- 1Department of Pediatric Rheumatology and Immunology, Dongguan Children’s Hospital Affiliated to Guangdong Medical University, Dongguan, Guangdong, China
- 2Rare Diseases Diagnosis & Treatment Center, Dongguan Children’s Hospital Affiliated to Guangdong Medical University, Dongguan, Guangdong, China
- 3Accurate Diagnosis & Key Laboratory of Rare Disease in Children, Dongguan Children’s Hospital Affiliated to Guangdong Medical University, Dongguan, Guangdong, China
- 4Department of Pediatric Allergy, Immunology & Rheumatology, Women and Children’s Medical Center, Guangzhou Medical University, Center and South National Pediatric Medical Center, Guangzhou, China
Background: Chronic graft-vs.-host disease (cGVHD) is a major complication of allogeneic hematopoietic cell transplantation. It is a leading cause of long-term morbidity, non-relapse mortality, and impaired health-related quality of life. cGVHD is a multifactorial syndrome that can manifest with articular involvement. Approximately 50% of cGVHD survivors do not respond to glucocorticoid therapy used for arthritis. Subsequently, we shall present a case of a juvenile patient with arthritis and cGVHD, who responded well to intra-articular injection of tocilizumab, after bone marrow transplantation.
Case pressentation: A male adolescent with acute myeloid leukemia successfully underwent marrow stem cell transplantation. However, he developed arthritis in the elbow and knee joints and had difficulty walking more than 3 months after transplantation. He was administered anti-rejection drugs with cyclosporine, ruxolitinib, and methylprednisolone by his physician, which did not work. He was subsequently treated with intravenous tocilizumab under the supervision of his rheumatologist. Although his clinical symptoms showed remission at early stages, his knee joints were more swollen, and he could not stand after being infected with COVID-19. Both of his knee joints was injected with tocilizumab at 0, 2, 4, 6, 7, 11, and 19 weeks. Interleukin (IL)-6 levels in the peripheral blood continuously decreased. After treatment for 4 months, the patient could walk a few hundred meters with minimal exertion.
Conclusion: An intra-articular injection of tocilizumab could be a viable treatment option for arthritis; however, large-scale clinical trials are warranted to confirm its efficacy.
1 Introduction
Allogeneic hematopoietic stem cell transfer is usually the only curative treatment for hematologic malignancies. However, it is associated with an elevated risk of developing acute or chronic graft-vs.-host disease (GVHD). Acute GVHD (aGVHD) occurs early after transplantation and primarily affects the skin, liver, and gastrointestinal tract, whereas chronic GVHD (cGVHD) occurs later after transplantation and is a complex syndrome involving multiple organ damage, including joints and fascia, lungs, liver, and skin fibrosis. cGVHD largely affects the quality of life of transplant recipients (1). The pathogenesis of cGVHD encompasses a complex multistage process, initiating with inflammatory cytokine-mediated tissue injury in the initial phase, and progressing to dysregulated tissue repair and fibrosis in later stages (2). This study reports a case of a juvenile patient with arthritis and cGVHD following bone marrow transplantation. The patient displayed a good clinical response to intra-articular injection of tocilizumab.
2 Case presentation
The patient was a 12-year-old Chinese adolescent boy. He was diagnosed with acute myeloid leukemia in January 2021. He successfully underwent marrow stem cell transplantation in August 2021. Unfortunately, he experienced intestinal rejection two months post-transplantation. He received anti-rejection drugs, including cyclosporine, ruxolitinib, and methylprednisolone from his physician.
He developed arthralgia in the elbow and knee joints with difficulty walking more than 3 months after transplantation. The antinuclear antibody (ANA), ANA profile, human leukocyte antigen B27 (HLA-B27), and cyclic citrullinated peptide (CCP) were negative. The levels of rheumatoid factors (RF) were 14.9 IU/ml (reference range: <14 IU/ml). Based on clinical manifestations and imaging results, we considered that he developed cGVHD with joint involvement (3). He was treated with intravenous tocilizumab under the supervision of his rheumatologist. After 1.5 years of treatment, his symptoms of diarrhea and arthralgia gradually improved.
However, he was infected with COVID-19 in January 2022, and his knee pain recurred and became worse than before, although he was still taking prednisone orally, ruxolitinib, cyclosporine, and intravenous tocilizumab. His knee joints are more swollen, and he could not stand by himself (Figure 1a and Supplementary File: Video 1). Thereafter, an ultrasound revealed abundant collection of fluid in the knee joint cavity. Magnetic resonance imaging (MRI) revealed abnormal signals in the bone marrow of the bilateral femur, patella, upper tibia, and fibula. After over 6 months of intravenous tocilizumab (dosage: 80 mg, intravenous injection, every 4 weeks) and oral ruxolitinib (dosage: 20 mg, oral, twice one day), the swelling and pain of bilateral knees did not improve. We next performed a puncture under ultrasound positioning in the joint cavity and extracted the pale-yellow fluid, revealing a significant increase in IL-6 levels in the fluid (13,940.84 pg/ml). Biochemical examination of the joint fluid demonstrated 14,045 nucleated cells, mononuclear cells accounted for 14.9%, and multiple nucleated cells accounted for 85.1%. No microbial growth was observed in synovial fluid cultures and the erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) levels were normal. We attempted intra-articular injection of tocilizumab to treat arthritis. The off-label drug treatment was recorded by the Ethics Committee of Dongguan Children's Hospital. In addition, we described the purposes and risks of intra-articular injection of tocilizumab to the patient and his parents. We both signed the risk notification for off-label drug using. The previous regimen of intravenous tocilizumab was terminated and 40 mg of tocilizumab was injected into the cavity of each knee joint under ultrasound localization. The patient was observed in the ward for 24 h after surgery. Methylprednisolone and ruxolitinib were continued for the remainder of the treatment. The same dose and treatment method were executed at 2, 4, 6, 7, 11, and 19 weeks. The level of IL-6 in peripheral blood decreased continuously (Figure 2). The patient did not experience any discomfort during intra-articular injection. After treatment for 4 months, the patient could walk a few hundred meters with minimal exertion (Supplementary File: Video 2), and the swelling of the knee joint improved (Figure 1b).
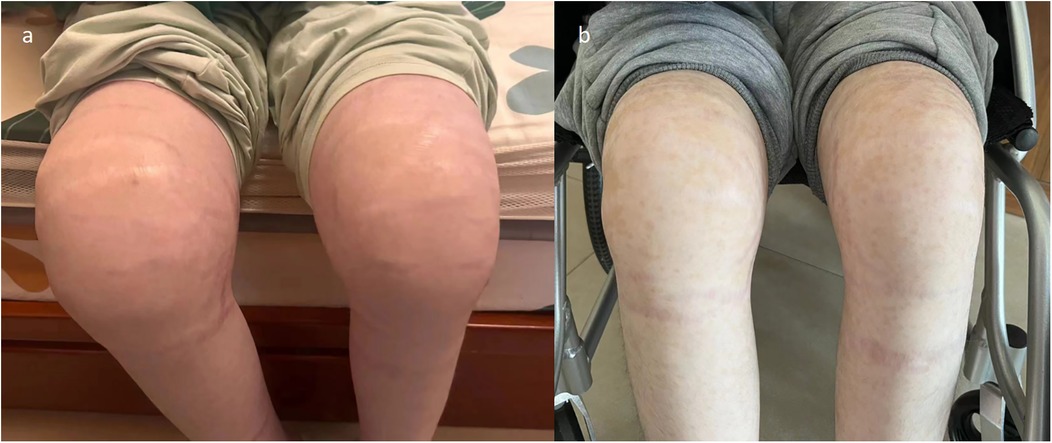
Figure 1. (a) knee joints are swollen before intra-articular injection of tocilizumab. (b) Knee joints swelling improved following intra-articular injection of tocilizumab.
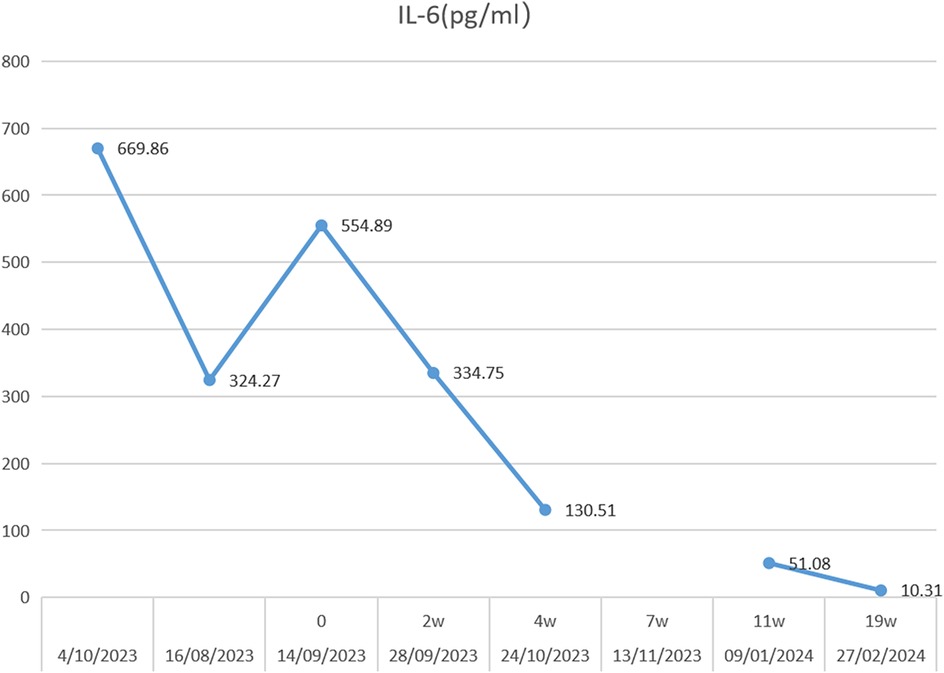
Figure 2. Serum IL-6 concentration trends during 19 weeks of intra-articular injection of tocilizumab.
3 Discussion
cGVHD majorly contributes to long-term morbidity and late mortality in allogeneic hematopoietic stem cell transplant survivors (4, 5). Patients with cGVHD show involvement of multiple organs, including the skin, mouth, eyes, a small number of joints, gastrointestinal tract, lungs, liver, and genital tract (5). An epidemiological study reported that over 40% of cGVHD survivors suffer from arthropathy (6). GVHD is essentially a syndrome of autoimmune multi-organ dysfunction caused by immunological differences between the host and the donor. The pathomechanism underlying cGVHD is not completely understood. Some theories explaining the pathophysiology of cGVHD include thymic dysfunction, regulatory T cell deficiency, autoantibody production by abnormal B cells, and the formation of profibrotic lesions (7). Arthritis due to GVHD has been speculated to have a similar principle to that of rheumatoid arthritis. Glucocorticoids remain the mainstay treatment for GVHD. However, approximately 50% of patients do not respond to glucocorticoid therapy (8). No standard second-line therapy exists for steroid-nonresponsive patients. IL-6 is a pleiotropic cytokine produced by different cell types and is elevated in the serum of patients with ongoing GVHD (9–12). Tocilizumab is a humanized anti-IL-6 receptor antibody recommended for the treatment of severe rheumatoid arthritis (13). Recent studies have reported tocilizumab for the treatment of GVHD (14–16). These studies indicated that tocilizumab could be used as a remedial measure for steroid-refractory GVHD with good clinical response. However, our case study indicated that intravenous administration of tocilizumab exhibited a poor clinical effect on cGVHD accompanied by arthritis.
Intra-articular corticosteroid injections (IACIs) are largely used to treat chronic rheumatoid arthritis and osteoarthritis of the knee. IAICs aimed at joints can significantly decrease systemic adverse reactions while still achieving local anti-inflammatory effects (17). The Clinical guidelines of the American College of Rheumatology recommend IAICs as the first-line treatment for juvenile idiopathic arthritis (JIA) affecting a few joints (17). Previous studies have reported that intra-articular injection of a tumor nuclear factor (TNF) inhibitor is an effective method for treating arthromeningitis (18). We detected a significant presence of IL-6 in the synovial fluid of the patient's articular cavity. These information provide serves as a reference for envisioning the treatment of arthritis through intra-articular injection of tocilizumab. Thus, we decided to use intra-articular injection of tocilizumab to relieve knee pain.
We administered the first dose of tocilizumab in the bilateral knee cavities under ultrasound guidance. The patient exhibited no reaction following the bilateral knee joint cavity injections. Throughout the ongoing treatment, the patient's symptoms progressively improved. Serum IL-6 levels exhibited a downward trend, joint effusion was significantly reduced compared to previous instances. In a previous study, it was found that the concentration of IL-6 in the joint synovial fluid of various types of arthritis was significantly higher than that in the serum (19). This might likely be due to IL-6 being primarily secreted by synovial fibroblasts in the synovium of arthritis patients (20). IL-6 promotes the differentiation and activation of inflammatory cells, osteoclast activation, and periarticular inflammation, often working in conjunction with other pro-inflammatory cytokines. In arthrosynovitis, IL-6 significantly amplifies inflammatory responses and triggers the production of various cytokines and chemokines by acting on monocytes in the peripheral blood and synovial fluid (21). Intra-articular injection of tocilizumab may directly neutralize interleukin-6, potentially reducing local inflammation. However, additional experiments are necessary to confirm this effect.
4 Conclusion
Our case offers a viable treatment option for intractable arthritis. Intra-articular injection of tocilizumab could be a viable treatment for monoarticular immune arthritis, although larger clinical trials are warranted to confirm its efficacy.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Ethics Committee of Dongguan Children's Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin. Written informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
MX: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Writing – original draft, Writing – review & editing. QL: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Supervision, Writing – original draft. HY: Investigation, Methodology, Project administration, Supervision, Writing – original draft. YC: Data curation, Investigation, Methodology, Supervision, Writing – original draft. XZ: Investigation, Resources, Software, Supervision, Writing – original draft. ZZ: Funding acquisition, Methodology, Resources, Supervision, Writing – original draft. BZ: Data curation, Investigation, Methodology, Resources, Software, Supervision, Writing – review & editing. HuZ: Data curation, Formal analysis, Investigation, Methodology, Resources, Software, Writing – review & editing. HaZ: Formal analysis, Investigation, Methodology, Resources, Software, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The study was supported by the Key Projects of Science and Technology Plan of Dongguan (project number: 20211800904872).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2025.1515706/full#supplementary-material
References
1. Arai S, Arora M, Wang T, Spellman SR, He W, Couriel DR, et al. Graft-vs-Host disease working committee of the CIBMTR. Increasing incidence of chronic graft-versus-host disease in allogeneic transplantation: a report from the center for international blood and marrow transplant research. Biol Blood Marrow Transplant. (2015) 21(2):266–74. doi: 10.1016/j.bbmt.2014.10.021
2. Vadakkel G, Eng S, Proli A, Ponce DM. Updates in chronic graft-versus-host disease: novel treatments and best practices in the current era. Bone Marrow Transplant. (2024) 59(10):1360–8. doi: 10.1038/s41409-024-02370-8
3. Cuvelier GDE, Schoettler M, Buxbaum NP, Pinal-Fernandez I, Schmalzing M, Distler JHW, et al. Toward a better understanding of the atypical features of chronic graft-versus-host disease: a report from the 2020 national institutes of health consensus project task force. Transplant Cell Ther. (2022) 28(8):426–45. doi: 10.1016/j.jtct.2022.05.038
4. Martin PJ, Counts GW Jr, Appelbaum FR, Lee SJ, Sanders JE, Deeg HJ, et al. Life expectancy in patients surviving more than 5 years after hematopoietic cell transplantation. J Clin Oncol. (2010) 28(6):1011–6. doi: 10.1200/JCO.2009.25.6693
5. Wingard JR, Majhail NS, Brazauskas R, Wang Z, Sobocinski KA, Jacobsohn D, et al. Long-term survival and late deaths after allogeneic hematopoietic cell transplantation. J Clin Oncol. (2011) 29(16):2230–9. doi: 10.1200/JCO.2010.33.7212
6. Hamilton BK, Storer BE, Wood WA, Pidala JA, Cutler CS, Martin PJ, et al. Disability related to chronic graft-versus-host disease. Biol Blood Marrow Transplant. (2020) 26(4):772–7. doi: 10.1016/j.bbmt.2019.10.019
7. Min CK. The pathophysiology of chronic graft-versus-host disease: the unveiling of an enigma. Korean J Hematol. (2011) 46(2):80–7. doi: 10.5045/kjh.2011.46.2.80
8. Berger M, Biasin E, Saglio F, Fagioli F. Innovative approaches to treat steroid-resistant or steroid refractory GVHD. Bone Marrow Transplant. (2008) 42(Suppl 2):S101–5. doi: 10.1038/bmt.2008.294
9. Cavet J, Dickinson AM, Norden J, Taylor PR, Jackson GH, Middleton PG. Interferon-gamma and interleukin-6 gene polymorphisms associate with graft-versus-host disease in HLA-matched sibling bone marrow transplantation. Blood. (2001) 98(5):1594–600. doi: 10.1182/blood.v98.5.1594
10. Pihusch M, Pihusch R, Fraunberger P, Pihusch V, Andreesen R, Kolb HJ, et al. Evaluation of C-reactive protein, interleukin-6, and procalcitonin levels in allogeneic hematopoietic stem cell recipients. Eur J Haematol. (2006) 76(2):93–101. doi: 10.1111/j.0902-4441.2005.00568.x
11. Chen X, Das R, Komorowski R, Beres A, Hessner MJ, Mihara M, et al. Blockade of interleukin-6 signaling augments regulatory T-cell reconstitution and attenuates the severity of graft-versus-host disease. Blood. (2009) 114(4):891–900. doi: 10.1182/blood-2009-01-197178
12. Tawara I, Koyama M, Liu C, Toubai T, Thomas D, Evers R, et al. Interleukin-6 modulates graft-versus-host responses after experimental allogeneic bone marrow transplantation. Clin Cancer Res. (2011) 17(1):77–88. doi: 10.1158/1078-0432.CCR-10-1198
13. Sanmartí R, Ruiz-Esquide V, Bastida C, Soy D. Tocilizumab in the treatment of adult rheumatoid arthritis. Immunotherapy. (2018) 10(6):447–64. doi: 10.2217/imt-2017-0173
14. Melgarejo-Ortuño A, Escudero-Vilaplana V, Revuelta-Herrero JL, Bailen R, Collado-Borrell R, Gomez-Centurión I, et al. Tocilizumab as salvage treatment of refractory pulmonary acute graft-versus-host disease. J Oncol Pharm Pract. (2021) 27(3):751–5. doi: 10.1177/1078155220948934
15. Kattner AS, Holler E, Holler B, Klobuch S, Weber D, Martinovic D, et al. IL6-receptor Antibody tocilizumab as salvage therapy in severe chronic graft-versus-host disease after allogeneic hematopoietic stem cell transplantation: a retrospective analysis. Ann Hematol. (2020) 99(4):847–53. doi: 10.1007/s00277-020-03968-w
16. Ganetsky A, Frey NV, Hexner EO, Loren AW, Gill SI, Luger SM, et al. Tocilizumab for the treatment of severe steroid-refractory acute graft-versus-host disease of the lower gastrointestinal tract. Bone Marrow Transplant. (2019) 54(2):212–7. doi: 10.1038/s41409-018-0236-z
17. Li S, Zhang W, Lin Y. Application of intra-articular corticosteroid injection in juvenile idiopathic arthritis. Front Pediatr. (2022) 10:822009. doi: 10.3389/fped.2022.822009
18. Chen Y, Yuan J, Cai Z, Ma Y. Efficacy of tumor necrosis factor inhibitor combined with intra-articular injection of triamcinolone acetonide in the treatment of refractory rheumatoid arthritis synovitis: a retrospective study. Clin Rheumatol. (2023) 42(7):1793–9. doi: 10.1007/s10067-023-06530-x
19. Mihailova A. Interleukin 6 concentration in synovial fluid of patients with inflammatory and degenerative arthritis. Curr Rheumatol Rev. (2022) 18(3):230–3. doi: 10.2174/1874471015666220128113319
20. Zhang F, Wei K, Slowikowski K, Fonseka CY, Rao DA, Kelly S, et al. Defining inflammatory cell states in rheumatoid arthritis joint synovial tissues by integrating single-cell transcriptomics and mass cytometry. Nat Immunol. (2019) 20(7):928–42. doi: 10.1038/s41590-019-0378-1
Keywords: cGVHD, tocilizumab, arthritis, intra-articular injection, Case Report
Citation: Xie M, Liu Q, Yuan H, Chen Y, Zou X, Zhang Z, Zhong B, Zeng H and Zeng H (2025) Case Report: Intra-articular injection of tocilizumab for arthritis treatment in chronic graft-vs.-host disease. Front. Pediatr. 13:1515706. doi: 10.3389/fped.2025.1515706
Received: 23 October 2024; Accepted: 17 June 2025;
Published: 2 July 2025.
Edited by:
Adriano La Vecchia, University of Milano-Bicocca, ItalyReviewed by:
Mikhail Kostik, Saint Petersburg State Pediatric Medical University, RussiaJyoti Singhal, King Edward Memorial Hospital Research Centre, India
Copyright: © 2025 Xie, Liu, Yuan, Chen, Zou, Zhang, Zhong, Zeng and Zeng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Baimao Zhong, emJvbWFvQDE2My5jb20=; Huasong Zeng, aHVhc29uZ3h1cWluZ0AxNjMuY29t; Haisheng Zeng, aGFpc2hlbmcyMDA2QDEyNi5jb20=
†These authors have contributed equally to this work
 Mingyu Xie
Mingyu Xie Qin Liu1,†
Qin Liu1,† Zhenhong Zhang
Zhenhong Zhang Huasong Zeng
Huasong Zeng