- Department No. 2 of Respiratory Medicine, National Children’s Medical Center, Beijing Children’s Hospital, Capital Medical University, Beijing, China
Background: A rapid, easy, and accurate method for screening Chronic Granulomatous Disease (CGD) is crucial. This study aimed to propose and evaluate the effectiveness of the DHR-Enzyme-linked immunosorbent assay (DHR-ELISA) for assessing NADPH oxidase complex activity in the early screening of CGD.
Methods: We conducted a retrospective analysis of 72 children suspected of having CGD who underwent NADPH oxidase activity assessment and genetic testing at Beijing Children's Hospital between July 2015 and January 2022.
Results: Of the subjects, 57 were male and 15 were female, resulting in a male-to-female ratio of 3.8:1. The median age at onset was 6 months, and the median age at diagnosis was 15 months. Thirty-eight patients were diagnosed with CGD based on typical clinical manifestations and genetic testing, exhibiting symptoms such as left lymphadenopathy or calcification (65.8%), a large Calmette-Guérin scar (60.5%), a history of skin or other lymph node infections (52.6%), and specific pulmonary infections (23.7%). Thirty-one patients exhibited normal enzyme activity, whereas 41 showed reduced activity. The DHR-ELISA method demonstrated a specificity of 90% and a sensitivity ranging from 90.5% to 100% in detecting CGD.
Conclusion: The DHR-ELISA is a rapid, easy, cost-effective, and efficient method for screening CGD, making it suitable for early diagnosis and potentially improving prognosis.
1 Introduction
Chronic granulomatous disease (CGD) was initially described by Janeway in 1954 and later characterized as “fatal childhood granulomatous disease” in 1959 (1). It constitutes a rare inborn error of immunity. The estimated prevalence of CGD is approximately 1 in 200,000–250,000 live births in the United States (2). Incidence rates vary among different ethnic groups, and precise data for China have yet to be established. Advances in treatment have significantly improved the overall survival rates for patients with CGD. However, in China, the disease is characterized by early onset and delayed diagnosis. Our team has identified eight diagnostic indicators that facilitate the early detection of CGD in Chinese patients, aiding clinicians in timely disease recognition (3). Patients suspected of having CGD require comprehensive diagnostic testing to confirm the diagnosis. Given that genetic testing is time-consuming, costly, and occasionally inconclusive, the dihydrorhodamine 123 (DHR) flow cytometric assay is recognized as the standard screening method due to its rapidity and high efficiency. However, the DHR-flow cytometric assay is unsuitable for primary hospitals lacking flow cytometry equipment. We have found that the DHR-Enzyme-linked immunosorbent assay (DHR-ELISA) can be used to evaluate the activity of the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase complex in neutrophils, offering significant benefits in terms of diagnostic efficiency, cost, and detection time, thereby meriting broader application.
2 Materials and methods
2.1 Participant profile
A cohort of 72 pediatric patients suspected of having CGD was subjected to an assessment for CGD using the DHR-ELISA method to evaluate NADPH oxidase complex function and whole exome sequencing (WES) with parental segregation analysis (07/2015–01/2022). This study was approved by the Ethics Committee of Beijing Children's Hospital (No.2024-E-012-R).
2.2 Indicators for CGD suspicion
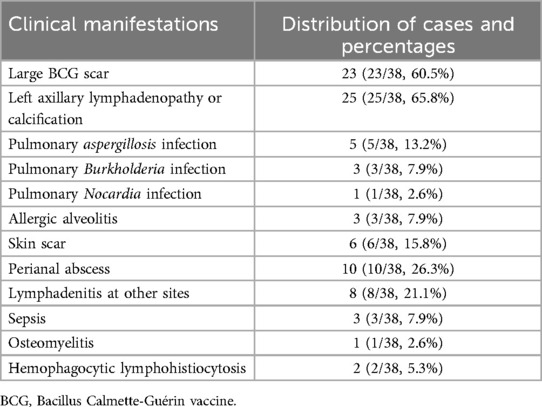
Based on literature, eight indicators have been established for suspecting CGD, which include a large BCG scar, left axillary lymphadenopathy or calcification, skin or other lymph node infections, skin scars, multiple lung nodules, perianal abscesses, pulmonary Aspergillus infection and Burkholderia infection, and repetitive respiratory infections (3, 4).
2.3 CGD diagnostic benchmarks
Patients presenting with symptoms indicative of chronic granulomatous disease and possessing any variants in the CYBB, CYBA, NCF1, NCF2, NCF4, or CYBC1 gene, as confirmed by WES, with the variant's pathogenicity classified as either pathogenic or likely pathogenic according to the ACMG clinical practice guideline, meet the diagnostic criteria for CGD.
2.4 DHR-ELISA of NADPH oxidase complex activity
Fresh EDTA-anticoagulated leukocytes from patients were collected and diluted 10 times with a buffer solution (PBS). Then, 80 μl of this diluted solution was added to a fluorescence detection enzyme plate. Next, 2 μl of dihydrorhodamine 123 (DHR123, 0.5 mg/ml, Thermo) was mixed in and incubated at 37°C for 5 min. Subsequently, 1 μl of PMA (100 μg/ml, Sigma) was added and incubated again at 37°C for 30 min. The fluorescence intensity was measured with an ELISA reader. The results were expressed in terms of enzymatic activity (F/μg) and relative enzymatic activity (the ratio of the sample's enzymatic activity to the average normal enzymatic activity). The reference range (1,332–9,312 F/μg) was established by testing 100 healthy individuals, yielding a mean NADPH oxidase activity of 4,311 F/μg (±2 SD). Relative enzymatic activity spanned 31%–216%. Quality control performed in accordance with the ELISA methodology. The reliability of our assay was confirmed by testing 5 patients with CGD and their parents using DHR-ELISA and DHR-flow cytometry, with both methods showing concordant results.
2.5 Statistical analysis
Clinical profiles and laboratory test outcomes were gathered from an extensive review of hospitalized cases, outpatient records, and telephonic follow-ups for the subjects. Data were presented as median values with interquartile ranges [M (P25, P75)]. Analyses of specificity and sensitivity regarding the NADPH oxidase complex activity, as determined by DHR-ELISA, and the confirmation of CGD were conducted.
The study was approved by the Ethics Committee of Beijing Children's Hospital, Beijing, China. Written consent was obtained from the parents of the study subjects.
3 Results
3.1 Demographic profile
Among the group of 72 patients, there were 57 males and 15 females, resulting in a male-to-female ratio of approximately 3.8:1. The median age at initial presentation was 6 months, with an interquartile range (IQR) from 11 to 20 months. The average age at the time of diagnosis was 15 months, with an IQR spanning from 6 to 47 months. The admission figures were distributed as follows: 21 cases in 2015–2016, 29 cases in 2017–2018, 13 cases in 2019–2020, and 9 cases in 2021–2022.
3.2 Presentation in CGD-affected children
Of the 72 cases, 38 were confirmed to have CGD according to the CGD Diagnostic Benchmarks stated earlier. Among the 38 patients with CGD, 23 presented with extensive scarring (23/38, 60.5%), 25 had lymphadenopathy or calcification in the left axillary region (25/38, 65.8%), 9 had pulmonary infections attributed to specific pathogens (9/38, 23.7%), 3 had allergic alveolitis (3/38, 7.9%), 20 had skin or other lymph node infections (20/38, 52.6%), 4 had infections at miscellaneous sites (4/38, 10.5%), and 2 had hemophagocytic lymphohistiocytosis (2/38, 5.3%), as detailed in Table 1.
3.3 WES findings in CGD
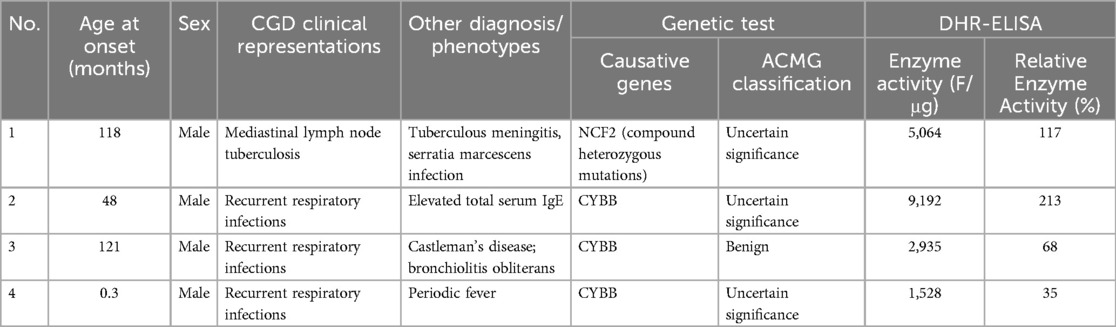
Thirty-eight patients were diagnosed with CGD according to the CGD Diagnostic Benchmarks mentioned above. Among this cohort of 38 patients with CGD, mutations in the CYBB gene were identified in 27 individuals (27/38, 71.1%). Mutations in the NCF1 gene were present in 6 children (6/38, 15.8%), CYBA gene alterations were found in 3 patients (3/38, 7.9%), and NCF2 gene mutations were detected in 2 cases (2/38, 5.3%), as presented in Table 2. Additionally, 4 patients exhibited atypical clinical signs of CGD, and their genetic testing revealed variants in genes associated with CGD; however, the pathogenicity level did not meet the criteria for a definitive diagnosis, thereby categorizing them as cases with uncertain significance, as detailed in Table 3.
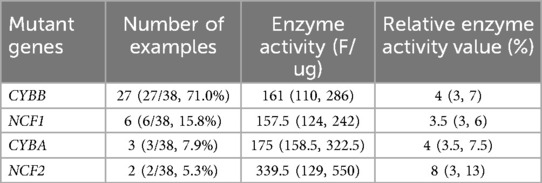
Table 2. The distribution of mutant genes and the detection results of NADPH oxidase complex activity by DHR-ELISA in 38 children with CGD.
3.4 Results of NADPH oxidase complex activity test
Among the 31 normal cases (including 4 cases detected with variants in CGD genes), the median value of enzyme activity units was 2,875 F/μg, with an interquartile range (IQR) of 2,286–3,705 F/μg; the median relative enzyme activity was 67%, with an IQR of 53%–86%. There were 41 cases with reduced results (3 of which were non-CGD patients), with a median enzyme activity unit of 170 F/μg and an IQR of 122.5–325 F/μg; the median relative enzyme activity was 4%, with an IQR of 3%–7.5%.
3.5 Analytical outcomes
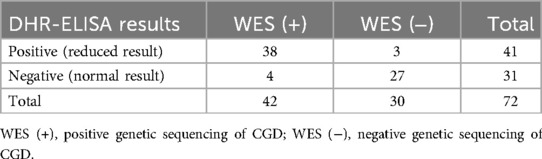
Four individuals exhibiting normal DHR-ELISA assay readings, yet with genetic testing indicating the presence of CGD-associated genes, were categorized within the CGD-genetic sequencing group (refer to Table 4). The specificity (true negative fraction) was calculated at 90% (27/30), and the sensitivity (true positive fraction) at 90.5% (38/42). Excluding the four subjects with indeterminate CGD status, the specificity and sensitivity of the DHR-ELISA in assessing NADPH oxidase complex activity were maintained at 90% (27/30) and elevated to 100% (38/38), respectively.
4 Discussion
Chronic granulomatous disease (CGD) is a rare human inborn error of immunity stemming from an anomaly in the subunits of the NADPH oxidase complex. This complex is composed of five protein subunits: two transmembrane proteins (gp91phox and p22phox, encoded by CYBB and CYBA genes, respectively) and three cytosolic proteins (p47phox, p67phox, and p40phox, encoded by the NCF1, NCF2, and NCF4 genes, respectively). The primary mutations occur in the first four subunits. Mutations in the CYBB gene are inherited in an X-linked recessive manner, representing approximately 70% of cases, while the remaining mutations follow an autosomal recessive inheritance pattern. Moreover, the CYBC1 gene encodes the Eros protein, essential for the formation of gp91phox-p22phox heterodimers, and mutations in CYBC1 are also linked to CGD, presenting with diverse phenotypes (5, 6). Phagocytic cells (monocytes, macrophages, neutrophils, etc.) rely on the NADPH oxidase complex to generate superoxide anions and other reactive oxygen species during pathogen phagocytosis, a process termed the “respiratory burst.” The byproducts of this process, together with lysosomes, play a role in neutralizing the ingested microorganisms. Consequently, patients with CGD exhibit impairments in their phagocytic respiratory bursts, leading to recurrent, life-threatening bacterial and fungal infections, granuloma formation due to excessive inflammatory responses, and sometimes, autoimmune diseases. Compared to autosomal recessive CGD (AR-CGD), X-linked CGD manifests earlier and has a poorer prognosis (2); survival rates are closely associated with the production of reactive oxygen intermediates (R) (7).
Advances in treatment have notably increased the overall survival rates for patients with CGD (8). Statistical data indicate that nearly 90% of patients with CGD can reach early adulthood, with some undergoing bone marrow transplantation and others maintained on lifelong prophylactic medication (antibacterial, antifungal, and immunomodulatory agents) (9). However, the clinical presentation of patients with CGD is widely variable, affecting multiple organ systems such as the lungs, skin, lymph nodes, liver, bones, and gastrointestinal tract. Patients may be infected with various catalase-positive pathogens (e.g., Staphylococcus aureus, Aspergillus, Serratia, Burkholderia, Nocardia) and, in severe cases, may develop macrophage activation syndrome or hemophagocytic lymphohistiocytosis (4, 10–12), complicating the diagnosis. Our team suggests that events related to BCG vaccination, historical skin or site-specific lymph node infections, bilateral lung nodules, perianal abscesses, and infections by Aspergillus or Burkholderia (singly or in combination) serve as early diagnostic indicators for CGD in Chinese children (3), aiding clinicians in prompt identification and diagnosis through rapid, accurate, and cost-effective tests following a suspected CGD diagnosis.
The DHR-flow cytometric assay is the laboratory gold standard for screening CGD, with genetic testing available to confirm CGD when mutations are found in CYBB, CYBA, NCF1, NCF2, NCF4, and CYBC1 (13). Although genetic testing can pinpoint mutations and aid in prognostication, it is not ideal for screening due to its cost, duration, and potential for indeterminate results. As a globally recognized standard, DHR-flow cytometric assays require samples to be preserved in EDTA tubes and analyzed preferably within 24–48 h (14, 15). They offer high sensitivity and a short testing cycle, operating on the principle that DHR123, after entering cells and oxidizing to rhodamine 123 (R123) in the mitochondria, emits green fluorescence upon blue light excitation (16). However, this method demands expensive equipment and skilled laboratory technicians, which many hospitals, particularly smaller ones, may lack, leading to delayed diagnosis. While the nitroblue tetrazolium (NBT) test, a cost-effective screening method for CGD, has been largely replaced by the DHR assay, it still requires the expertise of skilled laboratory technicians and is associated with numerous additional challenges (17). The DHR-ELISA method utilized in this study shares the same detection principle as DHR-flow cytometry but has lower operational requirements, demonstrating a specificity of 90% and a sensitivity between 90.5% and 100%. The low rates of misdiagnosis and missed diagnosis make it comparable to DHR-flow cytometry and thus suitable for widespread use. Additionally, the DHR-flow cytometric assay could be normal or mildly impaired in patients with NCF4/p40phox deficiency (18), and a similar result might occur with DHR-ELISA.
Three children exhibited clinical signs of left axillary lymphadenopathy and reduced DHR-ELISA results, yet genetic tests did not confirm CGD (one was diagnosed with Shwachman-Diamond syndrome, one showed no significant genetic abnormalities, and one was confirmed to have X-linked hyper IgM syndrome), indicating that DHR-ELISA assays may yield false positives. Previous reports suggest that false positives in DHR-flow cytometry may occur in cases lacking myeloperoxidase (19) or may be influenced by acetaminophen intake within 24 h prior to sampling (20), and acute disease phases can also lead to false positives (21). Therefore, caution is necessary when diagnosing suspected CGD in children with atypical clinical features and positive DHR-ELISA assays. In this study, four cases had normal DHR-ELISA results and lacked typical clinical signs of CGD at follow-up, with genetic tests revealing CGD-related genes but inadequate pathogenicity classification, thus classifying them as indeterminate. This suggests that residual NADPH enzyme function may account for the absence of clinical phenotype manifestation, necessitating continued monitoring and protein function testing for clarification, as current literature highlights that adult-diagnosed patients with CGD often exhibit milder phenotypes (22). Previous reports also indicate that low normal values in DHR-flow cytometry do not entirely exclude the possibility of CGD (17).
In summary, the DHR-ELISA technique, employed to assess NADPH oxidase complex function, stands out for its rapid processing time, affordability, simplicity in execution, and high efficacy in detection. This method is recommended as an initial screening approach for potential CGD cases and is particularly advantageous for broader implementation, including in primary healthcare settings lacking flow cytometric capabilities, to facilitate early CGD diagnosis and improve prognosis.
Data availability statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Ethics committee of Beijing Children's hospital is affiliated to our hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants' legal guardians/next of kin because This study is a retrospective study, which does not involve additional blood tests on children and does not increase the additional risk of children. Only clinical data of children are collected, and the real identity information of children is erased and de-identified during the statistical data, which does not involve the disclosure of privacy of children.
Author contributions
WX: Data curation, Methodology, Writing – original draft. ZS: Writing – review & editing. ZX: Methodology, Writing – review & editing. TX: Writing – review & editing. SY: Writing – review & editing. LJ: Writing – review & editing. LiHui: Writing – review & editing. LiuH: Writing – review & editing. YH: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. Respiratory Rehabilitation Management and Efficacy Evaluation for Bronchiolitis Obliterans in Children (PX2020052).
Acknowledgments
Thanks to my supervisor and colleagues for their guidance and strong support in this research and thesis collaboration. Thanks to Beijing Chigene Translational Medicine Research Center Co., Ltd.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Almutairi A, Zaman F, Day-Lewis M, Tsitsikov E, Reiter A, Xue K, et al. Acetaminophen inhibits the neutrophil oxidative burst: implications for diagnostic testing. J Allergy Clin Immunol Pract. (2020) 8:3543–8. doi: 10.1016/j.jaip.2020.07.012
2. Ang YN, Soh JY, Liew WK, Lee BW. Reliability of acute illness dihydrorhodamine-123 testing for chronic granulomatous disease. Clin Lab. (2013) 59:203–6. doi: 10.7754/Clin.Lab.2012.120501
3. Barkai T, Somech R, Broides A, Gavrieli R, Wolach B, Marcus N, et al. Late diagnosis of chronic granulomatous disease. Clin Exp Immunol. (2020) 201:297–305. doi: 10.1111/cei.13474
4. Bortoletto P, Lyman K, Camacho A, Fricchione M, Khanolkar A, Katz BZ. Chronic granulomatous disease: a large, single-center US experience. Pediatr Infect Dis J. (2015) 34:1110–4. doi: 10.1097/INF.0000000000000840
5. Bridges RA. A fatal granulomatous disease of childhood. AMA J Dis Child. (1959) 97:387. doi: 10.1001/archpedi.1959.02070010389004
6. Chiriaco M, De Matteis A, Cifaldi C, Di Matteo G, Rivalta B, Passarelli C, et al. Characterization of AR-CGD female patient with a novel homozygous deletion in CYBC1 gene presenting with unusual clinical phenotype. Clin Immunol. (2023) 251:109316. doi: 10.1016/j.clim.2023.109316
7. Connelly JA, Marsh R, Parikh S, Talano JA. Allogeneic hematopoietic cell transplantation for chronic granulomatous disease: controversies and state of the art. J Pediatric Infect Dis Soc. (2018) 7:S31–9. doi: 10.1093/jpids/piy015
8. Donko A, Kuhns DB, Cousin MA, Smith MJ, Sacco KA, Klee EW, et al. Interpretation of dihydrorhodamine-1,2,3 flow cytometry in chronic granulomatous disease: an atypical exemplar. J Clin Immunol. (2022) 42:986–99. doi: 10.1007/s10875-022-01217-5
9. Freihorst J, Lohmann L, Emmend A. Diagnosis of chronic granulomatous disease and of its mode of inheritance by dihydrorhodamine 123 and flow microcytofluorometry. Pediatrics. (1991) 9:161–5. doi: 10.1007/BF01963557
10. Gennery AR. Progress in treating chronic granulomatous disease. Br J Haematol. (2021) 192:251–64. doi: 10.1111/bjh.16939
11. Jirapongsananuruk O, Malech HL, Kuhns DB, Niemela JE, Brown MR, Anderson-Cohen M, et al. Diagnostic paradigm for evaluation of male patients with chronic granulomatous disease, based on the dihydrorhodamine 123 assay. J Allergy Clin Immunol. (2003) 111:374–9. doi: 10.1067/mai.2003.58
12. Kuhns DB, Alvord WG, Heller T, Feld JJ, Pike KM, Marciano BE, et al. Residual NADPH oxidase and survival in chronic granulomatous disease. N Engl J Med. (2010) 363:2600–10. doi: 10.1056/nejmoa1007097
13. Liu H, Li H, Liu J, Yang H, Xu H, Tang X, et al. Clinical features and early diagnostic clues of chronic granulomatous disease. Zhonghua Er Ke Za Zhi. (2021) 59:777–81. doi: 10.3760/cma.j.cn112140-20210426-00348
14. Marzollo A, Conti F, Rossini L, Rivalta B, Leonardi L, Tretti C, et al. Neonatal manifestations of chronic granulomatous disease: MAS/HLH and necrotizing pneumonia as unusual phenotypes and review of the literature. J Clin Immunol. (2022) 42:299–311. doi: 10.1007/s10875-021-01159-4
15. Milligan KL, Mann D, Rump A, Anderson VL, Hsu AP, Kuhns DB, et al. Complete myeloperoxidase deficiency: beware the “false-positive” dihydrorhodamine oxidation. J Pediatr. (2016) 176:204–6. doi: 10.1016/j.jpeds.2016.05.047
16. Oikonomopoulou Z, Shulman S, Mets M, Katz B. Chronic granulomatous disease: an updated experience, with emphasis on newly recognized features. J Clin Immunol. (2022) 42:1411–9. doi: 10.1007/s10875-022-01294-6
17. Rider NL, Jameson MB, Creech CB. Chronic granulomatous disease: epidemiology, pathophysiology, and genetic basis of disease. J Pediatric Infect Dis Soc. (2018) 7:S2–5. doi: 10.1093/jpids/piy008
18. Thomas DC, Charbonnier LM, Schejtman A, Aldhekri H, Coomber EL, Dufficy ER, et al. EROS/CYBC1 mutations: decreased NADPH oxidase function and chronic granulomatous disease. J Allergy Clin Immunol. (2019) 143:782–5.e1. doi: 10.1016/j.jaci.2018.09.019
19. Van De Geer A, Nieto-Patlán A, Kuhns DB, Tool ATJ, Arias AA, Bouaziz M, et al. Inherited p40 phox deficiency differs from classic chronic granulomatous disease. J Clin Invest. (2018) 128:3957–75. doi: 10.1172/JCI97116
20. Winkelstein JA, Marino MC, Johnston RB, Boyle J, Curnutte J, Gallin JI, et al. Chronic granulomatous disease: report on a national registry of 368 patients. Medicine. (2000) 79:155–69. doi: 10.1097/00005792-200005000-00003
21. Yu HH, Yang YH, Chiang BL. Chronic granulomatous disease: a comprehensive review. Clin Rev Allergy Immunol. (2021) 61:101–13. doi: 10.1007/s12016-020-08800-x
Keywords: chronic granulomatous disease, children, DHR-enzyme-linked immunosorbent assay, NADPH oxidase complex activity detection, respiratory burst
Citation: Xiaohui W, Shunying Z, Xiaoyan Z, Xiaolei T, Yuelin S, Jinrong L, Huimin L, Hui L and Haiming Y (2025) The value of DHR-enzyme-linked immunosorbent assay in the diagnosis of chronic granulomatous disease by detecting NADPH oxidase complex activity. Front. Pediatr. 13:1483173. doi: 10.3389/fped.2025.1483173
Received: 19 August 2024; Accepted: 20 May 2025;
Published: 5 June 2025.
Edited by:
Srdjan Pasic, The Institute for Health Protection of Mother and Child Serbia, SerbiaReviewed by:
Ekaterini Simoes Goudouris, Federal University of Rio de Janeiro, BrazilDingle Yu, Shenzhen Children's Hospital, China
Edyta Heropolitańska-Pliszka, Department of Immunology CMHI, Poland
Copyright: © 2025 Xiaohui, Shunying, Xiaoyan, Xiaolei, Yuelin, Jinrong, Huimin, Hui and Haiming. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liu Hui, bGl1aHVpMDIwOUAxMjYuY29t; Yang Haiming, eWFuZ2hhaW1pbmc4MzBAMTYzLmNvbQ==
 Wen Xiaohui
Wen Xiaohui Zhao Shunying
Zhao Shunying Zhang Xiaoyan
Zhang Xiaoyan Tang Xiaolei
Tang Xiaolei Shen Yuelin
Shen Yuelin Liu Jinrong
Liu Jinrong Li Huimin
Li Huimin Liu Hui
Liu Hui Yang Haiming*
Yang Haiming*