- 1Department of Pediatric Surgery, Zigong First People’s Hospital, Zigong, China
- 2Zigong First People’s Hospital, Zigong, China
- 3STU-CUHK Joint Shantou International Eye Center, Shantou, China
Gliomas are primary central nervous system tumors characterized by a high recurrence rate and poor prognosis, especially in high-grade forms such as glioblastoma (GBM). Radiotherapy remains a cornerstone in glioma management, particularly following surgical resection. Recent advancements in technology—including intensity-modulated radiotherapy (IMRT), proton therapy, carbon-ion radiotherapy, intraoperative radiotherapy, and ultra-high dose rate FLASH radiotherapy—have improved treatment precision and tumor control. However, clinical challenges persist due to tumor heterogeneity, imaging limitations, and planning variability. In the era of artificial intelligence (AI), novel tools such as radiomics, deep learning, and predictive modeling are increasingly being integrated into glioma radiotherapy workflows. These AI-driven approaches have shown potential to enhance imaging interpretation, automate contouring, optimize treatment planning, and predict clinical outcomes. This review highlights the evolution of glioma radiotherapy, explores the emerging role of AI across various stages of radiotherapy, and discusses future directions for implementing personalized, adaptive, and data-driven strategies in clinical practice.
1 Introduction
Gliomas are solid tumors originating from glial cells of the central nervous system, with an annual incidence of approximately 5–8 per 100,000 individuals (1), and they represent the most frequently diagnosed intracranial neoplasms in pediatric populations (2). According to the fifth edition of the World Health Organization (WHO) classification of central nervous system tumors released in 2021, gliomas are categorized into grades I to IV (3). Grade IV glioblastoma (GBM) is the most aggressive form, and despite comprehensive multimodal therapies—including surgery, concurrent chemoradiotherapy, and adjuvant chemotherapy—the median overall survival remains less than two years, with a 5-year survival rate of approximately 10% (4). Radiotherapy is a critical component in the therapeutic management of glioma, particularly for controlling residual disease post-surgery (5, 6). Nonetheless, challenges such as imprecise target delineation and suboptimal dose distribution continue to hinder treatment outcomes. The rapid advancement of artificial intelligence (AI) has enabled its application across several stages of radiotherapy—including image processing, contour automation, treatment planning, and outcome prediction—creating new opportunities for enhancing precision and personalization (7, 8). This review provides an overview of current AI applications in glioma radiotherapy, examines key technical challenges, and outlines future prospects for clinical integration.
2 Role and limitations of radiotherapy in glioma treatment
Figure 1 illustrates the integration of conventional radiotherapy techniques with emerging AI applications in glioma management. The synergy between advanced physical delivery modalities (e.g., Intensity-Modulated Radiotherapy [IMRT], proton and carbon-ion therapy, and FLASH radiotherapy) and AI-driven technologies (e.g., auto-segmentation, treatment planning optimization, radiomics, and prognostic modeling) aims to enhance tumor control and enable truly personalized treatment strategies.
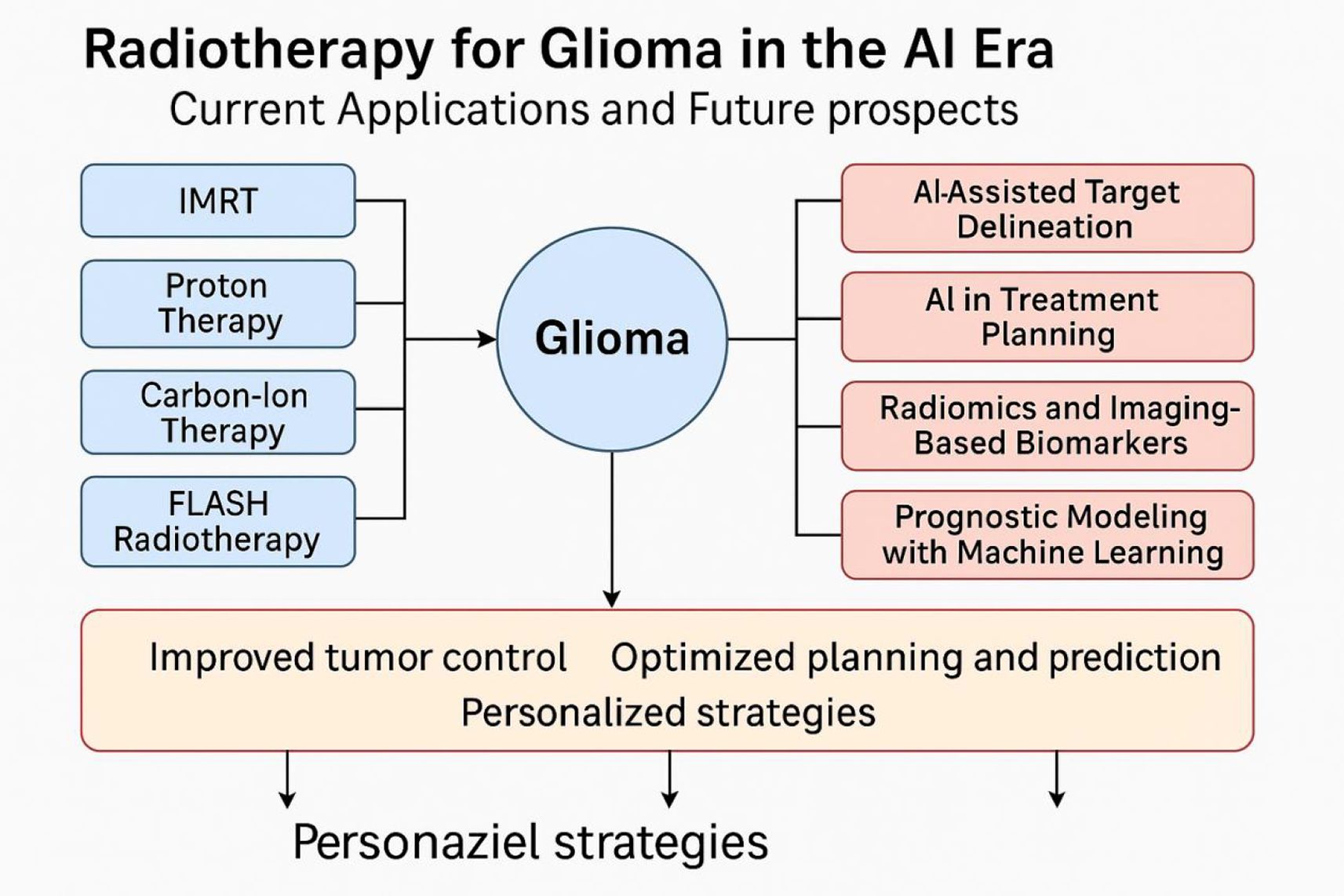
Figure 1. Overview of radiotherapy strategies and artificial intelligence applications in glioma treatment.
With the development of radiotherapy techniques, modalities such as IMRT, volumetric-modulated arc therapy (VMAT), proton therapy, carbon-ion therapy, and FLASH radiotherapy are increasingly being used in clinical practice (9–14). Enhancing local tumor control while minimizing damage to surrounding healthy tissue remains a central challenge in the radiotherapeutic management of gliomas.
Currently, the standard adjuvant radiotherapy protocol for high-grade gliomas (HGG) is the Stupp regimen (15, 16). This involves initiating fractionated radiotherapy approximately four weeks after surgery, delivering a total dose of 60 Gy in daily fractions of 1.8–2 Gy, concurrently with temozolomide (TMZ) chemotherapy at a dose of 75 mg/m². Approximately one month after completing concurrent chemoradiotherapy, patients proceed with six cycles of adjuvant TMZ chemotherapy.
For elderly patients or those with poor performance status, hypofractionated radiotherapy (e.g., 40 Gy in 15 fractions or 34 Gy in 10 fractions) can provide comparable efficacy to conventional fractionation.
Despite technological advances, therapeutic gains remain modest. Primary obstacles include: (1) intra- and inter-patient tumor heterogeneity, with diverse genetic drivers affecting radiosensitivity and recurrence patterns; (2) limitations in target delineation, as conventional MRI often fails to fully characterize infiltrative tumor margins, leading to under- or over-treatment; (3) static treatment planning, which cannot adapt to anatomical or pathological changes during therapy, such as edema or tumor shrinkage; and (4) variability in contouring and plan quality among clinicians and treatment centers.
These challenges collectively hinder the realization of precision radiotherapy and underscore the need for personalized and adaptive approaches.
2.1 AI in real-time adaptive planning
While radiotherapy has been a cornerstone in glioma treatment, traditional methods face significant limitations due to tumor heterogeneity, anatomical shifts, and edema changes during treatment (17). Real-time adaptive planning—the ability to dynamically adjust the treatment plan based on tumor changes during radiotherapy—has emerged as a promising avenue, and AI plays a critical role in facilitating this process (18).
AI can assist in real-time adaptive planning in several key ways:
Real-Time Imaging and Analysis: AI algorithms, particularly deep learning models, can process imaging data in real time, providing up-to-date tumor delineation and identifying changes in tumor volume or location (19). This ensures that the treatment plan is dynamically adjusted to account for tumor motion, edema, or anatomical shifts during radiotherapy sessions.
Treatment Adaptation: AI models can guide adjustments to the radiation dose distribution during radiotherapy, continuously optimizing the treatment plan based on updated tumor and organ-at-risk (OAR) positions (20). Reinforcement learning algorithms, for instance, can be used to learn from each radiotherapy session and make adjustments for future treatments.
Clinical Decision Support: Integrating AI with treatment delivery systems allows clinicians to receive real-time feedback on tumor changes (21). This feedback facilitates timely decisions on radiation dose adaptation, improving treatment precision and enhancing the effectiveness of radiotherapy, particularly for gliomas, where accurate tumor tracking and adaptability are crucial.
2.2 IMRT
IMRT has become a widely accepted standard for glioma treatment due to its ability to deliver highly conformal radiation doses to complex target volumes while sparing adjacent OARs such as the optic nerves, brainstem, and hippocampus. Through inverse planning algorithms and multileaf collimator (MLC) modulation, IMRT enhances dose conformity in irregularly shaped lesions typical of HGGs, especially those located near eloquent brain regions (22).
For WHO grade II high-risk low-grade glioma, Wang et al. (23) reported that both IMRT alone and in combination with TMZ significantly improved median progression-free survival (mPFS) and overall survival (mOS) compared with observation alone. Specifically, the mPFS was 59 months in the observation group, 82 months in the radiotherapy group, and not reached in the STUPP group; for OS, the median was 96 months in the observation group, while both RT and STUPP groups did not reach a median OS.
VMAT, a time-efficient evolution of IMRT, delivers intensity-modulated beams during continuous gantry rotation. By simultaneously varying gantry speed, dose rate, and MLC positions, VMAT significantly reduces treatment time while maintaining or exceeding the dosimetric quality of fixed-field IMRT (24). Navarria et al. (25) studied 341 patients with newly diagnosed high-grade glioma and demonstrated that VMAT achieved better dosimetric conformity and significantly improved mPFS (1.29 vs. 0.99 years, P = 0.02) and mOS (1.56 vs. 1.21 years, P < 0.01) compared to 3D-conformal radiotherapy (3DCRT).
2.3 Proton beam therapy
Proton therapy leverages the Bragg peak phenomenon to deposit most of the radiation dose at a defined depth with minimal exit dose. This feature allows superior sparing of healthy brain tissue, making PBT particularly advantageous in pediatric gliomas or recurrent HGGs located near critical structures (26). Several dosimetric and prospective trials suggest reduced neurocognitive decline and lower integral doses with proton therapy, although access remains limited due to high costs and restricted facility availability (27–29).
While clinical evidence for PBT in gliomas is currently limited, its potential appears promising (30). In GBM, due to its aggressive nature and rapid progression, the potential long-term neuroprotective advantages of PBT may be diminished. Future studies should focus on identifying subgroups of patients most likely to benefit from PBT. Younger, functionally independent individuals with high- or low-grade gliomas (HGG or LGG) and favorable molecular profiles may derive more benefit from the reduced normal tissue toxicity associated with PBT. Notably, preliminary small-scale studies in LGG have shown milder acute toxicities with PBT (31).
2.4 Carbon-ion radiotherapy
Carbon-ion therapy exhibits a higher relative biological effectiveness (RBE) than photon and proton therapy. RBE, which quantifies biological damage relative to 250 keV X-rays, ranges from 1.1 to 3.74 for carbon ions in vitro, depending on cell type (32, 33). Carbon ions induce more complex and lethal DNA damage than photons, with decreased repair efficiency in tumor cells (33–36). One study observed that carbon ion exposure caused pronounced G2/M cell cycle arrest in approximately 79.9% of cells, persisting for at least 48 hours (37, 38). In contrast to photon radiotherapy, where cytotoxicity is dose-dependent, carbon ions appear to exert lethal effects independent of dose duration (39).
In a phase I trial, Qiu et al. (40) enrolled 18 HGG patients to assess the feasibility and safety of carbon ion radiotherapy before proton therapy. Results showed that a pre-proton carbon ion dose of 15 Gy in 3 fractions was well tolerated and potentially beneficial, with a median OS of 17.9 months. No grade ≥3 acute or late toxicities were reported.
2.5 FLASH Radiotherapy
FLASH radiotherapy is an emerging technique delivering ultra-high dose rates (>40 Gy/s) and has shown preclinical evidence of reduced normal tissue toxicity while maintaining tumoricidal effects (41). Unlike conventional radiotherapy, which delivers radiation at lower doses over several minutes, FLASH radiotherapy irradiates the tumor in milliseconds. Preclinical studies have shown that FLASH radiotherapy can significantly reduce damage to normal tissues while preserving its tumoricidal effects, making it a promising technique for improving the therapeutic ratio (42). This has potential clinical implications for glioma treatment, as it could reduce neurocognitive side effects typically associated with radiation, while maintaining or improving treatment efficacy. Its application in gliomas remains experimental, but initial murine models demonstrate preserved neurocognitive function compared to conventional dose-rate irradiation (43). Iturri et al. (44) compared proton FLASH radiotherapy (257 ± 2 Gy/s) with conventional-dose-rate proton therapy (4 ± 0.02 Gy/s) in glioma-bearing rats using a single 25 Gy dose. FLASH notably preserved cognitive function and triggered a robust lymphoid immune response in tumors.
2.6 Intraoperative radiotherapy
The peritumoral area is a high-risk zone for glioma recurrence. Studies indicate that residual tumor volume significantly increases within the first two weeks post-surgery (45). IORT delivers a single high dose of radiation directly to the tumor bed during surgical resection. This approach minimizes treatment delays and reduces the risk of tumor repopulation between surgery and postoperative radiotherapy (46). Although still investigational in gliomas, early results suggest potential for reducing local recurrence when combined with external-beam RT (47, 48).
3 Artificial intelligence in glioma radiotherapy: a transformative role across the treatment spectrum
AI is revolutionizing glioma radiotherapy by enhancing precision, efficiency, and personalization throughout the treatment continuum. From target delineation and treatment planning to imaging biomarker development and prognostic modeling, AI-driven tools are increasingly integrated into clinical workflows (49). These technologies reduce inter-observer variability, automate complex tasks, and offer data-driven insights for individualized treatment strategies. The adoption of radiomics, machine learning, and explainable AI frameworks bridges the gap between imaging data and actionable clinical decisions, ultimately aiming to improve patient outcomes and standardize care.
3.1 AI-assisted target delineation
Manual delineation of gross tumor volume (GTV) and clinical target volume (CTV) is time-consuming and prone to inter-observer variability, especially in gliomas with diffuse or infiltrative borders. AI algorithms trained on large imaging datasets have shown high accuracy in auto-segmentation of tumor regions on MRI, reducing variability and standardizing contouring practices. Pehrson et al. (50) reviewed 48 studies and found that AI algorithms showed good concordance with clinicians in GTV delineation across various tumors, with Dice similarity coefficients ranging from 0.62 to 0.92, particularly in encoder–decoder architecture models.
3.2 AI in treatment planning
AI is increasingly employed to optimize radiotherapy planning, generating high-quality plans while reducing clinician workload. Knowledge-based planning (KBP) systems use historical treatment data to predict optimal dose distributions, while reinforcement learning models iteratively improve plan quality. These tools enhance conformity indices and spare organs at risk, particularly valuable for gliomas near critical structures such as the optic pathway and brainstem (51).
3.3 Radiomics and imaging-based biomarkers
Radiomics transforms standard imaging into high-dimensional, mineable data, enabling extraction of quantitative features that may reflect tumor heterogeneity, infiltration, and treatment response (52). In glioma, radiomics signatures correlate with IDH mutation status, MGMT promoter methylation, and progression patterns. Integrating radiomics with clinical and molecular data enhances risk stratification and supports personalized radiotherapy planning.
3.4 Prognostic modeling with machine learning
Machine learning models, including random forests (RF), support vector machines (SVM), XGBoost, and neural networks, have been utilized to predict survival, recurrence, and treatment response in glioma patients (53). Compared to traditional models, these machine learning approaches offer enhanced performance and adaptability in complex clinical scenarios.
3.4.1 RF
Robust and Reliable: RF is an ensemble learning method that combines multiple decision trees to produce highly reliable predictions, reducing the risk of overfitting.
Feature Importance Ranking: RF can identify the most relevant features, providing valuable insights for clinicians to understand the factors influencing prognosis.
Handles High-Dimensional Data: It works effectively with complex datasets like radiomics and genomics, handling large numbers of variables without requiring extensive data preprocessing.
Resistant to Missing Data: RF can handle missing data well, making it robust for clinical datasets with incomplete records (54).
3.4.2 SVM
Effective in High-Dimensional Spaces: SVM excels at handling high-dimensional data, especially when the number of features exceeds the number of samples, making it ideal for radiomics and genomic data.
Strong Generalization: SVM is less prone to overfitting, especially when the data is complex or noisy, and is effective in binary classification tasks.
Clear Margin of Separation: SVM works well when there is a clear boundary between different classes, such as distinguishing between high-grade and low-grade gliomas (55).
3.4.3 XGBoost
High Accuracy: XGBoost is known for its excellent performance, often providing state-of-the-art results in classification and regression tasks.
Handles Missing Data Efficiently: XGBoost can automatically manage missing values, making it ideal for clinical data where missing records are common.
Feature Importance: Like RF, XGBoost provides insights into the importance of different features, which is useful for understanding the key drivers behind predictions.
Scalable and Fast: XGBoost is highly efficient, handling large datasets and providing quick training times, making it scalable for big data applications (56).
3.4.4 Neural networks
Powerful for Complex Data: Neural networks, especially deep learning models, excel at extracting complex patterns from large, high-dimensional datasets like images and multi-omics data.
Adaptable to Various Data Types: They can process a wide range of data types, from structured clinical data to unstructured imaging data, making them versatile in clinical applications.
High Accuracy: When trained on large datasets, neural networks can achieve exceptional accuracy, often outperforming traditional machine learning models (57).
3.4.5 Machine learning applications
Samara et al. (58) developed a classification model using integrated feature selection (Boruta, LASSO, SHAP), identifying 13 key predictors such as IDH1, TP53, and ATRX. XGBoost achieved the highest AUC (0.93), while logistic regression showed the highest testing accuracy (88.09%), with strong model calibration and clinical utility.
Li et al. (59) created an MRI-based radiomics model for high-grade glioma classification using various machine learning algorithms; the Stacking fusion model showed the best performance (AUC = 0.95, sensitivity = 0.84, accuracy = 0.85, F1 score = 0.85). By incorporating imaging, genomic, and clinical data, these models surpass traditional prognostic methods and help identify patients who may benefit from intensified or alternative treatments. Additionally, explainable AI (XAI) frameworks are being developed to enhance transparency and clinical trust in model predictions (60, 61).
4 Model interpretability and explainable ai in glioma radiotherapy
One of the major challenges limiting the clinical adoption of AI models in glioma radiotherapy is the lack of interpretability. While AI models, particularly deep learning models, have demonstrated remarkable performance, their black-box nature makes it difficult for clinicians to understand how these models arrive at their predictions. This lack of transparency poses a significant barrier to their acceptance in clinical decision-makingl.
4.1 SHAP
SHAP values offer a unified measure of feature importance by quantifying the contribution of each feature to a model’s prediction. In glioma radiotherapy, SHAP can be used to explain which clinical, radiomic, or genomic features have the most significant impact on predicted outcomes, such as patient survival or recurrence. This provides clinicians with valuable insights into the factors driving the model’s predictions (62).
4.2 LIME
LIME is another powerful technique that generates interpretable explanations for individual predictions by approximating the AI model with a simpler, interpretable model in the local region around the prediction. This method is particularly useful in radiotherapy when explaining individual patient outcomes, such as why a certain treatment plan is recommended over others (63).
In summary, artificial intelligence holds transformative potential in glioma radiotherapy, providing tools to enhance workflow efficiency and clinical precision. Future directions include multi-omics integration, real-time adaptive planning, and prospective validation of AI models in large-scale clinical trials.
Author contributions
XW: Conceptualization, Writing – original draft, Software, Writing – review & editing. ZQ: Software, Writing – original draft, Writing – review & editing, Conceptualization. QZ: Writing – original draft, Writing – review & editing, Conceptualization, Software. DG: Software, Writing – original draft, Writing – review & editing, Conceptualization. TL: Conceptualization, Software, Writing – review & editing, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Siegel RL, Kratzer TB, Giaquinto AN, Sung H, and Jemal A. Cancer statistics, 2025. CA Cancer J Clin. (2025) 75:10–45. doi: 10.3322/caac.21871
2. Budha RR, Khan SW, Sharma DC, Kulchandani Y, and Gsn KR. Pediatric diffuse high-grade gliomas: A comprehensive review of ad-vanced methods of diagnosis and treatment. Curr Cancer Drug Targets. (2025) 52:101–30. doi: 10.2174/0115680096365252250618115641
3. Global Nutrition Target Collaborators. Global, regional, and national progress towards the 2030 global nutrition targets and forecasts to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet. (2025) 404:2543–83. doi: 10.1016/S0140-6736(24)01821-X
4. Gonzalez N, Pérez Küper M, Garcia Fallit M, Agudelo JAP, Nicola Candia A, Suarez Velandia M, et al. Integrated workflow for drug repurposing in glioblastoma: computational prediction and preclinical validation of therapeutic candidates. Brain Sci. (2025) 15:637. doi: 10.3390/brainsci15060637
5. La Rosa A, Mittauer KE, Rzepczynski AE, Chuong MD, Bassiri-Gharb N, McAllister NC, et al. Temporospatial tumor dynamic changes in glioblastoma during radiotherapy. J Neurooncol. (2025) 174:493–501. doi: 10.1007/s11060-025-05060-7
6. Pandey K, Mishra S, Garg K, Garg A, Singh M, and Kale SS. Patterns of response following gamma knife radiosurgery for tectal plate gliomas. J Neurooncol. (2025) 174:77–84. doi: 10.1007/s11060-025-05034-9
7. Liu Z, Ren S, Zhang H, Liao Z, Liu Z, An X, et al. Multiparametric MRI-based machine learning system of molecular subgroups and prognosis in medulloblastoma. Eur Radiol. (2025) 35:5053–63. doi: 10.1007/s00330-025-11385-8
8. Raymond C, Yao J, Kolkovsky ALL, Feiweier T, Clifford B, Meyer H, et al. Super-resolution sodium MRI of human gliomas at 3T using physics-based generative artificial intelligence. J Neurooncol. (2025) 174(3):653–65. doi: 10.1007/s11060-025-05094-x
9. Baumert BG, Jaspers J PM, Keil VC, Galldiks N, Izycka-Swieszewska E, Timmermann B, et al. ESTRO-EANO guideline on target delineation and radiotherapy for IDH-mutant WHO CNS grade 2 and 3 diffuse glioma. Radiother Oncol. (2025) 202:110594. doi: 10.1016/j.radonc.2024.110594
10. Hong WJ, Ho HW, Lin HM, Lin T, Chow WH, Yang CC, et al. A dosimetric comparison of hyperArc therapy planning and volumetric modulated arc therapy planning in treating patients with glioblastoma multiforme. In Vivo. (2025) 39:1009–21. doi: 10.21873/invivo.13906
11. Rejimon AC, Trivedi AG, Huang V, Ramesh KK, Esiashvilli N, Schreibmann E, et al. Longitudinal overlap and metabolite analysis in spectroscopic MRI-guided proton beam therapy in pediatric high-grade glioma. Tomography. (2025) 11:71. doi: 10.3390/tomography11060071
12. Padilla O, Minns HE, Wei HJ, Fan W, Webster-Carrion A, Tazhibi M, et al. Immune response following FLASH and conventional radiation in diffuse midline glioma. Int J Radiat Oncol Biol Phys. (2024) 119:1248–60. doi: 10.1016/j.ijrobp.2024.01.219
13. Chiranth S, Fougner V, Christensen IJ, Trip AK, Christiansen T, Nørøxe DS, et al. Identifying targetable alterations predictive of distant progression in glioblastoma patients undergoing standard therapy. Neurooncol Adv. (2025) 7:vdaf092. doi: 10.1093/noajnl/vdaf092
14. Konradsson E, Liljedahl E, Gustafsson E, Adrian G, Beyer S, Ilaahi SE, et al. Comparable long-term tumor control for hypofractionated FLASH versus conventional radiation therapy in an immunocompetent rat glioma model. Adv Radiat Oncol. (2022) 7:101011. doi: 10.1016/j.adro.2022.101011
15. Nguyen DV, Nguyen NTT, Nguyen PH, Nguyen HT, and Do TC. Evaluating treatment outcome of Glioblastoma with Stupp’s regimen: an experienced in single Institute. Chin Clin Oncol. (2025) 14:18. doi: 10.21037/cco-24-103
16. Zinsz A, Ahrari S, Becker J, Mortada A, Roch V, Doriat L, et al. Amino-acid PET as a prognostic tool after post Stupp protocol temozolomide therapy in high-grade glioma patients. J Neurooncol. (2024) 169:241–5. doi: 10.1007/s11060-024-04722-2
17. Baumert BG, Hegi ME, van den Bent MJ, von Deimling A, Gorlia T, Hoang-Xuan K, et al. Temozolomide chemotherapy versus radiotherapy in high-risk low-grade glioma (EORTC 22033-26033): a randomised, open-label, phase 3 intergroup study. Lancet Oncol. (2016) 17:1521–32. doi: 10.1016/S1470-2045(16)30313-8
18. Niyazi M, Andratschke N, Bendszus M, Chalmers AJ, Erridge SC, Galldiks N, et al. ESTRO-EANO guideline on target delineation and radiotherapy details for glioblastoma. Radiother Oncol. (2023) 184:109663. doi: 10.1016/j.radonc.2023.109663
19. Voon NS, Manan HA, and Yahya N. Remote assessment of cognition and quality of life following radiotherapy for glioma: deep-learning-based predictive models and MRI correlates. J Neurooncol. (2023) 162:407–15. doi: 10.1007/s11060-023-04303-9
20. Alzahrani N, Henry A, Clark A, Murray L, Nix M, and Al-Qaisieh B. Geometric evaluations of CT and MRI based deep learning segmentation for brain OARs in radiotherapy. Phys Med Biol. (2023) 68(17):245–62. doi: 10.1088/1361-6560/acf023
21. Yang X, Li S, Shao Q, Cao Y, Yang Z, and Zhao YQ. Uncertainty-guided man-machine integrated patient-specific quality assurance. Radiother Oncol. (2022) 173:1–9. doi: 10.1016/j.radonc.2022.05.016
22. Vargas López AJ. Glioblastoma in adults: a Society for Neuro-Oncology (SNO) and European Society of Neuro-Oncology (EANO) consensus review on current management and future directions. Neuro Oncol. (2021) 23:502–3. doi: 10.1093/neuonc/noaa287
23. Wang J, Yan L, Ai P, He Y, Guan H, Wei Z, et al. Observation versus radiotherapy with or without temozolomide in postoperative WHO grade II high-risk low-grade glioma: a retrospective cohort study. Neurosurg Rev. (2021) 44:1447–55. doi: 10.1007/s10143-020-01326-y
24. Palma D, Vollans E, James K, Nakano S, Moiseenko V, Shaffer R, et al. Volumetric modulated arc therapy for delivery of prostate radiotherapy: comparison with intensity-modulated radiotherapy and three-dimensional conformal radiotherapy. Int J Radiat Oncol Biol Phys. (2008) 72:996–1001. doi: 10.1016/j.ijrobp.2008.02.047
25. Navarria P, Pessina F, Cozzi L, Ascolese AM, Lobefalo F, Stravato A, et al. Can advanced new radiation therapy technologies improve outcome of high grade glioma (HGG) patients? analysis of 3D-conformal radiotherapy (3DCRT) versus volumetric-modulated arc therapy (VMAT) in patients treated with surgery, concomitant and adjuvant chemo-radiotherapy. BMC Cancer. (2016) 16:362. doi: 10.1186/s12885-016-2399-6
26. Mizumoto M, Oshiro Y, Yamamoto T, Kohzuki H, and Sakurai H. Proton beam therapy for pediatric brain tumor. Neurol Med Chir (Tokyo). (2017) 57:343–55. doi: 10.2176/nmc.ra.2017-0003
27. Grosshans DR, Mohan R, Gondi V, Shih HA, Mahajan A, and Brown PD. The role of image-guided intensity modulated proton therapy in glioma. Neuro Oncol. (2017) 19:ii30–7. doi: 10.1093/neuonc/nox002
28. Badiyan SN, Ulmer S, Ahlhelm FJ, Fredh ASM, Kliebsch U, Calaminus G, et al. Clinical and radiologic outcomes in adults and children treated with pencil-beam scanning proton therapy for low-grade glioma. Int J Part Ther. (2017) 3:450–60. doi: 10.14338/IJPT-16-00031.1
29. Adeberg S, Harrabi SB, Verma V, Bernhardt D, Grau N, Debus J, et al. Treatment of meningioma and glioma with protons and carbon ions. Radiat Oncol. (2017) 12:193. doi: 10.1186/s13014-017-0924-7
30. Qiu X, Gao J, Hu J, Yang J, Hu W, Huang Q, et al. Proton radiotherapy in the treatment of IDH-mutant diffuse gliomas: an early experience from shanghai proton and heavy ion center. J Neurooncol. (2023) 162:503–14. doi: 10.1007/s11060-022-04202-5
31. Wilkinson B, Morgan H, Gondi V, Larson GL, Hartsell WF, Laramore GE, et al. Low levels of acute toxicity associated with proton therapy for low-grade glioma: A proton collaborative group study. Int J Radiat Oncol Biol Phys. (2016) 96:E135. doi: 10.1016/j.ijrobp.2016.06.930
32. Combs SE, Kessel K, Habermehl D, Haberer T, Jäkel O, and Debus J. Proton and carbon ion radiotherapy for primary brain tumors and tumors of the skull base. Acta Oncol. (2013) 52:1504–9. doi: 10.3109/0284186X.2013.818255
33. Zhang G, Cai X, Cao H, Yu Z, Wang W, Xing Y, et al. Prospective phase II clinical trial of carbon ion radiotherapy combined with chemotherapy for locally advanced pancreatic carcinoma. Int J Radiat Oncol Biol Phys. (2025) S0360-3016(25)04522-5\\. doi: 10.1016/j.ijrobp.2025.06.3881
34. Shiba S, Tsuchida K, Mizoguchi N, Kawashiro S, Shima S, Kano K, et al. Carbon-ion radiotherapy as a local treatment option for hepatocellular carcinoma with child-pugh class B cirrhosis. Adv Radiat Oncol. (2025) 10:101812. doi: 10.1016/j.adro.2025.101812
35. Takakura R, Ota Y, Yamazaki Y, Iwamoto S, Kurouchi K, Moriyoshi K, et al. First report of metabolic complete response in hepatic sarcomatoid carcinoma achieved with carbon-ion radiotherapy. Clin J Gastroenterol. (2025) 55(4):353–64. doi: 10.1007/s12328-025-02172-5
36. Adachi A, Oike T, Kambe R, Yoshida Y, Takahashi A, Hirota Y, et al. Enhanced DNA double-strand break induction by carbon ions under intratumoral hypoxia. Anticancer Res. (2025) 45:2329–37. doi: 10.21873/anticanres.17606
37. Du TQ, Liu R, Zhang Q, Luo H, Chen Y, Tan M, et al. Does particle radiation have superior radiobiological advantages for prostate cancer cells? A systematic review of in vitro studies. Eur J Med Res. (2022) 27:306. doi: 10.1186/s40001-022-00942-2
38. Choi C, Lee GH, Son A, Yoo GS, Yu JI, and Park HC. Downregulation of mcl-1 by panobinostat potentiates proton beam therapy in hepatocellular carcinoma cells. Cells. (2021) 10:554. doi: 10.3390/cells10030554
39. Perréard M, Florent R, Divoux J, Bastit V, Lecouflet L, Desmartin G, et al. Use of patient-derived tumor organoids from head and neck squamous cell carcinoma for the evaluation of the differential effect of carbon ions over X-rays. Radiother Oncol. (2025) 3:111026. doi: 10.1016/j.radonc.2025.111026
40. Qiu X, Gao J, Yang J, Hu J, Hu W, Huang Q, et al. Carbon-ion radiotherapy boost with standard dose proton radiation for incomplete-resected high-grade glioma: a phase 1 study. Ann Transl Med. (2022) 10:1193. doi: 10.21037/atm-20-7750
41. Laurent PA, André F, Bobard A, Deandreis D, Demaria S, Depil S, et al. Pushing the boundaries of radiotherapy-immunotherapy combinations: highlights from the 7th immunorad conference. Oncoimmunology. (2025) 14:2432726. doi: 10.1080/2162402X.2024.2432726
42. Luo H, Yang C, Yue J, and Ge H. Consensus statement on the exploration of clinical translation and application of electron ultra-high dose rate FLASH radiotherapy. Prec Radiat Oncol. (2025) 9:4–12. doi: 10.1002/pro6.70001
43. Montay-Gruel P, Acharya MM, Petersson K, Alikhani L, Yakkala C, Allen BD, et al. Long-term neurocognitive benefits of FLASH radiotherapy driven by reduced reactive oxygen species. Proc Natl Acad Sci U S A. (2019) 116:10943–51. doi: 10.1073/pnas.1901777116
44. Iturri L, Bertho A, Lamirault C, Juchaux M, Gilbert C, Espenon J, et al. Proton FLASH radiation therapy and immune infiltration: evaluation in an orthotopic glioma rat model. Int J Radiat Oncol Biol Phys. (2023) 116:655–65. doi: 10.1016/j.ijrobp.2022.12.018
45. Stensjøen AL, Solheim O, Kvistad KA, Håberg AK, Salvesen Ø, and Berntsen EM. Growth dynamics of untreated glioblastomas in vivo. Neuro Oncol. (2015) 17:1402–11. doi: 10.1093/neuonc/nov029
46. Ji X, Ding W, Wang J, Zhou B, Li Y, Jiang W, et al. Application of intraoperative radiotherapy for Malignant glioma. Cancer Radiother. (2023) 27:425–33. doi: 10.1016/j.canrad.2023.01.007
47. Sarria GR, Sperk E, Han X, Sarria GJ, Wenz F, Brehmer S, et al. Intraoperative radiotherapy for glioblastoma: an international pooled analysis. Radiother Oncol. (2020) 142:162–7. doi: 10.1016/j.radonc.2019.09.023
48. Giordano FA, Brehmer S, Mürle B, Welzel G, Sperk E, Keller A, et al. Intraoperative radiotherapy in newly diagnosed glioblastoma (INTRAGO): an open-label, dose-escalation phase I/II trial. Neurosurgery. (2019) 84:41–9. doi: 10.1093/neuros/nyy018
49. Wang Y, Jian W, Yuan Z, Guan F, and Carlson D. Deep learning with attention modules and residual transformations improves hepatocellular carcinoma (HCC) differentiation using multiphase CT. Prec Radiat Oncol. (2025) 9:13–22. doi: 10.1002/pro6.70003
50. Pehrson LM, Petersen J, Panduro NS, Lauridsen CA, Carlsen JF, Darkner S, et al. AI-guided delineation of gross tumor volume for body tumors: A systematic review. Diagnostics (Basel). (2025) 15:846. doi: 10.3390/diagnostics15070846
51. Chong PL, Vaigeshwari V, Mohammed Reyasudin BK, Noor Hidayah BRA, Tatchanaamoorti P, Yeow JA, et al. Integrating artificial intelligence in healthcare: applications, challenges, and future directions. Future Sci OA. (2025) 11:2527505. doi: 10.1080/20565623.2025.2527505
52. Li Z, Su Y, Cui Y, Yin Y, and Li Z. Multi-sequence MRI-based clinical-radiomics models for the preoperative prediction of microsatellite instability-high status in endometrial cancer. Prec Radiat Oncol. (2025) 9:43–53. doi: 10.1002/pro6.70000
53. Booth TC, Williams M, Luis A, Cardoso J, Ashkan K, and Shuaib H. Machine learning and glioma imaging biomarkers. Clin Radiol. (2020) 75:20–32. doi: 10.1016/j.crad.2019.07.001
54. Suk Y and Kang H. Tuning random forests for causal inference under cluster-level unmeasured confounding. Multivariate Behav Res. (2023) 58:408–40. doi: 10.1080/00273171.2021.1994364
55. Huang S, Cai N, Pacheco PP, Narrandes S, Wang Y, and Xu W. Applications of support vector machine (SVM) learning in cancer genomics. Cancer Genomics Proteomics. (2018) 15:41–51. doi: 10.21873/cgp.20063
56. Liang D, Wang L, Zhong P, Lin J, Chen L, Chen Q, et al. Perspective: global burden of iodine deficiency: insights and projections to 2050 using XGBoost and SHAP. Adv Nutr. (2025) 16:100384. doi: 10.1016/j.advnut.2025.100384
57. Soffer S, Ben-Cohen A, Shimon O, Amitai MM, Greenspan H, and Klang E. Convolutional neural networks for radiologic images: A radiologist’s guide. Radiology. (2019) 290:590–606. doi: 10.1148/radiol.2018180547
58. Samara M and Harry K. Integrating boruta, LASSO, and SHAP for clinically interpretable glioma classification using machine learning. BioMedInformatics. (2025) 5:34. doi: 10.3390/biomedinformatics5030034
59. Li X, Huang X, Shen Y, Yu S, Zheng L, Cai Y, et al. Machine learning for grading prediction and survival analysis in high grade glioma. Sci Rep. (2025) 15:16955. doi: 10.1038/s41598-025-01413-4
60. Dokare I and Gupta S. Shap-driven explainable AI with simulated annealing for optimized seizure detection using multichannel EEG signal. Cognit Neurodyn. (2025) 19:85. doi: 10.1007/s11571-025-10269-3
61. Lagap U and Ghaffarian S. Digital twin-enabled post-disaster damage and recovery monitoring with deep learning: leveraging transfer learning, attention mechanisms, and explainable AI. Geomat Nat Haz Risk. (2025) 16(5):102–133. doi: 10.1080/19475705.2025.2485329
62. Qi X, Wang S, Fang C, Jia J, Lin L, and Yuan T. Machine learning and SHAP value interpretation for predicting comorbidity of cardiovascular disease and cancer with dietary antioxidants. Redox Biol. (2025) 79:103470. doi: 10.1016/j.redox.2024.103470
Keywords: artificial intelligence, glioma, radiotherapy, prospects, clinical practice
Citation: Wang X, Qi Z, Zeng Q, Gu D and Li T (2025) Radiotherapy for glioma in the AI era: current applications and future prospects. Front. Oncol. 15:1673752. doi: 10.3389/fonc.2025.1673752
Received: 26 July 2025; Accepted: 29 August 2025;
Published: 11 September 2025.
Edited by:
Yunwei Han, The Affiliated Hospital of Southwest Medical University, ChinaReviewed by:
Ying Jiang, Peking Union Medical College Hospital (CAMS), ChinaCopyright © 2025 Wang, Qi, Zeng, Gu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tianliang Li, dGlhbmxpYW5nbGk1Nzk0QDE2My5jb20=
 Xin Wang1
Xin Wang1 Tianliang Li
Tianliang Li