- Qingdao University, Department Thorac Surg, Affiliated Hospital, Qingdao, China
EGFR Exon 19 deletions and exon 21 point mutations of EGFR are the most prevalent alterations in lung adenocarcinoma, and patients with these mutations derive substantial clinical benefit from EGFR tyrosine kinase inhibitors (TKIs). Nevertheless, the therapeutic efficacy of TKIs in rare compound EGFR mutations remains unclear. Here, we describe a case of early-stage non-small cell lung cancer (NSCLC) harboring a G719C+S768I compound mutation that achieved complete remission following treatment with furmonertinib. These findings suggest that furmonertinib may represent a promising therapeutic option to improve cure rates in this subset of patients.
1 Introduction
The prevalence of epidermal growth factor receptor (EGFR) mutations in non-small cell lung cancer (NSCLC) is notably high, ranging from 30% to 60% in Asian populations (1). In 2021, researchers at MD Anderson Cancer Center published a landmark study in Nature, in which they proposed a novel classification of EGFR kinase domain mutations into four distinct subtypes: classical-like, T790M-like, PACC, and exon 20 insertions (2). Among these, PACC mutations constitute a distinct subset, accounting for approximately 12.5% of all EGFR mutations, and include point mutations such as G719X, S768I, and L858R.
At the 2024 World Conference on Lung Cancer (WCLC), the FURTHER study evaluated the efficacy and safety of furmonertinib monotherapy as first-line treatment in patients with EGFR PACC–mutant NSCLC. Patients were randomized into two groups to receive furmonertinib 160 mg QD or 240 mg QD. The results showed that the objective response rate (ORR) was 81.8% in the 240 mg group and 47.8% in the 160 mg group (3).
2 Case presentation
65-year-old Chinese man was admitted on April 16, 2025, with a four-month history of cough with sputum and chest discomfort, which had worsened over the previous two weeks. He had a 30-year smoking history but quit 10 years earlier, and a 30-year history of alcohol use, also discontinued 10 years earlier. His medical history included cerebral infarction 10 years prior, leaving mild mobility limitations, and a 10-year history of hypertension managed with sustained-release nifedipine. He reported no family history of hereditary disease or infection. Over the past four months, he experienced unexplained chest tightness accompanied by cough and white sputum.
The patient presented to the Department of Thoracic Surgery at our hospital. A chest CT performed on April 15, 2025, revealed a 3-cm mass in the left lower lobe (Figure 1). Bronchoscopy on April 16, 2025, showed obstruction of the bronchial orifice of the dorsal segment of the left lower lobe by a neoplasm, while the remaining bronchi were patent and unremarkable (Figure 2). A biopsy specimen was obtained and submitted for pathological examination.

Figure 1. (A) Cross-section before treatment. (B) Sagittal plane before treatment. (C) Coronal surface before treatment. (D) Mid-treatment cross-section. (E) Mid-treatment sagittal plane. (F) Mid-stage coronal surface treatment. (G) Preoperative cross-section. (H) Preoperative sagittal plane. (I) Preoperative coronal plane.
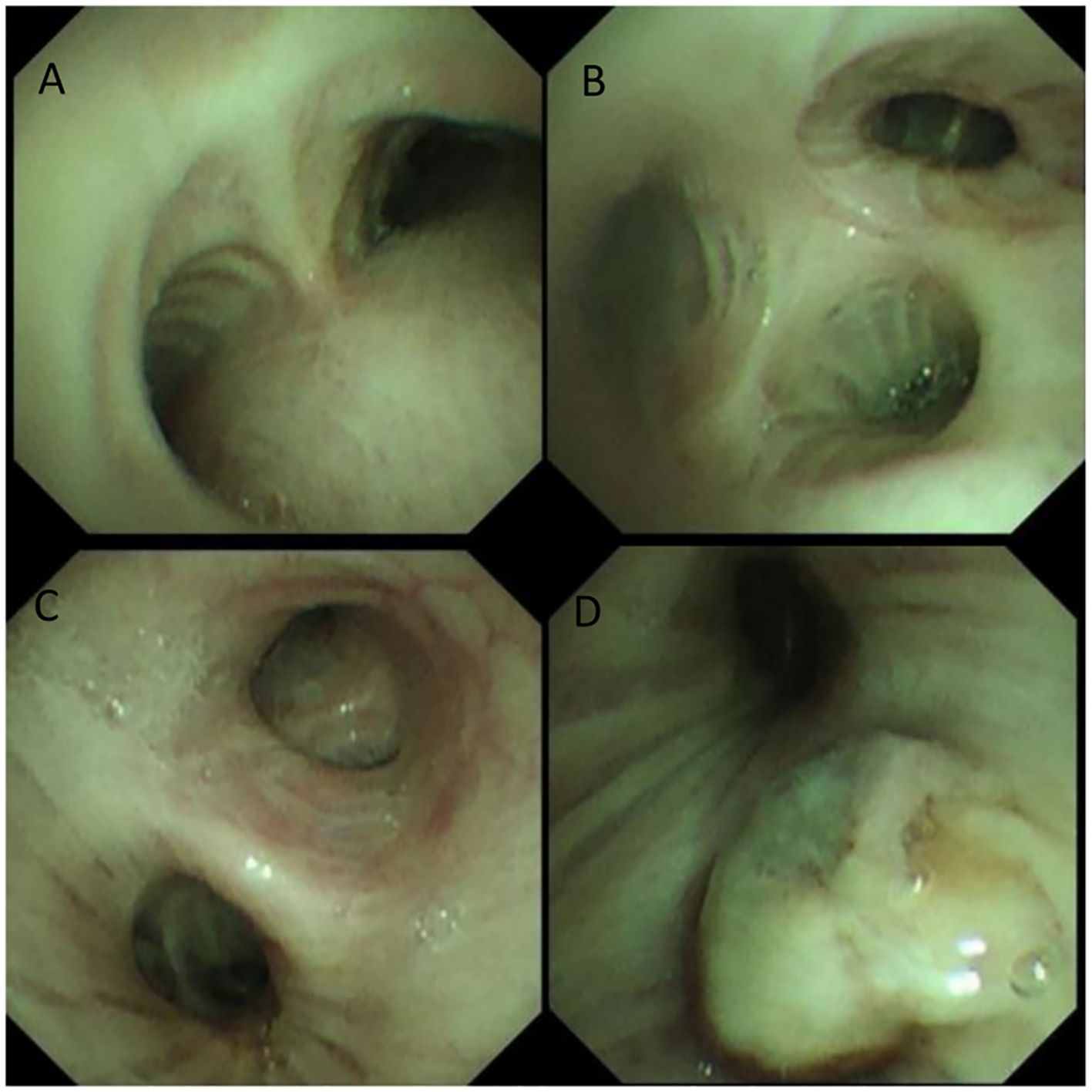
Figure 2. (A) tracheal carina (B) orifice of the right upper lobe bronchus (C) left main bronchus (D) orifice of the left lower lobe bronchus.
Pathology revealed non-small cell carcinoma with spindle and giant cell features. H&E–stained sections showed nested tumor growth with a high proportion of giant cells, abundant eosinophilic cytoplasm, variable nuclear size, irregular polygonal nuclear membranes, and increased nuclear-to-cytoplasmic ratio. Immunohistochemistry was positive for TTF-1, CK, CK5/6, GATA3, and Napsin A, and negative for P40, CgA, CD56, and Pax-8 (Figure 3).
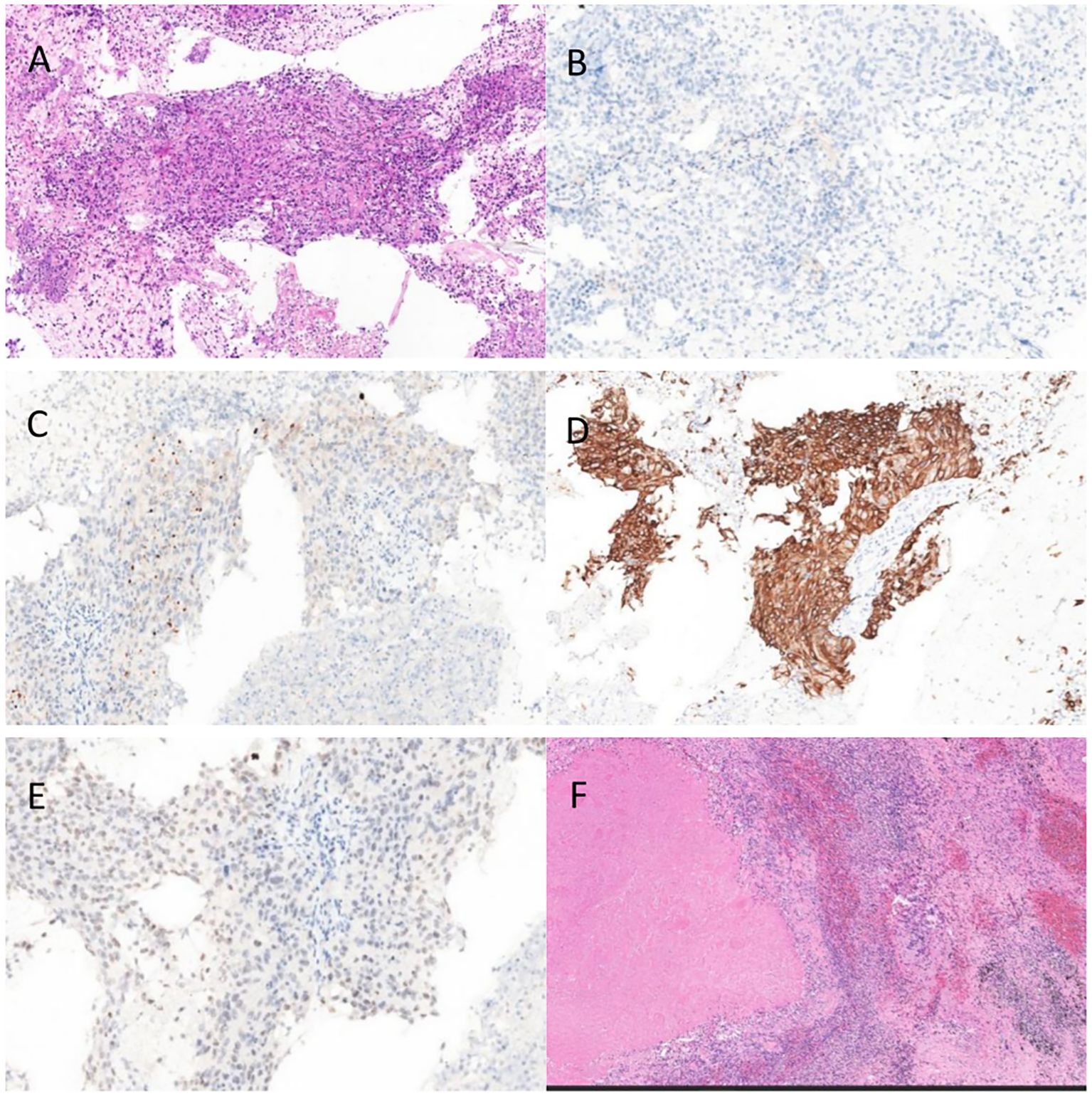
Figure 3. (A) Pre-EGFR TKI,HE (B) Pre-EGFR TKI P40 (C) Pre-EGFR TKI NaspinA (D) Pre-EGFR TKI CK5/6 (E) Pre-EGFR TKI TTF-1 (F) Post-EGFR TKI HE.
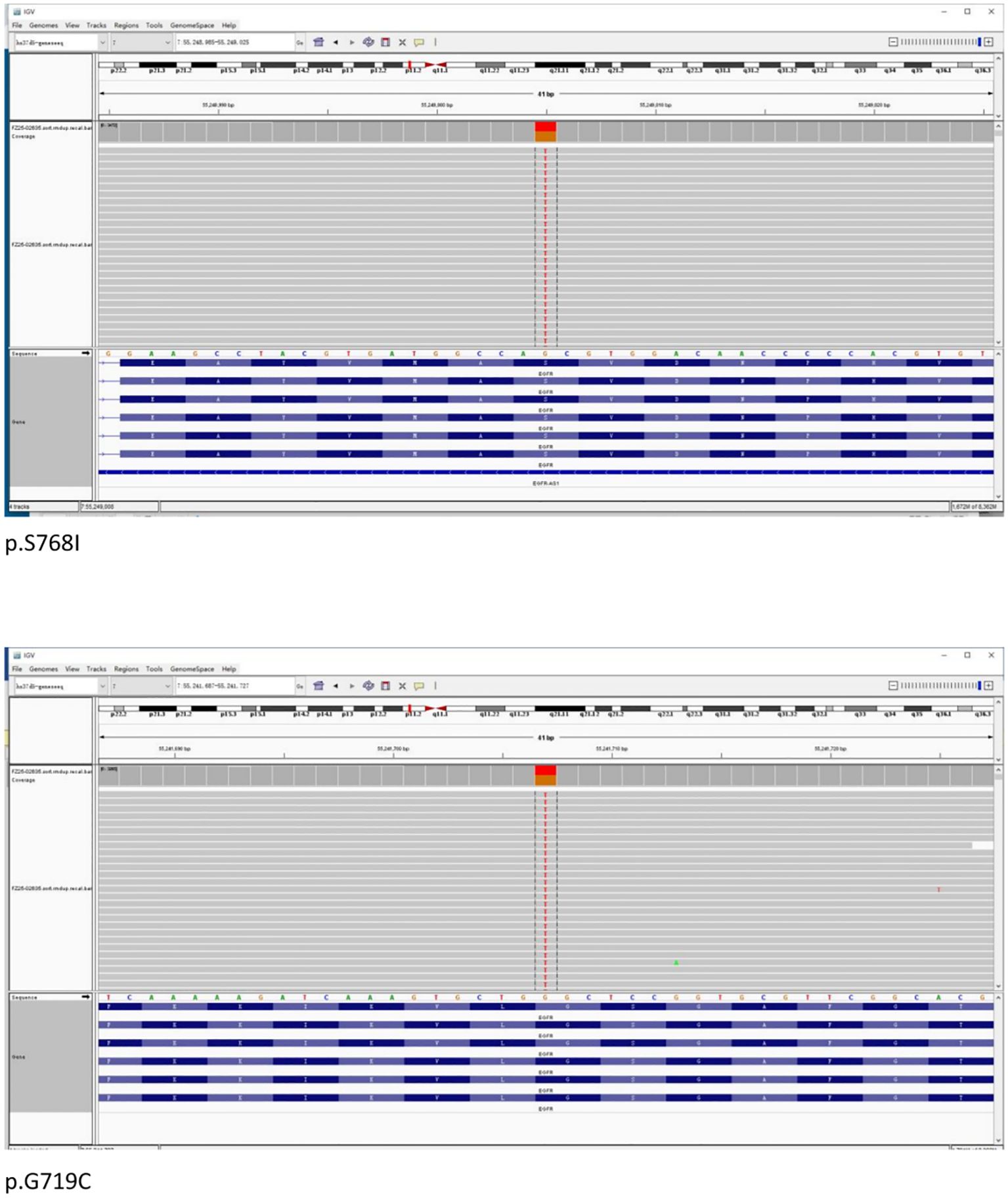
Formalin-fixed paraffin-embedded (FFPE) samples with an 80% tumor cell percentage were prepared from the patient’s left lung biopsy tissue under bronchoscopy for next-generation sequencing (NGS) based on targeted DNA. NGS identified an EGFR exon 18 G719C mutation with an allele frequency of 44.24% and an EGFR exon 20 S768I mutation with an allele frequency of 47.71%. No additional mutations were found in other key driver genes (Figure 4).
Cranial MRA revealed a suspected aneurysmal protrusion in the C5 segment of the right internal carotid artery. Electrocardiography showed atrial flutter with tachycardia. After multidisciplinary discussion, the patient was deemed unfit for surgery. Oral Furmonertinib 160 mg QD, and cardiology managed his cardiac condition.
The patient started oral furmonertinib mesylate (160 mg QD) on May 1, 2025. Follow-up on May 12 showed marked tumor shrinkage without adverse effects, and therapy was continued (Figure 1). By July 1, imaging demonstrated complete remission. After multidisciplinary discussion, the patient underwent thoracoscopic left lower lobectomy with systematic lymph node dissection. Pathology revealed no residual viable tumor cells, with 40% necrosis and 60% stroma (fibrogranulation, histiocytic infiltration, and calcification) (Figure 3F). All lymph nodes were negative for metastasis, confirming CPR.
3 Discussion
The EGFR S768I mutation is a point mutation in exon 20, accounting for approximately 1.5%–3% of all EGFR mutations. At codon 768, the AGC sequence is replaced by ATC, resulting in a serine-to-isoleucine substitution (S768I) (4). The G719X mutation affects codon 719, replacing glycine with alanine (G719A), cysteine (G719C), or serine (G719S) (5). Different G719X variants show variable sensitivity to EGFR inhibitors, with G719C reportedly more responsive to erlotinib than G719A (6). Among rare compound mutations, G719X+S768I is the most frequently observed combination (7, 8).
A database analysis of 693 patients with rare EGFR mutations showed that those with major uncommon mutations (G719X, L861Q, and S768I) achieved a 77% objective response rate (ORR) and a median response duration of 16.6 months with afatinib treatment (9).
Third-generation EGFR TKIs, such as Osimertinib, have been used to treat NSCLC with rare EGFR mutations (10), though their efficacy is generally lower than that of Afatinib. Ongoing global studies, including the FURTHER trial, are evaluating Furmonertinib in EGFR PACC–mutant NSCLC, showing promising results (3). Furmonertinib, a novel third-generation EGFR TKI, is approved in China as first-line therapy for advanced EGFR-mutant NSCLC (11). Several multicenter trials are underway, including a phase III randomized, double-blind study (NCT04853342) and a study in EGFR-mutant, PD-L1–positive advanced NSCLC (NCT05255406). However, data on Furmonertinib in patients with rare or compound EGFR mutations remain limited.
Pan reported a case of NSCLC with brain metastases in a patient harboring the EGFR G719X/S768I compound mutation. After 14 months of treatment with furmonertinib 80 mg daily, bilateral pulmonary lesions showed marked regression, and cranial metastatic lesions were reduced (12).
Among 31 NSCLC patients with rare EGFR mutations and CNS metastases, Furmonertinib yielded an overall response rate (ORR) of 38.7%, with 50.0% ORR in those with compound rare mutations (13). Patients with Rare exon 18 G719A and exon 21 L833V compound EGFR mutations achieved progression-free survival for 12.5 months after first-line treatment with Furmonertinib (14).
Preclinical studies indicate that NSCLC patients with rare compound EGFR mutations show variable sensitivity to different TKIs (15). Chiu et al. reported that patients with the G719X+S768I compound mutation had longer progression-free survival (PFS) on EGFR-TKI therapy than those with single mutations (16). G719X frequently occurs with other rare mutations, and these compound mutations generally yield better outcomes than G719X alone (17, 18).
We report a case of early-stage NSCLC with a G719C+S768I compound mutation that achieved complete remission with Furmonertinib. This case may offer valuable insights into managing NSCLC patients with this rare compound mutation.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: Contact the corresponding author for information.
Ethics statement
The studies involving humans were approved by Ethics Committee of the Affiliated Hospital of Qingdao University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
SL: Methodology, Writing – review & editing, Conceptualization, Writing – original draft. JZ: Data curation, Writing – original draft, Conceptualization. YD: Methodology, Writing – review & editing. YW: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declared that financial support was not received for this work and/or its publication.
Conflict of interest
The authors declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declared that generative AI was not used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Wu JY, Yu CJ, Chang YC, Yang CH, Shih JY, and Yang PC. Effectiveness of tyrosine kinase inhibitors on "uncommon" epidermal growth factor receptor mutations of unknown clinical significance in non-small cell lung cancer. Clin Cancer Res. (2011) 17:3812–21. doi: 10.1158/1078-0432.CCR-10-3408
2. Robichaux JP, Le X, Vijayan RSK, Hicks JK, Heeke S, Elamin YY, et al. Structure-based classification predicts drug response in EGFR-mutant NSCLC. Nature. (2021) 597:732–7. doi: 10.1038/s41586-021-03898-1
3. Le XN, Yu Y, Zhao Y, Planchard D, Cheng Y, Li X, et al. FURTHER: A global, randomized study of firmonertinib at two dose levels in TKI-naive, advanced NSCLC with EGFR PACC mutations. (2024).
4. Banno E, Togashi Y, Nakamura Y, Chiba M, Kobayashi Y, Hayashi H, et al. Sensitivities to various epidermal growth factor receptor-tyrosine kinase inhibitors of uncommon epidermal growth factor receptor mutations L861Q and S768I: What is the optimal epidermal growth factor receptor-tyrosine kinase inhibitor? Cancer Sci. (2016) 107:1134–40. doi: 10.1111/cas.12980
5. Li K, Yang M, Liang N, and Li S. Determining EGFR-TKI sensitivity of G719X and other uncommon EGFR mutations in non-small cell lung cancer: Perplexity and solution (Review). Oncol Rep. (2017) 37:1347–58. doi: 10.3892/or.2017.5409
6. Klughammer B, Brugger W, Cappuzzo F, Ciuleanu T, Mok T, Reck M, et al. Examining treatment outcomes with erlotinib in patients with advanced non-small cell lung cancer whose tumors harbor uncommon EGFR mutations. J Thorac Oncol. (2016) 11:545–55. doi: 10.1016/j.jtho.2015.12.107
7. Xu H, Yang G, Liu R, Yang Y, Li W, Li J, et al. EGFR uncommon alterations in advanced non-small cell lung cancer and structural insights into sensitivity to diverse tyrosine kinase inhibitors. Front Pharmacol. (2022) 13:976731. doi: 10.3389/fphar.2022.976731
8. Si J, Gu X, Wang W, Ying S, and Song Z. Clinical outcomes of lung adenocarcinoma patients harboring uncommon epidermal growth factor receptor (EGFR) mutations treated with EGFR-tyrosine kinase inhibitors (TKIs). Ann Palliat Med. (2022) 11:1624–34. doi: 10.21037/apm-21-2828
9. Yang JC, Schuler M, Popat S, Miura S, Heeke S, Park K, et al. Afatinib for the treatment of NSCLC harboring uncommon EGFR mutations: A database of 693 cases. J Thorac Oncol. (2020) 15:803–15. doi: 10.1016/j.jtho.2019.12.126
10. Pizzutilo EG, Agostara AG, Oresti S, Signorelli D, Stabile S, Lauricella C, et al. Activity of osimeRTInib in non-small-cell lung Cancer with UNcommon epidermal growth factor receptor mutations: retrospective Observational multicenter study (ARTICUNO). ESMO Open. (2024) 9:103592. doi: 10.1016/j.esmoop.2024.103592
11. Shi Y, Chen G, Wang X, Liu Y, Wu L, Hao Y, et al. Furmonertinib (AST2818) versus gefitinib as first-line therapy for Chinese patients with locally advanced or metastatic EGFR mutation-positive non-small-cell lung cancer (FURLONG): a multicenter, double-blind, randomized phase 3 study. Lancet Respir Med. (2022) 10:1019–28. doi: 10.1016/S2213-2600(22)00168-0
12. Pan X and Shi M. Successful therapy of a critically ill non-small cell lung cancer patient with compound mutations in EGFR G719X and S768I genes using furmonertinib: A case report. Heliyon. (2024) 10:e27106. doi: 10.1016/j.heliyon.2024.e27106
13. Xie Y, Fang H, Cheng W, Xu T, Xu S, Yu C, et al. Furmonertinib in uncommon EGFR-mutated non-small cell lung cancer with central nervous system metastases: A retrospective cohort study. Int J Cancer. (2025) 157:954–63. doi: 10.1002/ijc.35460
14. Yuejian, Zhao J, Wu T, and Zhang D. Rare exon 18 G719A and exon 21 L833V compound EGFR mutations show favorable response to Third-Generation TKI Furmonertinib: A case report and literature review. Invest New Drugs. (2025) 43:425–32. doi: 10.1007/s10637-025-01521-y
15. Suda K, Sakai K, Obata K, Ohara S, Fujino T, Koga T, et al. Inter- and intratumor heterogeneity of EGFR compound mutations in non-small cell lung cancers: analysis of five cases. Clin Lung Cancer. (2021) 22:e141–5. doi: 10.1016/j.cllc.2020.09.009
16. Chiu CH, Yang CT, Shih JY, Huang MS, Su WC, Lai RS, et al. Epidermal growth factor receptor tyrosine kinase inhibitor treatment response in advanced lung adenocarcinomas with G719X/L861Q/S768I mutations. J Thorac Oncol. (2015) 10:793–9. doi: 10.1097/JTO.0000000000000504
17. Yang JC, Sequist LV, Geater SL, Tsai CM, Mok TSK, Schuler M, et al. Clinical activity of afatinib in patients with advanced non-small-cell lung cancer harboring uncommon EGFR mutations: a combined post-hoc analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6. Lancet Oncol. (2015) 16:830–8. doi: 10.1016/S1470-2045(15)00026-1
Keywords: EGFR, EGFR Exon 18 G719C, EGFR exon 20 S768I, EGFR-TKI, furmonertinib, non-small cell lung cancer
Citation: Liu S, Zhang J, Dong Y and Wang Y (2026) Favorable response to third-generation TKI furmonertinib in a patient with early-stage non-small cell lung cancer harboring rare compound EGFR mutations: Exon 18 G719C and Exon 20 S768I — A Case Report. Front. Oncol. 15:1714432. doi: 10.3389/fonc.2025.1714432
Received: 27 September 2025; Accepted: 15 December 2025; Revised: 04 December 2025;
Published: 16 January 2026.
Edited by:
Carlos Gil Ferreira, Instituto Oncoclínicas, BrazilReviewed by:
Hongru Li, Fujian Medical University, ChinaMaxime Borgeaud, Hôpitaux Universitaires de Genève (HUG), Switzerland
Copyright © 2026 Liu, Zhang, Dong and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yongjie Wang, d3lqdGd6eUAxNjMuY29t
 Shihu Liu
Shihu Liu Yongjie Wang
Yongjie Wang