- 1Department of Thoracic Surgery, Tianjin First Central Hospital, Tianjin, China
- 2Nankai University School of Medicine, Tianjin, China
Background: Lung cancer is one of the leading causes of cancer incidence and mortality worldwide. At the molecular level, its development involves aberrations in multiple oncogenic pathways. Here, we report a rare case of lung adenocarcinoma with sarcomatoid differentiation and profound thrombocytosis (1,350 × 109/L), harboring concurrent TP53, JAK2 V617F, and MET exon 14 skipping mutations.
Case presentation: A 75-year-old female underwent VATS wedge resection for pathological stage IB lung adenocarcinoma. Platelet counts dropped from 1,350 to 572 × 109/L within 3 weeks postoperatively and remained stable during the 6-month follow-up period. No adjuvant therapy was administered. Molecular profiling of the resected tumor revealed concurrent TP53, JAK2, and MET mutations.
Conclusion: This case represents a rare coexistence of JAK2 V617F, MET, and TP53 mutations in lung adenocarcinoma. A marked postoperative decline in platelet count was observed without adjuvant therapy, suggesting a possible tumor-associated reactive thrombocytosis rather than a primary hematologic disorder. Recognition of such platelet dynamics may aid in the clinical assessment and follow-up of lung cancer patients.
Introduction
Reactive thrombocytosis in solid tumors is typically mediated by IL-6-driven thrombopoietin production. While JAK2 V617F mutation is well-established in myeloproliferative neoplasms (>95% polycythemia vera), its occurrence in non-hematopoietic malignancies is exceptionally rare. However, the biological significance of concurrent JAK2/TP53/MET alterations in NSCLC remains unexplored.
TP53 mutations are among the most frequent genetic alterations in lung cancer and are linked to genomic instability and aggressive tumor behavior (1, 2).The JAK2 p.V617F mutation is a gain-of-function alteration well recognized in myeloproliferative neoplasms (MPNs) but rarely detected in solid tumors. Its presence may cause constitutive activation of JAK-STAT signaling and excessive thrombopoiesis (3, 4). In classical MPNs, the mutation is detected in approximately 70% with essential thrombocythemia (5).Several signaling interactions, including PI3K/AKT/mTOR and MAPK, have been implicated in platelet production and inflammatory regulation, though their relevance in lung cancer remains unclear (6, 7).
Such dysregulated signaling may underlie paraneoplastic hematologic phenomena observed in solid tumors. In particular, thrombocytosis—a peripheral platelet count exceeding 450 × 109/L—is frequently reported in malignancies and is thought to reflect tumor-driven cytokine release, inflammation, and overall tumor burden (8).
Here, we report a case of lung adenocarcinoma with sarcomatoid differentiation and marked thrombocytosis harboring concurrent JAK2 V617F, TP53, and MET exon 14 skipping mutations, aiming to describe its clinical course and explore the potential association between tumor activity and platelet dynamics.
Case presentation
A 75-year-old female patient presented with a one-week history of cough and productive sputum with intermittent hemoptysis. Chest computed tomography (CT) demonstrated a patchy soft tissue density mass in the apicoposterior segment of the left upper lobe, measuring approximately 30.1×22.9 mm (Figure 1). The lesion exhibited ill-defined margins, spiculation, and partial ground-glass opacity, without evidence of lymphadenopathy or distant metastasis. The patient had no history of smoking, chronic inflammatory disease, autoimmune disorders, or bleeding diathesis.
On admission, physical examination was unremarkable. Laboratory tests revealed marked thrombocytosis, with a platelet count of 1,350 × 109/L (reference range: 120–350 × 109/L) and plateletcrit of 1.044%. Leukocytosis was noted with a total white blood cell count of 15.96 ×109/L and neutrophil count of 12.53 × 109/L, while lymphocytes, red blood cells, and hemoglobin remained within normal ranges. Serum tumor markers were as follows: alpha-fetoprotein (AFP), 8.51 ng/mL; carcinoembryonic antigen (CEA), 5.79 ng/mL; neuron-specific enolase (NSE), 34.40 ng/mL; and pro-gastrin-releasing peptide (ProGRP), 76.50 ng/mL. Common causes of thrombocytosis, including iron deficiency and inflammatory disorders, were excluded based on clinical and laboratory findings. However, no bone marrow biopsy or peripheral smear was performed.
The patient underwent video-assisted thoracoscopic wedge resection of the left upper lobe with systematic lymph node dissection. Intraoperative frozen section analysis revealed invasive non-mucinous adenocarcinoma, predominantly lepidic with a mixed acinar growth pattern. Foci of pleomorphic/spindle cell carcinoma were also identified, consistent with sarcomatoid carcinoma. Focal invasion of the visceral pleura was present. The pathological stage was pT2aN0M0 (stage IB).No adjuvant therapy was administered postoperatively. Postoperative molecular profiling (NGS) identified TP53 c.673-1G>T, JAK2 p.V617F, and MET exon 14 skipping mutations.
Three weeks after surgery, follow-up laboratory testing showed a reduction in platelet count to 572 × 109/L (Figure 2). Chest CT demonstrated interval shrinkage of the left upper lobe consolidation (Figure 1), and tumor markers normalized (CEA: 3.31 ng/mL; NSE: 12.40 ng/mL). The patient received hydroxyurea and enteric-coated aspirin for six months postoperatively, after which treatment was discontinued as the platelet count progressively declined and stabilized. No adjuvant chemotherapy or targeted therapy was administered. The patient has since remained under surveillance with no evidence of tumor recurrence.
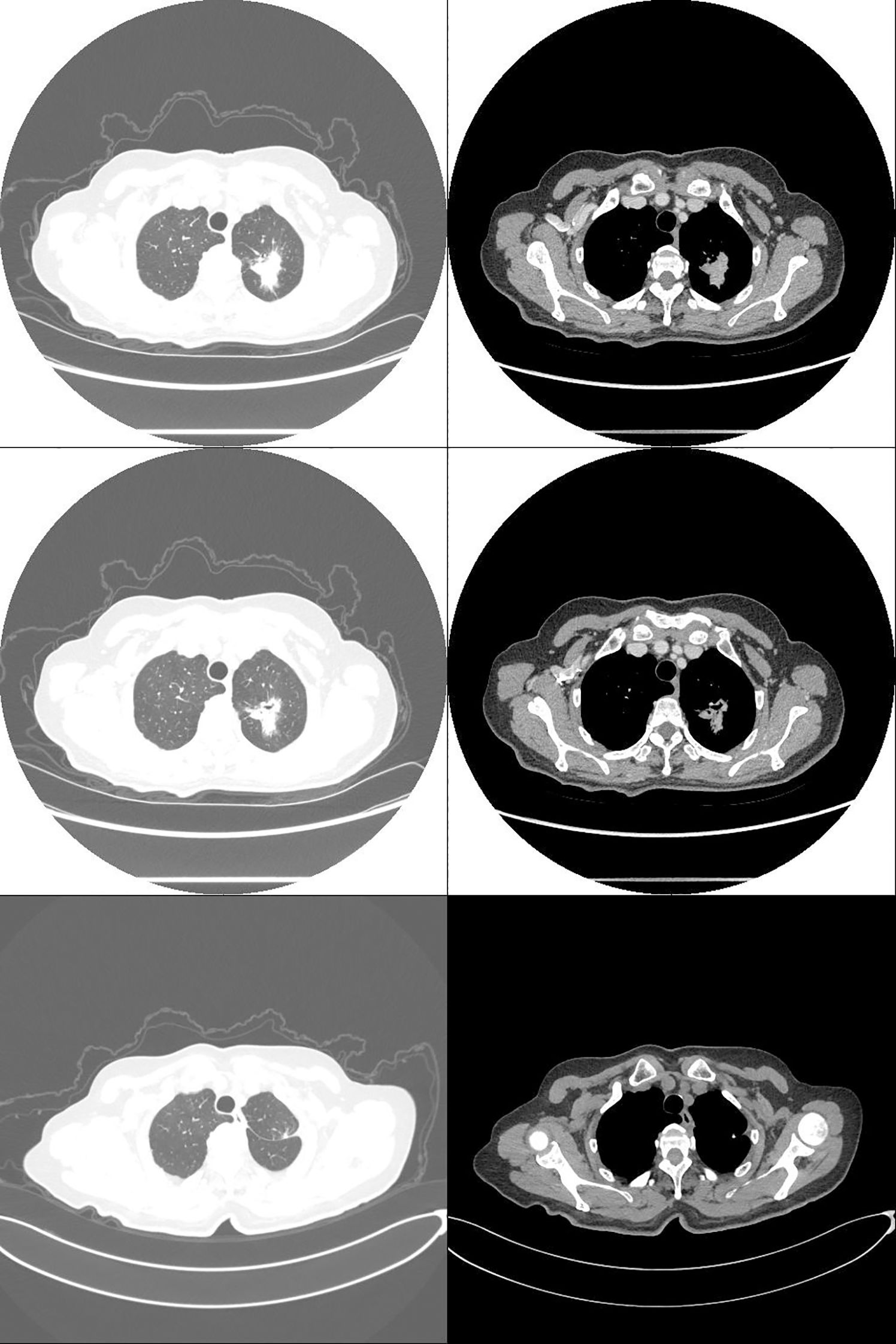
Figure 1. Computed tomography: non-small cell lung cancer (NSCLC) in the left upper lobe (pT2aN0M0, stage IB; 30.1 × 22.9 mm). Preoperative (top two rows) and postoperative (bottom row) images.

Figure 2. Dynamic changes in platelet counts (the red dashed line indicates the date of surgery on February 24, 2024).
Discussion
This report describes a rare case of lung adenocarcinoma with concurrent TP53, MET exon 14 skipping mutations and JAK2 V617F mutations, accompanied by marked preoperative thrombocytosis that decreased following tumor resection.
The TP53 c.673-1G>T splice-site alteration likely disrupted normal splicing and contributed to tumor progression (2). The marked preoperative thrombocytosis observed in this patient was most likely secondary to tumor-related inflammatory activation, potentially amplified by JAK2 signaling. The presence of a MET exon 14 skipping mutation—a known oncogenic driver frequently detected in pulmonary sarcomatoid carcinoma (PSC)—further supports an aggressive histologic subtype (9, 10). The postoperative decline in platelet count is consistent with a reactive process rather than a primary hematologic disorder. Dynamic platelet fluctuations in this context may serve as a clinical indicator—or “mirror”—of tumor activity, provided that other reactive causes such as iron deficiency, infection, or autoimmune disorders are excluded. This observation may also be considered in the context of tumor-educated platelets (TEPs), which have been associated with cancer-related thrombosis and inflammatory activation (11).
Given this mutational profile and the dynamic hematologic changes, several plausible mechanisms may be proposed to explain the observed thrombocytosis.
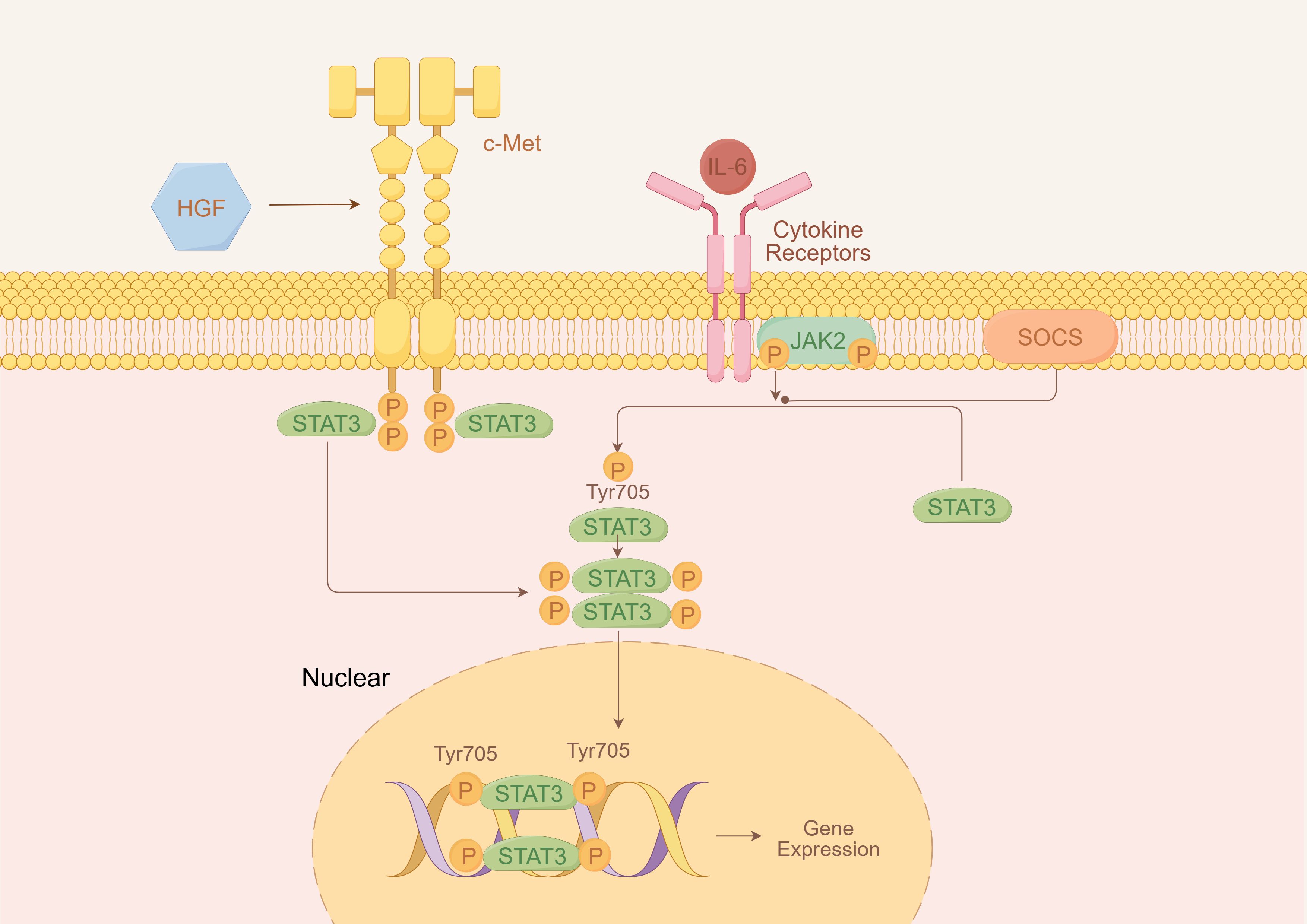
Among these, one plausible mechanism involves cytokine-mediated activation of the JAK/STAT pathway through interleukin-6 (IL-6) signaling. Notably, both TP53 and MET mutations have been reported to upregulate interleukin-6 (IL-6) expression (12, 13). IL-6, as a key upstream regulator of JAK/STAT signaling, can activate STAT3 and related downstream molecules, enhance thrombopoietin expression, and drive excessive myeloid proliferation, ultimately leading to secondary (reactive) thrombocytosis (14).
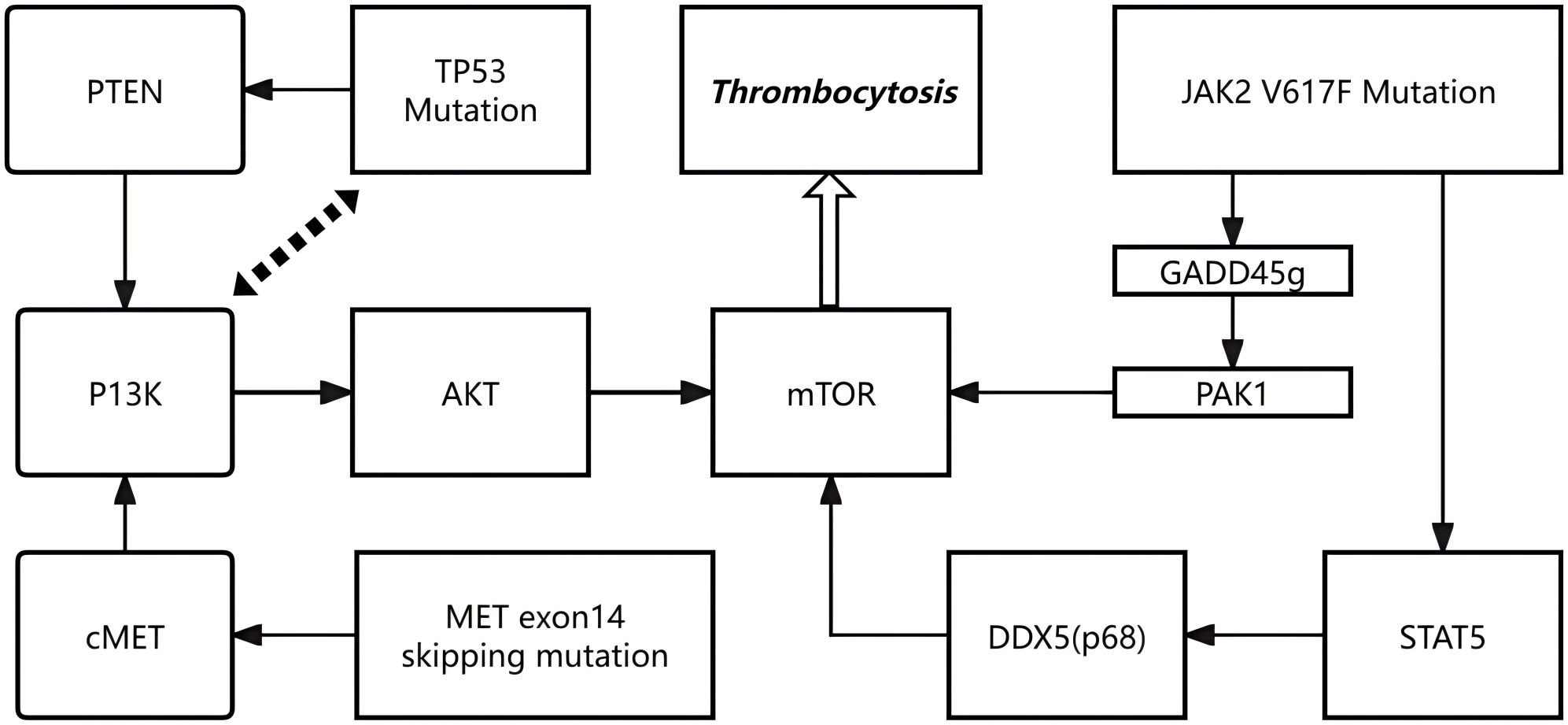
Beyond cytokine signaling, intracellular oncogenic pathways may also play an important role in promoting excessive megakaryopoiesis. Mutations in TP53, MET and JAK2 may aberrantly activate the PI3K/AKT/mTOR signaling cascade, thereby driving megakaryocytic expansion and thrombocytosis. TP53 mutations impair the tumor suppressor function of p53, weakening its negative regulatory influence on the PI3K/AKT/mTOR axis, for example through PTEN downregulation, which facilitates unchecked mTOR activation and enhances megakaryocyte proliferation and survival (15, 16). MET exon 14 skipping mutations stabilize the c-MET receptor by preventing its degradation, resulting in sustained activation of the PI3K/AKT/mTOR pathway and accelerated hematopoietic progenitor proliferation with megakaryocytic differentiation (10). The JAK2 V617F mutation exerts multifaceted effects: it suppresses GADD45g, releasing its inhibitory interaction with RAC2 and thereby activating PAK1–PI3K/AKT signaling (17); in addition, constitutive STAT5 activation promotes DDX5 (p68) expression, which maintains mTOR activity and amplifies cellular responsiveness to growth signals (18). Collectively, these mechanisms converge to sustain aberrant PI3K/AKT/mTOR activation, leading to excessive megakaryopoiesis and pathological thrombocytosis in patients carrying such mutations.
It is conceivable that the TP53 splice-site and MET exon 14 skipping mutations primarily contributed to tumorigenesis and cytokine dysregulation, whereas the JAK2 V617F mutation amplified inflammatory and thrombopoietic signaling. These combined alterations may have created a self-reinforcing loop in which tumor-derived cytokines activated the JAK/STAT pathway, while concurrent activation of PI3K/AKT/mTOR signaling promoted megakaryocytic proliferation and platelet production.
These findings suggest—but do not confirm—a possible interplay between tumor-associated oncogenic signaling and hematopoietic activation. The precise molecular mechanisms remain speculative in the absence of IL-6 quantification or phosphorylated STAT3 analysis, and further functional studies are warranted to validate these hypotheses.
Limitations of this report include the lack of hematologic confirmation by bone marrow biopsy and absence of cytokine measurements. Thus, the observed association should be interpreted as descriptive rather than causal. Despite these limitations, the postoperative drop in platelet count provides indirect clinical evidence supporting a reactive process secondary to tumor removal.
Figures 3, 4 are retained to conceptually illustrate potential signaling interactions; these hypotheses require further experimental validation.
Conclusion
This case illustrates a rare coexistence of JAK2 V617F, TP53, and MET exon 14 skipping mutations in NSCLC, associated with marked preoperative thrombocytosis that declined after surgery. Although a tumor-associated mechanism is plausible, further studies are needed to confirm this relationship and to determine whether platelet trends may serve as indicators of disease activity.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for this case report because it describes a single patient’s clinical course and treatment, without any experimental intervention or prospective data collection. Written informed consent for publication was obtained from the patient. The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from routine clinical diagnostic tests of the patient. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
Author contributions
LD: Writing – original draft. XL: Writing – review & editing, Supervision.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. National Natural Science Foundation of China (Grant No. 82372915, “Mechanistic study of hsa_circ_0030998 promoting non-small cell lung cancer progression and paclitaxel resistance through regulation of tumor-associated macrophage immune suppression via lactylation modification”).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Wang R, Pan S, and Song X. Research advances of EGFR-TP53 co-mutation in advanced non-small cell lung cancer. Zhongguo Fei Ai Za Zhi. (2022) 25:174–82. doi: 10.3779/j.issn.1009-3419.2022.101.06
2. Soussi T and Wiman KG. TP53: an oncogene in disguise. Cell Death Differ. (2015) 22:1239–49. doi: 10.1038/cdd.2015.53
3. Kralovics R, Passamonti F, Buser AS, Teo SS, Tiedt R, Passweg JR, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. (2005) 352:1779–90. doi: 10.1056/NEJMoa051113
4. Thomas SJ, Snowden JA, Zeidler MP, and Danson SJ. The role of JAK/STAT signalling in the pathogenesis, prognosis and treatment of solid tumours. Br J Cancer. (2015) 113:365–71. doi: 10.1038/bjc.2015.233
5. Greenfield G, McMullin MF, and Mills K. Molecular pathogenesis of the myeloproliferative neoplasms. J Hematol Oncol. (2021) 14:103. doi: 10.1186/s13045-021-01116-z
6. Zhang Y, Zhao Y, Liu Y, Zhang M, and Zhang J. New advances in the role of JAK2 V617F mutation in myeloproliferative neoplasms. Cancer. (2024) 130:4229–40. doi: 10.1002/cncr.35559
7. Blaquier JB and Recondo G. Non-small-cell lung cancer: how to manage MET exon 14 skipping mutant disease. Drugs Context. (2022) 11:2022–2–2. doi: 10.7573/dic.2022-2-2
8. Li SD, Ma M, Li H, Waluszko A, Sidorenko T, SChadt EE, et al. Cancer gene profiling in non-small cell lung cancers reveals activating mutations in JAK2 and JAK3 with therapeutic implications. Genome Med. (2017) 9:89. doi: 10.1186/s13073-017-0478-1
9. Gong C, Xiong H, Qin K, Wang J, Cheng Y, Zhao J, et al. MET alterations in advanced pulmonary sarcomatoid carcinoma. Front Oncol. (2022) 12:1017026. doi: 10.3389/fonc.2022.1017026
10. Organ SL and Tsao MS. An overview of the c-MET signaling pathway. Ther Adv Med Oncol. (2011) 3:S7–S19. doi: 10.1177/1758834011422556
11. Najafi S, Asemani Y, Majidpoor J, Mahmoudi R, Aghaei-Zarch SM, and Mortezaee K. Tumor-educated platelets. Clin Chim Acta. (2024) 552:117690. doi: 10.1016/j.cca.2023.117690
12. Huber A, Allam AH, Dijkstra C, Thiem S, Huynh J, Poh AR, et al. Mutant TP53 switches therapeutic vulnerability during gastric cancer progression within interleukin-6 family cytokines. Cell Rep. (2024) 43:114616. doi: 10.1016/j.celrep.2024.114616
13. Hov H, Tian E, Holien T, Holt RU, Våtsveen TK, Fagerli UM, et al. c-Met signaling promotes IL-6-induced myeloma cell proliferation. Eur J Haematol. (2009) 82:277–87. doi: 10.1111/j.1600-0609.2009.01212.x
14. Johnson DE, O’Keefe RA, and Grandis JR. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat Rev Clin Oncol. (2018) 15:234–48. doi: 10.1038/nrclinonc.2018.8
15. Stambolic V, MacPherson D, Sas D, Lin Y, Snow B, Jang Y, et al. Regulation of PTEN transcription by p53. Mol Cell. (2001) 8:317–25. doi: 10.1016/s1097-2765(01)00323-9
16. He Y, Sun MM, Zhang GG, Yang J, Chen KS, Xu WW, et al. Targeting PI3K/Akt signal transduction for cancer therapy. Signal Transduct Target Ther. (2021) 6:425. doi: 10.1038/s41392-021-00828-5
17. Zhang P, You N, Ding Y, Zhu W, Wang N, Xie Y, et al. Gadd45g insufficiency drives the pathogenesis of myeloproliferative neoplasms. Nat Commun. (2024) 15:2989. doi: 10.1038/s41467-024-47297-2
Keywords: lung adenocarcinoma, sarcomatoid carcinoma, TP53, JAK2, Met, reactive thrombocytosis
Citation: Deng L and Li X (2025) Rapid postoperative platelet decline in a lung adenocarcinoma with sarcomatoid differentiation and concomitant JAK2 V617F mutation: a case report. Front. Oncol. 15:1722712. doi: 10.3389/fonc.2025.1722712
Received: 11 October 2025; Accepted: 21 November 2025; Revised: 13 November 2025;
Published: 08 December 2025.
Edited by:
Mauricio Rodriguez-Dorantes, National Institute of Genomic Medicine (INMEGEN), MexicoReviewed by:
Vilma Araceli Maldonado Lagunas, National Institute of Genomic Medicine (INMEGEN), MexicoMiguel Morales, National Institute of Genomic Medicine (INMEGEN), Mexico
Copyright © 2025 Deng and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoping Li, aG9yYWNlbGlAbmFua2FpLmVkdS5jbg==
 Luojie Deng
Luojie Deng Xiaoping Li
Xiaoping Li