- 1Allergy Immunology Unit, Department of Paediatrics, Advances Paediatrics Centre, Post Graduate Institute of Medical Education and Research, Chandigarh, India
- 2Department of Cardiology, Advances Cardiac Centre, Post Graduate Institute of Medical Education and Research, Chandigarh, India
Kawasaki disease (KD) is now a common cause of acquired heart disease in children. Coronary artery involvement is the most serious complication in children with KD. Several non-coronary complications have now been identified in this condition but these are often overlooked. Myocarditis is an integral component of KD and may be more common than coronary artery abnormalities. Pericardial involvement and valvular abnormalities have also been observed in patients with KD. KD shock syndrome is now being increasingly recognized and may be difficult to differentiate clinically from toxic shock syndrome. Endothelial dysfunction has been reported both during acute stage and also on follow-up. This may be a potentially modifiable cardiovascular risk factor.
Introduction
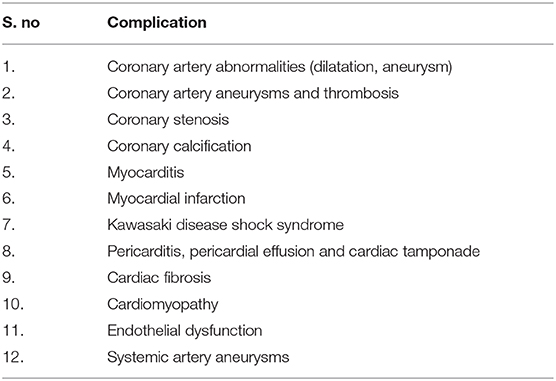
Kawasaki disease (KD) is one of the commonest vasculitides in children (1, 2). At time of its first recognition in 1967 by Dr. Tomisaku Kawasaki, it was described as “mucocutaneous lymph node syndrome” and was considered as a benign disease with self-limiting course (3). However, autopsy studies later revealed the coronary artery complications associated with KD (4). Over time, it has now been realized that KD may cause several other cardiac complications as well (5, 6) (Table 1). It has been shown that myocarditis in KD is, in fact, more common than coronary artery involvement and may be almost universal (7). In this review, we have discussed various non-coronary cardiac complications in patients with KD.
Myocarditis
Myocarditis appears to be an integral part of KD and may be seen in all patients (7, 8). Fujiwara et al. in 1978 have reported autopsy studies on 20 patients with KD (4). The authors classified pathology of KD into four clinico-pathological stages and noted pancarditis on histology. In the first 9 days of illness, predominant finding was carditis associated with edema and inflammatory cell infiltrate in all three layers of heart (4). Yutani et al. have performed right ventricular biopsy in 201 patients with KD after periods ranging from 1 month to 11 years of diagnosis of KD (9). They showed that myocarditis and fibrotic changes were seen in all patients. Sequelae of myocarditis were evident even during follow-up (9). Similarly, Yonesaka et al. have performed subendocardial myocardial biopsies and showed that findings of myocyte disarray, interstitial fibrosis and myocardial cell degeneration persist in patients with KD on follow-up (10). Necropsies have shown that patients with KD developed diffuse myocardial fibrosis and increased expression of transforming growth factor (TGF)-β in wall of coronary artery aneurysm (11). These studies help explain the pathogenesis of myocardial fibrosis/cardiomyopathy in children with KD on follow-up (9–12).
In 1995, Anderson et al. had published the long term effects of KD on cardiac function in 67 patients. Authors performed serial M-mode echocardiograms at baseline, 1–3, 3–12 months, and after 1 year of diagnosis. This study showed that left atrial and left ventricular dimensions continued to be abnormal in more than 50% of patients even 1 year after KD. Fractional shortening was abnormal initially but normalized at 3 months of follow-up. Left ventricular emptying was significantly reduced. Moreover, almost a third of patients evaluated beyond 1 year had diastolic dysfunction. This was amongst the first few studies showing that patients with KD had abnormalities in cardiac functions even in absence of CAAs (13).
Nakaoka et al. have recently reported on cardiac function in patients with KD having asymptomatic coronary artery disease by cardiac magnetic resonance (CMR) imaging. It was found that transmural extent of late gadolinium enhancement in this subgroup was ≤50% and these patients had subendocardial infarction with normal left ventricular function (14).
Pathophysiology
KD myocarditis often develops as a result of acute or subacute inflammation of interstitial tissue of myocardium and is usually concentrated around the coronaries. Myocardial inflammation peaks by day 10 of illness and gradually subsides by end of 3 weeks. During this stage, there is inflammation of small arteries of the myocardium including perivasculitis. Inflammation in interstitial tissue of the myocardium develops as a result of spill of inflammatory cells from perivasculitis. This explains prompt recovery of myocardial function following administration of intravenous immunoglobulin (IVIg) in these patients. Pathophysiology of KD myocarditis, therefore, differs in several aspects from viral myocarditis. While viral myocarditis is characterized by predominant lymphocytic interstitial cell infiltrates, edema and myocyte or myocardial fiber bundle necrosis, KD myocarditis on other hands is characterized by myocardial interstitial edema, vasodilatation and inflammatory cell infiltration. Severe myocarditis in patents with KD can manifest independent of coronary artery involvement. Rarely, patents with KD and severe inflammatory myocardial inflammation can have degenerative changes resulting in cardiomyopathy (8, 15, 16).
Clinical Characteristics
Myocarditis, which is one of the earliest presentations of KD, usually presents within first 10 days of illness in contrast to coronary artery vasculitis and coronary artery abnormalities (CAAs) that usually develop after day 10 of illness (8, 15). Audible gallop, tachycardia and hyperdynamic precordium are the subtle clinical correlates of KD myocarditis. Strain abnormalities and evidence of systolic and diastolic dysfunction are correlates of KD myocarditis on echocardiography (8).
Asymptomatic Myocarditis
Myocarditis in patients with KD is often asymptomatic and can easily be missed (6, 8).
Symptomatic Myocarditis
Myocarditis in KD can present clinically as unexplained tachycardia, congestive cardiac failure, hemodynamic instability, requirement of inotropic support and arrhythmias (17–23). Symptomatic myocarditis remains a significant cause of morbidity and mortality during the initial phase of the KD (15, 24–26).
Viral Myocarditis vs. KD Associated Myocarditis
Myocarditis of KD needs to be differentiated from viral myocarditis:
• Viral myocarditis generally follows a prodrome and at time of clinical presentation patients are usually afebrile. Myocarditis in KD develops in the early phase of disease and is usually accompanied by high grade fever (8).
• Myocardial dysfunction in KD is usually transient and responds dramatically to anti-inflammatory therapy with intravenous immunoglobulin (IVIg) (8, 15). Response to IVIg in viral myocarditis is, at best, modest (27, 28).
• The pathological changes in KD largely consist of interstitial edema and inflammatory cell infiltrate, while in viral myocarditis cell necrosis is the predominant finding (15).
Biomarkers for Myocarditis
Several biomarkers have been proposed in patients with KD to evaluate myocardial dysfunction and injury. The biomarker that has shown clinical promise is N-terminal pro B-type natriuretic peptide (NT-proBNP) (8, 29, 30).
NT-proBNP
In response to volume and pressure cardiac overload, pre-pro-BNP is synthesized and processed to pro-BNP. Pro-BNP is then processed to biologically active BNP fragment, and NT-pro-BNP which is inert. NT-ProBNP is preferred to BNP as a biomarker for laboratory assays as it has a longer half-life. Synthesis of pro-BNP from cardiac myocytes is controlled by many factors including mechanical factors like dilatation and strain of cardiac chambers, various neurohormonal factors and cytokines (e.g., interleukin-1 β or tumor necrosis factor α). Interpretation of pro-BNP levels is difficult as it can be affected by several factors other than myocardial damage. Pro-BNP levels are age dependent and are highest in infancy and early childhood (31). Presence of acute kidney injury and decreased glomerular filtration is also associated with falsely elevated pro-BNP levels (30). Several studies have found NT-ProBNP to be a useful marker for diagnosis as well as for assessment of disease severity in KD (29, 32, 33). Age specific cut-off values have been calculated and Z scores are also available for assessment of elevated levels of pro-BNP (29, 31). Reddy et al. have assayed levels of pro-BNP during the acute stage of KD. The authors reported that levels above 1025 pg/ml have a specificity of 96% and sensitivity of 88% for diagnosis of KD (33).
Studies have shown a positive correlation of NT-pro-BNP with C-reactive protein and hypoalbuminemia in children with KD during initial phase of disease. NT-pro-BNP levels are significantly raised during the acute phase of KD when compared to controls (32, 34). Levels of NT-pro-BNP showed negative correlation with left ventricular (LV) ejection fraction, fractional shortening, cardiac index values, diastolic function and positive correlation with impairment in ventricular relaxation (32, 34).
NT-pro-BNP is a valuable assessment tool in clinical evaluation of patients with incomplete forms of KD (29, 35, 36). Dionne et al. have proposed a diagnostic algorithm based on NT-pro-BNP. This is very useful in patients with incomplete forms of KD (29).
Cardiac Troponins
Serum cardiac troponins are superior to creatine kinase (CK)-MB for detection of myocardial damage in myocarditis (37). Kim et al. have compared cardiac troponin I and CK-MB levels in 45 patients with KD. Authors showed that levels of cardiac troponin I were elevated in 18 (40%) patients, while CK-MB levels were elevated in 11 (24%) patients (38).
Sato et al. have measured cardiac troponin by a highly sensitive assay and shown that cardiac troponin levels are elevated in 1/3rd of children with KD during the acute phase. These levels may continue to remain elevated during the convalescent phase as well (34). However, levels of cardiac troponin have very weak correlation with NT-pro-BNP and there was no significant correlation with systolic or diastolic function or CAAs in patients with KD (34). Checchia et al. have shown that elevation of cardiac troponin I in patients with KD was not significant and there was no significant correlation with development of CAAs (39).
Other Cardiac Biomarkers
Soluble suppression of tumorigenicity-2 (ST2) belongs to the IL-1 receptor family. It is released by cardiomyocytes and fibroblasts during stress phase of KD myocarditis. It has been reported to be positively correlated with impairment of ventricular relaxation in patients with KD (27). Gamma-glutamyl transferase and alanine transferase, however, have not been found to be useful in establishing a clinical diagnosis of KD (40).
Imaging in Myocarditis
Echocardiographic Features
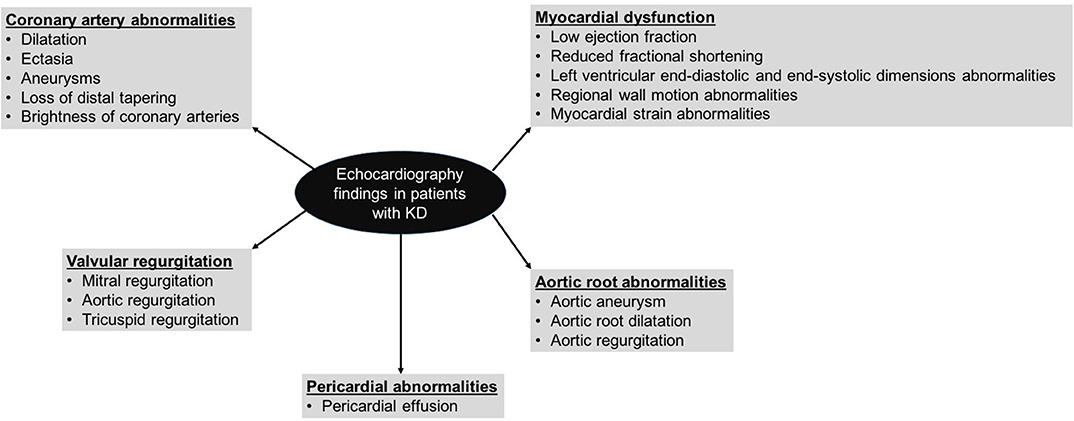
2D-echocardiography remains the mainstay of imaging for cardiovascular assessment in KD both during acute phase and long-term follow-up (1, 2, 36, 41, 42). It cannot be overemphasized that cardiac assessment in patients with KD is just not limited to coronary artery assessment and detailed cardiac assessment for ventricular functions, wall motion abnormalities, valvular functions, and pericardial effusion need to be performed as well (Figure 1). Traditional echocardiographic evaluation of KD myocarditis by M mode includes parameters for left ventricular systolic dysfunction and left ventricular dilatation. The diastolic function of the heart is assessed by inflow parameters across both atrio-ventricular valves by pulse wave doppler and tissue doppler.
Myocarditis is universal in almost all patients with KD during the acute phase of disease. Transient left ventricular dysfunction can occur in more than 50% patients (43). Newburger et al. showed that left ventricular dysfunction in patients with KD appears unrelated to development of CAAs (44). Normal systolic function is restored after recovery from acute illness in most of the patients with KD. However, diastolic dysfunction has also been found in many studies. Therefore, ventricular function assessment should be an integral part of echocardiographic evaluation in children with KD. This should include assessment of regional wall motion abnormalities that are often a surrogate marker for coronary artery involvement (1, 41).
Speckle tracking echocardiography (STE) is a sensitive tool that can accurately detect myocardial strain and can quantify myocardial function with high reproducibility. More sensitive measures of myocardial deformation, such as global longitudinal strain, circumferential strain, and strain rate have been reported to be decreased in KD (45). Strain abnormalities in patients with KD have been seen even in absence of apparent systolic function abnormalities.
Xu et al. have shown that left ventricular systolic strain decreased significantly in children with KD during acute phase of disease. However, it improved rapidly after IVIg therapy and normalized by 6–8 weeks (46). Regional left ventricular strain was found to be impaired in basal infero-septal, basal anterolateral, apical septal, and apical inferior segments in patients with KD during midterm follow up when compared with controls (47). Left atrial strain is a well-recognized surrogate marker for raised left ventricular end diastolic pressure and left ventricular diastolic dysfunction. Lower values of left atrial strain have been reported during the acute stage in KD and this may improve during follow-up (48). Studies have reported correlation between depressed strain and disease severity (49, 50). Strain imaging may also be useful during follow-up of these patients (51). Newburger et al. showed that velocity of circumferential fiber shortening corrected for wall stress, was reduced in patients with KD during and up to 3 months after acute illness and improved spontaneously by 1 year (44).
Dedeoglu et al. have evaluated myocardial deformation at 6 months follow-up and measured global as well as regional myocardial strain by STE and showed impaired left ventricular strain in patients with KD in basal and apical segments. However, there was no association between LV dysfunction and CAAs (47). Wang et al. compared STE findings in patients with IVIg resistant KD and IVIg responsive patients with KD. It was found that the former had more severe ventricular dysfunction (52).
To conclude, mere assessment of “Z” scores of coronary arteries in children with KD is not enough. Attempts should be made to look for abnormalities of myocardial function. It is also apparent that myocardial dysfunction in KD can occur independent of coronary artery involvement.
Other Imaging Modalities
There are several inherent limitations associated with 2D-echocardiography. It is highly observer dependent and the results are not always reproducible (36, 41). Studies using nuclear scans have shown that myocardial inflammation can be seen in more than 50% of patients. We have published our experience on exercise myocardial perfusion scintigraphy on 84 patients with KD at least 1 month after onset of illness (53). In this study, 12 (14.3%) patients showed reversible perfusion defects and these can be seen even in patients with no demonstrable CAAs on echocardiography (53).
Tacke et al. have evaluated cardiac function in children with KD at follow-up and showed that there was no significant difference in cardiac function and fibrosis in patients with KD compared to controls while using CMR at long-term follow-up (54). In a more recent study, Bratis et al. have reported LV myocardial deformation indices using CMR and found that there was reduced myocardial strain values during the convalescent phase and this was irrespective of coronary artery involvement (55). Clearly, we need more studies to fully comprehend the residual effects of KD on the myocardium.
ECG Features
Several conduction and repolarization abnormalities have been reported in patients with KD. These include non-specific ST and T-wave changes, PR interval prolongation, QT dispersion abnormalities and arrhythmias. In presence of severe myocarditis/pericarditis, low voltage complexes, and symptomatic arrhythmias may be seen (56–58). Bifid T-wave in limb leads have also been noted during the acute phase of KD (59). QT dispersion abnormalities may persist for several months (56, 57). Persistence of repolarization abnormalities in follow-up may indicate higher risk of ventricular arrhythmia during follow-up of patients with KD even in absence of obvious echocardiographic abnormalities (60).
Long Term Complications of Myocarditis
In conclusion, while it is likely that most children with KD myocarditis would remain well on follow-up and attain normal systolic function, a few patients may go on to develop myocardial dysfunction, fibrosis, myocardial infarction later in life. Further, these manifestations may occur even in patients who have had no obvious CAAs (7, 8, 11).
KD Shock Syndrome (KDSS)
KDSS is said to be occur when a patient with clinical diagnosis of KD develops systolic hypotension or shock. Although shock during acute stage of KD was recognized more than two decades ago (61–63), Kanegaye et al. defined KDSS for the first time in 2009 (64). In this study, hemodynamic instability was observed in 13/187 (7%) patients with KD (64). Since then, this entity has been reported from several centers across the world (65–67). These patients are often misdiagnosed as toxic shock syndrome (TSS) and this led to delays in institution of appropriate therapy (66, 67). KDSS is usually seen in older children and is more commonly reported in boys (66, 68, 69), although some studies have also noted a female predominance (70). Some authorities believe that KDSS may, in fact, be a unique subtype of KD (65). KDSS still remains an under-recognized complication (71).
The etiopathogenesis of KDSS remains poorly understood. It involves a combination of myocardial dysfunction (secondary to myocarditis) and distributive shock (caused by increased vascular permeability which is secondary to dysregulated cytokine storm) (8, 66, 72).
Patients with KDSS have been reported to have increased incidence of gastrointestinal manifestations, hyponatremia, anemia, thrombocytopenia, hypoalbuminemia, elevated inflammatory markers (e.g., neutrophila, high CRP, ESR), IVIg resistance, CAAs (up to 65%), morbidity and mortality (up to 6.8%) (65, 68, 69, 73). In addition, levels of several cytokines (e.g., TNF-α, interferon-γ) are found to be elevated in patients with KDSS (74). Whether these biomarkers can be considered for early diagnosis of KDSS remains conjectural.
How Does One Differentiate Between TSS and KDSS?
The clinical presentation of KDSS and TSS may appear similar and it may be very difficult to differentiate the two conditions at bedside. Lin et al. retrospectively analyzed 16 patients with KDSS and 17 patients with TSS (69). It was found that patients with KDSS were usually younger and had less prominent gastrointestinal symptoms. While anemia and thrombocytosis were more commonly seen in patients with KD, lymphopenia was characteristic of TSS. Further, CAAs and valvular abnormalities were seen only in KDSS (69, 75).
Hyper-Inflammatory Syndrome Associated With COVID-19—A Novel Syndrome
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection has rapidly spread worldwide since it was first identified in Wuhan, China in November 2019. Initial reports suggested that SARS-CoV-2 causes milder disease in children. However, by late April 2019a hyper-inflammatory syndrome had been identified (76). This was characterized by high grade persistent fever and multisystemic clinical manifestations suggesting a delayed hyperimmune response to SARS-CoV-2 infection. This novel syndrome has been variably termed as “multisystem inflammatory disorder in children and adolescents,” “multisystem inflammatory syndrome in children (MIS-C),” “pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS)” (77). Clinical features of this syndrome include cardiovascular collapse (e.g., hypotension, myocarditis, and myocardial dysfunction), predominant gastrointestinal symptoms (e.g., diarrhea, vomiting, and pain abdomen), features similar to KD (e.g., rash, conjunctival injection, and extremity changes), and neurological manifestations (e.g., headache, irritability, and encephalopathy). Reported data suggest that patients with MIS-C are usually older, have predominant gastrointestinal manifestations (some may present with acute surgical abdomen) and have myocardial dysfunction. Clinical findings in this syndrome may mimic those of KDSS and TSS (77–79). MIS-C and KD are probably two distinct entities as they have differences in demographic, laboratory and clinical findings (80, 81). Whittaker et al. have compared patients with PIMS-TS with KD, KDSS and TSS (82). Authors reported that patients with PIMS-TS are older than the ones in latter three categories. Further, patients with PIMS-TS were found to have higher inflammatory markers, more pronounced lymphopenia, and higher levels of troponins and NT-pro-BNP (82).
Pericarditis
Pericarditis is a common but an under-reported manifestation in patients with KD. Gowin et al. (83) reported pericarditis in 6/30 (20%) patients while Hamza et al. (84) found pericardial involvement in 7.8% of patients with KD.
Pericardial involvement is usually mild and not clinically significant. Septate pericarditis (85) and tamponade (86–88) that may occasionally be fatal has also been reported. Cardiac tamponade may be a component of polyserositis syndrome in patients with KD or may follow rupture of one of the coronary artery aneurysms in the pericardial cavity (89). While polyserositis leading to tamponade would manifest during the acute stage, tamponade caused by rupture of aneurysm may appear at any time (90, 91) and may even manifest several years after acute KD (92). Printz et al. reported transient pericardial effusion in 3% patients of KD and it resolved by 5 weeks (93).
Mild pericarditis may resolve with IVIg and aspirin. Patients with more severe forms of pericarditis may require additional immunomodulatory therapy (94). Cardiac tamponade from coronary artery aneurysmal rupture may require urgent pericardial window and emergency coronary artery bypass grafting (89, 90).
Valvular Abnormalities
Valvular regurgitation in acute phase has been ascribed to pancarditis, while patients having persistent valvular abnormalities are likely to have valvular dysfunction and papillary muscle dysfunction due to coronary ischemia (95). Myocardial inflammation can lead to valvular regurgitation. The most common abnormality is mitral regurgitation (MR) during acute phase of KD—this usually resolves on follow-up. It is seen commonly in patients with KD who have wall motion abnormalities or reduced ejection fraction (1, 8, 41, 93, 96). Some patients can go on to develop severe MR due to rupture of chorda tendinae. This complication may result in rapid clinical deterioration and even fatalities if not recognized and treated in time (97–99) Cardiac auscultation is, therefore, very important in patients with KD especially during the convalescent phase. Aortic regurgitation (AR) has also been reported (100, 101).
de La Harpe et al. have recently published 30 years of experience in KD and showed that 20% of patients in their cohort had valvular involvement and of these 88% had mitral valve dysfunction (96). Printz et al. have prospectively performed 2D-echocardiogtaphy on 198 patients with KD at baseline, 1 and 5 weeks of onset of illness (93). They showed that 27% of patients with KD had mild mitral regurgitation at baseline echocardiography during acute phase. Although MR had resolved significantly on follow-up at 5 weeks, 9% patients continued to have residual valvular dysfunction (93). It has, therefore, been suggested that Doppler evaluation should be a part of echocardiography evaluation in children with KD for assessment of valvular regurgitation abnormalities.
Aortic Root Involvement
KD is a systemic vasculitis and affects several non-coronary arteries in the body. Aortic root dilatation during acute stage of KD has been reported in up to 10% patients with KD (93). Ravekes et al. assessed aortic root abnormalities in patients with KD (102). Aortic root diameters were assessed during mid-systole and at four different time points (i.e., within first 10 days, at 2, 6 weeks, and 1 year). It was observed that aortic root diameters were significantly higher as compared to controls. A significant increase in aortic root diameter was noted at 2 weeks of follow-up. Subsequently, no significant increase in aortic root diameter was noted at 6 weeks and at 1 year follow-up but the aortic root continued to remain dilated (102). Printz et al. reported aortic root dilatation at baseline, at 1 week and at 5 weeks in patients with KD (93). These authors did not report any significant change in aortic root diameters when assessed at different time intervals. Size of aortic root was found to correlate with coronary artery diameters but not with inflammatory parameters. Both studies used body surface area adjusted Z scores for assessment of aortic root diameter. AR was reported in 1–4% patients and was more common at 1 year of follow-up. Patients with AR may require valve replacement later in life (62, 103).
Increase in stiffness of aorta and decrease in elasticity has also been reported by several authors leading to functional impairment of aorta. This has been observed both during the acute stage and several years after the diagnosis of KD (104–106).
Systemic Artery Involvement
Kato et al. first time performed angiography studies in patients with KD and revealed that 13/594 (2.2%) patients had systemic artery aneurysm (SAAs) in addition to CAAs (107). It was reported that SAAs were present only in patients who had multiple giant CAAs. Since than there have been reports of SAAs involving large arteries (e.g., iliac, femoral, subclavian, axillary). Recently Zhao et al. have reported full-body magnetic resonance angiography (MRA) or peripheral angiography in patients with KD for identification of SAAs (108). MRA (n = 110) was performed in patients with KD who had presumed risk factors for SAAs (e.g., patients having giant coronary aneurysm, increasing size aneurysm during acute phase or IVIg resistant KD). Peripheral angiography (n = 52) was performed along with CT coronary angiography in patients with giant or medium sized coronary aneurysms. Authors reported that 23/162 (14.2%) patients with KD having CAAs had SAAs, while overall prevalence was 23/1148 (2%). Most commonly affected arteries were axillary and common iliac. Risk factors for development of SAAs were young age and pronged fever (108). It appears that KD may also have a component of systemic vasculitis but this needs more detailed evaluation (107–109).
Endothelial Dysfunction in Patients With KD
Endothelial dysfunction in KD often goes unrecognized. It is attributed to release of pro-inflammatory mediators that lead to production of reactive oxygen species. Brachial artery flow mediated dilatation (FMD) evaluation is a reliable marker for endothelial dysfunction. FMD depicts the capacity of brachial artery to increase its diameter in response to increase in blood flow. Studies have shown endothelial dysfunction of brachial artery in patients with KD that may persist even a decade after the acute stage (105, 110–115). Dietz et al. has observed the increased stiffness index patients with KD and CAAs (116). These abnormalities have also been reported in patients who have no obvious CAAs detected during acute stage of KD (117). Pulse wave velocity (PWV) is also a simple and non-invasive tool for assessment of arterial stiffness. Studies have shown that PWV is higher in patients with history of KD as compared to controls (118). Carotid intima-media thickness (cIMT), well-recognized as a surrogate marker for atherosclerosis, has been found to be significantly higher in children with KD on follow-up. These studies emphasize the need for long-term follow-up of children with KD even in situations wherein there have been no CAAs (116, 119–122).
It is conjectural whether endothelial dysfunction correlates with occurrence of CAAs (123–126). Patients with KD and CAAs have been reported to have an increased risk of myocardial infarction and early deaths (11, 116, 127–130). Recent literature has, however, suggested that long term cardiovascular morbidity in patients with KD may not only be restricted to patients who have been detected to have CAAs (11, 131). Autopsy studies have demonstrated luminal myofibroplastic proliferation in both aneurysmal as well as non-aneurysmal coronary arteries (16). Therefore, it is prudent to counsel patients with KD with regard to modifiable cardiovascular risk factors.
To conclude, it is clear that KD is associated with several cardiovascular sequelae. While CAAs are the most well-recognized complications of this condition, other affectations like myocarditis, KDSS, valvular abnormalities, and endothelial dysfunction are now being increasingly recognized. Early identification and appropriate treatment of these complications is of paramount importance.
Author Contributions
RP and AJ: writing of initial draft of manuscript, editing and revision of manuscript at all stages of its production, and review of literature. DB and SN: contributed to editing of manuscript and review of literature. SS: inception of idea, critically revision of the manuscript at all stages of its production, final approval of manuscript, and review of literature. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. McCrindle BW, Rowley AH, Newburger JW, Burns JC, Bolger AF, Gewitz M, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation. (2017) 135:e927–9. doi: 10.1161/CIR.0000000000000484
2. Singh S, Jindal AK, Pilania RK. Diagnosis of Kawasaki disease. Int J Rheum Dis. (2018) 21:36–44. doi: 10.1111/1756-185X.13224
3. Kawasaki T. Acute febrile mucocutaneous syndrome with lymphoid involvement with specific desquamation of the fingers and toes in children. Arerugi Allergy. (1967) 16:178–222.
4. Fujiwara H, Hamashima Y. Pathology of the heart in Kawasaki disease. Pediatrics. (1978) 61:100–7.
5. Kato H. Cardiovascular complications in Kawasaki disease: coronary artery lumen and long-term consequences. Prog Pediatr Cardiol. (2004) 19:137–45. doi: 10.1016/j.ppedcard.2004.08.007
6. Jindal AK, Pilania RK, Prithvi A, Guleria S, Singh S. Kawasaki disease: characteristics, diagnosis and unusual presentations. Expert Rev Clin Immunol. (2019) 15:1089–104. doi: 10.1080/1744666X.2019.1659726
7. Dahdah N. Not just coronary arteritis, Kawasaki disease is a myocarditis, too. J Am Coll Cardiol. (2010) 55:1507–8. doi: 10.1016/j.jacc.2009.11.067
8. Dionne A, Dahdah N. Myocarditis and Kawasaki disease. Int J Rheum Dis. (2018) 21:45–9. doi: 10.1111/1756-185X.13219
9. Yutani C, Go S, Kamiya T, Hirose O, Misawa H, Maeda H, et al. Cardiac biopsy of Kawasaki disease. Arch Pathol Lab Med. (1981) 105:470–3.
10. Yonesaka S, Nakada T, Sunagawa Y, Tomimoto K, Naka S, Takahashi T, et al. Endomyocardial biopsy in children with Kawasaki disease. Acta Paediatr Jpn Overseas Ed. (1989) 31:706–11. doi: 10.1111/j.1442-200X.1989.tb01384.x
11. Gordon JB, Kahn AM, Burns JC. When children with Kawasaki disease grow up. J Am Coll Cardiol. (2009) 54:1911–20. doi: 10.1016/j.jacc.2009.04.102
12. Yonesaka S, Takahashi T, Eto S, Sato T, Otani K, Ueda T, et al. Biopsy-proven myocardial sequels in Kawasaki disease with giant coronary aneurysms. Cardiol Young. (2010) 20:602–9. doi: 10.1017/S1047951109991132
13. Anderson TM, Meyer RA, Kaplan S. Long-term echocardiographic evaluation of cardiac size and function in patients with Kawasaki disease. Am Heart J. (1985) 110(1 Pt 1):107–15. doi: 10.1016/0002-8703(85)90523-X
14. Nakaoka H, Tsuda E, Morita Y, Kurosaki K. Cardiac function by magnetic resonance imaging in coronary artery occlusions after Kawasaki disease. Circ J. (2020) 84:792–8. doi: 10.1253/circj.CJ-19-0511
15. Harada M, Yokouchi Y, Oharaseki T, Matsui K, Tobayama H, Tanaka N, et al. Histopathological characteristics of myocarditis in acute-phase Kawasaki disease. Histopathology. (2012) 61:1156–67. doi: 10.1111/j.1365-2559.2012.04332.x
16. Orenstein JM, Shulman ST, Fox LM, Baker SC, Takahashi M, Bhatti TR, et al. Three linked vasculopathic processes characterize Kawasaki disease: a light and transmission electron microscopic study. PLoS ONE. (2012) 7:e38998. doi: 10.1371/journal.pone.0038998
17. Yoshikawa H, Nomura Y, Masuda K, Hazeki D, Yotsumoto K, Arata M, et al. Four cases of Kawasaki syndrome complicated with myocarditis. Circ J. (2006) 70:202–5. doi: 10.1253/circj.70.202
18. Peduzzi TL, Pitetti RD. Myocardial infarction and atypical Kawasaki disease in a 3-month-old infant. Pediatr Emerg Care. (2002) 18:E16–9. doi: 10.1097/00006565-200210000-00008
19. Aggarwal P, Suri D, Narula N, Manojkumar R, Singh S. Symptomatic myocarditis in Kawasaki disease. Indian J Pediatr. (2012) 79:813–4. doi: 10.1007/s12098-011-0552-4
20. De Rosa G, Andreozzi L, Piastra M, Castelli B, Rigante D. Acute myocarditis as a revealing clue of complete Kawasaki disease. Reumatismo. (2018) 70:115–6. doi: 10.4081/reumatismo.2018.1101
21. Madhusudan S, Singh S, Rohit M, Gupta A, Suri D, Rawat A. Late symptomatic myocarditis in Kawasaki disease: an unusual manifestation. Indian J Pediatr. (2014) 81:404–5. doi: 10.1007/s12098-014-1339-1
22. Haney I, Beghetti M, McCrindle BW, Gow RM. Ventricular arrhythmia complicating Kawasaki disease. Can J Cardiol. (1995) 11:931–3.
23. Fujino M, Hata T, Kuriki M, Horio K, Uchida H, Eryu Y, et al. Inflammation aggravates heterogeneity of ventricular repolarization in children with Kawasaki disease. Pediatr Cardiol. (2014) 35:1268–72. doi: 10.1007/s00246-014-0926-2
24. Singh S, Bhattad S, Gupta A, Suri D, Rawat A, Rohit M. Mortality in children with Kawasaki disease: 20 years of experience from a tertiary care centre in North India. Clin Exp Rheumatol. (2016) 34:S129–33.
25. Gupta K, Rohit M, Sharma A, Nada R, Jain S, Varma S. An adolescent with kawasaki disease. Indian Pediatr. (2016) 53:51–6. doi: 10.1007/s13312-016-0791-6
26. Pucci A, Martino S, Tibaldi M, Bartoloni G. Incomplete and atypical kawasaki disease: a clinicopathologic paradox at high risk of sudden and unexpected infant death. Pediatr Cardiol. (2012) 33:802–5. doi: 10.1007/s00246-012-0186-y
27. Robinson J, Hartling L, Vandermeer B, Klassen TP. Intravenous immunoglobulin for presumed viral myocarditis in children and adults. Cochrane Database Syst Rev. (2015) CD004370. doi: 10.1002/14651858.CD004370.pub3
28. Jensen LD, Marchant DJ. Emerging pharmacologic targets and treatments for myocarditis. Pharmacol Ther. (2016) 161:40–51. doi: 10.1016/j.pharmthera.2016.03.006
29. Dionne A, Meloche-Dumas L, Desjardins L, Turgeon J, Saint-Cyr C, Autmizguine J, et al. N-terminal pro-B-type natriuretic peptide diagnostic algorithm versus American Heart Association algorithm for Kawasaki disease. Pediatr Int. (2017) 59:265–70. doi: 10.1111/ped.13154
30. Dionne A, Dahdah N. A decade of NT-proBNP in acute kawasaki disease, from physiological response to clinical relevance. Children (Basel). (2018) 5:141. doi: 10.3390/children5100141
31. Jun H, Ko KO, Lim JW, Yoon JM, Lee GM, Cheon EJ. Age-adjusted plasma N-terminal pro-brain natriuretic peptide level in Kawasaki disease. Korean J Pediatr. (2016) 59:298–302. doi: 10.3345/kjp.2016.59.7.298
32. Sun Y, Wei C, Wang W, Zheng X, Wang Y, Ma S, et al. Serum brain natriuretic peptide in children with Kawasaki disease. World J Emerg Med. (2010) 1:114–7.
33. Reddy M, Singh S, Rawat A, Sharma A, Suri D, Rohit MK. Pro-brain natriuretic peptide (ProBNP) levels in North Indian children with Kawasaki disease. Rheumatol Int. (2016) 36:551–9. doi: 10.1007/s00296-016-3430-6
34. Sato YZ, Molkara DP, Daniels LB, Tremoulet AH, Shimizu C, Kanegaye JT, et al. Cardiovascular biomarkers in acute Kawasaki disease. Int J Cardiol. (2013) 164:58–63. doi: 10.1016/j.ijcard.2011.06.065
35. Rawat A, Singh S. Biomarkers for diagnosis of Kawasaki Disease. Indian Pediatr. (2015) 52:473–4. doi: 10.1007/s13312-015-0658-2
36. Pilania RK, Bhattarai D, Singh S. Controversies in diagnosis and management of Kawasaki disease. World J Clin Pediatr. (2018) 7:27–35. doi: 10.5409/wjcp.v7.i1.27
37. Bachmaier K, Mair J, Offner F, Pummerer C, Neu N. Serum cardiac troponin T and creatine kinase–MB elevations in murine autoimmune myocarditis. Circulation. (1995) 92:1927–32. doi: 10.1161/01.CIR.92.7.1927
38. Kim M, Kim K. Elevation of cardiac troponin i in the acute stage of Kawasaki disease. Pediatr Cardiol. (1999) 20:184–8. doi: 10.1007/s002469900437
39. Checchia PA, Borensztajn J, Shulman ST. Circulating cardiac troponin I levels in Kawasaki disease. Pediatr Cardiol. (2001) 22:102–6. doi: 10.1007/s002460010170
40. Chaudhary H, Nameirakpam J, Kumrah R, Pandiarajan V, Suri D, Rawat A, et al. Biomarkers for Kawasaki disease: clinical utility and the challenges ahead. Front Pediatr. (2019) 7:242. doi: 10.3389/fped.2019.00242
41. McCrindle BW, Cifra B. The role of echocardiography in Kawasaki disease. Int J Rheum Dis. (2018) 21:50–5. doi: 10.1111/1756-185X.13216
42. Pilania RK, Jindal AK, Guleria S, Singh S. An update on treatment of Kawasaki disease. Curr Treat Options Rheumatol. (2019) 5:36–55. doi: 10.1007/s40674-019-00115-z
43. Ajami G, Borzouee M, Amoozgar H, Ashnaee F, Kashef S, Nesar MS, et al. Evaluation of myocardial function using the Tei index in patients with Kawasaki disease. Cardiol Young. (2010) 20:44–8. doi: 10.1017/S1047951109991910
44. Newburger JW, Sanders SP, Burns JC, Parness IA, Beiser AS, Colan SD. Left ventricular contractility and function in Kawasaki syndrome. Effect of intravenous gamma-globulin. Circulation. (1989) 79:1237–46. doi: 10.1161/01.CIR.79.6.1237
45. McCandless RT, Minich LL, Wilkinson SE, McFadden ML, Tani LY, Menon SC. Myocardial strain and strain rate in Kawasaki disease. Eur Heart J Cardiovasc Imaging. (2013) 14:1061–8. doi: 10.1093/ehjci/jet041
46. Xu Q-Q, Ding Y-Y, Lv H-T, Zhou W-P, Sun L, Huang J, et al. Evaluation of left ventricular systolic strain in children with Kawasaki disease. Pediatr Cardiol. (2014) 35:1191–7. doi: 10.1007/s00246-014-0915-5
47. Dedeoglu R, Barut K, Oztunc F, Atik S, Adrovic A, Sahin S, et al. Evaluation of myocardial deformation in patients with Kawasaki disease using speckle-tracking echocardiography during mid-term follow-up. Cardiol Young. (2017) 27:1377–85. doi: 10.1017/S1047951117000580
48. Wang H, Ruan L, Shang J, Song Y, Tong M, Wu T. Left atrial subclinical dysfunction in children with Kawasaki disease: a two-dimensional speckle tracking echocardiograpy study. Minerva Pediatr. (2019). doi: 10.23736/S0026-4946. [Epub ahead of print].
49. Gaur L, Waloff K, Schiller O, Sable CA, Frank LH. Noncoronary inflammation in Kawasaki disease is associated with abnormal myocardial deformation in the acute phase. J Am Soc Echocardiogr. (2014) 27:1329–35. doi: 10.1016/j.echo.2014.09.014
50. Frank B, Davidson J. Myocardial strain and strain rate in Kawasaki disease: range, recovery, and relationship to systemic inflammation/coronary artery dilation. J Clin Exp Cardiolog. (2016) 7:432. doi: 10.4172/2155-9880.1000432
51. Azak E, Cetin II, Gursu HA, Kibar AE, Surucu M, Orgun A, et al. Recovery of myocardial mechanics in Kawasaki disease demonstrated by speckle tracking and tissue Doppler methods. Echocardiography. (2018) 35:380–7. doi: 10.1111/echo.13773
52. Wang H, Shang J, Tong M, Song Y, Ruan L. Evaluation of left ventricular function in immunoglobulin-resistant children with Kawasaki disease: a two-dimensional speckle tracking echocardiography study. Clin Cardiol. (2019) 42:753–9. doi: 10.1002/clc.23213
53. Kashyap R, Mittal BR, Bhattacharya A, Manojkumar R, Singh S. Exercise myocardial perfusion imaging to evaluate inducible ischaemia in children with Kawasaki disease. Nucl Med Commun. (2011) 32:137–41. doi: 10.1097/MNM.0b013e3283411c67
54. Tacke CE, Romeih S, Kuipers IM, Spijkerboer AM, Groenink M, Kuijpers TW. Evaluation of cardiac function by magnetic resonance imaging during the follow-up of patients with Kawasaki disease. Circ Cardiovasc Imaging. (2013) 6:67–73. doi: 10.1161/CIRCIMAGING.112.976969
55. Bratis K, Hachmann P, Child N, Krasemann T, Hussain T, Mavrogeni S, et al. Cardiac magnetic resonance feature tracking in Kawasaki disease convalescence. Ann Pediatr Cardiol. (2017) 10:18–25. doi: 10.4103/0974-2069.197046
56. Ghelani SJ, Singh S, Manojkumar R. QT interval dispersion in North Indian children with Kawasaki disease without overt coronary artery abnormalities. Rheumatol Int. (2011) 31:301–5. doi: 10.1007/s00296-009-1252-5
57. Reddy S, Rai M, Singh Chouhan RR, Rao S, Kamath N. Electrocardiographic analysis of repolarization changes in south Indian children with kawasaki disease after the acute phase of illness. Int J Pediatr. (2018) 2018:1062154. doi: 10.1155/2018/1062154
58. Parihar M, Singh S, Vignesh P, Gupta A, Rohit M. Mid-term risk for subclinical atherosclerosis and chronic myocarditis in children with kawasaki disease and transient coronary abnormalities. Pediatr Cardiol. (2017) 38:1123–32. doi: 10.1007/s00246-017-1626-5
59. Oyamada J, Shimizu C, Kim J, Williams MR, Png E, Hibberd ML, et al. Bifid T waves on the ECG and genetic variation in calcium channel voltage-dependent beta 2 subunit gene (CACNB2) in acute Kawasaki disease. Congenit Heart Dis. (2019) 14:213–20. doi: 10.1111/chd.12696
60. Dahdah N, Jaeggi E, Fournier A. Long-term changes in depolarization and repolarization after kawasaki disease. Pediatr Res. (2003) 53:162. doi: 10.1203/00006450-200301000-00049
61. Gamillscheg A, Zobel G, Karpf EF, Dacar D, Beitzke A, Stein JI, et al. Atypical presentation of Kawasaki disease in an infant. Pediatr Cardiol. (1993) 14:223–6. doi: 10.1007/BF00795375
62. Fuse S, Tomita H, Ohara T, Iida K, Takamuro M. Severely damaged aortic valve and cardiogenic shock in an infant with Kawasaki disease. Pediatr Int. (2003) 45:110–3. doi: 10.1046/j.1442-200X.2003.01666.x
63. Dominguez SR, Friedman K, Seewald R, Anderson MS, Willis L, Glodé MP. Kawasaki disease in a pediatric intensive care unit: a case-control study. Pediatrics. (2008) 122:e786–90. doi: 10.1542/peds.2008-1275
64. Kanegaye JT, Wilder MS, Molkara D, Frazer JR, Pancheri J, Tremoulet AH, et al. Recognition of a Kawasaki disease shock syndrome. Pediatrics. (2009) 123:e783–9. doi: 10.1542/peds.2008-1871
65. Gamez-Gonzalez LB, Moribe-Quintero I, Cisneros-Castolo M, Varela-Ortiz J, Muñoz-Ramírez M, Garrido-García M, et al. Kawasaki disease shock syndrome; a unique and severe subtype of kawasaki disease. Pediatr Int. (2018) 60:781–90. doi: 10.1111/ped.13614
66. Taddio A, Rossi ED, Monasta L, Pastore S, Tommasini A, Lepore L, et al. Describing Kawasaki shock syndrome: results from a retrospective study and literature review. Clin Rheumatol. (2017) 36:223–8. doi: 10.1007/s10067-016-3316-8
67. Pilania RK, Jindal AK, Srikrishna VV, Samprathi M, Singhal M, Singh S. Hypotension in a febrile child-beware of Kawasaki disease shock syndrome. J Clin Rheumatol. (2020) 26:e130–1. doi: 10.1097/RHU.0000000000001002
68. Gámez-González LB, Murata C, Muñoz-Ramírez M, Yamazaki-Nakashimada M. Clinical manifestations associated with Kawasaki disease shock syndrome in Mexican children. Eur J Pediatr. (2013) 172:337–42. doi: 10.1007/s00431-012-1879-1
69. Lin Y-J, Cheng M-C, Lo M-H, Chien S-J. Early differentiation of Kawasaki disease shock syndrome and toxic shock syndrome in a pediatric intensive care unit. Pediatr Infect Dis J. (2015) 34:1163–7. doi: 10.1097/INF.0000000000000852
70. Zhang Q, Liao Y, Du J. Kawasaki disease shock syndrome: a report of two cases and literature review. Pediatr Investig. (2019) 3:81–5. doi: 10.1002/ped4.12127
71. Derespina KR, Kaushik S, Medar SS. Pediatric shock: an uncommon and underrecognized etiology. J Pediatr Intensive Care. (2020) 9:210–2. doi: 10.1055/s-0039-1700964
72. Gatterre P, Oualha M, Dupic L, Iserin F, Bodemer C, Lesage F, et al. Kawasaki disease: an unexpected etiology of shock and multiple organ dysfunction syndrome. Intensive Care Med. (2012) 38:872–8. doi: 10.1007/s00134-012-2473-8
73. Schuster JE, Palac HL, Innocentini N, Rowley AH, Young LT, Shulman ST. Hyponatremia is a feature of kawasaki disease shock syndrome: a case-control study. J Pediatr Infect Dis Soc. (2017) 6:386–8. doi: 10.1093/jpids/piw081
74. Li Y, Zheng Q, Zou L, Wu J, Guo L, Teng L, et al. Kawasaki disease shock syndrome: clinical characteristics and possible use of IL-6, IL-10 and IFN-γ as biomarkers for early recognition. Pediatr Rheumatol. (2019) 17:1. doi: 10.1186/s12969-018-0303-4
75. Brain AN, Frame JD, Eve MD. Early lymphopenia in burned children with and without the toxic shock syndrome. Burns. (1988) 14:120–4. doi: 10.1016/0305-4179(88)90216-1
76. Verdoni L, Mazza A, Gervasoni A, Martelli L, Ruggeri M, Ciuffreda M, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. (2020) 395:1771–8. doi: 10.1016/S0140-6736(20)31103-X
77. Bhat CS, Gupta L, Balasubramanian S, Singh S, Ramanan AV. Hyperinflammatory syndrome in children associated with COVID-19: need for awareness. Indian Pediatr. (2020). [Epub ahead of print].
78. Pouletty M, Borocco C, Ouldali N, Caseris M, Basmaci R, Lachaume N, et al. Paediatric multisystem inflammatory syndrome temporally associated with SARS-CoV-2 mimicking Kawasaki disease (Kawa-COVID-19): a multicentre cohort. Ann Rheum Dis. (2020) 79:999–1006. doi: 10.1136/annrheumdis-2020-217960
79. Koné-Paut I, Cimaz R. Is it Kawasaki shock syndrome, Kawasaki-like disease or pediatric inflammatory multisystem disease? The importance of semantic in the era of COVID-19 pandemic. RMD Open. (2020) 6:e001333. doi: 10.1136/rmdopen-2020-001333
80. Rowley AH. Understanding SARS-CoV-2-related multisystem inflammatory syndrome in children. Nat Rev Immunol. (2020) 20:453–4. doi: 10.1038/s41577-020-0367-5
81. Rowley AH. Diagnosing SARS-CoV-2 related multisystem inflammatory syndrome in children (MIS-C): focus on the gastrointestinal tract and the myocardium. Clin Infect Dis. (2020). doi: 10.1093/cid/ciaa1080. [Epub ahead of print].
82. Whittaker E, Bamford A, Kenny J, Kaforou M, Jones CE, Shah P, et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. (2020) 324:259–69. doi: 10.1001/jama.2020.10369
83. Gowin E, Małecka I, Stryczyńska-Kazubska J, Michalak M, Wysocki J, Górzna-Kamińska H. Cardiac complications in children with Kawasaki disease in our own experience. Kardiol Pol. (2015) 74:75–82. doi: 10.5603/KP.a2015.0136
84. Hamza HS, Raouf WA, Zaher AZ, Agha HM. Acute Kawasaki disease with emphasis on the echocardiographic profile: a single center experience. Glob Cardiol Sci Pract. (2017) 2017:e201727. doi: 10.21542/gcsp.2017.27
85. Sonçagi A, Devrim I, Karagöz T, Dilber E, Celiker A, Ozen S, et al. Septated pericarditis associated with Kawasaki disease: a brief case report. Turk J Pediatr. (2007) 49:312–4.
86. Ozdogu H, Boga C. Fatal cardiac tamponade in a patient with Kawasaki disease. Heart Lung. (2005) 34:257–9. doi: 10.1016/j.hrtlng.2004.12.003
87. Kuppuswamy M, Gukop P, Sutherland G, Venkatachalam C. Kawasaki disease presenting as cardiac tamponade with ruptured giant aneurysm of the right coronary artery. Interact Cardiovasc Thorac Surg. (2010) 10:317–8. doi: 10.1510/icvts.2009.215731
88. Dahlem PG, von Rosenstiel IA, Lam J, Kuijpers TW. Pulse methylprednisolone therapy for impending cardiac tamponade in immunoglobulin-resistant Kawasaki disease. Intensive Care Med. (1999) 25:1137–9. doi: 10.1007/s001340051025
89. Miyamoto T, Ikeda K, Ishii Y, Kobayashi T. Rupture of a coronary artery aneurysm in Kawasaki disease: a rare case and review of the literature for the past 15 years. J Thorac Cardiovasc Surg. (2014) 147:e67–9. doi: 10.1016/j.jtcvs.2014.02.035
90. Mok GC, Sung RY, Yam MC, Arifi AA, Lam WW, Fok TF. A child with Kawasaki disease who survived after rupture of a coronary artery aneurysm. Eur J Pediatr. (2003) 162:634–6. doi: 10.1007/s00431-003-1265-0
91. Hwong TM., Arifi AA, Wan IY., Thung K., Wan S, Sung RY., et al. Rupture of a giant coronary artery aneurysm due to Kawasaki disease. Ann Thorac Surg. (2004) 78:693–5. doi: 10.1016/j.athoracsur.2003.06.015
92. Koike R, Oku T, Satoh H, Sawada Y, Suma H, Takeuchi A, et al. Right ventricular myocardial infarction and late cardiac tamponade due to right coronary artery aneurysm–a case report. JPN J Surg. (1990) 20:463–7. doi: 10.1007/BF02470833
93. Printz BF, Sleeper LA, Newburger JW, Minich LL, Bradley T, Cohen MS, et al. Noncoronary cardiac abnormalities are associated with coronary artery dilation and with laboratory inflammatory markers in acute Kawasaki disease. J Am Coll Cardiol. (2011) 57:86–92. doi: 10.1016/j.jacc.2010.08.619
94. Okada S, Hasegawa S, Suzuki Y, Matsubara T, Shimomura M, Okuda M, et al. Acute pericardial effusion representing the TNF-α-mediated severe inflammation but not the coronary artery outcome of Kawasaki disease. Scand J Rheumatol. (2015) 44:247–52. doi: 10.3109/03009742.2014.956140
95. Akagi T, Kato H, Inoue O, Sato N, Imamura K. Valvular heart disease in Kawasaki syndrome: incidence and natural history. Am Heart J. (1990) 120:366–72. doi: 10.1016/0002-8703(90)90081-8
96. de La Harpe M, di Bernardo S, Hofer M, Sekarski N. Thirty years of Kawasaki disease: a single-center study at the University Hospital of Lausanne. Front Pediatr. (2019) 7:11. doi: 10.3389/fped.2019.00011
97. Kitamura S, Kawashima Y, Kawachi K, Harima R, Ihara K, Nakano S, et al. Severe mitral regurgitation due to coronary arteritis of mucocutaneous lymph node syndrome. a new surgical entity. J Thorac Cardiovasc Surg. (1980) 80:629–36. doi: 10.1016/S0022-5223(19)37751-7
98. Akagi T, Kato H, Inoue O, Sato N. Valvular heart disease in Kawasaki syndrome. Incidence and natural history. Kurume Med J. (1989) 36:137–49. doi: 10.2739/kurumemedj.36.137
99. Shiraishi I, Nishimura K, Sakaguchi H, Abe T, Kitano M, Kurosaki K, et al. Acute rupture of chordae tendineae of the mitral valve in infants: a nationwide survey in japan exploring a new syndrome. Circulation. (2014) 130:1053–61. doi: 10.1161/CIRCULATIONAHA.114.008592
100. Nakano H, Nojima K, Saito A, Ueda K. High incidence of aortic regurgitation following Kawasaki disease. J Pediatr. (1985) 107:59–63. doi: 10.1016/S0022-3476(85)80615-6
101. Liu FF, Liu HH, Qiu Z, Wang JJ, Samadli S, Wu Y, et al. Clinical observation of noncoronary cardiac abnormalities in Chinese children with Kawasaki disease. Eur J Clin Invest. (2020) 50:e13210. doi: 10.1111/eci.13210
102. Ravekes WJ, Colan SD, Gauvreau K, Baker AL, Sundel RP, van der Velde ME, et al. Aortic root dilation in Kawasaki disease. Am J Cardiol. (2001) 87:919–22. doi: 10.1016/S0002-9149(00)01541-1
103. Fukunaga S, Egashira A, Arinaga K, Akasu I, Kai E, Higashi T, et al. Aortic valve replacement for aortic regurgitation due to Kawasaki disease. Report of two cases. J Heart Valve Dis. (1996) 5:231–4.
104. Gupta A, Singh S, Gupta A, Suri D, Rohit M. Aortic stiffness studies in children with Kawasaki disease: preliminary results from a follow-up study from North India. Rheumatol Int. (2014) 34:1427–32. doi: 10.1007/s00296-014-3000-8
105. Pinto FF, Gomes I, Loureiro P, Laranjo S, Timóteo AT, Carmo MM. Vascular function long term after Kawasaki disease: another piece of the puzzle? Cardiol Young. (2017) 27:488–97. doi: 10.1017/S1047951116000780
106. Schäfer M, Truong U, Ivy DD, Fonseca B, Malone L, DiMaria M, et al. Children with Kawasaki disease present elevated stiffness of great arteries: phase-contrast MRI study: elevated stiffness of great arteries in KD. J Magn Reson Imaging. (2018) 48:1228–36. doi: 10.1002/jmri.26167
107. Kato H, Sugimura T, Akagi T, Sato N, Hashino K, Maeno Y, et al. Long-term consequences of Kawasaki disease. a 10- to 21-year follow-up study of 594 patients. Circulation. (1996) 94:1379–85. doi: 10.1161/01.CIR.94.6.1379
108. Zhao Q, Chu C, Wu L, Liang X, Sun S, He L, et al. Systemic artery aneurysms and Kawasaki disease. Pediatrics. (2019) 144:e20192254. doi: 10.1542/peds.2019-2254
109. Bray M, Ramirez A, Muscal E, De Guzman M. Systemic vascular involvement in Kawasaki disease: a single center cohort. In: 2019 ACR/ARP Annual Meeting (2019). p. 71.
110. Deng Y-B, Li T-L, Xiang H-J, Chang Q, Li C-L. Impaired endothelial function in the brachial artery after Kawasaki disease and the effects of intravenous administration of vitamin C. Pediatr Infect Dis J. (2003) 22:34–9. doi: 10.1097/00006454-200301000-00011
111. Dhillon R, Clarkson P, Donald AE, Powe AJ, Nash M, Novelli V, et al. Endothelial dysfunction late after kawasaki disease. Circulation. (1996) 94:2103–6. doi: 10.1161/01.CIR.94.9.2103
112. Ikemoto Y, Ogino H, Teraguchi M, Kobayashi Y. Evaluation of preclinical atherosclerosis by flow-mediated dilatation of the brachial artery and carotid artery analysis in patients with a history of Kawasaki disease. Pediatr Cardiol. (2005) 26:782–6. doi: 10.1007/s00246-005-0921-8
113. Noto N, Okada T, Karasawa K, Ayusawa M, Sumitomo N, Harada K, et al. Age-related acceleration of endothelial dysfunction and subclinical atherosclerosis in subjects with coronary artery lesions after Kawasaki disease. Pediatr Cardiol. (2009) 30:262–8. doi: 10.1007/s00246-008-9329-6
114. Ghelani SJ, Singh S, Manojkumar R. Endothelial dysfunction in a cohort of North Indian children with Kawasaki disease without overt coronary artery involvement. J Cardiol. (2009) 53:226–31. doi: 10.1016/j.jjcc.2008.11.006
115. Liu X, Huang G, Liang X, Ma X. Endothelial progenitor cells and arterial functions in the late convalescence period of Kawasaki disease. Acta Paediatr. (2009) 98:1355–9. doi: 10.1111/j.1651-2227.2009.01334.x
116. Dietz SM, Tacke CEA, Hutten BA, Kuijpers TW. Peripheral endothelial (dys)function, arterial stiffness and carotid intima-media thickness in patients after Kawasaki disease: a systematic review and meta-analyses. PLoS ONE. (2015) 10:e0130913. doi: 10.1371/journal.pone.0130913
117. Routhu SK, Singhal M, Jindal AK, Kumar V, Yadav AK, Singh S. Assessment of endothelial dysfunction in acute and convalescent phases of Kawasaki disease using automated edge detection software: a preliminary study From North India. J Clin Rheumatol. (2019). doi: 10.1097/RHU.0000000000001233. [Epub ahead of print].
118. Iwazu Y, Minami T, Kotani K. Pulse wave velocity in Kawasaki disease. Angiology. (2017) 68:189–95. doi: 10.1177/0003319716651789
119. Meena RS, Rohit M, Gupta A, Singh S. Carotid intima-media thickness in children with Kawasaki disease. Rheumatol Int. (2014) 34:1117–21. doi: 10.1007/s00296-013-2820-2
120. Gopalan K, Singh S, Vignesh P, Gupta A, Rohit M, Attri SV. Carotid intima-media thickness and lipid profile in children with Kawasaki disease: a single-center follow-up study after a mean duration of 6.9 years. J Clin Rheumatol. (2018) 24:385–9. doi: 10.1097/RHU.0000000000000754
121. Chen KYH, Curtis N, Dahdah N, Kowalski R, Cheung M, Burgner DP. Kawasaki disease and cardiovascular risk: a comprehensive review of subclinical vascular changes in the longer term. Acta Paediatr. (2016) 105:752–61. doi: 10.1111/apa.13367
122. Dusad S, Singhal M, Pilania RK, Suri D, Singh S. CT coronary angiography studies after a mean follow-up of 3.8 years in children with Kawasaki disease and spontaneous defervescence. Front Pediatr. (2020) 8:274. doi: 10.3389/fped.2020.00274
123. Ishikawa T, Iwashima S. Endothelial dysfunction in children within 5 years after onset of Kawasaki disease. J Pediatr. (2013) 163:1117–21. doi: 10.1016/j.jpeds.2013.04.046
124. Niboshi A, Hamaoka K, Sakata K, Yamaguchi N. Endothelial dysfunction in adult patients with a history of Kawasaki disease. Eur J Pediatr. (2008) 167:189–96. doi: 10.1007/s00431-007-0452-9
125. Mori Y, Katayama H, Kishi K, Ozaki N, Shimizu T, Tamai H. Persistent high fever for more than 10 days during acute phase is a risk factor for endothelial dysfunction in children with a history of Kawasaki disease. J Cardiol. (2016) 68:71–5. doi: 10.1016/j.jjcc.2015.08.008
126. Ding Y-Y, Ren Y, Feng X, Xu Q-Q, Sun L, Zhang J-M, et al. Correlation between brachial artery flow-mediated dilation and endothelial microparticle levels for identifying endothelial dysfunction in children with Kawasaki disease. Pediatr Res. (2014) 75:453–8. doi: 10.1038/pr.2013.240
127. Brogan P, Burns JC, Cornish J, Diwakar V, Eleftheriou D, Gordon JB, et al. Lifetime cardiovascular management of patients with previous Kawasaki disease. Heart Br Card Soc. (2020) 106:411–20. doi: 10.1136/heartjnl-2019-315925
128. Tsuda E, Hirata T, Matsuo O, Abe T, Sugiyama H, Yamada O. The 30-year outcome for patients after myocardial infarction due to coronary artery lesions caused by Kawasaki disease. Pediatr Cardiol. (2011) 32:176–82. doi: 10.1007/s00246-010-9838-y
129. Tsuda E, Hamaoka K, Suzuki H, Sakazaki H, Murakami Y, Nakagawa M, et al. A survey of the 3-decade outcome for patients with giant aneurysms caused by Kawasaki disease. Am Heart J. (2014) 167:249–58. doi: 10.1016/j.ahj.2013.10.025
130. Fukazawa R, Kobayashi T, Mikami M, Saji T, Hamaoka K, Kato H, et al. nationwide survey of patients with giant coronary aneurysm secondary to Kawasaki disease 1999–2010 in Japan. Circ J. (2017) 82:239–46. doi: 10.1253/circj.CJ-17-0433
Keywords: cardiac biomarkers, echocardiography, Kawasaki disease, Kawasaki disease shock syndrome, myocarditis, pericarditis
Citation: Pilania RK, Jindal AK, Bhattarai D, Naganur SH and Singh S (2020) Cardiovascular Involvement in Kawasaki Disease Is Much More Than Mere Coronary Arteritis. Front. Pediatr. 8:526969. doi: 10.3389/fped.2020.526969
Received: 15 January 2020; Accepted: 25 August 2020;
Published: 24 September 2020.
Edited by:
Xupei Huang, Florida Atlantic University, United StatesReviewed by:
Hiromichi Hamada, Tokyo Women's Medical University Yachiyo Medical Center, JapanMoshe Arditi, Cedars Sinai Medical Center, United States
Chisato Shimizu, University of California, San Diego, United States
Copyright © 2020 Pilania, Jindal, Bhattarai, Naganur and Singh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Surjit Singh, c3Vyaml0c2luZ2hwZ2lAcmVkaWZmbWFpbC5jb20=
 Rakesh Kumar Pilania
Rakesh Kumar Pilania Ankur Kumar Jindal
Ankur Kumar Jindal Dharmagat Bhattarai1
Dharmagat Bhattarai1 Surjit Singh
Surjit Singh