- 1Maternal and Child Health Hospital of Hubei Province, Wuhan, China
- 2Hubei University of Chinese Medicine, Wuhan, China
- 3Hubei Provincial Hospital of Traditional Chinese Medicine, Wuhan, China
- 4Hubei Shizhen Laboratory, Wuhan, China
- 5Affiliated Hospital of Hubei University of Chinese Medicine, Wuhan, China
Background: We aimed to explore the duration of IgM antibodies against Mycoplasma pneumoniae.
Methods: Data from two children who consistently tested positive for M. pneumoniae IgM antibodies were retrospectively analyzed. Moreover, we examined the etiological data and drug use of these cases. Serologic testing using the colloidal gold method, direct chemiluminescence technique, and specific immune agglutination test were utilized. Quantitative PCR was used to detect M. pneumoniae in bronchoalveolar lavage fluid and antigen tests and nucleic acid detection were conducted for other respiratory pathogens.
Results: The serological positivity of M. pneumoniae IgM antibody persisted for nearly ten months in one child and more than fifteen months in the other child. Furthermore, the persistently positive M. pneumoniae IgM antibody tests led to the inappropriate use of macrolides during multiple hospitalizations.
Conclusions: IgM antibodies against M. pneumoniae may remain positive for an extended duration. Therefore, a positive Mycoplasma pneumoniae IgM test does not necessarily indicate the presence of an acute infection.
1 Introduction
Mycoplasma pneumoniae is known to cause upper and lower respiratory tract infections in humans, particularly in children. Previous studies have indicated that M. pneumoniae accounts for 7% to 20% of cases of community-acquired pneumonia among children aged three to fifteen years, with outbreaks occurring globally every three to four years (1). In recent years, the incidence of mycoplasma pneumoniae pneumonia among children in China has been on the rise (2). Mycoplasma pneumoniae can also result in bronchial remodeling changes, atelectasis, destroyed lung (3), chronic interstitial fibrosis, bronchiolitis obliterans and bronchiectasis (4), as well as extrapulmonary complications such as myocarditis, pericarditis, nephritis, and meningitis (5). Due to the poor immune response and safety issues, there is no effective vaccine for mycoplasma infection currently (6). Serological measurement of IgM antibody levels is one of the methods employed for the clinical diagnosis of M. pneumoniae infection. Generally, compared to IgG, which indicates a chronic disease or a prior infection, IgM suggests a recent and acute infection (7). Therefore, many researchers utilize a single IgM level, sometimes combined with polymerase chain reaction (PCR), to diagnose acute infection (8–12). Studies have demonstrated that positive IgM antibody results can persist for weeks, months, or even longer after an M. pneumoniae infection (13). In China, a large-sample study showed that the age distribution of 70,259 children positive for MP-IgM antibody was as follows: preschool group (43.15%)> school-age group (29.80%)> preschool group (22.92%)> infant group (4.13%) (14). However, there have been rare case reports about persistently positive M. pneumoniae IgM antibody titers. Although IgM tests are clinically useful, they can result in misdiagnoses and inappropriate treatment (15). Notably, a positive M. pneumoniae IgM antibody test often leads to the misuse of macrolide antibiotics. Recently, the resistance of M. pneumoniae to macrolides has continuously increased. It is reported that the region with the highest proportion of macrolide-resistant Mycoplasma pneumoniae (MRMP) infection is the Western Pacific region, with a proportion of 53.4% (16). In China, the highest prevalence of MRMP infection was 97.4% in 2019 (17). It is considered an important cause of severe and refractory pneumonia caused by M. pneumoniae in some reports (18, 19).
In this study, we aimed to provide a deeper understanding of the clinical importance of positive M. pneumoniae IgM antibody titers by conducting a systematic review of data from the multiple hospitalizations of two children who consistently tested positive for IgM antibodies against M. pneumoniae.
2 Materials and methods
2.1 Study subjects
This study retrospectively collected the medical records of two children with persistent positive IgM antibodies against M. pneumoniae at our hospital and other hospitals from 2018 to 2023. We compared and serially analyzed pathogen detection data and drug use.
2.2 Pathogen detection method
The detection methods used for the etiological tests in this study are described below.
2.2.1 Serologic testing for Mycoplasma pneumoniae
IgM antibodies against M. pneumoniae were qualitatively measured using the colloidal gold method, following the instructions provided by the manufacturer (Sangon Biotech Co., Ltd., Shanghai, China). The sensitivity and specificity were 97.4% and 100.0% respectively (20). The result was defined as positive when both the detection and control lines displayed color. The result was considered negative when only the control line displayed color, or invalid if only the detection line displayed color. The test was repeated for invalid results.
Quantitative detection of IgM and IgG antibodies against M. pneumoniae was conducted using the direct chemiluminescence technique. The kits used in this study were provided by Shenzhen Yahui Long Biological Technology Co., Ltd. (Shenzhen, Guangdong, China). The procedures strictly followed the instructions provided. Samples with IgG concentration >36.0 Au/ml and IgM cutoff index (COI) >1.1 were considered positive.
A specific immune agglutination test (SERODIA-MYCO II Kit; Fujirebio Co., Ltd., Tokyo, Japan) was used to detect trace peripheral serum IgM antibodies against M. pneumoniae following the manufacturer's instructions. A positive result was defined as a titer of M. pneumoniae antibodies greater than 1:80.
2.2.2 Quantitative polymerase chain reaction assay for the nucleic acids of Mycoplasma pneumoniae
Bronchoalveolar lavage fluid (BALF) samples were analyzed using the M. pneumoniae nucleic acid quantitative detection kit (PCR fluorescent probe assay; DaAn Gene Co., Ltd., Guangzhou, Guangdong, China), following the instructions provided by the manufacturer. The linear range for quantitative detection is 400–4 × 109 copies/ml. A result greater than 400 copies/ml is defined as positive.
2.2.3 Antigen tests for seven respiratory viruses
Antigens for seven respiratory viruses, including adenovirus, respiratory syncytial virus (RSV), influenza A (InfA); influenza B (InfB), and parainfluenza types 1, 2, and 3, were tested using direct immunofluorescence. Fluorescently labeled monoclonal antibodies were visualized using a fluorescence microscope. The reagents used for this analysis were provided by Shanghai B&C Biological Technology Co., Ltd. (Shanghai, China), and the instructions for the tests were strictly followed.
2.2.4 Multiplex detection of nucleic acids from 13 respiratory pathogens
Various strains of InfA, influenza A H1N1 (InfA H1N1), InfA H3N2, InfB, human parainfluenza, human adenovirus, human bocavirus, human rhinovirus (HRV), human metapneumovirus, human coronavirus (HCoV), human respiratory syncytial virus (HRSV), Chlamydia pneumoniae, and M. pneumoniae were measured using primers that were included in the nucleic acid detection kit (Ningbo HEALTH Gene Technologies Co., Ltd. Zhejiang, China). The multiplex PCR system and procedures were conducted based on the instructions recommended by the manufacturer.
2.2.5 Next-generation sequencing
Nasopharyngeal swabs and BALF samples were sent to a third-party testing facility for targeted next-generation sequencing (tNGS) (KingMed Diagnostics Group Co., Ltd., Guangzhou, Guangdong, China). In addition, pathogen metagenomic next-generation sequencing (mNGS) was done by Vision Medicals Co., Ltd. (Guangzhou, Guangdong, China).
2.3 Statistical analysis
Pathogen detection data and drug use were comprehensively analyzed.
3 Results
3.1 Case 1
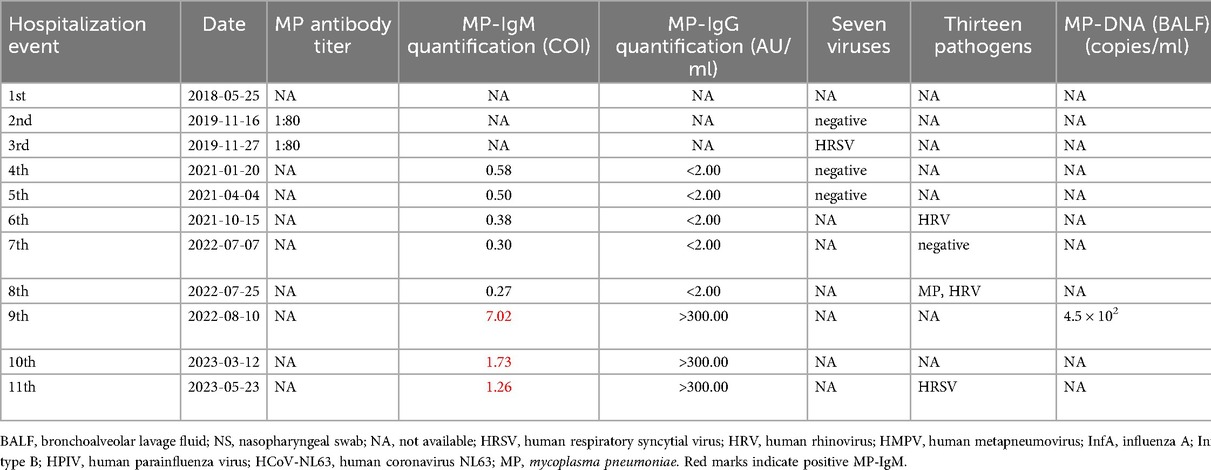
The first case was a girl who was born on August 24, 2017, through a G1P1 term Cesarean section with a birth weight of 2.7 kg. There was no history of familial genetic diseases, and her growth and development were normal. However, the child was hospitalized 11 times due to recurrent pulmonary infections. The relevant pathogenic detection data and drug use information are shown in Table 1.
In this case, we observed a gradual decrease in the serological levels of IgM antibodies against M. pneumoniae from August 10, 2022 (COI 7.02) to May 23, 2023 (COI 1.26). Despite having only one mycoplasma infection, the tests for M. pneumoniae IgM antibodies remained positive for nearly ten months. In six out of the eleven hospitalizations, macrolide antibiotics were administered.
Medication analysis revealed that the M. pneumoniae antibody titer was 1:80 during the third hospitalization, and macrolide antibiotics were prescribed. However, the tests for seven respiratory viruses suggested HRSV infection. During the fourth hospitalization, the M. pneumoniae IgM antibody titer revealed a COI of 0.58, and macrolide antibiotics were administered. In the seventh hospitalization, analysis of the BALF indicated that the sequence count of M. pneumoniae DNA was 19. In the eighth hospitalization, multiplex nucleic acid tests for 13 respiratory pathogens revealed a positive result for M. pneumoniae and the sequence count of M. pneumoniae DNA was 61,352 on tNGS. In the ninth hospitalization, the M. pneumoniae IgM antibody titer exhibited a 7.02 COI, the BALF M. pneumoniae DNA was positive at 4.5 × 102 copies/ml, and the tNGS revealed a sequence count of M. pneumoniae DNA of 534. During the 10th hospitalization, the M. pneumoniae IgM antibody titer was 1.73 COI, and macrolide antibiotics were administered. Although tNGS detected InfA H1N1 but not M. pneumoniae.
3.2 Case 2
Case 2 was a girl who was born on February 12, 2016. She was the firstborn of his parents delivered at full term. She weighed 3.45 kg at birth. She had no history of familial genetic diseases, and her growth and development were normal. The child was hospitalized six times due to medical problems, such as plastic bronchitis, bronchial occlusion, and atelectasis secondary to mycoplasma infection. Table 2 shows pathogen detection data and medication information.
In case 2, the qualitative serological tests for M. pneumoniae IgM antibodies showed positive results from February 21, 2022, to May 30, 2023 (i.e., more than 15 months). The M. pneumoniae IgM antibody titers gradually decreased from a COI of 6.33 on March 29, 2022, to a COI of 2.44 on May 30, 2023. These findings indicated a single M. pneumoniae infection. During the six hospitalizations, macrolide antibiotics were prescribed five times.
The reason for the first and second hospitalizations was a recurring condition. The child was readmitted with a fever five days after the first hospitalization. During the second hospitalization, the pulmonary CT scan revealed infections in the right middle lobe and the left lingual lobe, accompanied by localized atelectasis in the right middle lobe. Moreover, the lesion area had expanded compared to the previous pulmonary CT findings during the first hospitalization. Qualitative tests for M. pneumoniae IgM antibodies were positive on both occasions, suggesting a mycoplasma infection. In the third hospitalization, the M. pneumoniae IgM antibody titer exhibited a COI of 6.33. The BALF mNGS was positive for Haemophilus influenzae (Hin) and human cytomegalovirus (HCMV) and did not detect mycoplasma. In the fifth hospitalization, the M. pneumoniae IgM antibody titer had a COI of 3.19, and the tests for 13 pathogens detected HRV infection. During the sixth hospitalization, the M. pneumoniae IgM antibody titer exhibited a COI of 2.44. tNGS was positive for HCoV-OC43, Streptococcus pneumoniae, and Hin, but negative for mycoplasma.
4 Discussion
4.1 Dureation of M. Pneumoniae IgM positivity in children
In this study, two children underwent multiple serological tests for M. pneumoniae. One patient had prolonged serological positivity for M. pneumoniae IgM antibodies for nearly ten months, whereas the other showed persistent positivity for over fifteen months. After M. pneumoniae infection, the body can produce specific IgM, IgG, and IgA class antibodies. Previous studies have shown that IgM antibodies usually emerge one week after an M. pneumoniae infection, peak at two to three weeks, decrease at four weeks (21–23), and may persist in low levels for months or even years (24). In clinical practice, most children who were positive for M. pneumoniae IgM antibodies after effective treatment had generally no need for long-term follow-up examinations. Therefore, patients with persistent positivity for IgM antibodies against M. pneumoniae have rarely been reported.
4.2 Conventional understanding of M. Pneumoniae IgM positivity
IgM antibodies are primarily considered indicators of acute inflammation (25–27), appearing early during the host immune response to pathogens and showing decreased levels shortly after the appearance of IgG antibodies (28). IgM antibodies against M. pneumoniae are generally believed to be a diagnostic indicator for early infection (22, 29–31). Moreover, some researchers have reported that among patients with cough, fever, and expectoration, positive results for M. pneumoniae IgM antibodies can be used to diagnose recent M. pneumoniae pneumonia necessitating antibiotics (32). However, the use of macrolide antibiotics based solely on a positive IgM test as an indicator of a recent infection can increase the risk of drug misuse. Furthermore, many cases of refractory M. pneumoniae pneumonia have been recently reported among children (33–35).
4.3 Persistent M. Pneumoniae IgM positivity does not imply ongoing infection
In this study, detecting IgM antibodies against M. pneumoniae was the basis for prescribing macrolide antibiotics in 6 out of 11 hospitalizations of case 1 and 5 out of 6 hospitalizations of case 2. As can be seen in the two tables, the values obtained using the MP-IgM quantitative detection of the two patients gradually decreased. During the ninth hospitalization, case 1 tested positive for MP-IgM and DNA, which was the primary infection. In the tenth and eleventh hospitalizations, the patient was positive for MP-IgM, but the tNGS test was negative for MP while positive for InfA H1N1 and HRSV-A, which did not support persistent or active M. pneumoniae infection. Case 2 was positive for MP-IgM during the third to sixth hospitalizations, but the DNA test was negative and did not support persistent or active infection.
4.4 Positivity of M. Pneumoniae IgM antibodies and the risk of macrolide misuse
For case 1, using macrolide antibiotics in the seventh, eighth, and ninth hospitalizations was deemed appropriate based on the positive test results for M. pneumoniae DNA and IgM antibody. However, the use of macrolides was unreasonable during the third, fourth, and tenth hospitalizations based on the low M. pneumoniae antibody titer, decreased levels of M. pneumoniae IgM antibody titers, and the presence of other etiological results. For case 2, using macrolide antibiotics during the first and second hospitalizations was appropriate based on the positive tests for M. pneumoniae IgM antibody and other negative etiological results. However, during the third, fifth, and sixth hospitalizations, the use of macrolides was deemed inappropriate based on the positive M. pneumoniae IgM and IgG antibody titers because M. pneumoniae DNA result was negative and other etiological factors were present. Upon analysis, the excessive use of macrolides was problematic for both cases.
Based on these results and other similar cases (36–39), the use of macrolide antibiotics based on positive IgM antibody titers against M. pneumoniae has likely contributed to the development of M. pneumoniae resistance. In fact, the excessive use of macrolide antibiotics has been identified as an important factor contributing to the rising global prevalence of MRMP cases (1, 40), which is a serious issue that requires attention. Moreover, it is reported that azithromycin can also lead to gut microbiota imbalance (41, 42).
4.5 Reconceptualizing the clinical relevance of M. pneumoniae IgM antibodies
This study underscored the importance of dynamic monitoring of M. pneumoniae IgM antibody titers to ascertain the presence of an acute mycoplasma infection. Comparing the antibody levels before and after an infection is essential for this purpose. A rapid increase in M. pneumoniae IgM antibody titers within a short term is typically indicative of an acute infection. In these two cases, the IgM antibody titers against M. pneumoniae consistently decreased. Thus, observing the antibody titer trend, in conjunction with data obtained for other etiological factors, can provide a more comprehensive understanding and help distinguish acute infections from other conditions. Many researchers have emphasized the reliability of employing two short-term evaluations of serological IgM titers to screen patients for acute infection and minimize the selection bias encountered in studies on M. pneumoniae (43–45). Guidelines or protocols should pay more attention to standardize the interpretation of IgM antibody titers in clinical practice.
Although several studies and guidelines (31, 44, 46, 47) have indicated that IgM antibodies against M. pneumoniae may persist for an extended period, the specific duration has yet to be determined. Despite the demonstrated value of IgM level in numerous cases, many clinicians and laboratory personnel lack awareness about the importance of M. pneumoniae IgM antibodies in diagnosing acute or recent infections. This study highlighted the possibility of persistent positive IgM antibody titers against M. pneumoniae for more than one year and indicated that a positive Mycoplasma pneumoniae IgM test does not necessarily indicate the presence of an acute infection. Furthermore, these results indicated that macrolide antibiotics should not be misused solely based on M. pneumonia IgM test.
Studies on different Etiologies have also shown that specific IgM antibodies do not always indicate acute infection. Lassa virus-specific IgM serostatus cannot be considered a diagnostic marker of acute infection (48). The presence of Toxoplasma gondii-specific IgM antibodies also does not necessarily suggest an acute infection (49). Furthermore, it has been reported that specific IgM antibodies against parvovirus B19 infection in pregnant women can be persistently detected for up to nine months (50). A prospective observational study revealed that Zika virus IgM antibodies can persist for 237.7 days (128.7–459.5) following the estimated time of detectable infection via plasma nucleic acid amplification testing (51). West Nile virus(WNV) IgM antibodies can persist for more than three years in 12% of patients with WNV infection (52). A study described the persistence of IgM against SARS-CoV-2 for up to one year, so the authors believed that the use of IgM antibodies to identify the infection stage needs to be evaluated with caution (53).
4.6 Limitations
The main limitations of this study were the small number of cases and retrospective data analysis. While, in clinical practice, most children who were positive for M. pneumoniae IgM antibodies after effective treatment had generally no need for long-term follow-up examinations. Therefore, patients with persistent positivity for IgM antibodies against M. pneumoniae have rarely been reported. Further studies with long-term monitoring of larger sample sizes are needed to confirm these findings. Additionally, it is critical to consider whether the current positive cut-off value for the M. pneumoniae IgM antibody test is appropriate.
5 Conclusions
Positive IgM antibody titers for M. pneumoniae can persist for an extended period and even last over one year. Thus, positive M. pneumoniae IgM levels do not necessarily suggest a recent infection. Therefore, it is esential to highlight the implications of IgM antibody for clinical decision-making and the need for further research to establish standardized criteria for interpreting IgM antibody titers. When considering the use of macrolide antibiotics, it is crucial to not rely solely on M. pneumoniae IgM levels to ascertain the presence of a recent infection. Instead, a comprehensive approach should be taken by comparing the trend of M. pneumoniae IgM titers and dynamically analyzing the data. Furthermore, the results of other detection methods for relevant etiological factors should be considered to ascertain an accurate diagnosis and comprehensively confirm the presence of M. pneumoniae.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
This study was approved by Medical Ethics Committee of the Maternal and Child Health Hospital of Hubei Province (approval number: 2023IEC075). This study was conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the participants' legal guardians for the publication of this case report.
Author contributions
HW: Conceptualization, Funding acquisition, Methodology, Project administration, Supervision, Writing – original draft. XL: Conceptualization, Funding acquisition, Methodology, Project administration, Supervision, Writing – review & editing. YW: Writing – review & editing, Project administration, Supervision. XC: Data curation, Formal analysis, Investigation, Resources, Software, Validation, Visualization, Writing – review & editing. JL: Data curation, Formal analysis, Investigation, Resources, Software, Validation, Visualization, Writing – review & editing. WL: Data curation, Formal analysis, Investigation, Resources, Software, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We thank the guardians of the children who consented to include their children in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
M. pneumoniae, Mycoplasma pneumoniae; IgM, immunoglobulin M; MRMP, macrolide-resistant Mycoplasma pneumoniae; PCR, polymerase chain reaction, tNGS, targeted next-generation sequencing; mNGS, metagenomic next-generation sequencing, BALF, bronchoalveolar lavage fluid.
References
1. Shim JY. Current perspectives on atypical pneumonia in children. Clin Exp Pediatr. (2020) 63:469–76. doi: 10.3345/cep.2019.00360
2. Shuo Y, Xinying L, Huizhe W, Huanmin L, Xinmin L. Risk factors for severe Mycoplasma Pneumoniae pneumonia in children:a meta-analysis. Chin Gen Pract. (2024) 27:1750–60. doi: 10.12114/j.issn.1007-9572.2023.0737
3. Zuo M, Wang H, Zhu H. A left-sided destroyed lung in a 11-year-old girl: a rare sequela after Mycoplasma pneumoniae infection. Pediatr Pulm. (2024) 59:1765–8. doi: 10.1002/ppul.26942
4. Jiang Z, Zhou R, Leung P, Deng Z, Li S. An attenuated multiple genetic mutant of Mycoplasma pneumoniae imparts good immuno-protection against M. pneumoniae pneumonia in BALB/c mice. Microb Pathog. (2022) 165:105463. doi: 10.1016/j.micpath.2022.105463
5. Qin L, Liu L, Wu Y, Chen Y, Wu Y, Luo H, et al. Mycoplasma pneumoniae downregulates RECK to promote matrix metalloproteinase-9 secretion by bronchial epithelial cells. Virulence. (2022) 13:1270–84. doi: 10.1080/21505594.2022.2101746
6. Chen Y, Wu Y, Qin L, Yu L, Luo H, Li Y, et al. T-B cell epitope peptides induce protective immunity against Mycoplasma pneumoniae respiratory tract infection in BALB/c mice. Immunobiology. (2021) 226:152077. doi: 10.1016/j.imbio.2021.152077
7. Al-Saeedi AS, Abdulamir AS, Alubaidi GT. Development of a cost-effective quantitative in-house ELISA assay for screening anti-S1 IgG antibodies targeting SARS-CoV-2. J Med Life. (2023) 16:883–9. doi: 10.25122/jml-2023-0047
8. Zou CC, Liang L. Multiple hypoechoic lesions in spleen and Mycoplasma pneumoniae infection. Indian Pediatr. (2005) 42:379–82.15876602
9. Zeng XD, Chen H, Hu WG. Myelin oligodendrocyte glycoprotein (MOG) antibody-associated meningoencephalitis due to Mycoplasma pneumoniae infection. Neurol Res. (2023) 45:124–6. doi: 10.1080/01616412.2022.2124794
10. Qingzhan L, Bingru L, Yingying H, Tingting L. Investigation of Mycoplasma pneumoniae infection in 16948 pediatric patients. Chin J Front Health Quar. (2025) 48:174–6. doi: 10.16408/j.1004-9770.2025.02.016
11. Geyan X, Sha X, Jiaxin S, Xujing Z, Shasha L. Etiological characteristics analysis of community-acquired pneumonia in infants and young children. J Pathog Biol. (2025) 20:512–6. doi: 10.13350/j.cjpb.250421
12. Yue H. Diagnostic value of C-reactive protein in Mycoplasma pneumoniae pneumonia in adults. Chin J Mod Drug Appl. (2022) 16:1–5. doi: 10.14164/j.cnki.cn11-5581/r.2022.07.001
13. Expert Committee on Rational Use of Medicines for Children Pharmaceutical Group NHaFPC. Expert consensus on laboratory diagnostics and clinical practice of Mycoplasma pneumoniae infection in children in China (2019). Chin J Pediatr. (2020) 58:366–73. doi: 10.3760/cma.j.cn112140-20200304-00176
14. Shuzhi D, Weiya W, Ying W, Wen Z, Lijuan M. Clinical characteristics of pediatric patients with mycoplasma pneumonia antibody positive in clinic under different age groups. Chin J Front Med Sci (E). (2022) 14:42–6. doi: 10.12037/YXQY.2022.05-08
15. Landry ML. Immunoglobulin M for acute infection: true or false? Clin Vaccine Immunol. (2016) 23:540–5. doi: 10.1128/CVI.00211-16
16. Kim K, Jung S, Kim M, Park S, Yang HJ, Lee E. Global trends in the proportion of macrolide-resistant Mycoplasma pneumoniae infections: a systematic review and meta-analysis. JAMA Netw Open. (2022) 5:e2220949. doi: 10.1001/jamanetworkopen.2022.20949
17. Jiang Y, Dou H, Xu B, Xu B, Zhou W, Wang H, et al. Macrolide resistance of Mycoplasma pneumoniae in several regions of China from 2013 to 2019. Epidemiol Infect. (2024) 152:e75. doi: 10.1017/S0950268824000323
18. Ishiguro N, Koseki N, Kaiho M, Ariga T, Kikuta H, Togashi T, et al. Therapeutic efficacy of azithromycin, clarithromycin, minocycline and tosufloxacin against macrolide-resistant and macrolide-sensitive Mycoplasma pneumoniae pneumonia in pediatric patients. PLoS One. (2017) 12:e0173635. doi: 10.1371/journal.pone.0173635
19. Zhou Y, Wang J, Chen W, Shen N, Tao Y, Zhao R, et al. Impact of viral coinfection and macrolide-resistant mycoplasma infection in children with refractory Mycoplasma pneumoniae pneumonia. Bmc Infect Dis. (2020) 20:633. doi: 10.1186/s12879-020-05356-1
20. Li W, Liu Y, Zhao Y, Tao R, Li Y, Shang S. Rapid diagnosis of Mycoplasma pneumoniae in children with pneumonia by an immuno-chromatographic antigen assay. Sci Rep-Uk. (2015) 5:15539. doi: 10.1038/srep15539
21. Xiaochen B, Hongmei S. Current status and research progress of serological antibody detection of Mycoplasma pneumonia. Int J Pediat. (2017) 44(3):147–51. doi: 10.3760/cma.j.issn.1673-4408.2017.03.001
22. CADTH. Serum IgM and Molecular Tests for Mycoplasma pneumoniae Detection: A Review of Diagnostic Test Accuracy, Clinical Effectiveness, Cost-Effectiveness, and Guidelines. Ottawa (oN): Canadian Agency for Drugs and Technologies in Health (2015).
23. Atkinson TP, Duffy LB, Pendley D, Dai Y, Cassell GH. Deficient immune response to Mycoplasma pneumoniae in childhood asthma. Allergy Asthma Proc. (2009) 30:158–65. doi: 10.2500/aap.2009.30.3207
24. Sillis M. The limitations of IgM assays in the serological diagnosis of Mycoplasma pneumoniae infections. J Med Microbiol. (1990) 33:253–8. doi: 10.1099/00222615-33-4-253
25. Bachmann MF, Zinkernagel RM. Neutralizing antiviral B cell responses. Annu Rev Immunol. (1997) 15:235–70. doi: 10.1146/annurev.immunol.15.1.235
26. Zinkernagel RM, LaMarre A, Ciurea A, Hunziker L, Ochsenbein AF, McCoy KD, et al. Neutralizing antiviral antibody responses. Adv Immunol. (2001) 79:1–53. doi: 10.1016/s0065-2776(01)79001-3
27. Zinkernagel RM. On natural and artificial vaccinations. Annu Rev Immunol. (2003) 21:515–46. doi: 10.1146/annurev.immunol.21.120601.141045
28. Skountzou I, Satyabhama L, Stavropoulou A, Ashraf Z, Esser ES, Vassilieva E, et al. Influenza virus-specific neutralizing IgM antibodies persist for a lifetime. Clin Vaccine Immunol. (2014) 21:1481–9. doi: 10.1128/CVI.00374-14
29. Rao H, Ericson JE, O'Hara C. Mycoplasma pneumoniae presenting with pericardial tamponade. Clin Pediatr. (2020) 59:198–200. doi: 10.1177/0009922819885662
30. de Barbeyrac B, Obeniche F, Ratsima E, Labrouche S, Morate C, Renaudin H, et al. Serologic diagnosis of chlamydial and Mycoplasma pneumoniae infections. Ann Biol Clin-Paris. (2006) 64:409–19. doi: 10.1684/abc.2006.0002
31. National Health Commission of the People’s Republic of China. Guidelines for the diagnosis and treatment of Mycoplasma pneumoniae pneumonia in children (2023 edition). Int J Epidemiol Infect Dis. (2023) 50(2):79–85. doi: 10.3760/cma.j.cn331340-20230217-00023
32. Yonggang Y, Li L, Yuan L, Xiaoxi Z. Value of Mycoplasma pneumoniae antigen combined with Mycoplasma pneumoniae immunoglobulin M antibody in the diagnosis of Mycoplasma pneumoniae pneumonia. Int J Immunol. (2023) 46(3):283–7. doi: 10.3760/cma.j.issn.1673-4394.2023.03.009
33. Zhu J, Liu X, Zhan X, Wang M, Zhang Y, Na L, et al. Predictive value of chemokines (CCL 2) in bronchoalveolar lavage fluid for refractory mycoplasma pneumonia in children. Ital J Pediatr. (2023) 49:125. doi: 10.1186/s13052-023-01528-2
34. Huang W, Hao J, Zhang Y. Intracardiac and cerebral thrombosis complicated with Mycoplasma pneumonia. Cureus J Med Sci. (2024) 16:e60563. doi: 10.7759/cureus.60563
35. Shen HX, Liu C, Lin HJ, Xu LJ, Wang GY, Yan MX. The efficacy and safety of minocycline as adjuvant therapy in refractory mycoplasma pneumonia in Chinese children: a meta-analysis. Ital J Pediatr. (2022) 48(1):176. doi: 10.1186/s13052-022-01362-y
36. Youfang H, Huihua L, Yi L, Ling Z. Epidemiological investigation of Mycoplasma Pneumoniae and Chlamydia Pneumoniae infection in patients with acute respiratory tract infection. Int Med Health Guid News. (2024) 30:825–8. doi: 10.3760/cma.j.issn.1007-1245.2024.05.024
37. Zhibing G, Jian W, Guangping W, Zhuqiang W. Effect of azithromycin combined with low-dose glucocorticoid on inflammation related indexes of children with lobar pneumonia. Chin J Nosociol. (2017) 27:4012–5. doi: 10.11816/cn.ni.2017-171092
38. Zhe S, Guangyuan J, Xiao W, Baoqing Z. Clinical observation on the therapeutic effect of Yinqin Qingfei Granules combined with conventional western medicine therapy in the treatment of severe Mycoplasma pneumoniae pneumonia in children with damp-toxin blocking lung syndrome. China J Tradit Chin Med. (2024) 39:2662–7.
39. Aguilera-Alonso D, Lopez RR, Centeno RJ, Morell GM, Valero GI, Ocete MM, et al. Epidemiological and clinical analysis of community-acquired Mycoplasma pneumonia in children from a Spanish population, 2010–2015. An Pediatr (Engl Ed). (2019) 91:21–9. doi: 10.1016/j.anpede.2019.01.003
40. Tsai TA, Tsai CK, Kuo KC, Yu HR. Rational stepwise approach for Mycoplasma pneumoniae pneumonia in children. J Microbiol Immunol. (2021) 54:557–65. doi: 10.1016/j.jmii.2020.10.002
41. Ubeda C, Pamer EG. Antibiotics, microbiota, and immune defense. Trends Immunol. (2012) 33:459–66. doi: 10.1016/j.it.2012.05.003
42. Wu J, Lin Z, Wang X, Zhao Y, Zhao J, Liu H, et al. Limosilactobacillus reuteri SLZX19-12 protects the colon from infection by enhancing stability of the gut Microbiota and barrier integrity and reducing inflammation. Microbiol Spectr. (2022) 10:e0212421. doi: 10.1128/spectrum.02124-21
43. Youn YS, Lee SC, Rhim JW, Shin MS, Kang JH, Lee KY. Early additional immune-modulators for Mycoplasma pneumoniae pneumonia in children: an observation study. Infect Chemother. (2014) 46:239–47. doi: 10.3947/ic.2014.46.4.239
44. Lee SC, Youn YS, Rhim JW, Kang JH, Lee KY. Early serologic diagnosis of Mycoplasma pneumoniae pneumonia: an observational study on changes in titers of specific-IgM antibodies and cold agglutinins. Medicine (Baltimore). (2016) 95:e3605. doi: 10.1097/MD.0000000000003605
45. Yang EA, Kang HM, Rhim JW, Kang JH, Lee KY. Early corticosteroid therapy for Mycoplasma pneumoniae pneumonia irrespective of used antibiotics in children. J Clin Med. (2019) 8(5):726. doi: 10.3390/jcm8050726
46. Waites KB, Xiao L, Liu Y, Balish MF, Atkinson TP. Mycoplasma pneumoniae from the respiratory tract and beyond. Clin Microbiol Rev. (2017) 30:747–809. doi: 10.1128/CMR.00114-16
47. Tabassum I, Chaudhry R, Chourasia BK, Malhotra P. Identification of an N-terminal 27 kDa fragment of Mycoplasma pneumoniae P116 protein as specific immunogen in M. pneumoniae infections. Bmc Infect Dis. (2010) 10:350. doi: 10.1186/1471-2334-10-350
48. Branco LM, Grove JN, Boisen ML, Shaffer JG, Goba A, Fullah M, et al. Emerging trends in Lassa fever: redefining the role of immunoglobulin M and inflammation in diagnosing acute infection. Virol J. (2011) 8:478. doi: 10.1186/1743-422X-8-478
49. Petersen E. Toxoplasmosis. Semin Fetal Neonatal Med. (2007) 12:214–23. doi: 10.1016/j.siny.2007.01.011
50. Searle K, Guilliard C, Wallat S, Schalasta G, Enders G. Acute parvovirus B19 infection in pregnant women–an analysis of serial samples by serological and semi-quantitative PCR techniques. Infection. (1998) 26:139–43. doi: 10.1007/BF02771838
51. Stone M, Bakkour S, Lanteri MC, Brambilla D, Simmons G, Bruhn R, et al. Zika Virus RNA and IgM persistence in blood compartments and body fluids: a prospective observational study. Lancet Infect Dis. (2020) 20:1446–56. doi: 10.1016/S1473-3099(19)30708-X
52. Papa A, Anastasiadou A, Delianidou M. West Nile virus IgM and IgG antibodies three years post- infection. Hippokratia. (2015) 19:34–6.26435644
Keywords: Mycoplasma pneumoniae, pneumonia, immunoglobulin M, microbial infection, drug resistance, macrolides, case report
Citation: Wang H, Liu X, Wu Y, Cao X, Liu J and Li W (2025) Case Report: Positive Mycoplasma pneumoniae IgM does not necessarily indicate acute infection: two case studies. Front. Pediatr. 13:1520021. doi: 10.3389/fped.2025.1520021
Received: 30 October 2024; Accepted: 9 May 2025;
Published: 30 May 2025.
Edited by:
Luis Garcia-Marcos, University of Murcia, SpainReviewed by:
Peng Liu, University of South China, ChinaTu-Hsuan Chang, Chi Mei Medical Center, Taiwan
Copyright: © 2025 Wang, Liu, Wu, Cao, Liu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoying Liu, bHh5bHpqMTFAMTYzLmNvbQ==; Yabin Wu, d3V5YWJpbjQ1OEBzb2h1LmNvbQ==
 Hao Wang
Hao Wang Xiaoying Liu
Xiaoying Liu Yabin Wu1*
Yabin Wu1*