- 1Department of Nuclear Medicine, The Third Hospital of Mianyang (Sichuan Mental Health Center), Mianyang, China
- 2School of Clinical Medicine, North Sichuan Medical College, Nanchong, China
- 3Department of Oncology, The Third Hospital of Mianyang (Sichuan Mental Health Center), Mianyang, China
- 4Department of Orthopedics, The Third Hospital of Mianyang(Sichuan Mental Health Center), Mianyang, China
Introduction: Primary extranodal diffuse large B-cell lymphoma (PE-DLBCL) originates in extranodal organs and accounts for approximately 30%–40% of all DLBCL cases. PE-DLBCL involving multiple organs without lymph node involvement is uncommon. We present a case of DLBCL in a patient who initially presented with abdominal pain. Abdominal computed tomography (CT) revealed suspicious neoplastic lesions in the liver and ileum. Histopathological examination of an ileal biopsy confirmed the diagnosis of DLBCL. The patient subsequently underwent [18F]FDG PET/CT for disease staging. PET/CT demonstrated multiple hypermetabolic lesions in the liver, right kidney, and ileum, notably in the absence of any lymph node involvement throughout the body. After four cycles of R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) chemotherapy, [18F]FDG PET/CT evaluation revealed significant shrinkage of the abdominal lesions, indicating a favorable prognosis.
Conclusion: This case demonstrates a rare presentation of multisystem PE-DLBCL without lymphadenopathy and underscores the vital role of [18F]FDG PET/CT in identifying disease extent and guiding accurate staging and management, as well as evaluating treatment response.
Introduction
DLBCL is the most common subtype of non-Hodgkin lymphoma, characterized by highly aggressive behavior and heterogeneous clinical manifestations, which pose significant diagnostic and therapeutic challenges (1). While most DLBCL cases primarily involve the lymph nodes, a subset originates in extranodal organs. The gastrointestinal tract is the most common primary site of PE-DLBCL (2), while primary extranodal disease may also arise in the skin (3), breast (4), central nervous system (5), and other locations (6).
According to DLBCL clinical guidelines and SEER database analyses, PE-DLBCL accounts for approximately 30%-40% of all DLBCL cases (7, 8). Large-cohort epidemiological studies from multiple regions also provide important insights into the distribution of extranodal DLBCL (EN-DLBCL). Babu SM et al. (9) reported 526 DLBCL cases in India, among which 202 were EN-DLBCL. Economopoulos T et al. (10) analyzed 810 Non-Hodgkin Lymphoma (NHL) cases in Greece and identified 23 cases of multiple EN-DLBCL. Candelaria M et al. (11) reported 51 PE-DLBCL cases among 637 DLBCL patients in Mexico. More recently, Chen SY et al. (12) conducted a large Chinese cohort study involving 4,785 EN-DLBCL cases, including 3,163 single-organ and 1,621 multiple-organ presentations. As shown in Table 1.
PE-DLBCL should be distinguished from extranodal involvement of DLBCL (ENI-DLBCL), as the former refers to lymphoma originating in an extranodal organ, whereas the latter represents secondary extension of nodal disease into extranodal sites. Involvement of multiple extranodal organs can mimic metastatic carcinoma, potentially leading to diagnostic challenges, and definitive diagnosis often relies on histopathological confirmation through biopsy. Due to the low incidence and lack of large-scale studies, the clinical behavior and prognostic characteristics of such cases remain unclear. Most reports in the literature are individual case studies; for instance, some researchers have described cases of primary extranodal lymphoma predominantly involving the kidneys (13, 14). In these cases, initial staging was performed using [18F]FDG PET/CT prior to chemotherapy, followed by post-treatment PET/CT for response evaluation. The lesions showed marked reduction in size and metabolic activity, indicating a significant therapeutic response.
[18F]FDG PET/CT demonstrates high sensitivity for detecting hypermetabolic lesions and is particularly valuable in assessing the extent of disease in atypical cases with extranodal involvement. It plays a crucial role in the staging and treatment response evaluation of extranodal lymphomas (15). Moreover, semiquantitative PET/CT parameters, such as metabolic tumor volume (MTV) and total lesion glycolysis (TLG), may provide useful information for risk stratification and prognostic assessment (16, 17), thereby offering essential guidance for treatment decision-making.
Here, we present a case of DLBCL with simultaneous involvement of multiple extranodal organs without lymph node infiltration, as identified by [18F]FDG PET/CT. This case further highlights the diagnostic precision of PET/CT in DLBCL and its substantial value in guiding clinical decision-making.
Case presentation
A 78-year-old male presented with abdominal pain and underwent contrast-enhanced CT at an outside hospital, which revealed a large hepatic mass with internal hemorrhage and significant thickening of the ileal wall, both highly suspicious for malignancy. The patient exhibited no B symptoms such as fever, night sweats, or weight loss, and routine laboratory tests, including blood counts, liver and renal function, electrolytes, and tumor markers, were unremarkable. The patient had no significant past medical history and no family history of malignancy. The patient initially received interventional treatment to control hepatic bleeding. After achieving hemodynamic stabilization, an ileal biopsy was performed via colonoscopy, and pathological examination confirmed the diagnosis of diffuse large B-cell lymphoma (Figure 1A). Immunohistochemical staining (Figures 1B, C) showed the following profile: CD20(+), CD79α(+), CD3 (scattered+), CD45RO (scattered+), CD5 (scattered+), CD23(−), Bcl-2 (90%+), Bcl-6 (90%+), C-MYC (50%+), CD10 (+), MUM-1(+), CD56(−), TIA-1(−), P-CK(−), and Ki-67 (90%+). Immunohistochemical analysis revealed positivity for CD20 and CD79α, confirming B-cell lineage. High expression levels of Bcl-2, Bcl-6, and C-MYC, along with a Ki-67 index of 90%, indicated high tumor aggressiveness. The CD10(+)/MUM-1(+) profile suggested a germinal center B-cell (GCB) subtype, supporting the histopathological diagnosis of DLBCL. For disease staging, the patient was referred to our hospital for [18F]FDG PET/CT. [18F]FDG was administered intravenously at a dose of approximately 5.5 MBq/kg, and imaging was performed using a United Imaging uMI 550 PET/CT system. PET/CT (Figures 2A–J) revealed markedly increased FDG uptake in both hepatic and ileal lesions. Additionally, PET/CT identified a slightly hyperdense nodule in the right kidney—missed on prior CT—with significantly elevated FDG metabolism. The patient underwent biopsies of both the liver and kidney lesions at another hospital prior to receiving further treatment, and the pathological results confirmed the diagnosis of lymphoma. This effectively ruled out the possibility of multiple primary malignancies.The final stage of lymphoma was determined to be stage IV, with an International Prognostic Index(IPI) score of 3. Three months later, the patient completed four cycles of R-CHOP chemotherapy. Follow-up [18F]FDG PET/CT (Figures 3A–J) demonstrated significant reduction in the size of hepatic, ileal, and renal lesions with markedly decreased FDG metabolism, indicating an excellent treatment response. The patient's disease timeline is shown in Table 2.
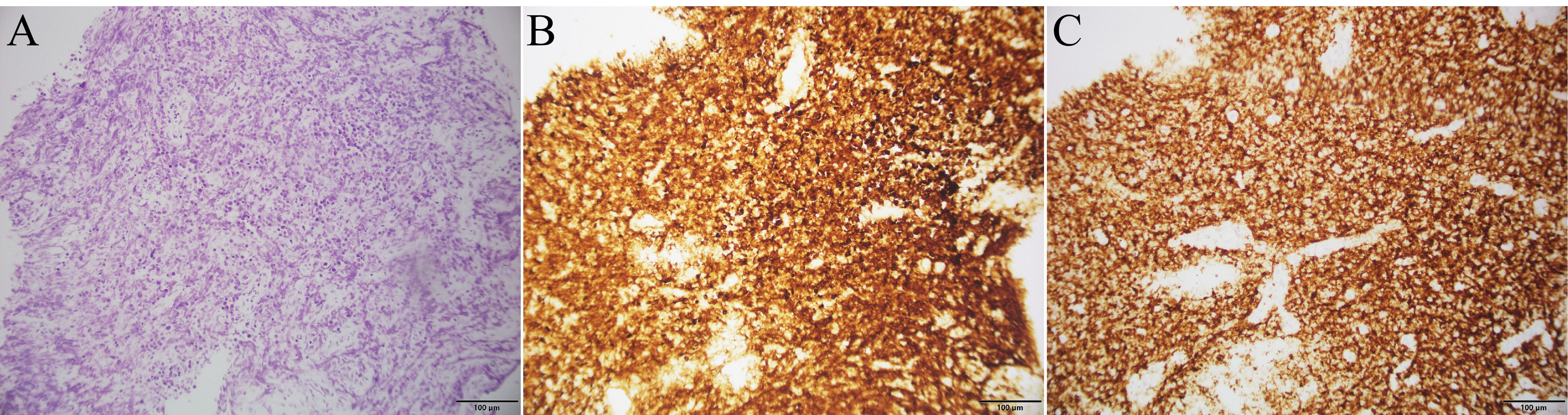
Figure 1. Histopathological and immunohistochemical findings of ileal biopsy: (A) Histopathological image of ileal tissue stained with hematoxylin and eosin; (B) Immunohistochemical image of Bcl-2; (C) Immunohistochemical image of CD20.
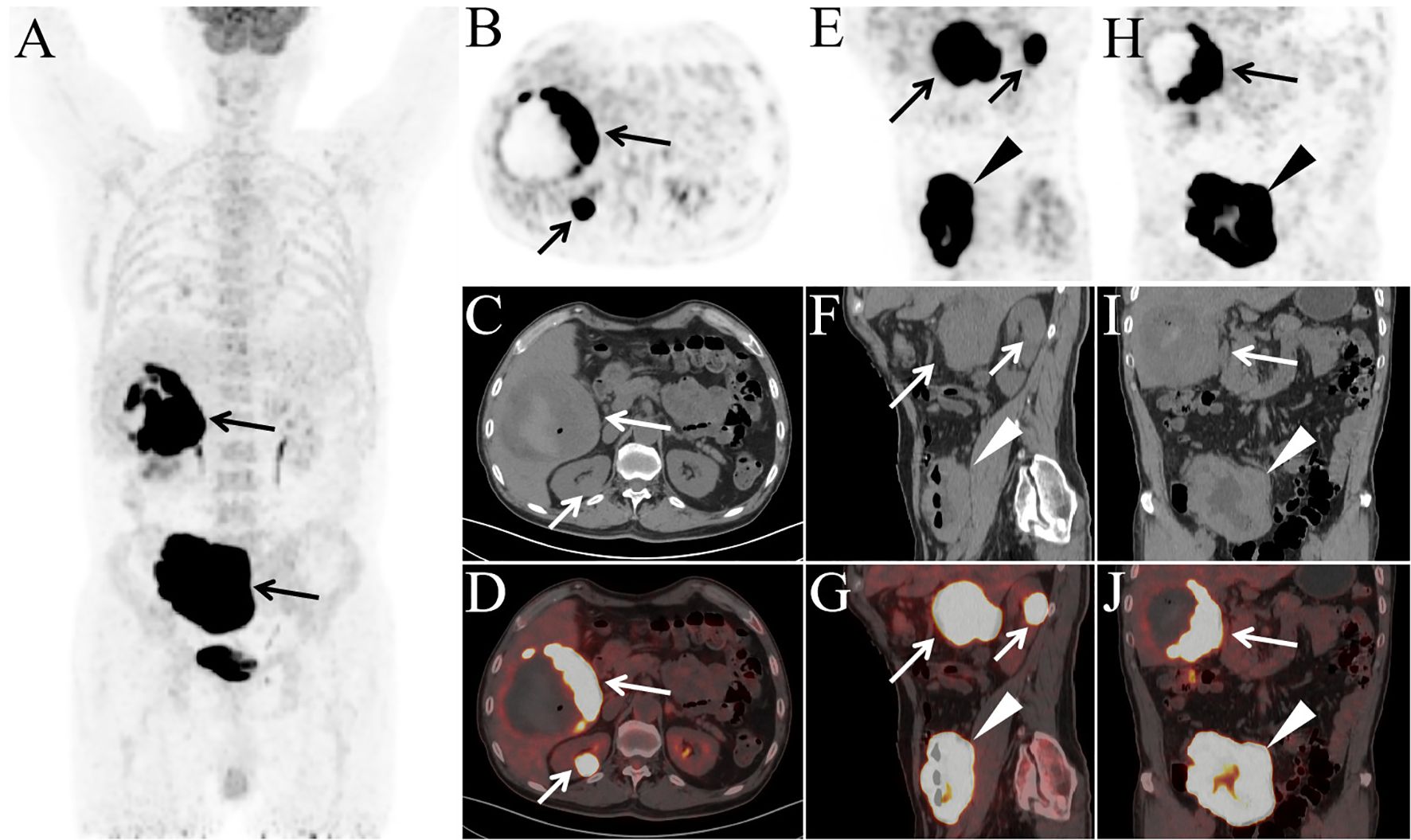
Figure 2. The patient underwent PET/CT examination for staging [(A) MIP; (B, E, H) PET; (C, F, I) CT; (D, G, J) PET/CT]. MIP image shows abnormally increased FDG uptake in the right upper and lower abdominal regions (A, arrows). Axial (B–D), sagittal (E–G), and coronal (H–J) PET/CT images demonstrate a large mass in the posterior segment of the right hepatic lobe measuring 11.0 cm × 10.2 cm × 10.5 cm (B–J, long arrows), a slightly hyperdense nodule in the right kidney measuring 2.6 cm × 2.2 cm × 2.6 cm (B–G, short arrows), and diffuse thickening of the ileal wall with a maximum wall thickness of 4.3 cm (E–J, arrowheads), all exhibiting markedly elevated FDG uptake (SUVmax: hepatic lesion, 32.3; renal lesion, 34.1; ileal lesion, 34.3). Central necrosis, hemorrhage, and a small amount of intralesional gas were observed within the hepatic mass. Notably, there was no evidence of lymphadenopathy or abnormal FDG uptake in any lymph nodes throughout the body.

Figure 3. PET/CT images after four cycles of chemotherapy [(A) MIP; (B, E, H) PET; (C, F, I) CT; (D, G, J) PET/CT]. The MIP image (A) reveals nodular and patchy areas of abnormally increased FDG uptake in the liver and right abdomen. Axial (B–D), sagittal (E–G), and coronal (H–J) PET/CT images demonstrate a hepatic lesion (B–J, long arrows) measuring 7.3 cm × 7.6 cm × 7.9 cm, with nodular areas of increased FDG uptake at the lesion margins (SUVmax: 13.9), while FDG uptake is absent in the remaining portions of the lesion. The ileal lesion (E–J, arrowheads) shows a markedly reduced extent compared to baseline, with a maximum wall thickness of 2.1 cm and abnormally increased FDG uptake (SUVmax: 21.2). The previously noted right renal lesion (B–D, short arrows) has completely resolved, with no abnormal FDG uptake observed.
Discussion
Diffuse large B-cell lymphoma (DLBCL) is the most prevalent subtype of non-Hodgkin lymphoma. In this case, although initial CT scans revealed hepatic and ileal abnormalities, the renal lesion was missed due to its small size and subtle density changes. [18F]FDG PET/CT, owing to its superior sensitivity in detecting lymphoma involvement, successfully identified focal hypermetabolism in the renal cortex, thereby improving staging accuracy. This observation aligns with findings from Le Dortz L et al., who emphasized the role of PET/CT in refining lymphoma staging and avoiding both under- and overtreatment (18). Notably, a maximum standardized uptake value (SUVmax) greater than 10 is suggestive of high-grade or aggressive lymphoma (19). In this patient, SUVmax values exceeded 30 in all three involved organs—liver, kidney, and ileum—indicating high tumor aggressiveness. These metabolic findings correlate with the pathological results, particularly the high expression levels of Bcl-2, Bcl-6, and C-MYC (20), reinforcing the utility of SUVmax in assessing lymphoma aggressiveness.Reports of multisystem extranodal lymphoma are rare, and most involve some degree of lymph node infiltration (21, 22). Cases with a complete absence of lymphadenopathy are exceptionally uncommon (23). This case highlights a unique presentation of DLBCL with simultaneous involvement of the liver, kidney, and ileum, without any lymph node involvement. It further underscores the indispensable role of [18F]FDG PET/CT in the accurate staging of lymphoma. At a three-month follow-up conducted via telephone, the patient reported good overall health, no B symptoms, and stable general condition. [18F]FDG PET/CT demonstrated a marked reduction in the size of hepatic, renal, and ileal lesions, indicating a favorable treatment response and prognosis. At a ten-month telephone follow-up, the patient had completed eight cycles of chemotherapy and did not report any specific discomfort.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Ethics Committee of The Third Hospital of Mianyang. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
Author contributions
TL: Writing – original draft, Writing – review & editing. HD: Writing – review & editing, Writing – original draft. YL: Writing – review & editing. JH: Writing – review & editing. LL: Writing – review & editing, Writing – original draft. CX: Writing – review & editing, Writing – original draft. JL: Writing – review & editing, Writing – original draft.
Funding
The author(s) declared that financial support was received for work and/or its publication. This work was supported by the Clinical Research Project of Sichuan Anti-Cancer Association (Qilu), Grant Number: XH2022-101. The funder was involved in the peer review and editing of the manuscript.
Conflict of interest
The authors declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declared that generative AI was not used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1697944/full#supplementary-material
References
1. Chapuy B, McKeown MR, Lin CY, Monti S, Roemer MG, Qi J, et al. Discovery and characterization of super-enhancer-associated dependencies in diffuse large B cell lymphoma [published correction appears in Cancer Cell. Cancer Cell. (2013) 24:777–90. doi: 10.1016/j.ccr.2013.11.003
2. Psyrri A, Papageorgiou S, and Economopoulos T. Primary extranodal lymphomas of stomach: clinical presentation, diagnostic pitfalls and management. Ann Oncol. (2008) 19:1992–9. doi: 10.1093/annonc/mdn525
3. Olsen EA, Whittaker S, Willemze R, Pinter-Brown L, Foss F, Geskin L, et al. Primary cutaneous lymphoma: recommendations for clinical trial design and staging update from the ISCL, USCLC, and EORTC. Blood. (2022) 140:419–37. doi: 10.1182/blood.2021012057
4. Radkani P, Joshi D, Paramo JC, and Mesko TW. Primary breast lymphoma: 30 years of experience with diagnosis and treatment at a single medical center. JAMA Surg. (2014) 149:91–3. doi: 10.1001/jamasurg.2013.2283
5. Ferreri AJM, Calimeri T, Cwynarski K, Dietrich J, Grommes C, Hoang-Xuan K, et al. Primary central nervous system lymphoma. Nat Rev Dis Prime. (2023) 9:29. doi: 10.1038/s41572-023-00439-0
6. Yılmaz F and Önner H. A rare involvement of diffuse large B-cell lymphoma: Peritoneal lymphomatosis with a peritoneal super-scan appearance on 18F-FDG PET/CT. Hell J Nucl Med. (2022) 25:103–5. doi: 10.1967/s002449912439
7. Vitolo U, Seymour JF, Martelli M, Illerhaus G, Illidge T, Zucca E, et al. Extranodal diffuse large B-cell lymphoma (DLBCL) and primary mediastinal B-cell lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. (2016) 27:v91–v102. doi: 10.1093/annonc/mdw175
8. Gupta V, Singh V, Bajwa R, Meghal T, Sen S, Greenberg D, et al. Site-specific survival of extra nodal diffuse large B-cell lymphoma and comparison with gastrointestinal diffuse large B-cell lymphoma. J Hematol. (2022) 11:45–54. doi: 10.14740/jh984
9. Babu SM, Garg S, Kanakasetty GB, Kuntegowdanahalli LC, Dasappa L, and Rao SA. Diffuse large B-cell lymphoma: A retrospective study from a regional care center in South India. Indian J Can. (2018) 55:66–9. doi: 10.4103/ijc.IJC_450_16
10. Economopoulos T, Papageorgiou S, Rontogianni D, Kaloutsi V, Fountzilas G, Tsatalas C, et al. Multifocal extranodal non-hodgkin lymphoma: a clinicopathologic study of 37 cases in Greece, a Hellenic Cooperative Oncology Group study. Oncologist. (2005) 10:734–8. doi: 10.1634/theoncologist.10-9-734
11. Candelaria M, Oñate-Ocaña LF, Corona-Herrera J, Barrera-Carmona C, Ponce-Martínez M, Gutiérrez-Hernández O, et al. Clinical characteristics of primary extranodal versus nodal diffuse large b-cell lymphoma: a retrospective cohort study in a cancer center. Rev Invest Clin. (2019) 71:349–58. doi: 10.24875/RIC.19003027
12. Chen SY, Xu PP, Feng R, Cui GH, Wang L, Cheng S, et al. Extranodal diffuse large B-cell lymphoma: Clinical and molecular insights with survival outcomes from the multicenter EXPECT study. Cancer Commun (Lond). (2025) 45:919–35. doi: 10.1002/cac2.70033
13. Benameur Y, Hammani A, Doghmi K, and Doudouh A. Bilateral renal involvement in diffuse large B-cell lymphoma on fluorodeoxyglucose positron emission tomography/computed tomography. World J Nucl Med. (2021) 20:195–7. doi: 10.4103/wjnm.WJNM_111_20
14. Arslan E, Aksoy T, Alçın G, Ermantaş N, Özlük Y, Yeğen G, et al. Diffuse large B-cell non-hodgkin lymphoma involving multiple different organs in a young adult with 18F-FDG PET/CT. Mol Imaging Radionucl Ther. (2022) 31:72–4. doi: 10.4274/mirt.galenos.2021.28190
15. Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. (2014) 32:3059–68. doi: 10.1200/JCO.2013.54.8800
16. Ceriani L, Milan L, Pirosa MC, Martelli M, Ruberto T, Cascione L, et al. PET-based risk stratification in primary mediastinal B-cell lymphoma: A comparative analysis of different segmentation methods in the IELSG37 trial patient cohort. J Nucl Med. (2025) 66:209–14. doi: 10.2967/jnumed.124.268874
17. Ceriani L, Milan L, Johnson PWM, Martelli M, Presilla S, Giovanella L, et al. Baseline PET features to predict prognosis in primary mediastinal B cell lymphoma: a comparative analysis of different methods for measuring baseline metabolic tumour volume. Eur J Nucl Med Mol Imag. (2019) 46:1334–44. doi: 10.1007/s00259-019-04286-8
18. Le Dortz L, De Guibert S, Bayat S, Devillers A, Houot R, Rolland Y, et al. Diagnostic and prognostic impact of 18F-FDG PET/CT in follicular lymphoma. Eur J Nucl Med Mol Imag. (2010) 37:2307–14. doi: 10.1007/s00259-010-1539-5
19. Ngeow JYY, Quek RHH, Ng DCE, Hee SW, Tao M, Lim LC, et al. High SUV uptake on FDG-PET/CT predicts for an aggressive B-cell lymphoma in a prospective study of primary FDG-PET/CT staging in lymphoma. Ann Oncol. (2009) 20:1543–7. doi: 10.1093/annonc/mdp030
20. Rosenthal A and Younes A. High grade B-cell lymphoma with rearrangements of MYC and BCL2 and/or BCL6: Double hit and triple hit lymphomas and double expressing lymphoma. Blood Rev. (2017) 31:37–42. doi: 10.1016/j.blre.2016.09.004
21. Song T, Rong R, Su Y, Liang Z, and Lu J. Extranodal involvement of multiple organs in diffuse large B cell lymphoma detected on 18F-FDG PET/CT. Eur J Nucl Med Mol Imag. (2020) 47:734–5. doi: 10.1007/s00259-019-04666-0
22. Dhull VS, Sharma P, Singla S, Faizi NA, Thulkar S, Bal C, et al. Extensive extranodal involvement of rare sites in non hodgkin’s lymphoma detected on (18)F- FDG PET-CT: A case report. Nucl Med Mol Imag. (2013) 47:125–9. doi: 10.1007/s13139-012-0183-3
Keywords: [18F]FDG, case report, diffuse large B-cell lymphoma, extranodal, PET/CT
Citation: Liao T, Deng H, Long Y, He J, Li L, Xiao C and Li J (2025) [18F]FDG PET/CT detection and therapeutic response assessment of primary multisystem extranodal diffuse large B-cell lymphoma without lymph node involvement: a case report. Front. Oncol. 15:1697944. doi: 10.3389/fonc.2025.1697944
Received: 03 September 2025; Accepted: 02 December 2025; Revised: 30 November 2025;
Published: 15 December 2025.
Edited by:
Carmelo Caldarella, Fondazione Policlinico Universitario A. Gemelli IRCCS, ItalyReviewed by:
Nataliya Lutay, Skåne University Hospital, SwedenMarzieh Nejabat, MODUL University Vienna, Austria
Copyright © 2025 Liao, Deng, Long, He, Li, Xiao and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lingxiao Li, Mjc4MTM2MTEwQHFxLmNvbQ==; Cong Xiao, NDE5MjkzMjM0QHFxLmNvbQ==; Jun Li, OTg3MTI3NTE2QHFxLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Taiping Liao
Taiping Liao Huan Deng2†
Huan Deng2† Jun He
Jun He
