- 1School of Public Health, Suzhou Medical College of Soochow University, Suzhou, China
- 2Medical Research Center, Sichuan Bingzhe Technology Co., Ltd., Chengdu, China
- 3School of Public Health, Hangzhou Normal University, Hangzhou, Zhejiang, China
- 4Jiangsu Key Laboratory of Preventive and Translational Medicine for Major Chronic Non-communicable Diseases, Suzhou Medical College of Soochow University, Suzhou, China
- 5MOE Key Laboratory of Geriatric Diseases and Immunology, Suzhou Medical College of Soochow University, Suzhou, China
Introduction: Only a few studies have reported the relationship between the sleep duration of pregnant women and preterm birth (PTB), and the findings are inconsistent. This study aimed to examine the association of maternal sleep duration in pregnancy with PTB in China.
Methods: A cross-sectional survey. Sleep duration in pregnancy and PTB status were self-reported via a validated questionnaire. The association of sleep duration in pregnancy with PTB was examined by logistic regression models.
Results: The overall prevalence of PTB was 16.6%. Compared to the women with total sleep duration in pregnancy of >8 h/day, those with sleep duration of 7–8 h/day (OR = 1.43; 95% CI: 1.20, 1.70) and <7 h/day (OR = 4.28; 95% CI: 3.06, 6.00) had higher odds of reporting PTB after adjustment for gender of baby, maternal age at delivery, educational level of mother, educational level of father, diabetes history, gestation diseases including gestational hyperglycemia, gestational hypertension, anemia during pregnancy and anxiety or depression during pregnancy, and pregnancy behaviors including smoking or passive smoking during pregnancy, alcohol drinking during pregnancy, prenatal education, exercise during pregnancy and folic acid supplementation daily during pregnancy. The risk associations were similar in subgroups stratified by sex, gestational hyperglycemia, anemia during pregnancy and status of folic acid supplementation.
Discussion: Sleep duration in pregnancy was inversely associated with PTB in China. Our novel findings suggest the importance of sufficient sleep in pregnant women for the prevention of PTB. Further studies are warranted to confirm and elucidate the observed association.
1 Introduction
Preterm birth (PTB) is defined by the World Health Organization as births before 37 completed weeks of gestation or less than 259 days from the first date of a woman's last menstrual period (1). It is one of the leading causes of newborn death (2, 3). Additionally, premature infants who survive have higher rates of long-term morbidity, including neurologic and developmental disabilities (4). In the United States, the prevalence of cerebral palsy among preterm infants is about 2‰, and the prevalence is as high as 3.4‰ in other regions, which can bring an enormous burden to patients, families and society (5, 6). The PTB rate has become an important indicator of perinatal health in a country or region. Overall, the prevalence of PTB in the world is on the rise, and the global PTB rate increased from 9.8% in 2000 to 10.6% in 2014 (7, 8). It is reported that China has the second highest number of preterm birth in the world, with more than one million premature infants each year (9).
Previous studies have shown multiple factors associated with PTB, including maternal demographic characteristics, nutritional status, psychological characteristics, adverse behaviors, infection, and biological markers (10). However, there are only a few studies on the relationship between sleep duration in pregnant women and PTB, and the existing results are inconsistent (11, 12). For example, as shown in a research, participants with short duration of sleep during the third trimester were more likely to report preterm birth (13). In contrast, other researches showed that the relationship between long/short sleep duration and preterm birth had no significant (14, 15). Thus, we aimed to examine the association of sleep duration during pregnancy with PTB among Chinese women, provide more evidence for the association between sleep duration and preterm birth.
2 Materials and methods
2.1 Study participants
The present study was based on a cross-sectional survey in October-December 2021. A total of 30 kindergartens in Chengdu, a provincial capital in Southwest China, were selected by a cluster random sampling method. Data were collected by a validated questionnaire administered by the mothers of preschool children via WJX (an online survey platform, https://www.wjx.cn). Overall, 4,360 mothers participated in the survey and were finally included in the analysis.
The study protocol was approved by the Ethics Committee of Soochow University (Approval NO. SUDA20210820H01). The questionnaire opens with the statement “answering the questionnaire constitutes consent to participate” in the study.
2.2 Assessment of sleep duration in pregnancy
Information on sleep duration in pregnancy was obtained via the following question: “For how long did you sleep including siesta per day during pregnancy? (a) <7 h/day, (b) 7–8 h/day, (c) >8 h/day” (16, 17).
2.3 Definition of PTB
The PTB status was determined by the following question in the questionnaire: “How many gestational weeks did you have when you delivered your child? (a) ≥37 weeks, (b) <37 weeks”. Gestational weeks of <37 weeks were defined as PTB (1).
2.4 Covariates
Gender of baby (male and female), maternal age at delivery (<25, 25–29, 30–34, and ≥35 years), educational level of mother (lower secondary education or below, high school or secondary vocational education, associate degree, bachelor's degree or above), educational level of father (lower secondary education or below, high school or secondary vocational education, associate degree, bachelor's degree or above), diabetes history (yes and no), gestation diseases including gestational hyperglycemia (yes and no), gestational hypertension (yes and no), anemia during pregnancy (yes and no) and anxiety or depression during pregnancy (yes and no), and pregnancy behaviors including smoking or passive smoking during pregnancy (0, 1–2, 3–4, and ≥5 days/week), alcohol drinking during pregnancy (0, 1–2, 3–4, and ≥5 days/week), prenatal education (0, 1–2, 3–4, and ≥5 times/week), exercise during pregnancy (0, <20, 20–40, and >40 min/day), and folic acid supplementation daily during pregnancy (yes and no) were all collected by self-report via the questionnaire (18, 19).
2.5 Statistical analyses
The basic characteristics of the participants by PTB status and sleep duration in pregnancy are presented as percentages (%) and were compared using the chi-square test or Fisher's exact test.
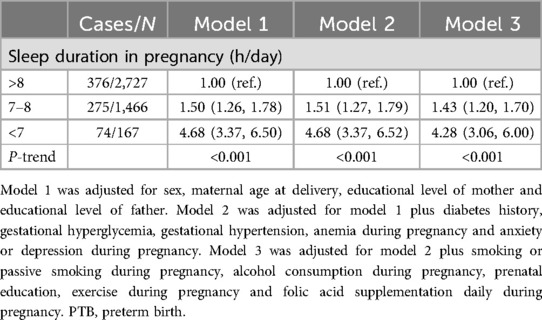
We used logistic regression to evaluate the association of sleep duration in pregnancy with PTB. Three models were constructed: model 1 was the basic model adjusted for sex of the baby, maternal age at delivery, educational level of mother and educational level of father; model 2 was adjusted as model 1 plus diabetes history and gestation diseases, including gestational hyperglycemia, gestational hypertension, anemia during pregnancy and anxiety or depression during pregnancy; and model 3 was adjusted as model 2 plus pregnancy behaviors, including smoking or passive smoking during pregnancy, alcohol drinking during pregnancy, prenatal education, exercise during pregnancy and folic acid supplementation daily during pregnancy. The P-trend was calculated by modelling the independent variable as a continuous variable in the regression models.
Effect modification of the association between sleep duration in pregnancy and PTB by gender of baby, gestational hyperglycemia, anemia during pregnancy and folic acid supplementation daily during pregnancy was examined by stratified analyses, and significance of interactions was evaluated on the first-degree multiplicative models for each stratification variable separately.
The statistical analyses were performed using SPSS 26.0 statistical software (IBM Corp., USA). All tests were two-tailed, and a P value <0.05 was considered to indicate statistical significance.
3 Results
Of the 4,360 women, there were 725 (16.6%) premature babies. The proportions of participants whose total sleep duration in pregnancy was <7, 7–8, and >8 h/day were 3.8%, 33.6%, and 62.6%, respectively.
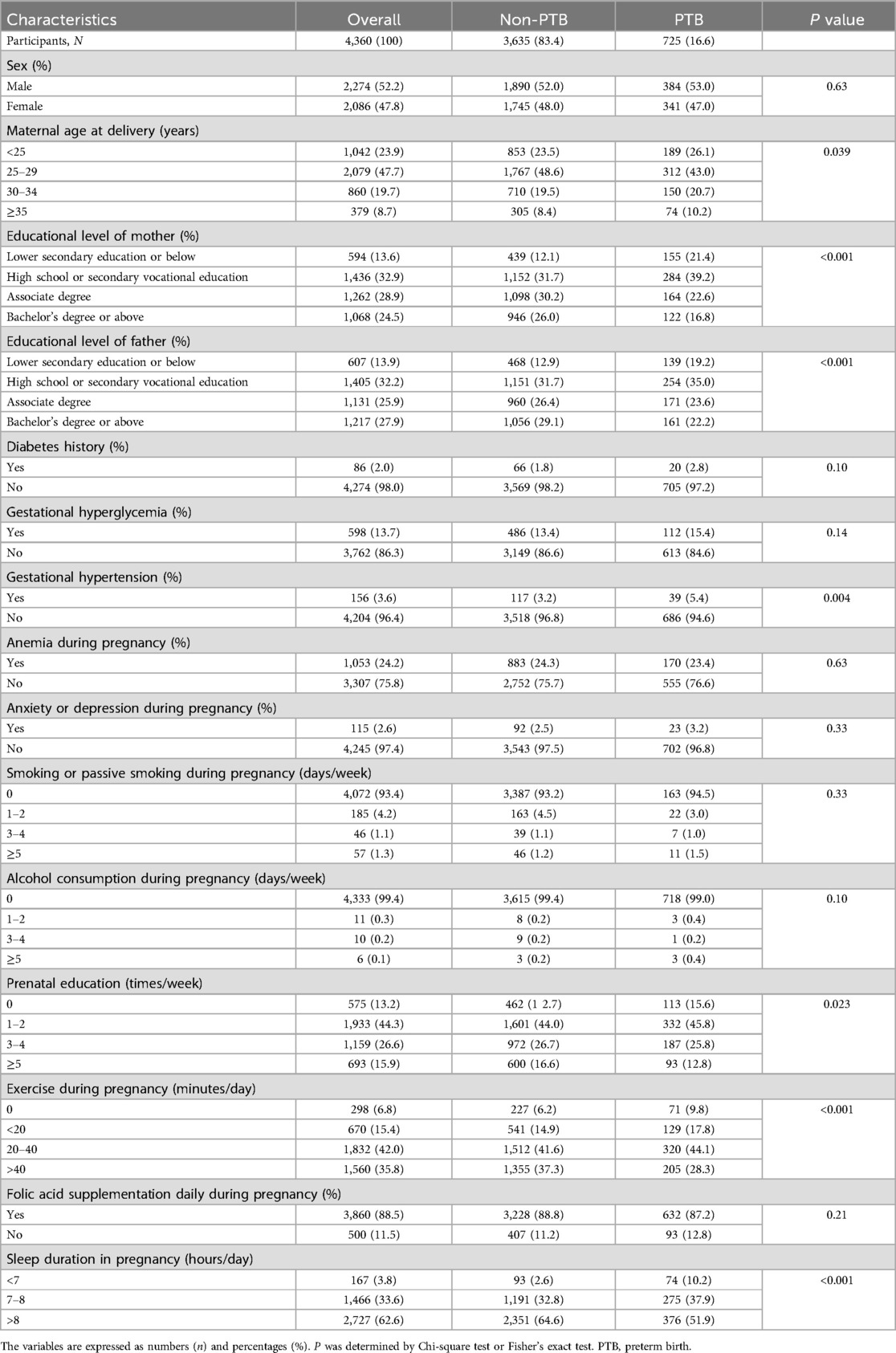
Table 1 shows the characteristics of the participating women by PTB status. The likelihood of PTB was highest in women whose delivery age was ≥35 years old (P = 0.039). The prevalence of preterm birth was significantly higher for infants whose mother's or father's educational attainment was lower secondary education or below (P < 0.001). Pregnant women with gestational hypertension were more likely to have PTB (P = 0.004). Moreover, the prevalence of PTB was higher in women with less prenatal education (P = 0.023) and exercise during pregnancy (P < 0.001).
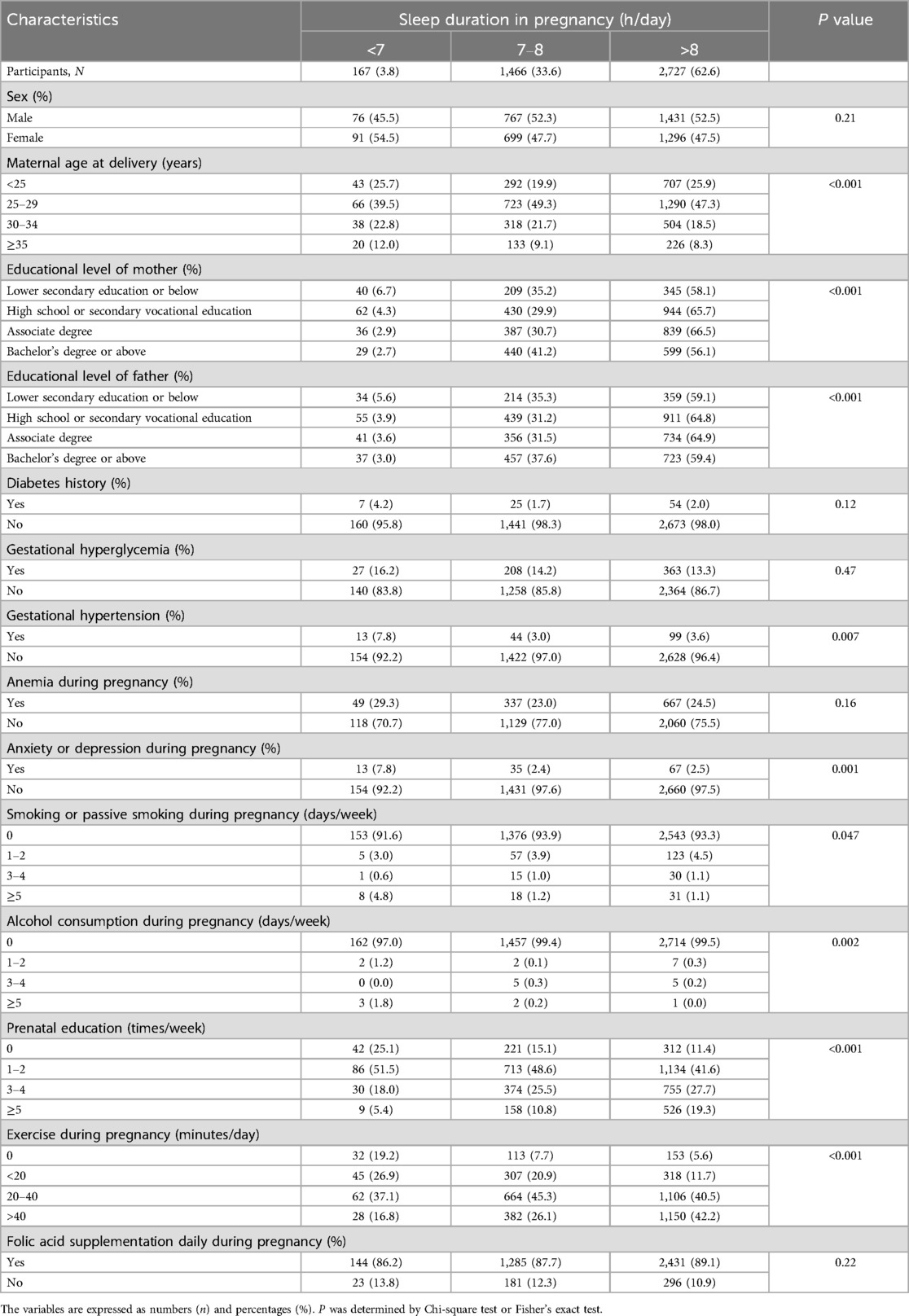
The characteristics of the participants in terms of sleep duration in pregnancy are presented in Table 2. Maternal age at delivery (P < 0.001), educational level of mother (P < 0.001), educational level of father (P < 0.001), gestational hypertension (P = 0.007), anxiety or depression during pregnancy (P = 0.001), smoking or passive smoking during pregnancy (P = 0.047), alcohol consumption during pregnancy (P = 0.002), prenatal education (P < 0.001), and exercise during pregnancy (P < 0.001) were different between the groups.
Compared with the women whose total sleep duration in pregnancy was >8 h/day, the multivariable adjusted ORs were 1.43 (95% CI: 1.20, 1.70) and 4.28 (95% CI: 3.06, 6.00) for those whose sleep duration in pregnancy was 7–8 and <7 h/day, respectively (P-trend <0.001) (Table 3).
The associations between sleep duration in pregnancy and PTB were similar in subgroups stratified by sex, gestational hyperglycemia, anemia during pregnancy and folic acid supplementation daily during pregnancy at baseline (all P-interaction >0.05) (Figure 1).
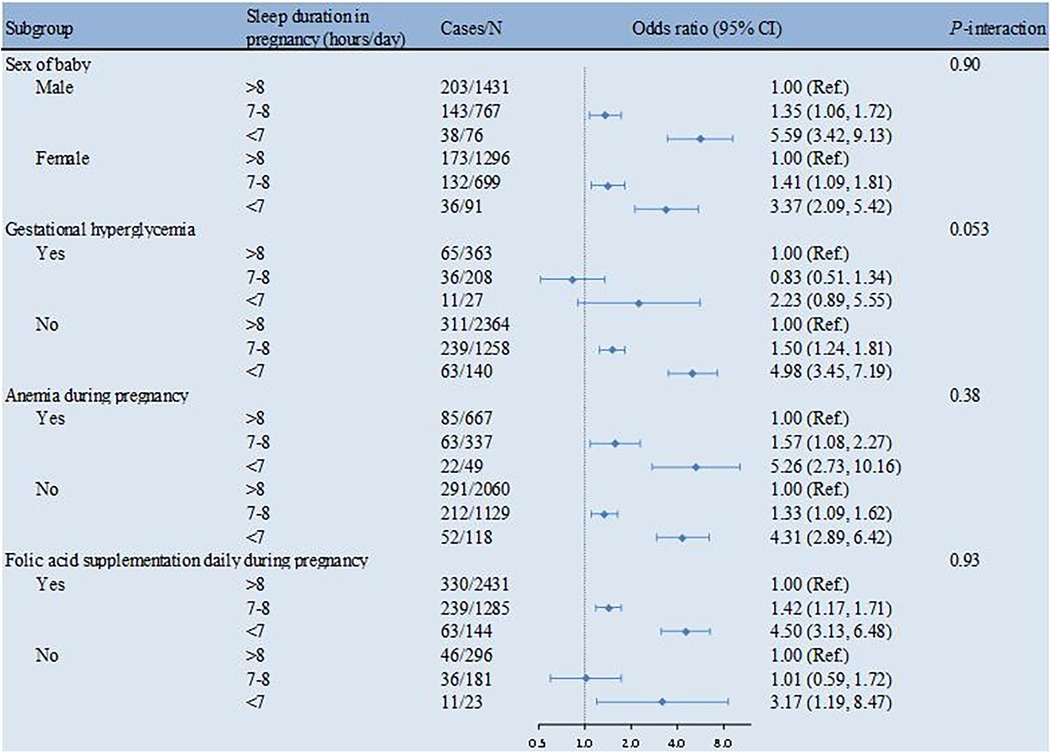
Figure 1. Sleep duration in pregnancy in association with PTB by strata. The multivariable model was adjusted for sex of the baby, maternal age at delivery, diabetes history, gestational hyperglycemia, gestational hypertension, anemia during pregnancy, anxiety or depression during pregnancy, smoking or passive smoking during pregnancy, alcohol consumption during pregnancy, prenatal education, exercise during pregnancy and folic acid supplementation daily during pregnancy. PTB, preterm birth.
4 Discussion
4.1 Principal findings
In this cross-sectional study, we observed that short sleep duration in pregnancy was independently associated with increased odds of PTB among Chinese women. The association between sleep duration in pregnancy and PTB was independent of confounding factors and similar across subgroups stratified by gender of baby, gestational hyperglycemia, anemia during pregnancy and folic acid supplementation daily during pregnancy.
4.2 Sleep duration in pregnancy and PTB
Several studies have demonstrated that insufficient sleep is potentially associated with an increased risk of preterm birth among pregnant women, which is consistent with our conclusion. A study from India showed that sleep duration in full-term women was longer than that in preterm women (20). In a cohort study, compared to pregnant women with sleep duration >8 h/day in the nighttime, those with sleep duration ≤5 h/day were more inclined to give preterm birth (21). In a case‒control study based on pregnant women in the first 6 months of pregnancy, compared to women with sleep duration in pregnancy of 7–8 h/day, those with sleep duration 6 h/day were at higher risk of PTB (22). In two prospective studies and one meta-analysis, sleep duration of <7 h/day significantly increased the odds of PTB (11, 13, 23).
Other studies have shown that both insufficient and excessive sleep duration increases the risk of preterm birth. A dose-response meta-analysis demonstrated a strong association between extreme sleep duration during pregnancy and preterm birth, characterized by a U-shaped relationship (24). Meanwhile, a Mendelian randomized analysis revealed that pregnant women sleeping less than 5 h per night or more than 10 h per night exhibited a higher risk of preterm birth (25).
However, a study from the USA showed no associations between the sleep duration of pregnant women at night and PTB, whether in the first, second or third trimester (12). Generally, the current research on preterm birth and sleep duration is inconclusive, which is consistent with the findings of another review (26).
Different study designs, study populations and methods of data collection may explain inconsistent findings.
4.3 Potential mechanisms
The underlying mechanism of sleep duration in pregnancy with PTB remains unclear. Most scholars believe that the immune system plays a mediating role between them (12, 27–30). Sleep disorders, either poor sleep quality or short sleep duration, interfere with the normal immune process, elevated levels of proinflammatory cytokines and enhanced inflammatory responses (30).
The dynamic equilibrium between Th1 (cell-mediated immunity) and Th2 (humoral immunity) at the fetal-maternal interface plays a key role in women's successful pregnancy. Th1 cells produce proinflammatory cytokines such as interleukin-1, 2, 6, 8, 12 and tumor necrosis factor α. Th2 produced anti-inflammatory cytokines such as interleukin-4, 5, 10, and 13 (31–33). During normal pregnancy, Th2 activity was much stronger than Th1 activity, which had potential protective effects in the fetal-maternal relationship. For instance, the injection of IL-10, a Th2-type cytokine, prevents fetal wastage in mice prone to fetal resorption via conspicuous downregulatory effects on Th1-type cytokines (34). However, immune dysregulation by a progressive shift toward Th1 predominance may initiate and intensify the cascade of proinflammatory cytokine release, including IL-1, 2, 6, 8 and TNF-α (31), which induces the production of prostaglandins, an important inducer of uterine contraction (35, 36).
In addition, other physical and psychological conditions, such as gestational diabetes (37, 38), gestational hypertension (39, 40), obesity (41, 42), poor psychological status including depression (43, 44) and hyperhomocysteinaemia (45, 46), may also play a role in the association between sleep duration in pregnancy and PTB.
4.4 Strengths and limitations
The main strengths of our study include a relatively large sample size and consideration of adjustment for a number of potential confounders. Meanwhile, the findings of the existing research are inconsistent. Our study provides additional reference evidence for research in this field and is meaningful. Sleep duration was collected by subjective estimation of the participants instead of objective measurement, which may lead to self-reporting bias. The gestational status in our study was retrospectively estimated; thus, recall bias might have occurred. Quality of sleep, such as sleep-disordered breathing, was not collected, which limited further exploration (47).
5 Conclusion
In this cross-sectional study, sleep duration in pregnancy was inversely associated with PTB among Chinese women. Our findings underline the importance of sufficient sleep (>8 h/day) for pregnant women in the prevention of PTB.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Soochow University (Approval No. SUDA20210820H01). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
HJ: Project administration, Writing – original draft. BY: Data curation, Resources, Writing – original draft. YL: Formal analysis, Writing – original draft. HG: Investigation, Writing – original draft. RS: Investigation, Writing – original draft. ST: Investigation, Writing – original draft. SH: Writing – review & editing. HZ: Conceptualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
BY and YL were employed by Sichuan Bingzhe Technology Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. WHO: recommended definitions, terminology and format for statistical tables related to the perinatal period and use of a new certificate for cause of perinatal deaths. Modifications recommended by FIGO as amended October 14, 1976. Acta Obstet Gynecol Scand. (1977) 56(3):247–53. doi: 10.3109/00016347709162009
2. Walani SR. Global burden of preterm birth. Int J Gynaecol Obstet. (2020) 150(1):31–3. doi: 10.1002/ijgo.13195
3. Frey HA, Klebanoff MA. The epidemiology, etiology, and costs of preterm birth. Semin Fetal Neonatal Med. (2016) 21(2):68–73. doi: 10.1016/j.siny.2015.12.011
4. Marlow N, Wolke D, Bracewell MA, Samara M, EPICure Study Group. Neurologic and developmental disability at six years of age after extremely preterm birth. N Engl J Med. (2005) 352:9–19. doi: 10.1056/NEJMoa041367
5. Jahan I, Muhit M, Hardianto D, Laryea F, Chhetri AB, Smithers-Sheedy H, et al. Epidemiology of cer- ebral palsy in low- and middle-income countries:preliminary findings from an international multi-centre cerebral palsy register. Dev Med Child Neurol. (2021) 63:1327. doi: 10.1111/dmcn.14926
6. McIntyre S, Goldsmith S, Webb A, Ehlinger V, Hollung SJ, McConnell K, et al. Global prevalence of eerebral palsy: a systematic analysis. Dev Med Child Neurol. (2022) 64:1494–506. doi: 10.1111/dmcn.15346
7. Lawn JE, Gravett MG, Nunes TM, Rubens CE, Stanton C, GAPPS Review Group. Global report on preterm birth and stillbirth (1 of 7): definitions, description of the burden and opportunities to improve data. BMC Pregnancy Childbirth. (2010) 10(Suppl 1):S1. doi: 10.1186/1471-2393-10-S1-S1
8. Chawanpaiboon S, Vogel JP, Moller A-B, Lumbiganon P, Petzold M, Hogan D, et al. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob Health. (2019) 7(1):e37–46. doi: 10.1016/S2214-109X(18)30451-0
9. Deng K, Liang J, Mu Y, Liu Z, Wang Y, Li M, et al. Preterm births in China between 2012 and 2018: an observational study of more than 9 million women. Lancet Glob Health. (2021) 9(9):e1226–41. doi: 10.1016/S2214-109X(21)00298-9
10. Goldenberg RL, Goepfert AR, Ramsey PS. Biochemical markers for the prediction of preterm birth. Am J Obstet Gynecol. (2005) 192(5 Suppl):S36–46. doi: 10.1016/j.ajog.2005.02.015
11. Li R, Zhang J, Zhou R, Liu J, Dai Z, Liu D, et al. Sleep disturbances during pregnancy are associated with cesarean delivery and preterm birth. J Matern Fetal Neonatal Med. (2017) 30(6):733–8. doi: 10.1080/14767058.2016.1183637
12. Okun ML, Luther JF, Wisniewski SR, Sit D, Prairie BA, Wisner KL. Disturbed sleep, a novel risk factor for preterm birth? J Womens Health. (2012) 21(1):54–60. doi: 10.1089/jwh.2010.2670
13. Li R, Zhang J, Gao Y, Zhang Y, Lan X, Dong H, et al. Duration and quality of sleep during pregnancy are associated with preterm birth and small for gestational age: a prospective study. Int J Gynaecol Obstet. (2021) 155(3):505–11. doi: 10.1002/ijgo.13584
14. Wang R, Xu M, Yang W, Xie G, Yang L, Shang L, et al. Maternal sleep during pregnancy and adverse pregnancy outcomes: a systematic review and meta-analysis. J Diabetes Investig. (2022) 13(7):1262–76. doi: 10.1111/jdi.13770
15. Loy SL, Cheung YB, Cai S, Colega MT, Godfrey KM, Chong Y-S, et al. Maternal night-time eating and sleep duration in relation to length of gestation and preterm birth. Clin Nutr. (2020) 39(6):1935–42. doi: 10.1016/j.clnu.2019.08.018
16. Healthy China Action Promotion Committee. Healthy China Action (2019-2030). Beijing: The State Council, (2019).
17. National Sleep Foundation. Sleep time duration recommendations (n.d.). Available at: https://www.sleepfoundation.org/ (Accessed March 6, 2025).
18. Ncube CN, McCormick SM, Badon SE, Riley T, Souter VL. Antepartum and intrapartum stillbirth rates across gestation: a cross-sectional study using the revised foetal death reporting system in the US. BMC Pregnancy Childbirth. (2022) 22(1):885. doi: 10.1186/s12884-022-05185-x
19. Nouri F, Feizi A, Keshteli AH, Roohafza H, Afshar H, Adibi P. Personality traits are differently associated with depression and anxiety: evidence from applying bivariate multiple binary logistic regression on a large sample of general adults. Psychiatr Danub. (2019) 31(4):448–56. doi: 10.24869/psyd.2019.448
20. Dolatian M, Mehraban Z, Sadeghniat K. The effect of impaired sleep on preterm labour. West Indian Med J. (2014) 63(1):62–7. doi: 10.7727/wimj.2012.305
21. Micheli K, Komninos I, Bagkeris E, Roumeliotaki T, Koutis A, Kogevinas M, et al. Sleep patterns in late pregnancy and risk of preterm birth and fetal growth restriction. Epidemiology. (2011) 22(5):738–44. doi: 10.1097/EDE.0b013e31822546fd
22. Kajeepeta S, Sanchez SE, Gelaye B, Qiu C, Barrios YV, Enquobahrie DA, et al. Sleep duration, vital exhaustion, and odds of spontaneous preterm birth: a case–control study. BMC Pregnancy Childbirth. (2014) 14:337. doi: 10.1186/1471-2393-14-337
23. Lu Q, Zhang X, Wang Y, Li J, Xu Y, Song X, et al. Sleep disturbances during pregnancy and adverse maternal and fetal outcomes: a systematic review and meta-analysis. Sleep Med Rev. (2021) 58:101436. doi: 10.1016/j.smrv.2021.101436
24. Shi F, Ji C, Wu Q, Zhao Y. Association between sleep duration during pregnancy and preterm birth: a dose-response meta-analysis. J Matern Fetal Neonatal Med. (2022) 35(25):7617–28. doi: 10.1080/14767058.2021.1957821
25. Yang Q, Magnus MC, Kilpi F, Santorelli G, Soares AG, West J, et al. Investigating causal relations between sleep duration and risks of adverse pregnancy and perinatal outcomes: linear and nonlinear Mendelian randomization analyses. BMC Med. (2022) 20(1):295. doi: 10.1186/s12916-022-02494-y
26. Delgado A, Louis JM. Sleep deficiency in pregnancy. Sleep Med Clin. (2023) 18(4):559–71. doi: 10.1016/j.jsmc.2023.06.011
27. Okun ML, Roberts JM, Marsland AL, Hall M. How disturbed sleep may be a risk factor for adverse pregnancy outcomes a hypothesis. Obstet Gynecol Surv. (2009) 64(4):273–80. doi: 10.1097/OGX.0b013e318195160e
28. Okun ML, Luther JF, Wisniewski SR, Wisner KL. Disturbed sleep and inflammatory cytokines in depressed and nondepressed pregnant women: an exploratory analysis of pregnancy outcomes. Psychosom Med. (2013) 75(7):670–81. doi: 10.1097/PSY.0b013e31829cc3e7
29. Felder JN, Baer RJ, Rand L, Jelliffe-Pawlowski LL, Prather AA. Sleep disorder diagnosis during pregnancy and risk of preterm birth. Obstet Gynecol. (2017) 130(3):573–81. doi: 10.1097/AOG.0000000000002132
30. Irwin M. Effects of sleep and sleep loss on immunity and cytokines. Brain Behav Immun. (2002) 16:503–12. doi: 10.1016/S0889-1591(02)00003-X
31. Challis JR, Lockwood CJ, Myatt L, Norman JE, Strauss JF, Petraglia F. Inflammation and pregnancy. Reprod Sci. (2009) 16(2):206–15. doi: 10.1177/1933719108329095
32. Makhseed M, Raghupathy R, Azizieh F, Omu A, Al-Shamali E, Ashkanani L. Th1 and Th2 cytokine profiles in recurrent aborters with successful pregnancy and with subsequent abortions. Hum Reprod. (2001) 16(10):2219–26. doi: 10.1093/humrep/16.10.2219
33. Poole JA, Claman HN. Immunology of pregnancy implications for the mother. Clin Rev Allergy Immunol. (2004) 26(04):161–70. doi: 10.1385/CRIAI:26:3:161
34. Chaouat G, Menu E, de Smedt D, Khrihnan L, Hui L, Assal Meliani A, et al. The emerging role of IL-10 in pregnancy. Am J Reprod Immunol. (1996) 35:325–9. doi: 10.1111/j.1600-0897.1996.tb00488.x
35. Makhseed M, Raghupathy R, El-Shazly S, Azizieh F, Al-Harmi JA, Al-Azemi MM. Pro-inflammatory maternal cytokine profile in preterm delivery. Am J Reprod Immunol. (2003) 49:308–18. doi: 10.1034/j.1600-0897.2003.00038.x
36. Gravett MG, Witkin SS, Haluska GJ, Edwards JL, Cook MJ, Novy MJ. An experimental model for intraamniotic infection and preterm labor in rhesus monkeys. Am J Obstet Gynecol. (1994) 171(6):1660–7. doi: 10.1016/0002-9378(94)90418-9
37. Reutrakul S, Zaidi N, Wroblewski K, Kay HH, Ismail M, Ehrmann DA, et al. Sleep disturbances and their relationship to glucose tolerance in pregnancy. Diabetes Care. (2011) 34(11):2454–7. doi: 10.2337/dc11-0780
38. HAPO Study Cooperative Research Group, Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. (2008) 305(19):1991–2002. doi: 10.1056/NEJMoa0707943
39. Williams MA, Miller RS, Qiu C, Cripe SM, Gelaye B, Enquobahrie D. Associations of early pregnancy sleep duration with trimester-specific blood pressures and hypertensive disorders in pregnancy. Sleep. (2010) 33(10):1363–71. doi: 10.1093/sleep/33.10.1363
40. Chen Y, Li G, Ruan Y, Zou L, Wang X, Zhang W. An epidemiological survey on low birth weight infants in China and analysis of outcomes of full-term low birth weight infants. BMC Pregnancy Childbirth. (2013) 13:242. doi: 10.1186/1471-2393-13-242
41. Cappuccio FP, Taggart FM, Kandala NB, Currie A, Peile E, Stranges S, et al. Meta-analysis of short sleep duration and obesity in children and adults. Sleep. (2008) 31(5):619–26. doi: 10.1093/sleep/31.5.619
42. McDonald SD, Han Z, Mulla S, Beyene J. Overweight and obesity in mothers and risk of preterm birth and low birth weight infants: systematic review and meta-analyses. Br Med J. (2010) 341:c3428. doi: 10.1136/bmj.c3428
43. Li L, Wu C, Gan Y, Qu X, Lu Z. Insomnia and the risk of depression: a meta-analysis of prospective cohort studies. BMC Psychiatry. (2016) 16(1):375. doi: 10.1186/s12888-016-1075-3
44. Dayan J, Creveuil C, Marks MN, Conroy S, Herlicoviez M, Dreyfus M, et al. Prenatal depression, prenatal anxiety, and spontaneous preterm birth: a prospective cohort study among women with early and regular care. Psychosom Med. (2006) 68(6):938–46. doi: 10.1097/01.psy.0000244025.20549.bd
45. Rajendiran S, Swetha Kumari A, Nimesh A, Ananthanarayanan PH, Dhiman P. Markers of oxidative stress in pregnant women with sleep disturbances. Oman Med J. (2015) 30(4):264–9. doi: 10.5001/omj.2015.53
46. Kramer MS, Kahn SR, Rozen R, Evans R, Platt RW, Chen MF, et al. Vasculopathic and thrombophilic risk factors for spontaneous preterm birth. Int J Epidemiol. (2009) 38(3):715–23. doi: 10.1093/ije/dyp167
Keywords: sleep duration, preterm birth, pregnant women, cross-sectional survey, China
Citation: Jiang H, Yu B, Liu Y, Gao H, Song R, Tan S, Han S and Zuo H (2025) Association of sleep duration in pregnancy with preterm birth in China: a cross-sectional survey. Front. Pediatr. 13:1493248. doi: 10.3389/fped.2025.1493248
Received: 8 September 2024; Accepted: 23 May 2025;
Published: 9 June 2025.
Edited by:
Zhangbin Yu, Department of Neonatology, Shenzhen People's Hospital, The Second Clinical Medical College of Jinan University, First Affiliated Hospital of Southern University of Science and Technology Shenzhen, ChinaReviewed by:
Meng-Bin Tang, China Medical University Hospital, TaiwanHao-Yuan Lee, Fu Jen Catholic University, Taiwan
Copyright: © 2025 Jiang, Yu, Liu, Gao, Song, Tan, Han and Zuo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shufen Han, c2ZoYW5AaHpudS5lZHUuY24=; Hui Zuo, enVvaHVpQHN1ZGEuZWR1LmNu
 Hui Jiang
Hui Jiang Bin Yu2
Bin Yu2 Shufen Han
Shufen Han Hui Zuo
Hui Zuo