- 1Department of Neonatology, Hunan Provincial Maternal and Child Health Care Hospital, University of South China, Changsha, Hunan, China
- 2Department of Obstetrics, Hunan Provincial Maternal and Child Health Care Hospital, University of South China, Changsha, Hunan, China
- 3Department of Clinical Laboratory, Hunan Provincial Maternal and Child Health Care Hospital, University of South China, Changsha, Hunan, China
Objective: Early-onset sepsis (EOS) remains an important issue in neonatal units. Characteristics of EOS from China have not been fully revealed yet. Our aim is to investigate epidemiology, microbiology and clinical feature of EOS in a Chinese maternal and child healthcare centre.
Methods: This is a retrospective observational study of EOS infants born in or admitted to Hunan Provincial Maternal and Child Health Care Hospital from January 1, 2017 to December 31, 2023.
Results: During the study period, there were 131 neonatal infections. The incidence of EOS was 1.12 (95% CI 0.94–1.33) per 1,000 live births or 3.85 (95% CI 3.22–4.56) per 1,000 admissions. Coagulase-negative staphylococci (CoNS) (n = 43, 32.3%), group B Streptococcus (GBS) (n = 24, 18.0%) and Escherichia coli (n = 18, 13.5%) were the predominant pathogens. GBS screening test was performed before delivery in 77.7% mothers of all infants with EOS, and 12.9% of screening results were positive. Among the main pathogens causing EOS, 86% of CoNS strains were resistant to penicillin, while all GBS strains were susceptible to penicillin.
Conclusions: We report a high burden of EOS among infants in the maternal and child healthcare centre from China. CoNS was the most frequent pathogen causing EOS. Longitudinal epidemiologic surveillance is required to improve empiric antibiotic treatment of EOS.
Introduction
At present, developed countries such as the United States and the United Kingdom have established population-based surveillance network for bacterial infections among infants (1, 2). Monitoring the epidemiological characteristics of early-onset sepsis (EOS), such as incidence rate and pathogen distribution patterns, is essential to guide empirical antibiotic treatment in infants with suspected sepsis. With the implementation of management strategies for perinatal infections such as intrapartum antibiotic prophylaxis (IAP), the incidence of early-onset group B Streptococcus (GBS) disease has significantly decreased (3). In addition, the antimicrobial resistance (AMR) rate in neonatal units remains high. Recent application of antimicrobial stewardship programs in neonatal units showed the association with reduction in the initiation and duration of antimicrobial use (4).
Previous studies reported distinct patterns of pathogen distribution and clinical features in infection surveillance in different medical setting models (5, 6). Maternal and child healthcare centres are women and children specialized health facilities charged with basic labor services in China. These centres are first choice of pregnant women to labor, and provides basic medical services and physical examinations for women and children. They are classified as provincial, municipal and county-level. Our study gave a brief insight to epidemiology, microbiology and clinical feature of EOS in a provincial maternal and child healthcare centre from China.
Methods
Study design and population
This study retrospectively collected data on neonatal EOS from January 1, 2017 to December 31, 2023 in Hunan Provincial Maternal and Child Health Care Hospital. The microbiological and clinical data of neonates with EOS admitted to the neonatal unit, whether inborn (born in the hospital) or outborn (outside the hospital or in other hospitals), were involved in the study. Infants were transferred from the maternity unit to the neonatal unit when suspected sepsis. Our hospital is the largest maternal and child healthcare centre in Hunan province, China, and has 150 beds for neonatal hospitalization. The study was approved by the Ethics Committee of Hunan Provincial Maternal and Child Health Care Hospital (Fast no.2024037).
Data collection and definitions
EOS was defined as a positive blood culture or cerebrospinal fluid (CSF) culture to a pathogen within 72 h after birth with clinical and laboratory findings consistent with infection. Cultures of Coagulase-negative staphylococci (CoNS) considered possible contaminants were excluded by clinical judgment (7). Antimicrobial susceptibility testing was followed by Clinical Laboratory Standards Institute (CLSI) guidelines (8). Data of demographics of infants and mothers, maternal risk factors, comorbidity and resource use in the neonatal unit of newborns and antibiotic resistance of main strains were recorded. Premature rupture of membranes was defined as rupture of membranes prior to delivery. Clinical chorioamnionitis was defined by the presence of maternal fever >38.0°C during labor with at least two of the following criteria: uterine tenderness (without another cause), fetal tachycardia, maternal leukocytosis, maternal heart rate >100 bpm, foul-smelling amniotic fluid (9). Bacterial meningitis was defined as positive CSF culture, Gram staining, or neutrophilic leukocytosis, with or without low sugar (less than 50% of plasma glucose level) and high protein content in CSF samples. Septic shock was defined as hypotension requiring catecholamine treatment (9).
Statistical analysis
Data were analysed using Stata V.16 (Stata Corp., College Station, Texas, USA). The incidence rates were calculated as cases per 1,000 live births (by dividing the number of inborn infants with sepsis by the number of live births in the hospital) or cases per 1,000 admissions (by dividing the number of inborn and outborn infants with sepsis by the number of infants admitted to the neonatal unit), and the 95% confidence intervals (CIs) for the incidence rates were calculated using the Poisson distribution. The incidence per 1,000 live births of a certain pathogen was calculated by dividing the number of inborn infants with the pathogen-caused sepsis by the number of live births in the hospital. The incidence of EOS by 1,000 live births was only calculated in inborn infants. The incidence of EOS by 1,000 admissions was calculated in infants admitted to the neonatal unit, inborn or outborn. The χ2 test was used to evaluate the differences in proportion, and χ2 for linear trend was used to test for trends in the incidence of EOS over the study period. P < 0.05 was considered significant.
Results
Incidence of neonatal EOS
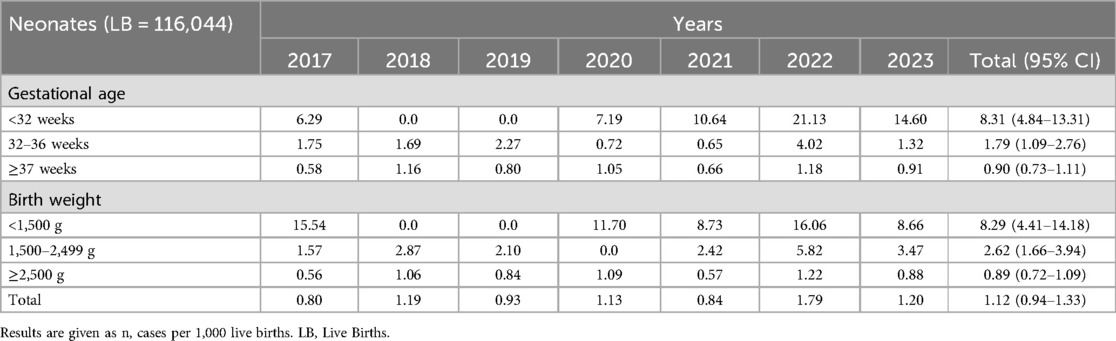
During the seven-year study period, a total of 116,044 live births were recorded in Hunan Provincial Maternal and Child Health Care Hospital. There were 130 cases of neonatal EOS occurred among inborn neonates, therefore the incidence was 1.12 (95% CI 0.94–1.33) per 1,000 live births. The incidence of EOS did not change over the study period (p = 0.085). After stratification by gestational age and birth weight, the incidence of EOS was significantly higher in neonates <32 weeks of gestation (p < 0.001) and <1,500 g (p < 0.001) (Table 1).
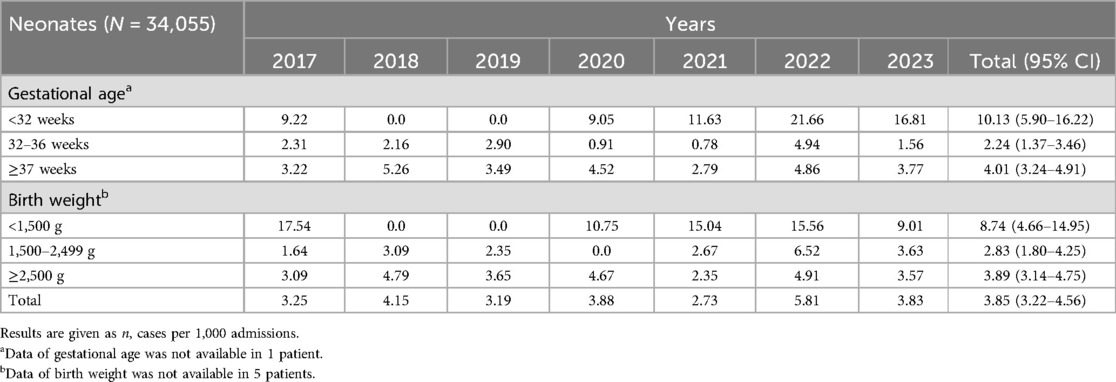
The yearly incidence of EOS among inborn and outborn infants out of admissions is shown in Table 2. There were 131 neonatal infections, of which 130 (99.2%) occurred among inborn and one (0.8%) among outborn infants. The incidence of EOS for admissions was 3.85 (95% CI 3.22–4.56) per 1,000 admissions. The incidence of EOS was significantly higher in very preterm infants (<32 weeks) (p < 0.001) and very low birth weight infants (<1,500 g) (p = 0.003). The incidence of EOS for admissions did not change over the study period (p = 0.329).
Pathogen distribution
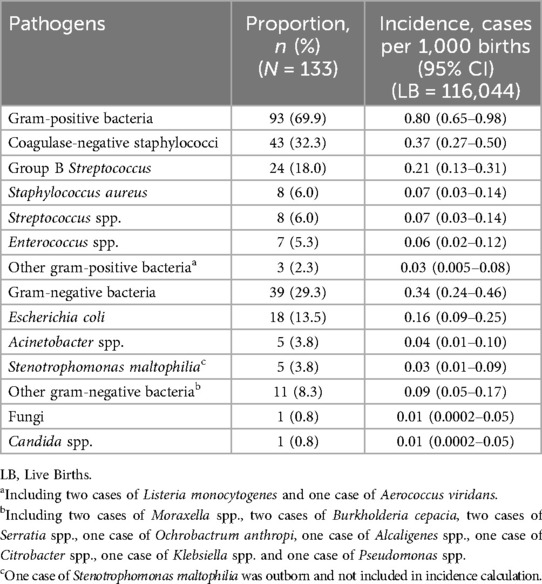
A total of 133 isolates were identified in 131 infants admitted to the neonatal unit (Table 3). Gram-positive bacteria accounted for 93 (69.9%) of 133 isolates. The most frequent pathogens were CoNS (n = 43, 32.3%), GBS (n = 24, 18.0%) and Escherichia coli (n = 18, 13.5%). Among these, 132 pathogens were isolated from inborn neonates. The incidence of CoNS EOS was 0.37 per 1,000 live births, while the incidence of GBS and E. coli EOS was 0.21 and 0.16 per 1,000 live births.
The online Supplementary Figure S1 shows the spectrum of pathogens causing EOS according to gestational age. CoNS was the predominant pathogen in infants <32 weeks, 32–36 weeks and ≥37 weeks, accounting for 4 (23.5%), 7 (35%), 32 (33.3%) infections separately. The online Supplementary Table S1 describes the clinical characteristics of infants developing CoNS-induced sepsis. CoNS has been classified as Staphylococcus epidermidis, Staphylococcus hominis, Staphylococcus capitis and other CoNS. E. coli accounted for 4 (23.5%) infections in very preterm infants. GBS accounted for 24 (25%) infections in term infants. One case of Candida glabrata was reported in infants <32 weeks. Three (15%) cases of Acinetobacter baumannii were reported in infants born at 32–36 weeks. Staphylococcus aureus accounted for two infections in neonates at 32–36 weeks and six in neonates ≥37 weeks. Stenotrophomonas maltophilia accounted for one infection in neonates <32 weeks, one in neonates at 32–36 weeks and three in neonates ≥37 weeks.
Characteristics of mothers and neonates
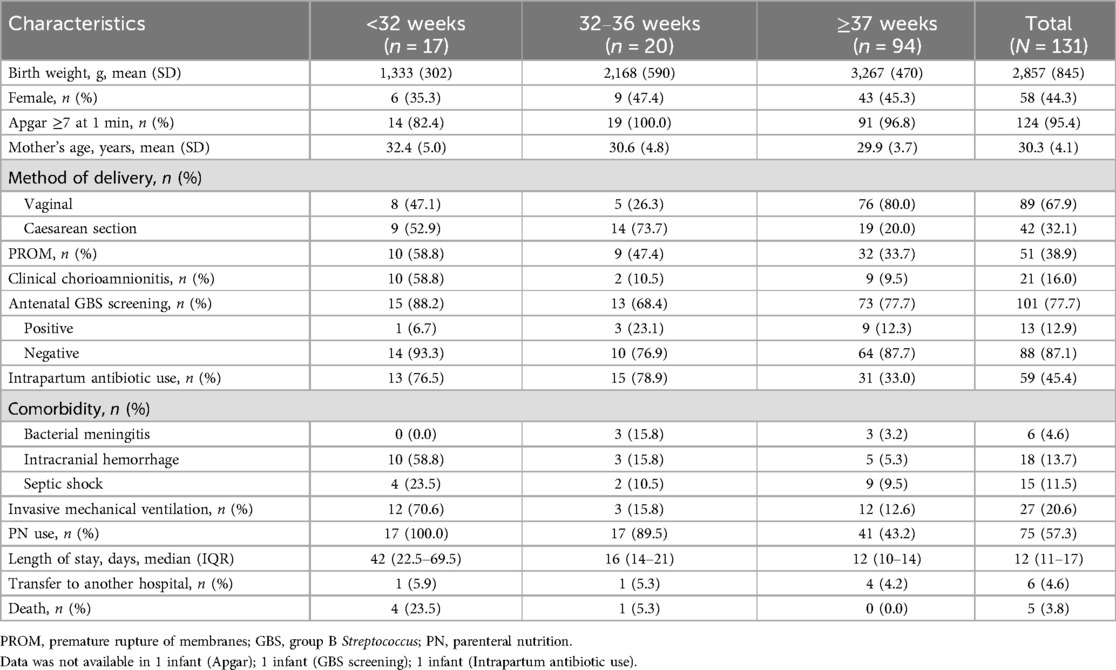
The characteristics of mothers and infants are described according to gestational age in Table 4. 80% mothers of term infants gave birth through vaginal delivery. GBS screening test was performed in 77.7% mothers of all infants and 77.7% mothers of term infants, in which 12.9% and 12.3% were positive results, respectively. Ten (58.8%) mothers of neonates <32 weeks developed clinical chorioamnionitis, with four of them leading to neonatal E. coli infection. A total of 59 (45.4%) women received intrapartum antibiotic use, mostly piperacillin (14/59, 23.7%) or second-generation cephalosporins (29/59, 49.2%).
The most frequent antibiotic use of initial treatment of infants were piperacillin (54/131, 41.2%) or third-generation cephalosporins (21/131, 16.0%). The median length of stay in neonatal unit was 12 (11–17) days. Six infants (4.6%) were transferred to another hospital for further treatment. Of the 131 infants with EOS, five (3.8%) died during hospitalization, including four born <32 weeks and one born at 32–36 weeks.
Antimicrobial resistance
Table 5 shows the AMR pattern of the main pathogens causing EOS, including CoNS, GBS and E. coli. Resistance to penicillin and ampicillin were found in 86% and 100% CoNS strains, respectively. All GBS strains were susceptible to penicillin. Among the eight E. coli isolates tested, three (37.5%) were resistant to ceftriaxone. No E. coli strain was found resistant to meropenem. Two of eight (25%) S aureus strains were identified as methicillin-resistant Staphylococcus aureus (MRSA).
Discussion
We firstly provide the incidence of neonatal EOS in China by cases per 1,000 live births or admissions in detail, due to the convenience to reach the data on live births as a maternal and child healthcare centre. Furthermore, we also firstly show the epidemiology, microbiology and clinical feature of EOS in a model of maternal and child healthcare centre, which provides basic medical services and physical examinations for women and children. The pattern of pathogen distribution and AMR rate may be a guidance for clinical management of newborns at high risk of early-onset neonatal infection in the rooming-in ward of the maternity unit or neonatal unit in similar medical model in developing countries with high birth rates.
Al-Taiar et al. (10) reported an incidence of EOS of 0.62 (95% CI 0.45–0.82) per 1,000 live births or 4.91 (95% CI 4.22–5.68) per 1,000 admissions in a prospective cohort study between 2006 and 2009 in neonatal care units in China, Malaysia, Hong Kong and Thailand. Comparatively, our results showed a higher incidence of 1.12 (95% CI 0.94–1.33) per 1,000 live births but a lower incidence of 3.85 (95% CI 3.22–4.56) per 1,000 admissions. The incidence of EOS was also reported 0.78 per 1,000 live births (95% CI 0.61–0.97) in a retrospective study from South China (11). Therefore, we showed a high burden of EOS among infants in the maternal and child healthcare centre in China. Consistent with our results, several studies reported the difference in incidence of EOS according to gestational age or birth weight (9, 12, 13).
In a prospective surveillance study in the Paris area, France from 2019 to 2021, Sikias et al. (13) revealed the incidence of EOS of different pathogen infection in neonates born at ≥34 weeks of gestation. In our study, GBS infections were all detected from term infants. The incidence of GBS EOS was 0.21 (95% CI 0.13–0.31) per 1,000 live births. We also showed that CoNS was the predominant pathogen in neonatal EOS. CoNS was reported the main pathogen responsible for neonatal sepsis in neonatal units from China or other countries, especially in regions with a low prevalence of MRSA using an aminoglycoside-beta-lactam antibiotic combination as the first-line empirical antimicrobial regimen to cover Gram-negative bacteria and methicillin-sensitive S. aureus (MSSA) (14). In our study, the clinical outcomes of neonates with CoNS infection were mostly good. CONS are not so virulent as Gram-negative bacteria and fungi, and CoNS infection was associated with the lower rate of short-term infectious complications as well as mortality. However, CONS are capable to exert a long-term detrimental effect on the host, particularly on infants <1,000 g (15). In our report, we also raise the awareness of CoNS infection in neonatal EOS. Further study should be considered in investigating the role of CONS in EOS.
The Chinese expert consensus for prevention of perinatal GBS disease was first issued in 2021 (16). The consensus standardized the timing and detection methods of GBS screening, and intrapartum antibiotic prophylaxis. The Neonatal Health Care Committee of Chinese Maternal and Child Health Association developed another expert consensus on the clinical management of newborns at high risk of early-onset neonatal infection in the rooming-in ward of the maternity unit (17). Our study described the antenatal risk factors of infants with EOS at <32 weeks, 32–36 weeks and ≥37 weeks. GBS screening test was performed in 77.7% mothers of infants with EOS, and 45.4% of the mothers received intrapartum antibiotic use. The practice of generalization of GBS screening and peripartum antibiotic prophylaxis is being performed in our centre in recent years. We would monitor and assess the effect of such practice in future studies.
Zhang et al. (18) reviewed the rates of AMR patterns for bloodstream isolates from Chinese neonates. We firstly provide the AMR pattern in the maternal and child healthcare centre in China. Recent Chinese consensus on management of neonatal sepsis recommends ampicillin combined with third-generation cephalosporin as first-line initial antibiotic use (19). However, our results showed high resistance rate to ampicillin and ceftriaxone in CoNS and E. coli strains. It might raise particular concern of considering novel combination of initial treatment for recommendation.
The strength of this study is the generalization of the EOS rate by cases per 1,000 live births or admissions. The incidence was less reported in previous studies of Chinese neonates, because most patients were admitted to specialized children's hospitals which lack of data on live births. We have provided data of seven years since the electronic medical recording system was introduced. The limitation of the study is mainly the retrospective design. In addition, multi-centre cooperation is needed for further regional or national surveillance in EOS infants.
In conclusion, the incidence of EOS was 1.12 per 1,000 live births or 3.85 per 1,000 admissions. CoNS, GBS and E. coli were the most common pathogens. The incidence of GBS EOS was 0.21 per 1,000 live births. Our data encourage the generalization of GBS screening, and raise the concern of CoNS infection in neonatal EOS. Longitudinal surveillance is required to monitor pathogen distribution and AMR pattern for adjusting EOS empiric treatment in initial management of infants with suspected infection.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by The Ethics Committee of Hunan Provincial Maternal and Child Health Care Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin because data were retrospectively collected from the electronic medical recording system, and were not identifiable.
Author contributions
WW: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. ZW: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. LW: Data curation, Formal analysis, Methodology, Software, Validation, Visualization, Writing – review & editing. SL: Data curation, Formal analysis, Methodology, Software, Validation, Visualization, Writing – review & editing. JL: Investigation, Project administration, Resources, Writing – review & editing. YH: Investigation, Project administration, Resources, Writing – review & editing. JL: Investigation, Project administration, Resources, Writing – review & editing. CZ: Investigation, Project administration, Resources, Writing – review & editing. JZ: Investigation, Project administration, Validation, Visualization, Writing – review & editing. CL: Investigation, Project administration, Validation, Visualization, Writing – review & editing. CY: Investigation, Project administration, Validation, Visualization, Writing – review & editing. QC: Investigation, Project administration, Validation, Visualization, Writing – review & editing. WW: Investigation, Project administration, Validation, Visualization, Writing – review & editing. SD: Investigation, Project administration, Validation, Visualization, Writing – review & editing. YD: Investigation, Project administration, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2025.1521908/full#supplementary-material
References
1. Schrag SJ, Farley MM, Petit S, Reingold A, Weston EJ, Pondo T, et al. Epidemiology of invasive early-onset neonatal sepsis, 2005–2014. Pediatrics. (2016) 138(6):e20162013. doi: 10.1542/peds.2016-2013
2. Cailes B, Kortsalioudaki C, Buttery J, Pattnayak S, Greenough A, Matthes J, et al. Antimicrobial resistance in UK neonatal units: neonIN infection surveillance network. Arch Dis Child Fetal Neonatal Ed. (2018) 103(5):F474–8. doi: 10.1136/archdischild-2017-313238
3. Nanduri SA, Petit S, Smelser C, Apostol M, Alden NB, Harrison LH, et al. Epidemiology of invasive early-onset and late-onset group B streptococcal disease in the United States, 2006–2015: multistate laboratory and population-based surveillance. JAMA Pediatr. (2019) 173(3):224–33. doi: 10.1001/jamapediatrics.2018.4826
4. Mascarenhas D, Ho MSP, Ting J, Shah PS. Antimicrobial stewardship programs in neonates: a meta-analysis. Pediatrics. (2024) 153(6):e2023065091. doi: 10.1542/peds.2023-065091
5. Buetti N, Marschall J, Atkinson A, Kronenberg A, Swiss Centre for Antibiotic Resistance (ANRESIS). National bloodstream infection surveillance in Switzerland 2008–2014: different patterns and trends for university and community hospitals. Infect Control Hosp Epidemiol. (2016) 37(9):1060–7. doi: 10.1017/ice.2016.137
6. Yu Y, Dong Q, Li S, Qi H, Tan X, Ouyang H, et al. Etiology and clinical characteristics of neonatal sepsis in different medical setting models: a retrospective multi-center study. Front Pediatr. (2022) 10:1004750. doi: 10.3389/fped.2022.1004750
7. Russell AR B, Kumar R. Early onset neonatal sepsis: diagnostic dilemmas and practical management. Arch Dis Child Fetal Neonatal Ed. (2015) 100(4):F350–4. doi: 10.1136/archdischild-2014-306193
8. CLSI. M100-S30 Performance Standards for Antimicrobial Susceptibility Testing. Thirtieth Informational Supplement. Wayne, PA: Clinical and Laboratory Standards Institute (2020).
9. Giannoni E, Agyeman PKA, Stocker M, Posfay-Barbe KM, Heininger U, Spycher BD, et al. Neonatal sepsis of early onset, and hospital-acquired and community-acquired late onset: a prospective population-based cohort study. J Pediatr. (2018) 201:106–14. doi: 10.1016/j.jpeds.2018.05.048
10. Al-Taiar A, Hammoud MS, Cuiqing L, Lee JK, Lui KM, Nakwan N, et al. Neonatal infections in China, Malaysia, Hong Kong and Thailand. Arch Dis Child Fetal Neonatal Ed. (2013) 98(3):F249–55. doi: 10.1136/archdischild-2012-301767
11. Gao K, Fu J, Guan X, Zhu S, Zeng L, Xu X, et al. Incidence, bacterial profiles, and antimicrobial resistance of culture-proven neonatal sepsis in south China. Infect Drug Resist. (2019) 12:3797–805. doi: 10.2147/IDR.S223597
12. Jiang S, Hong L, Gai J, Shi J, Yang Y, Lee SK, et al. Early-onset sepsis among preterm neonates in China, 2015–2018. Pediatr Infect Dis J. (2019) 38(12):1236–41. doi: 10.1097/INF.0000000000002492
13. Sikias P, Biran V, Foix-L'Hélias L, Plainvert C, Boileau P, Bonacorsi S, et al. Early-onset neonatal sepsis in the Paris area: a population-based surveillance study from 2019 to 2021. Arch Dis Child Fetal Neonatal Ed. (2023) 108(2):114–20. doi: 10.1136/archdischild-2022-324080
14. Li JY, Chen SQ, Yan YY, Hu YY, Wei J, Wu QP, et al. Identification and antimicrobial resistance of pathogens in neonatal septicemia in China-A meta-analysis. Int J Infect Dis. (2018) 71:89–93. doi: 10.1016/j.ijid.2018.04.794
15. Dong Y, Speer CP. Late-onset neonatal sepsis: recent developments. Arch Dis Child Fetal Neonatal Ed. (2015) 100(3):F257–63. doi: 10.1136/archdischild-2014-306213
16. Society of Perinatal Medicine, Chinese Medical Association, Obstetrics Subgroup, Society of Obstetrics and Gynecology, Chinese Medical Association. Chinese Experts consensus on prevention of perinatal group B streptococcal disease. Chin J Perinat Med. (2021) 24(8):561–6. doi: 10.3760/cma.j.cn113903-20210716-00638
17. Neonatal Health Care Committee of Chinese Maternal and Child Health Association, Neonatology Society of Chinese Medical Doctor Association. Expert consensus on clinical management of newborns at high risk of early-onset infection at rooming-in ward. Chin J Perinat Med. (2021) 24(8):567–75. doi: 10.3760/cma.j.cn113903-20210310-00202
18. Zhang J, Folgori L, Hsia Y, Sharland M, Yang Y. Pattern of antimicrobial resistance in bloodstream isolates from Chinese neonates. Pediatr Infect Dis J. (2019) 38(6):600–4. doi: 10.1097/INF.0000000000002246
19. The Subspecialty Group of Neonatology, the Society of Pediatrics, Chinese Medical Association, the Editorial Board, Chinese Journal of Pediatrics. Expert consensus on diagnosis and management of neonatal bacteria sepsis (2024). Chin J Pediatr. (2024) 62(10):931–40. doi: 10.3760/cma.j.cn112140-20240505-00307
Keywords: early-onset sepsis, incidence, antimicrobial resistance, surveillance, pathogens
Citation: Wang W, Wang Z, Wang L, Li S, Liu J, Huang Y, Liu J, Zhu C, Zhang J, Li C, Yang C, Chen Q, Wang W, Deng S and Du Y (2025) Early-onset neonatal sepsis in a Chinese maternal and child healthcare centre, 2017–2023. Front. Pediatr. 13:1521908. doi: 10.3389/fped.2025.1521908
Received: 3 November 2024; Accepted: 24 March 2025;
Published: 15 April 2025.
Edited by:
Adi Klein, Hillel Yaffe Medical Center, IsraelReviewed by:
Rinawati Rohsiswatmo, RSUPN Dr. Cipto Mangunkusumo, IndonesiaTakayuki Hoshina, University of Occupational and Environmental Health Japan, Japan
Copyright: © 2025 Wang, Wang, Wang, Li, Liu, Huang, Liu, Zhu, Zhang, Li, Yang, Chen, Wang, Deng and Du. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weilin Wang, Y2Flc2FyaW5ld2FuZ3dsQDE2My5jb20=
†These authors have contributed equally to this work
 Weilin Wang
Weilin Wang Zhenhui Wang2,†
Zhenhui Wang2,†