- 1Primary Child Care Department (Psychological Behavior Department), Children’s Hospital of Hebei Province, Shijiazhuang City, Hebei, China
- 2Department of Pediatric Neurology, Children’s Hospital of Hebei Province, Shijiazhuang City, Hebei, China
- 3Department of Anesthesiology, Children’s Hospital of Hebei Province, Shijiazhuang City, Hebei, China
Tic disorders (TD) represent a prevalent neurodevelopmental condition in children, characterised by involuntary, sudden motor or vocal tics. Dysfunction of the dopamine system plays a pivotal role in the pathogenesis of TD. Recent findings indicate that deep brain stimulation, by modulating striatal dopamine release, substantially alleviates tic symptoms. Neuroimaging studies have shown increased dopamine transporter binding and decreased serotonin levels in patients with TD. The presence of anti-dopamine D2 receptor autoantibodies, which correlate with disease severity, suggests immune involvement in the onset of TD. Nutritional factors influence the dopaminergic system's functionality by affecting neurotransmitter synthesis and metabolism, modulating gut microbiota and contributing to neuroinflammation. Clinical studies have demonstrated that interventions combining probiotics and fructooligosaccharides can help regulate neurotransmitter metabolism, whereas dietary patterns such as the ketogenic, Mediterranean and Mediterranean-DASH intervention for neurodegenerative delay diets exhibit anti-inflammatory and neuroprotective effects. The risk of TD in offspring is significantly associated with maternal autoimmune diseases and inflammatory states, with metabolic syndrome further affecting the dopaminergic system via AT1 receptor autoantibodies. Nutritional intervention-based treatment strategies present promising directions for TD management, warranting further investigation into the nutrition–immune–neurotransmitter network, the development of personalised nutritional plans and the validation of their clinical efficacy through large-scale randomised controlled trials. This review summarises the alterations in the dopaminergic system in TD, the regulatory effects of nutritional factors on dopamine levels, the interactions between neuroinflammation and the dopaminergic system and treatment strategies based on nutritional interventions, laying a theoretical foundation for understanding TD pathogenesis and advancing novel therapeutic approaches.
1 Introduction
Tic disorders (TD) manifest as neuropsychiatric conditions primarily characterised by involuntary, sudden motor or vocal tics. Studies indicate that TD typically begins in childhood and may co-occur with psychiatric and behavioural disorders such as attention deficit hyperactivity disorder, obsessive–compulsive disorder (OCD), anxiety disorders and depression (1). Epidemiological studies in China have reported prevalence rates of 1.7% for transient TD, 1.2% for chronic TD and 0.3% for Tourette syndrome (TS), estimating that approximately 10 million children and adolescents are affected by TD, including around 2 million cases of TS (2).
The pathogenesis of TD is multifactorial, involving genetic, immune, psychological and environmental factors. The link between its pathophysiology and clinical symptoms primarily manifests through disinhibition within the cortico-striato-thalamo-cortical loop (3). Recent research has highlighted the central role of dopaminergic system dysfunction in the pathogenesis of TD. Studies on deep brain stimulation have demonstrated that modulating striatal dopamine release can substantially alleviate tic symptoms, providing direct evidence of dopamine's key role in TD onset (4). Advanced imaging studies further reveal that patients with TD exhibit increased dopamine transporter (DAT) binding and reduced serotonin levels, underscoring the importance of neurotransmitter imbalance in disease progression (5).
Recent findings have identified anti-dopamine D2 receptor autoantibodies in patients with TD, with levels correlating to disease severity. This immunological evidence further elucidates the link between TD and dopaminergic dysfunction (6). Moreover, maternal autoimmune diseases and inflammatory states have been significantly associated with an increased risk of TD in offspring, suggesting inflammation as a critical mediator between genetic predisposition and environmental influences (7).
Nutritional factors, as modifiable environmental elements, may play a substantial role in the pathogenesis and treatment of TD. Research has shown that specific nutrients are involved in dopamine synthesis and metabolism, whereas dietary patterns can influence the gut–brain axis and neuroinflammation, thereby affecting dopaminergic system function (8). Emerging studies indicate that nutritional interventions targeting gut microbiota can influence brain dopamine levels and related behavioural manifestations (9). In addition, metabolic disorders such as obesity have been closely linked to alterations in dopamine signalling pathways, suggesting a complex interaction among the metabolic, immune and neurotransmitter systems in TD pathogenesis (10).
Conventional TD treatments primarily rely on dopamine receptor antagonists, which are often associated with notable side effects. In recent years, attention has shifted towards nutritional and anti-inflammatory treatment strategies (11). Studies have shown that certain natural compounds, such as Gastrodin, can alleviate TD symptoms by inhibiting neuroinflammation. Additionally, dietary modifications and specific nutritional supplements have emerged as promising approaches for influencing the dopaminergic system in TD therapy (12).
Given the complexity of TD pathogenesis and the limitations of current treatment options, understanding the interactions between nutrition, inflammation and the dopaminergic system is of considerable importance. This study presents a narrative review of how nutritional factors and neuroinflammation affect the dopaminergic system in TD and explores their potential therapeutic implications.
2 Altered function of the dopaminergic system in Tic disorders
The dopaminergic system plays a central role in the pathogenesis of TD. Advanced deep brain stimulation studies have shown that stimulation of the centromedian–parafascicular (CMPf) complex in the thalamus improves tic symptoms by regulating striatal dopamine release. In rat models, deep brain stimulation of the CMPf complex induces synaptic dopamine release and elevates baseline levels while reducing motor tic behaviours. The improvement in tic symptoms has been confirmed to be mediated by D2 receptor activation, as demonstrated through selective blockade (13).
Neuroimaging studies have found that patients with TD who are not on medication exhibit increased DAT binding in the caudate nucleus, putamen and the entire neostriatum. In patients receiving medication, DAT levels in the putamen remain normal, though increased DAT binding persists in the caudate nucleus. Additionally, there is a reduction in D2 receptor binding in the striatum and frontal cortex, accompanied by increased 5-HT2A receptor binding in the neocortex and limbic regions (14).
Research on genetic mutations further underscores the importance of the dopamine system in TD. Studies involving mice with mutations in high-confidence TD genes CELSR3 and WWC1 show that these mutations lead to abnormal sensorimotor behaviour, altered reward learning and increased striatal dopamine release. Prepulse inhibition tests reveal sensory gating deficits in Celsr3 mutant mice, whereas Wwc1 mutations produce this deficit only in women. Notably, aripiprazole—a partial agonist of the dopamine D2 receptor—corrects sensory gating deficits and abnormal upright behaviour (15).
Autoimmune responses also contribute to dopaminergic dysfunction in TD. Clinical studies indicate that 8% of patients test positive for anti-D2 receptor antibodies during symptom exacerbation, with an additional 6.6% becoming positive at later stages. Statistical analysis reveals a strong association between anti-D2 receptor antibodies and tic exacerbation (McNemar's odds ratio = 11, p = 0.003), a relationship that remains significant after adjusting for demographic variables and psychotropic medication use (16).
Magnetoencephalography studies reveal abnormal magnetic error-related negativity (mERN) patterns in patients with TD. Compared with controls, patients with TD do not show response-type-dependent amplitude modulation (error or correct) within 70–105 ms post-response. However, substantial mERN amplitudes are detected in both groups within the 105–160 ms window, suggesting delayed error processing in patients with TD. It is hypothesised that early error-related processing in TD is influenced by enhanced motor control triggered by the conflict between task execution and tic suppression (17).
Neuroinflammation plays a key role in dopaminergic dysfunction in TD. Experimental studies have shown that rats in a TD model induced by 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane display pronounced neuroinflammatory responses. Elevated levels of interleukin (IL)-6, IL-1β and tumour necrosis factor-α are observed in the striatum and serum. Western blot analysis reveals activation of the TLR/NF-κB and TLR/MAPK signalling pathways in the striatum (18).
These findings suggest that alterations in the dopaminergic system in TD encompass multiple aspects, including changes in neurotransmitter release, abnormal receptor expression, genetic mutations, autoimmune responses and neuroinflammation. Gaining a deeper understanding of these mechanisms is essential for developing new therapeutic strategies.
3 Influence of nutritional factors on dopamine levels
Nutritional status plays a crucial role in modulating brain dopamine levels. Studies have shown that gut microbiota and dietary interventions substantially influence central nervous system function. Clinical research on autism spectrum disorders has found that interventions combining probiotics and fructooligosaccharides considerably increase levels of beneficial bacteria such as Bifidobacteria and inhibit the growth of potential pathogens such as Clostridium. Following intervention, patients' serum levels of acetate, propionate and butyrate rise substantially to levels observed in control groups, whereas serum serotonin levels decrease and vanillic acid levels increase, highlighting the importance of the gut microbiota–neurotransmitter metabolic axis in neurological disease (19).
Dietary structure has a notable impact on the brain's reward system. Exposure to a high-fat diet restructures the feeding circuit and alters the motivational response to food. Animal experiments confirm that long-term high-calorie diets weaken dopaminergic neurons' response to standard food but not to high-fat food. Longitudinal recordings show that this adaptive change occurs at the level of hypothalamic agouti-related protein neurons and midbrain peripheral dopamine signalling (20).
There is considerable individual variability in the brain's response to nutritional signals. Random crossover studies have shown that, in both healthy-weight and obese individuals, intragastric glucose and lipid infusion have different effects on brain neural activity and striatal dopamine release. Healthy individuals exhibit specific neural responses and dopamine release, whereas obese individuals display severely impaired responses. Notably, diet-induced weight loss does not restore the impaired neural response in obese individuals, suggesting long-term adaptive changes in the nutrition–brain pathway (21).
Dopamine subsystems in the brain function to track homeostatic changes. Dopaminergic neurons in the ventral tegmental area (VTA) detect nutrient or water intake at different stages. Some neurons track systemic hydration changes within minutes after thirsty mice drink water, whereas others respond to nutrients in the gastrointestinal tract. Information on fluid balance, transmitted through the hypothalamic pathway, is relayed to the VTA and rerouted to downstream circuits that monitor intake stages in the mouth, gastrointestinal tract and post-absorption (22).
Dietary fat restriction also affects the activity of brain reward regions. In randomised crossover trials, selective restriction of dietary fat or carbohydrates, compared with an isocaloric baseline diet, produces different effects on dopamine D2/3 receptor binding potential and neural activity. After 5 days of fat restriction, both D2 receptor binding and neural responses to food cues in reward-related brain regions decrease, whereas no comparable changes are seen with carbohydrate restriction. Following fat restriction, spontaneous intake tends to shift towards high-fat, high-carbohydrate foods (23).
The insulin signalling pathway within the brain is closely linked to dopamine function. Studies show that central insulin action plays a key regulatory role in food intake, reward and emotional behaviours. Brain insulin resistance contributes to overeating, anxiety- and depression-like behaviours and impairs dopaminergic function. Insulin receptor sensitisers and dopamine receptor agonists have shown benefits in improving obesity and mental health conditions in both rodents and humans (24).
4 Interactions between neuroinflammation and the dopaminergic system
Neuroinflammation plays a pivotal role in the pathogenesis of neuropsychiatric disorders. Maternal autoimmune diseases and inflammatory states have been shown to be closely associated with the onset of TD and OCD in children. Studies report that the incidence of autoimmune diseases in mothers of children with TD is higher than in mothers of children with other autoimmune neurological disorders (p = 0.054) and significantly higher than in healthy control mothers (p = 0.0004). Furthermore, the incidence of autoimmune diseases is also markedly elevated among first- and second-degree maternal relatives of children with TD (p < 0.0001 and p = 0.014, respectively). Transcriptome analysis indicates that differentially expressed genes upregulated in the brains of patients with TD and maternal autoimmune disease are enriched in innate immune processes (25).
Metabolic syndrome exerts its effects on the dopaminergic system via mediation by AT1 receptor autoantibodies. Experimental data suggest that metabolic syndrome enhances activity of the pro-inflammatory renin–angiotensin system axis in the substantia nigra, leading to increased oxidative stress and neuroinflammation, which in turn exacerbate dopaminergic neuron degeneration. Administration of AT1 receptor blockers has been shown to mitigate these pathological changes. In rats with metabolic syndrome, serum levels of LIGHT and other key pro-inflammatory cytokines, as well as 27-hydroxycholesterol, are elevated alongside a marked increase in pro-inflammatory AT1 and ACE2 autoantibodies (26).
The dopaminergic pathway is also critical in the context of obesity-related inflammation. Research has found that dopamine not only regulates immune function but is also produced endogenously by immune cells. In the nigrostriatal and mesocorticolimbic systems, the inflammatory environment alters dopaminergic signalling by promoting the release of pro-inflammatory cytokines (including IL-1β, IL-6 and TNF-α). These inflammatory factors, once in systemic circulation, initiate widespread inflammation that further affects brain function and dopamine signalling (27).
Metabolic syndrome and obesity affect the occurrence, symptomatic presentation and progression of TD through a variety of mechanisms. Studies have shown that obesity-related chronic low-grade inflammatory states may lead to dysfunction of the brain's dopaminergic system, thereby promoting the development of TD or worsening its symptoms (3). For example, hyperprolactinaemia promotes weight gain, obesity and metabolic syndrome by inhibiting physiological dopaminergic tone and impairing glucose–insulin and lipid metabolism (14). This endocrine metabolic disorder not only affects overall health but may also indirectly influence TD symptoms by altering dopamine levels in the brain. In addition, insulin resistance associated with metabolic syndrome may affect the pathological process of TD by interfering with dopamine signalling (17). Regulating these metabolic abnormalities may represent a new direction in the treatment of TD.
Regulation of inflammatory responses also presents a promising avenue for TD management. Gastrodin, an active constituent of traditional Chinese medicine, is widely used for its sedative, anticonvulsant and neuroprotective effects. In TD animal models, gastrodin has been shown to substantially reduce abnormal stereotypic behaviours by decreasing the number of D2 receptors and increasing DT expression. Additionally, gastrodin reduces serum transporter density, thereby indirectly lowering dopamine release (28).
Combined treatment with glucagon-like peptide-1 (GLP-1) and nicotine has been shown to improve obesity by acting on the hypothalamic and mesocorticolimbic pathways. Studies report that the combination of the GLP-1 receptor agonist liraglutide with nicotine suppresses food intake and increases energy expenditure, resulting in reduced body weight in obese mice. This drug combination activates multiple brain areas, with liraglutide enhancing the excitability of hypothalamic pro-opiomelanocortin neurons and VTA dopaminergic neurons. Using genetically encoded dopamine sensors, researchers have shown that liraglutide can inhibit nicotine-induced dopamine release in the nucleus accumbens of freely behaving mice (29).
Brain insulin action also interacts with the dopamine system in the context of schizophrenia. Studies have shown that central nervous system insulin is involved in regulating striatal dopamine levels, peripheral glucose homeostasis and feeding behaviour. Insulin receptors and dopamine D2 receptors are co-expressed on human pancreatic β-cells and adipocytes, supporting the critical role of both insulin and dopamine in peripheral metabolic regulation. Clinical evidence confirms that dopaminergic drugs can improve the clinical manifestations of metabolic syndrome and obesity, substantially enhancing glucose–insulin metabolism and lipid profiles (30).
5 Nutritional intervention-based treatment strategies
Nutritional interventions have demonstrated notable efficacy in the treatment of neurological diseases. The ketogenic diet (KD) is well established as a therapy for refractory epilepsy and is currently under investigation for its potential use in febrile infection-related epilepsy syndrome and epileptic encephalopathies. Research has substantiated that KD acts by specifically targeting dysregulated adaptive and innate immune responses in refractory epilepsy and status epilepticus. The diet has also shown anti-inflammatory and neuroprotective effects in models of multiple sclerosis, Parkinson's disease, pain and spinal cord injury. Ketone bodies, caloric restriction, polyunsaturated fatty acids and alterations in gut microbiota all contribute to KD's anti-inflammatory effects (31).
The Mediterranean diet (MD) has shown substantial efficacy in the secondary prevention of cardiovascular disease. Results from randomised controlled trials indicate that, compared with a low-fat diet, the MD is associated with a reduced incidence of major cardiovascular events. After multivariate adjustment, the hazard ratio ranged from 0.719 to 0.753, consistently favouring the MD group. The effect was more pronounced in men, with a major endpoint event rate of 16.2% in the Mediterranean group compared with 22.8% in the low-fat group (multifactor-adjusted HR 0.669, p = 0.013). Research has also confirmed that the MD enhances neurological function through anti-inflammatory and neuroprotective mechanisms (32).
The Mediterranean-DASH intervention for neurodegenerative delay (MIND) diet combines features of the Mediterranean and DASH diets. In a randomised controlled trial, 604 elderly individuals without cognitive impairment but with a family history of dementia were assigned to either the MIND diet or a control diet with caloric restriction. After a 3-year follow-up, both groups showed improvements in global cognitive function scores, with the MIND diet group reporting an increase of 0.205 standard units and the control group 0.170. MRI results showed comparable changes in white matter hyperintensities, hippocampal volume and total grey and white matter volumes between the groups (33).
Dietary interventions in Parkinson's disease research indicate that protein-restricted diet, KD, MD and MIND diet all influence disease risk, progression and severity. Protein-restricted diet primarily improves medication absorption by reducing competition between levodopa and dietary protein. Ketogenic diet provides an alternative energy source and exerts neuroprotective effects through its anti-inflammatory action. Mediterranean diet, rich in antioxidants and omega-3 fatty acids, helps mitigate neurodegenerative changes (34).
Research on nutritional interventions for cognitive decline in elderly individuals suggests that changes in dietary patterns can have a protective effect on brain health. Anti-inflammatory diets—particularly the Mediterranean, Okinawan and MIND diets—have been shown to support nervous system health. Omega-3 fatty acids, antioxidants and polyphenolic compounds inhibit neuroinflammation associated with Alzheimer's disease. Additionally, anti-inflammatory diets reduce neuroinflammation through indirect immune pathways involving the gut microbiota and systemic circulation (35).
The influence of dietary patterns on migraines has been extensively investigated. Clinical observations have shown that certain dietary factors can trigger or exacerbate migraine attacks. Dietary intervention strategies include elimination diets, ketogenic diets and comprehensive diets. Despite inconsistencies in the literature and a lack of consensus, existing data support the potential benefits of dietary interventions for some patients with migraine. Factors such as age, gender, genetics and environmental conditions all play a role in determining the outcomes (36).
6 The influence of nutritional factors on the occurrence and development of Tic disorders
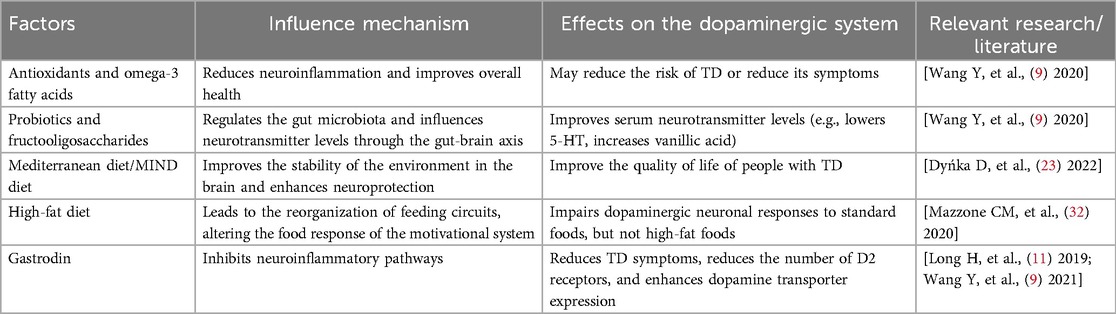
Nutritional status is closely related to the pathophysiology of TD. Studies have shown that specific nutrients can affect TD by modulating neuroinflammation, improving gut microbiota composition and directly influencing dopamine metabolism pathways in the brain. For example, antioxidants and omega-3 fatty acids are thought to reduce neuroinflammation, which may lower the risk of TD or help alleviate its symptoms (34). In addition, certain dietary patterns, such as the MD and the MIND diet, not only support overall health but may also be particularly beneficial for patients with TD by optimising environmental stability within the brain and enhancing neuroprotection (32, 33).
Recent studies have shown that adjusting the diet to include more beneficial ingredients can be used as an adjunct therapy to ease symptoms and improve the quality of life for people with TD. One study found that the use of probiotics and fructooligosaccharide interventions in the TD model could modulate the gut microbiota, which in turn affected serum neurotransmitter levels through the gut–brain axis—including a decrease in serum serotonin levels and an increase in vanillic acid levels—providing a new perspective on the role of nutritional factors in TD pathophysiology (9).
Research on the effectiveness of nutritional interventions for TD is still in its early stages, but there is evidence that some dietary patterns may offer therapeutic value. For example, the KD, as a high-fat, low-carbohydrate diet, has shown anti-inflammatory and neuroprotective effects in various neurological disorders. Although fewer studies have directly focused on TD, its positive effects in similar conditions suggest potential for future research (23). In addition, it has been shown that Gastrodin, a traditional Chinese medicine ingredient, can reduce TD symptoms by inhibiting neuroinflammatory pathways (11, 21). Although most of these findings are based on animal models, they provide a theoretical foundation for developing nutrition-based treatments.
In summary, although the current understanding of how nutritional factors specifically affect TD is still incomplete, existing research suggests that dietary modification and the introduction of specific nutrient supplements may be an effective strategy to improve TD symptoms. Further clinical trials are needed to validate these findings and to better elucidate the role of nutritional factors in TD management (Table 1).
7 Effect of neuroimmune factors on Tic disorders
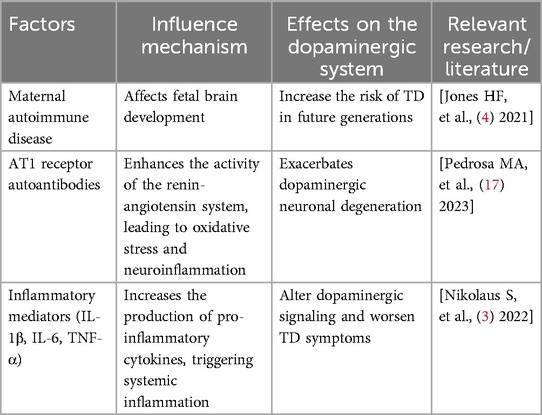
Neuroimmune factors influence the development of TD through a variety of mechanisms. Studies have shown that the autoimmune or inflammatory state experienced by the mother during pregnancy is associated with an increased risk of TD in offspring (4). This type of immune activation may lead to abnormal brain development during the fetal period, particularly in regions involving the dopaminergic system, thereby increasing the risk of TD later in life. Additionally, the presence of anti-dopamine D2 receptor antibodies may be associated with chronic TD, suggesting that immune-mediated mechanisms play an important role in the pathophysiology of TD (6).
Neuroimmune factors not only affect the onset of TD but also have a substantial impact on symptom expression. For instance, insulin resistance associated with metabolic syndrome may influence the symptomatic presentation of TD by interfering with the dopamine signalling pathway (17). Inflammatory mediators such as IL-1β, IL-6 and TNF-α can exacerbate neuroinflammation and alter dopaminergic function in the brain, thereby worsening TD symptoms (32). This inflammatory state not only affects localised brain regions but can also spread systemically through blood circulation, further impacting overall health. As the condition progresses, neuroimmune factors continue to play a key role. Long-term exposure to high levels of inflammatory cytokines may lead to the degeneration of dopaminergic neurons, a major factor in the exacerbation of TD (3).
Regarding prognosis, modulating the inflammatory response is considered a potential strategy to improve long-term outcomes. Gastrodin, a traditional Chinese medicine ingredient, has been shown to substantially reduce aberrant behaviour by decreasing D2 receptor numbers and enhancing DT expression in animal models of TD (11, 21). It also reduces serum transporter density and indirectly lowers dopamine release, helping to alleviate TD symptoms and improve overall outcomes.
In summary, neuroimmune factors influence the onset, symptomatology, progression and prognosis of TD through a range of complex mechanisms. Understanding these processes not only aids in the development of new treatments but also provides a theoretical foundation for personalised medicine. Future research should focus more closely on the interaction between neuroimmunity and the dopaminergic system to improve the management of TD (Table 2).
8 Prospects for future studies
Further exploration is needed to integrate nutritional interventions with immune modulation in treatment strategies for nervous system diseases. Current research indicates that the KD improves neurological function by modulating metabolism and the immune system. The KD has shown promising therapeutic effects in refractory epilepsy, multiple sclerosis and neurodegenerative diseases. Mechanistic studies suggest that its anti-inflammatory action is mediated by multiple factors, including ketone bodies, caloric restriction, polyunsaturated fatty acids and changes in gut microbiota composition (37).
The role of the gut microbiota–metabolite–brain axis in nervous system diseases warrants in-depth investigation. A low-protein, high-carbohydrate diet has demonstrated neuroprotective effects in MPTP-induced Parkinson's disease mouse models. This dietary intervention alters gut microbiota composition, increasing beneficial bacteria such as Bifidobacterium and Ileibacterium, whereas reducing the abundance of Bilophila and Alistipes. PICRUSt-predicted faecal microbiome functions indicate that this diet suppresses lipopolysaccharide biosynthesis and the tricarboxylic acid cycle while enhancing amino acid and carbohydrate metabolism (38).
Deoxyribonucleic acid methylation, as an epigenetic modification, is influenced by both metabolic and nutritional factors. Studies have shown that bioactive nutrients and gut microbiota can alter DNA methylation in the central nervous system via the gut–brain axis, thereby affecting neural function and behaviour. Deoxyribonucleic acid hydroxymethylation, in particular, is prevalent in the adult brain, and dietary interventions aimed at modulating this epigenetic process represent a novel therapeutic approach (39).
Studies on nutritional and exercise interventions for patients with sarcopenia and obesity offer novel insights into improving metabolic health. Meta-analyses indicate that aerobic exercise reduces body weight and fat mass, resistance exercise reduces fat mass and improves grip strength and their combination reduces fat mass and enhances walking speed. Nutritional interventions, particularly low-calorie, high-protein diets, reduce fat mass without affecting muscle mass or grip strength. However, nutritional supplementation combined with exercise does not confer additional benefits (40).
Endocrine metabolic disorders are closely linked to the neurotransmitter system. Hyperprolactinaemia promotes weight gain, obesity and the development of metabolic syndrome by inhibiting physiological dopaminergic tone and disrupting glucose–insulin and lipid metabolism. Human pancreatic β-cells and adipocytes express both prolactin receptors and dopamine D2 receptors, highlighting the key role of prolactin and dopamine in peripheral metabolic regulation. Dopamine receptor agonists, including bromocriptine and cabergoline, have been shown to reduce the prevalence of metabolic syndrome and obesity while improving metabolic indicators (41).
The brain's reward system plays a vital role in metabolic regulation. Dopamine neurons in the VTA track internal state changes and respond to nutrients at various stages of ingestion. Hypothalamic pathways transmit fluid balance information to the dopamine system, which redistributes it to downstream circuits that monitor oral, gastrointestinal and post-absorptive stages. A deeper understanding of the relationship between the reward system and metabolic regulation may offer new approaches for treating metabolic diseases (42).
9 Conclusion
The dopaminergic system's pivotal role in TD, and its intricate interplay with nutritional factors and neuroinflammation, has been highlighted by recent research. Deep brain stimulation studies have validated the mechanism of improving tic symptoms by modulating striatal dopamine release. Neuroimaging studies have identified abnormal changes in DT and receptor expression in patients with TD, whereas genetic studies have clarified the connection between high-confidence TD genes and dopaminergic system functionality. Nutritional factors influence the dopaminergic system by affecting neurotransmitter synthesis and metabolism, modulating gut microbiota and contributing to neuroinflammation. The association between maternal immune-inflammatory states and increased TD risk in offspring underscores the importance of immune-inflammatory factors in disease occurrence. These findings suggest that nutritional interventions may offer a novel therapeutic approach for TD. Specific dietary patterns, including the KD, MD and MIND diet, have been shown to improve neurological function through anti-inflammatory and neuroprotective effects. Future research should explore the detailed mechanisms underlying the nutrition–immune–neurotransmitter network, develop personalised nutritional intervention plans and investigate the synergistic effects of nutritional strategies alongside existing treatment methods. Further studies into the gut microbiota–metabolite–brain axis, the regulation of DNA methylation and the relationship between the reward system and metabolic regulation may reveal new mechanisms of TD pathogenesis and provide potential targets for prevention and treatment. Long-term, large-scale randomised controlled trials are essential for validating the clinical efficacy of nutritional interventions and clarifying their mechanisms of action.
Author contributions
LB: Conceptualization, Formal analysis, Supervision, Writing – original draft, Writing – review & editing. MJ: Data curation, Investigation, Writing – review & editing. QZ: Data curation, Investigation, Writing – review & editing. SS: Formal analysis, Investigation, Writing – review & editing, Data curation.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Nasello C, Poppi LA, Wu J, Kowalski TF, Thackray JK, Wang R, et al. Human mutations in high-confidence Tourette disorder genes affect sensorimotor behavior, reward learning, and striatal dopamine in mice. Proc Natl Acad Sci U S A. (2024) 121(19):e2307156121. doi: 10.1073/pnas.2307156121
2. Rusheen AE, Rojas-Cabrera J, Goyal A, Shin H, Yuen J, Jang DP, et al. Deep brain stimulation alleviates tics in Tourette syndrome via striatal dopamine transmission. Brain. (2023) 146(10):4174–90. doi: 10.1093/brain/awad142
3. Nikolaus S, Mamlins E, Antke C, Dabir M, Müller HW, Giesel FL. Boosted dopamine and blunted serotonin in Tourette syndrome—evidence from in vivo imaging studies. Rev Neurosci. (2022) 33(8):859–76. doi: 10.1515/revneuro-2022-0035
4. Jones HF, Han VX, Patel S, Gloss BS, Soler N, Ho A, et al. Maternal autoimmunity and inflammation are associated with childhood tics and obsessive-compulsive disorder: transcriptomic data show common enriched innate immune pathways. Brain Behav Immun. (2021) 94:308–17. doi: 10.1016/j.bbi.2020.12.035
5. Metzlaff J, Finis J, Münchau A, Müller-Vahl K, Schnitzler A, Bellebaum C, et al. Altered performance monitoring in Tourette syndrome: an MEG investigation. Sci Rep. (2022) 12(1):8300. doi: 10.1038/s41598-022-12156-x
6. Addabbo F, Baglioni V, Schrag A, Schwarz MJ, Dietrich A, Hoekstra PJ, et al. Anti-dopamine D2 receptor antibodies in chronic tic disorders. Dev Med Child Neurol. (2020) 62(10):1205–12. doi: 10.1111/dmcn.14613
7. Falk S, Petersen J, Svendsen C, Romero-Leguizamón CR, Jørgensen SH, Krauth N, et al. GLP-1 and nicotine combination therapy engages hypothalamic and mesolimbic pathways to reverse obesity. Cell Rep. (2023) 42(5):112466. doi: 10.1016/j.celrep.2023.112466
8. van Galen KA, Schrantee A, Ter Horst KW, la Fleur SE, Booij J, Constable RT, et al. Brain responses to nutrients are severely impaired and not reversed by weight loss in humans with obesity: a randomized crossover study. Nat Metab. (2023) 5(6):1059–72. doi: 10.1038/s42255-023-00816-9
9. Wang Y, Li N, Yang JJ, Zhao DM, Chen B, Zhang GQ, et al. Probiotics and fructo-oligosaccharide intervention modulate the microbiota-gut brain axis to improve autism spectrum reducing also the hyper-serotonergic state and the dopamine metabolism disorder. Pharmacol Res. (2020) 157:104784. doi: 10.1016/j.phrs.2020.104784
10. Leite F, Ribeiro L. Dopaminergic pathways in obesity-associated inflammation. J Neuroimmune Pharmacol. (2020) 15(1):93–113. doi: 10.1007/s11481-019-09863-0
11. Long H, Wang C, Ruan J, Zhang M, Huang Y. Gastrodin attenuates neuroinflammation in DOI-induce Tourette syndrome in rats. J Biochem Mol Toxicol. (2019) 33(5):e22302. doi: 10.1002/jbt.22302
12. McGrattan AM, McGuinness B, McKinley MC, Kee F, Passmore P, Woodside JV, et al. Diet and inflammation in cognitive ageing and Alzheimer's disease. Curr Nutr Rep. (2019) 8(2):53–65. doi: 10.1007/s13668-019-0271-4
13. Geisler CE, Hayes MR. Metabolic hormone action in the VTA: reward-directed behavior and mechanistic insights. Physiol Behav. (2023) 268:114236. doi: 10.1016/j.physbeh.2023.114236
14. Pirchio R, Graziadio C, Colao A, Pivonello R, Auriemma RS. Metabolic effects of prolactin. Front Endocrinol (Lausanne). (2022) 13:1015520. doi: 10.3389/fendo.2022.1015520
15. Low AYT, Goldstein N, Gaunt JR, Huang KP, Zainolabidin N, Yip AKK, et al. Reverse-translational identification of a cerebellar satiation network. Nature. (2021) 600(7888):269–73. doi: 10.1038/s41586-021-04143-5
16. DiFeliceantonio AG, Small DM. Dopamine and diet-induced obesity. Nat Neurosci. (2019) 22(1):1–2. doi: 10.1038/s41593-018-0304-0
17. Pedrosa MA, Labandeira CM, Valenzuela R, Quijano A, Sanchez-Andrade M, Suarez-Quintanilla JA, et al. AT1 Receptor autoantibodies mediate effects of metabolic syndrome on dopaminergic vulnerability. Brain Behav Immun. (2023) 108:255–68. doi: 10.1016/j.bbi.2022.12.009
18. Agarwal SM, Caravaggio F, Costa-Dookhan KA, Castellani L, Kowalchuk C, Asgariroozbehani R, et al. Brain insulin action in schizophrenia: something borrowed and something new. Neuropharmacology. (2020) 163:107633. doi: 10.1016/j.neuropharm.2019.05.010
19. Allison J, Kaliszewska A, Uceda S, Reiriz M, Arias N. Targeting DNA methylation in the adult brain through diet. Nutrients. (2021) 13(11):3979. doi: 10.3390/nu13113979
20. Rapp C, Hamilton J, Blum K, Thanos PK. The long-term interaction of diet and dopamine D2 gene expression on brain microglial activation. Psychiatry Res Neuroimaging. (2022) 320:111430. doi: 10.1016/j.pscychresns.2021.111430
21. Wang Y, Zhao L, Li AY. Gastrodin—a potential drug used for the treatment of Tourette syndrome. J Pharmacol Sci. (2021) 145(3):289–95. doi: 10.1016/j.jphs.2021.01.005
22. Barnes LL, Dhana K, Liu X, Carey VJ, Ventrelle J, Johnson K, et al. Trial of the MIND diet for prevention of cognitive decline in older persons. N Engl J Med. (2023) 389(7):602–11. doi: 10.1056/NEJMoa2302368
23. Dyńka D, Kowalcze K, Paziewska A. The role of ketogenic diet in the treatment of neurological diseases. Nutrients. (2022) 14(23):5003. doi: 10.3390/nu14235003
24. Knight E, Geetha T, Burnett D, Babu JR. The role of diet and dietary patterns in Parkinson’s disease. Nutrients. (2022) 14(21):4472. doi: 10.3390/nu14214472
25. Wells J, Swaminathan A, Paseka J, Hanson C. Efficacy and safety of a ketogenic diet in children and adolescents with refractory epilepsy-A review. Nutrients. (2020) 12(6):1809. doi: 10.3390/nu12061809
26. Abate G, Marziano M, Rungratanawanich W, Memo M, Uberti D. Nutrition and AGE-ing: focusing on Alzheimer’s disease. Oxid Med Cell Longev. (2017) 2017:7039816. doi: 10.1155/2017/7039816
27. Hsu KJ, Liao CD, Tsai MW, Chen CN. Effects of exercise and nutritional intervention on body composition, metabolic health, and physical performance in adults with sarcopenic obesity: a meta-analysis. Nutrients. (2019) 11(9):2163. doi: 10.3390/nu11092163
28. Koh S, Dupuis N, Auvin S. Ketogenic diet and neuroinflammation. Epilepsy Res. (2020) 167:106454. doi: 10.1016/j.eplepsyres.2020.106454
29. Delgado-Lista J, Alcala-Diaz JF, Torres-Peña JD, Quintana-Navarro GM, Fuentes F, Garcia-Rios A, et al. Long-term secondary prevention of cardiovascular disease with a Mediterranean diet and a low-fat diet (CORDIOPREV): a randomised controlled trial. Lancet. (2022) 399(10338):1876–85. doi: 10.1016/S0140-6736(22)00122-2
31. Ogbodo JO, Agbo CP, Njoku UO, Ogugofor MO, Egba SI, Ihim SA, et al. Alzheimer’s disease: pathogenesis and therapeutic interventions. Curr Aging Sci. (2022) 15(1):2–25. doi: 10.2174/1874609814666210302085232
32. Mazzone CM, Liang-Guallpa J, Li C, Wolcott NS, Boone MH, Southern M, et al. High-fat food biases hypothalamic and mesolimbic expression of consummatory drives. Nat Neurosci. (2020) 23(10):1253–66. doi: 10.1038/s41593-020-0684-9
33. Darcey VL, Guo J, Courville AB, Gallagher I, Avery JA, Simmons WK, et al. Dietary fat restriction affects brain reward regions in a randomized crossover trial. JCI Insight. (2023) 8(12):e169759. doi: 10.1172/jci.insight.169759
34. Grove JCR, Gray LA, La Santa Medina N, Sivakumar N, Ahn JS, Corpuz TV, et al. Dopamine subsystems that track internal states. Nature. (2022) 608(7922):374–80. doi: 10.1038/s41586-022-04954-0
35. Chu C, Li T, Yu L, Li Y, Li M, Guo M, et al. A low-protein, high-carbohydrate diet exerts a neuroprotective effect on mice with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinson’s disease by regulating the Microbiota-metabolite-brain axis and fibroblast growth factor 21. J Agric Food Chem. (2023) 71(23):8877–93. doi: 10.1021/acs.jafc.2c07606
36. Melchionda N, Cuzzolaro M. Parkinson’s disease, dopamine, and eating and weight disorders: an illness in the disease? Eat Weight Disord. (2019) 24(3):383–4. doi: 10.1007/s40519-019-00684-x
37. Schütteler C, Gerlach AL. Metakognitionen und interozeptive sensibilität bei der wahrnehmung des vorgefühls bei tic-störungen über die lebensspanne [metacognitions and interoceptive sensibility in the perception of premonitory urges in tic disorders across the lifespan]. Z Kinder Jugendpsychiatr Psychother. (2023) 51(4):275–82. (German). doi: 10.1024/1422-4917/a000910
38. Kleinridders A, Pothos EN. Impact of brain insulin signaling on dopamine function, food intake, reward, and emotional behavior. Curr Nutr Rep. (2019) 8(2):83–91. doi: 10.1007/s13668-019-0276-z
39. Long H, Ruan J, Zhang M, Wang C, Huang Y. Rhynchophylline attenuates Tourette syndrome via BDNF/NF-κB pathway in vivo and in vitro. Neurotox Res. (2019) 36(4):756–63. doi: 10.1007/s12640-019-00079-x
40. Hongyan L, Mengjiao Z, Chunyan W, Yaruo H. Rhynchophylline attenuates neurotoxicity in Tourette syndrome rats. Neurotox Res. (2019) 36(4):679–87. doi: 10.1007/s12640-019-00059-1
41. Blagotinšek Cokan K, Mavri M, Rutland CS, Glišić S, Senćanski M, Vrecl M, et al. Critical impact of different conserved endoplasmic retention motifs and dopamine receptor interacting proteins (DRIPs) on intracellular localization and trafficking of the D2 dopamine receptor (D2-R) isoforms. Biomolecules. (2020) 10(10):1355. doi: 10.3390/biom10101355
Keywords: Tic disorders, dopamine, nutrition, neuroinflammation, treatment
Citation: Bai L, Jin M, Zhang Q and Sun S (2025) Progress in research on nutrition, neuroinflammation and dopaminergic alterations in Tic disorders. Front. Pediatr. 13:1526117. doi: 10.3389/fped.2025.1526117
Received: 11 November 2024; Accepted: 5 May 2025;
Published: 20 May 2025.
Edited by:
Piero Pavone, University of Catania, ItalyCopyright: © 2025 Bai, Jin, Zhang and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Suzhen Sun, c3Vuc3V6aGVuX3NzekAxMjYuY29t
 Lu Bai1
Lu Bai1 Suzhen Sun
Suzhen Sun