Abstract
Peritoneal recurrence is the most common form of recurrence after radical gastrectomy for gastric cancer (GC) and is the main cause of death. The median survival period is 3–6 months, and the 5-year survival rate is almost zero. So far, the treatment methods recommended by various clinical guidelines for peritoneal metastasis (PM) of GC are all palliative systemic chemotherapy (SC). However, the therapeutic effect is not ideal. One possible reason is the existence of the blood-peritoneal barrier, which makes it difficult for drugs to reach the peritoneal metastatic foci fully. Based on this, surgeons have gradually attempted the intraperitoneal administration mode in clinical practice. Hyperthermic intraperitoneal chemotherapy (HIPEC) improves the anti-tumor efficacy through the synergistic effect of heat and chemotherapy drugs and has achieved good therapeutic effects in the treatment of PM of GC. Raltitrexed is mainly used for intravenous administration and intraperitoneal chemotherapy for advanced colorectal cancer. HIPEC with raltitrexed for the treatment of PM after radical gastrectomy for GC is extremely rare. This article introduces a case of advanced GC with peritoneal recurrence and a large amount of ascites 11 months after radical gastrectomy. After 5 cycles of HIPEC with raltitrexed, a favorable therapeutic effect was achieved. The patient experienced significant clinical improvement, characterized by the resolution of ascites, alleviation of symptoms, and prolonged survival, thereby creating an opportunity for subsequent comprehensive treatment. It is hoped that this case can provide a treatment option for patients with peritoneal recurrence after radical gastrectomy for GC.
Introduction
Gastric cancer (GC) ranks among the most prevalent malignancies globally and is the fifth leading cause of cancer-related mortality (1). Its poor 5-year survival rate is primarily attributed to tumor progression and recurrence. Following radical resection, the peritoneum represents the most frequent site of metastasis in GC patients, particularly those with serosal infiltration or lymphatic involvement. Peritoneal metastasis (PM), occurring in 20%–30% of gastric cancer patients, arises predominantly from the exfoliation of free cancer cells within the abdominal cavity. The prognosis for PM is dismal, with a median overall survival of 3–6 months without treatment and no reported long-term survival (2, 3). Current clinical guidelines recommend only systemic palliative chemotherapy (pSC) and best supportive care as treatment options. However, outcomes with chemotherapy alone are suboptimal, largely due to the limited penetration of chemotherapeutic agents across the blood-peritoneal barrier to reach peritoneal implants (4).
Since the 1990s, the concepts of cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) have been developed to address the limitations of pSC. CRS involves the surgical removal of all macroscopically visible disease. HIPEC concurrently delivers heated, high-concentration chemotherapeutic agents directly into the peritoneal cavity. This approach sustains a thermotherapeutic effect and maximizes drug exposure to residual cancer cells, thereby enhancing antitumor efficacy. The combination of CRS and HIPEC is now established as the standard treatment for certain peritoneal surface malignancies (5, 6). Consequently, the integration of HIPEC with systemic chemotherapy is emerging as a potential strategy for both preventing and treating PM in various cancers. Raltitrexed is a specific thymidylate synthase inhibitor characterized by a prolonged plasma terminal half-life and demonstrated activity against gastrointestinal malignancies. This extended half-life positions raltitrexed as a promising candidate drug for intraperitoneal administration.
Case presentation
The timeline of the 76-year-old male patient in this study is shown in Figure 1.
Figure 1
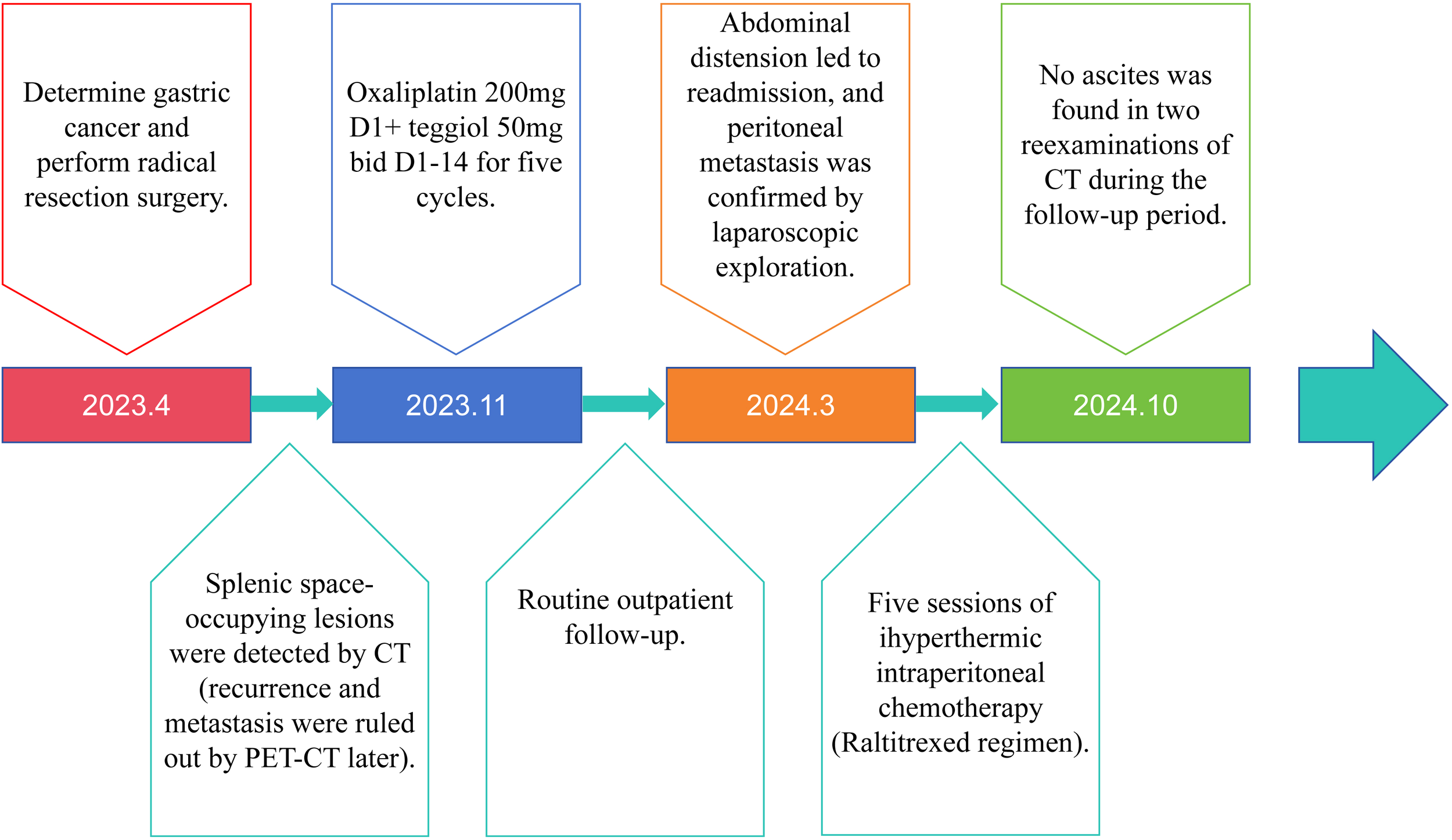
Timeline of patient clinical events.
On April 10, 2023, the patient was admitted to our hospital due to “abdominal fullness with dull pain and discomfort for 10 days”. Physical examination revealed mild upper abdominal tenderness only, with no other positive signs. Gastroscopy identified an ulcerated tumor in the gastric body. Pathological examination of the biopsy specimen confirmed poorly differentiated adenocarcinoma. Contrast-enhanced abdominal computed tomography (CT) demonstrated gastric carcinoma with regional lymphadenopathy. No significant abnormalities were detected on further auxiliary investigations. The initial diagnosis was gastric body adenocarcinoma (cT3N0M0, Stage II). Following a multidisciplinary team (MDT) discussion, surgical intervention was indicated. The patient underwent radical total gastrectomy under general anesthesia on April 13, 2023. Final histopathological staging revealed gastric body adenocarcinoma (pT4aN3bM0, Stage IIIC). The patient’s postoperative recovery was uneventful, and he was discharged. Adjuvant chemotherapy was subsequently initiated, consisting of six cycles administered on the following dates: May 29, 2023; June 28, 2023; July 27, 2023; August 31, 2023; October 14, 2023; and November 13, 2023. The regimen comprised: Oxaliplatin 200mg ivgtt D1+ S-1 50mg po bid D1-14. Notably, a surveillance CT scan performed during the fourth chemotherapy cycle (August 2023) revealed a splenic mass, suggestive of metastatic disease. However, a subsequent positron emission tomography-computed tomography (PET-CT) scan showed no evidence of tumor recurrence (Figure 2A).
Figure 2
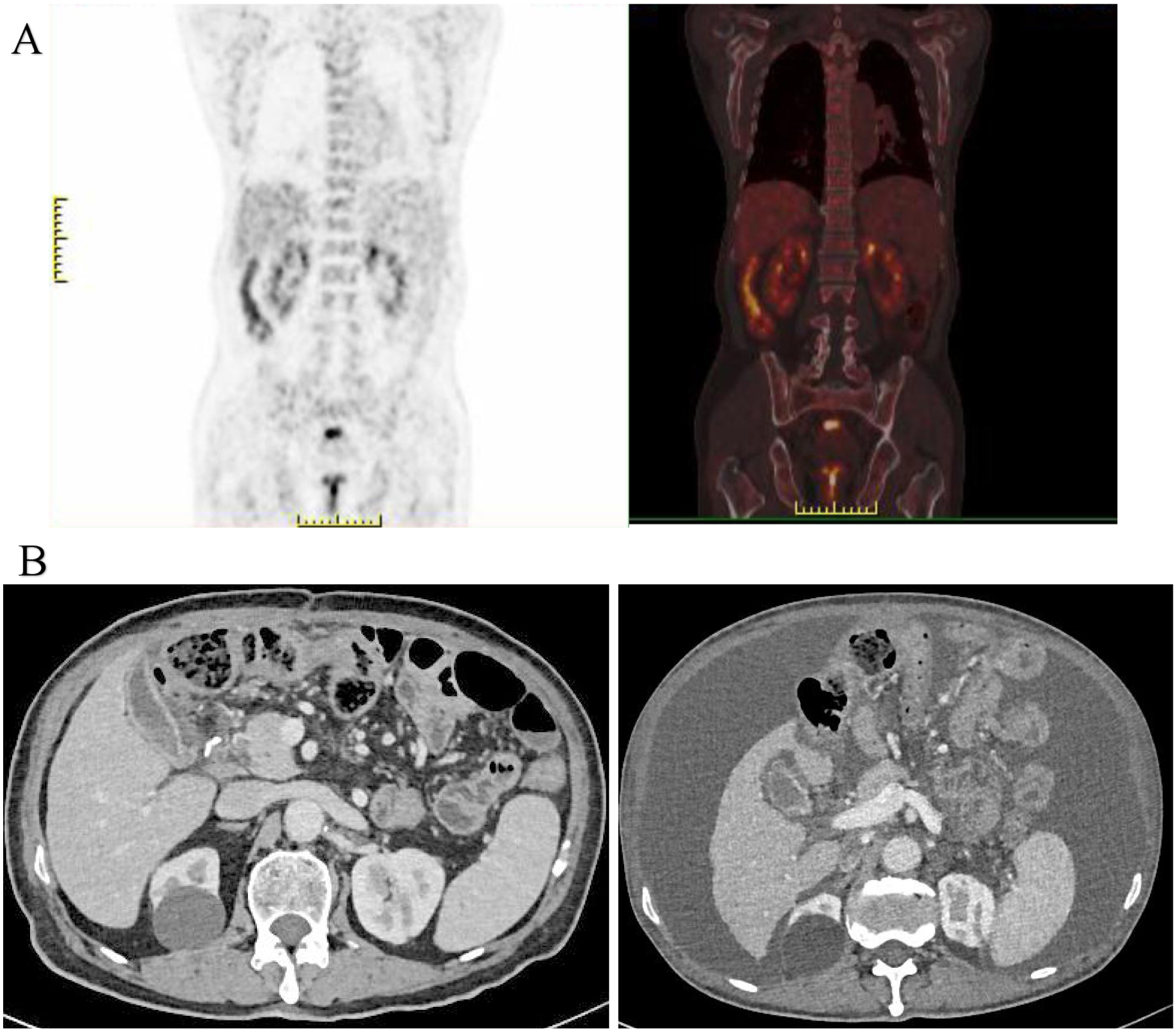
(A) PET-CT imaging demonstrated no findings suggestive of tumor recurrence; (B) Enhanced abdominal CT revealed a significant accumulation of fluid in the abdominal cavity compared to previous findings (August 2023).
Following regular outpatient surveillance, the patient was readmitted to the hospital due to “abdominal distension for 7 days” on March 12, 2024. Physical examination on admission showed a distended abdomen, frog-like abdomen, old surgical scars on the abdomen, soft and non-tender, and other abnormalities. Abnormal laboratory findings included: hemoglobin, 94g/L (normal 130-175g/L); Albumin, 30.9g/L (normal 40-55g/L); K, 2.77mmol/L (normal 3.5-5.5mmol/L); CA-125: 59.7U/ml (normal 0-30.2U/mL). Enhanced abdominal CT revealed a massive peritoneal fluid collection (Figure 2B). Due to the late stage of the tumor, the possibility of tumor recurrence was considered, and the exfoliative cytology of the tumor was performed twice; the results were negative. After the MDT discussion, laparoscopic exploration was recommended. The reasons included: First, after radical total gastrectomy, the stage was adenocarcinoma of the gastric body (pT4aN3bM0, III stage), and the recurrence rate was very high; Second, no malignant tumor cells were found in two exfoliative cytology examinations; Third, laparoscopic exploration can help to confirm whether it is recurrent gastric cancer and guide the follow-up strategy; Fourth, patients may benefit from HIPEC treatment of recurrent GC.
Consequently, laparoscopic exploration was performed on March 21, 2024. During the operation, it was found that the small intestine and its mesentery, the colon and its mesentery, and the pelvic peritoneum had multiple yellow-white nodules with tough texture. Part of the wall peritoneum was red, and a small amount of blood oozed. A large volume of clear yellow ascites was present within the abdominal and pelvic cavities (Figure 3). The white matter hard tissue was taken from the abdominal cavity and the top of the pelvic cavity and sent for intraoperative frozen. And the final pathological examination suggested adenocarcinoma. An intraperitoneal perfusion catheter system was placed for subsequent HIPEC. Postoperative HIPEC was administered on March 25, 26, 28, 30, and April 3, 2024. The procedure of hyperthermic intraperitoneal chemotherapy (HIPEC) was performed using the BR-TRG Body Cavity Hyperthermic Perfusion Therapy System (Bright Medical). Specifically, 4 mg of raltitrexed was added to 4000 mL of normal saline. The solution was heated by the circulation unit of the hyperthermic perfusion device and then infused into the abdominal cavity through four abdominal drainage tubes connected to the therapeutic perfusion circuit. During the treatment, approximately 1500-3500 mL of perfusate was maintained in the peritoneal cavity, with a flow rate controlled between 400 and 600 mL/min. The perfusion was continuously carried out at a constant temperature of 43 °C for 60 minutes. During the perfusion procedure, the patient reported mild abdominal distension. Following treatment, the patient developed grade 1 myelosuppression (as graded by CTCAE (Common Terminology Criteria for Adverse Events) v5.0), representing a mild adverse event, which improved after symptomatic management. An abdominal ultrasound performed on day 5 post-treatment revealed no significant fluid collection, and the drainage tubes were subsequently removed prior to hospital discharge.
Figure 3
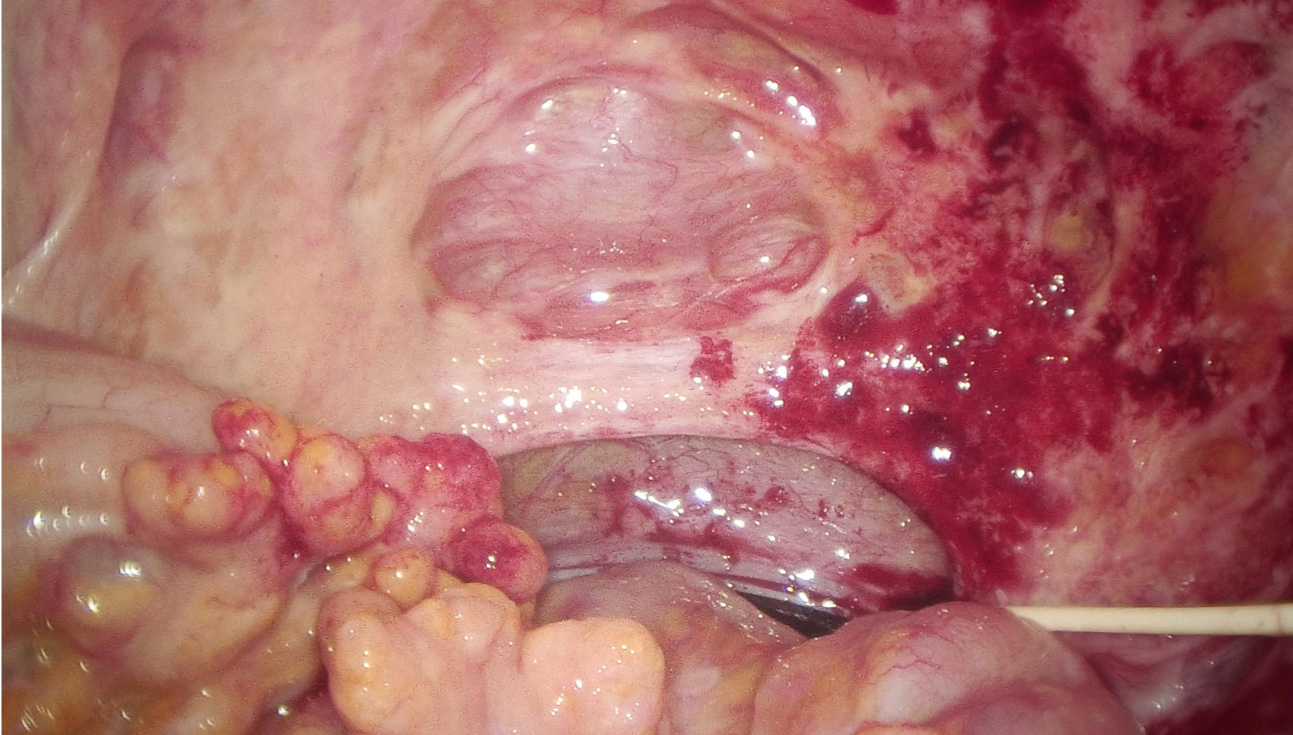
Laparoscopic exploration revealed recurrence of abdominal tumors.
Five days after the final HIPEC session, abdominal ultrasound revealed no residual ascites, and the perfusion catheters were removed before discharge. The patient was very satisfied with the treatment effect and would continue with systemic palliative chemotherapy in the oncology department subsequently. Surveillance abdominal CT scans performed in May and October 2024 showed no recurrence of ascites (Figure 4).
Figure 4
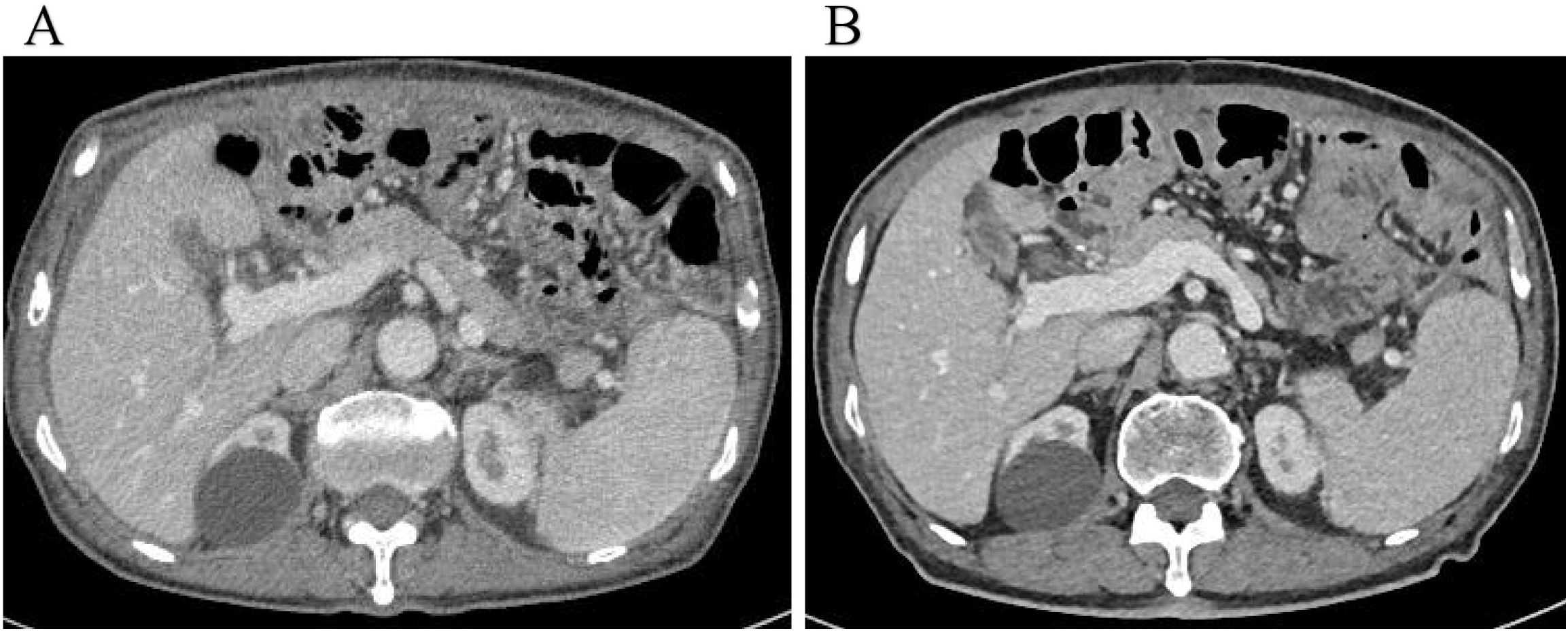
(A, B) Surveillance abdominal CT scans performed in May and October 2024 showed no recurrence of ascites.
Discussion
GC ranks as the fifth most common malignancy and leading cause of cancer-related mortality worldwide, with an overall 5-year survival rate of approximately 25%. Synchronous peritoneal metastases are present in up to 40% of patients at initial diagnosis (1, 4). Peritoneal recurrence following gastric cancer surgery carries a dismal prognosis, with a median survival not exceeding 6 months and a 5-year survival rate approaching zero (7). With ongoing research, initial recommendations for peritoneal dissemination of GC involved pSC or best supportive care. However, for selected patients, the latest NCCN guidelines suggest that CRS+HIPEC is also a viable option. A key advantage of CRS+HIPEC is its ability to overcome the peritoneal-plasma barrier, enabling effective drug delivery into the peritoneal cavity (8, 9). Current studies indicate that compared with pSC, CRS+HIPEC offers a significant survival advantage for patients with PM (10–12).
Research on treating advanced malignancies continues to advance. Evidence suggests that intraoperative lavage combined with perioperative intraperitoneal chemotherapy (IPC) effectively eliminates free cancer cells, with reported efficacy potentially 10 to 1000 times greater than intravenous chemotherapy (13). This regimen has been shown to significantly improve five-year survival rates. Furthermore, studies indicate a strong correlation between the plasma concentration of chemotherapeutic agents and their cytotoxic efficacy; specifically, an increase in intratumoral drug concentration at a critical time point can enable the elimination of a tenfold greater number of cancer cells (14).
While the efficacy of IPC is increasingly well-established, optimal agent selection remains contentious. Numerous drugs, including melphalan, 5-fluorouracil (5-FU), mitoxantrone, doxorubicin, and topotecan, have been administered intraperitoneally. In GC management, cisplatin, paclitaxel, 5-FU, and carboplatin are commonly utilized, with cisplatin being the most extensively studied agent based on comprehensive clinical data. Nevertheless, cisplatin’s substantial adverse effect profile poses significant clinical challenges. Moreover, prolonged use frequently induces resistance in most patients, leading to elevated recurrence rates. Compounding these issues, cisplatin’s relatively low molecular weight promotes rapid systemic absorption, potentially intensifying systemic toxicity (15, 16). Consequently, identifying optimal agents for IPC is paramount.
Peritoneal drug clearance is governed by both drug characteristics and peritoneal physiology. Generally, agents possessing higher molecular weights typically display lower lipophilicity, resulting in slower clearance from the peritoneal cavity. This pharmacokinetic profile extends local drug exposure, enhancing tumor tissue penetration and consequently augmenting cytotoxicity against malignant cells. Raltitrexed (molecular weight: 458), significantly larger than cisplatin or 5-FU, possesses an extended plasma half-life of 196 hours. This characteristic facilitates prolonged interaction with cancer cells without requiring sustained-release formulations. Additionally, raltitrexed is a specific, water-soluble inhibitor of thymidylate synthase. Its reduced folate metabolite undergoes active cellular transport via membrane carriers and is subsequently metabolized intracellularly to polyglutamate derivatives, which exhibit sustained intracellular activity. Collectively, these attributes—slower clearance, prolonged half-life, specific mechanism of action, and sustained intracellular activation—position raltitrexed as a highly promising IPC candidate. Emerging evidence suggests raltitrexed may offer advantages over 5-FU for IPC, supported by favorable safety profiles observed with its intraperitoneal administration (17). Raltitrexed also plays a significant role in other tumors. Studies have indicated that Raltitrexed can serve as a reliable option for chemotherapy in locally advanced or metastatic colorectal cancer, particularly for patients intolerant to 5-FU (18). Evidence also supports its reliability, efficacy, and safety in the intraperitoneal chemotherapy of colon cancer (19). Furthermore, Chen et al. demonstrated the efficacy and safety of Raltitrexed plus Oxaliplatin via hepatic arterial infusion chemotherapy combined with Apatinib as a second-line treatment in patients with advanced hepatocellular carcinoma and extrahepatic metastases (20). Similarly, Lu et al. confirmed that the aforementioned regimen could improve survival and safety outcomes in elderly patients with unresectable hepatocellular carcinoma (21).
While HIPEC with raltitrexed is primarily investigated for preventing PM post-radical gastrectomy, its application for treating established peritoneal recurrence in advanced GC remains unexplored. In this case, the patient received five HIPEC with raltitrexed cycles following peritoneal recurrence. Treatment resulted in significant clinical improvement: complete resolution of ascites, increased appetite, improved nutritional indices, and no ascites recurrence during 6 months of follow-up. This outcome substantially exceeds the expected median survival of 3–6 months for untreated PM, suggesting HIPEC with raltitrexed may offer meaningful disease control, symptom palliation, and survival prolongation in this setting. Study Limitations: This report describes a single patient experience. Consequently, the findings may lack broad generalizability, and validation through larger prospective studies is warranted.
Conclusion
Treatment options for postoperative peritoneal recurrence of advanced GC are limited. In this study, we present the case of a 76-year-old male patient with locally advanced GC that progressed to peritoneal carcinomatosis, who underwent five sessions of HIPEC with raltitrexed, which resulted in the complete disappearance of ascites. Notably, no recurrence of ascites was observed during the 6-month follow-up period, contributing to prolonged survival and improved quality of life. In conclusion, HIPEC with raltitrexed represents a viable therapeutic option for managing peritoneal recurrence in advanced GC and may achieve favorable clinical outcomes.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
Author contributions
WX: Investigation, Writing – original draft, Writing – review & editing, Data curation, Resources. LD: Investigation, Writing – review & editing. RM: Investigation, Writing – review & editing. KL: Writing – review & editing, Investigation, Writing – original draft.
Funding
The author(s) declared that financial support was not received for this work and/or its publication.
Conflict of interest
The author(s) declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declared that generative AI was not used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
HIPEC, Hyperthermic intraperitoneal chemotherapy; GC, gastric cancer; SC, systemic chemotherapy, PM, Peritoneal metastasis; pSC, systemic palliative chemotherapy; CRS, concepts of cytoreductive surgery; CT, computed tomography; MDT, multidisciplinary team; PET-CT, positron emission tomography-computed tomography; IPC, perioperative intraperitoneal chemotherapy; 5-FU, 5-fluorouracil;
References
1
Bray F Laversanne M Sung H Ferlay J Siegel RL Soerjomataram I et al . Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
2
Thomassen I van Gestel YR van Ramshorst B Luyer MD Bosscha K Nienhuijs SW et al . Peritoneal carcinomatosis of gastric origin: a population-based study on incidence, survival and risk factors. Int J Cancer. (2014) 134:622–8. doi: 10.1002/ijc.28373
3
Fujitani K Yang HK Mizusawa J Kim YW Terashima M Han SU et al . Gastrectomy plus chemotherapy versus chemotherapy alone for advanced gastric cancer with a single non-curable factor (REGATTA): a phase 3, randomised controlled trial. Lancet Oncol. (2016) 17:309–18. doi: 10.1016/S1470-2045(15)00553-7
4
Bang YJ Van Cutsem E Feyereislova A Chung HC Shen L Sawaki A et al . Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet (London England). (2010) 376:687–97. doi: 10.1016/S0140-6736(10)61121-X
5
Brenkman HJF Päeva M van Hillegersberg R Ruurda JP Haj Mohammad N . Prophylactic hyperthermic intraperitoneal chemotherapy (HIPEC) for gastric cancer-A systematic review. J Clin Med. (2019) 8. doi: 10.3390/jcm8101685
6
Cai Z Cai Z He T Zhao Z Yin Y Shen C et al . Comparative effectiveness of hyperthermic intraperitoneal chemotherapy for gastric cancer: A systematic review and network meta-analysis protocol. Medicine. (2018) 97:e11949. doi: 10.1097/MD.0000000000011949
7
Yonemura Y Canbay E Li Y Coccolini F Glehen O Sugarbaker PH et al . A comprehensive treatment for peritoneal metastases from gastric cancer with curative intent. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol. (2016) 42:1123–31. doi: 10.1016/j.ejso.2016.03.016
8
Ajani JA D'Amico TA Bentrem DJ Chao J Cooke D Corvera C et al . Gastric cancer, version 2.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Network: JNCCN. (2022) 20:167–92. doi: 10.6004/jnccn.2022.0008
9
Di Giorgio A Macrì A Ferracci F Robella M Visaloco M De Manzoni G et al . 10 years of pressurized intraperitoneal aerosol chemotherapy (PIPAC): A systematic review and meta-analysis. Cancers (Basel). (2023) 15. doi: 10.3390/cancers15041125
10
Martins M Santos-Sousa H Araújo F Nogueiro J Sousa-Pinto B . Impact of cytoreductive surgery with hyperthermic intraperitoneal chemotherapy in the treatment of gastric cancer with peritoneal carcinomatosis: A systematic review and meta-analysis. Ann Surg Oncol. (2022) 29:7528–37. doi: 10.1245/s10434-022-12312-7
11
Coccolini F Cotte E Glehen O Lotti M Poiasina E Catena F et al . Intraperitoneal chemotherapy in advanced gastric cancer. Meta-analysis of randomized trials. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol. (2014) 40:12–26. doi: 10.1016/j.ejso.2013.10.019
12
Liu YW Du Y Chen BA . Effect of hyperthermic intraperitoneal chemotherapy for gastric cancer patients: a meta-analysis of the randomized controlled trials. J Int Med Res. (2019) 47:5926–36. doi: 10.1177/0300060519882545
13
Hadi R Saunders V Utkina O Clingan P Kam P Links M et al . Review of patients with peritoneal Malignancy treated with peritonectomy and heated intraperitoneal chemotherapy. ANZ J Surg. (2006) 76:156–61. doi: 10.1111/j.1445-2197.2006.03579.x
14
Pohlen U Rieger H Kunick-Pohlen S Berger G Buhr HJ . Phase II study of regional chemotherapy using the hypoxic abdominal perfusion technique in advanced abdominal carcinoma. 5-FU pharmacokinetics, complications and outcome. Anticancer Res. (2007) 27:667–74.
15
Poon MAP O'Connell MJ Moertel CG Wieand HS Cullinan SA Everson LK et al . Biochemical modulation of fluorouracil: evidence of significant improvement of survival and quality of life in patients with advanced colorectal carcinoma. J Clin Oncol Off J Am Soc Clin Oncol. (1989) 7:1407–18. doi: 10.1200/JCO.1989.7.10.1407
16
Nordlinger B Sorbye H Glimelius B Poston GJ Schlag PM Rougier P et al . Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet (London England). (2008) 371:1007–16. doi: 10.1016/S0140-6736(08)60455-9
17
Zhao P Ding Z Tang L Zhou X . Preliminary investigation of intraperitoneal raltitrexed in patients with gastric cancer. World J Surg Oncol. (2014) 12:403. doi: 10.1186/1477-7819-12-403
18
Cheng Y Teng Z Zhang Y Jin B Zheng Z Man L et al . Irinotecan plus raltitrexed as second-line treatment in locally advanced or metastatic colorectal cancer patients: a prospective open-label, single-arm, multi-center, phase II study. BMC Cancer. (2024) 24:1082. doi: 10.1186/s12885-024-12831-4
19
Adnan K Hussain S Amir M Ahmed S Akbar A Jadoon SK et al . A clinical study of intraoperative perfusion chemotherapy with raltitrexed in colon cancer: A prospective cohort study. Cureus. (2024) 16:e58481. doi: 10.7759/cureus.58481
20
Chen S Wang X Yuan B Peng J Zhang Q Yu W et al . Apatinib plus hepatic arterial infusion of oxaliplatin and raltitrexed for hepatocellular carcinoma with extrahepatic metastasis: phase II trial. Nat Commun. (2024) 15:8857. doi: 10.1038/s41467-024-52700-z
21
Lu H Gao Y Xia X Fu Q Xiang D . RALOX-HAIC (raltitrexed + oxaliplatin) combined with lenvatinib improves survival and safety in elderly patients with unresectable hepatocellular carcinoma. BMC Cancer. (2025) 25:882. doi: 10.1186/s12885-025-14274-x
Summary
Keywords
case report, gastric cancer, hyperthermic intraperitoneal chemotherapy, peritoneal recurrence, raltitrexed
Citation
Xie W, Deng L, Mou R and Liu K (2026) Hyperthermic intraperitoneal chemotherapy with raltitrexed for peritoneal recurrence presenting with massive ascites following radical surgery for advanced gastric cancer: a case report. Front. Oncol. 16:1701376. doi: 10.3389/fonc.2026.1701376
Received
08 September 2025
Revised
02 January 2026
Accepted
26 January 2026
Published
17 February 2026
Volume
16 - 2026
Edited by
Zhen Liu, Zhejiang University, China
Reviewed by
Zu Ye, University of Chinese Academy of Sciences, China
Giorgio Ammerata, Magna Græcia University, Italy
Updates
Copyright
© 2026 Xie, Deng, Mou and Liu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kuan Liu, 464001503@qq.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.