- Department of Pediatrics, Division of Endocrinology, Sheikh Khalifa Medical City (SKMC), Abu Dhabi, United Arab Emirates
Early diagnosis of the pathological cause, if any, of short stature in children can lead to prompt intervention with recombinant growth hormone (rGH) treatment, potentially allowing them to achieve their true genetic height potential. However, it is crucial to identify children most likely to benefit from rGH treatment.
Methods: This cross-sectional, retrospective study provides a broad overview of rGH prescribing patterns and evaluates both short- and long-term treatment outcomes in children treated at the Pediatric Endocrinology Clinic, Sheikh Khalifa Medical City, Abu Dhabi, UAE, between January 2011 and December 2022. One- and three-year outcome data for children treated with rGH for different diagnoses of short stature were assessed.
Results: Idiopathic short stature (ISS) accounted for 34.8% of the cases for which rGH was prescribed. A significant response [mean height gain of ≥0.3 standard deviation score (SDS)/year] was seen across all assessed short-stature diagnoses, with the highest gain seen in the growth hormone deficiency (GHD) diagnosis group at the 1-year and 3-year treatment time points. More than 90% of the children diagnosed with GHD and ISS achieved normal final adult height. Younger age at rGH initiation, lower height SDS at baseline, and pre-pubertal status were associated with better outcomes post 1 and 3 years of rGH therapy. Greater response at 1 year of rGH therapy was associated with better final adult height outcome.
Conclusions: ISS was the most common indication for which rGH was prescribed in this study. A favorable increment in the height SDS of the rGH-treated children during their 1- and 3-year follow-ups was observed. Age, pubertal status, baseline height SDS, and rGH response at 1 year were directly associated with significantly improved short- and long-term response to rGH treatment. These findings provide a broad overview of the baseline and therapeutic response characteristics of rGH-treated children with short stature in the UAE and can help in optimizing and personalizing treatment strategies.
Introduction
Short stature, defined as “a condition in which the height of an individual is two standard deviations (SD) below the corresponding mean height of a given age, sex, and population group.” (1) Differentiating between the pathological and non-pathological etiologies of short stature is the key to determining the necessity and course of treatment. In most cases, familial genetic makeup and constitutional delay in growth and puberty are responsible for short stature (2). It may also be attributed to underlying endocrine disorders like growth hormone deficiency (GHD) and hypothyroidism. Several genetic disorders leading to short stature, such as Turner syndrome (TS), Noonan syndrome, and Prader-Willi syndrome (PWS), have also been identified (1, 2).
Given the limited window during which growth occurs, early identification of the underlying conditions can facilitate timely intervention, enabling children to achieve their full genetic height potential. Initially approved for treating GHD, recombinant growth hormone (rGH) is now approved for other conditions that negatively impact growth. These include TS, PWS, chronic renal disease (CKD), small for gestational age (SGA), Noonan's Syndrome, short stature homeobox-containing gene (SHOX) deficiency, and idiopathic short stature (ISS) (3, 4).
Although rGH has been approved for several indications, its use must be carefully targeted towards those most likely to benefit. The application of rGH for treating children with ISS remains somewhat controversial (5). The diagnosis of ISS, defined as short stature in the absence of any known endocrine, systemic, genetic, or nutritional defects, is primarily by exclusion. Children diagnosed with ISS represent a genotypically and phenotypically diverse group. Furthermore, a constitutional delay of growth and puberty is often a contributing factor in the diagnosis of ISS (6).
A study by Al-Abdulrazzaq et al. analyzed the pattern of use and outcomes associated with 1 year of rGH therapy in 60 children treated at a single hospital in Kuwait. The study identified GHD as the most common indication for prescribing rGH therapy. It also showed a significant 1-year response to rGH therapy in children diagnosed with GHD, SGA, and TS (and variants), but the response was not statistically significant in children diagnosed with ISS (7).
Our study aimed to get an overview of the rGH prescribing pattern and assess the short-term and long-term therapy outcomes among children treated with rGH at the Pediatric Endocrinology Clinic at our center, SKMC, Abu Dhabi, in UAE. Our primary objectives were to (1) ascertain the main indications for which rGH is prescribed, (2) determine the mean age at which rGH treatment is initiated and compare it with international recommendations, and (3) assess the short-term rGH treatment response, (measured as height gained after 1 and 3 years of therapy). The secondary objectives of this study were to (1) assess the long-term rGH treatment response upon reaching final adult height (FAH) and (2) investigate the potential predictors for favorable outcomes at 1 and 3 years of therapy.
Methods
This cross-sectional retrospective study assessed the short—and long-term outcomes in children treated with rGH at the Pediatric Endocrine Clinic, Sheikh Khalifa Medical City (SKMC), Abu Dhabi, UAE, between January 2011 and December 2022.
Inclusion and exclusion criteria
The inclusion criteria were children diagnosed with short stature and the availability of their 1-year and 3-year rGH treatment data. Children with chronic and active use of systemic steroids at baseline and those with missing baseline or missing 1-year treatment follow-up data were excluded from this analysis.
GHD diagnosis
A diagnosis of GHD was established if a patient failed to pass at least one of two GH stimulation tests (combined sequential same-day tests: Clonidine/Arginine). Additional indicators included slow growth velocity for age, delayed bone age (BA), and low or low-normal insulin-like growth factor (IGF)-1 levels. Sex steroid priming was not performed before GH stimulation in this study. GHD was confirmed if the peak GH level was <7 ng/ml. The stringent cut-off of <7 ng/ml was chosen due to the use of advanced immunochemiluminescent and newer monoclonal-based assays at our center.
An MRI of the brain was conducted for all children diagnosed with GHD before initiating GH therapy. While most children with constitutional delays in growth and puberty exhibited normal growth velocity and strong family history indicators, GH stimulation was performed in those with subnormal growth velocity and/or severe short stature to rule out GHD.
Relevant data such as patient demographics, anthropometric measurements [height standard deviation scores (SDS), BMI SDS, growth velocity], puberty status, bone age (BA) at baseline, indications for rGH administration and doses administered, mid-parental height (MPH) and FAH were extracted from the electronic medical records and analyzed statistically.
The study was approved by the ethical committee of Sheikh Khalifa Medical City, Abu Dhabi, UAE. Statistical analysis of the data was conducted using Stata version 17, StataCorp LLC, Texas, USA.
The short-term response of the rGH therapy was evaluated using Bang and Ranke criteria by calculating the difference between the height SDS at 1 year or 3 years and the height SDS at baseline. A height gain of ≥ 0.3 SDS per year was considered a “good” response (5, 8).
The long-term response of rGH therapy was assessed from the FAH SDS of the study participants. An FAH SDS of ≥−2 (corresponding to a height of ≥ 162.5 cm in males and ≥ 150.3 cm in females according to CDC estimates) was categorized as a “good/acceptable” response, and an FAH SDS of <−2 was graded as a suboptimal ‘ response. Participants achieving an FAH of ≥ −2 SDS were categorized as having ‘normal’ FAH.
For statistical inferences, the Stata 18 packages were used (StataCorp, College Station, Texas, USA). Mean, median, and standard deviations were calculated using the package modules. Discrete variables were tested using Pearson's Chi-Square, and continuous variables were compared using a t-test. Uni- and multivariable analytics were performed using the same package, setting the p-value at 0.05 significance.
Results
Baseline characteristics and mean age at rGH treatment initiation
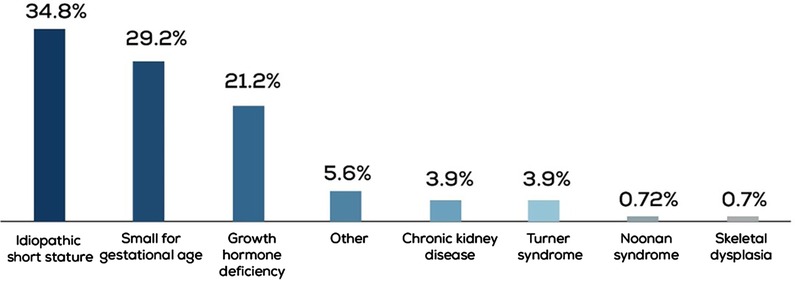
Data from 414 children treated for short stature between 2011 and 2022 were obtained. ISS was the most common diagnosis in 34.8% of the cases, followed by SGA and GHD in 29.2% and 21.2% of the cases, respectively (Figure 1). In the GHD cohort, 27.7% (n = 23) of patients had a peak GH level of <5 ng/ml.
Of the 414 children screened, 394 were identified as rGH-treatment naïve at baseline. Based on the inclusion criteria, 370 rGH-treated children were selected for further analysis. The children were stratified based on their diagnosis (GHD, ISS, SGA, TS, or CKD). Their auxological characteristics at baseline are shown in Table 1. The majority of the children diagnosed with Turner syndrome (TS) had a 45, X karyotype (n = 8), while four had an isochromosome, and the remaining four exhibited mosaicism. Children diagnosed with Prader-Willi syndrome, Kabuki syndrome, and Silver-Russel syndrome were categorized as “Other.”
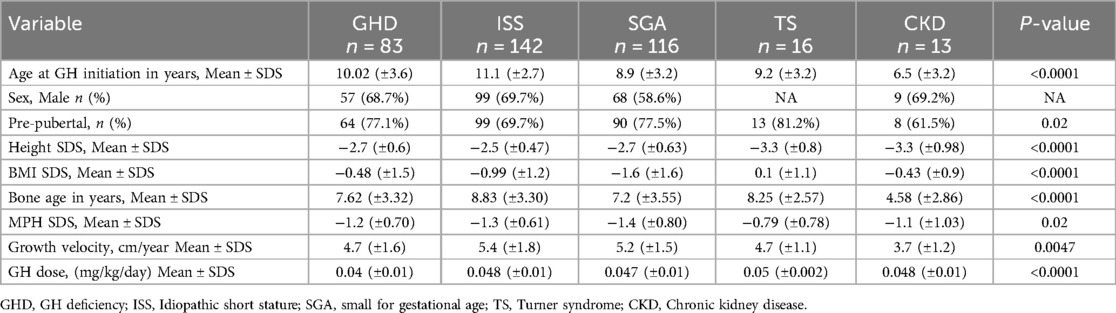
Table 1. Auxological characteristic of the study population at baseline based on diagnosis (n = 370).
The mean age at which rGH therapy was initiated varied according to the diagnosis (Figure 2). The rGH therapy was started earlier, at a mean age of 6.5 years in the CKD group, and much later in the ISS group at a mean age of 11.1 years. However, it should be noted that compared to 142 children in the ISS diagnosis group, the CKD cohort had only 13 children. For GHD, SGA, and TS, the mean age for rGH therapy initiation ranged between 8 and 10 years.
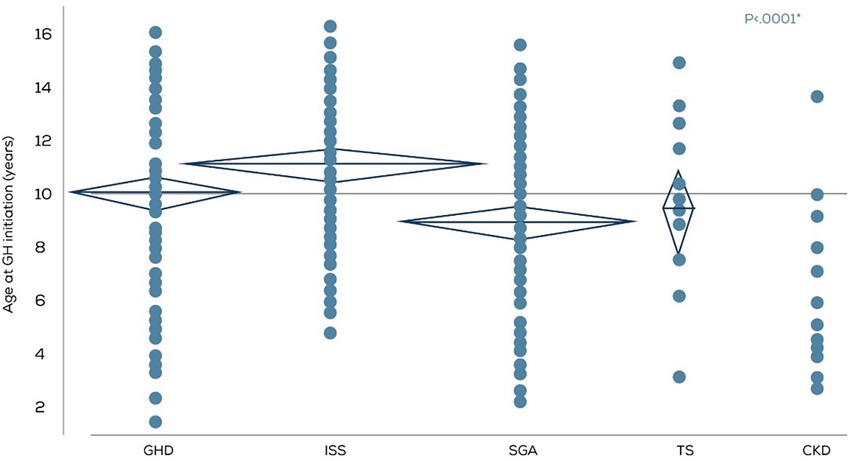
Figure 2. Age in years at rGH therapy initiation according to the diagnosis (n = 370). GHD, GH deficiency; ISS, Idiopathic short stature; SGA, small for gestational age; TS, Turner syndrome; CKD, Chronic kidney disease.
Short-term rGH response
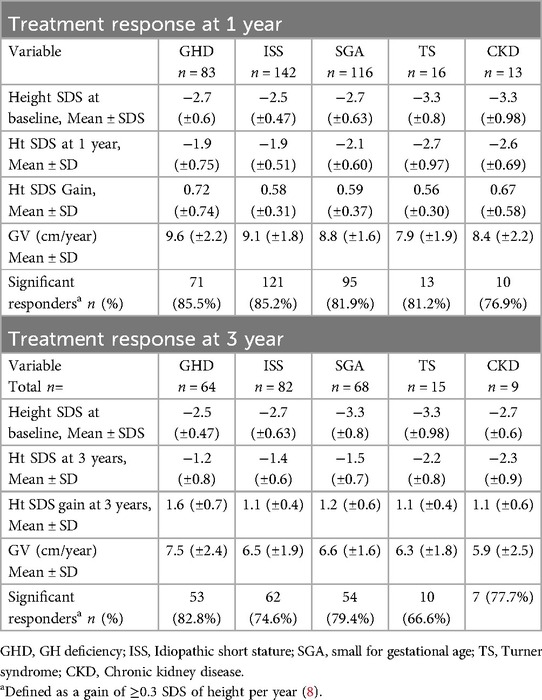
Short-term rGH response was evaluated by calculating the height gained after 1 year and 3 years of therapy (Table 2 and Figure 3). A significant response, defined as a mean height gain of ≥0.3 SDS/year, was seen across all diagnoses, with the highest gain seen in the GHD group at both the 1-year and 3-year treatment time points. Similarly, the mean growth velocity (cm/year) was the highest in the GHD group compared to other groups at both the tested time points. In this context, it should be noted that the number of children diagnosed with TS and CKD used for this analysis was relatively lower than those diagnosed with GHD, ISS, and SGA.

Figure 3. Height SDS at baseline, year-1 and year-3 of rGH therapy. GHD, GH deficiency; ISS, Idiopathic Short stature; SGA, small for gestational age; TS, Turner syndrome CKD, Chronic kidney disease.
Long-term rGH treatment response
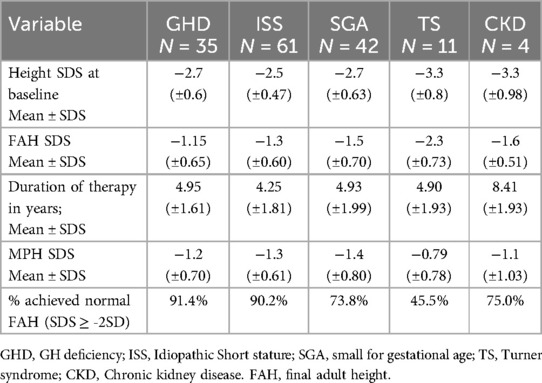
The final adult height outcomes of 153 out of 370 treated children were available and used for this analysis (Table 3 and Figure 4). The mean FAH SDS ≥ −2, which was considered a good/acceptable response, was seen in children with all diagnoses except TS. Moreover, more than 90% of the children diagnosed with GHD and ISS achieved normal FAH (SDS ≥ −2).
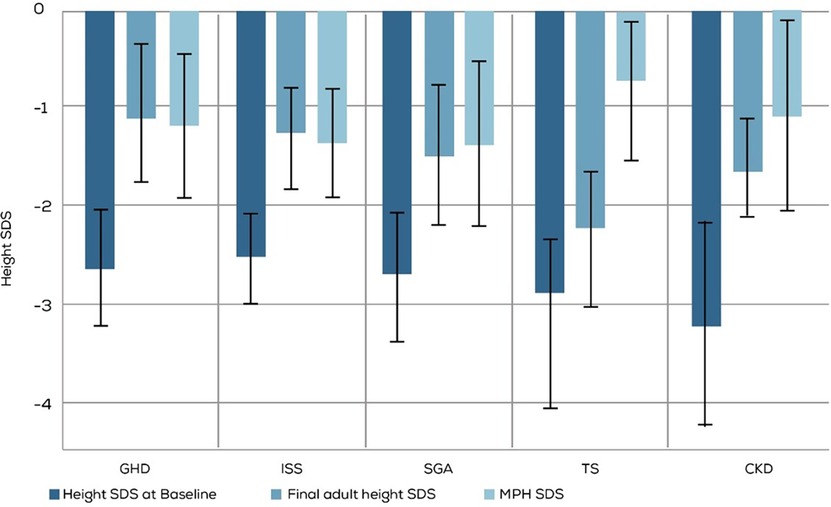
Figure 4. Comparison of baseline height SDS and MPH SDS with long-term rGH treatment outcomes assessed by FAH SDS. GHD, GH deficiency; ISS, Idiopathic Short stature; SGA, small for gestational age; TS, Turner syndrome; CKD, Chronic kidney disease. MPH, mid parenteral height.
Clinical predictors of improved short-term and long-term rGH treatment response
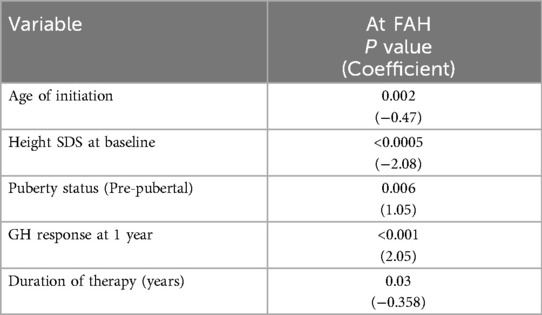
Multiple parameters — diagnosis/indication, age at rGH therapy initiation, rGH response post-1-year treatment, gender, puberty status at baseline, height SDS at baseline, BMI SDS at baseline, bone age (BA) status, and MPH SDS — were evaluated to identify potential predictors of improved response to rGH therapy. Among the tested parameters, younger age at rGH initiation, lower height SDS at baseline, and pre-pubertal status were associated with better outcome to rGH therapy (Table 4). Apart from these factors, a greater response after 1 year of rGH therapy and duration of therapy were associated with a better FAH outcome (Table 5).
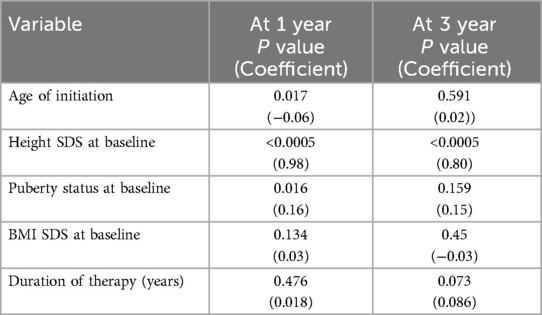
Table 4. Multi-regression analysis: clinical predictors for a significant response post 1 and 3 years of therapy.
Discussion
Significant heterogeneity exists in regulatory approvals of growth hormone replacement therapy in different countries, influencing physician prescribing patterns. Also, the variation in the quality of primary care and availability of genetic testing affect diagnoses for short stature and subsequent referrals to secondary care. These differences were highlighted by the two large studies from Europe and the US — NordiNet IOS (a multicenter, longitudinal, observational cohort study on data collected on Norditropin® using a web-based system) and ANSWER (a post-marketing registry of Norditropin® that was later converted to a noninterventional observation study). Both studies identified GHD as the most common indication for prescribing Norditropin®. SGA, TS, and ISS were the other indications for which Norditropin® was commonly prescribed, with SGA being more common than ISS in the European NordiNet IOS and ISS being more common than SGA in the US-based ANSWER study (9).
In our pediatric endocrinology clinic, ISS was the most common indication for rGH therapy, followed by SGA and GHD (Figure 5). ISS represents a heterogeneous group of patients, potentially involving underlying genetic etiologies that remain yet to be identified. Cultural factors, such as a high incidence of consanguinity and the distinct genetic profile of the region, may contribute to the increased prevalence of ISS diagnoses compared to GHD. Furthermore, the availability of comprehensive healthcare services and broad insurance coverage may facilitate earlier identification and management of ISS. This reflects a systemic pattern rather than a limitation, supporting the validity and relevance of our findings within our healthcare setting.
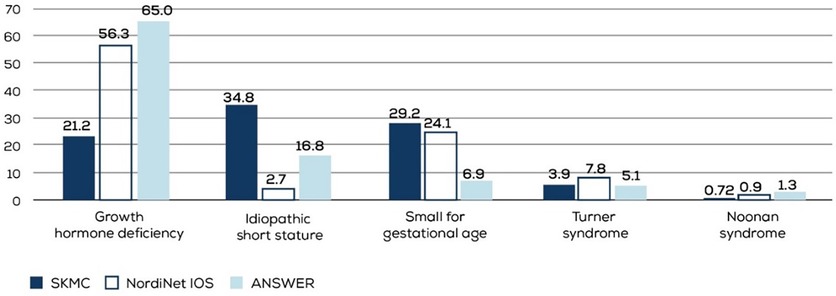
Figure 5. Indications for which rGH was commonly prescribed in the NordiNet IOS, ANSWER, and our study populations. SKMC, Sheikh Khalifa Medical City; NordiNet IOS, NordiNet International Outcome Study; ANSWER, American Norditropin Studies, Web-Enabled Research Program.
Confirming several observations from previous studies and those from the NordiNet IOS and ANSWER (9), we found more boys in our study population across all diagnoses. This gender imbalance is probably due to sociocultural pressure leading to more short-statured boys than girls being referred to specialists.
Most patients, over 75% of those diagnosed with GHD, SGA, and TS and over 65% of those diagnosed with ISS, were pre-pubertal when rGH therapy was initiated. The mean age at rGH initiation was the highest in the ISS diagnosis group. Height velocity in healthy children is age-dependent, and studies have shown children are more likely to achieve higher height velocity in the first year of rGH therapy if it is started at a young age (10). A study by Ranke et al. to identify factors likely to affect rGH outcome in children diagnosed with ISS found that younger age was associated with better first-year response to treatment (11). Age at which rGH therapy is initiated has also been shown to favorably affect growth response in children diagnosed with GHD, SGA, and TS (12).
The mean age of rGH therapy initiation across the four major diagnosis groups (GHD, SGA, TS, and ISS) was similar between our study population and the NordiNet IOS and ANSWER study populations (Figure 6) (9). However, the age at which rGH therapy was initiated is much higher than that recommended for SGA (>2 years in the US, > 3 years in Japan, and >4 years in Europe) and TS (4–6 years) (13, 14). A survey of 450 pediatricians and family medicine physicians from the Arabian Gulf region (KSA, UAE, Kuwait, Oman, Qatar, and Bahrain) has identified several gaps in short stature assessment, leading to delayed referrals (15). Reasons contributing to such sub-optimal diagnostic and referral processes need to be investigated. Additionally, steps must be taken to ensure children are appropriately and timely diagnosed, referred, and treated.
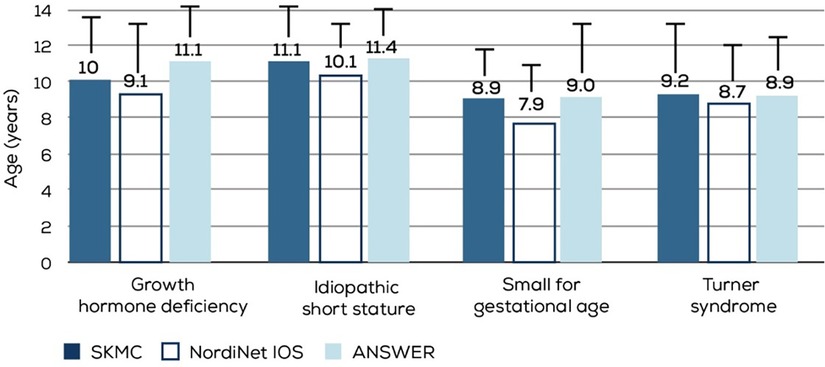
Figure 6. Comparison of mean age at rGH initiation in different diagnosis groups from the NordiNet IOS, ANSWER, and our study populations. SKMC, sheikh khalifa medical city; nordiNet IOS, NordiNet international outcome study; ANSWER, American Norditropin studies, Web-enabled research program.
At 1-year and 3-year follow-ups, the mean height increment in all the diagnosis groups was ≥0.3 SDS, which qualified as a good response. Moreover, more than 80% of the children with all diagnoses except CKD showed a significant response to rGH treatment after 1 year, and almost 75% of the children with all diagnoses except TS showed a significant response to rGH treatment after 3 years of treatment. The highest response to rGH treatment after 1 and 3 years was seen in the children diagnosed with GHD, hinting that rGH may be more effective in GHD conditions than non-GHD conditions. Studies have also shown that children with severe GHD respond better to rGH than those with milder deficiency (5). Whether a similar trend exists in our study population needs further evaluation.
The target mean FAH of more than −2 SDS was observed in all the diagnosis groups except TS. Less than half of the children with TS reached the target FAH SDS. Also, a greater percentage of children diagnosed with GHD and ISS than those diagnosed with SGA and CKD achieved the target FAH SDS. Multiple factors like the dose, age at which therapy is initiated, and its duration affect growth response to rGH in TS. Favorable growth outcomes are observed when rGH therapy is initiated before the age of 4 years (5). Apart from other factors, the sub-optimal response to rGH in TS observed in our study could be because the therapy was initiated late; the mean age of therapy initiation in our study was 9.2 years.
Growth disorders manifest in a continuum, ranging from severe to mild GH deficiency at one end to mild to severe GH insensitivity at the other, with short stature due to idiopathic etiologies lying somewhere in between (16). Thus, it is not surprising to observe substantial variability in response to rGH therapy. The pleiotropic effects of the growth hormone on various physiological processes also add to rGH response variability. Although challenging, it is vital to identify parameters that can predict favorable outcomes and assist in making critical therapy-related decisions, like how long to continue treatment and dosage to use.
Previous studies have identified several predictors for favorable rGH response in children diagnosed with ISS, such as first-year growth response, younger age at therapy initiation, the difference between baseline and target height, and GH dose (17). An analysis of the data from the ANSWER program registry identified height velocity at 4 months, baseline age, baseline height SDS, baseline BMI SDS, and baseline IGF-I SDS as significant predictive factors for rGH response in children diagnosed with GHD (17).
Clinical predictors for a significant short-term (after 1 and 3 years of therapy) rGH response across all diagnoses in our study were — the age at rGH therapy initiation, height SDS at baseline, and puberty status at baseline. The younger the age at rGH therapy initiation and pre-pubertal status were associated with a significantly greater response to therapy. In addition to the above factors, the rGH response at 1 year and duration of therapy were also found to predict long-term response to therapy. Other clinical parameters that were evaluated — the indication for which rGH was prescribed, gender, BA status, and MPH SDS — did not influence short-term or long-term treatment response in our cohort.
Conclusions
Compared to GHD reported in most other studies, ISS was the most common indication for which rGH was prescribed at our center. Irrespective of the diagnosis, we noticed a favorable increment in the height SDS of the rGH-treated children during their 1- and 3-year follow-ups. Age, pubertal status, baseline height SDS, and rGH response at 1 year were directly associated with significantly improved short- and long-term response to treatment.
The strengths of this study include a relatively large sample size and long-term follow-up through to adult height. While this study provides valuable insights into rGH prescribing patterns and treatment outcomes, limitations and some potential biases and confounders must be considered. As this is a single-center retrospective study, the results might not necessarily be applicable to be generalized to a larger scale, which represents a key limitation.
Referral and diagnosis biases may have played a role, as most children were referred by a primary care physician who was aware of the center's treatment options or by a family/friend member who experienced a similar condition. In such cases, many other short stature cases might be overlooked if they happen to be in a limited-resource area. Additionally, age at rGH therapy initiation and pubertal status significantly impact growth outcomes, making it difficult to determine the true effect of rGH across different diagnosis groups.
Lastly, long-term outcomes were assessed in a limited subset of patients, necessitating further research with larger, multicenter cohorts to validate these findings and improve our understanding of rGH treatment effectiveness in children with short stature. Although additional studies are required to further validate and extend our findings, they provide a broad overview of the baseline and therapeutic response characteristics of rGH-treated children with short stature in the UAE. Closing the gaps in the diagnosis and referral processes and identifying children most likely to respond to rGH therapy using clinical predictors can help optimize outcomes in rGH-eligible children from the UAE. Moreover, existing tools, such as digitally generated adherence reports, could be utilized to evaluate rGH therapy outcomes, offering real-world insights to help personalize dosing and treatment strategies.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Ethics committee approval number: REC-31.01.2023 [RS-795]. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin. Written informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
SAJ: Conceptualization, Data curation, Formal analysis, Project administration, Supervision, Writing – original draft, Writing – review & editing. FT: Writing – review & editing. MH: Writing – review & editing. ZA: Writing – review & editing. SW: Writing – review & editing. JA: Writing – review & editing. SAR: Conceptualization, Data curation, Formal analysis, Project administration, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This publication received funding from Merck Serono Middle East FZ-LTD. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication. This publication is done via an unrestricted medical writing grant by Merck Serono Middle East FZ-LTD, an affiliate of Merck KGaA, Darmstadt, Germany.
Acknowledgments
Dr. Preeti Nerurkar from Medcytes, Dubai, provided medical writing services for the development of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Rani D, Shrestha R, Kanchan T, Krishan K. Short Stature. Treasure Island (FL): StatPearls Publishing. (2023).
2. Barstow C, Rerucha C. Evaluation of short and tall stature in children. Am Fam Physician. (2015) 92(1):43–50.26132126
3. Ben-Ari T, Chodick G, Shalev V, Goldstein D, Gomez R, Landau Z. Real-world treatment patterns and outcomes of growth hormone treatment among children in Israel over the past decade (2004–2015). Front Pediatr. (2021) 9:711979. doi: 10.3389/fped.2021.711979
4. Kemp S, Frindik JP. Emerging options in growth hormone therapy: an update. Drug Des Devel Ther. Published online August. (2011) 5:411–9. doi: 10.2147/DDDT.S23140
5. Al Herbish AS, Almutair A, Bin Abbas B, Alsagheir A, Alqahtani M, Kaplan W, et al. Diagnosis and management of growth disorders in gulf cooperation council (GCC) countries: current procedures and key recommendations for best practice. Int J Pediatr Adolesc Med. (2016) 3(3):91–102. doi: 10.1016/j.ijpam.2016.07.002
6. Inzaghi E, Reiter E, Cianfarani S. The challenge of defining and investigating the causes of idiopathic short stature and finding an effective therapy. Horm Res Paediatr. (2019) 92(2):71–83. doi: 10.1159/000502901
7. Al-Abdulrazzaq D, Al-Taiar A, Hassan K, Al-Basari I. Recombinant growth hormone therapy in children with short stature in Kuwait: a cross-sectional study of use and treatment outcomes. BMC Endocr Disord. (2015) 15(1):76. doi: 10.1186/s12902-015-0073-7
8. Ranke MB, Lindberg A. Observed and predicted growth responses in pre-pubertal children with growth disorders: guidance of growth hormone treatment by empirical variables. J Clin Endocrinol Metab. (2010) 95(3):1229–37. doi: 10.1210/jc.2009-1471
9. Sävendahl L, Polak M, Backeljauw P, Blair J, Miller B, Rohrer T, et al. Treatment of children with GH in the United States and Europe: long-term follow-up from NordiNet® IOS and ANSWER program. J Clin Endocrinol Metab. (2019) 104(10):4730–42. doi: 10.1210/jc.2019-00775
10. Bang P, Ahmed SF, Argente J, Backeljauw P, Bettendorf M, Bona G, et al. Identification and management of poor response to growth-promoting therapy in children with short stature. Clin Endocrinol (Oxf). (2012) 77(2):169–81. doi: 10.1111/j.1365-2265.2012.04420.x
11. Ranke MB, Lindberg A, Price DA, Darendeliler F, Albertsson-Wikland K, Wilton P, et al. Age at growth hormone therapy start and first-year responsiveness to growth hormone are Major determinants of height outcome in idiopathic short stature. Horm Res Paediatr. (2007) 68(2):53–62. doi: 10.1159/000098707
12. Wit JM, Deeb A, Bin-Abbas B, Al Mutair A, Koledova E, Savage MO. Achieving optimal short- and long-term responses to paediatric growth hormone therapy. J Clin Res Pediatr Endocrinol. (2019) 11(4):329–40. doi: 10.4274/jcrpe.galenos.2019.2019.0088
13. Gravholt CH, Andersen NH, Conway GS, Dekkers OM, Geffner ME, Klein KO, et al. Clinical practice guidelines for the care of girls and women with turner syndrome: proceedings from the 2016 cincinnati international turner syndrome meeting. Eur J Endocrinol. (2017) 177(3):G1–G70. doi: 10.1530/EJE-17-0430
14. Hokken-Koelega ACS, van der Steen M, Boguszewski MCS, Cianfarani S, Dahlgren J, Horikawa R, et al. International consensus guideline on small for gestational age: etiology and management from infancy to early adulthood. Endocr Rev. (2023) 44(3):539–65. doi: 10.1210/endrev/bnad002
15. Kaplan W, Al Amiri E, Attia N, Al Basiri I, Romany I, Al Shehri E, et al. Assessment and referral of patients with short stature by primary care physicians in the Arabian gulf region: current perspectives from a regional survey. Front Pediatr. (2022) 10:988614. doi: 10.3389/fped.2022.988614
16. Savage MO, Storr HL, Backeljauw PF. The continuum between GH deficiency and GH insensitivity in children. Rev Endocr Metab Disord. (2021) 22(1):91–9. doi: 10.1007/s11154-020-09590-5
Keywords: growth hormone, short stature, recombinant, children, deficiency, endocrine
Citation: Al Jneibi SS, Taha F, Hammouri M, Allami Z, Weber S, Aljubeh J and Al Remeithi S (2025) Recombinant growth hormone therapy in children with short stature in Abu Dhabi: a cross-sectional study of indications and treatment outcomes. Front. Pediatr. 13:1516967. doi: 10.3389/fped.2025.1516967
Received: 25 October 2024; Accepted: 5 May 2025;
Published: 20 May 2025.
Edited by:
Eli Hershkovitz, Soroka Medical Center, IsraelReviewed by:
Chiara Guzzetti, Binaghi Hospital, ItalyTiago Jeronimo Dos Santos, University of Almeria, Spain
Copyright: © 2025 Al Jneibi, Taha, Hammouri, Allami, Weber, Aljubeh and Al Remeithi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sara Salem Al Jneibi, c2FyYS5zLmoyMkBnbWFpbC5jb20=
 Sara Salem Al Jneibi
Sara Salem Al Jneibi Fatima Taha
Fatima Taha Marwa Hammouri
Marwa Hammouri Jamal Aljubeh
Jamal Aljubeh