- 1Department of Pediatric Surgery, Children’s Hospital of Guizhou, Affiliated Hospital of Zunyi Medical University, Zunyi, Guizhou, China
- 2Guizhou Children’s Hospital, Affiliated Hospital of Zunyi Medical University Zunyi, Zunyi, Guizhou, China
Background: Biallelic mutations in the neuroblastoma amplified sequence (NBAS) gene can cause recurrent acute liver failure (RALF) and multi-systemic disease.
Case presentation: Herein, we report a 3-year-old Chinese boy with RALF due to a novel heterozygote mutation c.3596G>A(p.C1199Y)/c.1028G>A(p.S343N) in the NBAS gene, identified by whole-exome sequencing. The missense mutation c.3596G>A(p.C1199Y) was inherited from his father, and c.1028G>A(p.S343N) was inherited from his mother. He had suffered six acute liver crises triggered by fever. He eventually underwent living donor liver transplantation (LDLT) at 44 months, with his father donating the left lateral lobe liver, and is now healthy with no recurrence of ALF.
Conclusion: We describe a novel pathogenic mutation in the NBAS gene of a patient with RALF and report that LDLT is a safe and efficient treatment for RALF caused by the NBAS gene mutation.
Introduction
Pediatric acute liver failure (ALF) is a life-threatening condition with different phenotypes according to age and underlying etiology, which includes infection, inborn errors of metabolism, drug poisoning, abnormal perfusion, and autoimmune diseases (1). Although etiology is an important factor in determining prognosis and treatment, the etiology of up to 50% of children with ALF remains unknown (1). In 2015, whole-exome sequencing (WES) identified neuroblastoma amplified sequence (NBAS) gene mutations as a novel etiology of recurrent ALF (RALF) (2). The NBAS gene is located on chromosome 2p24.3 and has 52 exons encoding a protein called NBAS, which contains 2371 amino acids and is involved in intracellular vesicular trafficking (3). The NBAS protein is broadly expressed in various organs, including the liver, heart, skeletal muscle, and leukocytes. Many studies reported that NBAS gene mutations were associated with infantile failure syndrome 2 (ILFS2), a fever-induced multi-systemic syndrome known as short stature with optic atrophy and Pelger-Huët anomaly (SOPH) syndrome, and immunodeficiency (4, 5). Here, we report the possible first case of RALF caused by a novel compound heterozygous mutation in the NBAS gene that underwent living donor liver transplantation (LDLT) in China. We have described in detail the clinical features, genetic variants, and longer-term outcomes and discussed the similarities and differences with cases reported in the literature.
Case presentation
A 3-year-old boy with a history of RALF was admitted to our hospital due to a high fever (39.0℃) for six hours. He had no symptoms of cough, runny nose, headache, dizziness, coma, shortness of breath, chest tightness, dyspnea, or incontinence. This was the sixth time that he had RALF, which began at 11 months and recurred at 14, 26, 31, and 39 months, respectively. Physical examination (PE) revealed mild malaise and drowsiness, normal intelligence and behavior, hemorrhage-free skin, slight tenderness in the upper abdomen, mild percussion in the liver area, and no visual or hearing impairment, liver enlargement, or rebound pain. A neurological examination showed normal muscle tension and strength and a normal Babinski sign. Laboratory examination of the liver function tests revealed the following results: alanine transaminase (ALT) 4,682 U/L, aspartate transaminase (AST) 8,352 U/L, total bilirubin (TB) 20.2 μmol/L, direct bilirubin (DB) 10.4 μmol/L, alkaline phosphatase 343 U/L, γ-glutamyl-transferase (γ-GGT) 35 U/L, and total bile acid 387.69 μmol/L. Coagulation parameters included a prothrombin time (PT) of 16.6 s, an international normalized ratio (INR) of 1.45, thrombin time (TT) of 17.4 s, and blood ammonia of 85 μmol/L. Test for Epstein–Barr virus, cytomegalovirus, and other hepatitis viruses were all negative. Autoimmune hepatitis-related autoantibodies were also negative. An ultrasound showed no abnormalities in the heart, liver, or spleen. Brain magnetic resonance imaging was normal.
Immunologic evaluation of the patient showed that serum levels of immunoglobulin (Ig) A (0.62 g/L) and IgG (6.3 g/L) were slightly decreased, and IgE (224 IU/ml) was slightly increased. The serum level of complement C3 was 0.70 g/L. The serum immune cell count showed the absolute values of total immune cells, B lymphocytes, and T helper cells as 4,108 μl, 732 μl, and 1,635 μl, respectively, which were considerably higher. However, the absolute value of natural killer (NK) cells was normal.
Genomic DNA extracted from peripheral blood cells of the patient was subjected to WES. The data showed that the patient had a novel compound heterozygote mutation of c.3596G>A (p.C1199Y) and c.1028G>A (p.S343N) in the NBAS gene. Parental genotyping confirmed the mutation c.3596G>A (p.C1199Y) in the father and the c.1028G>A (p.S343N) mutation in the mother. The c.3596G>A (p.C1199Y) mutation has been previously reported in many Chinese patients with ALF, which is characterized by a highly conserved amino acid residue that affects the structure and function of the NBAS protein and is categorized as pathogenic (6). However, the c.1028G>A (p.S343N) is a novel mutation that has not been reported in the referencing or ClinVar databases so far. According to the MutationTaster database, all the mutations were predicted to be pathogenic. The PolyPhen database predicted that each parental variant would have a deleterious effect on the NBAS protein function.
The patient and his father underwent percutaneous core needle liver biopsy. The patient's liver biopsy was performed after his sixth liver crisis, when he was three years and six months old. Transaminases were normal (ALT/AST = 36/34), until five days prior to biopsy. On routine hematoxylin-eosin (H&E) staining, the patient's liver architecture showed hepatocytes preserved with vacuolization, infiltration of lymphocytes and neutrophils, and mild fibrosis in the portal area. These findings were compared with his father's hepatocytes (Figure 1A). The NBAS immunohistochemistry staining in the liver showed that the level of NBAS was significantly lower in the patient than in the his father's liver (Figure 1B). Cytokeratin 7 staining showed positive results that highlighted ductular proliferations, indicating the sign of cholestasis (Figure 1C). The perilipin 2 immunohistochemistry staining showed that the patient's liver had mild microvesicular steatosis compared to his father's liver (Figure 1D).
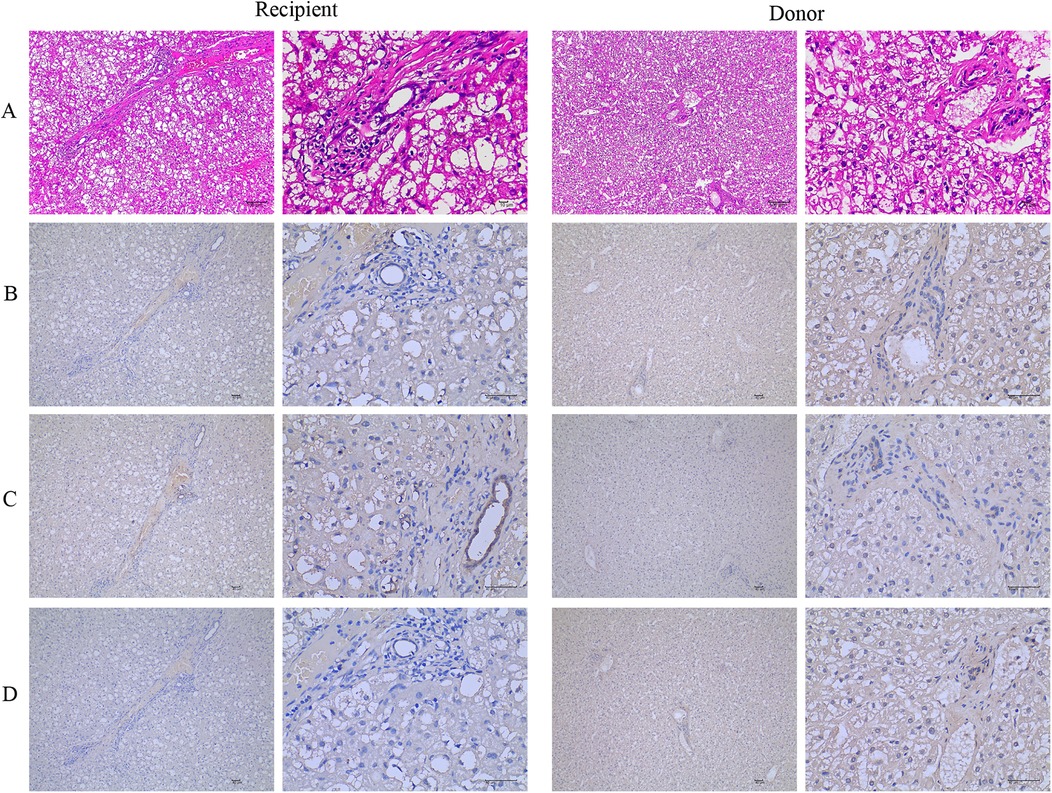
Figure 1. The NBAS mutation causes microvesicular steatosis and fibrosis. (A) Hematoxylin-eosin (H&E) stained liver biopsy shows hepatocytes with vacuolization, many lymphocytes and neutrophils infiltrated, and mild fibrosis in the portal area on routine compared with his father's hepatocytes. (B) The NBAS immunohistochemistry stained in the liver showed that the level of NBAS was significantly decreased in the patient compared with his father's hepatocytes. (C) Positive cytokeratin 7 stained ductular proliferations. (D) The perilipin 2 immunohistochemistry stain shows mild microvesicular steatosis. NBAS, neuroblastoma amplified sequence.
Results
After a thorough discussion with his family, the patient's father, aged 31 years with a height and body weight of 165.0 cm and 63.0 kg, respectively, wanted to donate a portion of his liver for LDLT. The donor's blood test results indicated the liver function, coagulopathy, and immunologic evaluation were normal. The donor's liver biopsy showed normal results.
Pretransplant liver volumetry as measured using IQQA-3D liver (EDDA Technology, lnc., Shanghai, China) showed that the donor's whole liver volume was 1,728 ml and the left lateral segment volume was 340 ml. The donor underwent left lateral segmentectomy for LDLT. The operation time was 2 h and 20 min, and the blood loss was 120 ml. No transfusions were administered during the operation. Finally, the donor's left lateral segment graft weight was 310 g. He was discharged from the hospital on postoperative day (POD) 8.
The recipient's height and body weight were 100.0 cm and 15.0 kg, respectively, and the standard liver volume was 480 ml. The recipient underwent LDLT using the donor's left lateral segment graft. First, the hepatic outflow of graft implantation was anastomosed to the open end of the recipient's hepatic vein, which was extended with an incision at the inferior vena cava, and then the portal vein was reconstructed. Under a microscope, the graft hepatic artery was anastomosed to the recipient's left hepatic artery. The biliary reconstruction was a duct-to-duct biliary anastomosis without T-tube insertion. The operative time was 6 h and 30 min, and the blood loss was 150 ml. There were no blood transfusions during the operation. The cold and warm ischemic times of the graft were 20 and 35 min, respectively. The graft to recipient weight ratio (GRWR) calculated by the weight of the patient was 2.1%.
The immunosuppressive regimen consisted of tacrolimus, mycophenolate mofetil, and methylprednisolone. The liver function measured immediately after operation showed the levels of serum transaminase were very high at POD 1 (ALT 1,269 U/L and AST 1,473 U/L), but gradually returned to normal at POD 19. The level of GGT rose to 112 U/L at POD 1 and returned to normal at POD 7. The coagulation parameters, including PT, APTT, and TT, were abnormal immediately after the operation but gradually returned to normal at POD 5. The abdominal drainage tube was pulled out at POD 6. However, the ascites appeared again at POD 10, and the ascites improved after another abdominal puncture, tube drainage, and infusion of human albumin for three weeks. While monitored using Doppler ultrasound and enhanced computed tomography scans, there were no strictures in the hepatic arteries, portal veins, or hepatic veins,. There were no episodes of rejection or surgical complications during recovery or follow-up. Currently, the patient's liver functions are normal when fever after LDLT.
Discussion and conclusions
The NBAS gene encodes a protein that includes two leucine zipper domains, namely a ribosomal protein S14 motif and a Sec39 domain (1). NBAS protein is thought to be a component of an endoplasmic reticulum (ER) tethering complex that interacts with the t-SNAREs p31, BNIPI, and STX18 at the ER and v-SNARE, which regulate the docking and fusion between target membrane and transport vesicles (7). Because of this, the mechanism of fever-dependent ALF in the NBAS mutation is related to altered golgi-ER retrograde transport and ER stress.
We presented a Chinese pediatric RALF case with two mutations, c.3596G>A (p.C1199Y) and c.1028G>A (p.S343N), in the NBAS gene. As far as it is understood, the c.3596G>A(p.C1199Y) mutation is located in the strictly conserved Sec39 domain of the NBAS gene, which can lead to structural changes of the whole Sec39 region or the entire protein (8). Sec39 was first found to be a component of the Dsl1p complex involved in the reverse transcriptional transport from the golgi apparatus to the ER in yeast as a protein. Further study identified that NBAS contains a Sec39 domain, which is a direct homologous sec39 protein (7). In humans, NBAS protein participates in retrograde transport from the golgi apparatus to the ER via the formation of the synaptic fusion protein 18 complex (7). In silico analysis predicated on the c.1028G>A(p.S343N) mutation had a deleterious effect on protein function, which was also reported in this study. However, further studies with a larger sample size are needed to confirm our observation.
Literature review on NBAS mutation with liver transplantation
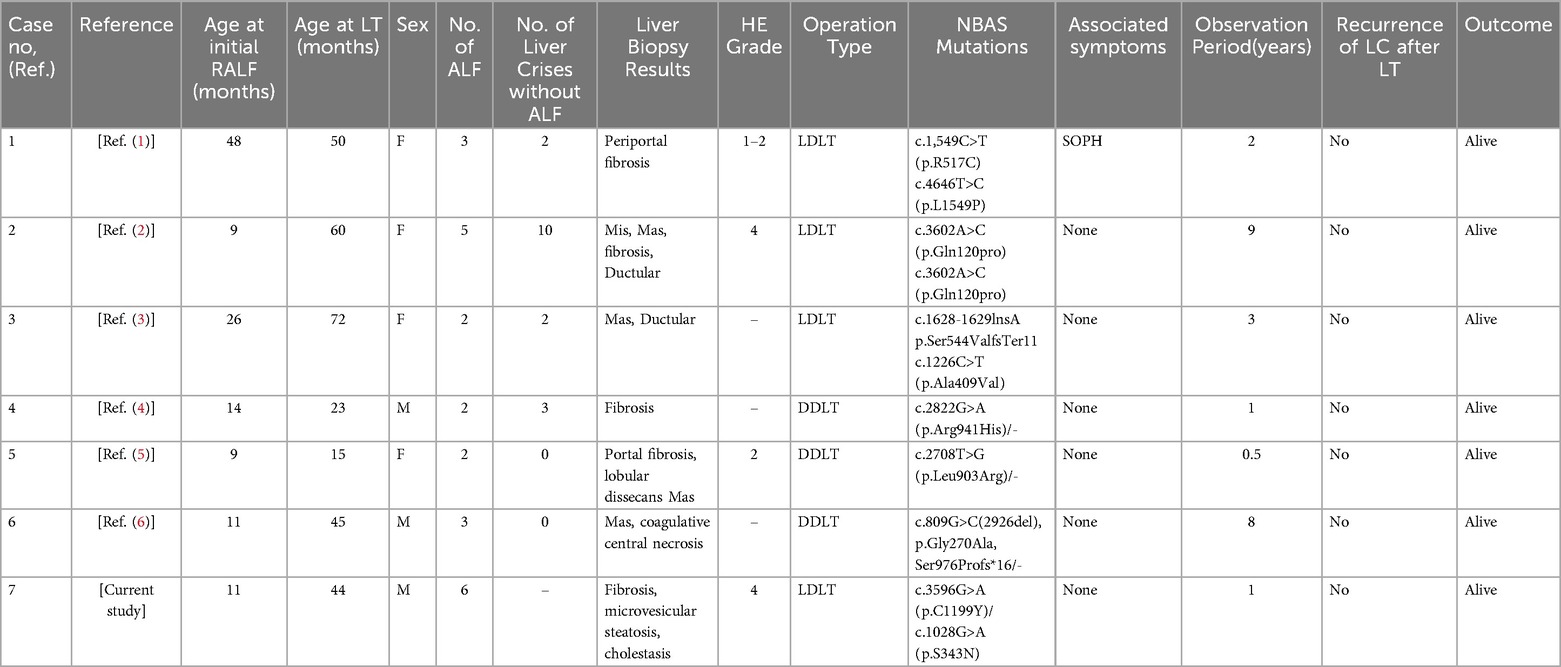
There are currently 101 patients with NBAS mutations described in the literature (including the one patient in our study), with an overall survival rate of 89% (90/101). To date, seven (6.9%, four females and three males; six in the previous literature and one patient from our study) out of 101 patients underwent LT. The median age at the initial RALF was 18.3 months (interquartile range, IQR 9–48), and the median age at the LT was 43.9 months (IQR 15–72). One patient was associated with SOPH syndrome. The average number of ALFs was 3.3 (IQR 2–6). Four patients underwent LDLT operations, and three underwent deceased donor LT (DDLT) operations. All the patients who underwent LT experienced no RALF and were alive at the time of the respective study (Table 1).
The livers of seven LT patients were analyzed histologically. The most common observations were fibrosis, macrovesicular steatosis, microvesicular steatosis, and coagulative central necrosis. Four of the LT patients had hepatic encephalopathy (HE), ranging from grade 1–4.
Clinical implications and recommendations
Although the severity of RALF episodes varies, most of the research reported cases were recovered with individualized supportive treatment and close follow-up. The overall survival rate was 89% (90/101) and only 6.9% (7/101) underwent LT. At present, there is a lack of detailed criteria for evaluating RALF LT indicators because of the low rates of LT, as well as their role and use in preventing further attacks and prognosis after LT. Calvo et al. reported that LT was required during one of the fulminant crises, but the final diagnosis was later confirmed by using genetic analysis (9). Geem et al. reported that LT should be considered when a patient develops a cerebral hemorrhage and it becomes difficult to manage with medical treatment (5). Many patients with RALF who present with extremely severe crises, polyvisceral failure, and severely impaired liver function suggest that patients require LT. In our series, the patient presented with six liver crises, and the most serious one was a coma in the intensive care unit (ICU) for 10 days due to HE at 31 months. At the age of three years, we evaluated the degree of liver damage by liver biopsy that showed liver with steatosis, hepatocyte swelling, and H&E staining showed mild periportal fibrosis. The immunohistochemistry showed cholestasis and microvesicular steatosis in the liver tissue. The results of the liver biopsy are consistent with the pathological findings of the NBAS mutation that have already been reported. Staufner et al. observed that patients with NBAS mutations who were followed up without LT were noted to have ongoing liver fibrosis (3). Suzuki et al. reported a 34-year-old patient with the NBAS mutations who had a cessation of acute attacks in early childhood but later developed symptoms of cirrhosis (10). Therefore, we recommend that the NBAS mutation with extremely recurrent liver crisis, HE, ongoing hepatic fibrosis, and polyvisceral failure, which is difficult to manage with medical treatment, be treated with LT as soon as possible.
In Europe, about 15%–20% of children with liver disease die every year while waiting for LT (11). At present, LDLT and DDLT are the most commonly used LT methods for the treatment of NBAS mutations (5). The emergence of LDLT has greatly eased the tension between supply and demand. Around 50% of the mutated genes come from one of their parents in RALF, caused by the NBAS mutation. Therefore, the gene phenotype and appropriate donor liver volume of their parents should be identified before adopting LDLT. A liver biopsy can be performed to determine whether there is fibrosis in the liver and the expression of NBAS protein, to reduce the occurrence of postoperative complications and avoid the recurrence of postoperative liver failure. In our series, the patient's donor was his father, who was evaluated by gene sequencing and liver biopsy before the LT operation. The results indicated that the donor was carrying the c.3596G>A (p.C1199Y) mutation, with normal levels of NBAS protein and without liver fibrosis. At the follow-up after LD-LT, the recipient's liver functions were normal but he had a fever. Many studies reported cases in which the liver remained healthy after transplantation, with no further episodes of ALF (5, 12, 13). With the continuous development of LT, more and more children with ALF may be treated.
In summary, we have reported that a novel compound heterozygote mutation in the NBAS gene caused RALF in a Chinese child and the resolution of RALF episodes after LDLT. The patient continues to be healthy during regular post-transplant follow-up. We also put forward the advice of LT for this kind of patient with our current experience of LT for the NBAS mutation.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Ethics Committee of Affiliated Hospital of Zunyi Medical University (20211028042), Guizhou, China. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
SH: Formal analysis, Investigation, Writing – original draft, Writing – review & editing. YZ: Data curation, Resources, Writing – review & editing. Zj: Data curation, Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing. XX: Conceptualization, Formal analysis, Software, Validation, Writing – review & editing. CT: Funding acquisition, Methodology, Resources, Validation, Writing – review & editing. YG: Formal analysis, Investigation, Resources, Validation, Visualization, Writing – review & editing. LH: Formal analysis, Investigation, Resources, Software, Validation, Writing – review & editing. QD: Formal analysis, Investigation, Software, Validation, Writing – review & editing. DZ: Data curation, Formal analysis, Methodology, Resources, Writing – review & editing. WZ: Conceptualization, Investigation, Validation, Visualization, Writing – review & editing. YL: Data curation, Project administration, Resources, Supervision, Writing – review & editing. ZZ: Data curation, Funding acquisition, Investigation, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the National Natural Science Foundation of China (No. 82060100), the Fund of the Department of Guizhou Science and Technology of China (No. ZK2021361), the Guizhou provincial basic research program (natural science) (No. MS-2025-406), and the Zunyi Science and Technology Program (Science and Technology Cooperation) (No. HZ-2023-319).
Acknowledgments
We are grateful to the patient and family for their kind cooperation in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Haack TB, Staufner C, Kopke MG, Straub BK, Kolker S, Thiel C, et al. Biallelic mutations in NBAS cause recurrent acute liver failure with onset in infancy. Am J Hum Genet. (2015) 97:163–9. doi: 10.1016/j.ajhg.2015.05.00926073778
2. Segarra NG, Ballhausen D, Crawford H, Perreau M, Campos-Xavier B, van Spaendonck-Zwarts K, et al. NBAS Mutations cause a multisystem disorder involving bone, connective tissue, liver, immune system, and retina. Am J Med Genet A. (2015) 167A:2902–12. doi: 10.1002/ajmg.a.3733826286438
3. Staufner C, Haack TB, Kopke MG, Straub BK, Kolker S, Thiel C, et al. Recurrent acute liver failure due to NBAS deficiency: phenotypic spectrum, disease mechanisms, and therapeutic concepts. J Inherit Metab Dis. (2016) 39:3–16. doi: 10.1007/s10545-015-9896-726541327
4. Dayan RR, Bignall Ii ONR, Johnson S, Flores F, Volovelsky O. Neuroblastoma amplified sequence gene mutations inducing acute kidney and liver injury in an adolescent female. Case Rep Nephrol Dial. (2020) 10:117–23. doi: 10.1159/00050878433173785
5. Geem D, Jiang W, Rytting HB, Chandrakasan S, Salem A, Stevens JP, et al. Resolution of recurrent pediatric acute liver failure with liver transplantation in a patient with NBAS mutation. Pediatr Transplant. (2021) 25:e14084. doi: 10.1111/petr.1408434288298
6. Li JQ, Qiu YL, Gong JY, Dou LM, Lu Y, Knisely AS, et al. Novel NBAS mutations and fever-related recurrent acute liver failure in Chinese children: a retrospective study. Bmc Gastroenterol. (2017) 17:77. doi: 10.1186/s12876-017-0636-328629372
7. Aoki T, Ichimura S, Itoh A, Kuramoto M, Shinkawa T, Isobe T, et al. Identification of the neuroblastoma-amplified gene product as a component of the syntaxin 18 complex implicated in Golgi-to-endoplasmic reticulum retrograde transport. Mol Biol Cell. (2009) 20:2639–49. doi: 10.1091/mbc.e08-11-110419369418
8. Li ZD, Abuduxikuer K, Zhang J, Yang Y, Qiu YL, Huang Y, et al. NBAS Disease: 14 new patients, a recurrent mutation, and genotype-phenotype correlation among 24 Chinese patients. Hepatol Res. (2020) 50:1306–15. doi: 10.1111/hepr.1355932812336
9. Calvo PL, Tandoi F, Haak TB, Brunati A, Pinon M, Olio DD, et al. NBAS Mutations cause acute liver failure: when Acetaminophen is not a culprit. Ital J Pediatr. (2017) 43:88. doi: 10.1186/s13052-017-0406-428946922
10. Suzuki S, Kokumai T, Furuya A, Nagamori T, Matsuo K, Ueda O, et al. A 34-year-old Japanese patient exhibiting NBAS deficiency with a novel mutation and extended phenotypic variation. Eur J Med Genet. (2020) 63:104039. doi: 10.1016/j.ejmg.2020.10403932805445
11. Kasahara M, Sakamoto S, Fukuda A. Pediatric living-donor liver transplantation. Semin Pediatr Surg. (2017) 26:224–32. doi: 10.1053/j.sempedsurg.2017.07.00828964478
12. Chavany J, Cano A, Roquelaure B, Bourgeois P, Boubnova J, Gaignard P, et al. Mutations in NBAS and SCYL1, genetic causes of recurrent liver failure in children: three case reports and a literature review. Arch Pediatr. (2020) 27:155–9. doi: 10.1016/j.arcped.2020.01.00332146038
Keywords: neuroblastoma amplified sequence, acute liver failure, living donor liver transplantation, pediatric liver, gene mutation
Citation: He S, Zhao Y, jin Z, Xia X, Tang C, Gong Y, Huang L, Du Q, Zhu D, Zhou W, Liu Y and Zheng Z (2025) Case Report: Living donor liver transplantation for the treatment of recurrent pediatric acute liver failure with neuroblastoma amplified sequence gene mutation: a literature review. Front. Pediatr. 13:1527759. doi: 10.3389/fped.2025.1527759
Received: 13 November 2024; Accepted: 11 April 2025;
Published: 30 April 2025.
Edited by:
Maria Immacolata Spagnuolo, Federico II University Hospital, ItalyReviewed by:
Juan A. Tovar, Department de Cirugía Pediátrica Hospital Universitario La Paz, SpainNikolaos Zavras, University General Hospital Attikon, Greece
Chao Sun, Tianjin First Central Hospital, China
Copyright: © 2025 He, Zhao, jin, Xia, Tang, Gong, Huang, Du, Zhu, Zhou, Liu and Zheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zebing Zheng, emViaW5nMTk4OEBzaW5hLmNvbQ==
 Shengying He
Shengying He Yi Zhao1,2
Yi Zhao1,2 Zhu jin
Zhu jin Xinrong Xia
Xinrong Xia Chengyan Tang
Chengyan Tang Yuan Gong
Yuan Gong Lu Huang
Lu Huang Zebing Zheng
Zebing Zheng