- 1Medical Genetic Diagnosis and Therapy Center, Fujian Maternity and Child Health Hospital College of Clinical Medicine for Obstetrics & Gynecology and Pediatrics, Fujian Medical University, Fujian Key Laboratory for Prenatal Diagnosis and Birth Defect, Fuzhou, China
- 2The Clinical Laboratory Center of the Second Affiliated Hospital of Fujian Medical University, Quanzhou, Fujian, China
- 3The Graduate School of Fujian Medical University, Fuzhou, Fujian, China
Background: Fetal hyperechoic kidney is an important soft marker in prenatal ultrasonography; however, the causes of this phenomenon are unclear. Therefore, we analyzed genetic diagnosis results, assessed pregnancy outcomes, and conducted postnatal follow-up to provide evidence for prenatal eugenics.
Methods: We retrospectively analyzed data from 94 cases with fetal hyperechoic kidneys identified between November 2017 and January 2024. Chromosome karyotyping and chromosomal microarray analysis (CMA) were performed on fetuses displaying this phenotype on prenatal ultrasound. For cases with normal results from karyotyping and CMA, whole-exome sequencing (WES) was applied.
Results: Among 94 fetuses with hyperechoic kidneys, five were not subject to chromosome karyotyping owing to gestational age constraints, and one sample failed to culture. Of the remaining 88, karyotyping helped detect six cases with abnormal karyotypes. Among 94 fetuses with hyperechoic kidneys, CMA analysis was performed on 90 fetuses, and 17 cases of abnormal copy number variations (CNVs) were detected. Furthermore, among 82 fetuses with normal karyotypes, 10 additional abnormal CNVs were identified. WES, performed on 13 fetuses with normal chromosomal karyotypes and CMA, helped identify three cases of mutations in HNF1B, NPHP3, and KMT2D. Follow-up of 94 fetuses indicated that 16 were lost to follow-up. Of the 78 followed-up, 25 pregnancies were terminated, and one fetus died in utero. Post-birth follow-up of 52 live births revealed an adverse outcome incidence of 3.85% (2/52), consisting of one neonatal death within 24 h and one case of intellectual disability.
Conclusions: CMA is recommended when prenatal ultrasound indicates fetal hyperechoic kidneys. For fetuses with normal CNVs and persistent hyperechoic kidneys, WES is advisable to exclude rare monogenic disorders. In cases of hyperechoic kidneys alongside other ultrasound abnormalities, the live birth rate and prognosis tend to be poor; thus, early genetic screening is essential to guide pregnancy management effectively.
Introduction
Fetal hyperechoic kidney is a common soft marker of prenatal ultrasound abnormalities, defined by a renal echo more brighter than the liver (1). This condition may result from congenital urinary system malformations, chromosomal abnormalities, monogenic mutations, normal kidney variations, or other conditions (2–6). Clinical manifestations and prognoses differ depending on the underlying causes. Currently, hyperechoic kidney is primarily categorized into isolated and non-isolated types, depending on the presence of additional abnormalities (7). Isolated hyperechoic kidney may only manifest as hyperechoic kidney prior to delivery and could be associated with renal cystic lesions, abnormal amniotic fluid volume, and renal volume changes (8). Non-isolated hyperechoic kidney frequently results in more severe conditions that may lead to neurological and cardiovascular malformations, consequently increasing the rate of pregnancy termination (9). Yulia et al. (9) recommended chromosome karyotyping analysis, chromosomal microarray analysis, or genetic testing when fetal hyperechoic kidney is observed in prenatal testing after 20 weeks of pregnancy, which is consistent with current prenatal guidance protocols. Genetic testing and postpartum follow-up of fetuses with hyperechoic kidneys enhance our understanding of the various causes of hyperechoic kidney.
In prenatal examinations, numerous cases with fetal hyperechoic kidney, such as those with abnormal chromosome numbers, are encountered. These cases do not alter the adverse outcomes of fetal illness or death, whether occurring intrauterine or postnatally. Therefore, screening for chromosomal and genetic abnormalities upon detection of fetal hyperechoic kidney during prenatal testing is crucial. Accordingly, we retrospectively analyzed data from ultrasound examinations, genetic factors, and postnatal follow-ups for 94 fetuses with hyperechoic kidneys to provide accurate etiological diagnoses for clinical practice, comprehensive genetic counseling, prognostic analysis, postnatal medical guidance, and to prevent unnecessary terminations of pregnancy.
Materials and methods
Patient data
A retrospective analysis was conducted on 94 cases with fetal hyperechoic kidneys identified at Fujian Maternal and Child Health Hospital from November 2017 to January 2024. Cases further treated at a prenatal diagnosis center that provided informed consent for interventional prenatal diagnosis were included. The mean age of the pregnant women was 29.6 years (range, 23–41 years); the mean gestational age was 25.83 weeks (range, 15–34 + 4 weeks). According to whether other ultrasound abnormalities were combined, cases were categorized into isolated (16 cases) and non-isolated (78 cases) hyperechoic kidney groups. Postpartum follow-up included collection of pregnancy information, results of postpartum ultrasound examinations, and determination of the need for surgical treatment. This is a secondary analysis of data (Ethics Approval No.: 2014042), where all participants provided written informed consent explicitly permitting future research use of their de-identified data. The current study was separately approved by the Ethics Committee of Fujian Maternal and Child Health Hospital.
Ultrasound examination
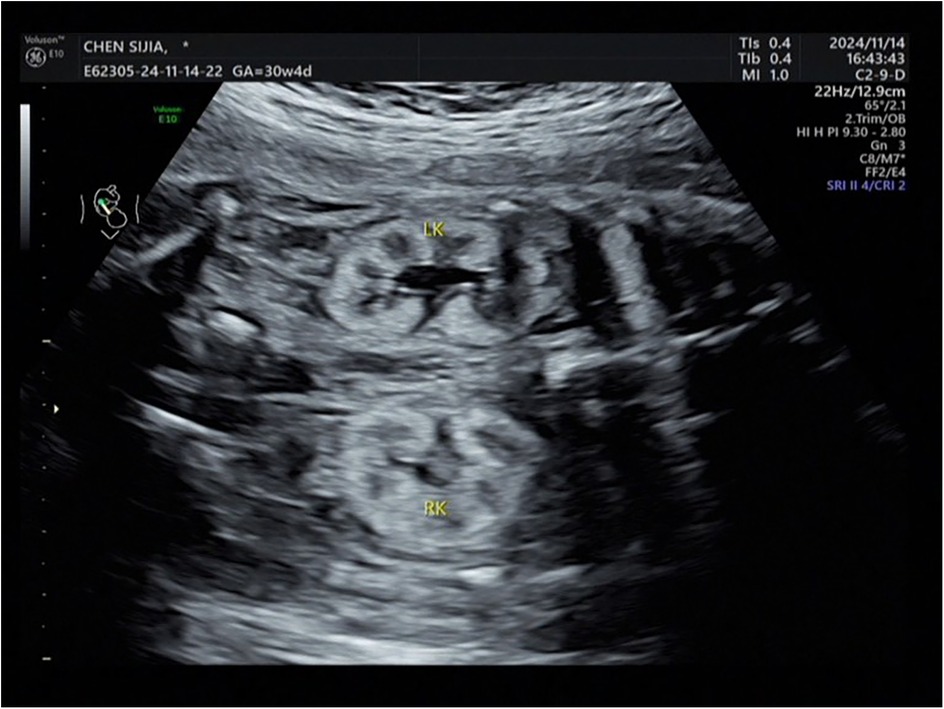
The Voluson E8 ultrasound diagnostic instrument from GE in the United States, with an abdominal probe frequency of 2.0–5.0 MHz. was employed. A systematic examination of the entire body and accessory structures of the fetus was performed along with routine biological measurements. Examination of fetal kidneys included assessments of kidney size and shape, echo, collecting system, a clear boundary between the cortex and medulla, and the presence of cysts. The criterion for diagnosing fetal hyperechoic kidney was renal echo during mid-to-late pregnancy brighter than the liver (Figure 1). The deepest vertical pocket and amniotic fluid index were used to evaluate amniotic fluid volume during mid and late pregnancy, respectively. Cases were categorized as isolated or non-isolated hyperechoic kidney based on the presence of additional abnormalities.
Preparation and analysis of chromosome karyotypes
Under the guidance of B-ultrasound, we performed amniocentesis to extract amniotic fluid from pregnant women. Amniotic fluid specimens were placed in two sterile centrifuge tubes and centrifuged at 2,000 rpm for 10 min. The supernatant was discarded, and the precipitate was inoculated into two bottles of 4 ml amniotic fluid cell culture medium. The culture was then incubated at 37°C in a 5% CO2 incubator for 8 days. After the cells grew well, the medium was changed, and the culture was continued for an additional day. Upon observing multiple cell clones under an inverted microscope and refractive amniotic fluid cells constituting ≥90% of the cloned cells, colchicine was added to arrest the culture. The adherent cells were digested with trypsin, and hypotonic, fixed, droplet, band, and Giemsa staining were performed. We counted ≥20 split cells cultured in double lines per sample, analyzed ≥5 split cells, and increased the count when chimerism was observed. The karyotype results are described according to the requirements detailed in the reference (10).
CMA technical standard operating procedure
Genomic DNA extraction was performed on fetal samples using the column method with the Qiamp DNA Blood MiniKit (Qiagen, Germany). After extraction, the purity and concentration of the DNA were measured using a NanoDrop 2000 ultra microspectrophotometer. Employing the Affymetrix CYTOSSCAN 750K single nucleotide polymorphism microarray detection platform (Affymetrix), 300 ng of genomic DNA was subjected to enzymatic digestion, ligation, PCR, purification, fragmentation, labeling, hybridization, washing, and scanning according to the standard experimental procedure. Analysis was performed using Chromosome Analysis Suite software version 4.2. Interpretation involved a comprehensive analysis of the Online Mendelian Inheritance in Man (OMIM) database, Clinical Genomic Variation Database (ClinVar), DECIPHER database, Database of Genomic Variants, and Clinical Genomic Resources (ClinGen). Following guidelines from the American Society for Medical Genetics and Genomics (11, 12), CNVs were classified into five levels: benign CNV, pathogenic CNV, likely pathogenic CNV, likely benign CNV, and variant of uncertain significance (VUS).
WES technical standard operating procedure
WES was performed by Beijing BioChain Medical Laboratory. Fetal DNA was cut into millions of small DNA fragments to construct a genomic library, obtain exon sequences using targeted hybridization probes, and sequence the DNA. Following sequencing, the raw data were aligned using BWA software. Mutations, including single nucleotide polymorphisms (SNPs), insertions, and deletions, were identified and analyzed using GATK and VarScan software for detection and annotation. Annovar software was used to annotate variant sites from external databases and evaluate the impact of target sequence mutations. Mutations detected using whole-exome technology were classified according to the guidelines of the American College of Medical Genetics and Genomics (ACMG) into pathogenic mutations, suspected pathogenic mutations, mutations of unknown significance, suspected benign mutations, and benign mutations (13). The DNA sequence obtained through the WES of the family was compared with that of the reference human genome hg19, and the coverage and sequencing quality of the target area were evaluated. Bioinformatics analysis was performed on the variations, and potential pathogenic homozygous and compound heterozygous single nucleotide variations and small variations were screened under quality control standards of target area coverage >99% and average depth >120×. Based on the site alignment of family sequencing data, the genetic patterns of sample variations were analyzed. The median turnaround time for WES results was 25 days (range: 20–28), with results typically available at a median gestational age of 28 weeks (range: 24–34).
Pregnancy outcome and postnatal follow-up
The internal clinical information registration system of the hospital and telephone follow-ups were used to track fetal pregnancy outcomes and the postnatal growth and neurobehavioral development of infants. Outcomes included live births, fetal deaths in utero, pregnancy terminations, spontaneous abortions, and infant deaths. Follow-ups, including evaluations of postpartum imaging, surgical interventions, effectiveness of the surgery, and growth and intellectual development of the neonates, were conducted on all cases after birth.
Statistical analysis
The obtained data were analyzed using SPSS 25.0 statistical software, and Fisher's exact probability method was used for rate comparisons. All statistical tests were conducted using a two-sided design, with a significance level of α = 0.05. A P < 0.05 is considered statistically significant.
Results
Chromosome karyotype analysis
Among the 94 fetuses with hyperechoic kidneys, five were excluded from karyotype analysis owing to advanced gestational age, and one sample failed to culture. We analyzed karyotypes in 88 cases and detected six abnormal karyotypes (6.82%, 6/88), including four cases with abnormal chromosome numbers (three cases of trisomy 21 and one case of XXY) and two cases with abnormal chromosome structures. Chromosomal structural abnormalities comprised one case of a large segment deletion [46, X, del (X) (q28)] and one case of an unbalanced chromosomal translocation [46, X, add (13) (p11)]. In follow-up, except for cases carrying 46, X, del (X) (q28) who refused follow-up, all pregnancies involving the five fetuses with identified karyotype abnormalities were terminated.
CMA analysis
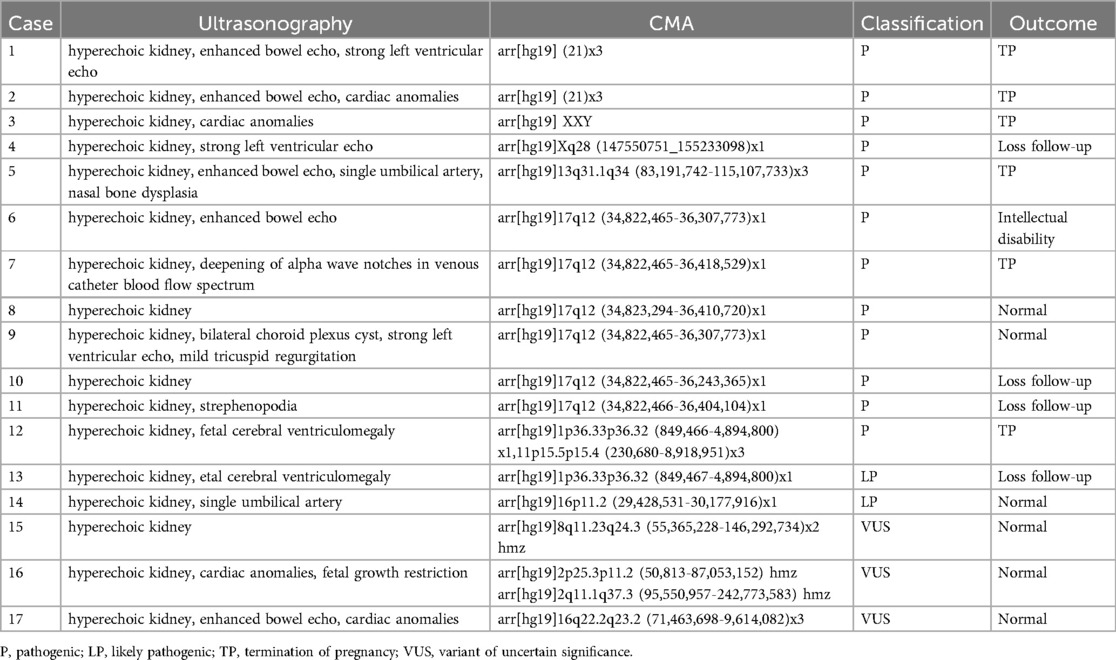
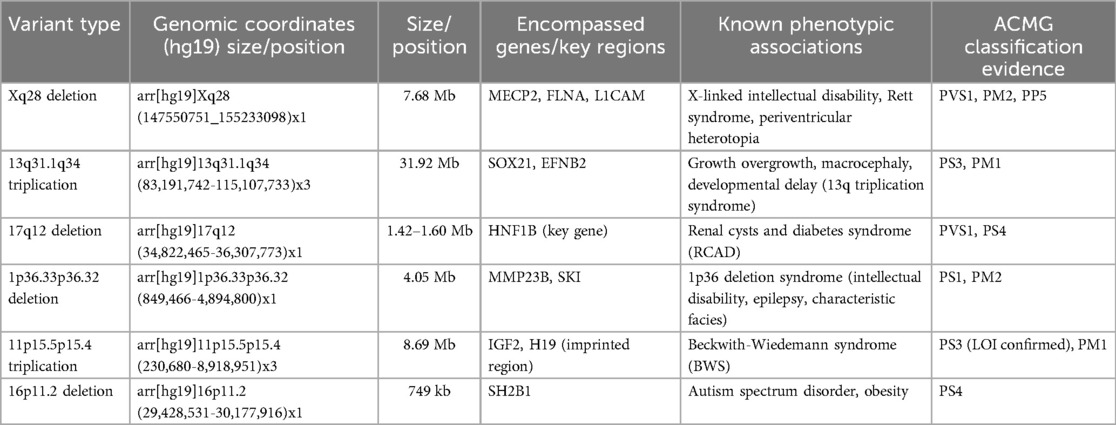
Among the 94 cases, four were not assessed, and the results from 90 fetuses revealed 17 with abnormal CNVs (18.89%, 17/90), including 12 pathogenic CNVs (13.33%, 12/90), two likely pathogenic CNVs (2.22%, 2/90), and three VUS (3.33%, 3/90). Identified abnormalities included three cases of aneuploidy (two trisomy 21, one 47, XXY), six cases of 17q12 microdeletion, two cases of 1p36.33p36.32 microdeletion, and one case each of 16p11.2 microdeletion, Xq28 deletion, 13q31.1q34 duplication, 16q22.2q23.2 microdeletion, 8q11.23q24.3 uniparental disomy, and 2p25.3p11.2 uniparental disomy (Tables 1, 2). In the follow-up of pregnancy outcomes of 17 fetuses with hyperechoic kidneys carrying abnormal CNVs, follow-up was refused for four cases, six pregnancies were terminated, and seven fetuses with normal clinical phenotypes were successfully followed up after birth.
Conventional karyotype analysis and CMA both helped effectively detect chromosomal numerical abnormalities and large fragment deletions or duplications. We employed these methods to identify two simultaneous cases of trisomy 21: one involved an abnormal sex chromosome number, another a chromosome duplication, and a third involved a chromosome deletion. Routine karyotype analysis revealed trisomy 21 in a patient who did not undergo CMA testing. CMA helped detect an additional 10 abnormal CNVs in 82 fetuses with normal chromosome karyotypes, including seven pathogenic CNVs, one likely pathogenic CNV, and two VUS. This increased the detection rate of chromosomal abnormalities by 12.20% (10/82). Additionally, CMA helped detect two additional abnormal CNVs in four fetuses without chromosomal karyotyping, including one harboring pathogenic CNV and one with a likely pathogenic CNV.
WES results
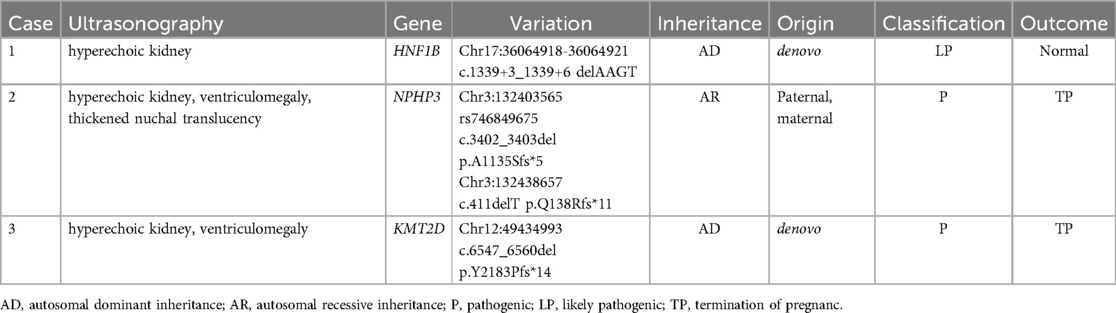
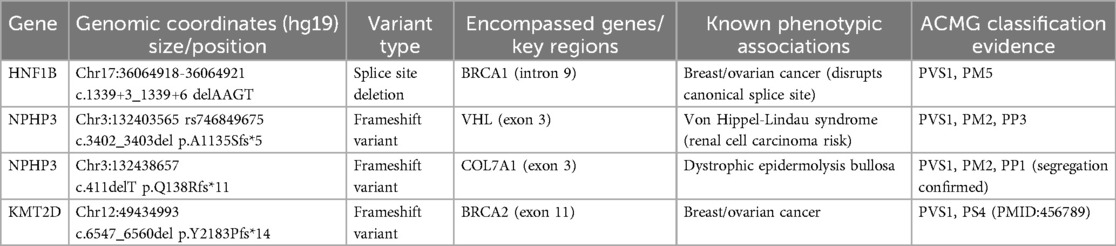
Among the 94 fetuses with hyperechoic kidneys, 13 cases with normal conventional karyotype and CMA results were further subjected to WES; two cases of pathogenic mutations and one likely pathogenic gene mutation involving genes, HNF1B, NPHP3, and KMT2D, were identified (Tables 3, 4). The key clinical distinction between fetal hyperechoic kidneys with normal vs. abnormal whole-exome sequencing (WES) results primarily manifests in the presence of concurrent extrarenal anomalies (Table 5).
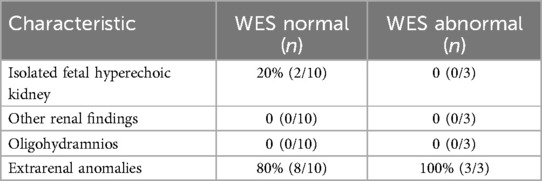
Table 5. Clinical characteristics of other renal findings and other anomalies for fetal hyperechoic kidneys with normal vs. abnormal WES.
Analysis of genetic abnormalities in each group with hyperechoic kidney
We categorized 94 cases into isolated and non-isolated fetal hyperechoic kidneys groups based on the presence of other ultrasound abnormalities. In the isolated fetal hyperechoic kidneys group (including 16 cases), we detected two cases of pathogenic CNV, one VUS, and one potential pathogenic gene mutation, resulting in a genetic abnormality detection rate of 25.00% (4/16). In the non-isolated fetal hyperechoic kidneys group (including 78 cases), we found 10 cases of pathogenic CNVs, two cases of potentially pathogenic CNVs, two cases of VUS, and two cases of pathogenic gene mutations. The detection rate of genetic abnormalities was 20.51% (16/78), with no statistically significant difference in the detection rate of genetic abnormalities between the two groups (P > 0.05).
Pregnancy outcome
Out of 94 cases of fetuses with hyperechoic kidneys, 78 completed pregnancy outcome follow-up; 16 were lost to follow-up. Of the followed-up cases, pregnancies were terminated in 25 cases, one fetal death occurred in utero, and 52 live births were recorded.
Postnatal follow-up
After follow-up observation of 52 live births, 2 cases experienced adverse outcomes post-birth, with an incidence rate of 3.85% (2/52). One fetus died within 24 h of birth despite normal genomic testing and an intrauterine ultrasound phenotype of hyperechoic kidney and oligohydramnios. Another exhibited fetal intellectual developmental delay post-birth with CMA, revealing a 17q12 microdeletion and intrauterine ultrasound phenotype of hyperechoic kidney and strong intestinal echo. Among the remaining 50 live births, 21 refused post-birth ultrasound follow-up, 24 had normal renal ultrasound results, and six maintained the same ultrasound conditions as before (these six cases within the non-isolated hyperechoic kidney).
Discussion
Fetal hyperechoic kidneys is an important manifestation of congenital renal dysplasia, with a detection rate of approximately 0.16% (1, 14). While often a nonspecific normal variation, hyperechoic kidney also serves as a clinically instructive ultrasound indicator (15). We categorized hyperechoic kidney into isolated and non-isolated types based on the presence of other ultrasonic abnormalities. Hyperechoic kidney is frequently detected in trisomy 21, trisomy 18, and trisomy 13 syndromes (16). The detection rate of chromosomal abnormalities in isolated fetal hyperechoic kidneys ranges from 21.4%–28.1%, with trisomy 9, trisomy 13, and 17q12 chromosome microdeletion as predominant pathogenic factors (16). In our study, we found that the detection rate of chromosomal abnormalities in isolated hyperechoic kidney was 25.00% (4/16), aligning with that reported in the literature.
Our findings highlight 17q12 microdeletion as a primary genetic pathogenic factor for fetal hyperechoic kidneys (6.52%, 6/92), often resulting from low copy number non-allelic homologous recombination (17). The core pathogenic gene within the 17q12 microdeletion is the hepatocyte nuclear factor-1β (HNF1B), a DNA-binding transcription factor essential for normal renal development. Deletion of HNF1B primarily contributes to simple renal echo enhancement (11–13, 18, 19). In this study, we observed genetic abnormality detection rates of 25% (4/16) in isolated hyperechoic kidney cases and 20.51% (16/78) in non-isolated cases, with no significant difference between them (P > 0.05). Consequently, we advocate for karyotype analysis and CMA irrespective of additional ultrasonic abnormalities when fetal hyperechoic kidney is evident.
Recent advances in WES have enhanced the diagnosis of monogenic diseases, identifying specific gene mutation sites causing fetal hyperechoic kidney (16, 20). Shuster et al. (7) identified an HNF1B mutation linked to hyperechoic kidney. Additional gene mutations, such as those found in PKHD1, PKD, PAX2, and RET, have been implicated in recent findings related to hyperechoic kidney (21). We conducted WES on 13 of 94 cases, discovering mutations in HNF1B, NPHP3, and KMT2D. HNF1B is predominantly associated with renal tubulointerstitial nephropathy, which is characterized by renal interstitial fibrosis, tubular atrophy, and basal membrane thickness alteration (4). NPHP3 is implicated in nephropathy involving significant yet nonspecific pathologies, with occasional extra-renal system involvement (22). Variations in KMT2D are associated with Kabuki syndrome (KS) (23). KS is a group of autosomal dominant genetic disorders characterized by distinctive facial and skeletal abnormalities, abnormal skin texture, congenital visceral abnormalities, postnatal growth restriction, and mild-to-moderate intellectual impairment (24). In this study, we observed that the ultrasound phenotypes of a fetus with HNF1B mutation included hyperechoic kidney and polycystic kidney, whereas the ultrasound phenotypes of the fetus with NPHP3 and KMT2D gene mutations included hyperechoic kidney and abnormal nervous system structure. Collectively, we recommend comprehensive WES to exclude rare monogenic genetic diseases in fetuses displaying hyperechoic kidney with normal CMA and chromosomal karyotype analysis.
The outcome of isolated hyperechoic kidney is significantly better than that of non-isolated hyperechoic kidney, with most cases of isolated enhancement showing favorable outcomes (9, 25). While our results showed no significant difference in genetic detection rates between isolated vs. non-isolated renal hyperechogenicity groups, clinically we observed better overall outcomes in cases with isolated findings. This suggests that even when genetic conditions are present, those with isolated renal manifestations may have less severe phenotypic expression. The analysis of clinical characteristics in fetal hyperechoic kidneys with normal or abnormal WES showed extrarenal anomalies. In this study, two cases of non-isolated hyperechoic kidney exhibited poor prognosis, which aligns with the findings reported in the literature. We observed that in clinical scenarios where hyperechoic kidney combined with other ultrasound abnormalities, the live birth rate and prognosis are generally poor. Early screening for genetic abnormalities is imperative to guide pregnancy management effectively, inform risk assessment, and facilitate informed decision-making regarding the continuation of pregnancy. Moreover, not all cases of hyperechoic kidney indicate underlying disease; therefore, meticulous observation and follow-up are crucial to accurately assess prognosis and prevent unnecessary terminations of pregnancy.
Consistent with previous prenatal cohorts, our study identified a VUS detection rate of 3.33% through CMA (26). In prenatal genetic diagnosis, the interpretation of VUS remains a critical challenge, primarily due to: limited parental verification, heterogeneity in detection platforms, and divergent classification criteria across laboratories. This study identified 3 VUS cases (representing 3.33% of all detected variants), all of which lacked parental origin data due to refusal of follow-up testing. Future studies should prioritize: longitudinal tracking of such VUS through international databases, functional assays to assess the impact of non-coding VUS, standardized reporting frameworks for prenatal VUS.
However, our study has the following limitations: retrospective nature over an extended period, possible omission of some cases, and selective application of WES owing to its high costs. While cost remains a primary barrier to WES implementation, the temporal constraints may represent an equally significant limitation in clinical practice. Additionally, the follow-up period for live births was insufficiently brief; therefore, we plan extended follow-up to acquire more accurate clinical information in the future.
Conclusions
When the fetal intrauterine ultrasound phenotype presents as a hyperechoic kidney, chromosome karyotype analysis, and CMA examination should be performed, regardless of the presence of other ultrasound abnormalities. In cases with normal karyotyping and CMA results but persistent hyperechoic kidney, we recommend further testing to exclude rare monogenic inherited diseases. The live birth rate and prognosis of fetuses exhibiting hyperechoic kidney alongside abnormal ultrasound structures are often unfavorable. Therefore, we recommend prioritizing early screening for genetic abnormalities to effectively guide pregnancy management, accurately inform risk, and facilitate informed decisions on continuing the pregnancy. Furthermore, not all hyperechoic kidney phenotypes indicate disease; hence, close observation and follow-ups should be conducted to accurately assess prognosis and prevent unnecessary pregnancy terminations.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Ethics Committee of Fujian Maternal and Child Health Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
MC: Writing – original draft. NL: Writing – review & editing. ZX: Writing – original draft. HH: Supervision, Writing – review & editing. LZ: Methodology, Writing – review & editing. LX: Funding acquisition, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was funded by the Fujian Provincial Natural Science Foundation (2021J01407), the Fujian Provincial Health Technology Project (2020CXB008), the Fujian Provincial Natural Science Foundation (2019J01509), Joint Funds for the Innovation of Science and Technology, Fujian Province (2020Y9159), Innovation Platform Project of Science and Technology, Fujian province (2021Y2012), National Key Clinical Specialty Construction Program of China (Obstetric), Key Project on the Integration of Industry, Education and Research Collaborative Innovation of Fujian Province (grant No. 2021YZ034011), and Key Project on Science and Technology Program of Fujian Health Commission (grant No. 2021ZD01002).
Acknowledgments
Thank you to all patients who participated in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Mashiach R, Davidovits M, Eisenstein B, Kidron D, Meizner I. Fetal hyperechogenic kidney with normal amniotic fluid volume: a diagnostic dilemma. Prenat Diagn. (2010) 25(7):553–8. doi: 10.1002/pd.1185
2. Hureaux M, Molin A, Jay N, Saliou AH, Spaggiari E, Salomon R, et al. Prenatal hyperechogenic kidneys in three cases of infantile hypercalcemia associated with SLC34A1 mutations. Pediatr Nephrol. (2018) 33(10):1723–9. doi: 10.1007/s00467-018-3998-z
3. Chaumoitre K, Brun M, Cassart M, Maugey-Laulom B, Eurin D, Didier F, et al. Differential diagnosis of fetal hyperechogenic cystic kidneys unrelated to renal tract anomalies: a multicenter study. Ultrasound Obst Gyne. (2006) 28(7):911–7. doi: 10.1002/uog.3856
4. Gondra L, Décramer S, Chalouhi GE, Muller FO, Salomon R, Heidet L. Hyperechogenic kidneys and polyhydramnios associated with HNF1B gene mutation. Pediatr Nephrol. (2016) 31(10):1–4. doi: 10.1007/s00467-016-3421-6
5. Vivante A, Hwang D, Kohl S, Chen J, Shril S. Exome sequencing discerns syndromes in patients from consanguineous families with congenital anomalies of the kidneys and urinary tract. J Am Soc Nephrol. (2017) 28(1):69–75. doi: 10.1681/ASN.2015080962
6. Chen Y, Liu W. Impact of prenatal ultrasound diagnosis for fetal renal abnormalities: study protocol. Medicine (Baltimore). (2019) 98(45):e17907. doi: 10.1097/MD.0000000000017907
7. Shuster S, Keunen J, Shannon P, Watkins N, Chong K, Chitayat D. Prenatal detection of isolated bilateral hyperechogenic kidneys: etiologies and outcomes. Prenat Diagn. (2019) 39(9):693–700. doi: 10.1002/pd.5418
8. Dias T, Sairam S, Kumarasiri S. Ultrasound diagnosis of fetal renal abnormalities. Best Pract Res Cl Ob. (2014) 28(3):403–15. doi: 10.1016/j.bpobgyn.2014.01.009
9. Yulia A, Napolitano R, Aiman A, Desai D, Johal N, Whitten M, et al. Perinatal and infant outcome of fetuses with prenatally diagnosed hyperechogenic kidneys. Ultrasound Obstet Gynecol. (2021) 57(6):953–8. doi: 10.1002/uog.22121
10. Liehr T. International system for human cytogenetic or cytogenomic Nomenclature (ISCN): some thoughts. Cytogenet Genome Res. (2021) 161(5):223–4. doi: 10.1159/000516654
11. Gilboa Y, Perlman S, Pode-Shakked N, Alon B, Einat S. Prenatal diagnosis of 17q12 deletion syndrome: from fetal hyperechogenic kidneys to high risk for autism. Prenatal Diag. (2016) 36(11):1027–32. doi: 10.1002/pd.4926
12. Wang F, Yao Y, Yang HX, Shi CY, Zhang XX, Xiao HJ, et al. Clinical phenotypes of hepatocyte nuclear factor 1 homeobox b-associated disease. Zhonghua Er Ke Za Zhi. (2017) 55(9):658–62. doi: 10.3760/cma.j.issn.0578-1310.2017.09.006
13. Zhou CX, Zhu XY, Gu YJ, He LL, Liu LL, Yang W, et al. Prenatal features of 17q12 microdeletion and microduplication syndromes: a retrospective case series. Taiwan J Obstet Gyne. (2021) 60(2):232–7. doi: 10.1016/j.tjog.2021.01.001
14. De LVA, Torres E. Prenatal diagnosis of renal disease. P R Health Sci J. (2005) 24(24):141–4.16116932
15. Stonebrook E, Hoff M, Spencer JD. Congenital anomalies of the kidney and urinary tract: a clinical review. Curr Treat Options Pediatr. (2019) 5(3):223–35. doi: 10.1007/s40746-019-00166-3
16. Deng L, Liu Y, Yuan M, Meng M, Yang Y, Sun L. Prenatal diagnosis and outcome of fetal hyperechogenic kidneys in the era of antenatal next-generation sequencing. Clin Chim Acta. (2022) 528:16–28. doi: 10.1016/j.cca.2022.01.012
17. George AM, Love DR, Hayes I, Tsang B. Recurrent transmission of a 17q12 microdeletion and a variable clinical spectrum. Mol Syndromol. (2012) 2(2):72–5. doi: 10.1159/000335344
18. Jing XY, Huang LY, Zhen L, Han J, Li DZ. Prenatal diagnosis of 17q12 deletion syndrome: a retrospective case series. J Obstet Gynaecol. (2019) 39(3):323–7. doi: 10.1080/01443615.2018.1519693
19. Li H, Chen C, Tu J, Geng H, Lin TT. Two cases of fetal hyperechogenic kidneys who had HNF1-β gene wariation. Clin Nephrol. (2022) 98(6):309–16. doi: 10.5414/CN110895
20. Monaghan KG, Leach NT, Pekarek D, Prasad P, Rose NC, ACMG Professional Practice and Guidelines Committee. The use of fetal exome sequencing in prenatal diagnosis: a points to consider document of the American College of Medical Genetics and Genomics (ACMG). Genet Med. (2020) 22(4):675–80. doi: 10.1038/s41436-019-0731-7
21. Klemens R, Eva K, Martin H, Theda W, Angela G, Dorothee D, et al. Expanded clinical spectrum in hepatocyte nuclear factor 1b-maturity-onset diabetes of the young. J Clin Endocr Metab. (2009) 94(7):2658–64. doi: 10.1210/jc.2008-2189
22. Zhang X, Zhi X, Wang X, Dong Y, Shu J, Wang W, et al. Identification of a splicing variant c.3813-3A>G in NPHP3 by reanalysis of whole exome sequencing in a Chinese boy with nephronophthisis. Nephron. (2023) 147(9):572–82. doi: 10.1159/000529472
23. Xin C, Wang C, Wang Y, Zhao J, Wang L, Li R, et al. Identification of novel KMT2D mutations in two Chinese children with Kabuki syndrome: a case report and systematic literature review. BMC Med Genet. (2018) 19(1):31. doi: 10.1186/s12881-018-0545-5
24. Wang YR, Xu NX, Wang J, Wang XM. Kabuki syndrome: review of the clinical features, diagnosis and epigenetic mechanisms. World J Pediatr. (2019) 15(6):528–35. doi: 10.1007/s12519-019-00309-4
25. Ruibin H, Fang F, Hang Z, Lu Z, Tingying L, Ken C, et al. Prenatal diagnosis in the fetal hyperechogenic kidneys: assessment using chromosomal microarray analysis and exome sequencing. Hum Genet. (2023) 142(6):835–47. doi: 10.1007/s00439-023-02545-1
Keywords: fetal hyperechoic kidneys, chromosomal microarray analysis, whole-exome sequencing, prenatal ultrasonography, genetic diagnosis in pregnancy
Citation: Cai M, Lin N, Xiao Z, Huang H, Zheng L and Xu L (2025) Genetic etiology and pregnancy outcomes of fetal hyperechoic kidneys: a retrospective analysis. Front. Pediatr. 13:1496381. doi: 10.3389/fped.2025.1496381
Received: 14 September 2024; Accepted: 25 June 2025;
Published: 20 August 2025.
Edited by:
Jordi Pérez-Tur, Spanish National Research Council (CSIC), SpainReviewed by:
Cristina Skrypnyk, Arabian Gulf University, BahrainAngie Jelin, Johns Hopkins University, United States
Copyright: © 2025 Cai, Lin, Xiao, Huang, Zheng and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lin Zheng, emxpbjE1MzZAMTI2LmNvbQ==; Liangpu Xu, eGlsaWFuZ3B1QGZqbXUuZWR1LmNu
†These authors have contributed equally to this work
 Meiying Cai
Meiying Cai Na Lin
Na Lin Ziheng Xiao2,3,†
Ziheng Xiao2,3,† Hailong Huang
Hailong Huang