioning, confirming the instrument’s cross-cultural utility (65). Integrating this EC-specific tool into routine follow-up therefore enables clinicians to detect symptom clusters, functional deficits and psychosocial needs after hospital discharge, informing tailored self-management support. This internationally validated scale is instrumental in identifying deficiencies in post-discharge self-management, thereby informing interventions for continuity of care. The establishment of a continuous improvement cycle—comprising evidence generation, outcome evaluation, and service optimization—facilitates the enhancement of quality throughout the patient management journey in EC.
7 Conclusion
This review systematically synthesizes existing evidence on discharge readiness services for patients undergoing EC surgery, highlighting its essential role in facilitating a seamless transition from hospital to home and enhancing long-term outcomes. The key findings are as follows (1): The variability of assessment tools necessitates adaptation to the specific characteristics of EC; (2) The effectiveness of service implementation is contingent upon the synergy of multidisciplinary collaboration, nurse-led education (e.g., the teach-back method), and the integration of technology (e.g., remote monitoring digital platforms); (3) Systems for evaluating outcomes must incorporate both clinical indicators (e.g., 30-day readmission rates) and patient-reported outcomes (e.g., the Health Education Impact Questionnaire [HeiQ] scale) to comprehensively assess multidimensional recovery status. Persistent challenges include the lack of standardized pathways, fragmented community resources, and insufficient patient engagement. To address these issues, we propose the following at the policy level: the development of guideline-based and culturally adapted standardized discharge pathways. At the research level, conducting prospective trials to compare various implementation models is essential for advancing evidence-based nursing management practices. For future prospective trials, the proposed flowchart can serve as the Standard Operating Procedure for the intervention group. This approach ensures that the complex intervention—the Discharge Preparedness Service Program—is delivered in a standardized and systematic manner, minimizing potential bias arising from inconsistent interpretations of the intervention by different researchers. The flowchart outlines clear operational details, including specified time points (e.g., initial screening within 24 hours, comprehensive re-evaluation within 72 hours), designated personnel (a multidisciplinary healthcare team), core methods (integration of qualitative interviews and machine learning algorithms), and intervention content (assessment of both in-hospital and post-discharge patient needs). By providing a detailed and structured blueprint, this flowchart significantly enhances the reproducibility of the trial and improves the comparability of results across studies. Moreover, the flowchart inherently highlights critical processes and outcome measures requiring attention in future trials. This ensures that subsequent studies maintain a consistent focus on evaluating the efficacy of the intervention and its impact on patient outcomes, thereby strengthening the evidence base for nursing management strategies.
The flowchart provided (Figure 1) serves as a practical and actionable framework for clinicians, highlighting the necessity for local adaptation in light of disparities in healthcare resource allocation. Future research should aim to advance evidence-based standardized practices while maintaining flexibility rooted in patient-centered principles.
8 Limitations
This study has several limitations. First, as a narrative review, its methodology inherently has inherent limitations. We did not conduct a systematic and exhaustive literature search, nor did we use standardized quality assessment tools to perform rigorous risk of bias assessment on all included studies. Literature screening and inclusion relied more on the researchers’ subjective judgments and considerations regarding the representativeness and heuristic value of the content. This may introduce selection bias and undermine the robustness of the conclusions. Second, the literature search was restricted to Chinese and English databases, excluding gray literature and studies in other languages. This may limit the comprehensiveness of the evidence obtained. Therefore, the conclusions of this paper are intended to provide a comprehensive overview and practical framework for this field, and its viewpoints and recommendations need to be verified and supported by more rigorous systematic reviews, meta-analyses, or original studies in the future.
Author contributions
XC: Conceptualization, Formal Analysis, Methodology, Software, Validation, Visualization, Writing – original draft. PT: Conceptualization, Formal Analysis, Methodology, Software, Validation, Visualization, Writing – original draft. CM: Conceptualization, Formal Analysis, Methodology, Software, Validation, Visualization, Writing – original draft. SM: Conceptualization, Formal Analysis, Methodology, Software, Validation, Visualization, Writing – original draft. JS: Data curation, Investigation, Resources, Writing – original draft. WC: Data curation, Investigation, Resources, Writing – original draft. YL: Conceptualization, Funding acquisition, Writing – original draft. YC: Investigation, Resources, Writing – original draft. YTW: Investigation, Resources, Writing – original draft. YH: Investigation, Resources, Writing – original draft. JW: Conceptualization, Project administration, Supervision, Writing – review & editing. YYW: Conceptualization, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declared that financial support was received for work and/or its publication. The study was supported by the Science and Technology Program of the Joint Fund of Scientific Research for the Public Hospitals of Inner Mongolia Academy of Medical Sciences (2024GLLH0016), the Evidence-Based Nursing Practice Application Project (Nmyyjd202417) and the Science and Technology Program of the Joint Fund of Scientific Research for the Public Hospitals of Inner Mongolia Academy of Medical Sciences (2023GLLH0084).
Acknowledgments
The authors thanks all the participants in this study.
Conflict of interest
The author(s) declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declared that generative AI was not used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Chinese Anti-Cancer Association GOC. Guidelines for diagnosis and treatment of endometrial carcinoma (2021 edition). China Oncol. (2021) 31:501–12. doi: 10.19401/j.cnki.1007-3639.2021.06.08
3. Zhang X and Wang JD. Research progress on epidemiological risk factors related to endometrial cancer. Med Recapitulate. (2021) 27:2995. doi: 10.3969/j.issn.1006-2084.2021.15.016
4. Zhou S, Qin YZ, Zhang L, Zhang HQ, Liu X, and Jiang JF. Status and influencing factors of body image in gynecological cancer patients receiving postoperative chemotherapy. Nurs Res China. (2021) 35:306. doi: 10.12102/j.issn.1009-6493.2021.02.022
5. Jiang Z. Incidence and influencing factors of postoperative lower limb lymphedema in cervical cancer patients. Modern Nurse. (2021) 28:55. doi: 10.19791/j.cnki.1006-6411.2021.25.017
6. Xia LL, Dai LN, and Deng C. Application of preoperative prehabilitation nursing model based on ERAS concept in gynecological tumor patients. China Modern Med. (2024) 31:162. doi: 10.3969/j.issn.1674-4721.2024.02.039
7. Wang L and Xu L. Role of reducing average length of stay in improving hospital operational efficiency. China Health Standard Management. (2017) 8:17. doi: 10.3969/j.issn.1674-9316.2017.12.010
8. Mäkelä P, Stott D, Godfrey M, Ellis G, Schiff R, and Shepperd S. The work of older people and their informal caregivers in managing an acute health event in a hospital at home or hospital inpatient setting. Age Ageing. (2020) 49:856–64. doi: 10.1093/ageing/afaa085
9. Landeiro F, Roberts K, Gray AM, and Leal J. Delayed hospital discharges of older patients: A systematic review on prevalence and costs. Gerontologist. (2019) 59:e86–97. doi: 10.1093/geront/gnx028
10. Newton C, Nordin A, Rolland P, Ind T, Larsen-Disney P, Martin-Hirsch P, et al. British Gynaecological Cancer Society recommendations and guidance on patient-initiated follow-up (PIFU). Int J Gynecol Cancer. (2020) 30:695–700. doi: 10.1136/ijgc-2019-001176
11. Fenwick AM. An interdisciplinary tool for assessing patients’ readiness for discharge in the rehabilitation setting. J Adv Nurs. (1979) 4:9–21. doi: 10.1111/j.1365-2648.1979.tb02984.x
12. Weiss ME and Piacentine LB. Psychometric properties of the readiness for hospital discharge scale. J Nurs Meas. (2006) 14:163–80. doi: 10.1891/jnm-v14i3a002
13. Galvin EC, Wills T, and Coffey A. Readiness for hospital discharge: A concept analysis. J Adv Nurs. (2017) 73:2547–57. doi: 10.1111/jan.13324
14. Naylor MD, Aiken LH, Kurtzman ET, Olds DM, and Hirschman KB. The care span: The importance of transitional care in achieving health reform. Health Aff (Millwood). (2011) 30:746–54. doi: 10.1377/hlthaff.2011.0041
15. Weiss ME, Bobay KL, Bahr SJ, Costa L, Hughes RG, and Holland DE. A model for hospital discharge preparation: from case management to care transition. J Nurs Adm. (2015) 45:606–14. doi: 10.1097/nna.0000000000000273
16. Coleman EA, Parry C, Chalmers S, and Min SJ. The care transitions intervention: results of a randomized controlled trial. Arch Intern Med. (2006) 166:1822–8. doi: 10.1001/archinte.166.17.1822
17. Mennuni M, Gulizia MM, Alunni G, Francesco Amico A, Maria Bovenzi F, Caporale R, et al. ANMCO Position Paper: hospital discharge planning: recommendations and standards. Eur Heart J Suppl. (2017) 19:D244–d55. doi: 10.1093/eurheartj/sux011
18. Yam CH, Wong EL, Cheung AW, Chan FW, Wong FY, and Yeoh EK. Framework and components for effective discharge planning system: a Delphi methodology. BMC Health Serv Res. (2012) 12:396. doi: 10.1186/1472-6963-12-396
19. Mui J, Cheng E, and Salindera S. Enhanced recovery after surgery for oncological breast surgery reduces length of stay in a resource limited setting. ANZ J Surg. (2024) 94:1096–101. doi: 10.1111/ans.18901
20. Ortiz Vazquez EF, Londoño Victoria JC, Castillo López GA, Hidalgo Tapia EC, Mena Acosta FI, and Puchaicela Namcela SDR. Minimally invasive gynecologic surgery and enhanced recovery and outcomes: A literature review. Cureus. (2025) 17:e84814. doi: 10.7759/cureus.84814
21. Bertsimas D, Li ML, Paschalidis IC, and Wang T. Prescriptive analytics for reducing 30-day hospital readmissions after general surgery. PloS One. (2020) 15:e0238118. doi: 10.1371/journal.pone.0238118
22. Pham H, Hitos K, Pawaskar R, Sinclair JL, Mathuthu H, Nahm CB, et al. Multidisciplinary protocol to reduce surgical readmissions in Australia: American College of Surgeons National Surgical Quality Improvement Program. ANZ J Surg. (2025) 95:335–41. doi: 10.1111/ans.19252
23. Gibbons C, Porter I, Gonçalves-Bradley DC, Stoilov S, Ricci-Cabello I, Tsangaris E, et al. Routine provision of feedback from patient-reported outcome measurements to healthcare providers and patients in clinical practice. Cochrane Database Syst Rev. (2021) 10:Cd011589. doi: 10.1002/14651858.CD011589.pub2
24. Nandhini RS and Lakshmanan R. QCNN_BaOpt: multi-dimensional data-based traffic-volume prediction in cyber-physical systems. Sensors (Basel). (2023) 23:1485. doi: 10.3390/s23031485
25. Gaboury I, Lapierre LM, Boon H, and Moher D. Interprofessional collaboration within integrative healthcare clinics through the lens of the relationship-centered care model. J Interprof Care. (2011) 25:124–30. doi: 10.3109/13561820.2010.523654
26. Irwin RS, Flaherty HM, French CT, Cody S, Chandler MW, Connolly A, et al. Interdisciplinary collaboration: the slogan that must be achieved for models of delivering critical care to be successful. Chest. (2012) 142:1611–9. doi: 10.1378/chest.12-1844
27. Hahn B, Ball T, Diab W, Choi C, Bleau H, and Flynn A. Utilization of a multidisciplinary hospital-based approach to reduce readmission rates. SAGE Open Med. (2024) 12:20503121241226591. doi: 10.1177/20503121241226591
28. Patel H, Yirdaw E, Yu A, Slater L, Perica K, Pierce RG, et al. Improving early discharge using a team-based structure for discharge multidisciplinary rounds. Prof Case Manage. (2019) 24:83–9. doi: 10.1097/ncm.0000000000000318
29. Kraun L, De Vliegher K, Vandamme M, Holtzheimer E, Ellen M, and van Achterberg T. Older peoples’ and informal caregivers’ experiences, views, and needs in transitional care decision-making: a systematic review. Int J Nurs Stud. (2022) 134:104303. doi: 10.1016/j.ijnurstu.2022.104303
30. Better together – ASSIST hospital discharge scheme. Nottinghamshire, UK: Mansfield District Council (MDC) (2016). Available online at: https://www.nice.org.uk/sharedlearning/better-together-assist-hospital-discharge-scheme-ahds (Accessed March 2025).
31. Mao H, Xie Y, Shen Y, Wang M, and Luo Y. Effectiveness of nurse-led discharge service on adult surgical inpatients: A meta-analysis of randomized controlled trials. Nurs Open. (2022) 9:2250–62. doi: 10.1002/nop2.1268
32. Oaknin A, Bosse TJ, Creutzberg CL, Giornelli G, Harter P, Joly F, et al. Endometrial cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. (2022) 33:860–77. doi: 10.1016/j.annonc.2022.05.009
33. Aubrey C and Nelson G. Enhanced recovery after surgery (ERAS) for minimally invasive gynecologic oncology surgery: A review. Curr Oncol. (2023) 30:9357–66. doi: 10.3390/curroncol30100677
34. Lin L, Fang Y, Wei Y, Huang F, Zheng J, and Xiao H. The effects of a nurse-led discharge planning on the health outcomes of colorectal cancer patients with stomas: A randomized controlled trial. Int J Nurs Stud. (2024) 155:104769. doi: 10.1016/j.ijnurstu.2024.104769
35. Alem N, Rinehart J, Lee B, Merrill D, Sobhanie S, Ahn K, et al. A case management report: a collaborative perioperative surgical home paradigm and the reduction of total joint arthroplasty readmissions. Perioper Med (Lond). (2016) 5:27. doi: 10.1186/s13741-016-0051-2
36. Po HW, Chu YC, Tsai HC, Chen CY, and Chiu YW. Evaluate the differential effectiveness of the case management and primary nursing models in the implementation of discharge planning. J Clin Nurs. (2024) 34:3753–3775. doi: 10.1111/jocn.17550
37. Jesus TS, Stern BZ, Lee D, Zhang M, Struhar J, Heinemann AW, et al. Systematic review of contemporary interventions for improving discharge support and transitions of care from the patient experience perspective. PloS One. (2024) 19:e0299176. doi: 10.1371/journal.pone.0299176
38. Optimal care pathway for women with endometrial cancer. Australia: Cancer Council Victoria and Department of Health Victoria (2021). Available online at: https://www.cancer.org.au/ (Accessed March 2025).
39. Couturier B, Carrat F, and Hejblum G. Comparing patients’ Opinions on the hospital discharge process collected with a self-reported questionnaire completed via the internet or through a telephone survey: an ancillary study of the SENTIPAT randomized controlled trial. J Med Internet Res. (2015) 17:e158. doi: 10.2196/jmir.4379
40. Liu S, Li J, Wan DY, Li R, Qu Z, Hu Y, et al. Effectiveness of eHealth self-management interventions in patients with heart failure: systematic review and meta-analysis. J Med Internet Res. (2022) 24:e38697. doi: 10.2196/38697
41. Bukke SPN, Kumarachari RK, Rajasekhar ESK, Dudekula JB, and Kamati M. Computational intelligence techniques for achieving sustainable development goals in female cancer care. Discover Sustainability. (2024) 5:390. doi: 10.1007/s43621-024-00575-x
42. Zhang K, Zhang Y, and Xiao Q. Development and usability evaluation of a WeChat mini program for home management of patients with gynecological Malignancies. Chin Nurs Management. (2024) 24:1256–60. doi: 10.3969/j.issn.1672-1756.2024.08.014
43. Delanerolle G, Jouaiti M, Pathiraja V, Mudalige T, Eleje GU, Mbwele B, et al. A rapid scoping review on the use of artificial intelligence applications in women’s health (MARIE WP1). doi: 10.20944/preprints202503.1920.v1
44. Khudhur YS. Artificial intelligence in obstetrics and gynecology: Current applications and future perspectives. Int J Obstetrics Gynaecology. (2025). doi: 10.33545/26648334.2025.v7.i1a.36
45. Hu HT, Hong SS, Jia YY, and Song JP. Advances in application of machine learning in discharge readiness services. Chin J Nursing. (2024) 59:378. doi: 10.3761/j.issn.0254-1769.2024.03.018
46. Meng Q, Liu W, Gao P, Zhang J, Sun A, Ding J, et al. Novel deep learning technique used in management and discharge of hospitalized patients with COVID-19 in China. Ther Clin Risk Manage. (2020) 16:1195–201. doi: 10.2147/tcrm.S280726
47. Safavi KC, Khaniyev T, Copenhaver M, Seelen M, Zenteno Langle AC, Zanger J, et al. Development and validation of a machine learning model to aid discharge processes for inpatient surgical care. JAMA Netw Open. (2019) 2:e1917221. doi: 10.1001/jamanetworkopen.2019.17221
48. Oh EG, Lee HJ, Yang YL, and Kim YM. Effectiveness of discharge education with the teach-back method on 30-day readmission: A systematic review. J Patient Saf. (2021) 17:305–10. doi: 10.1097/pts.0000000000000596
49. Thum A, Ackermann L, Edger MB, and Riggio J. Improving the discharge experience of hospital patients through standard tools and methods of education. J Healthc Qual. (2022) 44:113–21. doi: 10.1097/jhq.0000000000000325
50. Zurlo A and Zuliani G. Management of care transition and hospital discharge. Aging Clin Exp Res. (2018) 30:263–70. doi: 10.1007/s40520-017-0885-6
51. Ma MZ, Fan YY, Yang PP, Du X B, and Li Y. Translation and application of blaylock risk assessment screening scale. Nurs Res China. (2022) 36:1901. doi: 10.12102/j.issn.1009-6493.2022.11.004
52. Louis Simonet M, Kossovsky MP, Chopard P, Sigaud P, Perneger TV, and Gaspoz JM. A predictive score to identify hospitalized patients’ risk of discharge to a post-acute care facility. BMC Health Serv Res. (2008) 8:154. doi: 10.1186/1472-6963-8-154
53. Leong MQ, Lim CW, and Lai YF. Comparison of Hospital-at-Home models: a systematic review of reviews. BMJ Open. (2021) 11:e043285. doi: 10.1136/bmjopen-2020-043285
54. Stoller JK. COPD exacerbations: Prognosis, discharge planning, and prevention. Available online at: https://sso.uptodate.com/contents/zh-Hans/copd-exacerbations-prognosis-discharge-planning-and-prevention?search=COPD%20exacerbations%3A%20Prognosis%2C%20discharge%20planning%2C%20and%20prevention&source=search_result&selectedTitle=1%7E150&usage_type=default&display_rank=1 (Accessed February 1, 2025).
55. Wang H, Wang Y, Liu Y, Ying SY, Le X, Ke J, et al. Construction of core practice indicator system and related forms for discharge planning. Nurs Res China. (2022) 36:189. doi: 10.12102/j.issn.1009-6493.2022.02.001
56. Alper E, O’Malley TA, and Greenwald J. Discharge and readmission. Available online at: https://sso.uptodate.com/contents/zh-Hans/hospital-discharge-and-readmission?search=%E5%87%BA%E9%99%A2%E4%B8%8E%E5%86%8D%E5%85%A5%E9%99%A2&source=search_result&selectedTitle=1%7E150&usage_type=default&display_rank=1 (Accessed March 8, 2025).
57. Burdette ER, Pelletier A, Flores MN, Hinchcliff EM, St Laurent JD, and Feltmate CM. Same-day discharge after minimally invasive hysterectomy for endometrial cancer and endometrial intraepithelial neoplasia in patients with morbid obesity: Safety and potential barriers. Int J Gynecol Cancer. (2025) 35:100042. doi: 10.1016/j.ijgc.2024.100042
58. Lamiman K, Silver M, Goncalves N, Kim M, and Alagkiozidis I. Impact of robotic assistance on minimally invasive surgery for type II endometrial cancer: A national cancer database analysis. Cancers. (2024) 16:9. doi: 10.3390/cancers16142584
59. Liang MI, Rosen MA, Rath KS, Clements AE, Backes FJ, Eisenhauer EL, et al. Reducing readmissions after robotic surgical management of endometrial cancer: a potential for improved quality care. Gynecol Oncol. (2013) 131:508–11. doi: 10.1016/j.ygyno.2013.09.033
60. Mendivil AA, Busch JR, Richards DC, Vittori H, and Goldstein BH. The impact of an enhanced recovery after surgery program on patients treated for gynecologic cancer in the community hospital setting. Int J Gynecol Cancer. (2018) 28:581–5. doi: 10.1097/igc.0000000000001198
61. Chau JPC, Liu X, Lo SHS, Chien WT, Hui SK, Choi KC, et al. Perioperative enhanced recovery programmes for women with gynaecological cancers. Cochrane Database Syst Rev. (2022) 3:Cd008239. doi: 10.1002/14651858.CD008239.pub5
62. Skorstad M, de Rooij BH, Jeppesen MM, Bergholdt SH, Ezendam NPM, Bohlin T, et al. Self-management and adherence to recommended follow-up after gynaecological cancer: results from the international InCHARGE study. Int J Gynecol Cancer. (2021) 31:1106–15. doi: 10.1136/ijgc-2020-002377
63. Sobočan M, Gašpar D, Gjuras E, and Knez J. Evaluation of patient-reported symptoms and functioning after treatment for endometrial cancer. Curr Oncol. (2022) 29:5213–22. doi: 10.3390/curroncol29080414
64. Warring S, Yost KJ, Cheville AL, Dowdy SC, Faubion SS, Kumar A, et al. The quality of life after endometrial cancer study: baseline characteristics and patient-reported outcomes. Curr Oncol. (2024) 31:5557–72. doi: 10.3390/curroncol31090412
65. Li CC, Chang TC, Chang CW, Huang CH, Tsai YF, Huang CL, et al. Quality of life and climacteric symptoms in women with endometrial cancer: examining the impact of lower limb lymphedema. J Patient Rep Outcomes. (2025) 9:66. doi: 10.1186/s41687-025-00895-0
66. Archbold PG, Stewart BJ, Greenlick MR, and Harvath T. Mutuality and preparedness as predictors of caregiver role strain. Res Nurs Health. (1990) 13:375–84. doi: 10.1002/nur.4770130605
67. Henriksson A, Andershed B, Benzein E, and Arestedt K. Adaptation and psychometric evaluation of the Preparedness for Caregiving Scale, Caregiver Competence Scale and Rewards of Caregiving Scale in a sample of Swedish family members of patients with life-threatening illness. Palliat Med. (2012) 26:930–8. doi: 10.1177/0269216311419987
68. Hauksdóttir A, Valdimarsdóttir U, Fürst CJ, Onelöv E, and Steineck G. Health care-related predictors of husbands’ preparedness for the death of a wife to cancer--a population-based follow-up. Ann Oncol. (2010) 21:354–61. doi: 10.1093/annonc/mdp313
69. Weiss ME, Piacentine LB, Lokken L, Ancona J, Archer J, Gresser S, et al. Perceived readiness for hospital discharge in adult medical-surgical patients. Clin Nurse Spec. (2007) 21:31–42. doi: 10.1097/00002800-200701000-00008
70. Yang MM, Liang W, Zhao HH, and Zhang Y. Quality analysis of discharge instruction among 602 hospitalized patients in China: a multicenter, cross-sectional study. BMC Health Serv Res. (2020) 20:647. doi: 10.1186/s12913-020-05518-6
71. Blaylock A and Cason CL. Discharge planning predicting patients’ needs. J Gerontol Nurs. (1992) 18:5–10. doi: 10.3928/0098-9134-19920701-05
72. Leonetti A, Peroni M, Agnetti V, Pratticò F, Manini M, Acunzo A, et al. Thirty-day mortality in hospitalised patients with lung cancer: incidence and predictors. BMJ Support Palliat Care. (2023) 14:e2003–e2010. doi: 10.1136/spcare-2023-004558
73. Holland DE, Harris MR, Leibson CL, Pankratz VS, and Krichbaum KE. Development and validation of a screen for specialized discharge planning services. Nurs Res. (2006) 55:62–71. doi: 10.1097/00006199-200601000-00008
74. Socwell CP, Bucci L, Patchell S, Kotowicz E, Edbrooke L, and Pope R. Utility of Mayo Clinic’s early screen for discharge planning tool for predicting patient length of stay, discharge destination, and readmission risk in an inpatient oncology cohort. Support Care Cancer. (2018) 26:3843–9. doi: 10.1007/s00520-018-4252-8
75. Moy NY, Lee SJ, Chan T, Grovey B, Boscardin WJ, Gonzales R, et al. Development and sustainability of an inpatient-to-outpatient discharge handoff tool: a quality improvement project. Jt Comm J Qual Patient Saf. (2014) 40:219–27. doi: 10.1016/s1553-7250(14)40029-1
76. Prince M, Allen D, Chittenden S, Misuraca J, and Hockenberry MJ. Improving transitional care: the role of handoffs and discharge checklists in hematologic Malignancies. Clin J Oncol Nurs. (2019) 23:36–42. doi: 10.1188/19.Cjon.36-42
77. Li S, Luo C, Xie M, Lai J, Qiu H, Xu L, et al. Factors influencing readiness for hospital discharge among patients undergoing enterostomy: A descriptive, cross-sectional study. Adv Skin Wound Care. (2024) 37:319–27. doi: 10.1097/asw.0000000000000159
78. Nurhayati N, Songwathana P, and Vachprasit R. Surgical patients’ experiences of readiness for hospital discharge and perceived quality of discharge teaching in acute care hospitals. J Clin Nurs. (2019) 28:1728–36. doi: 10.1111/jocn.14764
79. Weiss M, Yakusheva O, and Bobay K. Nurse and patient perceptions of discharge readiness in relation to postdischarge utilization. Med Care. (2010) 48:482–6. doi: 10.1097/MLR.0b013e3181d5feae
80. Bobay KL, Weiss ME, Oswald D, and Yakusheva O. Validation of the registered nurse assessment of readiness for hospital discharge scale. Nurs Res. (2018) 67:305–13. doi: 10.1097/nnr.0000000000000293
81. Kamau EB, Foronda C, Hernandez VH, and Walters BA. Reducing length of stay and hospital readmission for orthopedic patients: A quality improvement project. J Dr Nurs Pract. (2021). doi: 10.1891/jdnp-d-20-00060
82. Re-Engineered Discharge(RED). Toolkit: agency for healthcare research and quality. Available online at: https://www.ahrq.gov/hai/red/toolkit/postdischarge-doc.html (Accessed March 19, 2025).
83. Vogel TR, Kruse RL, Schlesselman C, Doss E, Camazine M, and Popejoy LL. A qualitative study evaluating the discharge process for vascular surgery patients to identify significant themes for organizational improvement. Vascular. (2024) 32:395–406. doi: 10.1177/17085381221135267
84. Parker C and Griffith DH. Reducing hospital readmissions of postoperative patients with the martin postoperative discharge screening tool. J Nurs Adm. (2013) 43:184–6. doi: 10.1097/NNA.0b013e3182895902
85. Toich L. Novel tool could predict hospital readmissions (2016). Available online at: https://www.pharmacytimes.com/view/novel-tool-could-predict-hospital-readmissions (Accessed April 1, 2025).
86. van Walraven C, Dhalla IA, Bell C, Etchells E, Stiell IG, Zarnke K, et al. Derivation and validation of an index to predict early death or unplanned readmission after discharge from hospital to the community. Cmaj. (2010) 182:551–7. doi: 10.1503/cmaj.091117
87. Wang H, Robinson RD, Johnson C, Zenarosa NR, Jayswal RD, Keithley J, et al. Using the LACE index to predict hospital readmissions in congestive heart failure patients. BMC Cardiovasc Disord. (2014) 14:97. doi: 10.1186/1471-2261-14-97
88. Donzé JD, Williams MV, Robinson EJ, Zimlichman E, Aujesky D, Vasilevskis EE, et al. International validity of the HOSPITAL score to predict 30-day potentially avoidable hospital readmissions. JAMA Intern Med. (2016) 176:496–502. doi: 10.1001/jamainternmed.2015.8462
89. da Silva NC, Albertini MK, Backes AR, and das Graças Pena G. Validation of the HOSPITAL score as predictor of 30-day potentially avoidable readmissions in pediatric hospitalized population: retrospective cohort study. Eur J Pediatr. (2023) 182:1579–85. doi: 10.1007/s00431-022-04795-z
90. Holland DE, Hansen DC, Matt-Hensrud NN, Severson MA, and Wenninger CR. Continuity of care: a nursing needs assessment instrument. Geriatr Nurs. (1998) 19:331–4. doi: 10.1016/s0197-4572(98)90119-7
91. Kim CS and Flanders SA. In the clinic. Transitions of care. Ann Intern Med. (2013) 158:Itc3–1. doi: 10.7326/0003-4819-158-5-201303050-01003
92. Strategy 4: care transitions from hospital to home: IDEAL discharge planning. US: Agency for Healthcare Research and Quality (2013). Available online at: https://www.ahrq.gov/professionals/systems/hospital/engagingfamilies/strategy4/index.html (Accessed April 2025).
93. Luther B, Wilson RD, Kranz C, and Krahulec M. Discharge processes: what evidence tells us is most effective. Orthop Nurs. (2019) 38:328–33. doi: 10.1097/nor.0000000000000601
Keywords: discharge readiness service, endometrial cancer, narrative review, surgical patients, transitional care
Citation: Chen X, Tang P, Ma C, Su M, Sun J, Chang W, Li Y, Cui Y, Wang Y, Hu Y, Wang J and Wei Y (2026) Research progress on discharge readiness service practices for patients undergoing endometrial cancer surgery. Front. Oncol. 15:1664064. doi: 10.3389/fonc.2025.1664064
Received: 11 July 2025; Accepted: 17 December 2025; Revised: 03 December 2025;
Published: 15 January 2026.
Edited by:
Federico Ferrari, University of Brescia, ItalyReviewed by:
Andrea Giannini, University of Pisa, ItalyIbrahim Gomaa, Mayo Clinic, United States
Jacopo Conforti, University of Brescia, Italy
Copyright © 2026 Chen, Tang, Ma, Su, Sun, Chang, Li, Cui, Wang, Hu, Wang and Wei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jia Wang, d2oxODA0Nzk1Mzc2OEAxNjMuY29t; Yinyi Wei, NDc2NTczNzE1QHFxLmNvbQ==
†These authors have contributed equally to this work
 Xia Chen
Xia Chen Peijuan Tang1†
Peijuan Tang1† Mei Su
Mei Su Jiaxin Sun
Jiaxin Sun Jia Wang
Jia Wang