- 1Department of Ultrasonography, Shanxi Bethune Hospital, Shanxi Academy of Medical Sciences, Third Hospital of Shanxi Medical University, Tongji Shanxi Hospital, Taiyuan, China
- 2Department of Interventional Therapy, Shanxi Province Cancer Hospital, Shanxi Hospital Affiliated to Cancer Hospital, Chinese Academy of Medical Sciences, Cancer Hospital Affiliated to Shanxi Medical University, Taiyuan, China
Ectopic breast cancer (EBC) is a rare type of breast cancer that originates from ectopic breast tissue. This article describes a case with left breast cancer complicated by a second primary breast cancer of the left upper extremity, and summarizes the relevant literature to review the epidemiology, clinical presentation, pathologic features, imaging tests, treatment strategies, and prognosis of EBC with the aim of providing clinicians with a comprehensive reference to improve the understanding and management of this disease.
Introduction
Breast cancer is one of the most common malignant tumors among women worldwide. Ectopic breast cancer (EBC), as a specific type of breast cancer, has a low incidence (1, 2). The presence of ectopic breast tissue provides a potential “breeding ground” for breast cancer, and its rarity and complexity make it challenging to diagnose and treat. The most common site of ectopic breast tissue is the axilla, and there are also reports of EBC occurring in rare sites such as the face, neck, inguinal area, and vulva (3, 4). In this article, we report a case of EBC occurring in the left upper arm, and review the relevant literature in order to improve clinicians’ understanding of the clinical features, imaging characteristics, and key diagnostic points of this rare type of tumor.
Case report
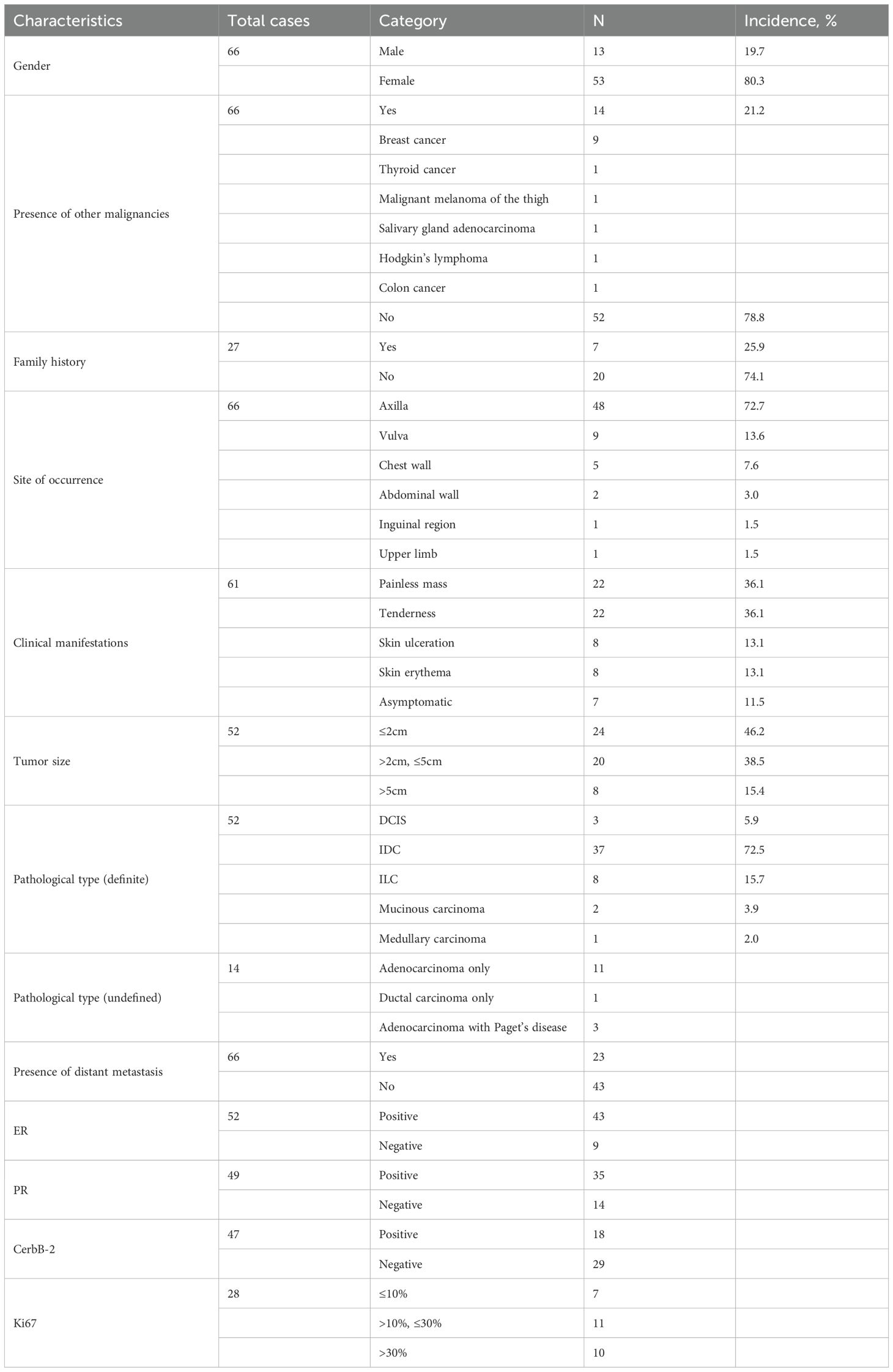
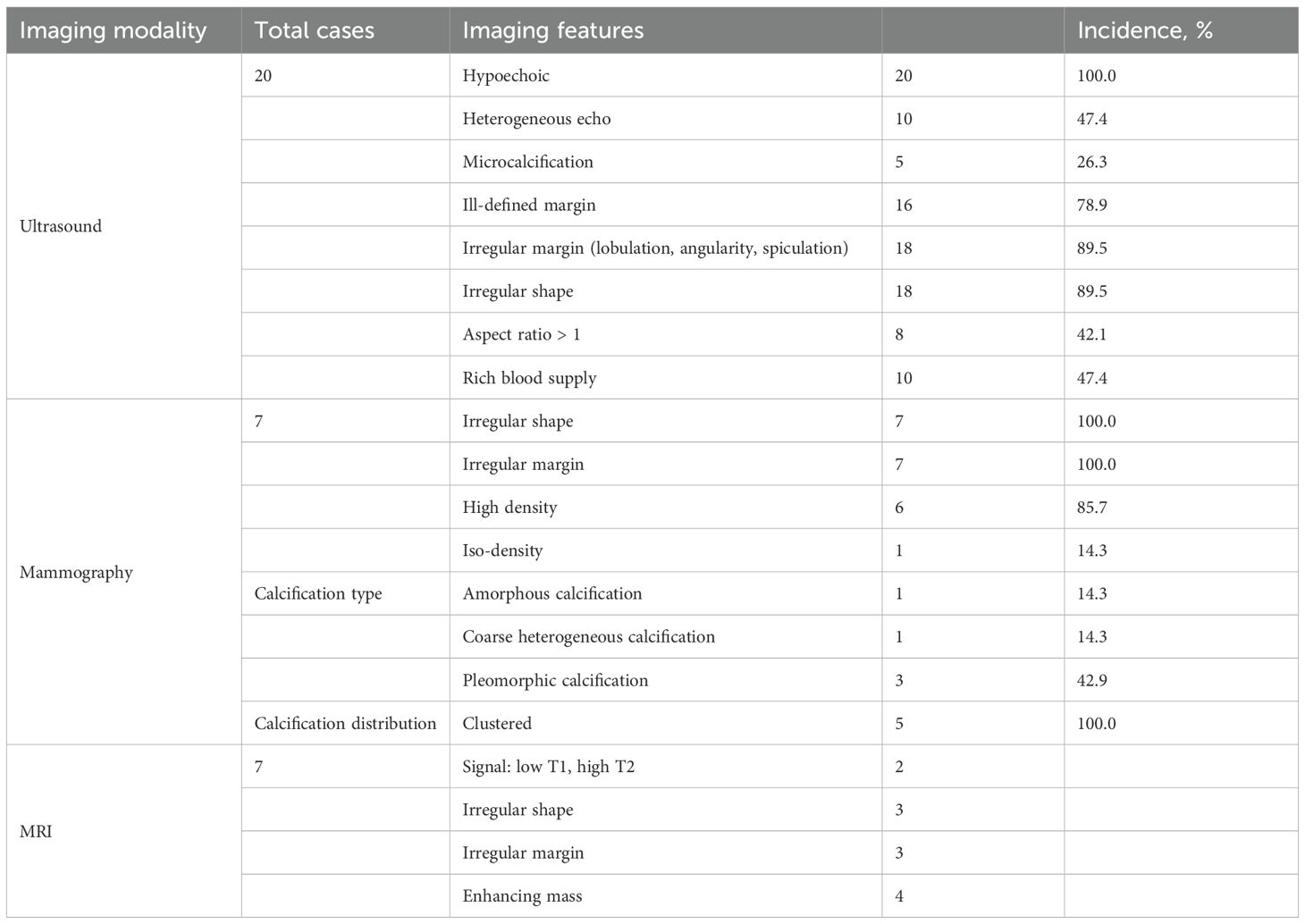
A 58-year-old female patient with no family history of breast cancer was found to have a left upper limb mass of about 2cm in size 30 years ago, which was not accompanied by redness, swelling and pain, so she did not pay attention to it and did not receive any treatment. During this period, the left upper extremity mass gradually increased to about 3 cm. In June 2024, the patient discovered a rapidly increasing mass in the left upper limb with a length of about 15cm, accompanied by pain, local redness, swelling, and ulceration. The patient sought medical attention at our hospital. Physical examination showed a raised red mass of about 15 × 8cm in the left upper limb, with scabs on the surface, hard texture, unclear boundaries, irregular shape, and obvious tenderness. (Figure 1A). Ultrasound examination showed multiple solid hypoechoic masses under the skin of the left upper arm, with unclear boundaries, blurred edges, irregular shapes, and abundant blood flow signals visible inside (Figures 1B, C). No obvious space occupying lesions were observed in both breasts. Multiple lymph node enlargement can be seen in the left axilla, with larger ones measuring 1.0 × 0.7cm in size, clear boundaries, regular morphology, and no obvious lymph node structure (Figure 1D). Ultrasound indicates multiple solid masses in the left upper arm, suggesting malignancy. Multiple lymph node swelling in the left axilla, suspected of metastasis. Chest CT shows multiple solid nodules in both lungs, with a high possibility of metastasis. The patient’s whole-body bone imaging, abdominal ultrasound, clavicle area ultrasound, and head MRI did not show any other tumor lesions. On January 2, 2025, the patient underwent ultrasound-guided biopsy of the left upper limb mass and left axillary mass. The pathological results of the left upper limb mass biopsy, combined with immunohistochemical markers, were consistent with ectopic breast tissue, with some areas being adenoma and others showing malignant transformation (metaplastic carcinoma). Immunohistochemistry results: ER (focal +), PR (focal weak +), HER-2 (0), Ki67 (approximately 80% +). PD-L1 CPS 80. The pathological results of the left axillary lymph node biopsy revealed malignant tumor, which was considered to be metastasis from the left upper limb malignant tumor (metaplastic carcinoma) (Figure 2).
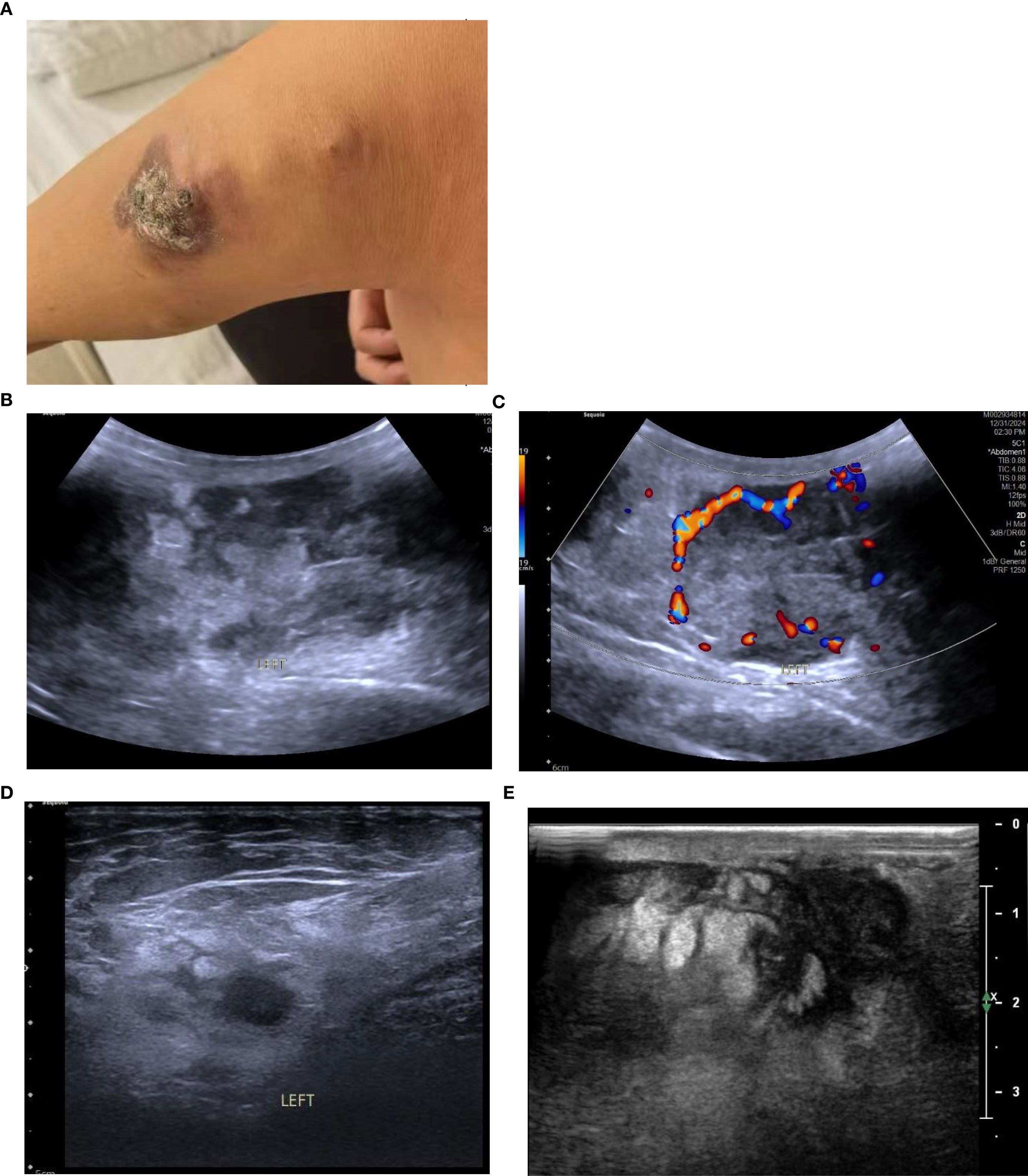
Figure 1. (A) The patient had a protruding red mass on the left upper limb, with a crust on the surface, hard in texture, ill-defined margins, and an irregular shape. (B) Conventional ultrasound revealed multiple hypoechoic solid masses in the subcutaneous tissue of the left upper arm, with ill-defined margins, indistinct edges, and irregular shapes; the largest measures 8.2 × 3.4 cm. (C) CDFI showed relatively rich blood flow signals within the masses. (D) Enlarged lymph nodes were visible in the left axilla, with clear margins and no obvious lymph node structure. (E) After two cycles of chemotherapy, the largest mass measures approximately 4.0 × 2.6 cm (a marked reduction from its pre-treatment size).
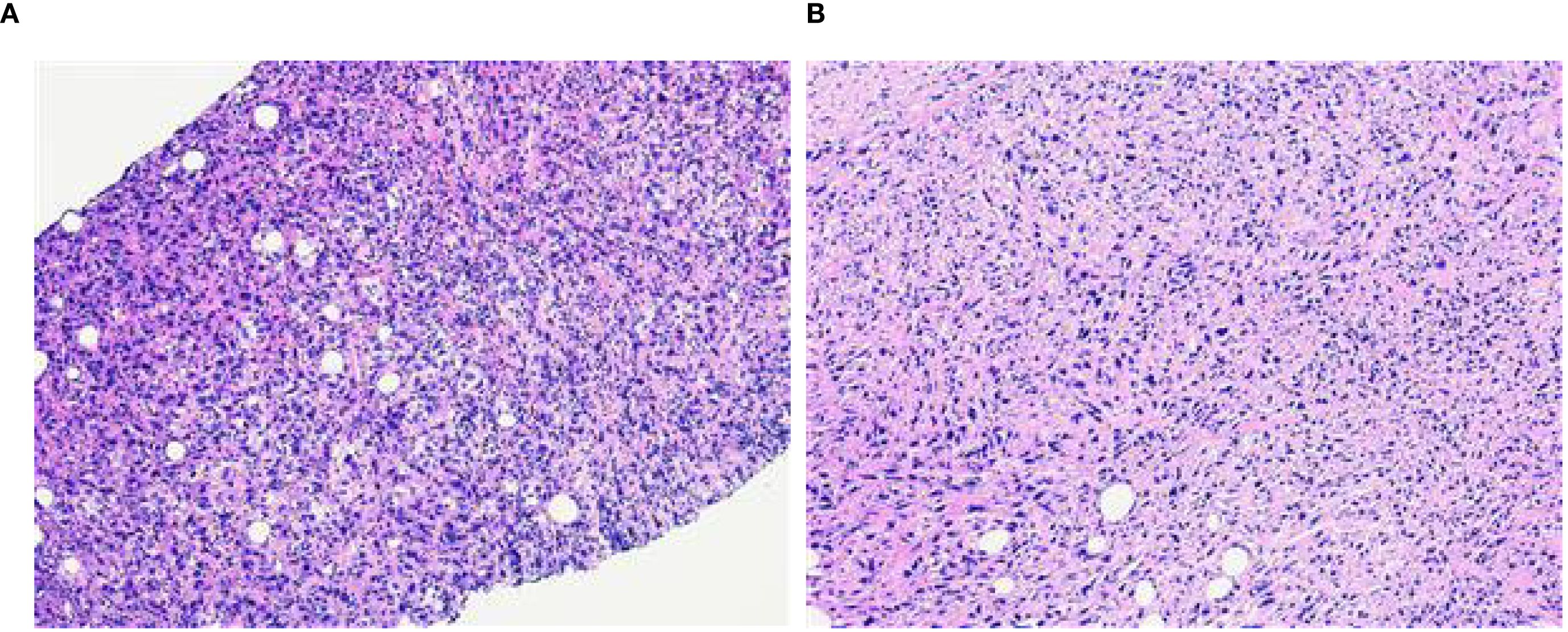
Figure 2. (A) The puncture pathology of the mass in the left upper limb showed that, in combination with the results of immunohistochemical staining, it was consistent with ectopic breast tissue, with some areas being tubular adenoma and some regions undergoing malignant transformation (metaplastic carcinoma) (HE×100). (B) The puncture pathology of the left axillary lymph node revealed a malignant tumor, which was considered to be a metastasis of the malignant component from the upper limb tumor (metaplastic carcinoma) (HE×100).
Medical History:5 years ago, she underwent lumpectomy (breast-conserving surgery) for left breast cancer, and the postoperative pathology returned grade III invasive ductal carcinoma of the left breast (9 points), with immunohistochemistry: ER (+, about 80%), PR (+, about 90%), CerbB-2 (1+), and Ki67 (about 60%+). Intensive EC-P regimen chemotherapy was performed after surgery, and 4 cycles of EC regimen chemotherapy (epirubicin 130 mg + cyclophosphamide 850 mg) were completed from September 1, 2021 to October 12, 2021, and 4 cycles of P regimen chemotherapy (paclitaxel liposome 230 mg for injection) were performed from October 26, 2021 to December 8, 2021. At the end of chemotherapy, left breast and clavicular area, axillary and internal breast lymphatic drainage areas + tumor bed additive intensity-modulated radiotherapy was performed (PTV 50.40Gy/28 times, PGTVtb 65.80Gy/28 times). Since the completion of radiotherapy, the patient has been consistently taking letrozole for endocrine therapy.
Due to the patient’s refusal to undergo surgery, a combination of chemotherapy, radiotherapy, and endocrine therapy was used. Chemotherapy began on January 16, 2025. The specific medication was 200mg of albumin bound paclitaxel (Day 1 and Day 8) and 1.5g of capecitabine twice a day (Day 1 to Day 14), with a cycle of 21 days. The patient has completed two chemotherapy cycles and the left upper limb mass has shrunk compared to before (Figure 1E).
Discussion
A total of 46 articles related to EBC from January 2005 to April 2025 in the PubMed database were retrieved and summarized together with this case. The general clinical and pathological data of the patient are shown in Table 1. The imaging manifestations of the patient are shown in Table 2. The collected data includes the patient’s gender, age, personal history, family history, location of occurrence, clinical manifestations, pathological type, metastasis, prognosis, and imaging findings. A total of 65 cases from 64 patients were collected (one case was bilateral axillary EBC). Because the integrity of clinical data obtained in each case was different, not every patient was included in the analysis of each variable.
Epidemiology
The formation of ectopic breast tissue is related to the incomplete regression of the milk line during embryonic development. In the 6th week of the embryo, the milk line extends from the axilla to the inguinal region. Normally, the milk line will regress, leaving only the breast tissue in the chest. If the regression is incomplete, ectopic breast tissue may form (5). The incidence of ectopic breast tissue in the general population is approximately 0.22% to 6.0%, and it is more common in females, with a male-to-female ratio of about 1:5 (6). In this study, the average age of the patients was 55.7 ± 13.5 years (ranging from 27 to 83 years), including 13 males (20.0%) and 53 females (80.0%). The number of female patients was significantly higher than that of male patients, which is consistent with previous reports. Meanwhile, the age distribution of patients with EBC is similar to that of patients with breast cancer. In the cases collected in this study, 14 patients had other malignancies, among which breast cancer was the most common (accounting for 64.3%), indicating that the occurrence of EBC may be similar to the genetic and environmental risk factors of in situ breast cancer.
Literature reported that the risk of breast cancer increased with the number of first-degree relatives (mother, daughter, or sister) who had been diagnosed. Approximately 13% to 19% of breast cancer patients had affected first-degree relatives (mother, daughter, or sister). If one first-degree relative had breast cancer, the risk increased by 1.5 to 4 times (7). In this study, the proportion of patients with a first-degree relative having breast cancer was 25.9%, which was similar to the reported figures for breast cancer, indicating that family history was of great importance for patients. The most common site for ectopic breast cancer was the axilla, accounting for 70% to 90% of all EBC (8). Other possible sites included the chest wall, abdominal wall, vulva, neck, face, etc (3, 4, 8). In this study, 72.7% of EBC were located in the axilla, 13.6% in the vulva, 7.6% in the chest wall, 3.0% in the abdominal wall, and only 1 case was found in the inguinal region (1.5%). This case report presented a case of ectopic breast cancer occurring in the subcutaneous tissue of the left upper limb, for which no similar reports had been found in the literature so far.
Clinical manifestation
The clinical manifestations of EBC were diverse. Common symptoms included palpable masses, skin erythema, ulcers, and pain (3, 9). Because ectopic breast tissue usually did not contain the nipple-areola complex and was located in special positions, these symptoms might have been mistaken for other diseases, such as lipomas, sebaceous cysts, or lymph node lesions (10). Moreover, EBC might not have had obvious symptoms in the early stage, leading to delayed diagnosis (11).
Pathological features
Histopathological examination is the gold standard for the diagnosis of EBC. It is necessary to have the presence of both normal breast tissue and cancerous tissue to confirm a diagnosis of EBC (5, 9, 12). Immunohistochemical testing could serve as an auxiliary diagnostic tool, with commonly used markers including ER, PR, CerbB-2/HER2, and Ki67 (12–14).
The pathological features of EBC were similar to those of normal breast cancer and could manifest as a variety of histological subtypes. Infiltrating ductal carcinoma was the most common subtype (accounting for 72.5% in this article), followed by lobular carcinoma, medullary carcinoma, mucinous carcinoma, and so on (6, 9, 14). The histological diagnosis needed to take into account the presence of ectopic breast tissue and the morphological characteristics of the tumor, while also ruling out metastasis from breast cancer in other locations (13, 14). This case was diagnosed as metaplastic breast carcinoma (MBC). MBC is a rare and highly heterogeneous subtype of invasive breast cancer, accounting for 0.2–5% of all breast cancers. The tumor undergoes “metaplasia” from epithelial toward non-glandular lineages and may exhibit diverse components such as squamous, spindle, chondroid, or osseous elements. MBC typically presents as a large mass, behaves aggressively, and carries a high risk of local recurrence and pulmonary metastasis (15, 16). As with the normal breast, ectopic breast tissue could also undergo both benign and malignant pathological changes, that is, the progression from fibroadenoma or intraductal papilloma to carcinoma (5, 17). The pathological results reported in this case showed that the patient’s left upper limb mass was partially a tubular adenoma, with some areas having undergone malignant transformation (metaplastic carcinoma or myoepithelial carcinoma), and the pathological type of the left axillary lymph node tissue was consistent with that of the left upper limb mass, suggesting malignant transformation of ectopic breast adenoma with left axillary lymph node metastasis.
Imaging examination
Ultrasound was the preferred diagnostic method for ectopic breast tumors. When the tumor was located in the breast line area and glandular-like echoes could be detected around it, it was helpful for the diagnosis of ectopic breast tumors. The criteria for determining benign or malignant tumors on ultrasound images were the same as those for breast tumors. Generally speaking, irregular morphology, unclear boundaries, rough or angular edges, and abundant blood flow signals were the ultrasound signs of malignant tumors. However, some ectopic breast tissues were located superficially, with thin glandular bodies and not in the breast line area, making it difficult to diagnose (4, 12, 18). In this case, the tumor was located in the left upper limb and there was no normal glandular echo around it, making it difficult to distinguish from other malignant tumors.
Sometimes, heterotopic breast cancer in the axilla could be displayed in the internal and external oblique mammography, but the rate of missed diagnosis was high, and heterotopic breast cancer in other locations could not be displayed (12, 18). In this article, a total of 7 patients were retrieved for mammography, and the location of EBC was the axilla, which was consistent with the X-ray findings of breast cancer. The findings included irregular shape, uneven edges, cluster distribution of polymorphic calcification, rough heterogeneous calcification, and amorphous calcification. These findings could be used as an auxiliary diagnostic method for ultrasound detection.
Magnetic Resonance Imaging (MRI) could reveal the characteristics of malignant tumors, such as irregular shape, uneven margins, and enhancement on contrast-enhanced scans (4, 6, 11). In addition, some patients underwent PET-CT scans, which could be used to assess the primary tumor, lymph node metastasis, and distant metastasis (9, 11, 13, 19).
Treatment strategies
The treatment of most EBC was based on the standard treatment protocols for breast cancer (9, 10, 20). Surgery was the main treatment, supplemented by chemotherapy, radiotherapy, endocrine therapy, and molecular targeted therapy. Surgical treatment included local excision and lymph node dissection in the drainage area. Postoperative radiotherapy could reduce the risk of local recurrence, especially for patients with lymph node involvement or larger tumors. The indications for chemotherapy followed those for primary breast cancer and were usually determined based on the tumor stage and molecular subtype. For patients with hormone receptor-positive tumors, endocrine therapy could lower the risk of recurrence (9–12, 20–22). In addition, targeted therapy could be administered, with indications being the same as those for primary breast cancer. For example, patients with HER2-positive tumors could receive trastuzumab (20–22).
Prognosis
The prognosis of EBC was worse than that of primary breast cancer due to its rarity and delayed diagnosis. However, recent studies had shown that the prognosis of EBC was not necessarily worse than that of primary breast cancer if it was diagnosed and treated at similar disease stages. The prognostic factors included tumor staging, lymph node involvement, and treatment plan.
Conclusion
EBC is a rare but challenging disease that requires multidisciplinary collaboration, including radiology, pathology, surgery, and medical oncology. Increasing awareness of EBC, early diagnosis, and standardized treatment are key to improving patient prognosis. More research is needed in the future to further explore its pathogenesis and optimize treatment strategies.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Shanxi Bethune Hospital, Shanxi Academy of Medical Sciences. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
S-NJ: Writing – original draft, Writing – review & editing. DW: Methodology, Conceptualization, Writing – original draft. T-TX: Writing – review & editing, Writing – original draft. Z-XZ: Supervision, Investigation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kim JH. Concurrent invasive carcinoma and fibroadenoma arising from bilateral ectopic breast tissue in the chest wall: A case report and literature review. J Korean Soc Radiol. (2024) 85:813–9. doi: 10.3348/jksr.2023.0137
2. Mansour M, Zahra O, Nabulsi D, Alhamwi A, Chahin M, Alani WR, et al. Ectopic primary ductal breast carcinoma of the vulva: a case report and literature review. Ann Med Surg (Lond). (2023) 85:5138–44. doi: 10.1097/MS9.0000000000001160
3. Lerttiendamrong B and Vongsaisuwon M. First report of bilateral synchronous male accessory breast cancer. BMJ Case Rep. (2022) 15:e250927. doi: 10.1136/bcr-2022-250927
4. Aramin H, Koirala P, Shah A, Adams K, Buza N, Desai S, et al. Metachronous vulvar ectopic breast cancer, a case report and literature review. Gynecol Oncol Rep. (2019) 30:100515. doi: 10.1016/j.gore.2019.100515
5. Thasanabanchong P and Vongsaisuwon M. Unexpected presentation of accessory breast cancer presenting as a subcutaneous mass at costal ridge: a case report. J Med Case Rep. (2020) 14:45. doi: 10.1186/s13256-020-02366-0
6. Lee KE, Kang J, Kang WY, and Woo OH. A case report on ductal carcinoma in situ arising from axillary accessory breast tissue. J Korean Soc Radiol. (2025) 86:154–9. doi: 10.3348/jksr.2024.0029
7. Łukasiewicz S, Czeczelewski M, Forma A, Baj J, Sitarz R, and Stanisławek A. Breast cancer-epidemiology, risk factors, classification, prognostic markers, and current treatment strategies-an updated review. Cancers (Basel). (2021) 13:4287. doi: 10.3390/cancers13174287
8. Miles RC, Amornsiripanitch N, and Scheel J. Inflammatory breast cancer in accessory abdominal breast tissue. Radiol Case Rep. (2017) 12:639–41. doi: 10.1016/j.radcr.2017.08.008
9. Bansal VY, Bansal VV, Shah SV, and Bellige AR. Primary ectopic breast carcinoma arising in the inguinal region in a male patient. J Cancer Res Ther. (2022) 18:837–9. doi: 10.4103/jcrt.JCRT_777_20
10. Marques-Antunes J, Cardoso F, Santos T, Nora M, and Scigliano H. Invasive lobular carcinoma arising in ectopic breast tissue: A case report. Cureus. (2022) 14:e24055. doi: 10.7759/cureus.24055
11. Addae JK, Genuit T, Colletta J, and Schilling K. Case of second primary breast cancer in ectopic breast tissue and review of the literature. BMJ Case Rep. (2021) 14:e241361. doi: 10.1136/bcr-2020-241361
12. Zhong GB, Ye XQ, Liu JL, Xiao SZ, Huang QH, and Wei W. Male accessory breast cancer on the abdominal wall: a case report and literature review. Onco Targets Ther. (2018) 11:6625–31. doi: 10.2147/OTT.S184185
13. Lee GY, Park YM, Kim D, Park HY, and Shin GW. Male breast cancer presenting in accessory axillary breast. J Clin Ultrasound. (2022) 50:955–7. doi: 10.1002/jcu.23250
14. Nardello SM, Kulkarni N, Aggon A, Boraas M, Sigurdson ER, and Bleicher RJ. Invasive mucinous carcinoma arising in ectopic axillary breast tissue: a case report and literature review. Am J Case Rep. (2015) 16:153–9. doi: 10.12659/AJCR.892650
15. Chaudhary D, Balhara K, Mandal S, Mallya V, Tomar R, Khurana N, et al. Metaplastic breast carcinoma: Analysis of clinical and pathologic features, a five-year study. J Cancer Res Ther. (2023) 19:1226–30. doi: 10.4103/jcrt.jcrt_1229_21
16. Drekolias D and Mamounas EP. Metaplastic breast carcinoma: Current therapeutic approaches and novel targeted therapies. Breast J. (2019) 25:1192–7. doi: 10.1111/tbj.13416
17. Mandal S, Bethala MG, Dadeboyina C, Khadka S, and Kasireddy V. A rare presentation of an invasive ductal carcinoma of ectopic axillary breast tissue. Cureus. (2020) 12:e9928. doi: 10.7759/cureus.9928
18. Patel BK, Jafarian N, Abbott AM, Khazai L, and Lee MC. Imaging findings and management of primary breast cancer in accessory axillary breast tissue. Clin Breast Cancer. (2015) 15:e223–9. doi: 10.1016/j.clbc.2015.04.003
19. Park JS, Lee AY, Bae SG, and Lee SM. Hypermetabolic axillary mass on (18)F FDG PET/CT: breast cancer arising from accessory breast tissue. Nucl Med Mol Imaging. (2010) 44:300–3. doi: 10.1007/s13139-010-0053-9
20. Nihon-Yanagi Y, Ueda T, Kameda N, and Okazumi S. A case of ectopic breast cancer with a literature review. Surg Oncol. (2011) 20:35–42. doi: 10.1016/j.suronc.2009.09.005
21. Zhang S, Yu YH, Qu W, Zhang Y, and Li J. Diagnosis and treatment of accessory breast cancer in 11 patients. Oncol Lett. (2015) 10:1783–8. doi: 10.3892/ol.2015.3388
Keywords: ectopic breast cancer, case report, diagnosis, literature review, imaging examination
Citation: Jia S-n, Wang D, Xue T-t and Zhao Z-x (2025) A case report of ectopic breast cancer of the left upper extremity with literature review. Front. Oncol. 15:1674230. doi: 10.3389/fonc.2025.1674230
Received: 27 July 2025; Accepted: 16 September 2025;
Published: 02 October 2025.
Edited by:
Robert Wesolowski, The Ohio State University, United StatesReviewed by:
Arya Mariam Roy, University at Buffalo, United StatesHasanain Jasim, College of Medicine Mustansiriyah University, Iraq
Copyright © 2025 Jia, Wang, Xue and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ting-ting Xue, eHVldGluZ3RpbmdAc3hicWVoLmNvbS5jbg==
 Shu-ni Jia
Shu-ni Jia Dong Wang2
Dong Wang2