Abstract
Objective:
The purpose of this systematic review was to explore the value of the expression level of the triggering receptor expressed on myeloid cell-1 (TREM-1) in the diagnosis and prognosis of neonatal sepsis.
Methods:
A comprehensive search was performed to identify the diagnostic and prognostic predictive values of the TREM-1 expression level in neonatal sepsis. Based on the retrieval strategy, Cochrane Library, Embase, Ovid, ProQuest, PubMed, Scopus, and Web of Science databases were searched from inception to February 2022. Studies were included if they assessed the accuracy of TREM-1 expression in the diagnosis of neonatal sepsis and distinguished survival and death in neonatal sepsis. Two authors independently evaluated the study and extracted the data, including the first author of the literature, country, total study population, basic population characteristics of the study group and the control group, study design (observational studies), type of sample, sepsis onset, type of biomarker, assay method, cut-off, sensitivity, specificity, true positives (TP), false positives (FP), false negatives (FN), and true negatives (TN). A third party will be consulted if disputed. The accuracy of TREM-1 expression in the diagnosis and prognostic prediction of neonatal sepsis was evaluated by a bivariate mixed-effects model. The source of heterogeneity was explored through meta-regression analysis.
Results:
Thirteen articles that met the research criteria were included in qualitative analysis, and 11 of them were included in quantitative analysis. The pooled sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), diagnostic odds ratio (DOR), and the area under the summary receiver operator characteristic (SROC) curve of soluble TREM-1 (sTREM-1) were 0.94 (95% CI: 0.82, 0.98), 0.87 (95% CI: 0.70, 0.95), 7.36 (95% CI: 2.75, 19.74), 0.07 (95% CI: 0.02, 0.24), 111.71 (95% CI: 13.24, 942.92), and 0.96 (95% CI: 0.94, 0.98), respectively. Meta-regression and subgroup analysis were used to investigate the heterogeneity, owing to non-threshold effects caused by types of test sample and research design. sTREM-1 as a biomarker for distinguishing survival and death in neonates with sepsis had pooled sensitivity, specificity, area under the SROC curve, PLR, NLR, and DOR of 0.95 (95% CI: 0.83, 0.99), 0.98 (95% CI: 0.68, 1.00), 0.99 (95% CI: 0.97, 0.99), 39.28 (95% CI: 2.13, 723.99), 0.05 (95% CI: 0.01, 0.19), and 789.61 (95% CI: 17.53, 35,560.72), respectively.
Conclusion:
The study showed that TREM-1 was a potential biomarker for the diagnosis and prognosis of neonatal sepsis. The biggest advantage of this study is that it is the first to comprehensively explore the role of TREM-1 expression in the diagnosis and prognosis of neonatal sepsis. However, there are some limitations in this study, such as the reduced number of clinical studies on TREM-1 expression as a biomarker of neonatal sepsis, regional bias, and differences in detection methods. Hence, more large-scale and high-quality studies are needed to improve diagnostic accuracy.
Systematic review registration:
https://www.crd.york.ac.uk/PROSPERO/, identifier: CRD42022338041.
Introduction
Neonatal sepsis is considered to be a systemic inflammatory response syndrome (SIRS) induced by bacteria, viruses, or fungi (yeast) infections (1). It can be divided into early-onset sepsis (EOS, ≤ 72 h of age) and late-onset sepsis (LOS, >72 h of age) depending on the onset time (2, 3). Neonatal sepsis is a major disease threatening the life of newborns, which is also one of the major challenges facing global public health. The occurrence of neonatal sepsis was found in 2,202 newborns per 100,000 live births, with a mortality rate of 11–19% (4). In 2015, 336,300 newborns died of neonatal sepsis, accounting for 12.8 % of neonatal deaths (5). Therefore, the timely diagnosis and effective evaluation of the prognosis of neonatal sepsis is particularly important, which may greatly and effectively reduce its morbidity and mortality.
To date, blood culture is still considered as the “-Gold Standard-” for the diagnosis of neonatal sepsis, but the results are often delayed for 24–48 h and vulnerability to multiple factors, which may greatly limit its application to the early diagnosis and treatment of neonatal sepsis (6). Moreover, laboratory indicators available including white blood cell count, immature neutrophil to total neutrophil ratio, C-reactive protein, etc., are not fully applicable to the diagnosis of neonatal sepsis (1, 2, 7). Besides, the clinical symptoms and signs of neonatal sepsis are often non-specific (e.g., unstable temperature, hypotension, metabolic acidosis, tachycardia or bradycardia, apnea, etc.), making it easy to miss the optimal treatment opportunity and usually leading to adverse outcomes (1). Therefore, it is urgent to find valuable biomarkers for the early diagnosis of neonatal sepsis.
The triggering receptor expressed on myeloid cell-1 (TREM-1) is a novel transmembrane protein discovered by Swiss researchers in 2000, which is a new receptor of the immunoglobulin superfamily (8). It is expressed both on the surface of immune cells (e.g., neutrophils, monocytes, macrophages, T lymphocytes, B lymphocytes, natural killer cells, monocyte-derived dendritic cells, etc.) (8–10) and on non-immune cells (e.g., gastric epithelial cells, liver endothelial cells, etc.) (11, 12). TREM-1 exists in two forms: membrane-bound TREM-1 (mTREM-1) and soluble TREM-1 (sTREM-1). When binding to its ligand, mTREM-1 can promote cell activation through an associated signal transduction molecule DNAX activation protein of 12 kDa (DAP12), leading to tyrosine phosphorylation in the immunoreceptor tyrosine-based activation motif (ITAM) chain and providing a binding site, spleen tyrosine kinase (SYK) and zeta-chain-associated protein kinase 70 (ZAP70) (13–18). After that, SYK regulates the activation of downstream signaling pathways and ultimately induces the activation of transcription factors, which in turn regulates the expression of inflammatory responses (18–20). TREM-1 plays multiple roles in regulating the host's antimicrobial immune responses, on the one hand, it can cooperate with toll-like receptor (TLR) to promote the release of proinflammatory cytokines/chemokines upon the infection of Mycobacterium tuberculosis, which contributes to the host defense against microbial challenges during both the early-induced and adaptive phases (21), and on the other hand, the release of sTREM-1 was also reported to be an amplifier of the SIRS associated with sepsis (22). Currently, TREM-1 is considered a potential therapeutic target in inflammatory diseases (15, 17). It was demonstrated that a fusion protein named mTREM-1/IgG1 containing murine TREM-1 and human IgG1 Fc played a protective role in mice with septic shock induced by lipopolysaccharide (LPS), lethal E. coli, or cecum ligation and puncture (CLP) (23). The production of proinflammatory cytokines and infiltration of neutrophils or monocytes/macrophages into the peritoneum were significantly reduced upon the pretreatment of TREM-1/IgG1 in LPS injection mice compared with controls (23). It is suggested that TREM-1 is closely related to the occurrence and development of inflammatory responses.
Two meta-analyses revealed that sTREM-1 had a moderate ability (area under curve (AUC): 0.89 and 0.88, respectively) to diagnose sepsis in adults (24, 25). sTREM-1 had a certain diagnostic value not only for adult sepsis but also for neonatal sepsis. A previous meta-analysis suggested that sTREM-1 might be a useful biomarker for predicting neonatal sepsis (26). However, this study might have overestimated the actual predictive ability of sTREM-1 in diagnosing neonatal sepsis, owing to the lack of analysis of the heterogeneity (26). At the same time, not all of the participants in one of the studies included were 28 days or younger (27). In recent years, with the novel discovery of the role of TREM-1 expression in neonatal sepsis, an update of its diagnostic values is extremely necessary to meet the demands of clinical work. In this study, we had enlarged the sample size and explored the source of potential heterogeneity. In addition, the mRNA transcription and protein expression level of TREM-1 on the cell surface were qualitatively analyzed in the diagnostic value of neonatal sepsis. Moreover, meta-analyses showed that sTREM-1 also had a moderate predictive capability (AUC: 0.82 and 0.78, respectively) in assessing adult sepsis mortality (25, 28); however, there was no involved data to evaluate the prognostic value of TREM-1 expression in neonatal sepsis in those studies. We hope to investigate the application of TREM-1 expression in clinical practice. Therefore, it was necessary to quantitatively and qualitatively analyze the value of TREM-1 expression in the diagnosis and prognosis of neonatal sepsis.
Methods
The present study was conducted based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (29). The study has also been registered in PROSPERO (CRD42022338041).
Search strategy
Databases including the Cochrane Library, Embase, Ovid, ProQuest, PubMed, Scopus, and Web of science were searched from inception to February 2022 for the role of TREM-1 expression in the diagnosis and prognosis of neonatal sepsis. Our search strategy used the following keywords: “neonatal sepsis,” “newborn,” “septic,” “septicemia,” “newborn sepsis,” “triggering receptor expressed on myeloid cells-1,” “TREM-1,” “soluble triggering expressed receptor on myeloid cells-1,” “sTREM-1,” “TREM-1 protein, human.” Search strategies for all databases are shown in Supplementary Material. Meanwhile, we obtained relevant literature through other means, such as a list of references to obtained articles.
Study selection
The inclusion criteria were as follows: (1) the purpose of the study was to evaluate the value of TREM-1 expression (including sTREM-1, mTREM-1, and TREM-1 mRNA) as a biomarker in the diagnosis and/or distinguishing survival and death of neonatal sepsis; (2) a 2 × 2 contingency table containing TP, FP, FN, and TN could be obtained; (3) participants: newborns. Subjects were culture-positive and/or clinically diagnosed with sepsis; (4) study types: relevant clinical observational studies (both prospective and retrospective).
Studies were excluded based on the following criteria: (1) review, editorial, commentary, research protocol, animal experiments, case reports, meta-analysis, and systematic review; (2) articles not published in English; (3) patients older than 28 days (e.g., children, adolescents, and adults); (4) repeated articles; (5) incomplete reporting of original data or the inability to obtain full-text literature.
Two researchers (QG and GD) independently screened the literature according to inclusion criteria and exclusion criteria. First, a preliminary screening was conducted by reading the title and abstract. After excluding irrelevant literature, the remaining were further screened by reading the full text to determine the final included literature. In case of disagreement between two researchers (QG and GD), a third party (HZ) could be invited for a consultation to ensure the reliability of included literature and reduce publication bias and heterogeneity.
Data extraction and quality assessment
Two researchers (QG and GD) extracted literature data, including the first author of the literature, country, total study population, basic population characteristics (gestational age, birth weight, gender) of the study group and the control group, study design, type of sample, sepsis onset, type of biomarker, assay method, cut-off, sensitivity, specificity, TP, FP, FN, and TN. For literature with incomplete data, it was tried to be obtained directly from the original author by email. If no response was received after sending the reminder, the literature was excluded.
We evaluated the risk of bias in research and diagnostic criterion suitability by using the Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2 tool) (30). QUADAS-2 consisted of four parts: patient selection, index test, reference standard, and flow and timing (30). These four items were used to assess the risk of bias in the included literature, and the first 3 items could also be used to evaluate clinical applicability. For specific evaluation criteria, refer to the references (30). Quality assessment was carried out by two researchers (QG and GD) and negotiated by a third party (HZ) in case of disagreement.
Statistical analysis
The quality evaluation results of the included literature were drawn by RevMan 5.3 software. MIDAS module of STATA16.0 was used for statistical analysis, and P < 0.05 was considered statistically significant. Sensitivity, specificity, DOR, PLR, NLR, and SROC were analyzed and summarized using the bivariate mixed-effects model (31). The overall diagnostic performance of sTREM-1 in the diagnosis and prognosis of neonatal sepsis was measured by the SROC curve (32). Heterogeneity between studies was assessed by Q-test and I2 index (33, 34). When P-value of Q-test < 0.05 and I2 index ≥50%, moderate heterogeneity existed, and the source of heterogeneity needed to be discussed. In addition to the proportion of heterogeneity that might be due to threshold effects, univariate meta-regression analysis and subgroup analysis (including type of sample, study design, and sample size) were used to explore the sources of potential heterogeneity. Cook's Distance was used for sensitivity analysis to assess the stability of the study results. Deek's funnel plot asymmetry test was drawn to evaluate the publication bias of the included literature (35). If the result of Deek's symmetry test was P < 0.05, publication bias was suggested.
Results
According to the literature retrieval strategy set in this study, 976 related literature were retrieved, and three related literature were obtained through other means, totaling 979 literature. By reading the titles and abstracts of included literature and eliminating duplicate literature, we finally re-screened the remaining 103 literature and read them in full. Among them, 84 studies included patients older than 28 days, four studies could not obtain full-texts, and two studies could not extract a 2 × 2 contingency table. Finally, 13 studies (36–48) that met our research criteria were included in qualitative analysis and 11 studies (36, 38–47) were included in quantitative analysis. The literature screening process is shown in Figure 1.
Figure 1
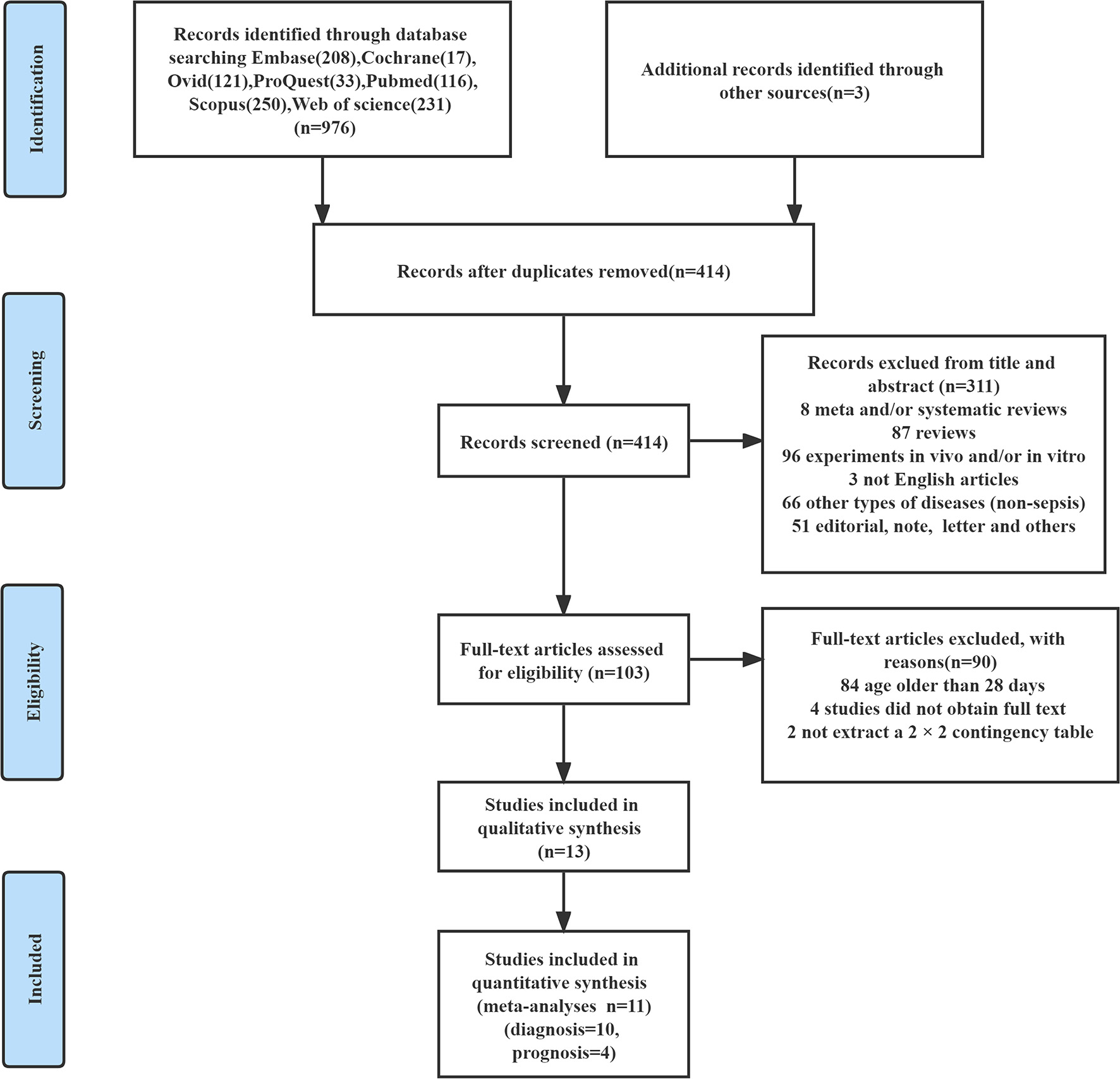
The detailed flow chart for study selection.
Characteristics of included studies
The 13 included studies were published between 2010 and 2021, and all were published in English (36–48). Among them, 12 studies discussed the characteristics of articles on the diagnostic value of TREM-1 expression for neonatal sepsis (Table 1) (36–39, 41–48). Table 2 showed the data related to the diagnostic accuracy of TREM-1 expression in neonatal sepsis. The expression of TREM-1 was sTREM-1 in 10 studies (36, 38, 39, 41–47), one about TREM-1 mRNA (48), and another one about mTREM-1 (37). Six studies were conducted in Egypt (39, 41, 43, 44, 46, 48), three in Turkey (42, 45, 47), and the remaining three studies were conducted in European countries (Italy, Switzerland, and Greece) (36–38). The study group included newborns with EOS in one study (38), newborns with LOS in six studies (36, 37, 42, 43, 45, 47), EOS+LOS in four studies (39, 44, 46, 48), and one study was not mentioned (41). Ten studies measured TREM-1 expression levels by enzyme-linked immunosorbent assay (ELISA) (36, 38, 39, 41–47), one by flow cytometry (37), and another one by reverse transcription-polymerase chain reaction (RT-PCR) (48). Considering the different detection methods, 10 studies were included in the meta-analysis, with a total of 743 newborns (36, 38, 39, 41–47).
Table 1
| References | Country | No. of cases | Patients/control ( n ) | GA (weeks) | BW (g) | Sex male % | Study design | Type of sample | Onset |
|---|---|---|---|---|---|---|---|---|---|
| Sarafidis et al. (36) | Greece | 52 | 31 infected neonates/ 21 non-infected newborns | 35 (24–40)/30 (24–39) | 1,990 (890–3,600)/1,190 (720–4,785) | 61.29/57.14 | Prospective | Serum | LOS |
| Schlapbach et al. (38) | Switzerland | 137 | 33 infected neonates/ 104 non-infected newborns | 39.9 (34.0–41.6)/38.9 (34.0–42.0) | 3,335 (1,950–4,400)/3,060(1,630–4,750) | 54.55/65.38 | Prospective | Serum | EOS |
| Mazzucchelli et al. (37) | Italy | 32 | 16 septic group/16 control group | 27.5 ± 2.7/26.9 ± 1.0 | 1,055 ± 492/1,155 ± 211 | 56.25/43.75 | Case-control | Whole blood | LOS |
| Adly et al. (39) | Egypt | 152 | 112 neonatal sepsis/ 40 healthy controls | N/A | N/A | 52.68/55 | Prospective | Serum | EOS+LOS |
| Saldir et al. (42) | Turkey | 50 | 30 septic group/20 non-septic group | 37.9 ± 1.7/38.3 ± 1.6 | 3,201 ± 313/3,328 ± 383 | 40/55 | Prospective | Serum | LOS |
| El-Gendy et al. (41) | Egypt | 60 | 40 neonatal sepsis/ 20 healthy controls | 35.8 ± 2.94/36.5 ± 2.31 | 2,580 ± 640/2,910 ± 610 | 52.5/50 | Case-control | Serum | N/A |
| El-Khier et al. (43) | Egypt | 59 | 30 sepsis group/ 29 control group | 31.7 ± 3.1/32 ± 3.1 | 1,520 (1,117.5–1,925)/1,470 (1,090–2,450) | 43.33/55.17 | Prospective | Serum | LOS |
| Zidan et al. (44) | Egypt | 45 | 35 septic neonates/10 healthy newborns | N/A | N/A | N/A | Prospective | Serum | EOS+LOS |
| Alkan Ozdemir et al. (45) | Turkey | 62 | 31 septic group/31 control group | 28.6 ± 3.2/29.7 ± 3.0 | 1,114 ± 439/1,226 ± 382 | 58.06/54.84 | Prospective | Urine | LOS |
| El-Madbouly et al. (46) | Egypt | 60 | 30 septic group/ 30 control group | 37.9 ± 1.7/39 ± 1.4 | 2,900 ± 700/3,000 ± 300 | 96.7/86.7 | Prospective | Serum | EOS+LOS |
| Ozdemir et al. (47) | Turkey | 66 | 31 septic group/ 35 control group | 31.9 ± 5.0/34.1 ± 4.6 | 1,771 ± 1,069/2,190 ± 1,015 | 70.97/62.86 | Prospective | Urine | LOS |
| Ghonaim et al. (48) | Egypt | 100 | 75 neonatal sepsis/25 healthy newborns | N/A | N/A | 54.67/56 | Case-control | Whole blood | EOS+LOS |
Characteristics of the included studies that investigated the diagnostic value of TREM-1 expression in neonatal sepsis.
GA, gestational age; BW, birth weight; EOS, early onset sepsis; LOS, late onset sepsis; N/A, not applicable; No, number.
Table 2
| References | Biomarkers | Assay method | Cut-off | Sensitivity/ Specificity (%) | TP | FP | FN | TN | AUC |
|---|---|---|---|---|---|---|---|---|---|
| Sarafidis et al. (36) | sTREM-1 | ELISA | 143.35 Pg /ml | 70/71 | 22 | 6 | 9 | 15 | 0.733 |
| Schlapbach et al. (38) | sTREM-1 | ELISA | 1,250 Pg /ml | 75/52 | 25 | 50 | 8 | 54 | 0.62 |
| Mazzucchelli et al. (37) | mTREM-1 | Routine flow cytometry | 62.12% | 56.2/93.5 | 9 | 1 | 7 | 15 | 0.8 |
| Adly et al. (39) | sTREM-1 | ELISA | 310 Pg /ml | 100/100 | 112 | 0 | 0 | 40 | 1 |
| Saldir et al. (42) | sTREM-1 | ELISA | 450 Pg /ml | 93.3/90 | 28 | 2 | 2 | 18 | 0.97 |
| El-Gendy et al. (41) | sTREM-1 | ELISA | 1,707.35 Pg /ml | 100/100 | 40 | 0 | 0 | 20 | 1 |
| El-Khier et al. (43) | sTREM-1 | ELISA | 77.5 Pg /ml | 90/51.7 | 27 | 14 | 3 | 15 | N/A |
| Zidan et al. (44) | sTREM-1 | ELISA | 250 Pg /ml | 97.1/90 | 34 | 1 | 1 | 9 | 0.97 |
| Ozdemir et al. (45) | sTREM-1 | ELISA | 78.5 Pg /ml | 90/78 | 28 | 7 | 3 | 24 | 0.87 |
| El-Madbouly et al. (46) | sTREM-1 | ELISA | 69.8 Pg /ml | 96.7/86.7 | 29 | 4 | 1 | 26 | N/A |
| Alkan Ozdemir et al. (47) | sTREM-1 | ELISA | 129 Pg /ml | 63.64/84.85 | 20 | 5 | 11 | 30 | N/A |
| Ghonaim et al. (48) | • TREM-1 • mRNA | RT-PCR | 0.631 | 65.33/96 | 49 | 1 | 26 | 24 | 0.708 |
The data related to the diagnostic accuracy of TREM-1 expression in neonatal sepsis.
TREM-1, triggering receptor expressed on myeloid cell-1; sTREM-1, soluble triggering expressed receptor on myeloid cells-1; ELISA, enzyme-linked immunosorbent assay; TP, true positives; FP, false positives; FN, false negatives; TN, true negatives; RT-PCR, reverse transcription-polymerase chain reaction.
Table 3 reported the characteristics of five studies that explored the prognostic value of TREM-1 expression in neonatal sepsis (39–41, 43, 48). Among them, the expression of TREM-1 was sTREM-1 in four studies (39–41, 43) and TREM-1 mRNA was expressed in the other study (48). Four studies (39, 41, 43, 48) were conducted in Egypt and one (40) in Mexico. The study group in two studies was newborns with LOS (40, 43), newborns with EOS+LOS in two studies (39, 48), and the study group was not mentioned in one study (41). TREM-1 expression was measured by ELISA in four studies (39–41, 43) and by RT-PCR in one (48). Due to different detection methods, four studies were included in the meta-analysis, with a total of 204 newborns (39–41, 43).
Table 3
| References | Country | No. | Survivors/non- survivors ( n ) | Study design | Type of sample | Onset | Biomarkers | Assay method | Cut-off | Sensitivity/ specificity (%) | TP | FP | FN | TN | AUC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adly et al. (39) | Egypt | 63 | 47/16 | Prospective | Serum | EOS+LOS | sTREM-1 | ELISA | 1,100 Pg /ml | 100/97 | 47 | 0 | 0 | 16 | 0.978 |
| El-Gendy et al. (41) | Egypt | 40 | 23/17 | Case-control | Serum | N/A | sTREM-1 | ELISA | 2,902.2 Pg/ml | 88.2/78.3 | 20 | 4 | 3 | 13 | 0.92 |
| Arízaga-Ballesteros et al. (40) | Mexico | 71 | 62/9 | Cross-sectional | Plasma | LOS | sTREM-1 | ELISA | 300 Pg/ml | 97/78 | 60 | 2 | 2 | 7 | 0.884 |
| El-Khier et al. (43) | Egypt | 30 | 27/3 | Prospective | Serum | LOS | sTREM-1 | ELISA | 91.5 Pg /ml | 96.3/100 | 26 | 0 | 1 | 3 | 0.988 |
| Ghonaim et al. (48) | Egypt | 75 | 34/41 | Case-control | Whole blood | EOS+LOS | TREM-1 mRNA | RT-PCR | 0.369 | 87.8/97.06 | 30 | 1 | 4 | 40 | 0.902 |
Characteristics of the included studies that explored the prognostic value of TREM-1 expression in neonatal sepsis.
TREM-1, triggering receptor expressed on myeloid cell-1; sTREM-1, soluble triggering expressed receptor on myeloid cells-1; ELISA, enzyme-linked immunosorbent assay; RT-PCR, reverse transcription-polymerase chain reaction; TP, true positives; FP, false positives; FN, false negatives; TN, true negatives; AUC, area under the curve; N/A, not applicable; No, number.
Quality assessment
Since one study did not involve the diagnostic value of TREM-1 expression in neonatal sepsis (40), we only evaluated the quality of the remaining 12 studies (36–39, 41–48). The results of the assessment of bias risk and suitability of the remaining 12 studies is shown in Figure 2. The reason why a high risk of bias was detected in the domain of index test was that the threshold value for the detection of neonatal sepsis by TREM-1 expression was not predetermined, but determined by the receiver operating characteristic (ROC) curve. The overall bias risk of the 12 studies was within an acceptable range, with high applicability and in line with QUADAS-2 evaluation criteria.
Figure 2
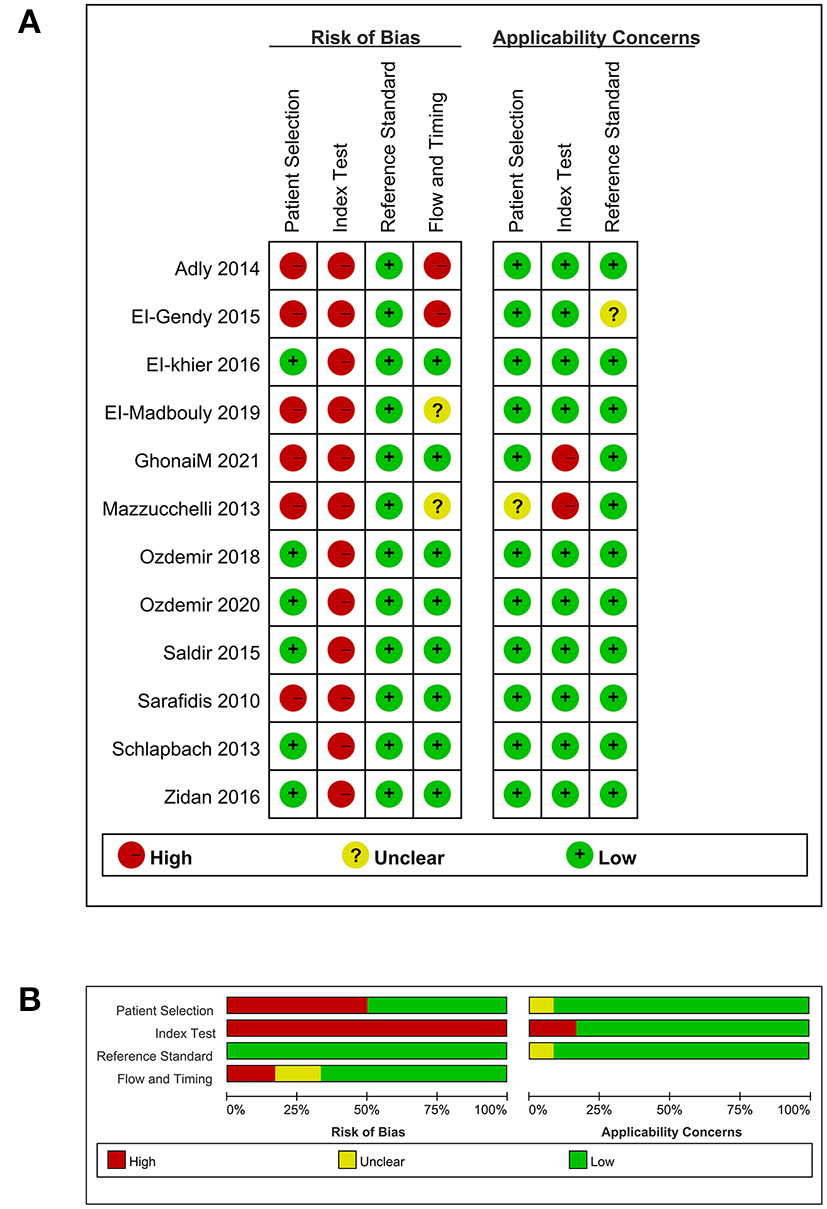
Quality assessment. (A) Methodological quality summary. (B) Methodological quality graph.
Data analysis in diagnostic value
In terms of the diagnostic value of sTREM-1 in neonatal sepsis, the aggregate sensitivity and specificity were 0.94 (95% CI: 0.82, 0.98) and 0.87 (95% CI: 0.70, 0.95), respectively (Figure 3A). The pooled PLR and NLR were 7.36 (95% CI: 2.75, 19.74) and 0.07 (95% CI: 0.02, 0.24), respectively (Figure 3B). The DOR was 111.71 (95% CI: 13.24, 942.92) and the area under the SROC curve was 0.96 (95% CI: 0.94, 0.98; Figure 4A). Deek's funnel plot asymmetry test was used to evaluate publication bias, and the P = 0.49, indicating that the funnel plot had no obvious asymmetry or publication bias, which further indicated that the results of this meta-analysis were reliable (Figure 5A).
Figure 3
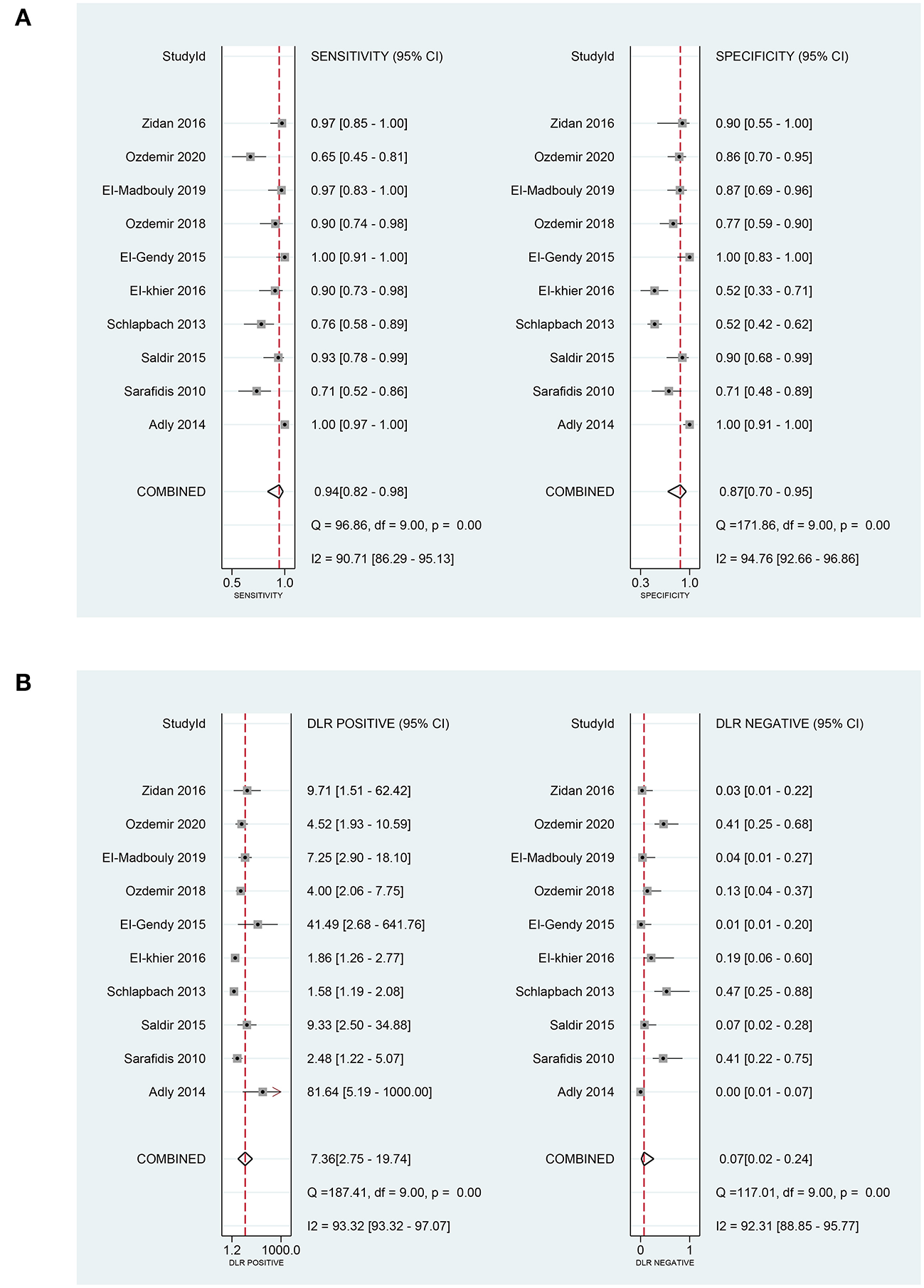
The pooled sensitivity, specificity, PLR, and NLR of sTREM-1 in diagnosing neonatal sepsis. (A) The pooled sensitivity and specificity. (B) The pooled PLR and NLR. PLR, positive likelihood ratio; NLR, negative likelihood ratio; sTREM-1, soluble triggering receptor expressed on myeloid cell-1.
Figure 4
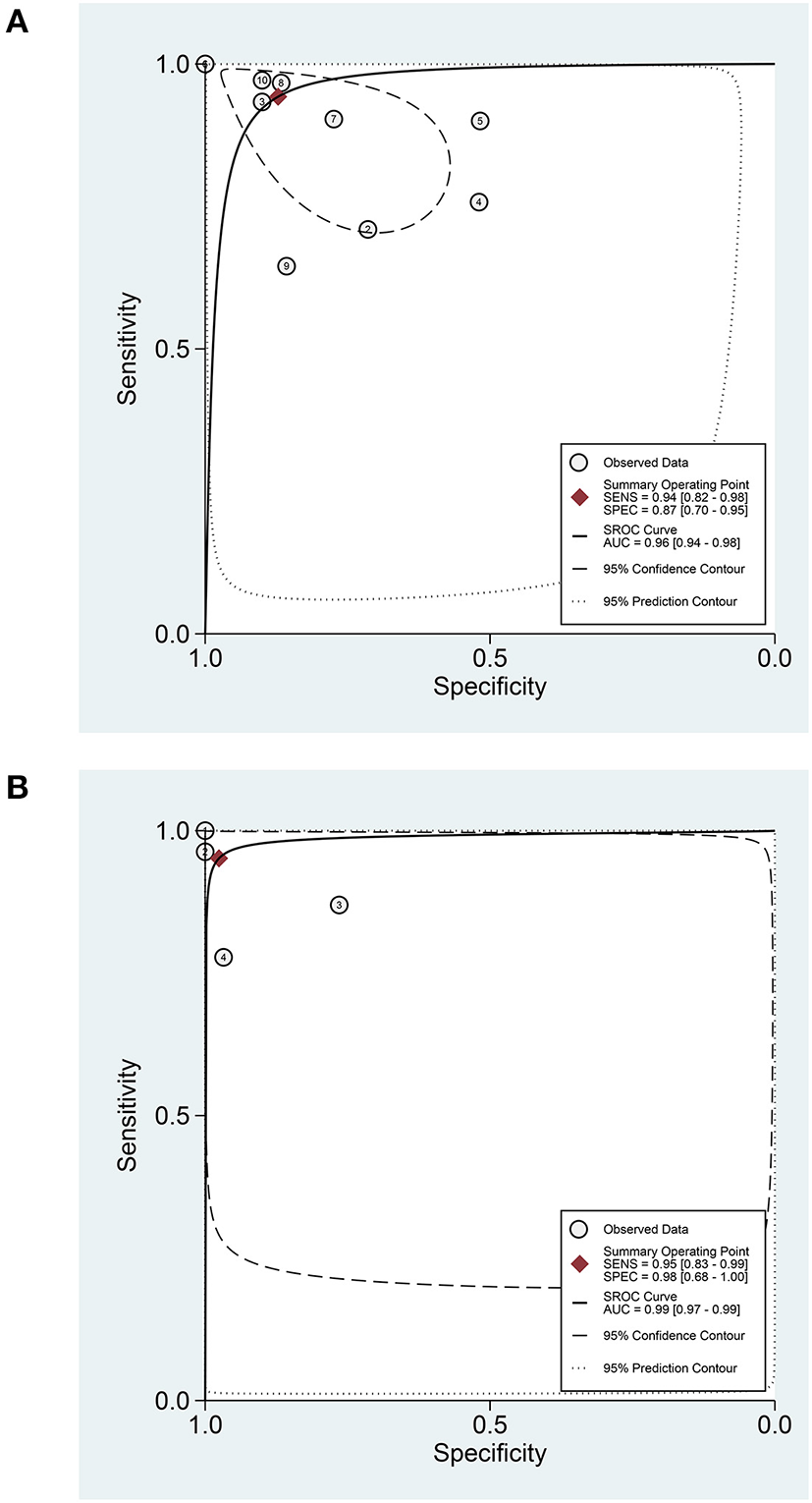
The SROC curve of sTREM-1 in diagnosing neonatal sepsis and distinguishing survival and death in neonatal sepsis. (A) In diagnosing neonatal sepsis. (B) In distinguishing survival and death in neonatal sepsis. sTREM-1, soluble triggering receptor expressed on myeloid cell-1; SROC, summary receiver operator characteristic.
Figure 5
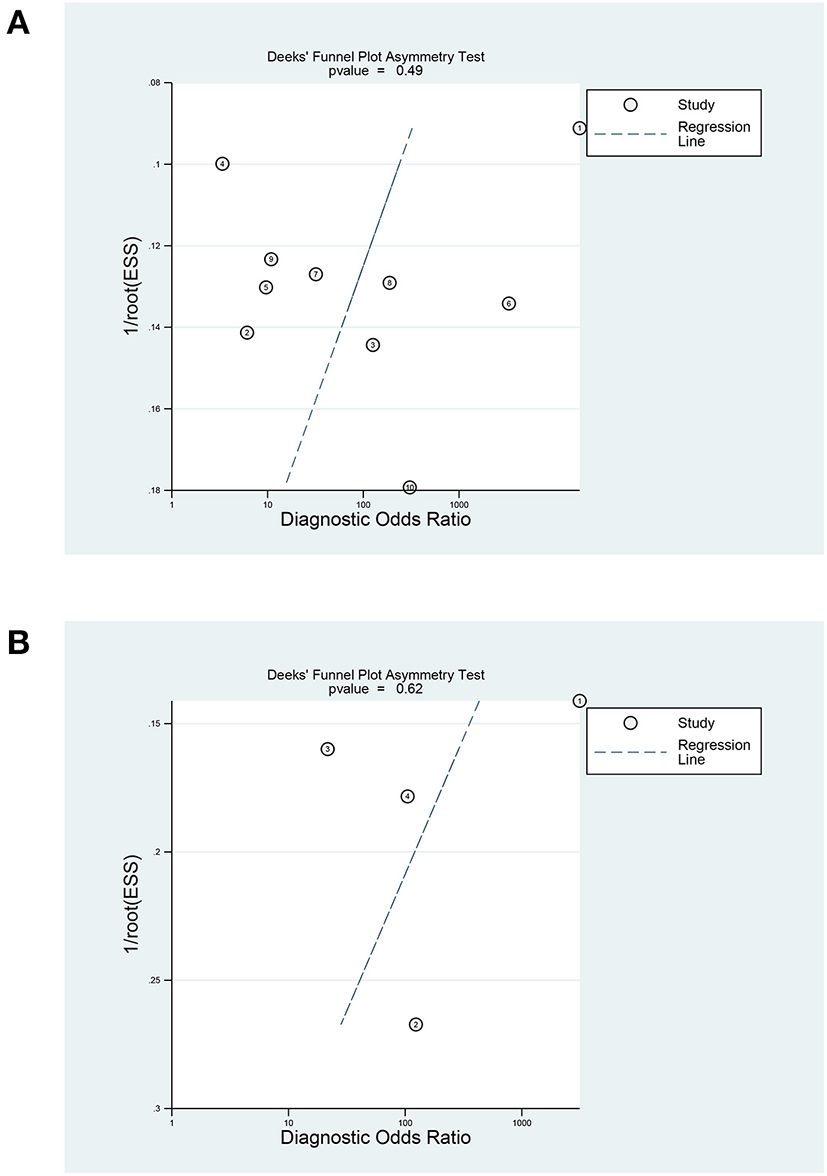
Deeks' funnel plot of sTREM-1 in diagnosing neonatal sepsis and distinguishing survival and death in neonatal sepsis. (A) In diagnosing neonatal sepsis. (B) In distinguishing survival and death in neonatal sepsis. sTREM-1, soluble triggering receptor expressed on myeloid cell-1.
There was significant heterogeneity among studies (overall I2 for bivariate mixed-effects model 63%, 95% CI: 17, 100). The I2-test results for the pooled sensitivity and specificity were 90.71% (P < 0.05) and 94.76% (P < 0.05). The proportion of heterogeneity that could be caused by the threshold effect was 0.76. Due to testing using STATA's MIDAS module, we did not record evidence of threshold effects. To explore the source of potential heterogeneity, we used univariate meta-regression analysis and subgroup analysis. The covariables of meta-regression included type of sample (blood sample or not), study design (prospective study or not), and sample size (≥60 newborns or not). We found that type of sample and study design was possibly correlated with the heterogeneity of sensitivity (Figure 6). The results of the subgroup analysis are shown in Supplementary Table 1.
Figure 6
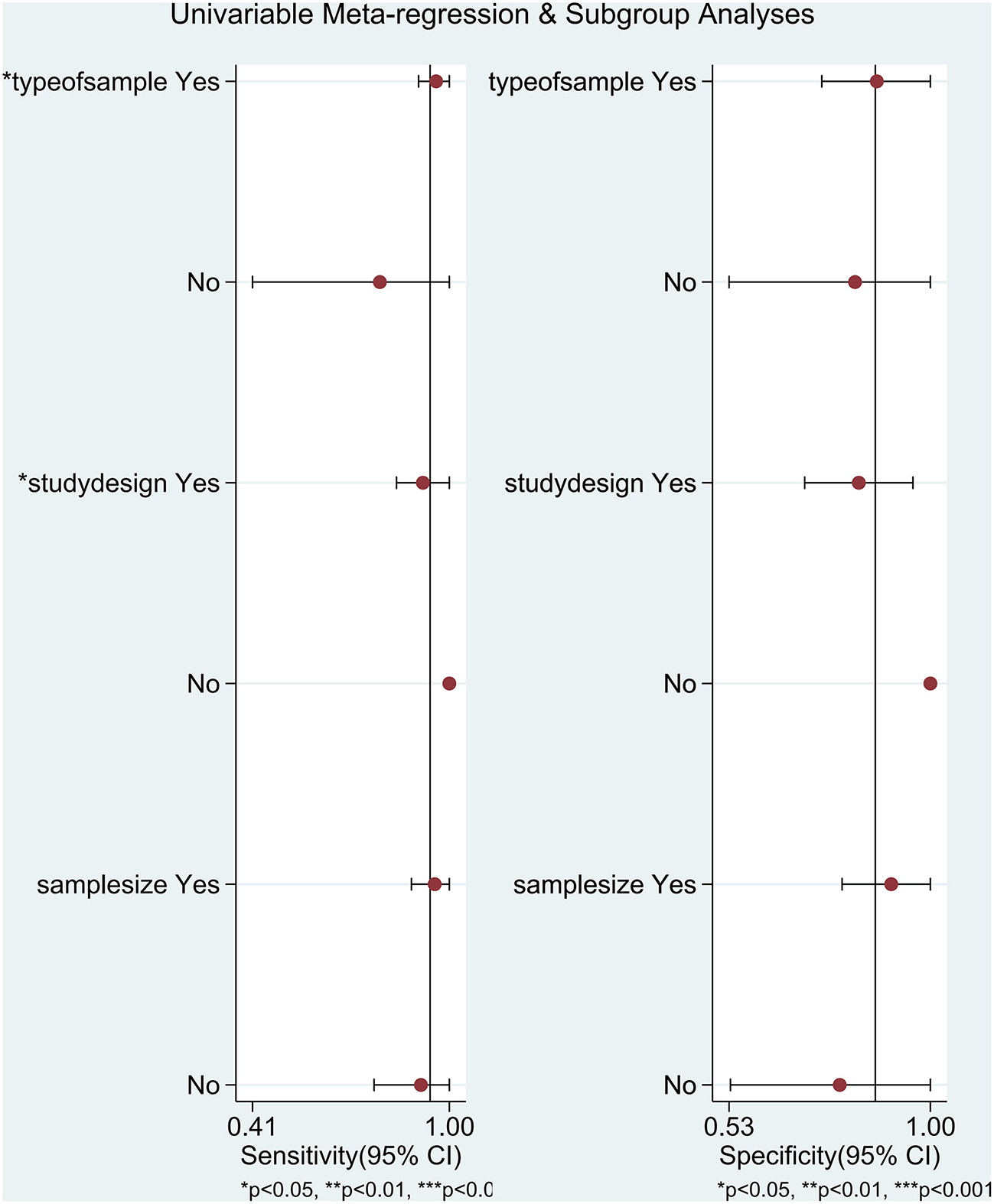
Univariate meta-regression analysis and subgroup analysis in diagnosing neonatal sepsis. **P < 0.01, ***P < 0.001.
The results of sensitivity analysis revealed that the studies of Adly et al. (39) and Ozdemir et al. (47) had a greater influence on the results (Figure 7). After the exclusion of these two studies, the pooled sensitivity and specificity of sTREM-1 decreased from 0.94 to 0.93 and 0.87 to 0.81, respectively.
Figure 7
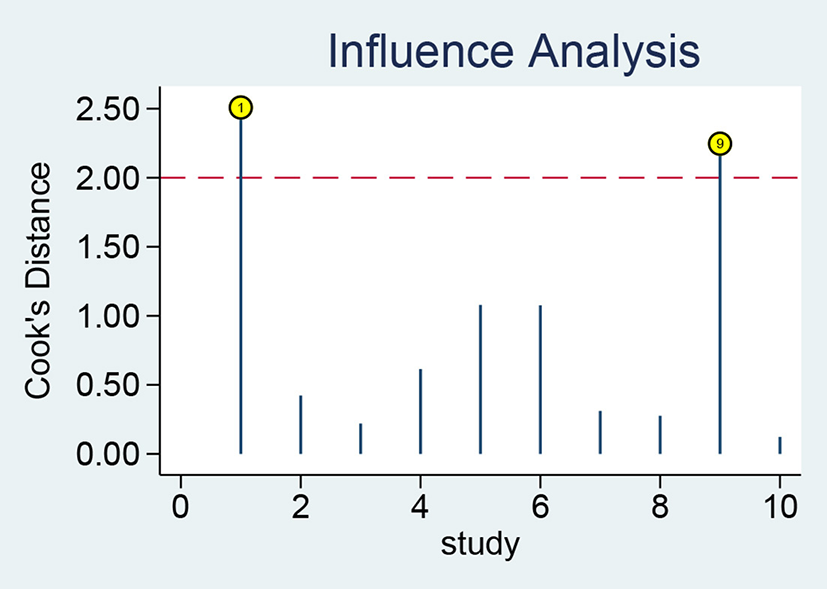
Sensitivity analysis of sTREM-1 in diagnosing neonatal sepsis. sTREM-1, soluble triggering receptor expressed on myeloid cell-1.
Data analysis in prognostic value
In terms of the prognostic value of sTREM-1 in neonatal sepsis, the aggregate sensitivity and specificity were 0.95 (95% CI: 0.83, 0.99) and 0.98 (95% CI: 0.68, 1.00), respectively (Figure 8A). The pooled PLR and NLR were 39.28 (95% CI: 2.13, 723.99) and 0.05 (95% CI: 0.01, 0.19), respectively (Figure 8B). The DOR was 789.61 (95% CI: 17.53, 35560.72) and the area under the SROC curve was 0.99 (95% CI: 0.97, 0.99; Figure 4B).
Figure 8
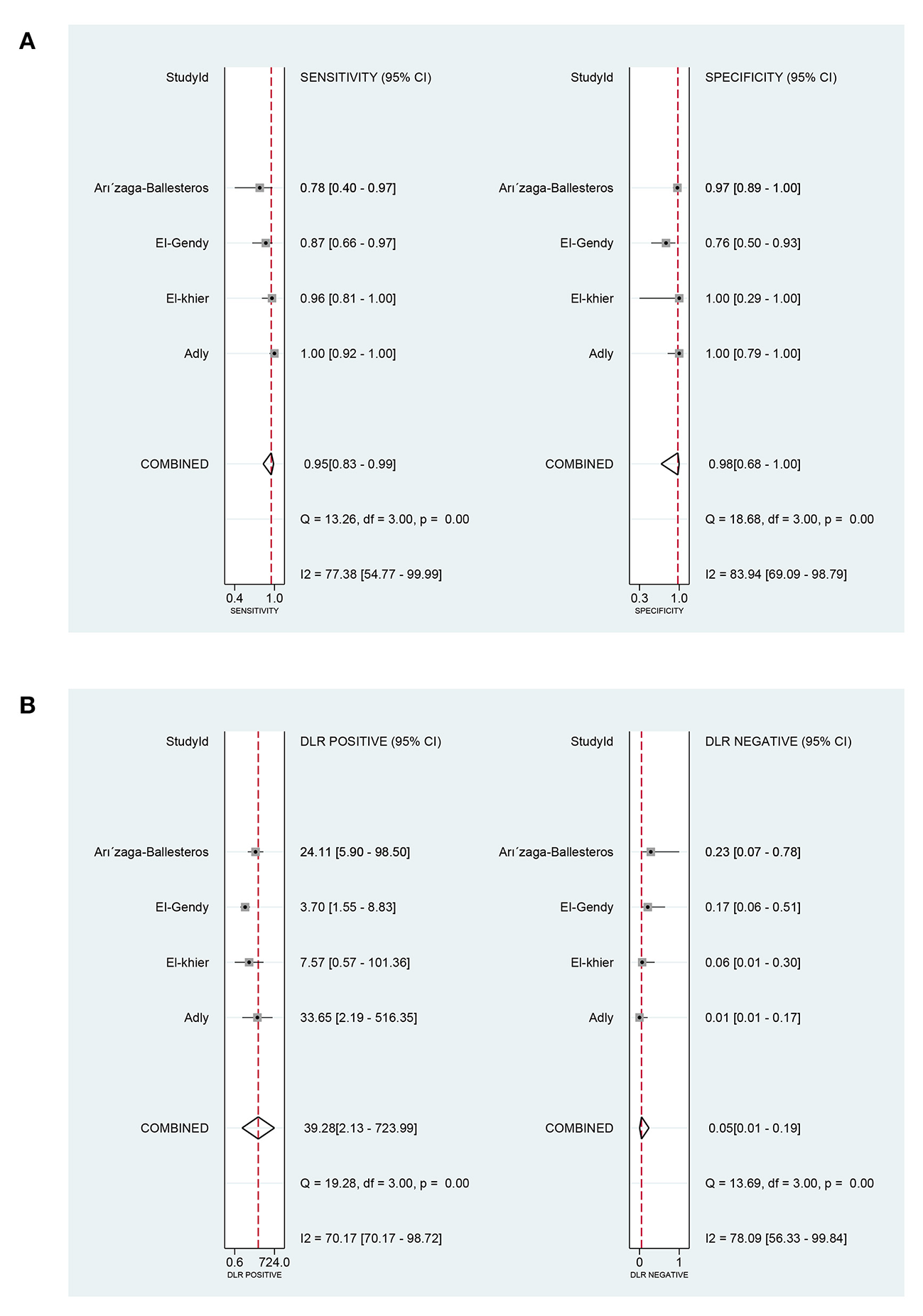
The pooled sensitivity, specificity, PLR, and NLR of the prognostic value of sTREM-1 in neonatal sepsis. (A) The pooled sensitivity and specificity. (B) The pooled PLR and NLR. PLR, positive likelihood ratio; NLR, negative likelihood ratio; sTREM-1, soluble triggering receptor expressed on myeloid cell-1.
The I2-test results for the pooled sensitivity and specificity were 77.38% (P < 0.05) and 83.94% (P < 0.05), respectively, indicating the existence of heterogeneity. Considering the small number of included studies, meta-regression analysis and subgroup analysis were not used to explore the heterogeneity caused by non-threshold effects.
The Deek's funnel plot of the included studies suggested there was no significant publication bias in the prognostic value of sTREM-1 (Figure 5B), P = 0.62). Sensitivity analysis showed that the results were stable and reliable (Supplementary Figure 1).
Discussion
Neonatal sepsis is the third most common cause of neonatal death, which is also one of the major factors leading to neonatal disability (5). In that case, early diagnosis and treatment are essential to reduce mortality and disability rate. Currently, in view of the limitations of blood culture, clinical symptoms and signs, and laboratory indicators in the diagnosis of neonatal sepsis, there are no recommended biomarkers for the diagnosis of neonatal sepsis. The exploration of convenient and effective biomarkers for neonatal sepsis has become a research hotspot. The purpose of this study is to investigate the role of TREM-1 as a biomarker in the diagnosis and prognosis of neonatal sepsis.
The results of our meta-analysis including 10 studies (36, 38, 39, 41–47) manifested that the pooled sensitivity and specificity of sTREM-1 in the diagnosis of neonatal sepsis were 0.94 (95% CI: 0.82, 0.98) and 0.87 (95% CI: 0.70, 0.95), respectively. A previous meta-analysis by Bellos et al. showed that sTREM-1 as a predictor of neonatal sepsis had a pooled sensitivity of 0.95 (95% CI: 0.81, 0.99) and specificity of 0.87 (95% CI: 0.56, 0.97) (26), which further indicated that sTREM-1 had high sensitivity and specificity as a diagnostic biomarker. The area under the SROC curve summarized in our study was 0.96 (95% CI: 0.94, 0.98), indicating high accuracy of sTREM-1 in the diagnosis of neonatal sepsis. The heterogeneity of the non-threshold effect was mainly derived from the type of sample and study design, which was explored by meta-regression analysis and subgroup analysis. Subgroup analysis showed that the sensitivity of sTREM-1 in the diagnosis of neonatal sepsis was significantly different between the blood subgroup and the urine subgroup (P < 0.05), indicating that type of sample may affect the accuracy of the diagnostic test. In addition, the sensitivity of the urine subgroup was significantly lower than that of the blood subgroup (0.79 vs. 0.96), which may be due to the dilution of sTREM-1 during urine production (49). Meta-regression analysis indicated that the sensitivity of sTREM-1 was significantly different between the prospective and non-prospective studies (P < 0.05), suggesting that study design may affect the accuracy of diagnostic tests. However, this conclusion was not supported by strong evidence, because there was only one non-prospective study. More studies are needed to investigate the effect of study design on the accuracy of diagnostic tests.
In previous studies, Ghonai et al. found that TREM-1 mRNA had a moderate ability (AUC: 0.708) to diagnose neonatal sepsis, similar to its accuracy (AUC: 0.75) in the diagnosis of adult sepsis (48, 50). While, compared with the diagnosis of neonatal sepsis, TREM-1 mRNA had higher accuracy (AUC: 0.902 vs. 0.708) in predicting the mortality of neonatal sepsis (48). In addition, the expression level of TREM-1 mRNA in neonates with septic shock was significantly lower than that in EOS and LOS, and the difference was statistically significant (48). This indicated that TREM-1 mRNA expression level was negatively correlated with the severity of sepsis, which was consistent with the study from Atef et al. (48, 51). However, Tao et al. found that there were no differences in TREM-1 mRNA expression level between severe sepsis and septic shock groups (52). It should be noticed that the relationship between TREM-1 mRNA expression level and the severity of disease was still controversial, therefore, accurate conclusions could not be drawn. Besides, the mTREM-1 was also demonstrated to be a potential marker for the diagnosis of neonatal sepsis. In the study of Mazzucchelli et al., TREM-1 expressed on the surface of polymorphonuclear neutrophils was moderately associated with the diagnosis of neonatal sepsis (37).
The expression form of TREM-1 included in our meta-analysis was sTREM-1. It was inferred that the sTREM-1 form may be derived from the translation of the splicing variant of TREM-1 mRNA, or a proteolytic cleavage of the cell-surface anchored TREM-1 (16, 53, 54). Studies had found that two other forms of TREM-1, namely, the mRNA transcription level and the protein expression level of TREM-1 on the cell surface had a certain clinical value in the identification and/or prognosis of sepsis (50, 51, 55). In that case, we also qualitatively analyzed the role of TREM-1 mRNA and mTREM-1 in the diagnosis and/or prognosis of neonatal sepsis.
Our meta-analysis included four literature (39–41, 43) about the prognostic value of sTREM-1 in neonatal sepsis. It indicated that the pooled sensitivity, specificity and area under the SROC curve were 0.95, 0.98, and 0.99, respectively. These results suggested that sTREM-1 had high sensitivity and specificity in the prediction of prognosis of neonatal sepsis, which made it a creditable biomarker for distinguishing survival and death from neonatal sepsis.
From the evaluation results of the risk of bias, it was found that the high-risk items were mainly index test and patient selection. On the one hand, high risk of bias was found in the index test domain because in most studies the threshold value for TREM-1 expression to diagnose neonatal sepsis was determined by ROC curves rather than predetermined (36, 38, 39, 41–43, 45–48). On the other hand, the high risk of bias in patient selection was mainly because some of the studies were not continuous or random cases within a certain time range (36, 37, 39, 41). In the evaluation of applicability, two studies scored as high risk in the index test domain as they used TREM-1 mRNA or mTREM-1 as biomarkers, which limited their clinical applicability (37, 48). In general, the overall risk of bias was within the acceptable range and the applicability was high.
In recent years, the search for an ideal biomarker with sufficient diagnostic accuracy in neonatal sepsis is still ongoing. At present, biomarkers that have been studied extensively include procalcitonin (PCT), interleukin-6 (IL-6), presepsin, and serum amyloid A (SAA). In four meta-analysis studies, the pooled sensitivity of PCT, IL-6, presepsin, and SAA were 0.81, 0.76, 0.91, and 0.84, respectively, and the pooled specificity was 0.79, 0.79, 0.91, and 0.89, respectively (56–59). Obviously, the pooled sensitivity (0.95) of sTREM-1 was higher than that of PCT, IL-6, presepsin, and SAA, and the pooled specificity (0.87) was higher than that of PCT and IL-6. However, we cannot conclude that sTREM-1 has higher diagnostic efficacy than them in neonatal sepsis. Due to the influence of population selection, gold standard selection and sample selection time on sensitivity and specificity cannot be excluded. If multiple single biomarkers can be compared in the same study and the study design can be standardized, it may avoid the incorrect evaluation of the diagnostic performance of a single biomarker and increase the reliability of the results. No single biomarker was found to have sufficient diagnostic accuracy to diagnose neonatal sepsis (60). The combination of biomarkers may be a strategy to improve diagnostic accuracy (60). Therefore, we should systematically evaluate the independent diagnostic efficacy of sTREM-1, hoping to provide clinical evidence for sTREM-1 in combination with other sensitive biomarkers. Currently, there is no systematic review of the co-diagnosis of sTREM-1 in neonatal sepsis. Studies on the prognostic value and severity of sTREM-1 in neonatal sepsis are also relatively scarce. All of these provide a new direction for our future research.
This study has the following advantages: (1) this study is the first comprehensive study to investigate the role of TREM-1 expression in the diagnosis and prognosis of neonatal sepsis; (2) meta-regression analysis and subgroup analysis were used to explore the heterogeneity caused by non-threshold effect; (3) sensitivity analysis was used to evaluate the stability of the results of TREM-1 expression in the diagnostic and prognostic value of neonatal sepsis.
However, due to the limitations of meta-analysis, the deficiencies of this study are as follows: (1) the number of clinical studies on sTREM-1 as a biomarker of neonatal sepsis that could be included in the meta-analysis was relatively small; (2) the sample size of the included population in some studies was small, which may lead to selection bias (36, 37, 40–47); (3) the threshold value for the detection of TREM-1 expression to diagnose and evaluate the prognosis of neonatal sepsis was not set in advance, but the optimal threshold value was determined by ROC curve; (4) this study included all literatures published in English, so some research data may be omitted, affecting its comprehensiveness; (5) for the detection of TREM-1 expression in this study, differences caused by the technician's own technology, usage method, and surroundings could not be excluded; (6) included studies were mostly published by Egyptian scholars, which might have regional bias (39, 41, 43, 44, 46, 48); (7) in the review process, only English databases were retrieved, and no other language databases were retrieved. In addition, we manually retrieved three identified studies (41, 43, 44), which would lead to incomplete retrieval and certain selection bias.
Conclusion
With a comprehensive analysis, sTREM-1 was demonstrated to be a credible biomarker for the diagnosis and prognosis of neonatal sepsis; TREM-1 mRNA and mTREM-1 may be potential markers for the diagnosis and/or prognosis of neonatal sepsis. Due to the limited number and quality of included studies, larger-scale and high-quality studies are still needed to improve diagnostic accuracy.
Funding
This work was supported financially by the funding of the Project of Science and Technology plan of the Jiangxi Health Committee (20204503), the Natural Science Foundation of Jiangxi Province (20202BAB216002), and the Key Research Project of Gannan Medical University (ZD201906).
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
CC and HZ: conception and design of the research, analysis and interpretation of data, and drafting of the manuscript. QG and GD: performed the database search, acquisition of data, study selection, and data extraction. HZ and KL: statistical analysis and revision of the manuscript for important intellectual content. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2022.929665/full#supplementary-material
References
1.
Shane AL Sanchez PJ Stoll BJ . Neonatal sepsis. Lancet. (2017) 390:1770–80. 10.1016/S0140-6736(17)31002-4
2.
Kim F Polin RA Hooven TA . Neonatal sepsis. BMJ. (2020) 371:m3672. 10.1136/bmj.m3672
3.
Simonsen KA Anderson-Berry AL Delair SF Davies HD . Early-onset neonatal sepsis. Clin Microbiol Rev. (2014) 27:21–47. 10.1128/CMR.00031-13
4.
Fleischmann-Struzek C Goldfarb DM Schlattmann P Schlapbach LJ Reinhart K Kissoon N . The global burden of paediatric and neonatal sepsis: a systematic review. Lancet Respir Med. (2018) 6:223–30. 10.1016/S2213-2600(18)30063-8
5.
GBD 2015 Mortality and Causes of Death Collaborators . Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. (2016) 388:1459–544. 10.1016/S0140-6736(16)31012-1
6.
Chiesa C Panero A Osborn JF Simonetti AF Pacifico L . Diagnosis of neonatal sepsis: a clinical and laboratory challenge. Clin Chem. (2004) 50:279–87. 10.1373/clinchem.2003.025171
7.
Polin RA . Management of neonates with suspected or proven early-onset bacterial sepsis. Pediatrics. (2012) 129:1006–15. 10.1542/peds.2012-0541
8.
Bouchon A Dietrich J Colonna M . Cutting edge: inflammatory responses can be triggered by TREM-1, a novel receptor expressed on neutrophils and monocytes. J Immunol. (2000) 164:4991–5. 10.4049/jimmunol.164.10.4991
9.
Bosco MC Pierobon D Blengio F Raggi F Vanni C Gattorno M et al . Hypoxia modulates the gene expression profile of immunoregulatory receptors in human mature dendritic cells: identification of TREM-1 as a novel hypoxic marker in vitro and in vivo. Blood. (2011) 117:2625–39. 10.1182/blood-2010-06-292136
10.
Matesanz-Isabel J Sintes J Llinàs L de Salort J Lázaro A Engel P . New B-cell CD molecules. Immunol Lett. (2011) 134:104–12. 10.1016/j.imlet.2010.09.019
11.
Chen LC Laskin JD Gordon MK Laskin DL . Regulation of TREM expression in hepatic macrophages and endothelial cells during acute endotoxemia. Exp Mol Pathol. (2008) 84:145–55. 10.1016/j.yexmp.2007.11.004
12.
Schmausser B Endrich S Beier D Moran AP Burek CJ Rosenwald A et al . Triggering receptor expressed on myeloid cells-1 (TREM-1) expression on gastric epithelium: implication for a role of TREM-1 in Helicobacter pylori infection. Clin Exp Immunol. (2008) 152:88–94. 10.1111/j.1365-2249.2008.03608.x
13.
McVicar DW Taylor LS Gosselin P Willette-Brown J Mikhael AI Geahlen RL et al . DAP12-mediated signal transduction in natural killer cells. A dominant role for the Syk protein-tyrosine kinase. J Biol Chem. (1998) 273:32934–42. 10.1074/jbc.273.49.32934
14.
Cohen J . TREM-1 in sepsis. Lancet. (2001) 358:776–8. 10.1016/S0140-6736(01)06007-X
15.
Colonna M Facchetti F . TREM-1 (triggering receptor expressed on myeloid cells): a new player in acute inflammatory responses. J Infect Dis. (2003) 187(Suppl.2):S397–401. 10.1086/374754
16.
Klesney-Tait J Turnbull IR Colonna M . The TREM receptor family and signal integration. Nat Immunol. (2006) 7:1266–73. 10.1038/ni1411
17.
Tessarz AS Cerwenka A . The TREM-1/DAP12 pathway. Immunol Lett. (2008) 116:111–6. 10.1016/j.imlet.2007.11.021
18.
Dantas P Matos AO da Silva Filho E Silva-Sales M Sales-Campos H . Triggering receptor expressed on myeloid cells-1 (TREM-1) as a therapeutic target in infectious and noninfectious disease: a critical review. Int Rev Immunol. (2020) 39:188–202. 10.1080/08830185.2020.1762597
19.
Tammaro A Derive M Gibot S Leemans JC Florquin S Dessing MC . TREM-1 and its potential ligands in non-infectious diseases: from biology to clinical perspectives. Pharmacol Ther. (2017) 177:81–95. 10.1016/j.pharmthera.2017.02.043
20.
de Oliveira Matos A Dos Santos Dantas PH Figueira Marques Silva-Sales M Sales-Campos H . The role of the triggering receptor expressed on myeloid cells-1 (TREM-1) in non-bacterial infections. Crit Rev Microbiol. (2020) 46:237–52. 10.1080/1040841X.2020.1751060
21.
Ford JW McVicar DW . TREM and TREM-like receptors in inflammation and disease. Curr Opin Immunol. (2009) 21:38–46. 10.1016/j.coi.2009.01.009
22.
Bleharski JR Kiessler V Buonsanti C Sieling PA Stenger S Colonna M et al . A role for triggering receptor expressed on myeloid cells-1 in host defense during the early-induced and adaptive phases of the immune response. J Immunol. (2003) 170:3812–8. 10.4049/jimmunol.170.7.3812
23.
Bouchon A Facchetti F Weigand MA Colonna M . TREM-1 amplifies inflammation and is a crucial mediator of septic shock. Nature. (2001) 410:1103–7. 10.1038/35074114
24.
Chang W Peng F Meng SS Xu JY Yang Y . Diagnostic value of serum soluble triggering expressed receptor on myeloid cells 1 (sTREM-1) in suspected sepsis: a meta-analysis. BMC Immunol. (2020) 21:2. 10.1186/s12865-020-0332-x
25.
Qin Q Liang L Xia Y . Diagnostic and prognostic predictive values of circulating sTREM-1 in sepsis: a meta-analysis. Infect Genet Evol. (2021) 96:105074. 10.1016/j.meegid.2021.105074
26.
Bellos I Fitrou G Daskalakis G Thomakos N Papantoniou N Pergialiotis V . Soluble TREM-1 as a predictive factor of neonatal sepsis: a meta-analysis. Inflamm Res. (2018) 67:571–8. 10.1007/s00011-018-1149-4
27.
Stein M Schachter-Davidov A Babai I Tasher D Somekh E . The accuracy of C-reactive protein, procalcitonin, and s-TREM-1 in the prediction of serious bacterial infection in neonates. Clin Pediatr. (2015) 54:439–44. 10.1177/0009922814553435
28.
Su L Liu D Chai W Liu D Long Y . Role of sTREM-1 in predicting mortality of infection: a systematic review and meta-analysis. BMJ Open. (2016) 6:e010314. 10.1136/bmjopen-2015-010314
29.
Liberati A Altman DG Tetzlaff J Mulrow C Gøtzsche PC Ioannidis JP et al . The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. Bmj. (2009) 339:b2700. 10.1136/bmj.b2700
30.
Whiting PF Rutjes AW Westwood ME Mallett S Deeks JJ Reitsma JB et al . QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. (2011) 155:529–36. 10.7326/0003-4819-155-8-201110180-00009
31.
Reitsma JB Glas AS Rutjes AW Scholten RJ Bossuyt PM Zwinderman AH . Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol. (2005) 58:982–90. 10.1016/j.jclinepi.2005.02.022
32.
Moses LE Shapiro D Littenberg B . Combining independent studies of a diagnostic test into a summary ROC curve: data-analytic approaches and some additional considerations. Stat Med. (1993) 12:1293–316. 10.1002/sim.4780121403
33.
Whitehead A Whitehead J . A general parametric approach to the meta-analysis of randomized clinical trials. Stat Med. (1991) 10:1665–77. 10.1002/sim.4780101105
34.
Higgins JP Thompson SG . Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21:1539–58. 10.1002/sim.1186
35.
Deeks JJ Macaskill P Irwig L . The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol. (2005) 58:882–93. 10.1016/j.jclinepi.2005.01.016
36.
Sarafidis K Soubasi-Griva V Piretzi K Thomaidou A Agakidou E Taparkou A et al . Diagnostic utility of elevated serum soluble triggering receptor expressed on myeloid cells (sTREM)-1 in infected neonates. Intensive Care Med. (2010) 36:864–8. 10.1007/s00134-010-1819-3
37.
Mazzucchelli I Garofoli F Ciardelli L Borghesi A Tzialla C Di Comite A et al . Diagnostic performance of triggering receptor expressed on myeloid cells-1 and CD64 index as markers of sepsis in preterm newborns. Pediatr Crit Care Med. (2013) 14:178–82. 10.1097/PCC.0b013e31826e726d
38.
Schlapbach LJ Graf R Woerner A Fontana M Zimmermann-Baer U Glauser D et al . Pancreatic stone protein as a novel marker for neonatal sepsis. Intensive Care Med. (2013) 39:754–63. 10.1007/s00134-012-2798-3
39.
Adly AA Ismail EA Andrawes NG El-Saadany MA . Circulating soluble triggering receptor expressed on myeloid cells-1 (sTREM-1) as diagnostic and prognostic marker in neonatal sepsis. Cytokine. (2014) 65:184–91. 10.1016/j.cyto.2013.11.004
40.
Arízaga-Ballesteros V Alcorta-García MR Lázaro-Martínez LC Amézquita-Gómez JM Alanís-Cajero JM Villela L et al . Can sTREM-1 predict septic shock and death in late-onset neonatal sepsis? A pilot study. Int J Infect Dis. (2015) 30:27–32. 10.1016/j.ijid.2014.10.013
41.
El-Gendy FM El-Nemr FM Midan DAR Habib MSE Metwally MMI . Value of measurement of sTREM-1 factor in diagnosis and prognosis in neonatal sepsis. J Med Medical Sci Res. (2015) 1:2052–1480. Available online at: https://www.researchgate.net/publication/316148053
42.
Saldir M Tunc T Cekmez F Cetinkaya M Kalayci T Fidanci K et al . Endocan and soluble triggering receptor expressed on myeloid cells-1 as novel markers for neonatal sepsis. Pediatr Neonatol. (2015) 56:415–21. 10.1016/j.pedneo.2015.03.006
43.
El-Khier N Nassar DK Elaal E Abdel-Hady ES . Soluble triggering receptor expressed on myeloid cells-1 (sTREM-1) as a diagnostic and prognostic marker of late-onset sepsis in preterm neonates. Int J Adv Res. (2016) 8:123–31. 10.21474/IJAR01/1193
44.
Zidan M Kamal H El-Behisy M Haie O Nasser M . Serum triggering receptor expressed on myloid cells-1 (sTREM-1) and its role in diagnosis of neonatal sepsis. Med J Cairo Univ. (2016) 84:17–25.
45.
Alkan Ozdemir S Ozer EA Ilhan O Sutcuoglu S Tatli M . Diagnostic value of urine soluble triggering receptor expressed on myeloid cells (sTREM-1) for late-onset neonatal sepsis in infected preterm neonates. J Int Med Res. (2018) 46:1606–16. 10.1177/0300060517749131
46.
El-Madbouly AA El Sehemawy AA Eldesoky NA Abd Elgalil HM Ahmed AM . Utility of presepsin, soluble triggering receptor expressed on myeloid cells-1, and neutrophil CD64 for early detection of neonatal sepsis. Infect Drug Resist. (2019) 12:311–9. 10.2147/IDR.S191533
47.
Ozdemir SA Colak R Ergon EY Calkavur S . Diagnostic value of urine sTREM-1 and urine C-reactive protein for infants with late onset neonatal sepsis. J Pediatric Infect Dis. (2019) 15:72–8. 10.1055/s-0039-3400468
48.
Ghonaim MM Makled AF Youssef AE El-Brolosy AM . Value of triggering receptor expressed on myeloid cells-1 (TREM-1) as a diagnostic marker for neonatal sepsis. J Clin Diagnost Res. (2021) 15:DC22–DC9. 10.7860/JCDR/2021/46634.14472
49.
Determann RM Schultz MJ Geerlings SE . Soluble triggering receptor expressed on myeloid cells-1 is not a sufficient biological marker for infection of the urinary tract. J Infect. (2007) 54:e249–50. 10.1016/j.jinf.2007.01.010
50.
How CK Chern CH Wu MF Wang LM Huang CI Lee CH et al . Expression of the triggering receptor expressed on myeloid cells-1 mRNA in a heterogeneous infected population. Int J Clin Pract. (2009) 63:126–33. 10.1111/j.1742-1241.2006.01193.x
51.
Atef DM Ghonaim RA Nada EN . The role of TREM-1 gene expression and soluble TREM-1 as prognostic markers of sepsis. J Medical Microbiol Diagn. (2016) 5:211. 10.4172/2161-0703.1000211
52.
Tao F Peng L Li J Shao Y Deng L Yao H . Association of serum myeloid cells of soluble triggering receptor-1 level with myocardial dysfunction in patients with severe sepsis. Mediators Inflamm. (2013) 2013:819246. 10.1155/2013/819246
53.
Gómez-Piña V Soares-Schanoski A Rodríguez-Rojas A Del Fresno C García F Vallejo-Cremades MT et al . Metalloproteinases shed TREM-1 ectodomain from lipopolysaccharide-stimulated human monocytes. J Immunol. (2007) 179:4065–73. 10.4049/jimmunol.179.6.4065
54.
Palazzo SJ Simpson T Schnapp LM . Triggering receptor expressed on myeloid cells type 1 as a potential therapeutic target in sepsis. Dimens Crit Care Nurs. (2012) 31:1–6. 10.1097/DCC.0b013e31823a5298
55.
Qian L Weng XW Chen W Sun CH Wu J . TREM-1 as a potential therapeutic target in neonatal sepsis. Int J Clin Exp Med. (2014) 7:1650–8. Available online at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4132125/
56.
Vouloumanou EK Plessa E Karageorgopoulos DE Mantadakis E Falagas ME . Serum procalcitonin as a diagnostic marker for neonatal sepsis: a systematic review and meta-analysis. Intensive Care Med. (2011) 37:747–62. 10.1007/s00134-011-2174-8
57.
Yuan H Huang J Lv B Yan W Hu G Wang J et al . Diagnosis value of the serum amyloid A test in neonatal sepsis: a meta-analysis. Biomed Res Int. (2013) 2013:520294. 10.1155/2013/520294
58.
Bellos I Fitrou G Pergialiotis V Thomakos N Perrea DN Daskalakis G . The diagnostic accuracy of presepsin in neonatal sepsis: a meta-analysis. Eur J Pediatr. (2018) 177:625–32. 10.1007/s00431-018-3114-1
59.
Eichberger J Resch B . Reliability of interleukin-6 alone and in combination for diagnosis of early onset neonatal sepsis: systematic review. Front Pediatr. (2022) 10:840778. 10.3389/fped.2022.840778
60.
Celik IH Hanna M Canpolat FE Mohan P . Diagnosis of neonatal sepsis: the past, present and future. Pediatr Res. (2022) 91:337–50. 10.1038/s41390-021-01696-z
Summary
Keywords
TREM-1, STREM-1, neonatal sepsis, diagnosis, prognosis
Citation
Chang C, Gao Q, Deng G, Luo K and Zhu H (2022) Diagnostic and prognostic predictive values of triggering receptor expressed on myeloid cell-1 expression in neonatal sepsis: A meta-analysis and systematic review. Front. Pediatr. 10:929665. doi: 10.3389/fped.2022.929665
Received
27 April 2022
Accepted
28 June 2022
Published
22 July 2022
Volume
10 - 2022
Edited by
Tatiana Rodrigues de Moura, Federal University of Sergipe, Brazil
Reviewed by
Helioswilton Sales-Campos, Universidade Federal de Goiás, Brazil; Lavinia Teixeira-Machado, Federal University of Sergipe, Brazil
Updates
Copyright
© 2022 Chang, Gao, Deng, Luo and Zhu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kaiyuan Luo luokaiyuan2008@126.comHuifang Zhu zhuhuifang1985@163.com
This article was submitted to Neonatology, a section of the journal Frontiers in Pediatrics
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.