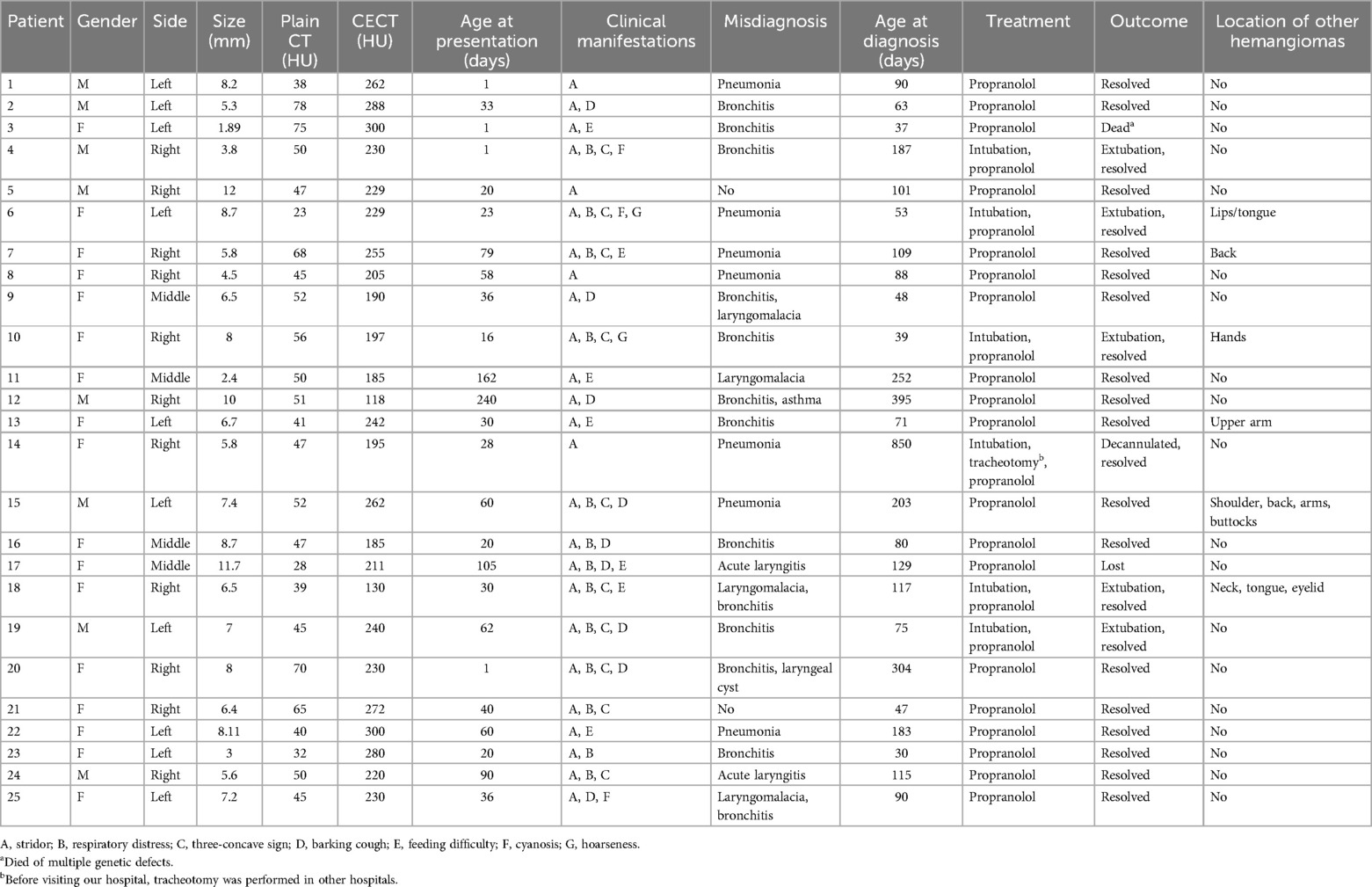
- 1Department of Otolaryngology-Head and Neck Surgery, Shanghai Children's Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China
- 2Department of Radiology, Shanghai Children's Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China
Objectives: This study aims to explore the clinical appearances of infantile subglottic hemangioma (SGH) and the diagnostic value of flexible fiberoptic laryngoscopy (FFL) combined with contrast-enhanced CT (CECT).
Methods: We retrospectively analyzed the data of 25 children diagnosed with SGH from January 2012 to January 2022.
Results: FFL showed a smooth, rounded, vascular-appearing submucosal lesion in the subglottic wall, while CECT revealed an enhancing lesion, obscuring the airway lumen. Among the 25 cases (8 males and 17 females; 10 left-sided, 11 right-sided, and 4 middle), the clinical appearances contained stridor (25), respiratory distress (13), three-concave sign (10), barking cough (9), feeding difficulty (8), cyanosis (2), and hoarseness (2). SGH with cutaneous hemangiomas accounted for 24% (6/25). The age at presentation ranged from 1 day to 8 months (median, 33 days), including 96% (24/25) of cases aged <6 months. Moreover, 92% (23/25) of cases had a history of misdiagnosis, 22 respiratory infections, 5 laryngomalacia, 1 laryngeal cyst, and 1 asthma, individually or in combination. Except for one case that died of polygenic abnormality and another case lost to follow-up, the remaining 23 cases were cured after oral propranolol.
Conclusions: For an infant with respiratory symptoms, who has repeated condition or poor effect after routine treatment, SGH should be considered, especially in infants under 6 months old. FFL combined with CECT is recommended to make a definite diagnosis of SGH.
1 Introduction
Subglottic hemangioma (SGH) is a rare form of infantile hemangioma and occupies barely 1.5% of all congenital laryngeal abnormalities (1). Despite its self-limiting natural course, SGH can damage the child's quality of life and even be life-threatening (2, 3). Clinical features are non-specific and mainly include recurrent stridor, respiratory distress, barking cough, repeated respiratory infections, cyanosis, thoracic, feeding difficulty, and abdominal retractions (4–7). Clinically, it is often misdiagnosed as a respiratory infection, but conventional anti-inflammatory treatment has poor effect or is repeated (8). Therefore, rapid and accurate diagnosis of SGH has extremely important significance (9, 10).
Clinically, SGH is prone to misdiagnosis before coming to our hospital, as many clinicians are not yet familiar with its clinical and imaging features. As a tertiary children's hospital, our Department of Otolaryngology—Head and Neck Surgery has certain advantages in treatment with SGH. Hence, we performed a retrospective analysis of the clinical data of SGH in the last 10 years to guide clinical practice.
2 Materials and methods
2.1 General information
In this study, we retrospectively analyzed 25 infants presenting with respiratory obstruction who were finally diagnosed with SGH by laryngoscopy combined with contrast-enhanced computed tomography (CECT) and treated with propranolol between January 2012 and January 2022. We collected data including clinical symptoms, gender, side, size, age at presentation, age at diagnosis, history of misdiagnosis, presence of other hemangiomas, diagnostic methods, treatment, and outcome. This study was approved by our institutional Research Ethics Board (2021R120-E01).
Inclusion criteria: (1) under 16 years old; (2) hemangioma located in subglottic alone or accompanied by other locations; (3) patients who underwent flexible fiberoptic laryngoscopy (FFL) and contrast-enhanced computed tomography (CECT); and (4) those who were treated with oral propranolol and followed up. Exclusion criteria: (1) patients with incomplete hospitalization and follow-up information; (2) repeated cases admitted to our hospital; (3) and those with other respiratory tract, thoracic, or pleural diseases.
2.2 Flexible fiberoptic laryngoscopy (FFL)
In our series, there were 22 cases initially misdiagnosed as respiratory infections (alone or combined with laryngomalacia, laryngeal cyst, or asthma), and they had poor effect or repeated condition after routine anti-inflammatory treatment. One case was misdiagnosed as merely laryngomalacia, and no improvement was found after treatment with calcium supplement and a small amount of multi-meal feeding. After otolaryngology consultation, the 23 infants underwent flexible fiberoptic laryngoscopy (FFL, Olympus Medical Systems, Tokyo, Japan). The remaining two cases were treated in the otolaryngology clinic and then underwent FFL.
2.3 Contrast-enhanced computed tomography (CECT)
The CT scan (GE LightSpeed VCT, USA) was performed on the neck, with a layer thickness of 0.625 mm, pitch of 0.984, interval of 2.5 mm, tube current of 240 mA, and tube voltage of 100 kV. Contrast enhancement was performed via iohexol administration, and CECT images were obtained in all 25 patients. Coronal and transverse multiplanar reconstruction CT images were reconstructed with a 3 mm section thickness. All cases were given 10% chloral hydrate (0.5 ml/kg) orally before examinations.
3 Results
3.1 Patient characteristics
Among the 25 cases, there were 17 females and 8 males. The age at presentation ranged from 1 day to 8 months, including 96% (24/25) of cases aged <6 months and 16% (4/25) of cases present at birth, with a median age of 33 days. The age at diagnosis ranged from 1 to 28.3 months, with a median of 3 months. The demographic details are listed in Table 1. There were 11 right-sided, 10 left-sided, and 4 middle SGH. Upper respiratory tract obstruction was the main clinical manifestation (Figure 1A), which included stridor (25/25), respiratory distress (13/25), three-concave sign (10/25), barking cough (9/25), feeding difficulty (8/25), cyanosis (2/25), and hoarseness (2/25), individually or in combination. The history of misdiagnosis was found in 23 cases, 22 respiratory infections (bronchitis/pneumonia/acute laryngitis), 5 laryngomalacia, 1 laryngeal cyst, and 1 asthma, alone or in combination. The cases of SGH combined with other multiple hemangiomas took up 24% (6/25), which were localized on the neck (1/25), back/buttocks (2/25), hands/ arms/ shoulder (3/25), and lips/tongue/eyelid (2/25).

Figure 1. (A) A female of 40 days with stridor, respiratory distress, and three-concave sign (red arrow). (B) Flexible fiberoptic laryngoscopy (FFL) revealed a smooth, protruded mucosa with a pinkish hue lesion (black arrow) in the right lateral subglottic wall, obscuring the airway lumen.
3.2 Diagnostic methods
The final diagnosis of SGH was made by FFL and CECT. SGH was highly suspected if FFL showed a smooth, rounded, vascular-appearing submucosal lesion in the subglottic wall, obscuring the airway lumen (Figure 1B). The mucosa covering the SGH appeared pink (normal) in 21 (84%), a purple or bluish livid color in two (8%), and bright red in two (8%). CECT was performed subsequently, which showed an enhancing lesion in the submucosal location of the subglottic region, inducing significant airway obstruction likely SGH (Figure 2). The maximum diameter size of SGH ranged from 1.89 to 12 mm, with an average of (6.77 ± 2.53) mm. The mean plain CT value was (49.36 ± 13.66) HU, with a range of 23–78 HU, while the CECT value ranged from 118 to 300 HU, with an average of (227.40 ± 46.45) HU.
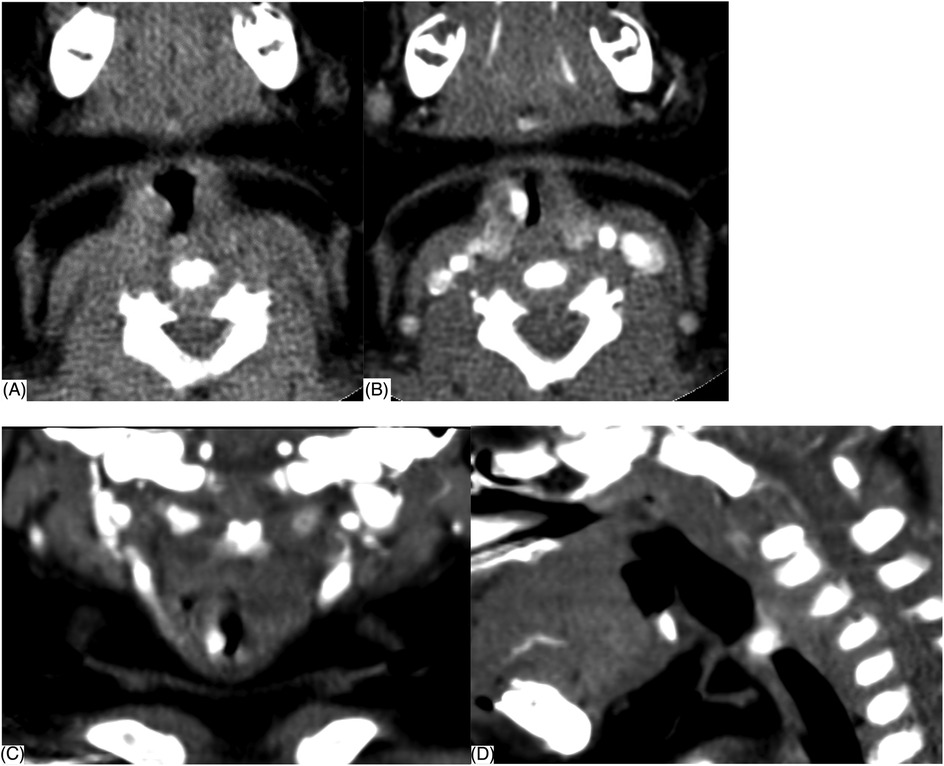
Figure 2. Contrast-enhanced CT (CECT) scan showing a well-defined enhancing lesion (arrow) in the submucosal location of the right lateral subglottic region. (A) Plain CT (B) transverse section, (C) coronal section, and (D) median sagittal section.
3.3 Treatment and follow-up
All 25 cases were subsequently treated with oral propranolol under adequate risk assessment after the diagnosis was confirmed without delay. The parents were advised to stop treatment when the case had no respiratory symptoms and the hemangioma disappeared by FFL (and/or) CECT. In this study, 24% (6/25) of patients presented with a history of intubation. Cases 4, 16, 18, and 19 underwent endotracheal intubation due to respiratory distress before diagnosis of SGH. Subsequently, extubation was performed successfully after taking propranolol for 3–7 days. In case 6, respiratory symptoms recurred on the 4th day after oral propranolol, endotracheal intubation was performed on the 7th day, and she was subsequently extubated on the 13th day. Case 14 underwent endotracheal intubation (42 days and 4 months, respectively), tracheotomy in other hospitals (5 months), oral propranolol (28 months), and then decannulated (30 months). Except for one case that died of polygenic abnormality and another case lost to follow-up, the remaining 23 cases were cured after oral propranolol.
4 Discussion
Although the SGH is a benign condition, it could be associated with a fatal outcome (2, 3). Clinically, the most common symptoms of SGH include biphasic stridor (8, 11, 12), followed by respiratory distress, barking cough (13), dysphagia (11, 13), thoracic and abdominal retractions, and hoarseness (5, 8). In our study, the symptoms included stridor (25/25), respiratory distress (13/25), three-concave sign (10/25), barking cough (9/25), feeding difficulty (8/25), cyanosis (2/25), and hoarseness (2/25), which were consistent with the above reports. Early clinical diagnosis of SGH is difficult, since the respiratory symptoms such as stridor may be misdiagnosed as respiratory infection, laryngomalacia, or asthma and may show improvement with anti-inflammatory therapy (8, 14). The symptoms of SGH may typically worsen in the presence of upper respiratory infection, therefore, mistaken for common disorders, such as infectious or inflammatory croup (8, 13). Chen (6) suggested we should consider SGH in infants under 2 years old presenting with respiratory symptoms, who had poor effect or repeated condition after anti-inflammatory treatment. In our series, 92% (23/25) had a history of misdiagnosis, 22 respiratory infections (bronchitis/pneumonia/acute laryngitis), 5 laryngomalacia, 1 laryngeal cyst, and 1 asthma, individually or in combination. Our research results were consistent with literature reports.
Laryngoscopy is a proven diagnostic tool for identifying airway narrowing, including at the subglottic level. Laryngoscopy can visualize the supraglottic and glottic regions to exclude causes of stridor such as severe laryngomalacia, glottic webs, and vocal cord palsy (15). Diagnosis is suggested by endoscopic observation of a unilateral smooth and soft lesion with a color that ranges from red to blue depending on the extent of the submucosal vascular proliferation (16). FFL demonstrates a classical mucosal blush and “bean-like” swelling in the subglottis (15), which is usually submucosal, asymmetric, and smooth (6). The endoscopic features of the tumor are a soft vascular-type swelling, the latter is mostly limited to the subglottis, between the mucus membrane and the perichondrium (2). FFL is less invasive and can be performed during awake respiration and has been proven to be an effective tool for visualizing the pediatric airway (10). In this study, FFL showed a smooth, rounded, vascular-appearing submucosal lesion in the subglottic wall, obscuring the airway lumen (Figure 1B). The mucosa covering the SGH appeared pink (normal) in 21 (84%), a purple or bluish livid color in 2 (8%), and bright red in 2 (8%). Our findings were consistent with the above reports.
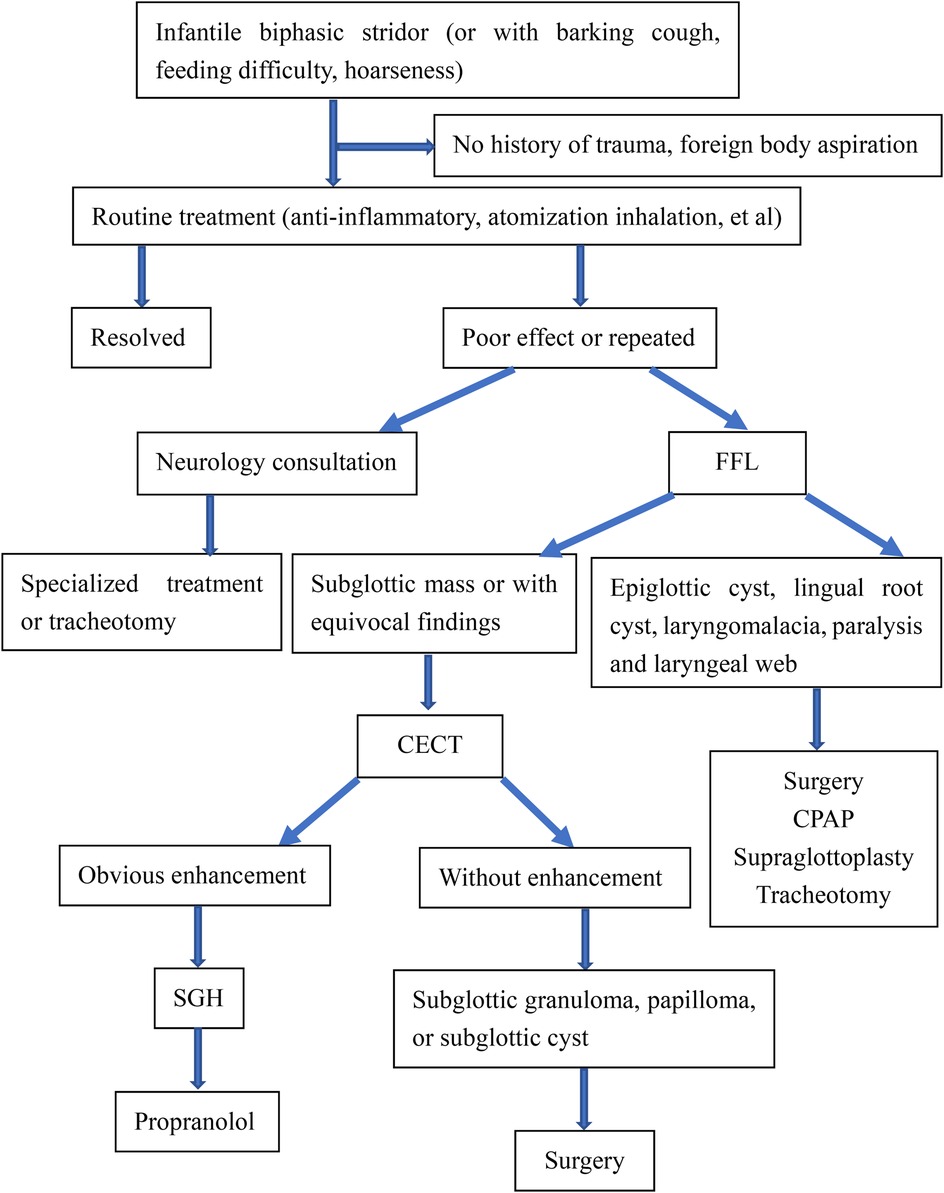
However, the endoscopic appearance is non-specific, and other primary tracheal tumors or highly vascularized metastatic tumors must be considered (17). The differentiated diagnosis of SGH in the infant should be expanded to include subglottic granuloma, papilloma, and subglottic cyst (6), and it is often difficult to differentiate by traditional endoscopic examination. Inflammatory change of the subglottic mucosa, as in spasmodic croup or severe gastroesophageal reflux, may mimic the endoscopic appearance of SGH. Therefore, complementary diagnostic techniques may provide benefits compared with laryngoscopy alone (14). When a child presents with stridor, laryngoscopy should be performed, followed by CT to rule out any type of vascular ring or mass (18). The diagnosis is confirmed by upper airway endoscopy (19) as well as the radiological examination and often obviates the need for biopsy (2). Imaging by CECT is helpful to appreciate the vascular nature of the lesion and determine the depth of invasion and extension of tracheal involvement (9, 11). Imaging can play a significant adjunct role in the evaluation of subglottic narrowing in an infant and exclude any external causes of compression (9, 15). CECT reveals SGH as a subglottic mass with intense, rapid enhancement as the cause of airway narrowing. The advantages of CECT are easy availability, multiplanar reconstructions, and non-invasive (8). In our study, CECT of the neck showed an isolated, well-defined enhancing lesion in the submucosal location of the subglottic region causing significant airway compromise (Figure 2). Here, we propose the diagnostic thinking, and advocate FFL combined with CECT to make a definitive diagnosis of SGH Figure 3. However, due to radiation risks and the need for sedation, CECT also has several drawbacks. Laryngeal US seems to be a rapid, tolerable, and highly reliable method worth further investigating (9).
The natural history of these tumors includes a stage of rapid proliferation during a 6- to 12-month period, followed by a stage of slow involution spreading over the following 18 months (20). Most SGH become symptomatic and could be life-threatening during their proliferative period. They grow quickly within the first few months following birth and lead to progressive airway obstruction (11, 21). Moreover, 80%–90% of affected babies present within the first 6 months of life, with a mean age of 3.6 months at diagnosis (22). The mean and median ages at diagnosis were 2.56 months and 2.0 months, respectively, with a range of 0.7–9 months (23). In our study, the age at presentation ranged from 1 day to 8 months, with a median age of 33 days. In addition, 96% (24/25) of cases presented <6 months old and 16% (4/25) of cases at birth. The median ages at diagnosis were 3 months, with a range of 1–28.3 months. Our research results were consistent with the above literature.
SGH may occur independently or accompany other cutaneous hemangiomas (19). Skin hemangiomas are present in 50% of cases of SGH at the time of diagnosis, with the head and neck being the most common location (23). In our study, the cases combined with other multiple hemangiomas accounted for 24% (6/25), which were localized on the neck (1/25), back/ buttocks (2/25), hands/arms/shoulder (3/25), and lips/tongue/eyelid (2/25). For reasons unknown, the incidence of SGH is lower in males than in females (24). There is a female preponderance (4, 13, 25). Schwartz et al. (21) found that 73% (36/49) of SGH cases published between 2009 and 2016 were female patients. Our series had 17 females and 8 males, which was inconsistent with the above reports.
Since the advent of propranolol therapy in 2008, the treatment of SGH has undergone a dramatic transformation (26). In 2009, Jephson et al. (27) reported the first two cases of SGH successfully treated with propranolol. Propranolol has since become the first-line treatment for SGH (5, 23, 28, 29) and has been shown to reduce the need for tracheostomy in these children (8, 30). In this study, except for one case that died of polygenic abnormality and another case lost to follow-up, the remaining 23 cases were cured after oral propranolol. The intubated patients received oral therapy through nasogastric tubes. Our results supported propranolol as the first-line therapeutic modality for SGH.
5 Conclusion
We advocate a strong index of suspicion for SGH presenting with respiratory symptoms, especially infants under 6 months old who have repeated condition or poor effect after routine treatment. We recommend FFL combined with CECT to make a definite diagnosis of SGH.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Approval Letter of Ethics Review Committee, Children's Hospital of Shanghai/Shanghai Children's Hospital, Shanghai Jiao Tong University (Approval No. 2021R120-E01). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin. Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
XL: Data curation, Methodology, Conceptualization, Formal analysis, Writing – original draft. RX: Data curation, Methodology, Conceptualization, Formal analysis, Software, Writing – original draft. HX: Data curation, Funding acquisition, Writing – review & editing. JC: Data curation, Writing – review & editing. XL: Conceptualization, Funding acquisition, Writing – review & editing.
Funding
The authors declare that financial support was received for the research and/or publication of this article. This study is supported by the Fundamental Research Funds for the Central Universities (YG2023ZD23) and National Natural Science Foundation of China (82171121).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Piram M, Hadj-Rabia S, Boccara O, Couloigner V, Hamel-Teillac D, Bodemer C. Beard infantile hemangioma and subglottic involvement: are median pattern and telangiectatic aspect the clue? J Eur Acad Dermatol Venereol. (2016) 30(12):2056–9. doi: 10.1111/jdv.13812
2. Ajmi H, Mama N, Hassayoun S, Karmani W, Zouari N, Abdelkefi M, et al. Life-threatening subglottic hemangioma in an infant successfully treated with propranolol. Arch Pediatr. (2018):S0929-693X(18)30114-3. doi: 10.1016/j.arcped.2018.05.008
3. McCormick AA, Tarchichi T, Azbell C, Grunwaldt L, Jabbour N. Subglottic hemangioma: understanding the association with facial segmental hemangioma in a beard distribution. Int J Pediatr Otorhinolaryngol. (2018) 113:34–7. doi: 10.1016/j.ijporl.2018.07.019
4. Yang W, Wolter NE, Cushing SL, Pope E, Wolter JK, Propst EJ. Propranolol versus nadolol for treatment of pediatric subglottic hemangioma. Int J Pediatr Otorhinolaryngol. (2021) 144:110688. doi: 10.1016/j.ijporl.2021.110688
5. Li XY, Wang Y, Jin L, Chen JR. Role of oral propranolol in the treatment of infantile subglottic hemangioma. Int J Clin Pharmacol Ther. (2016) 54(9):675–81. doi: 10.5414/CP202536
6. Liu Z, Song D, Wang L, Zhou J, Wang C, Li J, et al. Transarterial arterial sclerosing embolization for the treatment of propranolol-resistant subglottic hemangioma: feasibility and efficacy. Front Oncol. (2023) 13:1062510. doi: 10.3389/fonc.2023.1062510
7. Ke LQ, Shi MJ, Zhang FZ, Wu HJ, Wu L, Tang LF. The clinical application of flexible bronchoscopy in a neonatal intensive care unit. Front Pediatr. (2022) 10:946579. doi: 10.3389/fped.2022.946579
8. Chen W, Zhu P, Xu M, Chen S, Wang Y, Shen C, et al. Diagnosis of infantile subglottic hemangioma and the effect of oral propranolol. Am J Otolaryngol. (2022) 43(6):103610. doi: 10.1016/j.amjoto.2022.103610
9. Ezeh UC, Tesema N, Hasnie S, Ben-Dov T, Gallant SC, Gaffey MM, et al. Diagnostic techniques for infantile subglottic hemangiomas: a scoping review. Laryngoscope. (2025) 135(4):1287–94. doi: 10.1002/lary.31886
10. Ezeh UC, Ben-Dov T, Taufique ZM, Gaffey MM, Blei F, April MM. A new approach for diagnosis and surveillance of infantile subglottic hemangioma in the era of propranolol use: a case series. Ann Otol Rhinol Laryngol. (2024) 133(2):145–51. doi: 10.1177/00034894231191831
11. Rahbar R, Nicollas R, Roger G, Triglia JM, Garabedian EN, McGill TJ, et al. The biology and management of subglottic hemangioma: past, present, future. Laryngoscope. (2004) 114(11):1880–91. doi: 10.1097/01.mlg.0000147915.58862.27
12. Ozeki M, Nozawa A, Hori T, Kanda K, Kimura T, Kawamoto N, et al. Propranolol for infantile hemangioma: effect on plasma vascular endothelial growth factor. Pediatr Int. (2016) 58(11):1130–5. doi: 10.1111/ped.12981
13. Darrow DH. Management of infantile hemangiomas of the airway. Otolaryngol Clin North Am. (2018) 51(1):133–46. doi: 10.1016/j.otc.2017.09.001
14. Rossler L, Rothoeft T, Teig N, Koerner-Rettberg C, Deitmer T, Rieger CH, et al. Ultrasound and colour Doppler in infantile subglottic haemangioma. Pediatr Radiol. (2011) 41(11):1421–8. doi: 10.1007/s00247-011-2213-1
15. Liu Z, Yeo YH, Jackson C, Trimble K. Treatment failure with propranolol for subglottic haemangioma. BMJ Case Rep. (2019) 12(5):e227135. doi: 10.1136/bcr-2018-227135
16. Azman M. Subglottic hemangioma: now you see it, now you don’t. J Coll Physicians Surg Pak. (2022) 32(4):S67–9. doi: 10.29271/jcpsp.2022.Supp1.S67
17. Robitaille C, Fortin M, Trahan S, Delage A, Simon M. Subglottic hemangioma. J Bronchology Interv Pulmonol. (2016) 23(3):232–5. doi: 10.1097/LBR.0000000000000282
18. Richardson MA, Cotton RT. Anatomic abnormalities of the pediatric airway. Pediatr Clin North Am. (1984) 31(4):821–34. doi: 10.1016/S0031-3955(16)34647-8
19. Kumar P, Kaushal D, Garg PK, Gupta N, Goyal JP. Subglottic hemangioma masquerading as croup and treated successfully with oral propranolol. Lung India. (2019) 36(3):233–5. doi: 10.4103/lungindia.lungindia_200_18
20. Rutter MJ. Evaluation and management of upper airway disorders in children. Semin Pediatr Surg. (2006) 15(2):116–23. doi: 10.1053/j.sempedsurg.2006.02.009
21. Schwartz T, Faria J, Pawar S, Siegel D, Chun RH. Efficacy and rebound rates in propranolol-treated subglottic hemangioma: a literature review. Laryngoscope. (2017) 127(11):2665–72. doi: 10.1002/lary.26818
22. Bitar MA, Moukarbel RV, Zalzal GH. Management of congenital subglottic hemangioma: trends and success over the past 17 years. Otolaryngol Head Neck Surg. (2005) 132(2):226–31. doi: 10.1016/j.otohns.2004.09.136
23. Hardison S, Wan W, Dodson KM. The use of propranolol in the treatment of subglottic hemangiomas: a literature review and meta-analysis. Int J Pediatr Otorhinolaryngol. (2016) 90:175–80. doi: 10.1016/j.ijporl.2016.09.012
24. Parkes WJ, Propst EJ. Advances in the diagnosis, management, and treatment of neonates with laryngeal disorders. Semin Fetal Neonatal Med. (2016) 21(4):270–6. doi: 10.1016/j.siny.2016.03.003
25. Choa DI, Smith MC, Evans JN, Bailey CM. Subglottic haemangioma in children. J Laryngol Otol. (1986) 100(4):447–54. doi: 10.1017/S0022215100099461
26. Léauté-Labrèze C, Dumas de la Roque E, Hubiche T, Boralevi F, Thambo JB, Taïeb A. Propranolol for severe hemangiomas of infancy. N Engl J Med. (2008) 358(24):2649–51. doi: 10.1056/NEJMc0708819
27. Jephson CG, Manunza F, Syed S, Mills NA, Harper J, Hartley BE. Successful treatment of isolated subglottic haemangioma with propranolol alone. Int J Pediatr Otorhinolaryngol. (2009) 73(12):1821–3. doi: 10.1016/j.ijporl.2009.08.020
28. Chinnadurai S, Fonnesbeck C, Snyder KM, Sathe NA, Morad A, Likis FE, et al. Pharmacologic interventions for infantile hemangioma: a meta-analysis. Pediatrics. (2016) 137(2):e20153896. doi: 10.1542/peds.2015-3896
29. Wedgeworth E, Glover M, Irvine AD, Neri I, Baselga E, Clayton TH, et al. Propranolol in the treatment of infantile haemangiomas: lessons from the European propranolol in the treatment of complicated haemangiomas (PITCH) taskforce survey. Br J Dermatol. (2016) 174(3):594–601. doi: 10.1111/bjd.14233
Keywords: subglottic hemangioma, flexible fiberoptic laryngoscopy, contrast-enhanced computed tomography, infant, diagnosis
Citation: Liang X, Xu R, Xu H, Chen J and Li X (2025) Diagnosis of infantile subglottic hemangioma: a 10-year experience of 25 cases. Front. Pediatr. 13:1499656. doi: 10.3389/fped.2025.1499656
Received: 21 September 2024; Accepted: 7 May 2025;
Published: 30 May 2025.
Edited by:
Lin Huang, University of Electronic Science and Technology of China, ChinaReviewed by:
Marta Filauro, San Martino Hospital (IRCCS), ItalyYanting Wen, Chongqing University of Posts and Telecommunications, China
Copyright: © 2025 Liang, Xu, Xu, Chen and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoyan Li, Y2hoc2hlbnRzQDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
 Xiaoben Liang1,†
Xiaoben Liang1,† Xiaoyan Li
Xiaoyan Li