- 1Mycobacterial Research Laboratory, University of Basel Children's Hospital, Basel, Switzerland
- 2Faculty of Medicine, University of Basel, Basel, Switzerland
- 3School of Health Professions, Zürich University of Applied Sciences, Winterthur, Switzerland
- 4Paediatric Infectious Diseases and Vaccinology Unit, University of Basel Children's Hospital, Basel, Switzerland
- 5UCL Great Ormond Street Institute of Child Health, University College London, London, United Kingdom
- 6Department of Paediatric Infectious Diseases and Immunology, Evelina London Children's Hospital, Guy's and St. Thomas' NHS Foundation Trust, London, United Kingdom
- 7Royal Children's Hospital Melbourne, Department of Paediatrics, University of Melbourne, Melbourne, VIC, Australia
Background: Interferon-gamma release assays (IGRA) are well-established immunodiagnostic tests for tuberculosis (TB) in adults. In children these tests are associated with higher rates of false-negative and indeterminate results. Age is presumed to be one factor influencing cytokine release and therefore test performance. The aim of this study was to systematically review factors associated with indeterminate IGRA results in pediatric patients.
Methods: Systematic literature review guided by the preferred reporting items for systematic reviews and meta-analyses (PRISMA) searching PubMed, EMBASE, and Web of Science. Studies reporting results of at least one commercially available IGRA (QuantiFERON-TB, T-SPOT.TB) in pediatric patient groups were included. Random effects meta-analysis was used to assess proportions of indeterminate IGRA results. Heterogeneity was assessed using the I2 value. Risk differences were calculated for studies comparing QuantiFERON-TB and T-SPOT.TB in the same study. Meta-regression was used to further explore the influence of study level variables on heterogeneity.
Results: Of 1,293 articles screened, 133 studies were included in the final analysis. These assessed QuantiFERON-TB only in 77.4% (103/133), QuantiFERON-TB and T-SPOT.TB in 15.8% (21/133), and T-SPOT.TB only in 6.8% (9/133) resulting in 155 datasets including 107,418 participants. Overall 4% of IGRA results were indeterminate, and T-SPOT.TB (0.03, 95% CI 0.02–0.05) and QuantiFERON-TB assays (0.05, 95% CI 0.04–0.06) showed similar proportions of indeterminate results; pooled risk difference was−0.01 (95% CI −0.03 to 0.00). Significant differences with lower proportions of indeterminate assays with T-SPOT.TB compared to QuantiFERON-TB were only seen in subgroup analyses of studies performed in Africa and in non-HIV-infected immunocompromised patients. Meta-regression confirmed lower proportions of indeterminate results for T-SPOT.TB compared to QuantiFERON-TB only among studies that reported results from non-HIV-infected immunocompromised patients (p < 0.001).
Conclusion: On average indeterminate IGRA results occur in 1 in 25 tests performed. Overall, there was no difference in the proportion of indeterminate results between both commercial assays. However, our findings suggest that in patients in Africa and/or patients with immunocompromising conditions other than HIV infection the T-SPOT.TB assay appears to produce fewer indeterminate results.
Introduction
Tuberculosis (TB) remains the leading cause of mortality by a single infectious agent, accounting for an estimated 1.6 million deaths worldwide. According to the latest report by the World Health Organization 10 million people are estimated to have developed TB disease in 2017 (1). However, the majority of individuals infected with Mycobacterium tuberculosis are asymptomatic and remain in a latent stage of infection. Data on infected individuals is not included in the World Health Organization TB report as TB infection is not a notifiable disease. Therefore, only estimates exist with one of the most recent estimates suggesting that in 2014 a total of 1.7 billion individuals, equivalent to 23% of the global population, had latent TB infection (2).
Progression from latent TB infection to active TB disease occurs in approximately one in ten adults. Children, however, progress more frequently to active TB and progression may be particularly rapid in the first 2 years of life (3–5). Early diagnosis and treatment are therefore key to reduce the burden of active TB in children.
Immuno-diagnostic tests are the main tools for the diagnosis of latent TB infection and both the tuberculin skin test (TST) and interferon-gamma release assays (IGRA) are used in the clinical setting (6, 7). The latter have been developed to overcome the limited specificity of the TST (8, 9). In adults the two commercially available IGRA, the QuantiFERON-TB and T-SPOT.TB—both existing in several test generations—have replaced the TST in many settings, primarily in an attempt to improve specificity (10).
In children, there is evidence that IGRA may have limited sensitivity and therefore the TST is still advocated by most experts (11–14). In addition, indeterminate IGRA results—due to either high interferon-γ background concentration in the negative control or low interferon-γ response in the positive control—have been shown to be more frequent in children compared to adults (15–18).
Underlying reasons for higher proportions of indeterminate IGRA results in children are largely speculative, but several contributing factors including age, concomitant infections and malnutrition have been postulated (18–20).
The aim of this study was to summarize the existing data on indeterminate IGRA results in children and determine key influencing variables.
Methods
Study Selection
A systematic literature search of studies reporting IGRA results in children was performed using PubMed, Embase, and Web of Science. Studies published until October 1st, 2018 were considered. The study was done according to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement (21) (Supplementary Material 1 PRISMA Checklist). The following search terms were used: (tuberculosis OR TB) AND [(((t-spot.tb) OR t-spot) OR quantiferon-tb) OR quantiferon] AND (children OR pediatric OR pediatric). The following inclusion criteria were used (i) patients in the pediatric age range with a mean or median age <18 years and a maximum upper age range of 24 years, (ii) results of at least one of the commercially available IGRA detailed (including a statement about indeterminate test results), (iii) publication in English, French, or German. Case reports, case series, conference abstracts and studies involving fewer than 10 participants, commentaries and reviews were excluded. The search and selection of included studies was done by MG, NM, and NR. In unclear cases a joint decision for inclusion or exclusion of the study was made.
Data Extraction
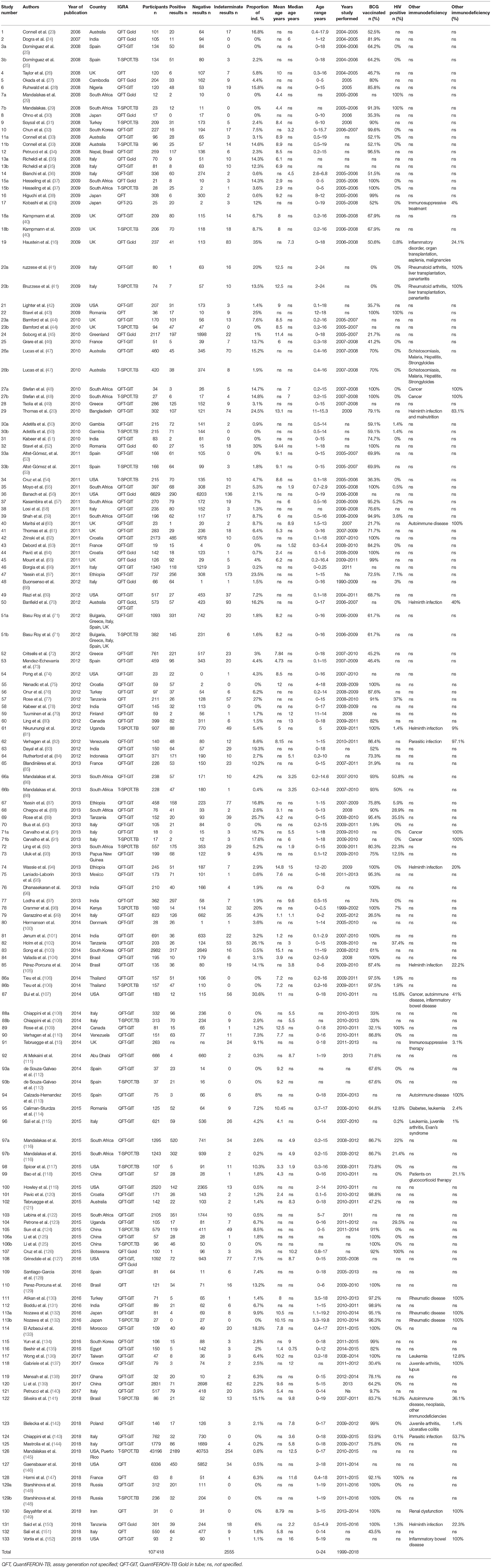
Data were extracted using a standard form including the following variables: year of publication, country in which the study was done, number of participants, mean or median age of participants, age range of participants, type of test performed, number of positive, negative and indeterminate results, definition of indeterminate result, Bacillus Calmette-Guérin (BCG) vaccination status, human immunodeficiency virus (HIV) infection status and information on other potential immunocompromising conditions (e.g., rheumatic diseases, cancer) and concomitant infections (e.g., helminth or other parasitic infections).
Statistical Analysis
The primary outcome was the proportion of indeterminate IGRA results, which was calculated as the number of indeterminate test results divided by the total number of valid test results. Stratified meta-analyses for proportions were performed using a random effects model and the DerSimonian and Laird method, with the estimate of heterogeneity taken from the inverse-variance fixed-effect model. Stratification variables comprised type of IGRA used (QuantiFERON-TB and T-SPOT.TB), age groups (0–7 ≥ 8 years), geographical location of the population under study (Africa, Australia, North America, South America, Asia, Europe) and immune status (HIV infection rate groups, and presence of other immunocompromising factors). Heterogeneity was determined using the I2 statistic.
In studies comparing both QuantiFERON-TB and T-SPOT.TB, additional stratified meta-analyses for risk differences were performed. Risk differences were defined as the difference in the proportion of indeterminate results between the two IGRA tests and were calculated according to Newcombe and Altman (22). For comparison of pooled risk difference, we applied the DerSimonian and Laird risk difference method. Study weight was indicated by using random effect models for the individual studies to account for the different study characteristics. For risk difference analysis stratification for age groups were done in two groups (0–7 ≥ 8 years) because of the limited number of available datasets.
To further explore potential sources of heterogeneity, we used meta-regression if I2 was higher than 30%. We considered the following variables as potentially explanatory in a multivariable model: type of IGRA used, age group, geographic location of the population under study, immune status (HIV or other immunocompromising conditions) were considered as explanatory variables in a multivariable model.
We used GraphPad Prism Version 7.02 (GraphPad Software, San Diego, CA, USA) and Stata Version 15.1 (StataCorp, College Station, TX, USA) to generate figures and perform meta-analyses. We reported estimated effect sizes with corresponding 95% confidence intervals (95% CI). A p < 0.05 was considered statistically significant.
Results
Demographical Data of the Studies Included
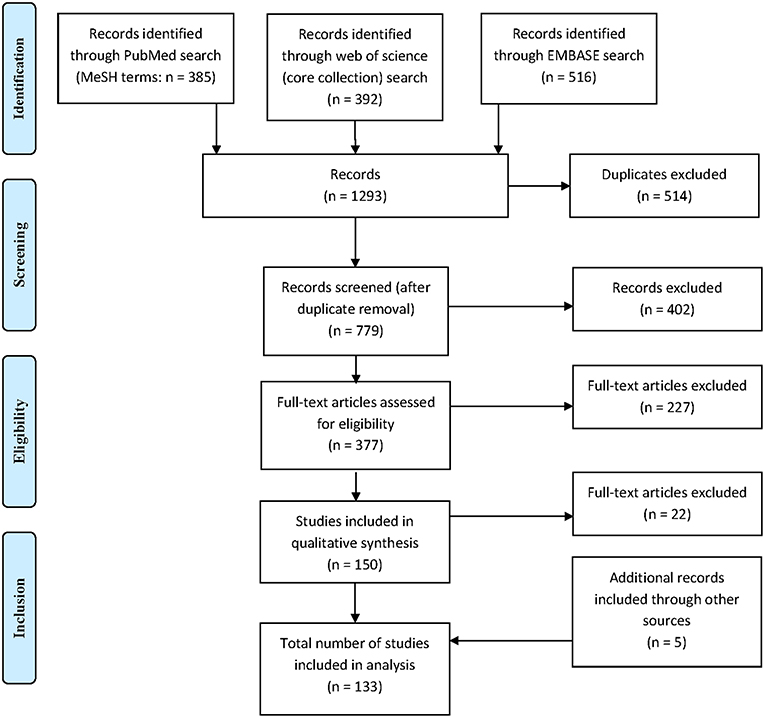
A total of 1,293 citations were identified, of which 379 publications were eligible for full-text assessment and 133 (5 of which were found through additional sources) were included in the final analysis (Figure 1). As 21 publications included data on both QuantiFERON-TB and T-SPOT.TB and one study included data on two different QuantiFERON-TB tests a total of 155 datasets were generated. Table 1 provides an overview of the studies included and summarizes their key characteristics.
The 155 datasets included a total of 107,418 participants with a median number of participants of 166 (range 12–43,196) per dataset. The mean or median age was specified in 69% (107/155) of datasets and reported to be 7.6 and 6 years, respectively. Upper age range was 18 years in 87.2% (116/133), 19 years in 5.3% (7/133), 24 years in 2.3% (3/133), and not specified in 5.3% (7/133) of studies. The studies were done in 45 countries with 36.8% (49/133) in Europe, 21.8% (29/133) in Asia, 20.3% (27/133) in Africa, 11.3% (15/133) in North America, 4.5% (6/133) in Australia, 4.5% (6/133) in South America, and 0.75% (1/133) recruited children in two continents (Asia and South America).
The BCG vaccination rates were reported in 80% (124/155) of datasets and varied from 0 to 100% with a median of 82%. HIV infection rates were reported in 49% (76/155) and varied from 0 to 100% with the median infection rate of 0.05%.
In 33 datasets additional information on immunocompromising or other factors potentially influencing IGRA results was reported: rheumatic or autoimmune diseases in 12.3% (19/155), various forms of cancer in 4.5% (7/155), and parasitic infections in 6.5% (10/155) of datasets. The range of participants included with additional factors varied from 1 to 100% with a median of 83.1% (not specified in 2 datasets).
Definition of Indeterminate Results of Interferon-Gamma Release Assays
A definition for indeterminate results was included in 88% (117/133) of studies with definitions provided for QuantiFERON-TB in 85.7% (108/126) and for T-SPOT.TB in 96.7% (29/30) of datasets. Of those that included a definition for indeterminate results most datasets 49.7% (77/155) simply stated to have used the manufacturers' definition [QuantiFERON-TB 47.6% (60/126) and T-SPOT.TB 56.7% (17/30)]. Further to this for the definition of indeterminate results in the QuantiFERON-TB assay three studies used their own definitions for failed nil controls [nil tube interferon-γ concentration of > 0.7 IU/ml (56) and > 2.0 IU/ml (63, 147), respectively]; five studies stated presence of high background response without reporting specific values (23, 36, 47, 70, 74).
Definition of indeterminate results for the T-SPOT.TB most commonly referred to low mitogen and/or high nil responses in combination with negative antigen response without stating specific values. Some studies indicated the absolute number of spots as cut-offs, others defined the number of spots in relation to the nil and/or mitogen response. In four studies a nil control of more than 10 spots was considered indeterminate, as opposed to the manufacturer's definition of ≥ 6 spots (92, 98, 112, 145).
Type of Interferon-Gamma Release Assays
Of the 133 studies, 77.4% (103/133) assessed QFT only, 15.8% (21/133) assessed both QuantiFERON-TB and T-SPOT.TB, and 6.8% (9/133) assessed T-SPOT.TB only. The proportions of indeterminate results ranged from 0 to 35% in the included studies. The overall pooled effect size (equivalent to the pooled proportion of indeterminate results) was 0.04 (95% CI 0.03–0.05, I2 = 96.32%) for both IGRAs combined.
QuantiFERON-TB was used in 124 studies including 57,183 participants. The pooled proportion of indeterminate results of QuantiFERON-TB was 0.05 (95% CI 0.04–0.06, I2 = 96.06%) (Figure 2). T-SPOT.TB was analyzed in 30 studies including 50,235 participants. The pooled proportion of indeterminate results of T-SPOT.TB was 0.03 (95% CI 0.02–0.05, I2 = 95.02%).
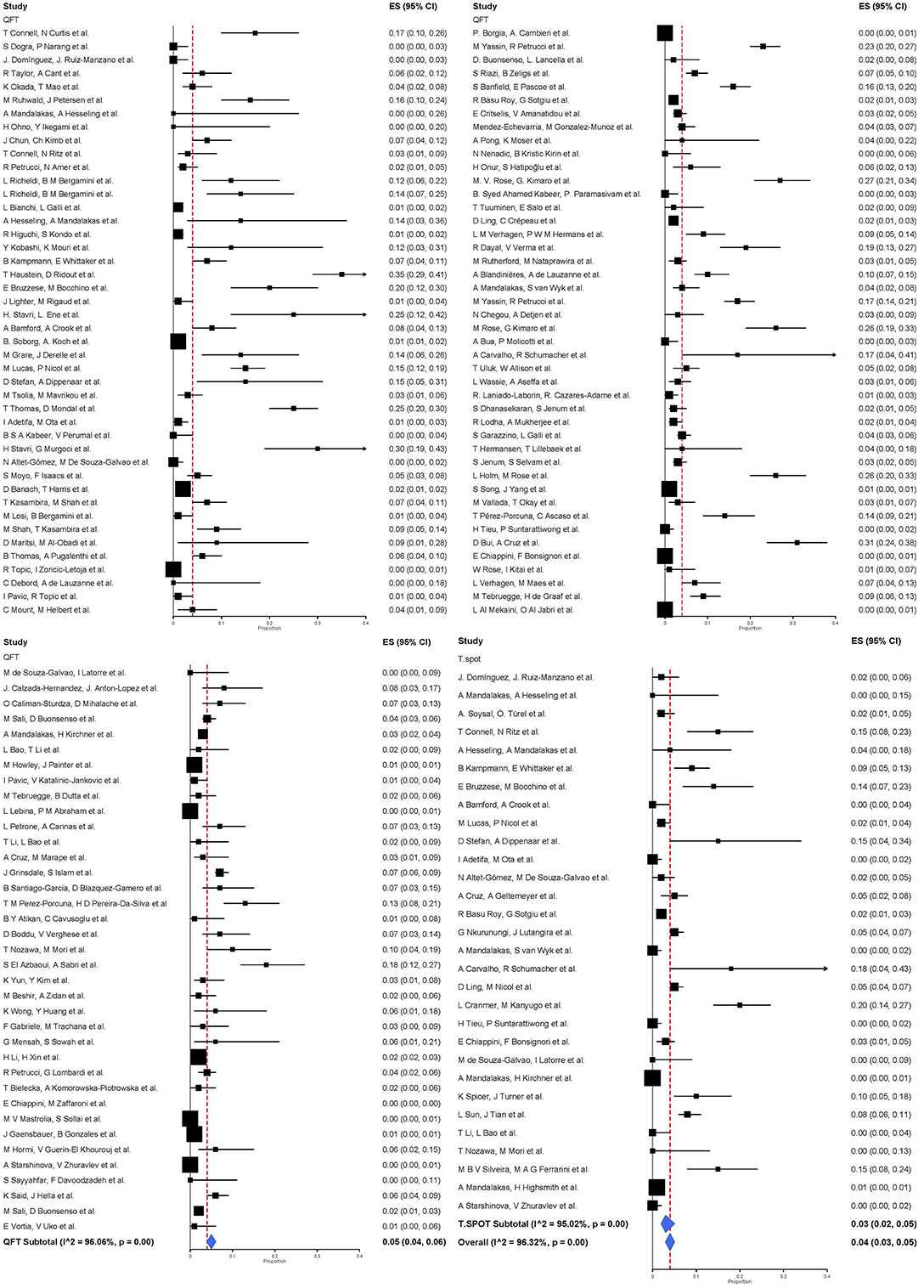
Figure 2. Proportion of indeterminate results with 95% CI by type of IGRA. Studies arranged according to year of publication.
A total of 21 studies assessed QuantiFERON-TB and T-SPOT.TB in the same study which allowed calculation of risk differences for the proportion of indeterminate results. The pooled proportion of indeterminate results was lower for T-SPOT.TB compared to QuantiFERON-TB (risk difference −0.01, 95% CI −0.03 to −0.00, I2 = 87.7%), but did not reach statistical significance (Figure 3).
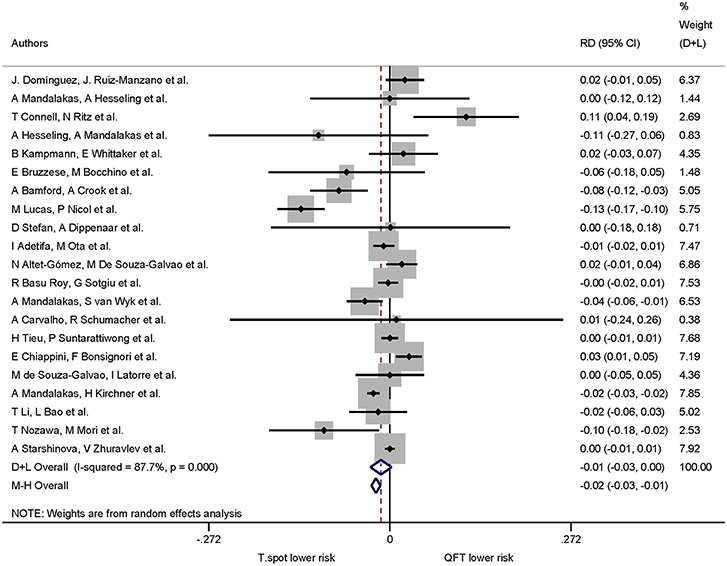
Figure 3. Risk difference (RD) with 95% CI in studies that included a head-to-head comparison of QuantiFERON-TB and T-SPOT.TB assays. Studies arranged according to year of publication.
Indeterminate Results According to Age
The mean or median age was specified in 108 datasets; of those 55 datasets had median or mean ages 0–7 years 52 datasets had median or mean ages ≥8years. The pooled proportions of indeterminate results were 0.04 (95% CI 0.03–0.06, I2 = 94.46%) for the age group 0–7 years and 0.04 (95% CI 0.03–0.05, I2 = 95.19%) for ≥8years. For the 48 datasets in which the mean or median age was not specified the proportions of indeterminate results were 0.05 (95% CI 0.03–0.06, I2 = 97.35%).
Of the 21 studies comparing both QuantiFERON-TB and T-SPOT.TB, 16 studies specified mean or median age. The pooled risk difference (negative values indicating lower risk of indeterminate results in the T-SPOT.TB) for 0–7 years was −0.01 (95% CI −0.03 to −0.01, I2 = 79.6%) and for ≥8 years −0.01 (95% CI −0.04 to −0.02, I2 = 76.7%). For studies which did not specify mean or median age the pooled risk difference was −0.03 (95% CI −0.10 to −0.05, I2 = 98.5%) (Figure 4). Risk differences within age groups for both assays were not statistically significant.
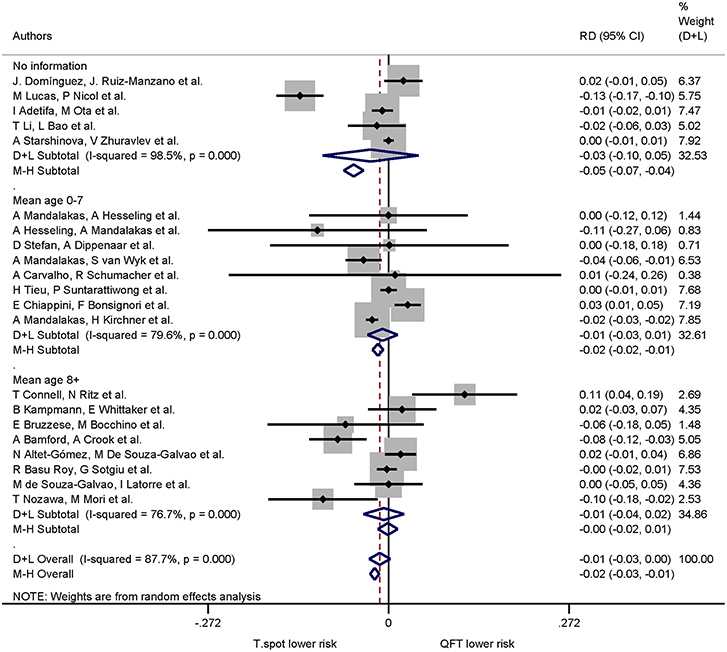
Figure 4. Risk differences (RD) with 95% CI in studies that included a head-to-head comparison of QuantiFERON-TB and T-SPOT.TB assays stratified by age. Studies sorted according to year of publication.
Indeterminate Results According to Geographical Location of the Study Population
A stratified analysis according to continents showed the following proportions for indeterminate IGRA results: Europe 0.03 (95% CI 0.02–0.05, I2 = 93.49%), Africa 0.07 (95% CI 0.04–0.10, I2 = 97.02%), Australia 0.08 (95% CI 0.04–0.14, I2 = 94.33%), Asia 0.03 (95% CI 0.01–0.04, I2 = 93.12%), North America 0.03 (95% CI 0.02–0.05, I2 = 97.48%), South America 0.09 (95% CI 0.06–0.14, I2 = 77.03%). One report with study sites in Asia and South America was excluded from this particular analysis, as the data could not be separated according to site of recruitment (34).
When continent of the study was included in the risk differences analysis the proportion of indeterminate results for T-SPOT.TB was significantly lower compared to QuantiFERON-TB in studies performed in Africa only (p < 0.001). The pooled risk difference for African studies was −0.022 (95% CI −0.032 to −0.011, I2 = 15.4%). Risk differences for studies performed on all other continents were not statistically significant (Figures 5, 6).
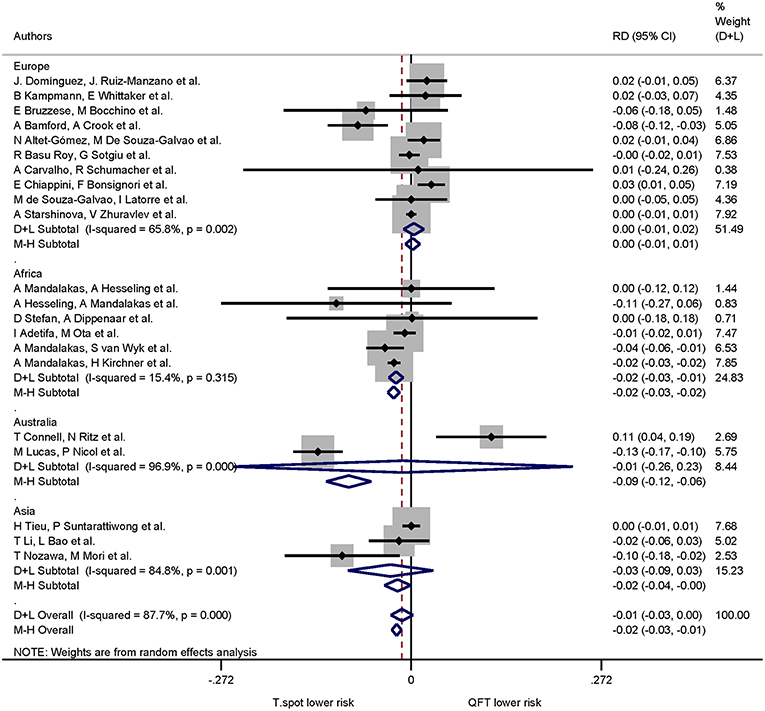
Figure 5. Risk differences (RD) with 95% CI in studies that included a head-to-head comparison of QuantiFERON-TB and T-SPOT.TB assays stratified by continent. Studies arranged according to year of publication.
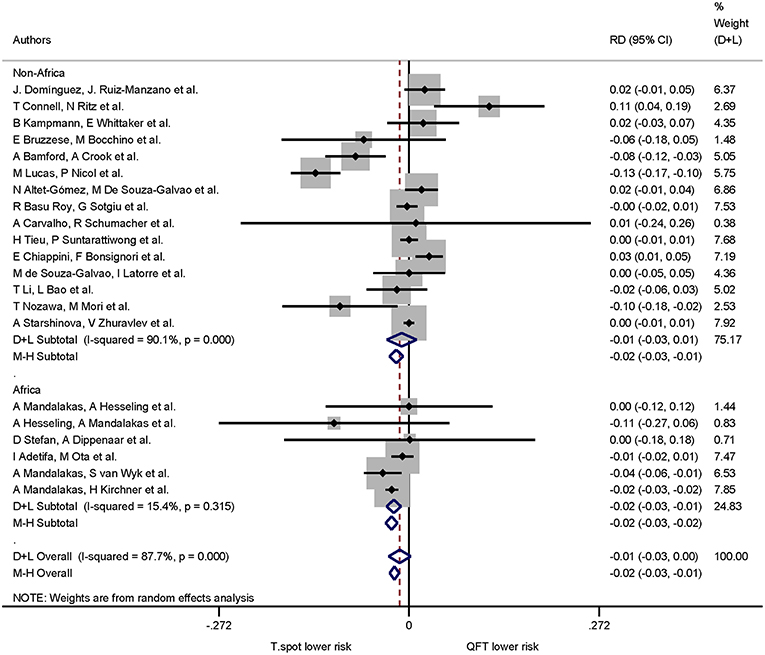
Figure 6. Risk differences (RD) with 95% CI in studies that included a head-to-head comparison of QuantiFERON-TB and T-SPOT.TB assays stratified by African/Non-African origin of the study. Studies arranged according to year of publication.
Indeterminate Results by Immune Status
The pooled proportion of indeterminate IGRA results for the 0% HIV+, 0 < 51% HIV+, 51–100% HIV+, immunocompromised/HIV− and no information were 0.03 (95% CI 0.02–0.05, I2 = 94.45%), 0.07 (95% CI 0.04–0.11, I2 = 97.70%), 0.03 (95% CI 0.01–0.05, I2 = 73.96%), 0.12 (95% CI 0.07–0.18, I2 = 47.12%), 0.03 (95% CI 0.03–0.04, I2 = 94.78%), respectively.
When immune status was included in the risk difference analysis of indeterminate results the T-SPOT.TB was associated with lower proportions of indeterminate results only in studies that included immunocompromised, HIV-uninfected participants: the pooled risk difference was −0.071(95% CI −0.133 to −0.010, I2 = 0.0%) and statistically significant (p = 0.022). The risk differences in the remaining groups were not statistically significant (Figure 7).
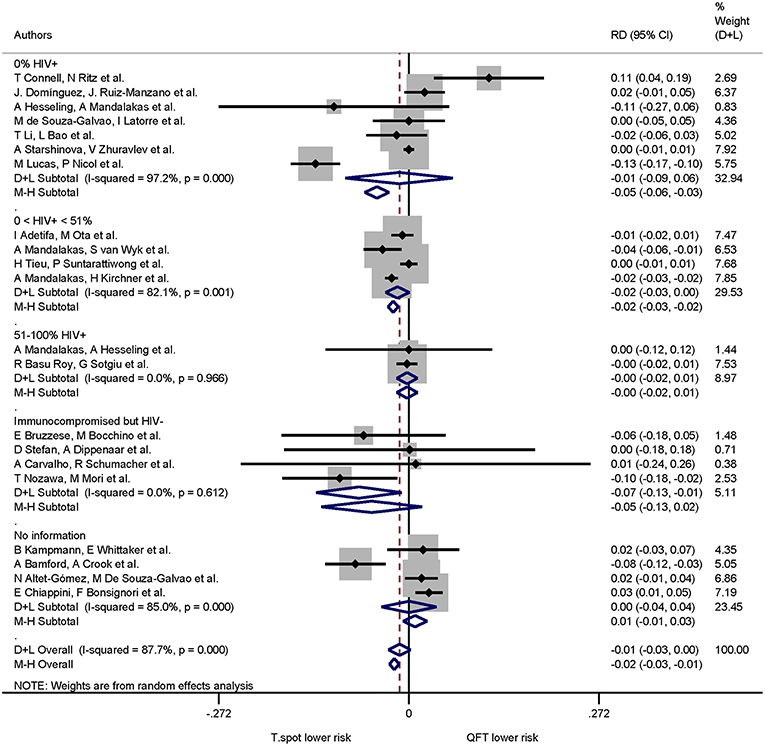
Figure 7. Risk differences (RD) with 95% CI in studies that included a head-to-head comparison of QuantiFERON-TB and T-SPOT.TB assays stratified by immune status. Studies arranged according to year of publication.
Meta-Regression of Indeterminate Results
Of the four variables in the model (type of IGRA, age group, continent where study was performed, immune status), only studies including non-HIV-infected immunocompromised patients had a statistically significant contribution to the heterogeneity in the multiple regression model (p = 0.0003).
Discussion
Indeterminate IGRA results have been reported shortly after introduction of these tests in routine clinical use. Despite this, analysis of indeterminate IGRA results has commonly been neglected in the literature, with those results either having been excluded from previous systematic literature reviews or only having been included in very limited subgroup analyses (11, 153, 154). To our knowledge, this systematic review is the first to comprehensively analyse the occurrence of indeterminate IGRA results in children and adolescents. We found that 4% of IGRA results are indeterminate, suggesting that 1 in 25 tests will not produce a conclusive result. The main factor associated with indeterminate results identified in this meta-analysis was the presence of an immunocompromising condition other than HIV infection.
In our analysis T-SPOT.TB assays were associated with a similar risk of indeterminate results compared to various generations of QuantiFERON-TB tests. T-SPOT.TB assays require lymphocyte adjustment which may reduce the risk of an indeterminate result particularly in patients with reduced lymphocyte count, such as HIV infection or immunocompromising conditions associated with lymphopenia. This assumption is confirmed by results from a meta-analysis including studies in adult HIV-infected patients showing that low CD4 cell counts increased indeterminate rates of QuantiFERON-TB but not of T-SPOT.TB assays (155). Our results contrast with another earlier meta-analysis by Diel et al that reported fewer indeterminate results for QFT-GIT (2.1%) compared to T-SPOT.TB (3.8%) (154). The authors concluded that the more demanding laboratory work for the T-SPOT.TB was likely the reason for higher indeterminate rates. However, their analysis predominately included studies in adults, did not include random effects models, and only included studies published until 2009.
Immunocompromising conditions, including HIV infection, have been identified in earlier studies as a major contributing factor to indeterminate results (16). A study by Oni et al. showed that HIV infection in adults increased the risk of indeterminate results, either through low positive control responses or high interferon-γ background concentrations in the negative control (156). In another study by Mandalakas et al. indeterminate results were more frequent in children infected with HIV than in HIV-uninfected children (116). The previously reported lower sensitivity of QuantiFERON-TB assays in HIV-infected individuals may be linked to a higher rate of indeterminate results, as the difference between the assays was negligible in a study after exclusion of indeterminate results in one analysis (157). Diel et al. reported in their meta-analysis that the rates of indeterminate results for QuantiFERON-TB and T-SPOT.TB assays were higher in immunocompromised compared to immunocompetent individuals, with 6.1 and 4.4%, respectively (154).
Further factors have been shown to influence IGRA results, particularly chronic rheumatic or auto-inflammatory diseases (158, 159). IGRA performance depends on intact cellular Th1 responses. Helminth infections, which primarily induce Th2 responses, may alter cytokine production and thereby increase the rate of indeterminate results (20, 150, 160, 161).
Importantly, in our analysis younger age was not associated with indeterminate results, reflected in similar proportions of indeterminate IGRA results in all age groups. This conflicts with several studies that have reported a clear correlation between IGRA performance, proportions of indeterminate results and age (15, 16, 18, 27, 33, 158). It is well-established that young children have a maturing immune system that may result in diminished cytokine release (162, 163). The link between age and cytokine concentrations has also been shown in numerous studies in healthy children unrelated to TB diagnostics (162, 163). One potential reason for not detecting a significant association between age and indeterminate IGRA results in this meta-analysis is that aggregate data based on the reported mean/median ages rather than individual patient data were used for this analysis.
There were several factors we were unable to analyse in the datasets that have been reported in some of the included studies, which mainly concern pre-analytical factors. Several studies in children and in adults found a decrease in interferon-γ production and indeterminate IGRA results to be associated with delayed sample incubation, shipping of samples, variation in environmental temperatures, and poor phlebotomy technique (164–168). In addition, co-medication may influence results as a recent ex vivo study showed that both corticosteroids and anti-TNF-alpha agents can cause false-negative IGRA results, and potentially also increase the rate of indeterminate results (169).
One potential limitation of our meta-analysis is the considerable heterogeneity of the included studies. Despite using empirical random effects weighting, excluding studies with < 10 participants, and using only data of the two commercially available IGRAs, heterogeneity remained. Moreover, it is possible that studies with poor IGRA performance and higher proportion of indeterminate results were less likely to be published, leading to publication bias. In addition, details on the type of QuantiFERON-TB assays used were often not reported in the publications, precluding a comparison of different test generations.
Conclusions
In children, indeterminate IGRA results occur in 1 in 25 tests performed on average. Overall, there was no difference in the proportion of indeterminate results between both commercial assays. However, the data of this meta-analysis indicate that in patients in Africa and/or children with immunocompromising conditions other than HIV infection the T-SPOT.TB assay appears to produce fewer indeterminate results than the QuantiFERON-TB assays.
Author Contributions
NR, MT, and UH conceptualized the study. NM and MG designed the search strategy and searched the literature, selected the studies and extracted the data. NR reviewed and approved the search strategy. NM, MG, and TV performed the data analysis. All authors performed the data interpretation. NM, MG, and NR wrote the draft manuscript. All authors reviewed, provided intellectual input into and approved the final manuscript.
Conflict of Interest Statement
MT has received support from Cepheid for conference attendance. MT also received QuantiFERON-TB Gold assays at reduced cost for another research project from the manufacturer (Cellestis/Qiagen). The manufacturer had no influence on the study design, the data interpretation, the writing of the manuscript or the decision to submit the data for publication.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
NM was supported by the following funding bodies: Bangerter Rhyner Stiftung, Lunge Zürich, Nora van Meeuwen-Häftliger Stiftung, and Schweizerische Lungenstiftung. MT was supported by a grant by the Technology Strategy Board/Innovate UK.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2019.00208/full#supplementary-material
Abbreviations
BCG, Bacille Calmette-Guérin; CI, Confidence interval; HIV, Human immunodeficiency virus; IGRA, Interferon-gamma release assay; IFN-γ, Interferon-gamma; ns, not specified; QFT, QuantiFERON-TB; TB, Tuberculosis; TST, Tuberculin skin test. *QFT used representative for the two reported generations of QFT [QFT Gold and QFT-GIT (Gold In-Tube)].
References
2. Houben RM, Dodd PJ. The global burden of latent tuberculosis infection: a re-estimation using mathematical modelling. PLoS Med. (2016) 13:e1002152. doi: 10.1371/journal.pmed.1002152
3. Diel R, Loddenkemper R, Niemann S, Meywald-Walter K, Nienhaus A. Negative and positive predictive value of a whole-blood interferon-gamma release assay for developing active tuberculosis: an update. Am J Respir Crit Care Med. (2011) 183:88–95. doi: 10.1164/rccm.201006-0974OC
4. Sloot R, Schim van der Loeff MF, Kouw PM, Borgdorff MW. Risk of tuberculosis after recent exposure. A 10-year follow-up study of contacts in Amsterdam. Am J Respir Crit Care Med. (2014) 190:1044–52. doi: 10.1164/rccm.201406-1159OC
5. Ritz N, Curtis N. Novel concepts in the epidemiology, diagnosis and prevention of childhood tuberculosis. Swiss Med Weekly. (2014) 144:14000. doi: 10.4414/smw.2014.14000
6. Starke JR, Byington CL, Maldonado YA, Barnett ED, Davies HD, Edwards KM, et al. Interferon-γ release assays for diagnosis of tuberculosis infection and disease in children. Pediatrics. (2014) 134:e1763–e73. doi: 10.1542/peds.2014-2983
7. Tebruegge M, Ritz N, Curtis N, Shingadia D. Diagnostic tests for childhood tuberculosis: past imperfect, present tense and future perfect? Pediatric Infect Dis J. (2015) 34:1014–9. doi: 10.1097/INF.0000000000000796
8. Lalvani A, Pareek M. A 100 year update on diagnosis of tuberculosis infection. Br Med Bull. (2010) 93:69–84. doi: 10.1093/bmb/ldp039
9. Lalvani A, Pathan AA, McShane H, Wilkinson RJ, Latif M, Conlon CP, et al. Rapid detection of Mycobacterium tuberculosis infection by enumeration of antigen-specific T cells. Am J Respir Crit Care Med. (2001) 163:824–8. doi: 10.1164/ajrccm.163.4.2009100
10. World Health Organization W. Latent TB Infection: Updated and consolidated guidelines for programmatic management. (2018).
11. Sollai S, Galli L, de Martino M, Chiappini E. Systematic review and meta-analysis on the utility of Interferon-gamma release assays for the diagnosis of Mycobacterium tuberculosis infection in children: a 2013 update. BMC Infect Dis. (2014) 14:S6. doi: 10.1186/1471-2334-14-S1-S6
12. Mandalakas AM, Detjen AK, Hesseling AC, Benedetti A, Menzies D. Interferon-gamma release assays and childhood tuberculosis: systematic review and meta-analysis. Int J Tubercul Lung Dis. (2011) 15:1018–32. doi: 10.5588/ijtld.10.0631
13. Sun L, Xiao J, Miao Q, Feng WX, Wu XR, Yin QQ, et al. Interferon gamma release assay in diagnosis of pediatric tuberculosis: a meta-analysis. Fems Immunol Med Microbiol. (2011) 63:165–73. doi: 10.1111/j.1574-695X.2011.00838.x
14. Machingaidze S, Wiysonge CS, Gonzalez-Angulo Y, Hatherill M, Moyo S, Hanekom W, et al. The utility of an interferon gamma release assay for diagnosis of latent tuberculosis infection and disease in children a systematic review and meta-analysis. Pediatric Infect Dis J. (2011) 30:694–700. doi: 10.1097/INF.0b013e318214b915
15. Tebruegge M, de Graaf H, Sukhtankar P, Elkington P, Marshall B, Schuster H, et al. Extremes of age are associated with indeterminate QuantiFERON-TB gold assay results. J Clin Microbiol. (2014) 52:2694–7. doi: 10.1128/JCM.00814-14
16. Haustein T, Ridout DA, Hartley JC, Thaker U, Shingadia D, Klein NJ, et al. The likelihood of an indeterminate test result from a whole-blood interferon-gamma release assay for the diagnosis of Mycobacterium tuberculosis infection in children correlates with age and immune status. Pediatric Infect Dis J. (2009) 28:669–73. doi: 10.1097/INF.0b013e3181a16394
17. Clifford V, Zufferey C, Germano S, Ryan N, Leslie D, Street A, et al. The impact of anti-tuberculous antibiotics and corticosteroids on cytokine production in QuantiFERON-TB Gold In Tube assays. Tuberculosis. (2015) 95:343–9. doi: 10.1016/j.tube.2015.02.039
18. Connell TG, Tebruegge M, Ritz N, Bryant PA, Leslie D, Curtis N. Indeterminate interferon-gamma release assay results in children. Pediatric Infect Dis J. (2010) 29:285–6. doi: 10.1097/INF.0b013e3181c4822f
19. Jeong SJ, Han SH, Kim CO, Baek JH, Jin SJ, Ku NS, et al. Predictive factors for indeterminate result on the QuantiFERON test in an intermediate tuberculosis-burden country. J Infect. (2011) 62:347–54. doi: 10.1016/j.jinf.2011.03.004
20. Thomas TA, Mondal D, Noor Z, Liu L, Alam M, Haque R, et al. Malnutrition and helminth infection affect performance of an interferon gamma-release assay. Pediatrics. (2010) 126:e1522–9. doi: 10.1542/peds.2010-0885
21. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
22. Altman DG, Machin D, Bryant TN, Gardner MJ. Statistics with Confidence: Confidence Intervals and Statistical Guidelines. 2nd ed. BMJ Books (2011).
23. Connell TG, Curtis N, Ranganathan SC, Buttery JP. Performance of a whole blood interferon gamma assay for detecting latent infection with Mycobacterium tuberculosis in children. Thorax. (2006) 61:616–20. doi: 10.1136/thx.2005.048033
24. Dogra S, Naraing P, Mendiratta DK, Chaturvedi P, Reingold AL, Colford JM, et al. Comparison of a whole blood interferon-gamma assay with tuberculin skin testing for the detection of tuberculosis infection in hospitalized children in rural India. J Infect. (2007) 54:267–76. doi: 10.1016/j.jinf.2006.04.007
25. Dominguez J, Ruiz-Manzano J, De Souza-Galvao M, Latorre I, Mila C, Blanco S, et al. Comparison of two commercially available gamma interferon blood tests for immunodiagnosis of tuberculosis. Clin Vacc Immunol. (2008) 15:168–71. doi: 10.1128/CVI.00364-07
26. Taylor REB, Cant AJ, Clark JE. Potential effect of NICE tuberculosis guidelines on paediatric tuberculosis screening. Archi Dis Childhood. (2008) 93:200–3. doi: 10.1136/adc.2006.106617
27. Okada K, Mao TE, Mori T, Miura T, Sugiyama T, Yoshiyama T, et al. Performance of an interferon-gamma release assay for diagnosing latent tuberculosis infection in children. Epidemiol Infect. (2008) 136:1179–87. doi: 10.1017/S0950268807009831
28. Ruhwald M, Petersen J, Kofoed K, Nakaoka H, Cuevas LE, Lawson L, et al. Improving T-cell assays for the diagnosis of latent tb infection: potential of a diagnostic test based on IP-10. PLoS ONE. (2008) 3:02858. doi: 10.1371/journal.pone.0002858
29. Mandalakas AM, Hesseling AC, Chegou NN, Kirchner HL, Zhu X, Marais BJ, et al. High level of discordant IGRA results in HIV-infected adults and children. Int J Tubercul Lung Dis. (2008) 12:417–23.
30. Ohno H, Ikegami Y, Kishida K, Yamamoto Y, Ikeda N, Taniguchi T, et al. A contact investigation of the transmission of Mycobacterium tuberculosis from a nurse working in a newborn nursery and maternity ward. J Infect Chemother. (2008) 14:66–71. doi: 10.1007/s10156-007-0565-0
31. Soysal A, Turel O, Toprak D, Bakir M. Comparison of positive tuberculin skin test with an interferon-gamma-based assay in unexposed children. Jpn J Infect Dis. (2008) 61:192–5.
32. Chun JK, Kim CK, Kim HS, Jung GY, Lee TJ, Kim KH, et al. The role of a whole blood interferon-γ assay for the detection of latent tuberculosis infection in Bacille Calmette-Guérin vaccinated children. Diagnos Microbiol Infect Dis. (2008) 62:389–94. doi: 10.1016/j.diagmicrobio.2008.08.022
33. Connell TG, Ritz N, Paxton GA, Buttery JP, Curtis N, Ranganathan SC. A three-way comparison of tuberculin skin testing, quantiFERON-TB Gold and T-SPOT. TB in Children. PLoS ONE. (2008) 3:02624. doi: 10.1371/journal.pone.0002624
34. Petrucci R, Amer NA, Gurgel RQ, Sherchand JB, Doria L, Lama C, et al. Interferon gamma, interferon-gamma-induced-protein 10, and tuberculin responses of children at high risk of tuberculosis infection. Pediatric Infect Dis J. (2008) 27:1073–7. doi: 10.1097/INF.0b013e31817d05a3
35. Richeldi L, Bergamini BM, Vaienti F. Prior tuberculin skin testing does not boost QuantiFERON-TB results in paediatric contacts. Eur Respi J. (2008) 32:524–5. doi: 10.1183/09031936.00014508
36. Bianchi L, Galli L, Moriondo M, Veneruso G, Becciolini L, Azzari C, et al. Interferon-gamma release assay improves the diagnosis of tuberculosis in children. Pediatric Infect Dis J. (2009) 28:510–4. doi: 10.1097/INF.0b013e31819abf6b
37. Hesseling AC, Mandalakas AM, Kirchner HL, Chegou NN, Marais BJ, Stanley K, et al. Highly discordant T cell responses in individuals with recent exposure to household tuberculosis. Thorax. (2009) 64:840–6. doi: 10.1136/thx.2007.085340
38. Higuchi K, Kondo S, Wada M, Hayashi S, Ootsuka G, Sakamoto N, et al. Contact investigation in a primary school using a whole blood interferon-gamma assay. J Infect. (2009) 58:352–7. doi: 10.1016/j.jinf.2009.02.019
39. Kobashi Y, Mouri K, Miyashita N, Okimoto N, Matsushima T, Kageoka T, et al. QuantiFERON TB-2G test for patients with active tuberculosis stratified by age groups. Scand J Infect Dis. (2009) 41:841–6. doi: 10.3109/00365540903186215
40. Kampmann B, Whittaker E, Williams A, Walters S, Gordon A, Martinez-Alier N, et al. Interferon-γ release assays do not identify more children with active tuberculosis than the tuberculin skin test. Eur Respir J. (2009) 33:1374–82. doi: 10.1183/09031936.00153408
41. Bruzzese E, Bocchino M, Assante LR, Alessio M, Bellofiore B, Bruzzese D, et al. Gamma interferon release assays for diagnosis of tuberculosis infection in immune-compromised children in a country in which the prevalence of tuberculosis is low. J Clin Microbiol. (2009) 47:2355–7. doi: 10.1128/JCM.01320-08
42. Lighter J, Rigaud M, Eduardo R, Peng CH, Pollack H. Latent tuberculosis diagnosis in children by using the QuantiFERON-TB gold in-tube test. Pediatrics. (2009) 123:30–7. doi: 10.1542/peds.2007-3618
43. Stavri H, Ene L, Popa GL, Duiculescu D, Murgoci G, Marica C, et al. Comparison of tuberculin skin test with a whole-blood interferon gamma assay and ELISA, in HIV positive children and adolescents with TB. Roumanian Arch Microbiol Immunol. (2009) 68:14–9.
44. Bamford ARJ, Crook AM, Clark JE, Zohreh N, Dixon G, Paton JY, et al. Comparison of interferon-γ release assays and tuberculin skin test in predicting active tuberculosis (TB) in children in the UK: A paediatric TB network study. Arch Dis Childhood. (2010) 95:180–6. doi: 10.1136/adc.2009.169805
45. Soborg B, Koch A, Thomsen VO, Ladefoged K, Andersson M, Wohlfahrt J, et al. Ongoing tuberculosis transmission to children in Greenland. Eur Respir J. (2010) 36:878–84. doi: 10.1183/09031936.00015510
46. Grare M, Derelle J, Dailloux M, Laurain C. QuantiFERON (R)-TB Gold In-Tube as help for the diagnosis of tuberculosis in a French pediatric hospital. Diag Microbiol Infect Dis. (2010) 66:366–72. doi: 10.1016/j.diagmicrobio.2009.11.002
47. Lucas M, Nicol P, McKinnon E, Whidborne R, Lucas A, Thambiran A, et al. A prospective large-scale study of methods for the detection of latent Mycobacterium tuberculosis infection in refugee children. Thorax. (2010) 65:442–8. doi: 10.1136/thx.2009.127555
48. Stefan DC, Dippenaar A, Detjen AK, Schaaf HS, Marais BJ, Kriel B, et al. Interferon-gamma release assays for the detection of Mycobacterium tuberculosis infection in children with cancer. International J Tuberculosis Lung Dis. (2010) 14:689–94.
49. Tsolia MN, Mavrikou M, Critselis E, Papadopoulos NG, Makrinioti H, Spyridis NP, et al. Whole blood interferon-γ release assay is a useful tool for the diagnosis of tuberculosis infection particularly among bacille calmette guèrin-vaccinated children. Pediatric Infect Dis J. (2010) 29:1137–40. doi: 10.1097/INF.0b013e3181ebfe8a
50. Adetifa IM, Ota MO, Jeffries DJ, Hammond A, Lugos MD, Donkor S, et al. Commercial interferon gamma release assays compared to the tuberculin skin test for diagnosis of latent Mycobacterium tuberculosis infection in childhood contacts in the Gambia. Pediatric Infect Dis J. (2010) 29:439–43. doi: 10.1097/INF.0b013e3181cb45da
51. Kabeer BS, Perumal V, Paramasivam P, Raja A. Yield of QuantiFERON-TB gold in tube assay and tuberculin skin test in healthy persons from a tuberculosis endemic population. J Pediatric Infect Dis. (2010) 5:125–9. doi: 10.3233/JPI-2010-0236
52. Stavri HR, Murgoci G, Ulea I, Popa LG, Popa M. Prospective comparison of two brands of tuberculin skin tests and quantiferon-TB gold in-tube assay performances for tuberculosis infection in hospitalized children. Maedica. (2010) 5:271–6.
53. Altet-Gomez N, De Souza-Galvao M, Latorre I, Mila C, Jimenez MA, Solsona J, et al. Diagnosing TB infection in children: analysis of discordances using in vitro tests and the tuberculin skin test. Eur Respir J. (2011) 37:1166–74. doi: 10.1183/09031936.00022710
54. Cruz AT, Geltemeyer AM, Starke JR, Flores JA, Graviss EA, Smith KC. Comparing the tuberculin skin test and T-SPOT.TB blood test in children. Pediatrics. (2011) 127:E31–8. doi: 10.1542/peds.2010-1725
55. Moyo S, Isaacs F, Gelderbloem S, Verver S, Hawkridge AJ, Hatherill M, et al. Tuberculin skin test and QuantiFERON® assay in young children investigated for tuberculosis in South Africa. Int J Tubercul Lung Dis. (2011) 15:1176–81. doi: 10.5588/ijtld.10.0770
56. Banach DB, Harris TG. Indeterminate QuantiFERON (R)-TB Gold results in a public health clinic setting. Int J Tubercul Lung Dis. (2011) 15:1623–9. doi: 10.5588/ijtld.11.0017
57. Kasambira TS, Shah M, Adrian PV, Holshouser M, Madhi SA, Chaisson RE, et al. QuantiFERON®-TB gold in-tube for the detection of Mycobacterium tuberculosis infection in children with household tuberculosis contact. Int J Tubercul Lung Dis. (2011) 15:628–34. doi: 10.5588/ijtld.10.0555
58. Losi M, Bergamini BM, Venturelli C, Del Giovane C, Sighinolfi G, Rumpaneisi F, et al. Tuberculosis infection in foreign-born children: a screening survey based on skin and blood testing. Int J Tubercul Lung Dis. (2011) 15:1182–4. doi: 10.5588/ijtld.10.0736
59. Shah M, Kasambira TS, Adrian PV, Madhi SA, Martinson NA, Dorman SE. Longitudinal analysis of quantiFERON-TB gold in-tube in children with adult household tuberculosis contact in South Africa: a prospective cohort study. PLoS ONE. (2011) 6:026787. doi: 10.1371/journal.pone.0026787
60. Maritsi D, Al-Obadi M, Brogan PA, Eleftheriou D, Dixon GL. The performance of quantiferon tb gold in-tube as a screening tool in paediatric rheumatology prior to initiation of infliximab: a single centre's experience. ISRN Rheumatol. (2011) 2011:505171. doi: 10.5402/2011/505171
61. Thomas B, Pugalenthi A, Patel H, Woltmann G, Bankart J, Hoskyns W. Concordance between tuberculin skin test and interferon-γ assay and interferon-γ response to mitogen in pediatric tuberculosis contacts. Pediatric Pulmonol. (2011) 46:1225–32. doi: 10.1002/ppul.21494
62. Topic RZ, Zoricic-Letoja I, Pavic I, Dodig S. Indeterminate results of QuantiFERON-TB gold in-tube assay in nonimmunosuppressed children. Arch Med Res. (2011) 42:138–43. doi: 10.1016/j.arcmed.2011.02.001
63. Debord C, De Lauzanne A, Gourgouillon N, Guérin-El Khourouj V, Pédron B, Gaudelus J, et al. Interferon-gamma release assay performance for diagnosing tuberculosis disease in 0-to 5-year-old children. Pediatric Infect Dis J. (2011) 30:995–7. doi: 10.1097/INF.0b013e3182272227
64. Pavić I, Topić RZ, Raos M, Aberle N, Dodig S. Interferon-γ release assay for the diagnosis of latent tuberculosis in children younger than 5 years of age. Pediatric Infect Dis J. (2011) 30:866–70. doi: 10.1097/INF.0b013e318220c52a
65. Mount C, Helbert M, Bell C, Murray C, Child F. Mantoux or gamma interferon (IGRA)-which test is best in children? Thorax. (2011) 66:A138–A9. doi: 10.1136/thoraxjnl-2011-201054c.175
66. Borgia P, Cambieri A, Chini F, Coltella L, Delogu G, Di Rosa E, et al. Suspected transmission of tuberculosis in a maternity ward from a smear-positive nurse: preliminary results of clinical evaluations and testing of neonates potentially exposed, Rome, Italy, 1 January to 28 July 2011. Euro Surveill. (2011) 16:19984. doi: 10.2807/ese.16.40.19984-en
67. Yassin MA, Petrucci R, Garie KT, Harper G, Arbide I, Aschalew M, et al. Can interferon-gamma or interferon-gamma-induced-protein-10 differentiate tuberculosis infection and disease in children of high endemic areas? PLoS ONE. (2011) 6:23733. doi: 10.1371/journal.pone.0023733
68. Buonsenso D, Lancella L, Delogu G, Krzysztofiak A, Testa A, Ranno O, et al. A twenty-year retrospective study of pediatric tuberculosis in two tertiary hospitals in Rome. Pediatric Infect Dis J. (2012) 31:1022–6. doi: 10.1097/INF.0b013e3182615270
69. Riazi S, Zeligs B, Yeager H, Peters SM, Benavides GA, Di Mita O, et al. Rapid diagnosis of Mycobacterium tuberculosis infection in children using interferon-gamma release assays (IGRAs). Allergy Asthma Proc. (2012) 33:217–26. doi: 10.2500/aap.2012.33.3574
70. Banfield S, Pascoe E, Thambiran A, Siafarikas A, Burgner D. Factors associated with the performance of a blood-based interferon-γ release assay in diagnosing tuberculosis. PLoS ONE. (2012) 7:38556. doi: 10.1371/journal.pone.0038556
71. Roy RB, Sotgiu G, Altet-Gomez N, Tsolia M, Ruga E, Velizarova S, et al. Identifying predictors of interferon-gamma release assay results in pediatric latent tuberculosis: a protective role of bacillus calmette-guerin? A pTB-NET collaborative study. Am J Respi Crit Care Med. (2012) 186:378–84. doi: 10.1164/rccm.201201-0026OC
72. Critselis E, Amanatidou V, Syridou G, Spyridis NP, Mavrikou M, Papadopoulos NG, et al. The effect of age on whole blood interferon-gamma release assay response among children investigated for latent tuberculosis infection. J Pediatrics. (2012) 161:632–8. doi: 10.1016/j.jpeds.2012.04.007
73. Méndez-Echevarría A, González-Muñoz M, Mellado MJ, Baquero-Artigao F, Blázquez D, Penín M, et al. Interferon-γ release assay for the diagnosis of tuberculosis in children. Archi Dis Childhood. (2012) 97:514–6. doi: 10.1136/adc.2010.202069
74. Pong A, Moser KS, Park SM, Magit A, Garcia MI, Bradley JS. Evaluation of an interferon gamma release assay to detect tuberculosis infection in children in San Diego, California. J Pediatric Infect Dis Soc. (2012) 1:74–7. doi: 10.1093/jpids/pis013
75. Nenadić N, Kirin BK, Letoja IZ, Plavec D, Topić RZ, Dodig S. Serial interferon-γ release assay in children with latent tuberculosis infection and children with tuberculosis. Pediatric Pulmonol. (2012) 47:401–8. doi: 10.1002/ppul.21555
76. Onur H, Hatipoglu S, Arica V, Hatipoglu N, Arica SG. Comparison of quantiferon test with tuberculin skin test for the detection of tuberculosis infection in children. Inflammation. (2012) 35:1518–24. doi: 10.1007/s10753-012-9466-1
77. Rose MV, Kimaro G, Nissen TN, Kroidl I, Hoelscher M, Bygbjerg IC, et al. QuantiFERON(R)-TB gold in-tube performance for diagnosing active tuberculosis in children and adults in a high burden setting. PLoS ONE. (2012) 7:e37851. doi: 10.1371/journal.pone.0037851
78. Kabeer BSA, Paramasivam P, Raja A. Interferon gamma and interferon gamma inducible protein-10 in detecting tuberculosis infection. J Infect. (2012) 64:573–9. doi: 10.1016/j.jinf.2012.02.013
79. Tuuminen T, Salo E, Kotilainen H, Ruhwald M. Evaluation of the filter paper IP-10 tests in school children after exposure to tuberculosis: a prospective cohort study with a 4-year follow-up. BMJ Open. (2012) 2:001751. doi: 10.1136/bmjopen-2012-001751
80. Ling DI, Crépeau CA, Dufresne M, Khan S, Quach C, Dendukuri N, et al. Evaluation of the impact of interferon-gamma release assays on the management of childhood tuberculosis. Pediatric Infect Dis J. (2012) 31:1258–62. doi: 10.1097/INF.0b013e318269d10c
81. Nkurunungi G, Lutangira JE, Lule SA, Akurut H, Kizindo R, Fitchett JR, et al. Determining mycobacterium tuberculosis infection among BCG-immunised ugandan children by T-SPOT.TB and tuberculin skin testing. PLoS ONE. (2012) 7:47340. doi: 10.1371/journal.pone.0047340
82. Verhagen LM, Hermans PW, Warris A, de Groot R, Maes M, Villalba JA, et al. Helminths and skewed cytokine profiles increase tuberculin skin test positivity in Warao Amerindians. Tuberculosis. (2012) 92:505–12. doi: 10.1016/j.tube.2012.07.004
83. Dayal R, Verma V, Sharma B, Kumar G, Kumar N, Gupta R, et al. Diagnostic value of interferon- Gamma release assays (QuantiFERON-TB Gold® in tube) in childhood tuberculosis. Indian J Pediatrics. (2012) 79:183–7. doi: 10.1007/s12098-011-0469-y
84. Rutherford ME, Nataprawira M, Yulita I, Apriani L, Maharani W, Van Crevel R, et al. QuantiFERON®-TB Gold in-Tube assay vs. tuberculin skin test in Indonesian children living with a tuberculosis case. Int J Tubercul Lung Dis. (2012) 16:496–502. doi: 10.5588/ijtld.11.0491
85. Blandinières A, de Lauzanne A, Guérin-El Khourouj V, Gourgouillon N, See H, Pédron B, et al. QuantiFERON to diagnose infection by Mycobacterium tuberculosis: Performance in infants and older children. J Infect. (2013) 67:391–8. doi: 10.1016/j.jinf.2013.06.011
86. Mandalakas AM, van Wyk S, Kirchner HL, Walzl G, Cotton M, Rabie H, et al. Detecting tuberculosis infection in HIV-infected children: a study of diagnostic accuracy, confounding and interaction. Pediatric Infect Dis J. (2013) 32:E111–E8. doi: 10.1097/INF.0b013e31827d77b7
87. Yassin MA, Petrucci R, Garie KT, Harper G, Teshome A, Arbide I, et al. Use of tuberculin skin test, IFN-γ release assays and IFN-γ-induced protein-10 to identify children with TB infection. Eur Respir J. (2013) 41:644–8. doi: 10.1183/09031936.00012212
88. Chegou NN, Detjen AK, Thiart L, Walters E, Mandalakas AM, Hesseling AC, et al. Utility of host markers detected in quantiferon supernatants for the diagnosis of tuberculosis in children in a high-burden setting. PLoS ONE. (2013) 8:64226. doi: 10.1371/journal.pone.0064226
89. Rose MV, Kimaro G, Kroidl I, Hoelscher M, Bygbjerg IC, Mfinanga SM, et al. Evaluation of QuantiFERON microtube, using 0.9 mL blood, for diagnosing tuberculosis infection. Eur Respi J. (2013) 41:909–16. doi: 10.1183/09031936.00194311
90. Bua A, Molicotti P, Cannas S, Ruggeri M, Olmeo P, Zanetti S. Tuberculin skin test and QuantiFERON in children. N Microbiol. (2013) 36:153–6.
91. Carvalho AC, Schumacher RF, Bigoni S, Soncini E, Notarangelo L, Apostoli A, et al. Contact investigation based on serial interferon-gamma release assays (IGRA) in children from the hematology-oncology ward after exposure to a patient with pulmonary tuberculosis. Infection. (2013) 41:827–31. doi: 10.1007/s15010-013-0450-y
92. Ling DI, Nicol MP, Pai M, Pienaar S, Dendukuri N, Zar HJ. Incremental value of T-SPOT.TB for diagnosis of active pulmonary tuberculosis in children in a high-burden setting: a multivariable analysis. Thorax. (2013) 68:860–6. doi: 10.1136/thoraxjnl-2012-203086
93. Uluk T, Allison WE, Vince J, Wand H, Tefuarani N, Causer LM, et al. Evaluation of an interferon-gamma release assay in children with suspected tuberculosis in papua new guinea. Pediatric Infect Dis J. (2013) 32:187–9. doi: 10.1097/INF.0b013e31827412fc
94. Wassie L, Aseffa A, Abebe M, Gebeyehu MZ, Zewdie M, Mihret A, et al. Parasitic infection may be associated with discordant responses to QuantiFERON and tuberculin skin test in apparently healthy children and adolescents in a tuberculosis endemic setting, Ethiopia. BMC Infect Dis. (2013) 13:265. doi: 10.1186/1471-2334-13-265
95. Laniado-Laborín R, Cazares-Adame R, Volker-Soberanes ML, Del Portillo-Mustieles C, Villa-Rosas C, Oceguera-Palao L, et al. Latent tuberculous infection prevalence among paediatric contacts of drug-resistant and drug-susceptible cases. Int J Tubercu Lung Dis. (2014) 18:515–9. doi: 10.5588/ijtld.13.0840
96. Dhanasekaran S, Jenum S, Stavrum R, Ritz C, Faurholt-Jepsen D, Kenneth J, et al. Identification of biomarkers for Mycobacterium tuberculosis infection and disease in BCG-vaccinated young children in Southern India. Genes Immun. (2013) 14:356–64. doi: 10.1038/gene.2013.26
97. Lodha R, Mukherjee A, Saini D, Saini S, Singh V, Singh S, et al. Role of the QuantiFERON(R)-TB Gold in-tube test in the diagnosis of intrathoracic childhood tuberculosis. Int J Tubercul Lung Dis. (2013) 17:1383–8. doi: 10.5588/ijtld.13.0348
98. Cranmer LM, Kanyugo M, Jonnalagadda SR, Lohman-Payne B, Sorensen B, Maleche Obimbo E, et al. High prevalence of tuberculosis infection in HIV-1 exposed Kenyan infants. Pediatric Infect Dis J. (2014) 33:401–6. doi: 10.1097/INF.0000000000000124
99. Garazzino S, Galli L, Chiappini E, Pinon M, Bergamini BM, Cazzato S, et al. Performance of interferon-gamma release assay for the diagnosis of active or latent tuberculosis in children in the first 2 years of age a multicenter study of the italian society of pediatric infectious diseases. Pediatric Infect Dis J. (2014) 33:E226–E31. doi: 10.1097/INF.0000000000000353
100. Hermansen T, Lillebaek T, Hansen ABE, Andersen PH, Ravn P. QuantiFERON-TB Gold In-Tube test performance in Denmark. Tuberculosis. (2014) 94:616–21. doi: 10.1016/j.tube.2014.09.003
101. Jenum S, Selvam S, Mahelai D, Jesuraj N, Cárdenas V, Kenneth J, et al. Influence of age and nutritional status on the performance of the tuberculin skin test and QuantiFERON-TB Gold in-Tube in young children evaluated for tuberculosis in Southern India. Pediatric Infect Dis J. (2014) 33:e260–e9. doi: 10.1097/INF.0000000000000399
102. Holm LL, Rose MV, Kimaro G, Bygbjerg IC, Mfinanga SG, Ravn P, et al. A comparison of interferon-γ and IP-10 for the diagnosis of tuberculosis. Pediatrics. (2014) 134:e1568–e75. doi: 10.1542/peds.2014-1570
103. Song SE, Yang J, Lee KS, Kim H, Kim YM, Kim S, et al. Comparison of the tuberculin skin test and interferon gamma release assay for the screening of tuberculosis in adolescents in close contact with tuberculosis TB patients. PLoS ONE. (2014) 9:100267. doi: 10.1371/journal.pone.0100267
104. Vallada MG, Okay TS, Del Negro GMB, Antonio CA, Yamamoto L, Ramos SRTS. Accuracy of the QuantiFERON-TB gold in tube for diagnosing tuberculosis in a young pediatric population previously vaccinated with bacille calmette-Guérin. Revista Paulista de Pediatria. (2014) 32:4–10. doi: 10.1590/S0103-05822014000100002
105. Pérez-Porcuna TM, Ascaso C, Malheiro A, Abellana R, Martins M, Sardinha JFJ, et al. Mycobacterium tuberculosis infection in young children: analyzing the performance of the diagnostic tests. PLoS ONE. (2014) 9:97992. doi: 10.1371/journal.pone.0097992
106. Tieu HV, Suntarattiwong P, Puthanakit T, Chotpitayasunondh T, Chokephaibulkit K, Sirivichayakul S, et al. Comparing interferon-gamma release assays to tuberculin skin test in Thai children with tuberculosis exposure. PLoS ONE. (2014) 9:e105003. doi: 10.1371/journal.pone.0105003
107. Bui DH, Cruz AT, Graviss EA. Indeterminate QuantiFERON-TB gold in-tube assay results in children: possible association with procedural specimen collection. Pediatric Infect Dis J. (2014) 33:220–2. doi: 10.1097/INF.0000000000000001
108. Chiappini E, Bonsignori F, Mazzantini R, Sollai S, Venturini E, Mangone G, et al. Interferon-gamma release assay sensitivity in children younger than 5 years is insufficient to replace the use of tuberculin skin test in western countries. Pediatric Infect Dis J. (2014) 33:1291–3. doi: 10.1097/INF.0000000000000432
109. Rose W, Kitai I, Kakkar F, Read SE, Behr MA, Bitnun A. Quantiferon Gold-in-tube assay for TB screening in HIV infected children: influence of quantitative values. BMC Infect Dis. (2014) 14:516. doi: 10.1186/1471-2334-14-516
110. Verhagen LM, Maes M, Villalba JA, d'Alessandro A, Rodriguez LP, Espana MF, et al. Agreement between QuantiFERON (R)-TB Gold In-Tube and the tuberculin skin test and predictors of positive test results in Warao Amerindian pediatric tuberculosis contacts. BMC Infect Dis. (2014) 14:383. doi: 10.1186/1471-2334-14-383
111. Mekaini LAA, Jabri ONA, Narchi H, Kamal SM, Mabrook A, Kuwaiti MMA, et al. The use of an interferon-gamma release assay to screen for pediatric latent tuberculosis infection in the eastern region of the Emirate of Abu Dhabi. Int J Infect Dis. (2014) 23:4–7. doi: 10.1016/j.ijid.2013.12.020
112. De Souza-Galvão ML, Latorre I, Altet-Gómez N, Jiménez-Fuentes MT, Milà C, Solsona J, et al. Correlation between tuberculin skin test and IGRAs with risk factors for the spread of infection in close contacts with sputum smear positive in pulmonary tuberculosis. BMC Infect Dis. (2014) 14:258. doi: 10.1186/1471-2334-14-258
113. Calzada-Hernandez J, Anton-Lopez J, Bou-Torrent R, Iglesias-Jimenez E, Ricart-Campos S, Martin de Carpi J, et al. Tuberculosis in pediatric patients treated with anti-TNFalpha drugs: a cohort study. Pediatric Rheumatol Online J. (2015) 13:54. doi: 10.1186/s12969-015-0054-4
114. Caliman-Sturdza OA, Mihalache D, Luca CM. Performance of an interferon-gamma release assay in the diagnosis of tuberculous meningitis in children. Revista Romana De Medicina De Labor. (2015) 23:199–212. doi: 10.1515/rrlm-2015-0016
115. Sali M, Buonsenso D, Goletti D, D'Alfonso P, Zumbo A, Fadda G, et al. Accuracy of QuantiFERON-TB gold test for tuberculosis diagnosis in children. PLoS ONE. (2015) 10:138952. doi: 10.1371/journal.pone.0138952
116. Mandalakas AM, Kirchner HL, Walzl G, Gie RP, Schaaf HS, Cotton MF, et al. Optimizing the detection of recent tuberculosis infection in children in a high tuberculosis-HIV burden setting. Am J Respir Crit Care Med. (2015) 191:820–30. doi: 10.1164/rccm.201406-1165OC
117. Spicer KB, Turner J, Wang SH, Koranyi K, Powell DA. Tuberculin skin testing and T-SPOT.TB in internationally adopted Children. Pediatric Infect Dis J. (2015) 34:599–603. doi: 10.1097/INF.0000000000000680
118. Bao L, Li T, Diao N, Shen Y, Shao L, Zhang Y, et al. Fluctuating behavior and influential factors in the performance of the QuantiFERON-TB gold in-tube assay in the diagnosis of tuberculosis. PLoS ONE. (2015) 10:e0103763. doi: 10.1371/journal.pone.0103763
119. Howley MM, Painter JA, Katz DJ, Graviss EA, Reves R, Beavers SF, et al. Evaluation of QuantiFERON-TB gold in-tube and tuberculin skin tests among immigrant children being screened for latent tuberculosis infection. Pediatric Infect Dis J. (2015) 34:35–9. doi: 10.1097/INF.0000000000000494
120. Pavić I, Katalinić-Janković V, Čepin-Bogović J, Rešić A, Dodig S. Discordance between tuberculin skin test and interferon-γ release assay in children younger than 5 years who have been vaccinated with bacillus Calmette-Guérin. Labor Med. (2015) 46:200–6. doi: 10.1309/LMCQLO8PG0IZ5APX
121. Tebruegge M, Dutta B, Donath S, Ritz N, Forbes B, Camacho-Badilla K, et al. Mycobacteria-specific cytokine responses detect tuberculosis infection and distinguish latent from active tuberculosis. Am J Respi Crit Care Med. (2015) 192:485–99. doi: 10.1164/rccm.201501-0059OC
122. Lebina L, Abraham PM, Milovanovic M, Motlhaoleng K, Chaisson RE, Rakgokong M, et al. Latent tuberculous infection in schoolchildren and contact tracing in Matlosana, North West Province, South Africa. Int J Tubercul Lung Dis. (2015) 19:1290–2. doi: 10.5588/ijtld.15.0370
123. Petrone L, Cannas A, Aloi F, Nsubuga M, Sserumkuma J, Nazziwa RA, et al. Blood or Urine IP-10 cannot discriminate between active tuberculosis and respiratory diseases different from tuberculosis in children. Biomed Res Int. (2015). doi: 10.1155/2015/589471
124. Sun L, Tian JL, Yin QQ, Xiao J, Li JQ, Guo YJ, et al. Performance of the interferon gamma release assays in tuberculosis disease in children five years old or less. PLoS ONE. (2015) 10:0143820. doi: 10.1371/journal.pone.0143820
125. Li T, Bao L, Diao N, Sun F, Gao Y, Wong KW, et al. Influencial factors of the performance of interferon-gamma release assays in the diagnosis of childhood tuberculosis. Clin Experi Med. (2015) 15:303–9. doi: 10.1007/s10238-014-0296-3
126. Cruz AT, Marape M, Graviss EA, Starke JR. Performance of the QuantiFERON-TB gold interferon gamma release assay among HIV-infected children in Botswana. J Int Assoc Prov AIDS Care. (2015) 14:4–7. doi: 10.1177/2325957414547432
127. Grinsdale JA, Islam S, Tran OC, Ho CS, Kawamura LM, Higashi JM. Interferon-gamma release assays and pediatric public health tuberculosis screening: the san francisco program experience 2005 to 2008. J Pediatric Infect Dis Soc. (2016) 5:122–30. doi: 10.1093/jpids/piu119
128. Santiago-Garcia B, Blazquez-Gamero D, Baquero-Artigao F, Ruiz-Contreras J, Bellon JM, Munoz-Fernandez MA, et al. Pediatric extrapulmonary tuberculosis: clinical spectrum, risk factors and diagnostic challenges in a low prevalence region. Pediatric Infect Dis J. (2016) 35:1175–81. doi: 10.1097/INF.0000000000001270
129. Perez-Porcuna TM, Pereira-da-Silva HD, Ascaso C, Malheiro A, Buhrer S, Martinez-Espinosa F, et al. Prevalence and diagnosis of latent tuberculosis infection in young children in the absence of a gold standard. PLoS ONE. (2016) 11:e0164181. doi: 10.1371/journal.pone.0164181
130. Atikan BY, Cavusoglu C, Dortkardesler M, Sozeri B. Assessment of tuberculosis infection during treatment with biologic agents in a BCG-vaccinated pediatric population. Clin Rheumatol. (2016) 35:427–31. doi: 10.1007/s10067-014-2842-5
131. Boddu D, Verghese VP, Michael JS, Chacko A, Jeyaseelan V. Utility of the quantiferon-TB gold in-tube test (QFT) compared with the tuberculin skin test (TST) in diagnosing tuberculosis in Indian children with malnutrition: a prospective study. Int J Infect Dis. (2016) 45:339. doi: 10.1016/j.ijid.2016.02.732
132. Nozawa T, Mori M, Nishimura K, Sakurai N, Kikuchi M, Hara R, et al. Usefulness of two interferon-γ release assays for rheumatic disease. Pediatrics Int. (2016) 58:347–52. doi: 10.1111/ped.12885
133. El Azbaoui S, Sabri A, Ouraini S, Hassani A, Asermouh A, Agadr A, et al. Utility of the QuantiFERON®-TB gold in-tube assay for the diagnosis of tuberculosis in moroccan children. Int J Tubercul Lung Dis. (2016) 20:1639–46. doi: 10.5588/ijtld.16.0382
134. Yun KW, Kim YK, Kim HR, Lee MK, Lim IS. Usefulness of interferon-γ release assay for the diagnosis of latent tuberculosis infection in young children. Korean J Pediatrics. (2016) 59:256–61. doi: 10.3345/kjp.2016.59.6.256
135. Beshir MR, Zidan AE, El-Saadny HF, Ramadan RA, Karam NA, Amin EK, et al. Evaluation of the immune response to interferon gamma release assay and tuberculin skin test among BCG vaccinated children in east of egypt a cross-sectional study. Medicine. (2016) 95:3470. doi: 10.1097/MD.0000000000003470
136. Wong KS, Huang YC, Hu HC, Huang YC, Wen CH, Lin TY. Diagnostic utility of QuantiFERON–TB gold in-tube test in pediatric tuberculosis disease in taiwanese children. J Microbiol Immunol Infect. (2017) 50:349–54. doi: 10.1016/j.jmii.2015.07.012
137. Gabriele F, Trachana M, Simitsopoulou M, Pratsidou-Gertsi P, Iosifidis E, Pana ZD, et al. Performance of QuantiFERON®-TB gold in-tube assay in children receiving disease modifying anti-rheumatic drugs. World J Pediatrics. (2017) 13:472–8. doi: 10.1007/s12519-017-0050-5
138. Mensah GI, Sowah SA, Yeboah NYA, Addo KK, Jackson-Sillah D. Utility of QuantiFERON Tuberculosis gold-in-tube test for detecting latent tuberculosis infection among close household contacts of confirmed tuberculosis patients in Accra, Ghana. Int J Mycobacteriol. (2017) 6:27–33. doi: 10.4103/2212-5531.201891
139. Li HJ, Xin HN, Qian SK, Li XW, Zhang HR, Li MF, et al. Testing of tuberculosis infection among Chinese adolescents born after terminating the Bacillus Calmette-Gu,rin booster vaccination: subgroup analysis of a population-based cross-sectional study. Front Med. (2017) 11:528–35. doi: 10.1007/s11684-017-0573-0
140. Petrucci R, Lombardi G, Corsini I, Bacchi Reggiani ML, Visciotti F, Bernardi F, et al. Quantiferon-TB gold in-tube improves tuberculosis diagnosis in children. Pediatric Infect Dis J. (2017) 36:44–9. doi: 10.1097/INF.0000000000001350
141. Silveira MBV, Ferrarini MAG, Viana PO, Succi RC, Terreri MT, Costa-Carvalho B, et al. Contribution of the interferon-gamma release assay to tuberculosis diagnosis in children and adolescents. Int J Tubercul Lung Dis. (2018) 22:1172–8. doi: 10.5588/ijtld.17.0883
142. Bielecka T, Komorowska-Piotrowska A, Krenke K, Feleszko W, Kulus M. Is secretion of IFN-gamma in response to Mycobacterium tuberculosis antigens in youngest children sufficient to play a role in TB diagnostics? Pediatric Pulmonol. (2018) 53:181–8. doi: 10.1002/ppul.23910
143. Chiappini E, Zaffaroni M, Bianconi M, Veneruso G, Grasso N, Garazzino S, et al. Italian multicentre study found infectious and vaccine-preventable diseases in children adopted from Africa and recommends prompt medical screening. Acta Paediatr. (2018) 107:1581–6. doi: 10.1111/apa.14237
144. Mastrolia MV, Sollai S, Totaro C, Putignano P, de Martino M, Galli L, et al. Utility of tuberculin skin test and IGRA for tuberculosis screening in internationally adopted children: Retrospective analysis from a single center in Florence, Italy. Travel Med Infect Dis. (2018) 28:64–7. doi: 10.1016/j.tmaid.2018.07.009
145. Mandalakas AM, Highsmith HY, Harris NM, Pawlicka A, Kirchner HL. T-SPOT.TB Performance in routine pediatric practice in a low TB burden setting. Pediatric Infect Dis J. (2018) 37:292–7. doi: 10.1097/INF.0000000000001792
146. Gaensbauer J, Gonzales B, Belknap R, Wilson ML, O'Connor ME. Interferon-Gamma Release Assay-based screening for pediatric latent tuberculosis infection in an urban primary care network. J Pediatrics. (2018) 200:202–9. doi: 10.1016/j.jpeds.2018.04.034
147. Hormi M, Guerin-El Khourouj V, Pommelet V, Jeljeli M, Pedron B, Diana JS, et al. Performance of the QuantiFERON-TB gold assay among HIV-infected children with active tuberculosis in france. Pediatric Infect Dis J. (2018) 37:339–44. doi: 10.1097/INF.0000000000001774
148. Starshinova A, Zhuravlev V, Dovgaluk I, Panteleev A, Manina V, Zinchenko U, et al. A Comparison of intradermal test with recombinant tuberculosis allergen (diaskintest) with other immunologic tests in the diagnosis of tuberculosis infection. Int J Mycobacteriol. (2018) 7:32–9. doi: 10.4103/ijmy.ijmy_17_18
149. Sayyahfar S, Davoodzadeh F, Hoseini R, Rahimzadeh N, Otukesh H. Comparison of tuberculin skin test and interferon gamma release assay for diagnosis of latent tuberculosis infection in pediatric candidates of renal transplantation. Pediatr Transl. (2018) 22:13148. doi: 10.1111/petr.13148
150. Said K, Hella J, Ruzegea M, Solanki R, Chiryamkubi M, Mhimbira F, et al. Immunologic-based diagnosis of latent tuberculosis among children less than 5 years of age exposed and unexposed to tuberculosis in tanzania: implications for tuberculosis infection screening. Pediatric Infect Dis J. (2018) 38:333–9. doi: 10.1097/INF.0000000000002131
151. Sali M, Buonsenso D, D'Alfonso P, De Maio F, Ceccarelli M, Battah B, et al. Combined use of Quantiferon and HBHA-based IGRA supports tuberculosis diagnosis and therapy management in children. J Infect. (2018) 77:526–33. doi: 10.1016/j.jinf.2018.09.011
152. Vortia E, Uko VE, Yen-Lieberman B, Frawley J, Worley SE, Danziger-Isakov L, et al. Low Indeterminate rates associated with use of the QuantiFERON-TB gold in-tube test in children with inflammatory bowel disease on long-term infliximab. Inflamm Bowel Dis. (2018) 24:877–82. doi: 10.1093/ibd/izx077
153. Chang KC, Leung ECC, Leung CC. Interferon-gamma release assays in childhood tuberculosis: a systematic review. Hong Kong J Paediatrics. (2009) 14:86–95.
154. Diel R, Loddenkemper R, Nienhaus A. Evidence-Based comparison of commercial interferon-gamma release assays for detecting active TB. Chest. (2010) 137:952–68. doi: 10.1378/chest.09-2350
155. Cattamanchi A, Smith R, Steingart KR, Metcalfe JZ, Date A, Coleman C, et al. Interferon-gamma release assays for the diagnosis of latent tuberculosis infection in HIV-infected individuals: a systematic review and meta-analysis. J Acquir Immune Defic Syndr. (2011) 56:230–8. doi: 10.1097/QAI.0b013e31820b07ab
156. Oni T, Gideon HP, Bangani N, Tsekela R, Seldon R, Wood K, et al. Risk factors associated with indeterminate gamma interferon responses in the assessment of latent tuberculosis infection in a high-incidence environment. Clin Vaccine Immunol. (2012) 19:1243–7. doi: 10.1128/CVI.00166-12
157. Aabye MG, Ravn P, PrayGod G, Jeremiah K, Mugomela A, Jepsen M, et al. The impact of HIV infection and CD4 cell count on the performance of an interferon gamma release assay in patients with pulmonary tuberculosis. PLoS ONE. (2009) 4:e4220. doi: 10.1371/journal.pone.0004220
158. Sharninghausen JC, Shapiro AE, Koelle DM, Kim HN. Risk factors for indeterminate outcome on interferon gamma release assay in non-us-born persons screened for latent tuberculosis infection. Open Forum Infect Dis. (2018) 5:ofy184. doi: 10.1093/ofid/ofy184
159. Kordy F, Waters V, Den-Hollander C, Read S, Lam R, Kitai I. Optimizing blood collection and processing for quantiferon-tb gold in-tube testing gives low rates of indeterminate results: clinical implications. Pediatr Infect Dis J. (2018) 37:e22–e4. doi: 10.1097/INF.0000000000001732
160. Borkow G, Leng Q, Weisman Z, Stein M, Galai N, Kalinkovich A, et al. Chronic immune activation associated with intestinal helminth infections results in impaired signal transduction and anergy. J Clin Invest. (2000) 106:1053–60. doi: 10.1172/JCI10182
161. Resende Co T, Hirsch CS, Toossi Z, Dietze R, Ribeiro-Rodrigues R. Intestinal helminth co-infection has a negative impact on both anti-Mycobacterium tuberculosis immunity and clinical response to tuberculosis therapy. Clin Exper Immunol. (2007) 147:45–52.
162. Decker ML, Gotta V, Wellmann S, Ritz N. Cytokine profiling in healthy children shows association of age with cytokine concentrations. Sci Rep. (2017) 7:17842. doi: 10.1038/s41598-017-17865-2
163. Decker ML, Grobusch MP, Ritz N. Influence of age and other factors on cytokine expression profiles in healthy children-a systematic review. Front Pediatrics. (2017) 5:255. doi: 10.3389/fped.2017.00255
164. Fabre V, Shoham S, Page KR, Shah M. High Proportion of Indeterminate QuantiFERON-TB gold in-tube results in an inpatient population is related to host factors and preanalytical steps. Open Forum Infect Dis. (2014) 1:ofu088. doi: 10.1093/ofid/ofu088
165. Herrera V, Yeh E, Murphy K, Parsonnet J, Banaei N. Immediate incubation reduces indeterminate results for QuantiFERON-TB Gold in-tube assay. J Clin Microbiol. (2010) 48:2672–6. doi: 10.1128/JCM.00482-10
166. Doberne D, Gaur RL, Banaei N. Preanalytical delay reduces sensitivity of QuantiFERON-TB gold in-tube assay for detection of latent tuberculosis infection. J Clin Microbiol. (2011) 49:3061–4. doi: 10.1128/JCM.01136-11
167. Doherty TM, Demissie A, Menzies D, Andersen P, Rook G, Zumla A, et al. Effect of sample handling on analysis of cytokine responses to Mycobacterium tuberculosis in clinical samples using ELISA, ELISPOT and quantitative PCR. J Immunol Methods. (2005) 298:129–41. doi: 10.1016/j.jim.2005.01.013
168. Jarvis J, Gao Y, de Graaf H, Hughes S, Allan RN, Williams A, et al. Environmental temperature impacts on the performance of QuantiFERON-TB Gold In-Tube assays. J Infect. (2015) 71:276–80. doi: 10.1016/j.jinf.2015.04.004
Keywords: Clinical studies, IGRA, latent, pediatrics, risk difference, QuantiFERON, T-SPOT.TB, T cell response
Citation: Meier NR, Volken T, Geiger M, Heininger U, Tebruegge M and Ritz N (2019) Risk Factors for Indeterminate Interferon-Gamma Release Assay for the Diagnosis of Tuberculosis in Children—A Systematic Review and Meta-Analysis. Front. Pediatr. 7:208. doi: 10.3389/fped.2019.00208
Received: 14 November 2018; Accepted: 08 May 2019;
Published: 29 May 2019.
Edited by:
Dimitri Van der Linden, Cliniques Universitaires Saint-Luc, BelgiumReviewed by:
Delane Shingadia, Great Ormond Street Hospital, United KingdomTea Nieminen, Helsinki Children's Hospital, Finland
Anna Starshinova, Saint Petersburg State University, Russia
Copyright © 2019 Meier, Volken, Geiger, Heininger, Tebruegge and Ritz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nicole Ritz, bmljb2xlLnJpdHpAdW5pYmFzLmNo
 Noëmi R. Meier
Noëmi R. Meier Thomas Volken
Thomas Volken Marc Geiger
Marc Geiger Ulrich Heininger
Ulrich Heininger Marc Tebruegge
Marc Tebruegge Nicole Ritz
Nicole Ritz