- 1Department of Cardiology, Kunming Children's Hospital, Kunming City, Yunnan, China
- 2Department of Respiratory Medicine, Kunming Children's Hospital, Kunming City, Yunnan, China
- 3Department of Cardiology, The First Affiliated Hospital of Kunming Medical University, Kunming City, Yunnan, China
Introduction: Pediatric pulmonary arterial hypertension (PAH) is a rare and severe disorder characterized by obstructive vascular changes that can lead to right heart failure. The clinical presentation and underlying causes of pediatric PAH differ significantly from those in adults, often involving congenital heart disease and developmental lung disorders, such as bronchopulmonary dysplasia (BPD). Despite advances in treatment, pediatric PAH remains underrecognized globally.
Methods: This study analyzed global, regional, and national trends in pediatric PAH from 1990 to 2021 using data from the Global Burden of Disease (GBD) database.
Results: The findings indicate a stable prevalence rate globally, with a slight increase in the absolute number of cases. Significantly, reductions were observed in both mortality and disability-adjusted life years (DALYs) associated with pediatric PAH, with mortality decreasing by 57.66% and DALYs by 63.59% over the study period, indicating progress in mitigating the disease burden. Substantial regional disparities were identified, with low-income regions, particularly Low Socio-Demographic Index (SDI) areas, experiencing the highest mortality and DALY rates. In contrast, high-middle SDI regions showed the greatest reductions in disease burden. The highest prevalence and burden were observed in South Asia, the Caribbean, and parts of Sub-Saharan Africa, with China, India, and Haiti bearing the greatest national burdens.
Discussion: These findings highlight the necessity for targeted health interventions, especially in low-resource settings, to improve early diagnosis, intervention, and treatment.
Introduction
Pulmonary arterial hypertension (PAH) is a rare and severe disease characterized by progressive obstructive vascular lesions in the distal pulmonary arterial circulation, ultimately leading to right heart failure and death (1, 2). Recent studies have reported a mortality rate of 0.27 per 100,000 population in 2021 (3). The pathophysiological mechanisms of PAH are complex, often involving endothelial dysfunction, vascular remodeling, and inflammation (4). The presentation of this disease in children differs significantly from that in adults, with more diverse etiologies often associated with various underlying conditions (5). In contrast, PAH in adults is more commonly secondary to left heart disease or chronic lung disease, highlighting the distinct challenges in the management of pediatric PAH (6, 7). Pediatric PAH can occur at any age and is frequently associated with developmental or genetic disorders, with an etiological distribution distinct from that seen in adults (8, 9).
The major causes of pediatric PAH include idiopathic pulmonary arterial hypertension (IPAH), PAH associated with congenital heart disease (PAH-CHD) (10), and PAH related to developmental lung disease (5). Among children, PAH-CHD is one of the most common forms, particularly prevalent in those with congenital heart defects who have not undergone timely surgical intervention (11). Developmental lung diseases, such as bronchopulmonary dysplasia (BPD) and prematurity, also contribute significantly to the etiology of pediatric PAH (12).
Despite significant advances in the treatment of PAH in recent years, the prognosis for pediatric PAH remains poor, and its global burden has not been fully recognized (3). Utilizing data from the Global Burden of Disease (GBD) database, evaluating the global, regional, and national burden of pediatric PAH is crucial for understanding its epidemiology, formulating effective public health strategies, and improving patient care and management. This study aims to systematically assess the global, regional, and national burden of pediatric PAH from 1990 to 2021 using the GBD database, analyze its epidemiological trends, and provide a scientific basis for future interventions.
Globally, there are significant disparities in the disease burden between different regions, influenced not only by genetic factors but also by differences in access to healthcare resources and diagnostic capabilities (13). In many low- and middle-income countries, early identification and intervention for pediatric PAH remain challenging due to a lack of adequate diagnostic and treatment facilities (14). Additionally, the nonspecific symptoms of pediatric PAH frequently result in misdiagnosis or diagnostic delays, with most cases identified only during advanced disease stages (15, 16). Therefore, increasing awareness of pediatric PAH and enhancing early screening and diagnosis are critical for improving outcomes in affected children.
Methods
Overview and data collection
This study was conducted with approval from Kunming Children's Hospital. The Ethics Committee of Kunming Children's Hospital granted a waiver of informed consent, as the study involved only secondary data analysis of anonymized information without any personally identifiable data. Data on pediatric PAH in children aged 0–14 years were obtained from the Global Health Data Exchange (GHDx) query tool, developed by the GBD collaborators, using standardized disease definitions and prevalence estimates. To access similar data, visit the GHDx website at: https://ghdx.healthdata.org/.
The 2021 GBD study assessed prevalence, mortality, and disability-adjusted life years (DALYs), along with corresponding rates and uncertainty intervals, for 369 diseases and injuries across 204 countries and territories from 1990 to 2021 (17). The data on the prevalence and number of PAH cases, PAH-related mortality, and the burden of pediatric PAH-related comorbidities, as well as corresponding rates at the global, regional, and national levels, were collected.
The GBD database did not provide information on racial or ethnic data for participants, as race and ethnicity were not assigned during data collection. Linear regression analysis was performed to estimate the annual percentage change (EAPC) (18). This study adhered to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines (19).
Sociodemographic Index
The Socio-demographic Index (SDI) is a key metric that integrates fertility rate, educational attainment, and per capita income to gauge the level of development of a country or region, ranging from 0 to 1, with higher values indicating greater socio-economic advancement (20). Previous studies have demonstrated a significant association between SDI and both disease prevalence and mortality (21). In this study, countries and geographic regions are categorized into five SDI levels (low, low-middle, middle, high-middle, and high) to explore the relationship between socioeconomic development and the burden of pediatric PAH.
Definitions
Historically, PAH in children has been defined using the same criteria as in adults: a mean pulmonary arterial pressure (mPAP) of ≥25 mmHg (22, 23). In normal fetal circulation, pulmonary arterial pressure is comparable to systemic pressure, but it declines rapidly after birth, reaching levels similar to those of adults by the age of 2–3 months. Given the variability in pulmonary hemodynamics during the postnatal transition, pediatric PAH is defined as an mPAP of ≥25 mmHg after 3 months of age (5). Estimation of prevalence, incidence, and mortality followed the standard GBD workflow but incorporated paediatric-specific assumptions to address data sparsity. Briefly, DisMod-MR 2.1 Bayesian meta-regression pooled all available hospital discharge, administrative claims, and registry data (21 countries, 11 paediatric cohorts) across ages and imposed age-specific random effects. A zero-prevalence prior was applied from birth to 3 months, with a spline-based ramp from 3 months to 15 years to reflect the biological onset of disease. Covariates capturing national CHD prevalence, neonatal survival, HIV prevalence, and the SDI were introduced to borrow strength where pediatric data were scarce. Cause-specific mortality was modelled with CODEm, constrained to the all-cause mortality envelope, and uncertainty was propagated through 1,000 posterior draws. All estimates are presented with 95% uncertainty intervals (UIs) (24).
Statistical analysis
Prevalence, mortality, DALYs, and their respective ratios are key indicators of the burden of pediatric PAH. Using the GBD methodology, we report prevalence rates per 100,000 population, along with the corresponding 95% UI. To analyze temporal trends in pediatric PAH, we used the EAPC to assess shifts in disease burden over time, with linear modeling employed to derive the 95% confidence interval (CI) for the EAPC. If the upper bound of the EAPC and its 95% CI are negative, this suggests a declining trend in prevalence; conversely, if the lower bound of the EAPC and its 95% CI are positive, this indicates an increasing trend (25).
Results
PAH in children: global trends
Prevalence
In 2021, the estimated global prevalence of pediatric PAH was 8,973.59 cases (95% UI, 6,312.64–11,961.61), compared to 7,614.65 cases (95% UI, 5,374.55–10,085.40) in 1990. From 1990 to 2021, the number of prevalent cases increased by 17.85% (95% UI, 0.22–3.37). Despite this increase, the prevalence rate showed minimal change, rising slightly from 0.44 (95% UI, 0.31–0.58) per 100,000 in 1990 to 0.45 (95% UI, 0.31–0.59) per 100,000 in 2021. EAPC was −0.01 (95% CI, −0.05–0.03) (Table 1).
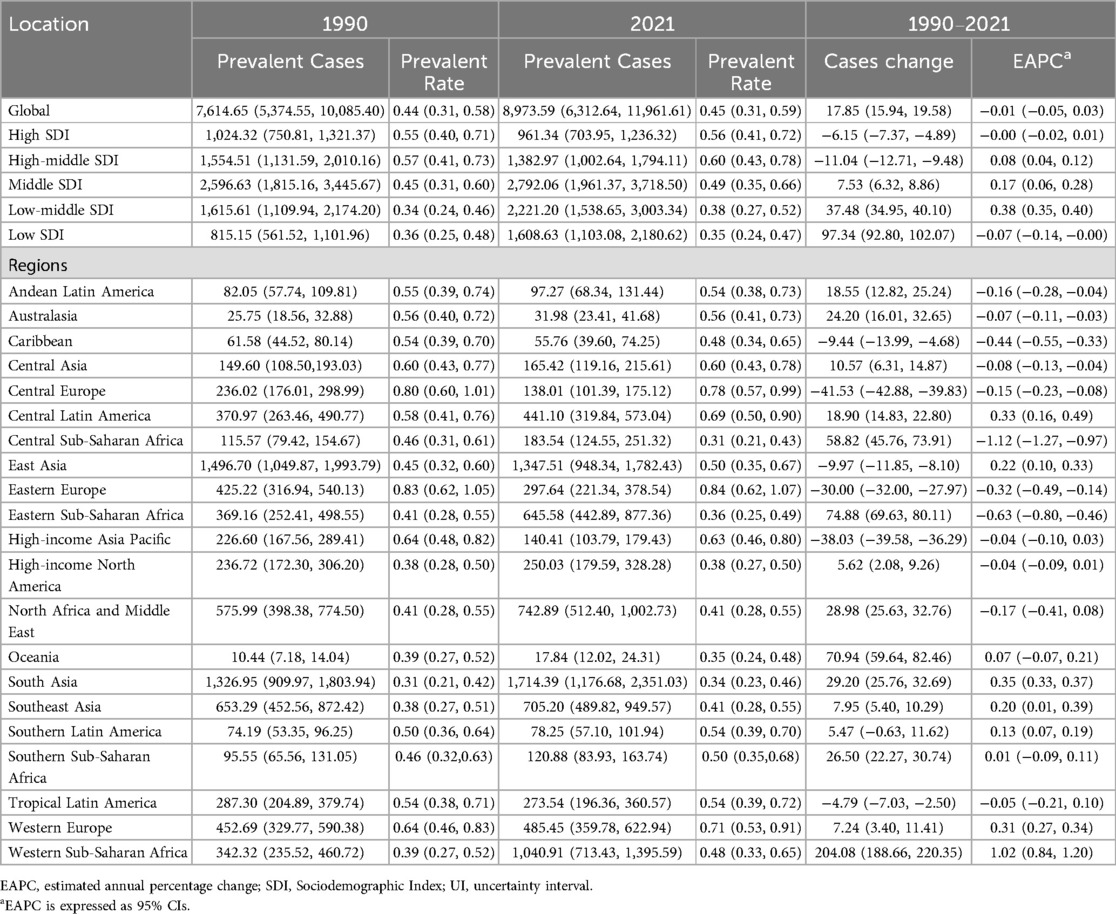
Table 1. Prevalence of pediatric pulmonary arterial hypertension at global and regional levels from 1990 to 2021.
Mortality
In 2021, an estimated 1,714.56 deaths (95% UI, 1,357.56–2,102.27) were attributed to pediatric PAH globally, marking a substantial decline from 4,049.32 deaths (95% UI, 2,365.91–5,629.40) reported in 1990. From 1990 to 2021, PAH-related mortality in children decreased by 57.66% (95% UI, −68.78 to −38.89). Similarly, the PAH-related mortality rate declined from 0.23 (95% UI, 0.14–0.32) per 100,000 in 1990 to 0.09 (95% UI, 0.07–0.10) per 100,000 in 2021, with an EAPC of −2.60 (95% CI, −2.83 to −2.39) (Supplementary Table S1).
DALYs
The burden of pediatric PAH, measured by DALYs, showed a marked reduction. In 2021, the number of DALYs attributable to pediatric PAH was 151,098.12 (95% UI, 119,751.94–185,128.35), significantly lower than the 358,741.23 (95% UI, 209,032.75–498,948.49) reported in 1990, representing a 63.59% reduction (95% UI, −73.18 to −47.38). The DALY rate also decreased from 20.63 (95% UI, 12.02–28.69) per 100,000 in 1990 to 7.51 (95% UI, 5.95–9.20) per 100,000 in 2021, with an EAPC of −2.62 (95% CI, −2.84 to −2.40) (Supplementary Table S2).
PAH in children: SDI regional trends
Prevalence
In 1990, the Middle SDI region reported the highest number of pediatric PAH cases, totaling 2,596.63 (95% UI, 1,815.16–3,445.67). This trend persisted in 2021, with the number of cases rising to 2,792.06 (95% UI, 1,961.37–3,718.50). Despite the increase in absolute case numbers, the prevalence rate in the Middle SDI region showed minimal change, shifting from 0.45 (95% UI, 0.31–0.60) per 100,000 in 1990 to 0.49 (95% UI, 0.35–0.66) per 100,000 in 2021. The Low-middle SDI region experienced the most pronounced increase in prevalence rate (EAPC, 0.38; 95% CI, 0.35–0.40), whereas the Low SDI region exhibited the largest decrease (EAPC, −0.07; 95% CI, −0.14 to −0.00) (Table 1 and Figure 1A).
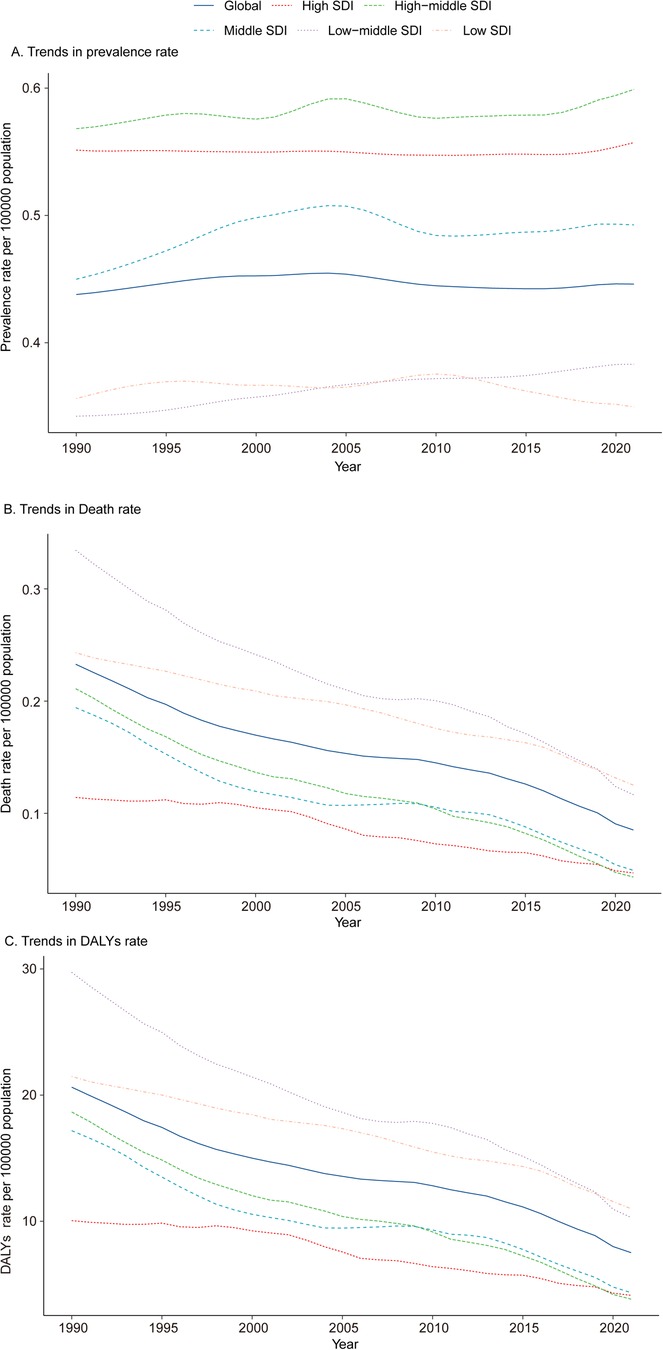
Figure 1. Epidemiologic trends in prevalence, mortality, and disability-adjusted life years (DALYs) of pediatric pulmonary arterial hypertension across five sociodemographic Index (SDI) regions from 1990 to 2021. (A) Trends in Prevalence Rate. (B) Trends in Mortality. (C) Trends in DALY Rate. DALYs, disability-adjusted life-years.
Mortality
Among the five SDI regions, only the Low SDI region exhibited an increase in mortality, rising by 3.48%. In 2021, the Low SDI region also had the highest number of PAH-related deaths, totaling 575.91 (95% UI, 420.09–796.75). In contrast, the High-middle SDI region demonstrated the largest decline in mortality, decreasing by 82.69%, and had the fewest PAH-related deaths in 2021, with 81.01 (95% UI, 73.34–87.79). The pediatric PAH-related mortality rate in 2021 was highest in the Low SDI region, at 0.13 (95% UI, 0.09–0.17) per 100,000, while the High-middle SDI region reported the lowest rate, at 0.04 (95% UI, 0.04–0.06) per 100,000. The most significant decline in mortality rate, as indicated by the lowest EAPC, was observed in the High-middle SDI region (−4.17; 95% CI, −4.54 to −3.80) (Supplementary Table S1 and Figure 1B). A heatmap further illustrates the shifts in the ranking of cardiovascular disease causes of death among children under 14 years old between 1990 and 2021 (per 100,000 population) (Figure 2).
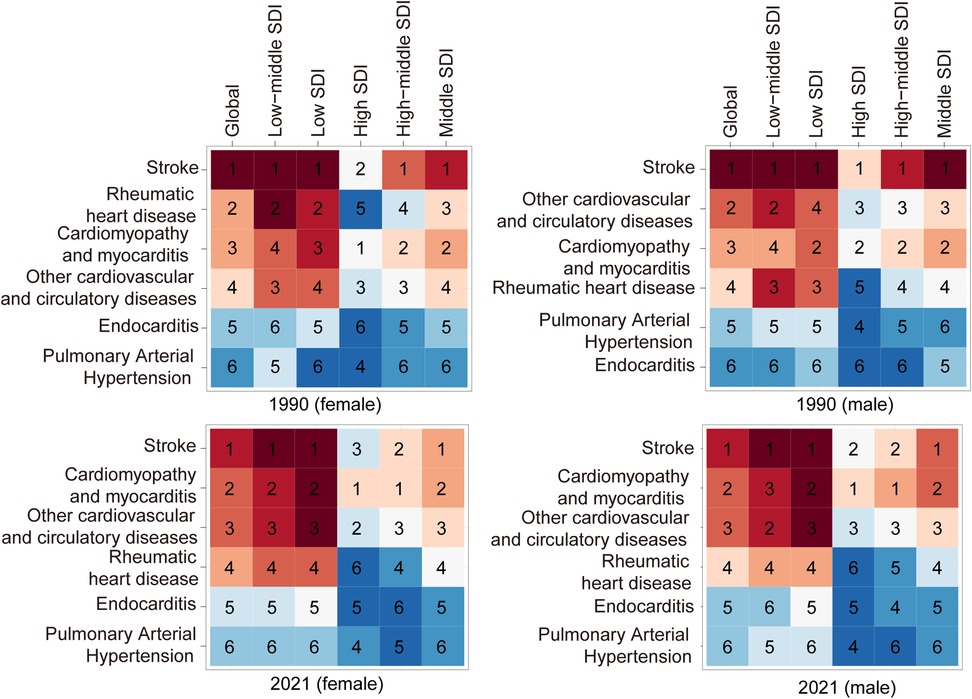
Figure 2. Changes in causes of cardiovascular mortality among children globally and across different sociodemographic Index (SDI) regions: a comparison Between 1990 and 2021. Heatmap representing a 6 × 6 data matrix with inverse intensity scaling (1 = highest value, 6 = lowest value).
DALYs
In 2021, the Low SDI region recorded the highest number of DALYs attributable to PAH, with 50,592.43 (95% UI, 36,915.79–69,903.99), representing a 48.83% reduction from 1990. The DALY rate for pediatric PAH in 2021 was also highest in the Low SDI region, at 10.99 (95% UI, 8.02–15.19) per 100,000, while the High-middle SDI region had the lowest DALY rate, at 3.83 (95% UI, 3.15–4.86) per 100,000. Notably, the High-middle SDI region exhibited the most significant reduction in DALY rate, as indicated by the highest EAPC (−1.89; 95% CI, −2.03 to −1.75) (Supplementary Table S2 and Figure 1C).
PAH in children: geographic regional trends
Prevalence
In 2021, South Asia reported the highest number of pediatric PAH cases among the 21 global regions, with an estimated 1,714.39 cases (95% UI, 1,176.68–2,351.03), while Oceania had the lowest number of cases, at 17.84 (95% UI, 12.02–24.31). The highest prevalence rate was observed in South Asia, whereas Oceania had the lowest. From 1990 to 2021, the most pronounced increases in pediatric PAH prevalence rates were seen in Western Sub-Saharan Africa (EAPC, 1.02; 95% CI, 0.84–1.20), South Asia (EAPC, 0.35; 95% CI, 0.16–0.49), and Central Latin America (EAPC, 0.33; 95% CI, −0.09–0.11). By contrast, Southern Sub-Saharan Africa exhibited the smallest increase in prevalence rate (EAPC, 0.01; 95% CI, −0.09–0.11) (Table 1 and Figure 3A).
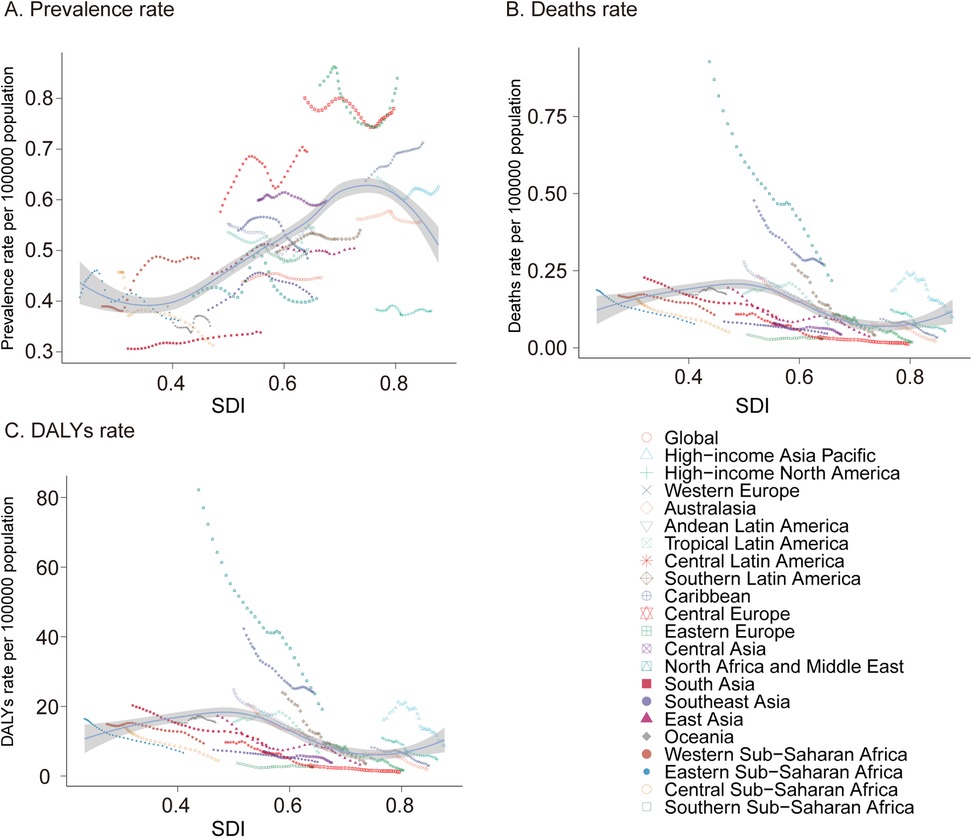
Figure 3. Prevalence, mortality, and disability-adjusted life years (DALYs) of pediatric pulmonary arterial hypertension from 1990 to 2021. (A) Prevalence rate. (B) Mortality. (C) DALYs rate. DALYs, disability-adjusted life-years.
Mortality
In 2021, South Asia also recorded the highest number of pediatric PAH-related deaths, with 508.77 cases (95% UI, 297.82–784.85), whereas Australasia had the lowest number, with 1.203 cases (95% UI, 0.98–1.48). The highest mortality rate was observed in the Caribbean (0.27; 95% UI, 0.10–0.49), while Central Europe had the lowest mortality rate (0.01; 95% UI, 0.01–0.02). Notably, Oceania was the only region that experienced an increase in the pediatric PAH-related mortality rate (EAPC, 0.16; 95% CI, −0.01–0.33), whereas Southern Sub-Saharan Africa showed the smallest decline (EAPC, −0.29; 95% CI, −0.67–0.09), and Southern Latin America exhibited the most substantial decrease (EAPC, −5.62; 95% CI, −5.90 to −5.34) (Supplementary Table S1 and Figure 3B).
DALYs
In terms of DALYs attributable to pediatric PAH in 2021, South Asia had the highest burden, with 45,121.64 DALYs (95% UI, 26,371.51–69,880.78), while Australasia had the lowest, with 107.39 DALYs (95% UI, 87.40–132.29). The Caribbean reported the highest DALY rate (24.09; 95% UI, 9.28–43.47), whereas Central Europe had the lowest rate (1.13; 95% UI, 0.99–1.47). Between 1990 and 2021, Southern Sub-Saharan Africa had the smallest decline in DALY rate (EAPC, −0.3; 95% CI, −0.68–0.08), while East Asia experienced the most marked reduction (EAPC, −5.65; 95% CI, −5.93 to −5.37) (Supplementary Table S2 and Figure 3C). These findings highlight significant regional disparities in the prevalence, mortality, and overall disease burden of pediatric PAH, with the greatest burden observed in South Asia and the Caribbean and the least in Australasia and Central Europe. The variations in trends across regions underscore the need for targeted interventions to address the specific challenges faced by each region.
PAH in children: national trends
Prevalence
In 2021, China reported the highest number of pediatric PAH cases among 204 countries, with an estimated 1,308 cases (95% UI, 920–1,730), reflecting a decrease of 8.95% since 1990 (95% UI, −10.81 to −7.04). In contrast, Niue and Tokelau reported no pediatric PAH cases. Switzerland had the highest prevalence rate (1.45 per 100,000; 95% UI, 1.03–1.88), whereas Angola had the lowest (0.26 per 100,000; 95% UI, 0.18–0.37). Nigeria experienced the largest increase in prevalence rate (EAPC, 1.75; 95% CI, 1.51–1.98), while Chile had the smallest increase (EAPC, 0.01; 95% CI, −0.14 to 0.15). Egypt demonstrated the most substantial decrease in prevalence rate (EAPC, −1.78; 95% CI, −2.10 to −1.47) (Supplementary Table S3, Figure 4A, and Supplementary Figure S1A). In 2021, Switzerland (SDI, 0.93) had the highest prevalence rate, while Angola (SDI, 0.45) had the lowest (Supplementary Table S3A).
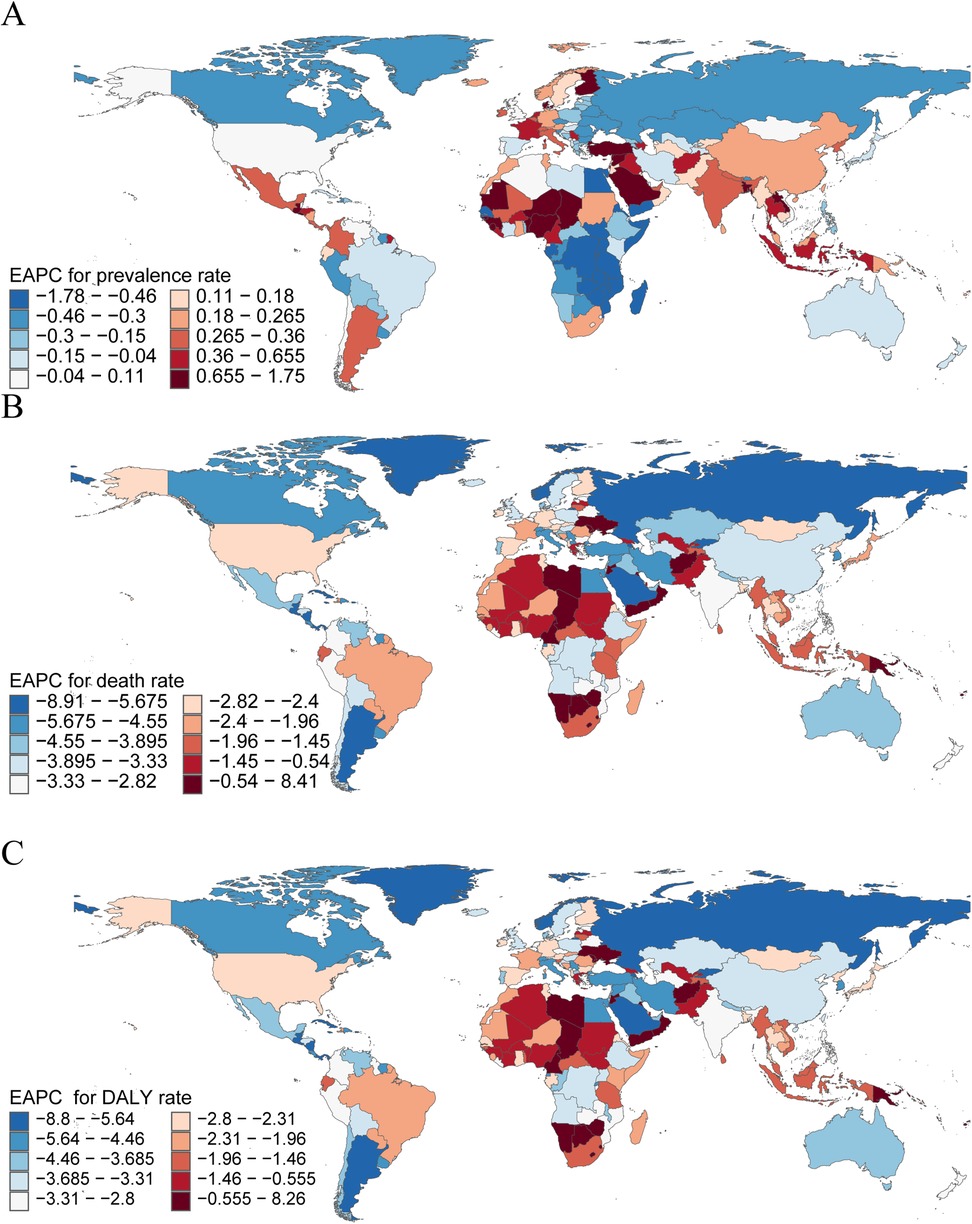
Figure 4. The national burden of pediatric pulmonary arterial hypertension in 204 countries and territories. (A) EAPC for Prevalence rate. (B) EAPC for Mortality. (C) EAPC for DALYs rate. disability-adjusted life-years; EAPC, estimated annual percentage change.
Mortality
In 2021, India recorded the highest number of pediatric PAH-related deaths, with 295 cases (95% UI, 178–472), representing a decrease of 57.86% since 1990 (95% UI, −70.00 to −32.43). San Marino reported the fewest deaths, with only 0.0002 cases (95% UI, 0.0001–0.0005). Haiti had the highest mortality rate (0.61 per 100,000; 95% UI, 0.20–1.17). The Republic of Mauritius experienced the largest increase in mortality rate (EAPC, 8.41; 95% CI, 5.76–11.13), while Puerto Rico (EAPC, −8.21; 95% CI, −9.36 to −7.05), Belize (EAPC, −8.91; 95% CI, −9.51 to −8.30), and Norway (EAPC, −8.38; 95% CI, −9.33 to −7.42) had the most significant declines (Supplementary Table S4, Figure 4B, and Supplementary Figure S1B). In 2021, Haiti (SDI, 0.45) had the highest mortality rate, while Slovenia (SDI, 0.84) had the lowest (Supplementary Figure S3B).
DALYs
In 2021, India had the highest number of DALYs attributable to pediatric PAH, with 26,219 DALYs (95% UI, 15,801–42,145), reflecting a decrease of 57.93% since 1990 (95% UI, −70.02 to −32.47). San Marino had the fewest DALYs, with 0.03 (95% UI, 0.01–0.05). Haiti reported the highest DALY rate (54.18 per 100,000; 95% UI, 17.26–103.28). Mauritius experienced the largest increase in DALY rate (EAPC, 8.26; 95% CI, 1.98–3.82), whereas Puerto Rico (EAPC, −8.80; 95% CI, −9.40 to −8.20) and Belize (EAPC, −8.42; 95% CI, −8.95 to −7.89) showed the most significant declines (Supplementary Table S5, Figure S3C, and Figure S1C). In 2021, Haiti (SDI, 0.45) had the highest DALY rate, while Slovenia (SDI, 0.84) had the lowest (Supplementary Figure S3C). These findings underscore substantial disparities in pediatric PAH prevalence, mortality, and disease burden across countries, with the highest burdens observed in China, India, and Haiti. The notable variations across countries and regions highlight the importance of targeted health interventions tailored to the specific challenges of each region.
Discussion
Over the past three decades, the prevalence of PAH among children aged 0–14 has steadily increased worldwide, reflecting an urgent public health concern given the associated medical and social costs. This study leverages data from the GBD database from 1990 to 2021 to examine the prevalence, mortality, and DALYs attributable to pediatric PAH across different regions and countries. The findings provide critical insights into the burden of pediatric PAH in countries with varying income levels, highlighting a consistent rise in prevalence in specific areas over the past thirty years. A comprehensive global assessment of the epidemiological trends of pediatric PAH offers valuable guidance for policymakers and clinicians to develop more effective prevention and management strategies, ultimately enhancing health outcomes for affected children.
Global analyses of pediatric PAH over the past three decades reveal that the overall prevalence of the disease has remained essentially unchanged, consistent with improved survival and earlier detection, even as mortality and disease burden have markedly decreased. Prevalence rates in 2021 were similar to those in 1990, indicating that the prevalence of pediatric PAH and the pool of affected children have not been drastically reduced over time. The number of pediatric PAH cases grew (reflecting population growth and improved detection), yet mortality and DALYs dropped by over 50%. This divergence suggests that while children continue to develop PAH at comparable rates, fewer are dying from it, and those affected are living longer, healthier lives. The data underscore a major success in pediatric PAH management: improved survival and reduced health losses, despite no substantial change in how often the condition occurs. These trends largely reflect advances in medical care, rather than the primary prevention of PAH. The stable prevalence implies that the root causes—congenital heart disease, heritable mutations, neonatal conditions, and other pediatric risk factors—remain prevalent worldwide. However, the steep reduction in mortality and DALYs signals that modern interventions are altering the disease's trajectory. Children with PAH today experience a more chronic course and longer survival than in the 1990s, when the diagnosis was often swiftly fatal. Notably, in the pre-prostacyclin era (before ∼1995), untreated idiopathic PAH in children carried a dismal median survival of only about 10 months, compared to ∼2.8 years in adults (26). By contrast, in the current era of targeted treatments, pediatric registries report 5-year survival rates on the order of 70%–85%—a remarkable improvement attributable to better therapies and multidisciplinary care (27). In essence, PAH has evolved from an acutely lethal pediatric disease to a more manageable chronic condition for many patients, thanks to progress in treatment and supportive care.
Regional differences in PAH etiologies significantly contribute to these observed disparities. For instance, in low-SDI regions, limited healthcare resources impede timely surgical interventions for PAH-CHD. Consequently, many pediatric patients develop severe pulmonary hypertension, including conditions such as Eisenmenger syndrome, greatly amplifying disease burden and mortality risk (28). In contrast, high-income regions, equipped with advanced pediatric cardiac surgical facilities, can perform timely interventions soon after birth, markedly reducing the progression of PAH. IPAH appears more frequently in high-SDI regions, likely reflecting enhanced diagnostic capabilities and increased clinical awareness. Robust pediatric cardiopulmonary specialty care systems in these regions facilitate early identification and diagnosis of IPAH, elevating its proportion among total PAH cases. Additionally, children in these areas benefit from prompt access to advanced pharmacological therapies, such as targeted therapies, significantly improving survival rates and reducing mortality (29). Developmental PAH predominantly emerges in regions with superior healthcare infrastructure, correlating with increased survival rates among premature infants and advancements in neonatal intensive care unit technologies. Due to the complexity and high medical costs associated with diagnosing and treating developmental PAH, its epidemiological characteristics remain inadequately captured in low-SDI regions.
Collectively, the significant correlation between pediatric PAH mortality and SDI is not coincidental; rather, it is the outcome of combined differences in etiology distribution and healthcare resource accessibility influenced by varied socioeconomic contexts. Hence, strengthening pediatric cardiac disease screening, diagnosis, and treatment capacities in low-SDI regions—especially enhancing early surgical intervention rates for congenital heart diseases—is critical to narrowing global disparities in pediatric PAH burdens. Furthermore, fostering international collaboration to standardize diagnosis and treatment approaches for idiopathic and developmental PAH and promoting equitable access to targeted pharmacological therapies are essential strategies for improving global pediatric PAH prognosis and disease burden.
In conclusion, despite considerable progress in reducing pediatric PAH mortality and morbidity globally, significant healthcare inequities persist, particularly affecting low-SDI regions. Continued efforts to enhance healthcare accessibility, coupled with targeted interventions, are necessary to ensure equitable and effective PAH treatment for all children, regardless of geographical location. Such initiatives are pivotal for achieving global health equity and meeting Sustainable Development Goals.
Limitations
This analysis inherits several methodological constraints intrinsic to GBD modelling, particularly salient for rare pediatric conditions such as PAH. First, primary data for PAH are sparse and geographically uneven: many low- and middle-income countries contribute few or no direct observations, compelling GBD to rely on Bayesian hierarchies and multiple-imputation procedures to borrow strength across covariates, locations, and time (13, 30). Although these techniques represent the current standard, they risk perpetuating undetected biases, and their 95% UIs may fail to adequately account for potential errors, particularly in regions with limited diagnostic infrastructure. Second, the GBD cause hierarchy aggregates all forms of PAH into a single category, precluding stratification by etiology (e.g., PAH secondary to congenital heart disease, idiopathic PAH, or developmentally mediated PAH). Because the necessary subtype-specific data were not available, our results reflect an averaged signal across heterogeneous phenotypes that differ in pathogeny, prognosis, and treatment response. This unavoidable aggregation limits the clinical granularity of the findings and should be considered when extrapolating the estimates to policy or practice.
Conclusions
The burden of pediatric PAH in 2021—and its trajectory since 1990—varies sharply by SDI quintile and region. Progress in high SDI settings contrasts with persistent or rising burden in low SDI regions, reflecting unequal access to diagnosis and care. Closing this gap requires: (1) universal neonatal and early childhood screening; (2) regional PAH registries with open data sharing; and (3) pooled procurement and capacity building to secure PAH specific drugs and multidisciplinary care. Implementing these measures would translate therapeutic advances into equitable health gains worldwide.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author contributions
LD: Software, Writing – original draft. JXi: Methodology, Writing – original draft. JXu: Validation, Writing – original draft. QL: Visualization, Writing – original draft. ZC: Methodology, Resources, Software, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2025.1527281/full#supplementary-material
Supplementary Figure S1 | Prevalence, Mortality, and Disability-Adjusted Life Year (DALY) Cases of Pediatric Pulmonary Arterial Hypertension Across 204 Countries and Territories. (A) Prevalent cases. (B) Death cases. (C) DALYs cases. DALYs=disability-adjusted life-years.
Supplementary Figure S2 | The Prevalence, Mortality, and DALY Rates of Pediatric Pulmonary Arterial Hypertension in 204 Countries and Territories. (A) Disease burden based on Prevalence rate. (B) Disease burden based on Mortality rate. (C) Disease burden based on DALY rate. DALY=Disability-Adjusted Life Year.
Supplementary Figure S3 | Prevalence, Mortality, and DALY Rates of Pediatric Pulmonary Arterial Hypertension Across 204 Countries by Sociodemographic Index (SDI) in 2021. (A) Prevalence rate. (B) Mortality. (C) DALY rate. DALY, disability-adjusted life year; SDI, socio-demographic index.
References
1. Aliotta JM, Pereira M, Wen S, Dooner MS, Del Tatto M, Papa E, et al. Exosomes induce and reverse monocrotaline-induced pulmonary hypertension in mice. Cardiovasc Res. (2016) 110(3):319–30. doi: 10.1093/cvr/cvw054
2. Semen K, Yelisyeyeva O, Jarocka-Karpowicz I, Kaminskyy D, Solovey L, Skrzydlewska E, et al. Sildenafil reduces signs of oxidative stress in pulmonary arterial hypertension: evaluation by fatty acid composition, level of hydroxynonenal and heart rate variability. Redox Biol. (2016) 7:48–57. doi: 10.1016/j.redox.2015.11.009
3. GBD 2021 Pulmonary Arterial Hypertension Collaborators. Global, regional, and national burden of pulmonary arterial hypertension, 1990-2021: a systematic analysis for the Global Burden of Disease Study 2021. Lancet Respir Med. (2025) 13(1):69–79. doi: 10.1016/s2213-2600(24)00295-9
4. Wauchope OR, Mitchener MM, Beavers WN, Galligan JJ, Camarillo JM, Sanders WD, et al. Oxidative stress increases M1dG, a major peroxidation-derived DNA adduct, in mitochondrial DNA. Nucleic Acids Res. (2018) 46(7):3458–67. doi: 10.1093/nar/gky089
5. Rosenzweig EB, Abman SH, Adatia I, Beghetti M, Bonnet D, Haworth S, et al. Paediatric pulmonary arterial hypertension: updates on definition, classification, diagnostics and management. Eur Respir J. (2019) 53(1):1801916. doi: 10.1183/13993003.01916-2018
6. Madonna R, Biondi F, Ghelardoni S, D'Alleva A, Quarta S, Massaro M, et al. Pulmonary hypertension associated to left heart disease: phenotypes and treatment. Eur J Intern Med. (2024) 129:1–15. doi: 10.1016/j.ejim.2024.07.030
7. Burgstaller G, Oehrle B, Gerckens M, White ES, Schiller HB, Eickelberg O, et al. The instructive extracellular matrix of the lung: basic composition and alterations in chronic lung disease. Eur Respir J. (2017) 50(1):1601805. doi: 10.1183/13993003.01805-2016
8. Barst RJ, McGoon MD, Elliott CG, Foreman AJ, Miller DP, Ivy DD, et al. Survival in childhood pulmonary arterial hypertension: insights from the registry to evaluate early and long-term pulmonary arterial hypertension disease management. Circulation. (2012) 125(1):113–22. doi: 10.1161/circulationaha.111.026591
9. Berger RM, Beghetti M, Humpl T, Raskob GE, Ivy DD, Jing ZC, et al. Clinical features of paediatric pulmonary hypertension: a registry study. Lancet. (2012) 379(9815):537–46. doi: 10.1016/s0140-6736(11)61621-8
10. Hoeper MM, Dwivedi K, Pausch C, Lewis RA, Olsson KM, Huscher D, et al. Phenotyping of idiopathic pulmonary arterial hypertension: a registry analysis. Lancet Respir Med. (2022) 10(10):937–48. doi: 10.1016/s2213-2600(22)00097-2
11. Beghetti M, Channick RN, Chin KM, Di Scala L, Gaine S, Ghofrani HA, et al. Selexipag treatment for pulmonary arterial hypertension associated with congenital heart disease after defect correction: insights from the randomised controlled GRIPHON study. Eur J Heart Fail. (2019) 21(3):352–9. doi: 10.1002/ejhf.1375
12. Agarwal S, Fineman J, Cornfield DN, Alvira CM, Zamanian RT, Goss K, et al. Seeing pulmonary hypertension through a paediatric lens: a viewpoint. Eur Respir J. (2024) 63(6):2301518. doi: 10.1183/13993003.01518-2023
13. Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the global burden of disease study. Lancet. (2020) 395(10219):200–11. doi: 10.1016/s0140-6736(19)32989-7
14. Faruk T, King C, Muhit M, Islam MK, Jahan I, Baset KU, et al. Screening tools for early identification of children with developmental delay in low- and middle-income countries: a systematic review. BMJ Open. (2020) 10(11):e038182. doi: 10.1136/bmjopen-2020-038182
15. Morales-Demori R, Coleman R, Mallory GB. Pediatric pulmonary hypertension. Pediatr Rev. (2024) 45(5):251–9. doi: 10.1542/pir.2023-006010
16. Jone PN, Ivy DD, Hauck A, Karamlou T, Truong U, Coleman RD, et al. Pulmonary hypertension in congenital heart disease: a scientific statement from the American Heart Association. Circ Heart Fail. (2023) 16(7):e00080. doi: 10.1161/hhf.0000000000000080
17. GBD 2021 Diabetes Collaborators. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet. (2023) 402(10397):203–34. doi: 10.1016/s0140-6736(23)01301-6
18. Lv B, Lan JX, Si YF, Ren YF, Li MY, Guo FF, et al. Epidemiological trends of subarachnoid hemorrhage at global, regional, and national level: a trend analysis study from 1990 to 2021. Mil Med Res. (2024) 11(1):46. doi: 10.1186/s40779-024-00551-6
19. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Bull World Health Organ. (2007) 85(11):867–72. doi: 10.2471/blt.07.045120
20. Feigin VL, Nguyen G, Cercy K, Johnson CO, Alam T, Parmar PG, et al. Global, regional, and country-specific lifetime risks of stroke, 1990 and 2016. N Engl J Med. (2018) 379(25):2429–37. doi: 10.1056/NEJMoa1804492
21. GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. (2020) 396(10258):1204–22. doi: 10.1016/s0140-6736(20)30925-9
22. Simonneau G, Montani D, Celermajer DS, Denton CP, Gatzoulis MA, Krowka M, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. (2019) 53(1):1801913. doi: 10.1183/13993003.01913-2018
23. Truong U, Patel S, Kheyfets V, Dunning J, Fonseca B, Barker AJ, et al. Non-invasive determination by cardiovascular magnetic resonance of right ventricular-vascular coupling in children and adolescents with pulmonary hypertension. J Cardiovasc Magn Reson. (2015) 17(1):81. doi: 10.1186/s12968-015-0186-1
24. GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. (2018) 392(10159):1789–858. doi: 10.1016/s0140-6736(18)32279-7
25. Caraceni A, Hanks G, Kaasa S, Bennett MI, Brunelli C, Cherny N, et al. Use of opioid analgesics in the treatment of cancer pain: evidence-based recommendations from the EAPC. Lancet Oncol. (2012) 13(2):e58–68. doi: 10.1016/s1470-2045(12)70040-2
26. D'Alonzo GE, Barst RJ, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, et al. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med. (1991) 115(5):343–9. doi: 10.7326/0003-4819-115-5-343
27. Avitabile CM, Vorhies EE, Ivy DD. Drug treatment of pulmonary hypertension in children. Paediatr Drugs. (2020) 22(2):123–47. doi: 10.1007/s40272-019-00374-2
28. Chaix MA, Gatzoulis MA, Diller GP, Khairy P, Oechslin EN. Eisenmenger syndrome: a multisystem disorder—do not destabilize the balanced but fragile physiology. Can J Cardiol. (2019) 35(12):1664–74. doi: 10.1016/j.cjca.2019.10.002
29. Shah AJ, Beckmann T, Vorla M, Kalra DK. New drugs and therapies in pulmonary arterial hypertension. Int J Mol Sci. (2023) 24(6):5850. doi: 10.3390/ijms24065850
30. Foreman KJ, Marquez N, Dolgert A, Fukutaki K, Fullman N, McGaughey M, et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: reference and alternative scenarios for 2016–40 for 195 countries and territories. Lancet. (2018) 392(10159):2052–90. doi: 10.1016/s0140-6736(18)31694-5
Keywords: pulmonary arterial hypertension (PAH), public health, GBD (2021) database, prevalence, mortaltity, DALYs—disability-adjusted life years
Citation: Deng L, Xiong J, Xu J, Li Q and Cheng Z (2025) Burden of pulmonary arterial hypertension in children globally, regionally, and nationally (1990–2021): results from the global burden of disease study. Front. Pediatr. 13:1527281. doi: 10.3389/fped.2025.1527281
Received: 13 November 2024; Accepted: 16 June 2025;
Published: 30 June 2025.
Edited by:
Emilia Maria Swietlik, University of Cambridge, United KingdomCopyright: © 2025 Deng, Xiong, Xu, Li and Cheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qinhong Li, MjAyNDA0NThAa21tdS5lZHUuY24=; Zugen Cheng, Y3pnMTg3MDI2OTM4NjJAb3V0bG9vay5jb20=
†These authors have contributed equally to this work
 Lili Deng
Lili Deng Jingxuan Xiong
Jingxuan Xiong Jiaoli Xu
Jiaoli Xu Qinhong Li
Qinhong Li Zugen Cheng
Zugen Cheng