- 1Department of Otorhinolaryngology, Head and Neck Surgery, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China
- 2Department of Otolaryngology, National Clinical Research Center for Child Health and Disorders, Ministry of Education Key Laboratory of Child Development and Disorders, Chongqing Key Laboratory of Pediatric Metabolism and Inflammatory Diseases, Chongqing Key Laboratory of Child Rare Diseases in Infection and Immunity, Chongqing Key Laboratory of Child Neurodevelopment and Cognitive Disorders, Chongqing Key Laboratory of Structural Birth Defect and Reconstruction, Chongqing Engineering Research Center of Stem Cell Therapy, Children’s Hospital of Chongqing Medical University, Chongqing, China
- 3West China Hospital, Sichuan University, Sichuan, China
- 4Department of Otorhinolaryngology, Head and Neck Surgery, The Second Affiliated Hospital of Chongqing Medical University, Chongqing, China
Objective: This study aims to analyze a genetic family with the GJB2 gene c.551G>A (p.R184Q) variant, exploring the relationship between its genotype and clinical phenotype, and summarizing the inheritance pattern and clinical features associated with this locus.
Methods: Detailed medical history collection and physical examinations were conducted for the proband and their family members. Audiological assessments and genetic sequencing analyses were performed on some members. Additionally, a review of existing literature concerning GJB2 c.551G>A (p.R184Q) was conducted.
Results: The proband, along with their father and paternal grandmother, carried the heterozygous mutation GJB2 c.551G>A, all exhibiting moderate to profound bilateral prelingual sensorineural deafness. Notably, the proband also presented symptoms of skin dryness and nail abnormalities characteristic of syndromic hearing loss.
Conclusion: The GJB2 c.551G>A mutation not only leads to severe hearing loss but may also be associated with syndromic hearing loss, expanding our understanding of the clinical spectrum associated with this variant.
Introduction
Hearing loss due to genetic factors is a major cause of hearing impairment in newborns. Recent epidemiological studies indicate that half of all newborn hearing losses can be attributed to genetic factors (1). Genetic hearing loss is categorized into syndromic and non-syndromic types. Currently, over 150 genes associated with hearing loss have been reported (2). GJB2 is the most common gene responsible for deafness in Chinese newborns (3). The discovery of rare variants within the GJB2 gene has marked a significant milestone in unraveling the genetic underpinnings of congenital deafness. GJB2 is responsible for producing connexin 26, a protein essential for the creation of gap junctions within the cochlea. Alterations in this gene can disrupt the normal operation of these junctions, causing hearing loss. Such mutations can give rise to a spectrum of genetic patterns and phenotypes, including autosomal recessive non-syndromic hearing loss, autosomal dominant non-syndromic hearing loss, and syndromic hearing loss (4). Syndromic hearing loss often presents when the auditory system is compromised in conjunction with the integumentary system, with related syndromes encompassing Keratitis-Ichthyosis-Deafness syndrome (KID), and Palmoplantar Keratoderma (PPK) linked to deafness (5, 6). Skin phenotypes associated with syndromic hearing loss due to GJB2 mutations encompass ichthyosis, palmoplantar keratoderma, joint pads, and nail abnormalities (7). The c.551G > A (p.R184Q) variant of the GJB2 gene is a rare mutation with limited documentation and some debate surrounding it. Nonetheless, the clinical phenotypes and genotypes linked to these rare variants are intricate and exhibit variability, necessitating a thorough analysis to clarify the underlying mechanisms.
In our study, we performed a comprehensive analysis of a genetic family to uncover the connection between the GJB2 c.551G>A mutation and syndromic hearing loss, concentrating on the phenotypic traits and inheritance patterns observed within this family. Our results indicate that the GJB2 c.551G>A mutation significantly contributes to the development of syndromic hearing loss in this genetic cohort, offering new insights that could enhance the diagnosis and genetic counseling for hereditary deafness.
Subjects and methods
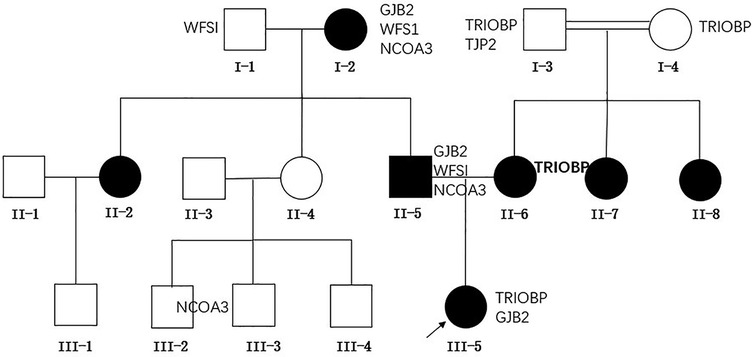
This study conducted an analysis on the family of a patient who had been deaf from birth, diagnosed with severe bilateral hearing impairment at the age of one at the Children's Hospital of Chongqing Medical University. The family tree is depicted in Figure 1. This study was approved by the Ethics Committee of Children's Hospital of Chongqing Medical University (Number 2024-383). This study adhered strictly to the ethical guidelines outlined in the Declaration of Helsinki and its subsequent amendments. Given the retrospective nature of the research, the institutional review board granted permission to dispense with the requirement for written informed consent.
Genomic DNA extraction and gene sequencing
Upon securing informed consent from the participants' family members, genomic DNA is extracted from peripheral venous blood samples. Utilizing whole-exome sequencing (WGS), the GJB2 gene and associated genes are sequenced to identify potential pathogenic mutations.
Medical history collection and physical examination
We perform a thorough collection of medical histories for patients with a family history of hearing loss, encompassing past instances of otitis media, meningitis, mumps, head injuries, a history of ototoxic medication use, maternal exposure to risk factors during pregnancy, and occurrences of hyperbilirubinemia, asphyxia, or hypoxia at birth. The physical examination zeroes in on evaluating the skin for signs of thickening, dryness, peeling, keratosis, erythema, or calluses, and also involves inspecting the nails for irregularities such as dysplasia, deep grooves, thickening, brittleness, or koilonychia, while also verifying the presence of digital pads without keratitis.
Audiological examination
The audiological examination includes otoscopic evaluation, pure tone audiometry (PTA), auditory brainstem response (ABR), distortion product evoked otoacoustic emissions (DPOAE), and tympanometry. Hearing impairment severity is categorized according to PTA outcomes, with classifications ranging from mild (20–40 dB), moderate (41–70 dB), severe (71–95 dB), to profound (>95 dB).
Results
Clinical manifestations of proband and family members
The proband was a 1-year-old female patient with no history of otitis media, meningitis, mumps, or head trauma. She had not been exposed to ototoxic drugs, and her mother had no exposure to risk factors during pregnancy. At birth, there were no instances of hyperbilirubinemia, asphyxia, or hypoxia. Cytomegalovirus screening was negative. No obvious abnormalities were found in the proband's ECG, urinalysis, or abdominal ultrasound. The family denied that the child had vision loss, double vision, or other problems with their eyes. The patient exhibited thickened, keratotic fingernails and toenails, with some having a spoon-shaped appearance (Figures 2a–d), and had dry skin on her face and feet, We invited a dermatologist to examine the proband's skin and nails during her hospital stay. After examining the patient, the dermatologist considered genetic factors. Auditory brainstem response (ABR) testing revealed air conduction was 95 dB nHL in the left ear and 95 dB nHL in the right ear, while bone conduction did not respond at 50 dB nHL in both ears. The auditory steady-state response (ASSR) was examined at nine months, as depicted in Figure 3a, and transient evoked otoacoustic emissions (TEOAE) were not elicited. No significant abnormalities were detected in acoustic immittance, otoscopy, inner ear MRI, or inner ear CT. The proband attempted to use a hearing aid but did not respond to it. At the age of two, she underwent cochlear implantation and is still undergoing speech rehabilitation.

Figure 2. Phenotypic characterization of selected family members. (a,b) The proband's hands exhibited thickened fingernails, some of which showed a dimple. (c,d) The proband's feet displayed similarly thickened and keratotic toenails. (e) The hands of the proband's grandmother showed a dimple on the left index finger (enlarged detail in lower right corner). (f) The feet of the proband's father exhibited thickening and hyperpigmentation at the distal end of the right great toenail (enlarged detail in lower right corner).
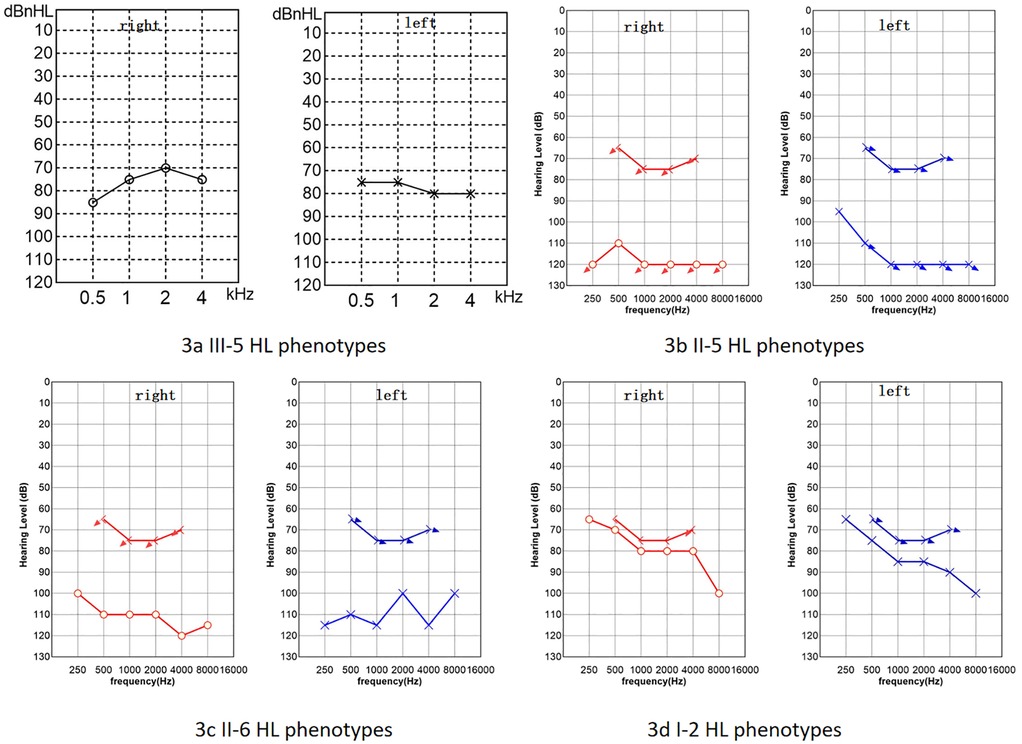
Figure 3. Hearing loss (HL) phenotypes of some family members. (a) The proband's ASSR. (b) PTA of the proband's father. (c) PTA of the proband's mother. (d) PTA of the proband's grandmother. Red circles represent air conduction audiometry for the right ear, blue Xs represent air conduction audiometry for the left ear, brackets represent bone conduction audiometry. The downward arrow indicates no response at the maximum audiometer output value (b–d).
The proband's paternal grandmother (I-2) has had poor hearing since childhood, with PTA results shown in Figure 3d, which indicates severe sensorineural deafness in both ears. She can comprehend common words in daily conversation, but struggles with long sentences. Her coherent speech is not intelligible, yet with context and lip-reading cues, her family can gradually understand individual words in her speech. There are no signs of skin thickening or dryness, but she reports cracks in the nail of her left ring finger following an injury, and a dimple is visible on her left index finger (Figure 2e). The proband's father (II-5) has PTA results indicating profound deafness (Figure 3b), and family members report poor sound response following high fever at ages 5–6, He is unable to respond to sounds in daily life; his words are not easily recognized. He communicates through gestures. The distal end of his right big toe nail is thickened and darkened (Figure 2f). The proband's mother (II-6) also shows profound deafness according to audiometry results (Figure 3c), She is unresponsive to sounds in her daily environment and unable to articulate meaningful words.Neither I-2, II-5, nor II-6 has a history of otitis media, meningitis, mumps, or head trauma, and no significant skin abnormalities were observed.
Genetic sequencing results
Genetic sequencing has uncovered that the proband possesses a heterozygous GJB2 c.551G>A mutation, aligning with the genotypes of the proband's father and paternal grandmother. Furthermore, the proband harbors a heterozygous TRIOBP c.2176C>T mutation, whereas the mother displays a homozygous mutation at this locus, and both the maternal grandfather and grandmother carry heterozygous mutations at this site.
The association between genotype and phenotype in this lineage
Through a detailed analysis of the genotypes and phenotypes of family members, we found a strong association between the GJB2 c.551G>A mutation and the hereditary deafness phenotype within the family. Furthermore, the TRIOBP c.2176C>T mutation was also detected in the mother of the affected individual and additional relatives, aligning with the characteristics of autosomal recessive non-syndromic deafness.
Summary of case characteristics for GJB2: c.551g>A (p.R184q)
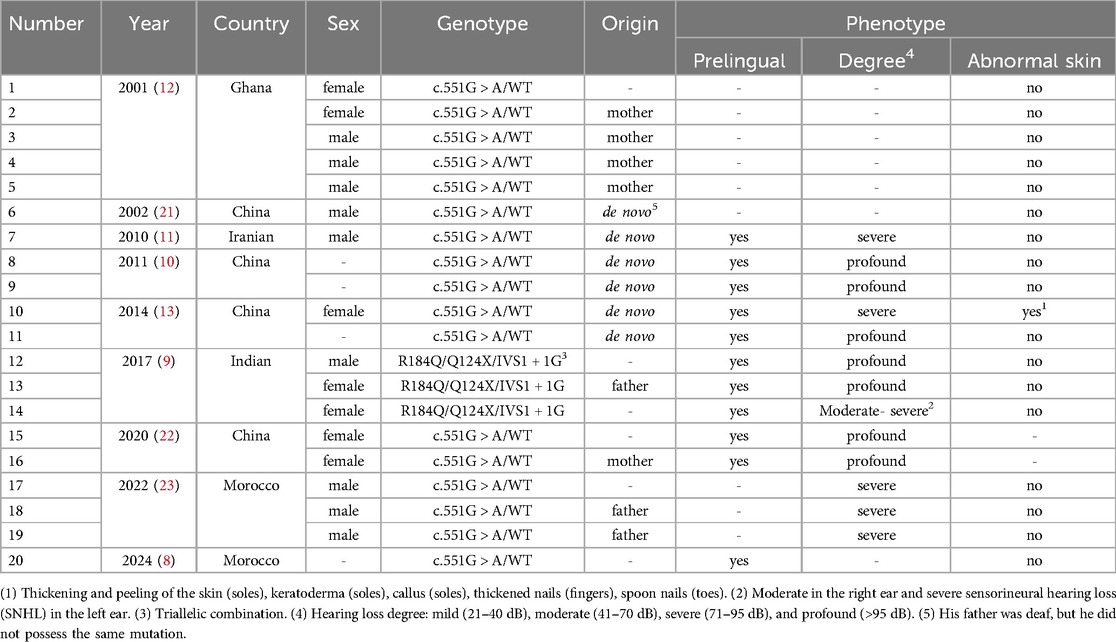
We summarized previous reports related to the GJB2 c.551G>A (p.R184Q) locus (Table 1), finding a total of 9 publications covering 20 patients, of which only one was diagnosed with syndromic deafness. All known auditory phenotypes were prelingual, ranging from severe to profound hearing loss. In some cases, the pathogenic gene occurred de novo, and all known inheritance patterns are autosomal dominant.
Discussion
The clinical significance of the GJB2 c.551g>A mutation
In this study, we document a family affected by GJB2-related prelingual severe to profound hearing loss, in which the proband also manifests characteristics of syndromic hearing impairment, including xeroderma and nail dystrophy. The GJB2 c.551G>A variant is a rare dominant mutation. As far as we are aware, this variant has been reported in only five countries: Ghana, Iran, China, India, and Morocco (8–12). Up to now, the GJB2 c.551G>A variant has been linked to autosomal dominant non-syndromic hearing impairment, with only a single case previously reported involving autosomal dominant syndromic hearing loss. The proband exhibits skin symptoms that are consistent with those described in a patient reported by Xiuhong Pang (13), who had a syndromic GJB2 c.551G>A heterozygous mutation. Our findings suggest that the GJB2 c.551G>A variant can result in both syndromic and non-syndromic forms of hearing loss.
GJB2 is located on chromosome 13q11-12 and encodes the human connexin 26 protein, a member of a multigene family comprising 20 structural proteins. This protein is extensively expressed across numerous human tissues, encompassing epithelial cells originating from the cochlear ectoderm, the cornea, and the skin. The GJB2 c.551G>A mutation represents a missense alteration, causing the replacement of glutamine with arginine at amino acid position 184. Research has shown that connexins with this mutation are incapable of forming functional gap junction channels (14). Furthermore, experiments conducted on HeLa cells have revealed that the mutated R184Q protein exerts a dominant negative impact on both wild-type connexin 26 and connexin 30 (15). Studies indicate that Cx26 mutations contribute to the development of various conditions by promoting cell death or exerting a dominant negative effect on co-expressed connexins, which can lead to skin disorders and hearing loss (16). Conversely, mutations that diminish channel function may exclusively result in hearing loss. Consequently, from a mechanistic standpoint, GJB2: c.551G>A is likely to induce syndromic hearing impairment.
The phenotypes of GJB2-related deafness are diverse, encompassing both syndromic and non-syndromic forms, with many locations being quite common. Patients with GJB2 p.R75Q-associated hearing loss may present with skin abnormalities or have no obvious skin conditions (17). The GJB2 c.250G>A variant is generally thought to be associated with post-lingual, severe to profound hearing loss, but there have been reported that this mutation lead to mild hearing loss and skin keratoderma syndromic deafness (18). Moreover, the skin phenotype in individuals with dominant syndromic deafness related to GJB2 varies in severity among different individuals. In the family we studied, the proband exhibited generalized dry skin and spoon-shaped nails, presenting more severe symptoms than their father and paternal grandmother, who shared the same genotype. Several speculations may explain this observation: (1) Additional heterozygous recessive mutations may alter the hearing loss and skin phenotype caused by the dominant GJB2 mutation. Research indicates that family members with p.R75W/c.235delC, where GJB2 c.235delC can cause autosomal recessive non-syndromic deafness, manifest more severe hearing loss and palmoplantar keratoderma than those with only the p.R75W mutation (13). (2) The influence of environmental and ethnic factors. The proband is only 1 year old and could not complete subjective hearing tests, so auditory evaluation was performed using ASSR. The father and grandmother underwent subjective hearing assessments, and the degree of hearing loss could not be compared. The hearing loss in II-5 is more severe than in I-2. We learned that II-5 experienced a severe fever at ages 5–6, after which he showed a poor response to sound. Whether environmental factors exacerbate GJB2-related deafness requires further validation in the future.
Diversity of genetic patterns
In our study, the pathogenic genes of the proband and the proband's father both originated from the grandmother of the proband. The variation at this locus exhibits an autosomal dominant inheritance pattern, consistent with previous reports. However, some studies suggest that mutations at this locus may be de novo mutations. One family reported by Amritkumar Pavithra includes three patients: a brother and sister, along with the sister's daughter, all of whom have compound heterozygous mutations, along with mutations in other recessive genes. Their parents have normal hearing, making it impossible to completely rule out the possibility of a gonadal mosaicism in either parent as the source of the P.R184Q mutation in the context of autosomal dominant inheritance (9). This diversity in inheritance patterns emphasizes the importance of considering multiple genetic mechanisms in hereditary hearing loss research.
Potential impact of TRIOBP: c.2176c>T mutation
Mutations in TRIOBP can cause autosomal recessive nonsyndromic deafness, which is associated with prelingual, severe to profound hearing loss (19). Very few mutations in the site manifest as postlingual deafness. More than 40 loci have been reported to cause disease in this gene (20). The proband's mother had profound sensorineural deafness and was homozygous for TRIOBP c.2176C>T. Both of her parents were heterozygous for TRIOBP c.2176C>T and were consanguineous. Her two younger sisters also had prelingual deafness. We were unable to obtain the results of genetic and hearing tests for her two younger sisters. There are no relevant literature reports on mutations in this site. We only found one record in the ClinVar database that mutations in this site may cause disease. The family we reported provided strong evidence that the TRIOBP c.2176C>T mutation caused autosomal recessive nonsyndromic deafness, which was associated with prelingual and profound hearing loss, and broadened the spectrum of pathogenic mutations in the TRIOBP gene.
Future research directions
The clinical phenotype of GJB2-related dominant syndromic deafness varies in severity among different individuals. In the same family, the same mutation site may cause different degrees of hearing loss and skin damage, and the mechanism behind it deserves further exploration. Furthermore, the two documented cases of syndromic deafness associated with the GJB2 c.551G>A mutation are both individuals of Chinese Han ethnicity. Whether this form of dominant syndromic deafness linked to GJB2 c.551G>A is exclusive to this demographic remains to be determined. Future research should delve into the prevalence and phenotypic variability of the GJB2 c.551G>A mutation across various populations. Concurrently, the functional implications of the TRIOBP c.2176C>T mutation and its role in causing deafness merit further investigation.
Conclusion
Our study reported a pedigree with syndromic deafness caused by the GJB2 p.R184Q mutation, summarized the phenotype and inheritance mode of GJB2 p.R184Q reported previously, and confirmed that GJB2 p.R184Q is associated with both syndromic and non-syndromic deafness. By understanding the genetic underpinnings and associated clinical presentations, we can improve diagnostic accuracy, provide more personalized management strategies, and ultimately enhance the quality of life for individuals affected by this form of hearing impairment. Future research should continue to unravel the intricate interplay between genetic variants and environmental factors to further advance our knowledge and treatment options for GJB2-related deafness.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Institutional review board of children's hospital of chongqing medical university. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin. Written informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
XZ: Data curation, Investigation, Writing – original draft, Writing – review & editing. HC: Data curation, Investigation, Writing – original draft, Writing – review & editing. YB: Resources, Methodology, Supervision, Writing – review & editing. YL: Resources, Writing – review & editing. WX: Writing – review & editing, Resources. HK: Resources, Writing – review & editing. CZ: Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Yoshimura H, Okubo T, Shinagawa J, Nishio SY, Takumi Y, Usami SI. Epidemiology, aetiology and diagnosis of congenital hearing loss via hearing screening of 153 913 newborns. Int J Epidemiol. (2024) 53(3):dyae052. doi: 10.1093/ije/dyae052
2. Ali A, Tabouni M, Kizhakkedath P, Baydoun I, Allam M, John A, et al. Spectrum of genetic variants in bilateral sensorineural hearing loss. Front Genet. (2024) 15:1314535. doi: 10.3389/fgene.2024.1314535
3. Feng J, Zeng Z, Luo S, Liu X, Luo Q, Yang K, et al. Carrier frequencies, trends, and geographical distribution of hearing loss variants in China: the pooled analysis of 2,161,984 newborns. Heliyon. (2024) 10(3):e24850. doi: 10.1016/j.heliyon.2024.e24850
4. Posukh OL, Maslova EA, Danilchenko VY, Zytsar MV, Orishchenko KE. Functional consequences of pathogenic variants of the Gjb2 gene (Cx26) localized in different Cx26 domains. Biomolecules. (2023) 13(10):1521. doi: 10.3390/biom13101521
5. Bedoukian EC, Rentas S, Skraban C, Shao Q, Treat J, Laird DW, et al. Palmoplantar keratoderma with deafness phenotypic variability in a patient with an inherited Gjb2 frameshift variant and novel missense variant. Mol Genet Genomic Med. (2021) 9(2):e1574. doi: 10.1002/mgg3.1574
6. Messmer EM, Kenyon KR, Rittinger O, Janecke AR, Kampik A. Ocular manifestations of keratitis-ichthyosis-deafness (kid) syndrome. Ophthalmology. (2005) 112(2):e1–6. doi: 10.1016/j.ophtha.2004.07.034
7. Mao L, Wang Y, An L, Zeng B, Wang Y, Frishman D, et al. Molecular mechanisms and clinical phenotypes of Gjb2 missense variants. Biology (Basel). (2023) 12(4):505. doi: 10.3390/biology12040505
8. El Fizazi K, Abbassi M, Nmer S, Laamarti H, ElAlami MN, Ouldim K, et al. Unraveling the diversity of Gjb2 mutations in nonsyndromic hearing loss: a comprehensive study in the Moroccan population. Audiol Neurootol. (2024) 29(3):216–23. doi: 10.1159/000535346
9. Pavithra A, Chandru J, Jeffrey JM, Karthikeyen NP, Srisailapathy CRS. Rare compound heterozygosity involving dominant and recessive mutations of Gjb2 gene in an assortative mating hearing impaired Indian family. Eur Arch Otorhinolaryngol. (2017) 274(1):119–25. doi: 10.1007/s00405-016-4229-5
10. Huang S, Yuan Y, Liu J, Han D, Kang D, Zhang X, et al. de novo dominant mutation of Gjb2 in two Chinese families with nonsyndromic hearing loss. Int J Pediatr Otorhinolaryngol. (2011) 75(10):1333–6. doi: 10.1016/j.ijporl.2011.07.033
11. Mahdieh N, Shirkavand A, Raeisi M, Akbari MT, Tekin M, Zeinali S. Unexpected heterogeneity due to recessive and de novo dominant mutations of Gjb2 in an Iranian family with nonsyndromic hearing loss: implication for genetic counseling. Biochem Biophys Res Commun. (2010) 402(2):305–7. doi: 10.1016/j.bbrc.2010.10.021
12. Hamelmann C, Amedofu GK, Albrecht K, Muntau B, Gelhaus A, Brobby GW, et al. Pattern of connexin 26 (Gjb2) mutations causing sensorineural hearing impairment in Ghana. Hum Mutat. (2001) 18(1):84–5. doi: 10.1002/humu.1156
13. Pang X, Chai Y, Sun L, Chen D, Chen Y, Zhang Z, et al. Characterization of Spectrum, de novo rate and genotype-phenotype correlation of dominant Gjb2 mutations in Chinese hans. PLoS One. (2014) 9(6):e100483. doi: 10.1371/journal.pone.0100483
14. Zhang Y, Tang W, Ahmad S, Sipp JA, Chen P, Lin X. Gap junction-mediated intercellular biochemical coupling in cochlear supporting cells is required for normal cochlear functions. Proc Natl Acad Sci USA. (2005) 102(42):15201–6. doi: 10.1073/pnas.0501859102
15. Yum SW, Zhang J, Scherer SS. Dominant Connexin26 mutants associated with human hearing loss have trans-dominant effects on Connexin30. Neurobiol Dis. (2010) 38(2):226–36. doi: 10.1016/j.nbd.2010.01.010
16. Press ER, Shao Q, Kelly JJ, Chin K, Alaga A, Laird DW. Induction of cell death and gain-of-function properties of Connexin26 mutants predict severity of skin disorders and hearing loss. J Biol Chem. (2017) 292(23):9721–32. doi: 10.1074/jbc.M116.770917
17. Dai X, Li J, Hu XJ, Tong J, Cai WQ. [Autosomal dominant hearing loss resulting from mutation in the Gjb2 gene:nonsyndromic presentation in a Chinese family]. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. (2016) 30(24):1939–41;45. doi: 10.13201/j.issn.1001-1781.2016.24.009
18. Hashimoto K, Miwa T, Ono C, Nara K, Mutai H, Seto T, et al. Gap junction Beta-2 P.Val84met can cause autosomal dominant syndromic hearing loss with keratoderma. Cureus. (2024) 16(2):e54992. doi: 10.7759/cureus.54992
19. Pollak A, Lechowicz U, Murcia Pieńkowski VA, Stawiński P, Kosińska J, Skarżyński H, et al. Whole exome sequencing identifies triobp pathogenic variants as a cause of post-lingual bilateral moderate-to-severe sensorineural hearing loss. BMC Med Genet. (2017) 18(1):142. doi: 10.1186/s12881-017-0499-z
20. Zhou C, Xiao Y, Xie H, Wang J, Liu S. Case report: novel compound heterozygous variants in triobp associated with congenital deafness in a Chinese family. Front Genet. (2021) 12:766973. doi: 10.3389/fgene.2021.766973
21. Wang YC, Kung CY, Su MC, Su CC, Hsu HM, Tsai CC, et al. Mutations of Cx26 gene (Gjb2) for prelingual deafness in Taiwan. European Journal of Human Genetics: EJHG. (2002) 10(8):495–8. doi: 10.1038/sj.ejhg.5200838
22. Liu XW, Wang JC, Wang SY, Li SJ, Zhu YM, Ding WJ, et al. The mutation frequencies of Gjb2, Gjb3, Slc26a4 and mt-Rnr1 of patients with severe to profound sensorineural hearing loss in Northwest China. Int J Pediatr Otorhinolaryngol. (2020) 136:110143. doi: 10.1016/j.ijporl.2020.110143
Keywords: GJB2, hereditary deafness, genotype-phenotype association, clinical characteristic analysis, congenital deafness
Citation: Zhao X, Chi H, Bai Y, Lu Y, Xiong W, Kang H and Zhang C (2025) Analysis of clinical phenotypes and genotypes of congenital deafness caused by rare variants in GJB2. Front. Pediatr. 13:1514369. doi: 10.3389/fped.2025.1514369
Received: 20 October 2024; Accepted: 3 April 2025;
Published: 23 April 2025.
Edited by:
Z. Jason Qian, University of California, San Diego, United StatesReviewed by:
Virginia Corazzi, University Hospital of Ferrara, ItalyKara Brodie, University of California, San Francisco, United States
Copyright: © 2025 Zhao, Chi, Bai, Lu, Xiong, Kang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cheng Zhang, Njk0MjYyODA1QHFxLmNvbQ==
†These authors have contributed equally to this work
 Xuxu Zhao
Xuxu Zhao Huan Chi
Huan Chi Yan Bai
Yan Bai Yu Lu
Yu Lu Wenyu Xiong3
Wenyu Xiong3 Houyong Kang
Houyong Kang