- 1Department of Neonatology, Shenzhen Baoan Women’s and Children’s Hospital, Shenzhen, Guangdong, China
- 2Department of Neonatology, Shenzhen People’s Hospital, (The Second Clinical Medical College, Jinan University, The First Affiliated Hospital, Southern University of Science and Technology), Shenzhen, Guangdong, China
Background: Persistent pulmonary hypertension of the newborn (PPHN) is a frequent neonatal emergency in the neonatal intensive care unit (NICU), representing a challenging condition that has not been extensively studied. PPHNremains associated with a high mortality and morbidity.
Objective: This scoping review was undertaken to provide a global overview of several key aspects: (1) the prevalence/incidence and etiologies of PPHN, (2) the mortality rate linked to PPHN during hospitalization and the primary causes of such mortality, (3) the risk factors related to PPHN, and (4) the approaches to managing PPHN. The aim of this scoping review was not to assess the methodological soundness of the identified studies, but instead to deliver a broad, comprehensive perspective on PPHN, identify gaps within the current literature, and outline potential avenues for future research. The results are anticipated to assist in developing public health strategies aimed at reducing the morbidity and mortality tied to PPHN globally.
Methods: We conducted a digital search in MEDLINE and the Cochrane Library, from January 1, 1993 to December 31, 2023.We incorporated observational studies, interventional studies, and reviews that provided adequate data on the incidence/prevalence, mortality rates, predictors, etiological factors, diagnosis, and management of PPHN among the general neonatal population (age 0–28 days old). This procedure followed the guidelines set by the Preferred Reporting Items for Systematic Reviews and Meta-Analysis Extensions for Scoping Reviews (PRISMA-ScR). Additionally, we utilized the methodological framework for scoping reviews as outlined by Arksey and O'Malley, which consists of formulating the research question, conducting a search for pertinent studies, selecting the studies, organizing the data, and compiling, summarizing and reporting the findings.
Results: A total of 128 research articles were collected from 27 countries categorized as either high-income or low- and middle-income countries (LMICs). The prevalence of PPHN ranges from 0.1%–8.1% in the different study populations. The highest global prevalence rates are observed in Europe and Asia, while lower prevalence rates are reported in the Americas and Africa. Neonatal infections are the leading cause of PPHN in Asia and the Americas, whereas meconium aspiration syndrome predominates in Europe. Several independent risk factors for PPHN include premature birth, male sex, ethnicity, extremes of birth weight, advanced maternal age, maternal obesity, multiple births, maternal smoking, pregestational/gestational diabetes mellitus, infectious history, caesarean delivery, antenatal drug exposure, fetal distress, APGAR score and meconium-stained amniotic fluid. The PPHN-related in-hospital mortality rate associated with PPHN ranges from 3.0%–57.9%, with the highest rates reported in Asia and the lowest in the United States of America (USA) and the United Kingdom (UK). It is advised that clinical evaluation incorporates the oxygenation index (OI) to assist in guiding medical practice.
Conclusion: PPHN has a high global burden, driven by neonatal infections and meconium aspiration syndrome, particularly pronounced in LIMCs where there is a pressing need for more intensive treatments and innovative solutions, ideally supported by region-specific subsidies, to address this concerning burden.
1 Introduction
Persistent pulmonary hypertension of the newborn (PPHN) refers to the inability to undergo the normal transition from intrauterine circulation at birth, resulting in right-to-left shunting of deoxygenated blood both intrapulmonary and extrapulmonary at the ductus arteriosus or atrium (1, 2). It is also called continuous fetal circulation. The hallmark of PPHN is persistent and severe hypoxemia. It is primarily a state of oxygenation failure. In the most severe and untreated cases, this can lead to heart failure (3) and even death. The overall incidence of PPHN ranges from 2–4 per 1,000 live births, but the proportion of all newborns with associated with respiratory failure accompanied can be as high as 10% (4).Typically, a newborn diagnosed with PPHN is a full-term or late preterm infant who shows no significant congenital anomalies and exhibits severe respiratory failure within hours after birth, necessitating intubation and mechanical ventilation. The incidence of PPHN in this group can be as high as 5.4 per1000 live births, with mortality rates varying between 4% and 33% (5). Recent research indicates that PPHN is increasingly observed in premature infants, primarily due to the underdeveloped pulmonary vasculature. As a result, PPHN is frequently encountered as a neonatal emergency in the neonatal intensive care unit (NICU), posing significant challenges linked to high morbidity and mortality rates.
PPHN is a clinical condition marked by increased pulmonary vascular resistance following birth, caused by various factors. The primary objective of PPHN therapy is to reduce pulmonary artery pressure and improve oxygenation. Inhaled nitric oxide (iNO) is currently the sole approved pulmonary vasodilator that does not significantly decrease systemic blood pressure and specifically targets PPHN (6).Research dating back to the 1990s has shown that iNO therapy notably enhances oxygenation and lessens the necessity for extracorporeal membrane oxygenation (ECMO) in critical instances (7, 8). The standard treatments are iNO and ECMO, but they are costly and often hard to obtain in most resource-limited nations (9). Even when iNO and ECMO are available, the mortality rate remains approximately 20%. Furthermore, the overall rate of neurological dysfunction among survivors at follow-up is up to 15% (10). An observational study conducted in the USA found that 46% of surviving infants exhibited composite neurodevelopmental and audiological impairments. Among them, 13% had major neurological abnormalities, 30% had cognitive delays, and 19% had hearing loss (11). To mitigate the high mortality rate linked with PPHN, especially in settings with limited resources, it is advisable to focus on preventing PPHN through early identification of its risk factors, aiming for anticipatory, preventive, and therapeutic management of its underlying causes.
After conducting an extensive literature search, we found that there was a lack of a comprehensive summary addressing this potentially highly fatal critical neonatal condition. There are wide regional variations in PPHN mortality rates and inconsistencies in management. It is imperative that healthcare providers-including neonatologists, pediatricians, obstetricians, and fetal medicine specialists have a thorough understanding of the major causes, diagnostic approaches, and treatment options for PPHN, especially in low- and middle-income countries and regions. In this regard, large-scale data studies, such as scoping reviews, can provide robust scientific evidence that may serve as a valuable resource for shaping recommendations, guidelines, and routine clinical practice.
Consequently, we undertook this scoping review to provide a global summary of: 1. the prevalence and incidence of PPHN along with its underlying causes, 2. the mortality rate related to PPHN during hospitalization and the primary causes of death, 3. the various risk factors tied to PPHN, and 4. the treatment approaches for PPHN. The objective of this scoping review was not to assess the methodological rigor of the relevant studies identified, but to offer a comprehensive global perspective on PPHN, identify gaps in current literature, and propose avenues for future investigation. The results are anticipated to assist in the development of public health strategies aimed at reducing the morbidity and mortality linked to PPHN on a global scale.
2 Methods
This scoping review was carried out and presented in line with the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analysis Extensions specifically for Scoping Reviews (PRISMA-ScR) (12), and can be accessed as Supplementary File S1. We utilized the methodological framework for scoping reviews established by Arksey and O'Malley, which encompasses the identification of the research question, the search for pertinent studies, the selection of studies, the visualization of data, as well as the synthesis, summarization, and presentation of the findings.
2.1 Data source, study eligibility criteria and search strategy
A digital search was performed on MEDLINE and The Cochrane Library covering the period from January 1, 1993–December 31, 2023.We included observational studies, interventional studies and reviews that provided adequate information concerning the prevalence/incidence, mortality, predictors, etiologies, diagnosis and management of PPHN in the general neonatal population (aged 0–28 days). The search strategy used the following keywords: “neonate”, “persistent fetal circulation syndrome”, “prevalence”, “mortality”, “risk factor”, “etiologies”, “diagnosis and management”, and these were cross-referenced with the names of all countries to maximize the retrieval of relevant research articles. No restrictions related to language or geographical location were imposed on the search (Supplementary File S2). Two reviewers independently screened the titles and abstracts of the retrieved records. They subsequently examined the full texts of articles relevant to the prevalence/incidence, mortality, risk factors, etiologies, diagnosis, and management of PPHN. References from the included articles were assessed as potential sources for further studies. We excluded commentaries, expert opinions, editorial letters, case reports, and case series that involved fewer than 30 participants. To ensure more uniformity in the data concerning prevalence, etiology, and mortality of PPHN, we also discarded studies focusing on neonates selected based on the presence of PPHN-specific diseases or conditions, such as respiratory distress syndrome (RDS), transient tachypnea of the newborn (TTN), meconium aspiration syndrome (MAS), COVID-19, and neonates born to high-risk pregnancies associated with PPHN (like gestational diabetes mellitus and mothers infected with COVID-19). A comprehensive description of the search strategy can be found in Supplementary File S2.
2.2 Data extraction and analysis
All relevant articles were imported into EndNote 21 reference management software to eliminate duplicates. Data extraction for the study was conducted using a standardized Microsoft Excel data extraction form. Title and abstract screening and full-text review of articles were performed independently by Yan Huang and Ting Yang. Any discrepancies were resolved through discussion and consensus, with consultation from a third author (L.Z.) if necessary. Authors of individual studies may be contacted to obtain additional data or to clarify results when required. A standardized and pre-tested data extraction form was utilized by the two reviewers to independently chart the following data from each included study: bibliometric information (including the name of the first author, the country in which the study was conducted, and the year of publication), study setting, diagnostic criteria used for PPHN, sample size, proportion of males, mean gestational age (GA), GA range, prevalence or incidence, in-hospital mortality rate, risk factors, etiologies, diagnostic criteria and management of PPHN. To ensure quality assurance, all extracted and charted data were reviewed for accuracy and completeness.
2.3 Critical appraisal of individual sources of evidence
The evaluation of bias risk was carried out utilizing the Newcastle-Ottawa Scale (NOS) for cohort and case-control studies, the criteria established by the Agency for Healthcare Research and Quality (AHRQ) for cross-sectional studies, and modified Jadad scores specifically designed for randomized controlled trials. This evaluation was conducted by a duo of researchers, Yan Huang and Ting Yang.
3 Results
3.1 Literature search and selection of studies
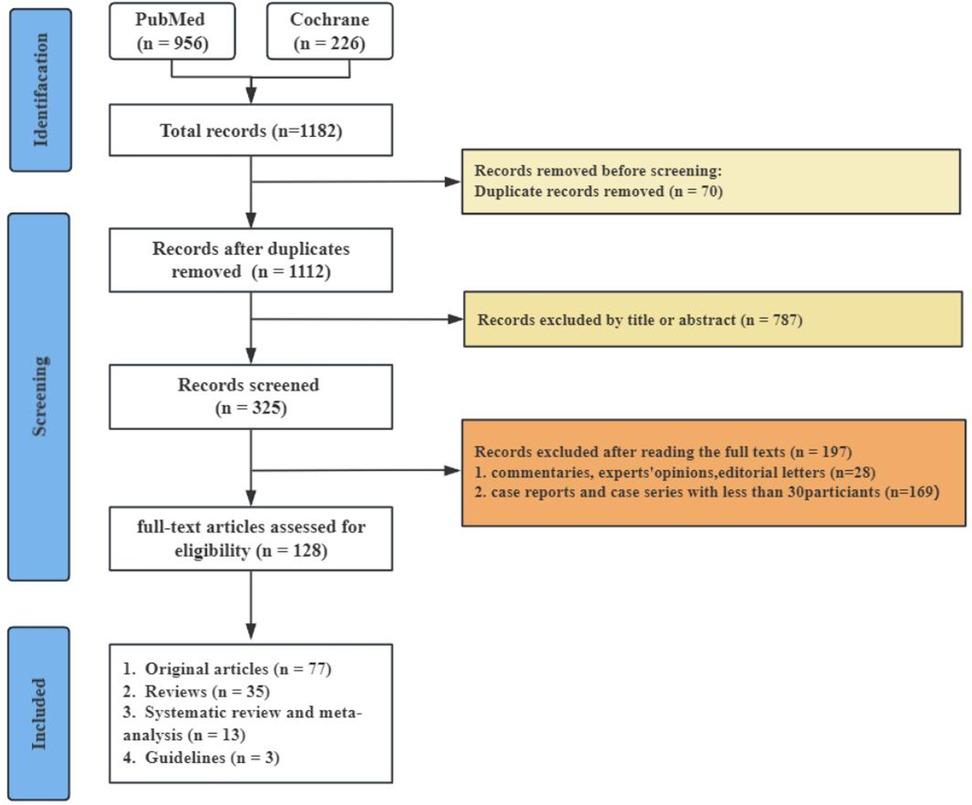
At the outset, we discovered 1,182 pertinent articles, removing 70 because of duplicates. We subsequently evaluated 1,112 articles according to established inclusion and exclusion criteria by examining the titles and abstracts, resulting in the exclusion of 787 articles. This left us with 325 articles, which were then assessed in full text. Following this full-text review, 197 articles were discarded for failing to satisfy the inclusion criteria (see Figure 1).
3.2 Characteristics of the selected studies
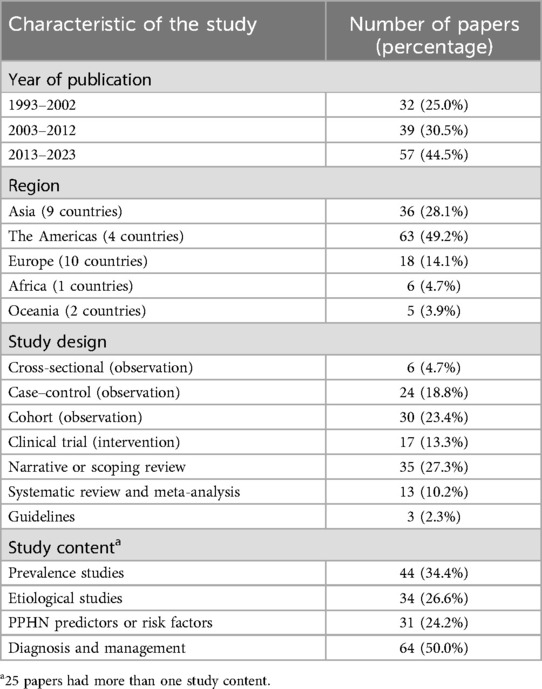
A total of 128 research articles were gathered from more than 600 centers spanning 27 different countries globally (see Figure 2). The largest proportion of these studies originated from the Americas (49.2%), with Asia following at 28.1%, Europe at 14.1%, Africa at 4.7%, and Oceania at 3.9% as detailed in Table 1. Specifically, the United States, China, and Thailand contributed 54, 13, and 8 citations, respectively (refer to Figure 2). Notably, most of the publications emerged within the previous decade, accounting for 44.5%. Additionally, there were 18 systematic reviews and 3 established guidelines (as shown in Table 1).
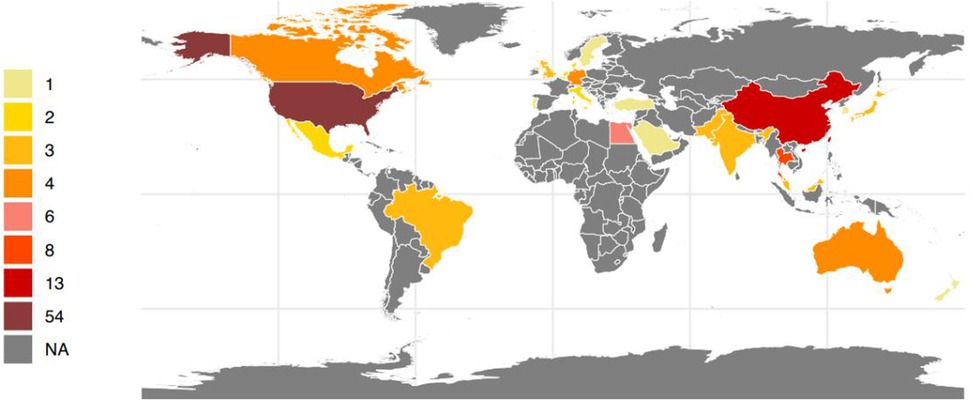
Figure 2. Number of research articles on persistent pulmonary hypertension of the newborn per country in the world between 1993 and 2023(created with R4.1.).
3.3 Prevalence of PPHN
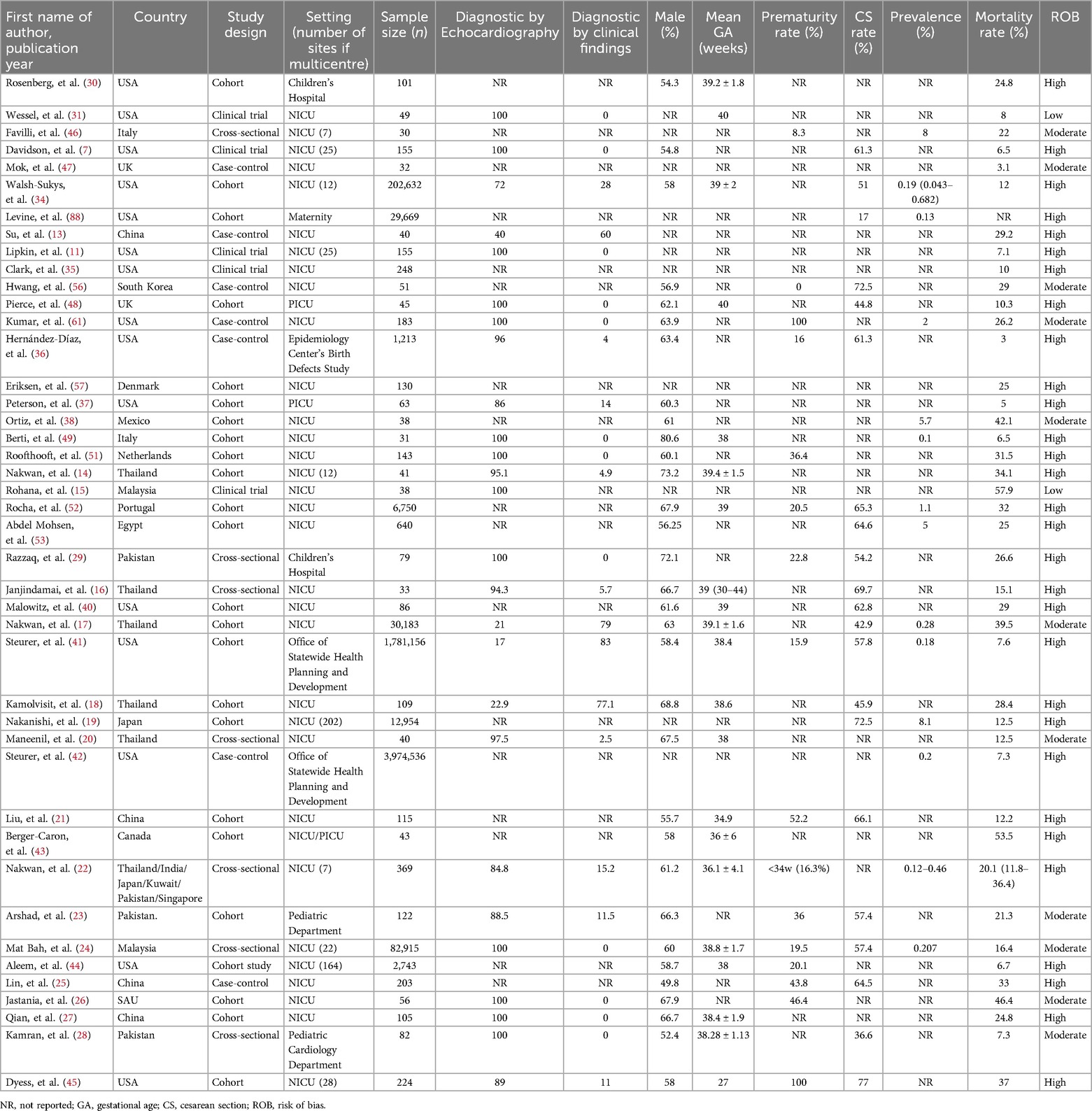
The prevalence of PPHN was obtained from 43 studies involving 6,128,630 neonates across 27 different countries (Table 2). In general, the worldwide prevalence of PPHN fluctuated between 0.1% and 8.1%. The prevalence varied by region as follows: Asia 0.12%–8.1% (13–28), the Americas 0.13%–5.7% (7, 11, 28–41), Europe 0.1%–8.0% (42–48), and Africa 5.0% (49, 50). More nationally representative prevalence data show that the highest prevalence of PPHN was 8.1% in Japan (19), and the lowest rate was 0.1% in a study published in Italy in 2010 (45), see Table 2.
3.4 Etiologies of PPHN
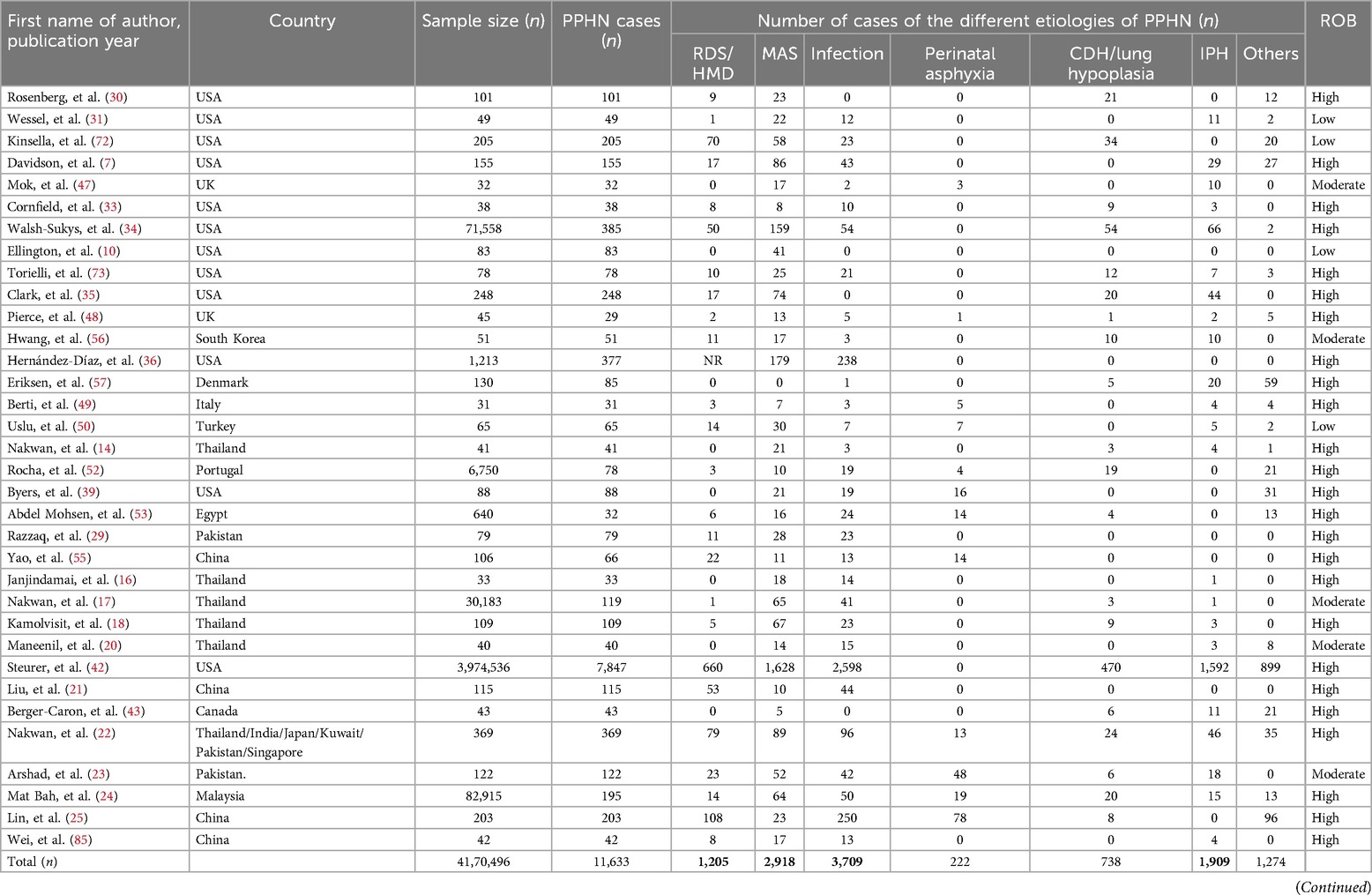
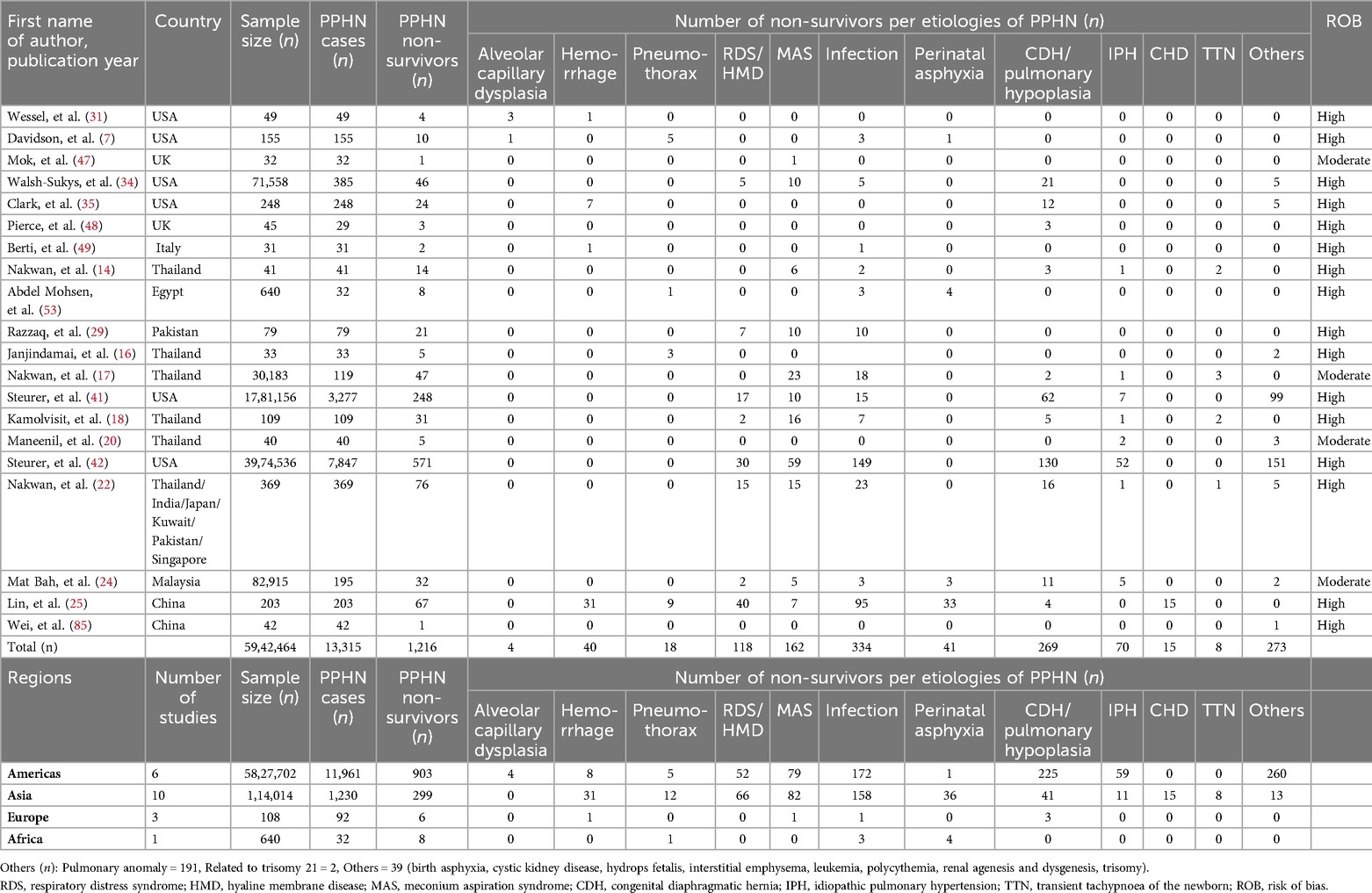
In our analysis of the causes of PPHN within the subgroup, we assessed information from 34 studies including 4,170,496 neonates from 17 countries (Table 3). Globally, the leading etiologies of PPHN were neonatal infection (n = 3,709), MAS (n = 2,918), idiopathic pulmonary hypertension (n = 1,909) and RDS (n = 1,205) (Table 3). The leading etiologies of PPHN varied regionally. Neonatal infections were the leading etiology of PPHN in Asia, the Americas and Africa,whereas MAS was the leading etiology in Europe, as indicated at the conclusion of Table 3.
3.5 PPHN-related death predictors, mortality rate and etiologies of death
Predictors associated with mortality in PPHN included a minimum PaO2/FIO2 ratio of less than 0.3 (OR = 8.26, 95% CI 1.78–38.41), a urine output of below 1.0 ml/kg/h within the initial 12 h (OR = 7.30, 95% CI 1.16–45.95), and a lowest mean blood pressure under 30 mm Hg (OR = 5.58, 95% CI 1.15–26.99). Infants presenting with SNAP-II score of 43 or higher faced the highest rist of mortality, reflected by an odds ratio (OR) of 10.00 (95% CI 1.03–97.50). Each one-point increase in the SNAP score was linked to a 1.04 increase in the odds of mortality (95% CI 1.01–1.07, P < 0.01) (14). The major risk factors for PPHN across various age groups encompassed male sex, cesarean deliveries, MAS, and RDS. Notably, RDS (RR = 5), birth asphyxia (RR = 2.5), and male gender (RR = 2) correlated with a heightened mortality risk in preterm infants in comparison to their term and post-term counterparts (51). A multicenter study in Malaysia identified that lower APGAR scores at five minutes and impaired cardiac function were linked to poorer outcomes. Key independent mortality risk factors comprised reverse flow detected in the descending aorta (OR = 15.91, 95% CI 5.64–44.92), APGAR scores of five or less at 5 min (OR = 6.72, 95% CI 2.04–22.15), and idiopathic pulmonary hypertension (OR = 6.46, 95% CI 1.52–27.43) (24). On a different note, pneumothorax (adjusted HR = 2.07, 95% CI 1.09–3.93, p = 0.03) and acute kidney injury (AKI) (adjusted HR = 2.99, 95% CI 1.59–5.61, p < 0.01) were significantly related to a higher likelihood of death. Conversely, conditions such as ventilator-associated pneumonia (VAP) (adjusted HR = 0.32, 95% CI 0.15–0.67) and the administration of total parenteral nutrition (TPN) (adjusted HR = 0.22, 95% CI 0.10–0.50) were linked to reduced mortality rates (17). Variations in plasma concentrations of atrial natriuretic peptide (ANP), endothelin-1 (ET-1) and von Willebrand factor (vWF) could indicate pulmonary artery systolic pressure (PASP) in newborns suffering from PPHN throughout their treatment. Ongoing assessment of these biomarkers may facilitate an evaluation of PPHN severity and direct appropriate treatment approaches (52).
Worldwide, the in-hospital mortality rates linked to PPHN fluctuated between 3.0 and 57.9%,showing regional variations as follows: Asia 7.3–57.9% (13–24, 28), the Americas 3.0–53.5% (7, 11, 28–40), Europe 3.1%–32% (42–48, 53) and Africa 25% (49). At the national level,this mortality rate was highest in Malaysia 57.9% (15) and Canada 53.5% (54) and lowest in the USA 3% (36)and the UK 3.1% (43), see Table 2. A California report focused on late preterm and term infants indicated that the one-year mortality associated with PPHN was significantly influenced by the underlying causes, with the highest rates seen in infants with other congenital respiratory anomalies (32%), followed by congenital diaphragmatic hernia (CDH) at 25.0%, respiratory distress syndrome (RDS) at 6.9%, and other causes at 8% (40).
Additionally, in a separate analysis of PPHN-related mortality, we evaluated data from 20 studies encompassing 5,942, 464 neonates across 13 countries (Table 4). Globally, the predominant causes of PPHN-related fatalities included neonatal infections (n = 334), CDH/lung hypoplasia (n = 269), MAS (n = 162) and RDS (n = 118). These trends showed minimal regional variation, with CDH/lung hypoplasia being the primary cause of PPHN-related in-hospital mortality in the Americas. In Asia, neonatal infections led as the primary cause of death. Conversely, fewer PPHN-related deaths were studied in Europe and Africa (Table 4).
3.6 Risk factors associated with PPHN
3.6.1 Fetal factor
Gestational age (GA) Numerous studies have consistently indicated that among the various causes of PPHN, premature newborns experience significantly higher rates than those born at term (35, 40, 55, 56). A retrospective, multicenter cohort study from Japan revealed that the prevalence of PPHN was 8.1% (95% CI 7.7%–8.6%), showing an upward trend each year, with an increased prevalence correlating with decreasing GA: for infants born at 22 weeks, the rate was 18.5% (ranging from 15.2%–22.4%), as opposed to 4.4% (ranging from 3.8%–5.2%) for those delivered at 27 weeks (19). A meta-analysis found that a gestational age of less than 37 weeks (OR = 4.34, CI 1.64–11.5) was one of the three key risk factors (57). Likewise, a prolonged gestational age (≥ 42 weeks) has become increasingly acknowledged as a significant risk element for PPHN (35, 49, 57).
3.6.1.1 Birth weight
In comparison to infants within the 10th and 90th percentiles of birth weight related to gestational age, the risk was heightened for those exceeding the 90th percentile. Both large and small for gestational age classifications were independently linked to PPHN (35, 40, 49). Low birth weight was significantly associated with death in preterm neonates with PH (58).
3.6.1.2 Gender
Gender The male sex has frequently been identified as an independent risk factor for PPHN (35, 40, 51). Additionally, male infants have shown a significant correlation with increased mortality in preterm neonates with PH (58). This was confirmed in the meta-analysis (OR = 1.84, 95% CI 1.28–2.63) (57).
3.6.1.3 Amniotic fluid (meconium stained amniotic fluid/MAS/oligohydramnios)
Meconium aspiration syndrome (MAS) has been reported as the predominant risk factor across multiple studies (22, 49, 51), a conclusion further supported by a corresponding meta-analys (57). Oligohydramnios has also been linked to the onset of PPHN in preterm infants (58).
3.6.1.4 APGAR scores/birth asphyxia
APGAR scores of 5 or lower at 5 min have been recognized as a standalone risk factor for mortality in PPHN, showing an adjusted odds ratio of 6.7 (95% CI 2.04–22.15) (24). In a similar vein, additional research has uncovered a link between low Apgar scores and a heightened risk of mortality (49, 51, 55, 58). Moreover, there is an increased mortality risk associated with birth asphyxia in preterm infants when compared to both term and postterm infants (51). A meta-analysis of 22 primary studies (n = 7,937 case records and 2,613,072 control cases), published by October 29, 2021, revealed a combined odds ratio of 3.9 (95% CI 2.87–5.31) relating perinatal asphyxia to the risk of PPHN (57).
3.6.2 Maternal risk factor
3.6.2.1 Maternal age
Advanced age in mothers has consistently been identified as a contributing factor to mortality related to PPHN (40, 57).
3.6.2.2 Maternal obesity
Numerous studies have indicated that obesity in mothers poses a risk factor for PPHN (35, 40, 55).
3.6.2.3 Maternal smoking
Research has revealed a notable risk of PPHN associated with maternal smoking both before and during pregnancy, with an odds ratio of 4.85 (95% CI 1.98–11.9) (57, 59). However, the study by Araujo did not report any interaction between smoking and PPHN (55).
3.6.2.4 Ethnicity
Research by Hernández-Díaz illustrated that being of black or Asian descent in mothers was linked to a heightened risk for PPHN (35). In contrast, Hispanic ethnicity was protective against PPHN (40).
3.6.3 Obstetrical risk factors
3.6.3.1 Gestational diabetes mellitus
Several studies without controversy have demonstrated that maternal diabetes was more common in all etiologies of PPHN (35, 40, 55). Newborns born to mothers with chronic diabetes mellitus, gestational diabetes mellitus (GDM) and pregestational diabetes mellitus (PGDM) face an elevated risk of PPHN (OR = 3.61, 95% CI 2.02–6.45) (57).
3.6.3.2 Chorioamnionitis
In a Japanese cohort study, clinical chorioamnionitis and premature rupture of membranes were correlated with PPHN (19).
3.6.3.3 Multiple gestation
A study involving late preterm and term infants in California found that multiple births offered a protective effect against PPHN (40).
3.6.3.4 Mode of delivery
Numerous studies have emphasized that the likelihood of PPHN post-cesarean section(CS) is significantly higher compared to that following vaginal delivery (35, 51, 60). In a retrospective cohort study, the raw relative risk (RR) of PPHN in elective cesareans performed before labor, vs. intended vaginal deliveries, was 2.0 (95% CI 1.3–3.1). In comparing elective cesareans to spontaneous labor resulting in vaginal deliveries, the RR of PPHN was observed to be 3.4 (95% CI 2.1–5.5). When gestational age at birth (less than vs. equal to or more than 37 weeks) was taken into account, the adjusted RRs for these delivery groups were 2.2 (95% CI 1.4–3.4) and 3.7 (95% CI 2.3–6.1,respectively. The rate of PPHN in the elective cesarean cohort was recorded as 6.9 per 1,000 deliveries. To prevent a single occurrence of PPHN in this population, it would be necessary to avoid 387 cesarean sections (number needed to harm, 95% CI 206.8–3,003.1) (61).
3.6.3.5 Antenatal drugs
As early as 1996, the intake of nonsteroidal anti-inflammatory drugs and aspirin by pregnant women, along with the reasons for their consumption, appeared to be linked to a heightened risk of PPHN (62). In 2006, advisories from Health Canada and the US Food and Drug Administration cautioned healthcare providers regarding a potential connection between the maternal use of selective serotonin reuptake inhibitors (SSRIs) during pregnancy and the incidence of PPHN. A large cohort study, involving roughly 30,000 women who took SSRIs during pregnancy in five Nordic nations, was carried out to assess whether SSRIs used by mothers elevate the risk of PPHN and to explore if this risk varies among different SSRIs. The findings suggested that while the overall risk of PPHN is comparatively low, the likelihood increases more than twofold when SSRIs are used in the later stages of pregnancy. This heightened risk appears to be a class effect, with the increased likelihood of PPHN being of similar magnitude across various SSRIs, including sertraline, citalopram, paroxetine, and fluoxetine (63). Nonetheless, high-quality evidence from a meta-analysis encompassing seven studies released before January 2014 indicated that the absolute risk difference for developing PPHN after being exposed to SSRIs in late pregnancy ranged from 2.9–3.5 per 1,000 infants. Consequently, it is estimated that 286–351 women would need to be treated with an SSRI during the late stages of pregnancy to yield an average of one additional PPHN case. Clinically, the absolute risk of PPHN remained low, and the increase in risk seems more modest than earlier research suggested (64). This finding was further validated by a meta-analysis of 11 studies (n = 156,978 women) published by January 2019, which revealed that the use of SSRIs or serotonin-norepinephrine reuptake inhibitors during pregnancy was associated with a greater risk of PPHN (OR = 1.82, 95% CI 1.31–2.54, I2 = 72%). Based on the results, sertraline appears to possess the lowest potential risk for PPHN when compared to other SSRIs, indicating it could offer the most favorable safety profile for use during pregnancy in this context. Additional research is required to validate these results (65).
3.7 Essentials of the diagnosis and management of PPHN
3.7.1 Diagnosis of PPHN
3.7.1.1 History
A detailed history guides in identifying the aforementioned maternal, fetal and/or obstetrical risk factors associated with the common etiologies of PPHN.
3.7.1.2 Clinical diagnosis
In the majority of included studies, the diagnosis of PPHN was primarily based on clinical or echocardiographic evidence of pulmonary hypertension (PH). The most frequent PPHN definition cited by 9 authors (14, 17, 18, 20, 22, 27, 33, 38, 66) was the presence of at least two of the following conditions: (1) documented pulmonary hypertension, as defined by echocardiographic evidence of elevated pulmonary pressure (right to left or bidirectional shunt), (2) a pre-to-post ductal partial pressure of oxygen gradient of 10–20 mmHg (1 mmHg = 0.133 kPa), and (3) a pulse oximetry oxygen saturation (SpO2) gradient ≥5%.
3.7.2 Assessment of the severity of PPHN
Various clinical scores have been suggested to classify the severity of PPHN into three categories: mild, moderate and severe. One score is determined based on pulmonary artery pressure values obtained through echocardiography, while another score is derived from the OI value, which is calculated using the formula: OI value = FiO2 × Mean airway pressure cmH2O/Arterial blood PO2 mmHg. Two authors categorized pulmonary hypertension as mild if the estimated pulmonary artery systolic pressure (PASP) was under 40 mmHg, moderate if ranged from 40–60 mmHg, and severe if it exceeded 60 mmHg (48, 50). In contrast, another author classified pulmonary hypertension as mild for PSAP values below 50 mmHg, moderate for values between 51 and 70 mmHg, and severe for values above 70 mmHg (23). An OI value of less than 15 was deemed mild PPHN, while values from 15–25 were classified as moderate PPHN, values between 25 and 40 as severe, and any value over 40 as very severe PPHN (47, 58).
3.7.3 Management of PPHN
The principles of treatment for confirmed PPHN include: 1. Ensuring adequate lung recruitment and alveolar ventilation with gentle ventilation, 2. Supporting of cardiovascular function, 3. Correcting severe acidosis while maintaining optimal blood pH and avoiding over-alkalinization, 4. Application of pulmonary vasodilators, and 5. Application of ECMO. This study summarizes the main treatments for PPHN in different studies and regions, see Table 5.
3.7.3.1 Ventilation
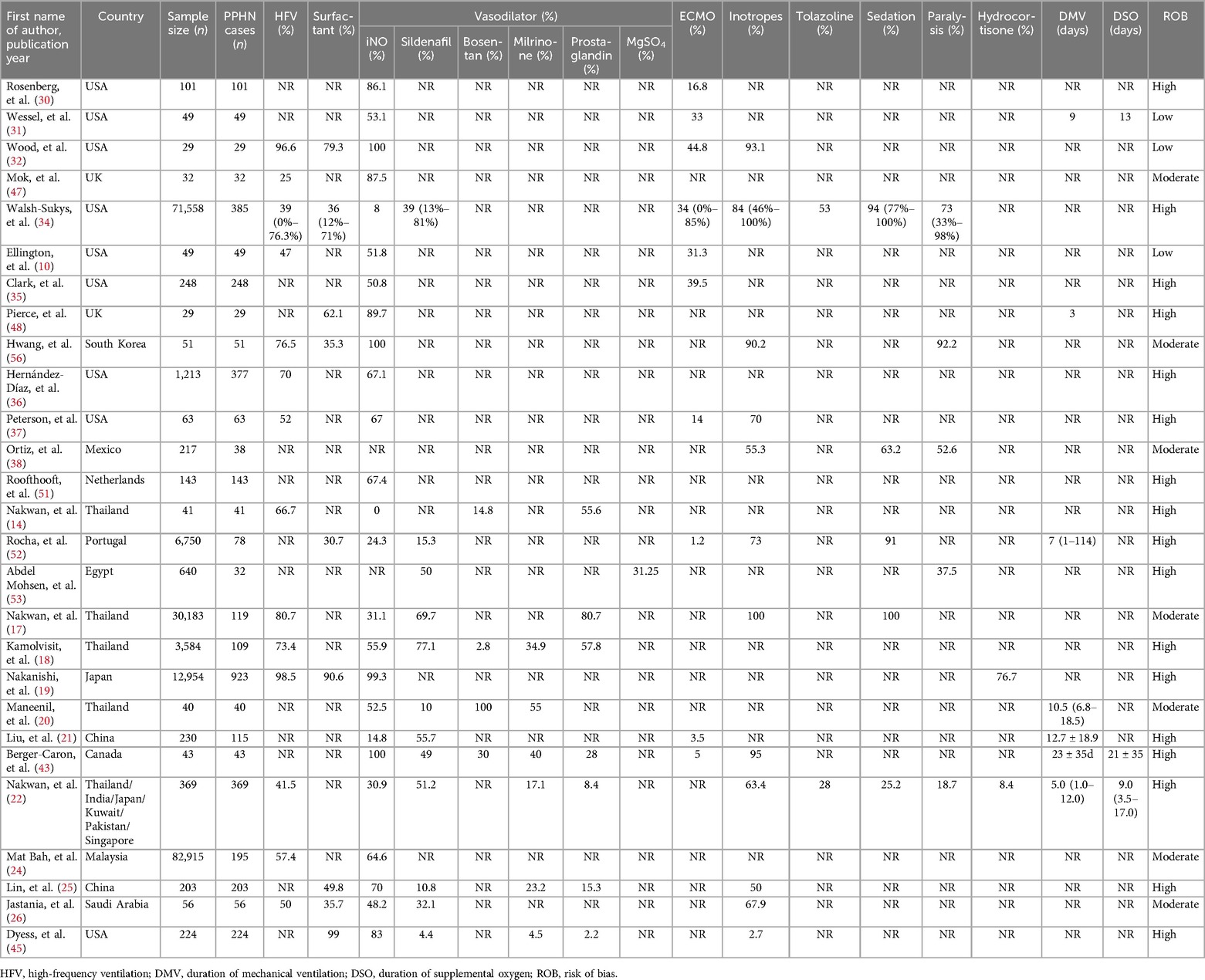
Gentle ventilation methods that utilize an optimal combination of positive end expiratory pressure (PEEP) and mean arterial pressure (MAP), alongside moderate tidal volume and permissive hypercapnia, should be implemented. In recent years, high-frequency ventilation (HFV) has been increasingly recommended for PPHN. The proportion of included treatment studies (n = 212,114) that included HFV ranged from 25%–98.2%, with slight variations by region. HFV was inaccessible in LMICs.
3.7.3.2 Surfactant therapy
For patients with parenchymal lung disease, such as RDS, MAS, pneumonia, etc., with primary or secondary surfactant inactivation, the concurrent PPHN emphasizes the use of pulmonary surfactant replacement therapy to recruit more alveoli and improve oxygenation (5). It is more effective in relatively mild PPHN (OI = 15–25) (67). In patients with non-parenchymal lung disease, surfactant is generally ineffective. In our studies (n = 212,114), the rate of PS therapy ranged from 30.7%–99%.
3.7.3.3 Supporting systemic blood pressure
PPHN is often accompanied by hypotension, which requires correction with positive inotropic drugs in addition to maintenance of blood volume. While increasing blood pressure above normal levels may temporarily ameliorate right-to-left shunting through the arterial conduit, thus potentially enhancing oxygenation in the short term, it fails to alleviate pulmonary artery pressure. Moreover, all such vasoactive treatments are associated with considerable side effects and require careful administration. An extensive array of studies explores the utility of functional echocardiography, near-infrared spectroscopy, and noninvasive impedance cardiometry as supplements to standard bedside hemodynamic assessments like blood pressure and heart rate. The ideal approach for managing hemodynamic injury in neonates is determined by the underlying pathophysiology of the condition (68).
3.8 Specific pulmonary hypertension therapies
3.8.1 Inhaled nitric oxide therapy
Inhaled nitric oxide (iNO) is the sole therapy sanctioned by the US Food and Drug Administration (FDA) for managing of PPHN in full-term or near-term neonates. This selective pulmonary vasodilator exerts minimal influence on systemic blood pressure. However, its availability is limited to a few centers within LMICs. iNO enhances systemic oxygenation in infants suffering from persistent pulmonary hypertension and has the potential to lessen the reliance on more invasive treatments (69). A randomized multicentre trial has demonstrated that treatment with high-frequency oscillatory ventilation (HFOV) combined with iNO is often more effective than treatment with either HFOV or iNO alone in cases of severe PPHN. Variation in response to treatment has been attributed in part to the specific diseases associated with PPHN (70). In our review, we found that the rate of iNO therapy for PPHN in published studies in the USA was as high as 86.1% as early as 1997 (30). Until the period of 2010–2013, it was still inconvenient in areas such as Mexico, Thailand, and Egypt (14, 37, 49). Meantime, the occurrence of residual pulmonary hypertension in term newborn infants treated with iNO for severe hypoxemic respiratory failure with associated persistent pulmonary hypertension is relatively low (71).
3.8.2 Sildenafil
Sildenafil is a phosphodiesterase 5 inhibitor. Multiple studies have indicated that sildenafil is both effective and well tolerated in neonates with PPHN, particularly when inhaled nitric oxide and extracorporeal membrane oxygenation are unavailable (72–74). In our findings, sildenafil emerged as the most favored pulmonary vasodilator (77.1%), particularly in LMICs. It often serves as the initial choice of pulmonary vasodilator for treating PPHN in developing nations (75).
3.8.3 Milrinone
Milrinone is classified as a phosphodiesterase 3A inhibitor, which induces pulmonary vasodilation by elevating levels of cyclic adenosine monophosphate (cAMP). Serving as both an inotropic agent and a vasodilator for both systemic and pulmonary circulation (76), it is regarded as the preferred treatment option for PPHN accompanied by left ventricular dysfunction. Additionally, milrinone is utilized for patients who do not respond to iNO and serves as a secondary option to sildenafil. Multiple studies indicate that the use of milrinone enhances oxygenation in cases of PPHN (77–81). This review included the use of milrinone in 6 trials (18, 20, 22, 25, 54, 66).
3.8.4 Bosentan
Bosentan was the subject of a randomized, double-blind, placebo-controlled, prospective investigation conducted in Egypt aimed at evaluating its efficacy in treating PPHN (66). The study concluded that bosentan might serve as a beneficial adjuvant therapy in managing PPHN (82), with comparable findings reported in additional trials. However, bosentan has been linked to several adverse effects, including abnormal liver function, anemia, and edema.
3.8.5 Prostaglandins
Prostaglandin E1, known for its pulmonary vasodilatory effects, is administered in both intravenous and nebulized forms for treating PPHN. A total of seven studies of prostaglandin treatment in PPHN were included in our review (14, 17, 18, 22, 25, 54, 66, 83). They had a role in improving oxygenation, but their safety and efficacy need to be further evaluation.
3.8.6 ECMO
The efficacy of ECMO is certain in severe PPHN with or without concomitant heart failure. With the widespread use of iNO and high frequency ventilation (HFV), there has been a relative decline in the number of cases requiring ECMO. The criteria for ECMO application can vary among different medical centers. According to the Guidelines for Neonatal Respiratory Failure from the Extracorporeal Life Support Organization (ELSO), the indications for ECMO include: (1) insufficient tissue oxygenation despite maximal therapeutic efforts; (2) severe hypoxic respiratory failure accompanied by acute decompensation (PaO2 < 40 mmHg); (3) sustained elevation of the oxygenation index without improvement; (4) significant pulmonary hypertension coupled with signs of right and/or left ventricular dysfunction. Contraindications encompass: (1) lethal chromosomal disorders (such as trisomy 13, 18, but not 21) or other lethal anomalies; (2) severe brain injury; (3) uncontrollable hemorrhage; (4) substantial intraventricular hemorrhage; (5) inadequate vessel size for cannulation. A systematic review involving 1,814 neonates indicated that ECMO can be effectively applied in neonates suffering from PPHN who have not responded to supportive cardiorespiratory care and conventional treatments, achieving a neonatal survival rate of 67.1% (84).
4 Discussion
This paper examines the current literature on PPHN to ascertain its worldwide prevalence, mortality rates, causes, risk factors, diagnostic methods, and treatment options. Our findings indicate that PPHN presents significant morbidity and mortality challenges in low-resource environments. Neonatal infections emerged as the primary contributor to PPHN and the leading cause of PPHN-related fatalities. Risk indicators for PPHN can be categorized into maternal, fetal, and obstetric factors. We outline the prevalent causes and existing treatments for PPHN and conclude by recommending a straightforward, practical algorithm for prompt diagnosis and management.
4.1 The global prevalence of PPHN
The global prevalence of PPHN The findings regarding the prevalence of PPHN indicate a significant variability across the 43 studies analyzed, influenced by multiple factors. Early investigations conducted in central Thailand identified an incidence of PPHN that fluctuated between 0.38 and 0.99 per 1,000 live births (85). In contrast, research from southern Thailand revealed a higher incidence of 2.8 per 1,000 live births (17). Prior to the extensive application of iNO therapy, data from a multicenter study in the United States reported a PPHN prevalence of 1.9 per 1,000 live births (from a cohort of 71,558 infants), with marked variation noted among different centers, ranging from 0.43–6.82 per 1,000 live births) (33). A separate investigation focusing on late preterm and term infants in California found an incidence of PPHN at 1.8 per 1,000 live births (0.18%).Notably, late preterm infants (gestational age between 34 and 36 weeks) had the highest incidence at 5.4 per 1,000 live births, whereas term infants presented a lower incidence of 1.6 per 1,000 live births (40). A retrospective review of medical records from several centers across six Asian nations (Japan, Kuwait, India, Pakistan, Singapore, and Thailand) conducted between January 1, 2014, and December 31, 2016, demonstrated that the incidence of PPHN varied from 1.2–4.6 per 1,000 live births (22). Additionally, a decade-long multicenter study involving extremely preterm infants (n = 12,954 cases) in Japan reported a prevalence of PPHN at 8.1% (95% CI 7.7%–8.6%), which showed an upward trend annually, and a notably higher prevalence with decreasing gestational age: 18.5% (range, 15.2%–22.4%) for those born at 22 weeks, vs. 4.4% (range, 3.8%–5.2%) for infants born at 27 weeks (19). The prevalence of PPHN is influenced by geographical locations, population characteristics, and gestational age. As illustrated by the aforementioned data, countries with the highest and lowest prevalence rates of PPHN suggest that low- and middle-income countries (LMICs), particularly in Africa and Asia, are disproportionately affected compared to high-income countries (HICs) in the Americas and Europe. Additionally, several advancements in neonatal care, including the creation of NICUs, the induction of fetal lung development through antenatal steroid administration, refinement of neonatal resuscitation protocols, the use of exogenous surfactant immature lungs, and the development of advanced respiratory support techniques like continuous positive airway pressure (CPAP), HFV, are often inadequate or lacking in LMICs. Moreover, the significant variability in prevalence rates can be attributed to differences in research methodologies, which report varying figures for the clinical diagnosis of PPHN. The diagnostic criteria for PPHN have shown inconsistencies, potentially leading to both underestimation and overestimation of its prevalence. Furthermore, study context might contribute to this variability; it was anticipated that single-center studies would report lower PPHN prevalence rates compared to multicenter studies. However, data revealed that PPHN prevalence in single-center investigations ranged from 0.1%–5.7%, while multicenter studies reported lower rates of 0.12%–8.1%. This discrepancy could be due to single-center studies being conducted in specialized neonatal care facilities, whereas multicenter studies were carried out across a broader range of institutions (see Table 2). Additionally, the prevalence variations across the 43 included studies may reflect the differing gestational ages of the newborns involved: from 8.3%–100% in premature infants (7, 19, 21–26, 32, 35, 38–40, 42, 47, 48, 51, 54, 58, 66, 86) compared to exclusively extremely preterm infants (19). Collectively, these results suggest that the incidence of PPHN diminishes with advancing gestational age, which is attributed to the functional and structural immaturity of the lungs in premature neonates.
4.2 Etiologies of PPHN and PPHN-related in-hospital mortality
Between 1993 and 2023, there has been no noteworthy reduction in mortality rates associated with PPHN. The fact that neonatal infections remain the primary cause of PPHN and contribute significantly to in-hospital fatalities suggests that, despite advancements in the management of PPHN over the last thirty years, additional therapeutic developments are still necessary. These initiatives should be especially focused on the Americas and Asia, where neonates experience varying impacts from neonatal infections. As expected, preventable neonatal infections remain a major global health problem, particularly in Asia and Africa. A preponderance of congenital diaphragmatic hernia and/or pulmonary dysplasia in the Americas may reflect a high local prevalence of congenital anomalies. More research is required to explore the role of environmental, genetic, and other factors alongside early prevention strategies.
4.3 Predictors or risk factors associated with PPHN
Factors that may predict the occurrence of PPHN include cesarean section, which is widely acknowledged as a contributing risk factor for this condition. A study conducted by Winovitch indicated that the rate of PPHN among those undergoing elective cesarean delivery was 6.9 per 1,000 births (61). Various research efforts have shown a greater prevalence of PPHN in infants born via cesarean section (CS) compared to those delivered vaginally (VD) (35, 55, 61, 87, 88). Specifically, the occurrence of PPHN was found to be around 0.37% in neonates delivered by CS, nearly five times higher than in those delivered vaginally (88). Elective cesarean sections, in particular, disrupt the physiological processes, such as the release of catecholamines and glucocorticoids that promote pulmonary fluid absorption, surfactant production, and pulmonary vasodilation, which are critical for the newborn's normal cardiorespiratory adjustment during labor (87, 89). Furthermore, fetal distress frequently leads to cesarean deliveries, which is another prevalent risk factor for PPHN. Consequently, the elevated rate of PPHN associated with CS may primarily stem from the activation of latent fetal factors rather than from a direct causal link with CS or the absence of VD. Notably, the incidence of PPHN in infants delivered through elective cesarean procedures before labor begins is significantly higher than in those born vaginally (OR = 4.9, 95% CI 1.7–14.0), indicating that cesarean delivery itself constitutes a risk factor for PPHN (60). CS has been linked to respiratory distress syndrome (RDS), which poses a higher mortality risk from PPHN in preterm infants compared to those born at term or post-term (51). Therefore, it is critical for obstetricians to conduct a comprehensive evaluation during CS procedures, particularly in cases of preterm labor. Meconium aspiration syndrome (MAS) is recognized as the leading contributor to PPHN. The presence of meconium interferes with pulmonary surfactants, triggering lung inflammation and causing alveolar hypoxia, which results in pulmonary vasoconstriction. Furthermore, meconium lodged in the airways can cause obstruction, gas trapping, and increased pulmonary vascular resistance. Nevertheless, the relationship between PPHN and meconium aspiration remains ambiguous; it is uncertain whether PPHN emerges directly from the aspiration or serves as an indirect indicator of in utero stress factors, such as hypoxia or infection. Additionally, a multicenter retrospective study of preterm infants indicated that clinical chorioamnionitis and premature rupture of membranes were correlated with PPHN (19). Inflammatory mediators leading to functional or structural pulmonary deficits, alongside severe infections that may compromise circulation or induce shock, could contribute to the emergence of PPHN, potentially accounting for the rising prevalence of this condition, particularly among preterm births.
Regarding the fetal risk factors related to delayed pulmonary biomechanics and vascular development, elevated levels of serum androgens in males compared to females contribute significantly (90, 91). Testosterone delays fetal lung maturation by regulating growth factors, and fetal androgens also inhibit fetal lung surfactant secretion. Meanwhile, estrogen promotes the synthesis of surfactant components, the growth of type II lung cells, and an overall increase in fetal lung surfactant secretion (92, 93). RDS is frequently a contributing factor to PPHN. Consequently, preterm infants face a greater risk of developing PPHN than their term counterparts. The insufficient synthesis and release of lung surfactant along with a limited number of respiratory units in preterm infants make them more susceptible to atelectasis and, ultimately, hypoxia associated with RDS. Factors such as poor lung compliance, reduced tidal volumes, increased physiological dead space, and inadequate alveolar ventilation lead to hypercapnia. The interplay of hypercapnia, hypoxia, and acidosis results in pulmonary vasoconstriction and enhanced right-to-left shunting through the patent foramen ovale and arterial ducts, as well as within the lungs, all of which exacerbate the risk of PPHN. GDM and PGDM result in intrauterine exposure to maternal hyperglycemia, which increases the transplacental transfer of glucose to the fetus, resulting in fetal hyperglycemia. This fetal hyperglycemia, in turn, inhibits the synthesis of fetal lung surfactant. Adverse outcomes are more prevalent in patients with inadequate blood glucose control, such as RDS (χ² = 13.373, P < 0.01). In addition, elevated fasting plasma glucose (FPG) has been deemed an independent risk factor for preterm birth, with an odds ratio (OR) of 1.460 (P < 0.001) (94). Simultaneously, there is a significant correlation between maternal obesity and advanced maternal age with an increased likelihood of experiencing obstetric complications, including maternal diabetes/GDM, pregnancy-related hypertensive disorders, and antepartum hemorrhage, all of which can predispose PPHN. All of these increase the probability of a cesarean section. Fetal distress and APGAR scores at birth often have interlocking or cascading mechanisms in predicting PPHN. Moreover, both hypoxia and acidosis are unequivocally powerful pulmonary vasoconstrictors, blocking changes in the cardiopulmonary circulation in normal newborns after birth, therefore, any factors leading to intrauterine hypoxia may play a role in the onset of PPHN.
The topic of using antidepressants during pregnancy remains contentious. Selective serotonin reuptake inhibitors (SSRIs) have been linked to a higher incidence of severe cardiac malformations as well as persistent pulmonary hypertension of the newborn (PPHN). Nevertheless, the majority of neurodevelopmental studies tracking follow-up have not uncovered significant cognitive impairments, observing only minor transient gross motor delays, slight language issues, and potential behavioral modifications. The dangers associated with halting treatment seem to surpass those tied to continuing it, as serious maternal depression could adversely affect a child's development. If deemed necessary, it is advisable to maintain treatment throughout pregnancy at the minimum effective dosage (95, 96).
It remains uncertain whether some of these risk factors are definitively a direct cause of persistent pulmonary hypertension in newborns or if they are merely co-contributors. Genetic variations have been identified in individuals with PPHN. Research within a single-center Chinese cohort has identified CPS1, NOTCH3, and SMAD9 as genetic risk factors for late preterm and term PPHN (21). These discoveries contribute further genetic support to the understanding of PPHN's pathogenesis and provide new perspectives on potential therapeutic strategies for the condition. Given the elevated risk of mortality and poor outcomes for survivors of PPHN, healthcare providers must be particularly attentive to the heightened need for monitoring and intervention in pregnant women or newborns presenting these risk factors. In addition, the influence of maternal factors, (e.g., maternal diabetes, maternal obesity, hypertensive disorders of pregnancy), on offspring warrant further exploration. In the future, in addition to databases of children with PPHN, more high-quality cohorts and databases of targeted therapeutic agents used could be established, especially in low- and middle-income settings, with closer and longer follow-up to assess the long-term impact.
The application of inhaled nitric oxide (iNO) for treating pulmonary hypertension (PH) in newborns delivered at less than 34 weeks of gestation has been extensively reviewed. Meta-analyses from randomized controlled trials focusing on the use of iNO for preventing bronchopulmonary dysplasia (BPD) have led groups from the National Institutes of Health (NIH) and the American Academy of Pediatrics (AAP) to determine that there is insufficient evidence to support the routine implementation of iNO in preterm infants. Nonetheless, existing research indicates that administering iNO can enhance oxygen levels in these infants, and the inclination to employ iNO is on the rise. The NIH consensus recommends that healthcare providers must evaluate the appropriateness of iNO treatment for preterm infants suffering from PH. Additionally, the American Heart Association, American Thoracic Society, and the Pediatric Pulmonary Hypertension Collaborative Network endorse the application of iNO in cases of persistent pulmonary hypertension of the newborn (PPHN) when standard respiratory and circulatory interventions fail to yield adequate results. It's important to note that both iNO and ECMO are not universally accessible and pose significant challenges in low- and middle-income countries (LMICs). Moreover, around 40% of neonates diagnosed with PPHN do not respond to iNO therapy (8). ECMO represents a resource-heavy treatment modality that necessitates a collaborative approach from a team of skilled healthcare professionals with specialized training in the setup, ongoing care, and cessation of ECMO for critically ill PPHN patients facing diverse causes and associated health complications. The principal risks linked to ECMO include bleeding, clot formation, and infections related to catheters. Comprehensive preparation, strategic resource distribution, and rigorous training of personnel to implement complex treatment strategies while following stringent infection control protocols are vital aspects of any ECMO operational strategy. There is a pressing need for additional prospective multi-institutional research to broaden the existing registry data.
Consequently, further interventions are under investigation. Recent years have seen significant progress in our comprehension of the physiopathology associated with PPHN, encompassing elements such as phosphodiesterase, endothelin receptors, and the supplementation of exogenous prostaglandins. These medications can be utilized individually or in conjunction with one another or with iNO. Sildenafil has undergone the most comprehensive research. A systematic review and network meta-analysis exploring various PPHN treatment comparisons indicated that a median concentration of 10–20 parts per million (ppm) of iNO (MNO), in combination with sildenafil given orally at a dose of 1–3 mg/kg every 6–8 h (OSID), showed the highest efficacy (OR = 27.53, 95% CI 2.36–321.75). In situations where iNO is unavailable, OSID paired with intravenous milrinone also demonstrated commendable efficacy (OR = 25.13, 95% CI 1.67–377.78) (97). PPHN is a complex and variable disease, and it is challenging for clinicians to make the right choice of individual drugs based on good evidence in routine practice. Meanwhile, most vasodilator drugs are still used outside the prescription instructions in neonates. Their clinical efficacy and potential and long-term adverse reactions are still being monitored and followed-up. At the same time, the treatment of PPHN is complex, expensive and limited in resource-limited regions. Alternative or cheaper treatments are being sought. Moreover, because the signaling pathways that control pulmonary vascular tone are intricate and highly interconnected, addressing only one particular pathway may not fully rectify vascular anomalies and could potentially upset the equilibrium between vasodilator and vasoconstrictor production. Each of the therapies mentioned may have significance, and a combined approach utilizing multiple treatments could be beneficial in certain severe cases.
Employing a combination of therapies could enhance outcomes in certain severe instances. A significant drawback of the present research is the variability among all studies included, which complicates the meta-analysis. Specifically, conducting a meta-analysis utilizing combined prevalence, mortality rates for PPHN, and risk assessments is unsuitable due to the diverse methodological approaches present in the studies examined. Nonetheless, this scoping review acts as a modern reference document that has been globally referenced for PPHN throughout the last three decades. It lays the foundation for systematic reviews and meta-analyses pertaining to epidemiological research on PPHN.
5 Conclusions
The prevalence of PPHN is particularly concerning in preterm infants and those with respiratory issues. Major contributors to PPHN include neonatal infections and meconium aspiration syndrome (MAS). Various identified risk factors (such as gestational age, birth weight, maternal smoking habits, diabetes mellitus-both pregestational and gestational, maternal obesity, prenatal infections, fetal distress, APGAR score, and meconium-stained amniotic fluid) are considered modifiable. Therefore, we suggest implementing changes in maternal lifestyle, ensuring appropriate antenatal, intrapartum, and postnatal care to mitigate these risks and improve the management strategies for PPHN. Additionally, the pressing demand for enhanced treatment methods and innovations aimed at PPHN, ideally supported in settings with limited resources, cannot be overstated as a crucial step to reduce the high mortality rates associated with PPHN.
Author contributions
YH: Writing – original draft. TY: Writing – review & editing. XL: Writing – review & editing. YC: Writing – review & editing. PZ: Writing – review & editing. ZY: Writing – review & editing. LZ: Writing – review & editing. GZ: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We thank the Shenzhen Neonatal Data Network (SNDN) for providing a learning and working platform for producing this review. And we thank all the authors whose studies were included in this review.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2025.1502385/full#supplementary-material
References
1. Nair J, Lakshminrusimha S. Update on Pphn: mechanisms and treatment. Semin Perinatol. (2014) 38(2):78–91. doi: 10.1053/j.semperi.2013.11.004
2. Steinhorn RH. Diagnosis and treatment of pulmonary hypertension in infancy. Early Hum Dev. (2013) 89(11):865–74. doi: 10.1016/j.earlhumdev.2013.09.012
3. Jain A, McNamara PJ. Persistent pulmonary hypertension of the newborn: advances in diagnosis and treatment. Semin Fetal Neonatal Med. (2015) 20(4):262–71. doi: 10.1016/j.siny.2015.03.001
4. Steinhorn RH. Neonatal pulmonary hypertension. Pediatr Crit Care Med. (2010) 11(2 Suppl):S79–84. doi: 10.1097/PCC.0b013e3181c76cdc
5. Lakshminrusimha S, Keszler M. Persistent pulmonary hypertension of the newborn. Neoreviews. (2015) 16(12):e680–e92. doi: 10.1542/neo.16-12-e680
6. Martinho S, Adão R, Leite-Moreira AF, Brás-Silva C. Persistent pulmonary hypertension of the newborn: pathophysiological mechanisms and novel therapeutic approaches. Front Pediatr. (2020) 8:342. doi: 10.3389/fped.2020.00342
7. Davidson D, Barefield ES, Kattwinkel J, Dudell G, Damask M, Straube R, et al. Inhaled nitric oxide for the early treatment of persistent pulmonary hypertension of the term newborn: a randomized, double-masked, placebo-controlled, dose-response. Pediatrics. (1998) 101(3 Pt 1):325–34. doi: 10.1542/peds.101.3.325
8. Clark RH, Kueser TJ, Walker MW, Southgate WM, Huckaby JL, Perez JA, et al. Low-dose nitric oxide therapy for persistent pulmonary hypertension of the newborn. N Engl J Med. (2000) 342(7):469–74. doi: 10.1056/nejm200002173420704
9. Evers PD, Critser PJ, Cash M, Magness M, Hoelle S, Hirsch R. Cost-utility of sildenafil for persistent pulmonary hypertension of the newborn. Am J Perinatol. (2021) 38(14):1505–12. doi: 10.1055/s-0040-1713819
10. Ellington M Jr, O'Reilly D, Allred EN, McCormick MC, Wessel DL, Kourembanas S. Child health status, neurodevelopmental outcome, and parental satisfaction in a randomized, controlled trial of nitric oxide for persistent pulmonary hypertension of the newborn. Pediatrics. (2001) 107(6):1351–6. doi: 10.1542/peds.107.6.1351
11. Lipkin PH, Davidson D, Spivak L, Straube R, Rhines J, Chang CT. Neurodevelopmental and medical outcomes of persistent pulmonary hypertension in term newborns treated with nitric oxide. J Pediatr. (2002) 140(3):306–10. doi: 10.1067/mpd.2002.122730
12. Tricco AC, Lillie E, Zarin W, O'Brien KK, Colquhoun H, Levac D, et al. Prisma extension for scoping reviews (Prisma-Scr): checklist and explanation. Ann Intern Med. (2018) 169(7):467–73. doi: 10.7326/m18-0850
13. Su BH, Lin TW, Lin HC, Tsai FJ, Peng CT. Inhaled nitric oxide in persistent pulmonary hypertension of the newborn: four-year experience in a single medical center. Acta Paediatr Taiwan. (2002) 43(5):259–64.12607481
14. Nakwan N, Nakwan N, Wannaro J. Predicting mortality in infants with persistent pulmonary hypertension of the newborn with the score for neonatal acute physiology-version Ii (Snap-Ii) in thai neonates. J Perinat Med. (2011) 39(3):311–5. doi: 10.1515/jpm.2011.011
15. Rohana J, Boo NY, Chandran V, Sarvananthan R. Neurodevelopmental outcome of newborns with persistent pulmonary hypertension. Malays J Med Sci. (2011) 18(4):58–62.22589673
16. Janjindamai W, Thatrimontrichai A, Maneenil G, Chanvitan P, Dissaneevate S. Effectiveness and safety of intravenous iloprost for severe persistent pulmonary hypertension of the newborn. Indian Pediatr. (2013) 50(10):934–8. doi: 10.1007/s13312-013-0263-1
17. Nakwan N, Pithaklimnuwong S. Acute kidney injury and pneumothorax are risk factors for mortality in persistent pulmonary hypertension of the newborn in thai neonates. J Matern Fetal Neonatal Med. (2016) 29(11):1741–6. doi: 10.3109/14767058.2015.1060213
18. Kamolvisit W, Jaroensri S, Ratchatapantanakorn B, Nakwan N. Factors and outcomes of persistent pulmonary hypertension of the newborn associated with acute kidney injury in thai neonates. Am J Perinatol. (2018) 35(3):298–304. doi: 10.1055/s-0037-1607213
19. Nakanishi H, Suenaga H, Uchiyama A, Kusuda S. Persistent pulmonary hypertension of the newborn in extremely preterm infants: a Japanese cohort study. Arch Dis Child Fetal Neonatal Ed. (2018) 103(6):F554–f61. doi: 10.1136/archdischild-2017-313778
20. Maneenil G, Thatrimontrichai A, Janjindamai W, Dissaneevate S. Effect of bosentan therapy in persistent pulmonary hypertension of the newborn. Pediatr Neonatol. (2018) 59(1):58–64. doi: 10.1016/j.pedneo.2017.02.003
21. Liu X, Mei M, Chen X, Lu Y, Dong X, Hu L, et al. Identification of genetic factors underlying persistent pulmonary hypertension of newborns in a cohort of Chinese neonates. Respir Res. (2019) 20(1):174. doi: 10.1186/s12931-019-1148-1
22. Nakwan N, Jain S, Kumar K, Hosono S, Hammoud M, Elsayed YY, et al. An asian multicenter retrospective study on persistent pulmonary hypertension of the newborn: incidence, etiology, diagnosis, treatment and outcome. J Matern Fetal Neonatal Med. (2020) 33(12):2032–7. doi: 10.1080/14767058.2018.1536740
23. Arshad MS, Adnan M, Anwar-Ul-Haq HM, Zulqarnain A. Postnatal causes and severity of persistent pulmonary hypertension of newborn. Pak J Med Sci. (2021) 37(5):1387–91. doi: 10.12669/pjms.37.5.2218
24. Mat Bah MN, Tan RYH, Razak H, Sapian MH, Abdullah N, Alias EY. Survival and associated risk factors for mortality among infants with persistent pulmonary hypertension of the newborn in Malaysia. J Perinatol. (2021) 41(4):786–93. doi: 10.1038/s41372-021-00962-6
25. Lin C, Mi J, Zhang Y, Duan S, Wu J, Li Y. A nomogram prediction model for early death in patients with persistent pulmonary hypertension of the newborn. Front Cardiovasc Med. (2022) 9:1077339. doi: 10.3389/fcvm.2022.1077339
26. Jastania EI, Alqarni MS, Abukhodair AW, Bukhari ZM, Bukhari RA, Khatrawi S, et al. Risk factors of persistent pulmonary hypertension in neonate in a tertiary care referral center. Cureus. (2022) 14(2):e22416. doi: 10.7759/cureus.22416
27. Qian AM, Zhu W, Yang Y, Lu KY, Wang JL, Chen X, et al. Early risk factors for death in neonates with persistent pulmonary hypertension of the newborn treated with inhaled nitric oxide. Zhongguo Dang Dai Er Ke Za Zhi. (2022) 24(5):507–13. doi: 10.7499/j.issn.1008-8830.2111191
28. Kamran A, Rafiq N, Khalid A, Amin F, Kumari V, Shaikh AS, et al. Effectiveness of oral sildenafil for neonates with persistent pulmonary hypertension of newborn (PPHN): a prospective study in a tertiary care hospital. J Matern Fetal Neonatal Med. (2022) 35(25):6787–93. doi: 10.1080/14767058.2021.1923003
29. Rosenberg AA, Kennaugh JM, Moreland SG, Fashaw LM, Hale KA, Torielli FM, et al. Longitudinal follow-up of a cohort of newborn infants treated with inhaled nitric oxide for persistent pulmonary hypertension. J Pediatr. (1997) 131(1 Pt 1):70–5. doi: 10.1016/s0022-3476(97)70126-4
30. Wessel DL, Adatia I, Van Marter LJ, Thompson JE, Kane JW, Stark AR, et al. Improved oxygenation in a randomized trial of inhaled nitric oxide for persistent pulmonary hypertension of the newborn. Pediatrics. (1997) 100(5):E7. doi: 10.1542/peds.100.5.e7
31. Wood KS, McCaffrey MJ, Donovan JC, Stiles AD, Bose CL. Effect of initial nitric oxide concentration on outcome in infants with persistent pulmonary hypertension of the newborn. Neonatology. (1999) 75(4):215–24. doi: 10.1159/000014098
32. Cornfield DN, Maynard RC, deRegnier RA, Guiang SF 3rd, Barbato JE, Milla CE. Randomized, controlled trial of low-dose inhaled nitric oxide in the treatment of term and near-term infants with respiratory failure and pulmonary hypertension. Pediatrics. (1999) 104(5 Pt 1):1089–94. doi: 10.1542/peds.104.5.1089
33. Walsh-Sukys MC, Tyson JE, Wright LL, Bauer CR, Korones SB, Stevenson DK, et al. Persistent pulmonary hypertension of the newborn in the era before nitric oxide: practice variation and outcomes. Pediatrics. (2000) 105(1 Pt 1):14–20. doi: 10.1542/peds.105.1.14
34. Clark RH, Huckaby JL, Kueser TJ, Walker MW, Southgate WM, Perez JA, et al. Low-dose nitric oxide therapy for persistent pulmonary hypertension: 1-year follow-up. J Perinatol. (2003) 23(4):300–3. doi: 10.1038/sj.jp.7210908
35. Hernández-Díaz S, Van Marter LJ, Werler MM, Louik C, Mitchell AA. Risk factors for persistent pulmonary hypertension of the newborn. Pediatrics. (2007) 120(2):e272–82. doi: 10.1542/peds.2006-3037
36. Peterson AL, Deatsman S, Frommelt MA, Mussatto K, Frommelt PC. Correlation of echocardiographic markers and therapy in persistent pulmonary hypertension of the newborn. Pediatr Cardiol. (2009) 30(2):160–5. doi: 10.1007/s00246-008-9303-3
37. Ortiz MI, Estévez-Castillo R, Bautista-Rivas MM, Romo-Hernández G, López-Cadena JM, Copca-García JA. Prevalence and treatment of persistent pulmonary hypertension in the newborn in a Mexican pediatric hospital. Proc West Pharmacol Soc. (2010) 53:39–41.22128450
38. Byers HM, Dagle JM, Klein JM, Ryckman KK, McDonald EL, Murray JC, et al. Variations in Crhr1 are associated with persistent pulmonary hypertension of the newborn. Pediatr Res. (2012) 71(2):162–7. doi: 10.1038/pr.2011.24
39. Malowitz JR, Forsha DE, Smith PB, Cotten CM, Barker PC, Tatum GH. Right ventricular echocardiographic indices predict poor outcomes in infants with persistent pulmonary hypertension of the newborn. Eur Heart J Cardiovasc Imaging. (2015) 16(11):1224–31. doi: 10.1093/ehjci/jev071
40. Steurer MA, Jelliffe-Pawlowski LL, Baer RJ, Partridge JC, Rogers EE, Keller RL. Persistent pulmonary hypertension of the newborn in late preterm and term infants in California. Pediatrics. (2017) 139(1):e20161165. doi: 10.1542/peds.2016-1165
41. Steurer MA, Baer RJ, Oltman S, Ryckman KK, Feuer SK, Rogers E, et al. Morbidity of persistent pulmonary hypertension of the newborn in the first year of life. J Pediatr. (2019) 213:58–65.e4. doi: 10.1016/j.jpeds.2019.06.053
42. Favilli S, De Simone L, Pollini I, Bettuzzi MG, Cianfrini D, Crepaz R, et al. [The prevalence and characteristics of persistent pulmonary hypertension of the newborn. A multicenter study. The study group of the società italiana di cardiologia pediatrica (sicp)]. G Ital Cardiol. (1998) 28(11):1247–52.9866802
43. Mok Q, Yates R, Tasker RC. Persistent pulmonary hypertension of the term neonate: a strategy for management. Eur J Pediatr. (1999) 158(10):825–7. doi: 10.1007/s004310051214
44. Pierce CM, Krywawych S, Petros AJ. Asymmetric dimethyl arginine and symmetric dimethyl arginine levels in infants with persistent pulmonary hypertension of the newborn. Pediatr Crit Care Med. (2004) 5(6):517–20. doi: 10.1097/01.Pcc.0000144715.03515.55
45. Berti A, Janes A, Furlan R, Macagno F. High prevalence of minor neurologic deficits in a long-term neurodevelopmental follow-up of children with severe persistent pulmonary hypertension of the newborn: a cohort study. Ital J Pediatr. (2010) 36:45. doi: 10.1186/1824-7288-36-45
46. Uslu S, Kumtepe S, Bulbul A, Comert S, Bolat F, Nuhoglu A. A comparison of magnesium sulphate and sildenafil in the treatment of the newborns with persistent pulmonary hypertension: a randomized controlled trial. J Trop Pediatr. (2011) 57(4):245–50. doi: 10.1093/tropej/fmq091
47. Roofthooft MT, Elema A, Bergman KA, Berger RM. Patient characteristics in persistent pulmonary hypertension of the newborn. Pulm Med. (2011) 2011:1. doi: 10.1155/2011/858154
48. Rocha G, Baptista MJ, Guimarães H. Persistent pulmonary hypertension of non cardiac cause in a neonatal intensive care unit. Pulm Med. (2012) 2012:1. doi: 10.1155/2012/818971
49. Abdel Mohsen AH, Amin AS. Risk factors and outcomes of persistent pulmonary hypertension of the newborn in neonatal intensive care unit of al-minya university hospital in Egypt. J Clin Neonatol. (2013) 2(2):78–82. doi: 10.4103/2249-4847.116406
50. El-Khazragy N, El Barbary M, Fouad H, Abdelgawad A, Rabie D. Association between genetic variations in carbamoyl-phosphate synthetase gene and persistent neonatal pulmonary hypertension. Eur J Pediatr. (2021) 180(9):2831–8. doi: 10.1007/s00431-021-04053-8
51. Razzaq A, Iqbal Quddusi A, Nizami N. Risk factors and mortality among newborns with persistent pulmonary hypertension. Pak J Med Sci. (2013) 29(5):1099–104. doi: 10.12669/pjms.295.3728
52. Yao AM, Hao YP, Zhang J, Sun XJ, Wang HY, Li B, et al. Changes in plasma levels of atrial natriuretic peptide, endothelin-1 and von willebrand factor among newborns with persistent pulmonary hypertension. Zhongguo Dang Dai Er Ke Za Zhi. (2013) 15(9):718–22.24034911
53. Eriksen V, Nielsen LH, Klokker M, Greisen G. Follow-up of 5- to 11-year-old children treated for persistent pulmonary hypertension of the newborn. Acta Paediatr. (2009) 98(2):304–9. doi: 10.1111/j.1651-2227.2008.01065.x
54. Berger-Caron F, Piedboeuf B, Morissette G, Simonyan D, Chétaille P, Pellerin A, et al. Inhaled epoprostenol for pulmonary hypertension treatment in neonates: a 12-year experience. Am J Perinatol. (2019) 36(11):1142–9. doi: 10.1055/s-0038-1676483
55. Araujo OR, Albertoni Ade C, Lopes VA, Louzada ME, Lopes AO, Cabral EA, et al. Cesarean deliveries and other risks for persistent pulmonary hypertension of the newborn. Rev Bras Ter Intensiva. (2008) 20(4):394–7.25307245
56. Naumburg E, Söderström L. Increased risk of pulmonary hypertension following premature birth. BMC Pediatr. (2019) 19(1):288. doi: 10.1186/s12887-019-1665-6
57. Zhou R, Zheng YN, Zhang XY, Cheng YY. A meta-analysis of the risk factors of persistent pulmonary hypertension in newborns. Front Pediatr. (2021) 9:659137. doi: 10.3389/fped.2021.659137
58. Kumar VH, Hutchison AA, Lakshminrusimha S, Morin FC 3rd, Wynn RJ, Ryan RM. Characteristics of pulmonary hypertension in preterm neonates. J Perinatol. (2007) 27(4):214–9. doi: 10.1038/sj.jp.7211673
59. Muraskas JK, Juretschke LJ, Weiss MG, Bhola M, Besinger RE. Neonatal-perinatal risk factors for the development of persistent pulmonary hypertension of the newborn in preterm newborns. Am J Perinatol. (2001) 18(2):87–91. doi: 10.1055/s-2001-13638
60. Wilson KL, Zelig CM, Harvey JP, Cunningham BS, Dolinsky BM, Napolitano PG. Persistent pulmonary hypertension of the newborn is associated with mode of delivery and not with maternal use of selective serotonin reuptake inhibitors. Am J Perinatol. (2011) 28(1):19–24. doi: 10.1055/s-0030-1262507
61. Winovitch KC, Padilla L, Ghamsary M, Lagrew DC, Wing DA. Persistent pulmonary hypertension of the newborn following elective cesarean delivery at term. J Matern Fetal Neonatal Med. (2011) 24(11):1398–402. doi: 10.3109/14767058.2010.551681
62. Van Marter LJ, Leviton A, Allred EN, Pagano M, Sullivan KF, Cohen A, et al. Persistent pulmonary hypertension of the newborn and smoking and aspirin and nonsteroidal antiinflammatory drug consumption during pregnancy. Pediatrics. (1996) 97(5):658–63. doi: 10.1542/peds.97.5.658
63. Kieler H, Artama M, Engeland A, Ericsson O, Furu K, Gissler M, et al. Selective serotonin reuptake inhibitors during pregnancy and risk of persistent pulmonary hypertension in the newborn: population based cohort study from the five nordic countries. Br Med J. (2011) 344:d8012. doi: 10.1136/bmj.d8012
64. Huybrechts KF, Bateman BT, Palmsten K, Desai RJ, Patorno E, Gopalakrishnan C, et al. Antidepressant use late in pregnancy and risk of persistent pulmonary hypertension of the newborn. Jama. (2015) 313(21):2142–51. doi: 10.1001/jama.2015.5605
65. Masarwa R, Bar-Oz B, Gorelik E, Reif S, Perlman A, Matok I. Prenatal exposure to selective serotonin reuptake inhibitors and serotonin norepinephrine reuptake inhibitors and risk for persistent pulmonary hypertension of the newborn: a systematic review, meta-analysis, and network meta-analysis. Am J Obstet Gynecol. (2019) 220(1):57.e1–.e13. doi: 10.1016/j.ajog.2018.08.030
66. Dyess NF, Palmer C, Soll RF, Clark RH, Abman SH, Kinsella JP. Practices and outcomes from a prospective, multicenter registry for preterm newborns with pulmonary hypertension. J Pediatr. (2023) 262:113614. doi: 10.1016/j.jpeds.2023.113614
67. Lotze A, Mitchell BR, Bulas DI, Zola EM, Shalwitz RA, Gunkel JH. Multicenter study of surfactant (beractant) use in the treatment of term infants with severe respiratory failure. J Pediatr. (1998) 132(1):40–7. doi: 10.1016/s0022-3476(98)70482-2
68. Wu TW, Noori S. Recognition and management of neonatal hemodynamic compromise. Pediatr Neonatol. (2021) 62(Suppl 1):S22–9. doi: 10.1016/j.pedneo.2020.12.007
69. Roberts JD Jr., Fineman JR, Morin FC 3rd, Shaul PW, Rimar S, Schreiber MD, et al. Inhaled nitric oxide and persistent pulmonary hypertension of the newborn. N Engl J Med. (1997) 336(9):605–10. doi: 10.1056/nejm199702273360902
70. Kinsella JP, Truog WE, Walsh WF, Goldberg RN, Bancalari E, Mayock DE, et al. Randomized, multicenter trial of inhaled nitric oxide and high-frequency oscillatory ventilation in severe, persistent pulmonary hypertension of the newborn. J Pediatr. (1997) 131(1 Pt 1):55–62. doi: 10.1016/s0022-3476(97)70124-0
71. Torielli F, Fashaw LM, Knudson O, Kinsella J, Ivy D, Valdes-Cruz L, et al. Echocardiographic outcome of infants treated as newborns with inhaled nitric oxide for severe hypoxemic respiratory failure. J Pediatr. (2001) 138(3):349–54. doi: 10.1067/mpd.2001.111328
72. Baquero H, Soliz A, Neira F, Venegas ME, Sola A. Oral sildenafil in infants with persistent pulmonary hypertension of the newborn: a pilot randomized blinded study. Pediatrics. (2006) 117(4):1077–83. doi: 10.1542/peds.2005-0523
73. Al Omar S, Salama H, Al Hail M, Al Rifai H, Bunahia M, El Kasem W, et al. Effect of early adjunctive use of oral sildenafil and inhaled nitric oxide on the outcome of pulmonary hypertension in newborn infants. J Neonatal Perinatal Med. (2016) 9(3):251–9. doi: 10.3233/npm-16161
74. Vargas-Origel A, Gómez-Rodríguez G, Aldana-Valenzuela C, Vela-Huerta MM, Alarcón-Santos SB, Amador-Licona N. The use of sildenafil in persistent pulmonary hypertension of the newborn. Am J Perinatol. (2010) 27(3):225–30. doi: 10.1055/s-0029-1239496
75. Singh P, Deshpande S, Nagpal R, Garegrat R, Gupta S, Suryawanshi P. Management of neonatal pulmonary hypertension-a survey of neonatal intensive care units in India. BMC Pediatr. (2023) 23(1):149. doi: 10.1186/s12887-023-03964-9
76. Singh Y, Lakshminrusimha S. Pathophysiology and management of persistent pulmonary hypertension of the newborn. Clin Perinatol. (2021) 48(3):595–618. doi: 10.1016/j.clp.2021.05.009
77. McNamara PJ, Shivananda SP, Sahni M, Freeman D, Taddio A. Pharmacology of milrinone in neonates with persistent pulmonary hypertension of the newborn and suboptimal response to inhaled nitric oxide. Pediatr Crit Care Med. (2013) 14(1):74–84. doi: 10.1097/PCC.0b013e31824ea2cd
78. McNamara PJ, Laique F, Muang-In S, Whyte HE. Milrinone improves oxygenation in neonates with severe persistent pulmonary hypertension of the newborn. J Crit Care. (2006) 21(2):217–22. doi: 10.1016/j.jcrc.2006.01.001
79. Patel N. Use of milrinone to treat cardiac dysfunction in infants with pulmonary hypertension secondary to congenital diaphragmatic hernia: a review of six patients. Neonatology. (2012) 102(2):130–6. doi: 10.1159/000339108
80. James AT, Corcoran JD, McNamara PJ, Franklin O, El-Khuffash AF. The effect of milrinone on right and left ventricular function when used as a rescue therapy for term infants with pulmonary hypertension. Cardiol Young. (2016) 26(1):90–9. doi: 10.1017/s1047951114002698
81. Bassler D, Kreutzer K, McNamara P, Kirpalani H. Milrinone for persistent pulmonary hypertension of the newborn. Cochrane Database Syst Rev. (2010) 2010(11):Cd007802. doi: 10.1002/14651858.CD007802.pub2
82. Mohamed WA, Ismail M. A randomized, double-blind, placebo-controlled, prospective study of bosentan for the treatment of persistent pulmonary hypertension of the newborn. J Perinatol. (2012) 32(8):608–13. doi: 10.1038/jp.2011.157
83. Wei E, Chen XH, Zhou SJ. Comparison of treprostinil and oral sildenafil for the treatment of persistent pulmonary hypertension of the newborn: a retrospective cohort study. Front Pediatr. (2023) 11:1270712. doi: 10.3389/fped.2023.1270712
84. Alhumaid S, Alnaim AA, Al Ghamdi MA, Alahmari AA, Alabdulqader M, Al HajjiMohammed SM, et al. International treatment outcomes of neonates on extracorporeal membrane oxygenation (Ecmo) with persistent pulmonary hypertension of the newborn (Pphn): a systematic review. J Cardiothorac Surg. (2024) 19(1):493. doi: 10.1186/s13019-024-03011-3
85. Khorana M, Yookaseam T, Layangool T, Kanjanapattanakul W, Paradeevisut H. Outcome of oral sildenafil therapy on persistent pulmonary hypertension of the newborn at queen sirikit national institute of child health. J Med Assoc Thai. (2011) 94(Suppl 3):S64–73.22043756
86. Aleem S, Robbins C, Murphy B, Elliott S, Akinyemi C, Paredes N, et al. The use of supplemental hydrocortisone in the management of persistent pulmonary hypertension of the newborn. J Perinatol. (2021) 41(4):794–800. doi: 10.1038/s41372-021-00943-9
87. Hansen AK, Wisborg K, Uldbjerg N, Henriksen TB. Elective caesarean section and respiratory morbidity in the term and near-term neonate. Acta Obstet Gynecol Scand. (2007) 86(4):389–94. doi: 10.1080/00016340601159256
88. Levine EM, Ghai V, Barton JJ, Strom CM. Mode of delivery and risk of respiratory diseases in newborns. Obstet Gynecol. (2001) 97(3):439–42. doi: 10.1016/s0029-7844(00)01150-9
89. Roth-Kleiner M, Wagner BP, Bachmann D, Pfenninger J. Respiratory distress syndrome in near-term babies after caesarean section. Swiss Med Wkly. (2003) 133(19-20):283–8. doi: 10.4414/smw.2003.10121
90. Ulizzi L, Zonta LA. Sex differential patterns in perinatal deaths in Italy. Hum Biol. (2002) 74(6):879–88. doi: 10.1353/hub.2003.0012
91. Whitehouse AJ, Mattes E, Maybery MT, Sawyer MG, Jacoby P, Keelan JA, et al. Sex-Specific associations between umbilical cord blood testosterone levels and language delay in early childhood. J Child Psychol Psychiatry. (2012) 53(7):726–34. doi: 10.1111/j.1469-7610.2011.02523.x
92. Bresson E, Seaborn T, Côté M, Cormier G, Provost PR, Piedboeuf B, et al. Gene expression profile of androgen modulated genes in the murine fetal developing lung. Reprod Biol Endocrinol. (2010) 8:1–14. doi: 10.1186/1477-7827-8-2
93. Seaborn T, Simard M, Provost PR, Piedboeuf B, Tremblay Y. Sex hormone metabolism in lung development and maturation. Trends Endocrinol Metab. (2010) 21(12):729–38. doi: 10.1016/j.tem.2010.09.001
94. Feng R, Liu L, Zhang YY, Yuan ZS, Gao L, Zuo CT. Unsatisfactory glucose management and adverse pregnancy outcomes of gestational diabetes mellitus in the real world of clinical practice: a retrospective study. Chin Med J (Engl). (2018) 131(9):1079–85. doi: 10.4103/0366-6999.230718
95. Robinson GE. Controversies about the use of antidepressants in pregnancy. J Nerv Ment Dis. (2015) 203(3):159–63. doi: 10.1097/nmd.0000000000000256
96. Ornoy A, Koren G. Selective serotonin reuptake inhibitor use in pregnant women; pharmacogenetics, drug-drug interactions and adverse effects. Expert Opin Drug Metab Toxicol. (2018) 14(3):247–59. doi: 10.1080/17425255.2018.1430139
Keywords: persistent pulmonary hypertension of the newborn (PPHN), neonate, epidemiology, mortality, etiologies
Citation: Huang Y, Yang T, Liang X, Chen Y, Zhou P, Yu Z, Zhong G and Zhang L (2025) Global, regional and national trends in the burden of persistent pulmonary hypertension of the newborn and essentials of its management from 1993 to 2023: a scoping review. Front. Pediatr. 13:1502385. doi: 10.3389/fped.2025.1502385
Received: 26 September 2024; Accepted: 20 May 2025;
Published: 4 June 2025.
Edited by:
Viraraghavan Vadakkencherry Ramaswamy, Ankura Hospital For Women and Children, IndiaReviewed by:
Xiaoling Guo, Wenzhou Medical University, ChinaChongbing Yan, Shanghai Children's Hospital, China
Copyright: © 2025 Huang, Yang, Liang, Chen, Zhou, Yu, Zhong and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guichao Zhong, emhvbmcuZ2NAc3pob3NwaXRhbC5jb20=; Lian Zhang, emhhbmdsaWFuZGVAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Yan Huang
Yan Huang Ting Yang1,†
Ting Yang1,† You Chen
You Chen Ping Zhou
Ping Zhou Zhangbin Yu
Zhangbin Yu